Abstract
Background
Robotic pancreaticoduodenectomy (RPD) is a novel type of minimally invasive surgery to treat tumors located at the head of the pancreas. This study aimed to construct a novel prediction model for predicting selection preference for RPD in a Chinese single medical center population.
Material/Methods
The clinical data from 451 pancreatic ductal adenocarcinoma patients were collected and analyzed from January 2013 to December 2016. Twenty-three items affecting clinical strategies were optimized by LASSO (least absolute shrinkage and selection operator) regression analysis and then were incorporated in multivariable logistic regression analysis. C-index was used for evaluating the discriminative ability. Decision curve was applied to determine clinical application of this model and the calibration of this nomogram was evaluated by calibration plot. The model was internally validated through bootstrapping validation.
Results
Clinicopathological factors included in the model were age, history of diabetes mellitus, history of hypertension, history of heart, brain and kidney disease, history of abdominal surgery, symptoms (jaundice, accidental discovery and weight loss), anemia, elevated carcinoembryonic antigen (CEA), smoking, alcohol intake, American Society of Anesthesiologists (ASA) scores, vascular invasion, overweight, preoperative lymph node metastasis and tumor size >3.5 cm. A C-index of 0.831 indicated good discrimination and calibration of this model. Interval validation generated an acceptable C-index of 0.787. When surgical approach was determined at the threshold of preference possibility less than 63%, decision curve analysis indicated that this model had good clinical application value in this range.
Conclusions
This new nomogram could be conveniently used to predict the selection preference of robotic surgery for patients with pancreatic head cancer.
MeSH Keywords: Nomograms, Pancreatic Neoplasms, Robotics
Background
One type of pancreatic cancer, pancreatic ductal adenocarcinoma, accounts for 90% of pancreatic cancers and is considered the fourth leading cause of cancer-related deaths in the world [1]. In 2018, an estimated 458 918 people will be diagnosed with pancreatic cancer, and approximately 432 242 people will die from this tumor [2]. Pancreatic cancer is characterized by its poor prognosis, with a median survival less than 6 months [3]. Surgical resection is the only possible curative and preferred treatment for pancreatic cancer, improving long-term survival, compared to chemotherapy, radiotherapy, or any other adjuvant therapy.
Pancreaticoduodenectomy is the radical operation for carcinoma of the head of the pancreas. It represents one of the most difficult and complex surgeries for the management of tumor resection and reconstruction of the gastrointestinal tract. With advances in minimally invasive techniques, pancreaticoduodenectomy now can be safely performed by a robotic approach [4,5]. It results in less surgical trauma and enables faster postoperative recovery [6]. A systemic review that including 62 studies and 1028 patients concluded that robotic pancreaticoduodenectomy (RPD) offers good perioperative and oncological outcomes [7]. However, it remains unclear whether RPD can be performed in all patients with carcinomas at the head of the pancreas, and which kinds of patients are better candidates for robotic surgery.
Many high-volume centers have chosen the robotic approach for pancreatic surgery. In 2010, Giulianotti et al. reported on 134 cases of robotic pancreatic surgery from 2000 to 2009 and concluded that robotic pancreatic surgery is feasible and safe [8]. Zureikat et al. performed 250 robot-assisted pancreatic resections with a low incidence of conversion from 2008 to 2012 [9]. In 2017, a Chinese surgical team shared a single center experience of robotic pancreatic resections in 1010 cases; they were convinced that robotic pancreatic surgery would gradually replace the open procedure and that a robotic procedure will become the primary choice of approach for pancreatectomy [10]. Nevertheless, the decision for robotic pancreaticoduodenectomy depends on the individual judgement of experienced surgeons and the willingness of patients in many high-volume centers. As no standard for selection of RPD has been set, different centers must set their own standards for selection.
Our study aimed to explore the predictive factors affecting the decision to choose robotic pancreaticoduodenectomy and to construct a standardized model of selection preference for RPD with the surgical experience of our center. We hope this model can provide a new predictive tool for selecting RPD candidates for our colleagues and will be validated in future studies.
Material and Methods
Patients
The research protocol for this study was reviewed and approved by the local ethics committee of Ruijin Hospital. Due to the retrospective nature of our study, the ethics committee of Ruijin Hospital waived the need for informed consent. Patients were recruited from Pancreatic Surgery of Ruijin Hospital from January 2013 to December 2016, and they were all diagnosed with pancreatic ductal adenocarcinoma. The patients were included if their cancer was considered to be resectable by a multidisciplinary team consultation with the use of pancreatic enhancement computed tomography or magnetic resonance imaging. All enrolled patients underwent pancreatoduodenectomy either by the open approach or the robotic approach. Patients with illiteracy, severe cognitive impairment, or severe physical disorders were excluded. Data regarding demographic, laboratory tests, imaging studies, and tumor features of the patients were collected from the Hospital Information System.
The exclusion criteria were as follows: 1) patients with heterogenous carcinoma (such as intra-ductal papillary mucinous tumor or pancreatic adenosquamous carcinoma); 2) patients considered as having distant metastasis by preoperative examination; 3) clinical data or follow-up data were insufficient; and 4) histopathological confirmation cancer was not pancreatic ductal adenocarcinoma. No patients received preoperative neoadjuvant therapy or radiotherapy in this study. The focus of the study was not the surgical procedure. Therefore, the details of surgical procedures and lymphadenectomy are not described.
Statistical analysis
R software was used for statistical analysis. The mathematical statistical methods we used in this study are the least absolute shrinkage and selection operator (LASSO) regression model, multivariable logistic regression analysis, calibration plot, C-index, decision curve analysis, and bootstrapping validation. The LASSO method is a regression model processing procedure for high dimensional data to generate a model with relatively lower dimension [11,12]. In this study, it was used to select potential prediction features in demographic and clinical features of pancreatic ductal adenocarcinoma patients. In the LASSO method, those features with nonzero coefficients were considered as significant predictors [13]. The result is evaluated by an odds ratio (OR), 95% confidence interval (CI) and corresponding P-values. Predictors with P-value <0.05 were considered statistically significant and involved in the nomogram. The calibration of this nomogram was assessed by calibration curves [14]. Harrell’s C-index was applied to measure the apparent performance of this prediction nomogram. The C-index was then corrected by bootstrapping validation (1000 bootstrap resamples) [15]. By comparing the net benefit of RPD under different threshold probabilities, clinical usefulness was evaluated by decision curve analysis [16]. The net benefit was calculated by separating people who did not benefit from RPD from all patients who did benefit from RPD, which represents the possibility that a patient would benefit from the RPD by application of this nomogram [17].
Results
Patient characteristics
Four hundred and fifty-one patients with pancreatic ductal adenocarcinoma treated in our center from January 2013 to December 2016 were enrolled in our study. All patients were sorted into robotic pancreaticoduodenectomy (RPD) group and open pancreaticoduodenectomy (OPD) group according to the surgical approach (285 males and 166 females; mean age 63.64±9.18 years; range 25–85 years). All the demographic and clinical features in both groups are given in Table 1.
Table 1.
General Clinical information of patients in robotic pancreaticoduodenectomy group and open OPD pancreaticoduodenectomy group.
| Clinical parameters | n (%) | ||
|---|---|---|---|
| RPD (n=139) | OPD (n=312) | Total (n=451) | |
| Age (years) <50 | |||
| Yes | 16 (0.12) | 24 (0.08) | 40 (0.09) |
| No | 123 (0.88) | 288 (0.92) | 411 (0.91) |
| Male | |||
| Yes | 88 (0.63) | 197 (0.63) | 285 (0.63) |
| No | 51 (0.37) | 115 (0.37) | 166 (0.37) |
| Overweight (BMI >24) | |||
| Yes | 32 (0.23) | 56 (0.18) | 88 (0.20) |
| No | 107 (0.77) | 256 (0.82) | 363 (0.80) |
| HT | |||
| Yes | 58 (0.42) | 54 (0.17) | 112 (0.25) |
| No | 81 (0.58) | 258 (0.83) | 339 (0.75) |
| DM | |||
| Yes | 41 (0.29) | 41 (0.13) | 82 (0.18) |
| No | 98 (0.71) | 271 (0.87) | 369 (0.82) |
| Heart/brain/kidney disease | |||
| Yes | 10 (0.07) | 24 (0.08) | 34 (0.08) |
| No | 129 (0.93) | 288 (0.92) | 417 (0.92) |
| History of abdominal surgery | |||
| Yes | 0 (0.00) | 32 (0.10) | 32 (0.07) |
| No | 139 (1.00) | 280 (0.90) | 419 (0.93) |
| Symptoms | |||
| None | 40 (0.29) | 97 (0.31) | 137 (0.30) |
| Jaundice | 67 (0.48) | 171 (0.55) | 238 (0.53) |
| Accidental discovery | 26 (0.19) | 42 (0.13) | 68 (0.15) |
| Weight loss | 6 (0.04) | 2 (0.01) | 8 (0.02) |
| Biliary drainage | |||
| Yes | 38 (0.27) | 88 (0.28) | 126 (0.28) |
| No | 101 (0.73) | 224 (0.72) | 325 (0.72) |
| Anemia | |||
| Yes | 17 (0.12) | 47 (0.15) | 64 (0.14) |
| No | 122 (0.88) | 265 (0.85) | 387 (0.86) |
| Plt >350×109/L | |||
| Yes | 8 (0.06) | 20 (0.06) | 28 (0.06) |
| No | 131 (0.94) | 292 (0.94) | 423 (0.94) |
| Alb < 30 g/L | |||
| Yes | 8 (0.06) | 28 (0.09) | 36 (0.08) |
| No | 131 (0.94) | 284 (0.91) | 415 (0.92) |
| TB >24 μmol/L | |||
| Yes | 77 (0.55) | 184 (0.59) | 261 (0.58) |
| No | 62 (0.45) | 128 (0.41) | 190 (0.42) |
| Elevated CEA μg/L | |||
| Yes | 30 (0.22) | 116 (0.37) | 146 (0.32) |
| No | 109 (0.78) | 196 (0.63) | 305 (0.68) |
| Elevated CA-199 U/mL | |||
| Yes | 106 (0.76) | 234 (0.75) | 340 (0.75) |
| No | 33 (0.24) | 78 (0.25) | 111 (0.25) |
| Elevated CA-125 U/mL | |||
| Yes | 20 (0.14) | 54 (0.17) | 74 (0.16) |
| No | 119 (0.86) | 258 (0.83) | 377 (0.84) |
| Smoking | |||
| Yes | 40 (0.29) | 77 (0.25) | 117 (0.26) |
| No | 99 (0.71) | 235 (0.75) | 334 (0.74) |
| Alcohol intake | |||
| Yes | 30 (0.22) | 54 (0.17) | 84 (0.19) |
| No | 109 (0.78) | 258 (0.83) | 367 (0.81) |
| ASA score | |||
| 0 | 81 (0.58) | 82 (0.26) | 163 (0.36) |
| 1 | 41 (0.29) | 141 (0.45) | 182 (0.40) |
| 2 | 12 (0.09) | 70 (0.22) | 82 (0.18) |
| 3 | 5 (0.04) | 19 (0.06) | 24 (0.05) |
| Imaging vascular invasion | |||
| Yes | 13 (0.09) | 35 (0.11) | 48 (0.11) |
| No | 126 (0.91) | 277 (0.89) | 403 (0.89) |
| Imaging LNM | |||
| Yes | 16 (0.12) | 57 (0.18) | 73 (0.16) |
| No | 123 (0.88) | 255 (0.82) | 378 (0.84) |
| Tumor size >3.5 cm | |||
| Yes | 36 (0.26) | 103 (0.33) | 139 (0.31) |
| No | 103 (0.74) | 209 (0.67) | 312 (0.69) |
| AJCC stage >IIB | |||
| Yes | 61 (0.44) | 162 (0.52) | 223 (0.49) |
| No | 78 (0.56) | 150 (0.48) | 228 (0.51) |
RPD – robotic pancreaticoduodenectomy; OPD – open pancreaticoduodenectomy; BMI – body mass index; HT – hypertension; DM – diabetes mellitus; Plt – platelets; Alb – albumin; TB – total bilirubin; CEA – carcinoembryonic antigen; CA – carbohydrate antigen; ASA – American Society of Anesthesiologists; LNM – lymph node metastasis; AJCC – American Joint Committee on Cancer.
Feature selection and construction of a standardized prediction model
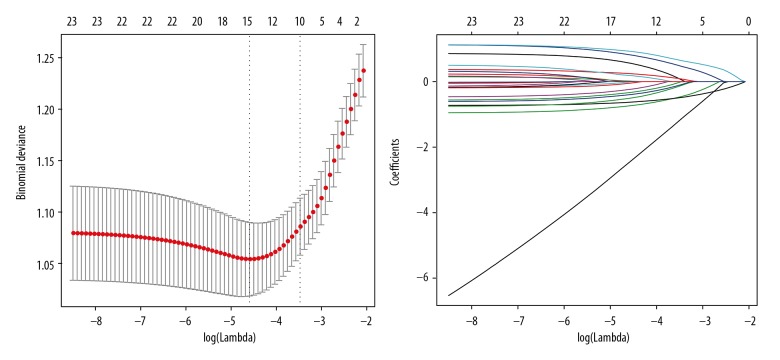
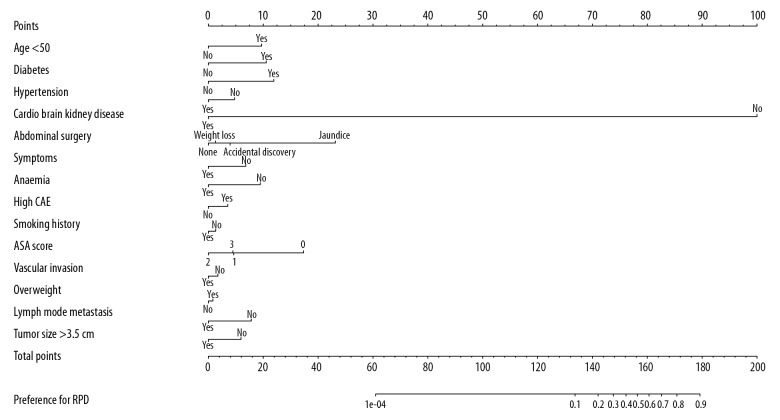
Of all potential features, 23 features were reduced to 15 significant predictors by LASSO method (Figure 1), including age, history of diabetes mellitus, history of hypertension, history of heart, brain and kidney disease, history of abdominal surgery, symptoms, anemia, elevated carcinoembryonic antigen (CEA), smoking, alcohol intake, American Society of Anesthesiologists (ASA) scores, imaging vascular invasion, overweight, preoperative imaging lymph node metastasis, and tumor size (Table 2). These predictors were selected and incorporated to the nomogram as shown in Figure 2.
Figure 1.
General clinical information and characteristic selection using the LASSO (least absolute shrinkage and selection operator) regression model.
Table 2.
Prediction factors for selection preference of robotic pancreaticoduodenectomy.
| Intercept and variable | Multivariate logistic regression model | ||
|---|---|---|---|
| β | Odds ratio (95% CI) | P-value | |
| Intercept | −0.1183 | 0.888 (0.451–1.747) | 0.731 |
| Age <50 years old | 0.9364 | 2.551 (1.121–5.783) | 0.024* |
| DM | 1.0174 | 2.766 (1.492–5.197) | 0.001** |
| HT | 1.1510 | 3.161 (1.834–5.516) | <0.001*** |
| Heart/brain/kidney disease | −0.4601 | 0.631 (0.251–1.496) | 0.308 |
| Abdominal surgery | −16.7719 | 5.201×10−8 (9.196×10−89–556649) | 0.978 |
| Symptom | 0.1260 | 1.134 (0.640–2.030) | 0.668 |
| Anemia | −0.6574 | 0.518 (0.240–1.074) | 0.084 |
| CEA abnormity | −0.9116 | 0.402 (0.228–0.691) | 0.001** |
| Smoking | 0.3407 | 1.406 (0.735–2.681) | 0.300 |
| Alcohol intake | −0.1289 | 0.879 (0.425–1.802) | 0.726 |
| ASA scores | −1.2436 | 0.288 (0.085–0.831) | 0.029 |
| Vascular invasion | −0.1676 | 0.846 (0.370–1.853) | 0.682 |
| Overweight | 0.0791 | 1.082 (0.589–1.961) | 0.796 |
| Imaging LNM | −0.7540 | 0.470 (0.226–0.933) | 0.036* |
| Tumor size >3.5 cm | −0.5732 | 0.564 (0.322–0.969) | 0.041* |
β is the regression coefficient. DM – diabetes mellitus; HT – hypertension; CEA – carcinoembryonic antigen; ASA – American Society of Anesthesiologists; LNM – lymph node metastasis.
Figure 2.
Developed selection preference nomogram.
Apparent performance of the selection preference nomogram in the cohort
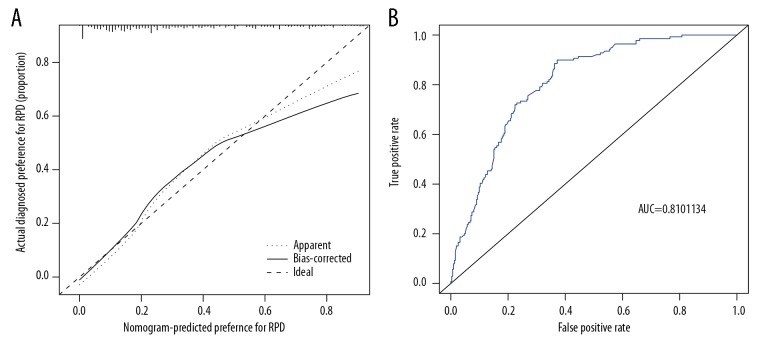
The calibration plot showed a good coherence in the cohort (Figure 3A). The C-index was 0.831 (95% CI: 0.807–0.907) for the primary cohort and validation cohort through bootstrapping also showed good accuracy with C-index of 0.787, which indicated the good discrimination of this nomogram. The accuracy rate of this prediction model is relatively high. The AUC [area under the ROC (receiver operating characteristic) curve] for the prediction model was 0.810 (95% CI: 0.66–0.76), as shown in Figure 3B.
Figure 3.
Calibration curves and receiver operating characteristic (ROC) curves of predicting selection preference in the cohort. (A) Calibration curves to measure the coherence of nomogram. (B) ROC curves plotted to measure the discriminative capacity of nomogram.
Clinical use
The practicability of this nomogram was analyzed with a decision curve, as shown in Figure 4. The preference possibility of a patient is <63%, which means that within this range, more benefits could be gained by application of this preference nomogram to predict selection preference, compared with the scheme.
Figure 4.
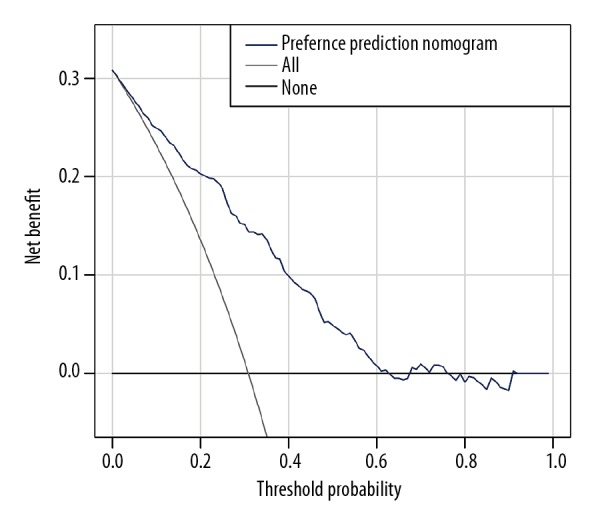
Clinical usefulness measured by decision curve analysis. The horizontal line indicates that all patients are not suitable for robotic pancreaticoduodenectomy (RPD), while the thin light color line represents oppositely that all patients are suitable for RPD.
Discussion
Nomogram is a multi-index graphical calculating device, which is widely used in cancer research to predict prognosis and clinical events [18–21]. Our study might be the first study applied to robotic surgery selection preference. In this study, approximately 31% of the pancreatic cancer patients chose RPD for treatment. The age, history of diabetes mellitus, history of hypertension, history of heart, brain and kidney disease, history of abdominal surgery, symptoms, anemia, elevated CEA, smoking, alcohol intake, ASA scores, vascular invasion, overweight, preoperative lymph node metastasis, and tumor size were associated with selection preference in robotic pancreatic surgery. We included all 15 characteristics into the nomogram rather than only the 6 factors with P values <0.05 by multivariate logistic analysis, because more risk factors are related to increased sensitivity and specificity of this nomogram. In addition, these 6 factors should be paid more attention.
The risk factors were then investigated for the selection preference model of RPD. First, previous abdominal surgery may be the most decisive factors for RPD, as few patients with previous abdominal surgery chose RPD in our study. It is widely believed that previous abdominal surgery increases the chance of intra-abdominal adhesions, necessitates changes in the insertion site of the surgical instruments and is even considered a relative contraindication due to risk of bowel injury during the removal of adhesions [22]. Another feature affecting the selection preference for RPD is the ASA score. The ASA score is designed to assess the physiological status of patients, which provides information for clinicians to predict operative risk [23]. A higher ASA score indicates increased morbidity and mortality. In our study, most patients in the RPD group had a lower ASA score compared with patients in the OPD group, which indicated that patients with a higher ASA score because of heart/brain/kidney disease might not be good candidates for RPD. After all, patients with those diseases might find it difficult to tolerate pneumoperitoneum.
As for selection tendency predictors, patients of younger ages tend to select RPD for enhanced recovery and better postoperative life quality. Patients discovered by accidental examination or jaundice might pay more attention to their health status, which leads to the early detection of the tumor. Those patients with early diagnosis are better candidates for RPD than patients with a complaint of weight loss, who tend to seek treatment in advanced stages of cancer. Patients with hypertension and diabetes mellitus tend to select RPD, probably due to their higher expectancy of reduced complications with minimally invasive techniques. Minimally invasive surgery has the characteristics of a precision intraoperative operation, which can reduce intraoperative stress, maintain blood pressure stability and maximize organ retention.
Smoking status is considered as an independent predictor of postoperative pancreatic fistula after pancreaticoduodenectomy [24]; in addition, those patients with smoking history select RPD for enhanced recovery with their concerns of avoiding pulmonary infection. Alcohol consumption is a negative predictor for RPD, for it increases the risk of acute pancreatitis and chronic pancreatitis [25], which causes inflammation and adhesion in the surgical site, making it difficult to operate with a robotic approach. Obesity is a risk factor for surgical site infection after elective surgery [26], while it is defined as a preference feature for RPD. The laparoscopic system is more suitable for obese patients with limited abdominal space to perform the surgery. Many studies suggested that patients with higher body mass index had more postoperative complications such as pancreatic fistula, but no significant association with mortality [27–31]. Some studies showed that obesity increases postoperative complications regardless of approach, but a robotic approach can mitigate some of the increased complications, such as decreased operation time and less wound infection, while preserving other perioperative outcomes [32,33].
For tumor biomarkers, CEA was used for selection for RPD instead of CA 19-9. Some studies considered that the independent risk predictors of postoperative serum CA19-9, CA125, and CEA indicated poor surgical outcome in pancreatic ductal adenocarcinoma [34]. CA 19-9 is a good indicator for the screening of pancreatic cancer [35] but was not a good predictor of selection for RPD in our study. This is probably because the levels of CA 19-9 are evaluated in most pancreatic ductal adenocarcinoma patients. Cases with larger tumor size, preoperative imaging lymph node metastasis, or imaging vascular involvement are all unsuitable for RPD. Not surprisingly, the size of pancreatic tumors is associated with tumor stage and overall survival [36], and lymph node metastasis and vascular involvement of pancreatic cancer are associated with more advanced stage and poorer prognosis [37–39]. Patients with these tumor features seldom benefited from robotic surgery.
Since Giulianotti et al. successfully performed the first RPD, robotic surgery has been widely applied to pancreatic surgery [40]. Compared with laparoscopic surgery, robotic surgery has comparable perioperative outcomes but more flexibility and reduced learning curve [41–43]. In Asian countries, RPD has currently become common. In our hospital, surgeons have been performing RPD since 2010 and laparoscopic pancreaticoduodenectomy (LPD) has rarely been performed in recent years. That is why our study aimed to establish a prediction model by nomogram to evaluate the preference of receiving RPD, instead of comparing the clinical outcomes between RPD and LPD. Hopefully, our studies will provide useful enlightenment for the selection of these 2 minimally invasive surgical procedures.
There are some deficiencies in this study. First, the data were collected and processed between 2013 and 2016 retrospectively, which inevitably will cause some selection bias. In addition, the cohort cannot represent all resectable Chinese pancreatic ductal adenocarcinoma patients. Some patients who are unaware of the disease or are unable to afford surgery were not included in our study. Second, due to our limited energy, not all potential risk factors that affected the selection preference were analyzed in our study. Third, we used bootstrap testing to validate the reliability of our nomogram, but due to the lack of external validation, we cannot determine the universality of our model. Further studies are needed to externally evaluate the usefulness of this nomogram in wider populations.
Conclusions
Our study aimed to construct a relatively accurate model to predict the selection preference of robotic pancreaticoduodenectomy in pancreatic ductal adenocarcinoma patients using a nomogram. This approach only requires preoperative clinical data, and after preliminary evaluation with our nomogram, surgeons and patients can make an easier decision regarding the surgical approach. However, external validation is required in future research to determine whether this nomogram of selection preference can help surgeons select candidates for robotic surgery more accurately and create a wider indication of robotic surgery in pancreatic cancer patients.
Footnotes
Source of support: This study was supported by grants from the Shanghai Anti-cancer Association (EYAS PROJECT; no. SACA-CY1C19)
Conflicts of interest
None.
References
- 1.Adamska A, Domenichini A, Falasca M. Pancreatic ductal adenocarcinoma: Current and evolving therapies. Int J Mol Sci. 2017;18 doi: 10.3390/ijms18071338. pii: E1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 3.Ilic M, Ilic I. Epidemiology of pancreatic cancer. World J Gastroenterol. 2016;22:9694–705. doi: 10.3748/wjg.v22.i44.9694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boggi U, Signori S, De Lio N, et al. Feasibility of robotic pancreaticoduodenectomy. Br J Surg. 2013;100:917–25. doi: 10.1002/bjs.9135. [DOI] [PubMed] [Google Scholar]
- 5.Lai EC, Tang CN. Current status of robot-assisted laparoscopic pancreaticoduodenectomy and distal pancreatectomy: A comprehensive review. Asian J Endosc Surg. 2013;6:158–64. doi: 10.1111/ases.12040. [DOI] [PubMed] [Google Scholar]
- 6.Zhan Q, Deng X, Weng Y, et al. Outcomes of robotic surgery for pancreatic ductal adenocarcinoma. Chin J Cancer Res. 2015;27:604–10. doi: 10.3978/j.issn.1000-9604.2015.05.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liao CH, Wu YT, Liu YY, et al. Systemic review of the feasibility and advantage of minimally invasive pancreaticoduodenectomy. World J Surg. 2016;40:1218–25. doi: 10.1007/s00268-016-3433-1. [DOI] [PubMed] [Google Scholar]
- 8.Giulianotti PC, Sbrana F, Bianco FM, et al. Robot-assisted laparoscopic pancreatic surgery: single-surgeon experience. Surg Endosc. 2010;24:1646–57. doi: 10.1007/s00464-009-0825-4. [DOI] [PubMed] [Google Scholar]
- 9.Zureikat AH, Moser AJ, Boone BA, et al. 250 robotic pancreatic resections: Safety and feasibility. Ann Surg. 2013;258:554–59. doi: 10.1097/SLA.0b013e3182a4e87c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu R, Zhao GD, Tang WB, et al. [A single-team experience with robotic pancreatic surgery in 1010 cases]. Nan Fang Yi Ke Da Xue Bao. 2018;38:130–34. doi: 10.3969/j.issn.1673-4254.2018.02.02. [in Chinese] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sauerbrei W, Royston P, Binder H. Selection of important variables and determination of functional form for continuous predictors in multivariable model building. Stat Med. 2007;26:5512–28. doi: 10.1002/sim.3148. [DOI] [PubMed] [Google Scholar]
- 12.Friedman J, Hastie T, Tibshirani R. Regularization paths for generalized linear models via coordinate descent. J Stat Softw. 2010;33:1–22. [PMC free article] [PubMed] [Google Scholar]
- 13.Kidd AC, McGettrick M, Tsim S, et al. Survival prediction in mesothelioma using a scalable LASSO regression model: Instructions for use and initial performance using clinical predictors. BMJ Open Respir Res. 2018;5:e000240. doi: 10.1136/bmjresp-2017-000240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iasonos A, Schrag D, Raj GV, Panageas KS. How to build and interpret a nomogram for cancer prognosis. J Clin Oncol. 2008;26:1364–70. doi: 10.1200/JCO.2007.12.9791. [DOI] [PubMed] [Google Scholar]
- 15.Kramer AA, Zimmerman JE. Assessing the calibration of mortality benchmarks in critical care: The Hosmer-Lemeshow test revisited. Crit Care Med. 2007;35:2052–56. doi: 10.1097/01.CCM.0000275267.64078.B0. [DOI] [PubMed] [Google Scholar]
- 16.Pencina MJ, D’Agostino RB. Overall C as a measure of discrimination in survival analysis: Model specific population value and confidence interval estimation. Stat Med. 2004;23:2109–23. doi: 10.1002/sim.1802. [DOI] [PubMed] [Google Scholar]
- 17.Vickers AJ, Cronin AM, Elkin EB, Gonen M. Extensions to decision curve analysis, a novel method for evaluating diagnostic tests, prediction models and molecular markers. BMC Med Inform Decis Mak. 2008;8:53. doi: 10.1186/1472-6947-8-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang YQ, Liang CH, He L, et al. Development and validation of a radiomics nomogram for preoperative prediction of lymph node metastasis in colorectal cancer. J Clin Oncol. 2016;34:2157–64. doi: 10.1200/JCO.2015.65.9128. [DOI] [PubMed] [Google Scholar]
- 19.Liang W, Zhang L, Jiang G, et al. Development and validation of a nomogram for predicting survival in patients with resected non-small-cell lung cancer. J Clin Oncol. 2015;33:861–69. doi: 10.1200/JCO.2014.56.6661. [DOI] [PubMed] [Google Scholar]
- 20.Brockman JA, Alanee S, Vickers AJ, et al. Nomogram predicting prostate cancer–specific mortality for men with biochemical recurrence after radical prostatectomy. Eur Urol. 2015;67:1160–67. doi: 10.1016/j.eururo.2014.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu S, Zheng J, Li Y, et al. A radiomics nomogram for the preoperative prediction of lymph node metastasis in bladder cancer. Clin Cancer Res. 2017;23:6904–11. doi: 10.1158/1078-0432.CCR-17-1510. [DOI] [PubMed] [Google Scholar]
- 22.Figueiredo MN, Campos FG, D’Albuquerque LA, et al. Short-term outcomes after laparoscopic colorectal surgery in patients with previous abdominal surgery: A systematic review. World J Gastrointest Surg. 2016;8:533–40. doi: 10.4240/wjgs.v8.i7.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Doyle DJ, Garmon EH. American Society of Anesthesiologists classification (ASA class) StatPearls Publishing; 2019. [PubMed] [Google Scholar]
- 24.Rozich NS, Landmann A, Butler CS, et al. Tobacco smoking associated with increased anastomotic disruption following pancreaticoduodenectomy. J Surg Res. 2019;233:199–206. doi: 10.1016/j.jss.2018.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alsamarrai A, Das SL, Windsor JA, et al. Factors that affect risk for pancreatic disease in the general population: A systematic review and meta-analysis of prospective cohort studies. Clin Gastroenterol Hepatol. 2014;12:1635–44.e5. doi: 10.1016/j.cgh.2014.01.038. [DOI] [PubMed] [Google Scholar]
- 26.Waisbren E, Rosen H, Bader AM, et al. Percent body fat and prediction of surgical site infection. J Am Coll Surg. 2010;210:381–89. doi: 10.1016/j.jamcollsurg.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 27.Chen YT, Deng Q, Che X, et al. Impact of body mass index on complications following pancreatectomy: Ten-year experience at National Cancer Center in China. World J Gastroenterol. 2015;21:7218–24. doi: 10.3748/wjg.v21.i23.7218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aoki S, Miyata H, Konno H, et al. Risk factors of serious postoperative complications after pancreaticoduodenectomy and risk calculators for predicting postoperative complications: A nationwide study of 17,564 patients in Japan. J Hepatobiliary Pancreat Sci. 2017;24:243–51. doi: 10.1002/jhbp.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Greenblatt DY, Kelly KJ, Rajamanickam V, et al. Preoperative factors predict perioperative morbidity and mortality after pancreaticoduodenectomy. Ann Surg Oncol. 2011;18:2126–35. doi: 10.1245/s10434-011-1594-6. [DOI] [PubMed] [Google Scholar]
- 30.Dumitrascu T, Barbu ST, Purnichescu-Purtan R, et al. Risk factors for surgical complications after central pancreatectomy. Hepatogastroenterology. 2012;59:592–98. doi: 10.5754/hge11758. [DOI] [PubMed] [Google Scholar]
- 31.Wiltberger G, Muhl B, Benzing C, et al. Preoperative risk stratification for major complications following pancreaticoduodenectomy: Identification of high-risk patients. Int J Surg. 2016;31:33–39. doi: 10.1016/j.ijsu.2016.04.034. [DOI] [PubMed] [Google Scholar]
- 32.Girgis MD, Zenati MS, Steve J, et al. Robotic approach mitigates perioperative morbidity in obese patients following pancreaticoduodenectomy. HPB (Oxford) 2017;19:93–98. doi: 10.1016/j.hpb.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 33.Chan JK, Gardner AB, Taylor K, et al. Robotic versus laparoscopic versus open surgery in morbidly obese endometrial cancer patients – a comparative analysis of total charges and complication rates. Gynecol Oncol. 2015;139:300–5. doi: 10.1016/j.ygyno.2015.09.006. [DOI] [PubMed] [Google Scholar]
- 34.Takahashi C, Shridhar R, Huston J, et al. Correlation of tumor size and survival in pancreatic cancer. J Gastrointest Oncol. 2018;9:910–21. doi: 10.21037/jgo.2018.08.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Honda K, Katzke VA, Hüsing A, et al. CA19-9 and apolipoprotein-A2 isoforms as detection markers for pancreatic cancer: A prospective evaluation. Int J Cancer. 2019;144:1877–87. doi: 10.1002/ijc.31900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ansari D, Bauden M, Bergström S, et al. Relationship between tumour size and outcome in pancreatic ductal adenocarcinoma. Br J Surg. 2017;104:600–7. doi: 10.1002/bjs.10471. [DOI] [PubMed] [Google Scholar]
- 37.Kasumova GG, Conway WC, Tseng JF. The role of venous and arterial resection in pancreatic cancer surgery. Ann Surg Oncol. 2018;25:51–58. doi: 10.1245/s10434-016-5676-3. [DOI] [PubMed] [Google Scholar]
- 38.van Rijssen LB, Narwade P, van Huijgevoort NC, et al. Prognostic value of lymph node metastases detected during surgical exploration for pancreatic or periampullary cancer: A systematic review and meta-analysis. HPB (Oxford) 2016;18:559–66. doi: 10.1016/j.hpb.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taki K, Hashimoto D, Nakagawa S, et al. Significance of lymph node metastasis in pancreatic neuroendocrine tumor. Surg Today. 2017;47:1104–10. doi: 10.1007/s00595-017-1485-y. [DOI] [PubMed] [Google Scholar]
- 40.Giulianotti PC, Mangano A, Bustos RE, et al. Operative technique in robotic pancreaticoduodenectomy (RPD) at University of Illinois at Chicago (UIC): 17 steps standardized technique: Lessons learned since the first worldwide RPD performed in the year 2001. Surg Endosc. 2018;32:4329–36. doi: 10.1007/s00464-018-6228-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu M, Ji S, Xu W, et al. Laparoscopic pancreaticoduodenectomy: Are the best times coming? World J Surg Oncol. 2019;17:81. doi: 10.1186/s12957-019-1624-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang T, Zhao ZM, Gao YX, et al. The learning curve for a surgeon in robot-assisted laparoscopic pancreaticoduodenectomy: A retrospective study in a high-volume pancreatic center. Surgical Endosc. :2018. doi: 10.1007/s00464-018-6595-0. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 43.Zhang Y, Hong D, Zhang C, Hu Z. Total laparoscopic versus robot-assisted laparoscopic pancreaticoduodenectomy. Biosci Trends. 2018;12:484–90. doi: 10.5582/bst.2018.01236. [DOI] [PubMed] [Google Scholar]