Abstract
Solvent extraction of soybean creates soybean meal (SBM), but an array of other soybean products can be created using further processing of SBM or soybean. For accurate inclusion of these products in pig feed, characterization of digestible AA profile and energy value is required. Soybean products from processes such as extrusion (EX) of soybean and thermo-mechanical (TM) treatment, bioconversion using fermentation or enzymes (BC), and ethanol-water extraction (EW) of soybean meal were collected together with SBM. These 9 soybean products were tested in cornstarch-based diets together with an N-free diet for a total of 10 diets. Ten ileal-cannulated barrows (30.4 ± 0.7 kg initial BW) were fed 10 diets at 2.8 times maintenance DE for six 9-d periods with a 6 (periods) × 10 (pigs) Youden square. The control SBM contained 47.0% CP, 1.4% ether extract, and ADF 6.0%. The 9 soybean products contained 35.6% to 66.4% CP, 0.9% to 21.6% ether extract, and 4.4% to 8.0% ADF. The EW soybean products were high in CP (>61%), whereas the 2 EX soybean products were low in CP (<36%) but high in ether extract (≥19%). Chemically available Lys ranged from 92.6% to 100% of total Lys, indicating that minor Lys damage occurred during processing. The apparent total tract digestibility (ATTD) of energy was lower (P < 0.05) for soybean products with greater ether extract and ADF content than SBM, and varied among soybean products. The standardized ileal digestibility (SID) did not differ (P > 0.05) among soybean products for most AA, except for lower SID of Arg, Ile, Leu, Lys, Phe, and Tyr (P < 0.05) for EX2 and BC1 than other soybean products. The DE and predicted NE value did not differ (P > 0.05) among soybean products. The greater SID AA content (P < 0.05) in EW, BC, and TM1 soybean products than SBM was mainly a result of greater total AA content due to removal of other macronutrients. In conclusion, extrusion of soybean creates soybean products with a greater energy value but lower ATTD of energy and lower SID AA content than SBM. Further processing of SBM creates soybean products with greater CP and SID AA content but similar SID of AA than SBM. Thus, new technologies to process SBM or soybean create high-value ingredients to be included in pig diets, especially for young pigs with high nutritional requirements.
Keywords: amino acid, digestibility, energy, soybean product, pig
Introduction
Soybean meal (SBM) is a popular feedstuff for swine diets around the world, because SBM is nutritionally superior to many other plant-based protein sources (Shelton et al., 2001), due to a greater protein content and better amino acid balance. In commercial diets fed to young pigs, SBM cannot be included in large quantities because it may cause digestive disturbances due to presence of residual trypsin inhibitors (Lallès, 2000; Woyengo et al., 2017), carbohydrate complexes, and antigens (Li et al., 1990). To avoid negative effects of feeding SBM to young pigs, diets include animal-based protein sources such as dried whey, spray-dried plasma protein, or fish meal; however, those are expensive (Lenehan et al., 2007).
Heat used to dry SBM after oil extraction also reduces antinutritional factors (ANF) in SBM, but does not eliminate ANF completely (Baker, 2000). The oil extraction industry introduced new processes while plant genetic companies introduced new cultivars of soybean containing less oligosaccharides (Woyengo et al., 2014a). These novelties enhanced oil extraction and simultaneously created new soybean products, e.g., protein concentrates produced from fermentation and enzyme-treated SBM with reduced ANF that allowed complete substitution of SBM or animal-based protein sources (Lenehan et al., 2007; Yang et al., 2007; Cervantes-Pahm and Stein, 2010). Application of these new soybean products depends on their digestible nutrient profile, ANF, price, and value-attributes such as stimulating feed intake in weaned pigs (Zijlstra et al., 2009). Characterizing the digestible nutrient value of new feedstuffs is an important step for the successful introduction of novel feedstuffs into swine diets (Gunawardena et al., 2010). However, limited information exists about the digestible nutrient profile of a wide array of samples of new soybean products.
We hypothesized that novel soybean products obtained from various processing methods would vary in chemical composition and digestibility of energy and AA. The objective of the present study was to determine the digestible energy (DE) and predicted net energy (NE) values and digestible AA profile of 9 new soybean products in ileal-cannulated grower-finisher pigs.
Materials and Methods
Experimental Diets and Design
Soybean products were produced using extrusion (EX) of full-fat soybean and thermo-mechanical (TM) treatment, bioconversion using fermentation or enzymes (BC), and ethanol-water extraction (EW) of SBM. Organized according to processing method (Table 1), test ingredients were SBM (47% CP), EX1 (Danex soybean; Danis NV, Koolskamp, Belgium), EX2 (Pigletsoy 2000MSA; SCA Ibérica, Mequinenza, Zaragoza, Spain), TM1 (HTM-96; SCA Ibérica, Mequinenza, Zaragoza, Spain), TM2 (Provisoy; Provimi, Brookville, OH), BC1 (Pepsoygen; Nutraferma, North Sioux City, SD) produced using fermentation, BC2 (HP-300; Hamlet Protein Inc., Findlay, OH) produced using enzymes, EW1 (SPC-60-IP; Imcopa, ‘s-Hertogenbosch, Netherlands), and EW2 (Soycomil P; Archer Daniels Midland, Decatur, IL). Together with an N-free diet, in total 10 diets were tested using 10 pigs for 6 periods in a 10 × 6 Youden square design (Table 2). Diets contained cornstarch, canola oil, and sucrose as energy sources, and contained 1 of 9 soybean products as sole source of crude protein (CP) and amino acids (AA). The ratio of inclusion among energy sources was identical between soybean product diets and the N-free diet (Stein et al., 2006). Diets were fortified to meet vitamin and mineral requirements (NRC, 2012). As an indigestible marker, Cr2O3 was included in diets.
Table 1.
Analyzed nutrient content of soybean products (as-is basis)
| Item, % | Soybean products1 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| SBM | EX1 | EX2 | TM1 | TM2 | BC1 | BC2 | EW1 | EW2 | |
| Moisture | 9.0 | 7.4 | 8.1 | 12.5 | 6.5 | 6.0 | 8.2 | 7.0 | 8.4 |
| CP, % | 47.0 | 38.2 | 35.6 | 44.9 | 54.4 | 51.8 | 56.1 | 61.4 | 66.4 |
| GE, Mcal/kg | 4.23 | 5.12 | 4.91 | 4.10 | 4.39 | 4.44 | 4.44 | 4.48 | 4.41 |
| Ether extract, % | 1.4 | 21.6 | 19.0 | 1.7 | 1.1 | 1.2 | 1.9 | 1.4 | 0.9 |
| Ash, % | 6.7 | 5.0 | 5.1 | 5.9 | 7.2 | 6.9 | 7.0 | 6.5 | 6.3 |
| Crude fiber, % | 3.8 | 4.7 | 5.4 | 4.9 | 2.2 | 4.3 | 2.3 | 4.8 | 3.4 |
| ADF, % | 6.0 | 7.3 | 8.0 | 6.3 | 4.5 | 5.6 | 4.4 | 7.7 | 5.4 |
| Starch, % | 1.2 | 0.4 | 1.9 | 2.7 | 0.2 | 0.9 | 0.8 | 0.6 | 0.1 |
| P, % | 0.67 | 0.54 | 0.55 | 0.61 | 0.70 | 0.76 | 0.74 | 0.73 | 0.74 |
| Ca, % | 0.34 | 0.24 | 0.33 | 0.32 | 0.33 | 0.28 | 0.32 | 0.39 | 0.45 |
| Indispensable AA, % | |||||||||
| Arg | 3.35 | 2.79 | 2.49 | 3.20 | 3.90 | 3.45 | 3.92 | 4.46 | 4.86 |
| His | 1.21 | 0.99 | 0.89 | 1.16 | 1.36 | 1.30 | 1.37 | 1.61 | 1.73 |
| Ile | 2.17 | 2.00 | 1.72 | 2.26 | 2.71 | 2.59 | 2.72 | 3.14 | 3.36 |
| Leu | 3.61 | 3.06 | 2.73 | 3.50 | 4.24 | 4.08 | 4.31 | 4.89 | 5.29 |
| Lys | 2.87 | 2.36 | 2.07 | 2.72 | 3.23 | 3.02 | 3.26 | 3.83 | 4.21 |
| Met | 0.70 | 0.52 | 0.51 | 0.63 | 0.75 | 0.79 | 0.77 | 0.87 | 0.97 |
| Phe | 2.34 | 2.05 | 1.79 | 2.32 | 2.81 | 2.62 | 2.88 | 3.18 | 3.41 |
| Thr | 1.80 | 1.39 | 1.32 | 1.64 | 1.99 | 1.95 | 2.09 | 2.30 | 2.49 |
| Trp | 0.64 | 0.47 | 0.47 | 0.61 | 0.73 | 0.72 | 0.73 | 0.82 | 0.89 |
| Val | 2.32 | 2.02 | 1.80 | 2.34 | 2.75 | 2.69 | 2.75 | 3.20 | 3.47 |
| Dispensable AA, % | |||||||||
| Ala | 2.00 | 1.63 | 1.50 | 1.91 | 2.30 | 2.27 | 2.38 | 2.64 | 2.82 |
| Asp | 5.17 | 4.30 | 3.84 | 4.97 | 6.09 | 5.67 | 6.12 | 6.90 | 7.49 |
| Cys | 0.80 | 0.67 | 0.69 | 0.64 | 0.90 | 0.85 | 0.85 | 1.03 | 1.01 |
| Glu | 7.60 | 6.19 | 5.57 | 7.44 | 9.08 | 8.22 | 8.97 | 10.14 | 11.20 |
| Gly | 1.94 | 1.60 | 1.47 | 1.90 | 2.21 | 2.24 | 2.24 | 2.55 | 2.74 |
| Pro | 2.21 | 1.78 | 1.65 | 2.13 | 2.55 | 2.46 | 2.61 | 2.95 | 3.22 |
| Ser | 2.07 | 1.55 | 1.50 | 1.90 | 2.32 | 2.25 | 2.47 | 2.59 | 2.82 |
| Tyr | 1.65 | 1.40 | 1.27 | 1.58 | 1.94 | 1.82 | 2.01 | 2.09 | 2.27 |
| Available Lys, % | 2.89 | 2.22 | 2.03 | 2.55 | 3.16 | 2.91 | 3.10 | 3.69 | 3.90 |
| Lys, % of CP | 6.11 | 6.19 | 5.82 | 6.06 | 5.94 | 5.83 | 5.80 | 6.24 | 6.34 |
| Lys availability, % | 100.4 | 94.0 | 98.0 | 93.5 | 97.9 | 96.5 | 95.2 | 96.3 | 92.6 |
1SBM = Soybean meal 47; EX1 = Danex soybeans; EX2 = Pigletsoy 2000MSA; TM1 = HTM-96; TM2 = Provisoy; BC1 = Pepsoygen; BC2 = HP-300; EW1 = SPC-60-IP; and EW2 = Soycomil P.
Table 2.
Ingredient composition (as-fed basis) of the experimental diets1
| Item, % | Soybean products1 | N-free | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SBM | EX1 | EX2 | TM1 | TM2 | BC1 | BC2 | EW1 | EW2 | ||
| Cornstarch2 | 55.15 | 47.29 | 47.29 | 47.29 | 61.17 | 61.17 | 61.17 | 63.48 | 65.80 | 85.50 |
| Soybean meal3 | 36.50 | – | – | – | – | – | – | – | – | – |
| Danex soybeans4 | – | 45.00 | – | – | – | – | – | – | – | – |
| Pigletsoy 2000SMA5 | – | – | 45.00 | – | – | – | – | – | – | – |
| HTM-966 | – | – | – | 30.00 | – | – | – | – | – | – |
| Provisoy7 | – | – | – | – | 30.00 | – | – | – | – | – |
| Pepsoygen8 | – | – | – | – | – | 30.00 | – | – | – | – |
| HP3009 | – | – | – | – | – | – | 30.00 | – | – | – |
| SPC-60-IP10 | – | – | – | – | – | – | – | 27.50 | – | – |
| Soycomil P11 | – | – | – | – | – | – | – | – | 25.00 | – |
| Sugar, sucrose | 3.25 | 2.79 | 2.79 | 2.79 | 3.59 | 3.59 | 3.59 | 3.73 | 3.86 | 5.00 |
| Solka-Floc12 | – | – | – | – | – | – | – | – | – | 3.00 |
| Canola oil | 1.30 | 1.12 | 1.12 | 1.12 | 1.44 | 1.44 | 1.44 | 1.49 | 1.54 | 2.00 |
| Limestone | 1.20 | 1.20 | 1.20 | 1.20 | 1.20 | 1.20 | 1.20 | 1.20 | 1.20 | 1.00 |
| Mono-dicalcium phosphate | 0.80 | 0.80 | 0.80 | 0.80 | 0.80 | 0.80 | 0.80 | 0.80 | 0.80 | 1.20 |
| Salt | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 |
| Vitamin premix13 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 |
| Mineral premix14 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 |
| Cr2O3 | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 |
| K2CO3 | – | – | – | – | – | – | – | – | – | 0.40 |
| MgO | – | – | – | – | – | – | – | – | – | 0.10 |
1BC = Bioconversion using fermentation (BC1) or enzymes (BC2); EW = Ethanol-water extraction; EX = Extrusion; SBM = Soybean meal; TM = Thermo-mechanical treatment.
2Melojel (National Starch and Chemical Co., Bridgewater, NJ).
347% CP (Commercial supplier).
4EX1 = Danex soybeans (Danis NV, Koolskamp, Belgium).
5EX2 = Pigletsoy 2000MSA (SCA Ibérica, Mequinenza, Zaragoza, Spain).
6TM1 = HTM-96 (SCA Ibérica, Mequinenza, Zaragoza, Spain).
7TM2 = Provisoy (Provimi, Brookville, OH).
8BC1 = Pepsoygen (Nutraferma, North Sioux City, SD).
9BC2 = HP-300 (Hamlet Protein Inc., Findlay, OH).
10EW1 = SPC-60-IP (Imcopa, ‘s-Hertogenbosch, Netherlands).
11EW2 = Soycomil P (Archer Daniels Midland, Decatur, IL).
12International Fiber Corp., North Tonawanda, NY.
13Provided the following per kilogram of diet: vitamin A, 8,250 IU; vitamin D3, 825 IU; vitamin E, 40 IU; niacin, 35 mg; D-pantothenic acid, 15 mg; riboflavin, 5 mg; menadione, 4 mg; folic acid, 2 mg; thiamine, 1 mg; D-biotin 0.2 mg; and vitamin B12, 0.025 mg.
14Provided the following per kilogram of diet: Zn, 100 mg as ZnSO4; Fe, 80 mg as FeSO4; Cu, 50 mg as CuSO4; Mn, 25 mg as MnSO4; I, 0.5 mg as Ca(IO3)2; and Se, 0.1 mg as Na2SeO3.
Experimental Procedures
Animal protocols were reviewed and approved by the University of Alberta Animal Care and Use Committee for Livestock and followed guidelines established by the Canadian Council on Animal Care (CCAC, 2009). The experiment was conducted at the Swine Research and Technology Center at the University of Alberta (Edmonton, AB, Canada).
Ten crossbred barrows (initial BW, 30.4 ± 0.7 kg; Duroc sire × Large White/Landrace F1; Genex Hybrid, Hypor, Regina, SK, Canada) were housed in raised individual metabolism pens that allowed freedom of movement (1.2 m wide, 1.2 m long, and 0.9 m high). Pens were equipped with a stainless-steel self-feeder attached to the front of the pen, a cup drinker next to the feeder, plastic walls, and slatted flooring in a temperature-controlled room (22 ± 2.5 °C). During a 10-d preoperative adaptation, barrows had free access to an 18%-CP grower diet. Pigs were then fitted with a simple T-cannula at the distal ileum, circa 5 cm prior to the ileocecal sphincter. The preparation of the cannulas, surgical procedure, and modifications were described previously (Sauer et al., 1983; de Lange et al., 1989). Pre- and post-operative care was done as described previously (Li et al., 1993). After surgery, barrows recovered for 7 d with a gradual increase in feed allowance and were then switched to the experimental diets. Daily feed allowance was adjusted to 2.8 times the maintenance requirement for DE (2.8 × 110 kcal of DE/kg of BW0.75; NRC, 2012) that was fed in 2 equal meals at 0800 and 1500 h. Each 9-d experimental period consisted of a 5-d acclimation to experimental diets, followed sequentially by a 2-d collection of feces and a 2-d collection of ileal digesta. Pigs had free access to water throughout the experiment.
Feces were collected using plastic bags attached to the skin around the anus (van Kleef et al., 1994). Digesta samples were collected for 2 d using soft plastic bags (length, 20 cm; i.d., 4 cm) from 0800 to 2000 h containing 15 mL of 5% formic acid that were attached to the opened barrel of the cannula with a rubber band. Tubes were replaced as soon as filled or after 20 min (Li et al., 1993). Collected feces and digesta were pooled for each pig within experimental period and frozen at −20 °C. Prior to analyses, feces and digesta were thawed, homogenized, sub-sampled, and freeze-dried.
Chemical Analyses
Diets, test feedstuffs, and lyophilized digesta and feces were ground in a centrifugal mill (model ZM 200; Retsch Co., Newton, PA) through a 1.0-mm sieve. Feedstuffs, diets, feces, and digesta were analyzed for moisture (method 930.15; AOAC, 2006), ash (method 942.05; AOAC, 2006), acid detergent fiber (ADF; method 973.18; AOAC, 2006), crude fiber (method 962.09; AOAC, 2006), and ether extract (method 920.39; AOAC, 2006). Diets, feedstuffs, and digesta were analyzed for CP (method 990.03; AOAC, 2006), for AA by high-pressure liquid chromatography (HPLC; method 982.30E; AOAC, 2006), and for chemically available Lysine (Lys; method 975.44; AOAC, 2006). The phosphorus (P) and calcium (Ca) in feedstuffs were analyzed spectrophotometrically at 400 nm using the vanadate–molybdate method (method 946.06; AOAC, 2006). Starch in feedstuffs was analyzed using the amyloglucosidase and α-amylase method with a final glucose analysis using a spectrophotometer at 510 nm (method 996.11; AOAC, 2006). The gross energy (GE) of diets, feedstuffs, feces, and digesta was analyzed by an adiabatic calorimeter (model AC-300, Leco Corp., St. Joseph, MI). After ashing, Cr2O3 in diets, feces, and digesta was analyzed spectrophotometrically at 440 nm (Fenton and Fenton, 1979).
Calculations
Apparent ileal digestibility (AID; %) and apparent total tract digestibility (ATTD; %) of components in the diet were calculated using the following equation (Adeola, 2001):
The basal ileal endogenous loss (Iend) of an AA or CP (g/kg of DM intake) was calculated by the equation for the nitrogen (N) free diet (Stein et al., 2007):
Standardized ileal digestibility (SID; %) for each AA was then calculated by correcting the AID for basal ileal endogenous losses by the equation (Stein et al., 2007):
Content of SID CP and AA was calculated by multiplying SID measured in digesta by total CP and AA content of soybean products. The DE value of soybean products was calculated by subtracting the DE in the N-free diet that was provided by energy sources from the DE in each of soybean product-containing diets using the difference method (Adeola, 2001).
The NE value of feedstuffs was predicted from the determined DE values (kcal/kg of DM) and analyzed macronutrient (g/kg of DM) of feedstuffs using equation 5 that was developed by Noblet et al. (1994) and adopted by NRC (2012).
Statistical Analyses
The N-free diet was solely used for calculations and was excluded from statistical analyses. Data were analyzed using the MIXED procedure of SAS version 9.4 (SAS Inst. Inc., Cary, NC). The model included diet or ingredient as fixed effect and pig and period as random effects. The treatment means were reported as least squares means that were separated using the LSD method with the LSMEANS statement and PDIFF option in case treatments effects was significant. Individual pig was considered the experimental unit. Differences were considered significant if P < 0.05. To observe associations among chemical composition, digestibility of energy and nutrients, and digestible content of CP and AA of soybean co-products, data were analyzed using the PLS procedure of SAS with chemical composition as predictor variables and digestibility of energy and nutrients, and digestible content of CP and AA as response variables.
Results
Pigs remained healthy throughout the experiment, and no pigs were removed from the study. No orts were collected as all pigs consumed their daily allotments of feed throughout the experiment.
The CP content of soybean products varied depending on processing technique and averaged 36.9% for EX, 49.6% for TM, 53.9% for BC, and 63.9% for EW with similar patterns for dispensable and indispensable AA (Table 1). Chemically available Lys was 5% units greater for SBM than the other soybean products. The GE value of SBM was 140 kcal/kg lower than the average of soybean products obtained by TM, BC, and EW. With 18.9% units greater ether extract content, soybean products obtained by EX had a 670 kcal/kg greater GE value than the other soybean products. Content of fiber (crude fiber and ADF), P, and Ca was similar among soybean products. Analyzed nutrient composition of the 10 diets followed patterns in accordance with the ingredient nutrient values, indicating proper diet mixing (Table 3).
Table 3.
Analyzed nutrient composition (as-fed basis) of experimental diets containing soybean products
| Item, % | Soybean products1 | N-free | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SBM | EX1 | EX2 | TM1 | TM2 | BC1 | BC2 | EW1 | EW2 | ||
| Moisture | 8.2 | 7.9 | 8.2 | 9.7 | 7.8 | 7.6 | 8.1 | 8.1 | 8.5 | 8.1 |
| CP, % | 15.9 | 17.1 | 16.2 | 19.3 | 16.4 | 14.4 | 16.8 | 15.8 | 15.7 | 0.6 |
| GE, Mcal/kg | 3.93 | 4.30 | 4.28 | 3.88 | 3.93 | 3.94 | 3.94 | 3.90 | 3.89 | 3.70 |
| Ether extract, % | 1.31 | 10.50 | 9.26 | 1.36 | 1.42 | 1.50 | 1.37 | 1.07 | 1.60 | 0.75 |
| Ash, % | 4.74 | 4.84 | 4.90 | 5.27 | 4.51 | 4.26 | 4.69 | 3.98 | 3.84 | 2.54 |
| Crude fiber, % | 1.49 | 2.08 | 2.29 | 1.75 | 0.61 | 1.31 | 0.81 | 1.38 | 0.92 | 1.54 |
| Indispensable AA, % | ||||||||||
| Arg | 1.14 | 1.23 | 1.11 | 1.42 | 1.16 | 0.96 | 1.13 | 1.07 | 1.14 | 0.01 |
| His | 0.42 | 0.44 | 0.40 | 0.51 | 0.41 | 0.37 | 0.41 | 0.39 | 0.41 | 0.00 |
| Ile | 0.77 | 0.80 | 0.72 | 0.92 | 0.82 | 0.69 | 0.81 | 0.75 | 0.77 | 0.01 |
| Leu | 1.25 | 1.36 | 1.23 | 1.54 | 1.31 | 1.15 | 1.29 | 1.22 | 1.28 | 0.03 |
| Lys | 1.00 | 1.05 | 0.93 | 1.21 | 0.99 | 0.85 | 0.96 | 0.96 | 1.00 | 0.01 |
| Met | 0.22 | 0.22 | 0.27 | 0.27 | 0.21 | 0.21 | 0.22 | 0.21 | 0.22 | 0.01 |
| Phe | 0.82 | 0.90 | 0.80 | 1.03 | 0.86 | 0.73 | 0.85 | 0.79 | 0.83 | 0.02 |
| Thr | 0.59 | 0.66 | 0.61 | 0.74 | 0.59 | 0.56 | 0.60 | 0.58 | 0.61 | 0.01 |
| Trp | 0.19 | 0.23 | 0.21 | 0.24 | 0.21 | 0.18 | 0.22 | 0.17 | 0.18 | 0.04 |
| Val | 0.83 | 0.83 | 0.77 | 0.98 | 0.84 | 0.73 | 0.84 | 0.79 | 0.79 | 0.02 |
| Dispensable AA, % | ||||||||||
| Ala | 0.70 | 0.75 | 0.70 | 0.86 | 0.72 | 0.66 | 0.72 | 0.67 | 0.70 | 0.02 |
| Asp | 1.82 | 1.98 | 1.77 | 2.26 | 1.89 | 1.64 | 1.84 | 1.74 | 1.84 | 0.02 |
| Cys | 0.31 | 0.31 | 0.45 | 0.33 | 0.25 | 0.29 | 0.31 | 0.28 | 0.30 | 0.01 |
| Glu | 2.79 | 2.98 | 2.67 | 3.50 | 2.85 | 2.47 | 2.80 | 2.62 | 2.86 | 0.05 |
| Gly | 0.69 | 0.73 | 0.68 | 0.85 | 0.69 | 0.65 | 0.68 | 0.65 | 0.68 | 0.01 |
| Pro | 0.81 | 0.82 | 0.76 | 1.00 | 0.79 | 0.70 | 0.77 | 0.73 | 0.79 | 0.02 |
| Ser | 0.67 | 0.82 | 0.72 | 0.86 | 0.68 | 0.66 | 0.70 | 0.65 | 0.72 | 0.01 |
| Tyr | 0.52 | 0.58 | 0.53 | 0.67 | 0.51 | 0.45 | 0.52 | 0.43 | 0.47 | 0.01 |
| Available Lys, % | 0.87 | 0.99 | 0.92 | 1.16 | 0.98 | 0.77 | 0.91 | 0.97 | 0.90 | 0.01 |
| Lys, % of CP | 6.25 | 6.12 | 5.74 | 6.27 | 6.03 | 5.91 | 5.75 | 6.05 | 6.38 | 1.82 |
| Lys availability, % | 87.6 | 94.0 | 98.5 | 96.1 | 98.9 | 90.4 | 94.4 | 100.9 | 89.5 | 90.7 |
1SBM = Soybean meal 47; EX1 = Danex soybeans; EX2 = Pigletsoy 2000MSA; TM1 = HTM-96; TM2 = Provisoy; BC1 = Pepsoygen; BC2 = HP-300; EW1 = SPC-60-IP; and EW2 = Soycomil P.
Among diets, the ATTD of GE was greater (P < 0.001; Table 4) for diets containing SBM, TM, BC, or EW than that for diets containing EX1 or EX2. However, diet DE value had a range of 0.12 Mcal/kg of DM and did not differ among soybean products. Among ingredients, the ATTD of GE was greater (P = 0.01) for TM1 than EW1, EX1, and EX2, but was intermediate for SBM, TM2, BC1, BC2, and EW2.
Table 4.
Apparent total tract digestibility (ATTD) of energy of diets and soybean products and diet DE value (DM basis)
| Item, % | Soybean products1 | SEM | P | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SBM | EX1 | EX2 | TM1 | TM2 | BC1 | BC2 | EW1 | EW2 | |||
| ATTD of energy, % | |||||||||||
| Diet | 94.1a | 86.6b | 86.8b | 93.5a | 94.6a | 94.6a | 94.5a | 92.5a | 94.3a | 1.31 | < 0.001 |
| Ingredient | 91.0ab | 76.4c | 76.8c | 91.7a | 90.1ab | 90.1ab | 90.3ab | 81.9bc | 88.7ab | 3.68 | 0.010 |
| Diet DE, Mcal/kg | 4.03 | 4.05 | 4.04 | 4.01 | 4.03 | 4.03 | 4.05 | 3.92 | 4.01 | 0.06 | 0.649 |
1SBM = Soybean meal 47; EX1 = Danex soybeans; EX2 = Pigletsoy 2000MSA; TM1 = HTM-96; TM2 = Provisoy; BC1 = Pepsoygen; BC2 = HP-300; EW1 = SPC-60-IP; and EW2 = Soycomil P.
The AID of CP ranged from 79.6% to 89.0% and did not differ (P > 0.05; Table 5) among diets containing soybean products. Diet AID of Lys was greater (P < 0.05) for TM1, TM2, and EW1 than that for EX2 and BC1, and was intermediate for SBM, EX1, BC2, and EW2. Diet AID of Arg, Ile, Leu, Phe, Val, Glu, Tyr, and chemically available Lys differed (P < 0.05) among diets. Diet AID of His, Met, Thr, Trp, Ala, Asp, Cys, Gly, Pro, and Ser for soy products did not differ.
Table 5.
Apparent ileal digestibility of CP and AA in diets containing soybean meal (SBM) and soy products
| Item, % | Soybean products1 | SEM | P | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SBM | EX1 | EX2 | TM1 | TM2 | BC1 | BC2 | EW1 | EW2 | |||
| CP | 80.8 | 85.0 | 79.6 | 89.0 | 88.4 | 82.1 | 86.6 | 85.6 | 83.4 | 2.96 | 0.229 |
| Indispensable AA | |||||||||||
| Arg | 90.2b | 90.3b | 84.7c | 95.3a | 94.6ab | 91.1ab | 93.6ab | 91.9ab | 92.5ab | 1.70 | 0.007 |
| His | 84.8 | 88.5 | 82.7 | 92.6 | 92.7 | 88.9 | 91.3 | 84.4 | 89.7 | 2.82 | 0.101 |
| Ile | 86.6cd | 88.2bc | 82.1d | 93.0ab | 94.4a | 88.9bc | 92.5ab | 89.7abc | 90.8abc | 2.09 | 0.007 |
| Leu | 85.4bc | 88.1abc | 82.4c | 92.9a | 93.8a | 88.8ab | 92.2a | 89.3ab | 90.6ab | 2.16 | 0.011 |
| Lys | 85.1ab | 88.0ab | 82.9b | 92.7a | 93.2a | 82.6b | 87.2ab | 92.1a | 89.3ab | 2.64 | 0.019 |
| Met | 89.8 | 89.1 | 87.8 | 94.5 | 94.7 | 90.5 | 93.6 | 91.1 | 91.1 | 1.81 | 0.072 |
| Phe | 85.6cd | 88.2bcd | 82.6d | 93.2ab | 94.0a | 89.1abc | 92.9ab | 90.1abc | 91.4abc | 2.11 | 0.005 |
| Thr | 78.0 | 84.8 | 78.9 | 87.4 | 87.4 | 81.5 | 84.8 | 83.4 | 83.2 | 3.35 | 0.332 |
| Trp | 85.3 | 91.1 | 85.1 | 91.1 | 92.9 | 89.0 | 91.1 | 88.8 | 90.7 | 2.29 | 0.178 |
| Val | 83.5bc | 86.3abc | 80.0c | 90.8a | 91.9a | 85.8abc | 89.6ab | 87.4ab | 88.1abc | 2.54 | 0.046 |
| Dispensable AA | |||||||||||
| Ala | 81.5 | 86.0 | 80.0 | 89.9 | 89.3 | 82.4 | 87.4 | 84.2 | 85.5 | 2.90 | 0.154 |
| Asp | 82.8 | 86.9 | 81.6 | 89.2 | 90.8 | 85.6 | 87.9 | 85.9 | 82.3 | 2.71 | 0.215 |
| Cys | 80.4 | 88.3 | 88.5 | 86.1 | 88.2 | 85.1 | 87.1 | 85.4 | 85.4 | 3.07 | 0.559 |
| Glu | 86.0c | 89.2abc | 84.1c | 91.5ab | 94.3a | 87.9bc | 91.8ab | 89.3abc | 90.7abc | 2.15 | 0.044 |
| Gly | 69.6 | 79.0 | 70.1 | 82.9 | 79.6 | 73.3 | 76.0 | 73.0 | 71.3 | 5.21 | 0.455 |
| Pro | 65.8 | 69.1 | 65.8 | 80.3 | 55.0 | 72.5 | 75.5 | 78.4 | 62.9 | 8.34 | 0.265 |
| Ser | 82.5 | 87.3 | 80.9 | 90.8 | 90.2 | 85.5 | 88.3 | 87.0 | 87.4 | 2.56 | 0.110 |
| Tyr | 85.4bc | 88.0abc | 81.8c | 92.7a | 93.2a | 87.4abc | 91.0ab | 87.8abc | 89.3abc | 2.27 | 0.024 |
| Available Lys | 84.9bc | 88.4abc | 83.6bc | 92.8a | 92.9a | 82.6c | 86.9abc | 89.4ab | 88.5abc | 2.37 | 0.019 |
1SBM = Soybean meal 47; EX1 = Danex soybeans; EX2 = Pigletsoy 2000MSA; TM1 = HTM-96; TM2 = Provisoy; BC1 = Pepsoygen; BC2 = HP-300; EW1 = SPC-60-IP; and EW2 = Soycomil P.
a–cMeans without a common superscript differ at P < 0.05.
The SID of CP ranged from 88.1% to 96.8% and did not differ (P > 0.05; Table 6) among soybean products. The SID of Lys was greater (P < 0.01) for TM1, TM2, and EW1 than that for EX2 and BC1, and was intermediate for SBM, EX1, BC2, and EW2. The SID of Arg, Ile, Leu, Phe, and Tyr differed (P < 0.05) among soybean products and was consistently lower for EX2 (P < 0.05). The SID of His, Met, Thr, Trp, Val, Ala, Asp, Cys, Glu, Gly, Ser, and chemically available Lys did not differ (P > 0.05) among soybean products.
Table 6.
Standardized ileal digestibility of CP and AA in soybean meal (SBM) and soybean products
| Item, % | Soybean products1,2 | SEM | P | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SBM | EX1 | EX2 | TM1 | TM2 | BC1 | BC2 | EW1 | EW2 | |||
| CP | 89.4 | 93.0 | 88.1 | 95.9 | 96.8 | 91.7 | 94.8 | 94.3 | 92.1 | 2.96 | 0.391 |
| Indispensable AA | |||||||||||
| Arg | 95.9a | 95.6a | 90.6b | 99.8a | 100.0a | 98.0a | 99.4a | 98.0a | 98.2a | 1.70 | 0.015 |
| His | 89.5 | 93.0 | 87.6 | 96.3 | 97.4 | 94.3 | 96.1 | 89.4 | 94.4 | 2.78 | 0.133 |
| Ile | 89.4cd | 90.9bcd | 85.1d | 95.3ab | 97.0a | 92.1abc | 95.2ab | 92.6abc | 93.7abc | 2.09 | 0.012 |
| Leu | 88.6bc | 91.1abc | 85.6c | 95.5a | 96.9a | 92.3ab | 95.3a | 92.6ab | 93.7ab | 2.16 | 0.015 |
| Lys | 90.3ab | 91.0ab | 86.3b | 95.3a | 96.4a | 86.3b | 90.5ab | 95.4a | 92.4ab | 2.64 | 0.033 |
| Met | 92.1 | 91.4 | 89.8 | 96.4 | 97.2 | 93.0 | 95.9 | 93.6 | 93.4 | 1.81 | 0.069 |
| Phe | 88.6cd | 90.9bcd | 85.7d | 95.5ab | 97.3a | 92.5abc | 95.8ab | 93.2abc | 94.4abc | 2.11 | 0.007 |
| Thr | 85.2 | 91.3 | 86.0 | 93.1 | 96.4 | 89.1 | 91.9 | 90.8 | 90.1 | 3.35 | 0.444 |
| Trp | 89.8 | 94.7 | 89.1 | 94.4 | 96.9 | 93.7 | 95.0 | 93.6 | 95.4 | 2.29 | 0.254 |
| Val | 88.0 | 90.7 | 84.8 | 94.5 | 96.3 | 90.8 | 94.0 | 92.1 | 92.8 | 2.54 | 0.072 |
| Dispensable AA | |||||||||||
| Ala | 89.1 | 93.2 | 87.7 | 96.0 | 96.9 | 90.6 | 94.9 | 92.3 | 93.2 | 2.90 | 0.280 |
| Asp | 86.1 | 89.9 | 85.0 | 91.9 | 94.1 | 89.3 | 91.2 | 89.4 | 85.6 | 2.71 | 0.266 |
| Cys | 85.0 | 92.9 | 91.6 | 90.3 | 93.9 | 90.0 | 91.8 | 90.6 | 90.3 | 3.07 | 0.547 |
| Glu | 88.6 | 91.6 | 86.8 | 93.5 | 96.8 | 90.9 | 94.3 | 92.1 | 93.1 | 2.15 | 0.058 |
| Gly | 88.5 | 97.2 | 89.4 | 98.1 | 98.9 | 93.7 | 95.5 | 93.3 | 90.6 | 5.21 | 0.723 |
| Ser | 88.6 | 92.2 | 86.5 | 95.5 | 96.2 | 91.6 | 94.1 | 93.2 | 93.0 | 2.56 | 0.157 |
| Tyr | 88.7bc | 90.9abc | 84.4c | 95.1a | 96.5a | 91.1abc | 94.2ab | 91.7ab | 92.9abc | 2.27 | 0.032 |
| Available Lys | 88.9 | 92.0 | 87.4 | 95.7 | 96.4 | 87.2 | 90.7 | 93.0 | 92.3 | 2.37 | 0.051 |
1SBM = Soybean meal 47; EX1 = Danex soybeans; EX2 = Pigletsoy 2000MSA; TM1 = HTM-96; TM2 = Provisoy; BC1 = Pepsoygen; BC2 = HP-300; EW1 = SPC-60-IP; and EW2 = Soycomil P.
2Standardized ileal digestibility of CP and AA was calculated by correcting apparent ileal digestibility of CP and AA with measured basal endogenous losses (g/kg dry matter intake): CP, 14.9; Arg, 0.71; His, 0.21; Ile, 0.24; Leu, 0.44; Lys, 0.34; Met, 0.06; Phe, 0.27; Thr, 0.47; Trp, 0.09; Val, 0.40; Ala, 0.59; Asp, 0.66; Cys, 0.16; Glu, 0.78; Gly, 1.43; Ser, 0.44, and Tyr, 0.18.
a–cMeans without a common superscript differ at P < 0.05.
Ingredient DE value ranged from 3.94 to 4.37 Mcal/kg and NE value ranged from 2.71 to 3.01 Mcal/kg, and did not differ among soybean products (Table 7). The SID content of CP was greatest (P < 0.05) for EW1 and EW2, followed by TM2 and BC2, then SBM, TM1, and BC1, and was lowest (P < 0.05) for EX1 and EX2, with a similar pattern for SID content of indispensable and dispensable AA.
Table 7.
The DE and NE value and standardized ileal digestible CP and AA content in soybean products
| Item, DM basis | Soybean products1 | SEM | P | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SBM | EX1 | EX2 | TM1 | TM2 | BC1 | BC2 | EW1 | EW2 | |||
| DE, Mcal/kg | 4.23 | 4.23 | 4.11 | 4.29 | 4.23 | 3.96 | 4.37 | 3.94 | 4.27 | 0.18 | 0.621 |
| NE, Mcal/kg | 2.92 | 2.96 | 2.88 | 2.97 | 2.92 | 2.73 | 3.01 | 2.71 | 2.93 | 0.13 | 0.587 |
| CP, % | 46.2d | 38.3e | 34.2e | 49.2cd | 56.3b | 50.6c | 57.9b | 62.3a | 66.8a | 1.62 | < 0.001 |
| Indispensable AA, % | |||||||||||
| Arg | 3.53d | 2.88e | 2.45f | 3.64d | 4.18c | 3.59d | 4.24c | 4.70b | 5.20a | 0.06 | < 0.001 |
| His | 1.19e | 0.99f | 0.85g | 1.28de | 1.42c | 1.30d | 1.44bc | 1.55b | 1.79a | 0.04 | < 0.001 |
| Ile | 2.13e | 1.96f | 1.60g | 2.46d | 2.81c | 2.54d | 2.81c | 3.13b | 3.44a | 0.05 | < 0.001 |
| Leu | 3.52e | 3.01f | 2.55g | 3.81d | 4.40c | 4.00d | 4.48c | 4.87b | 5.41a | 0.09 | < 0.001 |
| Lys | 2.85d | 2.32e | 1.96f | 2.96d | 3.33c | 2.77d | 3.21c | 3.93b | 4.26a | 0.09 | < 0.001 |
| Met | 0.70d | 0.51e | 0.50e | 0.69d | 0.78c | 0.78c | 0.81c | 0.88b | 0.99a | 0.01 | < 0.001 |
| Phe | 2.28e | 2.02f | 1.68g | 2.53d | 2.92c | 2.58d | 3.01c | 3.19b | 3.51a | 0.06 | < 0.001 |
| Thr | 1.69e | 1.37f | 1.24f | 1.75e | 2.01cd | 1.85de | 2.09bc | 2.25ab | 2.45a | 0.07 | < 0.001 |
| Trp | 0.63d | 0.48e | 0.45e | 0.65d | 0.76c | 0.72c | 0.75c | 0.83b | 0.93a | 0.02 | < 0.001 |
| Val | 2.24e | 1.97f | 1.67g | 2.52d | 2.83c | 2.60d | 2.82c | 3.17b | 3.52a | 0.07 | < 0.001 |
| Dispensable AA, % | |||||||||||
| Ala | 1.95e | 1.64f | 1.43g | 2.09de | 2.38c | 2.19d | 2.46bc | 2.62b | 2.87a | 0.07 | < 0.001 |
| Asp | 4.89d | 4.18e | 3.57f | 5.21cd | 6.13b | 5.39c | 6.08b | 6.63a | 7.00a | 0.16 | < 0.001 |
| Cys | 0.74d | 0.68de | 0.68de | 0.66e | 0.90b | 0.81bc | 0.85bc | 1.00a | 1.00a | 0.03 | < 0.001 |
| Glu | 7.39e | 6.13f | 5.27g | 7.94d | 9.41c | 7.95d | 9.21c | 10.04b | 11.40a | 0.19 | < 0.001 |
| Gly | 1.89cd | 1.68de | 1.44e | 2.12bc | 2.33ab | 2.24b | 2.33ab | 2.55a | 2.72a | 0.12 | < 0.001 |
| Pro | 2.86bcd | 2.45cd | 2.01d | 3.00abc | 3.20abc | 3.43ab | 3.41ab | 3.79a | 3.69ab | 0.35 | 0.012 |
| Ser | 2.02e | 1.54f | 1.42f | 2.07de | 2.38c | 2.19d | 2.53bc | 2.60b | 2.86a | 0.06 | < 0.001 |
| Tyr | 1.79c | 1.40d | 1.22e | 1.76c | 2.12b | 1.84c | 2.16ab | 2.25a | 2.32a | 0.05 | < 0.001 |
| Available Lys | 2.82c | 2.21d | 1.94e | 2.78c | 3.26b | 2.70c | 3.06b | 3.69a | 3.94a | 0.08 | < 0.001 |
1SBM = Soybean meal 47; EX1 = Danex soybeans; EX2 = Pigletsoy 2000MSA; TM1 = HTM-96; TM2 = Provisoy; BC1 = Pepsoygen; BC2 = HP-300; EW1 = SPC-60-IP; and EW2 = Soycomil P.
a-fMeans without a common superscript differ at P < 0.05.
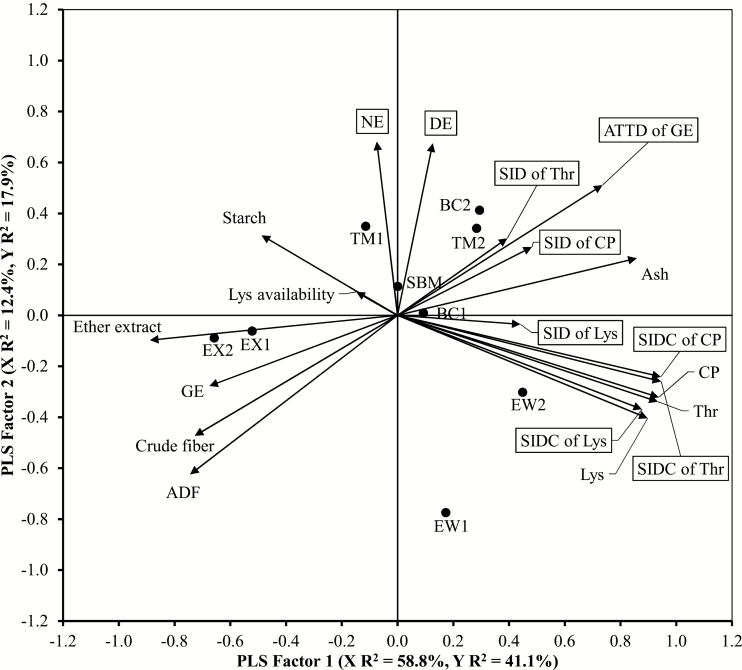
Using partial least square (PLS) analysis, the first two PLS factors explained 71.2% variation in predictor variables (chemical composition and GE) and 59.0% variation in response variables (digestibility and digestible content of nutrients or GE) of the soy co-products (Figure 1). The variation in SID content of CP, Lys, or Thr, and ATTD of GE was explained well with the two PLS factors. The PLS analysis revealed that ATTD of GE was strongly negatively correlated with ADF and crude fiber content. The EX1 and EX2 characterized by greater ether extract content, but that was negatively correlated with the ATTD of GE. The SID content of CP, Lys, and Thr was more correlated with total content of CP, Lys, and Thr than SID of CP, Lys, and Thr. The SID of Lys did not correlate with Lys availability. The EW1 was far from and in an opposite direction to the DE and NE loading.
Figure 1.
Correlation loading plot of chemical components (predictor variables; without box) and digestibility of energy and nutrients and digestible content of crude protein and amino acids (response variables; labeled with solid-line boxes) following PLS analyses. The 9 soybean products were labeled with solid round dots. Lines pointing in similar direction with sharp angles indicate that variables were strongly correlated, but lines in opposite directions indicate that variables were strongly negatively correlated, whereas lines perpendicular to each other indicate that variables were not correlated. Placing of soybean products closer to each other or to predictor or response variables indicates a stronger association. SBM = Soybean meal 47; EX1 = Danex soybeans; EX2 = Pigletsoy 2000MSA; TM1 = HTM-96; TM2 = Provisoy; BC1 = Pepsoygen; BC2 = HP-300; EW1 = SPC-60-IP and EW2 = Soycomil P; and SIDC = SID content.
Discussion
The present study was focused on novel soybean products that were produced by processing soybean or SBM using various technologies. Consequently, these soybean products varied in macronutrient composition especially for ether extract, CP, and fiber. Changes in macronutrients and in factors that restrict digestibility resulted in differences in digestibility of energy and AA.
Composition of Ingredients
The GE value and CP and AA content of SBM in the present study were similar to the published data for SBM (NRC, 2012; Yáñez et al., 2014). The EX1 and EX2 soybean products from extrusion of full fat soybean had ether extract, CP, and AA profile close to the previously reported for full fat soybeans (Cervantes-Pahm and Stein, 2008; NRC, 2012). However, novel cultivars of full fat soybeans may contain high protein or low oligosaccharide and may contain slightly more ether extract (NRC, 2012). Because soybean oil was not extracted, the EX1 and EX2 contained more ether extract than SBM and other soybean products, and thus had a greater GE value. Processed using a thermo-mechanical treatment to increase nutrient digestibility of SBM, the TM soybean products contained CP and AA in average similar to regular SBM with 47% CP. The BC process uses either fermentation (BC1) or enzymes (BC2) to hydrolyze carbohydrates and protein from SBM (Rosenthal et al., 1996). Consequently, the BC soybean products had ether extract, CP, and AA profile similar to fermented SBM and enzyme-treated SBM (Cervantes-Pahm and Stein, 2010; NRC, 2012). The EW process uses SBM as starting material, which is then under liquid–solid extraction, removal and recovery of solvent from liquid extract, removal and recovery of solvent from extracted flakes, and drying and grinding of flakes to extract the soluble sugar fraction of defatted SBM without solubilizing its proteins (Berk, 1992). Little is known about the nutrient composition of soybean products processed using EW, but they are expected to be similar to soybean protein concentrates due to their greater CP content (NRC, 2012). In the present study, soybean products that had low fat content contained more CP. The processes used to create the soybean products in the present study may reduce ANF, such as trypsin inhibitors, oligosaccharides, and allergenic proteins, which are not tolerated well by young pigs (Sissons et al., 1982; Cromwell, 2000; Cervantes-Pahm and Stein, 2010). Removal or reduction of these ANF may increase digestibility of energy and nutrients. Consequently, novel soybean products with low ANF might be attractive for inclusion in diets for young pigs (Jones et al., 2010; Kim et al., 2010).
Digestibility of Energy
In the present study, full-fat soybean had lower energy digestibility than SBM (Kim et al., 2013), which could be due to its fat within a fibrous matrix and its external hull that may hinder energy digestibility (Dilger et al., 2004). The extrusion step in EX1 and EX2 may open the fibrous matrix (Lundblad et al., 2011). However, energy digestibility remained lower for EX than for SBM. Following dehulling and oil extraction, SBM contains less fiber than full-fat soybean. Energy digestibility and energy value of dehulled-expelled SBM have been well established. In the present study, energy value of SBM was similar to NRC (2012) values of DE at 4.21 Mcal/kg and NE at 2.60 Mcal/kg, and other reported values (Baker and Stein, 2009), but greater than the reported 3.38 Mcal DE/kg (van Kempen et al., 2006). Fiber analyses used in the present study especially measure insoluble fiber and not soluble fiber (Goering and van Soest, 1970). Treatments used to process SBM in the present study may cause marginal changes in insoluble fiber content among the soy products; however, these changes were associated with changes of ATTD of energy compared with SBM. Treatments used in BC and EW of SBM may cause changes in soluble fiber and especially the oligosaccharides content (Cervantes-Pahm and Stein, 2010). However, these potential changes did not alter ATTD of energy too much when comparing with SBM in the present study, likely because soluble fiber components in SBM were fermented well within the gastrointestinal tract (Jha and Berrocoso, 2016). Overall, similar GE with similar ATTD of GE or greater GE with lower ATTD of GE for soybean products in the present study resulted in similar DE and predicted NE value among soybean products.
Digestibility of Amino Acids
Soybean products are rich in protein overall, and thus their protein quality is important (Sauer and Ozimek, 1986). In the present study, soybean products varied substantially in protein content and differed in AID and SID of AA, similar to previous studies (e.g., Stein et al., 2007). Some ingredient factors may have influenced AA digestibility. For example, the fiber-rich soybean hulls reduce digestibility of AA (Dilger et al., 2004). The ANF in raw soybean or remaining ANF in processed soybean can reduce digestibility of AA (Qin et al., 1996). Finally, excessive heat processing may reduce digestibility and availability of AA (Fontaine et al., 2007). As an indicator of protein quality, Lys chemical-availability was slightly lower than 100% for soybean products other than SBM in the present study, indicating heat damage to protein was minor, similar to soybean products such as dry extruded-expelled SBM (Opapeju et al., 2006).
In full-fat soybean and other oilseeds, AA might be trapped in the protein–fiber–fat matrix that may make digestion of AA difficult and thereby reduce digestibility of AA (Woyengo et al., 2014b; Park et al., 2017). Increased fat in diets may reduce passage rate, thereby allowing for more time for AA digestion and absorption (Albin et al., 2001); however, the effect on increasing AA digestibility was not apparent for the EX soybean products containing the extra oil likely because the oil was contained within the ingredient matrix and not free oil. Due to greater oil and fiber content, AA content in EX soybean products was lower; therefore, SID AA content was lower in EX soybean products than SBM, similar to the previously reported (Cervantes-Pahm and Stein, 2008).
The novel technologies used to process SBM may remove components that reduce digestibility of AA including fibrous components (Cromwell, 2000). Consequently, novel soybean products with lower ANF may be included to diets for monogastric species, especially for young pigs, to meet their high requirements for digestible AA. These novel soybean products might therefore substitute animal origin protein sources. In the present study, digestibility of the first- to fourth-limiting AA did not differ between SBM and novel soybean products. However, digestibility of branched-chain AA was greater in novel soybean products processed from SBM. With the increased growth rate of young pigs, meeting the requirements of the fifth- to seventh-limiting AA such as the branched-chain AA is becoming increasingly important (NRC, 2012). Therefore, these novel soybean meal products are attractive for inclusion into diets for young pigs. Moreover, the novel processing procedures such as treatments using solvents, fermentation, or enzymes increase the digestible content of essential AA in the novel soybean products compared with SBM. Principally, these treatments open the protein matrix, reduce ANF, or enhance access to digestive enzymes, thereby making these novel soybean products interesting feedstuffs to consider in diets for young pigs (Cervantes-Pahm and Stein, 2008; Oliveira and Stein, 2016).
In conclusion, soybean products developed from soybean had unique chemical characteristics. Soybean products with high residual oil increased GE value, but not DE or NE value. Finally, soybean products with enriched CP through removing fractions other than CP would provide greater SID CP content to pigs.
Literature Cited
- Adeola O. 2001. Digestion and balance techniques in pigs. In: Lewis A. L. and Southern L. L., editors, Swine nutrition. CRC Press, Boca Raton, FL: p. 903–916. [Google Scholar]
- Albin D. M., Smiricky M. R., Wubben J. E., and Gabert V. M.. . 2001. The effect of dietary level of soybean oil and palm oil on apparent ileal amino acid digestibility and postprandial flow patterns of chromic oxide and amino acids in pigs. Can. J. Anim. Sci. 81:495–503. doi: 10.4141/A00-104 [DOI] [Google Scholar]
- AOAC 2006. Official methods of analysis. 18th ed AOAC Int., Gaithersburg, MD. [Google Scholar]
- Baker D. H. 2000. Nutritional constraints to the use of soy products by animals. In: Drackley J. K., editor, Soy in animal nutrition. Fed. Anim. Sci. Soc., Savoy, IL: p. 1–12. [Google Scholar]
- Baker K. M., and Stein H. H.. . 2009. Amino acid digestibility and concentration of digestible and metabolizable energy in soybean meal produced from conventional, high-protein, or low-oligosaccharide varieties of soybeans and fed to growing pigs. J. Anim. Sci. 87:2282–2290. doi: 10.2527/jas.2008-1414 [DOI] [PubMed] [Google Scholar]
- Berk Z. 1992. Technology of production of edible flours and protein products from soybeans. FAO, Rome, Italy. [Google Scholar]
- CCAC 2009. The care and use of farm animals in research, teaching and testing. Can. Counc. Anim. Care Sci., Ottawa, ON, Canada. [Google Scholar]
- Cervantes-Pahm S. K., and Stein H. H.. . 2008. Effect of dietary soybean oil and soybean protein concentration on the concentration of digestible amino acids in soybean products fed to growing pigs. J. Anim. Sci. 86:1841–1849. doi: 10.2527/jas.2007-0721 [DOI] [PubMed] [Google Scholar]
- Cervantes-Pahm S. K., and Stein H. H.. . 2010. Ileal digestibility of amino acids in conventional, fermented, and enzyme-treated soybean meal and in soy protein isolate, fish meal, and casein fed to weanling pigs. J. Anim. Sci. 88:2674–2683. doi: 10.2527/jas.2009-2677 [DOI] [PubMed] [Google Scholar]
- Cromwell G. L. 2000. Utilization of soy products in swine diets. In: Drackley J. K., editor, Soy in animal nutrition. Fed. Anim. Sci. Soc., Savoy, IL: p. 258–282. [Google Scholar]
- Dilger R. N., Sands J. S., Ragland D., and Adeola O.. . 2004. Digestibility of nitrogen and amino acids in soybean meal with added soyhulls. J. Anim. Sci. 82:715–724. doi: 10.2527/2004.823715x [DOI] [PubMed] [Google Scholar]
- Fenton T. W., and Fenton M.. . 1979. An improved procedure for the determination of chromic oxide in feed and feces. Can. J. Anim. Sci. 59:631–634. doi: 10.4141/cjas79-081 [DOI] [Google Scholar]
- Fontaine J., Zimmer U., Moughan P. J., and Rutherfurd S. M.. . 2007. Effect of heat damage in an autoclave on the reactive lysine contents of soy products and corn distillers dried grains with solubles. Use of the results to check on lysine damage in common qualities of these ingredients. J. Agric. Food Chem. 55:10737–10743. doi: 10.1021/jf071747c [DOI] [PubMed] [Google Scholar]
- Goering H. K., and van Soest P. J.. . 1970. Forage fibre analysis (apparatus, reagents, procedures, and some applications). Agric. ARS-USDA, Washington, DC, p. 379. [Google Scholar]
- Gunawardena C. K., Zijlstra R. T., Goonewardene L. A., and Beltranena E.. . 2010. Protein and starch concentrates of air-classified field pea and zero-tannin faba bean for weaned pigs. J. Anim. Sci. 88:2627–2636. doi: 10.2527/jas.2009-2291 [DOI] [PubMed] [Google Scholar]
- Jha R., and Berrocoso J. F. D.. . 2016. Dietary fiber and protein fermentation in the intestine of swine and their interactive effects on gut health and on the environment: a review. Anim. Feed Sci. Technol. 212:18–28. doi.org/10.1016/j.anifeedsci.2015.12.002 [Google Scholar]
- Jones C. K., DeRouchey J. M., Nelssen J. L., Tokach M. D., Dritz S. S., and Goodband R. D.. . 2010. Effects of fermented soybean meal and specialty animal protein sources on nursery pig performance. J. Anim. Sci. 88:1725–1732. doi: 10.2527/jas.2009-2110 [DOI] [PubMed] [Google Scholar]
- van Kempen T. A. T. G., van Heughten E., Moeser A. J., Muley N. S., and Sewalt V. J. H.. . 2006. Selecting soybean meal characteristics preferred for swine nutrition. J. Anim. Sci. 84:1387–1395. doi: 10.2527/2006.8461387x [DOI] [PubMed] [Google Scholar]
- Kim S. W., van Heugten E., Ji F., Lee C. H., and Mateo R. D.. . 2010. Fermented soybean meal as a vegetable protein source for nursery pigs: I. Effects on growth performance of nursery pigs. J. Anim. Sci. 88:214–224. doi: 10.2527/jas.2009-1993 [DOI] [PubMed] [Google Scholar]
- Kim B. G., Kil D. Y., and Stein H. H.. . 2013. In growing pigs, the true ileal and total tract digestibility of acid hydrolyzed ether extract in extracted corn oil is greater than in intact sources of corn oil or soybean oil. J. Anim. Sci. 91:755–763. doi: 10.2527/jas.2011-4777 [DOI] [PubMed] [Google Scholar]
- van Kleef D. J., Deuring K., and van Leeuwen P.. . 1994. A new method of faeces collection in the pig. Lab. Anim. 28:78–79. doi: 10.1258/002367794781065942 [DOI] [PubMed] [Google Scholar]
- Lallès J. P. 2000. Soy products as protein sources for preruminants and young pigs. In: Drackley J. K., editor, Soy in animal nutrition. Fed. Anim. Sci. Soc., Champaign, IL: p. 106− 126. [Google Scholar]
- de Lange C. F., Sauer W. C., Mosenthin R., and Souffrant W. B.. . 1989. The effect of feeding different protein-free diets on the recovery and amino acid composition of endogenous protein collected from the distal ileum and feces in pigs. J. Anim. Sci. 67:746–754. doi: 10.2527/jas1989.673746x [DOI] [PubMed] [Google Scholar]
- Lenehan N. A., DeRouchey J. M., Goodband R. D., Tokach M. D., Dritz S. S., Nelssen J. L., Groesbeck C. N., and Lawrence K. R.. . 2007. Evaluation of soy protein concentrates in nursery pig diets. J. Anim. Sci. 85:3013–3021. doi: 10.2527/jas.2007-0071 [DOI] [PubMed] [Google Scholar]
- Li D. F., Nelssen J. L., Reddy P. G., Blecha F., Hancock J. D., Allee G. L., Goodband R. D., and Klemm R. D.. . 1990. Transient hypersensitivity to soybean meal in the early-weaned pig. J. Anim. Sci. 68:1790–1799. doi: 10.2527/1990.6861790x [DOI] [PubMed] [Google Scholar]
- Li S., Sauer W. C., and Fan M. Z.. . 1993. The effect of dietary crude protein level on ileal and fecal amino acid digestibility in early weaned pigs. J. Anim. Physiol. Anim. Nutr. 70:117–128. doi: 10.1111/j.1439-0396.1993.tb00314.x [DOI] [Google Scholar]
- Lundblad K. K, Issa S., Hancock J. D., Behnke K. C., McKinney L. J., Alavi S., Prestlokken E., Fledderus J., and Sorensen J.. . 2011. Effects of steam conditioning at low and high temperature, expander conditioning and extruder processing prior to pelleting on growth performance and nutrient digestibility in nursery pigs and broiler chickens. Anim. Feed Sci. Technol. 169:208–217. doi: 10.1016/j.anifeedsci.2011.06.008. [DOI] [Google Scholar]
- Noblet J., Fortune H., Shi X. S., and Dubois S.. . 1994. Prediction of net energy value of feeds for growing pigs. J. Anim. Sci. 72:344–354. doi: 10.2527/1994.722344x [DOI] [PubMed] [Google Scholar]
- NRC 2012. Nutrient requirements of swine. 11th rev. ed Natl. Acad. Press, Washington, DC. [Google Scholar]
- Oliveira M. S., and Stein H. H.. . 2016. Digestibility of energy, amino acids, and phosphorus in a novel source of soy protein concentrate and in soybean meal fed to growing pigs. J. Anim. Sci. 94:3343–3352. doi: 10.2527/jas2016-0505 [DOI] [PubMed] [Google Scholar]
- Opapeju F. O., Golian A., Nyachoti C. M., and Campbell L. D.. . 2006. Amino acid digestibility in dry extruded-expelled soybean meal fed to pigs and poultry. J. Anim. Sci. 84:1130–1137. doi: 10.2527/2006.8451130x [DOI] [PubMed] [Google Scholar]
- Park C. S., Helmbrecht A., Htoo J. K., and Adeola O.. . 2017. Comparison of amino acid digestibility in full-fat soybean, two soybean meals, and peanut flour between broiler chickens and growing pigs. J. Anim. Sci. 95:3110–3119. doi: 10.2527/jas.2017.1404 [DOI] [PubMed] [Google Scholar]
- Qin G., ter Elst E. R., Bosch M. W., and van der Poel A. F. B.. . 1996. Thermal processing of whole soy beans: studies on the inactivation of antinutritional factors and the effects on ileal digestibility in piglets. Anim. Feed Sci. Technol. 57:313–324. doi: 10.1016/0377-8401(95)00863-2 [DOI] [Google Scholar]
- Rosenthal A., Pyle D. E., and Niranjan K.. . 1996. Aqueous and enzymatic process for edible oil extraction. Enzyme Microb. Technol. 19:402–420. [DOI] [PubMed] [Google Scholar]
- Sauer W. C., Jorgensen H., and Berzins R.. . 1983. A modified nylon bag technique for determining apparent digestibilities of protein in feedstuffs for pigs. Can. J. Anim. Sci. 63:233–237. doi: 10.4141/cjas83-027 [DOI] [Google Scholar]
- Sauer W. C., and Ozimek L.. . 1986. Digestibility of amino acids in swine: results and their practical applications. A review. Livest. Prod. Sci. 15:367–388. doi: 10.1016/0301-6226(86)90076-X [DOI] [Google Scholar]
- Shelton J. L., Hemann M. D., Strode R. M., Brashear G. L., Ellis M., McKeith F. K., Bidner T. D., and Southern L. L.. . 2001. Effect of different protein sources on growth and carcass traits in growing-finishing pigs. J. Anim. Sci. 79:2428–2435. doi: 10.2527/2001.7992428x [DOI] [PubMed] [Google Scholar]
- Sissons J. W., Nyrup A., Kilshaw P. J., and Smith R. H.. . 1982. Ethanol denaturation of soya bean protein antigens. J. Sci. Food Agric. 33:706–710. doi: 10.1002/jsfa.2740330804 [DOI] [Google Scholar]
- Stein H. H., Gibson M. L., Pedersen C., and Boersma M. G.. . 2006. Amino acid and energy digestibility in ten samples of distillers dried grain with solubles fed to growing pigs. J. Anim. Sci. 84:853–860. doi: 10.2527/2006.844853x [DOI] [PubMed] [Google Scholar]
- Stein H. H., Sève B., Fuller M. F., Moughan P. J., and de Lange C. F. M.. . 2007. Invited review: amino acid bioavailability and digestibility in pig feed ingredients: terminology and application. J. Anim. Sci. 85:172–180. doi: 10.2527/jas.2005-742 [DOI] [PubMed] [Google Scholar]
- Woyengo T. A., Beltranena E., and Zijlstra R. T.. . 2017. Effect of anti-nutritional factors of oilseed co-products on feed intake of pigs and poultry. Anim. Feed Sci. Technol. 233:76–86. doi: 10.1016/j.anifeedsci.2016.05.006 [DOI] [Google Scholar]
- Woyengo T. A., Jha R., Beltranena E., Pharazyn A., and Zijlstra R. T.. . 2014a. Nutrient digestibility of lentil and regular and low-oligosaccharide full-fat soybean fed to grower pigs. J. Anim. Sci. 92:229–237. doi: 10.2527/jas.2013-6555 [DOI] [PubMed] [Google Scholar]
- Woyengo T. A., Yánez J., Young M. G., Lanz G., Beltranena E., and Zijlstra R. T.. . 2014b. Nutritional value of full-fat green canola seed fed to growing-finishing pigs. J. Anim. Sci. 92:3449–3459. doi: 10.2527/jas.2013-6730 [DOI] [PubMed] [Google Scholar]
- Yáñez J. L., Beltranena E., and Zijlstra R. T.. . 2014. Dry fractionation creates fractions of wheat distillers dried grains and solubles with highly digestible nutrient content for grower pigs. J. Anim. Sci. 92:3416–3425. doi: 10.2527/jas.2013-6820 [DOI] [PubMed] [Google Scholar]
- Yang Y. X., Kim Y. G., Lohakare J. D., Yun J. H., Lee J. K., Kwon M. S., Park J. I., Choi J. Y., and Chae B. J.. . 2007. Comparative efficacy of different soy protein sources on growth performance, nutrient digestibility, and intestinal morphology in weaned pigs. Asian-Australas. J. Anim. Sci. 20:775–783. doi: 10.5713/ajas.2007-775 [DOI] [Google Scholar]
- Zijlstra R. T., Tibble S., and van Kempen T. A. T. G.. . 2009. Feed manufacturing technology and feed intake in young pigs. In: D. Torrallardona and E. Roura, editors, Voluntary feed intake in pigs. Wageningen, The Netherlands: Wageningen Academic Publishers, p.275–290. [Google Scholar]