Abstract
The voltage-gated sodium channel is critical for cardiomyocyte function and consists of a protein complex comprising a pore-forming α subunit and two associated β subunits. It has been shown previously that the associated β2 subunits promote cell surface expression of the α subunit. The major α isoform in the adult human heart is NaV1.5, and germline mutations in the NaV1.5-encoding gene, sodium voltage-gated channel α subunit 5 (SCN5A), often cause inherited arrhythmias. Here, we investigated the mechanisms that regulate β2 trafficking and how they may determine proper NaV1.5 cell surface localization. Using heterologous expression in polarized Madin–Darby canine kidney cells, we show that β2 is N-glycosylated in vivo and in vitro at residues 42, 66, and 74, becoming sialylated only at Asn-42. We found that fully nonglycosylated β2 was mostly retained in the endoplasmic reticulum, indicating that N-linked glycosylation is required for efficient β2 trafficking to the apical plasma membrane. The nonglycosylated variant reached the cell surface by bypassing the Golgi compartment at a rate of only approximately one-third of that of WT β2. YFP-tagged, nonglycosylated β2 displayed mobility kinetics in the plane of the membrane similar to that of WT β2. However, it was defective in promoting surface localization of NaV1.5. Interestingly, β2 with a single intact glycosylation site was as effective as the WT in promoting NaV1.5 surface localization. In conclusion, our results indicate that N-linked glycosylation of β2 is required for surface localization of NaV1.5, a property that is often defective in inherited cardiac arrhythmias.
Keywords: sodium channel, N-linked glycosylation, protein trafficking (Golgi), protein sorting, protein targeting, cardiac arrhythmia, NaV1.5, SCN2B, voltage-gated sodium channel
Introduction
Genetic alterations leading to channelopathies are frequently found in the voltage-gated sodium (NaV)3 channel (1). A well-known ion channel disorder causing ventricular fibrillation is Brugada syndrome (BrS). In this regard, ∼20% of BrS cases are caused by mutations in SCN5A, the gene encoding NaV1.5 (i.e. the pore-forming α subunit of the major cardiac NaV channel) (2). The NaV channel allows fast influx of sodium ions, thus generating the rapid upward deflection of the action potential. Therefore, it plays a central role in myocardial cell excitability. The abnormal electrocardiogram observed in BrS is due to NaV channel loss-of-function, often caused by defective NaV1.5 trafficking and localization to the cell surface (3).
NaV1.5 is localized at the sarcolemma (i.e. the cardiomyocytes' plasma membrane). The differential localization of NaV channel pools at sarcolemma subregions is important for conduction velocity and cardiac impulse propagation (4). Much evidence shows that localization and function of the α subunit are regulated by NaV channel auxiliary β subunits and other associated proteins (5). Analysis of NaV1.5 trafficking can be envisaged from at least three standpoints: first, to address how NaV1.5 is targeted to the plasma membrane; second, how Nav1.5 is retained at certain surface domains or subregions; and third, how NaV1.5 endocytosis and turnover are regulated. In this work, we mainly focused on the first two aspects, addressing the contribution of one of the associated β subunits. Five β subunits are known in mammals: β1, β2, β3, β4, and β1B (the latter is an alternative splice variant of β1) (6). Interacting with NaV1.5 through their extracellular region (7) or even with their transmembrane domain (TMD) (8), β subunits are thought to assist α for effective transport to the plasma membrane (3). In fact, various mutations in β subunits have been found associated with BrS, thereby causing loss-of-function of the NaV channel (9–12).
We focused here on β2, whose case is of particular interest, because it is believed to influence NaV1.5 localization in post-Golgi compartments just before or during its targeting to the cell surface (13, 14). In fact, we previously described the first BrS-associated mutation in SCN2B, the gene encoding β2. Such a mutation, D211G (substitution of Asp for Gly), causes a 40% decrease in sodium current density due to reduced cell surface levels of NaV1.5 (10). Moreover, we have shown that exogenously expressed β2 is transported in a polarized fashion, namely to the apical domain in polarized Madin–Darby canine kidney (MDCK) cells. Both in MDCK cells and in cardiomyocyte-derived HL-1 cells, surface localization of NaV1.5 was promoted by WT but not D211G β2 (15).
It is not known how β2 is targeted to the cell surface and, more specifically, how it preferentially reaches the apical surface in MDCK cells. Indeed, it is the subject of intense study to understand how apical targeting signals are recognized. Recognition can take place by association of the protein's TMD to lipid rafts. It can also occur via N- or O-linked glycosylation of the luminal domain and consequent interaction with sugar-binding galectins. In addition, Ras-related Rab GTPases, microtubule motors, and the actin cytoskeleton have been implicated (16, 17).
β2 is a type I transmembrane protein with an extracellular, immunoglobulin-like loop, likely performing a cell adhesion function (5), a single TMD, and a short cytoplasmic tail (18). The extracellular loop, maintained by an intramolecular disulfide bond between Cys-50 and Cys-127 (7), has three potential N-glycosylation sites (i.e. Asn-42, Asn-66, and Asn-74) (19). Within this region, a third cysteine, Cys-55, establishes a disulfide bond with the α subunit (7). In addition, the short C-terminal intracellular domain has two potential phosphorylation sites (i.e. Ser-192 and Thr-204) (20); see UniProtKB accession number O60939.
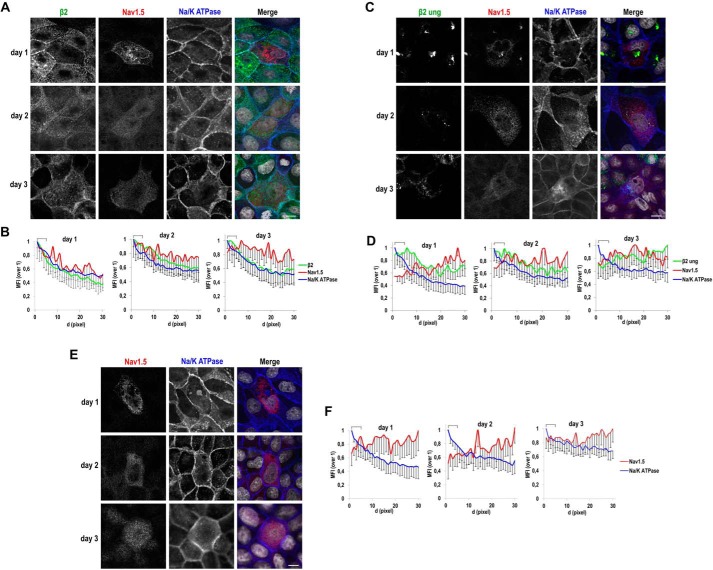
Glycosylation, and more specifically sialylation, appears important for regulating channel biophysical properties. Thus, changes in sodium current density at the plasma membrane have been related with the sialic acid content of β2 (19). For the β1 subunit, which interacts noncovalently with α, it has been proposed that its glycosylation level, including its sialylation, may be differentially regulated in a tissue-specific and developmentally specific manner. Hence, different α/β1 subunit combinations would be differentially sialylated in various tissues throughout development, thereby contributing, to a different degree, to NaV channel gating. Such differences could even be linked to pathological alterations (21). Despite this evidence, to our knowledge, the contribution of β2 glycosylation on its own trafficking and, importantly, how such posttranslational modification may influence trafficking of the α subunit have not been addressed in detail. Here, we found that N-linked glycosylation of β2 is required for its efficient trafficking to the plasma membrane. Importantly, unglycosylated β2 was defective in promoting surface localization of NaV1.5.
Results
β2 is N-glycosylated and sialylated in vitro and in vivo
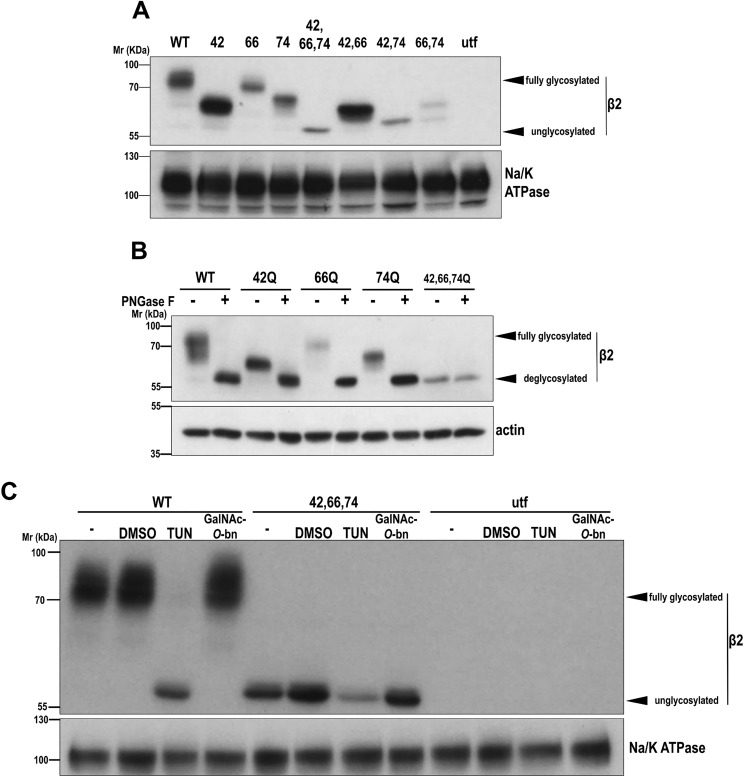
We previously showed that exogenously expressed β2 localizes almost exclusively at the apical domain in polarized MDCK cells (15). Here, we addressed how β2 is preferentially targeted to this surface domain. Both N- and O-linked glycosylation are common apical sorting signals (16, 17). The extracellular domain of β2 has three predicted N-glycosylation sites (i.e. Asn-42, Asn-66, and Asn-74) (18) that follow the Asn-Xaa-Ser/Thr (NX(S/T)) motif, X being any amino acid except Pro (22). We thus systematically mutated these to Gln, which is never glycosylated due to its different conformation, and transiently expressed YFP-tagged β2 in MDCK cells. Consequently, all mutants showed increased electrophoretic mobility, with N42Q displaying the highest increase, followed by N74Q and N66Q, the latter with a minor, albeit measurable, shift. This variable mobility may be due to different degrees of glycosylation on each site and/or changes in glycoprotein size or charge due to the sugar chain; the triple (fully) unglycosylated mutant showed complete reduction in apparent mass, no longer appearing as a smear, with double mutants migrating in between (Fig. 1A). To verify that β2 variants were indeed N-glycosylated, cells were lysed and treated with peptide:N-glycosidase F (PNGase F), which cleaves off the bond between Asn and the first GlcNAc moiety, liberating the entire N-glycan (23). Upon treatment, WT and mutants displayed identical mobility to that of fully unglycosylated β2 (Fig. 1B). To confirm that β2 glycosylation takes place in vivo, cells were treated with tunicamycin (TUN) or with benzyl-2-acetamido-2-deoxy-α-d-galactopyranoside (GalNAc-O-bn) to block N- or O-glycosylation, respectively. As a result, β2 WT became fully deglycosylated only with TUN, remaining unaffected with GalNAc-O-bn (Fig. 1C). These data show that β2 is N-glycosylated in vitro and in vivo but does not undergo O-glycosylation.
Figure 1.
β2 is N-glycosylated at positions Asn-42, Asn-66, and Asn-74. MDCK cells were transiently transfected with the SCN2B-yfp vector to express WT or partially or fully unglycosylated β2 or left untransfected (utf). A and B, cells were grown for 2 days in wells. Representative Western blots are shown with the same amount of protein lysate loaded into each lane. A, all glycosylation-defective mutants display increased electrophoretic mobility, with N42Q as the single mutant with the greatest change, and triple (fully) unglycosylated β2 showing complete shift. B, denatured protein from cell lysates was treated overnight at 37 °C with PNGase F to cleave off all N-glycans. C, cells were treated with TUN or with GalNAc-O-bn, to block N- or O-glycosylation, respectively, and grown for 1 day in wells; β2 WT remains unglycosylated only with TUN. DMSO, cells with the equivalent volume of solvent added; −, untreated cells. Blots for Na/K-ATPase or actin are included as loading controls. Molecular mass markers are in kDa.
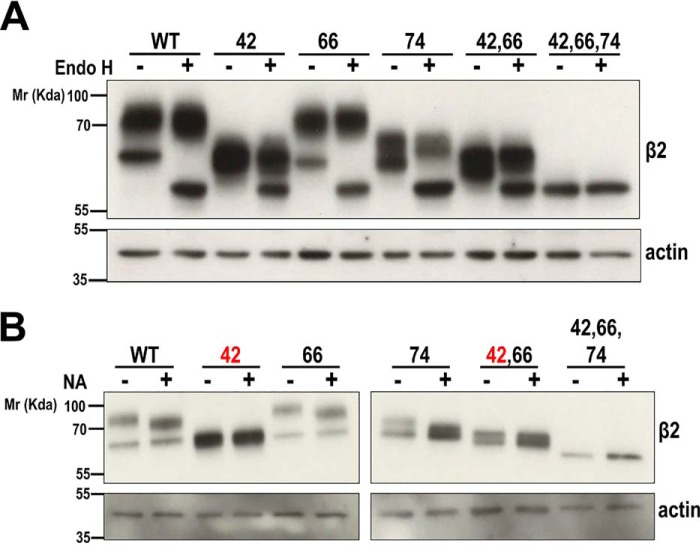
We next investigated the complexity of β2 N-glycosylation with endoglycosidase H (Endo H), which cleaves on high-mannose and hybrid, but not complex, glycans, typically generated at late stages of Golgi glycosylation (23). When cells were analyzed early (1 day) after transfection, a faster-migrating band, also visible in single and double mutants, suggested the presence of immature β2-YFP still unprocessed in the endoplasmic reticulum (ER). Endo H treatment effectively increased the mobility of this band, which then coincided with unglycosylated β2, without affecting mature β2 (Fig. 2A and Fig. S1A). Thus, at that moment, a considerable fraction of β2 had not yet undergone processing by Golgi α-mannosidase II (23).
Figure 2.
β2 undergoes complex N-glycosylation and is sialylated at Asn-42. MDCK cells were transiently transfected with the SCN2B-yfp vector to express WT or partially or fully unglycosylated β2 and grown for 1 day in wells. Representative Western blots are shown with the same amount of protein lysate loaded into each lane. Note that immature (unprocessed) β2 is clearly discernible from the slowly migrating mature form (compare with Fig. 1). A, denatured protein from cell lysates was treated overnight at 37 °C with Endo H to cleave off immature N-glycans (faster-migrating band) in β2. B, lysates were treated overnight at 37 °C with NA to cleave off all terminal sialic acids. The upper band displays a slight increase in mobility in WT and single and double mutants not including the N42Q mutation (red type). Blots for actin are included as loading controls. Molecular mass markers are in kDa. The dividing line in B separates different blots (taken from the same exposure) conveniently put together for clear display.
To further assess N-glycans complexity, cells were treated with broad-specificity sialidase (i.e. α2–3,6,8-neuraminidase (NA)), which cleaves terminal sialic acids from both N- and O-glycans (23). In consequence, the slower-migrating band displayed a noticeable increase in mobility in β2 WT and N66Q and N74Q mutants, but interestingly not in β2 N42Q. Similarly, no effect was seen in double mutants, including the N42Q mutation, but it was clear in β2 N66Q/N74Q (Fig. 2B and Fig. S1B). Because all variants with the N42Q mutation were insensitive to NA, we conclude that β2 is sialylated uniquely at Asn-42.
N-Glycosylation is required for efficient cell surface localization of β2
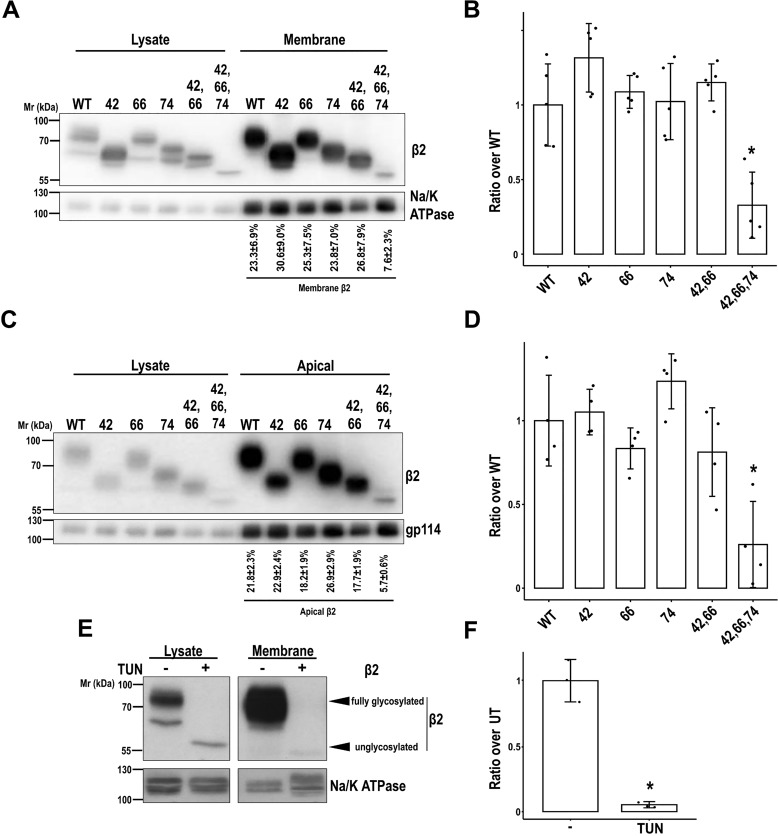
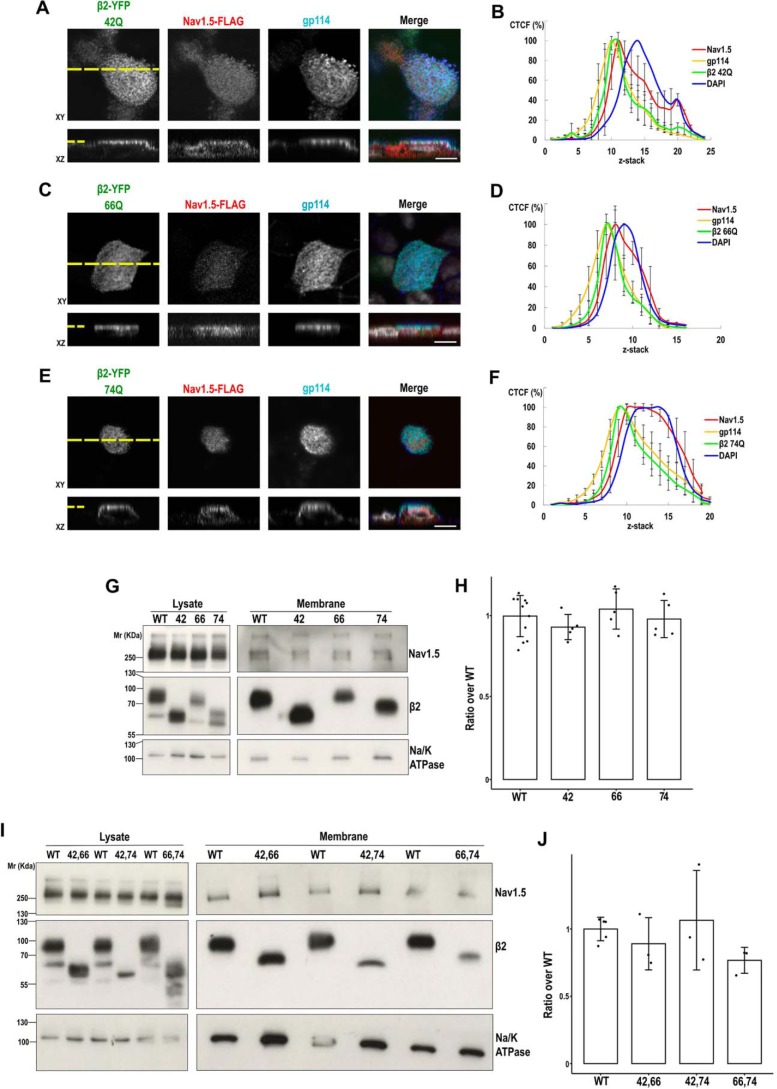
Because glycosylation is a well-known mechanism for many proteins to efficiently reach the plasma membrane (16), we tested by protein biotinylation whether partially or fully unglycosylated β2 properly localizes to the cell surface. Uniquely the triple mutant displayed a substantial defect, and band quantitation showed that it reaches the surface at a rate of approximately one-third compared with the WT (Fig. 3, A and B). Moreover, the portion of unglycosylated mutant at the surface was around 8% of total cellular β2 protein, contrasting with 25–30% by the WT and single or double mutants. A comparable defect was found in fully polarized cells. In these, the rate by which unglycosylated β2 reached the apical surface was also approximately one-third when compared with β2 WT or the partial mutants (Fig. 3, C and D). To note, all variants of β2 remained nearly undetected at the basolateral surface or at least clearly de-enriched compared with lysates (Fig. S2).
Figure 3.
N-Glycosylation is required for efficient cell surface localization of β2. MDCK cells were transiently transfected with the SCN2B-yfp vector to express WT or partially or fully unglycosylated β2. Cells were grown for 2 days in wells (A and B) or polarized in Transwells (C and D) and surface-biotinylated at 4 °C. Representative Western blots (A and C) and band quantitation (B and D) show that levels of fully unglycosylated β2 were reduced compared with the WT and partially glycosylated mutants in biotin-NeutrAvidin pulldowns, both in subconfluent and in polarized cells. One-way ANOVA with Tukey's HSD post hoc test highlighted these differences (B, *, p < 0.001; D, *, p < 0.05). A and C, values below blots show the percentage of each β2 variant at the surface over total cellular β2 protein. E and F, cells were treated with TUN to block N-glycosylation, grown for 1 day in wells, and surface-biotinylated at 4 °C (UT, untreated cells). Representative Western blots (E) and band quantitation (F) show the absence of unglycosylated β2 in pulldowns (two-tailed Student's t test shows significant differences; *, p = 0.009). The same amount of protein was used to process each lysate (∼130 μg), and the corresponding portion (nine-tenths) was subjected to overnight pulldown. Na/K-ATPase (A and E) or gp114 (C) were blotted as surface markers to correct for quantitations in pulldowns. All data are mean ± S.D. (error bars) (n ≥ 3). Molecular mass markers are in kDa. The dividing line in E separates different parts of the same blot (taken from the same exposure) conveniently put together for clear display.
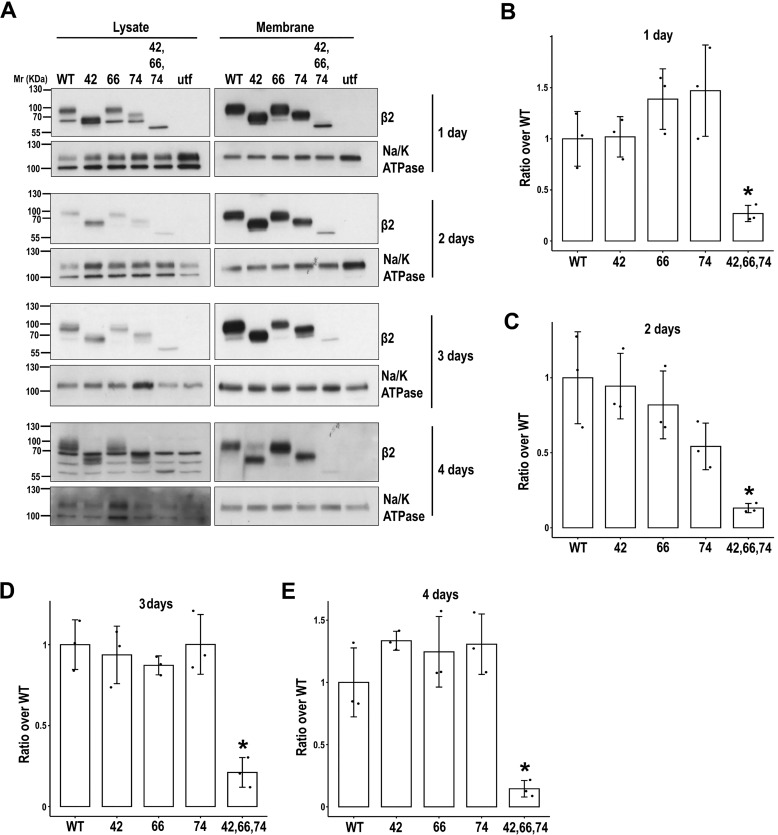
To determine the magnitude of glycosylation loss in trafficking deficiency of β2 overtime, we analyzed its surface levels along various days from transfection. Indeed, the defect was maintained throughout time. Therefore, these data show that total lack of glycosylation significantly prevents β2 localization to the surface (Fig. 4). Whereas a single glycosylation site appeared sufficient for proper surface localization of β2, TUN treatment further confirmed that unglycosylated β2 virtually does not reach the plasma membrane in vivo (Fig. 3, E and F).
Figure 4.
Defect over time in β2 surface localization due to lack of N-glycosylation. MDCK cells were transiently transfected with the SCN2B-yfp vector to express WT or partially or fully unglycosylated β2 (UNG). Cells were grown in wells for the indicated number of days and surface-biotinylated at 4 °C. Representative Western blots (A) and band quantitation (B–E) show that levels of fully unglycosylated β2 were reduced compared with the WT and partially glycosylated mutants in biotin-NeutrAvidin pulldowns (Membrane). One-way ANOVA with Tukey's HSD post hoc test highlighted these differences, with a few exceptions (B, all p < 0.05, except 42 versus UNG (p = 0.054); C, all p < 0.05 except 42 versus UNG (p = 0.052) and 74 versus UNG (p = 0.189); D, p < 0.002; E, p < 0.005). The same amount of protein was used to process each lysate (∼130 μg), and the corresponding portion (nine-tenths) was subjected to overnight pulldown. Na/K-ATPase was blotted as surface marker to correct for quantitations in pulldowns. Data are mean ± S.D. (error bars) (n ≥ 3). Molecular mass markers are in kDa.
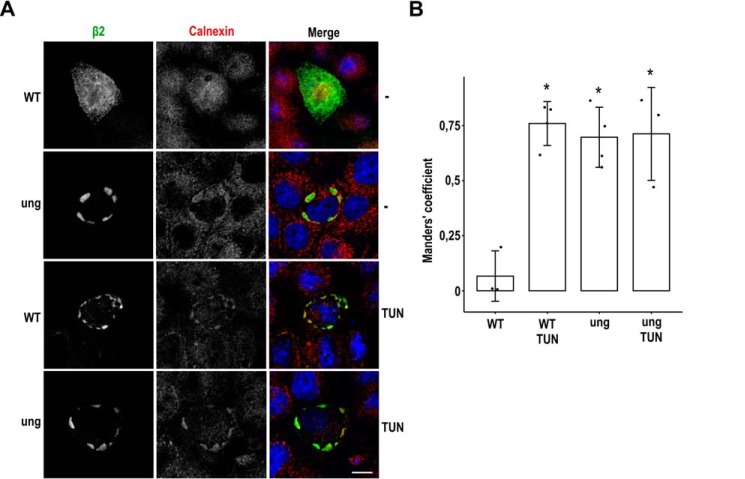
We next determined in what subcellular compartment trafficking of unglycosylated β2 becomes interrupted. To this end, cells were immunostained for detection of various subcellular markers of the endocytic and exocytic pathways. These included the early endosome marker EEA1, the late endosomal lysobisphosphatidic acid, the lysosome-associated membrane protein LAMP2, the cis-Golgi marker GM130 (Golgi matrix protein of 130 kDa), and the trans-Golgi network (TGN) marker TGN46. None of them overlapped markedly with unglycosylated β2 (Fig. S3). However, an apparent overlap found with the ER chaperone calnexin indicates that a large portion of the triple mutant is retained in the ER membranes. Moreover, its pattern was highly comparable with that of β2 WT in cells treated with TUN (Fig. 5A). Indeed, the Manders' coefficient was around 0.7 in cells expressing unglycosylated β2 and in TUN-treated cells, in contrast with negligible overlap in untreated cells expressing β2 WT (Fig. 5B). In the latter, β2 outlined the cell end, also displaying an obvious dotted pattern, which likely corresponds to β2 getting positioned at the developing apical surface (i.e. its final location in polarized cells) (15). Because of its preponderant surface localization, no manifested overlap was observed between β2 WT and any of the markers tested (Fig. S3). Altogether, these data indicate that unglycosylated β2 becomes retained in the ER.
Figure 5.
Unglycosylated β2 is retained in the ER. MDCK cells were transiently transfected with the SCN2B-yfp vector to express WT or fully unglycosylated β2 (ung) and grown for 1 day in wells. Cells were treated with TUN 2 h after transfection or left untreated (−), fixed, and immunostained with a rabbit polyclonal antibody against calnexin (red). A, representative xy sections show that unglycosylated β2 (green) is intracellular and overlaps with calnexin, as does the WT in TUN-treated cells. This contrasts with the localization of β2 WT at the cell end in untreated cells, also displaying a scattered pattern. To focus more accurately where β2 is found in each condition, sections were taken right above the nucleus (WT −) or at the nuclear level (for the rest). Nuclear staining by DAPI is in blue. Scale bar, 10 μm. B, line chart showing Manders' coefficients calculated along the z axis and indicating the fraction of β2 overlapping to compartments labeled with calnexin. The high overlap in TUN-treated cells and in those expressing unglycosylated β2 contrasts with negligible overlap in untreated cells expressing β2 WT. One-way ANOVA with Tukey's HSD post hoc test revealed differences among means (*, p < 0.0005). Data are mean ± S.D. (error bars) (n ≥ 3).
Unglycosylated β2 can reach the cell surface by bypassing the Golgi compartment
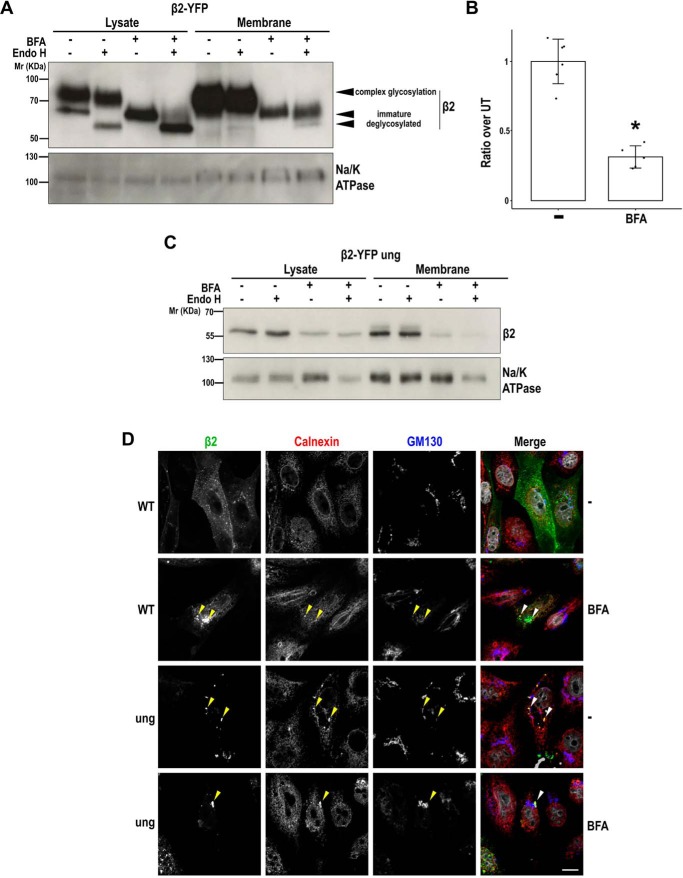
Although unglycosylated β2 was seen retained in the ER, a small fraction reached the cell surface and, in polarized MDCK cells, even properly localized to the apical surface. We therefore tested whether blocking the ER-to-Golgi pathway with brefeldin A (BFA) would analogously prevent the arrival of immature β2 to the plasma membrane. Here, transfected cells were treated overnight with BFA, and both lysates and pulldowns were then deglycosylated with Endo H. As expected, mature, fully glycosylated β2 WT was not visible in cells treated with BFA, confirming lack of processing by Golgi enzymes (Fig. 6A). Upon Endo H treatment, the faster-migrating band (immature β2) increased its mobility, coinciding with unglycosylated β2 (Fig. 6A; see Fig. 2A for comparison). Remarkably, this immature form was the only constituent of pulldowns from BFA-treated cells, indicating that it can reach the plasma membrane by bypassing Golgi glycosylation. Subsequently, pulldowns were also treated with Endo H, which again shifted a small fraction of immature β2 to the position of (faster-moving) unglycosylated β2 (Fig. 6A).
Figure 6.
BFA prevents complex glycosylation of β2, a fraction of which can reach the cell surface. MDCK cells were transiently transfected with the SCN2B-yfp vector to express WT or fully unglycosylated β2 (ung) and then treated 2 h later with BFA (+) or left untreated (−) and grown overnight in wells. A and C, cells were surface-biotinylated at 4 °C. The same amount of protein was used to process each lysate (∼100 μg), and the corresponding portion (nine-tenths) was subjected to overnight pulldown. Denatured protein from cell lysates and pulldowns was treated overnight at 37 °C with Endo H to cleave off immature N-glycans or left untreated (−). Representative Western blots show that the (lower) faster-migrating band of β2 WT is the only one visible in cells treated with BFA and increases its mobility with the Endo H treatment; this band coincides with unglycosylated β2 (C; compare with Fig. 2A). Note that Endo H digestion in pulldowns is only partial, either due to saturation of the enzyme or to suboptimal conditions for enzyme action. Blots for Na/K-ATPase are included as loading controls. Molecular mass markers are in kDa. B, band quantitation shows reduced levels of immature β2 in biotin-NeutrAvidin pulldowns (Membrane) of BFA-treated cells. Two-tailed Student's t test shows significant difference (*, p < 0.05). Data are mean ± S.D. (error bars) (n ≥ 3). D, cells were fixed and immunostained with a rabbit polyclonal antibody against calnexin (red) and a mouse monoclonal to GM130 (blue). Representative xy sections show that, in BFA-treated cells, β2 WT displays an intracellular accumulation comparable with mutated β2 (green), grossly overlapping with calnexin in enlarged structures (arrowheads). This contrasts with its apparent localization in the plasma membrane in untreated cells, displaying also a scattered pattern that does not overlap with calnexin (sections were taken at the cell level where β2 is mainly found in each case). Nuclear staining by DAPI is shown in gray. Scale bar, 10 μm.
Albeit to a lesser extent, β2 WT was still detected in pulldowns of BFA-treated cells. However, quantitation of Western blots indicated that, similarly to unglycosylated β2, the ratio at which immature β2 can reach the cell surface in BFA-treated cells is only approximately one-third as compared with untreated cells (Fig. 6B). Proper validation that the drug produced β2 accumulation in the ER was seen by its considerable overlap with calnexin (Fig. 6D), whose pattern clearly differed from that of the cis-Golgi marker GM130, which became more tubulated and disperse in the presence of BFA (see also Fig. S4A). Thus, a large portion of β2 WT now appeared accumulated in enlarged calnexin-positive structures, often undistinguishable from buildup of unglycosylated β2 (arrowheads in Fig. 6D). Moreover, in untreated cells expressing low levels of unglycosylated β2, the mutated protein largely overlapped with calnexin, further confirming its retention in the ER (Fig. S4B).
Dynamics of β2 in the plane of the membrane is not influenced by N-glycosylation
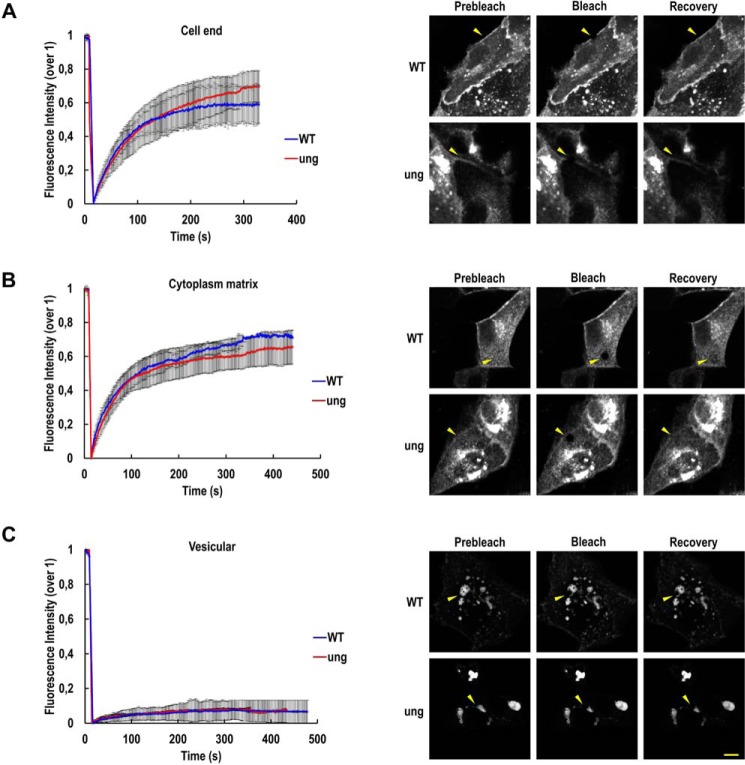
The data above provide strong evidence that N-glycosylation is required for β2 to reach the plasma membrane. It is plausible to contemplate that N-glycans ensure correct folding and oligomerization of β2 to exit the ER properly. Glycosylation may favor β2 clustering at the TGN, which in turn may increase affinity to lipid rafts, for subsequent inclusion into apical transport carriers (24). Thus, we hypothesized that β2 dynamics in the plane of the membrane may be influenced by its glycosylation, which could have important functional implications. Movement of fluorescently tagged β2 was monitored by fluorescence recovery after photobleaching (FRAP). The mobile fraction (MF) (i.e. the portion of molecules undergoing diffusion) differed depending on the cell's location where the measurement was taken. Hence, we chose three representative regions for analysis (i.e. at the cell end, mostly representing cell surface β2 (Fig. 7A); within the cytoplasm matrix, likely including the dispersed ER network as well as clusters of β2 already at the surface (Fig. 7B); and in vesicular structures of unknown nature, which may represent large perinuclear ER elements with β2 in transit to the cell surface (Fig. 7C)). At the cell end, ∼60% of β2 WT molecules underwent diffusion 4–5 min after bleaching. MF at the cell end was slightly increased for unglycosylated β2, yet differences were not significant (Fig. 7A and Table S1). Similar data were found for cytoplasmic β2 (Fig. 7B). However, when β2 found in large vesicles was bleached, mean fluorescence never recovered above 10% of the total initial signal (Fig. 7C). These differences in the portion of freely diffusible molecules suggest that the molecular environment of β2 seemingly accumulated in these large vesicles differed from that present in the other areas analyzed.
Figure 7.
Dynamics of β2 is not influenced by N-glycosylation. MDCK cells were transiently transfected with the SCN2B-yfp vector to express WT or fully unglycosylated (ung) β2 and grown for 2 days on glass supports. Mobility of β2-YFP on three different cellular locations was monitored by FRAP with a confocal microscope. Line charts of fluorescence intensity (mean ± S.D. (error bars)) of at least three representative experiments show comparable mobile fraction between β2 WT (blue line) and mutant (red line) at the three regions analyzed (i.e. the cell end (A), the cytoplasm matrix (B), and in large vesicular structures (C)). For each, images on the right show a representative cell prebleached, just after bleaching, and after fluorescence recovery (arrowheads mark the bleached area); see the complete FRAP data in Table S1. Scale bar, 10 μm.
The FRAP data also showed that the mobility rate of WT and unglycosylated β2 is comparable, with a slight tendency of the mutant to move slower. Regardless of the location, half-time of recovery (τ½, the time point of half-fluorescence recovery) was ∼1 min in both β2 variants (Table S1). Consequently, a diffusion coefficient (D) of ∼0.02 μm2/s was found in general, although β2 in large vesicles moved even slower (i.e. at about one-fourth of this speed) (see “Experimental procedures”).
Unglycosylated β2 is defective in promoting surface localization of NaV1.5
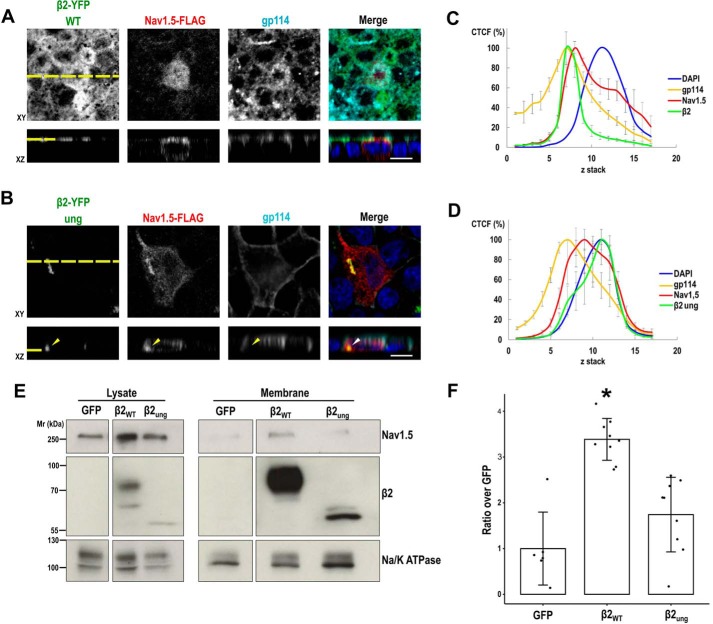
The comparable mobility of both WT and unglycosylated mutant β2 indicates that the sugar moiety does not influence β2 dynamics within the membrane bilayer. It is accepted that β subunits function in concert with the α subunit to promote channel trafficking to the plasma membrane and, in some cases, to modulate its biophysical properties (5). In this regard, it has been shown that the major function of β2 in vivo is to chaperone α subunits to the plasma membrane, both in the heart ventricle (25) and in neurons (26). Therefore, we tested whether unglycosylated β2 was defective in promoting surface localization of NaV1.5. As expected (15), a fraction of NaV1.5 colocalized with β2 and the apical marker gp114 (Fig. 8A). Although NaV1.5 distributed throughout the cell, calculation of the corrected total cell fluorescence (CTCF) along z-stacks showed its maximum fluorescence peak nearly overlapping with those of gp114 and β2, corresponding to the apical plasma membrane (Fig. 8C). In the presence of unglycosylated β2, NaV1.5 distribution was more widespread, mostly abounding at the nuclear level and right above the nucleus (Fig. 8, B and D). Moreover, a large portion of NaV1.5 colocalized with accumulations of mutated β2 (arrowhead in Fig. 8B).
Figure 8.
Surface localization of NaV1.5 is reduced with unglycosylated β2. A and B, MDCK cells stably expressing WT or fully unglycosylated (ung) β2-YFP were transiently transfected with the vector SCN5A-FLAG and grown polarized in Transwells. Cells were fixed and immunostained with a rabbit polyclonal antibody against NaV1.5 (red) and with a mouse mAb to gp114 (cyan). Images were obtained by confocal microscopy. In merged images, the YFP-emitted fluorescence is shown in green and DAPI is in blue. Representative xy sections taken at the apical (A) or nuclear (B) levels (sections taken at the cell level where NaV1.5 is mainly found in each case) and corresponding z axis reconstruction (reciprocal xz and xy sections marked by a yellow dashed line) show improved apical localization of NaV1.5 with β2 WT (A), which remains mostly intracellular in the presence of unglycosylated β2 (B); note the intracellular NaV1.5 accumulation with mutated β2 (arrowhead). Scale bars, 10 μm. C and D, line charts displaying the CTCF (mean percentage ± S.D. (error bars)) along an apical-to-basal z-stack (section 1: most apical; 0.5-μm optical slice thickness) show the NaV1.5 curve peak close to those of apical gp114 and β2 WT (C). In contrast, NaV1.5 is displaced toward the nuclear section with mutated β2, which overlays with DAPI (D), included as reference for the nuclear level (≥6 cells were analyzed per condition). E, MDCK cells stably expressing NaV1.5-YFP were transiently cotransfected with the SCN2B-yfp vector to express β2, WT or fully unglycosylated (ung), plus additional SCN5A-FLAG vector to ensure extensive NaV1.5 overexpression, and grown overnight in wells; the pEGFP-N1 vector was used as a control. Cells were surface-biotinylated at 4 °C. The same amount of protein was used to process each lysate (∼600 μg), 97% of which was subjected to overnight NeutrAvidin pulldown. Representative Western blots and band quantitation (F) show reduced levels of NaV1.5 in biotin-NeutrAvidin pulldowns (Membrane) in the presence of unglycosylated β2 or without β2 (GFP), when comparing with the WT. One-way ANOVA with Tukey's HSD post hoc test showed significant differences (*, p < 0.002). The percentage of NaV1.5 at the cell surface over total cellular NaV1.5 protein varied from 1.42 ± 0.98 in the WT to 0.73 ± 0.50% with unglycosylated β2. Data are mean ± S.D. (n ≥ 6). Na/K-ATPase was blotted as surface marker to correct for quantitations in pulldowns. Molecular mass markers are in kDa. For clear display, the blot in E shows lysates and pulldowns separated by division lines, which indicate different exposure between lysates and pulldowns but equal exposure within each group.
By biochemical means, a small portion of NaV1.5 can be effectively detected at the cell surface of MDCK cells in the presence of β2 (15). We thus biotinylated surface proteins to detect in pulldowns NaV1.5, whose levels were visibly reduced in cells expressing unglycosylated β2 (Fig. 8, E and F), thus supporting the data obtained by immunofluorescence.
Because we could measure the presence of NaV1.5 at the surface by biotinylation, we then wished to determine the magnitude of this defect over time. To this end, we first analyzed β2 function in promoting NaV1.5 arrival to the surface early from transfection. We thus performed this analysis in cells growing nonpolarized in wells. Here, we took advantage of our approach to quantify relative fluorescence levels (i.e. mean fluorescence intensity (MFI)) along a segment drawn from the cell end perpendicularly into the cytoplasm; by means of confocal microscopy, the cell end taken is a close approximation of the plasma membrane region (15). As expected, localization of NaV1.5 to the plasma membrane was not promoted by unglycosylated β2 throughout time, and the bulk of NaV1.5 label remained intracellular (Fig. 9, C and D), similarly as in cells not expressing β2 (Fig. 9, E and F). In contrast, the MFI of NaV1.5 was concentrated at the cell end in the presence of β2 WT, also in parallel with Na/K-ATPase, especially at day 1, displaying a more widespread distribution at days 2 and 3 (Fig. 9, A and B); a general defect in promoting surface localization of NaV1.5 at late time points was also verified by cell surface biotinylation, by which all β2 variants were ineffective, including the WT (Fig. S5).
Figure 9.
Defect over time of unglycosylated β2 in promoting surface localization of NaV1.5. MDCK cells stably expressing WT (A) or fully unglycosylated (ung) β2-YFP (C) or untransfected (parental) cells (E) were transiently transfected with the vector SCN5A-FLAG and grown in wells for the indicated number of days. Cells were fixed and immunostained with a rabbit polyclonal antibody against NaV1.5 (red) and with a mouse mAb to Na/K-ATPase (blue). Images were obtained by confocal microscopy. In merged images, the YFP-emitted fluorescence is shown in green and DAPI is in gray. A, C, and E, representative xy sections (sections taken at the cell level where NaV1.5 is mainly found in each case) show a general diffuse NaV1.5 pattern, intracellular and often perinuclear, except for a noticeable overlap with Na/K-ATPase, particularly at day 1, in the presence of β2 WT. Scale bars, 10 μm. Confocal images were analyzed by calculating the MFI along linear segments of 30 pixels in length (d, distance; 0.1 μm/pixel) drawn from the cell end perpendicularly into the cytoplasm. B, D, and F, line charts show MFIs with the first 5 pixels of the segments, equivalent to the plasma membrane region (cell end), marked with a square bracket. The highest MFI levels are at the cell end for Na/K-ATPase and for β2 WT, which progressively decrease intracellularly. The profile for NaV1.5 increases at the cell end only in the presence of β2 WT and especially at day 1 (B) but remains comparatively low within this region with unglycosylated β2 (D) or in the absence of β2 (F). Data are mean ± S.D. (error bars) (number of cells analyzed ≥3; 4 segments/cell).
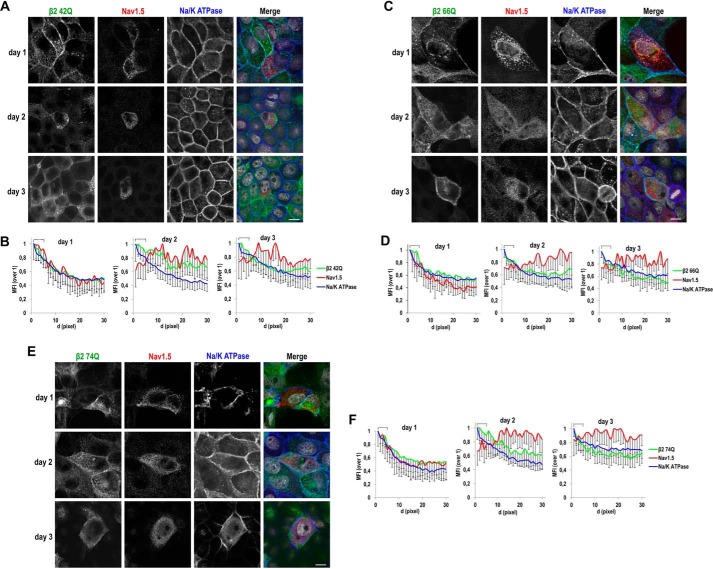
We have shown that a single intact glycosylation site in β2 is sufficient for its proper surface localization (see Figs. 3 and 4). Now, we asked whether incomplete glycosylation would affect β2 in promoting surface localization of NaV1.5. Interestingly, partial loss of glycosylation still allowed a positive effect; namely, only fully unglycosylated β2 is clearly defective in promoting surface localization of NaV1.5. Thus, we found that single β2 mutants maintain effectiveness at day 1 from transfection (Fig. 10), which we also verified by cell surface biotinylation (Fig. 11, G and H). By biochemical means, we also observed a comparable behavior in double mutants, appearing similarly effective as the WT in promoting surface localization of NaV1.5 (Fig. 11, I and J). Moreover, single mutants were also effective to promote apical localization of NaV1.5 in cells growing polarized in Transwells (Fig. 11, A–F; compare with Fig. 8, A–D).
Figure 10.
Single β2 glycosylation mutants can promote surface localization of NaV1.5; analysis over time. MDCK cells stably expressing the indicated single mutant for β2-YFP glycosylation were transiently transfected with the vector SCN5A-FLAG and grown in wells for the specified number of days. Cells were fixed and immunostained with a rabbit polyclonal antibody against NaV1.5 (red) and with a mouse mAb to Na/K-ATPase (blue). Images were obtained by confocal microscopy. In merged images, the YFP-emitted fluorescence is in green and DAPI is in gray. A, C, and E, Representative xy sections (taken at the level where NaV1.5 is mainly found in each case) show some areas of overlap of NaV1.5 with Na/K-ATPase at the cell end, particularly at day 1, in the presence of any of the mutants, while remaining mostly disperse throughout the cell at later time points. Scale bars, 10 μm. Confocal images were analyzed by calculating the MFI along linear segments of 30 pixels in length (d, distance; 0.1 μm/pixel) drawn from the cell end perpendicularly into the cytoplasm. B, D, and F, line charts show MFIs with the first 5 pixels of the segments, equivalent to the plasma membrane region (cell end), marked with a square bracket. The highest MFI levels are at the cell end for Na/K-ATPase and for the different β2 single mutants, all progressively decreasing intracellularly. The profile for NaV1.5 increases at the cell end at day 1 in all cases, remaining comparatively low within this region at later time points. Data are mean ± S.D. (error bars) (number of cells analyzed ≥3, 4 segments/cell).
Figure 11.
Single and double glycosylation mutants of β2 can promote surface localization of NaV1.5. MDCK cells (A, C, and E) stably expressing the indicated single mutant for β2-YFP glycosylation were transiently transfected with the vector SCN5A-FLAG and grown polarized in Transwells. Cells were fixed and immunostained with a rabbit polyclonal antibody against NaV1.5 (red), and with a mouse mAb to gp114 (cyan). Images were obtained by confocal microscopy. In merged images, the YFP-emitted fluorescence is in green, and DAPI is in gray. Representative xy sections taken at the apical level (section level chosen to assess presence of NaV1.5 at the apical surface) and corresponding z axis reconstruction (reciprocal xz and xy sections marked by a yellow dashed line) show noticeable apical localization of NaV1.5 with the different β2 variants. Scale bars, 10 μm. B, D, and F, line charts displaying the CTCF (mean percentage ± S.D. (error bars)) along an apical-to-basal z-stack (section 1: most apical; 0.5-μm optical slice thickness) show the NaV1.5 curve peak in close proximity to those of apical gp114 and any of the β2 mutants. DAPI is included as reference for the nuclear level (≥6 cells were analyzed per condition). G and I, MDCK cells stably expressing NaV1.5-YFP were transiently cotransfected with the SCN2B-yfp vector to express β2-YFP, WT, or any of the indicated single (G) or double (I) mutants, plus additional SCN5A-FLAG vector to ensure extensive NaV1.5 overexpression, and grown overnight in wells. Cells were surface-biotinylated at 4 °C. The same amount of protein was used to process each lysate (∼600 μg), 97% of which was subjected to overnight NeutrAvidin pulldown. Representative Western blots (G and I) and band quantitation (H and J) show comparable levels of NaV1.5 in biotin-NeutrAvidin pulldowns (Membrane) in the presence of any mutant variant of β2 as with the WT. One-way ANOVA revealed no differences among means. Data are mean ± S.D. (n ≥ 3). Na/K-ATPase was blotted as surface marker to correct for quantitations in pulldowns. Molecular mass markers are in kDa. For clear display, the blots in G and I show lysates and pulldowns separated by division lines, which indicate different exposure between each.
In summary, glycosylation is required for β2 to reach efficiently the plasma membrane and is important for β2 to promote surface localization of NaV1.5.
Discussion
In this work, we analyzed the mechanisms regulating β2 trafficking and how this may be determinant for proper localization at the cell surface of NaV1.5, the major cardiac NaV channel. We show that β2 is N-glycosylated in vivo and in vitro at residues 42, 66, and 74, becoming sialylated only at Asn-42, and that glycosylation is required for its efficient trafficking to the plasma membrane. We found that a comparatively small fraction of the fully unglycosylated mutant can reach the cell surface by bypassing the Golgi compartment, in fact, at only one-third the rate of the WT. In addition, it was defective in promoting surface localization of NaV1.5. We therefore propose that N-linked glycosylation of β2 is required for NaV1.5 trafficking to the surface.
NaV1.5 is often mislocalized in inherited channelopathies triggering cardiac arrhythmias. Defective trafficking is often responsible (27), although proper organization of macromolecular complexes is also important (28). In addition, association with adaptor proteins should ensure proper sorting, targeting, anchoring, and stabilization of the channel complex to certain plasma membrane subdomains (29). Such proteins may include auxiliary β subunits. In this regard, β2 association with the α subunit, at least in neurons, is important for proper targeting and subcellular localization of the α/β complex (5, 7).
By confocal microscopy and protein biotinylation, we observed that unglycosylated β2 was clearly defective in shifting the localization of NaV1.5 from the ER to the cell surface. In fact, a considerable portion of both proteins appeared stuck in the ER; to avoid excessive β2 levels due to overexpression, in most of these experiments we used cells stably expressing β2-YFP in moderate levels transiently transfected to express NaV1.5-FLAG. Virtually all exogenously expressed NaV1.5 actually remains intracellular in MDCK cells, a large portion being in the ER. This fits with the classic idea that the ER may serve as a reservoir for cardiac (14) and neuronal NaV channels, generating a pool potentially essential to regulate export of the α/β2 complex to appropriate surface locations (13). Yet, even in MDCK cells, β2 can promote NaV1.5 localization to the apical surface (15). The fully unglycosylated mutant was, however, defective and therefore lacked the most relevant function described for the β2 subunit to date, at least within the context of the NaV channel (5, 25). Remarkably, a single glycosylation site in β2 was sufficient to allow its trafficking to the apical surface and to promote surface localization of NaV1.5.
The implication of β subunits in promoting trafficking of the α subunit to the plasma membrane is a common finding seen in the literature (5, 30, 31). For β2 in particular, it has long been believed that covalent assembly of α/β2 takes place right before their arrival to the plasma membrane (13) or at least after the subunits have left the Golgi apparatus (14). This is consistent with data from Scn2b deletion in mice, which causes, both in ventricular myocytes (25) and in primary hippocampal neuron cultures (26), an approximately 40% reduction of α subunits at the cell surface. Interestingly, β2 must associate with the α subunit for its targeting to nodes of Ranvier and to the axon initial segment (7). A similar scenario is seen for β4 (32). Whereas it was concluded that trafficking to the plasma membrane of β1, but not β2, is altered by the α subunit (14), association of β2 with α actually determines β2 targeting to specialized neuron domains (7).
Regarding proper subcellular localization, this evidence may lead us to question whether β acts on the α subunit, or vice versa. Our data are consistent with the notion that β2 plays an important role in ensuring efficient surface localization of NaV1.5; this process is seen only defective when β2 remains mainly retained in the ER as a result of no glycosylation. The data from the present work thus challenge the view that β2 acts on NaV1.5 at a later stage, such as at the cell surface, or in a post-Golgi compartment, as we also proposed previously (15). Indeed, the unglycosylated mutant seemed to drag along a large portion of NaV1.5 to intracellular compartments, likely in the very ER. According to this observation, it is plausible that the β2 mutant causes NaV1.5 retention early in the secretory pathway in an attempt to chaperone it for proper folding on its way to the cell surface.
A minor fraction of unglycosylated β2, estimated to be no more than 10%, was detected at the cell surface, contrasting with 25–30% of the WT; this reduction to approximately one-third of the rate by β2 WT was similarly seen at the apical domain of polarized cells. By blocking ER-to-Golgi transport with the fungal drug BFA, we demonstrated that even immature β2 WT, which is Endo H–sensitive, could be detected at the plasma membrane. Based on this result, the most likely explanation for a small fraction of unglycosylated β2 being detected at the cell surface is that it bypassed the Golgi compartment. At least for NaV1.5, there is evidence that the immature protein may follow such a Golgi-independent, secretory pathway. For NaV1.5, the role of this alternative anterograde pathway is not clear, although it was proposed to be potentially useful for clearance of accumulating proteins in the ER as a constitutive response to relieve or prevent ER stress (33). In fact, it has been shown that a fraction of NaV1.5 remains Endo H–sensitive and associates with Kir2.1, the α subunit of the inward rectifying potassium channel, early in their biosynthetic pathway (34). In this regard, it has been hypothesized that NaV1.5 mutants associated with BrS and retained in the ER may still be delivered to the plasma membrane via an unconventional pathway (35).
We found that YFP-tagged, unglycosylated β2 displays similar kinetics of mobility in the plane of the membrane as β2 WT. Thus, N-glycosylation does not influence its lateral mobility as well as interactions with proteins and lipids within and across the membrane. Taking into account the relatively low D of around 0.02 μm2/s, or even less, it would be conceivable to consider that β2 diffuses inside lipid rafts, fitting with reported D ≤ 0.05 μm2/s (36). Yet, a considerably lower D would also agree with the possibility that the protein is tethered to cytoskeleton elements underlying the membrane (37).
We previously showed that proper localization of NaV1.5 to the cell surface is defective in the presence of β2 D211G (15), a missense mutation associated with BrS (10). In MDCK cells, β2 localizes in a polarized fashion, seen almost exclusively at the apical surface (15). In the present work, we found that fully unglycosylated β2 poorly reaches the apical plasma membrane. This agrees with previous data showing that N-glycans are required for polarized distribution of many apical membrane proteins in epithelia (24). As the WT, β2 D211G effectively localizes to the apical surface of MDCK cells and, similarly, to the plasma membrane of atria-derived HL-1 cells (15). Therefore, its defective action on NaV1.5 must be different from what we observed here for the fully unglycosylated mutant and may be related to a potential effect on posttranslational modifications, such as phosphorylation of the intracellular domain, as we suggested (15). However, previous work in heterologous systems actually showed that the cytoplasmic domain of β subunits does not have much influence on α/β interaction. Thus, a β1 chimera bearing the intracellular domain of β2 overlapped strongly with NaV1.5, supposedly in intracellular compartments (38), similarly as β1 does, but in contrast to β2 (14).
In summary, we found that β2 N-glycosylation is required for its efficient trafficking to the plasma membrane, although a small fraction of fully unglycosylated β2 can reach the cell surface by bypassing the Golgi compartment. Importantly, this mutant was defective in promoting surface localization of NaV1.5. These findings add to a better understanding of β2 function, which appears primarily relevant for proper NaV1.5 localization, thereby influencing cell excitability and electrical coupling in the heart and in turn contributing to an improved knowledge of how arrhythmias develop.
Experimental procedures
Plasmid vectors, cDNA cloning, and site-directed mutagenesis
The vector containing SCN2B-yfp, to express β2 with YFP fused to its C terminus, has been described (15). Following the manufacturer's instructions, the QuikChange Lightning Site-Directed Mutagenesis Kit (Agilent Technologies) was used to change Asn for Gln at predicted N-glycosylation sites (22), thus preventing potential N-glycosylation of the expressed protein. Human SCN2B (designated in the Consensus Coding Sequence database (CCDS) with ID 8390.1), containing the desired mutation, was used as a template. Complementary primer pairs for PCR were designed with the QuikChange® Primer Design Program (Agilent) and synthesized by Metabion International AG. Sequences were as follows: for N42Q, 5′-CCCTCAACGTCCTCCAGGGCTCTGACGCCCG-3′ (sense) and 5′-CGGGCGTCAGAGCCCTGGAGGACGTTGAGGG-3′ (antisense); for N66Q, 5′-CACAAACAGTTCTCCCTGCAGTGGACTTACCAGGAGTGC-3′ (sense) and 5′-GCACTCCTGGTAAGTCCACTGCAGGGAGAACTGTTTGTG-3′ (antisense); for N74Q, 5′-ACTTACCAGGAGTGCAACCAGTGCTCTGAGGAGATGTTC-3′ (sense) and 5′-GAACATCTCCTCAGAGCACTGGTTGCACTCCTGGTAAGT-3′ (antisense) (mutated bases are marked in boldface type and underlined). All possible combinations of mutant β2 were generated: N42Q, N66Q, N74Q, N42Q/N66Q, N42Q/N74Q, N66Q/N74Q, and N42Q/N66Q/N74Q. The constructs were then verified by sequencing.
The FLAG-tagged human SCN5A cloned in pcDNA3.1 has been described; the tag is located in the extracellular loop (right after Pro-154) between segments S1 and S2 of domain I (39).
Cell culture and transient transfection
MDCK cells II and transfectant derivatives were maintained in minimum essential medium with Earl's salts. To generate a fully polarized monolayer, cells were grown on polycarbonate Transwell filters of 12-mm diameter and 0.4-μm pore size for at least 3 days (Corning-Costar), as described (40); the medium was supplemented with GlutaMAXTM (Gibco). Transfections were performed according to the manufacturer's instructions. Cells to be split for transfection had been grown overnight until subconfluence. 400,000 cells/Transwell, 350,000 cells/22-mm well (12-well plates), or 1.2 × 106 cells/35-mm well (6-well plates) were seeded and immediately (co-)transfected, in suspension, with vector(s) to (co-)express NaV1.5, β2, and/or GFP, using Lipofectamine® 2000, at 1 μl of reagent/μg of DNA, in Gibco Opti-MEMTM I reduced-serum medium (Invitrogen). 2 μg of SCN2B-yfp vector were used per transfection in Transwells and 22-mm wells, and 4 μg in 35-mm wells; 6.5 μg of SCN5A-FLAG vector were transfected into β2 stable cells in Transwells; and 2 μg of the latter plus 3 μg of SCN2B-yfp vector were transfected into cells in 35-mm wells. In cotransfections of NaV1.5 and β2, the pEGFP-N1 vector (Clontech) was used as a control for β2-YFP.
For experiments of FRAP, 180,000 cells were seeded in ibidi® μ-slides (with four wells of Ph+ and a glass bottom) and transfected as above with 1.5 μg of SCN2B-yfp vector.
Generation of stable cell lines
Transfections were performed by calcium phosphate coprecipitation, as described (41), and single-cell clones were then selected with 200 μg/ml G418 (Sigma). Positive clones for WT and unglycosylated β2-YFP mutants were identified visually using the appropriate filter under a fluorescence microscope and then confirmed by anti-β2 Western blotting. Proper distribution of surface markers (gp114, apical; p58, basolateral) and tight junctions (ZO-1) was then verified by immunofluorescence, ensuring normal cell polarity. The cell line expressing NaV1.5-YFP has been described (15).
Pharmacological inhibition of glycosylation
To block N-linked protein glycosylation, cells were treated with TUN (Sigma T7765). TUN inhibits the initial events in glycosylation of Asn residues, resulting in the synthesis of totally unglycosylated proteins. TUN was first dissolved at 10 mg/ml in DMSO. Cells were treated 2 h after transfection with 0.3 μg/ml TUN for 24 h in complete medium; in untreated samples, an equivalent volume of solvent was added (0.003%).
To inhibit O-linked protein glycosylation, cells were treated with GalNAc-O-bn (Sigma B4894), a competitive inhibitor of O-glycan chain extension (42). GalNAc-O-bn was first dissolved at 100 mg/ml in DMSO. Cells were treated ∼2 h after transfection with 2 mm GalNAc-O-bn for 24 h in complete medium; in untreated samples, the equivalent volume of solvent was added (0.6%).
Treatment with BFA
To block transport from the ER to the Golgi, cells were treated with the fungal drug BFA (Thermo Fisher Scientific, 00-4506-51). BFA reversibly inhibits a GTPase activity necessary for coat formation on Golgi membranes, which ultimately induces a redistribution of Golgi components to the ER (43). Cells were treated ∼2 h after transfection with 1.5 μg/ml BFA overnight in Opti-MEM (BFA was purchased already dissolved at 3 mg/ml in methanol); in untreated samples, an equivalent volume of solvent was added (0.05%).
In vitro deglycosylation
Deglycosylation was performed in whole-cell lysates. Reactions were stopped with Laemmli buffer. To remove completely N-glycans, we used PNGase F (New England Biolabs, P0708), which cleaves off the bond between Asn and the first GlcNAc moiety, liberating the entire N-glycan. The protocol by New England Biolabs was used. Briefly, 10 μg of protein were denatured for 10 min at 100 °C in 10 μl of Glycoprotein Denaturing Buffer (0.5% SDS with 40 mm DTT). The reaction with 1 μl of PNGase F (500 units) was then performed in a 20-μl total volume, including GlycoBuffer 2 (50 mm sodium phosphate at pH 7.5) and containing 1% Nonidet P-40, by overnight incubation at 37 °C.
To discern between simple and complex N-glycosylation, we used Endo H (New England Biolabs, P0702), which cleaves N-glycans between the two GlcNAc moieties in the core region of the glycan chain on high-mannose and hybrid, but not complex, glycans. Similarly, 7.5 μg of protein were denatured for 10 min at 100 °C in 10 μl of Glycoprotein Denaturing Buffer. The reaction with 1 μl of Endo H (500 units) was then performed in a 20-μl total volume, including GlycoBuffer 3 (50 mm sodium acetate at pH 6), by overnight incubation at 37 °C.
To cleave terminal sialic acids, from N- and O-glycans, we used NA (New England Biolabs, P0720), which hydrolyzes α2–3-, α2–6-, and α2–8-linked sialic acid residues from glycoproteins and oligosaccharides. Here, 2 μl of NA (100 units) were added to 3.5 μg of protein in GlycoBuffer 1 (5 mm CaCl2 in 50 mm sodium acetate at pH 5.5) and incubated overnight at 37 °C. To ensure proper visibility in gels with samples from double mutants, twice the amount of protein and enzyme were used in digestions with Endo H and NA.
In experiments addressing the effect of BFA, material obtained by surface protein biotinylation (see below) was also digested with Endo H. Here, overnight NeutrAvidin pulldowns were resuspended in 20 μl of Glycoprotein Denaturing Buffer and denatured as above to release the protein from beads. Beads were then spun down, and 10 μl of supernatant was deglycosylated in GlycoBuffer 3 as above using 3 times as much enzyme.
Antibodies
Some antibodies were provided by other researchers, including the mouse monoclonal antibodies to gp114 (a cell adhesion molecule) and to p58 (the Na/K-ATPase β subunit) (44), as well as the rat mAb against ZO-l (45). The following are commercially available mouse monoclonal antibodies: to the early endosome marker EEA1 and GM130 (BD Transduction Laboratories 610457 and 610822, respectively) and to the Na/K-ATPase α1 subunit and the TGN marker TGN46 (Abcam ab7671 and ab50595, respectively). Commercial rabbit polyclonal antibodies used were from Alomone (ASC-013 to NaV1.5 and ASC-007 to anti-β2), from Abcam (anti-GFP (ab290) and anti-calnexin (ab75801)), and from Sigma (anti-actin (A 2066)).
Secondary antibodies horseradish peroxidase–conjugated for Western blotting were from Jackson ImmunoResearch (codes 111-035-003 and 115-035-003), and Alexa Fluor®-labeled for immunofluorescence were from Molecular Probes-Invitrogen (codes A11012 and A21050).
Sample preparation for Western blotting
Protein determination from cell lysates and preparation of samples for SDS-PAGE were done as previously (15, 46), with the following modifications in samples analyzing NaV1.5. These were prepared in Laemmli buffer by heating at 70 °C for 10 min, and protein transfer to polyvinylidene difluoride membranes was done for 30 h in the presence of 0.01% SDS to optimize NaV1.5 solubilization and transfer.
Cell surface biotinylation
Surface protein biotinylation was done with EZ-LinkTM Sulfo-NHS-SS-Biotin (Pierce 21331), a water-soluble and membrane-impermeable reagent. The procedure followed has been described in detail previously (15, 46). Unless otherwise specified, nine-tenths of cell lysate was subjected to overnight pulldown with NeutrAvidin (Pierce, 53150) and analyzed by Western blotting along with the remaining 10% (referred to as lysate). Quantitation of blotted protein bands in lysates and pulldowns was performed as described (15) using the ImageJ program.
Confocal immunofluorescence microscopy and quantitative image analysis
MDCK cells were analyzed at subconfluence on glass coverslips or grown polarized in 12-mm Transwells. Cells were fixed with paraformaldehyde and immunostained, essentially as described (15, 46).
High-magnification images were taken on a Nikon A1R confocal microscope at a minimum pixel resolution of 1,024 × 1,024 using the NIS-Elements AR software, as described (47). Images were exported to TIFF format, and three-dimensional colocalization was done without image preprocessing using Fiji, the ImageJ-based package that includes the JACoP plugin for colocalization analysis. Manders' colocalization coefficients were then calculated along apical-to-basal z-stacks to estimate the fraction of β2 present in compartments positive for a given subcellular marker, as described (15).
To measure cell fluorescence along z-stacks (optical slice thickness of 0.5 μm), confocal images were taken at 512 × 512-pixel resolution. As previously (47), we calculated along three-dimensional reconstructions the CTCF, which integrates fluorescence intensity and area. In nonpolarized cells, to measure relative fluorescence levels from the plasma membrane into the cytoplasm, we calculated for each channel the MFI, which shows the percentage of fluorescence intensity/pixel over the pixel with maximum intensity, as described (15).
FRAP
MDCK cells were transiently transfected and grown subconfluent (2 days) on ibidi® glass supports. Cells were placed in a live-cell imaging chamber at 37 °C and 5% CO2 and imaged through a water-immersion objective (Plan-Apo ×60, 1.2 numeric aperture) on a Nikon A1R confocal microscope. Confocal images were taken at 512 × 512-pixel resolution. An argon laser with emission at 514 nm was used to image the YFP fluorescence, and a 405-nm diode laser was used for photobleaching. The pinhole radius was set to 3 airy units, except when imaging perinuclear ER structures, when the pinhole was set to 1 airy unit. Three regions of interest were drawn: a bleached area, in which fluorescence recovery was recorded along time; a background area, outside obvious fluorescence labeling; and a nonbleached (reference) area, in a different cell displaying similar fluorescence intensity as the bleached cell. Both bleached and reference areas were circular regions with a nominal radius (rn) of 2 μm, except when imaging perinuclear ER structures, where rn was 1 μm.
Images were collected at a rate of 1 frame/s, as follows. First, 10 prebleaching images were taken, and then bleaching was done for 5 s at 100% laser transmission. Immediately, postbleaching images were captured until fluorescence recovery reached a plateau. Similarly as described (48), we used the NIS-Elements AR software to measure average fluorescence intensities and to correct for background and acquisition photobleaching, taking into account background and reference fluorescence values, respectively.
Next, data were normalized as follows. First, the lowest fluorescence value, obtained from the first postbleaching recording, was subtracted from each time point value to set bleach depth to zero. Then all values were divided by the value from the last prebleaching (10th) frame (i.e. right before photobleaching). From each curve, we then obtained three parameters: 1) the MF, determined by averaging the fluorescence values of the first 30 time points throughout which the curve reaches a plateau (30 s) (this value is expressed as a percentage of the maximum fluorescence at prebleaching and indicates the portion of molecules that can undergo diffusion during the experiment); 2) the half-time of recovery (τ½) (i.e. the time point in which half of total fluorescence recovery has occurred; this value inversely correlates to the rate of diffusion and, therefore, to the speed of molecule movement in the area analyzed); and 3) the diffusion coefficient (D), indicating rate of diffusion, calculated applying the simplified Soumpasis equation (49).
| (Eq. 1) |
Statistics
All experiments were performed a minimum of three times. Data are expressed as mean ± S.D., as indicated in the figure legends, and displayed as curves or bar graphs superimposed to scatterplots showing all of the individual data points. Statistical significance was calculated by the two-tailed Student's t test or by one-way ANOVA with Tukey's honest significant difference (HSD) post hoc test by using the R software for statistical computing (50), when differences among groups needed to be tested. p values are also specified in the figure legends.
Author contributions
E. C. carried out the experimental work and contributed in designing the work and writing up the manuscript. R. B. gave advice and provided financial support to carry out the project. M. V. conceived the project, designed the work, supervised the experiments, and wrote the manuscript.
Supplementary Material
Acknowledgments
DNA constructs kindly donated by other researchers were those to express NaV1.5-FLAG (Matteo Vatta and Jonathan Makielski) in addition to NaV1.5-YFP and β2-YFP (Thomas Zimmer). Antibodies given by other scientists were those against gp114 and p58 (Kai Simons). Antibodies for lysobisphosphatidic acid (Jean Gruenberg) and LAMP-2 (Enrique Rodriguez-Boulan) were also kind gifts. We appreciate the service and advice from the technicians at the confocal microscopy facility of the University of Girona-Research Technical Services and by Maria Buxó (IDIBGI Statistical Service).
This work was supported by “La Caixa” Foundation (to R. B.). This work was also supported by the University of Girona (to M. V.) and by the Spanish Instituto de Salud Carlos III (ISCIII). The CIBERCV is an initiative of the ISCIII from the Spanish Ministerio de Ciencia, Innovación y Universidades. The authors declare that they have no conflicts of interest with the contents of this article.
This article contains Table S1 and Figs. S1–S5.
- NaV
- voltage-gated sodium
- BrS
- Brugada Syndrome
- CTCF
- corrected total cell fluorescence
- D
- diffusion coefficient
- DAPI
- 4′,6-diamidino-2-phenylindole
- Endo H
- endoglycosidase H
- ER
- endoplasmic reticulum
- FRAP
- fluorescence recovery after photobleaching
- GalNAc-O-bn
- benzyl-2-acetamido-2-deoxy-α-d-galactopyranoside
- GM130
- Golgi matrix protein of 130 kDa
- τ½
- half-time of recovery
- HSD
- honest significant difference
- LAMP
- lysosomal-associated membrane protein
- MDCK
- Madin–Darby canine kidney
- MF
- mobile fraction
- MFI
- mean fluorescence intensity
- NA
- α2–3,6,8 neuraminidase
- rn
- nominal radius
- PNGase F
- peptide:N-glycosidase F
- TGN
- trans-Golgi network
- TMD
- transmembrane domain
- TUN
- tunicamycin
- BFA
- brefeldin A
- ANOVA
- analysis of variance.
References
- 1. Amin A. S., Asghari-Roodsari A., and Tan H. L. (2010) Cardiac sodium channelopathies. Pflugers Arch. 460, 223–237 10.1007/s00424-009-0761-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Napolitano C., and Priori S. G. (2006) Brugada syndrome. Orphanet J. Rare Dis. 1, 35 10.1186/1750-1172-1-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rook M. B., Evers M. M., Vos M. A., and Bierhuizen M. F. (2012) Biology of cardiac sodium channel Nav1.5 expression. Cardiovasc. Res. 93, 12–23 10.1093/cvr/cvr252 [DOI] [PubMed] [Google Scholar]
- 4. Petitprez S., Zmoos A. F., Ogrodnik J., Balse E., Raad N., El-Haou S., Albesa M., Bittihn P., Luther S., Lehnart S. E., Hatem S. N., Coulombe A., and Abriel H. (2011) SAP97 and dystrophin macromolecular complexes determine two pools of cardiac sodium channels Nav1.5 in cardiomyocytes. Circ. Res. 108, 294–304 10.1161/CIRCRESAHA.110.228312 [DOI] [PubMed] [Google Scholar]
- 5. O'Malley H. A., and Isom L. L. (2015) Sodium channel β subunits: emerging targets in channelopathies. Annu. Rev. Physiol. 77, 481–504 10.1146/annurev-physiol-021014-071846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brackenbury W. J., and Isom L. L. (2011) Na channel β subunits: overachievers of the ion channel family. Front. Pharmacol. 2, 53 10.3389/fphar.2011.00053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen C., Calhoun J. D., Zhang Y., Lopez-Santiago L., Zhou N., Davis T. H., Salzer J. L., and Isom L. L. (2012) Identification of the cysteine residue responsible for disulfide linkage of Na+ channel α and β2 subunits. J. Biol. Chem. 287, 39061–39069 10.1074/jbc.M112.397646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhu W., Voelker T. L., Varga Z., Schubert A. R., Nerbonne J. M., and Silva J. R. (2017) Mechanisms of noncovalent β subunit regulation of NaV channel gating. J. Gen. Physiol. 149, 813–831 10.1085/jgp.201711802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hu D., Barajas-Martinez H., Burashnikov E., Springer M., Wu Y., Varro A., Pfeiffer R., Koopmann T. T., Cordeiro J. M., Guerchicoff A., Pollevick G. D., and Antzelevitch C. (2009) A mutation in the β 3 subunit of the cardiac sodium channel associated with Brugada ECG phenotype. Circ. Cardiovasc. Genet. 2, 270–278 10.1161/CIRCGENETICS.108.829192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Riuró H., Beltran-Alvarez P., Tarradas A., Selga E., Campuzano O., Vergés M., Pagans S., Iglesias A., Brugada J., Brugada P., Vázquez F. M., Pérez G. J., Scornik F. S., and Brugada R. (2013) A missense mutation in the sodium channel β2 subunit reveals SCN2B as a new candidate gene for Brugada syndrome. Hum. Mutat. 34, 961–966 10.1002/humu.22328 [DOI] [PubMed] [Google Scholar]
- 11. Watanabe H., Koopmann T. T., Le Scouarnec S., Yang T., Ingram C. R., Schott J. J., Demolombe S., Probst V., Anselme F., Escande D., Wiesfeld A. C., Pfeufer A., Kääb S., Wichmann H. E., Hasdemir C., et al. (2008) Sodium channel β1 subunit mutations associated with Brugada syndrome and cardiac conduction disease in humans. J. Clin. Invest. 118, 2260–2268 10.1172/JCI33891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ishikawa T., Takahashi N., Ohno S., Sakurada H., Nakamura K., On Y. K., Park J. E., Makiyama T., Horie M., Arimura T., Makita N., and Kimura A. (2013) Novel SCN3B mutation associated with Brugada syndrome affects intracellular trafficking and function of Nav1.5. Circ. J. 77, 959–967 10.1253/circj.CJ-12-0995 [DOI] [PubMed] [Google Scholar]
- 13. Schmidt J. W., and Catterall W. A. (1986) Biosynthesis and processing of the α subunit of the voltage-sensitive sodium channel in rat brain neurons. Cell 46, 437–444 10.1016/0092-8674(86)90664-1 [DOI] [PubMed] [Google Scholar]
- 14. Zimmer T., Biskup C., Bollensdorff C., and Benndorf K. (2002) The β1 subunit but not the β2 subunit colocalizes with the human heart Na+ channel (hH1) already within the endoplasmic reticulum. J. Membr. Biol. 186, 13–21 10.1007/s00232-001-0131-0 [DOI] [PubMed] [Google Scholar]
- 15. Dulsat G., Palomeras S., Cortada E., Riuró H., Brugada R., and Vergés M. (2017) Trafficking and localization to the plasma membrane of Nav1.5 promoted by the β2 subunit is defective due to a β2 mutation associated with Brugada syndrome. Biol. Cell 109, 273–291 10.1111/boc.201600085 [DOI] [PubMed] [Google Scholar]
- 16. Stoops E. H., and Caplan M. J. (2014) Trafficking to the apical and basolateral membranes in polarized epithelial cells. J. Am. Soc. Nephrol. 25, 1375–1386 10.1681/ASN.2013080883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Weisz O. A., and Rodriguez-Boulan E. (2009) Apical trafficking in epithelial cells: signals, clusters and motors. J. Cell Sci. 122, 4253–4266 10.1242/jcs.032615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Isom L. L., Ragsdale D. S., De Jongh K. S., Westenbroek R. E., Reber B. F., Scheuer T., and Catterall W. A. (1995) Structure and function of the β2 subunit of brain sodium channels, a transmembrane glycoprotein with a CAM motif. Cell 83, 433–442 10.1016/0092-8674(95)90121-3 [DOI] [PubMed] [Google Scholar]
- 19. Johnson D., and Bennett E. S. (2006) Isoform-specific effects of the β2 subunit on voltage-gated sodium channel gating. J. Biol. Chem. 281, 25875–25881 10.1074/jbc.M605060200 [DOI] [PubMed] [Google Scholar]
- 20. UniProt Consortium (2015) UniProt: a hub for protein information. Nucleic Acids Res. 43, D204–D212 10.1093/nar/gku989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Johnson D., Montpetit M. L., Stocker P. J., and Bennett E. S. (2004) The sialic acid component of the β1 subunit modulates voltage-gated sodium channel function. J. Biol. Chem. 279, 44303–44310 10.1074/jbc.M408900200 [DOI] [PubMed] [Google Scholar]
- 22. Hart G. W. (1992) Glycosylation. Curr. Opin. Cell Biol. 4, 1017–1023 10.1016/0955-0674(92)90134-X [DOI] [PubMed] [Google Scholar]
- 23. Freeze H. H., and Kranz C. (2010) Endoglycosidase and glycoamidase release of N-linked glycans. Curr. Protoc. Mol. Biol. Chapter 17, Unit 17.13A 10.1002/0471142727.mb1713as89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vagin O., Kraut J. A., and Sachs G. (2009) Role of N-glycosylation in trafficking of apical membrane proteins in epithelia. Am. J. Physiol. Renal Physiol. 296, F459–F469 10.1152/ajprenal.90340.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bao Y., Willis B. C., Frasier C. R., Lopez-Santiago L. F., Lin X., Ramos-Mondragón R., Auerbach D. S., Chen C., Wang Z., Anumonwo J., Valdivia H. H., Delmar M., Jalife J., and Isom L. L. (2016) Scn2b deletion in mice results in ventricular and atrial arrhythmias. Circ. Arrhythm. Electrophysiol. 9, e003923 10.1161/CIRCEP.116.003923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chen C., Bharucha V., Chen Y., Westenbroek R. E., Brown A., Malhotra J. D., Jones D., Avery C., Gillespie P. J. 3rd, Kazen-Gillespie K. A., Kazarinova-Noyes K., Shrager P., Saunders T. L., Macdonald R. L., Ransom B. R., et al. (2002) Reduced sodium channel density, altered voltage dependence of inactivation, and increased susceptibility to seizures in mice lacking sodium channel β2-subunits. Proc. Natl. Acad. Sci. U.S.A. 99, 17072–17077 10.1073/pnas.212638099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Remme C. A. (2013) Cardiac sodium channelopathy associated with SCN5A mutations: electrophysiological, molecular and genetic aspects. J. Physiol. 591, 4099–4116 10.1113/jphysiol.2013.256461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Balse E., and Eichel C. (2018) The cardiac sodium channel and its protein partners. Handb. Exp. Pharmacol. 246, 73–99 10.1007/164_2017_45 [DOI] [PubMed] [Google Scholar]
- 29. Shy D., Gillet L., and Abriel H. (2013) Cardiac sodium channel Nav1.5 distribution in myocytes via interacting proteins: the multiple pool model. Biochim. Biophys. Acta 1833, 886–894 10.1016/j.bbamcr.2012.10.026 [DOI] [PubMed] [Google Scholar]
- 30. Veerman C. C., Wilde A. A., and Lodder E. M. (2015) The cardiac sodium channel gene SCN5A and its gene product NaV1.5: role in physiology and pathophysiology. Gene 573, 177–187 10.1016/j.gene.2015.08.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hull J. M., and Isom L. L. (2018) Voltage-gated sodium channel β subunits: the power outside the pore in brain development and disease. Neuropharmacology 132, 43–57 10.1016/j.neuropharm.2017.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Buffington S. A., and Rasband M. N. (2013) Na+ channel-dependent recruitment of Navβ4 to axon initial segments and nodes of Ranvier. J. Neurosci. 33, 6191–6202 10.1523/JNEUROSCI.4051-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mercier A., Clément R., Harnois T., Bourmeyster N., Bois P., and Chatelier A. (2015) Nav1.5 channels can reach the plasma membrane through distinct N-glycosylation states. Biochim. Biophys. Acta 1850, 1215–1223 10.1016/j.bbagen.2015.02.009 [DOI] [PubMed] [Google Scholar]
- 34. Ponce-Balbuena D., Guerrero-Serna G., Valdivia C. R., Caballero R., Diez-Guerra F. J., Jiménez-Vázquez E. N., Ramírez R. J., Monteiro da Rocha A., Herron T. J., Campbell K. F., Willis B. C., Alvarado F. J., Zarzoso M., Kaur K., Pérez-Hernandez M., et al. (2018) Cardiac Kir2.1 and NaV1.5 channels traffic together to the sarcolemma to control excitability. Circ. Res. 122, 1501–1516 10.1161/CIRCRESAHA.117.311872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pérez-Hernandez M., Matamoros M., Alfayate S., Nieto-Marín P., Utrilla R. G., Tinaquero D., de Andrés R., Crespo T., Ponce-Balbuena D., Willis B. C., Jiménez-Vazquez E. N., Guerrero-Serna G., da Rocha A. M., Campbell K., Herron T. J., et al. (2018) Brugada syndrome trafficking-defective Nav1.5 channels can trap cardiac Kir2.1/2.2 channels. JCI Insight 3, 96291 10.1172/jci.insight.96291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pralle A., Keller P., Florin E. L., Simons K., and Hörber J. K. (2000) Sphingolipid-cholesterol rafts diffuse as small entities in the plasma membrane of mammalian cells. J. Cell Biol. 148, 997–1008 10.1083/jcb.148.5.997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Peran M., Hicks B. W., Peterson N. L., Hooper H., and Salas R. (2001) Lateral mobility and anchoring of recombinant GABAA receptors depend on subunit composition. Cell Motil. Cytoskeleton 50, 89–100 10.1002/cm.1043 [DOI] [PubMed] [Google Scholar]
- 38. Zimmer T., and Benndorf K. (2007) The intracellular domain of the β2 subunit modulates the gating of cardiac Nav1.5 channels. Biophys. J. 92, 3885–3892 10.1529/biophysj.106.098889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Valdivia C. R., Medeiros-Domingo A., Ye B., Shen W. K., Algiers T. J., Ackerman M. J., and Makielski J. C. (2010) Loss-of-function mutation of the SCN3B-encoded sodium channel β3 subunit associated with a case of idiopathic ventricular fibrillation. Cardiovasc. Res. 86, 392–400 10.1093/cvr/cvp417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Vergés M., Sebastián I., and Mostov K. E. (2007) Phosphoinositide 3-kinase regulates the role of retromer in transcytosis of the polymeric immunoglobulin receptor. Exp. Cell Res. 313, 707–718 10.1016/j.yexcr.2006.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Breitfeld P. P., Casanova J. E., Harris J. M., Simister N. E., and Mostov K. E. (1989) Expression and analysis of the polymeric immunoglobulin receptor in Madin-Darby canine kidney cells using retroviral vectors. Methods Cell Biol. 32, 329–337 10.1016/S0091-679X(08)61178-4 [DOI] [PubMed] [Google Scholar]
- 42. Huet G., Hennebicq-Reig S., de Bolos C., Ulloa F., Lesuffleur T., Barbat A., Carrière V., Kim I., Real F. X., Delannoy P., and Zweibaum A. (1998) GalNAc-α-O-benzyl inhibits NeuAcα2–3 glycosylation and blocks the intracellular transport of apical glycoproteins and mucus in differentiated HT-29 cells. J. Cell Biol. 141, 1311–1322 10.1083/jcb.141.6.1311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lippincott-Schwartz J., and Liu W. (2006) Insights into COPI coat assembly and function in living cells. Trends Cell Biol. 16, e1–e4 10.1016/j.tcb.2006.08.008 [DOI] [PubMed] [Google Scholar]
- 44. Füllekrug J., Shevchenko A., Shevchenko A., and Simons K. (2006) Identification of glycosylated marker proteins of epithelial polarity in MDCK cells by homology driven proteomics. BMC Biochem. 7, 8 10.1186/1471-2091-7-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Stevenson B. R., Siliciano J. D., Mooseker M. S., and Goodenough D. A. (1986) Identification of ZO-1: a high molecular weight polypeptide associated with the tight junction (zonula occludens) in a variety of epithelia. J. Cell Biol. 103, 755–766 10.1083/jcb.103.3.755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cuartero Y., Mellado M., Capell A., Alvarez-Dolado M., and Verges M. (2012) Retromer regulates postendocytic sorting of β-secretase in polarized Madin-Darby canine kidney cells. Traffic 13, 1393–1410 10.1111/j.1600-0854.2012.01392.x [DOI] [PubMed] [Google Scholar]
- 47. Mellado M., Cuartero Y., Brugada R., and Verges M. (2014) Subcellular localization of retromer in postendocytic pathways of polarized Madin-Darby canine kidney cells. Biol. Cell 106, 377–393 10.1111/boc.201400011 [DOI] [PubMed] [Google Scholar]
- 48. Phair R. D., Gorski S. A., and Misteli T. (2004) Measurement of dynamic protein binding to chromatin in vivo, using photobleaching microscopy. Methods Enzymol. 375, 393–414 10.1016/s0076-6879(03)75025-3 [DOI] [PubMed] [Google Scholar]
- 49. Kang M., Day C. A., Kenworthy A. K., and DiBenedetto E. (2012) Simplified equation to extract diffusion coefficients from confocal FRAP data. Traffic 13, 1589–1600 10.1111/tra.12008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Dessau R. B., and Pipper C. B. (2008) [“R”–project for statistical computing]. Ugeskr. Laeger 170, 328–330 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.