Abstract
Purpose
To examine the associations of neighborhood characteristics with treatment and outcomes of ductal carcinoma in situ of the breast (DCIS).
Methods
From the Missouri Cancer Registry, we identified 9,195 women with DCIS diagnosed between 1996 and 2011. A composite index using US Census data and American Community Survey data was developed to assess census tract-level socioeconomic deprivation, and rural-urban commuting area codes were used to define rural census tracts. Odds ratios (ORs) and 95% confidence intervals (CIs) of treatment were estimated using logistic regression. Hazard ratios (HRs) of DCIS outcomes were estimated using Cox proportional hazards regression.
Results
Women in the most socioeconomically deprived census tracts were more likely than those in the least deprived to have mastectomy (OR 1.44, 95% CI 1.25–1.66, Ptrend<.0001), no surgery (OR 1.54, 95% CI 1.02–2.30, Ptrend=0.04), no radiation therapy post-breast conserving surgery (BCS) (OR 1.90, 95% CI 1.56–2.31, Ptrend<.0001), delayed radiation therapy (OR 1.26, 95% CI 1.01–1.57, Ptrend=0.02), and ipsilateral breast tumors (HR=1.59, 95% 1.07–2.38, Ptrend=0.03). There was no significant difference in the risk of contralateral breast tumors. Compared with urban women, rural women had significantly higher odds of underutilization of radiation therapy post-BCS (OR 1.29, 95% CI 1.08–1.53). However, rural locations were not associated with the risk of ipsilateral or contralateral breast tumors.
Conclusion
Neighborhood socioeconomic deprivation was associated with higher risks of suboptimal treatment and ipsilateral breast tumors. While DCIS treatment significantly varied by rural/urban locations, we did not observe any statistically significant rural-urban differences in the risks of second breast tumors.
Keywords: Breast cancer, ductal carcinoma in situ, rural health, socioeconomic factors, second primary cancer
INTRODUCTION
Ductal carcinoma in situ (DCIS) is a heterogeneous group of pre-invasive neoplastic lesions in the breast that has increased in clinical significance during recent years. The incidence of DCIS has grown drastically from 2% to 25–30% in the past several decades, resulting largely from widespread adoption of mammographic screening. Although 10-year survival is greater than 98% (1), 11–19% of women with DCIS experience recurrent breast tumors within 10 years of diagnosis (1, 2). Risk of recurrence is influenced by tumor characteristics as well as treatment modality, which includes mastectomy and breast conserving surgery (BCS) with or without radiation therapy (3, 4). Endocrine therapy is recommended with surgery for patients with estrogen receptor positive (ER+) DCIS or who cannot receive radiation therapy (5). Although mastectomy has a nearly 100% cure rate in the ipsilateral breast, it is associated with significant physical and psychological morbidity. BCS is typically accompanied by radiation therapy, which reduces recurrence by half (4). However, radiation therapy requires much greater coordination between patients, specialists, and healthcare providers and may not provide local control benefits to DCIS patients at low risk of recurrence (6). There is no consensus on best management of DCIS.
Both individual- and area-level factors influence breast cancer outcomes. Low socioeconomic status (SES), as measured either at the individual or area level, has been associated with later stage of diagnosis, suboptimal treatment, and lower survival in women with invasive breast cancer (7–11). Less is known about socioeconomic disparities in DCIS treatment and their impact on outcomes.
There are also disparities in breast cancer treatment and outcomes between rural and urban areas. Women residing in rural areas are less likely than those residing in urban areas to receive radiation therapy following BCS and more likely to receive mastectomy for both DCIS and invasive breast cancer (12–14). It is unclear whether urban-rural differences in breast cancer treatment are attributable to SES as poverty rates vary between rural and urban areas (15). Singh et al. (16) found that rural-urban location and neighborhood SES interacted in their contributions to breast cancer mortality and that socioeconomic gradients in mortality were steeper in non-metropolitan populations. Research on potential differences in DCIS outcomes between rural and urban areas is lacking. Here, we examined the associations of neighborhood socioeconomic deprivation and rural/urban residency with treatment utilization and risk of second breast tumors after DCIS using the population-based Cancer Registry data in Missouri.
PATIENTS AND METHODS
Data source
The Missouri Cancer Registry (MCR) has been involved in the National Program of Cancer Registries and complies with its data collecting and coding rules. It periodically audits to ensure case completeness and high-quality data. The MCR also uses the criteria for case reportability defined by the Surveillance, Epidemiology, and End Results (SEER) program of the National Cancer Institute. The MCR includes over 95% of incident cases of cancer diagnosed in Missouri and collects demographic and clinical information. The MCR also has information on residential census tract at the time of cancer diagnosis, start dates of surgical treatment, adjuvant therapies, and surgical margins, which is not available in the SEER database. Treatment dates were collected for over 90% of cases (17). This study was performed in accordance with the ethical guidelines including Belmont Report and U.S. Common Rule. Due to the use of de-identified data, this study was determined exempt by the Institutional Review Boards of the Missouri Department of Health and Senior Services and Washington University in St. Louis. Informed written consent from patients was not required.
Patient selection
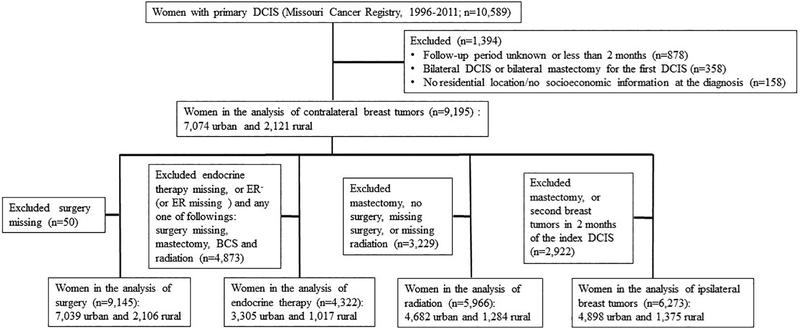
This study included women who were diagnosed with primary DCIS between 1996 and 2011, had no cancer history, and were followed through Dec 31, 2011 (n=10,589). Figure 1 shows the exclusion criteria and sample size. We excluded patients with missing geographic data at the time of diagnosis (n=158); unknown follow-up periods, or periods less than 2 months (n=878); and bilateral DCIS, or those who received bilateral mastectomies (n=358). The analytic sample consisted of 9,195 women of whom 7,074 lived in urban areas and 2,121 lived in rural areas at the time of diagnosis.
Figure 1.
Flow chart of cohort inclusion and exclusion criteria and the number of women with ductal carcinoma in situ (DCIS) in the Missouri Cancer Registry
Treatment
We used information on surgical and adjuvant treatment for the first diagnosis of DCIS, excluding treatment related to tumor progression or recurrence. The most invasive surgical procedure was recorded for the cases with more than one surgery on a primary site. Delayed radiation therapy was defined as an interval of at least 8 weeks between BCS and initiation of radiation therapy, as it was associated with an increased risk of recurrence in women with invasive breast cancer (18). Although aromatase inhibitors have not been approved by the US Food and Drug Administration for DCIS, they have been prescribed for a small group of women and demonstrated to have comparable efficacy (19). Thus, endocrine therapy included both tamoxifen and aromatase inhibitors.
Outcomes
Second breast tumor was defined as either an ipsilateral breast tumor or contralateral breast tumor identified two or more months after the first diagnosis of DCIS (17). Ipsilateral breast tumors included any histological type of carcinoma in situ or invasive tumors in the ipsilateral breast and contralateral breast tumors were defined analogously in the contralateral breast. Person-times were estimated from two months after the index DCIS through either the date of diagnosis of a second breast tumor, last follow-up, death, or Dec 31, 2011, whichever was earliest.
Neighborhood socioeconomic deprivation index
There are 1,320 census tracts in Missouri. The socioeconomic deprivation indices for 2000 and 2010 were assessed separately using a composite index based on 21 variables from the 2000 U.S. Census data and the 2005–2009 American Community Survey data, as described elsewhere (9, 20–22). The 2000 socioeconomic deprivation index was linked to the cases diagnosed between 1996 and 2004, and the 2010 socioeconomic deprivation index to the cases diagnosed after 2004. The selected variables reflected six aspects of socioeconomic deprivation, including education, occupation, housing environment, income/poverty, race/ethnicity, and residential stability, and were incorporated into a multivariate common factor analysis with the varimax rotation. Seven variables, accounting for 43.4% of overall variance, were identified in the first common factor with high loading coefficients and internal consistency (Cronbach alpha = 0.92). They included percentage of persons with an education level lower than high school, percentage of working class, percentage of vacant housing units, percentage of households in poverty, percentage of households with family income under $30,000 per year, income disparity, and percentage of population below federal poverty line. These variables were standardized and weighed by their loading coefficients. A socioeconomic deprivation index for a given census tract was obtained by summing the weighted variables. Socioeconomic deprivation index scores were divided into tertiles, with a higher tertile suggesting greater socioeconomic deprivation.
Rurality
Rural census tracts were determined using the rural-urban commuting area (RUCA) codes developed by the US Department of Agriculture. The RUCA codes are based on a combination of population density, proximity on an urban area as defined by the US Census Bureau, and daily commuting patterns. The 2000 RUCA codes were used for the cases diagnosed between 1996 and 2009, and the 2010 RUCA codes for the cases diagnosed after 2009. Rural areas were defined as census tracts coded as 4, 4.2, 5, 5.2, 6, 6.1, 7, 7.2, 7.3, 7.4, 8, 8.2, 8.3, 8.4, 9, 9.1, 9.2, 10, 10.2, 10.3, 10.4, 10.5, or 10.6, and urban areas as 1, 1.1, 2, 2.1, 3, 4.1, 5.1, 7.1, 8.1, or 10.1.
Covariates
Potential covariates included age (<50, 50–59, 60–69, or >=70), race (non-Hispanic white, non-Hispanic black, or others), year of diagnosis (1996–1999, 2000–2004, 2005–2009, or 2010–2011), health insurance (private, Medicare, Medicaid/uninsured, or other/unknown), tumor grade (well differentiated, moderately differentiated, poorly differentiated, or unknown), tumor size (<2 cm, ≥2 cm, or unknown), histology (comedo, papillary, cribriform, solid, or NOS), and ER status (positive, negative, or unknown).
Statistical analysis
We used the χ2 test to compare categorical variables and the t-test and the analysis of variance to compare continuous variables. Logistic regression analyses were performed to estimate the odds ratios (ORs) for treatment with surgery (mastectomy and no surgical treatment vs. BCS), radiation (delayed initiation and no radiation vs. timely initiation), and endocrine therapy. The models were adjusted for the aforementioned covariates. The analysis of radiation utilization was restricted to women with BCS and also adjusted for surgical margins (clear, positive, or unknown). The analysis of endocrine therapy utilization were restricted to women with ER+ DCIS and those who did not receive radiation after BCS. The ORs for endocrine therapy were additionally adjusted for surgical treatment (no surgery, BCS, mastectomy, or unknown) and radiation (yes, no, or unknown). We also assessed the interaction between neighborhood socioeconomic deprivation and rural locations in DCIS treatment by including a cross-product term in the multivariable-adjusted logistic regression models.
Since women undergoing mastectomy experience extremely low risk of ipsilateral recurrence, women who received mastectomy for DCIS were excluded from the analysis of ipsilateral breast tumors (n=2,929). The analysis of contralateral breast tumors included all patients, regardless of their surgical categories. We used Kaplan-Meier estimates with the log-rank test to compare 10-year cumulative probabilities of ipsilateral and contralateral breast tumors. The risks of second breast tumors were compared between the tertiles of neighborhood socioeconomic deprivation and between rural and urban residencies using hazard ratios (HRs) derived from Cox proportional hazards regression analyses. We tested the proportional hazards assumption by assessing the significance of an interaction term between socioeconomic deprivation and follow-up time and an interaction term between urban/rural location and follow-up time. The HRs were adjusted for age, race, and year of diagnosis as outlined above. Further covariates were added in succession and included insurance, tumor characteristics (grade, size, histology, ER status), and treatment modality (surgery, surgical margin, radiation, endocrine therapy). Trend across socioeconomic deprivation tertiles was tested using Wald statistic in multivariable models with the median scores of deprivation groups as continuous variables. All analyses were performed using SAS (version 9.4, SAS Institute, Cary, NC). Statistical significance was suggested by two-sided P < 0.05.
RESULTS
Eligible DCIS patients came from 1,245 census tracts of Missouri. Table 1 summarizes demographic and tumor characteristics of DCIS cases by neighborhood socioeconomic deprivation and rural/urban residency. Compared with women in the lowest tertile of socioeconomic deprivation, women in the highest tertile were older (mean age 62.3 vs. 59.5, P< .0001), comprised of a greater proportion of non-Hispanic African Americans (25.2% vs. 4.5%, P< .0001), had a shorter follow-up (71 vs. 77 months, P=0.0006), and were more likely to lack health insurance or have Medicaid (12.0% vs. 2.4%, P< .0001). There was a significant difference in histological subtypes across socioeconomic deprivation tertiles (P=0.01); more women in the most socioeconomically deprived tertile had the comedo (13.7% vs. 11.6) and papillary subtypes (8.7% vs. 6.8%). No difference was found in tumor grade, size, ER status, or year of first DCIS diagnosis across the neighborhood socioeconomic deprivation groups.
Table 1.
Characteristics of women with ductal carcinoma in situ by socioeconomic deprivation and urban/rural locations at the time of diagnosis in the Missouri Cancer Registry, 1996–2011
| Socioeconomic deprivation | Urban/rural location | |||||
|---|---|---|---|---|---|---|
| Lowest tertile N (%) |
Highest tertile N (%) |
P | Urban N (%) |
Rural N (%) |
P | |
| Number of cases | 4306 | 1997 | 7074 | 2121 | ||
| Age at diagnosis, y | ||||||
| Mean (SD) | 59.5 (12.5) | 62.3 (12.9) | <.0001 | 60.1 (12.6) | 61.7 (12.4) | <.0001 |
| <50 | 1021 (23.7) | 360 (18.0) | 1595 (22.6) | 372 (17.5) | ||
| 50–59 | 1234 (28.7) | 481 (24.1) | 1918 (27.1) | 563 (26.5) | ||
| 60–69 | 1070 (24.9) | 541 (27.1) | 1809 (25.6) | 570 (26.9) | ||
| ≥70 | 981 (22.8) | 615 (30.8) | <.0001 | 1752 (24.8) | 616 (29.0) | <.0001 |
| Race and ethnicity | ||||||
| Non-Hispanic white | 3989 (92.6) | 1458 (73.0) | 6013 (85.0) | 2052 (96.8) | ||
| Non-Hispanic black | 194 (4.5) | 503 (25.2) | 877 (12.4) | 38 (1.8) | ||
| Others | 123 (2.9) | 36 (1.8) | <.0001 | 184 (2.6) | 31 (1.5) | <.0001 |
| Length of follow-up, months | ||||||
| Median (range) | 77 (2, 191) | 71 (2, 191) | 0.0006 | 74 (2, 191) | 78 (2, 191) | 0.001 |
| 6–11 | 294 (6.8) | 123 (6.2) | 536 (7.6) | 88 (4.2) | ||
| 12–59 | 1362 (31.6) | 721 (36.1) | 2340 (33.1) | 709 (33.4) | ||
| 60–119 | 1473 (34.2) | 688 (34.5) | 2415 (34.1) | 768 (36.2) | ||
| ≥120 | 1177 (27.3) | 465 (23.3) | 0.001 | 1783 (25.2) | 556 (26.2) | <.0001 |
| Year of the first DCIS diagnosis | ||||||
| 1996–1999 | 932 (21.6) | 392 (19.6) | 1481 (20.9) | 418 (19.7) | ||
| 2000–2004 | 1411 (32.8) | 656 (32.9) | 2232 (31.6) | 755 (35.6) | ||
| 2005–2009 | 1446 (33.6) | 722 (36.2) | 2409 (34.1) | 796 (37.5) | ||
| 2010–2011 | 517 (12.0) | 227 (11.4) | 0.15 | 952 (13.5) | 152 (7.2) | <.0001 |
| Health insurance | ||||||
| Private | 966 (66.9) | 299 (44.3) | 1532 (61.4) | 301 (47.9) | ||
| Medicare | 445 (30.8) | 295 (43.7) | 842 (33.7) | 285 (45.3) | ||
| Medicaid or uninsured | 34 (2.4) | 81 (12.0) | <.0001 | 122 (4.9) | 43 (6.8) | <.0001 |
| Other or missing | 2861 | 1322 | 4578 | 1492 | ||
| Histological subtype | ||||||
| Not otherwise specified | 3101 (72.0) | 1397 (70.0) | 5062 (71.6) | 1484 (70.0) | ||
| Comedo | 499 (11.6) | 274 (13.7) | 824 (11.7) | 315 (14.9) | ||
| Papillary | 291 (6.8) | 174 (8.7) | 518 (7.3) | 167 (7.9) | ||
| Cribiform | 243 (5.6) | 82 (4.1) | 394 (5.6) | 87 (4.1) | ||
| Solid | 172 (4.0) | 70 (3.5) | 0.01 | 276 (3.9) | 68 (3.2) | <.0001 |
| Grade | ||||||
| Well differentiated | 628 (20.8) | 291 (22.6) | 1063 (21.4) | 273 (21.5) | ||
| Moderately differentiated | 1078 (35.7) | 452 (35.1) | 1800 (36.3) | 472 (37.1) | ||
| Poorly differentiated | 1314 (43.5) | 546 (42.4) | 0.05 | 2094 (42.2) | 526 (41.4) | 0.83 |
| Missing | 1286 | 708 | 2117 | 850 | ||
| Tumor size, cm | ||||||
| <2.0 | 927 (72.9) | 467 (70.3) | 1604 (71.6) | 487 (70.8) | ||
| ≥2.0 | 345 (27.1) | 197 (29.7) | 0.32 | 635 (28.4) | 201 (29.2) | 0.66 |
| Missing | 3034 | 1333 | 4835 | 1433 | ||
| Estrogen receptor | ||||||
| Negative | 304 (16.1) | 160 (18.9) | 538 (16.9) | 175 (20.3) | ||
| Positive | 1590 (84.0) | 686 (81.1) | 0.05 | 2648 (83.1) | 687 (79.7) | 0.02 |
| Missing | 2412 | 1151 | 3888 | 1259 | ||
| Surgery for first DCIS | ||||||
| None | 89 (2.1) | 64 (3.2) | 166 (2.4) | 55 (2.6) | ||
| BCS | 2965 (69.1) | 1191 (60.0) | 4717 (67.0) | 1307 (62.1) | ||
| Mastectomy | 1237 (28.8) | 729 (36.7) | <.0001 | 2156 (30.6) | 744 (35.3) | 0.0001 |
| Missing | 15 | 13 | 35 | 15 | ||
| Surgical margin† | ||||||
| Clear | 3874 (96.0) | 1730 (95.8) | 6337 (96.0) | 1794 (95.6) | ||
| Positive | 162 (4.0) | 75 (4.2) | 0.97 | 261 (4.0) | 82 (4.4) | 0.42 |
| Missing | 166 | 115 | 275 | 175 | ||
| Radiation therapy for first DCIS | ||||||
| No | 1980 (46.2) | 1207 (61.3) | 3489 (49.6) | 1274 (61.3) | ||
| Yes | 2303 (53.8) | 763 (38.7) | <.0001 | 3545 (50.4) | 803 (38.7) | <.0001 |
| Missing | 23 | 27 | 40 | 44 | ||
| Surgery and radiation therapy for first DCIS | ||||||
| No surgical treatment | 89 (2.1) | 64 (3.2) | 166 (2.4) | 55 (2.6) | ||
| BCS alone | 683 (16.0) | 442 (22.4) | 1220 (17.4) | 508 (24.4) | ||
| BCS and radiation | 2271 (53.1) | 738 (37.4) | 3479 (49.6) | 776 (37.3) | ||
| Mastectomy | 1237 (28.9) | 729 (37.0) | <.0001 | 2156 (30.7) | 744 (35.7) | <.0001 |
| Missing | 26 | 24 | 53 | 38 | ||
| Weeks from surgery to radiation, mean (SD)‡ | 6.2 (3.8) | 7.2 (4.7) | <.0001 | 6.5 (4.2) | 6.4 (3.8) | 0.53 |
| Guideline concordant use of endocrine therapy§ | ||||||
| No | 1171 (59.6) | 655 (67.1) | 2018 (61.1) | 689 (67.8) | ||
| Yes | 794 (40.4) | 321 (32.9) | 0.0002 | 1287 (38.9) | 328 (32.3) | 0.0001 |
| Missing | 113 | 48 | 214 | 37 | ||
The comparison was made in the patients with surgical treatment.
The analysis was limited to the patients who received radiation therapy following definitive breast-conserving surgery.
The analysis included the patients with estrogen receptor-positive DCIS and the patients with breast-conserving surgery alone.
Compared to urban women, rural women with DCIS were older (61.7 vs. 60.1, P < .0001), had a smaller proportion of racial minorities (1.8% vs. 12.4%, P < .0001), and were less likely to have private health insurance (47.9% vs. 61.4%, P < .0001). There were no significant urban-rural differences in tumor grade or size, although rural women were more likely to have the comedo subtype (14.9% vs. 11.7%, P < .0001) and less likely to have ER+ tumors (79.7% vs. 83.1%, P = 0.02). Median length of follow-up was 74 months in the urban group compared to 78 months in the rural group (P = 0.001). More urban women were diagnosed recently with DCIS than their rural counterparts (13.5% vs. 7.2%, P < .0001).
Treatment
Table 1 summarizes treatment characteristics in our sample. There were different patterns of surgical treatment and radiation therapy across the tertiles of socioeconomic deprivation (P < .0001). Compared with women from the least deprived tertile, more women from the most deprived tertile did not receive surgical treatment (3.2% vs. 2.1%) or underwent mastectomy (37.0% vs. 28.9%), and fewer women received radiation therapy following BCS (37.4% vs 53.1%). When they received radiation therapy, they waited on average 7.2 weeks after BCS versus 6.2 weeks in the least deprived tertile (P < .0001). There was no significant difference in surgical margins across neighborhood socioeconomic deprivation tertiles. Endocrine therapy use was lower in the most versus least deprived census tracts. (32.9% vs 40.4%, P =0.0002). Table 2 shows the odds of receiving treatment for DCIS. After adjustment for age, race, year of diagnosis, health insurance, and tumor characteristics, women from the most deprived tertile had greater odds of receiving mastectomies (OR 1.44, 95% CI 1.25–1.66, Ptrend<.0001) and lack of surgery (OR 1.54, 95% CI 1.02–2.30, Ptrend=0.04). After further adjusting for surgical factors, women in the most socioeconomically deprived census tracts had higher odds of radiation underutilization (OR 1.90, 95% CI 1.56–2.31, Ptrend<.0001) and delayed radiation therapy after BCS (OR 1.26, 95% CI 1.01–1.57, Ptrend=0.02). There was no significant difference in the odds of receiving endocrine therapy (OR 0.96, 95% CI 0.77–1.20, Ptrend=0.75).
Table 2.
Adjusted odds ratios (ORs) and their 95% confidence intervals (CIs) of treatment associated with neighborhood socioeconomic deprivation and urban/rural locations among women with ductal carcinoma in situ of the breast
| Surgical treatment† | Radiation therapy§ | Endocrine therapy‡ | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mastectomy | No surgery | Delayed | No radiation | |||||||
| N | OR (95% CI) |
N | OR (95% CI) |
N | OR (95% CI) |
N | OR (95% CI) |
N | OR (95% CI) |
|
| Socioeconomic deprivation | ||||||||||
| Tertile 1 (lowest) | 1237 | 1.00 (reference) | 89 | 1.00 (reference) | 516 | 1.00 (reference) | 668 | 1.00 (reference) | 794 | 1.00 (reference) |
| Tertile 2 | 934 | 1.16 (1.07, 1.35) | 68 | 1.18 (0.83, 1.67) | 322 | 1.16 (0.98, 1.39) | 600 | 1.57 (1.35, 1.84) | 500 | 1.03 (0.86, 1.23) |
| Tertile 3 (highest) | 729 | 1.44 (1.25, 1.66) | 64 | 1.54 (1.02, 2.30) | 227 | 1.26 (1.01, 1.57) | 437 | 1.90 (1.56, 2.31) | 321 | 0.96 (0.77, 1.20) |
| Ptrend<.0001 | Ptrend=0.04 | Ptrend=0.02 | Ptrend<.0001 | Ptrend=0.75 | ||||||
| Urban/rural location | ||||||||||
| Urban | 2156 | 1.00 (reference) | 166 | 1.00 (reference) | 871 | 1.00 (reference) | 1199 | 1.00 (reference) | 1287 | 1.00 (reference) |
| Rural | 744 | 1.09 (0.96, 1.24) | 55 | 1.02 (0.70, 1.49) | 194 | 0.95 (0.77, 1.18) | 506 | 1.29 (1.08, 1.53) | 328 | 1.17 (0.96, 1.44) |
Mastectomy and no surgical treatment were compared with breast-conserving surgery. The ORs were adjusted for age (<50, 50–59, 60–69, ≥70), race (white, black, other), year of diagnosis (1996–1999, 2000–2004, 2005–2009, 2010–2011), health insurance (private, Medicare, Medicaid/uninsured, others/unknown), tumor grade (well differentiated, moderately differentiated, poorly differentiated, unknown), tumor size (<2cm, ≥2cm, unknown), histology (comedo, papillary, cribriform, solid, NOS), and estrogen receptor status (positive, negative, unknown).
The analysis was restricted to women with breast-conserving surgery and complete data on timing of surgery and first radiation therapy. Radiation delay was defined as an interval of eight weeks or longer between breast conserving surgery and the start of radiation therapy. Delayed initiation of radiation therapy and no radiation therapy were compared with timely initiation of radiation therapy. The ORs were adjusted for age, race, surgery, surgical margins, year of diagnosis, health insurance, tumor grade, tumor size, histology, and estrogen receptor status.
The analysis included patients with estrogen receptor-positive DCIS and patients with breast conserving surgery only. The OR was adjusted for age, race, year of diagnosis, insurance, tumor grade, tumor size, histology, estrogen receptor status, surgery, surgical margin, and radiation therapy.
As shown in Table 1, there were different patterns of surgery and radiation therapy between rural women and urban women (P<.0001). More rural patients received mastectomy than urban ones (35.7% vs 30.7%) and fewer rural patients undertook radiation therapy after BCS (37.3% vs 49.6%), though rates of no surgery were similar (2.6% vs 2.4%). There was no significant difference in radiation delay or surgical margins between urban and rural groups. Fewer rural women underwent endocrine therapy in concordance with guidelines (32.3% vs 38.9%, P = 0.0001). After adjusting for the aforementioned covariates, rural patients were less likely than urban patients to receive radiation therapy following BCS (OR 1.29, 95% CI 1.08–1.53) (Table 2). There were no significant urban-rural differences in the odds of receiving mastectomy (OR 1.09, 95% CI 0.96–1.24), no surgery (OR 1.02, 95% CI 0.70–1.49), delayed radiation (OR 0.95, 95% CI 0.77–1.18), or endocrine therapy (OR 1.17, 95% CI 0.96–1.44). We did not observe any significant interactions between socioeconomic deprivation and rural locations in treatment for DCIS (Pinteraction=0.07 for mastectomy, Pinteraction=1.00 for no surgical treatment, Pinteraction=0.39 for delays in radiation therapy, Pinteraction=0.42 for no radiation therapy, Pinteraction=0.36 for endocrine therapy)
Outcomes
During an average 72-month follow-up period, the rate of ipsilateral breast tumors was 3.5% (218 cases in 6,273 women with BCS or no surgical treatment). The rate of contralateral breast tumors was 3.8% over an average 76-month follow-up period (354 cases in our entire cohort of 9,195 women). Figure 2 shows the cumulative incidences of second breast tumors by neighborhood socioeconomic deprivation. Women in the most deprived tertile had a 10-year ipsilateral breast tumor incidence of 8.0% compared to 5.2% and 4.3% in women in the middle and least deprived tertiles respectively (P=0.0005) (Figure 2 A). Ten-year incidences of contralateral breast tumors were comparable across neighborhood socioeconomic deprivation tertiles, with a rate of 5.2% in the least deprived tertile, 5.4% in the middle tertile, and 5.2% in the most deprived tertile (P=0.90) (Figure 2 B).
Figure 2.
Cumulative incidences of ipsilateral breast tumors (A) and contralateral breast tumors (B) in women with ductal carcinoma in situ by neighborhood socioeconomic deprivation
Table 3 shows adjusted HRs for second breast tumors by neighborhood socioeconomic deprivation and rural/urban location. After adjusting for age, race, and year of diagnosis, women in the most deprived tertile had a significantly higher risk of developing second breast tumors in the same breast than those in the least deprived tertile (HR 1.65, 95% CI 1.11–2.46, Ptrend=0.02). After further adjustment for health insurance, tumor characteristics, and treatment modality, the risk of ipsilateral breast tumors was still statistically significant (HR 1.59, 95% CI 1.07–2.38, Ptrend=0.03). The HR of contralateral breast tumors in women from the most deprived tertile was 0.87 (95% CI 0.62–1.21, Ptrend=0.43). Additional adjustment for insurance, tumor characteristics, and treatment did not affect the risk (HR 0.86, 95% CI 0.61–1.20, Ptrend=0.40).
Table 3.
Adjusted hazard ratios (HRs) and their 95% confidence intervals (CIs) of ipsilateral and contralateral breast tumors for neighborhood socioeconomic deprivation and urban/rural locations among women with primary ductal carcinoma in situ of the breast in the Missouri Cancer Registry, 1996–2011
| Neighborhood socioeconomic deprivation | Urban/rural location | ||||
|---|---|---|---|---|---|
| Tertile 1 (lowest) | Tertile 2 | Tertile 3 (highest) | Urban | Rural | |
| Ipsilateral breast tumors | |||||
| Person-years | 21060 | 12463 | 7927 | 32340 | 9110 |
| No. of events | 90 | 68 | 60 | 170 | 48 |
| HR | 1.00 | 1.33 | 1.65 | 1.00 | 0.88 |
| 95% CI | Reference | 0.95, 1.86 | 1.11, 2.46 | Reference | 0.60, 1.29 |
| Ptrend=0.02 | |||||
| Model 1+health insurance | |||||
| HR | 1.00 | 1.33 | 1.64 | 1.00 | 0.88 |
| 95% CI | Reference | 0.95, 1.86 | 1.10, 2.44 | Reference | 0.60, 1.28 |
| Ptrend=0.02 | |||||
| Model 1+health insurance+tumor characteristics‡ | |||||
| HR | 1.00 | 1.34 | 1.67 | 1.00 | 0.87 |
| 95% CI | Reference | 0.95, 1.87 | 1.12, 2.49 | Reference | 0.60, 1.28 |
| Ptrend=0.01 | |||||
| Model 1+health insurance+tumor characteristics+treatment§ | |||||
| HR | 1.00 | 1.29 | 1.59 | 1.00 | 0.85 |
| 95% CI | Reference | 0.92, 1.81 | 1.07, 2.38 | Reference | 0.58, 1.25 |
| Ptrend=0.03 | |||||
| Contralateral breast tumors | |||||
| Person-years | 30091 | 19361 | 13224 | 47692 | 14984 |
| No. of events | 169 | 113 | 72 | 269 | 85 |
| Model 1† | |||||
| HR | 1.00 | 0.99 | 0.87 | 1.00 | 1.08 |
| 95% CI | Reference | 0.76, 1.28 | 0.62, 1.21 | Reference | 0.80, 1.45 |
| Ptrend=0.43 | |||||
| Model 1+health insurance | |||||
| HR | 1.00 | 0.99 | 0.87 | 1.00 | 1.08 |
| 95% CI | Reference | 0.76, 1.28 | 0.62, 1.21 | Reference | 0.81, 1.45 |
| Ptrend=0.44 | |||||
| Model 1+health insurance and tumor characteristics‡ | |||||
| HR | 1.00 | 0.99 | 0.86 | 1.00 | 1.09 |
| 95% CI | Reference | 0.76, 1.28 | 0.62, 1.21 | Reference | 0.81, 1.46 |
| Ptrend=0.43 | |||||
| Model 1+health insurance, tumor characteristics, and treatment§ | |||||
| HR | 1.00 | 0.99 | 0.86 | 1.00 | 1.08 |
| 95% CI | Reference | 0.76, 1.28 | 0.61, 1.20 | Reference | 0.81, 1.46 |
| Ptrend=0.40 | |||||
The HRs were adjusted for age, race/ethnicity, and year of diagnosis of the index ductal carcinoma in situ.
Tumor characteristics included grade, size, histology, and estrogen receptor status.
Treatment included surgery, surgical margin status (in the analysis of ipsilateral breast tumors), radiation therapy, and endocrine therapy.
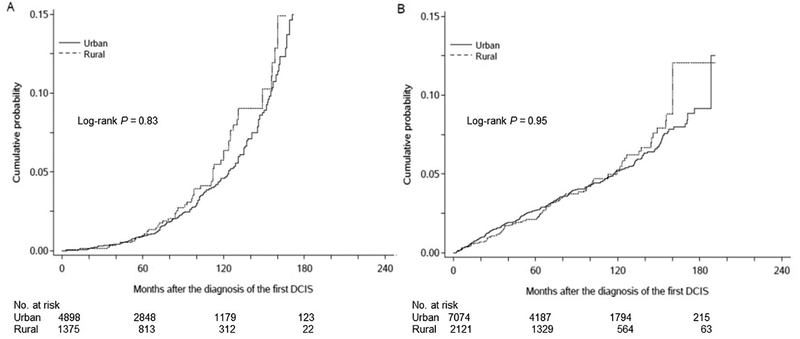
There were no significant urban-rural differences in the incidences of second breast tumors. The 10-year incidence of ipsilateral breast tumors was 5.3% in rural women and 5.2% in urban women (P=0.83) (Figure 3 A). The 10-year incidence of contralateral breast tumors was 5.2% in rural women and 5.2% in urban women (P=0.95) (Figure 3 B). After adjustment for age, race, and year of diagnosis, the HR of ipsilateral breast tumors in rural women was 0.88 (95% CI 0.60–1.29) (Table 3). This remained similar after further adjustment for insurance, tumor characteristics, and treatment (HR 0.85, 95% CI 0.58–1.25). The HR of contralateral breast tumors in rural women was 1.08 (95% CI 0.80–1.45). This was unaffected by further adjustment as mentioned above (HR 1.08, 95% CI 0.81–1.46).
Figure 3.
Cumulative incidences of ipsilateral breast tumors (A) and contralateral breast tumors (B) in women with ductal carcinoma in situ by rural/urban residency
Discussion
Using population-based Cancer Registry data, we observed significant differences in the treatment received by women with DCIS from different socioeconomic and rural-urban census tracts in Missouri. Both neighborhood socioeconomic deprivation and rural residency were independently associated with underutilization of radiation therapy. Women in the most socioeconomically deprived areas were also more likely than those in the least deprived areas to receive mastectomy if treated with surgery, lack surgical treatment, and have delays if receiving radiation therapy. Neighborhood socioeconomic deprivation was associated with a higher risk of ipsilateral breast tumors, but not with the risk of contralateral breast tumors, after DCIS. However, there was no significant difference in the risk of second breast tumors between rural and urban residencies.
Neighborhood-level socioeconomic differences in surgical treatment and radiation therapy have been reported for invasive breast cancer. Neighborhoods with lower socioeconomic levels was associated with higher odds of receiving mastectomy and lower odds of receiving radiation after BCS, though rural-urban residency was not taken in to account (8, 23, 24). Other studies have yielded similar results in women with disabilities and women on Medicaid (25, 26). Underutilization of radiation therapy after BCS in low SES populations may reflect financial barriers, including limited access to transportation (27), daycare (28), time off work (28), and ability to afford copayments (29). Though lack of adequate health insurance has been associated with poorer treatment (30), the variations we observed persisted despite adjustment for this factor. Patients’ health literacy and cultural preferences may also influence surgical treatment choices (31). Low SES women and women living in the socioeconomic deprived areas may prefer mastectomy to BCS probably due to the longer surveillance and higher degree of financial and logistical commitment in the latter. Women in the most deprived areas also have higher rates of comorbidities (32), resulting in contraindications to surgery or radiation therapy. Differences in cancer treatment may also reflect either disparities in patients’ access to providers or in the care provided by providers themselves (33, 34). We have demonstrated in the same cohort of DCIS patients that black women were more likely than white women to have mastectomy, underutilize radiation therapy after BCS, and delayed initiation of radiation therapy (17). Larger proportions of black women in more socioeconomically deprived areas did not explain the observed socioeconomic differences in DCIS treatment.
Rural areas generally have a lower level of geographic accessibility to cancer care (35, 36), which might explain in part the observed disparities in radiation therapy following BCS for DCIS in rural women. Underutilization of radiation therapy has been related to increased travel distances to radiation therapy facilities (37). Proximity to large National Comprehensive Cancer Network (NCCN) centers equipped with radiation therapy capabilities increased utilization of radiation therapy (38). In addition, urban-rural differences in radiation therapy use may represent less awareness of NCCN guidelines in rural areas. More than half of primary care physicians in rural North and South Carolina thought that BCS alone was sufficient for breast cancer treatment (39). Cultural differences, such as concern about radiation or preference for a shorter treatment course, may also influence rural women to decline radiation therapy after BCS (40).
While we identified large geographic variations in DCIS treatment, we did not observe significant associations between rural-urban residence and risks of second breast tumors after adjusting for demographic and clinical factors. In contrast, neighborhood socioeconomic deprivation was associated with a higher risk of ipsilateral breast tumors in women with DCIS. It corroborated with findings by Singh et al. (16) that rurality was not associated with breast cancer mortality after adjustment for SES. In that study, a higher level of neighborhood socioeconomic deprivation was associated with a higher risk of breast cancer mortality, which was stronger in non-metropolitan areas. Compared to SES, differences in adherence to treatment, comorbid health conditions, or high-risk behaviors such as alcohol consumption might be smaller in the rural-urban continuum. Due to a relatively small number of DCIS cases with second breast tumors, we were unable to examine the potential interaction between neighborhood socioeconomic deprivation and rural location in DCIS outcomes.
This study had several limitations. We utilized a rural-urban dichotomy which might have obscured variations in the rural-urban continuum. The MCR did not collect the information regarding comorbidities and risk behaviors (e.g. smoking and alcohol consumption), which might confound both treatment and outcomes. Data on completion of endocrine and radiation therapy were unavailable, and thus we could not further clarify the relationship between disparities in treatment and outcomes.
In conclusion, this study is the first to specifically examine both neighborhood-level socioeconomic factors and urban/rural residency in relation to DCIS outcomes. Neighborhood socioeconomic deprivation was associated with higher odds of underutilization of surgical treatment and radiation therapy after BCS and receiving mastectomy. However, sociodemographic and clinical factors could not explain the higher risk of ipsilateral breast tumors in women with DCIS living in socioeconomically disadvantaged areas. While rural women were less likely than urban women to receive radiation therapy after BCS, there were no significant urban-rural differences in the risks of second breast tumors. The results suggested that women in socioeconomically deprived areas and rural areas likely face barriers to care for DCIS, though the specific factors involved and their clinical significance may vary. Population-based studies with a larger sample size and a more nuanced conceptualization of rurality are needed to refine our understanding of the contributions of neighborhood characteristics to DCIS outcomes.
Acknowledgements
We thank Dr. Chester Schmaltz at the Missouri Cancer Registry and Research Center in University of Missouri for his assistance with data access.
Funding/support:
This work was supported by the Breast Cancer Research Foundation and the Foundation for Barnes-Jewish Hospital, St. Louis, Missouri. The Alvin J. Siteman Cancer Center at Washington University School of Medicine and Barnes-Jewish Hospital in St. Louis, MO., Biostatistics Shared Resource also provided support. The Siteman Cancer Center is supported in part by a NCI Cancer Center Support Grant #P30 CA091842, Eberlein, PI. Dr. Colditz (P20CA192966) and Dr. Lian (K07CA178331) are also supported by grants from the National Cancer Institute.
Footnotes
Conflict of Interest: The authors declare no potential conflicts of interest.
References
- 1.Virnig BA, Tuttle TM, Shamliyan T, Kane RL. Ductal carcinoma in situ of the breast: a systematic review of incidence, treatment, and outcomes. J Natl Cancer Inst 2010, 102:170–8. [DOI] [PubMed] [Google Scholar]
- 2.Ernster VL, Barclay J, Kerlikowske K, Wilkie H, Ballard-Barbash R. Mortality among women with ductal carcinoma in situ of the breast in the population-based surveillance, epidemiology and end results program. Arch Intern Med 2000, 160:953–8. [DOI] [PubMed] [Google Scholar]
- 3.Boyages J, Delaney G, Taylor R. Predictors of local recurrence after treatment of ductal carcinoma in situ: a meta-analysis. Cancer 1999, 85:616–28. [PubMed] [Google Scholar]
- 4.Garg PK, Jakhetiya A, Pandey R, Chishi N, Pandey D. Adjuvant radiotherapy versus observation following lumpectomy in ductal carcinoma in-situ: A meta-analysis of randomized controlled trials. Breast J 2017. [DOI] [PubMed] [Google Scholar]
- 5.Gradishar WJ, Anderson BO, Blair SL, Burstein HJ, Cyr A, Elias AD et al. Breast cancer version 3.2014. J Natl Compr Canc Netw 2014, 12:542–90. [DOI] [PubMed] [Google Scholar]
- 6.Smith GL. Toward Minimizing Overtreatment and Undertreatment of Ductal Carcinoma In Situ in the United States. J Clin Oncol 2016, 34:1172–4. [DOI] [PubMed] [Google Scholar]
- 7.Vona-Davis L, Rose DP. The influence of socioeconomic disparities on breast cancer tumor biology and prognosis: a review. J Womens Health (Larchmt) 2009, 18:883–93. [DOI] [PubMed] [Google Scholar]
- 8.Byers TE, Wolf HJ, Bauer KR, Bolick-Aldrich S, Chen VW, Finch JL et al. The impact of socioeconomic status on survival after cancer in the United States : findings from the National Program of Cancer Registries Patterns of Care Study. Cancer 2008, 113:582–91. [DOI] [PubMed] [Google Scholar]
- 9.Lian M, Perez M, Liu Y, Schootman M, Frisse A, Foldes E et al. Neighborhood socioeconomic deprivation, tumor subtypes, and causes of death after non-metastatic invasive breast cancer diagnosis: a multilevel competing-risk analysis. Breast Cancer Res Treat 2014, 147:661–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ward E, Jemal A, Cokkinides V, Singh GK, Cardinez C, Ghafoor A et al. Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J Clin 2004, 54:78–93. [DOI] [PubMed] [Google Scholar]
- 11.Ursem CJ, Bosworth HB, Shelby RA, Hwang W, Anderson RT, Kimmick GG. Adherence to adjuvant endocrine therapy for breast cancer: importance in women with low income. J Womens Health (Larchmt) 2015, 24:403–8. [DOI] [PubMed] [Google Scholar]
- 12.Martinez SR, Shah DR, Tseng WH, Canter RJ, Bold RJ. Rural-urban disparities in use of post-lumpectomy radiation. Med Oncol 2012, 29:3250–7. [DOI] [PubMed] [Google Scholar]
- 13.Markossian TW, Hines RB. Disparities in late stage diagnosis, treatment, and breast cancer-related death by race, age, and rural residence among women in Georgia. Women Health 2012, 52:317–35. [DOI] [PubMed] [Google Scholar]
- 14.Jacobs LK, Kelley KA, Rosson GD, Detrani ME, Chang DC. Disparities in urban and rural mastectomy populations : the effects of patient- and county-level factors on likelihood of receipt of mastectomy. Ann Surg Oncol 2008, 15:2644–52. [DOI] [PubMed] [Google Scholar]
- 15.Albrecht DE, Albrecht SL. Poverty in nonmetropolitan America: Impacts of industrial employment, and family structure variables. Rural Sociology 2000, 65:87–103. [Google Scholar]
- 16.Singh GK, Williams SD, Siahpush M, Mulhollen A. Socioeconomic, Rural-Urban, and Racial Inequalities in US Cancer Mortality: Part I-All Cancers and Lung Cancer and Part II-Colorectal, Prostate, Breast, and Cervical Cancers. J Cancer Epidemiol 2011, 2011:107497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Madubata CC, Liu Y, Goodman MS, Yun S, Yu J, Lian M et al. Comparing treatment and outcomes of ductal carcinoma in situ among women in Missouri by race. Breast Cancer Res Treat 2016, 160:563–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang J, Barbera L, Brouwers M, Browman G, Mackillop WJ. Does delay in starting treatment affect the outcomes of radiotherapy? A systematic review. J Clin Oncol 2003, 21:555–63. [DOI] [PubMed] [Google Scholar]
- 19.Forbes JF, Sestak I, Howell A, Bonanni B, Bundred N, Levy C et al. Anastrozole versus tamoxifen for the prevention of locoregional and contralateral breast cancer in postmenopausal women with locally excised ductal carcinoma in situ (IBIS-II DCIS): a double-blind, randomised controlled trial. Lancet 2016, 387:866–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lian M, Struthers J, Liu Y. Statistical Assessment of Neighborhood Socioeconomic Deprivation Environment in Spatial Epidemiologic Studies. Open J Stat 2016, 6:436–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yost K, Perkins C, Cohen R, Morris C, Wright W. Socioeconomic status and breast cancer incidence in California for different race/ethnic groups. Cancer Causes Control 2001, 12:703–11. [DOI] [PubMed] [Google Scholar]
- 22.Messer LC, Laraia BA, Kaufman JS, Eyster J, Holzman C, Culhane J et al. The development of a standardized neighborhood deprivation index. J Urban Health 2006, 83:1041–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boscoe FP, Johnson CJ, Henry KA, Goldberg DW, Shahabi K, Elkin EB et al. Geographic proximity to treatment for early stage breast cancer and likelihood of mastectomy. Breast 2011, 20:324–8. [DOI] [PubMed] [Google Scholar]
- 24.White A, Richardson LC, Krontiras H, Pisu M. Socioeconomic disparities in breast cancer treatment among older women. J Womens Health (Larchmt) 2014, 23:335–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Foley KL, Kimmick G, Camacho F, Levine EA, Balkrishnan R, Anderson R. Survival disadvantage among Medicaid-insured breast cancer patients treated with breast conserving surgery without radiation therapy. Breast Cancer Res Treat 2007, 101:207–14. [DOI] [PubMed] [Google Scholar]
- 26.McCarthy EP, Ngo LH, Roetzheim RG, Chirikos TN, Li D, Drews RE et al. Disparities in breast cancer treatment and survival for women with disabilities. Ann Intern Med 2006, 145:637–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goodwin JS, Hunt WC, Samet JM. Determinants of cancer therapy in elderly patients. Cancer 1993, 72:594–601. [DOI] [PubMed] [Google Scholar]
- 28.Sharrocks K, Spicer J, Camidge DR, Papa S. The impact of socioeconomic status on access to cancer clinical trials. Br J Cancer 2014, 111:1684–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wong YN, Hamilton O, Egleston B, Salador K, Murphy C, Meropol NJ. Understanding how out-of-pocket expenses, treatment value, and patient characteristics influence treatment choices. Oncologist 2010, 15:566–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ward E, Halpern M, Schrag N, Cokkinides V, DeSantis C, Bandi P et al. Association of insurance with cancer care utilization and outcomes. CA Cancer J Clin 2008, 58:9–31. [DOI] [PubMed] [Google Scholar]
- 31.Katz SJ, Lantz PM, Janz NK, Fagerlin A, Schwartz K, Liu L et al. Patient involvement in surgery treatment decisions for breast cancer. J Clin Oncol 2005, 23:5526–33. [DOI] [PubMed] [Google Scholar]
- 32.Schrijvers CT, Coebergh JW, Mackenbach JP. Socioeconomic status and comorbidity among newly diagnosed cancer patients. Cancer 1997, 80:1482–8. [PubMed] [Google Scholar]
- 33.Keating NL, Kouri EM, He Y, Freedman RA, Volya R, Zaslavsky AM. Location Isn’t Everything: Proximity, Hospital Characteristics, Choice of Hospital, and Disparities for Breast Cancer Surgery Patients. Health Serv Res 2016, 51:1561–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bach PB, Pham HH, Schrag D, Tate RC, Hargraves JL. Primary care physicians who treat blacks and whites. N Engl J Med 2004, 351:575–84. [DOI] [PubMed] [Google Scholar]
- 35.Chan L, Hart LG, Goodman DC. Geographic access to health care for rural Medicare beneficiaries. J Rural Health 2006, 22:140–6. [DOI] [PubMed] [Google Scholar]
- 36.Onega T, Hubbard R, Hill D, Lee CI, Haas JS, Carlos HA et al. Geographic access to breast imaging for US women. J Am Coll Radiol 2014, 11:874–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Athas WF, Adams-Cameron M, Hunt WC, Amir-Fazli A, Key CR. Travel distance to radiation therapy and receipt of radiotherapy following breast-conserving surgery. J Natl Cancer Inst 2000, 92:269–71. [DOI] [PubMed] [Google Scholar]
- 38.Buchholz TA, Theriault RL, Niland JC, Hughes ME, Ottesen R, Edge SB et al. The use of radiation as a component of breast conservation therapy in National Comprehensive Cancer Network Centers. J Clin Oncol 2006, 24:361–9. [DOI] [PubMed] [Google Scholar]
- 39.Hatzell TA, Ricketts TC, Tropman SE, Paskett ED, Cooper MR. Rural physicians’ understanding of the state-of-the-art in breast, colon and rectum cancer treatment. Cancer Causes Control 1999, 10:261–7. [DOI] [PubMed] [Google Scholar]
- 40.Stafford D, Szczys R, Becker R, Anderson J, Bushfield S. How breast cancer treatment decisions are made by women in North Dakota. Am. J. Surg 1998, 176. [DOI] [PubMed] [Google Scholar]