Abstract
Two sets of in vitro rumen fermentation experiments were conducted to determine effects of diets that included wet distiller’s grains plus solubles (WDGS) and tannin-rich peanut skin (PS) on the in vitro digestibility, greenhouse gas (GHG) and other gas emissions, fermentation rate, and microbial changes. The objectives were to assess associative effects of various levels of PS or WDGS on the in vitro digestibility, GHG and other gas emissions, fermentation rate, and microbial changes in the rumen. All gases were collected using an ANKOM Gas Production system for methane (CH4), carbon dioxide (CO2), nitrous oxide (N2O), and hydrogen sulfide (H2S) analyses. Cumulative ruminal gas production was determined using 250 mL ANKOM sampling bottles containing 50 mL of ruminal fluid (pH 5.8), 40 mL of artificial saliva (pH 6.8), and 6 g of mixed diets after a maximum of 24 h of incubation. Fermenters were flushed with CO2 gas and held at 39 °C in a shaking incubator for 24 h. Triplicate quantitative real-time polymerase chain reaction (qPCR) analyses were conducted to determine microbial diversity. When WDGS was supplied in the diet, in the absence of PS, cumulative CH4 production increased (P < 0.05) with 40% WDGS. In the presence of PS, production of CH4 was reduced but the reduction was less at 40% WDGS. In the presence of PS, ruminal lactate, succinate, and acetate/propionate (A/P) ratio tended to be less with a WDGS interaction (P < 0.01). In the presence of PS and with 40% WDGS, average populations of Bacteroidetes, total methanogens, Methanobrevibacter sp. AbM4, and total protozoa were less. The population of total methanogens (R2 = 0.57; P < 0.01), Firmicutes (R2 = 0.46: P < 0.05), and Firmicutes/Bacteroidetes (F/B) ratio (R2 = 0.46; P < 0.03) were strongly correlated with ruminal CH4 production. Therefore, there was an associative effect of tannin-rich PS and WDGS, which suppressed methanogenesis both directly and indirectly by modifying populations of ruminal methanogens.
Keywords: Bacteroidetes, distiller’s grain, Firmicutes, methanogens, peanut skin
Introduction
Wet distiller’s grains plus solubles (WDGS) are a byproduct of ethanol production from various grains, and contain high concentrations of protein, lipids, phosphorous (P), sulfur (S), and non-forage fiber (Klopfenstein et al., 2008). Inconsistent results, however, have been reported for feeding WDGS and dried distillers’ grains plus solubles (DDGS) in beef cattle on greenhouse gas (GHG) emissions. Beef cattle that received a diet containing DDGS had 16.4% lower CH4 emissions (McGinn et al., 2009), while little effect (Hales et al., 2012), or a 29% increase in CH4 production have also been reported (Behlke et al., 2008). Other studies reported that increasing DDGS also increased in vitro CH4 production in cattle (Hunerberg et al., 2013), sheep (Pecka-Keilb et al., 2015), and poultry (Li et al., 2014). Conversely, Miśta et al., (2014) reported that inclusion of DDGS reduced both CH4 and total in vitro gas production. A meta-analysis (Klofenstein et al., 2008) indicated that feedlot cattle fed DDGS produced higher average daily gain (ADG) and gain:feed (G:F) ratios compared with cattle fed corn-based diets. The authors reported that there were quadratic responses of ADG and dry matter intake (DMI) to distiller’s grain in the diet with ADG and DMI being maximized at about 20% to 30% of WDGS and DDGS, respectively. However, feed efficiency was maximized at 10% to 20% and 20 to 30% for DDGS and WDGS, respectively (Klofenstein et al., 2008). In addition, when distiller’s grains are fed as an energy source (above 20% to 30% of diet DM), then protein and P were overfed. Overfeeding of nitrogen (N) and P could lead to subsequent negative impacts on the environment due to N and P losses to air, soil, and water (Huntington and Archibeque, 1999; Koenig and Beauchemin, 2018).
Condensed tannins (CT) tend to precipitate with proteins in the rumen during the chewing, swallowing, and rumination processes, which protects soluble dietary proteins from rapid microbial digestion and deamination. The result is either more effective breakdown of amino acids in the abomasum and lower intestine, or the CT-protein complexes being excreted in manure (de Klein and Eckard, 2008; Min et al., 2003). In addition, tannins as dietary supplements or as tannin-containing forages have shown a potential for reducing methane (CH4) emissions by up to 20% (Woodward et al., 2001; Waghorn et al., 2002). However, the associative effects of WDGS and tannin-rich peanut skin (PS) supplementations on CH4 production associated with rumen fermentation and methanogenesis in cattle are not clear. Therefore, the objective of this study was to investigate the effects of various levels of PS or WDGS supplementation on in vitro ruminal fermentation, total gas production, GHG emissions, and to quantify major microbial changes.
Materials and Methods
The experiment was conducted at USDA-ARS/Texas A & M AgriLife Research Center, Bushland, TX. West Texas A & M University Animal Care and Use Committee approved animal care, handling, and sampling procedures for this experiment (# 03-06-18).
Experimental Design
Two sets of in vitro rumen fermentation experiments were conducted to determine: 1) effects of different levels of PS (0, 5, 10, 15, and 20% as-fed) at 15% WDGS and, 2) the effects of different levels of WDGS (0, 10, 20, 30, and 40% as-fed) with and without 15% PS inclusion on in vitro gas production, GHG emissions, rumen fermentation rate, in vitro dry matter disappearance rate (IVDMD), and microbial changes when diets incubated with pooled rumen fluid. The experimental design of the second experiment was a 2 × 4 factorial with two replications. Two levels of PS supplementation (control vs. 15% PS) and four levels of WDGS (0, 10, 20, 30, and 40% of total dietary components [as-fed basis]) were the factorial treatments. The WDGS and PS were used as sources of crude protein (CP; 31.6% CP dry matter [DM]) and condensed tannins (CT; 6.5% CT DM), respectively. Ingredients and rations are described in Tables 1 and 2. Cumulative ruminal gas production was determined, where 15% ground PS and 0, 10, 20, 30, or 40% WDGS were placed in 250 mL fermentation vessels containing 40 mL of ruminal fluid (pH 5.8), 50 mL of artificial saliva (pH 6.8), and 6 g of mixed diets for the in vitro experiments. Triplicate quantitative real-time polymerase chain reaction (qPCR) analyses were conducted to determine microbial diversity changes.
Table 1.
Nutrient profiles of ingredients used for diets tested in vitro
| Item1, % of DM | Ingredient | |||
|---|---|---|---|---|
| WDGS2 | Cotton-seed meal | Steam-flaked corn | Peanut skin | |
| CP | 31.0 | 43.0 | 9.8 | 18.0 |
| ADF | 15.4 | 21.0 | 4.7 | 20.5 |
| NDF | 32.0 | 33.0 | 14.4 | 37.6 |
| Crude fat | 3.3 | 2.5 | 4.4 | - |
| CT | 0.07 | 0.52 | 0.04 | 6.5 |
| Ca | 0.01 | 0.2 | 0.01 | 0.29 |
| P | 0.74 | 1.2 | 0.31 | 0.23 |
| Mg | 0.33 | 0.80 | 0.14 | 0.17 |
| S | 0.43 | 0.45 | 0.11 | 0.19 |
1CP, crude protein; ADF, acid detergent fiber; NDF, neutral detergent fiber; CT, condensed tannins.
2Wet-distiller’s grains plus solubles.
Table 2.
Ingredients and chemical composition of the experimental diets tested in vitro
| WDGS Content, %/ Ingredient, % of diet (as-fed) | Diet1, % | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| – PS | + PS | |||||||||
| 0 | 10 | 20 | 30 | 40 | 0 | 10 | 20 | 30 | 40 | |
| Steam-flaked corn | 92.5 | 82.5 | 72.5 | 62.5 | 52.5 | 77.5 | 67.5 | 57.5 | 47.5 | 37.5 |
| WDGS | 0 | 10 | 20 | 30 | 40 | 0 | 10 | 20 | 30 | 40 |
| Cotton-seed meal | 7.0 | 7.0 | 7.0 | 7.0 | 7.0 | 7.0 | 7.0 | 7.0 | 7.0 | 7.0 |
| Peanut skin (PS) | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 15.0 | 15.0 | 15.0 | 15.0 | 15.0 |
| Urea | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| Chemical composition2, % of DM | ||||||||||
| DM, % | 87.5 | 87.6 | 87.5 | 87.4 | 87.9 | 87.3 | 87.8 | 87.8 | 87.5 | 87.7 |
| CP, % | 14.2d | 18.4c | 20.6b | 21.5b | 23.9a | 16.3b | 19.8b | 21.4ab | 23.9a | 25.4a |
| Soluble CP, % CP | 23.0 | 22.5 | 19.5 | 21.0 | 20.5 | 20.5a | 20.0ab | 18.0b | 14.0b | 16.0b |
| ADF, % | 7.6 | 6.4 | 7.1 | 8.5 | 10.7 | 6.0b | 7.9b | 7.3b | 8.5ab | 10.6a |
| NDF, % | 9.6c | 14.1c | 18.7b | 15.9b | 20.7a | 12.6b | 15.9b | 14.9b | 20.5ab | 24.1a |
| TDN, % | 81.9 | 81.8 | 77.9 | 79.0 | 77.1 | 80.5 | 79.0 | 79.5 | 77.0 | 75.2 |
| CT3, % | 0.07 | 0.08 | 0.08 | 0.08 | 0.08 | 1.04 | 1.04 | 1.05 | 1.05 | 1.05 |
| Ca, % | 0.02c | 0.03c | 0.05c | 0.22b | 0.48a | 0.06b | 0.42a | 0.42a | 0.45a | 0.46a |
| P, % | 0.35c | 0.47c | 0.48c | 1.35b | 2.43a | 0.33b | 2.18a | 2.25a | 2.20a | 2.32a |
| Mg, % | 0.14b | 0.18b | 0.20b | 0.24ab | 0.35a | 0.15b | 0.29a | 0.31a | 0.32a | 0.33a |
| S, % | 0.16c | 0.23b | 0.24b | 0.26b | 0.36a | 0.17b | 0.28b | 0.31ab | 0.34a | 0.38a |
1Diet without (– peanut skin [PS]) or with peanut skin (+ PS) coupled with varying concentrations (0%–40% of as-fed) of wet distiller’s grains plus solubles (WDGS).
2CP, crude protein; ADF, acid detergent fiber; NDF, neutral detergent fiber; CT, condensed tannins.
3CT was predicted from Table 1.
a-dMeans within a row with different letters differ at P < 0.05.
Animals and Feeding Management
Six individual rumen cannulated steers were selected as donors (2 years of age Black Angus; Bos taurus) based on similar body weight (BW; 804.6 ± 37.3 kg). Rumen fluid was collected from each of the six steers 2h after feeding (Cone et al., 2002). Cattle had been receiving a total mixed ration (TMR) diet, based on steam-flaked corn (52%), WDGS (20%), wet corn gluten (10%), cotton-seed meal (10%), chopped corn stalks (7%), and mineral and vitamin premix (1%). Ruminal contents from individual steers were placed into pre-warmed insulated thermal bottles, transported to the laboratory, homogenized, filtered through four layers of cheesecloth, and used as an in vitro inoculum. Rumen fluid was kept in a water bath at 39 °C with CO2 saturation until inoculation (Bueno et al., 2005).
Experiments 1 and 2
The two in vitro experiments (experiments 1 and 2) were conducted to determine the effect of different levels of PS (0, 5, 10, 15, and 20% as-fed) with 15% WDGS inclusion or different levels of WDGS (0, 10, 20, 30, and 40% as-fed) with and without 15% PS inclusion on in vitro gas production, GHG emissions, rumen fermentation, and microbial changes when diets incubated with rumen fluid (Tables 1 and 2). Ten and twenty in vitro rumen incubations for experiments 1 and 2 were undertaken, respectively. Each incubation was done in duplicate.
Anaerobic condition was maintained by continuous flushing of bottle head space with CO2. For in vitro incubations, 6 g of mixed diets containing steam-flaked corn (62.5%), WDGS (30%), cotton-seed meal (7.0%), and urea (0.5% as-fed) were placed in 250 mL ANKOM (ANKOM Technology Corp. Macedon, NY) sample bottles containing 50 mL of pooled ruminal fluid (pH 5.8) and 40 mL artificial saliva (pH 6.8; Min et al., 2005). Bottles were flushed with CO2 gas before being capped with an ANKOM Gas Production module. One-liter FlexFoil PLUS sample bags (SKC Inc., Eighty Four, PA) were connected to the modules. Bottles were placed in a shaking incubator at 39 °C. Bottles containing only inoculum and buffer served as blanks. Cumulative ruminal gas production was recorded every 30 min for 24 h via the ANKOM system, which records pressure buildup in individual vessels.
After incubating for 24 h, two 10 mL, and two 3 mL samples were taken from each bottle for volatile fatty acid (VFA) and microbial community analyses, respectively, and stored at −80 °C. Gas sample bags were closed and separated from the module. The remaining contents of the sample bottle were used for estimating the IVDMD as follows: bottle contents were poured into pre-weighed 250 mL beakers, dried in a forced-air oven at 60 °C for approximately 48 h, then weighed.
After incubating for 24 h, two 6.0 mL of collected gas samples were taken from each sample bag for GHG and other gas analyses. Gas sample bags were closed and separated from the module and then GHG (CH4, CO2, and nitrous oxide [N2O]) concentrations were determined using an SRI gas chromatograph equipped with a methanizer, and electron capture detector (ECD) and flame ionization detector (FID) (SRI instruments, Torrance, CA). Analyzers were calibrated with GHG gases of known concentrations. Hydrogen sulfide (H2S) production was analyzed using a gas monitor (GX2012 model, RIKIN KEIKI Co. Ltd., Tokyo, Japan). Specific gas concentrations were plotted against total gas production to estimate total gas emission per hour, or gas produced on a DM basis.
Laboratory Analysis
Nutrient and mineral content of ingredients and DM was analyzed by Dairy One Forage Testing Laboratory (Ithaca, NY). Analytical DM concentrations of diet samples were determined by oven drying at 105 °C for 24 h (AOAC, 1998). Ash, ether extract, and minerals were analyzed according to the methods described by AOAC (2016). Concentrations of N were determined using an organic elemental analyzer (Flash 2000; CE Elantech Inc., Lakewood, NJ; AOAC, 1998). Concentrations of neutral detergent fiber (NDF) and acid detergent fiber (ADF) were sequentially determined using an ANKOM200/220 Fiber Analyzer (ANKOM Technology, Macedon, NY) according to the methodology supplied by the company, which was based on Van Soest et al. (1991). Sodium sulfite was used in the procedure for NDF determination and pre-treated with heat stable amylase (Type XI-A from Bacillus subtilis; Sigma-Aldrich Corporation, St. Louis, MO). Total digestible nutrient (TDN) concentration were calculated based on % NDF content (TDN = 86.2 – (% NDF × 0.513) × 0.88; Undersander et al., 1993). Ether extract was measured (AOAC, 1998) using a fat analyzer (XT20, ANKOM Technology).
Rumen VFA were analyzed according to the methods described by Boček et al., (1978). For VFA analyses, 5 mL of ruminal fluid was diluted with 1 ml of 0.012 M hydrochloric acid and 0.02 M urotropine at pH 4.9 as the leading electrolyte and 0.01 M sodium hydrogen carbonate as the terminator and quantified using a gas chromatograph (model 5890 series II; Hewlett Packard Co, Palo Alto, CA) with a capillary column (30 m × 0.32 mm i.d., 1 μm phase thickness, Zebron ZB-FAAP, Phenomenex, Torrance, CA). The samples of rumen fluid (2.60 µL) were injected with a micro-syringe. Aqueous solutions of 0.01 M oxalic, formic, acetic, propionic, and butyric acids and a 0.01 M solution of lithium acetate served as a standard solution. Acetone (70%) extractable CT in dietary samples were determined using a butanol-HCl colorimetric procedure with a quebracho (Schinopsis sp.) condensed tannins equivalent (Terrill et al., 1994). Specific gas concentrations were plotted against total gas production to estimate total gas emission per hour, or gas produced on a DM basis.
DNA Extraction and Quantitative PCR
One mL aliquots of rumen fluid samples were centrifuged at 17,000 × g for 1 min. DNA extractions were performed on approximately 100 mg of the collected pellets using the Quick-DNA Fungal/Bacterial Miniprep Kit (ZymoResearch, Irvine, CA) according to the manufacturer’s protocol. A PreCellys 24 Tissue Homogenizer (Bertin Instruments, Montigny-le-Bretonneux, France) was used for bead beating with three cycles of 5,000 rpm for 15 s each. DNA was quantified using the Quant-iT PicoGreen dsDNA Assay Kit (Invitrogen, Carlsbad, CA) using a Synergy 2 Microplate Reader (BioTek Instruments, Winooski, VT). Samples were diluted to 5 ng µL−1 concentrations. Quantitative PCR was carried out with primers specific to Firmicutes, Bacteroidetes, total protozoa, Methanobrevibacter sp. strain AbM4, and total methanogens as described previously (Sylvester et al., 2004; Guo et al., 2008; Zhou et al., 2009). Methanobrevibacter sp. strain AbM4 was chosen because this strain is one of the predominant methanogens in the rumen when animals are fed high-energy diet (Zhou et al., 2010; Belanche et al., 2014; Patra et al., 2017).
All reactions were carried out using a CFX96 Real-Time PCR Detection System (Bio-Rad, Hercules, CA). Total reaction volumes were 25 µL and included SYBR Green JumpStart Taq ReadyMix (Sigma-Aldrich, St. Louis, MO) (1.25 units Taq DNA polymerase, 10 mM Tris-HCl, 50 mM KCl, 3.5 mM MgCl2, 0.2 mM dNTPs final concentrations) and 200 nM of each primer. The specific DNA template that was added varied with the target: 10 ng was used for Firmicutes and Bacteroidetes, 100 ng was used for protozoa, and 50 ng was used for Methanobrevibacter sp. strain AbM4 and total methanogens. The qPCR conditions for Firmicutes, Bacteroidetes and total methanogens were 94 °C for 2 min, 40 cycles of 94 °C for 15 s and 60 °C for 1 min. The qPCR conditions for protozoa were 94 °C for 2 min, 40 cycles of 94 °C for 15 s, 50 °C for 30 s, 72 °C for 1 min. The qPCR conditions for Methanobrevibacter sp. strain AbM4 were 94 °C for 2 min, 40 cycles of 94 °C for 15 s and 55 °C for 1 min. A melt curve analysis (65 °C to 95 °C, 0.5 °C increments) was also performed. The qPCR standards were created using synthetic genes called gBlocks (Integrated DNA Technologies, Inc., Coralville, IA), that were created using partial GenBank sequences from 16S rDNA genes of bacteria and 18S rDNA genes of protozoa representative of the groups being analyzed (accession number KP944119.1 for Bacteroidetes, MH560569.1 for Firmicutes, AF550156.1 for Methanobrevibacter sp. strain AbM4 and total methanogens, and AM158471.1 for protozoa). Synthesized gBlocks were resuspended to a concentration of 10 ng/µL and serially diluted to create standard curves against which gene copies were quantified. A copy number per µL was determined by multiplying the concentration of the resuspended gBlock by Avogadro’s number (10,000 pg/µL × 6.0221 × 10″ copies/p mol) divided by the molecular weight of the gBlock (p g/mol). The resuspended gBlock was diluted to 1 × 1010 copies/µL, and serial dilutions to 1 × 103 copies/µL were created (Conte et al., 2018).
Data Analysis and Interpretation
All statistical analyses were conducted using the GLM procedure of SAS Institute (SAS Inst. Inc., Cary, NC), with the factors examined being PS, WDGS, and PS × WDGS interactions (INT). The PS and WDGS level effects were tested by polynomial regression (linear and quadratic effects) using orthogonal contrast for equally spaced treatments estimated by the Proc GLM procedure of SAS. Data were presented as least-square means, together with the standard error of the mean (SEM). The relationship between microorganisms and CH4 production per mL or per DM was determined among dietary supplementations (PS and WDGS) by linear regression using the PROC Reg procedure of SAS. In vitro gas production rates were calculated using the exponential equation without lag time of Ørskov and McDonald (1979).
where Y was defined as gas production in time t hours of rumen incubation. The values of a, b, and c were constants of the exponential equation, where a = gas production at time 0, b = the proportion of gas production during time t, and c = the rate of gas production of the b fraction. The constants b and c for each treatment were calculated with the method described by Min et al. (2000) using the nonlinear regression (NLIN) procedure from SAS. The responses of b and c to WDGS and PS and associated interactions were analyzed using the GLM procedure of SAS. Least squares mean are reported throughout, and significance was declared at P < 0.05.
Results
Nutrient Composition of Diets
The chemical composition of the dietary components and treatments are presented in Tables 1 and 2, respectively. Of the ingredients used in this study, WDGS, cotton-seed meal, steam-flaked corn, and PS had extractable CT contents of 0.07, 0.52, 0.04, and 6.5% DM, while CP contents of those ingredients were 31.0, 43.0, 9.8, and 18.0% DM, respectively (Table 1). Cotton-seed meal contained high levels of CP, ADF, fat, Ca, P, and Mg, while WDGS and cotton-seed meal contained high levels of S. A significant CT concentration was detected in PS (6.5% DM), compared to other treatments. Therefore, nutrient contents of the experimental diets (Table 2) increased in CP, NDF, CT, and various minerals (i.e. Ca, P, Mg, and S) with increasing WDGS and PS inclusion. Across the experimental diets, total CP, ADF, and NDF increased with increasing WDGS, while soluble CP decreased with increasing WDGS (Table 2).
Experiment 1
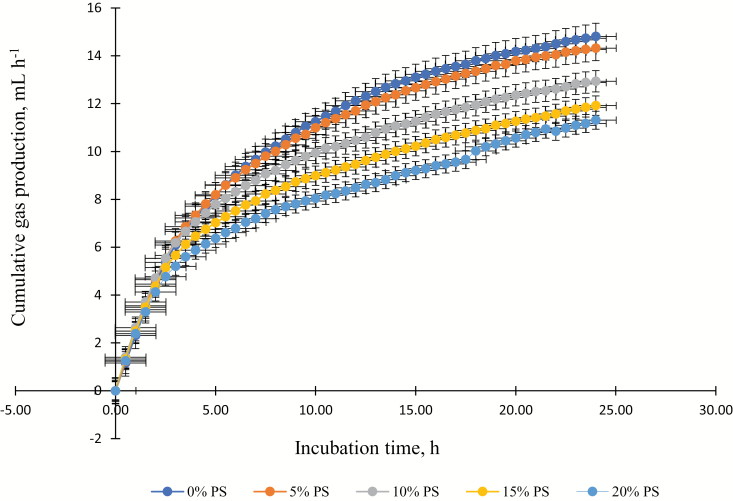
Cumulative in vitro gas and total gas production decreased (P < 0.01) with increasing PS (Figure 1 and Table 3). In addition, CH4, CO2, N2O, and H2S production decreased (P < 0.01) with increasing PS supplementation.
Figure 1.
Cumulative in vitro ruminal gas production with various levels of peanut skin (PS; 0, 5, 10, 15, 20, and 25% PS) plus 15% wet distiller’s grains plus solubles (WDGS; n = 2) supplementation incubated with combined rumen fluid for 24 h (Experiment 1).
Table 3.
In vitro ruminal total gas production and greenhouse gas (GHG) emissions as a function of addition of different levels of dietary peanut skin (PS) and fixed level (15%) of wet distiller’s grains plus solubles (WDGS)
| Item | Peanut skin treatment, % as-fed | ||||||
|---|---|---|---|---|---|---|---|
| 0 | 5 | 10 | 15 | 20 | SEM | P-value | |
| Total gas, mL 24 h−1 | 14.8a | 14.3ab | 12.9b | 11.9b | 11.3b | 0.245 | 0.001 |
| CH4, mL 24 h−1 | 0.56a | 0.52b | 0.48b | 0.43c | 0.39c | 0.012 | 0.001 |
| CO2, mL 24 h−1 | 4.40a | 3.86a | 3.94a | 3.64b | 3.35b | 0.214 | 0.01 |
| N2O, ppm 24 h−1 | 1.10a | 0.74b | 0.28c | 0.26c | 0.19c | 0.042 | 0.001 |
| H2S, ppm 24 h−1 | 13.1a | 10.4b | 9.3b | 8.2b | 5.8b | 0.92 | 0.001 |
a-cMeans within a row with different letters differ at the indicated P-value.
Experiment 2
In vitro dry matter disappearance (IVDMD) and gas production
Results from IVDMD and gas production are presented in Table 4. Increasing WDGS in the diets up to 20%, in the absence of PS, increased IVDMD (P < 0.01), but decreased IVDMD when PS was included (P < 0.01). There was a significant interaction (P < 0.001) between WDGS and PS for IVDMD. In the presence of PS, a quadratic response was observed for potential gas (a + b) production (P < 0.01) at varying WDGS concentrations. Rates of gas (c) production decreased linearly with increasing WDGS (P < 0.05). The WDGS x PS interaction was significant (P < 0.01) for potential (a + b) and rate of gas (c) production: PS addition decreased the average rate of gas production when WDGS was added to the rumen fluid.
Table 4.
In vitro dry matter disappearance (IVDMD) and kinetics of gas production as a function of addition of dietary peanut skin (PS) and wet distiller’s grains plus solubles (WDGS)
| Item | Treatment1 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WDGS | WDGS (– PS) | WDGS + PS | Significance of effect2 | |||||||||||
| 0 | 10 | 20 | 30 | 40 | 0 | 10 | 20 | 30 | 40 | SEM | PS | WDGS | INT | |
| IVDMD | 32.1b | 32.7b | 34.7a | 32.9b | 33.9ab | 32.0a | 27.4b | 26.9b | 27.5b | 27.7b | 0.40 | 0.01 | 0.01 | 0.01 |
| Gas production | Parameters3 | |||||||||||||
| a + b | 19.5b | 19.2b | 20.5a | 19.3b | 20.6a | 19.4a | 17.0b | 18.5ab | 19.0a | 19.2a | 0.24 | 0.01 | 0.01 | 0.01 |
| +PS; Q (P < 0.01) | ||||||||||||||
| c | 0.149a | 0.147a | 0.126b | 0.107b | 0.090b | 0.148a | 0.106b | 0.09b | 0.122ab | 0.108b | 0.07 | 0.05 | 0.01 | 0.01 |
| ± PS; L/Q | ||||||||||||||
| (P < 0.05) |
1Diet without (– PS) or with peanut skin (+ PS) coupled with varying concentrations (0% to 40% of as-feed) of wet distiller’s grains plus solubles (WDGS).
2PS, without versus with PS supplementation; WDGS, supplementation rate; NS, nonsignificant (P > 0.05); L, linear effect of WDGS supplementation; Q, quadratic effect of WDGS supplementation; PS × WDGS, interaction (INT) between PS and WDGS; IVDMD, in vitro dry matter disappearance rate.
3The values of a, b, and c were constants of the exponential equation, where a = gas production at time 0, b = the proportion of gas production during time t, a + b = potential gas production, and c = the rate of gas production of the b fraction.
a,bMeans with different superscripts, within column, are different (P < 0.05).
Gas production
In vitro ruminal GHG emissions as a function of addition of dietary PS and WDGS are presented in Table 5. In the absence of PS, there was an increase (P < 0.01) in total ruminal gas and H2S production with up to 30% to 40% WDGS. However, in the presence of PS, those trends quadratically reduced (P < 0.05) with increasing WDGS content. A diet with 15% PS with supplementation of 10% and 20% WDGS could reduce CH4 and H2S emissions by 12% and 33%, respectively. In the present study, N2O emission was not different (P > 0.10) between treatments, but substrates containing WDGS without addition of PS had greater (Linear; P < 0.01) H2S emission per gram of DM or total H2S production during 24 h than control diet (Table 6). Substrates containing 40% WDGS had greater (P < 0.01) H2S emission than those with control (0% WDGS) and 10% WDGS. In contrast, in the presence of PS, total H2S was quadratically reduced by 28% (quadratic: P < 0.01) or per gram of DM production by 29% (Linear and Quadratic; P < 0.01) at the ranges of 10% to 20% of WDGS.
Table 5.
In vitro ruminal total gas production and greenhouse gas (GHG) emissions as a function of addition of dietary peanut skin (PS) and wet distiller’s grains plus solubles (WDGS)
| Item | Treatment1 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WDGS | WDGS (– PS) | WDGS + PS | Significance of effect2 | |||||||||||
| 0 | 10 | 20 | 30 | 40 | 0 | 10 | 20 | 30 | 40 | SEM | PS | WDGS | INT | |
| Total gas, mL 24 h−1 | 20.4b | 20.6b | 21.9a | 21.8a | 22.7a | 20.2a | 16.7b | 17.3b | 19.7a | 20.4a | 0.553 | 0.001 | 0.01 | 0.01 |
| –PS; L (P < 0.01) | ||||||||||||||
| +PS; Q (P < 0.04) | ||||||||||||||
| CH4, mL | 1.00b | 0.96b | 1.03b | 0.95b | 1.23a | 1.10a | 0.82b | 0.85b | 1.02ab | 1.01ab | 0.051 | 0.08 | 0.01 | 0.01 |
| 24 h−1 | ±PS; L (P < 0.05) | |||||||||||||
| +PS; Q (P < 0.05) | ||||||||||||||
| CH4, mL g DM−1 | 0.16b | 0.18a | 0.17b | 0.16b | 0.19a | 0.17a | 0.12b | 0.13b | 0.15ab | 0.15ab | 0.008 | 0.001 | 0.02 | 0.01 |
| of feed | ±PS; L (P < 0.01) | |||||||||||||
| +PS; Q (P < 0.04) | ||||||||||||||
| CO2, mL 24 h−1 | 5.3 | 5.1 | 4.9 | 5.0 | 6.8 | 8.2 | 6.4 | 7.2 | 8.0 | 6.7 | 0.62 | 0.01 | 0.42 | 0.16 |
| CO2, mL g DM−1 of feed | 0.87 | 0.84 | 0.81 | 0.82 | 1.11 | 1.24 | 0.97 | 1.09 | 1.18 | 0.99 | 0.100 | 0.01 | 0.51 | 0.18 |
| N2O, ppm 24 h−1 | 3.7 | 3.6 | 2.7 | 3.9 | 6.1 | 5.2 | 5.1 | 4.8 | 4.6 | 4.9 | 0.96 | 0.16 | 0.47 | 0.49 |
| N2O, ppm DM−1 | 0.60 | 0.58 | 0.45 | 0.64 | 1.0 | 0.78 | 0.77 | 0.72 | 0.68 | 0.74 | 0.152 | 0.67 | 0.48 | 0.46 |
| H2S, ppm 24 h−1 | 4.95b | 4.89b | 6.21ab | 6.69a | 7.15a | 4.70a | 1.36b | 2.32b | 5.36a | 6.24a | 0.32 | 0.001 | 0.001 | 0.01 |
| ±PS; L (P < 0.01) | ||||||||||||||
| +PS; Q (P < 0.01) | ||||||||||||||
| H2S, ppm DM−1 of feed | 0.81b | 0.80b | 1.09ab | 1.10a | 1.16a | 0.71a | 0.21b | 0.35b | 0.79a | 0.92a | 0.049 | 0.001 | 0.01 | 0.001 |
| ±PS; L (P < 0.01) | ||||||||||||||
| +PS; Q (P < 0.01) |
1Diet without (– PS) or with peanut skin (+ PS) coupled with varying concentrations (0% to 40% of as-feed) of wet distiller’s grains plus solubles (WDGS).
2PS, without versus with PS supplementation; WDGS, supplementation rate; L, linear effect of WDGS supplementation; Q, quadratic effect of WDGS supplementation; PS × WDGS, interaction (INT) between PS and WDGS.
a,bMeans with different superscripts, within column, are different (P < 0.05).
Table 6.
In vitro ruminal volatile fatty acids (VFA; mol/100 mol) production as a function of addition of dietary peanut skin (PS) and wet distiller’s grains plus solubles (WDGS)
| Item | Treatment1 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| – PS | + PS | SEM | Significance of effect2 | |||||||||||
| 0 | 10 | 20 | 30 | 40 | 0 | 10 | 20 | 30 | 40 | PS | WDGS | INT | ||
| Acetate | 39.8a | 23.8b | 24.5b | 24.4b | 19.1b | 28.2 | 26.9 | 24.2 | 23.3 | 23.4 | 2.69 | 0.43 | 0.001 | 0.01 |
| Propionate | 16.3a | 8.3b | 8.9b | 11.0b | 9.2b | 12.6 | 11.4 | 11.1 | 10.8 | 12.1 | 1.45 | 0.28 | 0.01 | 0.06 |
| Butyrate | 16.2a | 9.8a | 12.8ab | 16.7a | 15.0ab | 13.9b | 12.4b | 13.9b | 14.9ab | 17.4a | 3.31 | 0.05 | 0.01 | 0.37 |
| Iso-butyrate | 0.24a | 0.10b | 0.12b | 0.14b | 0.12b | 0.25ab | 0.21b | 0.24ab | 0.23ab | 0.29a | 0.05 | 0.001 | 0.01 | 0.01 |
| Lactate | 4.2a | 4.7a | 3.4ab | 2.6b | 2.7b | 1.9ab | 2.7a | 2.1ab | 1.9ab | 1.7b | 0.26 | 0.05 | 0.01 | 0.12 |
| Valerate | 4.7b | 4.8b | 7.1b | 10.2a | 10.4a | 4.4b | 3.8b | 5.1b | 6.2a | 7.6a | 0.23 | 0.01 | 0.01 | 0.01 |
| Iso-valerate | 1.56a | 0.84b | 1.02b | 1.23b | 1.14b | 1.6ab | 1.42b | 1.54ab | 1.66ab | 1.79a | 0.01 | 0.01 | 0.01 | 0.02 |
| Caproate | 1.4b | 1.8b | 2.7a | 2.7a | 2.8a | 1.1b | 1.1c | 1.4b | 2.1a | 2.6a | 0.15 | 0.001 | 0.01 | 0.01 |
| Oxalate | 0.19a | 0.19a | 0.15ab | 0.12b | 0.11b | 0.09ab | 0.11a | 0.11a | 0.09ab | 0.08b | 0.02 | 0.01 | 0.01 | 0.01 |
| Succinate | 9.8a | 11.4a | 9.9a | 7.9b | 5.3c | 7.1a | 8.1a | 7.6a | 6.7b | 5.0c | 0.99 | 0.01 | 0.01 | 0.10 |
| A/P ratio | 2.53ab | 2.88a | 2.75a | 2.24b | 2.07b | 2.31a | 2.38a | 2.18b | 2.18b | 1.93b | 0.16 | 0.01 | 0.01 | 0.01 |
| Total VFA | 112.1a | 88.9b | 85.8b | 88.3b | 77.4b | 112.1a | 80.6b | 75.6c | 74.2c | 77.4b | 4.86 | 0.05 | 0.001 | 0.49 |
| Ruminal pH | 5.4b | 5.5b | 5.7a | 5.8a | 5.7a | 5.7a | 5.7a | 5.7a | 5.8a | 5.9b | 0.04 | 0.01 | 0.01 | 0.06 |
1Diet without peanut skin (– PS) or with peanut skin (+ PS) coupled with varying concentrations (0% to 40% of as-feed) of wet distiller’s grains plus solubles (WDGS).
2PS, without versus with PS supplementation; WDGS, supplementation rate; PS × WDGS, interaction (INT) between PS and WDGS.
a,bMeans with different superscripts in the same rows are different (P < 0.05).
Organic acid concentrations
In vitro molar proportion of VFA production as a function of addition of dietary PS and WDGS are presented in Table 6. In the absence of PS, acetate, propionate, lactate, iso-butyrate, oxalate, and succinate decreased (P < 0.01), with increasing WDGS levels. However, valerate and caproate increased (P < 0.01), with increasing WDGS. The A/P ratio showed a quadratic (P < 0.05) response with WDGS inclusion rate. In the presence of PS, no differences were found in rumen acetate and propionate concentrations among treatments. In the presence of WDGS and PS, the concentration of acetate, oxalate, succinate, total VFA concentrations, and A/P ratio decreased (P < 0.05–0.01); whereas, butyrate, iso-butyrate, valerate, iso-valerate, caproate concentrations, and ruminal pH increased (P < 0.01) with increasing WDGS. The PS x WDGS interactions were significant (P < 0.01) for acetate, propionate, iso-butyrate, valerate, iso-valerate, caproate, oxalate, and A/P ratio.
Microbial changes
The populations of Firmicutes, total methanogens, and Methanobrevibacter sp. AbM4 increased (linear; P < 0.05) with WDGS in the absence of PS (Table 7). There were decreased (P < 0.05) protozoa populations with increasing WDGS without PS addition. In the presence of PS, Bacteroidetes and Firmicutes populations were increased (P < 0.05) by incubation with 30% WDGS. The populations of total methanogens, Methanobrevibacter sp. AbM4, and total protozoa populations decreased (P < 0.05) at 40% WDGS with PS, with a significant PS × WDGS interaction (P < 0.01). However, the Firmicutes/Bacteroidetes (F/B) ratio increased (P < 0.05) at 40% WDGS compared to other treatment groups.
Table 7.
In vitro rumen microbial changes (10–7 mL−1) tested for peanut skin (PS) and wet distiller’s grains plus solubles (WDGS)
| Item | Treatment1 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| – PS | + PS | Significance of effect2 | ||||||||||||
| 0 | 10 | 20 | 30 | 40 | 0 | 10 | 20 | 30 | 40 | SEM | PS | WDGS | INT | |
| Bacteroidetes (B) | 32.7 | 35.1 | 32.3 | 32.8 | 35.8 | 32.2ab | 33.8ab | 32.0ab | 34.1a | 28.5b | 1.18 | 0.03 | 0.22 | 0.01 |
| +PS; L (P < 0.05) | ||||||||||||||
| Firmicutes (F) | 41.2c | 47.6b | 57.9ab | 52.6b | 61.4a | 42.2b | 41.2b | 41.8b | 47.1a | 42.3b | 2.35 | 0.001 | 0.001 | 0.001 |
| ±PS; L/Q (P < 0.05) | ||||||||||||||
| Total methanogens | 0.36b | 0.39b | 0.47a | 0.43b | 0.48a | 0.43a | 0.38b | 0.38b | 0.42ab | 0.39b | 0.014 | 0.01 | 0.01 | 0.01 |
| ±PS; L/Q (P < 0.05) | ||||||||||||||
| Methanobrevibacter sp. | 0.29b | 0.32b | 0.35a | 0.34ab | 0.38a | 0.33ab | 0.33ab | 0.33ab | 0.36a | 0.29b | 0.019 | 0.53 | 0.05 | 0.01 |
| AbM4 | ±PS; L (P < 0.05) | |||||||||||||
| Total protozoa | 10.2a | 9.3ab | 9.9a | 6.8b | 7.9b | 11.0a | 12.2a | 10.9b | 11.2ab | 9.7b | 0.85 | 0.01 | 0.05 | 0.198 |
| F/B ratio | 1.6b | 1.4b | 1.8a | 1.6b | 1.7b | 1.3b | 1.2b | 1.3b | 1.4ab | 1.5a | 0.05 | 0.001 | 0.01 | 0.001 |
| ±PS; L/Q (P < 0.01) |
1Diet without (– PS) or with peanut skin (+ PS) coupled with varying concentrations (0% to 40% of as-feed) of wet-distiller’s grains plus solubles (WDGS).
2PS, without versus with PS supplementation; WDGS, supplementation rate; NS, nonsignificant (P > 0.05); L, linear effect of WDGS supplementation; Q, quadratic effect of WDGS supplementation; PS × WDGS, interaction (INT) between PS and WDGS. Bacterial cell number × 10–6.
a,bMeans with different superscripts in the same rows are different (P < 0.05).
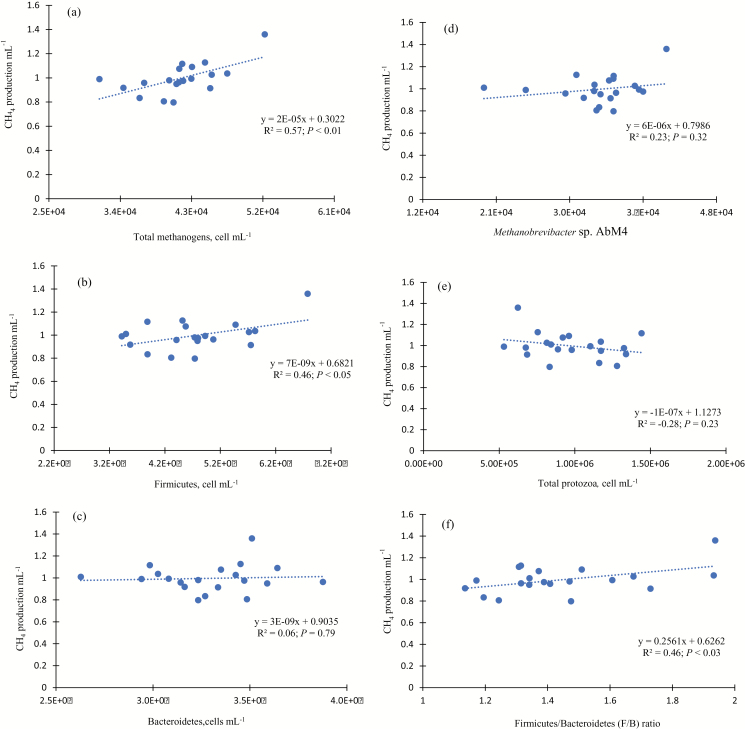
The relationship between CH4 emissions and total methanogens (a), Firmicutes (b), Bacteroidetes (c), strain of Methanobrevibacter sp. AbM4 (d), total protozoa (e), and F/B ratio (f) in an in vitro incubation with rumen fluid are presented in Figure 2. In the present study, there were no associations between methanogen (Methanobrevibacter sp.; b), total protozoa (c), and Bacteroidetes phylum (f) on ruminal CH4 production per mL of rumen fluid. Populations of total methanogens (R2 = 0.57; P < 0.01; a), Firmicutes (R2 = 0.46: P < 0.05; e), and the F/B ratio (R2 = 0.46; P < 0.03; d) were significantly correlated with ruminal CH4 production (Figure 2). Despite their fundamental role in methanogenesis, the richness of individual strain of Methanobrevibacter sp. AbM4 and total protozoa had weak or no correlation with CH4 emissions from in vitro rumen fluid incubations. Total methanogens, Firmicutes phylum and the F/B ratio seemed to be associated with high CH4 emissions across the diets.
Figure 2.
Relationship between CH4 emissions (CH4 mL−1) and total methanogens (a), Firmicutes (b), Bacteroidetes (c), Methanobrevibacter sp. AbM4 (d), total protozoa (e), and Firmicutes/Bacteroidetes (F/B) ratio (f) in an in vitro rumen incubation with pooled rumen fluid obtained from 6 cannulated steers.
Discussion
Experiment 1
Several in vitro (Bhatta et al., 2009; Jayanegara et al., 2012; Gemeda and Hassen, 2015) and in vivo (Beauchemin et al., 2007; Koenig and Beauchemin, 2018) studies showed that the addition of tannin-containing forages or tannin extracts in the diet-induced CH4 reduction. In the present study, in vitro total gas production was decreased by 23% when PS included at 20% of DM in the rumen incubation. Furthermore, CH4, CO2, N2O, and H2S production decreased with increasing PS supplementation (Table 3). These results suggested that the addition of PS decreased in vitro gas and GHG production. Bhatta et al. (2009) reported that plant tannins altered methanogenesis by reducing methanogenic activities in the rumen by either direct inhibition of methanogens or indirect restriction with the protozoa population.
Recently, Min et al. (2019) reported that the levels of PS supplementation in meat goats ranges from 15% to 20% PS (as-fed basis) included in a mixed ration, or CT levels of up to 2.4% to 4.9% dietary DM have been suggested. Previous in vivo and current in vitro experiments suggested that 15% to 20% PS supplementation in a mixed ration could potentially improve animal production, but also reduce GHG emission, hence 15% PS included in a mixed ration was chosen as a source of tannins for experiment 2.
Experiment 2
In vitro dry matter disappearance (IVDMD) and ruminal gas production
Plant tannins are largely distributed in the plant kingdom and are recognized to defend against infection, insects, or animal herbivory (Broucek, 2018). Increasing WDGS in the diets, in the absence of PS, increased IVDMD, but decreased IVDMD when PS was included (Table 4). This was similar to results reported from Koenig and Beauchemin (2018), in which increasing DDGS (0% to 40% DM) in feedlot diets reduced the digestibility of organic matter, starch and fiber (NDF and ADF) by heifers when fed a diet containing 2.5% CT extracts from black wattle (Acacia mearnsii). Plant tannins have the capability to form complexes with dietary proteins, mineral, and other polymers, such as cellulose, hemicellulose, and pectin, thus delayed digestion; therefore, tannins are considered anti-nutritive agents (McNabb et al., 1998; McSweeney et al., 1999).
In the presence of PS, quadratic and linear responses were observed for potential gas (a + b) and rate of gas (c) production at varying WDGS concentrations, respectively (Table 4). It has been reported that in vitro rates of gas production, potential gas production, and cumulative gas production decreased linearly in a dose-dependent manner for quebracho, mimosa (Acacia dealbata), and chestnut (Castanea dentata) tannin extracts (Min et al., 2015). Similarly, Gemeda and Hassen (2015) reported decreased in vitro gas production and digestibility (McSweeney et al., 1999) when various tannin-containing diets were fed. In the present study, rates of gas (c) production decreased linearly with increasing WDGS concentrations. Pecka-keilb et al. (2015) reported decreased total gas production when DDGS was included in an in vitro incubation with sheep rumen fluid (May et al., 2011)
Ruminal GHG and other gas production.
Numerous studies have demonstrated that tannins decrease CH4 production in ruminants (Puchala et al. 2005; Tavendale et al., 2005; Min et al., 2006). Experiments with feeding tannin-containing birdsfoot trefoil (Lotus corniculatus; 2.5% CT DM) and Sericea lespedeza (Lespedeza cuneate; 17.7% CT DM) reduced CH4 emissions in small ruminants (sheep, goats) by 30% (Pinares-Patiño et al., 2003; Puchala et al., 2005). In New Zealand, Friesian and Jersey dairy cows grazing sulla (Hedysarum coronarium), a tannin-containing legume, emitted less CH4 per unit of DMI (19.5 vs. 24.6 g CH4 kg DMI−1) compared to cows grazing perennial ryegrass pasture (Woodward et al., 2002). In the present study, a diet with 15% tannin-rich PS with supplementation of 10% and 20% WDGS reduced CH4 and H2S emissions by 12% and 33%, respectively (Table 5). This is a similar trend with results from a meta-analysis on feedlot cattle fed DGGS and WDGS, where G:F efficiency was improved by 10% to 20% and 20% to 30% for DDGS and WDGS, respectively (Klopfenstein et al., 2008). Liu et al. (2011) reported that the addition of 30 g CT kg−1 DM reduced CH4 emissions by 25% and 26% (g animal−1 d−1 and g kg−1 DMI d−1, respectively) compared to control diet in sheep. The inhibitory effect of CT on CH4 emission was reported by Bhatta et al. (2009), where CT lowered in vitro CH4 emission by 19.5%. However, high WDGS (40%) plus 15% PS could restore CH4 production. Similar results were observed in meat goats fed tannin-rich Sericea lespedeza (Lespedeza cuneate; 17.7% CT kg−1 DM) forage diets (Animut et al. 2008a, 2008b). Moreover, in the presence of commercial tannins and polyphenol extracts from quebracho, mimosa, chestnut, and yucca extracts, CH4 production decreased linearly (Min et al., 2015). The control of methanogenesis with tannin-rich PS supplementation may be accredited to decreasing the methanogenic archaea and protozoa concentrations in the rumen (Bhatta et al., 2009; Liu et al., 2011).
In the United States, feeding WDGS and DDGS to beef cattle has led to a corresponding increase in dietary S intake. In the present study, substrates containing WDGS without addition of PS had greater H2S emission per gram of DM or total H2S production during 24 h than control diet (Table 5). Substrates containing 40% WDGS had greater H2S emission than those with control (0% WDGS) and 10% WDGS. These results are consistent with other data (May et al., 2011). Their research indicates that higher S-containing WDGS (0.22% to 0.31% S) produced more H2S than lower S-containing DGGS (0.16% S; May et al., 2011). Increased ruminal H2S production in finishing beef cattle was observed when dietary S concentration increased from 0.42% to 0.65% in diets containing 30% DDGS (Uwituze et al., 2011). In contrast, in the presence of PS, total H2S was quadratically reduced by 28%, or per gram of DM production by 29%, at the ranges of 10% to 20% of WDGS. Ruminal H2S production results from S-reducing bacteria present in the rumen (Whitehead et al., 2013). These same authors reported that the addition of quebracho CT appeared to reduce S-reducing bacteria (Desulfobulbus propionicus and Desulfobulbus) qPCR copy numbers by 70% to 90% over the course of an experiment. The inhibitory influence on bacteria and methanogenic archaea is one of the reasons that tannins are useful for reducing H2S and CH4 gas emissions from cattle. However, there is very limited research available on the effect of dietary tannin inclusion on H2S emission from cattle.
In the present study, quadratic responses with PS by WDGS for CH4 and H2S production suggested that it may possible to have an optimum level of CT:protein interaction ratios (e.g. threshold levels of 1:13 to 1:14 for CT:protein ratios in the rumen) in the diet to suppress CH4 and H2S losses (Table 5). This is in agreement with a feedlot beef cattle study reported that up to 2.5% CT extract in a low protein TMR diets (13.0% to 16.8% CP) containing 0% to 20% DDGS (1:9 to 1:13 for CT:protein ratios, respectively) reduced DMI and rumen digestibility; however, diets containing high protein (20% to 40% DDGS; 1:15 for CT: protein ratio) with 2.5% CT extract (1.33% CT DM) supplementation had no effect on DMI and animal growth performance (Koenig and Beauchemin, 2018; Koenig et al., 2018). McNabb et al. (1998) reported that the amount of CT extract required to precipitate all the soluble protein in 10 µg of white clover was 25 to 50 µg.
Several reports on enteric CH4 and N2O emissions and mitigation strategies have been published (Beauchemin et al., 2008; Dalal et al., 2003; de Klein and Eckard, 2008; McAllister and Newbold, 2008). However, to fully assess the mitigation potential of using feed additives, each strategy needs to be subjected to both in vitro and in vivo analysis, as well as studied as part of a life-cycle assessment, to confirm that a reduction in one form of emissions does not create greater emissions elsewhere in the livestock production system (i.e. pollutant swapping). Fecal-nitrogen (N) is mainly in organic form and thus less volatile, whereas urinary-N is largely present as urea, which is readily hydrolyzed to NH4+ and vulnerable to volatilization as NH3. When altered through nitrification and denitrification to N2O, fecal-N could potentially be responsible for about 60% of N2O emissions from grazing land (de Klein and Ledgard 2005). Plant tannins are precipitated with dietary proteins in the rumen during rumination process, thereby protecting dietary proteins from fast microbial digestion (de Klein and Eckard 2008; Min et al., 2003). In the present study, we quantified N2O emissions in the rumen incubations as a part of a feedlot beef dietary trial, where there was no significance in N2O emissions among dietary treatments (Table 5). The mechanism leading to N2O emissions in the rumen is currently unknown, but the possible importance of dietary N2O recycling via blood, saliva, urea-N, and fecal-N should be examined (de Klein and Ledgard, 2005; Petersen et al., 2015). In general, a higher fecal-N:urinary-N ratio could be advantageous from an environmental perspective because of the slower mineralization rate of fecal organic matter in soil, as compared to readily volatilized urea-N (Dalal et al., 2003; de Klein and Ledgard 2005).
Rumen Fermentation Characteristics
In the presence of PS, the average percentage of produced lactate, succinate, and the A/P ratio decreased (P < 0.01); whereas, butyrate, iso-butyrate, valerate, iso-valerate, caproate increased (P < 0.01) with increasing WDGS (Table 6). The observed reductions in lactate and succinate were likely due to decreased dietary starch when more WDGS was incorporated into diet, as well as increased concentrations of lactate and succinate utilizing bacteria (e.g. Selenomonas ruminantium) (Ungerfeld and Kohn, 2006). The PS × WDGS interactions were significant for acetate, propionate, iso-butyrate, valerate, iso-valerate, caproate, oxalate, A/P ratio, and ruminal pH suggesting that PS addition increased feed efficiency (as a measured by A/P ratio) when WDGS was included in the rumen incubation. Concerning the effect of tannin-rich PS on A/P ratio, the results of the current study were comparable to those of Bhatta et al. (2009) who found that six different sources of hydrolyzable tannins (HT) and CT or its combinations (e.g., HT + CT) in TMR diets in vitro consistently reduced A/P ratio and total VFA with increasing CT concentrations. It has been reported that nutritional S in ruminant diets increased ruminal propionate production by altering the transformation of lactate to acryloyl-CoA (by 30%) via the acrylate pathway of rumen microorganisms from sheep fed a diet formulated to only supply adequate S (Russell, 2002; Whanger and Matrone, 1967). The acrylate pathway is an important means of producing propionate in the rumen that involves the lactate utilizing bacteria (Megasphaera elsdenii) when lactate is present (Russell and Wallace, 1997). In addition, mixed rumen microorganism has been shown to decarboxylate succinate to form propionate (Blackburn and Hungate, 1963), and the increase in ruminal propionate formation is stoichiometrically associated with a reduction in CH4 production (Lan and Yang, 2019). The succinate pathway is also propionate producing pathway in the rumen, which is conducted by propionate-forming bacteria (Selenomonas ruminantium, Wolinella succinogenes, Fibrobacter succinogenes, and Selenomonas ruminantium) (Ungerfeld and Kohn, 2006). Moreover, increased dietary S concentrations due to feeding WDGS affects S-reducing bacteria that partially oxidize lactate and increase concentrations of some fermentation products, such as lactate, ethanol, and propionate (Widdle, 1988). This could explain the declined lactate (P < 0.01) and suppression of succinate (P < 0.01) concentrations with increasing WDGS with PS substrates, while concomitantly decreasing A/P ratios in this study.
Rumen Microbial Community Changes
The mechanism by which tannin-rich PS supplementation reduces CH4 production in ruminants is not well understood. In the present study, qPCR analyses were conducted to evaluate the extent to which dietary WDGS levels were associated with PS supplementation, and how they were related to CH4 emissions and methanogenic archaea from ruminants (Table 7). Several studies have analyzed the relationship between dietary tannins/or tannin extracts on CH4 formation in ruminants in vivo and in vitro (Jayanegara et al., 2012; Patra and Yu, 2014; Tedeschi et al., 2014). The methanogens Methanobrevibacter smithii and M. ruminantium strains were inhibited by tannin extracts from black wattle (Mohammed et al., 2011) and Lotus pedunculatus (Big trefoil; Tavendale et al. 2005), respectively. In the presence of PS and with 40% WDGS in the present study, average populations of Bacteroidetes, total methanogens, Methanobrevibacter sp. AbM4, and total protozoa were less, while the F/B ratio was greater than for other treatments (Table 7). Results indicated that tannin-rich PS supplementation suppressed methanogenesis by reducing the populations of total methanogens and protozoa, thereby reducing CH4 production. To our knowledge, this is the first study to report effects of dietary tannin-rich PS with WDGS additions on anti-methanogenic activities associated with CH4 sink in the rumen.
According to Bhatta et al. (2009), the mixtures of HT plus CT extracts incubated with mixed rumen fluid obtained from cannulated, non-lactating, Holstein cows exhibited greater anti-protozoal activity than HT alone or the control diet, resulting in anti-methanogenic activity. Protozoa is one of the major H2 contributors for the reduction of CO2 to CH4 by methanogens associated with epi- and endo-symbiotic methanogens (Belenche et al., 2014). In the present study, the number of protozoa was reduced in the presence of PS and WDGS, which is similar to results of Bhatta et al. (2009). Mohammed et al. (2011) reported that a lower population of methanogens (Methanobrevibacter sp.) with diets containing in 200 and 400 g of DGGS kg−1 DM, as well as 400 DGGS + 25g CT kg−1 DM, compared to the control in their report assessing effects of DGGS and CT (Acasia mearnsii extract) effect on rumen methanogen diversity in cattle. However, dietary tannins can be anti-nutritional when dietary CP concentrations are limiting, as they reduce protein utilization in the rumen (Min et al., 2003; Waghorn et al., 2008). Generally, intake of forage containing high levels of CT (>5% DM) is reduced with negative effects on digestibility and animal productivity (Min et al., 2003; Min and Solaiman, 2018).
The presence of WDGS from 10% to 40% (range of 17% to 22% CP [DM]) in the present study necessarily increased dietary CP concentration compared with the control diet (13% to 15.2% DM). Increasing WDGS from 0% to 40% of DM, however, reduced soluble CP from 22 to 14% DM, respectively (Tables 1 and 2). This effect is similar to that reported by Koenig and Beauchemin (2018). The authors reported that with increasing DDGS, rumen degradable protein (RDP) was decreased from 76.2% to 47.1%. Alternatively, rumen undegradable protein (RUP) was raised from 23.8% to 52.9% of CP. Excessive dietary CP is digested and the majority of excess N is excreted in the form of urinary urea (Reynolds and Kristensen, 2008). However, Castillo-Lopez et al. (2017) reported that increased dietary CP with DDGS inclusion actually decreased ruminal ammonia concentrations due to slowing of ruminal CP digestibility in dairy cows. This could improve protein utilization (e.g. by-pass protein) in the rumen, and thereby alter rumen microbial diversity (e.g. F/B ratio) and the methanogenesis. It has been reported that including dietary WDGS decreased rate of gas and potential gas production, but increased H2S production (May et al., 2011).
In the present study, the population of total methanogens, Firmicutes, and F/B ratio were strongly correlated (Figure 2) with ruminal CH4 production, but the individual strain of Methanogens (Methanobrevibacter sp.), total protozoa, Bacteroidetes phylum, and pH in the rumen had no significant correlation with ruminal CH4 production per mL of rumen fluid. Controlling ruminal pH could also be a feasible approach to increase the competition of acetogens over methanogens. It was suggested that acetogens can survive well at pH 6.0 to 6.5, but the ruminal methanogens prefer pH above 6.5 (Van Kessel and Russell, 1996). However, Hünerberg et al. (2015) reported that CH4 production rates (g h−1) in heifers fed barley silage-based diets (55%) did not decrease when ruminal pH declined to threshold levels for subacute (pH 5.2 to 5.5) or acute ruminal acidosis (pH < 5.2), indicating that lowering ruminal pH alone may not an effective CH4-mitigation strategy. Despite their fundamental role in methanogenesis, the richness of individual strains of Methanobrevibacter sp. AbM4 and total protozoa had weak or no correlation with CH4 emissions from in vitro rumen fluid incubations. Total methanogens, Firmicutes phylum and F/B ratio seemed to be associated with high CH4 emissions across the diets. Wallace et al. (2014) reported that the archaea:bacteria ratio in rumen contents from ruminant animals exhibited greater relationship with CH4 emissions (R2 = 0.49). Min et al. (2019) reported that increased average daily gain (ADG) was strongly associated with higher percentages of Firmicutes, F/B ratio (R2 = 0.79), and lower A/P ratio (R2 = 0.72) in grazing meat goats. Among the major bacterial species, fibrolytic bacteria such as Ruminococcus and some Eubacterium spp. (Firmicutes) are well studied H2 producers (Kittelmann et al., 2014). However, the predominant fiber digesting bacteria, such as Fibrobacter spp., does not produce H2, while Bacteroides are net H2 utilizers (Stewart et al., 1997), indicating that CH4 emissions might depend on the abundance of Firmicutes and other H2-producing bacteria present in the rumen. It has been reported that goats receiving an anti-methanogenic agent (halogenated methane analog) had increased abundance of H2-consuming bacterial populations, such as Prevotella spp. (Bacteroidetes; Denman et al., 2015). Proteobacteria were 4-fold less abundant in high emitting beef cattle (Wallace et al., 2014) and dairy cows (Danielsson et al., 2017). Additional research on the phenotypes of these central ruminal bacteria is necessary. Therefore, the observed associative effect of tannin-rich PS and WDGS suppression of methanogenesis were due primarily by their combined anti-methanogenic activity and secondarily throughout their modification of microbial structures (e.g. F/B ratio).
Conclusion
Our study concluded that tannin-rich PS in the presence of WDGS suppressed methanogenesis directly via anti-methanogenic activity. The population of total methanogens, Firmicutes, and F/B ratio were strongly correlated with ruminal CH4 production. Further studies are needed to characterize the associative effect of specific dietary nutrient compositions and microbiome changes on methanogenesis and feed efficiency in ruminants. In addition, animal feeding studies are required to explore the long-term effects of PS supplementation on rumen microbial populations and animal growth performance.
Acknowledgments
The guidance of Drs. Robin C. Anderson, USDA/ARS, College Station, TX, and Bill Pinchak, Texas A & M AgriLife, Vernon, TX in editing the manuscript is gratefully acknowledged. Rumen fluid was provided by researchers with Texas A & M AgriLife located in Bushland, TX.
Footnotes
The mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the USDA.
Conflict of Interest
None declared.
Literature Cited
- Animut G., Puchala R., Goetsch A. L., Patra A. K., Sahlu T., Varel H., and Wells J.. . 2008a. Methane emission by goats consuming diets with different levels of condensed tannins from lespedeza. Anim. Feed Sci. Technol. 144:212–227. doi: 10.1016/j.anifeedsci.2007.10.014 [DOI] [Google Scholar]
- Animut G., Puchala R., Goetsch A. L., Patra A. K., Sahlu T., Varel H., and Wells J.. . 2008b. Methane emission by goats consuming different sources of condensed tannins. Anim. Feed Sci. Technol. 144:228–241. doi: 10.1016/j.anifeedsci.2007.10.015 [DOI] [Google Scholar]
- AOAC 1998. Association of official methods of analysis. 16th ed., Off. Anal. Chem. Int., Gaithersburg, MD. [Google Scholar]
- AOAC 2016. Official methods of analysis. 20th ed. Ass. Off. Anal. Chem., Int., Gaithersburg, MD. [Google Scholar]
- Beauchemin K. A., Kreuzer M., O’Mara F., and McAllister T. A.. . 2008. Nutritional management for enteric methane abatement: a review. Aust. J. Exp. Agri. 48:21–27. [Google Scholar]
- Beauchemin K. A., McGinn S. M., Martinez T. F., and McAllister T. A.. . 2007. Use of condensed tannin extract from quebracho trees to reduce methane emissions from cattle. J. Anim. Sci. 85:1990–1996. doi: 10.2527/jas.2006-686 [DOI] [PubMed] [Google Scholar]
- Behlke E. J., Sanderson T. G., Klopfenstein T. J., and Miner J. L.. . 2008. Ruminal methane production following the replacement of dietary corn with dried distillers’ grains. Nebraska Beef Cattle Reports. 50:130–132. [Google Scholar]
- Belanche A., de la Fuente G., and Newbold C. J.. . 2014. Study of methanogen communities associated with different rumen protozoal populations. FEMS Microbiol. Ecol. 90:663–677. doi: 10.1111/1574-6941.12423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatta R., Uyeno Y., Tajima K., Takenaka A., Yabumoto Y., Nonaka I., Enishi O., and Kurihara M.. . 2009. Difference in the nature of tannins on in vitro ruminal methane and volatile fatty acid production and on methanogenic archaea and protozoal populations. J. Dairy Sci. 92:5512–5522. doi: 10.3168/jds.2008-1441 [DOI] [PubMed] [Google Scholar]
- Blackburn T. H., and Hungate R. E.. . 1963. Succinic acid turnover and propionate production in the bovine rumen. Appl. Microbiol. 11:132–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boček P., Pavelka S., Grígelová K., Deml M., and Janák J.. . 1978. Determination of lactic and acetic acids in silage extracts by analytical isotachophoresis. J. Chroma. 154:356–359. [Google Scholar]
- Broucek J. 2018. Options to methane production abatement in ruminants: a review. J. Anim. Plant Sci. 28:348–364. [Google Scholar]
- Bueno, I. C., S. L. S. Cabral, L. S. Filho, S. P. Gobbo, H. Louvandini, D. M. S. S. Vitti, and A. L. Abdalla. 2005. Influence of inoculum source in a gas production method. Anim. Feed Sci. Technol. 123–124:95–105. [Google Scholar]
- Castillo-Lopez E., Jenkins C. J. R., Aluthge N. D., Tom W., Kononoff P. J., and Fernando S. C.. . 2017. The effect of regular or reduced-fat distillers grains with solubles on rumen methanogenesis and the rumen bacterial community. J. Appl. Microbiol. 123:1381–1395. doi: 10.1111/jam.13583 [DOI] [PubMed] [Google Scholar]
- Cone, J. W., A. H. van Gelder, and H. Beachemin. 2002. Influence of inoculum source on gas production profiles. Anim. Feed Sci. Technol. 99:221–231. [Google Scholar]
- Conte J., Potoczniak M. J., and Tobe S. S.. . 2018. Using synthetic oligonucleotides as standards in probe-based qPCR. Biotechniques 64:177–179. doi: 10.2144/btn-2018-2000 [DOI] [PubMed] [Google Scholar]
- Dalal R., Wang W., Robertson G., and Parton W.. . 2003. Nitrous oxide emission from Australian agricultural lands and mitigation options: a review. Aust. J. Soil Res. 41:165–195. [Google Scholar]
- Danielsson R., Dicksved J., Sun L., Gonda H., Müller B., Schnürer A., and Bertilsson J.. . 2017. Methane production in dairy cows correlates with rumen methanogenic and bacterial community structure. Front. Microbiol. 8:226. doi: 10.3389/fmicb.2017.00226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denman S. E., Martinez F. G., Shinkai T., Mitsumori M., and McSweeney C. S.. . 2015. Metagenomic analysis of the rumen microbial community following inhibition of methane formation by a halogenated methane analog. Front Microbiol. 6:1087: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemeda B. S., and Hassen A.. . 2015. Effect of tannin and species variation on in vitro digestibility, gas, and methane production of tropical browse plants. Asian-Australas. J. Anim. Sci. 28:188–199. doi: 10.5713/ajas.14.0325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X., Xia X., Tang R., Zhou J., Zhao H., and Wang K.. . 2008. Development of a real-time PCR method for Firmicutes and Bacteroidetes in faeces and its application to quantify intestinal population of obese and lean pigs. Lett. Appl. Microbiol. 47:367–373. doi: 10.1111/j.1472-765X.2008.02408.x [DOI] [PubMed] [Google Scholar]
- Hales K. E., Cole N. A., and Varel V. H.. . 2012. Effects of corn processing method and dietary inclusion of corn wet distillers grains with solubles on odor and gas production in cattle manure. J. Anim. Sci. 90:3988–4000. doi: 10.2527/jas.2011-4679 [DOI] [PubMed] [Google Scholar]
- Hünerberg M., McGinn S. M., Beauchemin K. A., Entz T., Okine E. K., Harstad O. M., and McAllister T. A.. . 2015. Impact of ruminal pH on enteric methane emissions. J. Anim. Sci. 93:1760–1766. doi: 10.2527/jas.2014-8469 [DOI] [PubMed] [Google Scholar]
- Hunerberg M., McGinn S. M., Beauchemin K. A., Okine E. K., Harstad O. M., and McAllister T. A.. . 2013. Effects of dried distillers’ grain plus solubles on enteric methane emissions and nitrogen excretion from growing beef cattle. J. Anim. Sci. 91:2846–2857. [DOI] [PubMed] [Google Scholar]
- Huntington G. B., and Archibeque S. L.. . 1999. Practical aspects of urea and ammonia metabolism in ruminants. Proc. Am. Soc. Anim. Sci. 1999:1–11. [Google Scholar]
- Jayanegara A., Leiber F., and Kreuzer M.. . 2012. Meta-analysis of the relationship between dietary tannin level and methane formation in ruminants from in vivo and in vitro experiments. J. Anim. Physiol. Anim. Nutr. (Berl). 96:365–375. doi: 10.1111/j.1439-0396.2011.01172.x [DOI] [PubMed] [Google Scholar]
- Kittelmann S., Seedorf H., Walters W. A., Clemente J. C., Knight R., and Gordon J. I.. . 2014. Simultaneous amplicon sequencing to explore co-occurrence patterns of bacterial, archaeal and eukaryotic microorganisms in rumen microbial communities. PLOS One. 2013:8:e103171. doi: 10.1371/journal.pone.0047879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Klein C. A. M., and Eckard R. J.. . 2008. Targeted technologies for nitrous oxide abatement from animal agriculture. Aust. J. Exp. Agric. 48:14–20. [Google Scholar]
- de Klein, C. A. M., and S. F. Ledgard. 2005. Nitrous oxide emissions from New Zealand agriculture—key sources and mitigation strategies. Nutr. Cycl Agroecosys. 72:77–85. [Google Scholar]
- Klopfenstein T. J., Erickson G. E., and Bremer V. R.. . 2008. BOARD-INVITED REVIEW: use of distillers by-products in the beef cattle feeding industry. J. Anim. Sci. 86:1223–1231. doi: 10.2527/jas.2007-0550 [DOI] [PubMed] [Google Scholar]
- Koenig K. M., and Beauchemin K. A.. . 2018. Effect of feeding condensed tannins in high protein finishing diets containing corn distillers grains on ruminal fermentation, nutrient digestibility, and route of nitrogen excretion in beef cattle. J. Anim. Sci. 96:4398–4413. doi: 10.1093/jas/sky273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig K. M., Beauchemin K. A., and McGinn S. M.. . 2018. Feeding condensed tannins to mitigate ammonia emissions from beef feedlot cattle fed high-protein finishing diets containing distillers grains. J. Anim. Sci. 96:4414–4430. doi: 10.1093/jas/sky274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan W., and Yang C.. . 2019. Ruminal methane production: associated microorganisms and the potential of applying hydrogen-utilizing bacteria for mitigation. Sci. Total Environ. 654:1270–1283. doi: 10.1016/j.scitotenv.2018.11.180 [DOI] [PubMed] [Google Scholar]
- Li W., Li O. F. Powers W., Karcher D., and Applegate T. J.. . 2014. Effects of distillers dried grains with solubles and mineral sources on gaseous emissions. J. Appl. Poultry Res. 23:41–50. [Google Scholar]
- Liu H., Vaddella V., and Zhou D.. . 2011. Effects of chestnut tannins and coconut oil on growth performance, methane emission, ruminal fermentation, and microbial populations in sheep. J. Dairy Sci. 94:6069–6077. doi: 10.3168/jds.2011-4508 [DOI] [PubMed] [Google Scholar]
- May M. L., Quinn M. J., Dilorenzo N., Smith D. R., and Galyean M. L.. . 2011. Effects of roughage concentration in steam-flaked corn-based diets containing wet distillers grains with solubles on feedlot cattle performance, carcass characteristics, and in vitro fermentation. J. Anim. Sci. 89:549–559. doi: 10.2527/jas.2010-3049 [DOI] [PubMed] [Google Scholar]
- McAllister T. A., and Newbold C. J.. . 2008. Redirecting rumen fermentation to reduce methanogenesis. Aust. J. Exp. Agric. 48:7–13. [Google Scholar]
- McGinn S. M., Chung Y. H., Beauchemin K. A., Iwaasa A. D., and Grainger C.. . 2009. Use of corn distillers’ dried grains to reduce enteric methane loss from beef cattle. Can. J. Anim. Sci. 89:409–413. [Google Scholar]
- McNabb W. C., Peters J. S., Foo L. Y., Waghorn G. C., and Jackson F. S.. . 1998. Effect of condensed tannin prepared from several forages on the in vitro precipitation of ribulose-1,5-bisphosphate carboxylase (Rubisco) protein and its digestion by trypsin (EC 2.4.21.4) and chymotrypsin (E.C. 2.4.21.1). J. Sci. Food Agric. 77:201–212. [Google Scholar]
- McSweeney C., Palmer B., Bunch R., and Krause D.. . 1999. In vitro quality assessment of tannin containing tropical shrub legumes: protein and fiber digestion. Anim. Feed Sci. Tech. 82:227–241. [Google Scholar]
- Min B. R., Barry T. N., Attwood G. T., and McNabb W. C.. . 2003. The effect of condensed tannins on the nutrition and health of ruminants fed fresh temperate forages: a review. Anim. Feed Sci. Technol. 106:3–19. doi: 10.1016/S0377-8401(03)00041-5 [DOI] [Google Scholar]
- Min B. R., Gurung N., Shange R., and Solaiman S.. . 2019. Potential role of rumen microbiota in altering average daily gain and feed efficiency in meat goats fed simple and mixed pastures using bacterial tag-encoded FLX amplicon pyrosequencing1. J. Anim. Sci. 97:3523–3534. doi: 10.1093/jas/skz193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min B. R., McNabb W. C., Peters J. S., and Barry T. N.. . 2000. Solubilization and degradation of ribulose-1,5-bisphosphate carboxylase (Rubisco) protein from white clover (Trifolium repens) and Lotus corniculatus by rumen micro-organisms and the effect of condensed tannins on those process. J. Agri. Sci. Cam. 134:305–317. [Google Scholar]
- Min B. R., Perkins D., Wright C., Dawod A., Min B. J., Terrill T. H., Eun J. S., Shange R., Yang S. Y., and Gurung N.. . 2015. Effects of two different tannin-containing diets on ruminal fermentation profiles and microbial community changes in meat goats. Agric. Food Anal. Bact. 5:153–165. [Google Scholar]
- Min B. R., Pinchak W. E., Anderson R. C., Fulford J. D., and Puchala R.. . 2006. Effects of condensed tannins supplementation level on weight gain and in vitro and in vivo bloat precursors in steers grazing winter wheat. J. Anim. Sci. 84:2546–2554. doi: 10.2527/jas.2005-590 [DOI] [PubMed] [Google Scholar]
- Min B. R., Pinchak W. E., Fulford J. D., and Puchala R.. . 2005. Wheat pasture bloat dynamics, in vitro ruminal gas production, and potential bloat mitigation with condensed tannins. J. Anim. Sci. 83:1322–1331. doi: 10.2527/2005.8361322x [DOI] [PubMed] [Google Scholar]
- Min B. R., and Solaiman S.. . 2018. Comparative aspects of plant tannins on digestive physiology, nutrition and microbial community changes in sheep and goats: a review. J. Anim. Physiol. Anim. Nutri. 2018:1–13. [DOI] [PubMed] [Google Scholar]
- Miśta S., Pecka E., Zachwieja A., Zawadzki W., Bodarski R., Paczyńska K., Tumanowicz J., Kupczyński R., and Adamski M.. . 2014. In vitro ruminal fluid fermentation as influenced by corn-derived dried distillers’ grains with solubles. Folia Biol. (Krakow). 62:345–351. [DOI] [PubMed] [Google Scholar]
- Mohammed R., Zhou M., Koenig K. M., Beauchemin K. A., and Guan L. L.. . 2011. Evaluation of rumen methanogen diversity in cattle fed diets containing dry corn distillers and condensed tannins using PCR-DGGE and qRT-PCR analyses. Anim. Feed Sci. Technol. 166–167:122–131. [Google Scholar]
- Ørskov E. R., and McDonald I.. . 1979. The estimation of protein degradability in the rumen from incubation measurements weighted according to rate of passage. J. Agric. Sci. 92:499–503. [Google Scholar]
- Patra A., Park T., Kim M., and Yu Z.. . 2017. Rumen methanogens and mitigation of methane emission by anti-methanogenic compounds and substances. J. Anim. Sci. Biotechnol. 8:13. doi: 10.1186/s40104-017-0145-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patra A. K., and Yu Z.. . 2014. Combinations of nitrate, saponin, and sulfate additively reduce methane production by rumen cultures in vitro while not adversely affecting feed digestion, fermentation or microbial communities. Bioresour. Technol. 155:129–135. doi: 10.1016/j.biortech.2013.12.099 [DOI] [PubMed] [Google Scholar]
- Petersen S. O., Hellwing A. L., Brask M., Højberg O., Poulsen M., Zhu Z., Baral K. R., and Lund P.. . 2015. Dietary nitrate for methane mitigation leads to nitrous oxide emissions from dairy cows. J. Environ. Qual. 44:1063–1070. doi: 10.2134/jeq2015.02.0107 [DOI] [PubMed] [Google Scholar]
- Pinares-Patiño C. S., Hickey S. M., Young E. A., Dodds K. G., MacLean S., Molano G., Sandoval E., Kjestrup H., Harland R., Hunt C., . et al. 2003. Heritability estimates of methane emissions from sheep. Animal. 7(Suppl 2):316–321. doi: 10.1017/S1751731113000864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puchala R., Min B. R., Goetsch A. L., and Sahlu T.. . 2005. The effect of a condensed tannin-containing forage on methane emission by goats. J. Anim. Sci. 83:182–186. doi: 10.2527/2005.831182x [DOI] [PubMed] [Google Scholar]
- Reynolds C. K., and Kristensen N. B.. . 2008. Nitrogen recycling through the gut and the nitrogen economy of ruminants: an asynchronous symbiosis. J. Anim. Sci. 86(14 Suppl):E293–E305. doi: 10.2527/jas.2007-0475 [DOI] [PubMed] [Google Scholar]
- Russell J. B. 2002. The rumen as a microbial habitat. In: J. B. Russell, editor, Rumen microbiology and its role in ruminant nutrition. Ithaca, NY: Cornell University; p. 37–51. [Google Scholar]
- Russell J. B., and Wallace R. J.. . 1997. Energy-yielding and energy-consuming reactions. In: Russell J. B., and Wallace R. J., editors. The rumen microbial ecosystem. Dordrecht (Netherlands): Springer; p. 246–282. [Google Scholar]
- Stewart C., Flint H., and Bryant M. P.. . 1997. The rumen bacteria. In: Hobson P. N., and Stewart C. S., editors. The rumen microbial ecosystem. London (UK): Blackie; p. 10–72. [Google Scholar]
- Sylvester J. T., Karnati S. K., Yu Z., Morrison M., and Firkins J. L.. . 2004. Development of an assay to quantify rumen ciliate protozoal biomass in cows using real-time PCR. J. Nutr. 134:3378–3384. doi: 10.1093/jn/134.12.3378 [DOI] [PubMed] [Google Scholar]
- Tavendale M. H., Meagher L. P., Pacheco D., Walker N., Attwood G. T., and Sivakumaran S.. . 2005. Methane production from in vitro rumen incubations with Lotus pendunculatus and Medicago sativa, and effects of extractable condensed tannin fractions on methanogenesis. Anim. Feed Sci. Technol. 123–124:403–419. [Google Scholar]
- Tedeschi L. O., Ramírez-Restrepo C. A., and Muir J. P.. . 2014. Developing a conceptual model of possible benefits of condensed tannins for ruminant production. Animal. 8:1095–1105. doi: 10.1017/S1751731114000974 [DOI] [PubMed] [Google Scholar]
- Terrill T. H., Waghorn G. C., Woolley D. J., McNabb W. C., and Barry T. N.. . 1994. Assay and digestion of 14C-labelled condensed tannins in the gastrointestinal tract of sheep. Br. J. Nutr. 72:467–477. doi: 10.1079/bjn19940048 [DOI] [PubMed] [Google Scholar]
- Undersander D, and Mertens D. E. R.. . 1993. Forage analysis procedure. Omaha (NE): National Forage Testing Association. [Google Scholar]
- Ungerfeld E. and Kohn R.. . 2006. The role of thermodynamics in the control of ruminal fermentation. In: Sejrsen K., Hyelplund T., and Nielsen M. O., editors, Ruminant physiology. Wageningen (The Netherlands): Wageningen Academic Publishers; p. 55–85. [Google Scholar]
- Uwituze S., Parsons G. L., Karges K. K., Gibson M. L., Hollis L. C., Higgins J. J., and Drouillard J. S.. . 2011. Effects of distillers grains with high sulfur concentration on ruminal fermentation and digestibility of finishing diets. J. Anim. Sci. 89:2817–2828. doi: 10.2527/jas.2010-3401 [DOI] [PubMed] [Google Scholar]
- Van Kessel J. A. S., and Russell J. B.. . 1996. The effect of pH on ruminal methanogenesis. FEMS Microbiol. Ecol. 20:205–210. [Google Scholar]
- Van Soest P. J., Robertson J. B., and Lewis B. A.. . 1991. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 74:3583–3597. doi: 10.3168/jds.S0022-0302(91)78551-2 [DOI] [PubMed] [Google Scholar]
- Waghorn G. C., Clark H., Taufa V., and Cavanagh A.. . 2008. Monensin controlled release capsules for methane mitigation in pasture-fed dairy cows. Aust. J. Exp. Agric. 48:65–68. doi: 10.1071/EA07299 [DOI] [Google Scholar]
- Waghorn G. C., Tavendale M. H., and Woodfield D. R.. . 2002. Methanogenesis from forages fed to sheep. Proc. N.Z. Grass. Assoc. 64:167–171. [Google Scholar]
- Wallace R. J., Rooke J. A., Duthie C. A., Hyslop J. J., Ross D. W., McKain N., de Souza S. M., Snelling T. J., Waterhouse A., and Roehe R.. . 2014. Archaeal abundance in post-mortem ruminal digesta may help predict methane emissions from beef cattle. Sci. Rep. 4:5892. doi: 10.1038/srep05892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whanger P. D., and Matrone G.. . 1967. Metabolism of lactic, succinic and acrylic acids by rumen microorganisms from sheep fed sulfur-adequate and sulfur-deficient diets. Biochim. Biophys. Acta. 136:27–35. doi: 10.1016/0304-4165(67)90317-0 [DOI] [PubMed] [Google Scholar]
- Whitehead T. R., Spence C., and Cotta M. A.. . 2013. Inhibition of hydrogen sulfide, methane, and total gas production and sulfate-reducing bacteria in in vitro swine manure by tannins, with focus on condensed quebracho tannins. Appl. Microbiol. Biotechnol. 97:8403–8409. doi: 10.1007/s00253-012-4562-6 [DOI] [PubMed] [Google Scholar]
- Widdle F. 1988. Microbiology and ecology of sulfate reducing bacteria. In: Zehnder A. J. B., editor. Biology of anaerobic microorganisms. New York (NY): Wiley; p. 120–469. [Google Scholar]
- Woodward S. L., Waghorn G. C., Lassy K. R., and Laboyrie P. G.. . 2002. Does feeding sulla (Hedysarum coronarium) reduce methane emission from dairy cows? Proc. N.Z. Soc. Anim. Sci. 62:227–230. [Google Scholar]
- Woodward S. L., Waghorn G. C., Ulyatt M. J., and Lassey K. R.. . 2001. Early indications that feeding Lotus will reduce methane emissions from ruminants. Proc. N. Z. Soc. Anim. Prod. 61:23–26. [Google Scholar]
- Zhou M., Hernandez-Sanabria E., and Guan L. L.. . 2009. Assessment of the microbial ecology of ruminal methanogens in cattle with different feed efficiencies. Appl. Environ. Microbiol. 75:6524–6533. doi: 10.1128/AEM.02815-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M., Hernandez-Sanabria E., and Guan L. L.. . 2010. Characterization of variation in rumen methanogenic communities under different dietary and host feed efficiency conditions, as determined by PCR-denaturing gradient gel electrophoresis analysis. Appl. Environ. Microbiol. 76:3776–3786. doi: 10.1128/AEM.00010-10 [DOI] [PMC free article] [PubMed] [Google Scholar]