Abstract
Some definitions of feed efficiency such as residual energy intake (REI) and residual gain (RG) may not truly reflect production efficiency. The energy sinks used in the derivation of the traits include metabolic live-weight; producers finishing cattle for slaughter are, however, paid on the basis of carcass weight, as opposed to live-weight. The objective of the present study was to explore alternative definitions of REI and RG which are more reflective of production efficiency, and quantify their relationship with performance, ultrasound, and carcass traits across multiple breeds and sexes of cattle. Feed intake and live-weight records were available on 5,172 growing animals, 2,187 of which also had information relating to carcass traits; all animals were fed a concentrate-based diet representative of a feedlot diet. Animal linear mixed models were used to estimate (co)variance components. Heritability estimates for all derived REI traits varied from 0.36 (REICWF; REI using carcass weight and carcass fat as energy sinks) to 0.50 (traditional REI derived with the energy sinks of both live-weight and ADG). The heritability for the RG traits varied from 0.24 to 0.34. Phenotypic correlations among all definitions of the REI traits ranged from 0.90 (REI with REICWF) to 0.99 (traditional REI with REI using metabolic preslaughter live-weight and ADG). All were different (P < 0.001) from one suggesting reranking of animals when using different definitions of REI to identify efficient cattle. The derived RG traits were either weakly or not correlated (P > 0.05) with the ultrasound and carcass traits. Genetic correlations between the REI traits with carcass weight, dressing difference (i.e., live-weight immediately preslaughter minus carcass weight) and dressing percentage (i.e., carcass weight divided by live-weight immediately preslaughter) implies that selection on any of the REI traits will increase carcass weight, lower the dressing difference and increase dressing percentage. Selection on REICW (REI using carcass weight as an energy sink), as opposed to traditional REI, should increase the carcass weight 2.2 times slower but reduce the dressing difference 4.3 times faster. While traditionally defined REI is informative from a research perspective, the ability to convert energy into live-weight gain does not necessarily equate to carcass gain, and as such, traits such as REICW and REICWF provide a better description of production efficiency for feedlot cattle.
Keywords: beef cattle, genetic correlations, residual energy intake, residual gain, slaughter traits
Introduction
Several studies exist on cattle comparing animals and production systems that differ in their feed efficiency metrics (Arthur et al., 2001a; Robinson and Oddy, 2004; Durunna et al., 2011), including studies with reported interanimal genetic differences (for review, see Berry and Crowley, 2013). Almost all such studies have been based on growing cattle (Arthur et al., 2001a; Crowley et al., 2010) and, in the vast majority of cases, these cattle were all purebred (Arthur et al., 2001b; Bouquet et al., 2010) and were undertaken on a single animal sex (Schenkel et al., 2004; Kayser and Hill, 2013). Furthermore, the carcass credentials of the animals on test were largely unknown, although more recent studies have documented the associations between efficiency metrics and some carcass measures (Mao et al., 2013; Torres-Vázquez et al., 2018).
Residual feed intake (RFI) is a popular scientific metric that attempts to describe interanimal differences in feed efficiency (Byerly, 1941; Koch et al., 1963). Residual feed intake in cattle was traditionally defined as the residuals from a multiple linear regression model, regressing some form of feed intake value on ADG and metabolic live-weight (Koch et al., 1963; Arthur et al., 2001a; Crowley et al., 2010). Basarab et al. (2003) subsequently recommended the inclusion of some measure of body fat in the multiple regression model in an attempt to ensure the observed differences in RFI were not simply due to differences in body fat, and to minimize the effects of selection for low RFI on carcass leanness in slaughter cattle and later fattening or maturing in replacement heifers. Savietto et al. (2014) progressed this recommendation further by stating that the interaction between body fat measures and both body weight and ADG should be considered in the model. Producers of the final beef product, however, are generally paid on the basis of carcass weight and carcass quality (Polkinghorne and Thompson, 2010). Therefore, because of the large interanimal variation in dressing percentage (Coyne et al., 2019), RFI defined using metabolic live-weight may not necessarily be a good reflection of production efficiency for producers fattening animals (i.e., feedlot cattle where concentrate constitutes 80 to 90% of the diet) who would be more concerned with the carcass weight of the animal rather than the metabolic live-weight.
The objective of the present study was to modify the status quo definition of both RFI and residual gain (RG) traits and to investigate their interrelationships with performance, ultrasound, and carcass traits in 3 different animal sexes (young bulls, steers, and heifers) of purebred and crossbred growing cattle. The novelty of the present study lies in the derivation of an extensive suite of feed efficiency traits which may have downstream applications in both management and breeding strategies to monitor and improve animal production efficiency.
Materials and Methods
The data used in the present study were obtained from a pre-existing database managed by the Irish Cattle Breeding Federation (ICBF). Therefore, it was not necessary to obtain animal care and use committee approval in advance of conducting this study.
All feed intake, live-weight, carcass, and ultrasound records originated from animals that were on test for feed intake at the ICBF Performance Test Station (1992 to 2011, inclusive) and later the ICBF Gene Ireland Progeny Test Center (2012 to present day), Tully, Co. Kildare, Ireland. Prior to 2012, the test center operated as a beef bull performance test center where details of the bull selection process, center practices, and management were described in detail by Crowley et al. (2010). In August 2012, the test center changed function to a progeny test center where bulls, steers, and heifers were purchased by the ICBF from Irish commercial producers, tested for feed intake and efficiency on a high energy concentrate-based diet, and subsequently slaughtered. No feed intake, live-weight, carcass, or ultrasound data were available during the transition period between October 2011 and July 2012.
Pre-2012
Prior to 2012, bulls entered the test station in, on average, 3 different groups annually, hereafter referred to as batches. There were 2 to 5 bulls per pen, assigned based on breed and live-weight, and all 40 pens were equipped with a Calan Broadbent gate system (American Calan, Northwood, NH) for recording individual bull feed intake. Initially bulls were fed 4.5 to 6 kg of concentrates, which was increased daily by 10% of the previous day’s allowance until ad libitum feed intake was reached. The test started once the bulls had entered the test station and had acclimatized to the facilities and diet; concentrate intake was recorded on a fresh weight basis once ad libitum levels of concentrate feeding were reached. To obtain total weekly concentrate intake, concentrate refusals were measured 1 d per week and subtracted from the cumulative concentrate offered over the previous 7 d. A daily allowance of 1.5 kg fresh weight of hay per bull was provided into the Calan Broadbent feeder throughout the bull’s residency in the test station. Access to clean, fresh water was also provided ad libitum to all bulls. Animals were weighed every 14 d between 1992 and 1995, every 21 d between 1995 and 2005, every 14 d between 2005 and 2008, and every 21 d between 2008 and 2011. From September 1992 to September 2011, all hay was assumed to have a DM of 85% and a metabolizable energy concentration of 8.6 MJ/kg DM. The concentrates offered to bulls between September 1992 and September 2002 was assumed to have DM of 87.5% and a metabolizable energy concentration of 12.1 MJ/kg DM, whereas the concentrates offered to bulls between October 2002 and September 2011 was assumed to have a DM of 86% and a metabolizable energy concentration of 14.5 MJ/kg DM. Daily metabolizable energy intake (MEI) for each bull tested pre-2012 was defined as the sum of daily hay DMI multiplied by the hay metabolizable energy concentration plus daily concentrate DMI multiplied by the concentrate metabolizable energy concentration.
Post-2011
From August 2012 onwards, all animals within each batch started their progeny test together and all animals within a batch were slaughtered within a week of each other at the end of their test period. Each batch was composed of one sex and was grouped by birth-date where the maximum range in age was 4 mo. On arrival at the test station, all cattle were assigned to pens based on breed and live-weight and then underwent an acclimatization period of between 21 and 30 d to adapt to the feeding system and environment. There were 4 to 6 animals per pen, across a total of 40 pens; 30 pens were equipped with 2 automatic feed stations (RIC Feed-Weigh Trough, Hokofarm Group BV, Marknesse, The Netherlands) and a further 10 pens were equipped with a Calan Broadbent gate system. While in the test station, all animals were weighed, on average, every 7 d between August 2012 and August 2013, every 21 d between September 2013 and December 2017, and every 7 d in 2018.
Each automatic feed station was mounted on 2 load cell and had a pneumatic access gate with an infrared sensor on one side that recorded the presence of an animal. An antenna directly above the access gate detected the radio frequency identification (RFID) tag (HDX EID Tag, Allflex Livestock Intelligence, Dallas, TX) in the animal’s ear to identify the individual animal in the feed station. A feed event commenced when an animal’s RFID tag was first detected and ended after interruption of the infrared sensor ended. All automatic feed stations provided ad libitum access to feed. Refusals were discarded in all feed stations daily before feed was refreshed. All steers, heifers, and some bulls were fed with this system. For every pen in the test center, access to clean, fresh water was provided ad libitum, with one water trough shared between 2 adjacent pens. Steers and heifers were fed a total mixed ration (TMR) with a concentrate, hay, and water fresh-weight ratio of 2:6:8, and 5:3:9 for days 1 to 7, and days 8 to 12 of the acclimatization period, respectively. A TMR with a concentrate, hay, and water fresh-weight ratio of 10:3:9 was fed, for the rest of the acclimatization period and subsequently throughout the test period, ad libitum once per day with a paddle mixer wagon. Daily feed intake of each animal fed through the automatic feed stations was calculated by summing, per day, the feed consumed in each feed event which was then averaged across all valid test days.
Young bulls entering the test center from the year 2012 onwards were fed a starting daily allocation of 5 kg fresh weight of concentrates. During the acclimatization period, the concentrate allowance of each bull was increased by 0.5 kg fresh weight per day until ad libitum levels were reached; a daily fixed rate of 2 kg fresh weight of hay was also fed to each bull during this period to maintain healthy rumen function. The recording of feed intake commenced when all animals reached ad libitum levels of feeding. Young bulls fed through the automated feed stations during the test period were fed both concentrates and hay once in the morning, 7 d per week; an allocation of 2 kg fresh weight per animal of hay was fed in one of the feed stations in the pen, while concentrates were fed ad libitum separately in the other feed station in the pen. Daily feed intake was calculated by summing, per day, the feed consumed in each feed event which was then averaged across all valid test days. Young bulls fed during the test period through the Calan Broadbent system from 2012 onwards were offered concentrates twice per day, 7 d per week; a fixed daily rate of 2 kg fresh weight of hay per animal was also provided, split into 2 feeds, 1 in the morning and 1 in the afternoon, 7 d per week. Concentrate intake was calculated weekly by recording concentrate refusals of each bull 1 d per week and subtracting from the cumulative feed offered over the previous 7 d; this sum was subsequently divided by 7 to obtain average daily concentrate intake within this time period.
From 2012 to 2018, all hay fed was assumed to have a DM of 85% and a metabolizable energy concentration of 8.6 MJ/kg DM. The concentrates offered to bulls between August 2012 and November 2018 was assumed to have a DM of 86% and a metabolizable energy concentration of 14.1 MJ/kg DM. Daily MEI for each bull tested post-2011 was defined as the sum of daily hay DMI multiplied by the hay metabolizable energy concentration and daily concentrate DMI multiplied by the concentrate metabolizable energy concentration. The TMR fed to all steers and heifers was assumed to have a DM of 51% and a metabolizable energy concentration of 12.1 MJ/kg DM; daily MEI per animal was calculated as the animal’s daily total DMI multiplied by the energy concentration of the TMR. Hay energy values were derived from feed tables (Sauvant et al., 2004) and concentrate energy values were obtained from the manufacturer.
Data Editing
The test period in the present study was defined as the last 70 d of test. For all animals, the most recent live-weight record before the 70-d cut-off was retained if it was recorded after the acclimatization period; all animals had to have at least 3 live-weight records during the test period. Additionally, for animals tested post-2011, the final live-weight of an animal preslaughter was also retained for use in the present study. Any animal tested after the year 2011 that did not have a live-weight record within 7 d preslaughter (n = 38) was removed from all analyses. Data from a further 161 animals were removed due to abnormal growth rates where the r-squared of a linear regression through their live-weight records was <0.90 (discussed later). All animals tested between the years 1992 and 2011 had to be between 8 and 16 mo of age when they started their test, while all animals tested between the years 2012 and 2018 had to be between 10 and 24 mo of age when they started their test. Five days of feed intake records from cattle fed through the automatic feed stations were removed due to a weight malfunction on those days. Thirteen animals were identified as sick from a combination of their growth and feed intake patterns; data from these animals were removed from all analyses. After all edits, feed intake and live-weight records were available on 5,172 animals of which 2,985 were bulls tested pre-2012, 1,402 were bulls tested post-2011, 542 were steers, and 243 were heifers; all post-2011 bulls, steers, and heifers (n = 2,187) also had carcass-related records.
Trait Definitions
Carcass data and final live-weight preslaughter were only available on 2,187 animals tested from the year 2012 onwards. Carcass weight (kg) was measured, on average, 2 h postslaughter. Carcass conformation and carcass fat class were obtained using video image analysis from a mechanical grading system (Pabiou et al., 2011). Carcass conformation was defined by the EUROP system and represented by the letters E, U, R, O, and P, where E represents the best conformation and P represents the worst conformation (Englishby et al., 2016). Each conformation class was subdivided into 3 divisions, specifying a 15-point scale for carcass conformation. Carcass fat classes were represented on a scale from 1 to 15, where 1 represents the least fat and 15 represents the greatest fat cover on the carcass. Dressing difference (kg) was calculated as the animal’s final live-weight, within 7 d preslaughter, minus its carcass weight (Coyne et al., 2019). Carcass dressing percentage (%) was calculated as the carcass weight divided by the final live-weight of an animal within 7 d preslaughter (Coyne et al., 2019) multiplied by 100. All 2,187 animals with carcass data had a record for both dressing difference and dressing percentage.
Ultrasound measurements were available on 3,726 animals. Bulls performance tested between 1992 and 2011 were scanned once, approximately half way through their test period. Of the animals scanned post-2011, 32 batches (1,370 animals) had their last ultrasound record within 30 d of slaughter, while 5 batches (200 animals) had their last ultrasound record between 35 and 75 d preslaughter; only the last recorded preslaughter ultrasound measurement was retained for each animal tested. An Esaote-Pie Medical Aquila PRO Vet ultrasound scanner with a 3.5 MHz transducer head was used to obtain all ultrasound measurements. Fat depth was measured in 2 areas; 1) at the third lumbar vertebrae in 3 locations approximately 2 cm apart, and 2) at the 13th thoracic rib in 4 locations approximately 2 cm apart. Ultrasound fat depth (mm) was calculated as the average of all fat depth records at the third lumbar vertebrae and fat depth records at the 13th thoracic rib; ultrasound fat depth records were available on 3,726 animals. An eye muscle depth (mm) record was available on 2,782 animals and was measured at the third lumbar vertebra on top of the loin, at a single point representing the deepest point of the muscle. Intramuscular fat (IMF; %) records were available on 1,446 animals and were estimated from images taken at a lateral position to the animal’s spine at the 13th thoracic rib; all animals with an intramuscular fat record also had a record for both eye muscle depth and fat depth.
Average daily gain was calculated, per animal, as the linear regression coefficient from a simple linear regression of individual live-weight on days on test. Mid-test metabolic live-weight (MBW; i.e., live-weight0.75) was represented as the predicted metabolic live-weight 35 d before the end of the test, derived from the intercept and linear regression coefficient of metabolic live-weight measures on days on test. Metabolic final live-weight (MFW) was represented as the final live-weight of an animal within 7 d preslaughter raised to the power of 0.75. Energy conversion ratio (ECR) was defined as MEI divided by ADG.
Several definitions of REI were derived. The traditional definition of REI (herein referred to as just REI) was calculated as the residuals from a multiple linear regression of MEI on MBW and ADG:
where represents the intercept and and represent the respective partial regression coefficients of MEI on MBW and ADG.
Where ultrasound records were available, a separate trait of REI adjusted for ultrasound fat depth (REIU) was calculated as already described for REI except ultrasound fat depth was itself included as a covariate but also in a 2-way interaction with both ADG and MBW. Residual energy intake using MFW (REIFW) was calculated as the residuals from a multiple linear regression of MEI on MFW and ADG:
where represents the intercept and and represent the respective partial regression coefficients of MEI on MFW and ADG.
Residual energy intake using carcass weight (REICW) was calculated as the residuals from a multiple linear regression of MEI on both carcass weight and ADG:
where represents the intercept and and represent the respective partial regression coefficients of MEI on carcass weight and ADG.
A separate trait of REICW adjusted for carcass fat score (REICWF) was calculated the same as for REICW except carcass fat score was itself included as a covariate but also in a 2-way interaction with carcass weight. The partial regression coefficients for each REI trait model within animal sex (bulls tested post-2011, steers, and heifers) are given in Table 1 of the Supplementary Material.
Several definitions of RG were also derived. The traditional definition of RG was calculated as the residuals from a multiple linear regression of ADG on MBW and MEI:
where represents the intercept and and represent the respective partial regression coefficients of ADG on MBW and MEI.
Where ultrasound records were available, a separate trait of RG adjusted for ultrasound traits (RGU) was calculated as already described for RG except ultrasound fat depth was itself included as a covariate but also in a 2-way interaction with both MEI and MBW. Residual gain using MFW (RGFW) was calculated as the residuals from a multiple linear regression of ADG on MFW and MEI:
where represents the intercept and and represent the respective partial regression coefficients of ADG on MFW and MEI.
Residual gain using carcass weight (RGCW) was calculated as the residuals from a multiple linear regression of ADG on both carcass weight and MEI:
where represents the intercept and and represent the respective partial regression coefficients of ADG on carcass weight and MEI.
A separate trait of RGCW adjusted for carcass fat score (RGCWF) was calculated as already described for RGCW except carcass fat score was itself included as a covariate but also in a 2-way interaction with carcass weight. All derivations of REI and RG were calculated within animal sex, with batch included as a fixed effect as illustrated.
The heterosis coefficient and recombination loss coefficient were calculated for each animal as:
and
respectively, where sirei and dami are the proportion of breed i in the sire and dam, respectively (Van Raden and Sanders, 2003). Heterosis coefficient was subsequently divided into 12 classes (0.0%, >0.0 to <0.1%, ≥0.1 to <0.2%,… ≥0.9 to <100.0%, and 100.0%), and recombination loss coefficient was divided into 7 classes (0.00%, >0.00 to <0.05%, ≥0.05 to <0.10%,… ≥0.45 to <0.50%, 0.50%, and >0.50%).
Statistical Analyses
Phenotypic and genetic variance components for the performance, efficiency, ultrasound, and carcass traits were estimated using a series of univariate animal linear mixed models in ASReml (Gilmour et al., 2009). Fixed effects for consideration in all models were batch (n = 118), age at the end of test (covariate), the 2-way interaction between age at the end of test and animal sex, heterosis coefficient class, recombination loss coefficient class, and dam parity (1, 2, 3, 4, ≥5, and missing). Animal was included as a random effect, and average genetic relationships among animals were considered by tracing the pedigree of each animal back to founder animals which were allocated to genetic groups based on breed; up to 22 ancestral generations were used in the generation of the relationship matrix. The pedigree file consisted of 59,682 animals. Phenotypic and genetic covariances among all traits were estimated using a series of bivariate animal linear mixed models; fixed effects in the model were those described for the univariate analyses. The numbers of records used in each bivariate analysis are presented in Tables 2, 3, and 4 of the Supplementary Material.
Results
Summary statistics by animal sex for each performance, efficiency, ultrasound, and carcass trait are listed in Table 1. Daily MEI ranged from 133.51 MJ/d for bulls tested pre-2012 to 180.17 MJ/d for bulls tested post-2011. Bulls tested post-2011, on average, grew faster, weighed more, had a heavier carcass weight and had a better dressing percentage compared to both steers and heifers. There was no difference (P > 0.05) in growth rate, energy intake, or ECR between steers and heifers, although, on average, heifers weighed less, had the lightest carcasses, and had the lowest dressing percentage. The mean of all derived residual traits was zero, due to the properties of least squares regression. Performance trait heritability estimates ranged from 0.29 for ADG to 0.66 for MBW. Heritability estimates for the REI traits ranged from 0.36 for REICWF to 0.50 for traditional REI. Heritability estimates for the RG traits varied from 0.24 for traditional RG to 0.34 for RGFW. The inclusion of body fat measures, such as ultrasound fat depth (UFD), reduced the genetic standard deviation from 7.31 MJ/d for REI to 6.69 MJ/d for REIU, while the genetic standard deviation reduced from 8.33 MJ/d for REICW to 7.34 MJ/d for REICWF with the inclusion of carcass fat measures in the regression model.
Table 1.
Raw means (standard deviations in parentheses), heritability estimates (h2; standard error in parentheses), and genetic standard deviations (σ g) of the performance, efficiency ultrasound, and carcass traits in bulls tested before 2012 (pre-2012 bulls), bulls tested post-2011 (post-2011 bulls), steers, and heifers1
| Trait2 | Pre-2012 Bulls | Post-2011 Bulls | Steers | Heifers | h2 | σ g |
|---|---|---|---|---|---|---|
| Performance | ||||||
| MEI, MJ/d | 133.51a (20.81) | 180.17b (17.63) | 149.05c (21.97) | 147.51c (24.37) | 0.54 (0.05) | 10.51 |
| ADG, kg/d | 1.71a (0.38) | 2.04b (0.34) | 1.44c (0.30) | 1.42c (0.30) | 0.29 (0.04) | 0.15 |
| MBW, kg0.75 | 113.3a (11.94) | 121.7b (10.56) | 122.8b (10.01) | 114.8a (9.23) | 0.66 (0.05) | 6.42 |
| MFW, kg0.75 | N/A | 133.22a (10.23) | 129.93b (10.10) | 122.15c (9.30) | 0.61 (0.08) | 6.62 |
| Efficiency | ||||||
| ECR | 80.78a (16.81) | 90.49b (14.78) | 106.54c (21.35) | 107.6c (26.37) | 0.24 (0.04) | 6.93 |
| REI, MJ/d | 0 (10.71) | 0 (9.87) | 0 (13.47) | 0 (18.21) | 0.50 (0.05) | 7.31 |
| REIU, MJ/d | 0 (9.59) | 0 (9.42) | 0 (13.92) | 0 (18.40) | 0.40 (0.06) | 6.69 |
| REIFW, MJ/d | N/A | 0 (9.95) | 0 (13.41) | 0 (18.20) | 0.40 (0.08) | 7.46 |
| REICW, MJ/d | N/A | 0 (11.29) | 0 (14.66) | 0 (19.21) | 0.43 (0.08) | 8.33 |
| REICWF, MJ/d | N/A | 0 (10.37) | 0 (13.96) | 0 (18.44) | 0.36 (0.07) | 7.34 |
| RG, kg/d | 0 (0.25) | 0 (0.24) | 0 (0.21) | 0 (0.19) | 0.24 (0.04) | 0.12 |
| RGU, kg/d | 0 (0.23) | 0 (0.24) | 0 (0.20) | 0 (0.20) | 0.26 (0.05) | 0.12 |
| RGFW, kg/d | N/A | 0 (0.24) | 0 (0.20) | 0 (0.18) | 0.34 (0.07) | 0.13 |
| RGCW, kg/d | N/A | 0 (0.24) | 0 (0.20) | 0 (0.19) | 0.34 (0.07) | 0.13 |
| RGCWF, kg/d | N/A | 0 (0.24) | 0 (0.20) | 0 (0.19) | 0.33 (0.07) | 0.13 |
| Ultrasound | ||||||
| UFD, mm | 3.1a (1.68) | 3.7b (1.13) | 5.2c (1.63) | 6.0d (1.92) | 0.49 (0.06) | 0.76 |
| UMD, mm | 84.1a (7.14) | 81.5b (7.43) | 74.7c (7.07) | 72.2d (7.83) | 0.30 (0.06) | 3.18 |
| IMF, % | N/A | 5.01a (1.43) | 5.97b (1.34) | 6.66c (1.05) | 0.25 (0.08) | 0.57 |
| Carcass | ||||||
| Carcass Weight, kg | N/A | 401.7a (45.47) | 360.6b (40.85) | 325.0c (38.70) | 0.62 (0.09) | 28.51 |
| Carcass Conformation, scale 1–15 | N/A | 11.8a (1.27) | 8.6b (1.68) | 8.2c (1.79) | 0.62 (0.08) | 0.89 |
| Carcass Fat, scale 1–15 | N/A | 5.8a (1.03) | 7.5b (1.48) | 8.7c (1.90) | 0.63 (0.09) | 0.87 |
| Dressing Difference, kg | N/A | 279.6a (31.83) | 298.4b (36.25) | 281.8a (32.41) | 0.66 (0.08) | 22.28 |
| Dressing Percentage,% | N/A | 58.94a (2.46) | 54.73b (2.71) | 53.55c (2.87) | 0.78 (0.08) | 1.78 |
1N/A = not available
2MEI = metabolizable energy intake; MBW = mid-test metabolic live-weight; MFW = metabolic final live-weight preslaughter; ECR = energy conversion ratio; REI = residual energy intake; REIU = REI adjusted for ultrasound fat depth; REIFW = residual energy intake using metabolic final live-weight preslaughter; REICW = residual energy intake using carcass weight; REICWF = REICW adjusted for carcass fat; RG = residual gain; RGU = RG adjusted for ultrasound fat depth; RGFW= residual gain using metabolic final live-weight preslaughter; RGCW = residual gain using carcass weight; RGCWF = RGCW adjusted for carcass fat score; UFD = ultrasound fat depth; UMD = ultrasound muscle depth; IMF= intramuscular fat percentage.
a-dMeans within a row with different subscripts differ (P < 0.05).
Correlations Between the Performance and Efficiency Traits
Phenotypic and genetic correlations between the performance and efficiency traits are summarized in Table 2. On average, animals with a higher energy intake grew faster, were heavier, and had an inferior ECR; this conclusion presented irrespective of whether the correlations were phenotypic or genetic. The phenotypic correlation between MBW and MFW was 0.98, while the respective genetic correlation was 0.99; both correlations were different (P < 0.001) from one. The phenotypic and genetic correlations between the performance and efficiency traits with the residual energy intake and RG traits are listed in Table 3. Neither the phenotypic nor the genetic correlations between all the REI traits and their respective component traits were different (P > 0.05) from zero. Similarly, the phenotypic and genetic correlations between all the RG traits and their respective components traits were not different (P > 0.05) from zero. The fact that the phenotypic correlations between either the REI traits or the RG traits with their component traits were not exactly zero was because fixed effects that were included in the bivariate mixed models, used to calculate the correlations, were not included in the regression equations to derive the REI and RG traits.
Table 2.
Phenotypic1 (below the diagonal) and genetic (above the diagonal with standard errors in parentheses) correlations between the performance and efficiency traits
| Trait2 | MEI | ADG | MBW | MFW | ECR |
|---|---|---|---|---|---|
| MEI | 0.61 (0.06) | 0.65 (0.04) | 0.63 (0.06) | 0.25 (0.09) | |
| ADG | 0.46 | 0.43 (0.07) | 0.41 (0.10) | −0.61 (0.06) | |
| MBW | 0.61 | 0.28 | 0.99 (0.003) | 0.15 (0.09) | |
| MFW | 0.60 | 0.51 | 0.98 | −0.12 (0.14) | |
| ECR | 0.14 | −0.75 | 0.08 | −0.15 |
1Standard errors of the phenotypic correlations were all <0.03.
2MEI = metabolizable energy intake; ADG = average daily gain; MBW = mid-test metabolic live-weight; MFW = metabolic final live-weight preslaughter; ECR = energy conversion ratio.
Table 3.
Phenotypic and genetic (standard errors in parentheses) correlations of the performance and efficiency traits with the residual energy intake and residual gain traits
| Trait2 | Phenotypic Correlations1 | Genetic Correlations | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MEI | ADG | MBW | MFW | ECR | MEI | ADG | MBW | MFW | ECR | |
| REI | 0.71 | −0.03 | −0.03 | −0.03 | 0.47 | 0.68 (0.04) | 0.12 (0.09) | −0.07 (0.07) | −0.20 (0.11) | 0.46 (0.08) |
| REIU | 0.65 | −0.02 | −0.03 | −0.01 | 0.46 | 0.62 (0.06) | 0.14 (0.11) | 0.02 (0.08) | −0.11 (0.13) | 0.44 (0.09) |
| REIFW | 0.69 | −0.02 | −0.01 | −0.03 | 0.52 | 0.64 (0.06) | −0.06 (0.14) | −0.15 (0.12) | −0.18 (0.13) | 0.39 (0.12) |
| REICW | 0.76 | −0.04 | 0.17 | 0.16 | 0.57 | 0.75 (0.05) | −0.03 (0.14) | 0.10 (0.10) | 0.08 (0.13) | 0.43 (0.11) |
| REICWF | 0.70 | −0.04 | 0.13 | 0.11 | 0.55 | 0.69 (0.06) | −0.07 (0.15) | 0.08 (0.12) | 0.06 (0.13) | 0.45 (0.12) |
| RG | 0.03 | 0.89 | 0.01 | 0.15 | −0.89 | 0.04 (0.10) | 0.80 (0.04) | 0.06 (0.09) | 0.15 (0.13) | −0.90 (0.02) |
| RGU | 0.02 | 0.87 | 0.03 | 0.16 | −0.86 | 0.02 (0.10) | 0.75 (0.05) | 0.06 (0.10) | 0.29 (0.14) | −0.84 (0.04) |
| RGFW | 0.02 | 0.83 | −0.12 | 0.01 | −0.83 | 0.08 (0.11) | 0.73 (0.06) | −0.20 (0.11) | −0.12 (0.12) | −0.84 (0.05) |
| RGCW | 0.01 | 0.83 | −0.09 | 0.04 | −0.85 | 0.11 (0.12) | 0.77 (0.05) | −0.15 (0.11) | −0.06 (0.12) | −0.87 (0.04) |
| RGCWF | 0.01 | 0.83 | −0.09 | 0.05 | −0.85 | 0.12 (0.12) | 0.78 (0.05) | −0.15 (0.11) | −0.06 (0.12) | −0.87 (0.04) |
1Phenotypic correlations ≤ |0.04| were not different (P > 0.05) from zero.
2REI = residual energy intake; REIU = REI adjusted for ultrasound fat depth; REIFW = residual energy intake using metabolic final live-weight preslaughter; REICW = residual energy intake using carcass weight; REICWF = REICW adjusted for carcass fat; RG = residual gain; RGU = RG adjusted for ultrasound fat depth; RGFW= residual gain using metabolic final live-weight preslaughter; RGCW = residual gain using carcass weight; RGCWF = RGCW adjusted for carcass fat score; MEI = metabolizable energy intake; ADG = average daily gain; MBW = mid-test metabolic live-weight; MFW = metabolic final live-weight preslaughter; ECR = energy conversion ratio.
Phenotypic correlations of all REI traits with MEI ranged from 0.65 (REI) to 0.76 (REICW), while genetic correlations between all REI traits and MEI varied from 0.62 (REIU) to 0.75 (REICW). Similarly, the phenotypic correlations between all RG traits and ADG ranged from 0.83 (RGFW, RGCW, and RGCWF) to 0.89 (RG), while the genetic correlations between all RG traits and ADG ranged from 0.73 (RGFW) to 0.80 (RG). Superior ECR was associated with both better REI (i.e., lower REI) and better RG (i.e., greater RG); the phenotypic correlations between the REI traits and ECR varied from 0.46 (REIU) to 0.57 (REICW) and were different (P < 0.01) from each other, while the phenotypic correlations between the RG traits and ECR were also different (P < 0.001) from each other and varied from −0.89 (RG) to −0.83 (RGFW).
Correlations Among and Between the REI and the RG traits
The phenotypic and genetic correlations among all REI traits and all RG traits, as well as between all the REI traits and RG traits, are listed in Table 4. The phenotypic correlations among all of the REI traits ranged from 0.88 (REIU with REICW) to 0.99 (REI with REIFW) but were all different (P < 0.001) from one. The genetic correlations among all REI traits were generally weaker than the respective phenotypic correlations and ranged from 0.82 (REIU with REICW) to 0.99 (REI with REIFW). Traditional REI had a phenotypic correlation of 0.95 with REICW and thus 9.75% of the phenotypic variation in REICW was not explained by traditional REI. Similarly, 19% of the phenotypic variation in REICWF was unexplained by REIU; the phenotypic and genetic correlations between REIU and REICWF were 0.90 and 0.89, respectively. The phenotypic correlations among all RG traits were >0.97, but were all different (P < 0.001) from one, while the genetic correlations ranged from 0.95 (RGCWF with RGU) to 0.99 (RGCW with RGCWF). The phenotypic correlations between all the REI traits and all the RG traits were generally stronger than the respective genetic correlations and varied from −0.44 (REICW with RG) to −0.20 (REIU with RGFW), while the genetic correlations ranged from −0.36 (REIU with RGU) to −0.12 (REI with RGCWF).
Table 4.
Phenotypic1 (below the diagonal) and genetic (above the diagonal with standard errors in parentheses) correlations among and between the residual energy intake and residual gain traits
| Trait2 | REI | REIU | REIFW | REICW | REICWF | RG | RGU | RGFW | RGCW | RGCWF |
|---|---|---|---|---|---|---|---|---|---|---|
| REI | 0.93 (0.01) | 0.99 (0.001) | 0.94 (0.01) | 0.89 (0.02) | −0.32 (0.09) | −0.29 (0.10) | −0.12 (0.11) | −0.13 (0.11) | −0.12 (0.11) | |
| REIU | 0.93 | 0.92 (0.02) | 0.82 (0.04) | 0.89 (0.03) | −0.26 (0.11) | −0.36 (0.10) | −0.13 (0.13) | −0.15 (0.13) | −0.15 (0.13) | |
| REIFW | 0.99 | 0.93 | 0.94 (0.01) | 0.89 (0.03) | −0.26 (0.14) | −0.32 (0.15) | −0.13 (0.13) | −0.13 (0.13) | −0.12 (0.14) | |
| REICW | 0.95 | 0.88 | 0.96 | 0.92 (0.02) | −0.32 (0.13) | −0.32 (0.15) | −0.27 (0.12) | −0.25 (0.12) | −0.24 (0.13) | |
| REICWF | 0.90 | 0.90 | 0.92 | 0.94 | −0.31 (0.14) | −0.35 (0.15) | −0.24 (0.13) | −0.23 (0.13) | −0.24 (0.13) | |
| RG | −0.36 | −0.35 | −0.36 | −0.44 | −0.40 | 0.98 (0.005) | 0.96 (0.01) | 0.96 (0.01) | 0.95 (0.01) | |
| RGU | −0.34 | −0.39 | −0.32 | −0.39 | −0.38 | 0.99 | 0.98 (0.01) | 0.99 (0.01) | 0.98 (0.01) | |
| RGFW | −0.21 | −0.20 | −0.24 | −0.35 | −0.32 | 0.99 | 0.97 | 0.99 (0.002) | 0.99 (0.003) | |
| RGCW | −0.25 | −0.25 | −0.28 | −0.37 | −0.34 | 0.99 | 0.97 | 0.99 | 0.99 (0.001) | |
| RGCWF | −0.25 | −0.26 | −0.29 | −0.37 | −0.36 | 0.98 | 0.97 | 0.99 | 0.99 |
1All phenotypic correlations are different (P < 0.001) from zero and different (P < 0.001) from |1.00|.
2REI = residual energy intake; REIU = REI adjusted for ultrasound fat depth; REIFW = residual energy intake and metabolic final live-weight preslaughter; REICW = residual energy intake and carcass weight; REICWF = REICW adjusted for carcass fat; RG = residual gain; RGU = RG adjusted for ultrasound fat depth; RGFW= residual gain and metabolic final live-weight preslaughter; RGCW = residual gain and carcass weight; RGCWF = RGCW adjusted for carcass fat score.
Correlations Among and Between the Ultrasound and Carcass Traits
Table 5 summarizes the phenotypic and genetic correlations between the ultrasound and the carcass traits; almost all of the genetic correlations were stronger than the respective phenotypic correlations. Ultrasound muscle depth (UMD) had a phenotypic correlation of 0.43 and 0.48 with carcass conformation and carcass weight, respectively, while UFD had a phenotypic correlation of 0.58 and 0.07 with carcass fat and carcass weight, respectively. Dressing percentage was both phenotypically and genetically correlated with reduced UFD (phenotypic and genetic correlations of −0.28 and −0.67, respectively), reduced IMF (phenotypic and genetic correlations of −0.18 and −0.64, respectively), and greater UMD (phenotypic and genetic correlations of 0.42 and 0.55, respectively). Dressing percentage was also genetically and phenotypically correlated with a heavier carcass weight, better carcass conformation, but a lower carcass fat score. Dressing difference was both phenotypically and genetically correlated with a heavier carcass weight but greater UFD, a greater carcass fat score, greater intramuscular fat, and reduced carcass conformation.
Table 5.
Phenotypic1 (below the diagonal) and genetic (above the diagonal with standard error in parentheses) correlations among and between the ultrasound and carcass traits
| Trait2 | UFD | UMD | IMF | CW | CC | CF | DD | DP |
|---|---|---|---|---|---|---|---|---|
| UFD | −0.49 (0.11) | 0.68 (0.12) | −0.17 (0.13) | −0.42 (0.12) | 0.84 (0.06) | 0.44 (0.10) | −0.67 (0.09) | |
| UMD | −0.07 | −0.01 (0.21) | 0.53 (0.11) | 0.60 (0.10) | −0.15 (0.14) | −0.05 (0.14) | 0.55 (0.10) | |
| IMF | 0.35 | 0.02 | −0.17 (0.17) | −0.39 (0.15) | 0.51 (0.15) | 0.37 (0.16) | −0.64 (0.13) | |
| CW | 0.07 | 0.48 | 0.06 | 0.52 (0.08) | −0.02 (0.11) | 0.55 (0.07) | 0.44 (0.08) | |
| CC | −0.14 | 0.43 | −0.08 | 0.48 | −0.26 (0.11) | −0.20 (0.11) | 0.77 (0.05) | |
| CF | 0.58 | −0.01 | 0.29 | 0.18 | −0.04 | 0.39 (0.09) | −0.46 (0.09) | |
| DD | 0.32 | 0.11 | 0.21 | 0.63 | −0.06 | 0.36 | −0.50 (0.08) | |
| DP | −0.28 | 0.42 | −0.18 | 0.41 | 0.64 | −0.21 | −0.44 |
1Phenotypic correlations ≤ |0.05| were not different (P > 0.05) from zero.
2UFD = ultrasound fat depth; UMD = ultrasound muscle depth; IMF = intramuscular fat percentage; CW = carcass weight; CC = carcass conformation score; CF = carcass fat score; DD = dressing difference; DP = dressing percentage.
Correlations Between Both the Performance and Efficiency Traits With Both the Ultrasound and Carcass Traits
The phenotypic correlations between both the performance and efficiency traits with both the ultrasound and carcass traits are presented in Table 6. Metabolic mid-test live-weight (MBW) had correlations of 0.92 with carcass weight and 0.83 with dressing difference. Likewise, there was a correlation of 0.93 between MFW and carcass weight, and a correlation of 0.87 between MFW and dressing difference. A greater MEI and faster ADG were both moderately correlated with both a heavier carcass and heavier dressing difference. Energy conversion efficiency was weakly negatively phenotypically correlated with carcass weight, but was not correlated with dressing percentage. Phenotypically, more efficient animals (i.e., lower REI) had heavier and better conformed carcasses, reduced dressing difference but increased dressing percentage; weak to moderate negative correlations existed between dressing percentage and all the REI traits, ranging from −0.37 (REICW) to −0.14 (REIU). Based on the slope of the phenotypic regression of REI on dressing difference, every 10 MJ reduction in REI was expected to be associated with, on average, a 2.45 kg lighter dressing difference. In contrast, every 10 MJ decrease in REICW was expected to be phenotypically associated with a 6.91 kg lighter dressing difference. Phenotypically, the RG traits were either not correlated (i.e., P>0.05), or weakly correlated with the ultrasound and carcass traits.
Table 6.
Phenotypic1 correlations among the performance, efficiency, ultrasound, and carcass traits
| Trait2 | UFD | UMD | IMF | CW | CC | CF | DD | DP |
|---|---|---|---|---|---|---|---|---|
| MEI | 0.24 | 0.10 | 0.18 | 0.48 | 0.02 | 0.32 | 0.63 | −0.17 |
| ADG | 0.03 | 0.15 | 0.02 | 0.44 | 0.19 | 0.15 | 0.49 | −0.05 |
| MBW | 0.12 | 0.29 | 0.16 | 0.92 | 0.26 | 0.27 | 0.83 | 0.08 |
| MFW | 0.20 | 0.35 | 0.14 | 0.93 | 0.27 | 0.28 | 0.87 | 0.05 |
| ECR | 0.11 | −0.06 | 0.10 | −0.14 | −0.17 | 0.03 | −0.11 | −0.03 |
| REI | 0.23 | −0.11 | 0.13 | −0.12 | −0.20 | 0.18 | 0.09 | −0.24 |
| REIU | −0.10 | −0.07 | 0.04 | −0.07 | −0.14 | 0.06 | 0.06 | −0.14 |
| REIFW | 0.23 | −0.12 | 0.16 | −0.13 | −0.22 | 0.20 | 0.10 | −0.26 |
| REICW | 0.30 | −0.11 | 0.21 | 0.01 | −0.26 | 0.26 | 0.32 | −0.37 |
| REICWF | 0.12 | −0.09 | 0.11 | −0.01 | −0.22 | −0.07 | 0.24 | −0.29 |
| RG | −0.11 | 0.10 | −0.11 | 0.15 | 0.18 | −0.04 | 0.13 | 0.04 |
| RGU | 0.02 | 0.08 | −0.06 | 0.15 | 0.16 | 0.01 | 0.12 | 0.03 |
| RGFW | −0.11 | 0.04 | −0.11 | 0.00 | 0.12 | −0.06 | 0.01 | 0.00 |
| RGCW | −0.09 | 0.01 | −0.10 | 0.00 | 0.08 | −0.03 | 0.08 | −0.09 |
| RGCWF | −0.07 | 0.01 | −0.08 | 0.00 | 0.07 | 0.01 | 0.09 | −0.09 |
1Phenotypic correlations ≤ |0.05| were not different (P > 0.05) from zero.
2MEI = metabolizable energy intake; MBW = mid-test metabolic live-weight; MFW = metabolic final live-weight preslaughter; ECR = energy conversion ratio; REI = residual energy intake; REIU = REI adjusted for ultrasound fat depth; REIFW = residual energy intake using metabolic final live-weight preslaughter; REICW = residual energy intake using carcass weight; REICWF = REICW adjusted for carcass fat; RG = residual gain; RGU = RG adjusted for ultrasound fat depth; RGFW= residual gain using metabolic final live-weight preslaughter; RGCW = residual gain using carcass weight; RGCWF = RGCW adjusted for carcass fat score; UFD = ultrasound fat depth; UMD = ultrasound muscle depth; IMF = intramuscular fat percentage; CW = carcass weight; CC = carcass conformation score; CF = carcass fat score; DD = dressing difference; DP = dressing percentage.
Genetic correlations between the REI traits with both the ultrasound and carcass traits were generally stronger, and in the same direction, as the respective phenotypic correlations (Table 7). Of all REI traits, REICW was the trait most strongly genetically correlated with a reduction in all fat-related traits such as, UFD, carcass fat, and IMF; REICW was also the efficiency trait most strongly genetically correlated with a lighter dressing difference, better dressing percentage and better carcass conformation. Every 10 MJ decrease in REI was genetically associated with a 2.53 kg lighter dressing difference and a 15.23 kg heavier carcass, whereas a 10 MJ decrease in REICW was genetically associated with a 10.80 kg lighter dressing difference and a 6.13 kg heavier carcass. Apart from the correlations between RG and either UFD, UMD, or carcass conformation, the genetic correlations between the RG traits and the ultrasound and carcass traits were not different (P > 0.05) from zero.
Table 7.
Genetic correlations (SE in parentheses) among the performance, efficiency, ultrasound, and carcass traits
| Trait1 | UFD | UMD | IMF | CW | CC | CF | DD | DP |
|---|---|---|---|---|---|---|---|---|
| MEI | 0.40 (0.08) | −0.22 (0.11) | 0.35 (0.15) | 0.41 (0.08) | −0.15 (0.11) | 0.44 (0.09) | 0.76 (0.05) | −0.39 (0.09) |
| ADG | 0.03 (0.10) | 0.09 (0.13) | 0.15 (0.19) | 0.30 (0.11) | 0.11 (0.12) | 0.24 (0.12) | 0.44 (0.10) | −0.13 (0.12) |
| MBW | 0.02 (0.08) | 0.18 (0.10) | 0.14 (0.16) | 0.91 (0.02) | 0.25 (0.09) | 0.19 (0.10) | 0.82 (0.03) | 0.07 (0.09) |
| MFW | 0.13 (0.12) | 0.30 (0.13) | 0.09 (0.17) | 0.91 (0.02) | 0.23 (0.11) | 0.18 (0.11) | 0.85 (0.03) | 0.03 (0.10) |
| ECR | 0.31 (0.10) | −0.23 (0.14) | 0.03 (0.20) | −0.08 (0.13) | −0.16 (0.13) | −0.08 (0.14) | −0.09 (0.13) | −0.02 (0.13) |
| REI | 0.54 (0.07) | −0.44 (0.11) | 0.31 (0.16) | −0.39 (0.10) | −0.48 (0.09) | 0.31 (0.10) | 0.08 (0.11) | −0.50 (0.08) |
| REIU | 0.21 (0.10) | −0.21 (0.13) | −0.01 (0.18) | −0.20 (0.12) | −0.31 (0.12) | 0.08 (0.12) | 0.02 (0.12) | −0.23 (0.11) |
| REIFW | 0.57 (0.11) | −0.38 (0.11) | 0.38 (0.18) | −0.38 (0.12) | −0.51 (0.10) | 0.29 (0.12) | 0.14 (0.12) | −0.55 (0.09) |
| REICW | 0.63 (0.10) | −0.36 (0.15) | 0.46 (0.16) | −0.20 (0.12) | −0.56 (0.10) | 0.37 (0.11) | 0.40 (0.11) | −0.66 (0.08) |
| REICWF | 0.33 (0.13) | −0.32 (0.16) | 0.23 (0.19) | −0.15 (0.13) | −0.48 (0.11) | −0.02 (0.13) | 0.30 (0.12) | −0.51 (0.10) |
| RG | −0.24 (0.10) | 0.27 (0.13) | −0.03 (0.21) | 0.15 (0.13) | 0.29 (0.13) | 0.05 (0.14) | 0.04 (0.13) | 0.14 (0.12) |
| RGU | −0.06 (0.11) | 0.05 (0.15) | 0.15 (0.22) | 0.24 (0.14) | 0.24 (0.14) | 0.15 (0.15) | 0.22 (0.14) | 0.01 (0.14) |
| RGFW | −0.12 (0.13) | 0.07 (0.15) | −0.09 (0.18) | −0.15 (0.12) | 0.09 (0.11) | −0.04 (0.12) | −0.09 (0.11) | −0.01 (0.11) |
| RGCW | −0.07 (0.12) | 0.04 (0.15) | −0.04 (0.18) | −0.14 (0.12) | 0.04 (0.12) | 0.01 (0.12) | 0.00 (0.11) | −0.10 (0.11) |
| RGCWF | −0.02 (0.13) | 0.04 (0.15) | −0.01 (0.19) | −0.14 (0.12) | 0.03 (0.12) | 0.05 (0.12) | 0.02 (0.11) | −0.12 (0.11) |
1MEI = metabolizable energy intake; MBW = mid-test metabolic live-weight; MFW = metabolic final live-weight preslaughter; ECR = energy conversion ratio; REI = residual energy intake; REIU = REI adjusted for ultrasound fat depth; REIFW = residual energy intake using metabolic final live-weight preslaughter; REICW = residual energy intake using carcass weight; REICWF = REICW adjusted for carcass fat; RG = residual gain; RGU = RG adjusted for ultrasound fat depth; RGFW= residual gain using metabolic final live-weight preslaughter; RGCW = residual gain using carcass weight; RGCWF = RGCW adjusted for carcass fat score; UFD = ultrasound fat depth; UMD = ultrasound muscle depth; IMF = intramuscular fat percentage; CW = carcass weight; CC = carcass conformation score; CF = carcass fat score; DD = dressing difference; DP = dressing percentage.
Discussion
Residual feed intake is a popular measure of feed efficiency in cattle and is often defined as an animal’s actual feed intake minus its predicted feed intake estimated from a multiple regression of feed intake on ADG, metabolic live-weight, and sometimes a measure of body fat. Coyne et al. (2019) documented phenotypic and genetic correlations of 0.92 and 0.93, respectively between live-weight immediately preslaughter and carcass weight in young cattle, and although these are strong correlations, they are both different (P < 0.001) from one suggesting these are not the same trait. As such, RFI defined using metabolic live-weight may not be an entirely true reflection of efficiency in systems where animals are being produced for slaughter, as the ability to convert energy into live-weight gain does not necessarily equate to carcass gain. Besides gut fill in the intestinal tract, the nonequivalence between live-weight gain and carcass gain may be partly due to the different rates of change in the proportions of fat, bone, and muscle, and morphological differences in internal organ size between animals (Albertí et al., 2008). The main objective of the present study was to evaluate other REI-type metrics by replacing metabolic live-weight (which producers of finishing cattle are not paid on) with carcass weight (for which they are paid) in the regression equation used to calculate REI and RG. Also of interest were the phenotypic and genetic correlations between the said efficiency metrics and carcass traits. The justification of the newly defined efficiency traits was to identify animals that partition a greater proportion of their daily energy intake into actual kilograms of carcass, as opposed to kilograms of animal live-weight.
Of the previous studies that have documented the genetic and phenotypic relationships between feed efficiency and carcass traits across multiple breeds of growing cattle (Hoque et al., 2006; Mao et al., 2013; Torres-Vázquez et al., 2018; Taussat et al., 2019), the present study is one of the largest. Furthermore, the present study is the first to relate measures of feed efficiency with dressing difference, defined as the final preslaughter live-weight of an animal minus the carcass weight (Coyne et al., 2019), where a larger positive value therefore indicates a heavier dressing difference. Nonetheless, the genetic parameters estimated for all traits in the present study were similar to those already reported in the literature for cattle (Arthur et al., 2001a; Robinson and Oddy, 2004; Bouquet et al., 2010; Berry and Crowley, 2013). Furthermore, the narrow range of heritability estimates (0.36 to 0.50) for the newly defined REI traits in the present study were within the range of estimates reported for REI by Berry and Crowley (2013) in their review of the literature on feed efficiency in growing dairy and beef cattle. Heritability estimates for the carcass traits in the present study, specifically dressing difference and dressing percentage, were greater than estimates for the same traits reported by Coyne et al. (2019) in crossbred cattle. For example, in the present study, the residual variance was 1.6 times lower, and the genetic variance was 2.2 times greater than the respective variances for dressing difference reported by Coyne et al. (2019). All animals in the present study originated from a single herd and batches were all fed and managed to the same standard operating procedure, thus contributing to more uniform management and data recording, reduced residual variance, and greater heritability.
Progressing the Definitions of REI and of RG
Of particular interest in the present study was the substitution of metabolic live-weight with carcass weight as an energy sink in the derivation of REI. Prior to doing this, what was first of interest was the impact of deriving REI using metabolic live-weight immediately preslaughter, rather than the traditionally used mid-test metabolic live-weight (Berry and Crowley, 2013). The near unity phenotypic and genetic correlations between traditional REI and REIFW suggest there was no impact of the latter. To our knowledge, no study in any species has investigated replacing MBW with carcass weight in the multiple regression to derive REI. Therefore, phenotypic correlations among feed intake, ADG, MBW, and carcass weight (Supplementary Material, Table 5) published in a range of studies (Nkrumah et al., 2004; Mao et al., 2013; Torres-Vázquez et al., 2018) were used to estimate the proportion of variation in feed intake explained by both ADG and carcass weight as (Berry and Crowley, 2013):
where R2 is the proportion of variation in the dependent variable explained by the predictor variables, V is the vector of phenotypic correlations between the dependent variable and the predictor variables, and C is the matrix of phenotypic correlations among the predictor variables. In the present study, using the same methodology, replacing MBW with carcass weight to derive REICW explained 5.67 percentage units less of the variance in energy intake compared to REI; this is slightly greater than the range of a reduction of 4.12 (Torres-Vázquez et al., 2018) to 5.47 percentage units (Taussat et al., 2019) in R2 based on the calculations from the parameters reported in the literature. Contrastingly, the proportion of variability in feed intake increased by 0.99 percentage units for Angus steers and 0.14 percentage units for Charolais steers when MBW was replaced by carcass weight using the phenotypic correlations reported by Mao et al. (2013); this was due to the fact that, in contrast to the present study and other studies reviewed, the phenotypic correlation between DMI and carcass weight in both Angus steers and Charolais steers was marginally stronger than the phenotypic correlation between DMI and metabolic live-weight.
Cattle can have a similar MEI, live-weight, and ADG but, if differences in body composition exist, then true differences in net feed efficiency are not realized without including some measure of body composition in the equation to derive REI and RG (Basarab et al., 2003; Savietto et al., 2014). As recommended by Basarab et al. (2003) and Savietto et al. (2014), UFD and its interactions with MBW and ADG were all included in the derivation of REIU to ensure any observed differences in REI were not due to interanimal differences in body fat. For cattle that had an UFD record, the inclusion of UFD and its interactions explained 2.85% more of the phenotypic variation in MEI compared to just MBW and ADG (i.e., traditional REI), which is within the range of the 2 to 4% increase in R2 reported by Arthur et al. (2003) in British bred bulls and heifers, and Basarab et al. (2003) in crossbred steers when ultrasound back-fat measures were included as independent variables to derive RFI. Similarly, cattle with a greater carcass fat cover may be unfairly categorized as less efficient compared to their counterparts that have leaner carcasses and thus, to limit bias in net production efficiency, the inclusion of carcass fat in the equation to derive REICW was justified. The R2 for the REICW model increased from 67.96% to 72.46% when carcass fat score was also included in the multiple regression model, which is marginally greater than the range of the increase in R2 already reported by Arthur et al. (2003) and Basarab et al. (2003) when UFD was used to derive REIU.
Furthermore, some retail markets and beef processors penalize overly fat carcasses (Fisher, 2007); overfat carcasses costs some processors in terms of the labor and waste associated with trimming excess fat off the carcass. Therefore, overfat cattle should be penalized to truly limit bias in net production efficiency defined using REICWF. One such approach is to determine the maximum desired carcass fat score and fix the carcass fat score of overfat cattle to this maximum in the dataset, prior to the calculation of REICWF. In the present study, 111 of the 2,187 animals with carcass data (5.08%) were overfat (had a carcass fat score greater than 9), and the phenotypic correlation between REICWF as defined in the present study and REICWF fixed to a maximum carcass fat was 0.99. Of the 2,187 animals with carcass data, 22.63% (495 out of 2,187 cattle) had a carcass fat score greater than the optimum of 7.12, but, the phenotypic correlation between REICWF as defined in the present study and REICWF fixed to an optimum carcass fat score was 0.98. Therefore, in the present study, there was a negligible impact to ranking animals on production efficiency by penalizing overfat carcasses in the definition of REICWF, whether carcass fat was fixed to an optimum or a maximum specification.
Few studies (Crowley et al., 2011; Torres-Vázquez et al., 2018; Taussat et al., 2019) have examined the phenotypic and genetic relationships between RG and carcass traits, and, to the best of our knowledge, no study has explicitly reported the contribution of measures of body composition to the variability in ADG in the regression model used to derive RG in growing cattle. Nevertheless, the proportion of variation in ADG explained by feed intake, ADG, MBW, and UFD was estimated as described previously based on the phenotypic correlations among feed intake, ADG, MBW, and UFD published in the aforementioned range of studies (Nkrumah et al., 2004; Robinson and Oddy, 2004; Schenkel et al., 2004; Barwick et al., 2009; Mao et al., 2013; Torres-Vázquez et al., 2018). For animals that had both carcass and ultrasound data in the present study, the inclusion of UFD in the derivation of RGU explained an additional 0.83 percentage units of phenotypic variation in ADG compared to just MBW and MEI, which is within the range of the 0.02 (Robinson and Oddy, 2004) to 2.54 percentage unit (Mao et al., 2013) increase in the coefficient of multiple determination estimated from phenotypic correlations reported in the literature for cattle.
Benefits of REI using carcass weight
As the phenotypic and genetic correlations between REI and REICW were 0.95 and 0.94, respectively, some reranking of animals would be expected depending on whether REI or REICW was used to classify animals on efficiency. For example, of the 1,402 bulls in the present study that had both MEI and carcass data, 49 bulls that ranked in the top 20% phenotypically for traditional REI (i.e., 49 out of 280 bulls) did not rank in the top 20% phenotypically for REICW. Furthermore, of the 1,045 bulls that had all of MEI, carcass, and ultrasound fat depth data, 49 bulls that ranked in the top 20% phenotypically for REIU (i.e., 49 out of 209 bulls) did not rank in the top 20% phenotypically for REICWF, indicating the difference between feed efficiency percentiles when using the different REI traits. There was minimal phenotypic reranking of animals based on the alternative definitions of RG, which is expected given the near unity correlations among the different RG traits.
Although several studies have documented the relationships between carcass traits and feed efficiency in cattle (Basarab et al., 2003; Nkrumah et al., 2004; Mao et al., 2013), few have reported correlations between feed efficiency traits and dressing percentage (Jensen et al., 1992; Taussat et al., 2019). The range in phenotypic and genetic correlations between the REI traits and dressing percentage in the present study were of the same sign but all stronger than the phenotypic and genetic correlations between RFI and dressing percentage reported in Charolais bulls (Taussat et al., 2019) and in young bulls sired by Holstein-Friesian or Brown Swiss sires (Jensen et al., 1992). Based on the genetic correlations between the derived REI traits and the carcass traits in the present study, selection on either REI or REICW will, on average, increase carcass weight, reduce dressing difference, and thus increase dressing percentage. However, based on the genetic regression of REI on both carcass weight and dressing difference as well as the genetic regression of REICW on both carcass weight and dressing difference, selection on REICW is expected to increase carcass weight 2.16 times slower and reduce dressing difference 4.3 times faster than selection on REI.
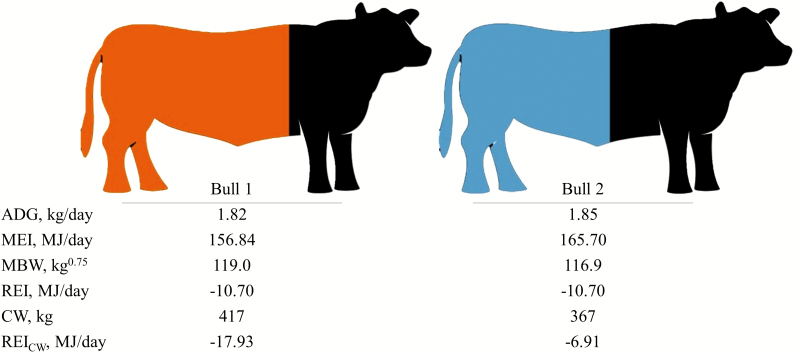
There is no doubt that residual energy intake is a very useful metric in research studies as it depicts the interanimal variability in net feed intake and therefore can be used to rank animals on net feed efficiency for further investigation. However, REI, as currently defined, is not the ideal metric to distinguish interanimal variation in true production efficiency as it does not take into account the carcass weight of the animal which is of greater monetary value to the finishing farmer than live-weight alone. To illustrate this point, 2 bulls were selected from the data used in this study (Fig. 1); both bulls had a similar ADG (1.82 kg/d vs. 1.85 kg/d), a similar MBW (119.0 kg0.75 vs. 116.9 kg0.75), and a similar MEI (156.84 MJ/d vs. 165.70 MJ/d) and were thus ranked equally as efficient using REI (both animals were −10.70 MJ/d). Nonetheless, 1 bull had a 50 kg heavier carcass (417 kg vs. 367 kg) and a 5.99 percentage unit higher dressing percentage (62.80% vs. 56.81%) and was thus differentiated from the second animal in terms of efficiency when using REICW (−17.93 MJ/d vs. −6.91 MJ/d). Additionally, when differences in carcass fat composition were accounted for by using REICWF, the bull with the heavier carcass was still ranked more efficient (−13.46 MJ/d vs. −7.45 MJ/d). Although there may not be a saving in feed costs between the 2 bulls, the bull with the heavier carcass (through better dressing percentage) will generate an extra profit of approximately €200 in comparison to the bull with the lighter carcass, assuming a price of €4.00 per kg carcass and all else being equal.
Figure 1.
Comparison of 2 actual young bulls in the dataset that both have similar energy intake (MEI), metabolic live-weight (MBW), and ADG and thus similar residual energy intake (REI), but have different carcass weights (CW; represented by the colored regions) and therefore different values for residual energy intake using carcass weight (REICW).
At a farmer level, combining carcass data and regular weighing can aid in identifying these production-efficient animals but the difficulties with measuring the live-weight of the animal are that it is generally time consuming for the farmer, the weighing scales may be costly, and the appropriate facilities to restrain and handle animals may not always be in place on farm. Furthermore, variation due to gut fill may inflate the measurement error of the associated weight measurements and result in inaccurate data which in turn may enter the residual component of the statistical model; it is this residual component (i.e., RFI) that is often cited to represent efficiency. A total of 1,018 animals in the present study had a live-weight measure on 2 consecutive days. The standard deviation of the per-animal difference between both live-weight measures was 5.34 kg with a range of −18 to 22 kg; this could be considered variation attributable to gut fill. Including such live-weight data in the models to derive REI and RG may result in these gut fill differences (or simply noise due to weighing) entering the efficiency metrics; using the metabolic live-weight equivalents and the regression coefficient from the REIU model (Supplementary Table 1), such variation accounts for up to 3 MJ/d of metabolizable energy. Nevertheless, deriving metabolic mid-test weight from the intercept and linear regression coefficient of metabolic live-weight measures on days on test minimizes the effect of this live-weight measurement error when modeling REI and RG. While the weight of carcass is likely to also suffer from random noise, it will be less influenced by gut fill; also, from a genetic evaluation perspective, a systematic error in weighing for a given day should enter the contemporary group effect.
In production systems where animals are being reared for slaughter, animals that partition a greater proportion of their daily energy consumption to carcass weight and less so to the dressing difference should be deemed more economically and feed efficient. Animals ranked less efficient (i.e., greater REICW or greater REICWF) partition a greater proportion of their daily MEI to maintain the dressing difference for which producers, in general, receive little to no tangible value (Coyne et al., 2019) and could be considered a measure of economic and production inefficiency. Moreover, while there is a large economic cost to grow and maintain the dressing difference, there is also a large associated carbon cost. Donoghue et al. (2016) reported phenotypic and genetic correlations of 0.61 and 0.86, respectively, between yearling live-weight and daily methane production in Angus cattle. Calculations from the data provided by Donoghue et al. (2016) suggest that a 10 kg increase in yearling live-weight was associated with a 2.47 g increase in daily methane production. Using the phenotypic standard deviation for dressing difference in the present study and assuming a 10 kg increase in dressing difference is associated with a 2.47 g increase in daily methane production, the carbon cost of a heavier dressing difference can be estimated. Animals in the upper 20% for heaviest dressing difference will, on average, produce 11.88 g (i.e., 1.755 standard deviation units × 27.4 kg × 0.247 regression coefficient) more daily methane per animal than animals with the average for dressing difference. This equates to a 1.43 kg increase in methane production per animal over a 120-d finishing period.
In the present study, the relationships between REI type traits has been presented but Van der Werf (2004) illustrated the mathematical equivalence of including a feed efficiency trait such as RFI as a trait in itself in a breeding goal versus including the individual component traits. Hence, assuming all parameters are known, there is no difference between including RFI or its individual component traits in a breeding goal and thus the approach actually undertaken is solely at the discretion of the relevant stakeholders. Terminal beef indexes, however, do not tend to include live-weight but instead include carcass weight (Amer et al., 1998; Connolly et al., 2016; Berry et al., 2019). Therefore, RFI defined using carcass weight maybe a better metric to complement current beef terminal indexes
Conclusions
Residual feed intake is a very useful metric in research studies to depict interanimal variability in net feed intake, but results from the present study suggest that using RFI as a measure of production efficiency is misleading. While the present study used carcass weight (adjusted to a common fat score) as one of the regressor variables in the definition of RFI to better represent true production efficiency, replacing carcass weight with saleable red meat yield or carcass weight weighted by the individual carcass retail cuts may be more appropriate. Judge et al. (2019) documented clear genetic variability in retail carcass cut yields in cattle, even after adjustment to a common carcass weight. The efficiency metric would then depict the ability of an animal to partition more of its energy intake into a higher value carcass. Nevertheless, REICW and REICWF are still useful phenotypic feed efficiency metrics, for example, to rank animals on genetic merit for production efficiency and thus group and feed accordingly; these traits could also be useful to select individuals for breeding lines divergent in net production efficiency. Furthermore, REICW and REICWF also have potential uses as standalone traits, separate to a breeding goal, to market animals as production efficient for producers fattening those animals for slaughter.
Supplementary Data
Supplementary data are available at Journal of Animal Science online.
Supplementary Material Table 1. Partial regression coefficients (standard errors in parentheses) of metabolizable energy intake (MEI) on mid-test metabolic live-weight (MBW), metabolic final live-weight preslaughter (MFW), ADG, ultrasound fat depth (UFD), carcass weight (CW), carcass fat score (CF), and their interactions for each of the respective alternative REI trait models1 within animal sex (bulls tested post-2011, steers, and heifers).
Supplementary Material Table 2. Summary of the number of records used in each bivariate analysis between the performance and the efficiency traits.
Supplementary Material Table 3. Summary of the number of records used each bivariate analysis between the ultrasound and the carcass traits.
Supplementary Material Table 4. Summary of the number of records used in each bivariate analysis of the performance and the efficiency traits with the ultrasound and the carcass traits.
Supplementary Material Table 5. Range of phenotypic correlations among feed intake, metabolic bodyweight, ADG, ultrasound fat depth, and carcass weight extracted from the literature and used in the coefficient of multiple determination calculations. Superscript beside the correlation is the number of studies included within the correlation range.
Footnotes
Funding from the Department of Agriculture, Food and the Marine (Dublin, Ireland) Research Stimulus Fund Ref: 17/S/235 (GreenBreed) is gratefully acknowledged as well as a research grant from Science Foundation Ireland and the Department of Agriculture, Food and the Marine on behalf of the Government of Ireland under the Grant 16/RC/3835 (VistaMilk).
Literature Cited
- Albertí P., Panea B., Sañudo C., Olleta J., Ripoll G., Ertbjerg P., Christensen M., Gigli S., Failla S., and Concetti S.. . 2008. Live weight, body size and carcass characteristics of young bulls of fifteen European breeds. Livest. Sci. 114:19–30. doi: 10.1016/j.livsci.2007.04.010. [Google Scholar]
- Amer P. R., Crump R., and Simm G.. . 1998. A terminal sire selection index for UK beef cattle. Anim. Sci. 67:445–454. doi: 10.1017/S1357729800032859 [DOI] [Google Scholar]
- Arthur P. F., Archer J. A., Johnston D. J., Herd R. M., Richardson E. C., and Parnell P. F.. . 2001a. Genetic and phenotypic variance and covariance components for feed intake, feed efficiency, and other postweaning traits in Angus cattle. J. Anim. Sci. 79:2805–2811. doi: 10.2527/2001.79112805x [DOI] [PubMed] [Google Scholar]
- Arthur P., Herd R. M., and Archer J.. . 2003. Should measures of body composition be included in the model for residual feed intake in beef cattle? Proc. Assoc. Adv. Anim. Breed. Genet.15: 306– 309. [Google Scholar]
- Arthur P. F., Renand G., and Krauss D.. . 2001b. Genetic and phenotypic relationships among different measures of growth and feed efficiency in young Charolais bulls. Livest. Prod. Sci. 68:131–139. doi: 10.1016/S0301-6226(00)00243-8 [DOI] [Google Scholar]
- Barwick S., Wolcott M., Johnston D., Burrow H., and Sullivan M.. . 2009. Genetics of steer daily and residual feed intake in two tropical beef genotypes, and relationships among intake, body composition, growth and other post-weaning measures. Anim. Prod. Sci. 49:351–366. doi: 10.1071/EA08249 [DOI] [Google Scholar]
- Basarab J. A., Price M. A., Aalhus J. L., Okine E. K., Snelling W. M., and Lyle K. L.. . 2003. Residual feed intake and body composition in young growing cattle. Can. J. Anim. Sci. 83:189–204. doi: 10.4141/A02-065 [DOI] [Google Scholar]
- Berry D. P., Amer P. R., Evans R. D., Byrne T., Cromie A. R., and Hely F.. . 2019. A breeding index to rank beef bulls for use on dairy females to maximize profit. J. Dairy Sci. 102:10056–10072. doi: 10.3168/jds.2019-16912 [DOI] [PubMed] [Google Scholar]
- Berry D. P., and Crowley J. J.. . 2013. Cell Biology Symposium: Genetics of feed efficiency in dairy and beef cattle. J. Anim. Sci. 91:1594–1613. doi: 10.2527/jas.2012-5862. [DOI] [PubMed] [Google Scholar]
- Bouquet A., Fouilloux M. N., Renand G., and Phocas F.. . 2010. Genetic parameters for growth, muscularity, feed efficiency and carcass traits of young beef bulls. Livest. Sci. 129: 38–48. doi: 10.1016/j.livsci.2009.12.010 [DOI] [Google Scholar]
- Byerly T. C. 1941. Feed and other costs of producing market eggs. Bull. A1 (Tech.), Univ. Maryland, Agric. Exp. Stn., College Park, MD. [Google Scholar]
- Connolly S. M., Cromie A. R., and Berry D. P.. . 2016. Genetic differences based on a beef terminal index are reflected in future phenotypic performance differences in commercial beef cattle. Animal 10:736–745. doi: 10.1017/S1751731115002827 [DOI] [PubMed] [Google Scholar]
- Coyne J. M., Evans R. D., and Berry D. P.. . 2019. Dressing percentage and the differential between live weight and carcass weight in cattle are influenced by both genetic and non-genetic factors1. J. Anim. Sci. 97:1501–1512. doi: 10.1093/jas/skz056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley J. J., Berry D. P., Mc Hugh N., Kenny D. A., McGee M., Evans R. D., Pabiou T., and Crews D. H. Jr.. 2011. Genetic associations between feed efficiency measured in a performance test station and performance of growing cattle in commercial beef herds. J. Anim. Sci. 89:3382–3393. doi: 10.2527/jas.2011-3836 [DOI] [PubMed] [Google Scholar]
- Crowley J. J., McGee M., Kenny D. A., Crews J. D. H., Evans R. D., and Berry D. P.. . 2010. Phenotypic and genetic parameters for different measures of feed efficiency in different breeds of Irish performance-tested beef bulls. J. Anim. Sci. 88:885–894. doi: 10.2527/jas.2009-1852 [DOI] [PubMed] [Google Scholar]
- Donoghue K. A., Bird-Gardiner T., Arthur P. F., Herd R. M., and Hegarty R. F.. . 2016. Genetic and phenotypic variance and covariance components for methane emission and postweaning traits in Angus cattle. J. Anim. Sci. 94:1438–1445. doi: 10.2527/jas.2015-0065. [DOI] [PubMed] [Google Scholar]
- Durunna O. N., Wang Z., Basarab J. A., Okine E. K., and Moore S. S.. . 2011. Phenotypic and genetic relationships among feeding behavior traits, feed intake, and residual feed intake in steers fed grower and finisher diets. J. Anim. Sci. 89:3401–3409. doi: 10.2527/jas.2011-3867 [DOI] [PubMed] [Google Scholar]
- Englishby T. M., Banos G., Moore K. L., Coffey M. P., Evans R. D., and Berry D. P.. . 2016. Genetic analysis of carcass traits in beef cattle using random regression models. J. Anim. Sci. 94:1354–1364. doi: 10.2527/jas.2015-0246. [DOI] [PubMed] [Google Scholar]
- Fisher A. V. 2007. Beef carcass classification in the EU: an historical perspective. In: Lazzaroni C., Gigli S. and Gabiña D., editors, Evaluation of carcass and meat quality in cattle and sheep. EAAP scientific series no. 123. Wageningen, Netherlands: Wageningen Academic Publishers; p. 19–31. [Google Scholar]
- Gilmour A. R., Gogel B. J., Cullis B. R., and Thompson R.. . 2009. ASReml user guide release 3.0. VSN International Ltd, Hemel Hempstead, UK. [Google Scholar]
- Hoque M. A., Arthur P. F., Hiramoto K., and Oikawa T.. . 2006. Genetic parameters for carcass traits of field progeny and their relationships with feed efficiency traits of their sire population for Japanese Black cattle. Livest. Sci. 100:251–260. doi: 10.1016/j.livprodsci.2005.09.006 [DOI] [Google Scholar]
- Jensen J., Mao I. L., Andersen B. B., and Madsen P.. . 1992. Phenotypic and genetic relationships between residual energy intake and growth, feed intake, and carcass traits of young bulls. J. Anim. Sci. 70:386–395. doi: 10.2527/1992.702386x [DOI] [PubMed] [Google Scholar]
- Judge M. M., Pabiou T., Murphy J., Conroy S. B., Hegarty P. J., and Berry D. P.. . 2019. Potential exists to change, through breeding, the yield of individual primal carcass cuts in cattle without increasing overall carcass weight. J. Anim. Sci. 97:2769–2779. doi: 10.1093/jas/skz152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayser W., and Hill R. A.. . 2013. Relationship between feed intake, feeding behaviors, performance, and ultrasound carcass measurements in growing purebred Angus and Hereford bulls. J. Anim. Sci. 91:5492–5499. doi: 10.2527/jas.2013-6611 [DOI] [PubMed] [Google Scholar]
- Koch R. M., Swiger L. A., Chambers D., and Gregory K. E.. . 1963. Efficiency of feed use in beef cattle. J. Anim. Sci. 22: 486–494. doi: 10.2527/jas1963.222486x [DOI] [Google Scholar]
- Mao F., Chen L., Vinsky M., Okine E., Wang Z., Basarab J., Crews D. H. Jr, and Li C.. . 2013. Phenotypic and genetic relationships of feed efficiency with growth performance, ultrasound, and carcass merit traits in Angus and Charolais steers. J. Anim. Sci. 91:2067–2076. doi: 10.2527/jas.2012-5470 [DOI] [PubMed] [Google Scholar]
- Nkrumah J. D., Basarab J. A., Price M. A., Okine E. K., Ammoura A., Guercio S., Hansen C., Li C., Benkel B., Murdoch B., . et al. 2004. Different measures of energetic efficiency and their phenotypic relationships with growth, feed intake, and ultrasound and carcass merit in hybrid cattle. J. Anim. Sci. 82:2451–2459. doi: 10.2527/2004.8282451x [DOI] [PubMed] [Google Scholar]
- Pabiou T., Fikse W. F., Cromie A. R., Keane M. G., Näsholm A., and Berry D. P.. . 2011. Use of digital images to predict carcass cut yields in cattle. Livest. Sci. 137:130–140. doi: 10.1016/j.livsci.2010.10.012 [DOI] [Google Scholar]
- Polkinghorne R. J., and Thompson J. M.. . 2010. Meat standards and grading: A world view. Meat Sci. 86:227–235. doi: 10.1016/j.meatsci.2010.05.010 [DOI] [PubMed] [Google Scholar]
- Robinson D. L., and Oddy V. H.. . 2004. Genetic parameters for feed efficiency, fatness, muscle area and feeding behaviour of feedlot finished beef cattle. Livest. Prod. Sci. 90: 255–270. doi: 10.1016/j.livprodsci.2004.06.011 [DOI] [Google Scholar]
- Savietto D., Berry D. P., and Friggens N. C.. . 2014. Towards an improved estimation of the biological components of residual feed intake in growing cattle. J. Anim. Sci. 92:467–476. doi: 10.2527/jas.2013-6894 [DOI] [PubMed] [Google Scholar]
- Sauvant D., Perez J. M., and Tran G.. . 2004. Tables of composition and nutritional value of feed materials: pigs, poultry, cattle, sheep, goats, rabbits, horses and fish. Wageningen Academic Publishers, Wageningen, Netherlands. [Google Scholar]
- Schenkel F. S., Miller S. P., and Wilton J. W.. . 2004. Genetic parameters and breed differences for feed efficiency, growth, and body composition traits of young beef bulls. Can. J. Anim. Sci. 84: 177–185. doi: 10.4141/A03-085 [DOI] [Google Scholar]
- Taussat S., Saintilan R., Krauss D., Maupetit D., Fouilloux M. N., and Renand G.. . 2019. Relationship between feed efficiency and slaughter traits of French Charolais bulls. J. Anim. Sci. 97:2308–2319. doi: 10.1093/jas/skz108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Vázquez J. A., van der Werf J. H. J., and Clark S. A.. . 2018. Genetic and phenotypic associations of feed efficiency with growth and carcass traits in Australian Angus cattle. J. Anim. Sci. 96:4521–4531. doi: 10.1093/jas/sky325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Werf J. H. J. 2004. Is it useful to define residual feed intake as a trait in animal breeding programs? Aus. J. Exp. Agric. 44:405–409. doi: 10.1071/EA02105 [DOI] [Google Scholar]
- Van Raden P. M., and Sanders A. H.. . 2003. Economic merit of crossbred and purebred US dairy cattle. J. Dairy Sci. 86: 1036–1044. doi: 10.3168/jds.S0022-0302(03)73687-X [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.