Abstract
Prebiotics and dietary fibers are nondigestible ingredients that may confer benefits to the host by selectively stimulating beneficial intestinal bacteria and microbial-derived metabolites that support gut and host health. This experiment evaluated the effects of a blend of prebiotics and dietary fibers on apparent total tract digestibility (ATTD) and fecal metabolites related to gastrointestinal health in adult dogs. Four diets containing either 5% cellulose (control; CT), 5% dietary fiber and prebiotic blend (FP), 0.02% saccharin and eugenol (SE), or 5% fiber blend plus 0.02% saccharin and eugenol (FSE) were formulated to meet or exceed the AAFCO (2017) nutritional requirements for adult dogs. Eight adult female beagles (mean age 4.2 ± 1.1 yr; mean BW = 10.8 ± 1.4 kg; mean BCS = 5.8 ± 0.6) were randomly assigned to 1 of the 4 dietary treatments using a replicated 4 × 4 Latin square design. Each experimental period consisted of 14 d (10 d of diet adaptation and 4 d of total and fresh fecal and total urine collection). All animals remained healthy throughout the study, with serum metabolites being within reference ranges for adult dogs. All diets were well accepted by the dogs, resulting in similar (P > 0.05) daily food intakes among treatments. Likewise, fecal output and scores did not differ (P > 0.05) among dietary treatments, with the latter being within the ideal range (2.5–2.9) in a 5-point scale. All diets were highly digestible and had similar (P > 0.05) ATTD of dry matter (81.6%–84.4%), organic matter (86.4%–87.3%), and crude protein (86.6%–87.3%). However, total dietary fiber (TDF) digestibility was greater for dogs fed the FSE diet (P < 0.05) in contrast with dogs fed the CT and SE diets, whereas dogs fed FP diets had intermediate TDF digestibility, but not different from all other treatments. Fecal acetate and propionate concentrations were greater (P < 0.05) for dogs fed FP and FSE diets. Fecal concentrations of isobutyrate and isovalerate were greater for dogs fed CT (P < 0.05) compared with dogs fed the other three treatments. No shifts in fecal microbial richness and diversity were observed among dietary treatments. Overall, the data suggest that dietary supplementation of fiber and prebiotic blend was well tolerated by dogs, did not cause detrimental effects on fecal quality or nutrient digestibility, and resulted in beneficial shifts in fecal metabolites that may support gut health.
Keywords: dietary fiber, dogs, gut health, microbiota, nutrient digestibility
Introduction
It is recognized that a nutritionally balanced diet and an appropriate microbial ecology are required for a healthy gut, as the latter assists with colon microenvironment homeostasis, immune system development, gut epithelial function, and systemic host health (Swanson et al., 2002a). As such, dietary strategies to support or maintain gut health maintained interest in both human and animal nutrition. Supplementation of prebiotics and dietary fibers has been a main focal point of research in this field for the past couple decades. Dietary supplementation of gut health promoters has not been studied to any extent in canine nutrition. The scientific literature on this topic is scarce, but the use of dietary additives such as anise oil, eugenol, and sweetener has been evaluated in poultry and swine nutrition.
Dietary fibers are heterogenous compounds and have different physiological benefits depending on their chemical structure and physical properties (e.g., viscosity, solubility, water-holding capacity, and fermentability; Schneeman, 1994). Although there are a few definitions for dietary fibers, in 2016, the Food and Drug Administration (FDA) redefined dietary fiber as “non-digestible soluble and insoluble carbohydrates (with 3 or more monomeric units), and lignin that are intrinsic and intact in plants; isolated or synthetic non-digestible carbohydrates (with 3 or more monomeric units) determined by FDA to have physiological effects that are beneficial to human health.”
In 1995, Gibson and Roberfroid first defined a prebiotic as a “non-digestible food ingredient that beneficially affects the host by selectively stimulating the growth and/or activity of one or a limited number of bacteria in the colon, and thus improves host health.” Ingredients can be classified as prebiotics if they fit the following criteria: 1) resist gastric acidity, hydrolysis by mammalian enzymes, and gastrointestinal absorption; 2) are fermented by the intestinal microbiota; and 3) stimulate selectively the growth and/or activity of intestinal bacteria associated with health and wellbeing (Gibson et al., 2004). More recently, the term prebiotic has been redefined by the International Scientific Association for Probiotics and Prebiotics consensus panel as “a substrate that is selectively utilized by the host microorganisms conferring a health benefit” (Gibson et al., 2017).
The use of eugenol, a polyphenol compound that is the major antimicrobial component present in the oil of cloves (Syzgium aromaticum), has been shown to exert both antimicrobial and anti-inflammatory properties in cell culture, and in vivo in swine and poultry (Friedman et al., 2002; Kim et al., 2003; Thapa et al., 2012; Upadhyay et al., 2017). Additionally, the use of artificial sweeteners has been evaluated in weaned pigs and it resulted in an increase in the abundance of cecal Lactobacillus, even though the mechanism(s) by which they would act are presently unknown (Daly et al., 2014).
Dietary fibers and prebiotics play an important role in the health of companion animals by modulating bowel movement, influencing immune function and gut microbiota profile, diluting caloric density, contributing to weight loss and, indirectly, ameliorating the incidence of obesity and diabetes mellitus in the pet population (de Godoy et al., 2013). Further studies evaluating the effects of artificial sweeteners and eugenol on canine gut health may be beneficial. Not only might they modulate the gut microbiome, but also may serve as a stimulus for mucus production and aid in the protection of the gastrointestinal tract.
Thus, the objective of this research was to evaluate the effects of dietary supplementation of a fiber and prebiotic blend alone or in combination with a food additive containing saccharin and eugenol as potential gut health promoters on parameters related to gastrointestinal health of adult dogs through the determination of apparent total tract macronutrient digestibility, and fecal characteristics, metabolites, and microbial communities.
Materials and Methods
Animals and Experimental Design
All animal procedures were approved by the University of Illinois Institutional Animal Care and Use committee prior to animal experimentation. Eight intact adult female dogs (mean age 4.2 ± 1.14 yr; mean body weight [BW] = 10.8 ± 1.38 kg; mean body condition score [BCS] = 5.6 ± 0.63) were used in a replicated 4 × 4 Latin square design, so each animal served as its own control. Each experimental period consisted of 10 d of diet adaptation and 4 d of total fecal and urine collection. The dogs were housed in a temperature- and light-controlled room (14 h light: 10 h dark) at the Veterinary Medicine Basic Sciences building at the University of Illinois at Urbana-Champaign. Dogs were housed individually (1.2 × 1.8 m) with nose to nose contact with dogs in adjacent runs and visual contact with all dogs in the room. Dog were socialized in groups at least twice a week with toy enrichment.
Dogs were randomly assigned to 1 of the 4 experimental diets and were fed to maintain BW and BCS, which were measured once a week during the experimental period. Food intake was determined based on previous individualized food intake and metabolizable energy (ME) requirement records. Water was available ad libitum and feeding was done twice daily at 0800 and 1600 h. Dogs had access to their assigned food until the next feeding time when food refusals, if present, were collected and recorded. During the collection phase, dogs were housed individually in metabolic cages, given the same access to food and water, and allowed individual social interaction daily.
Diets
Four diets were used: 1) 5% cellulose (CT); 2) 5% fiber and prebiotic blend (containing a mix of cellulose, beet pulp, inulin, mannan-oligosaccharide [MOS], and fructo-oligosaccharide [FOS]; [FP]); 3) 0.02% of saccharin (sweetener [SUCRAM]) and Eugenol (phytomolecule); TAK TIK, Pancosma, Geneva, Switzerland; (SE)]; and 4) 5% fiber and prebiotic blend plus 0.02% of saccharin and eugenol (FSE). Diets were formulated to meet or exceed the AAFCO (2017) nutritional requirements for adult dogs. All 4 experimental diets had similar ingredient composition except for the fiber and prebiotic blend inclusion on the FP and FSE diets, and the saccharin and eugenol inclusion in the SE and FSE diets (Table 2).
Table 2.
Ingredient composition of treatments containing selected fiber sources, prebiotics, and saccharin and eugenol fed to adult dogs
| Ingredient, % as-is | Treatment1 | |||
|---|---|---|---|---|
| CT | FP | FSE | SE | |
| Corn | 36.43 | 36.70 | 36.70 | 36.42 |
| Corn gluten meal | 10.00 | 10.00 | 10.00 | 10.00 |
| Rice | 5.00 | 5.00 | 5.00 | 5.00 |
| Flaxseed | 1.20 | 1.20 | 1.20 | 1.20 |
| Fish oil | 0.50 | 0.50 | 0.50 | 0.50 |
| Poultry fat | 9.00 | 9.00 | 9.00 | 9.00 |
| Poultry byproduct meal | 24.95 | 24.68 | 24.71 | 24.98 |
| Fish meal | 2.00 | 2.00 | 2.00 | 2.00 |
| Sodium chloride | 0.40 | 0.40 | 0.40 | 0.40 |
| Fiber and prebiotic blend | 0.00 | 2.83 | 2.83 | 0.00 |
| Brewer's yeast | 0.50 | 0.50 | 0.50 | 0.50 |
| Cellulose powder | 5.00 | 2.18 | 2.18 | 5.00 |
| Sodium hexametaphosphate | 0.30 | 0.30 | 0.30 | 0.30 |
| Yucca extract | 0.10 | 0.10 | 0.10 | 0.10 |
| Potassium chloride | 0.60 | 0.60 | 0.60 | 0.60 |
| Palatant − liquid | 2.00 | 2.00 | 2.00 | 2.00 |
| Vitamins mix | 0.46 | 0.46 | 0.46 | 0.46 |
| Minerals and trace elements mix | 0.41 | 0.41 | 0.41 | 0.41 |
| Palatant − powder | 1.15 | 1.15 | 0.00 | 0.00 |
| Palatant + saccharin and eugenol − powder | 0.00 | 0.00 | 1.13 | 1.13 |
1CT = Control; FP = fiber and prebiotic blend; SE = saccharin and eugenol additive; FSE = fiber and prebiotic blend + saccharin and eugenol additive.
Total Fecal and Urine Collection
Throughout the 4 d of total fecal and urine collections, all feces were collected and scored using the following 5-point scale: 1 = hard, dry pellets; small hard mass; 2 = hard formed, remains firm and soft; 3 = soft, formed and moist stool, retains shape; 4 = soft, unformed stool; assumes shape of container; 5 = watery, liquid that can be poured. All individual fecal samples identified by dog and period were stored at −20 °C until analysis. Similarly, total urine output was collected simultaneously with fecal collections, into vessels containing 10 mL 2 N hydrochloric acid for immediate acidification of samples. The volume and weight of acidified urine samples were recorded and approximately 25% of each sample was saved and stored frozen. Composited urine samples by dog and period were stored in separate containers and kept at −20 °C until analysis.
Fresh Fecal Collection
Within the 4-d fecal collection period, one fresh fecal sample from each dog was collected within 15 min of defecation and analyzed for dry matter (DM), phenols and indoles, short-chain fatty acids (SCFA), branched-chain fatty acids (BCFA), and microbiota. A pH reading, fecal score, and total sample weight also were recorded. Dry matter was measured by drying approximately 2 g of feces in duplicate in a 105 °C oven until all moisture was removed. Approximately 2 g of feces in duplicate were stored in plastic tubes covered in parafilm and frozen at −20 °C for subsequent indole and phenol analyses. Finally, 5 g of sample were stored in Nalgene bottles containing 5 mL of 2N hydrochloric acid and frozen at −20 °C to determine SCFA, BCFA, and ammonia concentrations.
Blood Collection
Additionally, after overnight fasting, 5 mL of blood were collected via jugular venipuncture from each dog at the end of each experimental period. Serum separator and EDTA vacutainer tubes (Becton, Dickinson and Company, Franklin Lakes, NJ) were used for serum chemistry (4 mL) and complete blood count (1 mL) analyses, respectively. These analyses were conducted by the Clinical Pathology Laboratory at the University of Illinois College of Veterinary Medicine (Urbana, IL).
Sample Preparation and Chemical Analyses
Food and fecal samples were used to determine apparent total tract digestibility (ATTD) of macronutrient digestibility. Fecal samples from each dog and period were pooled and dried at 57 °C in a forced-air oven before grinding in a Wiley Mill with a 10-mesh (2 mm) screen size and used for subsequent laboratory analyses. Diet samples were also ground in the same way for analysis. DM, organic matter (OM), and ash were determined for the diets and feces according to AOAC (2006; methods 934.01 and 942.05, respectively). Total lipid content was determined by acid hydrolysis followed by ether extraction according to the methods of the American Association of Cereal Chemists (1983) and Budde (1952). Crude protein (CP) content of the diets and fecal samples were done by measuring total nitrogen using a LECO TruMac (model 630-300-300) and following the Official Method of AOAC International (2002). Diet and fecal total dietary fiber (TDF) content were analyzed according to Prosky et al. (1992) and the Official Method of AOAC International, 2006 (Methods 985.29 and 991.43). Diet, fecal, and urine samples were analyzed for gross energy (GE) by bomb calorimeter (Model 6200, Parr Instruments Co., Moline, IL). Urine GE values were used to calculate ME.
Fecal SCFA and BCFA concentrations were analyzed using gas chromatography according to the methods of Erwin et al. (1961) and Goodall and Byers (1978). Gas chromatography also was used to measure phenols and indoles as cited in Flickinger et al. (2003). Fecal ammonia concentrations were determined according to the method of Chaney and Marbach (1962).
Statistical Analysis
All data were analyzed using SAS (SAS Institute Inc., version 9.4, Cary, NC), using MIXED Model procedures. The statistical model used diet as a fixed effect and dog as the random effect. Data normality was checked using the PROC UNIVARIATE procedure of SAS. Differences among treatments were determined using a Fisher-protected least significant difference test with a Tukey adjustment to control for type-1 experiment-wise error. A probability of P ≤ 0.05 was accepted as statistically significant and reported pooled standard errors of the mean (SEM) were determined according to the Mixed Models procedure of SAS.
DNA Extraction, Amplification, Sequencing, and Bioinformatics
Total DNA from fresh fecal samples was extracted using Mo-Bio PowerSoil kits (MO BIO Laboratories, Inc., Carlsbad, CA) and DNA concentration was quantified using a Qubit 2.0 Fluorometer (Life technologies, Grand Island, NY). Amplification of the 16S rRNA gene was completed using a Fluidigm Access Array (Fluidigm Corporation, South San Francisco, CA) in combination with Roche High Fidelity Fast Start Kit (Roche, Indianapolis, IN). The primers 515F (5′-GTGCCAGCMGCCGCGGTAA-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′) that target a 291 bp-fragment of V4 region were used for amplification (primers synthesized by IDT Corp., Coralville, IA; Caporaso et al., 2012). Fluidigm specific primer forward (CS1) and reverse (CS2) tags were added according to the Fluidigm protocol. Fragment Analyzer (Advanced Analytics, Ames, IA) was used to confirm the quality of amplicons' regions and sizes. A DNA pool was generated by combining equimolar amounts of the amplicons from each sample. The pooled samples were then size selected on a 2% agarose E-gel (Life Technologies, Grand Island, NY) and extracted using Qiagen gel purification kit (Qiagen, Valencia, CA). Cleaned size-selected pooled products were run on an Agilent Bioanalyzer to confirm appropriate profile and average size. Illumina sequencing was performed on a MiSeq using v3 reagents (Illumina Inc., San Diego, CA) at the W. M. Keck Center for Biotechnology at the University of Illinois. Fluidigm tags were removed using FASTX-Toolkit (version 0.0.14), and sequences were analyzed using QIIME 1.9.1 (Caporaso et al., 2010). High-quality (quality value ≥ 20) sequence data derived from the sequencing process were demultiplexed. Sequences were then clustered into operational taxonomic units (OTU) using opened-reference OTU picking against the Greengenes 13_8 reference OTU database with a 97% similarity threshold. Singletons (OTUs that were observed fewer than 2 times) and OTUs that had less than 0.01% of the total observation were discarded. A total of 2,174,997 reads were obtained, with an average of 67,968 reads (range = 40,335–88,936) per sample. Rarefaction curves based on observed species and phylogenetic distance whole tree measures plateaued, suggesting sufficient sequencing depth. The data set was rarified to 40,335 reads for analysis of diversity and species richness. Principal coordinates analysis (PCoA) was performed, using both weighted and unweighted unique fraction metric (UniFrac) distances that measured the phylogenetic distance between sets of taxa in a phylogenetic tree as the fraction of the branch length of the tree, on the 97% OTU composition and abundance matrix (Lozupone et al., 2005).
Results
Serum chemistry profiles of dogs fed all 4 diets were within the reference range for adult dogs and did not differ among treatments (P > 0.05; Table 1). Likewise, complete blood count results were normal among all dogs and dietary treatments (data not shown). All dogs remained healthy, without any signs of gastrointestinal discomfort or intolerance. All 4 diets were formulated targeting a similar nutrient profile and to be isonitrogenous and isocaloric (Table 2). Analyzed chemical composition of the experimental diets revealed that while all diets were isocaloric and had similar chemical composition, ash, OM, and AHF concentrations were a few percentage units higher in the CT and SE diets (Table 3). Among the 4 experimental diets, DM content ranged from 93.11% to 94.61%. On a DM basis (DMB), OM was higher in the diets FP and FSE (average 94.9%) compared with CT and SE diets (average 92.6%). In addition, analyzed chemical composition of the experimental diets revealed that CT and SE diets had a higher CP concentration of approximately 30.8% on DMB vs. 26.3% for FP and FSE diets, respectively. Lastly, TDF content of diets also revealed that CT and SE diets had lower TDF (12% and 12.7%, respectively) compared with FP and FSE (13.5% and 15.9%).
Table 1.
Fasted serum chemistry profiles for adult dogs fed diets containing selected fiber sources, prebiotics, and saccharin and eugenol
| Item | Reference range | Treatment1 | SEM2 | |||
|---|---|---|---|---|---|---|
| CT | FP | FSE | SE | |||
| Creatinine, mg/dL | 0.5–1.5 | 0.6 | 0.5 | 0.5 | 0.5 | 0.05 |
| Blood urea nitrogen, mg/dL | 6–30 | 12.5 | 10.6 | 10.5 | 11.7 | 1.03 |
| Total protein, g/dL | 5.1–7.0 | 6.0 | 6.1 | 6.0 | 6.0 | 0.13 |
| Albumin, g/dL | 2.5–3.8 | 3.3 | 3.4 | 3.3 | 3.3 | 0.08 |
| Globulin, g/dL | 2.7–4.4 | 2.8 | 2.7 | 2.7 | 2.7 | 0.09 |
| Calcium, mg/dL | 7.6–11.4 | 10.1 | 10.1 | 10.1 | 10.1 | 0.10 |
| Phosphorus, mg/dL | 2.7–5.2 | 3.9 | 4.0 | 3.9 | 3.9 | 0.18 |
| Sodium, mmol/L | 141–152 | 144.6 | 145.1 | 144.7 | 144.6 | 0.53 |
| Potassium, mmol/L | 3.9–5.5 | 4.5 | 4.5 | 4.3 | 4.3 | 0.10 |
| Sodium/potassium ratio | 28–36 | 32.6 | 32.6 | 33.6 | 33.9 | 0.77 |
| Chloride, mmol/L | 107–118 | 109.5 | 110.0 | 110.2 | 110.2 | 0.76 |
| Glucose, mg/dL | 68–126 | 90.2 | 90.4 | 92.2 | 90.2 | 2.13 |
| Alkaline phosphatase total, U/L | 7–92 | 38.9 | 45.5 | 47.0 | 40.5 | 7.10 |
| Corticosteroid-induced alkaline phosphatase, U/L | 0–40 | 11.1 | 14.1 | 15.0 | 12.4 | 4.83 |
| Alanine aminotransferase, U/L | 8–65 | 22.4 | 23.0 | 22.6 | 25.7 | 2.34 |
| Gamma-glutamyl transferase, U/L | 0–7 | 3.4 | 3.9 | 3.5 | 3.1 | 0.30 |
| Total bilirubin, mg/dL | 0.1–0.3 | 0.2 | 0.2 | 0.2 | 0.2 | 0.02 |
| Creatine kinase, U/L | 26–310 | 96.2 | 112.1 | 90.7 | 102.6 | 15.36 |
| Cholesterol total, mg/dL | 129–297 | 229.6 | 231.1 | 240.5 | 224.0 | 17.78 |
| Triglycerides, mg/dL | 35–154 | 70.9 | 82.9 | 71.9 | 60.7 | 9.21 |
| Bicarbonate (TCO2), mmol/L | 16–24 | 20.9 | 19.7 | 20.4 | 20.9 | 0.44 |
| Anion gap | 8–25 | 18.7 | 19.9 | 18.6 | 18.0 | 0.81 |
1CT = Control; FP = fiber and prebiotic blend; SE = saccharin and eugenol additive; FSE = fiber and prebiotic blend + saccharin and eugenol additive.
2Standard error of the mean.
Table 3.
Chemical composition of treatments containing selected fiber sources, prebiotics, and saccharin and eugenol for adult dogs
| Item | Treatment1 | |||
|---|---|---|---|---|
| CT | FP | FSE | SE | |
| Dry matter, % | 93.1 | 93.1 | 93.9 | 94.6 |
| % DM basis | ||||
| Organic matter | 92.6 | 94.9 | 94.9 | 92.5 |
| Ash | 7.3 | 5.1 | 5.1 | 7.5 |
| Acid hydrolyzed fat | 18.5 | 17.8 | 17.6 | 19.0 |
| Crude protein | 30.8 | 26.2 | 26.3 | 30.7 |
| Total dietary fiber | 12.0 | 13.5 | 15.9 | 12.8 |
| Soluble dietary fiber | 3.2 | 4.7 | 4.8 | 3.7 |
| Insoluble dietary fiber | 8.9 | 8.8 | 11.0 | 9.0 |
| Gross energy, kcal/g | 5.3 | 5.2 | 5.2 | 5.2 |
1CT = Control; FP = fiber and prebiotic blend; SE = saccharin and eugenol additive; FSE = fiber and prebiotic blend + saccharin and eugenol additive.
Daily food intake (DMB), fecal output g/d (as is), fecal output g/d (DMB), and fecal score did not differ (P > 0.05) among treatments (Table 4). Likewise, ATTD of DM, OM, CP, AHF, digestible energy, and metabolizable energy were not affected (P > 0.05) by treatment. However, TDF digestibility was greater for dogs fed the FSE diet in contrast with dogs fed the CT diet (P < 0.05) and SE diet (P < 0.05), whereas dogs fed FP had intermediate TDF digestibility but not different from either FSE, SE, or CT (Table 4).
Table 4.
Food intake, fecal characteristics, and total tract apparent macronutrient digestibility by adult dogs fed dietary treatments containing selected fiber sources, prebiotics, and saccharin and eugenol
| Item | Treatment1 | ||||
|---|---|---|---|---|---|
| CT | FP | FSE | SE | SEM2 | |
| Food intake, g/d (DM basis) | 148.1 | 146.4 | 149.9 | 149.5 | 7.03 |
| Fecal output, g/d (as is) | 64.5 | 62.4 | 62.9 | 65.7 | 5.41 |
| Fecal output, g/d (DM basis) | 27.1 | 23.1 | 23.5 | 26.7 | 1.78 |
| Fecal score3 | 3.0 | 3.0 | 2.7 | 2.8 | 0.29 |
| Digestibility, % | |||||
| Dry matter | 81.6 | 84.3 | 84.4 | 82.1 | 0.92 |
| % DM basis | |||||
| Organic matter | 86.4 | 87.3 | 87.2 | 86.7 | 0.72 |
| Acid hydrolyzed fat | 94.4 | 94.7 | 94.9 | 94.3 | 0.27 |
| Crude protein | 87.2 | 86.8 | 86.5 | 87.3 | 0.73 |
| Total dietary fiber | 25.6b | 36.3ab | 47.0a | 28.3b | 3.29 |
| Digestible energy | 87.4 | 88.1 | 88.1 | 87.7 | 0.65 |
| Digestible energy, kcal/g | 4.6 | 4.6 | 4.6 | 4.6 | 0.03 |
| Metabolizable energy, kcal/g | 4.3 | 4.4 | 4.3 | 4.3 | 0.04 |
1CT = Control; FP = fiber and prebiotic blend; SE = saccharin and eugenol additive; FSE = fiber and prebiotic blend + saccharin and eugenol additive.
2Standard error of the mean.
3Fecal scores: 1 = hard, dry pellets; small hard mass; 2 = hard formed, remains firm and soft; 3 = soft, formed and moist stool, retains shape; 4 = soft, unformed stool; assumes shape of container; 5 = watery, liquid that can be poured.
a,bSuperscripts with different letters in a row represent statistical differences (P < 0.05).
Dogs fed the SE diet had a higher fecal pH (P < 0.05) compared with dogs on FSE diet, whereas dogs fed CT and FP diets did not differ from either SE- or FSE- fed dogs (Table 5). Fecal total SCFA and acetate concentrations were greater (P < 0.05) in dogs fed FP and FSE diets compared with fecal samples of dogs fed CT and SE diets. Similarly, fecal concentrations of propionate was greater (P < 0.5) in FP and FSE treatments compared with SE, with CT being intermediate. Fecal concentration of butyrate did not differ among treatments (P > 0.05). Likewise, total BCFA acid concentrations did not vary among treatments (P > 0.05). However, fecal concentrations of isobutyrate and isovalerate were greater (P < 0.05) for dogs fed CT compared with dogs fed FP, SE, or FSE diets. Fecal concentration of valerate tended (P = 0.063) to be greater for FP compared with SE, and FSE and CT had intermediate values (Table 5). Total phenol and indole concentrations were the highest in CT (P < 0.05) compared with FP, FSE, and SE; FP, FSE, and SE did not differ from each other (P > 0.05). Fecal indole and ammonia concentrations were the greatest (P < 0.05) in CT in contrast with FP, FSE, and SE treatments. However, the latter 3 treatments did not differ from each other (P > 0.05). Lastly, phenol concentrations were not different among the 4 treatments (P > 0.05; Table 5).
Table 5.
Fecal pH and fermentative-end product concentrations of adult dogs fed diets containing selected fiber sources, prebiotics, and saccharin and eugenol
| Item, µmole/g DM | Treatment1 | SEM2 | |||
|---|---|---|---|---|---|
| CT | FP | FSE | SE | ||
| Fecal pH | 6.0ab | 5.8ab | 5.8b | 6.1a | 0.09 |
| Total short-chain fatty acids | 341.6b | 497.0a | 487.5a | 311.0b | 33.49 |
| Acetate | 205.5b | 327.0a | 332.9a | 195.9b | 19.99 |
| Propionate | 100.8ab | 126.3a | 130.5a | 81.1b | 9.56 |
| Butyrate | 35.3 | 36.8 | 39.2 | 34.1 | 4.27 |
| Total branched-chain fatty acids | 18.1 | 14.5 | 14.5 | 14.4 | 1.56 |
| Isobutyrate | 7.6a | 5.5b | 5.3b | 5.6b | 0.51 |
| Isovalerate | 11.2a | 8.3b | 8.0b | 8.2b | 0.80 |
| Valerate | 0.6xy | 0.7x | 0.7xy | 0.5y | 0.09 |
| Ammonia | 131.2a | 98.1b | 108.3b | 105.3b | 8.03 |
| Phenols and Indoles | |||||
| Total Phenols/Indoles | 3.6a | 1.9b | 1.9b | 2.1b | 0.36 |
| Phenol | 1.2 | 0.6 | 0.4 | 0.8 | 0.34 |
| Indole | 2.4a | 1.5b | 1.5b | 1.5b | 0.19 |
1CT = Control; FP = fiber and prebiotic blend; SE = saccharin and eugenol additive; FSE = fiber and prebiotic blend + saccharin and eugenol additive.
2Standard error of the mean.
a,b Superscripts with different letters in a row represent statistical differences (P < 0.05).
x,ySuperscripts with different letters in a row represent trending differences (0.06 < P < 0.10).
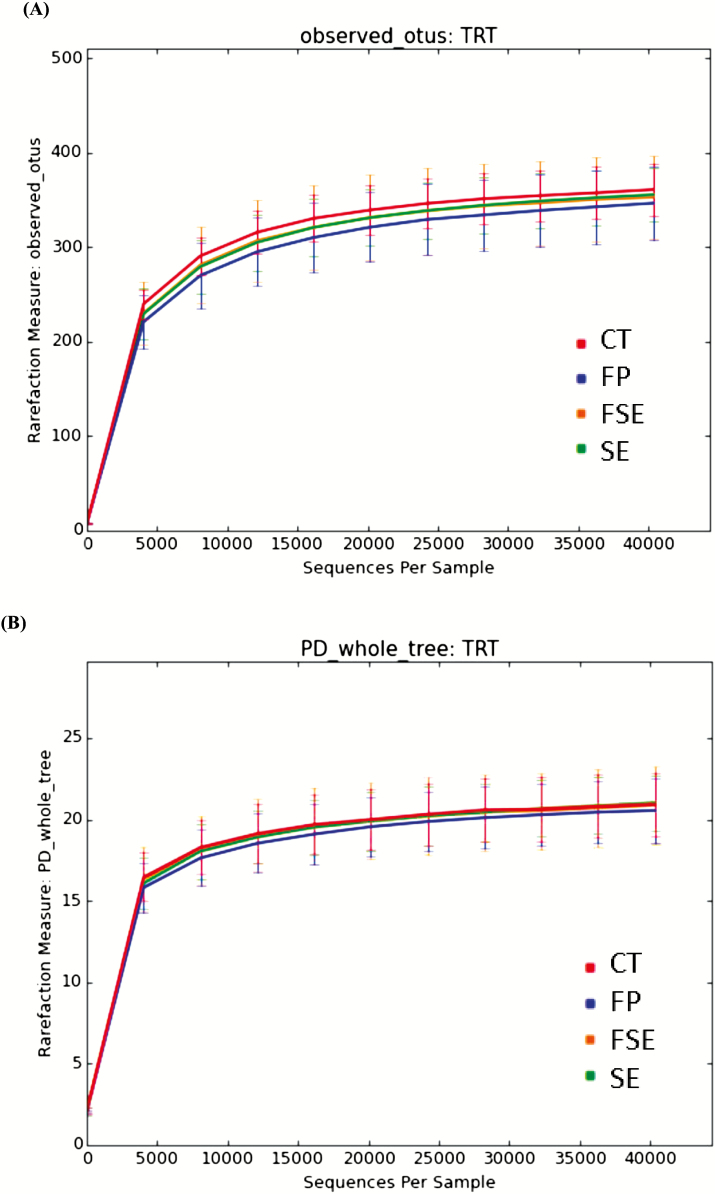
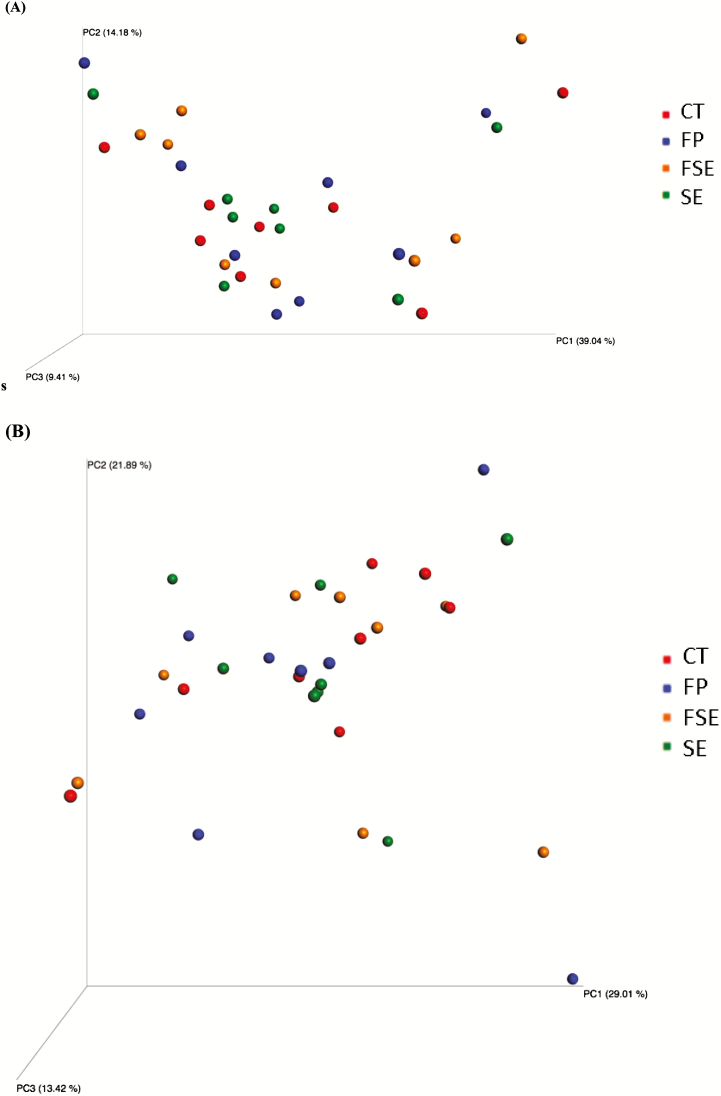
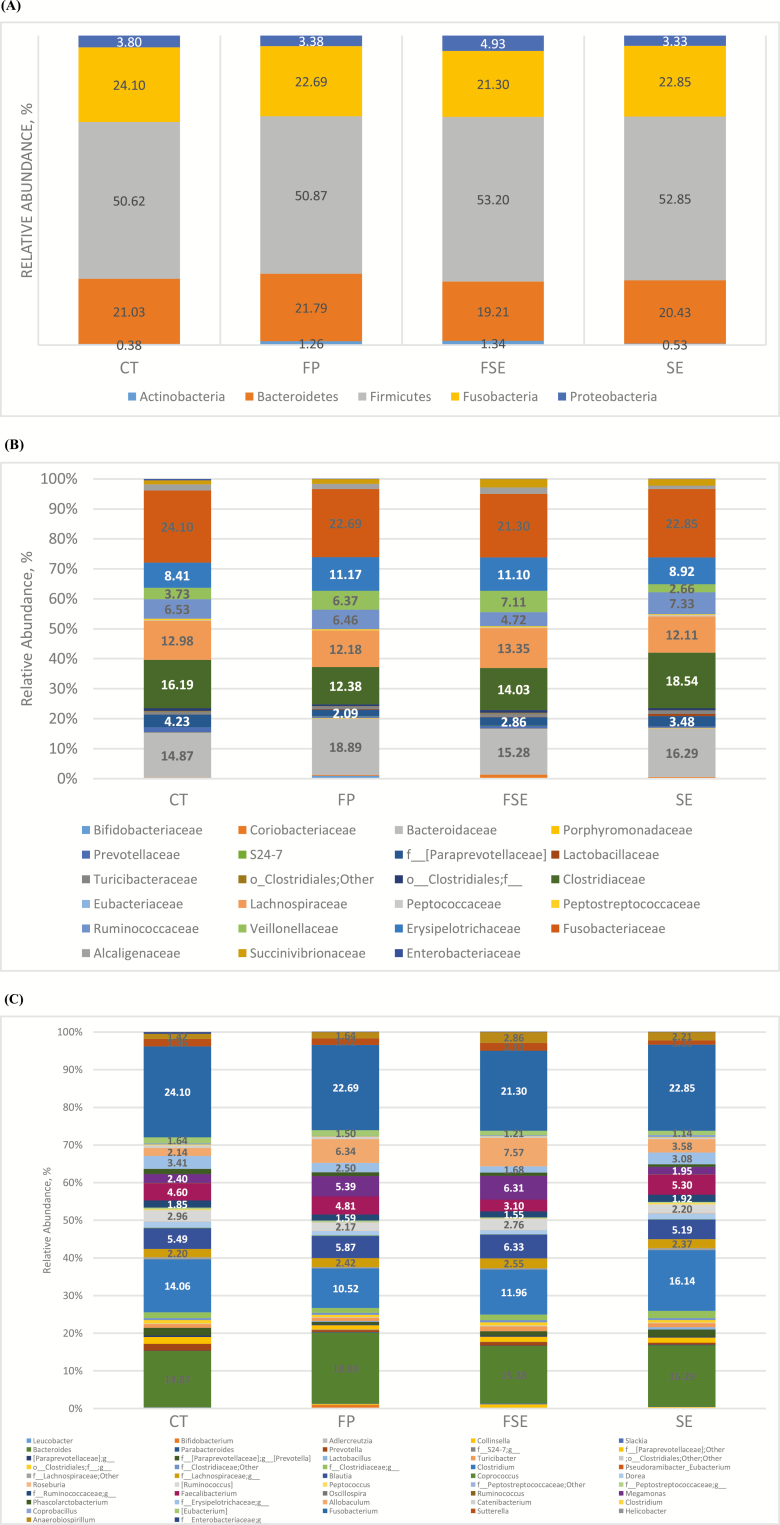
Alpha-diversity measures suggested that dietary supplementation of fiber and prebiotic blend (FP) and (or) saccharin and eugenol additive (SE or FSE) did not affect species richness; observed species at the 97% level OTUs and phylogenetic diversity whole tree matrix (Figure 1A and B, respectively). Likewise, PCoA of weighted and unweighted UniFrac distances performed on the 97% OTU abundance matrix revealed no distinct separation (P > 0.05) on the beta-diversity of gut microbial communities of dogs fed the experimental diets (Figure 2A and B, respectively). Notably, the first 3 axes of the unweighted and weighted PCoA accounted for over 60% of the variation in our study. Greengenes classifier assigned usable raw reads to 6 phyla, 26 families, and 48 genera (Figure 3A–C, respectively).
Figure 1.
Alpha diversity of observed OTUs (A) and phylogenetic distance whole tree (B) of fecal microbial communities of dogs fed diets containing selected fiber sources, prebiotics, and saccharin and eugenol.
Figure 2.
Principal coordinated plots of unweighted (A) and weighted (B) UniFrac distances of fecal microbial communities of dogs fed diets containing selected fiber sources, prebiotics, and saccharin and eugenol.
Figure 3.
Predominant fecal microbial communities at the phyla (A), family (B), and genera (C) levels of dogs fed diets containing selected fiber sources, prebiotics, and saccharin and eugenol.
The most abundant phyla included Firmicutes (52.0% of sequences), Fusobacteria (22.9% of sequences), Bacteroidetes (20.5% of sequences), and Proteobateria (3.8% of sequences). Actinobacteria (0.9%) and Deferribacteres (0.01%) were also present. Inclusion of fiber and prebiotic blend and (or) saccharin and eugenol additive did not alter the proportions of bacterial phyla in the canine fecal microbial community (P > 0.05).
Also, the most abundant families included Veillonellaceae (23.0%) and Bifidobacteriaceae (16.2%), and the relative abundance of both did not differ among treatments (P > 0.05). Relative abundance of Eubacteriaceae was higher in SE compared with FP (P < 0.05). Relative abundance of Peptostreptococcaceae was lower in SE compared with FSE (P < 0.05) and FP (P < 0.05), and CT had an intermediate value.
Finally, the most abundant genera were Fusobacterium (22.9%), Bacteroides (16.2%), and Clostridium (13.2%). There was no significant difference in these genera among the 4 dietary treatments. However, relative abundance of Prevotella was higher in CT compared with FP (P < 0.05). Peptococcus was higher in SE compared with FP (P < 0.05). Megamonas was higher in FSE (P < 0.05) and in FP (P < 0.05) compared with SE. Parabacteroides was higher in FP compared with FSE (P < 0.05). Finally, Peptostreptococcaceae was higher in FSE compared with SE (P < 0.05). Overall, relative abundance of fecal microbial communities was minimally impacted by dietary fiber source, prebiotic, and/or saccharin and eugenol supplementation, despite significant physiological alterations in fecal metabolites. Relative abundance of bacterial phyla, families, and genera affected by dietary treatment of dogs fed diets containing selected fiber sources, prebiotics, and saccharin and eugenol are illustrated in Table 6.
Table 6.
Relative abundance of bacterial phyla, families, and genera affected by dietary treatments of dogs fed diets containing selected fiber sources, prebiotics, and saccharin and eugenol
| Phylum and Family | Genus | Treatment1 | SEM2 | |||
|---|---|---|---|---|---|---|
| CT | FP | FSE | SE | |||
| Firmicutes | ||||||
| Eubacteriaceae | 0.2ab | 0.1b | 0.2ab | 0.4a | 0.11 | |
| Peptostreptococcaceae | 2.4ab | 2.9a | 2.9a | 2.1b | 0.27 | |
| Veillonellaceae | Megamonas | 1.9ab | 2.7a | 2.7a | 1.8b | 0.31 |
| Bacteroidetes | ||||||
| Prevotellaceae | Prevotella | 1.7a | 1.1b | 1.4ab | 1.6ab | 0.24 |
| Porphyromonadaceae | Parabacteroides | 0.8ab | 0.9a | 0.5b | 0.6ab | 0.10 |
1CT = Control; FP = fiber and prebiotic blend; SE = saccharin and eugenol additive; FSE = fiber and prebiotic blend + saccharin and eugenol additive.
2Standard error of the mean.
a,bSuperscripts with different letters in a row represent statistical differences (P < 0.05).
Discussion
Diet, Food Intake, and Fecal Characteristics
All 4 experimental diets were formulated targeting similar nutrient and ingredient composition, except for the source of dietary fiber (i.e., cellulose and beet pulp), prebiotic (i.e., inulin, MOS and FOS), and saccharin and eugenol additive. Both FP and FSE diets had the same sources of dietary fiber (i.e., beet pulp and cellulose) and prebiotics (i.e., inulin, FOS, and MOS); the only difference in the ingredient composition between the 2 diets was the addition of the saccharin and eugenol in the FSE treatment. Likewise, both CT and SE diets had the same sources of dietary fiber (i.e., cellulose) without inclusion of prebiotics in their formulation; the only difference in the ingredient composition between these 2 diets was the addition of the saccharin and eugenol additive in the SE treatment. The fact that the chemical composition of CT and SE was more similar than between FP and FSE could likely be explained by differences in the composition of dietary fibers.
Beet pulp and cellulose are traditional dietary fiber sources used in complete and balanced diets of companion animals. They differ in their chemical composition and physiochemical properties, which affect fiber fermentability and physiological outcomes (de Godoy et al., 2013). Plant byproducts may vary in chemical composition due to several factors. Thus, the variation in TDF concentration among the four diets is expected, especially for the FP and FSE that contained beet pulp as the main dietary fiber source, which is an known ingredient to have a wide variation in TDF content (Fahey et al., 1990a; Sunvold et al., 1995a, 1995c).
In the present study, the comparable chemical composition and caloric density of the 4 experimental diets resulted in similar food intakes among dogs fed these diets. Fahey et al. (1990a) reported that dogs consuming diets with increasing concentrations of beet pulp (from 2.5% to 12.5%) had a linear increase in daily DM intake. In agreement with our results, Swanson et al. (2002a, 2002b) did not find differences in daily food intake by supplementing FOS in extruded diets of adult dogs. More recently, Bosch et al. (2009) researched the effects of dietary fiber type on satiety-related hormones and voluntary food intake by dogs and reported that dogs fed high fermentable fiber diet tended to ingest less food than dogs fed a low fermentable fiber diet. In contrast with their findings, different fiber types of FP and FSE diets did not affect food intake.
Supplementation of fiber and prebiotic blend and (or) saccharin and eugenol did not affect fecal scores, and these were all within the acceptable range of 2.5 to 2.9 on a 5-point scale. Similarly results were reported by Kröger et al. (2017) in dogs fed 3 different diets containing either 12% sugar beet pulp (TDF 13.1% DM), 2.7% sugar beet pulp (TDF 5.71% DM), and 2.7% lignocellulose (TDF 6.72% DM). Additionally, supplementation of FOS or inulin did not affect fecal score of dogs (Swanson et al., 2002a; Alexander et al., 2018). Finally, as-is and DM fecal output did not differ in dogs fed the 4 treatments; however, FP and FSE treatments had the lowest numerical values, likely due to the greater concentration of soluble fibers and TDF digestibility. Beet pulp can increase fecal output at high levels of inclusion (Fahey et al., 1992).
Apparent Total Tract Macronutrient and Energy Digestibility
Nutrient digestibility is an important factor to be considered when adding fiber to a diet, since different fiber sources have been shown to affect nutrient digestibility depending on their quality and quantity (Fahey et al. 1990a, 1990b; Lewis et al., 1994; Silvio et al., 2000). Soluble fibers are generally more fermentable and better energy substrates for gastrointestinal microorganisms and the host than insoluble fibers. In 1996, Zentek reported a lower apparent digestibility of DM for dogs fed diets containing cellulose (insoluble fiber source; 80.3%) compared with pectin (soluble fiber source; 89.6%) and guar gum (solube fiber source; 88.1 ± 1.3%). However, in the experiment, the difference between soluble and insoluble fiber among treatments was greater (approximately 70% soluble fiber for pectin and guar gum diets and 5% for the cellulose diet) than in the present study, which could explain why digestibility of DM, OM, CP, and AHF from CT and SE did not differ from FP and FSE. Fahey et al. (1990a) fed dogs diets containing increasing inclusion levels of beet pulp and concluded that increasing beet pulp concentrations in the diet decreased linearly ATTD of DM, OM, and AHF. Similar results were reported by Kroger et al. (2017).
Although insoluble and poorly fermentable fibers may reduce transit time and total tract nutrient digestibility, highly fermentable oligosaccharides appear to have a smaller impact. Similar to our findings, Strickling et al. (2000) and Swanson et al. (2002b) reported no differences on ileal ATTD of DM and CP in dogs supplemented with prebiotics. In contrast, Zentek et al. (2002) reported lower digestibility of DM, CP, and N-free extract by dogs supplemented with MOS (1 g/kg BW/d) compared with other dietary treatments (i.e., transgalactooligosaccharides, lactose, or lactulose).
The greater TDF digestibility by dogs fed the FSE diet is not surprising, since this treatment contained the highest concentration of soluble dietary fiber. However, this finding also suggests a synergistic effect between the fiber and prebiotic blend with the saccharin and eugenol, enhancing microbial activity and improving fiber degradation by gut microbiota. As expected, dogs fed the CT and SE diets had the lowest TDF digestibility due to the greater ratio of insoluble to soluble fiber (8.9:3.2 and 9.0:3.7, respectively). Insoluble fibers are less fermentable in the large intestine of monogastric animals and they can accelerate digesta passage and relieve constipation (i.e., laxative effect). Sunvold et al. (1995a) evaluated single source and blends of dietary fibers in dog foods; they reported a wide range of TDF digestibilities depending on fiber source. Dietary fibers may affect satiety and dilute caloric density of the diet, consequently, decreasing digestible energy (DE) and ME of diets (Wenk 2001; Weber et al., 2007). The experimental diets tested herein, however, were isocaloric and had comparable ingredient and chemical composition, except for the dietary fiber composition, which were likely to result in similar daily food intake, and ATTD of DM, OM, CP, AHF, DE, and ME among treatments.
Fecal Fermentative End-Products and Serum Chemistry
Fecal fermentative end-products were affected by treatments. When dietary fiber enters the large intestine, microbial enzymes act on this substrate, resulting in the production of SCFA, which are the major end-products of microbial activity and saccharolytic fermentation. The chemical and physical characteristics of dietary fibers may modify the intestinal microbiota or its metabolic activity by influencing the fermentation pattern of SCFA (Sunvold et al., 1995a; Zentek, 1996). In the present study, fecal acetate, propionate, and butyrate concentrations represented 64.8%, 25.9%, and 9.3% of total SCFA, respectively. Swanson et al. (2002b) reported similar concentrations for dogs supplemented with 1 g of FOS and 1 g of MOS. In support of our findings, previous literature showed that supplementation of fermentable fibers might increase fecal concentrations of SCFA. Silvio et al. (2000) compared the use of cellulose (slowly fermented fiber) and pectin (soluble, rapidly fermented fiber) in dogs and reported that ileal concentrations of acetate increased and propionate decreased as pectin increased in the diet. Alexander et al. (2018) reported a total SCFA of 408.9 µmol/g when dogs were supplemented with 1% inulin, suggesting that a high dose of inulin-type prebiotic may modulate fecal metabolites.
Production of SCFA by microbial fermentation in the hindgut also lowers luminal pH and creates an environment less favorable for pathogenic species to flourish (Swanson et al., 2002b). In the present study, dogs that were fed diets containing fiber and prebiotic blend (FP and FSE) had greater total fecal SCFA concentration and lower fecal pH. Increased production, absorption, and metabolism of SCFA in the hindgut of dogs fed the fiber and prebiotic blend may support gut and host health. However, determination of fecal SCFA concentration is not an accurate representation of the total SCFA production in the hindgut, but it has been used and accepted as a proxy measurement in noninvasive animal studies. In addition, prebiotics are rapidly fermented by colonic bacteria, which may affect the microbial populations and metabolites in the proximal colon without many effects on microbial populations and/or metabolites in lower regions of the large bowel or in feces (Swanson et al., 2002c).
Ammonia, phenols and indoles, and BCFA are putrefactive components that are derived from protein fermentation (Miner and Hazen, 1969). These compounds cause foul-smelling feces, which can be an unappealing quality to a diet from the pet owner's point of view (O'Neill and Phillips, 1992). Silvio et al. (2000) reported higher concentrations of ammonia in dogs when cellulose was used as the main dietary fiber source compared with dogs fed a pectin-rich diet. Increased fecal ammonia concentration in dogs fed diets containing primarily cellulose may indicate higher protein fermentation. In the present study, the hypothesis of higher protein fermentation matches with the findings related to fecal BCFA concentrations, which were greater in dogs fed the CT diet. Another interesting finding is that dogs receiving the SE diet had similar concentrations of fecal ammonia and branched-chain fatty acids as dogs fed the FP and FSE diets. This finding suggests a beneficial effect of the saccharin and eugenol on substrate utilization by gut microbes that warrants further evaluation.
As was mentioned previously, undigested proteins in the small intestine are subjected to microbial fermentation in the large intestine, resulting in the formation of putrefactive compounds in the hindgut like phenols, indoles, ammonia, and BCFA. Given the high variability in phenol concentration among samples (SEM = 0.34), no significant differences were observed among treatments. Total indole and phenol concentration was significantly greater in dogs fed the CT diet vs. FP, FSE, and SE diets, which indicates a beneficial effect of the fiber and prebiotic blend and saccharin and eugenol. Similarly, dogs fed the CT diet had greater fecal indole concentrations than dogs fed the other 3 diets. These findings suggest a possible benefit of dietary supplementation of the saccharin and eugenol, since dogs fed the SE diet responded similarly to dogs fed the diets containing the fiber and prebiotic blend (FP and FSE). A potential mechanism that could explain the lower levels of BCFA and phenols and indoles in the SE group is due to its antimicrobial activity, since eugenol has been shown to exert both antimicrobial and anti-inflammatory properties (Friedman et al., 2002; Kim et al., 2003). Swanson et al. (2002a , 2002c) reported that FOS decreased fecal ammonia, BCFA, and total indole and phenol concentrations.
Fecal Microbial Composition
The gastrointestinal microbiota contains a complex population of microorganisms, and their role in health and disease is of importance. Nondigestible fibers such as fructo-oligosaccharides are fed to dogs to modulate microbial communities, increasing the abundance of beneficial taxa (e.g., lactobacillus and bifidobacterium; Garcia-Mazcorro et al., 2017; Redfern et al., 2017). In the present study, however, the inclusion of the fiber and prebiotic blend and (or) the saccharin and eugenol additive did not affect species richness and diversity. Similar to the findings reported by Suchodolski et al. (2008), the most abundant phyla found in the dog fecal microbiota were Firmicutes, Fusobacteria, and Bacteroidetes, which characterize the colon/fecal sample of healthy dogs. Thus, in the present study, supplementation of the blend of prebiotics and fibers and/or the saccharin and eugenol did not affect the proportions of bacterial phyla expected in a healthy canine fecal sample.
Although we hypothesized that supplementation with the fiber blend and prebiotic and the saccharin and eugenol would beneficially shift the fecal microbiota and increase the abundance of beneficial bacteria such as Bifidobacterium spp. and Lactobacillus spp., this was not observed in the present study. Similarly, Alexander et al. (2018) reported that supplementation of inulin-type prebiotic at 1% of the diet did not result in an increased relative abundance of either Bifidobacterium or Lactobacillus spp. In the present study, although phyla were not altered, there was a decrease in the Eubacteriaceae family for dogs fed FP compared with SE. Omori et al. (2017) reported that Eubacteriaceae was increased in dogs that had inflammatory bowel disease and intestinal lymphoma. In the present study, the family Peptostreptococcaceae was lower in dogs fed with SE. Harris et al. (2015) noted Peptostreptococcaceae as one of the most abundant family in gingivitis and mild periodontitis in cats and dogs. Diets containing the saccharin and eugenol (SE) might have a protective effect on the gut mucosa. However, this variable was not evaluated in the current study and warrants further evaluation.
Additionally, the relative abundance of Megamonas was greater in dogs fed FP and FSE diets. Megamonas is a predominant member of the family, Veillonellaceae. Members of Megamonas are known to produce acetic and propionic acids with fermentable fibers as the substrate (Kieler et al., 2017). Moreover, Beloshapka et al. (2013) fed six healthy female adult beagles a raw meat-based (i.e., beef or chicken) diet with or without inulin (1.4%) or yeast cell wall extract (YCW, 1.4%), and reported that dogs consuming diets containing inulin had the highest sequence percentage of Megamonas. Similarly, a study by Hidaka et al. (2008) stated that FOS is utilized by Megamonas. Likewise, Garcia-Mazcorro et al. (2017) reported dogs fed FOS and inulin at approximately 0.1% of DM intake showed a greater abundance of Megamonas. In that study, they also did not report a significant change in the abundance of most bacterial groups in feces of healthy dogs, except for this bacterial genus. In the present study, the higher amounts of fermentable fiber and prebiotic in the diets FP and FSE might explain the increased abundance of Megamonas. This greater abundance of Megamonas was associated with increased fecal concentrations of acetate and propionate in dogs fed the 2 diets. Based on these results, further studies might evaluate the effects of the Veillonellaceae family and/or the Megamonas genus on gastrointestinal health.
Studies about the significance of Prevotella and Parabacteroides in the canine gut health are scarce. However, the genus Prevotella has been related to fermentation of nonstarch polysaccharides and production of SCFA in ileal microbial communities of growing pigs (Ivarsson et al., 2014). In humans, this genus has been reported to synthesize enzymes involved in nonstarch polysaccharide degradation (e.g., glucanase, mannase, and xylanase; Flint and Bayer, 2008). More recently, increased relative abundance of over 44% in Prevotella was observed in fecal samples of healthy piglets as they transitioned from nursing to weaning diets (Guevarra et al., 2018). Those authors suggested the increased abundance of this genus as a possible adaptive strategy to new dietary conditions after weaning when the piglets are being fed diets with greater concentrations of polysaccharide-containing ingredients. A study in rats examining the effect of resistant starch on the gut microbiome and its protective effect against colitis-associated colorectal cancer reported an increased relative abundance of Parabacteroides in rats fed resistant starch. In that same study, resistant starch-fed rats had decreased expression of genes related to inflammation in the colon (Hu et al., 2016). Future studies are needed to explore additional benefits of the fiber blends and prebiotics and (or) saccharin and eugenol canine gut health, and a potential strategy would be integration of analytical tools such as microbiomics and metabolomics to determine possible associations between dietary interventions with modulation of specific microbial taxa and postbiotics at local and systemic levels.
Implications
Based on the results of this study, dogs fed fiber and prebiotic blend and saccharin and eugenol had no negative effects on nutrient digestibility or fecal quality, and had higher TDF digestibilities. In addition, dietary supplementation resulted in beneficial shifts in fecal fermentative end-products that may support gut health, since there was an increase in fecal SCFA concentrations and a decrease in phenols and indoles, BCFA, and ammonia concentrations. Although the test substances resulted in a modest change in fecal microbial communities of healthy adult dogs, it had a significant physiological effect on fecal metabolites, indicating a potentially better microbial fitness in dogs fed diets containing these ingredients. Overall, dietary supplementation of test ingredients were well tolerated by dogs without any indication of gastrointestinal disturbance while conferring potential gut health benefits. Future studies should evaluate similar nutritional strategies in therapeutic diets, focusing on gastrointestinal diseases and obesity.
Acknowledgments
We thank Olivier Capet, Director of Research & Development Petfood, and Neovia Company for the financial support. M.R.C.G. designed the experiment. J.N. and P.M.O. performed the animal trials and laboratory analyses. J.N. and M.R.C.G. performed the statistical analyses. F.H. and H.M. assisted in laboratorial analysis and methods. J.N. wrote the manuscript. All authors have collaborated writing and reviewing this manuscript.
Literature Cited
- Alexander C., Cross T. L., Devendran S., Neumer F., Theis S., Ridlon J. M., Suchodolski J. S., de Godoy M. R. C., and Swanson K. S.. . 2018. Effects of prebiotic inulin-type fructans on blood metabolite and hormone concentrations and faecal microbiota and metabolites in overweight dogs. Br. J. Nutr. 120:711–720. doi: 10.1017/S0007114518001952. [DOI] [PubMed] [Google Scholar]
- American Association of Cereal Chemists (AACC) 1983. Approved methods. 8th ed AACC, St. Paul, MN. [Google Scholar]
- Association of American Feed Control Officials (AAFCO) 2017. Official Publication. AAFCO, Oxford, IN. [Google Scholar]
- Association of Official Analytical Chemists (AOAC) 2006. Official methods of analysis. 17th ed Assoc. Off. Anal. Chem, Gaithersburg, MD. [Google Scholar]
- Beloshapka A. N., Dowd S. E., Suchodolski J. S., Steiner J. M., Duclos L., and Swanson K. S.. . 2013. Fecal microbial communities of healthy adult dogs fed raw meat-based diets with or without inulin or yeast cell wall extracts as assessed by 454 pyrosequencing. FEMS Microbiol. Ecol. 84:532–541. doi: 10.1111/1574-6941.12081. [DOI] [PubMed] [Google Scholar]
- Bosch G., Verbrugghe A., Hesta M., Holst J. J., van der Poel A. F., Janssens G. P., and Hendriks W. H.. . 2009. The effects of dietary fibre type on satiety-related hormones and voluntary food intake in dogs. Br. J. Nutr. 102:318–325. doi: 10.1017/S0007114508149194. [DOI] [PubMed] [Google Scholar]
- Budde E. F. 1952. The determination of fat in baked biscuit type of dog foods J. Assoc. Off. Agric. Chem. 35:799–805. [Google Scholar]
- Caporaso J. G., Kuczynski J., Stombaugh J., Bittinger K., Bushman F. D., Costello E. K., Fierer N., Peña A. G., Goodrich J. K., Gordon J. I., . et al. 2010. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso J. G., Lauber C. L., Walters W. A., Berg-Lyons D., Huntley J., Fierer N., Owens S. M., Betley J., Fraser L., Bauer M., . et al. 2012. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. Isme J. 6:1621–1624. doi: 10.1038/ismej.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaney A. L., and Marbach E. P.. . 1962. Modified reagents for determination of urea and ammonia. Clin. Chem. 8:130–132. [PubMed] [Google Scholar]
- Daly K., Darby A. C., Hall N., Nau A., Bravo D., and Shirazi-Beechey S. P.. . 2014. Dietary supplementation with lactose or artificial sweetener enhances swine gut Lactobacillus population abundance. Br. J. Nutr. 111 (Suppl 1):S30–S35. doi: 10.1017/S0007114513002274. [DOI] [PubMed] [Google Scholar]
- Erwin E. S., March G. J., and Emergy E. M.. . 1961. Volatile fatty acid analysis of blood and rumen fluid by gas chromatography. J. Dairy Sci. 44:1768–1771. [Google Scholar]
- Fahey G. C. Jr, Merchen N. R., Corbin J. E., Hamilton A. K., Bauer L. L., Titgemeyer E. C., and Hirakawa D. A.. . 1992. Dietary fiber for dogs: III. Effects of beet pulp and oat fiber additions to dog diets on nutrient intake, digestibility, metabolizable energy, and digesta mean retention time. J. Anim. Sci. 70:1169–1174. doi: 10.2527/1992.7041169x. [DOI] [PubMed] [Google Scholar]
- Fahey G. C. Jr, Merchen N. R., Corbin J. E., Hamilton A. K., Serbe K. A., and Hirakawa D. A.. . 1990b. Dietary fiber for dogs: II. Iso-total dietary fiber (TDF) additions of divergent fiber sources to dog diets and their effects on nutrient intake, digestibility, metabolizable energy and digesta mean retention time. J. Anim. Sci. 68:4229–4235. doi: 10.2527/1990.68124229x. [DOI] [PubMed] [Google Scholar]
- Fahey G. C. Jr, Merchen N. R., Corbin J. E., Hamilton A. K., Serbe K. A., Lewis S. M., and Hirakawa D. A.. . 1990a. Dietary fiber for dogs: I. Effects of graded levels of dietary beet pulp on nutrient intake, digestibility, metabolizable energy and digesta mean retention time. J. Anim. Sci. 68:4221–4228. doi: 10.2527/1990.68124221x. [DOI] [PubMed] [Google Scholar]
- Flickinger E. A., Schreijen E. M., Patil A. R., Hussein H. S., Grieshop C. M., Merchen N. R., and Fahey G. C. Jr.. 2003. Nutrient digestibilities, microbial populations, and protein catabolites as affected by fructan supplementation of dog diets. J. Anim. Sci. 81:2008–2018. doi: 10.2527/2003.8182008x. [DOI] [PubMed] [Google Scholar]
- Flint H. J., and Bayer E. A.. . 2008. Plant cell wall breakdown by anaerobic microorganisms from the Mammalian digestive tract. Ann. N. Y. Acad. Sci. 1125:280–288. doi: 10.1196/annals.1419.022. [DOI] [PubMed] [Google Scholar]
- Food and Drug Administration 2016. Food labeling: revision of the nutrition and supplement facts labels. Fed. Regist. 81(103):33741–33999. [PubMed] [Google Scholar]
- Friedman M., Henika P. R., and Mandrell R. E.. . 2002. Bactericidal activities of plant essential oils and some of their isolated constituents against Campylobacter jejuni, Escherichia coli, Listeria monocytogenes, and Salmonella enterica. J. Food Prot. 65:1545–1560. doi: 10.4315/0362-028x-65.10.1545. [DOI] [PubMed] [Google Scholar]
- Garcia-Mazcorro J. F., Barcenas-Walls J. R., Suchodolski J. S., and Steiner J. M.. . 2017. Molecular assessment of the fecal microbiota in healthy cats and dogs before and during supplementation with fructo-oligosaccharides (FOS) and inulin using high-throughput 454-pyrosequencing. PeerJ. 5:e3184. doi: 10.7717/peerj.3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson G. R., Hutkins R., Sanders M. E., Prescott S. L., Reimer R. A., Salminen S. J., Scott K., Stanton C., Swanson K. S., Cani P. D., . et al. 2017. Expert consensus document: the International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 14:491–502. doi: 10.1038/nrgastro.2017.75. [DOI] [PubMed] [Google Scholar]
- Gibson G. R., Probert H. M., Loo J. V., Rastall R. A., and Roberfroid M. B.. . 2004. Dietary modulation of the human colonic microbiota: updating the concept of prebiotics. Nutr. Res. Rev. 17:259–275. doi: 10.1079/NRR200479. [DOI] [PubMed] [Google Scholar]
- Gibson G. R., and Roberfroid M. B.. . 1995. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J. Nutr. 125:1401–1412. doi: 10.1093/jn/125.6.1401. [DOI] [PubMed] [Google Scholar]
- de Godoy M. R., Kerr K. R., and Fahey G. C. Jr.. 2013. Alternative dietary fiber sources in companion animal nutrition. Nutrients 5:3099–3117. doi: 10.3390/nu5083099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodall S. R., and Byers F. M.. . 1978. Automated micro method for enzymatic L(+) and D(-) lactic acid determinations in biological fluids containing cellular extracts. Anal. Biochem. 89:80–86. doi: 10.1016/0003-2697(78)90728-5. [DOI] [PubMed] [Google Scholar]
- Guevarra R. B., Hong S. H., Cho J. H., Kim B. R., Shin J., Lee J. H., Kang B. N., Kim Y. H., Wattanaphansak S., Isaacson R. E., . et al. 2018. The dynamics of the piglet gut microbiome during the weaning transition in association with health and nutrition. J. Anim. Sci. Biotechnol. 9:54. doi: 10.1186/s40104-018-0269-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris S., Croft J., O'Flynn C., Deusch O., Colyer A., Allsopp J., Milella L., and Davis I. J.. . 2015. A pyrosequencing investigation of differences in the feline subgingival microbiota in health, gingivitis and mild periodontitis. A. Al-Ahmad, editor. PLoS One. 10:e0136986. doi: 10.1371/journal.pone.0136986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidaka H., Adachi T., and Hirayama M.. . 2008. Development and beneficial effects of fructo-oligosaccharides (Neosugar®). In: Advanced dietary fibre technology. Blackwell Science Ltd, Oxford, UK: p. 471–479. doi: 10.1002/9780470999615 [DOI] [Google Scholar]
- Hu Y., Le Leu R. K., Christophersen C. T., Somashekar R., Conlon M. A., Meng X. Q., Winter J. M., Woodman R. J., McKinnon R., and Young G. P.. . 2016. Manipulation of the gut microbiota using resistant starch is associated with protection against colitis-associated colorectal cancer in rats. Carcinogenesis 37:366–375. doi: 10.1093/carcin/bgw019. [DOI] [PubMed] [Google Scholar]
- Ivarsson E., Roos S., Liu H. Y., and Lindberg J. E.. . 2014. Fermentable non-starch polysaccharides increases the abundance of Bacteroides-Prevotella-Porphyromonas in ileal microbial community of growing pigs. Animal 8:1777–1787. doi: 10.1017/S1751731114001827. [DOI] [PubMed] [Google Scholar]
- Kieler I. N., Shamzir Kamal S., Vitger A. D., Nielsen D. S., Lauridsen C., and Bjornvad C. R.. . 2017. Gut microbiota composition may relate to weight loss rate in obese pet dogs. Vet. Med. Sci. 3:252–262. doi: 10.1002/vms3.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. S., Oh O. J., Min H. Y., Park E. J., Kim Y., Park H. J., Nam Han Y., and Lee S. K.. . 2003. Eugenol suppresses cyclooxygenase-2 expression in lipopolysaccharide-stimulated mouse macrophage RAW264.7 cells. Life Sci. 73:337–348. doi: 10.1016/s0024-3205(03)00288-1. [DOI] [PubMed] [Google Scholar]
- Kröger S., Vahjen W., and Zentek J.. . 2017. Influence of lignocellulose and low or high levels of sugar beet pulp on nutrient digestibility and the fecal microbiota in dogs. J. Anim. Sci. 95:1598–1605. doi: 10.2527/jas.2016.0873. [DOI] [PubMed] [Google Scholar]
- Lewis L. D., Magerkurth J. H., Roudebush P., Morris M. L. Jr., Mitchell E. E., and Teeter S. M.. . 1994. Stool characteristics, gastrointestinal transit time and nutrient digestibility in dogs fed different fiber sources. J. Nutr. 124 (12 Suppl):2716S–2718S. doi: 10.1093/jn/124.suppl_12.2716S. [DOI] [PubMed] [Google Scholar]
- Lozupone C., and Knight R.. . 2005. UniFrac: a new phylogenetic method for comparing microbial communities. Appl. Environ. Microbiol. 71:8228–8235. doi: 10.1128/AEM.71.12.8228-8235.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miner J. R., and Hazen T. E.. . 1969. Ammonia and amines: components of swine building odor. Trans. ASAE 12:772–774. [Google Scholar]
- Omori M., Maeda S., Igarashi H., Ohno K., Sakai K., Yonezawa T., Horigome A., Odamaki T., and Matsuki N.. . 2017. Fecal microbiome in dogs with inflammatory bowel disease and intestinal lymphoma. J. Vet. Med. Sci. 79:1840–1847. doi: 10.1292/jvms.17-0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill D. H., and Phillips V. R.. . 1992. A review of the control of odour nuisance from livestock buildings: Part 3. Properties of the odorous substances which have been identified in livestock wastes or in the air around them. J. Agric. Eng. Res. 53:23–50. [Google Scholar]
- Prosky A., Asp N. G., Schweizer T. F., Devries J. W., and Furda I.. . 1992. Determination of insoluble and soluble dietary fiber in foods and food products: collaborative study. J. AOAC. 75:360–367. [PubMed] [Google Scholar]
- Redfern A., Suchodolski J., and Jergens A.. . 2017. Role of the gastrointestinal microbiota in small animal health and disease. Vet. Rec. 181:370–377. doi: 10.1136/vr.103826. [DOI] [PubMed] [Google Scholar]
- Schneeman B. O. 1994. Carbohydrates: significance for energy balance and gastrointestinal function. J. Nutr. 124 (9 Suppl):1747S–1753S. doi: 10.1093/jn/124.suppl_9.1747S. [DOI] [PubMed] [Google Scholar]
- Silvio J., Harmon D. L., Gross K. L., and McLeod K. R.. . 2000. Influence of fiber fermentability on nutrient digestion in the dog. Nutrition 16:289–295. [DOI] [PubMed] [Google Scholar]
- Suchodolski J. S., Camacho J., and Steiner J. M.. . 2008. Analysis of bacterial diversity in the canine duodenum, jejunum, ileum, and colon by comparative 16S rRNA gene analysis. FEMS Microbiol. Ecol. 66:567–578. doi: 10.1111/j.1574-6941.2008.00521.x. [DOI] [PubMed] [Google Scholar]
- Strickling J. A., Harmon D. L., Dawson K. A., and Gross K. L.. . 2000. Evaluation of oligosaccharide addition to dog diets: Influences on nutrient digestion and microbial populations. Anim. Feed Sci. Technol. 86:205–219. doi: 10.1016/S0377-8401(00)00175-9. [DOI] [Google Scholar]
- Sunvold G. D., Fahey G. C. Jr., Merchen N. R., and Reinhart G. A.. . 1995b. In vitro fermentation of selected fibrous substrates by dog and cat fecal inoculum: influence of diet composition on substrate organic matter disappearance and short-chain fatty acid production. J. Anim. Sci. 73:1110–1122. doi: 10.2527/1995.7341110x. [DOI] [PubMed] [Google Scholar]
- Sunvold G. D., Fahey G. C. Jr., Merchen N. R., Titgemeyer E. C., Bourquin L. D., Bauer L. L., and Reinhart G. A.. . 1995a. Dietary fiber for dogs: IV. In vitro fermentation of selected fiber sources by dog fecal inoculum and in vivo digestion and metabolism of fiber-supplemented diets. J. Anim. Sci. 73:1099–1109. doi: 10.2527/1995.7341099x. [DOI] [PubMed] [Google Scholar]
- Sunvold G. D., Hussein H. S., Fahey G. C. Jr., Merchen N. R., and Reinhart G. A.. . 1995c. In vitro fermentation of cellulose, beet pulp, citrus pulp, and citrus pectin using fecal inoculum from cats, dogs, horses, humans, and pigs and ruminal fluid from cattle. J. Anim. Sci. 73:3639–3648. doi: 10.2527/1995.73123639x. [DOI] [PubMed] [Google Scholar]
- Swanson K. S., Grieshop C. M., Flickinger E. A., Bauer L. L., Chow J., Wolf B. W., Garleb K. A., and Fahey G. C. Jr.. 2002a. Fructooligosaccharides and Lactobacillus acidophilus modify gut microbial populations, total tract nutrient digestibilities and fecal protein catabolite concentrations in healthy adult dogs. J. Nutr. 132:3721–3731. doi: 10.1093/jn/132.12.3721. [DOI] [PubMed] [Google Scholar]
- Swanson K. S., Grieshop C. M., Flickinger E. A., Bauer L. L., Healy H., Dawson K. A., Merchen N. R., and Fahey G. C. Jr. 2002b. Supplemental fructooligosaccharides and mannnanoligosaccharides influence immune function, ileal and total tract nutrient digestibilities, microbial populations and concentrations of protein catabolites in the large bowel of dogs. J. Nutr. 132:980–989. doi: 10.1093/jn/132.5.980. [DOI] [PubMed] [Google Scholar]
- Swanson, K. S., C. M. Grieshop , E. A. Flickinger , N. R. Merchen , and G. C. Fahey, Jr. 2002c. Effects of supplemental fructooligosaccharides and mannanoligosaccharides on colonic microbial populations, immune function and fecal odor components in the canine. J. Nutr. 132:1717S-1719S. doi: 10.1093/jn/132.6.1717S. [DOI] [PubMed] [Google Scholar]
- Thapa D., Losa R., Zweifel B., and Wallace R. J.. . 2012. Sensitivity of pathogenic and commensal bacteria from the human colon to essential oils. Microbiology 158(Pt 11):2870–2877. doi: 10.1099/mic.0.061127-0. [DOI] [PubMed] [Google Scholar]
- Upadhyay, A., K. Arsi, B. R. Wagle, I. Upadhyaya, S. Shrestha, A. M. Donoghue, and D. J. Donoghue. 2017. Trans-cinnamaldehyde, carvacrol, and eugenol reduce campylobacter jejuni colonization factors and expression of virulence genes in vitro. Front. Microbiol. 8:713. doi: 10.3389/fmicb.2017.00713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber M., Bissot T., Servet E., Sergheraert R., Biourge V., and German A. J.. . 2007. A high-protein, high-fiber diet designed for weight loss improves satiety in dogs. J. Vet. Intern. Med. 21:1203–1208. doi: 10.1892/07-016.1. [DOI] [PubMed] [Google Scholar]
- Wenk C. 2001. The role of dietary fibre in the digestive physiology of the pig. Anim. Feed Sci. Technol. 90:21–33. doi: 10.1016/S0377-8401(01)00194-8. [DOI] [Google Scholar]
- Zentek J. 1996. Cellulose, pectins and guar gum as fibre sources in canine diets. J. Anim. Physiol. Anim. Nutr. 75:36–45. [Google Scholar]
- Zentek J., Marquart B., and Pietrzak T.. . 2002. Intestinal effects of mannanoligosaccharides, transgalactooligosaccharides, lactose and lactulose in dogs. J. Nutr. 132 (6 Suppl 2):1682S–1684S. doi: 10.1093/jn/132.6.1682S. [DOI] [PubMed] [Google Scholar]