Abstract
Pancreatic cancer (PC), currently the third leading cause of cancer-related deaths in the USA, is projected to become the second leading cause, behind lung cancer, by 2020. The increasing incidence, low survival rate, and limited treatment opportunities necessitate the use of alternative approaches such as chemoprevention, to tackle PC. In this study, we report significant synergistic chemoprevention efficacy for the first time from a low-dose combination of a classical antihistaminic drug, Loratadine (LOR) and a neutraceutical compound, Sulforaphane (SFN) using a self-microemulsifying drug delivery system (SMEDDS) formulation. The formulation was developed using Quality by Design approach (globule size, 95.13 ± 7.9 nm; PDI, 0.17 ± 0.04) and revealed significant (p < 0.05) enhancement in the in vitro dissolution profile confirming the enhanced solubility of BCS class II drug LOR with SMEDDS formulation. The LOR-SFN combination revealed ~ 40-fold reduction in IC50 concentration compared to LOR alone in MIA PaCa-2 and Panc-1 cell lines respectively, confirming the synergistic enhancement in chemoprevention. Further, the nanoformulation resulted in ~ 7-fold and ~ 11-fold reduction in IC50 values compared to LOR-SFN combination. Hence, our studies successfully demonstrate that a unique low-dose combination of LOR encapsulated within SMEDDs with SFN shows significantly enhanced chemopreventive efficacy of PC.
Keywords: Pancreatic cancer, Chemoprevention, Loratadine, Sulforaphane, Synergism, Self-microemulsifying drug delivery system
Introduction
Pancreatic cancer (PC), currently the third leading cause of cancer-related deaths in the USA, is expected to claim about 44,330 deaths in the year 2018. The increasing incidence and mortality related to PC has projected it to become the second leading cause of deaths in the USA by 2020 exceeding breast and colorectal cancer [1]. Unfortunately, most patients with PC exhibit symptoms after the disease becomes metastatic thus making it very difficult to treat which has led to very meager 5-year life expectancy (< 14% for stage I, < 7% for stage II; <3% for stage III, and < 1% for stage IV). It is also reported that the life expectancy in patients treated with surgery is more than those treated without it [2].
The increasing incidence, low survival rate, and limited treatment opportunities necessitate concentrated efforts to tackle PC; thus, alternative approaches are being investigated extensively [3]. In this context, chemoprevention is gaining wide attention as a potential approach. Chemoprevention refers to preventive strategy implemented to lessen the risk and/or to delay the development or progression of cancer. The literature reports a wide range of such actives that have shown chemopreventive potential. Some reported examples include antioxidants like resveratrol, NSAIDS like aspirin, sulindac, celecoxib, and other actives like sulforaphane and genistein [4, 5].
A multi-target approach using drug combinations (drugs with different mechanism of action and target) offers benefits over a single drug approach in terms of enhanced efficacy, low-dose regimen, and hence reduced side effects and toxicity. Thus, use of drug combinations has wide scope for chemoprevention application and has made its way into various clinical trials [6]. Further, we believe that repurposing approved drugs for chemoprevention alone or in combination can allow relatively faster intervention for chemoprevention as the clinical safety and performance data are already available. With this rationale, our research group has focused on identifying and assessing the chemopreventive potential of various actives over a decade. We were the first group to report the synergistic chemopreventive efficacy using a combination drug approach with aspirin, curcumin, and sulforaphane, and with ferulic acid combined with aspirin against PC [7, 8].
After investigating a battery of drug molecules and their possible combinations to elicit chemopreventive action, recently, we have discovered significant synergistic chemoprevention efficacy from a classical antihistaminic drug loratadine (LOR) when combined with sulforaphane (SFN), which is reported for the first time in this paper [USA provisional patent (US, 62/596,380) granted for the invention].
LOR is a classical H1 receptor antagonist which belongs to the class of second-generation antihistaminic and is clinically prescribed for allergic manifestation (as Claritin ®). Interestingly, pharmacoepidemiological studies have shown potential of this class of antihistaminic drugs in PC, colon cancer, non-small-cell lung cancer (NSCLC), prostate, and breast cancer. Mechanistically, LOR is reported to cause lysosomal membrane destabilization in non-small-cell lung cancer cell lines, cell cycle progression dysregulation with enhanced radiation sensitivity in colon cancer cell lines, and reduce bone pain in breast cancer, leading to its anticancer activity [9–11].
SFN is an isothiocyanate derivative naturally present in vegetables belonging to cruciferous family for example broccoli, cabbage, and sprouts. SFN is reported to interact at multiple cell targets like nrf2, hypoxia-inducible factor-1α, c-Myc, and vascular endothelial growth factor causing interference in many cellular activities that include apoptosis and angiogenesis. Most, importantly, it interacts with NF-κB causing reduction in its activity and hence impedes the associated gene expression that codes for various inflammatory cytokines, adhesion molecules, etc. These could be the underlying mechanisms for its chemoprevention activity [7, 12–14]. SFN has also been reported to be used as an excipient to develop taxane-based lipid emulsified systems for anticancer treatment [15]. To summarize, LOR and SFN are reported to elicit the inhibitory action against various cancerous cells via multiple mechanisms. Hence, it can be hypothesized that the combination of LOR-SFN can simultaneously target multiple cellular pathways leading to synergistic enhancement in the chemoprevention activity which was the premise of this research.
Further, drug potency and its bioavailability are the two important factors that determine the overall efficacy of any chemoprevention and treatment regimen. Hence, after identifying the synergistic chemoprevention activity (high potency) of LOR-SFN combination, the next logical step was to ensure the bioavailability of the proposed regimen. For this, the reported physicochemical and pharmacokinetic properties of both the drugs were assessed. SFN belongs to BCS Class I and shows high oral bioavailability. The reported oral bioavailability of SFN in rats is 82% with dose-dependent kinetics. Hence, it was rationalized that SFN can be administered in its original form to elicit a pharmacological response [16]. However, LOR belongs to BCS Class II exhibiting low solubility and high permeability that results in poor oral bioavailability [17]. Therefore, to enhance the oral bioavailability of LOR, a suitable drug delivery system must be designed that will enhance the solubility and in turn the absorption and bioavailability of the drug upon oral administration.
In this context, lipid-based systems can be looked upon as promising drug delivery systems for BCS class II molecules as it allow incorporation of drugs in the lipid matrix leading to enhancement of bioavailability. Some lipid-based systems have been reported in literature for LOR. These include LOR lipid-based solid formulation prepared by adsorption and milling technique with droplet size of 1.2 μm; solid lipid microparticles of LOR prepared by emulsion congealing technique, and lipid-based formulations comprising natural or digested lipids wherein LOR was used as a model drug [18–21]. Among various lipid-based systems, we rationalized use of oil/water (O/W) self-microemulsifying drug delivery systems (SMEDDS) for effective LOR delivery. SMEDDS are isotropic mixtures of oil/s, surfactant/s, cosurfactant/s, stabilizer/s, and drug that form spontaneous microemulsion upon introduction in aqueous media under mild agitation. This allows spontaneous micro-emulsification of SMEDDS in the gastro intestinal (GI) milieu under mild agitation resulting from digestive motility of the GI tract making it suitable for oral delivery [22]. We selected SMEDDS as a potential delivery system as it exhibits added advantages over the earlier reported LOR lipid-based systems which include (1) singlestep formulation process involving simple mixing in contrast to reported multistep formulation process; (2) spontaneous emulsification leading to nano-droplet size which can enhance both rate and extent of dissolution and absorption compared to microparticulate systems; (3) high drug solubilization; (4) isotropic formulation; and (5) thermodynamic and kinetic stability. Additionally, the dosage form here is a preconcentrate (devoid of water) comprising drug, oil/s, surfactant/s, cosurfactant/s, and stabilizer/s which allow development of compact unit dosage forms, offering additional stability, and simplifying the formulation process to simple mixing operation making it industrially scalable [22].
Based on this rationale, the hypothesis of the present study was to investigate the pancreatic chemoprevention efficacy of combination drug regimen comprising LOR and SFN. In the study, we developed and optimized the LOR SMEDDS to enhance drug solubility. Further, we assessed the inhibitory activity of LOR and SFN combination and LOR SMEDDS and SFN combination in two human PC cell lines MIA PaCa-2 and Panc-1 to confirm the synergistic pancreatic chemoprevention efficacy.
Materials and methods
Materials
LOR and SFN were purchased from Tokyo Chemical Industries Co. Ltd. (Portland, OR, USA) and LKT Laboratories (St. Paul, MN, USA), respectively. Dimethyl sulfoxide (DMSO), hydrochloric acid (HCl), acetonitrile, and Tween 80 were ordered from Sigma Chemicals (St. Louis, MO, USA). Transcutol HP was obtained from Gattefosse Ltd. (Saint-Priest, Cedex, France) and Capmul MCM C8 was obtained from Abitec Corp. (Janesville, WI, USA). The Spectra/Por® Dialysis membrane-MWCO:50 kD was procured from Spectrum, Labs Inc. (Compton, CA, USA).
Human pancreatic cancer cell lines
Two human PC cell lines MIA PaCa-2 and Panc-1 were received from ATCC (Rockville, MD). To culture and maintain the cells, Dulbecco’s modified Eagle’s medium (DMEM) with 10% fetal bovine serum (FBS) was used. Cells were cultured in a humidifier at ambient temperature of 37 °C with 5% CO2 and 95% air.
Formulation development and optimization of LOR SMEDDS
The SMEDDS formulation was developed and optimized using a step by step approach as follows.
Optimization of SMEDDS formulation using water titration method and pseudo-ternary phase diagram
Based on the preliminary LOR saturation solubility studies (data not shown) Capmul MCM C8 (Cap C8) was selected as optimum oil phase as it presented the highest solubility for LOR. Similarly, among surfactants, Tween 80 (T80) and Tween 60 (T60) were shortlisted while among co-surfactants and stabilizers, PEG 200, PEG 400, and Transcutol HP (THP) were selected. To determine the possible composition of SMEDDS formulation (forming spontaneous microemulsion upon dilution with water), initially, blank SMEDDS formulations (devoid of LOR drug) were prepared using the water titration method. For this, mixtures of Cap C8 (oil) and surfactant mix (Smix) were prepared in 1:9 to 9:1 ratio. The mixtures were uniformly mixed and water was added in dropwise manner under vortex to determine the visual turbidity point. These observations in combination with composition of oil, Smix, and water phase were used to plot a pseudo-ternary phase diagram using Triplot® software and microemulsion region was identified. The Smix used for various trials included permutations and combinations of surfactant with co-surfactant and stabilizer (T80: PEG 200; T80: PEG 400; T80: THP; T60: PEG 200; T60: PEG 400; T60: THP in 1:1 w/w ratio). From the pseudo-ternary phase diagrams, the Smix composition resulting in maximum microemulsion area was selected. To determine the most favorable SMEDDS composition from the phase diagram, a composition leading to formation of clear microemulsion upon infinite dilution was considered as optimal.
Optimization of drug loading
To determine the maximum drug loading in the optimized SMEDDS formulation, the drug loading was increased from 0.5–4% w/w of LOR to obtain a clear isotropic formulation. Briefly, the shortlisted SMEDDS compositions with Smix T80-PEG 200 and T80-THP with varying amount of LOR (0.5–4% w/w) were accurately weighed and mixed uniformly under vortex to form a clear and isotropic one-phase system. The maximum drug loading was determined as LOR concentration that did not result in any drug precipitation and retained the clear, isotropic properties of the formulation.
Characterization of optimized LOR SMEDDS
Appearance
LOR SMEDDS were observed visually for color and clarity. Also the microemulsion formed by diluting (10× dilution) the LOR SMEDDS with purified water were observed for color and clarity.
Globule size and polydispersity index measurement
The globule size and the polydispersity index (PDI) of LOR SMEDDS were measured using Zetasizer Nanoseries Nano-ZS90 (Serial No: Mal1074171), Malvern Instruments Ltd. USA. The instrument specifications involved 4 mW standard laser at 633 nm with scattered light detection at 90° scattering angle (measuring range ~0.3 to 10 μm). Briefly, 100 mg of LOR SMEDDS was diluted to 1 g (10× dilution) using purified water and measurement was executed. The experiment was conducted in triplicate (n = 3) and results were reported as mean ± S.D.
Zeta potential
The zeta potential of LOR SMEDDS was measured using Zetasizer Nanoseries Nano-ZS90 (Serial No: Mal1074171), Malvern Instruments Ltd. USA. Briefly, 100 μL of LOR SMEDDS was diluted to 1 mL (10× dilution) using pH 7.4 buffer and measurement was executed. The experiment was conducted in triplicate (n = 3) and results were reported as mean ± S.D.
Effect of pH and dilution on LOR SMEDDS
To ensure the stability of LOR SMEDDS towards dilution, the globule size, PDI, and transmittance of LOR SMEDDS upon 10×, 100×, 250×, and 500× dilution using pH 1.2 buffer and pH 7.4 buffer were measured using Zetasizer Nanoseries Nano-ZS90 (Serial No: Mal1074171), Malvern Instruments Ltd. USA. The instrument specifications involved 4-mW standard laser at 633 nm with scattered light detection at 90° scattering angle (measuring range ~ 0.3 to 10 μm). The experiment was conducted in triplicate (n = 3) and results were reported as mean ± S.D. The transmittance was checked by measuring the transmittance at 650 nm with a UV spectrophotometer (Shimadzu, USA).
Assay of drug content
To determine assay of drug content, LOR SMEDDS equivalent to 10 mg of LOR was weighed and dissolved in 10 mL of methanol. The sample was diluted and analyzed by a validated HPLC method. The experiment was conducted in triplicate (n = 3) and results were reported as mean ± S.D.
In vitro drug release profile
Plain drug LOR-SFN and LOR SMEDDS-SFN were subjected to in vitro drug release studies using the dialysis bag method. The SMEDDS formulation equivalent to 10 mg of LOR with 10 mg of SFN was incorporated in donor compartment dialysis bag and was sealed using clips. For control, 10 mg of LOR-SFN suspended in the dissolution media (equivalent to SMEDDS formulation concentration) was incorporated in donor compartment dialysis bag and was sealed using clips. The dialysis bags were then introduced separately in dissolution media comprising 500 mL of 0.1 N HCl solution at 37 ± 0.5 °C and 100 rpm. At predetermined time intervals, 1 mL aliquot was retrieved from the dissolution chamber and was replaced with fresh dissolution medium. All samples were centrifuged at 5000 rpm for 10 min and the LOR and SFN content was analyzed using validated HPLC method. The experiment was conducted in triplicate (n = 3) and results were reported as mean ± S.D. The data was subjected to analysis using Graph Pad Prism software (San Diego, CA, USA) and was subjected to statistical analysis using unpaired t test. For result comparison, the probability value ≤ 0.05% was considered as significant.
In vitro cytotoxicity studies
In vitro cytotoxicity studies were performed using two PC cell lines Panc-1 and MIA PaCa-2 using MTS assay in accordance with the Promega CellTitre 96 Aqueous MTS reagent (Madison, WI) protocol [23]. This study was performed in two phases. In the first phase, 5 × 103 cells were seeded in 96-well plates for 24 h. At the end of 24 h, the individual formulation excipients Cap C8, T80, and THP were incubated for a period of 72 h. In the second phase, 7.5 × 103 cells were seeded in 96 well plates for 24 h. At the end of 24 h, LOR, SFN, LOR SMEDDS, LOR-SFN, and LOR SMEDDS-SFN were incubated for a period of 72 h. At the end point of incubation period, the DMEM medium was aspirated and 100 μL serum-free DMEM medium comprising 20% w/v MTS and 1% w/v phenazine methosulfate (PMS) was added to each well followed by incubation for 2 h. Principally, living cells convert the MTS to Formazan dye and hence the cell viability can be quantified proportional to the intensity of Formazan dye. Hence, at end of incubation, the formazan was quantified by absorbance measurement at 490 nm. The data was subjected to analysis using Graph Pad Prism software (San Diego, CA, USA) and IC50 values were determined. The experiments were conducted in triplicate (n = 3) and results were reported as mean ± S.D. The data was analyzed using Graph Pad Prism software (San Diego, CA, USA) and subjected to one-way ANOVA followed by Tukey’s post hoc test. For result comparison, the probability value ≤ 0.05% was considered as significant.
Results and discussion
Formulation development and optimization of LOR SMEDDS
The pseudo-ternary phase diagrams depicting the microemulsion region are represented in Fig. 1. It was observed that higher microemulsion region was observed with systems containing Tween 80 compared to those containing Tween 60. This can be explained based on their chemical and physical variations. Chemically, Tween 80 is Polyoxyethylene-sorbitan monooleate (HLB 15) while Tween 60 is Polyoxyethylene-sorbitan monostearate (HLB 14.9). Though there is no significant difference in HLB value of both surfactants, Tween 80 contains an unsaturated fatty acid and has less viscosity compared to Tween 60 which may have also played a role in better interaction and emulsification [24, 25]. Hence, Tween 80–based systems were considered superior over the Tween 60–based formulations. Among all, the combination of Cap C8 with Smix comprising T80-THP (1:1 w/w) and T80-PEG 200 (1:1 w/w) showed high microemulsion region and was shortlisted for LOR drug loading studies.
Fig. 1.
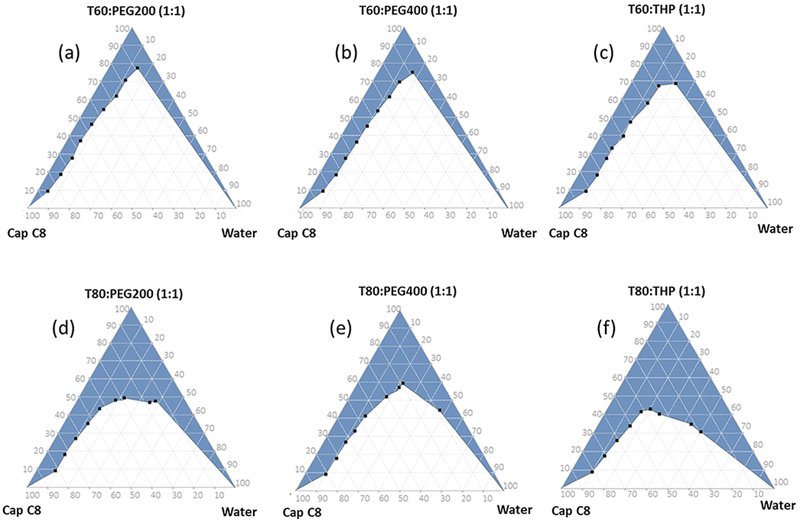
Pseudoternary phase diagram depicting the microemulsion region with various Smix a T60: PEG 200, bT60: PEG 400, cT60: THP, dT80: PEG 200, e T80: PEG 400, and f T80: THP in 1:1 w/w ratio
The maximum LOR drug loading that resulted in formation of stable, clear, and isotropic SMEDDS formulation without precipitate upon dilution with water was 2% w/w and 2.5% w/w for the SMEDDS comprising Smix T80-PEG200 and T80-THP, respectively. Hence, the SMEDDS formulation with Smix T80-THP was selected as a final optimum composition of proposed LOR SMEDDS. In this SMEDDS, 3% w/w drug loading resulted in slight turbidity while drug loading above that resulted in clear precipitation indicating formation of oversaturated and unstable LOR SMEDDS.
Based on the results, 2%w/w LOR drug loading was selected as a final optimum drug loading for the optimized SMEDDS. Here, slightly lower drug loading of 2% w/w was purposely selected over the maximum allowable LOR drug loading of 2.5% w/w to avoid development of saturated LOR SMEDDS that have higher probability of precipitation over the long-term stability.
Characterization of LOR SMEDDS
The LOR SMEDDS preconcentrate was an isotropic clear solution with slightly buff color. The formulation underwent spontaneous emulsification upon dilution with aqueous phase to form a clear solution with mild stirring using an overhead stirrer. The formed microemulsion exhibited a globule size of 95.13 ± 7.9 nm (PDI, 0.17 ± 0.04). This confirmed the kinetic and thermodynamic stability of the spontaneously emulsifying LOR-SEMDDS formulation. It is reported that microemulsions are thermodynamically and kinetically stable and undergo emulsification even with mild agitation [26, 27]. On the contrary, nanoemulsion is kinetically stable but is thermodynamically unstable which requires application of relatively high-shear energy to form nanoemulsion. Hence, the spontaneous emulsification of LOR SMEDSS confirmed the microemulsion formation [26, 27]. The microemulsion upon dilution of SMEDDS exhibited zeta potential of – 5.26 ± 0.28 mV. The low negative zeta potential can be justified by the composition of SMEDDS that comprise nonionic surfactant and stabilizers like T80 and THP. The stability of LOR SMEDDS with low zeta potential can be explained by steric stabilization offered by these nonionic surfactants as a predominant mechanism of stabilization [28–30].
It is important to determine the effect of pH and dilution on the microemulsion formed after dilution of SMEDDS. This is an imperative study as the SMEDDS formulation will witness variability in dilution and pH under various in vitro and in vivo conditions. For this, the globule size, PDI and transmittance of the SMEDDS formulation upon dilution using multiple buffers was assessed and the data is depicted in Table 1. The results indicated that the LOR SMEDDS were stable towards pH and dilution effects and an isotropic stable formulation was achieved. Further, it is important to note that the LOR formulated as SMEDDS not only offered an advantage of high drug loading but also assured high supersaturation, superior stability upon dilution, and lack of precipitation compared to the literature reported LOR lipid-based systems [18]. This can be attributed to the spontaneous emulsification and thermodynamic and kinetic stability of the SMEDDS.
Table 1.
Effect of pH and dilution on LOR SMEDDS (n = 3) results reported as mean ± S.D.
| Dilution factor | Globule size | PDI | % Transmittance |
|---|---|---|---|
| pH 1.2 | |||
| 10× | 89.18 ± 6.93 | 0.21 ± 0.02 | 99.18 ± 0.08 |
| 100× | 92.83 ± 9.29 | 0.2 ± 0.02 | 98.56 ± 0.12 |
| 250× | 97.71 ± 4.82 | 0.16 ± 0.03 | 99.03 ± 0.07 |
| 500× | 84.9 ± 7.23 | 0.21 ± 0.01 | 99.27 ± 0.09 |
| pH 7.4 | |||
| 10× | 95.13 ± 7.9 | 0.17 ± 0.04 | 99.73 ± 0.10 |
| 100× | 101.43 ± 5.83 | 0.18 ± 0.03 | 99.31 ± 0.05 |
| 250× | 92.49 ± 3.92 | 0.20 ± 0.02 | 99.49 ± 0.15 |
| 500× | 86.82 ± 4.98 | 0.18 ± 0.02 | 99.37 ± 0.07 |
During the formulation development and optimization study, the drug loading of LOR in LOR SMEDDS was optimized to 2% w/w. To ensure the incorporation of drug in SMEDDS, formulation assay of drug content was carried out. The LOR SMEDDS formulation revealed a drug content of 99.03 ± 0.013% w/w and hence confirmed successful incorporation of drug in the formulation and its stability. In vitro drug release studies were conducted with an objective to understand and assess the rate and extent of drug release from the SMEDDS formulation compared to free drugs at same drug concentration. The dissolution medium of 0.1 N HCl was selected as a recommended medium for LOR as per USFDA guidelines [31]. The in vitro drug release profile of LOR from LOR-SFN and LOR SMEDDS-SFN is depicted in Fig. 2(a). The results revealed that almost 100% drug LOR was released from the SMEDDS formulation within 1 h which was almost twofold higher than that of LOR alone (statistically significant p < 0.05). Also, the dissolution rate of LOR with LOR SMEDDS was observed to be higher compared to the lipid-based microparticulate systems reported in the literature [19]. This enhancement in rate and extent of drug dissolution was possible due to incorporation of LOR in SMEDDS formulation that formed an isotropic microemulsion with very small nano-droplet size (95.13 ± 7.9 nm) in the aqueous medium. This observed dissolution enhancement with LOR SMEDDS is very promising because LOR belongs to BCS class II (low solubility and high permeability) wherein the low solubility becomes the rate-limiting step in bioavailability. Herein, such significant enhancement in the LOR dissolution enables enhanced solubility and in turn enhanced bioavailability. The in vitro drug release profile of SFN from LOR-SFN and LOR SMEDDS-SFN is depicted in Fig. 2(b). The results revealed that almost 100% drug SFN was released from both the groups within 20 min and no significant difference in the in vitro dissolution profile was observed. This can be attributed to the fact that SFN belongs to BCS class I which has high solubility in aqueous medium and high permeability. This also corroborated our hypothesis that SFN could be administered in its original form as it has high aqueous solubility and reported high oral bioavailability [16].
Fig. 2.
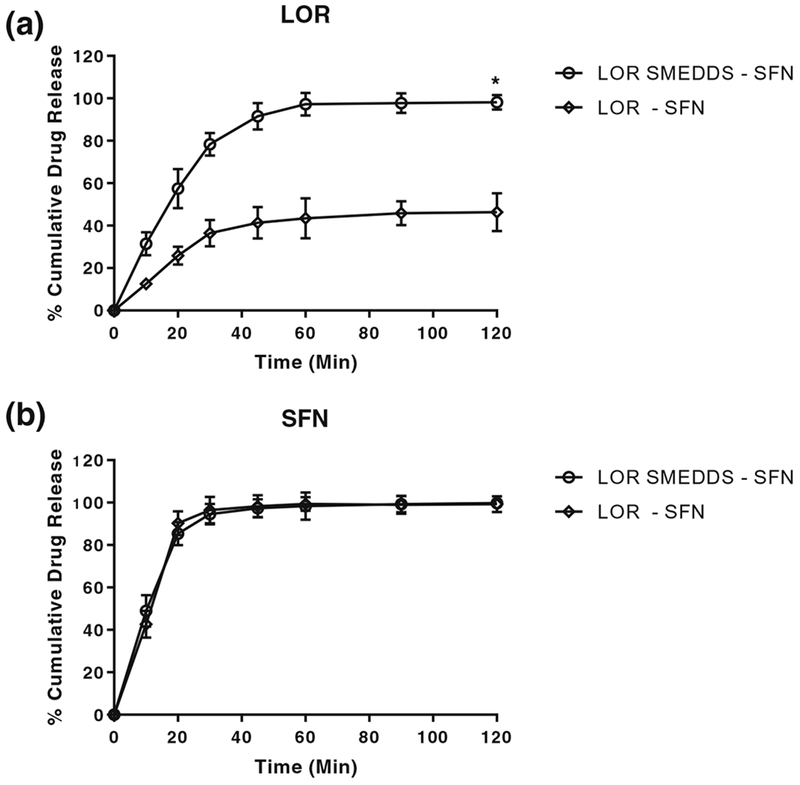
In vitro drug dissolution profile of a LOR and b SFN from LOR-SFN and LOR SMEDDS-SFN using dialysis bag method. The experiment was performed in triplicates (n = 3) and the data is expressed as mean ± S.D. Statistical significance determined using unpaired t test *p < 0.05 for LOR SMEDDS vs LOR
In vitro cytotoxicity studies
To determine the chemoprevention efficacy of LOR–SFN combination regimen, MTS assay was performed in two human pancreatic cell lines MIA PaCa-2 and Panc-1 and dose-dependent inhibition response was measured. The rationale behind using two human pancreatic cell lines was to confirm the consistency in the activity using multiple cell lines with similar homology. For this, MIA PaCa-2 and Panc-1 cells lines were selected because both cell lines present neuroendocrine differentiation. They have similar mutagenic origin involving KRAS and TP53 mutations with homozygous deletion that includes the first three exons of CDKN2A/p16INK4A but excludes SMAD4/DPC4 mutations. The observed variation in the IC50 values can be ascribed to the reported variation in their receptor expression [32].
In phase one, the impact of excipients of the SMEDDS formulation on the PC cell lines was assessed. The dose-dependent % inhibition data in MIA PaCa-2 and Panc-1 is depicted in Fig. 3. The excipients Cap C8, T80, and THP exhibited IC 50 values of 10.376 ± 0.862 mM, 0.858 ± 0.097 mM, and 12.287 ± 0.728 mM in MIA PaCa-2 cell line and 8.404 ± 0.396 mM, 0.996 ± 0.085 mM, and 13.334 ± 1.038 mM in Panc-1 cell line, respectively. It is important to note that the excipients showed inhibitory effect on PC cell lines at relatively higher concentration than what was employed in the formulation and hence could be considered to be safe. Furthermore, to assess the combined effect of the excipients in the form of SMEDDS formulation on the PC cell lines, the inhibitory effect of blank SMEDDS formulation was also assessed using MTS assay (Figs. 4 and 5). The studies concluded that the blank SMEDDS formulation did not have significant inhibitory effect on the PC cell lines in the concentrations used and hence can be considered as safe.
Fig. 3.
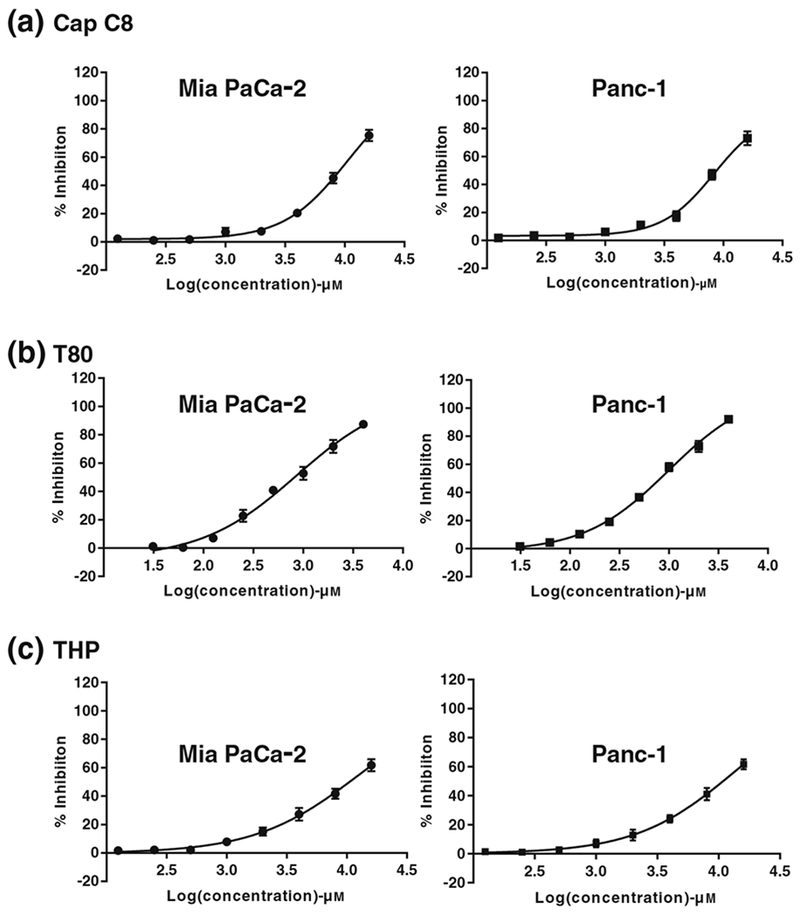
Dose-dependent cell inhibition study in human PC cell line MIA PaCa-2 and Panc-1 using MTS assay upon treatment with a Cap C8, b T80, and c THP. MTS assay was executed to determine the cell inhibition after treatment with various treatment groups over a wide concentration range. Graphpad Prism 7 software was used to analyze the data using non-linear regression analysis. The experiment was performed in triplicates (n = 3) and data is represented as mean inhibition in triplicate independent and parallel experiment
Fig. 4.
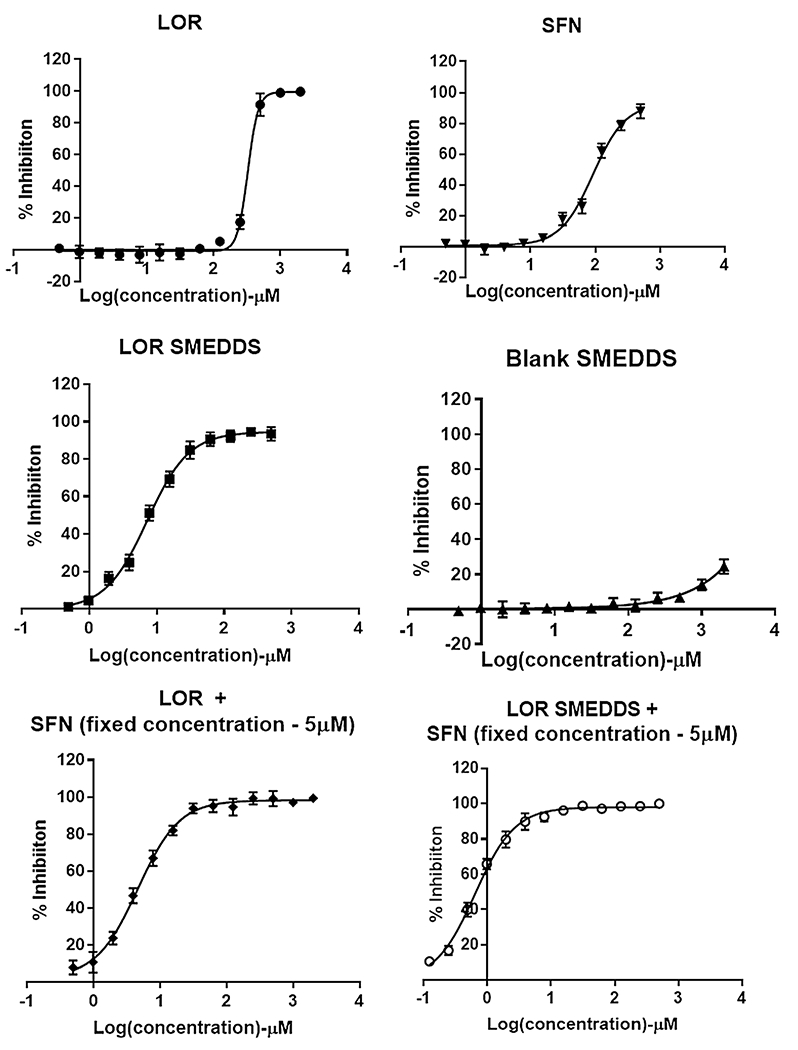
Dose-dependent cell inhibition study in human PC cell line MIA PaCa-2 using MTS assay upon treatment with a LOR, b SFN, c LOR SMEDDS, d Blank SMEDDS, e LOR-SFN, and f LOR SMEDDS-SFN. For the combination pancreatic chemoprevention study, the concentration of SFN was kept constant at 5 μM and concentration of LOR was varied. MTS assay was executed to determine the cell inhibition after treatment with various treatment groups over a wide concentration range. Graphpad Prism 7 software was used to analyze the data using non-linear regression analysis. The experiment was performed in triplicates (n = 3) and data is represented as mean inhibition in triplicate independent and parallel experiment
Fig. 5.
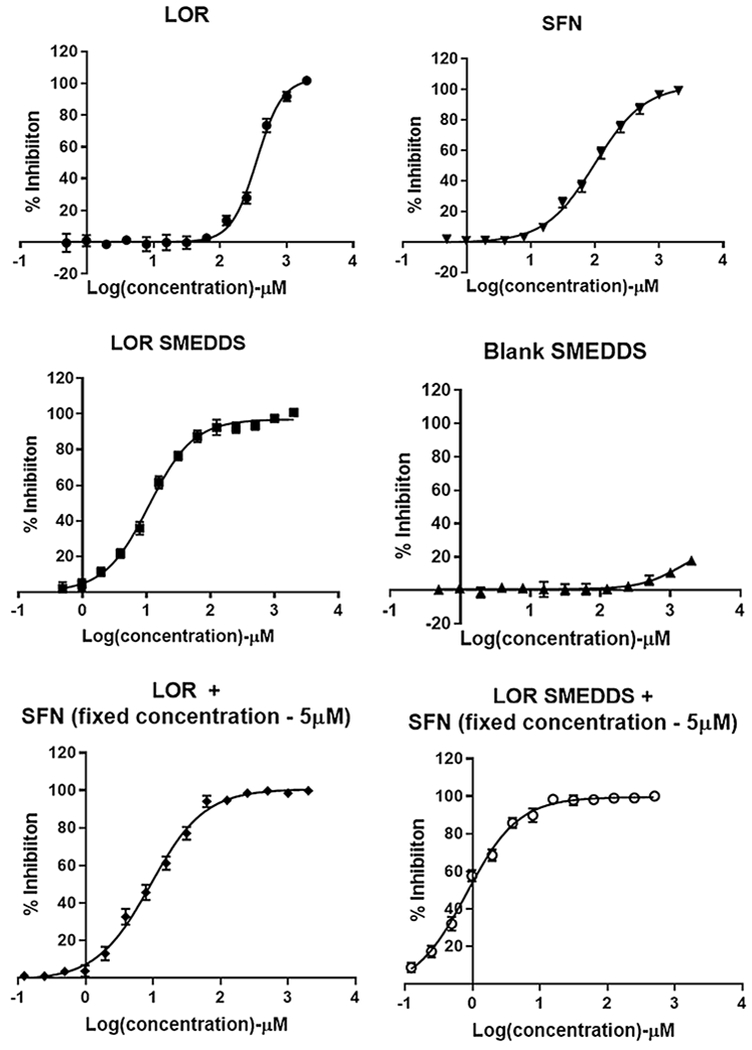
Dose-dependent cell inhibition study in human PC cell line Panc-1 using MTS assay upon treatment with a LOR, b SFN, c LOR SMEDDS, d Blank SMEDDS, e LOR-SFN, and f LOR SMEDDS-SFN. For the combination pancreatic chemoprevention study, the concentration of SFN was kept constant at 5 μM and concentration of LOR was varied. MTS assay was executed to determine the cell inhibition after treatment with various treatment groups over a wide concentration range. Graphpad Prism 7 software was used to analyze the data using non-linear regression analysis. The experiment was performed in triplicates (n = 3) and data is represented as mean inhibition in triplicate independent and parallel experiment
Further, the dose-dependent % inhibition data for LOR, SFN, and their formulations in MIA PaCa-2 and Panc-1 is depicted in Figs. 4 and 5, respectively. The IC50 concentrations were determined by subjecting the data to non-linear regression analysis using Graphpad prism 7 software and the IC50 values are represented in Fig. 6. It was observed that LOR and SFN exhibited IC50 values of 326.4 μM and 92.18 μM in MIA PaCa-2 cell line. The cell inhibition study showed that SFN did not elicit any measurable inhibition response below 15 μM and to assess the potential of LOR-SFN combination in chemoprevention, a threefold lower SFN concentration (5 μM) was selected (ensuring no inhibition response upon single drug SFN treatment) and MTS assay was performed by using varying concentration of LOR. The LOR–SFN (5-μM fixed concentration) exhibited an IC50 value of 4.6 μM in MIA PaCA-2 cell line. Hence, almost 70-fold reduction in IC50 concentration was observed when SFN was combined with LOR as compared to LOR treatment alone. This confirmed the synergistic PC chemoprevention potential of LOR-SFN combination. Similar results were observed in Panc-1 cell lines. Herein, LOR and SFN exhibited IC50 values of 356.8 μM and 99.59 μM in Panc-1 cell line. The LOR-SFN (5-μM fixed concentration) exhibited an IC50 value of 9.51 μM in Panc-1 cell line. Hence, almost 40-fold reduction in IC50 concentration was observed when SFN was combined with LOR as compared to LOR treatment alone. This corroborated the synergistic PC chemoprevention potential of LOR-SFN combination in multiple human PC cell lines.
Fig. 6.
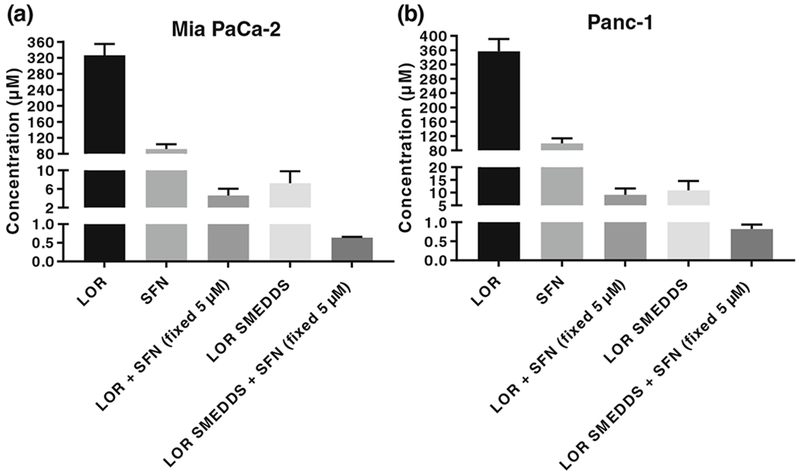
Comparison of IC50 values between LOR, SFN, LOR-SFN, and LOR SMEDDS-SFN in two human PC cell lines a MIA PaCa-2 and b Panc-1. For the combination pancreatic chemoprevention study, the concentration of SFN was kept constant at 5 μM and concentration of LOR was varied. MTS assay was executed to determine the cell inhibition after treatment with various treatment groups over a wide concentration range. Graphpad Prism 7 software was used to analyze the data using non-linear regression analysis. The experiment was performed in triplicates (n = 3) and data is represented as mean inhibition in triplicate independent and parallel experiment.
The second objective of the study was to enhance the performance of BCS class II drug LOR by incorporating it in a SMEDDS formulation. The studies showed that the LOR SMEDDS exhibited IC50 value of 7.25 μM and 10.90 μM in MIA PaCa-2 and Panc-1 cell lines, respectively. The results confirmed that incorporating LOR in SMEDDS formulation resulted in almost 45-fold and 32-fold reduction in IC50 values as compared to LOR alone in MIA PaCa-2 and Panc-1 cell lines, respectively. Interestingly, as stated earlier, the blank SMEDDS formulation did not elicit any measurable cell inhibition at the similar concentration confirming that the effect was observed due to LOR SMEDDS formulation approach only. This enhancement in the cell inhibitory activity can be attributed to the fact that the microemulsified LOR formulation with size less than 100 nm allowed enhanced solubility (significant enhancement as observed with in vitro dissolution studies (*p < 0.05) and better interaction with the cells that must have resulted in higher uptake and hence eliciting higher chemoprevention activity at relatively lower doses. Similar results have been reported in literature wherein enhancement in chemoprevention efficacy was confirmed using curcumin microemulsion in Panc-1 cell line (PC) and HT-29 (colon cancer cell line); gemcitabine-atorvastatin microemulsion in HCT 116 (colon cancer cell line) etc. [33–35].
With a positive lead from above studies, the next set of experiments were conducted wherein the chemoprevention efficacy of varying concentration of LOR SMEDDS in combination with fixed SFN concentration (5 μM) was studied in both the PC cell lines using MTS assay. The studies revealed an IC 50 concentration of 0.64 μM and 0.82 μM MIA PaCa-2 and Panc-1 cell lines, respectively. The results confirmed that the LOR SMEDDS-SFN combination resulted in sevenfold and 11-fold reduction in IC 50 value compared to LOR-SFN in MIA PaCa-2 and Panc-1 cell lines, respectively.
After confirming the chemoprevention activity in multiple PC cell lines, we conclude that the LOR-SFN combination elicits synergistic chemoprevention activity against PC. Further, formulating LOR in SMEDDS formulation resulted in additional enhancement in chemoprevention efficacy when used as LOR SMEDDS-SFN combination. Hence, we believe that a combination platform using the two drugs delivered in a nanocarrier system has great potential as a future PC chemoprevention regimen. This work is very timely because we believe that PC chemoprevention is possibly the best strategy for PC management in view of the very alarming facts that PC incidence is on rampant increase, diagnosis is late mostly after metastasis, life expectancy is very low, and limited success has been achieved in the treatment arena.
Conclusion
The incidence of PC is increasing exponentially and requires desperate preventive measures to arrest or lag the incidence and progression of disease. Our studies using a multimodal approach, successfully demonstrated that a unique combination of an antihistaminic drug with a neutraceutical compound encapsulated within a novel oral drug delivery system significantly enhanced chemoprevention efficacy of PC.
Acknowledgments
Funding This work was supported by the National Institutes of Health [grant num: 1R15CA182834-01].
Footnotes
Conflict of interest The authors declare that they have no conflict of interest.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59(4):225–49. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. [DOI] [PubMed] [Google Scholar]
- 3.Desai PAD, Wang J, Prabhu S. Pancreatic cancer: recent advances in nano-formulation based therapies. Critical Reviews™ in therapeutic drug carrier systems. 2018;Forthcoming Article. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller MS, Allen P, Brentnall TA, Goggins M, Hruban RH, Petersen GM, et al. Pancreatic cancer chemoprevention translational workshop: meeting report. Pancreas. 2016;45(8):1080–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stan SD, Singh SV, Brand RE. Chemoprevention strategies for pancreatic cancer. Nat Rev Gastroenterol Hepatol. 2010;7(6):347–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patterson SL, Colbert Maresso K, Hawk E. Cancer chemoprevention: successes and failures. Clin Chem. 2013;59(1):94–101. [DOI] [PubMed] [Google Scholar]
- 7.Sutaria D, Grandhi BK, Thakkar A, Wang J, Prabhu S. Chemoprevention of pancreatic cancer using solid-lipid nanoparticulate delivery of a novel aspirin, curcumin and sulforaphane drug combination regimen. Int J Oncol. 2012;41(6):2260–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thakkar A, Chenreddy S, Wang J, Prabhu S. Ferulic acid combined with aspirin demonstrates chemopreventive potential towards pancreatic cancer when delivered using chitosan-coated solid-lipid nanoparticles. Cell Biosci. 2015;5:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ellegaard AM, Dehlendorff C, Vind AC, Anand A, Cederkvist L, Petersen NHT, et al. Repurposing cationic amphiphilic antihistamines for cancer treatment. EBioMedicine. 2016;9:130–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kirshner JJ, McDonald MC 3rd, Kruter F, Guinigundo AS, Vanni L, Maxwell CL, et al. NOLAN: a randomized, phase 2 study to estimate the effect of prophylactic naproxen or loratadine vs no prophylactic treatment on bone pain in patients with early-stage breast cancer receiving chemotherapy and pegfilgrastim. Support Care Cancer. 2018;26(4):1323–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Soule BP, Simone NL, DeGraff WG, Choudhuri R, Cook JA, Mitchell JB. Loratadine dysregulates cell cycle progression and enhances the effect of radiation in human tumor cell lines. Radiat Oncol. 2010;5:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bertl E, Bartsch H, Gerhauser C. Inhibition of angiogenesis and endothelial cell functions are novel sulforaphane-mediated mechanisms in chemoprevention. Mol Cancer Ther. 2006;5(3):575–85. [DOI] [PubMed] [Google Scholar]
- 13.Liu P, Atkinson SJ, Akbareian SE, Zhou Z, Munsterberg A, Robinson SD, et al. Sulforaphane exerts anti-angiogenesis effects against hepatocellular carcinoma through inhibition of STAT3/HIF-1alpha/VEGF signalling. Sci Rep. 2017;7(1):12651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Russo M, Spagnuolo C, Russo GL, Skalicka-Wozniak K, Daglia M, Sobarzo-Sanchez E, et al. Nrf2 targeting by sulforaphane: a potential therapy for cancer treatment. Crit Rev Food Sci Nutr. 2018;58(8):1391–405. [DOI] [PubMed] [Google Scholar]
- 15.Kamal MM, Nazzal S. Novel sulforaphane-enabled self-microemulsifying delivery systems (SFN-SMEDDS) of taxanes: formulation development and in vitro cytotoxicity against breast cancer cells. Int J Pharm. 2018;536(1):187–98. [DOI] [PubMed] [Google Scholar]
- 16.Hanlon N, Coldham N, Gielbert A, Kuhnert N, Sauer MJ, King LJ, et al. Absolute bioavailability and dose-dependent pharmacokinetic behaviour of dietary doses of the chemopreventive isothiocyanate sulforaphane in rat. Br JNutr. 2008;99(3):559–64. [DOI] [PubMed] [Google Scholar]
- 17.Khan MZ, Rausl D, Zanoski R, Zidar S, Mikulcic JH, Krizmanic L, et al. Classification of loratadine based on the biopharmaceutics drug classification concept and possible in vitro-in vivo correlation. Biol Pharm Bull. 2004;27(10):1630–5. [DOI] [PubMed] [Google Scholar]
- 18.Gautschi N, Bergstrom CA, Kuentz M. Rapid determination of drug solubilization versus supersaturation in natural and digested lipids. Int J Pharm. 2016;513(1–2):164–74. [DOI] [PubMed] [Google Scholar]
- 19.Huang R, Tan Y, Shen L, Wang T, Quan D. A novel surfactant-free lipid-based formulation for improving oral bioavailability of loratadine using colloidal silicon dioxide as emulsifier and solid carrier. Curr Pharm Biotechnol. 2018;19(3):217–23. [DOI] [PubMed] [Google Scholar]
- 20.Stillhart C, Durr D, Kuentz M. Toward an improved understanding of the precipitation behavior of weakly basic drugs from oral lipid-based formulations. J Pharm Sci. 2014;103(4):1194–203. [DOI] [PubMed] [Google Scholar]
- 21.Üner M, Karaman E. Preliminary studies on solid lipid microparticles of loratadine for the treatment of allergic reactions via the nasal route. Trop J Pharm Res. 2013;12(3):287–93. [Google Scholar]
- 22.Desai PDA.; Patravale V. Overcoming poor oral bioavailability using nanoparticle formulations - opportunities and limitations. Drug Discov Today Technol. 2012;9(2):e71–e174. [DOI] [PubMed] [Google Scholar]
- 23.Dasgupta S, Mazumder B, Ghosh SK, Kaurav SS. Solid lipid nanoparticles (SLNs) for topical delivery of aceclofenac by using xanthan gum: ex vivo and in vivo evaluation. Curr Drug Deliv. 2012. [PubMed] [Google Scholar]
- 24.Berton-Carabin CRMH, Genot C Lipid oxidation in oil-in-water emulsions: involvement of the interfacial layer. Comprehensive Reviews in Food Science and Food Safety. 2014;13(5). [Google Scholar]
- 25.Mahdi ES, Sakeena MH, Abdulkarim MF, Abdullah GZ, Sattar MA, Noor AM. Effect of surfactant and surfactant blends on pseudoternary phase diagram behavior of newly synthesized palm kernel oil esters. Drug Des Devel Ther. 2011;5:311–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McClements DJ. Nanoemulsions versus microemulsions: terminology, differences, and similarities. Soft Matter. 2012;8(6):1719–29. [Google Scholar]
- 27.Mehta SK, Kaur G. Microemulsions: thermodynamic and dynamic properties Thermodynamics: INTECH Open Access Publisher; 2011. p. 381–406. [Google Scholar]
- 28.Nobel A Performance blends for emulsification: Akzo Nobel surface chemistry LLC; 2009. [cited 2018 June 15]. Available from: http://www.sc.akzonobel.com/en/agriculture/Documents/Letter_size/AkzoNobel_tb_71_Agro_Emulsion_Performance_Blends.pdf.
- 29.Beugin S, Edwards K, Karlsson G, Ollivon M, Lesieur S. New sterically stabilized vesicles based on nonionic surfactant, cholesterol, and poly(ethylene glycol)-cholesterol conjugates. Biophys J. 1998;74(6):3198–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stalidis G, Avranas A, Jannakoudakis D. Interfacial properties and stability of oil-in-water emulsions stabilized with binary mixtures of surfactants. J Colloid Interface Sci. 1990;135(2):313–24. [Google Scholar]
- 31.USFDA. Dissolution methods: USFDA; 2018. [cited 2018 June 15]. Available from: https://www.accessdata.fda.gov/scripts/cder/dissolution/dsp_getallData.cfm.
- 32.Gradiz R, Silva HC, Carvalho L, Botelho MF, Mota-Pinto A. MIA PaCa-2 and PANC-1 - pancreas ductal adenocarcinoma cell lines with neuroendocrine differentiation and somatostatin receptors. Sci Rep. 2016;6:21648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alkhatib MA-SD; Backer W Cytotoxic effect of the combination of gemcitabine and atorvastatin loaded in microemulsion on the HCT116 colon cancer cells. Int J Pharm Clin Res. 2017;9(2):146–55. [Google Scholar]
- 34.Chen YC, Chen BH. Preparation of curcuminoid microemulsions from Curcuma longa L. to enhance inhibition effects on growth of colon cancer cells HT-29. RSC Adv. 2018;8(5):2323–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Margulis K, Srinivasan S, Ware MJ, Summers HD, Godin B, Magdassi S. Active curcumin nanoparticles formed from a volatile microemulsion template. J Mater Chem B. 2014;2:3745–52. [DOI] [PMC free article] [PubMed] [Google Scholar]


