Abstract
Colony cages are commonly used in China for the natural mating of layer breeders. However, feather pecking (FP) is a major problem in this system, and feather damage mainly due to FP needs to be alleviated. The objective of this study was to investigate the effects of nest boxes provided in colony cages. Each colony cage confined 10 roosters and 90 laying hens. The use of nest boxes as it relates to age, feather damage, sexual behavior, fertility, and fearfulness was evaluated. Thyroid hormones, which are considered to be physiological indicators of various forms of stress in poultry and may be correlated with the quality of feather coverage, were also tested. The control group and the nest box group each had 12 replicates, totaling 24 identical cages. Analyses were conducted using the linear mixed models procedure of SPSS Statistics 22.0. The results showed that the control group had a significantly higher proportion of hens with feather damage to 4 specific body regions (back, rump, tail, and belly) compared to the nest box group (P < 0.05). Increasing the use of the nest boxes took place from weeks 41 to 47 and at 53 wk of age, as seen by the percentage of eggs and number of sitting events in the nests, number of hens using the nests, and frequency of visits. There were no significant differences in fertility, the occurrence of mounting, or full copulation behavior between the 2 groups. Hens in the control group showed a significantly longer duration of tonic immobility at 43, 49, and 55 wk of age (P < 0.05). No significant differences were found between groups for the concentration of triiodothyronine or thyroxine, but a significantly higher concentration of corticosterone was measured in the control group than in the nest box group (P < 0.05). In conclusion, hens with access to nest boxes during the laying period had a decreased FP frequency, fewer damaged feathers, lower plasma corticosterone secretion, and were less fearful. This information contributes to the understanding of the FP behavior and stress sensitivity of layer breeders, which will provide a basis for the development and optimization of the colony cage equipment.
Keywords: corticosterone, fearfulness, feather damage, nest box, poultry, welfare
Introduction
Labor costs and animal welfare concerns have generated the need to adopt a new management practice, termed the natural mating colony cage system, for layer breeders in China. Compared with conventional artificial insemination cages, this colony cage system can increase activity space and better satisfy the behavioral requirements of hens, reduce the stress caused by artificial insemination and damage to the cloaca, and alleviate infection and disease transmission. Nonetheless, this colony cage system lacks specialized environmental enrichment equipment and facilities compared with other alternative housing systems. Feather damage due to feather pecking (FP) and its associated vent pecking (VP) are a major problem in this system, which contributes to economic loss and diminished health and welfare of the hens (Pötzsch et al., 2001).
Feather pecking appears when one hen pecks at or pulls out the feathers from her conspecifics. It ranges from mild to severe and generates poor quality plumage, patches of feather loss, and damage to the skin (Rodenburg and Koene, 2003). Considerable studies have attempted to establish the underlying mechanism of the development of FP (Blokhuis and Arkes, 1984; Savory, 1995; Kjaer and Vestergaard, 1999). Several hypotheses have been proposed to account for FP, including fear (Hughes and Duncan, 1972), redirected ground pecking, more precisely, that FP might be related to foraging (Blokhuis, 1986) and misdirected pecks related to dust bathing (Vestergaard et al., 1993). In the case of FP in cages, it is quite clear that FP is a redirected behavior originating from the lack of floor substrate for foraging and dustbathing. However, in some cases, the development of FP among hens is still largely unclear and unpredictable, although numerous research studies have revealed possible factors contributing to the development of FP, including animal-related and environmental-related factors, such as genetics, hormones, nutrition, light conditions, early-life history, stocking density, and group size (Allen and Perry, 1975; Rodenburg et al., 2008).
Current measures to limit feather and tissue damage due to FP involve beak trimming, keeping the hens under dim light or altering the light color. Beak trimming has been associated with reduced plumage damage (Hartcher et al., 2015), which results from the less severe FP behavior performed by adult hens. Lee and Craig (1991) also reported that beak trimming could increase the survival rates of pullets and reduce mortality due to cannibalistic pecking. However, this procedure is criticized because it reduces animal welfare; there is neurological evidence that it causes both acute and chronic pain in hens (Jongman et al., 2008). In addition, it adversely affects beak function and sensitivity and diminishes the expression of normal behaviors (Dennis and Cheng, 2010; Freire et al., 2011). Due to this dilemma, there is an ongoing discussion in several European countries about whether to ban the beak trimming procedure, and in some countries (e.g., Norway, Sweden, Finland, Switzerland, Netherlands, and Germany), it is already banned, and a ban will be enforced in the near future in the United Kingdom (Riber and Hinrichsen, 2017). The objective of dimming the light or altering the light color is to diminish the birds’ perception of colors and the visual detection between each other (Bright, 2007). However, this control practice is also doubtful because it can result in eye abnormalities (Prescott et al., 2003) and skeletal dysplasia due to reduced activity of laying hens (Newberry, 1999). Nickla et al. (2001) reported that lower light intensity during the day could lead to disproportionate development of the eyes in the ratio of the axial length to choroidal thickness. In addition, other inverse influences of low light intensity may include the reduced latency for birds to move between different perches (Taylor et al., 2003). Moreover, it is inconvenient for farmers when inspecting flocks. However, dim light can be adopted temporarily to reduce FP and cannibalism until the situation is calmer.
Not much research has been carried out to investigate FP in layer breeders in natural mating colony cages. Generally, a barren and unvaried environment minimizes the opportunities for exploration behaviors and compromises birds’ welfare by increasing fearfulness and FP, as well as reducing productivity (Jones, 1996). In addition, previous research has indicated that the key stimulus for VP is exposure of the cloacal mucosal membrane of affected birds after oviposition (Lambton et al., 2015). Consequently, birds undergoing oviposition outside the nest boxes would have a higher risk of being pecked at the cloaca or surrounding areas (Gunnarsson et al., 1999). Lambton et al. (2015) indicated that VP is associated with FP, and they share common risk factors for the development of FP. The hypothesis of this study was that providing hens with nest boxes in colony cages may facilitate the expression of natural behaviors, improve feather conditions, and alleviate fearfulness and physiological stress. Measures to alleviate feather damage due to FP using natural mating colony cages are urgently required in China, and installing nests may be an efficient and welfare-oriented option. The aim of the present experiment was to investigate the application of nest boxes in colony cages and the effects on feather condition, mounting behavior, fertility, fearfulness, and physiological stress for layer breeders.
Materials and Methods
Ethics Statement
All birds were managed by trained staff under standard guidelines for Hy-Line Brown layer breeders of Hebei Huayu Poultry Breeding Co. Ltd., Handan, Hebei, China. The study procedure was approved by The Laboratory Animal Ethical Committee of China Agricultural University.
Animals and Housing
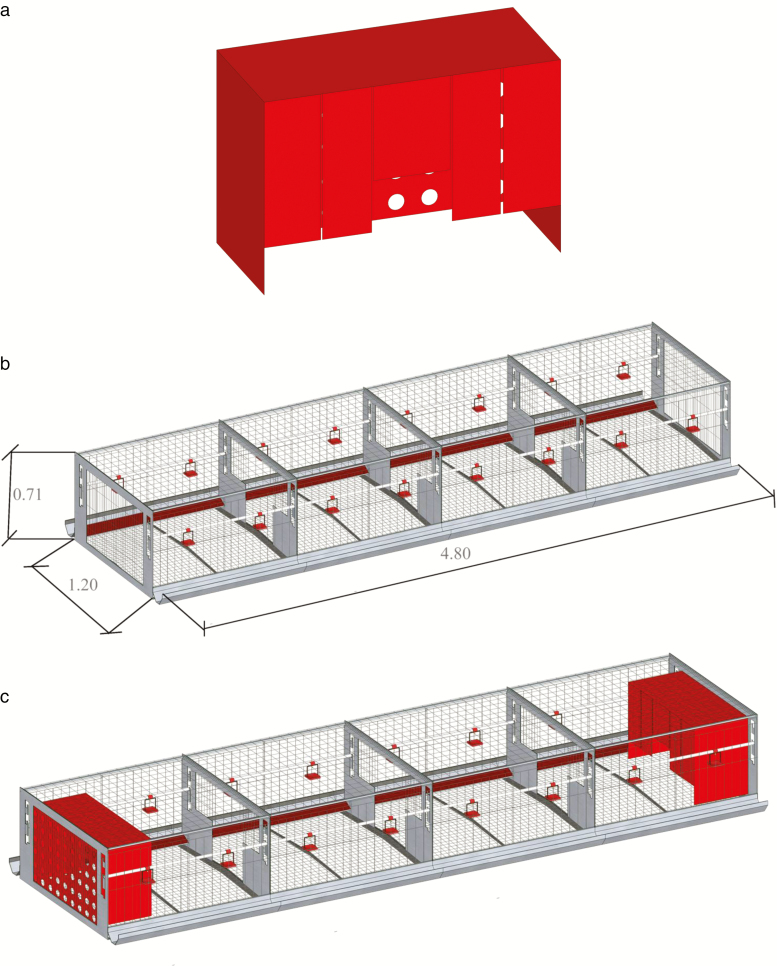
Beak-trimmed Hy-Line Browns layer breeders were subjects in the investigation, and the experiment was carried out from the age of 37 to 56 wk. Twenty-four double-sided colony cages were involved, and the size of each cage was 4.80 × 1.20 × 0.71 m (length × width × height). Each cage housed 10 males and 90 females, with approximately 576 cm2 floor area per bird. All experimental cages were arranged in 4 rows of 6 cages in a climate-controlled room and randomly divided into 2 groups (12 cages for each group). The treatment cages, named the nest box group, were provided 2 identical red gregarious nest boxes at the ends of each cage at the age of 37 wk. The nest box was 0.90 × 0.40 × 0.60 m (L × W × H) and made of polyethylene resin (Figs. 1 and 2). The control group was not equipped with nest boxes. Twelve randomly chosen birds from each experimental cage were marked with large plastic wing tags on both wings and were used as focal birds for measurement samplings. During the study, the photoperiod followed commercial recommendations with 16 L:8 D hours light–dark rhythm. The light intensity averaged approximately 5 lux and was measured at bird head height in the front of the colony cages facing outside. The dry bulb temperature was measured once every minute using data loggers (HOBO Pro v2, Onset Computer Corporation, Bourne, Massachusetts) installed at the height of birds’ head in the center of each aisle. Hens were ventilated with exhaust fans installed in one gable wall of the house. One fan ran continuously to provide the minimum ventilation, and the remaining fans were controlled to operate on or off to maintain the indoor temperature. Room temperature was maintained between approximately 16 °C and 23 °C. Water was provided ad libitum and commercial food was automatically distributed 4 times a day at 07:00, 11:00, 15:00, and 19:00 to ensure birds had permanent ad libitum access to feed. Eggs and excrement were collected once a day through conveyor belts. All birds were managed under the same standard guidelines for Hy-Line Brown layer breeders throughout the experiment.
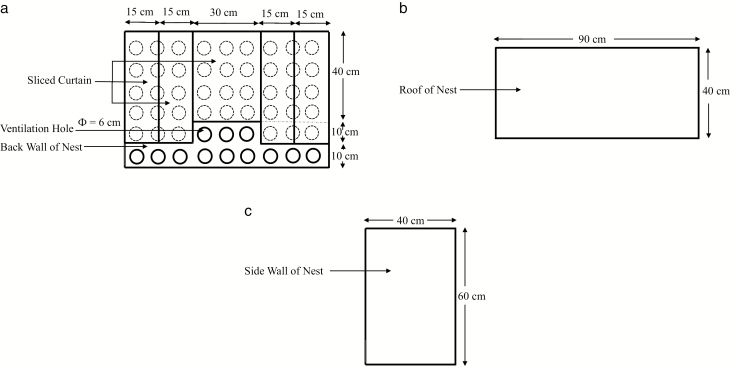
Figure 1.
(a) Front view, (b) top view, and (c) side view of the nest box.
Figure 2.
Axonometric views of (a) the nest box, (b) the colony cage with dimensions (unit: m), and (c) the colony cage with nest box and the installation position of the nest box.
Behavioral Observation
The numbers of floor eggs and eggs in each nest were collected for 16 wk. Video recording started at 41 wk of age to give hens enough time to habituate to the nests, followed by 2 stages of acquisition at the age of 47 and 53 wk. Video recording was performed on 3 consecutive days for 5 h after the lights were switched on in the morning at each age stage. The females and males were videotaped in the nest boxes to record and observe behavior. A camera with an infrared light source in the top center of each nest box was used for video recording. By employing a scan sampling approach, we recorded the numbers of females and males in all nest boxes every 10 min during the 5 h. We also recorded the numbers and duration of nest visits by females and males, the duration spent exploring the nest by females, the numbers of sitting events, and the numbers of eggs laid in nests. On weeks when nesting behavior was determined, eggs from each experimental cage of the nest box group and control group were incubated and candled at 14 d to determine fertility.
Observations of sexual behavior were made by 2 observers from an elevated seat in the corridor that allowed a clear view of the 2 experimental cages distributed in 2 adjacent rows. Observations were made for 6 consecutive days during weeks 42, 48, and 54. The observers sat in position and allowed 10 min for the birds to settle down. Behavioral data were collected between 08:00 and 12:00, 13:00 and 17:00, and 18:00 and 20:00 on measurement days. All occurrences of mounting behavior and full copulation behavior of all roosters in each experimental cage were recorded.
Tonic Immobility Test
The fear response was determined through the tonic immobility (TI) test as derived from Jones (1985a,b) and modified by Albentosa et al. (2003). Twelve focal hens were induced for TI at 3 different ages: 43, 49, and 55 wk. Hens were caught from their cages and carried to a table covered with several layers of cloth at the end of the shed. They were placed on their back and restrained for 15 s (one hand over the sternum and one over the head). Towards the end of the 15 s, the hands were released, and the observer sat 1 m from the hen and observed. If the hen jumped up or still moved, another induction period was needed, but the restraint attempts were made no more than 5 times. The number of inductions and head movements needed and TI duration and latency were recorded for each hen. If the hens were not put into TI after 5 inductions, scores of 0 s for the duration and latency were given to hens, whereas a maximum of 5 was given for the number of inductions. If a hen remained in TI for the maximum testing period of 5 min, a score of 600 s was given for the duration of TI.
Evaluation of the Feather Condition
At the age of 43, 49, and 55 wk, the feather condition of the 12 focal hens per cage was assessed using a scoring system derived from the Welfare Quality protocol for on-farm assessment of poultry welfare (2009) and modified by Bilcík and Keeling (1999). Each focal hen was taken individually from the cages to the end of the house for a careful examination of feather conditions. Based on areas of damaged and broken and missing feathers, a score from a to c was assigned to 11 body parts of the hens. We calculated the proportion of layer breeders with feather damage type b or c per region per flock. The details about how the scoring was done and how the calculation was conducted are given in Table 1.
Table 1.
Description of scoring method for feather condition evaluation and method of calculating the proportion1 of hens with feather damage
| Score | Description |
|---|---|
| a | No or slight wear, (nearly) complete feathering (only single feathers lacking) |
| b | Moderate wear, i.e., damaged feathers (worn, deformed) or one or more featherless areas <5 cm in diameter at the largest extent |
| c | At least one featherless area ≥5 cm in diameter at the largest extent |
1Proportion of layer breeders with feather damage b or c per region per flock was calculated as the ratio of the number of hens with feather damage b or c per region and the whole number of hens in per cage.
Blood Sampling
Brachial blood samples were obtained after the TI test and feather damage scoring, when the birds were 56 wk old. Three focal hens were randomly chosen from the tagged hens in each experimental cage for a total of 72 birds. The samples were collected at the same time each day (14:00 to 17:00). Ethylenediaminetetraacetate (EDTA) was used as an anticoagulant, and samples were collected into 2 mL tubes and held on ice immediately after collection. Studies have revealed that the hens were captured for more than 2 min would elevate the stress hormone levels. Therefore, blood was drawn from the brachial vein of each hen within 2 min of being caught. Samples were refrigerated and centrifuged at 2,500 × g for 20 min at 4 °C. Plasma was separated and stored at −20 °C in microcentrifuge tubes until analysis. Concentrations of thyroxine (T4), triiodothyronine (T3), and corticosterone (CORT) were determined using an enzyme-linked immunoassay kit (Awareness Technology Inc., Palm City, FL).
Statistical Analysis
Individual sample data within each of the replicate units (i.e., individual cage) were averaged before analysis, and the residuals were tested for normality and heterogeneity of variance. Analyses were conducted using the linear mixed models procedure of SPSS software (IBM SPSS Statistics 22.0, Armonk, NY). Cage was a random effect, whereas the nest treatment and age were the fixed effect. The individual cage was considered the experimental unit. The main effects of nest treatment, age, and the 2-way interaction were tested. When statistically significant effects occurred (P < 0.05), further analysis was conducted. For the use of nest boxes, mean comparisons were assessed on fertility of eggs, TI response, and blood parameters, by Duncan’s Multiple Range test. The Mann–Whitney U test was applied for post hoc group comparisons of feather condition and sexual behavior. Statistical significance was determined at P <0.05 unless otherwise stated.
Results
Behavioral Observation
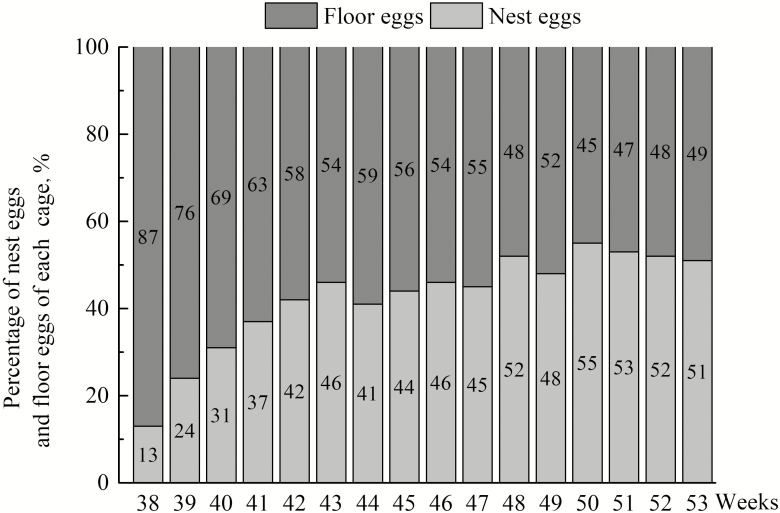
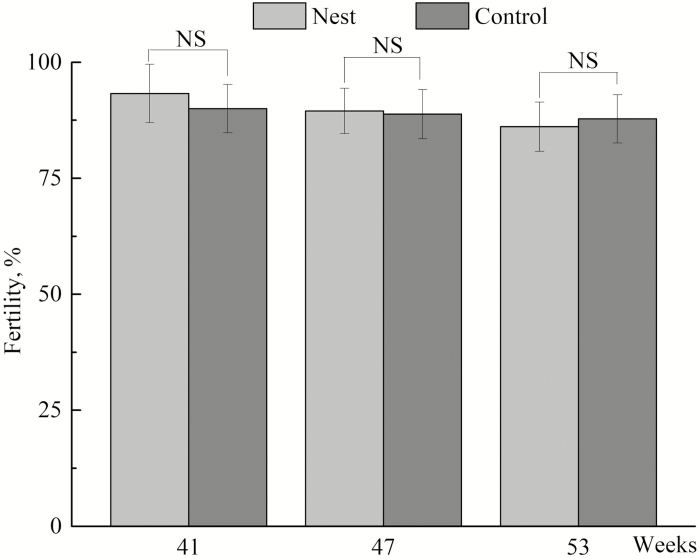
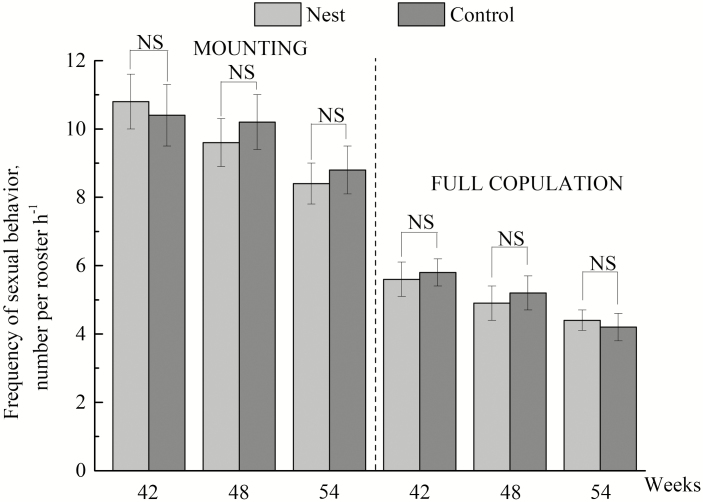
The first egg was collected from the nest boxes 2 d after they were installed, and approximately 50% of hens laid their eggs in the nests 42 d after they were provided. As shown in Table 2 and Fig. 3, increasing use of the nest boxes took place from weeks 41 to 47 and at 53 wk of age, as indicated by the percentage of eggs in the nests (P < 0.05), the number of hens using the nests (P < 0.05), the number of sitting events in the nests (P < 0.05), and the frequency of nest visits (P < 0.05). At the 3 different ages, the percentages of eggs in the nests were 36.75%, 44.75%, and 50.38%, respectively. There was no significant difference in the duration of laying or not laying among the hens, nor a significant difference in the duration of hens’ exploring or roosters’ staying in the nests among different ages. No significant difference was found in the fertility of the eggs for cages without nest boxes and with nest boxes at the 3 ages (Fig. 4). In addition, there was no significant effect of the nest box on the occurrence of sexual behavior of the birds (Fig. 5).
Table 2.
Hy-line breeder nest box use at 41, 47, and 53 wk of age1
| Age, weeks | ||||
|---|---|---|---|---|
| Test variables | 41 | 47 | 53 | LSD2 |
| Percentage of nest eggs, % | 36.8 ± 2.1b | 44.8 ± 2.1a | 50.6 ± 4.6a | 1.8 |
| Percentage of hens in nests, % | 23.3 ± 1.2b | 32.8 ± 1.2a | 36.7 ± 1.4a | 2.5 |
| Percentage of rooster in nests, % | 19.2 ± 1.3b | 20.0 ± 1.2a | 20.8 ± 1.5a | NS3 |
| Ratio of nest visits to nest eggs4 | 6.9 ± 2.6b | 7.8 ± 2.2ab | 8.5 ± 2.6a | 2.2 |
| Number of nest visits of hens per cage | 201.7 ± 11.3c | 280.2 ± 15.6b | 341.1 ± 17.2a | 6.1 |
| Number of nest visits of roosters per cage | 15.8 ± 2.4 | 12.7 ± 2.3 | 14.6 ± 2.3 | NS |
| Number of sitting events per cage | 112.5 ± 9.6b | 183.4 ± 10.3a | 178.0 ± 10.8a | 5.2 |
| Duration of laying per hen, min | 53.4 ± 10.6 | 52.8 ± 10.3 | 51.5 ± 12.4 | NS |
| Duration of without laying per hen, min | 32.3 ±11.2 | 36.4 ± 13.2 | 33.6 ± 12.8 | NS |
| Duration of exploring nests per hen, min | 19.6 ± 5.9 | 16.2 ± 4.1 | 17.3 ± 3.3 | NS |
| Duration of staying in nests per rooster, min | 9.6 ± 2.1 | 7.2 ± 1.4 | 8.1 ± 1.5 | NS |
1Nests were provided at 37 wk of age.The mean values per day and per 5-h observations are presented (12 cages for each group, 10 roosters and 90 hens per cage). Values shown are means ± SE.
2LSD = least significant difference.
3NS = not significant in analysis of variance.
4Ratio of nest visits to nest eggs: the number of visits required to produce an egg.
a–cMeans within a column with no common superscript letter are significantly different (P < 0.05).
Figure 3.
Percentage of nest eggs and floor eggs of each experimental cage for the nest box group at different ages.
Figure 4.
Fertility of eggs for cages without nest boxes (Control) and with nest boxes (Nest) at the ages of 41, 47, and 53 wk. NS = not significant in analysis of variance.
Figure 5.
Frequency of mounting behavior and full copulation behavior during 10-h observation of hens in cages without nest boxes (Control) and with nest boxes (Nest) at the age of 42, 48, and 54 wk. NS = not significant in analysis of variance.
Feather Condition
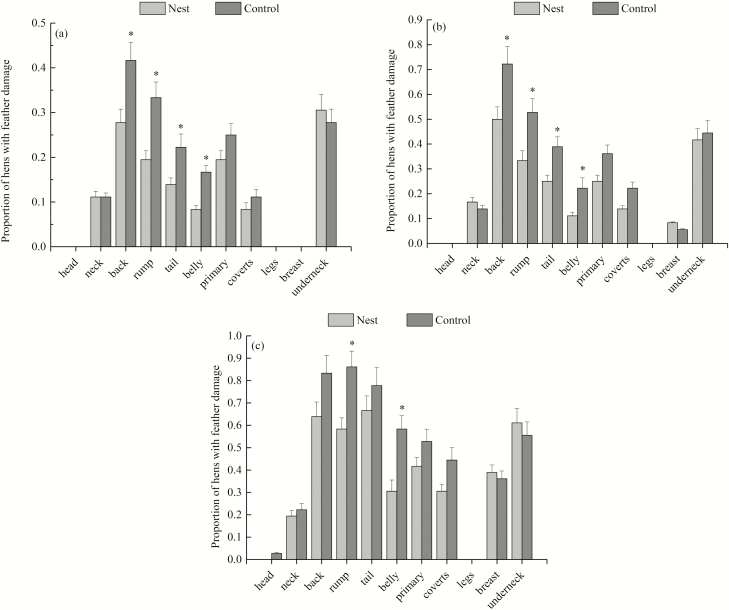
As shown in Fig. 6a–c, feather quality decreased from the first evaluation at 43 wk to the last evaluation at 55 wk in both groups. In the control group, the proportion of hens with a feather damage score b or c in the rump and belly was worse compared to the nest box groups at 43, 49, and 55 wk of age (P < 0.05). Additionally, the nest box group had higher feather quality in the back and tail regions than the control group at weeks 43 and 55 of age (P < 0.05). There were no significant differences between groups in other body regions.
Figure 6.
Proportion of feather damage on 11 body regions of hens in cages without nest boxes (Control) and with nest boxes (Nest) at the age of 43 (a), 49 (b), and 55 (c) wk. *P < 0.05.
Tonic Immobility Test
The results showed that having nest boxes reduced TI (Table 3). Birds in the control group showed a significantly longer duration than birds in the nest box group at the ages of 43, 49, and 55 wk (P < 0.05). The latency of birds in the control group was significantly greater than in the nest box group at 43 and 49 wk (P < 0.05), but there was no significant difference between the groups at 55 wk. The number of inductions and the number of head movements of birds in the nest box group were slightly more than in the control group, but did not reach statistical significance at any age. There was a significantly greater numbers of failed TI attempts at 49 and 55 wk (P < 0.05) and a smaller numbers of birds who remained in TI for a maximum of 5 min at 55 wk (P < 0.05) in the nest box group than in the control group.
Table 3.
Tonic immobility responses of Hy-line breeder hens at 43, 49, and 55 wk of age kept in colony cages with or without a nest box1
| 43 wk | 49 wk | 55 wk | |||||||
|---|---|---|---|---|---|---|---|---|---|
| TI test2 | Nest | Control | P-value | Nest | Control | P-value | Nest | Control | P-value |
| Duration, s | 64.8 ± 8.2 | 75.5 ± 12.6 | 0.042 | 68.9 ± 9.3 | 82.4 ± 11.3 | 0.037 | 74.4 ± 8.5 | 89.4 ± 11.6 | 0.033 |
| Latency3, s | 18.7 ± 1.5 | 20.6 ± 2.7 | 0.045 | 17.6 ± 2.6 | 19.9 ± 2.8 | 0.038 | 16.6 ± 1.9 | 18.2 ± 2.1 | 0.052 |
| Induction, n | 2.86 ± 0.24 | 2.64 ± 0.20 | 0.092 | 2.42 ± 0.20 | 2.18 ± 0.20 | 0.088 | 2.24 ± 0.18 | 2.12 ± 0.15 | 0.133 |
| Head movement, n | 5.83 ± 0.97 | 5.00 ± 0.64 | 0.483 | 7.36 ± 1.55 | 7.08 ± 1.26 | 0.937 | 7.85 ± 1.64 | 7.32 ± 1.35 | 0.846 |
| Failed TI attempts, n | 1.32 ± 0.15 | 1.13 ± 0.15 | 0.076 | 1.87 ± 0.13 | 0.96 ± 0.08 | 0.038 | 2.21 ± 0.18 | 1.24 ± 0.09 | 0.024 |
| 5-min TI, n | 1.45 ± 0.13 | 1.66 ± 0.12 | 0.162 | 1.38 ± 0.11 | 1.83 ± 0.12 | 0.073 | 1.54 ± 0.13 | 2.33 ± 0.12 | 0.043 |
1Nests were provided at 37 wk of age. Twelve cages for each group, 12 focal hens per cage. Values shown are means ± SE.
2TI = tonic immobility; s = seconds; n = number.
3Latency is the time till first head movement.
Blood Parameters
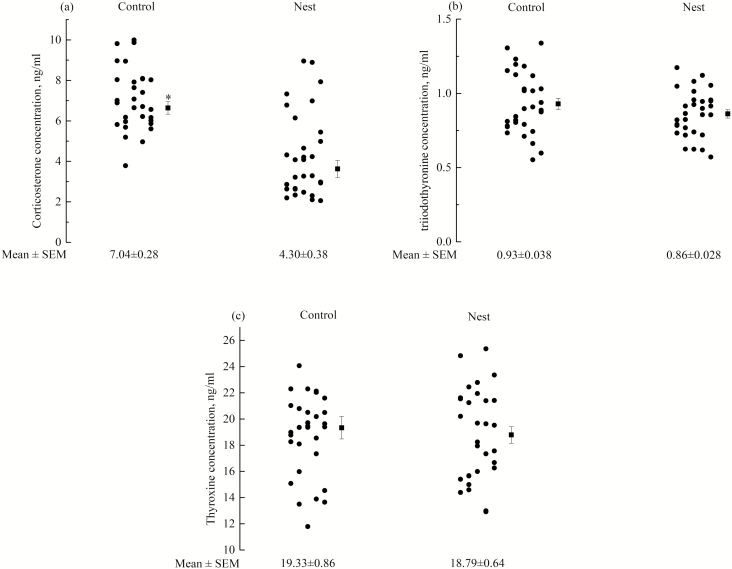
The plasma concentrations of the 3 hormones are given in Fig. 7a–c. The basal plasma CORT level was higher (P < 0.05) in the nest box group (4.30 ± 0.38 ng/mL) than in the control group (7.04 ± 0.28 ng/mL). However, there were no significant differences between the 2 groups for the concentrations of T3 and T4.
Figure 7.
Comparison of plasma corticosterone (a), triiodothyronine (b), and thyroxine (c) concentration (ng/mL) of hens in cages without nest boxes (Control) and with nest boxes (Nest) at 56 wk of age. *P < 0.05.
Discussion
The results of this experiment indicate that more than 50% of hens laid their eggs in the nests, and hens with access to nest boxes during the laying period had less damaged feathers, a lower plasma CORT secretion, and were less fearful. Such knowledge might help to understand the FP behavior, stress sensitivity, and production parameters of hens in natural mating colony cages and will provide a basis for the development and optimization of the cage equipment.
Behavioral Observation
Studies have shown that the absence of nests was probably the most serious welfare issue for laying hens (Cronin et al., 2013). Most hens are genetically predisposed to laying eggs in nest boxes, which are discrete and less intrusive as perceived by hens (Weeks and Nicol, 2006). A lack of an appropriate nesting site contributes to frustration, which is expressed by attempts to escape and stereotyped pacing prior to oviposition (Cooper and Albentosa, 2003). Therefore, in the colony cage, the implementation of the nest box, as one of the elements guaranteeing welfare, could generate the possibility of satisfying hens’ natural need for nesting. In this study, increasing use of the nests took place from their installation. Approximately half of the eggs were laid in nests, but the other half of the eggs were laid outside the nests. Several possible reasons could account for this. First, floor eggs were mostly found at the corners of the cage, which might be because the corners have nest-like features that were less intrusive and relatively enclosed compared to other places outside the nests. Second, the cage was equipped with the nest box after the onset of laying, so the hens may have already developed a habit of laying in a particular consistent location outside of a nest. Third, the high stocking density in this study may have resulted in limited space in and outside the nest boxes in the colony cage. The nest box may not be enough to accommodate all hens at the same time. If the nests were fully occupied, the hens might be forced to lay their eggs outside the nests.
As shown in Figs. 4 and 5, no significant difference was found between the control group and the nest box group in mounting behavior, full copulation behavior, and fertility. In this study, hens without access to nest boxes had more feather loss on their backs and had a higher stress level. It has long been assumed that hens with more feather loss on their backs and selected for low levels of stress will have a higher frequency of mating behavior and fertility than hens with more feather covering on their backs and hens that had high levels of stress (Jones and Prescott, 2000; Marin and Satterlee, 2003). However, this was contrary to our initial prediction and the results of this study. In addition, during our observation, although some of the roosters usually stayed in the nest boxes, they would leave the nests to mate with hens. Male libido is one of the most important factors affecting mating frequency in poultry, and males with low libido mate less frequently and fertilize fewer females (Craig et al., 1977). The presence of the nest box did not exert an effect on mounting behavior, full copulation behavior, and fertility, mainly because the mating motivation and libido of the males were not influenced by the presence of the nest box. Although the actual cause of the results in this study is not known, sexual behavior and fertility may be attributed to other factors, such as genetic, nutrition, and environmental factors. Further studies are needed to advance our understanding of this observation.
Feather Condition
In the present experiment, there was a significantly higher proportion of hens with feather damage to the 4 specific body regions (back, rump, tail, and belly) in the control group. The results confirmed our hypothesis that the feather coverage condition was improved by providing nest boxes in this colony cage system for layer breeders. Studies have shown that FP by conspecifics is a major reason for poor feather conditions (Bilcík and Keeling, 1999). Feather damage in PS breeder hens is partly caused by the roosters’ claws during copulation, especially damage to the back region. Four specific body regions (back, rump, tail, and belly) of hens are the most common indicators used to evaluate the cause of feather damage: damage to feathers of the back and rump usually indicates FP, and feather damage to the tail and belly is usually linked to VP (Welfare Quality, 2009). Environmental enrichment devices are being increasingly used to provide opportunities for hens to engage in exploratory behaviors and foraging behaviors. The rewards of such interventions include an elevated behavioral repertoire, an enhanced ability to deal with challenge, reduced occurrence of damage pecking, and decreased feather damage caused by FP (Jones, 1996; Chow and Hogan, 2005). Vestergaard et al. (1993) suggested that the involvement of enrichment devices in the cages of hens could reduce the frequency of FP, cannibalism, and aggressive behavior. Providing foraging materials and pecking substrates or devices preferably interchangeable to keep hens occupied and not frustrated or bored may be an effective approach to alleviating FP and improving feather conditions (McAdie et al., 2005; de Haas et al., 2014). Other inanimate stimuli, such as a perch and nest box, might also be regarded as putative enrichment to redirect pecks correlated with FP (Gunnarsson et al., 1999). The following reasons might account for the positive effect of the nest box on improving feather condition. First, the nest boxes in colony cages were intuitively attractive stimuli for hens and enriched the environment in cages. The hens showed no neophobic responses to the nest boxes, and they made contact with and pecked them after they had been installed. During behavioral observations, the nest boxes were pecked even after their continued existence for the following weeks in the cages, which confirmed the ability of the nest boxes to sustain interest (Shi et al., 2018). Second, the nest boxes created a possibility for hens to express nesting behaviors. It was observed that the hens spent more time exploring the nests and staying in the nests, leaving less time available (daylight hours) for pecking conspecifics. Third, hens in the nest boxes were isolated from their conspecifics, which would reduce conflict over resources and decrease feather abrasion. Another important reason for the positive effect of the nests is that they separate active and resting birds. Feather pecking is often directed from active to resting birds.
Fear and Blood Parameters
Tonic immobility is an important indicator of birds’ welfare and is thought to be associated with FP as well as feather conditions in commercial breeding. Many studies show that due to pecking, the duration of TI increases, and on an individual and flock level, having high levels of fear at a young age can become a risk factor for developing FP as an adult (Fraisse and Cockrem, 2006; Daigle et al., 2014). In this study, the duration of TI of hens without access to nest boxes was longer than in hens provided with nest boxes. This observation suggested that hens without access to nests were more prone to fearfulness and sensitive to the TI test. This result was consistent with Reed et al. (1993), who showed that exposure to enrichment stimuli during laying contributes to a significant reduction in the severity of fear reactions in caged hens. It is also suggested by Nicol (1992) that enrichment of the environment with a variety of attractive novel objects or the application of sounds can reduce fearfulness in hens. Environmental enrichment effectively decreases stress, fearfulness, aggression, and injurious pecking and improves both physical and psychological well-being of hens (Reed et al., 1993; Altan et al., 2013; Daigle et al., 2014), which demonstrate that confined in an ill-equipped cage environment, hens will show inadaptation to their surroundings. Fearfulness and FP are specific responses to the long-term boring and barren environment where hens are situated (Nicol, 1995). The nest boxes could be regarded as a rewarding environmental necessity because they attracted and maintained an intuitive interest by the hens and kept them in a favorable mental status (Jones and Carmichael, 1999). A possible reason for the greater fear in the control group could be the difficulty in starting and expressing natural nesting behaviors. These hens did not have access to relatively concealed nest sites for egg laying and were therefore more disturbed by other hens. This may lead to higher levels of fearfulness.
In this experiment, we found no significant differences in the concentrations of T3 and T4 between the 2 groups. However, the plasma concentration of CORT in hens without access to nest boxes was significantly higher than hens in cages with nest boxes. This result was in agreement with previous studies indicating that an elevated concentration of CORT correlated with greater fearfulness in hens reflected by the manual restraint test (Jones et al., 1994). Cockrem (2007) reported that the TI and CORT response results in hens were consistent with greater fearfulness accompanied by larger CORT responses to the increase in environmental pressure. If hens suffer fear when they react to a stressor, then fearfulness should increase when plasma concentrations of CORT increase during a CORT response. The exposure of hens to chronic stress stimuli or potentially threatening stimuli may lead to increased CORT secretion (Franciosini et al., 2010). Collectively, plasma CORT concentration is positively associated with the TI test. In this study, control group hens had no access to nest boxes and were confined in a barren and high-stress environment. This might make the hens bored, nervous, or depressed and subsequently increase their fearfulness and CORT secretion. However, fearfulness is not an easily measurable variable, and the correlation between fearfulness and concentrations of thyroid hormones is difficult to demonstrate. The level of fearfulness may not be reflected accurately in the concentration of T3 and T4, which may be because the situation is not straightforward in practice.
Conclusion
The results of this study suggest that increasing use of the nest boxes took place when nest boxes were provided at the age of 37 wk in the colony cages for layer breeders, and more than 50% of hens laid their eggs in nests. The fertility of eggs and mounting behavior were not affected by the nest box. Hens having access to nest boxes had less feather damage, less fearfulness, and lower baseline CORT. Less feather damage to the belly helped reduce mortality from VP. Nest boxes allowed expression of natural nesting by providing a microenvironment that may be perceived as safer, thereby providing comfort and refuge in the case of aggression or FP, thus stimulating the expression of comfort behaviors and ultimately increases welfare. Roosters can also use the nests as shelter to avoid FP by hens. In future studies, nest boxes should be provided for hens at the onset of laying.
Footnotes
This research was funded by the China Agricultural Research System (CARS-40) and National Natural Science Foundation of China (31601981).
We would like to thank the manager and staff of Hebei Huayu Poultry Breeding Co. Ltd., Handan, Hebei, China. We are grateful to our colleagues at the Department of Agricultural Engineering in Structure and Environment at China Agricultural University for their help and support during the project.
Mention of trade names or commercial products in this article is only for the purpose of providing specific information and does not imply recommendation. No conflicts of interest, financial, or otherwise are declared by the author(s).
Literature Cited
- Albentosa M. J., Kjaer J. B., and Nicol C. J.. . 2003. Strain and age differences in behaviour, fear response and pecking tendency in laying hens. Br. Poult. Sci. 44:333–344. doi: 10.1080/00071660310001598085 [DOI] [PubMed] [Google Scholar]
- Allen J., and Perry G. C.. . 1975. Feather pecking and cannibalism in a caged layer flock. Br. Poult. Sci. 16:441–451. doi: 10.1080/00071667508416212 [DOI] [PubMed] [Google Scholar]
- Altan O., Şeremet C., and Bayraktar H.. . 2013. The effects of early environmental enrichment on performance, fear and physiological responses to acute stress of broiler. Arch. Geflugelk. 77:23–28. doi: 10.4081/ijas.2013.e85 [DOI] [Google Scholar]
- Bilcík B., and Keeling L. J.. . 1999. Changes in feather condition in relation to feather pecking and aggressive behaviour in laying hens. Br. Poult. Sci. 40:444–451. doi: 10.1080/00071669987188 [DOI] [PubMed] [Google Scholar]
- Blokhuis H. J. 1986. Feather-pecking in poultry: Its relation with ground-pecking. Appl. Anim. Behav. Sci. 16:63–67. doi:10.1016/0168-1591(86)90040–7 [Google Scholar]
- Blokhuis H. J., and Arkes J. G.. . 1984. Some observations on the development of feather-pecking in poultry. Appl. Anim. Behav. Sci. 12:145–157. doi:10.1016/0168-1591(84)90104–7 [Google Scholar]
- Bright A. 2007. Plumage colour and feather pecking in laying hens, a chicken perspective? Br. Poult. Sci. 48:253–263. doi: 10.1080/00071660701370483 [DOI] [PubMed] [Google Scholar]
- Chow A., and Hogan J. A.. . 2005. The development of feather pecking in Burmese red jungle fowl: the influence of early experience with exploratory-rich environments. Appl. Anim. Behav. Sci. 93:283–294. doi: 10.1016/j.applanim.2005.01.004 [DOI] [Google Scholar]
- Cockrem J. F. 2007. Stress, corticosterone responses and avian personalities. J. Ornithol. 148:169–178. doi: 10.1007/s10336-007-0175-8 [DOI] [Google Scholar]
- Cooper J. J., and Albentosa M. J.. . 2003. Behavioural priorities of laying hens. Avian. Biol. Res. 14:127–149. doi: 10.3184/147020603783637508 [DOI] [Google Scholar]
- Craig J. V., Al-Rawi B., and Kratzer D. D.. . 1977. Social status and sex ration effects on mating frequency of cockerels. Poult. Sci. 56:767–772. doi: 10.3382/ps.0560767 [DOI] [PubMed] [Google Scholar]
- Cronin G. M., Barnett L. J. L., and Hemsworth P. H.. . 2013. The importance of pre-laying behaviour and nest boxes for laying hen welfare: a review. Anim. Prod. Sci. 52:398–405. doi: 10.1071/AN11258 [DOI] [Google Scholar]
- Daigle C. L., Rodenburg T. B., Bolhuis J. E., Swanson J. C., and Siegford J. M.. . 2014. Use of dynamic and rewarding environmental enrichment to alleviate feather pecking in non-cage laying hens. Appl. Anim. Behav. Sci. 161:75–85. doi: 10.1016/j.applanim.2014.10.001 [DOI] [Google Scholar]
- de Haas E. N., Bolhuis J. E., Kemp B., Groothuis T. G., and Rodenburg T. B.. . 2014. Parents and early life environment affect behavioral development of laying hen chickens. PLoS ONE 9:e90577. doi: 10.1371/journal.pone.0090577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis R. L., and Cheng H. W.. . 2010. Effects of beak trimming on pecking force. Int. J. Poult. Sci. 9:863–866. doi: 10.3923/ijps.2010.863.866 [DOI] [Google Scholar]
- Fraisse F., and Cockrem J. F.. . 2006. Corticosterone and fear behaviour in white and brown caged laying hens. Br. Poult. Sci. 47:110–119. doi: 10.1080/00071660600610534 [DOI] [PubMed] [Google Scholar]
- Franciosini M. P., Canali C., Proietti P. C., and Tarhuni O.. . 2010. Plasma corticosterone levels in laying hens from three different housing systems: preliminary results. Ital. J. Anim. Sci. 4:276–278. doi: 10.4081/ijas.2005.276 [DOI] [Google Scholar]
- Freire R., Eastwood M. A., and Joyce M.. . 2011. Minor beak trimming in chickens leads to loss of mechanoreception and magnetoreception. J. Anim. Sci. 89:1201–1206. doi: 10.2527/jas.2010-3129 [DOI] [PubMed] [Google Scholar]
- Gunnarsson S., Keeling L. J., and Svedberg J.. . 1999. Effect of rearing factors on the prevalence of floor eggs, cloacal cannibalism and feather pecking in commercial flocks of loose housed laying hens. Br. Poult. Sci. 40:12–18. doi: 10.1080/00071669987773 [DOI] [PubMed] [Google Scholar]
- Hartcher K. M., Tran K. T., Wilkinson S. J., Hemsworth P. H., Thomson P. C., and Cronin G. M.. . 2015. The effects of environmental enrichment and beak-trimming during the rearing period on subsequent feather damage due to feather-pecking in laying hens. Poult. Sci. 94:852–859. doi: 10.3382/ps/pev061 [DOI] [PubMed] [Google Scholar]
- Hughes B. O., and Duncan I. J.. . 1972. The influence of strain and environmental factors upon feather pecking and cannibalism in fowls. Br. Poult. Sci. 13:525–547. doi: 10.1080/00071667208415981 [DOI] [PubMed] [Google Scholar]
- Jones R. B. 1985a. Fear responses of individually-caged laying hens as a function of cage level and aisle. Appl. Anim. Behav. Sci. 14:63–74. doi: 10.1016/0168-1591(85)90038-3 [DOI] [Google Scholar]
- Jones R. B. 1985b. Fearfulness of hens caged individually or in groups in different tiers of a battery and the effects of translocation between tiers. Br. Poult. Sci. 26:399–408. doi: 10.1080/00071668508416828 [DOI] [PubMed] [Google Scholar]
- Jones R. B. 1996. Fear and adaptability in poultry: insights, implications and imperatives. World’s Poult. Sci. J. 52:131–174. doi: 10.1079/WPS19960013 [DOI] [Google Scholar]
- Jones R. B., and Carmichael N. L.. . 1999. Responses of domestic chicks to selected pecking devices presented for varying durations. Appl. Anim. Behav. Sci. 64:125–140. doi: 10.1016/s0168-1591(99)00031-3 [DOI] [Google Scholar]
- Jones R. B., Mills A. D., Faure J. M., and Williams J. B.. . 1994. Restraint, fear, and distress in Japanese quail genetically selected for long or short tonic immobility reactions. Physiol. Behav. 56:529–534. doi: 10.1016/0031-9384(94)90297-6 [DOI] [PubMed] [Google Scholar]
- Jones E. K. M., and Prescott N. B.. . 2000. Visual cues used in the choice of mate by fowl and their potential importance for the breeder industry. World’s Poult. Sci. J. 56:127–138. doi: 10.1079/WPS20000010 [DOI] [Google Scholar]
- Jongman E. C., Glatz P. C., and Barnett J. L.. . 2008. Changes in behaviour of laying hens following beak trimming at hatch and re-trimming at 14 week. Asian-Australas. J. Anim. Sci. 21:291–298. doi: 10.5713/ajas.2008.60152 [DOI] [Google Scholar]
- Kjaer J. B., and Vestergaard K. S.. . 1999. Development of feather pecking in relation to light intensity. Appl. Anim. Behav. Sci. 62:243–254. doi: 10.1016/s0168-1591(98)00217-2 [DOI] [Google Scholar]
- Lambton S. L., Knowles T. G., Yorke C., and Nicol C. J.. . 2015. The risk factors affecting the development of vent pecking and cannibalism in free-range and organic laying hens. Anim. Welf. 24:101–111. doi: 10.7120/09627286.24.1.101 [DOI] [Google Scholar]
- Lee H. Y., and Craig J. V.. . 1991. Beak trimming effects on behavior patterns, fearfulness, feathering, and mortality among three stocks of White Leghorn pullets in cages or floor pens. Poult. Sci. 70:211–221. doi: 10.3382/ps.0700211 [DOI] [PubMed] [Google Scholar]
- Marin R. H., and Satterlee D. G.. . 2003. Selection for contrasting adrenocortical responsiveness in Japanese quail (Coturnix japonica) influences sexual behaviour in males. Appl. Anim. Behav. Sci. 83:187–199. doi: 10.1016/s0168-1591(03)00129-1 [DOI] [Google Scholar]
- McAdie T. M., Keeling L. J., Blokhuis H. J., and Jones R. B.. . 2005. Reduction in feather pecking and improvement of feather condition with the presentation of a string device to chickens. Appl. Anim. Behav. Sci. 93:67–80. doi: 10.1016/j.applanim.2004.09.004 [DOI] [Google Scholar]
- Newberry R. C. 1999. Exploratory behaviour of young domestic fowl. Appl. Anim. Behav. Sci. 63:311–321. doi: 10.1016/s0168-1591(99)00016-7 [DOI] [PubMed] [Google Scholar]
- Nickla D. L., Wildsoet C. F., and Troilo D.. . 2001. Endogenous rhythms in axial length and choroidal thickness in chicks: implications for ocular growth regulation. Invest. Ophthalmol. Vis. Sci. 42:584–588. doi: 10.1097/00004397-200104000-00013 [DOI] [PubMed] [Google Scholar]
- Nicol C. J. 1992. Effects of environmental enrichment and gentle handling on behaviour and fear responses of transported broilers. Appl. Anim. Behav. Sci. 33:367–380. doi:10.1016/s0168-1591(05)80073–5 [Google Scholar]
- Nicol C. J. 1995. The social transmission of information and behaviour. Appl. Anim. Behav. Sci. 44:79–98. doi: 10.1016/0168-1591(95)00607-T [DOI] [Google Scholar]
- Pötzsch C. J., Lewis K., Nicol C. J., and Green L. E.. . 2001. A cross-sectional study of the prevalence of vent pecking in laying hens in alternative systems and its associations with feather pecking, management and disease. Appl. Anim. Behav. Sci. 74:259–272. doi: 10.1016/s0168-1591(01)00167-8 [DOI] [Google Scholar]
- Prescott N. B., Wathes C. M., and Jarvis J. R.. . 2003. Light, vision and the welfare of poultry. Anim. Welf. 12:269–288. doi: 10.1006/anbe.2003.2131 [DOI] [Google Scholar]
- Reed H. J., Wilkins L. J., Austin S. D., and Gregory N. J.. . 1993. The effect of environmental enrichment during rearing on fear reactions and depopulation trauma in adult caged hens. Appl. Anim. Behav. Sci. 36:39–46. doi:10.1016/0168-1591(93)90097–9 [Google Scholar]
- Riber A. B., and Hinrichsen L. K.. . 2017. Welfare consequences of omitting beak trimming in barn layers. Front. Vet. Sci. 4:222. doi: 10.3389/fvets.2017.00222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodenburg T. B., and Koene P.. . 2003. Comparison of individual and social feather pecking tests in two lines of laying hens at ten different ages. Appl. Anim. Behav. Sci. 81:133–148. doi: 10.1016/s0168-1591(02)00275-7 [DOI] [Google Scholar]
- Rodenburg T. B., Komen H., Ellen E. D., Uitdehaag K. A., and Arendonk J. A. M. V.. . 2008. Selection method and early-life history affect behavioural development, feather pecking and cannibalism in laying hens: a review. Appl. Anim. Behav. Sci. 110:217–228. doi: 10.1016/j.applanim.2007.09.009 [DOI] [Google Scholar]
- Savory C. J. 1995. Feather pecking and cannibalism. World’s Poult. Sci. J. 51:215–219. doi: 10.1079/WPS19950016 [DOI] [Google Scholar]
- Shi H. P., Zheng W. C., Tu J., and LI B.. 2018. Reducing feather pecking and cloacal cannibalism by providing layer breeders with nest boxes in colony cages for natural mating. Int. J. Agric. Bio. Eng. 11:27–32. doi:10.25165/j.ijabe.20181106.3323 [Google Scholar]
- Taylor P. E., Scott G. B., and Rose P.. . 2003. The ability of domestic hens to jump between horizontal perches: effects of light intensity and perch colour. Appl. Anim. Behav. Sci. 83:99–108. doi: 10.1016/s0168-1591(03)00127-8 [DOI] [Google Scholar]
- Vestergaard K. S., Kruijt J. P., and Hogan J. A.. . 1993. Feather pecking and chronic fear in groups of red jungle fowl: their relations to dustbathing, rearing environment and social status. Anim. Behav. 45:1127–1140. doi: 10.1006/anbe.1993.1137 [DOI] [Google Scholar]
- Weeks C. A., and Nicol C. J.. . 2006. Behavioural needs, priorities and preferences of laying hens. World’s Poult. Sci. J. 62:296-30. doi: 10.1079/WPS200598 [DOI] [Google Scholar]
- Welfare Quality 2009. Welfare Quality® assessment protocol for poultry (broilers, laying Hens). Welfare Quality® Consortium, Lelystad, the Netherlands. [Google Scholar]