Abstract
Dietary omega-3 polyunsaturated fatty acids (n-3 PUFA) are precursors for lipid metabolites that reduce inflammation. Two experiments were conducted to test the hypothesis that enriching the sow diet in n-3 PUFA during late gestation and throughout lactation reduces stress and inflammation and promotes growth in weaned pigs. A protected fish oil product (PFO; Gromega) was used to enrich the diet in n-3 PUFA. In the initial experiment, time-bred gilts were fed a gestation and lactation diet supplemented with 0% (control; n = 5), 0.25% (n = 4), 0.5% (n = 4), or 1% (n = 5) PFO from 101 ± 2 d of gestation to day 16 of lactation. Adding 1% PFO to the diet increased the n-3:n-6 PUFA ratio in colostrum and milk compared with controls (P = 0.05). A subsequent experiment was performed to determine whether supplementing the sow diet with 1% PFO improved growth and reduced circulating markers of acute inflammation and stress in the offspring. Plasma was harvested from piglets (16 per treatment group) on day 0 (d of weaning) and days 1 and 3 postweaning. Pigs from the 1% PFO treatment group weighed more (P = 0.03) on day 3 postweaning and had a greater (P ˂ 0.05) n-3:n-6 PUFA ratio in plasma on each day sampled compared with 0% PFO controls. There was an overall treatment effect on plasma total cortisol (P = 0.03) and haptoglobin (P = 0.04), with lesser concentrations in pigs on the 1% PFO diet. Plasma corticosteroid-binding globulin (CBG) concentrations were not different between treatment groups but were less (P ˂ 0.001) on days 1 and 3 when compared with day 0. The resultant free cortisol index [FCI (cortisol/CBG)] was less (P = 0.02) on days 1 and 3 for pigs from the 1% treatment group compared with the controls. An ex vivo lipopolysaccharide (LPS) challenge of whole blood collected on days 0 and 1 was used to determine whether 1% PFO attenuated release of inflammatory cytokines (IL-1β, IL-6, and TNF-α). Blood from pigs within the 1% PFO treatment group tended (P = 0.098) to have a lesser mean concentration of TNF-α in response to LPS compared with blood from controls. These results suggest that providing a PFO supplement as 1% of the diet to sows beginning in late gestation and during lactation can increase the n-3:n-6 PUFA ratio in their offspring, which may improve growth and reduce the acute physiological stress response in the pigs postweaning.
Keywords: fish oil, lactation, pig, sow, supplementation, weaning stress
Introduction
Pigs undergo a great amount of stress within the first 24-h postweaning. Unfamiliar pigs in conjunction with fighting increases stress and activates the inflammatory response which can result in reduced pig health, growth, and feed intake (Campbell et al., 2013). The hypothalamic-pituitary-adrenal (HPA) axis mediates this response in conjunction with proinflammatory cytokines to produce these physiological challenges (Elenkov and Chrousos, 2002; Goshen and Yirmiya, 2009). Dietary supplementation of preweaned pigs with a protected fish oil source (PFO) of omega-3 polyunsaturated fatty acids (n-3 PUFA) has been found to attenuate the inflammatory response in the HPA axis and immune system (Liu et al., 2013), reduce serum concentrations of tumor necrosis factor α (TNF-α) and cortisol (Carroll et al., 2003; Upadhaya et al., 2015), and improve ADG (Liu et al., 2003) following a lipopolysaccharide (LPS) challenge.
Piglets from sows fed diets supplemented with 0.2% PFO in late gestation and lactation had greater BW and BW gains during lactation compared with those fed a control diet (Mateo et al., 2009). Likewise, Rooke et al. (2000, 2001) noted higher pig weight gains at 7-d postweaning when their dams were fed 1.75% tuna oil during either mid or late gestation or lactation.
Therefore, in the present study, 2 experiments were carried out to test the hypothesis that feeding sows n-3 PUFA, in the form of a PFO in late gestation and throughout lactation would reduce the stress and inflammatory responses as well as promote growth in pigs upon weaning. The objective of Experiment 1 was to determine the PFO concentration which, when supplied in the diet to sows during late gestation and throughout lactation, would significantly increase the colostrum and milk n-3:n-6 PUFA ratio. The objective of Experiment 2 was to assess indicators of stress and growth in pigs postweaning from sows fed a diet supplemented with the percentage of PFO determined from Experiment 1.
Materials and Methods
The following experiments were conducted at the University of Kentucky swine facility (UK; Versailles, KY) and University of Tennessee Knoxville Johnson Animal Research and Teaching Unit (UTK JARTU; Knoxville, TN). Animal use and sample collection procedures used in this study were preapproved by the University of Kentucky and University of Tennessee Animal Care and Use Committees. Bred females, maximum of 8 that the JARTU facility can accommodate, are supplied by UK for UTK teaching purposes each Fall semester. On occasion, those females are fed dietary treatments that may be continued through farrowing and lactation at UTK.
Experimental Design, Animals, Housing, and Diets
Experiment 1
Eighteen pregnant gilts [(Yorkshire x Landrace) x Duroc] and [Yorkshire x Landrace]) with an average initial BW of 189.0 ± 11.6 kg (mean ± SD) from the UK swine herd were used to determine a sufficient dietary supplement of a fish oil (PFO) product (Gromega, JBS United, Inc., Sheridan, IN) that would significantly alter the colostrum and milk n-3:n-6 PUFA ratio. The Gromega supplement contained 39.2% fat (by acid hydrolysis) with eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) making up 13.8% and 11.4% of the total fat, respectively. Gilts were bred with purchased semen collected from Krskopolje, Duroc, or Bulgarian White breeds (Swine Genetics International [SGI], Cambridge, IA). Gilts determined to be pregnant and expected to farrow within 72 h of one another, were subject to a completely randomized design (CRD), and selected to receive a gestation and lactation diet supplemented with 0% (n = 5), 0.25% (n = 4), 0.5% (n = 4), or 1% (n = 5) added PFO from 101 ± 2 d of gestation to day 16 of lactation (Tables 1 and 2). All diets were formulated using National Research Council (NRC, 2012) requirements for gestating and lactating sows. Gilts were fed 1.82 kg/d of the gestation diet up to the day of farrowing. Beginning on day of farrowing, sows were fed 3.18 kg/d of the lactation diet and if fully consumed, 0.91 kg/d of feed was added up to a maximum 9.09 kg/d and allowed unlimited access to water through nipple waterers. On 104 ± 2 d of gestation, a tightly grouped subset of 8 females from the pool of bred females, representing an equal number from each PFO dietary treatment, were transported by commercial livestock trailer from UK to UTK JARTU and housed in individual farrowing crates. Farrowing room temperature was thermostatically maintained at 23 °C. All 8 gilts farrowed within a period of 3 d of one another with a litter number ranging between 7 and 15 piglets averaging 1.46 ± 0.51 kg. Piglets were provided supplemental heat via heating pads and processed within 3 d of birth. Processing consisted of spraying the naval cords with Betadine solution (Purdue Products L.P., Stamford, CT), administering 1 mL of iron dextran intramuscularly (INFeD; ACTAVIS, Parsippany, NJ) that provided 200 mg of Fe, clipping needle teeth, ear notching and tagging, tail docking, and castration of the males. Pigs were allowed free access to water and any feed remaining in the sow feeder.
Table 1.
Percentage composition of the gestation diet fed to gilts from late gestation up to farrowing (as-fed basis)
| Percent fish oil product | ||||
|---|---|---|---|---|
| Ingredient, % | 0% | 0.25% | 0.50% | 1% |
| Yellow corn, ground | 83.12 | 82.90 | 82.67 | 82.22 |
| Dehulled soybean meal, 48% CP | 10.05 | 10.03 | 10.00 | 9.95 |
| Alfalfa Meal | 2.50 | 2.50 | 2.50 | 2.50 |
| Choice white grease | 1.00 | 1.00 | 1.00 | 1.00 |
| Dicalcium phosphate | 1.55 | 1.55 | 1.55 | 1.55 |
| Limestone, ground | 0.83 | 0.83 | 0.83 | 0.83 |
| Salt, plain | 0.50 | 0.50 | 0.50 | 0.50 |
| Vitamin mix1 | 0.10 | 0.10 | 0.10 | 0.10 |
| Trace mineral premix2 | 0.05 | 0.05 | 0.05 | 0.05 |
| Choline Mix, 50%3 | 0.10 | 0.10 | 0.10 | 0.10 |
| Santoquin4 | 0.20 | 0.20 | 0.20 | 0.20 |
| Gromega, protected fish oil5 protected fish oil4 | 0.00 | 0.25 | 0.50 | 1.00 |
| Calculated composition | ||||
| ME content of diet, kcal/kg | 3,303 | 3,303 | 3,303 | 3,303 |
| CP, % | 12.11 | 12.11 | 12.11 | 12.11 |
| Lysine, % | 0.54 | 0.54 | 0.54 | 0.54 |
| Calcium, % | 0.75 | 0.75 | 0.75 | 0.75 |
| Phosphorous, % | 0.60 | 0.60 | 0.60 | 0.60 |
1Supplied per kilogram of diet: 6,600 IU vitamin A, 1,320 IU vitamin D3, 66 IU vitamin E, 6.6 mg vitamin K (menadione sodium bisulfate complex), 8.8 mg riboflavin, 22 mg d-pantothenic acid, 88 mg niacin, 6.6 mg vitamin B6, 33 μg vitamin B12, 220 μg d-biotin, and 1,320 μg folic acid.
2Supplied per kilogram of diet: 100 mg Zn as ZnO, 120 mg Fe as FeSO4·H2O, 45 mg Mn as MnO, 12 mg Cu as CuSO4·5H2O, 1.5 mg I as CaI2O6, and 0.30 mg Se as NaSeO3.
3Provided 500 mg/kg of choline chloride to the final diet.
4The Santoquin product (Novus International Inc., St. Louis, MO) supplied 130 mg of ethoxyquin per kilogram of basal diet.
5Gromega product (JBS United, Inc., Sheridan, IN) fatty acid profile was 39.2% total fat (by acid hydrolysis) with myristic (14:0) 8.1%, myristoleic (15:0) 0.76%, palmitic (16:0) 17.08%, palmitoleic [(16:1) 11.85%, (17:0) 0.61%, (17:1) 1.65%], stearic (18:0) 3.18%, elaidic (18:1n-9) 1.68%, oleic (18:1n-9) 5.46%, vaccenic (18:1n-7) 4.15%, linoleic (18:2) 1.56%, linolenic [(18:3) 1.43% and (18:4) 2.85%], arachidic [(20:0) 0.2% and (20:1n-9) 1.01%], arachidonic (20:4n-6) 1.15%, eicosapentaenoic (20:5n-3) 13.75%, docosanoic (22:0) 0.26%, erucic (22:1n-9) 0.22%, docosapentaenoic (22:5n-3) 2.46%, docosahexaenoic (22:6n-3) 11.39%, and nervonic (24:1n-9) 0.46%.
Table 2.
Percentage composition of the lactation diet fed to sows immediately after farrowing up to weaning (as-fed basis)
| Percent fish oil product | ||||
|---|---|---|---|---|
| Ingredient, % | 0% | 0.25% | 0.50% | 1% |
| Yellow corn, ground | 67.56 | 67.33 | 67.11 | 66.66 |
| Dehulled soybean meal, 48% CP | 25.60 | 25.58 | 25.55 | 25.50 |
| Alfalfa Meal | 2.50 | 2.50 | 2.50 | 2.50 |
| Choice white grease | 1.00 | 1.00 | 1.00 | 1.00 |
| Dicalcium phosphate | 1.21 | 1.21 | 1.21 | 1.21 |
| Limestone, ground | 0.89 | 0.89 | 0.89 | 0.89 |
| Salt, plain | 0.50 | 0.50 | 0.50 | 0.50 |
| Vitamin mix1 | 0.10 | 0.10 | 0.10 | 0.10 |
| Trace mineral premix2 | 0.05 | 0.05 | 0.05 | 0.05 |
| Choline Mix, 50%3 | 0.10 | 0.10 | 0.10 | 0.10 |
| Dynamate4 | 0.50 | 0.50 | 0.50 | 0.50 |
| Santoqiun5 | 0.20 | 0.20 | 0.20 | 0.20 |
| Gromega, protected fish oil6 | 0.00 | 0.25 | 0.50 | 1.00 |
| Calculated composition | ||||
| ME content of diet, kcal/kg | 3,290 | 3,290 | 3,290 | 3,290 |
| Crude protein, % | 18.19 | 18.19 | 18.19 | 18.19 |
| Lysine, % | 0.97 | 0.97 | 0.97 | 0.97 |
| Calcium, % | 0.75 | 0.75 | 0.75 | 0.75 |
| Phosphorous, % | 0.60 | 0.60 | 0.60 | 0.60 |
1Supplied per kilogram of diet: 6,600 IU vitamin A, 1,320 IU vitamin D3, 66 IU vitamin E, 6.6 mg vitamin K (menadione sodium bisulfate complex), 8.8 mg riboflavin, 22 mg d-pantothenic acid, 88 mg niacin, 6.6 mg vitamin B6, 33 μg vitamin B12, 220 μg d-biotin, and 1,320 μg folic acid.
2Supplied per kilogram of diet: 100 mg Zn as ZnO, 120 mg Fe as FeSO4·H2O, 45 mg Mn as MnO, 12 mg Cu as CuSO4·5H2O, 1.5 mg I as CaI2O6, and 0.30 mg Se as NaSeO3.
3Provided 500 mg/kg of choline chloride to the final diet.4The Dynamate product (Mosaic Feed Ingredients, South Riverview, FL) contained per kilogram: 180 g of K, 110 g of Mg, and 220 g of S.
5The Santoquin product (Novus International Inc., St. Louis, MO) supplied 130 mg of ethoxyquin per kilogram of basal diet.
6Gromega product (JBS United, Inc., Sheridan, IN) fatty acid profile was 39.2% total fat (by acid hydrolysis) with myristic (14:0) 8.1%, myristoleic (15:0) 0.76%, palmitic (16:0) 17.08%, palmitoleic [(16:1) 11.85%, (17:0) 0.61%, (17:1) 1.65%], stearic (18:0) 3.18%, elaidic (18:1n-9) 1.68%, oleic (18:1n-9) 5.46%, vaccenic (18:1n-7) 4.15%, linoleic (18:2) 1.56%, linolenic [(18:3) 1.43% and (18:4) 2.85%], arachidic [(20:0) 0.2% and (20:1n-9) 1.01%], arachidonic (20:4n-6) 1.15%, eicosapentaenoic (20:5n-3) 13.75%, docosanoic (22:0) 0.26%, erucic (22:1n-9) 0.22%, docosapentaenoic (22:5n-3) 2.46%, docosahexaenoic (22:6n-3) 11.39%, and nervonic (24:1n-9) 0.46%.
Experiment 2
Eight pregnant gilts (Yorkshire and Yorkshire x Landrace) with an average initial BW of 206.9 ± 40.9 kg (mean ± SD) and bred with Duroc Choice Semen purchased from SGI were transported at 105 ± 3 d of gestation from the UK swine facility to UTK JARTU. The gilts were randomly housed in individual farrowing crates where only the 0% (control) and 1% PFO-supplemented diets were used as dietary treatments following the results of Experiment 1. Diets were formulated and fed as described in Experiment 1. All gilts farrowed within 48 h of each other with 8 to 14 piglets per litter and birth weight of 2.16 ± 0.81 kg. Piglets were housed and processed as in Experiment 1 and were not cross-fostered within dietary treatments. One week prior to weaning (day −7), piglets were weighed and provided ad libitum access to their dam’s lactation diet using creep feeders. Upon weaning (31 ± 2 d of age), 32 pigs (8 males/trt and 8 females/trt) were selected from each litter (3 to 5 pigs/litter) of each dietary treatment group based upon a uniform weight (6.25 ± 0.9 kg) taken on day −7 and transferred relative to experimental diet to 1 of 2 nursery pens (3.05 × 3.05 m) located within the farrowing room. Pens contained nipple cup waterers and a large self-feeder providing ad libitum access to either the control or 1% PFO nursery feed consistent with their preweaning dietary treatment group. The 2 dietary treatments were formulated based upon NRC (2012) nutrition requirements for nursery feed (Table 3). All room conditions were the same in both experiments.
Table 3.
Percentage composition of the nursery diet fed to pigs 1 wk prior to weaning and 3 d postweaning (as-fed basis)
| Percent fish oil product | ||
|---|---|---|
| Ingredient, % | 0% | 1% |
| Corn | 49.73 | 48.73 |
| Soybean meal | 31.60 | 31.60 |
| Fish meal | 3.00 | 3.00 |
| Whey dried | 10.00 | 10.00 |
| Grease | 2.30 | 2.30 |
| Corn starch | 0.30 | 0.30 |
| L-Lysine | 0.23 | 0.23 |
| DL-Methionine | 0.24 | 0.24 |
| L-Threonine | 0.18 | 0.18 |
| L-Tryptophan | 0.01 | 0.01 |
| Dicalcium Phosphate | 0.76 | 0.76 |
| Limestone | 0.88 | 0.88 |
| Salt | 0.50 | 0.50 |
| Trace mineral premix1 | 0.15 | 0.15 |
| Vitamin mix2 | 0.10 | 0.10 |
| Santoquin3 | 0.02 | 0.02 |
| Gromega, protected fish oil4 | 0.00 | 1.00 |
| Calculated composition | 0% | 1% |
|---|---|---|
| SID amino acids, % | ||
| Lys | 1.35 | 1.35 |
| Met | 0.56 | 0.56 |
| Ile | 0.87 | 0.87 |
| Thr | 0.94 | 0.94 |
| Trp | 0.26 | 0.26 |
| ME content of diet, kcal/kg | 3,406 | 3,406 |
| CP, % | 22.82 | 22.82 |
| Calcium, % | 0.80 | 0.80 |
| Phosphorous, % | 0.65 | 0.65 |
| Available P, % | 0.40 | 0.40 |
1Supplied per kilogram of diet: 125 mg Zn as ZnSO4·H2O, 100 mg Fe as FeSO4·H2O, 50 mg Mn as MnSO4·H2O, 20 mg Cu as CuSO4·5H2O, 0.35 mg I as CaI2O6, and 0.30 mg Se as NaSeO3.
2Supplied per kilogram of diet: 6,600 IU vitamin A, 1,320 IU vitamin D3, 66 IU vitamin E, 6.6 mg vitamin K (menadione sodium bisulfate complex), 8.8 mg riboflavin, 22 mg d-pantothenic acid, 88 mg niacin, 6.6 mg vitamin B6, 33 μg vitamin B12, 220 μg d-biotin, and 1,320 μg folic acid.
3The Santoquin product (Novus International Inc., St. Louis, MO) supplied 130 mg of ethoxyquin per kilogram of basal diet.
4The Gromega product (JBS United Inc., Sheridan, IN) fatty acid profile was 39.2% total fat (by acid hydrolysis) with myristic (14:0) 8.1%, myristoleic (15:0) 0.76%, palmitic (16:0) 17.08%, palmitoleic [(16:1) 11.85%, (17:0) 0.61%, (17:1) 1.65%], stearic (18:0) 3.18%, elaidic (18:1n-9) 1.68%, oleic (18:1n-9) 5.46%, vaccenic (18:1n-7) 4.15%, linoleic (18:2) 1.56%, linolenic [(18:3) 1.43% and (18:4) 2.85%], arachidic [(20:0) 0.2% and (20:1n-9) 1.01%], arachidonic (20:4n-6) 1.15%, eicosapentaenoic (20:5n-3) 13.75%, docosanoic (22:0) 0.26%, erucic (22:1n-9) 0.22%, docosapentaenoic (22:5n-3) 2.46%, docosahexaenoic (22:6n-3) 11.39%, and nervonic (24:1n-9) 0.46%.
Tissue and Performance Measurements
Colostrum and milk samples ranging from 10 to 45 mL were collected in 50-mL falcon tubes following manual expression from multiple teats within 24 h of farrowing and 16 ± 2 d postfarrowing and were frozen at −80 °C until later analysis.
Blood samples (6 ± 2 mL) were collected via cranial vena cava puncture in heparinized vacutainer tubes (Becton Dickinson Vacutainer Systems; Becton, Dickinson and Company, Franklin Lakes, NJ) from the 32 selected pigs in Experiment 2 immediately prior to weaning (day 0) and days 1 and 3 postweaning. Blood samples were stored on ice and processed within 1 h following collection. Pigs were weighed 1 wk prior to weaning (day −7) and following blood collection on day 3 postweaning. Aliquots of whole blood (1 mL) collected on days 0 and 1 were designated for use in an ex vivo LPS challenge and cytokine assay. The remaining blood samples were centrifuged at 3134 × g for 20 min at 4 °C. Plasma was pipetted into cryogenic vials and stored at −80 °C until assayed for concentrations of cortisol, corticosteroid binding globulin (CBG), haptoglobin, and phospholipid analysis.
Phospholipid Extraction from Colostrum, Milk, and Plasma
Colostrum, milk, and plasma (200 µL) samples were extracted for phospholipid analysis following the procedure of Xiong et al. (2012). Samples were pipetted into 1.5-mL Eppendorf tubes containing 40 µL of internal standard (acetylcholine-d13: 0.0112 g, betaine-d11: 0.0386 g, choline-d9: 0.024 g, lysophosphatidylcholine-d3: 0.005 g, phosphatidylcholine-d9: 0.0096 g, phosphocholine-d9: 0.2404 g, phingomyelin-d3-13C: 0.021 g) dissolved in methanol. A HPLC grade extraction solvent containing chloroform, methanol, and water (1:2:0.8; 1 mL0) was added for a total of 1.24 mL of solution. Tubes were vortexed at 2.5 × g in 4 °C for 5 min and the resulting supernatant transferred to a clear glass vial. The extraction process was repeated 2 more times by adding 1 mL of extraction solvent to the pellet and then vortexed, centrifuged, and transferred to the glass vial. Collected supernatant was dried under a steady stream of nitrogen and re-dissolved in 3 mL of methanol. Solution volumes of 300 µL were pipetted into auto sample vials for phospholipid analysis by LC-MS/MS. The n-3:n-6 PUFA ratio was calculated as total n-3 being 18:3, 20:5, 22:5, 22:6 and n-6 being 20:4 and 18:2. Each total was then divided by the total amount of phosphatidylcholine. Individual PUFA were calculated in the same manner (Smit et al., 2013).
Ex Vivo Whole Blood Stimulation Assay and Cytokine Analysis
Whole blood collected from pigs on days 0 and 1 were designated for use in an ex vivo LPS challenge according to the procedure of Carstensen et al. (2005). Briefly, samples (1.0 mL) of blood were maintained at room temperature and processed within 1 h following collection. The samples were pipetted into micro-centrifuge tubes followed by the addition of 25 µg of LPS E. coli (L4391; Sigma Chemical, St. Louis, MO) diluted in 100-µL PBS (pH 7.4). Centrifuge tubes were placed in a 39 °C shaking water bath for 8 h while inverting tubes every 30 min to prevent separation. Tubes were centrifuged at 2000 × g at 4 °C for 10 min. Plasma was collected and stored at −80 °C until cytokine analysis.
Plasma cytokine concentrations (TNF-α, IL-1β, and IL-6) were determined by a custom porcine 3-plex sandwich-based chemiluminescence ELISA kit (Searchlight-Aushon BioSystems, Inc., Billerica, MA) according to the manufacturer’s directions. The intra-assay CVs from a pooled pig sample were less than 5.5% and inter-assay CVs were less than 3.5% for all assays.
Cortisol Analysis
Plasma samples were analyzed for total cortisol concentration by RIA (MP Biomedicals, LLC, Orangeburg, NY) as reported previously (Adcock et al., 2007). Intra- and inter-assay CV were 8.6% and 3.0% for the low control (41.8 nmol/L) and 5.4% and 8.8% for the high control (163.6 nmol/L) cortisol standards.
Corticosteroid-Binding Globulin Analysis
Plasma CBG concentrations were determined by a direct ELISA, as previously described by Roberts et al. (2003). Intra- and inter-assay CV of a pooled pig plasma sample were 6.1% and 11.6%, respectively.
Free Cortisol Index
The free cortisol index was calculated using the ratio of plasma total cortisol (nmol/L) to CBG (mg/L) concentration (le Roux et al., 2002) and reported in units of nmoL/mg.
Haptoglobin Analysis
Plasma haptoglobin concentration was determined by a radial immunodiffusion (RID) kit (Ecos Institute, Aasahi, Furukawa, Miyagi, Japan). Plasma samples (100 μL) were diluted 5-fold with PBS pH 7.4. Aliquots of diluted sample (5 μL) were pipetted into individual wells on agar test plates then incubated at 37 °C for 24 h. During incubation, a precipitin reaction occurred forming a visible ring in the gel. Measurements of sample ring diameter were performed under UV light. Ring diameter was proportional to the concentration of haptoglobin in the diluted sample. The intra-assay CV was 9.5% for duplicate haptoglobin samples (635 μg/mL). The inter-assay CV was 5.4% for the low (252 μg/mL) and 1.5% for the high (1512 μg/mL) haptoglobin controls.
Statistical Analysis
All data were analyzed using the mixed model ANOVA GLIMMIX procedure in SAS 9.4 (SAS Institute Inc., Cary, NC) with degrees of freedom adjusted using the Kenward–Roger method. Least squares means were compared using least significant difference mean ± SEM. For sow data, treatment and sample (colostrum or milk) were fixed effects in a CRD split-plot model, using sow as the whole-plot experimental unit. Plasma PUFA, cortisol, CBG, haptoglobin, and BW were analyzed utilizing a CRD with repeated measures (day) and AR (1) variance structure for the model. Fixed effects were treatment, sex, and day with pig as the experimental unit for subplot factors. Cytokine data were analyzed using a CRD with a split-split plot. Fixed effects were diet, sex, day, and endotoxin (LPS) or control (PBS). Log transformation was used to analyze cortisol, FCI, and cytokines. For all other variables, normality was not an issue (Shapiro–Wilk > 0.90), there were no outliers or influential points, and equal variance was acceptable. Sex was determined to be nonsignificant and was therefore removed from the model.
Results and Discussion
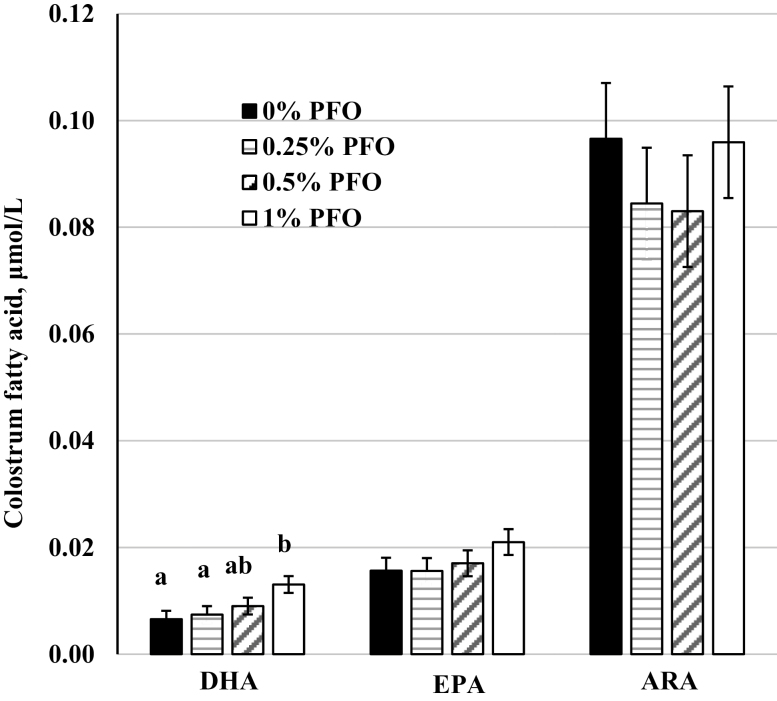
A multitude of fish oil varieties, from tuna oil to cod liver oil, have been used to supplement sow diets during gestation and lactation to decrease the n-6:n-3 PUFA ratio in the milk with varying results (Kim et al., 2006). Fish oil and PFO products, resistant to oxidation, are most common supplements to enhance the concentration of EPA and DHA in colostrum and milk (Horrocks and Yeo, 1999). However, these n-3 PUFA are unstable due to the amount of double bonds and are subject to oxidation reducing the amount of EPA and DHA present. The oxidation of these PUFA can be prevented and is done so by using fish oil protected against oxidation, like Gromega (Cameron-Smith et al., 2015). In the present study, DHA concentration measured in colostrum was greater (P < 0.05) when 1% PFO (Gromega) was added to the basal diet compared with the control or 0.25% PFO supplemented diets (Figure 1). The DHA concentration in the colostrum from sows supplemented with 0.25% and 0.5% PFO diets did not differ from the control. Colostrum EPA and arachidonic acid (ARA) concentrations did not differ (P = 0.36) due to dietary treatment. The concentration of DHA in milk samples collected on days 16 ± 2 postfarrowing tended (P = 0.09) to be greater in 1% PFO supplemented sows compared with that in the controls (data not shown). Milk EPA and ARA concentrations did not differ as a result of treatment. These results were fairly similar to those described by Gabler et al. (2007) as their 1.5% fish oil diet outperformed other similar n-3 PUFA supplements in reducing the n-6:n-3 PUFA ratio. Others have observed similar results with increased DHA and EPA in milk with no effect on ARA content while supplementing with PFO (Arbuckle and Innis, 1993; Rooke et al., 1998).
Figure 1.
Colostrum concentrations of docosahexaenoic acid (DHA), eicosapentaenoic acid (EPA), and arachidonic acid (ARA) from sows receiving a corn-soybean meal based diet supplemented with 0% (n = 5), 0.25% (n = 4), 0.5% (n = 4), or 1% (n = 5) of a protected fish oil product (PFO; Gromega, JBS United Inc., Sheridan, IN) from days 101 ± 2 of gestation to day 16 of lactation and collected within 24 h of farrowing. a,bMeans ± SEM with different letters differ (P = 0.05).
Based upon the results of Experiment 1, the 1% PFO supplemented diet was determined to be the most effective diet in increasing the n-3:n-6 PUFA ratio of the sows colostrum and milk and, as reported by others, this increase can be transferred to the piglet mostly through the milk (Arbuckle and Innis, 1993; Clouard et al., 2015). However, in Experiment 2, the n-3:n-6 PUFA ratio did not differ (P = 0.7) between the 1% PFO and control diet in either the colostrum or milk. A potential explanation for lack of differences seen here maybe due to low replication numbers among treatments. The n-3:n-6 PUFA ratio in the milk samples was noticeably higher between the 1% (0.042) and control (0.037) PFO-supplemented diet; however, this difference was not detectable statistically.
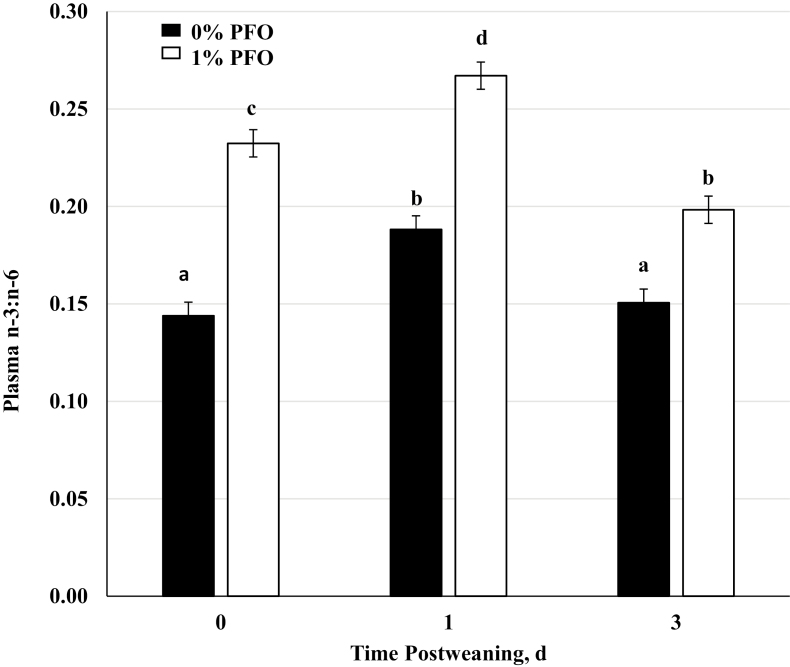
Although the sow colostrum and milk samples did not differ in n-3:n-6 PUFA ratio, the offspring did exhibit detectable differences in plasma n-3:n-6 PUFA ratio measured on day 0 (day of weaning), and days 1 and 3 postweaning (Figure 2). This would suggest that the sow’s milk did incorporate sufficient n-3 PUFA to alter the piglets FA profile. Weaned pigs consuming the 1% PFO diet had a greater (P < 0.001) overall plasma n-3:n-6 PUFA ratio than the control pigs. The difference in n-3:n-6 PUFA ratio for piglet plasma vs. sow milk could be explained by a difference in rate of tissue incorporation of total fatty acid and specific n-3 PUFA. Milk incorporation is 8 g/d of total fatty acid in the sow and pig intake of specific n-3 PUFA is estimated based upon grams of milk consumed and size of the piglet (Gabler et al., 2007). This could also be due to the size of the piglet in relation to the sow, as both obtain relatively the same PUFA. The increase in n-3 PUFA resulting from the effect of the 1% PFO diet represents increased n-3 PUFA in the membrane of cells. The increase in membrane concentration increases the substrate availability for cleavage by phospholipase A2. Thus, more free n-3 than n-6 PUFA is available for oxidation by COX-2, which produces a less potent series of prostaglandins, namely, PGE3, and other inflammatory mediators (Calder, 2006). The production of these less inflammatory eicosanoids and docosanoids does not as effectively stimulate the neurons leading to the HPA axis, which has been shown to result in less cortisol produced (Hong et al., 2003).
Figure 2.
Plasma n-3:n-6 PUFA ratio measured in samples collected immediately prior to weaning (day 0), and on days 1 and 3 postweaning in pigs nursed by sows receiving a corn-soybean meal based diet supplemented with 1% protected fish oil (1% PFO, n = 16 pigs; Gromega, JBS United Inc., Sheridan, IN) or no supplement (0% PFO, n = 16 pigs) 1 wk prior to farrowing until day of weaning (31 ± 2 d of age). a–dMeans ± SEM with different letters differ by diet (P < 0.001), day (P < 0.001), and diet x day (P < 0.05).
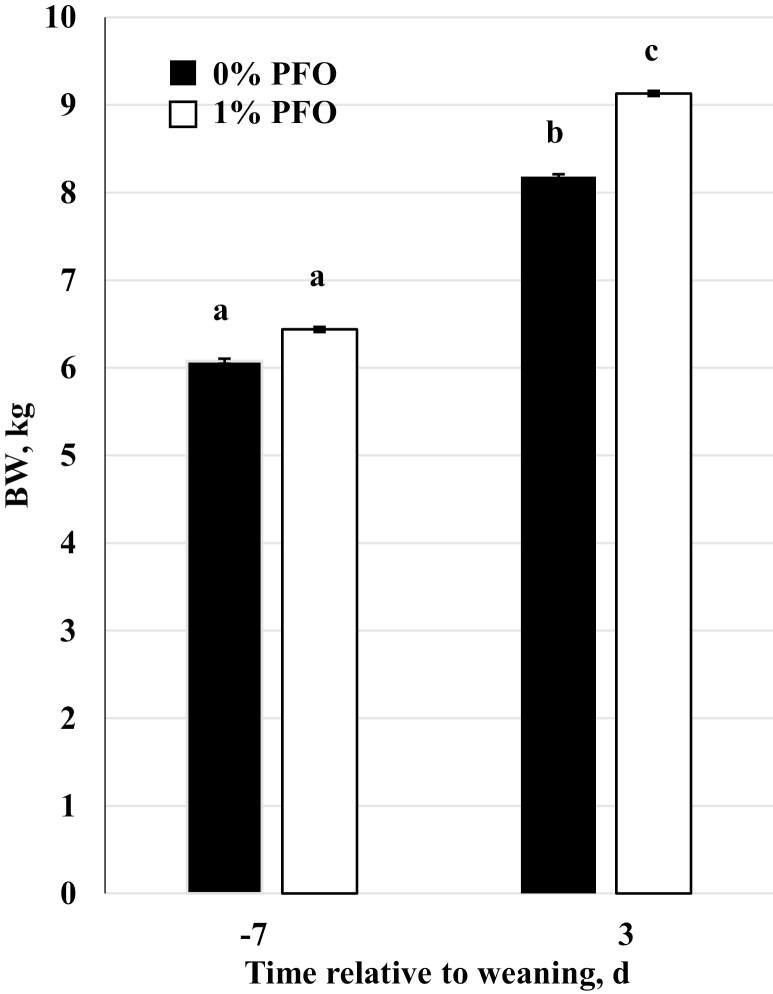
Body weight and weight gain of pigs measured on day 3 postweaning relative to day −7 preweaning were greater (P = 0.03) for the 1% PFO-supplemented diet group compared with the control group (Figure 3). Typically, the initial fighting after weaning causes inflammation, lethargy, and reduced feed intake (de Groot et al., 2001). Inflammation and stress due to weaning in swine decreases ADG and disrupts intestinal function, which decreases nutrient absorption (Gabler et al., 2007; Sutherland et al., 2014). A study by Giroux et al. (2000) showed that pigs with a passive reaction to stress (minimal action) had better weight gain than reacting pigs (squealing and constant movement) during the first week postweaning. The researchers suggested that this may be due to passive pigs expending less energy in their reaction to stress. It is possible that the pigs in our study receiving the 1% PFO-supplemented diet were indeed more passive. However, this cannot be confirmed without evidence from video recording.
Figure 3.
Body weight (BW) on day −7 preweaning and to day 3 postweaning in pigs nursed by sows receiving a corn-soybean meal based diet supplemented with 1 % protected fish oil (1% PFO, n = 16 pigs; Gromega, JBS United Inc., Sheridan, IN) or no supplement (0% PFO, n = 16 pigs) 1 wk prior to farrowing until day of weaning (31 ± 2 d of age) and provided ad libitum access to either the control or 1% PFO nursery feed consistent with their pre-weaning dietary treatment group. a–cMeans ± SEM with different letters differ for diet (P = 0.03) and day (P < 0.01).
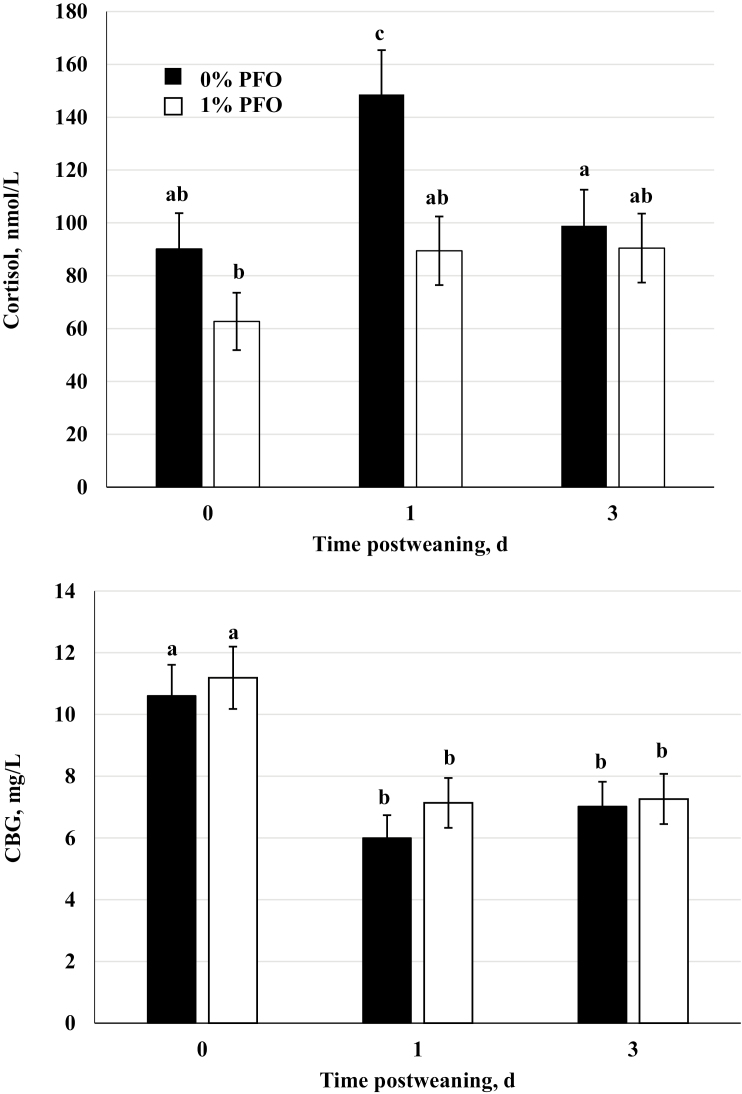
Total cortisol concentration has been shown to be a reliable predictor of a stress response but does not account for the biological activity of cortisol. Greater than 60% of circulating cortisol in swine is bound to its specific carrier glycoprotein, corticosteroid-binding globulin (CBG; Kattesh et al., 1990), which both transports and modulates cortisol availability in the circulation (Siiteri et al., 1982). In the present study, cortisol concentrations were greater (P < 0.001) on day 1 and CBG concentrations were lower (P < 0.001) on days 1 and 3 compared with the other sampling day(s) regardless of dietary treatment (Figure 4). This observation is consistent with previous work reported in pigs subjected to acute stressors such as transportation, social mixing, maternal separation, and elevated temperature (Heo et al., 2005; Kojima et al., 2008; Cooper et al., 2009). There was an overall treatment effect (P = 0.03) on plasma total cortisol, with lesser concentrations in pigs on the 1% PFO diet. The present study did not show a day by treatment effect for total plasma cortisol or CBG, however, measured concentrations of both constituents over the sampling period where either lower (cortisol) or greater (CBG) in pigs from the 1% PFO treatment group compared with the controls (Figure 4).
Figure 4.
Plasma concentrations of cortisol and corticosteroid-binding globulin (CBG) in samples collected immediately prior to weaning (day 0), and on days 1 and 3 postweaning in pigs nursed by sows receiving a corn-soybean meal based diet supplemented with 1% protected fish oil (1% PFO, n = 16 pigs; Gromega, JBS United Inc., Sheridan, IN) or no supplement (0% PFO, n = 16 pigs) 1 wk prior to farrowing until day of weaning (31 ± 2 d of age). a–cMeans ± SEM with different letters differ by diet (P = 0.03) and day (P < 0.01).
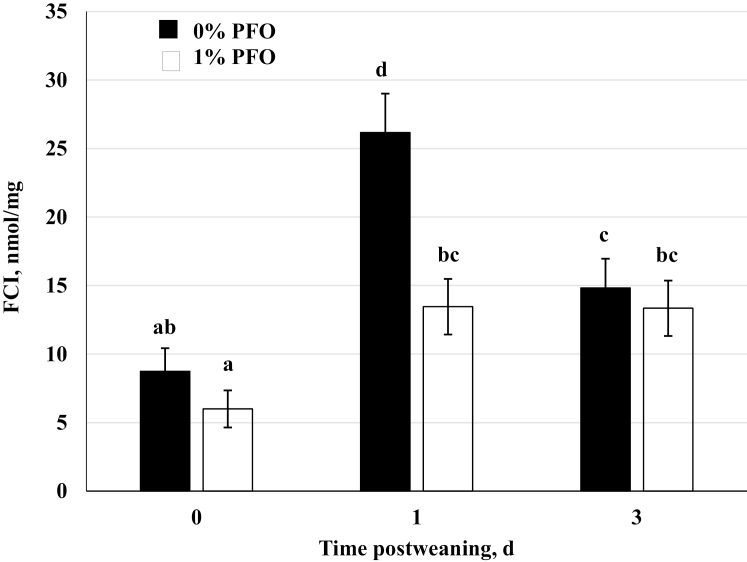
Rising concentrations of biologically available cortisol result from increased cleavage from CBG by neutrophil elastase present at the site of inflammation (Nguyen et al., 2014). The FCI has been shown to be a dependable measure of the amount of biologically active cortisol in the circulation and a far better index of an animals stress response (le Roux et al., 2003; Heo et al., 2005). Regardless of dietary treatment, FCI in the current study was greater (P < 0.01) on day 1 postweaning (Figure 5). The overall FCI for pigs supplemented with the 1.0% PFO was lower (P = 0.02) compared with that for pigs on the control diet, and a day x dietary treatment interaction was observed (P < 0.01) on day 1 postweaning such that FCI was particularly lower in the PFO-supplemented pigs. The day effect for the FCI indicates that all the pigs in the study experienced a similar acute stress response associated with weaning. These results are like those found by Kojima et al. (2008) who showed similar physiological responses to weaning with and without transport stress. The resultant lower FCI on day 1 in the 1.0% PFO group can be attributed to the lower total cortisol concentrations, since both groups of pigs exhibited similar plasma CBG concentrations. The present study is the first to report adding 1% PFO to the sow’s diet can lower FCI in pigs on d 1 postweaning.
Figure 5.
Free cortisol index (FCI) in samples collected immediately prior to weaning (day 0), and on days 1 and d 3 postweaning in pigs nursed by sows receiving a corn-soybean meal based diet supplemented with 1% protected fish oil (1% PFO, n = 16 pigs; Gromega, JBS United Inc., Sheridan, IN) or no supplement (0% PFO, n = 16 pigs) 1 wk prior to farrowing until day of weaning (31 ± 2 d of age). The FCI was calculated by dividing plasma cortisol concentrations (nmol/L) by corticosteroid-binding globulin concentrations at each day sampled. a-dMeans ± SEM with different letters differ by diet (P = 0.02), day (P < 0.001), and diet x day (P = 0.01).
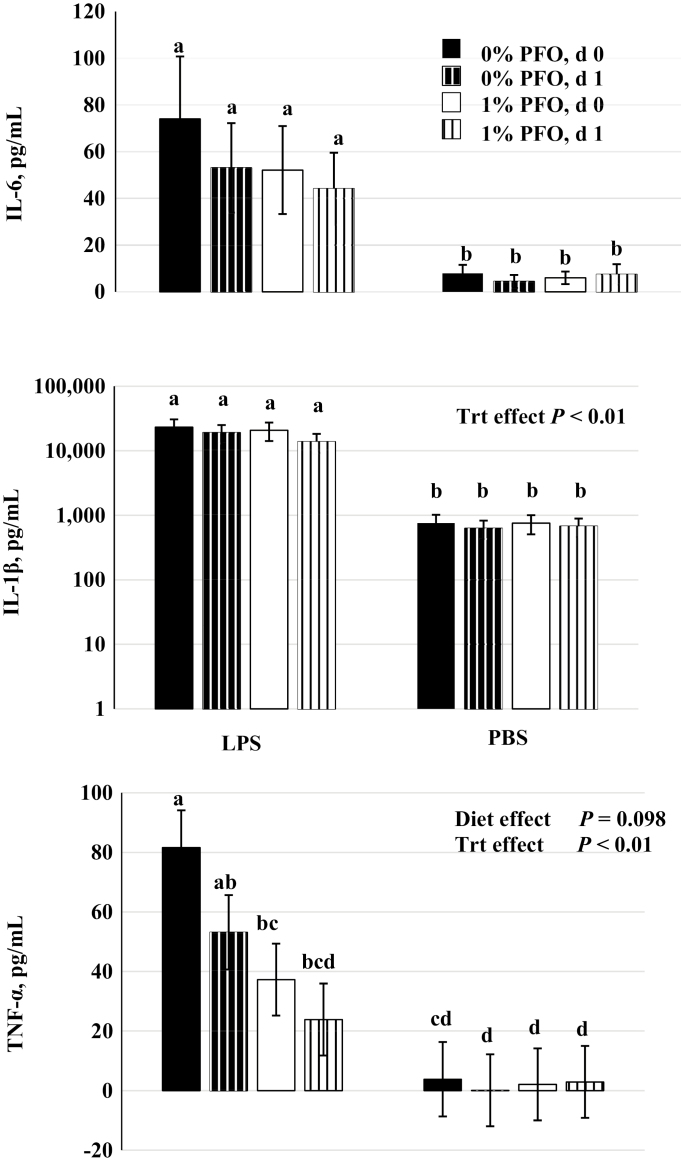
Basal pro-inflammatory cytokine concentrations are at or below the detectable limit of the assays (Carroll et al., 2003; Burdick Sanchez et al., 2019). Endotoxin challenges have been used in swine models to characterize production of acute phase cytokines IL-6, IL-1β, and TNF-α during an inflammatory response (as reviewed by Carroll et al., 2012). Direct administration of lipopolysaccharide (LPS) is a useful method to stimulate cytokine production but requires extra handling of pigs which can cause additional stress. An ex vivo whole blood LPS challenge can circumvent this additional stress. Using whole blood from healthy pigs, it has been shown that stimulation with LPS caused a marked TNF-α response and serve as an indicator of how acute stress such as weaning may prime the immune system (Carstensen et al., 2005). Additionally, the monocytes and other cytokine producing immune cells are in their natural environment and can more accurately represent physiological conditions within the animal, whereby producing more accurate concentrations of cytokines (Damsgaard et al., 2009). In the present study, all cytokines tested exhibited an increase in concentration when spiked with LPS, showing that the LPS had the desired effect of inducing inflammatory conditions (Figure 6). However, only TNF-α differed between the control pigs and the pigs on the 1% PFO-supplemented diet. Although others have reported the influence of LPS stimulation on cytokine concentrations, those studies were completed with an in vivo challenge using different strains (055:B5 vs K235), timing (3 times vs. 6 times), and doses (5 vs. 2 μg) of LPS (Myers et al., 2003; Llamas Moya et al., 2006). The cytokine TNF-α may have a negative correlation with IL-1β as observed by Upadhaya et al. (2015), making it likely to be the only cytokine detectable. Also, TNF-α may be the primary cytokine activated during LPS stimulation (Upadhaya et al., 2015). In our study, whole blood from pigs provided the 1% PFO tended (P = 0.098) to exhibit an overall lower concentration of TNF-α than did the control following ex vivo LPS challenge. This suggests that the n-3 PUFA in the 1% PFO diet may have had a protective effect and reduced the inflammatory response associated with acute postweaning stress. Increases in n-3 PUFA have been found to increase in the phospholipid bilayer. The effect of this increase has been shown to decrease the activity of PPARγ and NFκB, both of which are genetic pathways that increase the concentration of inflammatory cytokines produced in immune cells (Innis, 2003; Vandoros et al., 2006). In human studies, infusion of the cytokine TNF-α stimulates the production of IL-6 (Nielsen et al., 2013), which in turn has been shown to down-regulate CBG gene expression in hepatocytes (Bartalena et al., 1993), increase neutrophil elastase activity (Owen et al., 1997), and stimulate the HPA axis (Michie et al., 1988). The lower concentrations of TNF-α in the pigs on the 1% PFO diet reflect the cortisol and FCI results observed here. Likewise, elevated concentrations of TNF- α have been shown to suppress feed intake and growth (Webel et al., 1997) via the actions of leptin and IL-1β (Iwasa et al., 2011), which may explain the treatment differences we observed in BW. Therefore, dietary supplementation with PFO may have an indirect effect in reducing the HPA stimulation through reduced immune system activation as suggested by Carroll et al. (2003).
Figure 6.
Concentrations of cytokines IL-6, TNF-α, and IL-1β from media containing whole blood and subjected to ex vivo lipopolysaccharide (LPS) or PBS treatment (Trt). Whole blood was collected on day of weaning (day 0) and 1 d postweaning from pigs receiving a corn-soybean meal based diet supplemented with 1% protected fish oil (1% PFO, n = 16 pigs; Gromega, JBS United Inc., Sheridan, IN) or no supplement (0% PFO, n = 16 pigs) 1 wk prior to farrowing until day of weaning (31 ± 2 d of age). a–dMeans ± SEM with different letters within measured cytokine differ.
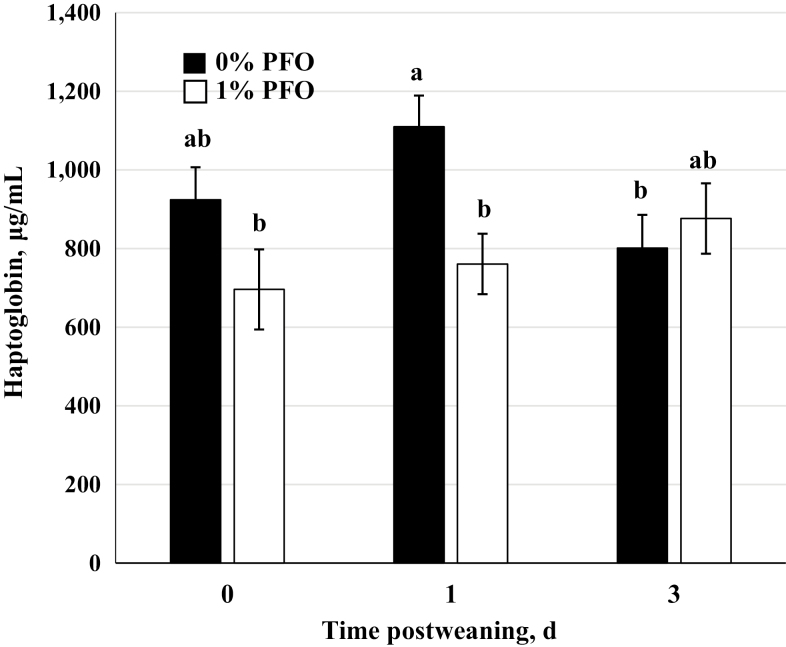
Plasma haptoglobin concentration was greater (P = 0.04) overall in pigs fed the control diet when compared with pigs on the 1% PFO diet (Figure 7). A diet x day interaction was detected for haptoglobin such that haptoglobin was greater (P = 0.05) in control pigs than in the PFO supplemented pigs on day 1 postweaning. Inflammation from fighting increases the TNF-α concentrations which then increases the expression of haptoglobin in the liver (Baumann and Gauldie, 1994; Llamas Moya et al., 2006). The timing of this increase in haptoglobin is consistent with the greater TNF-α response and FCI noted earlier for the control pigs.
Figure 7.
Plasma haptoglobin concentrations in samples collected immediately prior to weaning (day 0), and on days 1 and 3 postweaning in pigs nursed by sows receiving a corn-soybean meal based diet supplemented with 1% protected fish oil (1% PFO, n = 16 pigs; Gromega, JBS United Inc., Sheridan, IN) or no supplement (0% PFO, n = 16 pigs) 1 wk prior to farrowing until day of weaning (31 ± 2 d of age). a–cMeans ± SEM with different letters differ by diet (P = 0.04) and diet x day (P = 0.05).
The results of the present study suggest that the inclusion of a 1% PFO supplement in the sow diet from gestation into late lactation does have some effects on the phospholipid profile and indicators of stress and inflammation in their offspring postweaning. This study showed, albeit inconsistently, that supplementing a 1% PFO in the diet of sows does increase the n-3:n-6 PUFA ratio in colostrum and milk. This increase although statistically undetectable can still transfer n-3 PUFA to the piglets and decrease their n-6:n-3 PUFA ratio. Through this increase in n-3:n-6 PUFA ratio, the effects of the postweaning period on acute stress and inflammation may be mitigated. Not only did pigs fed the 1% PFO diet gain more weight but they also had lower concentrations of TNF-α, haptoglobin, and FCI all indicating a modification of the inflammatory and stress response associated with weaning.
Video recordings of behavior would be beneficial to view fighting bout length and frequency. Even though most of the fighting and stress happens within the first 24 h of weaning, we may consider extending the postweaning period to observe if the effects of the PFO on weight gain and performance continue after the initial 3-d period.
Footnotes
This research is published with the approval of the Dean of UTIA AgResearch and supported by State and Hatch Funds allocated to the College.
Mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the USDA. The USDA prohibits discrimination in all its programs and activities on the basis of race, color, national origin, age, disability, and where applicable, sex, marital status, familial status, parental status, religion, sexual orientation, genetic information, political beliefs, reprisal, or because all or part of an individual’s income is derived from any public assistance program. (Not all prohibited bases apply to all programs.) Persons with disabilities who require alternative means for communication of program information (Braille, large print, audiotape, etc.) should contact the USDA’s TARGET Center at (202) 720-2600 (voice and TDD). To file a complaint of discrimination, write to USDA, Director, Office of Civil Rights, 1400 Independence Avenue, S.W., Washington, DC 20250-9410, or call (800) 795-3272 (voice) or (202) 720-6382 (TDD). The USDA is an equal opportunity provider and employer.
Literature Cited
- Adcock R. J., Kattesh H. G., Roberts M. P., Carroll J. A., Saxton A. M., and Kojima C. J.. 2007. Temporal relationships between plasma cortisol, corticosteroid-binding globulin (CBG), and the free cortisol index (FCI) in pigs in response to adrenal stimulation or suppression. Stress 10:305–310. doi: 10.1080/10253890701248020. [DOI] [PubMed] [Google Scholar]
- Arbuckle L. D., and Innis S. M.. 1993. Docosahexaenoic acid is transferred through maternal diet to milk and to tissues of natural milk-fed piglets. J. Nutr. 123:1668–1675. doi: 10.1093/jn/123.10.1668. [DOI] [PubMed] [Google Scholar]
- Bartalena L., Hammond G. L., Farsetti A., Flink I. L., and Robbins J.. 1993. Interleukin-6 inhibits corticosteroid-binding globulin synthesis by human hepatoblastoma-derived (Hep G2) cells. Endocrinology 133:291–296. doi: 10.1210/endo.133.1.8391424. [DOI] [PubMed] [Google Scholar]
- Baumann H., and Gauldie J.. 1994. The acute phase response. Immunol. Today 15:74–80. doi: 10.1016/0167-5699(94)90137-6. [DOI] [PubMed] [Google Scholar]
- Burdick Sanchez N. C., Carroll J. A., Broadway P. R., Bass B. E., and Frank J. W.. 2019. Supplementation of a Lactobacillus acidophilus fermentation product can attenuate the acute phase response following a lipopolysaccharide challenge in weaned pigs. Animal 13:144–152. doi: 10.1017/S1751731118001222. [DOI] [PubMed] [Google Scholar]
- Calder P. C. 2006. Polyunsaturated fatty acids and inflammation. Prostaglandins. Leukot. Essent. Fatty Acids 75:197–202. doi: 10.1016/j.plefa.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Cameron-Smith D., Albert B. B., and Cutfield W. S.. 2015. Fishing for answers: is oxidation of fish oil supplements a problem? J. Nutr. Sci. 4:e36. doi: 10.1017/jns.2015.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell J. M., Crenshaw J. D., and Polo J.. 2013. The biological stress of early weaned piglets. J. Anim. Sci. Biotechnol. 4:19. doi: 10.1186/2049-1891-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll J. A., Burdick N. C., Chase C. C. Jr, Coleman S. W., and Spiers D. E.. 2012. Influence of environmental temperature on the physiological, endocrine, and immune responses in livestock exposed to a provocative immune challenge. Domest. Anim. Endocrinol. 43:146–153. doi: 10.1016/j.domaniend.2011.12.008. [DOI] [PubMed] [Google Scholar]
- Carroll J. A., Gaines A. M., Spencer J. D., Allee G. L., Kattesh H. G., Roberts M. P., and Zannelli M. E.. 2003. Effect of menhaden fish oil supplementation and lipopolysaccharide exposure on nursery pigs. I. Effects on the immune axis when fed diets containing spray-dried plasma. Domest. Anim. Endocrinol. 24:341–351. [DOI] [PubMed] [Google Scholar]
- Carstensen L., Røntved C. M., and Nielsen J. P.. 2005. Determination of tumor necrosis factor-alpha responsiveness in piglets around weaning using an ex vivo whole blood stimulation assay. Vet. Immunol. Immunopathol. 105:59–66. doi: 10.1016/j.vetimm.2004.12.014. [DOI] [PubMed] [Google Scholar]
- Clouard C., Souza A. S., Gerrits W. J., Hovenier R., Lammers A., and Bolhuis J. E.. 2015. Maternal fish oil supplementation affects the social behavior, brain fatty acid profile, and sickness response of piglets. J. Nutr. 145:2176–2184. doi: 10.3945/jn.115.214650. [DOI] [PubMed] [Google Scholar]
- Cooper T. A., Roberts M. P., Kattesh H. G., and Kojima C. J.. 2009. Effects of transport stress, gender, and weaning weight on post-weaning performance in pigs. Prof. Anim. Sci. 25:189–194. [Google Scholar]
- Damsgaard C. T., Lauritzen L., Calder P. C., Kjaer T. M., and Frøkiaer H.. 2009. Whole-blood culture is a valid low-cost method to measure monocytic cytokines - a comparison of cytokine production in cultures of human whole-blood, mononuclear cells and monocytes. J. Immunol. Methods 340:95–101. doi: 10.1016/j.jim.2008.10.005. [DOI] [PubMed] [Google Scholar]
- Elenkov I. J., and Chrousos G. P.. 2002. Stress hormones, proinflammatory and antiinflammatory cytokines, and autoimmunity. Ann. N. Y. Acad. Sci. 966:290–303. doi: 10.1111/j.1749-6632.2002.tb04229.x. [DOI] [PubMed] [Google Scholar]
- Gabler N. K., Spencer J. D., Webel D. M., and Spurlock M. E.. 2007. In utero and postnatal exposure to long chain (n-3) PUFA enhances intestinal glucose absorption and energy stores in weanling pigs. J. Nutr. 137:2351–2358. doi: 10.1093/jn/137.11.2351. [DOI] [PubMed] [Google Scholar]
- Giroux S., Martineau G., and Robert S.. 2000. Relationships between individual behavioural traits and post-weaning growth in segregated early-weaned piglets. Appl. Anim. Behav. Sci. 70:41–48. [DOI] [PubMed] [Google Scholar]
- Goshen I., and Yirmiya R.. 2009. Interleukin-1 (IL-1): a central regulator of stress responses. Front. Neuroendocrinol. 30:30–45. doi: 10.1016/j.yfrne.2008.10.001. [DOI] [PubMed] [Google Scholar]
- de Groot J., Ruis M. A., Scholten J. W., Koolhaas J. M., and Boersma W. J.. 2001. Long-term effects of social stress on antiviral immunity in pigs. Physiol. Behav. 73:145–158. doi: 10.1016/s0031-9384(01)00472-3. [DOI] [PubMed] [Google Scholar]
- Heo J., Kattesh H. G., Roberts M. P., Morrow J. L., Dailey J. W., and Saxton A. M.. 2005. Hepatic corticosteroid-binding globulin (CBG) messenger RNA expression and plasma CBG concentrations in young pigs in response to heat and social stress. J. Anim. Sci. 83:208–215. doi: 10.2527/2005.831208x. [DOI] [PubMed] [Google Scholar]
- Hong S., Gronert K., Devchand P. R., Moussignac R. L., and Serhan C. N.. 2003. Novel docosatrienes and 17S-resolvins generated from docosahexaenoic acid in murine brain, human blood, and glial cells. Autacoids in anti-inflammation. J. Biol. Chem. 278:14677–14687. doi: 10.1074/jbc.M300218200. [DOI] [PubMed] [Google Scholar]
- Horrocks L. A., and Yeo Y. K.. 1999. Health benefits of docosahexaenoic acid (DHA). Pharmacol. Res. 40:211–225. doi: 10.1006/phrs.1999.0495. [DOI] [PubMed] [Google Scholar]
- Innis S. M. 2003. Perinatal biochemistry and physiology of long-chain polyunsaturated fatty acids. J. Pediatr. 143(4 Suppl):S1–S8. doi: 10.1067/s0022-3476(03)00396-2. [DOI] [PubMed] [Google Scholar]
- Iwasa T., Matsuzaki T., Murakami M., Kinouchi R., Gereltsetseg G., Nakazawa H., Yamamoto S., Kuwahara A., Yasui T., and Irahara M.. 2011. Changes in responsiveness of appetite, leptin and hypothalamic IL-1β and TNF-α to lipopolysaccharide in developing rats. J. Neuroimmunol. 236:10–16. doi: 10.1016/j.jneuroim.2011.04.004. [DOI] [PubMed] [Google Scholar]
- Kattesh H. G., Charles S. F., Baumbach G. A., and Gillespie B. E.. 1990. Plasma cortisol distribution in the pig from birth to six weeks of age. Biol. Neonate 58:220–226. doi: 10.1159/000243271. [DOI] [PubMed] [Google Scholar]
- Kim S. W., Mateo R. D., Yin Y., and Wu G.. 2006. Functional amino acids and fatty acids for enhancing production performance of sows and piglets. Asian-Australasian J. Anim. Sci. 20:295–306. [Google Scholar]
- Kojima C. J., Kattesh H. G., Roberts M. P., and Sun T.. 2008. Physiological and immunological responses to weaning and transport in the young pig: modulation by administration of porcine somatotropin. J. Anim. Sci. 86:2913–2919. doi: 10.2527/jas.2008-1089. [DOI] [PubMed] [Google Scholar]
- Liu Y., Chen F., Li Q., Odle J., Lin X., Zhu H., Pi D., Hou Y., Hong Y., and Shi H.. 2013. Fish oil alleviates activation of the hypothalamic-pituitary-adrenal axis associated with inhibition of TLR4 and NOD signaling pathways in weaned piglets after a lipopolysaccharide challenge. J. Nutr. 143:1799–1807. doi: 10.3945/jn.113.179960. [DOI] [PubMed] [Google Scholar]
- Liu Y. L., Li D. F., Gong L. M., Yi G. F., Gaines A. M., and Carroll J. A.. 2003. Effects of fish oil supplementation on the performance and the immunological, adrenal, and somatotropic responses of weaned pigs after an Escherichia coli lipopolysaccharide challenge. J. Anim. Sci. 81:2758–2765. doi: 10.2527/2003.81112758x. [DOI] [PubMed] [Google Scholar]
- Llamas Moya S., Boyle L. A., Lynch P. B., and Arkins S.. 2006. Pro-inflammatory cytokine and acute phase protein responses to low-dose lipopolysaccharide (LPS) challenge in pigs. Anim. Sci. 82:527–534. [Google Scholar]
- Mateo R. D., Carroll J. A., Hyun Y., Smith S., and Kim S. W.. 2009. Effect of dietary supplementation of n-3 fatty acids and elevated concentrations of dietary protein on the performance of sows. J. Anim. Sci. 87:948–959. doi: 10.2527/jas.2008-0964. [DOI] [PubMed] [Google Scholar]
- Michie H. R., Spriggs D. R., Manogue K. R., Sherman M. L., Revhaug A., O’Dwyer S. T., Arthur K., Dinarello C. A., Cerami A., and Wolff S. M.. 1988. Tumor necrosis factor and endotoxin induce similar metabolic responses in human beings. Surgery 104:280–286. [PubMed] [Google Scholar]
- Myers M. J., Farrell D. E., Palmer D. C., and Post L. O.. 2003. Inflammatory mediator production in swine following endotoxin challenge with or without co-administration of dexamethasone. Int. Immunopharmacol. 3:571–579. doi: 10.1016/S1567-5769(03)00048-1. [DOI] [PubMed] [Google Scholar]
- Nguyen P. T., Lewis J. G., Sneyd J., Lee R. S., Torpy D. J., and Shorten P. R.. 2014. Development of a formula for estimating plasma free cortisol concentration from a measured total cortisol concentration when elastase-cleaved and intact corticosteroid binding globulin coexist. J. Steroid Biochem. Mol. Biol. 141:16–25. doi: 10.1016/j.jsbmb.2013.12.015. [DOI] [PubMed] [Google Scholar]
- Nielsen S. T., Lehrskov-Schmidt L., Krogh-Madsen R., Solomon T. P., Lehrskov-Schmidt L., Holst J. J., and Møller K.. 2013. Tumour necrosis factor-alpha infusion produced insulin resistance but no change in the incretin effect in healthy volunteers. Diabetes. Metab. Res. Rev. 29:655–663. doi: 10.1002/dmrr.2441. [DOI] [PubMed] [Google Scholar]
- NRC 2012. Nutrient requirements of swine. 11th ed Natl. Acad. Press, Washington, DC. doi: 10.17226/13298. [DOI] [Google Scholar]
- Owen C. A., Campbell M. A., Boukedes S. S., and Campbell E. J.. 1997. Cytokines regulate membrane-bound leukocyte elastase on neutrophils: a novel mechanism for effector activity. Am. J. Physiol. 272(3 Pt 1):L385–L393. doi: 10.1152/ajplung.1997.272.3.L385. [DOI] [PubMed] [Google Scholar]
- Roberts M. P., Kattesh H. G., Baumbach G. A., Gillespie B. E., Godkin J. D., Schneider J. F., and Saxton A. M.. 2003. Age-related changes in porcine corticosteroid-binding globulin (pCBG) as determined by an enzyme-linked immunosorbent assay. Domest. Anim. Endocrinol. 24:323–339. [DOI] [PubMed] [Google Scholar]
- Rooke J. A., Bland I. M., and Edwards S. A.. 1998. Effect of feeding tuna oil or soyabean oil as supplements to sows in late pregnancy on piglet tissue composition and viability. Br. J. Nutr. 80:273–280. doi: 10.1017/s0007114598001329. [DOI] [PubMed] [Google Scholar]
- Rooke J. A., Shanks M., and Edwards S. A.. 2000. Effect of offering maize, linseed or tuna oils throughout pregnancy and lactation on sow and piglet tissue composition and piglet performance. Anim. Sci. 71:289–299. [Google Scholar]
- Rooke J. A., Sinclair A. G., and Ewen M.. 2001. Changes in piglet tissue composition at birth in response to increasing maternal intake of long-chain n-3 polyunsaturated fatty acids are non-linear. Br. J. Nutr. 86:461–470. doi: 10.1079/bjn2001422. [DOI] [PubMed] [Google Scholar]
- le Roux C. W., Chapman G. A., Kong W. M., Dhillo W. S., Jones J., and Alaghband-Zadeh J.. 2003. Free cortisol index is better than serum total cortisol in determining hypothalamic-pituitary-adrenal status in patients undergoing surgery. J. Clin. Endocrinol. Metab. 88:2045–2048. doi: 10.1210/jc.2002-021532. [DOI] [PubMed] [Google Scholar]
- le Roux C. W., Sivakumaran S., Alaghband-Zadeh J., Dhillo W., Kong W. M., and Wheeler M. J.. 2002. Free cortisol index as a surrogate marker for serum free cortisol. Ann. Clin. Biochem. 39(Pt 4):406–408. doi: 10.1258/000456302760042182. [DOI] [PubMed] [Google Scholar]
- Siiteri P. K., Murai J. T., Hammond G. L., Nisker J. A., Raymoure W. J., and Kuhn R. W.. 1982. The serum transport of steroid hormones. Recent Prog. Horm. Res. 38:457–510. [DOI] [PubMed] [Google Scholar]
- Smit M. N., Patterson J. L., Webel S. K., Spencer J. D., Cameron A. C., Dyck M. K., Dixon W. T., and Foxcroft G. R.. 2013. Responses to n-3 fatty acid (LCPUFA) supplementation of gestating gilts, and lactating and weaned sows. Animal 7:784–792. doi: 10.1017/S1751731112002236. [DOI] [PubMed] [Google Scholar]
- Sutherland M. A., Backus B. L., and McGlone J. J.. 2014. Effects of transport at weaning on the behavior, physiology and performance of pigs. Animals (Basel). 4:657–669. doi: 10.3390/ani4040657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadhaya S. D., Kim J. C., Mullan B. P., Pluske J. R., and Kim I. H.. 2015. Vitamin E and omega-3 fatty acids independently attenuate plasma concentrations of proinflammatory cytokines and prostaglandin E3 in Escherichia coli lipopolysaccharide-challenged growing-finishing pigs. J. Anim. Sci. 93:2926–2934. doi: 10.2527/jas.2014-8330. [DOI] [PubMed] [Google Scholar]
- Vandoros G. P., Konstantinopoulos P. A., Sotiropoulou-Bonikou G., Kominea A., Papachristou G. I., Karamouzis M. V., Gkermpesi M., Varakis I., and Papavassiliou A. G.. 2006. PPAR-gamma is expressed and NF-kB pathway is activated and correlates positively with COX-2 expression in stromal myofibroblasts surrounding colon adenocarcinomas. J. Cancer Res. Clin. Oncol. 132:76–84. doi: 10.1007/s00432-005-0042-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webel D. M., Finck B. N., Baker D. H., and Johnson R. W.. 1997. Time course of increased plasma cytokines, cortisol, and urea nitrogen in pigs following intraperitoneal injection of lipopolysaccharide. J. Anim. Sci. 75:1514–1520. doi: 10.2527/1997.7561514x. [DOI] [PubMed] [Google Scholar]
- Xiong Y., Zhao Y. Y., Goruk S., Oilund K., Field C. J., Jacobs R. L., and Curtis J. M.. 2012. Validation of an LC-MS/MS method for the quantification of choline-related compounds and phospholipids in foods and tissues. J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 911:170–179. doi: 10.1016/j.jchromb.2012.10.038. [DOI] [PubMed] [Google Scholar]