Abstract
Many bacteria and some archaea produce the second messenger cyclic diadenosine monophosphate (c-di-AMP). c-di-AMP controls the uptake of osmolytes in Firmicutes, including the human pathogen Listeria monocytogenes, making it essential for growth. c-di-AMP is known to directly regulate several potassium channels involved in osmolyte transport in species such as Bacillus subtilis and Streptococcus pneumoniae, but whether this same mechanism is involved in L. monocytogenes, or even whether similar ion channels were present, was not known. Here, we have identified and characterized the putative L. monocytogenes' potassium transporters KimA, KtrCD, and KdpABC. We demonstrate that Escherichia coli expressing KimA and KtrCD, but not KdpABC, transport potassium into the cell, and both KimA and KtrCD are inhibited by c-di-AMP in vivo. For KimA, c-di-AMP–dependent regulation requires the C-terminal domain. In vitro assays demonstrated that the dinucleotide binds to the cytoplasmic regulatory subunit KtrC and to the KdpD sensor kinase of the KdpDE two-component system, which in Staphylococcus aureus regulates the corresponding KdpABC transporter. Finally, we also show that S. aureus contains a homolog of KimA, which mediates potassium transport. Thus, the c-di-AMP–dependent control of systems involved in potassium homeostasis seems to be conserved in phylogenetically related bacteria. Surprisingly, the growth of an L. monocytogenes mutant lacking the c-di-AMP–synthesizing enzyme cdaA is only weakly inhibited by potassium. Thus, the physiological impact of the c-di-AMP–dependent control of potassium uptake seems to be less pronounced in L. monocytogenes than in other Firmicutes.
Keywords: second messenger, cyclic diadenosine monophosphate (c-di-AMP), osmotic swelling, Gram-positive bacteria, Escherichia coli (E. coli), signal transduction, ion transport, osmoadaptation, regulation, transporter, osmoregulation, turgor, osmoadaptation
Introduction
Bacteria use complex signal transduction systems to adjust the cellular turgor to the environmental osmolarity (1–3). Under hyperosmotic growth conditions, potassium ions are imported to prevent water efflux from the cytosol and to increase the cellular turgor (4). The potassium ions are thereupon often replaced by compatible solutes such as glycine betaine and ectoine, osmolytes that do not disturb essential cellular processes (4). Depending on the external osmolarity, the import and export of osmolytes have to be tightly controlled to prevent osmotic swelling and shrinking of the cell, respectively (1, 5, 6). Although osmoregulation has been intensively studied, it is still rather unclear how a cell senses the environmental osmolarity to adjust the turgor accordingly. The second messenger cyclic diadenosine monophosphate (c-di-AMP),2 which is produced by specific diadenylate cyclases (DACs), plays a key role in regulating the turgor in Firmicute bacteria because it controls the uptake and export of osmolytes, including potassium (see below) (5, 7–15). c-di-AMP was discovered during the structural characterization of DNA integrity scanning protein DisA, which is involved in DNA damage response and in controlling sporulation initiation in the Gram-positive bacterium Bacillus subtilis (16–20). DisA is present in spore-forming Firmicutes, in actinobacteria (21), and in hyperthermophilic bacteria (17). Whereas DisA is the only c-di-AMP-producing enzyme in actinobacteria, bacteria like B. subtilis also contain the DACs CdaA and CdaS, of which the latter is required for efficient spore germination (22, 23). CdaA is attached to the membrane, and DisA and CdaS are soluble proteins (17, 22, 24). CdaA is the most abundant DAC, and many prominent apathogenic and pathogenic Gram-positive bacteria like Lactococcus lactis, Listeria monocytogenes, Staphylococcus aureus, and Streptococcus agalactiae rely only on this DAC for c-di-AMP synthesis (21). Because c-di-AMP is essential for growth of these bacteria (14, 25, 26), the DAC CdaA is an interesting target for novel antibiotics.
c-di-AMP is also intracellularly degraded by specific phosphodiesterases (PDEs), which can be assigned to three different groups (23, 27). The GdpP- and PgpH-type PDEs consist of domains that are involved in signaling and enzyme catalysis. Both PDEs are attached to the membrane, suggesting that the enzymes may sense and respond to extracellular cues. The DhhP-type PDEs, which are located in the cytosol, form the third group of c-di-AMP–degrading enzymes (27). Because the DACs and the PDEs determine the cellular c-di-AMP levels that are required for optimal growth in environments with changing osmolarities (5, 15), the activities of the enzymes have to be tightly regulated. Recently, it has been observed that the phosphoglucosamine mutase GlmM inhibits the DAC CdaA in L. lactis and S. aureus, suggesting a link between c-di-AMP metabolism and cell wall biosynthesis (28, 29). However, the molecular mechanisms by which the DACs, the PDEs, and GlmM sense the environmental osmolarity are unknown. Moreover, the sensing mechanisms may vary among the enzymes due to the different domain composition and cellular localization.
Several c-di-AMP targets have been identified. c-di-AMP activates the DNA-binding activity of the transcription factor DarR in Mycobacterium smegmatis (30). In L. monocytogenes, c-di-AMP inhibits the pyruvate carboxylase PycA (31, 32). Moreover, c-di-AMP binds to the CBS (cystathione-beta-synthase domain–containing) proteins CbpA and CbpB and the PII-like signal transduction DarA in this organism (31, 33). The DarA homologs from B. subtilis and S. aureus have been structurally and biochemically characterized (34–36). Whereas the biological functions of CbpA, CbpB, and DarA remain to be elucidated, several c-di-AMP targets are involved in the transport of osmolytes, such as potassium, glycine betaine, and carnitine (7, 8, 11–15, 26). c-di-AMP inhibits the KimA, KupA/KupB, KtrCD/KtrCB, and CabP-TrkH potassium uptake systems in B. subtilis, L. lactis, S. aureus, and S. pneumoniae, respectively (7, 8, 37–39). Moreover, c-di-AMP stimulates the S. aureus potassium and sodium transporter CpaA (9). In Bacillus thuringiensis and S. aureus, the synthesis of the KdpFABC potassium transporter is also inhibited by binding of c-di-AMP to the sensor kinase KdpD of the KdpDE two-component system (40, 41). In B. subtilis, the expression of the ktrAB and kimA genes, encoding the potassium transporters KtrAB and KimA, respectively, is negatively regulated by c-di-AMP (5). Thus, c-di-AMP plays a central role in osmolyte homeostasis in a variety of bacteria.
We are interested in the c-di-AMP–dependent control of osmolyte homeostasis in the food-borne pathogen L. monocytogenes (42). The ability of L. monocytogenes to thrive under adverse conditions including high osmolarity depends on the c-di-AMP–dependent control of osmolyte transport, such as carnitine (11). However, the involvement of c-di-AMP in potassium uptake or homeostasis in L. monocytogenes has remained elusive. Here we show that the L. monocytogenes KimA (Lmo2130) and KtrCD (Lmo1023 and Lmo0993) proteins are high- and low-affinity potassium transporters, respectively. We also show that the transporters are inhibited by c-di-AMP and that unregulated activity leads to rapid osmotic swelling of Escherichia coli cells synthesizing KimA from L. monocytogenes. The interaction between c-di-AMP and KtrC, as well as between c-di-AMP and KdpD, was also confirmed in vitro. Moreover, the C-terminal domain of KimA is important for the c-di-AMP–dependent regulation of potassium uptake. Finally, we show that the control of potassium uptake is not an essential function of c-di-AMP in L. monocytogenes.
Results
In silico identification of potassium transporters from L. monocytogenes
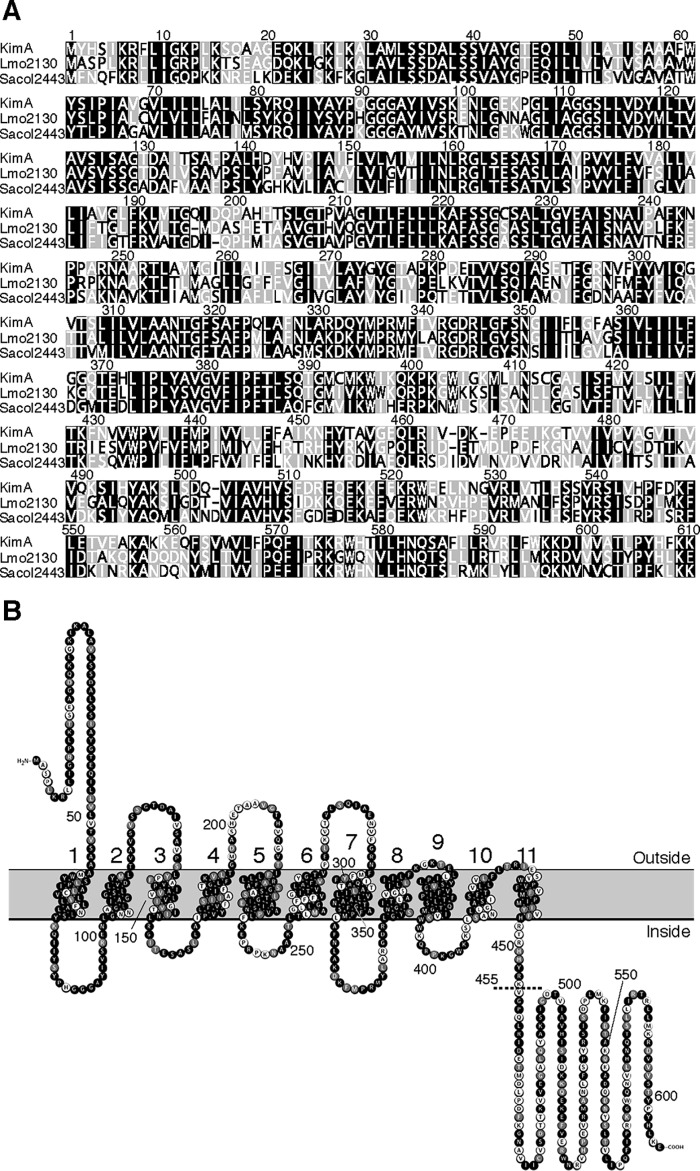
Both B. subtilis and S. aureus contain well-described potassium uptake systems. B. subtilis uses the high-affinity transporters KtrAB and KimA and the low-affinity transporter KtrCD (5, 43). By contrast, S. aureus relies on the high-affinity transporter KdpFABC, whose synthesis and activity is controlled by the two-component system KdpDE and c-di-AMP, respectively (41, 44, 45). S. aureus also contains the low-affinity potassium transport systems KtrCB and KtrCD sharing the accessory protein KtrC (44). A BLASTp sequence analysis revealed that the L. monocytogenes genome codes for the KdpABCDE (Lmo2682-Lmo2678) and KtrCD (Lmo1023, Lmo0993) proteins, which show about 31–56% and 51–64% overall amino acid identity with the homologs from S. aureus and B. subtilis, respectively. The kdpF gene that has been shown to be important for proper function of the Kdp system in E. coli does not exist in the L. monocytogenes genome (46). A homolog of the high-affinity potassium transporter KimA from B. subtilis is also present in L. monocytogenes and S. aureus (5). The KimA homologs from B. subtilis, L. monocytogenes, and S. aureus are from now on designated as KimABsu, KimALmo (Lmo2130), and KimASau (Sacol2443), respectively. KimALmo and KimASau show about 59 and 57% overall amino acid identity, respectively, with the B. subtilis homolog (Fig. 1A). The membrane topology was illustrated using the web-based tool Protter (47). Like KimABsu, KimALmo also contains an N-terminal extracellular domain, 11 transmembrane helices, and a C-terminal intracellular domain, which might be important for activity control of the transporter (Fig. 1B). To conclude, although B. subtilis, L. monocytogenes, and S. aureus are phylogenetically related, each species uses a different set of transporters for potassium uptake.
Figure 1.
Alignment of KimA homologs and domain organization of the KimALmo protein. A, MUSCLE alignment of the KimABsu, KimALmo, and KimASau homologs from B. subtilis, L. monocytogenes (Lmo2130), and S. aureus (Sacol2443), respectively, generated with the Geneious software package (63). Amino acids in black, gray, and white have an amino acid similarity of >80, 60–80, or <60%, respectively. B, predicted membrane topology of KimALmo overlaid with a MUSCLE alignment between KimABsu and KimALmo. The dashed line indicates the position at which the KimALmo protein was truncated. Amino acids in black are identical; amino acids in gray are similar, and amino acids in white are nonsimilar.
In vivo activities of the L. monocytogenes potassium transporters
To assess whether KdpABC, KimA, and KtrCD from L. monocytogenes are active in potassium transport, we cloned the kdpABC, kimA, and ktrCD genes using the plasmid pWH844, which allows IPTG-dependent expression of heterologous genes in E. coli (48). We also cloned a truncated kimALmo gene encoding the ΔC-KimALmo variant lacking 152 amino acids of the C-terminal cytosolic domain. Furthermore, we cloned the kimASau gene from S. aureus, to evaluate whether KimA homologs from other Firmicutes are involved in potassium uptake. The resulting plasmids were used to transform the E. coli strain LB650 that is unable to take up potassium via the native uptake systems Kup, KdpABC, TrkG, and TrkH (49). The strain is suitable to study potassium transporters because it is only viable in minimal medium supplemented with potassium concentrations above 15 mm KCl (see Fig. 4). The empty plasmid and a plasmid encoding the B. subtilis ktrAB genes, which were previously shown to mediate potassium transport in E. coli LB650 (5), served as negative and positive controls, respectively. The cells were grown during the day in M9 medium with 50 mm KCl and without IPTG induction, collected by centrifugation, washed in potassium-free medium, and propagated on M9 minimal medium plates without and with 10 mm IPTG. As shown in Fig. 2, with the exception of the strain harboring the plasmid for kimASau expression, the bacteria could not grow in the absence of IPTG. The weak growth of the cells containing the kimASau gene could be due to a leaky promoter and due to the high affinity of the encoded KimASau transporter for potassium (see below). By contrast, the strains carrying the ktrABBsu, kimALmo, and ktrCDLmo, could grow with low amounts of K+ when these genes were induced with IPTG (Fig. 2). Moreover, the kimALmo variant lacking the C-terminal domain (kimALmo ΔC) also supported growth of the strains, albeit less well than the full-length protein. These results indicate that the N-terminal extracellular domain and the 11 transmembrane helices of KimALmo from L. monocytogenes are sufficient for mediating potassium import in E. coli (Fig. 2). Expression of ktrABBsu from B. subtilis restores growth on low potassium concentrations, agreeing with previous reports that KtrABBsu is a high-affinity potassium transporter (5, 43). Expression of KimASau from S. aureus in the E. coli strain LB650 resulted in much better growth than those strains expressing KimALmo and KtrCD, indicating that KimASau is likely a high-affinity potassium transporter (Fig. 2). Thus, KimALmo and KtrCDLmo from L. monocytogenes as well as KimASau from S. aureus are indeed potassium transporters. The putative potassium transporter KdpABCLmo did not support growth of the E. coli strain LB650 irrespective of whether the kdpABCLmo genes were expressed from the IPTG- and arabinose-dependent plasmids pWH844 and pBAD24 (data not shown; see “Experimental procedures”). Therefore, the KdpABCLmo system was not further analyzed in regard to its affinity to potassium ions and in vivo inhibition by c-di-AMP.
Figure 4.
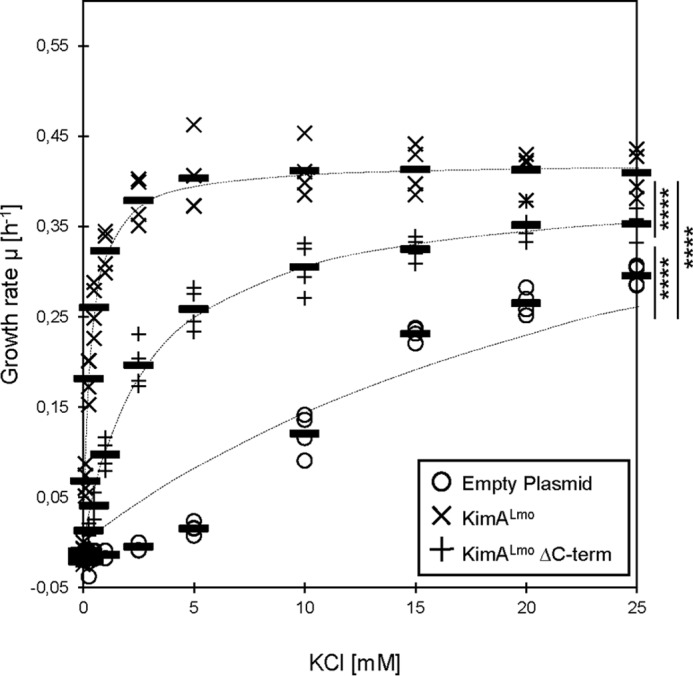
Potassium-dependent growth of E. coli synthesizing the full-length and the C-terminally truncated KimALmo protein. E. coli LB650 strains harboring plasmids pWH844 (empty plasmid), pBP384 (kimALmo), and pBP396 (kimALmo ΔC terminus) were grown to an OD600 of 0.3–0.5 in M9 minimal medium supplemented with 50 mm KCl. The cells were washed for 1 h in potassium-free M9 medium. Multiwell plate reader growth assays with different KCl concentrations were performed (n = 4). The growth rates were plotted against the KCl concentrations and fitted to the Michaelis–Menten equation. Bars, means; dashed lines, fitted curves. Significant differences between the fitted curves are shown (p < 0.0001 (****)) (F(4,2307) = 540.1, one-way ANOVA with Tukey's post hoc test).
Figure 2.
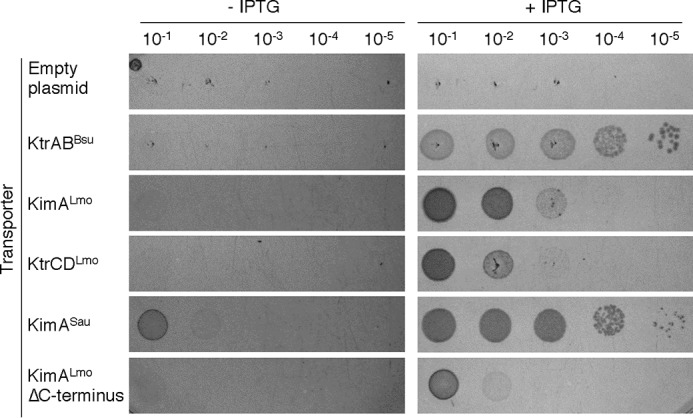
Drop dilution assay to assess the activities of putative potassium transporters. E. coli LB650 strains harboring plasmids pWH844 (empty plasmid), pBP372 (ktrABBsu, positive control), pBP384 (kimALmo), pBP396 (kimALmo ΔC terminus), pBP385 (KimSau), and pBP371 (ktrCDLmo) were grown to an OD600 of 0.3–0.5 in M9 minimal medium supplemented with 50 mm KCl. The cells were washed for 1 h in potassium-free M9 medium, the OD600 was adjusted to 0.1, the suspension was serially 10-fold diluted, and 5 μl of the diluted cell suspensions were plated on M9 plates containing 10 mm KCl. IPTG was added to a final concentration of 50 μm to induce the expression of the transporter genes. The plates were incubated for 24 h at 37 °C.
Apparent affinities of KimA and KtrCD for potassium
To determine the apparent affinities of KimALmo, the KimALmo ΔC terminus variant (ΔC-KimALmo) and KtrCD from L. monocytogenes and KimASau from S. aureus, we determined the growth rates of the E. coli strain LB650 synthesizing the potassium transporters in M9 minimal medium supplemented with different amounts of potassium. The strains carrying the empty plasmid and expressing the B. subtilis ktrAB genes served as negative and positive controls, respectively. The growth rates were plotted against the potassium concentrations and fitted to the Michaelis–Menten equation (5). The Vmax values and the apparent affinities are summarized in Table 1. As shown in Figs. 3 and 4, each E. coli strain required a different concentration of external potassium to reach half-maximal growth; the strains synthesizing the transporters KtrCDLmo, KimALmo, ΔC-KimALmo, KimASau, and KtrABBsu required 6.30 ± 2.06, 0.35 ± 0.12, 2.99 ± 0.65, 0.14 ± 0.02, and 0.03 ± 0.01 mm, respectively. These results demonstrate that KtrCDLmo and KimALmo from L. monocytogenes are transporters with low and moderately high affinities for potassium, respectively. Moreover, the C-terminal intracellular domain of KimALmo is important for full activity of the transporter (Figs. 1B and 4). In contrast to KtrCDLmo and KimALmo, KimASau from S. aureus is a high-affinity potassium transporter, which is in line with the observation that the E. coli strain LB650 synthesizing KimASau and KtrABBsu grew comparatively well with low amounts of potassium (Fig. 2).
Table 1.
Michaelis–Menten constants of the potassium transporters
Mean values of the Km and S.E. are shown (n = 4). p values were always <0.0001 (****) compared with empty plasmid alone (F(8,18) = 54.69, ANOVA with Dunnett's post hoc test). Mean values of the Vmax and S.E. are shown (n = 4). p < 0.01 (**), p < 0.001 (***), p < 0.0001 (****) compared with empty plasmid alone (F(5,18) = 14.5, ANOVA with Dunnett's post hoc test).
| Empty vector | KtrABBsu | KimALmo | KimALmo ΔC terminus | KtrCDLmo | KimASau | |
|---|---|---|---|---|---|---|
| Apparent Km (mm KCl) | 56.28 ± 14.62 | 0.03 ± 0.01 | 0.35 ± 0.12 | 2.99 ± 0.65 | 6.30 ± 2.06 | 0.14 ± 0.02 |
| Vmax (μ (h−1)) | 0.82 ± 0.13 | 0.73 ± 0.05 | 0.52 ± 0.04 (***) | 0.40 ± 0.01 (****) | 0.59 ± 0.12 (**) | 0.62 ± 0.05 (**) |
Figure 3.
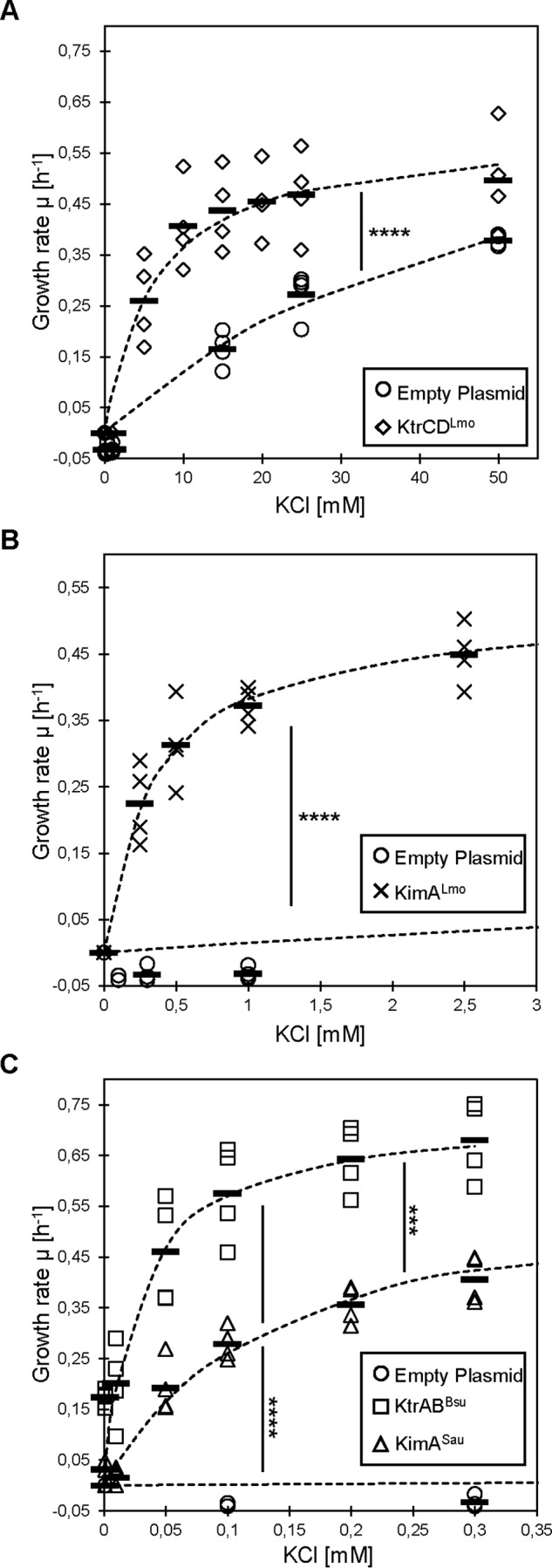
Potassium-dependent growth of E. coli synthesizing potassium transporters from L. monocytogenes and S. aureus. A, E. coli LB650 strain harboring the plasmids pWH844 (empty plasmid) and pBP371 (ktrCDLmo). B, E. coli LB650 strain harboring the plasmid pBP384 (kimALmo). C, E. coli LB650 strain harboring the plasmids pBP372 (ktrABBsu) and pBP385 (kimSau). The strains were grown to an OD600 of 0.3–0.5 in M9 minimal medium supplemented with 50 mm KCl. The cells were washed for 1 h in potassium-free M9 medium. Multiwell plate reader growth assays with different KCl concentrations were performed (n = 4). The growth rates were plotted against the KCl concentrations and fitted to the Michaelis–Menten equation. Bars, means; dashed lines, fitted curves. Significant differences between the fitted curves are shown (p < 0.001 (***) and p < 0.0001 (****)) (F(2,2997) = 1480, one-way ANOVA with Tukey's post hoc test).
Inhibition of KimA and KtrCD potassium transport activity by c-di-AMP
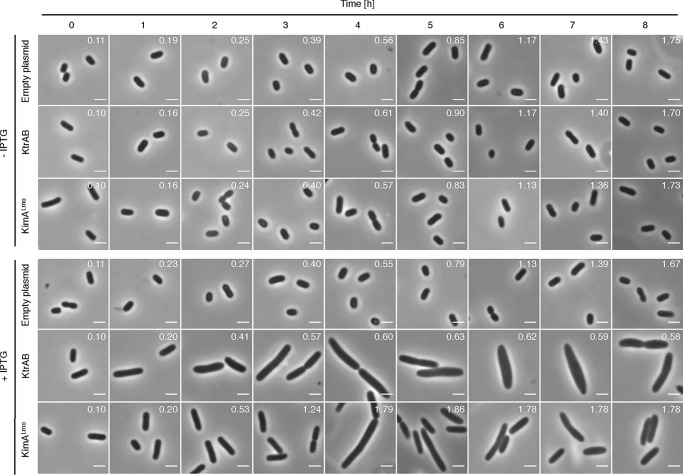
Several recent studies indicate that c-di-AMP is essential for viability of Gram-positive bacteria like B. subtilis, L. lactis, L. monocytogenes, S. agalactiae, and S. aureus because the nucleotide controls influx of osmolytes like potassium whose accumulation leads to cell lysis due to water uptake (5, 14, 15, 25, 26). Thus, either synthesis of the potassium transporters or their activity or both need to be tightly regulated. As shown in Fig. 5, the IPTG-dependent overexpression of the ktrABBsu and kimALmo genes encoding high-affinity potassium transporters KtrABBsu and KimALmo, respectively, in E. coli during growth in M9 minimal medium caused a strong increase of the cellular volume. Moreover, the growth of E. coli synthesizing the higher-affinity KtrABBsu transporter was in addition significantly reduced, as illustrated by the decline of the optical density (Fig. 5, top right corners). By contrast, in the absence of the inducer IPTG, the growth and the volume of the cells containing the ktrABBsu and kimALmo genes were indistinguishable from that of the cells carrying the empty vector. Thus, once sufficient potassium has been taken up by the bacteria to cope with the osmolarity of the environment, the activities of osmolyte transporters have to be reduced to prevent further ion uptake and cell lysis. It has indeed been demonstrated that the activity of the cytoplasmic gating component of the transporters KtrCB and KtrCD from S. aureus as well as the KimABsu transporter from B. subtilis are inhibited by c-di-AMP (7, 39). Like KimABsu from B. subtilis, the KimALmo homolog from L. monocytogenes belongs to a novel class of high-affinity potassium transporters (see above) (5). However, whether c-di-AMP directly binds to KimALmo and KtrCDLmo to inhibit the transport activity of the proteins has not been tested so far.
Figure 5.
Impact of unregulated potassium import on the cell volume of E. coli. Derivatives of the E. coli strain LB650 harboring plasmids pWH844 (empty plasmid), pBP372 (ktrABBsu), and pBP384 (kimALmo) were grown overnight in LB-K medium. The cells were washed and cultivated in M9 medium without and with 1 mm IPTG for the induction of the transporter genes. The OD600, which is shown in the top right corners of the microscopic pictures, was measured in hourly intervals. Scale bar, 2 μm.
To assess whether c-di-AMP affects the activity of KimALmo and KtrCDLmo, we established a co-expression system using the E. coli strain LB2003, which carried unmarked mutations in the kdp, kup, and trk genes, and enable the use of multiple plasmids encoding chloramphenicol and ampicillin resistance genes (49). Like the E. coli strain LB650, LB2003 is deficient in the Kdp, Kup, and Trk potassium uptake systems and is therefore only able to grow at low potassium concentrations when synthesizing a potassium transporter. Moreover, E. coli lacks c-di-AMP–producing and c-di-AMP–degrading enzymes, which is a prerequisite to assess the phenotypic effect of c-di-AMP on the activity of KimALmo and KtrCDLmo. The plasmids pBP384 (kimALmo), pBP396 (ΔC-kimALmo), and pBP371 (ktrCDLmo) were used for the IPTG-dependent expression of ΔC-KimALmo, KimALmo, and KtrCDLmo, respectively. The empty plasmid pWH844 served as a negative control. The L. monocytogenes DAC CdaA and the inactive CdaA* variant D171N (50) are encoded by the arabinose-inducible plasmids pBP370 and pBP373, respectively. The strains carrying pWH844, pBP384, pBP396, and pBP371 as well as either of the two DAC-encoding plasmids were grown in M9 minimal medium supplemented with 30, 0.35, 3, and 7 mm KCl, respectively, conditions that allow half-maximal growth of the bacteria. As shown in Fig. 6, growth of the strains carrying the empty plasmid pWH844, and synthesizing the active and the catalytically inactive CdaA and CdaA* variants, respectively, was not reduced. Thus, neither the DAC proteins nor c-di-AMP affect growth of the E. coli strain. By contrast, growth of the bacteria synthesizing KimALmo and KtrCDLmo was reduced when the active DAC CdaA was co-produced, indicating that c-di-AMP inhibits the transporter with a moderately high affinity for potassium and to a lesser extent also the low-affinity transporter (Fig. 6). Growth was not affected in the absence of a functional DAC and, thus, of c-di-AMP production. Moreover, c-di-AMP did not affect the activity of the C-terminally truncated ΔC-KimALmo variant, indicating that the C-terminal intracellular domain of the transporter contributes to c-di-AMP-dependent regulation (see “Discussion”). Surprisingly, c-di-AMP did not inhibit the activity of KimASau from S. aureus (data not shown). To conclude, the potassium transporters KimALmo and KtrCDLmo from L. monocytogenes are both inhibited by c-di-AMP.
Figure 6.
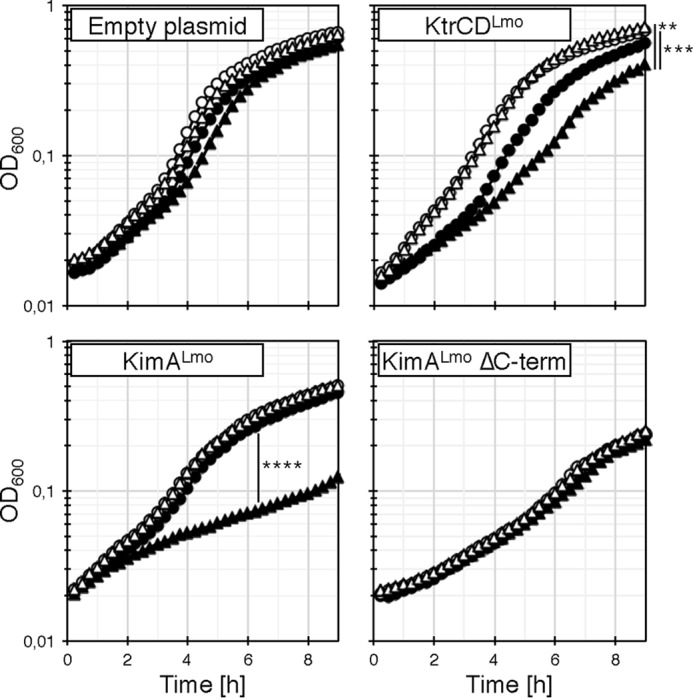
Inhibition of potassium transporters by c-di-AMP. The E. coli strain LB2003 harboring the plasmids pWH844 (empty plasmid), pBP371 (ktrCDLmo), pBP384 (kimALmo), pBP396 (kimALmo ΔC terminus) and either pBP370 (cdaA; filled symbols) or pBP373 (cdaA D171N; unfilled symbols) was grown to an OD600 of 0.3–0.5 in M9 medium and washed for 1 h in potassium-free M9 medium. The growth assays were performed with (triangles) or without (circles) 0.005% (w/v) l-arabinose and at KCl concentrations that are equal to the Km values of the transporters. Data are means (n = 3), and there were no significant differences between the strains harboring the empty plasmid (F(3,260) = 0.6496; p = 0.5838). The same is valid for the strains harboring the plasmid for the expression of the kimALmo ΔC terminus variant (F(3,260) = 0.5085; p = 0.6768). There were significant differences between the strains harboring the plasmid for the expression of the ktrCD (F(3,260) = 6.588; p = 0.0003) or kimA genes (F(3,260) = 12.43; p < 0.0001). p < 0.05 (**), p < 0.001 (***), and p < 0.0001 (****) (one-way ANOVA with Tukey's post hoc test).
Effect of potassium on growth of a c-di-AMP-free L. monocytogenes strain
Previously, it has been shown that c-di-AMP is essential in B. subtilis to control the uptake of potassium to toxic levels (5). To investigate whether c-di-AMP is also essential for the control of potassium uptake in L. monocytogenes, we constructed a markerless deletion of the cdaA gene, encoding the sole c-di-AMP–synthesizing enzyme. As described previously, we confirmed that the cdaA mutant is not viable on complex, but on chemically defined growth medium (10, 11). We prepared Listeria synthetic medium (LSM) (11) without potassium (LSM-K+) and observed that potassium concentrations below 1 mm impair the growth of the L. monocytogenes WT strain (Fig. 7). The growth behavior of the WT strain was not affected at potassium chloride concentrations higher than 1 mm. The L. monocytogenes cdaA mutant shows a slightly slower growth than the WT at potassium concentrations above 1 mm. However, high potassium concentrations did not fully inhibit growth of the L. monocytogenes cdaA mutant strain as it has been shown for a c-di-AMP-free strain of B. subtilis (see Fig. 7) (5).
Figure 7.
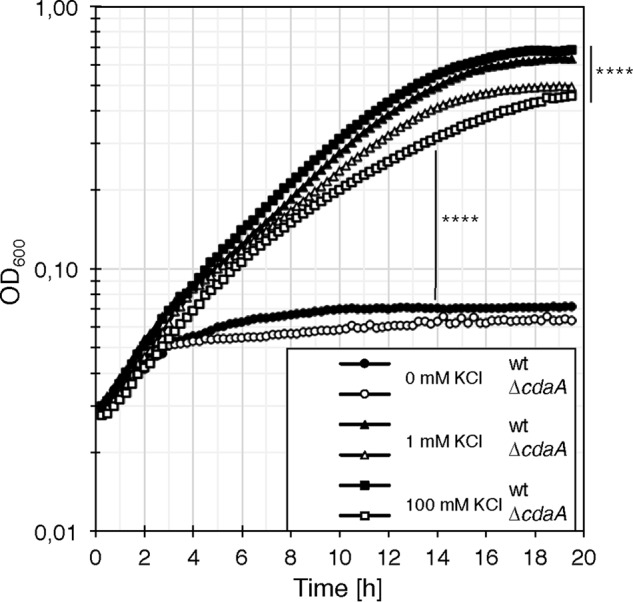
Effect of potassium on growth of the L. monocytogenes cdaA mutant. The L. monocytogenes WT strain EGD-e (filled symbols) and the cdaA mutant strain (nonfilled symbols) were grown overnight in LSM-K+ with 1 mm potassium shaking at 37 °C. Cells were washed in LSM-K+, grown in LSM-K+, washed again, and subsequently used to inoculate LSM-K+ medium with the indicated concentrations of KCl (0 mm (circles), 1 mm (triangles), and 100 mm (squares)). Bacteria were grown at 37 °C. Data are means (n = 3). Significant differences between the WT or the ΔcdaA strains (0 mm KCl) and the strains grown with KCl and between WT and the ΔcdaA strains grown with 100 mm KCl are depicted (p < 0.0001 (****)) (F(4,462) = 46.38, one-way ANOVA with Tukey's post hoc test).
c-di-AMP interaction with the KimA homologs and KtrCDLmo
To assess the interaction between c-di-AMP and the potassium transporters or their regulators, we performed a differential radial capillary action of ligand assay (DRaCALA) with the proteins KimALmo, ΔC-KimALmo, KimASau, KtrCLmo, KdpABCLmo, and KdpDLmo (see “Experimental procedures”). We also tested the interaction between c-di-AMP and the 156- and 158-amino-acid-long C-terminal cytosolic domains of KimALmo and KimASau, respectively. This domain could be involved in the c-di-AMP–dependent control of KimA potassium transport activity. The lysate of the E. coli strain DH5α containing the empty plasmid pWH844 or pGP172 served as a negative controls. Whereas the majority of the proteins showed no specific interaction with c-di-AMP in the DRaCALA assay, KtrCLmo, the cytosolic protein of the KtrCD potassium transporter, and KdpDLmo, the sensor kinase of the KdpDE two-component system, gave positive results (Fig. 8). To conclude, the potassium transport activity of KtrCDLmo from L. monocytogenes is inhibited by c-di-AMP in vivo, and the nucleotide binds to the KtrC subunit of the KtrCDLmo transporter and to KtrD of the KdpDELmo two-component system in vitro. Due to toxicity, we were unable to purify the full-length KimALmo protein. The failure of purification of the C-terminal part of KimA also precludes further in vitro characterization.
Figure 8.
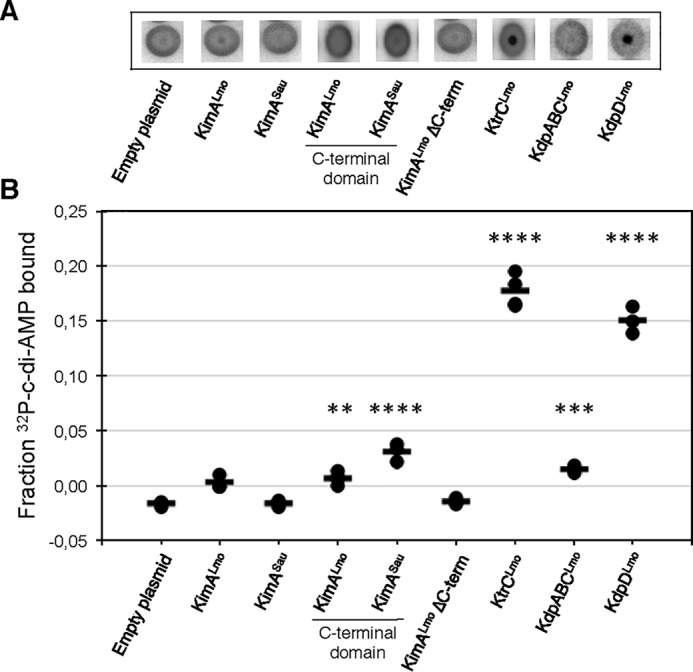
Interaction between c-di-AMP and potassium transporters determined by DRaCALA. A, autoradiographs showing the interaction between [32P]c-di-AMP and the potassium transporters as well as the truncated variants that are present in whole-cell lysates of the E. coli strains Rosetta (DE3) carrying the plasmid pGP172 (empty plasmid) or the derivatives pBP265 (kimALmo), pBP266 (kimALmo ΔC terminus), pBP267 (kimASau), or NEB T7 Express Iq carrying the plasmid pWH844 (empty plasmid) or the derivatives pBP346 (kimALmo C-terminal domain), pBP347 (kimASau C-terminal domain), pBP345 (ktrCLmo), pBP559 (kdpABCLmo), and pBP560 (kdpDLmo). Both empty vectors showed similar nonbinding (data not shown). B, fraction bound of [32P]c-di-AMP is shown for lysates from E. coli induced overnight for the expression of the indicated gene. Bars, means (n > 3). p < 0.01 (**), p < 0.001 (***), and p < 0.0001 (****) compared with empty plasmid alone (F(8,20) = 275.9, ANOVA with Dunnett's post hoc test).
Discussion
Here, we have identified and characterized the potassium transporters KtrCDLmo and KimALmo from L. monocytogenes. As stated above, the KtrCD homologs from L. monocytogenes and B. subtilis show 64% overall sequence identity and have similar affinities for potassium (see Table 1) (43). We also demonstrate that S. aureus possesses a homolog of KimA (Sacol2443). The KimA homologs from S. aureus and L. monocytogenes belong to a novel class of high-affinity potassium transporters that are active at low external potassium concentrations (5). Moreover, the potassium transport activity of KtrCDLmo and KimALmo from L. monocytogenes is inhibited by c-di-AMP (Fig. 6). Furthermore, we show that the C-terminal cytosolic domain is important for the c-di-AMP–mediated regulation of KimALmo in vivo because the C-terminally truncated variant lacking 156 amino acids did not respond to the nucleotide. Recently, it has been shown that c-di-AMP binds to the KimA homolog from B. subtilis and controls the uptake of potassium by the transporter in vivo (39). Unfortunately, we could not show the binding of c-di-AMP to the full-length KimALmo protein and to the C-terminal domain of KimALmo. However, we speculate that binding of c-di-AMP to the cytosolic domain is required of for regulation of KimALmo in vivo (Fig. 6). Therefore, it might be worthwhile to study the role of the C-terminal domain in controlling the activity of the high-affinity potassium transporter KimA. Surprisingly, the KimALmo transporter from L. monocytogenes has a much lower affinity for potassium than the homolog from B. subtilis (39, 43). As the external concentrations of potassium are rather low, it is tempting to speculate that L. monocytogenes possesses an additional high-affinity potassium transporter to be able to compete with other bacteria when the extracellular potassium is scarce. The phylogenetically related bacteria B. subtilis and S. aureus contain two high-affinity potassium transport systems that are active during growth at low potassium concentrations. B. subtilis employs the high-affinity potassium transporters KtrAB and KimA under potassium-limiting growth conditions (5, 39, 43). Previously, it has been shown that S. aureus relies on the high-affinity transporter KdpFABC, whose synthesis and activity is regulated by the two-component system KdpDE (38, 44, 45). KimASau could also be important for growth of S. aureus when the extracellular potassium concentrations are low. In contrast to S. aureus, the KdpABC homolog of L. monocytogenes does not seem to contribute to potassium uptake. It has been shown previously that the small membrane protein KdpF is required for proper function of the E. coli Kdp potassium transport system (46). As described above, no KdpF homolog is present in L. monocytogenes. Therefore, the lack of KdpF in L. monocytogenes could be the reason why the KdpABC system is not active in potassium transport. Interestingly, we found that c-di-AMP binds to the sensor kinases KdpD of the KdpDE two-component system that might be involved in controlling the expression of the kdpABC genes in L. monocytogenes (Fig. 8). However, our comparative RNA-Seq experiments using the WT strain and a c-di-AMP–free cdaA mutant strain in chemically defined medium revealed that the lack of c-di-AMP does not alter the expression of genes involved in potassium uptake (data not shown). In B. subtilis, it has been shown that the 5′-UTRs of the kimA and ktrAB genes contain ydaO riboswitches preventing synthesis of the transporters in the presence of c-di-AMP (5, 51). However, c-di-AMP–dependent riboswitches that could be involved in controlling the expression of potassium transporter genes are absent in L. monocytogenes. Therefore, it remains to be elucidated under which conditions the potassium transporter genes are transcribed. Moreover, it has to be investigated whether c-di-AMP controls the expression of the kimA, ktrC, ktrD, and kdpABC genes in L. monocytogenes at all.
As stated above, during growth under hyperosmotic conditions many bacteria take up potassium ions to prevent water efflux from the cytosol and to increase the cellular turgor (1–4). Once the cellular turgor has been adjusted to the environmental osmolarity, the transport of potassium ions across the cell membrane has to be reduced to prevent osmotic swelling and cell lysis (1–4). A reduction of the ion uptake might be achieved either by proteolytic degradation or by controlling the activity of the transporters through binding of low-molecular weight ligands. It has indeed been shown that transport systems are rapidly degraded when the respective substrates are not available (52). However, the cellular turgor is a physical variable that changes rapidly and needs to be tightly adjusted (1–4). Thus, it is obvious that the proteolytic degradation of transport systems would be too slow to allow the bacteria to prevent potassium uptake to toxic levels. However, the tight control of the cellular turgor requires the existence of low-molecular weight ligands, which specifically modulate the activity of potassium transporters and other osmolyte uptake systems. In S. aureus, it has been shown that the low-affinity potassium transporters KtrCB and KtrCD are inhibited by the second messenger c-di-AMP that binds to the RCK_C (regulator of conductance of K+) domain of the KtrC gating component (7). Moreover, c-di-AMP binds to the CabP protein and prevents potassium uptake by the CabP-TrkH protein complex in S. pneumoniae (8). The cytoplasmatic regulatory subunit KtrC of the KtrCD potassium transporter is also bound by c-di-AMP in Mycoplasma pneumoniae (53). Recently, it has been demonstrated that the potassium importers KupA and KupB of L. lactis are inhibited by c-di-AMP (38). Here, we show that the potassium transporters KtrCDLmo and KimALmo from L. monocytogenes are inhibited by the second messenger c-di-AMP. This study also revealed that the uncontrolled influx of potassium ions via the KtrABBsu and KimALmo results in osmotic swelling of E. coli (Fig. 5). Recently, it has been shown that c-di-AMP inhibits the potassium transport activity of the KimA homolog from B. subtilis (39). In this organism, c-di-AMP is required to reduce potassium uptake to toxic levels. As described above, c-di-AMP also controls the uptake of potassium at the level of transcription. For instance, c-di-AMP inhibits the sensor kinase KdpD of the KdpDE two-component system and thus reduces the expression of the kdpFABC operon encoding the high-affinity KdpFABC potassium transport system from S. aureus (41, 45). Moreover, c-di-AMP prevents the expression of the ktrAB and kimA mRNAs in B. subtilis, thereby reducing expression of the high-affinity potassium transporters KtrAB and KimA, respectively (5, 51). It should be noted that c-di-AMP also inhibits the uptake of other osmolytes, such as glycine betaine and carnitine (11–13, 15, 26). Thus, c-di-AMP plays a central role in controlling the activities of potassium transporters and other osmolyte uptake systems, and the c-di-AMP–dependent regulation can occur at two different levels in a variety of bacteria.
Recently, it has been demonstrated that the control of potassium uptake is an essential function of c-di-AMP in B. subtilis (5). A B. subtilis strain lacking all c-di-AMP–producing enzymes was only viable in medium containing low potassium concentrations. c-di-AMP is also essential in bacteria like L. monocytogenes, S. agalactiae, and S. aureus to prevent uptake of osmolytes to toxic levels (10, 15, 26). However, in these bacteria, the control of glycine betaine and amino acid uptake seems to be the essential function of c-di-AMP. This could explain why an increase of external osmolarity, either by sodium or potassium chloride, rescues the growth defect of a cdaA mutant strain in complex media, irrespective of the ion (11). We furthermore show that high amounts of potassium only slightly inhibit the growth of the cdaA mutant in defined medium (Fig. 7). Thus, the physiological impact of the c-di-AMP–dependent control of the potassium transporters seems to be less pronounced in L. monocytogenes than in bacteria like B. subtilis (5). In fact, phylogenetically related bacteria have evolved species-specific mechanisms to regulate the cellular turgor using different osmolytes, but they all use c-di-AMP in this essential process (6). It remains to be elucidated how c-di-AMP controls potassium homeostasis in L. monocytogenes. Moreover, it will be crucial to identify the osmo-signal–sensing mechanism of the c-di-AMP system, which could be conserved among different bacteria (6, 15).
Experimental procedures
Bacterial strains and growth conditions
The bacterial strains are listed in Table 2. The E. coli strains XL1-Blue (Stratagene), Rosetta (DE3) (Novagen), and T7 Express Iq (New England Biolabs) were used for cloning and protein overproduction. E. coli was grown in LB medium, and transformants were selected on LB plates (15 g/liter Bacto agar (Difco)) containing kanamycin (50 μg ml−1), ampicillin, carbenicillin (100 μg ml−1), or chloramphenicol (30 μg ml−1). The L. monocytogenes WT strain EGD-e (laboratory strain collection) was cultivated in brain heart infusion medium (Sigma-Aldrich, Darmstadt, Germany). For the deletion of the cdaA gene, the LSM was used as described previously (11), with the following minor changes (equimolar substitutions): riboflavin-5′-monophosphate instead of riboflavin; l-isoleucine, l-methionine, and l-valine instead of the dl-enantiomers; and l-cysteine·HCl·H2O instead of l-cysteine·2HCl. For experiments with defined potassium concentrations, the KH2PO4 component of the “phosphate stock solution” was replaced by equimolar concentrated NaH2PO4, and for the other 43 components, no chemicals containing potassium salts were used. The LSM without potassium was subsequently adjusted to the depicted concentrations, by the addition of KCl. Erythromycin and 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) were used in the deletion process at concentrations of 5 or 100 μg ml−1, respectively. For pouring minimal medium agar plates, 2-fold concentrated medium was prewarmed to 37 °C and mixed with 70 °C prewarmed 2-fold Bacto agar, directly before pouring the plates. The B. subtilis WT strain 168 (laboratory strain collection) was cultivated in LB medium. Potassium transporter–deficient E. coli strains LB650 and LB2003 were cultivated in LB-K medium (NaCl substituted by 1% KCl (w/v)) (49). M9 medium was used for E. coli growth experiments with the following composition: 37.85 mm Na2HPO4, 22.05 mm KH2PO4, 18.75 mm NH4Cl, 1 mm MgSO4, 0.1 mm CaCl2, 0.5 μm FeCl3, 28 mm d-glucose or glycerol as sources of carbon. For the E. coli strain LB650, the M9 medium was supplemented with amino acids l-valine, l-isoleucine, l-methionine, l-proline, and l-serine (each 0.02% (w/v)) and 3 μm thiamine. For the E. coli strain LB2003, the M9 medium was supplemented with 0.0066% (w/v) casein hydrolysate (acid) (Oxoid), 0.004% (w/v) l-proline, and 3 μm thiamine. For experiments with defined potassium concentrations, the KH2PO4 salt was replaced by NaH2PO4, and KCl was added as indicated. If not otherwise specified, IPTG was used at a concentration of 50 μm, and l-arabinose was used at 0.005% (w/v).
Table 2.
Strains
| Name | Genotype | Description | Reference |
|---|---|---|---|
| E. coli | |||
| XL1-Blue | recA1 endA1 gyrA96 thi-1 hsdR17 supE44 relA1 lac [F' proAB lacIq ZΔM15 Tn10 (Tetr) ] | Cloning | Stratagene |
| LB650 | F− thi lacZ gal rha kup1 (trkD1) ΔkdpABC5 ΔtrkH (Cmr) ΔtrkG (Kanr) | Potassium uptake studies | Ref. 54 |
| LB2003 | F− aroE rpsL metE thi gal rha kup1 (trkD1) ΔkdpABC5 ΔtrkA aroE+ | Potassium uptake studies | Ref. 54 |
| Rosetta (DE3) | F− ompT hsdSB(rB− mB−) gal dcm (DE3) pRARE (Cmr) | Protein expression | Novagen |
| NEB T7 Express Iq | MiniF lacIq(CamR)/fhuA2 lacZ::T7 gene1 [lon] ompT gal sulA11 R(mcr-73::miniTn10–TetS)2 [dcm] R(zgb-210::Tn10–TetS) endA1 Δ(mcrC-mrr)114::IS10 | Protein expression and DRaCALA | New England Biolabs |
| B. subtilis | |||
| 168 | trpC2 | WT | Laboratory collection |
| L. monocytogenes | |||
| EGD-e | WT | Serotype 1/2a strain | Laboratory collection |
| BPL77 | ΔcdaA | L. monocytogenes strain without a DAC | This work |
DNA manipulation
Transformation of E. coli was performed using standard procedures (54). Plasmid DNA was extracted using the NucleoSpin Plasmid Kit (Macherey and Nagel). Restriction enzymes, T4 DNA ligase, and DNA polymerases were used as recommended by the manufacturers. DNA fragments were purified using the PCR purification kit (Qiagen). DNA sequences were determined by the Microsynth sequencing laboratories (Göttingen, Germany). Chromosomal DNA of L. monocytogenes or B. subtilis was isolated using the NucleoSpin Microbial DNA Kit (Macherey and Nagel). Chromosomal DNA of S. aureus COL was kindly provided by Dr. Jan Pané-Farré (University of Greifswald, Germany). Oligonucleotides were purchased from Sigma-Aldrich (Darmstadt, Germany).
Plasmid construction
The genes encoding putative potassium transporters were introduced into the vector pWH844, allowing IPTG-dependent expression in E. coli (48). The kimALmo and kimASau genes were amplified using the oligonucleotide pairs JH95/JH96 and JH97/JH98, respectively (Table 3). The PCR products were EcoRI/BamHI-digested and ligated to pWH844. The resulting plasmids were designated as pBP384 and pBP385 (Table 4). To study the role of the C-terminal domain of KimALmo, the plasmid pBP396 was constructed. The truncated kimALmo gene was amplified with the oligonucleotide pair JH95/JH120, digested with EcoRI/BamHI, and ligated to pWH844. The plasmid pBP371 for the expression of the L. monocytogenes ktrCD genes was constructed as follows. The ktrC and ktrD genes were amplified using the oligonucleotide pairs JH59/JH60 and JH61/JH62, respectively, and fused by splicing by overhang extension (SOE) PCR using primer pair JH59/JH62 (55). The resulting PCR product was digested with EcoRI and BamHI and ligated to pWH844. The plasmids pBP559 and pBP563 were constructed for the expression of the L. monocytogenes kdpABC genes. The kdpABC genes were amplified using the oligonucleotide pairs MI1/MI2 and MI21/MI2, respectively. The PCR products were digested with BamHI/PstI and KpnI/PstI and ligated to the plasmids pWH844 and pBAD24, respectively. The plasmid pBP560 served for the expression of the L. monocytogenes kdpD gene. The kdpD gene was amplified by PCR with the oligonucleotide pair MI11/MI13. The BamHI/PstI-digested PCR product was ligated to the plasmid pWH844. The plasmids pBP370 and pBP373 were constructed for producing the WT CdaA enzyme and the inactive D171N variant (50). The cdaA gene was amplified using the oligonucleotide pair JH51/JH52 and introduced into the XbaI/PstI sites of pBAD33 (56). For the construction of plasmid pBP373, we used the oligonucleotide pair JH51/JH52 together with the 5′-phosphorylated oligonucleotide JR18 to introduce the D171N mutation via the combined chain reaction (57). The pBAD33 and pWH844 expression vectors have compatible selection markers and origin of replications, allowing the co-expression of potassium transporter (from pWH844) and cdaA genes (from pBAD33). The plasmids pBP345, pBP346, and pBP347 were constructed to study the binding of c-di-AMP to KtrC and the cytosolic domains of KimALmo (aa 452–607) and KimASau (aa 452–609). The respective genes were amplified using the oligonucleotide pairs GH5/GH6, GH7/GH8, and GH9/GH10, digested with BamHI/SalI, and ligated to pWH844 cut with the same enzymes. The genes encoding the full-length KimALmo and KimASau proteins as well as the C-terminally truncated KimALmo variant (aa 1–455) were amplified using oligonucleotide pairs JH142/JH96, JH143/JH98, and JH142/JH120, respectively. The PCR products were digested with SacI/BamHI and ligated to pGP172 (58). The resulting plasmids were designated as pBP265 (kimALmo), pBP267 (kimASau), and pBP266 (kimALmo ΔC terminus). The plasmids are suitable for the IPTG-dependent overproduction of the transporters with an N-terminal Strep-tag II in the E. coli strain Rosetta (DE3). For the chromosomal deletion of the cdaA gene, pBP352 was constructed (Table 4). The up- and downstream regions of cdaA, while leaving the cdaA ORF out, were amplified using oligonucleotide pairs JH05/JH06 and JH07/JH08, respectively (Table 3). The resulting PCR products were fused by SOE PCR using oligonucleotides JH05 and JH08, digested with EcoRI and BamHI, and ligated to pMAD (55, 59), which was digested using the same enzymes.
Table 3.
Oligonucleotides
Restriction sites are underlined, and complementary regions are in boldface type.
| Name | Sequence | Purpose |
|---|---|---|
| GH5 | 5′-AAAGGATCCATGAAAGAAGGATTTGCAGTCATCGGTCTTG | pBP345 |
| GH6 | 5′-TTTGTCGACTTATTGAATTTTTTCTTGTAGTCGTTCAATGTCATCATCC | pBP345 |
| GH7 | 5′-AAAGGATCCCATTACCGGAAAGTTGGACCACAACTTAG | pBP346 |
| GH8 | 5′-TTTGTCGACTTATTCTTTTAAATGATAAGGATATGTGGAAACTACTACATCC | pBP346 |
| GH9 | 5′-AAAGGATCCCATTATCGAGATATCGCAGAACAATTACGTTCTG | pBP347 |
| GH10 | 5′-TTTGTCGACCTATTTTTTAAGTTTAAATGGAATTGTACATACGTTAACATTCTTTTG | pBP347 |
| JH05 | 5′-AAAGAATTCAGAATTGCGTTCCACGGATACATTAAAAC | pBP352 |
| JH06 | 5′-CCTCCTTTCGTCGACGTGCCTCTTGAAAACCATTTATAATCAC | pBP352 |
| JH07 | 5′-AAGAGGCACGTCGACGAAAGGAGGCAAAAGCGAATGATG | pBP352 |
| JH08 | 5′-TTTGGATCCCACTTTCCGGCGTGCCTTCTTG | pBP352 |
| JH51 | 5′-AAATCTAGACACGGAGGTGAAGTGATGGATTTTTCCAATATGTCGATATTGCAT | pBP370/pBP373 |
| JH52 | 5′-TTTCTGCAGTCATTCGCTTTTGCCTCCTTTCCA | pBP370/pBP373 |
| JH59 | 5′-AAAGAATTCAAGGAGGTAACGTACACATGAAAGAAGG | pBP371 |
| JH60 | 5′-AATCTTCTGCTAAGTACGGCTTTTTATTGAATTTTTTCTTGTAGTCGTTCAATG | pBP371 |
| JH61 | 5′-CAATAAAAAGCCGTACTTAGCAGAAGATTAAAGCTTGTTTTGGCACG | pBP371 |
| JH62 | 5′-TTTGGATCCTTAACCAGTAATAATTTTCTCTTTTGGTAAACGAATC | pBP371 |
| JH95 | 5′-AAAGAATTCAAAGGTAGGGAATACAATGGCTTCGCC | pBP384/pBP396 |
| JH96 | 5′-TTTGGATCCCTCTTGTTATTCTTTTAAATGATAAGGATATGTGGAAAC | pBP384/pBP265 |
| JH97 | 5′-AAAGAATTCAAAGGAATAGGAGATTATGTTCAATCAATTTAAAAGAC | pBP385 |
| JH98 | 5′-TTTGGATCCGAATCTATTTTTTAAGTTTAAATGGAATTGTACATACGTTAAC | pBP385/pBP267 |
| JH120 | 5′-TTTGGATCCTTATTTCCGGTAATGATGTCTTGTACGATGGAAAAC | pBP396/pBP266 |
| JH142 | 5′-AAAGAGCTCGATGGCTTCGCCGCTAAAAAGACTATTAATCG | pBP265/pBP266 |
| JH143 | 5′-AAAGAGCTCGATGTTCAATCAATTTAAAAGACTTATTATAGGGCAACC | pBP267 |
| JR18 | 5′-P-GAATACACCGCTTCATAATGGAGCAGTTATTATTAA | pBP373 |
| MI1 | 5′-AAAGGATCCTAATAAGTTTAGAGGTGAGGATTTATGAAGTATATTGTGATG | pBP559 |
| MI2 | 5′-TTTCTGCAGTTACATTTTCAATCTATCTAATGCCAAATTCACTTGTAAG | pBP559 |
| MI11 | 5′-AAAGGATCCATGGAAACGAATCGTCCAAGTCCGG | pBP560 |
| MI12 | 5′-TTTCTGCAGTCATTTTCCATCTCCTCCGTCTAGTG | pBP560 |
| MI21 | 5′-AAAGGTACCGAAGTATATTGTGATGCAGGATGTG | pBP563 |
Table 4.
Plasmids
| Name | Insert/Features | Reference |
|---|---|---|
| pBAD24 | PBAD, ampicillin resistance gene | Ref. 56 |
| pBAD33 | PBAD, chloramphenicol resistance gene | Ref. 56 |
| pMAD | bgaB, pBR322 ori and ampicillin resistance gene for (E. coli); pE194ts ori and erythromycin resistance gene (L. monocytogenes) | Ref. 59 |
| pWH844 | PT5, ampicillin resistance gene | Ref. 48 |
| pGP172 | PT7, ampicillin resistance gene | Ref. 58 |
| pBP265 | pGP172, Strep-tag II-kimALmo | This work |
| pBP266 | pGP172, Strep-tag II- kimALmo ΔC terminus | This work |
| pBP267 | pGP172, Strep-tag II-kimASau | This work |
| pBP345 | pWH844, His6-ktrCLmo | This work |
| pBP346 | pWH844, His6-kimASau C-terminal domain | This work |
| pBP347 | pWH844, His6-kimASau C-terminal domain | This work |
| pBP352 | pMAD-ΔcdaA (cdaA up- and downstream region) | This work |
| pBP370 | pBAD33, cdaA | This work |
| pBP371 | pWH844, ktrCLmo and ktrDLmo | This work |
| pBP372 | pWH844, ktrABBsu | This work |
| pBP373 | pBAD33, cdaA (D171N) | This work |
| pBP384 | pWH844, kimALmo | This work |
| pBP385 | pWH844, kimASau | This work |
| pBP396 | pWH844, kimALmo ΔC terminus | This work |
| pBP559 | pWH844, kdpABCLmo | This work |
| pBP560 | pWH844, kdpDLmo | This work |
| pBP563 | pBAD24, kdpABCLmo | This work |
Deletion of the cdaA gene
The chromosomal deletion of the cdaA gene in strain BPL77 was performed as follows. The plasmid pBP352 (pMAD-ΔcdaA) was introduced into the WT strain EGD-e by electroporation, and the cells were plated on LSM with erythromycin and X-Gal at 30 °C for up to 72 h. Single blue colonies were streaked on the same medium and incubated for up to 72 h at 42 °C to facilitate the selection for integrants. Blue colonies were used to inoculate 5 ml of LSM without antibiotics at 30 °C for 4 h, and the temperature was shifted to 42 °C for 6 h, after which serial dilutions were plated on LSM with X-Gal and incubated at 37 °C for up to 72 h. Erythromycin-sensitive, X-Gal–negative bacteria that did grow on LSM but not on brain heart infusion were subjected to colony PCR as described previously (60). The cdaA deletion and the absence of ectopic suppressor mutations was confirmed by whole-genome sequencing and Sanger sequencing, and the strain was designated BPL77 (Table 2).
Growth of L. monocytogenes in LSM
Single colonies of the L. monocytogenes WT and the cdaA mutant strains were grown overnight in LSM-K+ with 1 mm KCl. Overnight cultures were harvested by centrifugation at 4000 × g for 5 min at room temperature and resuspended in LSM-K+. These cell suspensions were used to inoculate 10 ml of LSM-K+ to an OD600 of 0.1 and grown for about 4 h. Cells were washed again as described in LSM-K+, the OD600 was adjusted to 0.2, and 100 μl were used to inoculate wells of a 96-well plate (Microtest Plate 96 Well, F, Sarstedt), containing 100 μl of LSM-K+ with a 2-fold concentration of the indicated potassium concentrations. The 96-well plate was incubated at 37 °C with medium orbital shaking at 237 cpm (4 mm) in an Epoch 2 microplate spectrophotometer (BioTek Instruments), and growth was measured at an optical density (OD600) in 15-min intervals.
Drop dilution assay
Single colonies of the E. coli strain LB650 harboring the plasmid pWH844, pBP371, pBP372, pBP384, pBP385, or pBP396 were taken from LB-K plates and used to inoculate 4 ml of LB-K medium supplemented with kanamycin, ampicillin, and chloramphenicol. The cultures were incubated at 37 °C and 220 rpm. The precultures were used to inoculate 4 ml of M9 medium supplemented with glucose, antibiotics, and 50 mm KCl to an OD600 of 0.001. The cultures were incubated for about 16 h at 37 °C. The next day, the cultures were used to inoculate 10 ml of the same medium to an OD600 of 0.1. At an OD600 between 0.3 and 0.5, the cells were harvested by centrifugation at 4000 × g for 10 min at room temperature. The cell pellets were washed twice in 10 ml of M9 medium lacking KCl. The cell suspension was adjusted to an OD600 of 0.1, and 5 μl of the diluted cells were spotted onto M9 minimal medium plates, which were incubated for 24 h at 37 °C. M9 plates were prepared by mixing 2× M9 medium (prewarmed to 37 °C) and 2× Bacto agar (prewarmed to 70 °C before mixing). The finial medium contained glucose as a carbon source, 10 mm KCl, and 50 μm IPTG when required.
Determination of kinetic parameters of the potassium transporters
To determine the growth characteristics of the E. coli strain LB650 synthesizing potassium transporters from L. monocytogenes and S. aureus, the bacteria were grown until the early exponential phase and harvested by centrifugation at 4000 × g for 10 min. The pellet was resuspended in 10 ml of M9 medium with glucose, ampicillin, and 50 μm IPTG without KCl. The cells were incubated for 1 h at 37 °C, harvested by centrifugation, and washed twice. The cultures were adjusted to an OD600 of 0.2, and 50 μl were used to inoculate a 96-well plate (Microtest Plate 96 Well, F, Sarstedt) containing 50 μl of M9 medium with glucose, ampicillin, 50 μm IPTG, and KCl concentrations ranging from 0 to 100 mm. The 96-well plate was incubated at 37 °C with medium orbital shaking at 237 cpm (4 mm) in an Epoch 2 microplate spectrophotometer (BioTek Instruments). The growth rates were calculated (μ = (2.303·(log(OD2) − log(OD1)))/(t2 − t1)), plotted against the KCl concentrations, and fitted to the Michaelis–Menten equation using the solver tool of Excel 2013 (Microsoft) to calculate Vmax (μ (h−1)) and the apparent Km (mm KCl).
c-di-AMP in vivo inhibition assay
The potassium transporter–deficient E. coli strain LB2003 was co-transformed with the plasmid pWH844 or derivatives (pBP371, pBP384, or pBP396) and the pBAD33 derivatives (pBP370 or pBP373) on LB-K plates containing 0.5% (w/v) glucose, ampicillin, and chloramphenicol. Single colonies were used to inoculate 4 ml of LB-K medium containing 0.2% (w/v) glucose, ampicillin, and chloramphenicol, and the exponentially growing cultures were used to inoculate M9 medium containing 0.2% (w/v) glycerol and 0.02% (w/v) glucose to an OD600 of 0.001. The cultures were incubated overnight at 37 °C and used to re-inoculate the same medium (without glucose) to an OD600 of 0.1. After reaching early exponential phase (OD600 = 0.3–0.5), the cells were washed, and 50 μl of the suspensions were used to inoculate a 96-well plate. The M9 medium was supplemented with glycerol, 50 μm IPTG, ampicillin, chloramphenicol, and KCl with or without l-arabinose. Final concentrations of KCl were equal to the determined Km values (see Table 2), and either no or 0.005% (w/v) l-arabinose was present, as indicated. Growth was monitored in an Epoch 2 microplate spectrophotometer (BioTek Instruments).
Protein expression and DRaCALA
The binding of c-di-AMP to the potassium transporters was analyzed using the E. coli strain Rosetta (DE3) for pGP172 and derivatives or strain NEB T7 Express Iq for pWH844 and derivatives. Single colonies were used to inoculate 10 ml of LB-K medium containing carbenicillin and chloramphenicol. After incubation overnight at 30 °C, the precultures were used to inoculate 1.5 ml of LB-K medium to an OD600 of 0.1. 1 mm IPTG was added at an OD600 of 1.0–1.5 to induce gene expression. After incubation for 4 h, the cultures were harvested by centrifugation (4000 × g, 10 min, 4 °C), the cell pellets were resuspended in 150 μl of Tris-NaCl buffer (10 mm Tris, pH 8.0, 100 mm NaCl). Cells were lysed by three freeze/thaw cycles of −80 °C and room temperature. DRaCALA was performed by mixing 1 μl of [32P]c-di-AMP with 20 μl of cell lysates. After a 1-min incubation, 2 μl of the mixture was spotted on dry nitrocellulose, dried, exposed to a PhosphorImager screen, and imaged using an FLA-7000 PhosphorImager. The fraction bound was calculated using the inner and total areas and intensities, as described previously (61).
Microscopic analysis
Derivatives of the LB650 strain harboring the plasmids pWH844 (empty plasmid), pBP372, or pBP384 were in 4 ml of LB-K medium containing ampicillin, kanamycin, and chloramphenicol at 37 °C. The next day, the cultures were washed twice and used to inoculate 10 ml of M9 medium (containing 22.05 mm KH2PO4) with or without 1 mm IPTG to an OD600 of 0.1. Cells were transferred to standard microscope slides (Carl Roth) and examined using an Axioskop 40 FL fluorescence microscope, equipped with an Axio-Cam MRm digital camera, objectives of the Neofluar series at 1000-fold primary magnification, and the AxioVision Rel 4.8.2 software (Carl Zeiss). Images were later equally processed using ImageJ 1.48 software (62).
Statistical analysis
All data are presented as means with n representing the number of independent experiments. Data were statistically evaluated by analysis of variance (ANOVA) tests with post hoc Dunnett's or Tukey tests using the GraphPad Prism version 8.2.1 software (GraphPad Software, La Jolla, CA).
Author contributions
J. G., V. T. L., and F. M. C. conceptualization; J. G., V. T. L., and F. M. C. formal analysis; J. G., G. H., A. T., and M. W. investigation; J. G. methodology; F. M. C. supervision; F. M. C. funding acquisition; F. M. C. writing-original draft; F. M. C. project administration; F. M. C. writing-review and editing.
Acknowledgments
We are grateful to Jan Gunlach and Jörg Stülke for the fruitful discussions regarding potassium homeostasis in Gram-positive bacteria. We thank Jonathan Rosenberg and Jasmin Gömann for help with some experiments. We are grateful to Sabine Lentes for technical support.
This work was supported by Grant CO 1139/2–1 from the Deutsche Forschungsgemeinschaft via Priority Program SPP1879, the Fonds der Chemischen Industrie, and the Max-Buchner-Forschungsstiftung (MBFSt-Kennziffer 3381) (to F. M. C.). The authors declare that they have no conflicts of interest with the contents of this article.
- c-di-AMP
- cyclic diadenosine monophosphate
- DAC
- diadenylate cyclase
- DRaCALA
- differential radial capillary action of ligand assay
- SOE
- splicing by overhang extension
- PDE
- phosphodiesterase
- IPTG
- isopropyl 1-thio-β-d-galactopyranoside
- LB
- lysogeny broth
- aa
- amino acids
- LSM
- Listeria synthetic medium
- OD
- optical density
- ANOVA
- analysis of variance.
References
- 1. Wood J. M. (1999) Osmosensing by bacteria: signals and membrane-based sensors. Microbiol. Mol. Biol. Rev. 63, 230–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wood J. M. (2011) Bacterial osmoregulation: a paradigm for the study of cellular homeostasis. Annu. Rev. Microbiol. 65, 215–238 10.1146/annurev-micro-090110-102815 [DOI] [PubMed] [Google Scholar]
- 3. Sleator R. D., and Hill C. (2002) Bacterial osmoadaptation: the role of osmolytes in bacterial stress and virulence. FEMS Microbiol. Rev. 26, 49–71 10.1111/j.1574-6976.2002.tb00598.x [DOI] [PubMed] [Google Scholar]
- 4. Bremer E., and Krämer R. (2019) Responses of microorganisms to osmotic stress. Annu. Rev. Microbiol. 73, 313–334 10.1146/annurev-micro-020518-115504 [DOI] [PubMed] [Google Scholar]
- 5. Gundlach J., Herzberg C., Kaever V., Gunka K., Hoffmann T., Weiss M., Gibhardt J., Thürmer A., Hertel D., Daniel R., Bremer E., Commichau F. M., and Stülke J. (2017) Control of potassium homeostasis is an essential function of the second messenger cyclic di-AMP in Bacillus subtilis. Sci. Signal. 10, eaal3011 10.1126/scisignal.aal3011 [DOI] [PubMed] [Google Scholar]
- 6. Commichau F. M., Gibhardt J., Halbedel S., Gundlach J., and Stülke J. (2018) A delicate connection: c-di-AMP affects cell Integrity by controlling osmolyte transport. Trends Microbiol. 26, 175–185 10.1016/j.tim.2017.09.003 [DOI] [PubMed] [Google Scholar]
- 7. Corrigan R. M., Campeotto I., Jeganathan T., Roelofs K. G., Lee V. T., and Gründling A. (2013) Systematic identification of conserved bacterial c-di-AMP receptor proteins. Proc. Natl. Acad. Sci. U.S.A. 110, 9084–9089 10.1073/pnas.1300595110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bai Y., Yang J., Zarrella T. M., Zhang Y., Metzger D. W., and Bai G. (2014) Cyclic di-AMP impairs potassium uptake mediated by a cyclic di-AMP binding protein in Streptococcus pneumoniae. J. Bacteriol. 196, 614–623 10.1128/JB.01041-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chin K. H., Liang J. M., Yang J. G., Shih M. S., Tu Z. L., Wang Y. C., Sun X. H., Hu N. J., Liang Z. X., Dow J. M., Ryan R. P., and Chou S. H. (2015) Structural insights into the distinct binding mode of cyclic di-AMP with SaCpaA-RCK. Biochemistry 54, 4936–4951 10.1021/acs.biochem.5b00633 [DOI] [PubMed] [Google Scholar]
- 10. Whiteley A. T., Pollock A. J., and Portnoy D. A. (2015) The PAMP c-di-AMP is essential for Listeria monocytogenes growth in rich but not minimal media due to a toxic increase in (p)ppGpp. Cell Host Microbe 17, 788–798 10.1016/j.chom.2015.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Whiteley A. T., Garelis N. E., Peterson B. N., Choi P. H., Tong L., Woodward J. J., and Portnoy D. A. (2017) c-di-AMP modulates Listeria monocytogenes central metabolism to regulate growth, antibiotic resistance and osmoregulation. Mol. Microbiol. 104, 212–233 10.1111/mmi.13622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schuster C. F., Bellows L. E., Tosi T., Campeotto I., Corrigan R. M., Freemont P., and Gründling A. (2016) The second messenger c-di-AMP inhibits the osmolyte uptake system OpuC in Staphylococcus aureus. Sci. Signal. 9, ra81 10.1126/scisignal.aaf7279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Huynh T. N., Choi P. H., Sureka K., Ledvina H. E., Campillo J., Tong L., and Woodward J. J. (2016) Cyclic di-AMP targets the cystathionine β-synthase domain of the osmolyte transporter OpuC. Mol. Microbiol. 102, 233–243 10.1111/mmi.13456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zeden M. S., Schuster C. F., Bowman L., Zhong Q., Williams H. D., and Gründling A. (2018) Cyclic di-adenosine monophosphate (c-di-AMP) is required for osmotic regulation in Staphylococcus aureus but dispensable for viability in anaerobic conditions. J. Biol. Chem. 293, 3180–3200 10.1074/jbc.M117.818716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pham H. T., Nhiep N. T. H., Vu T. N. M., Huynh T. N., Zhu Y., Huynh A. L. D., Chakrabortti A., Marcellin E., Lo R., Howard C. B., Bansal N., Woodward J. J., Liang Z. X., and Turner M. S. (2018) Enhanced uptake and potassium or glycine betaine or export of cyclic-di-AMP restores osmoresistance in a high cyclic-di-AMP Lactococcus lactis mutant. PLoS Genet. 14, e1007574 10.1371/journal.pgen.1007574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bejerano-Sagie M., Oppenheimer-Shaanan Y., Berlatzky I., Rouvinski A., Meyerovich M., and Ben-Yehuda S. (2006) A checkpoint protein that scans the chromosome for damage at the start of sporulation in Bacillus subtilis. Cell 125, 679–690 10.1016/j.cell.2006.03.039 [DOI] [PubMed] [Google Scholar]
- 17. Witte G., Hartung S., Büttner K., and Hopfner K.-P. (2008) Structural biochemistry of a bacterial checkpoint protein reveals diadenylate cyclase activity regulated by DNA recombination intermediates. Mol. Cell 30, 167–178 10.1016/j.molcel.2008.02.020 [DOI] [PubMed] [Google Scholar]
- 18. Gándara C., and Alonso J. C. (2015) DisA and c-di-AMP act at the intersection between DNA-damage response and stress homeostasis in exponentially growing Bacillus subtilis cells. DNA Repair (Amst.) 27, 1–8 10.1016/j.dnarep.2014.12.007 [DOI] [PubMed] [Google Scholar]
- 19. Raguse M., Torres R., Seco E. M., Gándara C., Ayora S., Moeller R., and Alonso J. C. (2017) Bacillus subtilis DisA helps to circumvent replicative stress during spore revival. DNA Repair 59, 57–68 10.1016/j.dnarep.2017.09.006 [DOI] [PubMed] [Google Scholar]
- 20. Valenzuela-García L. I., Ayala-García V. M., Regalado-García A. G, Setlow P., and Pedraza-Reyes M. (2018) Transcriptional coupling (Mfd) and DNA damage scanning (DisA) coordinate excision repair events for efficient Bacillus subtilis spore outgrowth. Microbiologyopen 7, e00593 10.1002/mbo3.593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Corrigan R. M., and Gründling A. (2013) Cyclic di-AMP: another second messenger enters the fray. Nat. Rev. Microbiol. 11, 513–524 10.1038/nrmicro3069 [DOI] [PubMed] [Google Scholar]
- 22. Mehne F. M. P., Gunka K., Eilers H., Herzberg C., Kaever V., and Stülke J. (2013) Cyclic di-AMP homeostasis in Bacillus subtilis: both lack and high level accumulation of the nucleotide are detrimental for cell growth. J. Biol. Chem. 288, 2004–2017 10.1074/jbc.M112.395491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Commichau F. M., Heidemann J. L., Ficner R., and Stülke J. (2019) Making and breaking of an essential poison: the cyclases and phosphodiesterases that produce and degrade the essential second messenger cyclic di-AMP in bacteria. J. Bacteriol. 201, e00462–18 10.1128/JB.00462-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rismondo J., Gibhardt J., Rosenberg J., Kaever V., Halbedel S., and Commichau F. M. (2016) Phenotypes associated with the essential diadenylate cyclase CdaA and its potential regulator CdaR in the human pathogen Listeria monocytogenes. J. Bacteriol. 198, 416–426 10.1128/JB.00845-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Woodward J. J., Iavarone A. T., and Portnoy D. A. (2010) c-di-AMP secreted by intracellular Listeria monocytogenes activates a host type I interferon response. Science 328, 1703–1705 10.1126/science.1189801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Devaux L., Sleiman D., Mazzuoli M.-V., Gominet M., Lanotte P., Trieu-Cuot P., Kaminski P.-A., and Firon A. (2018) Cyclic di-AMP regulation of osmotic homeostasis is essential in Group B Streptococcus. PLoS Genet. 14, e1007342 10.1371/journal.pgen.1007342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Huynh T. N., and Woodward J. J. (2016) Too much of a good thing: regulated depletion of c-di-AMP in the bacterial cytoplasm. Curr. Opin. Microbiol. 30, 22–29 10.1016/j.mib.2015.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhu Y., Pham T. H., Nhiep T. H. N., Vu N. M. T., Marcellin E., Chakrabortti A., Wang Y., Waanders J., Lo R., Huston W. M., Bansal N., Nielsen L. K., Liang Z.-X., and Turner M. S. (2016) Cyclic-di-AMP synthesis by the diadenylate cyclase CdaA is modulated by the peptidoglycan biosynthesis enzyme GlmM in Lactococcus lactis. Mol. Microbiol. 99, 1015–1027 10.1111/mmi.13281 [DOI] [PubMed] [Google Scholar]
- 29. Tosi T., Hoshiga F., Millership C., Singh R., Eldrid C., Patin D., Mengin-Lecreulx D., Thalassinos K., Freemont P., and Gründling A. (2019) Inhibition of the Staphylococcus aureus c-di-AMP cyclase DacA by direct interaction with the phosphoglucoseamine mutase GlmM. PLoS Pathog. 15, e1007537 10.1371/journal.ppat.1007537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhang L., Li W., and He Z.-G. (2013) DarR, a TetR-like transcriptional factor, is a cyclic di-AMP-responsive repressor in Mycobacterium smegmatis. J. Biol. Chem. 288, 3085–3096 10.1074/jbc.M112.428110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sureka K., Choi P. H., Precit M., Delince M., Pensinger D. A., Huynh T. N., Jurado A. R., Goo Y. A., Sadilek M., Iavarone A. T., Sauer J.-D., Tong L., and Woodward J. J. (2014) The cyclic dinucleotide c-di-AMP is an allosteric regulator of metabolic enzyme function. Cell 158, 1389–1401 10.1016/j.cell.2014.07.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Choi P. H., Vu T. M. N., Pham H. T., Woodward J. J., Turner M. S., and Tong L. (2017) Structural and functional studies of pyruvate carboxylase regulation by cyclic di-AMP in lactic acid bacteria. Proc. Natl. Acad. Sci. U.S.A. 114, E7226–E7235 10.1073/pnas.1704756114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Choi P. H., Sureka K., Woodward J. J., and Tong L. (2015) Molecular basis for the recognition of cyclic-di-AMP by PstA, a PII-like signal transduction protein. Microbiologyopen 4, 361–374 10.1002/mbo3.243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Campeotto I., Zhang Y., Mladenov M. G., Freemont P. S., and Gründling A. (2015) Complex structure and biochemical characterization of the Staphylococcus aureus cyclic diadenylate monophosphate (c-di-AMP)-binding protein PstA, the founding member of a new signal transduction protein family. J. Biol. Chem. 290, 2888–2901 10.1074/jbc.M114.621789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gundlach J., Dickmanns A., Schröder-Tittmann K., Neumann P., Kaesler J., Kampf J., Herzberg C., Hammer E., Schwede F., Kaever V., Tittmann K., Stülke J., and Ficner R. (2015) Identification, characterization, and structure analysis of the cyclic di-AMP-binding PII-like signal transduction protein DarA. J. Biol. Chem. 290, 3069–3080 10.1074/jbc.M114.619619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Müller M., Deimling T., Hopfner K. P., and Witte G. (2015) c-di-AMP recognition by Staphylococcus aureus PstA. FEBS Lett. 589, 45–51 10.1016/j.febslet.2014.11.022 [DOI] [PubMed] [Google Scholar]
- 37. Kim H., Youn S.-J., Kim S. O., Ko J., Lee J.-O., and Choi B.-S. (2015) Structural studies of potassium transport protein KtrA regulator of conductance of K+ (RCK) C domain in complex with cyclic diadenosine monophosphate (c-di-AMP). J. Biol. Chem. 290, 16393–16402 10.1074/jbc.M115.641340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Quintana I. M., Gibhardt J., Turdiev A., Hammer E., Commichau F. M., Lee V. T., Magni C., and Stülke J. (2019) The KupA and KupB proteins of Lactococcus lactis IL1403 are novel c-di-AMP receptor proteins responsible for potassium uptake. J. Bacteriol. 201, e00028–19 10.1128/JB.00028-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gundlach J., Krüger L., Herzberg C., Turdiev A., Poehlein A., Tascón I., Weiss M., Hertel D., Daniel R., Hänelt I., Lee V. T., and Stülke J. (2019) Sustained sensing in potassium homeostasis: cyclic di-AMP controls potassium uptake by KimA at the levels of expression and activity. J. Biol. Chem. 294, 9605–9614 10.1074/jbc.RA119.008774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wang X., Cai X., Ma H., Yin W., Zhu L., Li X., Lim H. M., Chou S. H., and He J. (2019) A c-di-AMP riboswitch controlling kdpFABC operon transcription regulates the potassium transporter system in Bacillus thuringiensis. Commun. Biol. 2, 151 10.1038/s42003-019-0414-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Moscoso J. A., Schramke H., Zhang Y., Tosi T., Dehbi A., Jung K., and Gründling A. (2016) Binding of cyclic di-AMP to the Staphylococcus aureus sensor kinase KdpD occurs via the universal stress protein domain and downregulates the expression of the Kdp potassium transporter. J. Bacteriol. 198, 98–110 10.1128/JB.00480-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rolhion N., and Cossart P. (2017) How the study of Listeria monocytogenes has led to new concepts in biology. Fut. Microbiol. 12, 621–638 10.2217/fmb-2016-0221 [DOI] [PubMed] [Google Scholar]
- 43. Holtmann G., Bakker E. P., Uozumi N., and Bremer E. (2003) KtrAB and KtrCD: two K+ uptake systems in Bacillus subtilis and their role in adaptation to hypertonicity. J. Bacteriol. 185, 1289–1298 10.1128/JB.185.4.1289-1298.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Price-Whelan A., Poon C. K., Benson M. A., Eidem T. T., Roux C. M., Boyd J. M., Dunman P. M., Torres V. J., and Krulwich T. A. (2013) Transcriptional profiling of Staphylococcus aureus during growth in 2 m NaCl leads to clarification of physiological roles for Kdp and Ktr K+ uptake systems. MBio 4, e00407–13 10.1128/mBio.00407-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gründling A. (2013) Potassium uptake systems in Staphylococcus aureus: new stories about ancient systems. MBio 4, e00784–13 10.1128/mBio.00784-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gassel M., Möllenkamp T., Puppe W., and Altendorf K. (1999) The KdpF subunit is part of the K+-translocating Kdp complex of Escherichia coli and is responsible for stabilization of the complex in vitro. J. Biol. Chem. 274, 37901–37907 10.1074/jbc.274.53.37901 [DOI] [PubMed] [Google Scholar]
- 47. Omasits U., Ahrens C. H., Müller S., and Wollscheid B. (2014) Protter: interactive protein feature visualization and integration with experimental proteomic data. Bioinformatics 30, 884–886 10.1093/bioinformatics/btt607 [DOI] [PubMed] [Google Scholar]
- 48. Schirmer F., Ehrt S., and Hillen W. (1997) Expression, inducer spectrum, domain structure, and function of MopR, the regulator of phenol degradation in Acinetobacter calcoaceticus NCIB8250. J. Bacteriol. 179, 1329–1336 10.1128/jb.179.4.1329-1336.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Stumpe S., and Bakker E. P. (1997) Requirement of a large K+-uptake capacity and of extracytoplasmic protease activity for protamine resistance of Escherichia coli. Arch. Microbiol. 167, 126–136 10.1007/s002030050425 [DOI] [PubMed] [Google Scholar]
- 50. Rosenberg J., Dickmanns A., Neumann P., Gunka K., Arens J., Kaever V., Stülke J., Ficner R., and Commichau F. M. (2015) Structural and biochemical analysis of the essential diadenylate cyclase CdaA from Listeria monocytogenes. J. Biol. Chem. 290, 6596–6606 10.1074/jbc.M114.630418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Nelson J. W., Sudarsan N., Furukawa K., Weinberg Z., Wang J. X., and Breaker R. R. (2013) Riboswitches in eubacteria sense the second messenger c-di-AMP. Nat. Chem. Biol. 9, 834–839 10.1038/nchembio.1363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Horak J., and Wolf D. H. (1997) Catabolite inactivation of the galactose transporter in the yeast Saccharomyces cerevisiae: ubiquitination, endocytosis, and degradation in the vaculoe. J. Bacteriol. 179, 1541–1549 10.1128/jb.179.5.1541-1549.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Blötz C., Treffon K., Kaever V., Schwede F., Hammer E., and Stülke J. (2017) Identification of the components involved in cyclic di-AMP signaling in Mycoplasma. Front. Microbiol. 8, 1328 10.3389/fmicb.2017.01328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sambrook J., Maniatis T., and Fritsch E. F. (1989) Molecular Cloning: A Laboratory Manual, 2nd Ed., Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 55. Horton R. M., Cai Z. L., Ho S. N., and Pease L. R. (1990) Gene splicing by overlap extension: tailor-made genes using the polymerase chain reaction. BioTechniques 8, 528–535 [PubMed] [Google Scholar]
- 56. Guzman L. M., Belin D., Carson M. J., and Beckwith J. (1995) Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J. Bacteriol. 177, 4121–4130 10.1128/jb.177.14.4121-4130.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Bi W., and Stambrook P. J. (1997) CCR: a rapid and simple approach for mutation detection. Nucleic Acids Res. 25, 2949–2951 10.1093/nar/25.14.2949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Merzbacher M., Detsch C., Hillen W., and Stülke J. (2004) Mycoplasma pneumoniae HPr kinase/phosphorylase. Eur. J. Biochem. 271, 367–374 10.1046/j.1432-1033.2003.03935.x [DOI] [PubMed] [Google Scholar]
- 59. Arnaud M., Chastanet A., and Débarbouillé M. (2004) New vector for efficient allelic replacement in naturally nontransformable, low-GC-content, Gram-positive bacteria. Appl. Environ. Microbiol. 70, 6887–6891 10.1128/AEM.70.11.6887-6891.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Dussurget O., Cabanes D., Dehoux P., Lecuit M., Buchrieser C., Glaser P., Cossart P., and European Listeria Genome Consortium (2002) Listeria monocytogenes bile salt hydrolase is a PrfA-regulated virulence factor involved in the intestinal and hepatic phases of listeriosis. Mol. Microbiol. 45, 1095–1106 10.1046/j.1365-2958.2002.03080.x [DOI] [PubMed] [Google Scholar]
- 61. Roelofs K. G., Jones C. J., Helman S. R., Shang X., Orr M. W., Goodson J. R., Galperin M. Y., Yildiz F. H., and Lee V. T. (2015) Systematic identification of cyclic-di-GMP binding proteins in Vibrio cholerae reveals a novel class of cyclic-di-GMP-binding ATPases associated with type II secretion systems. PLoS Pathog. 11, e1005232 10.1371/journal.ppat.1005232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Schneider C. A., Rasband W. S., and Eliceiri K. W. (2012) NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675 10.1038/nmeth.2089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kearse M., Moir R., Wilson A., Stones-Havas S., Cheung M., Sturrock S., Buxton S., Cooper A., Markowitz S., Duran C., Thierer T., Ashton B., Meintjes P., and Drummond A. (2012) Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28, 1647–1649 10.1093/bioinformatics/bts199 [DOI] [PMC free article] [PubMed] [Google Scholar]