Structured Summary
Aim:
To systematically review and quantify the efficacy of tranexamic acid (TXA) use in reducing the risk of receiving a blood transfusion in patients undergoing orthopaedic trauma surgery, in reducing blood loss, and risk of thromboembolic events.
Methods:
A systematic literature search was performed using Medline, Embase, ClinicalTrials.gov, and conference proceeding abstracts from 2014–2016. A minimum of 2 reviewers screened each study and graded quality. The primary outcome measure was the risk of receiving a blood transfusion in the TXA group versus control. A meta-analysis was performed to construct a combined odds ratio (OR) of receiving a blood transfusion, mean difference (MD) of blood loss, and OR of thromboembolic events.
Results:
Sixteen studies met inclusion criteria and 12 contained sufficient data to be included in the quantitative analysis (1,333 patients). The risk of blood transfusion was significantly less in patients who were administered TXA compared to controls (OR 0.407; 95% confidence interval (CI) 0.278–0.594, I2=34, Q=17, p=<0.001). There was significantly less blood loss in the TXA group compared to controls, as the mean difference was 304 mL (95% CI 142–467 mL) (I2=94, Q-value=103, p <0.001). There was no significant difference in risk of symptomatic thromboembolic events (OR 0.968; 95% CI 0.530–1.766, I2=0, Q-value=5, p =0.684).
Conclusion:
In orthopaedic trauma patients, TXA reduces the risk of blood transfusion, reduces perioperative blood loss, and has no significant effect on the risk of symptomatic thromboembolic events. More high-quality studies are needed to ensure the safety of the drug in these patients.
Introduction
Blood loss in orthopaedic trauma patients is a major source of morbidity and mortality. Blood transfusions are associated with an increased risk of bacterial infection, increased hospital length of stay, as well as an increased cost of over $1,731 per admission. [1–3] A recent study demonstrated that a blood transfusion is associated with mortality even 90 days following hip fracture. [4] Between 20–60% of patients who sustain hip fractures will require a blood transfusion over the course of their hospitalization. [5–7] Blood loss and subsequent blood transfusion is not limited to hip fracture patients. In a series of trauma patients who underwent intramedullary nailing of 2 or more long bones, all 27 (100%) patients required a blood transfusion. [8]
Tranexamic acid (TXA) is a lysine analog that acts as an antifibrinolytic to stabilize blood clot and limit blood loss. Its use was associated with reduced overall mortality compared to control when administered acutely to trauma patients in the CRASH-2 study. [9,10] TXA has demonstrated efficacy in reducing transfusion rates without increasing complications in patients underoing elective orthopaedic surgery, including arthroplasty [11,12] and spine surgery, [13,14] as well as in patients undergoing surgery for maxillofacial trauma. [15,16] Despite the benefits established by this literature, TXA is not routinely used in treating orthopaedic trauma patients in the United States. Many clinicians are hesitant to administer TXA secondary to theoretical concern of increased thromboembolic complications. The preliminary studies of TXA use in patients undergoing hip fracture surgery are promising. [17] We hypothesized that the reports of TXA use in all orthopaedic trauma surgery would demonstrate a decreased risk of blood transfusion and total blood loss without altering the risk of thromboembolic events. The purpose of this study was to systematically review the use of TXA in orthopaedic trauma surgery and quantify the efficacy of TXA in reducing the risk of receiving a blood transfusion and perioperative blood loss in orthopaedic trauma patients.
Methods
A systematic review was performed in August 2016 using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist of Medline, and Embase databases. The search term was created from following phrases: “tranexamic acid,” “TXA,” “fracture,” “orthopaedic trauma,” “trauma surgery,” and “hemiarthroplasty.” This initial search yielded 524 studies (Figure 1). All 524 abstracts were reviewed by at least 2 reviewers for inclusion in the study. In cases of disagreement, a third reviewer helped achieve consensus. Inclusion criteria were English language, study performed using human subjects, cohort or randomized controlled trial that was performed in patients undergoing orthopaedic trauma surgery in which administration of TXA was clearly described as given to one group and placebo or standard of care to another, and studies that reported one of the following outcomes: blood loss, transfusion rate, or thromboembolic events. Studies were excluded if they were performed in facial trauma patients, polytrauma patients, or patients undergoing elective arthroplasty for osteoarthritis.
Figure 1:
PRISMA flow diagram of search and study selection.
An additional search was performed for unpublished data using the American Academy of Orthopaedic Surgeons (AAOS) and Orthopaedic Trauma Association (OTA) conference proceedings from 2014–2016 that identified abstracts from 3 unique studies that met our inclusion criteria. A final search through clinicaltrials.gov was also performed, yielding one additional study that met inclusion criteria.
Data Collection
Pre-defined study characteristics and data was abstracted from each of the selected studies by one reviewer and verified by a second reviewer. The study year, country of origin, design (RCT versus cohort), anatomic region of interest, route of TXA administration, dose, sample size, demographic description of the study population including age and gender, use and type of deep vein thrombosis (DVT) prophylaxis, transfusion rate, number of blood transfusions per patient, blood loss as well as method of calculated blood loss used in each study, rate of in-hospital, 30-day and 90-day mortality, length of stay, post-operative hemoglobin (Hb) level, and fall in Hb were all recorded. Differences between reviewers were resolved by joint re-review and consensus. Unless noted otherwise, data are reported as means and standard deviations (SDs). The primary outcome recorded was the incidence of blood transfusion, and secondary outcomes included total calculated blood loss, incidence of symptomatic thromboembolic events including DVT/pulmonary embolism (PE)/cerebrovascular accident (CVA)/transient ischemic attack (TIA) and mortality.
Quality Assessment
Risk of bias was assessed by two reviewers. As all of the studies were relatively small in number of patients and not adequately powered to detect differences in thromboembolic complications, the quality was graded as to whether the studies were described as double-blinded or unblinded. Any inconsistencies between reviewers were settled by consensus.
Statistical Analysis
Following data collection, the variables were analyzed with the use of Comprehensive Meta-Analysis version 3.0 (New Jersey, USA). Heterogeneity was assessed with Inconsistency (I2) and Q-values. The incidence of a patient receiving a blood transfusion or experiencing a thromboembolic event was treated as a dichotomous variable, and odds ratios (OR) with associated confidence intervals (CI) were calculated. Total calculated blood loss, a continuous variable, was assessed as mean difference with associated standard deviation and 95% CIs.
Funnel plots were utilized to assess for publication bias. Planned sub-group analyses were performed for studies by route (IV versus topical), anatomic region, and study quality. A planned meta-regression was performed to examine the potential effect of age on the efficacy of TXA in reducing risk of blood transfusion.
Results
There were 524 studies identified through the search after duplicates were removed. A total of 12 studies met inclusion criteria for the systematic review. Of the 12 studies included in the quantitative analysis, three were identified from conference proceedings between 2014–2016 and one study was identified on clinicaltrials.gov. A total of 1,333 patients were included in the 12 studies that qualified for quantitative analysis. The average age of patients in the studies ranged from 35 to 85 years (Table I). Eleven of the 12 studies included in the quantitative analysis were RCTs, and one was a cohort study. Ten of the studies were double-blinded and two were not. Three of the studies were limited to femoral neck fractures (FNF), 3 studies were performed in patients with intertrochanteric (IT) hip fractures, 3 studies included all hip fractures (IT and FNF), 1 study was performed in patients with calcaneus fractures, 1 study was performed in patients with femoral shaft fractures, and 1 study was performed in patients with acetabular fractures. In 9 studies, intravenous (IV) TXA was administered, in 2 studies topical TXA was used, and in 1 study topical TXA was given to a subset of patients and IV TXA was used in another subset.[6]
Table 1:
Description of included studies.
| Study Description | ||||||||
|---|---|---|---|---|---|---|---|---|
| Study | Year | Design | Full Text/ Abstract | Anatomic ROI | Average Age | Route TXA | Dose TXA | Total Size (n) |
| Lee | 2015 | Cohort | full text | Femoral neck | 85 | IV | 1 g preop | 271 |
| Watts | 2015 | RCT | abstract | Femoral neck | NR | IV | 15 mg/kg dose at time of incision and closure |
138 |
| Emara | 2014 | RCT | full text | Femoral neck | 55.8 | IV&topical | 10 mg/kg bolus, then 5 mg/kg/h infusion | 60 |
| Zuffrey | 2010 | RCT | full text | Hip fracture | 81.5 | IV | 15 mg/kg in 2 doses | 110 |
| Sadeghi | 2006 | RCT | full text | Hip fracture | 47.9 | IV | 15 mg/kg bolus preop | 67 |
| Vijay | 2013 | RCT | full text | Hip fracture | 49.1 | IV | 10 mg/kg at surgery | 90 |
| Mohib | 2015 | RCT | full text | IT | 69.5 | IV | 10 mg/kg x 2 (at surgery and 3 hours later) | 100 |
| Drakos | 2016 | RCT | full text | IT | 80.9 | topical | 3 grams subfascial upon closure | 200 |
| Tengberg | 2016 | RCT | full text | IT | 77.2 | IV | 1 gram at surgery, followed by 3 grams x 24h infusion | 72 |
| Xie | 2015 | RCT | full text | Calcaneus | 43.0 | IV | 15 mg/kg over 15 minutes | 83 |
| Kashyap | 2015 | RCT | abstract | Acetabulum | NR | topical | 3 gm | 61 |
| Clincal Trial Pfizer | 2011 | RCT | abstact | Femoral shaft | 35.1 | IV | 15 mg/kg at surgery followed, then additional doses at 3 h and 6 h from surgery | 81 |
These studies were not included in the quantitative analysis, as they did not provide the number of patients in the TXA versus control groups. NR=not reported.
Primary outcome: Blood Transfusion
Twelve studies presented data related to blood transfusion as an outcome measure (Table II). [5–7,18–26] There was relatively low heterogeneity identified in the studies (I2=34, Q=17, p=0.117). The summary OR for receiving a blood transfusion in patients given TXA compared to control was 0.407 (95% CI: 0.278–0.594, p <0.001) (Figure 2).
Table 2:
Studies that reported rate of blood transfusion in study population.
| Rate of Blood Transfusion | ||||||
|---|---|---|---|---|---|---|
| Study | Year | Route TXA | TXA Transfusion (n) | TXA total (n) | Control Transfusion (n) | Control total (n) |
| Lee | 2015 | IV | 5 | 84 | 35 | 187 |
| Emara | 2014 | IV&topical | 2 | 40 | 8 | 20 |
| Zuffrey | 2010 | IV | 24 | 57 | 32 | 53 |
| Sadeghi | 2006 | IV | 12 | 32 | 20 | 35 |
| Vijay | 2013 | IV | 7 | 45 | 18 | 45 |
| Mohib | 2015 | IV | 9 | 50 | 21 | 50 |
| Drakos | 2016 | topical | 22 | 100 | 29 | 100 |
| Tengberg | 2016 | IV | 27 | 33 | 33 | 39 |
| Xie | 2015 | IV | 0 | 41 | 1 | 42 |
| Watts | 2015 | IV | 12 | 69 | 18 | 69 |
| Kashyap | 2015 | topical | 13 | 31 | 29 | 30 |
| Clincal Trial Pfizer | 2011 | IV | 5 | 41 | 6 | 40 |
Figure 2:
Forest plot of TXA versus control in terms of rate of blood transfusion.
Secondary Outcomes:
Perioperative Blood Loss:
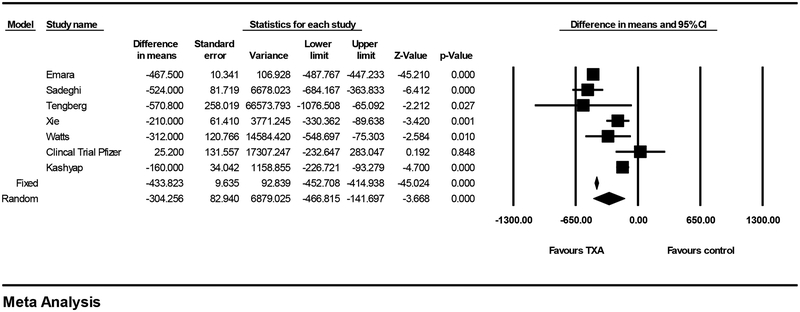
Seven studies reported calculated blood loss. [5,6,18,21–24] There was significant heterogeneity in terms of this outcome (I2=94, Q-value=103, p<0.001). This heterogeneity can likely be attributed to the varying methods used for calculating blood loss and the different time frames that were used (Table 3). Using the random-effects model, the mean difference in perioperative blood loss for patients given TXA compared to control was 304 mL (95% CI 142–467 mL) (p <0.001) (Figure 3a). The studies were further categorized by the type of blood loss reported (intraoperative blood loss, blood loss within the first 24 hour following surgery, and total calculated blood loss). The two studies that reported calculated blood loss for the first 24 hours following surgery reported the largest reduction in blood loss with the TXA group (346 mL, 95% CI 94–598 mL, p=0.007).[5,6]
Figure 3:
Forest plot of TXA versus control in terms of blood loss.
Thromboembolic Events:
Eight studies reported the rate of symptomatic thromboembolic events (DVT/PE/CVA/TIA). The combined OR was 0.968 (95% CI: 0.530–1.766), indicating that there was no significant difference in the risk of symptomatic thromboembolic events in the patients administered TXA as compared to control (see Figure, Supplemental Digital Content 1). There was no significant heterogeneity amongst the studies in terms of this outcome (I2=0, Q-value=5, p-value=0.684).
Subgroup Analyses:
Subgroup analyses were performed to evaluate whether the association between TXA and odds of blood transfusion varied by route of TXA administration, study quality, anatomic location of fracture or fracture type.
Route of TXA Administration:
The studies were grouped according to the route of TXA that was used in the study (IV versus topical administration). Emara et al. [6] reported three study groups, one that was given IV TXA, another topical TXA, and a control, and the results of this study were stratified for the purpose of this subgroup analysis (see Figure, Supplemental Digital Content 2). The subgroup analysis found that there was no significant difference in the reduction of risk of blood transfusion between the studies that utilized IV TXA compared to those that used topical TXA. (Q-value=0.067, p=0.795).
Fracture Location:
The 12 studies were then compared based on the anatomic region of fracture in each study (Table 1). TXA was associated with less blood loss in all subgroups, with no significant difference in the magnitude of the effect of TXA based on anatomic region of the fracture (Q-value=9, p=0.094).
This subgroup analysis was also done isolating the hip fracture studies. Six studies specifically reported on either patients with intertrochanteric fractures or femoral neck fractures (see Figure, Supplemental Digital Content 3). The OR for risk of blood transfusion was lower in studies limited to femoral neck fractures (combined OR 0.312, 95% CI: 0.128–0.765) compared to intertrochanteric fractures (combined OR 0.554, 95% CI: 0.316–0.973), but this difference was not statistically significant (Q-value=1, p-value=0.253).
Meta-regression
A meta-regression was performed to assess the effect modification of age. There was no significant effect modification by age on the relationship between TXA and reduction in risk of blood transfusion (coefficient 0.0067, p=0.4896).
Sensitivity & Publication Bias
A “one removed” meta-analysis was performed removing each individual study from the model, and there was no evidence that removal of any single study resulted in a change in the conclusion that TXA reduces the risk of blood transfusion. Furthermore, a total of 184 missing studies would be required in order for the finding of this meta-analysis to be overturned.
Four abstracts were identified in the search that met inclusion criteria, but either did not provide sufficient detail regarding the number of patients allocated to treatment and control groups [18,19,40] or did not provide numbers of patients who underwent blood transfusion from both treatment and control group [27] and were unable to be included in the meta-analysis. Angulo et al. [27] reported similar rate of blood transfusion in both groups of patients undergoing hemiarthroplasty, and 13% rate of thromboembolic event in the TXA group compared to 6% in the control group. Harris et al. [28] reported results of using IV TXA compared to historical controls and found no difference in the median number of blood transfusions between groups and a non-significant decrease in median intraoperative blood loss in patients undergoing pelvic, acetabular or femoral trauma surgery. These authors did identify a significant increase in rate of thromboembolism in the TXA group. Lack et al. [29] preliminarily reported no significant difference in the rate of blood transfusion between TXA and control groups in patients undergoing acetabular fracture surgery. Moghaddam et al. [30] reported a significant reduction in the rate of blood transfusion and blood loss in hip fracture patients who received TXA.
Discussion
This meta-analysis identified 1,333 patients derived from 12 studies and found that TXA significantly reduces the risk of blood transfusion and decreases blood loss in orthopaedic trauma surgery. These findings are consistent with the growing body of evidence that demonstrates the safety and efficacy of TXA in arthroplasty, spine, maxillofacial, and general surgery.
The effect of TXA in reducing blood loss was the strongest in the first 24 hours compared to total blood loss. As TXA has a half-life of 3 hours, it is logical that the association between TXA administration and decreased blood loss is strongest in the first 24 hours and begins to weaken over the subsequent days of hospital stay.
Additionally, no significant difference was identified between the effect of topical TXA and IV TXA in terms of reducing risk of blood transfusion. These findings are also in agreement with Shin et al. [12] and Chen et al. [31]who also recently reported no significant difference between topical and IV TXA in arthroplasty patients.
A recent meta-analysis by Farrow et al., which was limited to patients with hip fractures, drew similar conclusions. [17] The authors found decreased risk of blood transfusion in the patients who received TXA, as well as decreased blood loss, and no difference in incidence of postoperative complications. [17] They also reported that patients’ age did not modify the association between TXA and decreased risk of blood transfusion. Overall, they reported a 46% decreased rate of blood transfusion requirement in patients who received TXA. The separate analysis of hip fracture patients in the present study found similar results (see Figure, Supplemental Digital Content 3). As the incidence of hip fractures continues to rise as the population ages, the use of TXA in such patients has the potential to improve clinical outcomes, reduce length of stay, and lower costs.
One of the major hesitations to giving TXA to orthopaedic trauma patients is concern over the theoretical increased risk of thromboembolic events. Although multiple meta-analyses have demonstrated no increased rate of thromboembolic complications with TXA use in arthroplasty patients[32,33], orthopaedic trauma patients have a 0.4–7.5% increased risk of fatal pulmonary embolism compared to arthroplasty patients. [34] There was no significant increase in risk of symptomatic thromboembolic events identified in this meta-analysis for the total of 1,015 patients were included in the 8 studies that reported this outcome. Each individual study was underpowered to detect a significant difference in rate of thromboembolic event given the relatively low rate of this complication in patients who are properly anticoagulated. As the rate of thromboembolic events varies widely by study population and anticoagulation method used, it is difficult to estimate the sample size needed to power a significant finding for this outcome. It is likely that even with the 1,015 patients included for this outcome, this meta-analysis is underpowered to detect a significant difference, and even more patients should be studied before a definitive conclusion is made.
A potential weakness of this meta-analysis is the inclusion of studies that have not been published in full-text form (Table 1). Part of the slow dissemination of TXA use in trauma patients is likely related to the publication lag time, and including these studies in this review was intended to offset that delay. Furthermore, the strategy of including studies in abstract form was intended to mitigate the effect of publication bias towards positive findings. For example, we included one industry-sponsored unpublished study, which had full details available electronically, that found no significant relationship between TXA and blood loss or risk of blood transfusion in femoral shaft fractures. [18] An additional limitation to this meta-analysis was the heterogeneity in terms of patients included and reported outcomes. We combined the reported calculations of postoperative blood loss, although studies varied in what they defined as the postoperative period and in their calculation methods. For this reason, we chose to report the random-effects results for this analysis, which aims to accommodate for this heterogeneity, and to stratify in a subgroup analysis.
Overall, the body of evidence for TXA use in orthopaedic trauma surgery has not grown as rapidly as in arthroplasty surgery. Performing well-designed randomized controlled trials in the trauma setting requires 24-hour, 7-days/week research, and patients may be more reluctant to consent to randomization under emergent circumstances. [35] Furthermore, regulations imposed by the Federal Drug Administration may contribute to the slow uptake of using TXA in trauma patients in the United States. Only two of the studies included in this meta-analysis were performed in the United States. The time to operating room, techniques for fracture fixation, threshold for blood transfusion, and the underlying rate of thromboembolic events may vary widely based on country, making these results potentially less applicable to the U.S. population, which is another limitation of this study.
Larger well-designed RCTs will help clarify any potential association between TXA and thromboembolic events, and more research is needed to determine the optimal dose and timing for TXA in orthopaedic trauma patients. In this meta-analysis , TXA did not appear to have a significant effect on the rate of thromboembolic events. Despite its limitations, this meta-analysis indicates that TXA has the potential to reduce both the risk of blood transfusions and blood loss in orthopaedic trauma patients. The preliminary studies indicate that the benefits of TXA may apply broadly to orthopaedic trauma patients of various ages and fracture types, and that the route of administration does not alter the beneficial effect.
Supplementary Material
Acknowledgement
The authors would like to acknowledge Dr. Michael Stoto for his guidance in the study design.
Source of Funding
This study was funded by the Samuel and May Rudin Foundation.
Footnotes
Disclosure
Tranexamic acid use in preventing blood loss in fracture surgery is not FDA-approved and is off-label use.
Contributor Information
Elizabeth B. Gausden, Department of Orthopaedic Surgery, Hospital for Special Surgery, New York, NY 10021.
Rameez Qudsi, Harvard Combined Orthopaedic Residency Program, Brigham and Women’s Hospital Orthopaedic and Arthritis Center for Outcomes Research, Boston. MA.
Myles D. Boone, Department of Anesthesiology & Critical Care, Beth Israel Deaconess, Boston, MA.
Brian O’Gara, Department of Anesthesiology & Critical Care, Beth Israel Deaconess, Boston, MA.
Joseph Ruzbarsky, Department of Orthopaedic Surgery, Hospital for Special Surgery, New York, NY 10021.
Dean G. Lorich, Department of Orthopaedic Surgery, Hospital for Special Surgery, Weill Cornell Medical College/New York Presbyterian Hospital, New York, NY 10021.
References
- 1.Hill GE, Frawley WH, Griffith KE, et al. Allogeneic blood transfusion increases the risk of postoperative bacterial infection: a meta-analysis. J Trauma. 2003;54:908–914. [DOI] [PubMed] [Google Scholar]
- 2.Shokoohi A, Stanworth S, Mistry D, et al. The risks of red cell transfusion for hip fracture surgery in the elderly. Vox Sang. 2012;103:223–230. [DOI] [PubMed] [Google Scholar]
- 3.Saleh A, Small T, Chandran Pillai AL, et al. Allogenic blood transfusion following total hip arthroplasty: results from the nationwide inpatient sample, 2000 to 2009. J Bone Joint Surg Am. 2014;96:e155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Engoren M, Mitchell E, Perring P, et al. The effect of erythrocyte blood transfusions on survival after surgery for hip fracture. J Trauma. 2008;65:1411–1415. [DOI] [PubMed] [Google Scholar]
- 5.Sadeghi MMA. Does a single bolus dose of tranexamic acid reduce blood loss and transfusion requirements during hip fracture surgery? A prospective randomized double blind study in 67 patients. Acta Medica Iranica. 2007;45(6):437–442. [Google Scholar]
- 6.Emara WM, Moez KK, Elkhouly AH. Topical versus intravenous tranexamic acid as a blood conservation intervention for reduction of post-operative bleeding in hemiarthroplasty. Anesth Essays Res. 2014;8:48–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee C, Freeman R, Edmondson M, et al. The efficacy of tranexamic acid in hip hemiarthroplasty surgery: an observational cohort study. Injury. 2015;46:1978–1982. [DOI] [PubMed] [Google Scholar]
- 8.Sabboubeh A, Banaszkiewicz PA, McLeod I, et al. Intramedullary nailing of multiple long-bone fractures of the lower extremity at the same surgery: a single-center experience. J Orthop Sci. 2003;8:313–318. [DOI] [PubMed] [Google Scholar]
- 9.CRASH-2 trial collaborators, Shakur H, Roberts I, et al. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised, placebo-controlled trial. Lancet. 2010;376:23–32. [DOI] [PubMed] [Google Scholar]
- 10.CRASH-2 collaborators, Roberts I, Shakur H, et al. The importance of early treatment with tranexamic acid in bleeding trauma patients: an exploratory analysis of the CRASH-2 randomised controlled trial. Lancet. 2011;377:1096–101, 1101.e1–2. [DOI] [PubMed] [Google Scholar]
- 11.Yi Z, Bin S, Jing Y, et al. Tranexamic Acid Administration in Primary Total Hip Arthroplasty: A Randomized Controlled Trial of Intravenous Combined with Topical Versus Single-Dose Intravenous Administration. J Bone Joint Surg Am. 2016;98:983–991. [DOI] [PubMed] [Google Scholar]
- 12.Shin YS, Yoon JR, Lee HN, et al. Intravenous versus topical tranexamic acid administration in primary total knee arthroplasty: a meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2016. [DOI] [PubMed] [Google Scholar]
- 13.Li ZJ, Fu X, Xing D, et al. Is tranexamic acid effective and safe in spinal surgery? A meta-analysis of randomized controlled trials. Eur Spine J. 2013;22:1950–1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raksakietisak M, Sathitkarnmanee B, Srisaen P, et al. Two doses of tranexamic acid reduce blood transfusion in complex spine surgery: A prospective randomized study. Spine (Phila Pa 1976). 2015. [DOI] [PubMed] [Google Scholar]
- 15.Choi WS, Irwin MG, Samman N. The Effect of Tranexamic Acid on Blood Loss During Orthognathic Surgery: A Randomized Controlled Trial. J Oral Maxillofac Surg. 2009;67:125–133. [DOI] [PubMed] [Google Scholar]
- 16.Dakir A, Ramalingam B, Ebenezer V, et al. Efficacy of Tranexamic acid in reducing blood loss during maxillofacial trauma surgery-A pilot study. J Clin Diagn Res. 2014;8:6–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Farrow LS, Smith TO, Ashcroft GP, et al. A systematic review of Tranexamic acid in hip fracture surgery. Br J Clin Pharmacol. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pfizer. Study Of Tranexamic Acid For The Reduction Of Blood Loss In Patients Undergoing Surgery For Long Bone Fracture. 2011;August 16, 2016:https://clinicaltrials.gov/ct2/show/NCT00824564. [Google Scholar]
- 19.Vijay BS, Bedi V, Mitra S, et al. Role of tranexamic acid in reducing postoperative blood loss and transfusion requirement in patients undergoing hip and femoral surgeries. Saudi J Anaesth. 2013;7:29–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Drakos A, Raoulis V, Karatzios K, et al. Efficacy of Local Administration of Tranexamic Acid for Blood Salvage in Patients Undergoing Intertrochanteric Fracture Surgery. J Orthop Trauma. 2016;30:409–414. [DOI] [PubMed] [Google Scholar]
- 21.Tengberg PT, Foss NB, Palm H, et al. Tranexamic acid reduces blood loss in patients with extracapsular fractures of the hip: results of a randomised controlled trial. Bone Joint J. 2016;98-B:747–753. [DOI] [PubMed] [Google Scholar]
- 22.Xie B, Tian J, Zhou DP. Administration of Tranexamic Acid Reduces Postoperative Blood Loss in Calcaneal Fractures: A Randomized Controlled Trial. J Foot Ankle Surg. 2015;54:1106–1110. [DOI] [PubMed] [Google Scholar]
- 23.Watts C, Houdek M, Cross Willliam W., et al. Tranexamic Acid Safely Reduced Blood Loss: Randomized Clinical Trial of 138 Femoral Neck Fractures AAOS. 2016:Orlando. [DOI] [PubMed] [Google Scholar]
- 24.Kashyap S. Topical Tranexemic Acid Reduces Blood Loss and Transfusion Rates during Internal Fixation of Acetabular Fractures AAOS. 2015:Las Vegas. [Google Scholar]
- 25.Mohib Y, Rashid RH, Ali M, et al. Does tranexamic acid reduce blood transfusion following surgery for inter-trochanteric fracture? A randomized control trial. J Pak Med Assoc. 2015;65:S17–20. [PubMed] [Google Scholar]
- 26.Zufferey PJ, Miquet M, Quenet S, et al. Tranexamic acid in hip fracture surgery: A randomized controlled trial. Br J Anaesth. 2010;104:23–30. [DOI] [PubMed] [Google Scholar]
- 27.Angulo Tabernero M, Aguilar Ezquerra A, Cassinello Ojea C, et al. Tranexamic acid in osteoporotic hip fracture surgery. Osteoporosis Int. 2015;26:S379–S380. [Google Scholar]
- 28.Harris S, Mullis B, Blair M, et al. Assessment of a tranexamic acid protocol for orthopedic trauma surgery. Crit Care Med. 2015;43:292–293. [Google Scholar]
- 29.Lack W, Seymour R, Crist B, et al. Randomized Controlled Trial of Tranexamic Acid in Acetabular Fracture Surgery: Early Results AAOS. 2016:Las Vegas. [Google Scholar]
- 30.Moghaddam MJ, Darabi E, Sheikholeslamy F. Effect of tranexamic acid in decreasing need to transfusion in hip fracture surgery. Eur J Anaesthesiol. 2011;28:89. [Google Scholar]
- 31.Chen Y, Chen Z, Cui S, et al. Topical versus systemic tranexamic acid after total knee and hip arthroplasty: A meta-analysis of randomized controlled trials. Medicine (Baltimore). 2016;95:e4656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu Q, Zhang HA, Liu SL, et al. Is tranexamic acid clinically effective and safe to prevent blood loss in total knee arthroplasty? A meta-analysis of 34 randomized controlled trials. Eur J Orthop Surg Traumatol. 2015;25:525–541. [DOI] [PubMed] [Google Scholar]
- 33.Yang B, Li H, Wang D, et al. Systematic review and meta-analysis of perioperative intravenous tranexamic acid use in spinal surgery. PLoS One. 2013;8:e55436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Geerts WH, Bergqvist D, Pineo GF, et al. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133:381S–453S. [DOI] [PubMed] [Google Scholar]
- 35.Perry DC, Griffin XL, Parsons N, et al. Designing clinical trials in trauma surgery: overcoming research barriers. Bone Joint Res. 2014;3:123–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.