Abstract
Introduction
Radium-223 (Ra223) prolongs the survival and improves the quality of life of men with metastatic, castration-resistant prostate cancer (mCRPC) to bones. However, compared to other mCRPC therapies, using Ra223 comes with its unique challenges. Hence, we aimed to identify Ra223 utilization patterns under real-world conditions, as well as factors predicting treatment completion and outcome.
Methods
In this retrospective chart analysis, 198 mCRPC patients were identified that had received Ra223 outside of clinical trials or access programs from January 2015 to October 2016 at four cancer centres in Ontario. The main outcomes studied were Ra223 completion rate, reasons for early treatment discontinuation, overall survival, and survival differences in patients completing Ra223 therapy versus patients receiving <6 cycles of Ra223. In addition, patient and disease characteristics were analysed to identify predictors of treatment completion and survival.
Results
In this cohort of patients mostly pretreated with abiraterone and/or enzalutamide (92.4%), almost half of which had also received docetaxel (48.5%), the Ra223 completion rate was 46.5%, and the actuarial median survival was 13.3 months. The main reason for early Ra223 discontinuation was disease progression, and Ra223 non-completion was associated with poorer outcome (median survival 8.1 months [6.0–12.2] versus 18.7 months [15.3–22.3] in men completing Ra223, p<0.0001). Lymph node metastases and a high baseline prostate-specific antigen (PSA) were independent predictors of early treatment discontinuation. Multivariable Cox proportional hazards models revealed early Ra223 discontinuation, baseline anemia, high PSA, prior skeletal-related events, visceral metastases, and being referred to another centre for Ra223 therapy as predictors of worse outcome.
Conclusion
Despite a lower completion rate than observed under clinical trial conditions, the real-world results achieved with Ra223 are encouraging. If prospectively validated, predictive patient and disease characteristics identified in our cohort might become instrumental to identify mCRPC patients likely to complete and to most benefit from Ra223 therapy.
Keywords: radium 223, metastatic prostate cancer, real-world, predictive markers of outcome
Introduction
Bone is the most common site of metastasis in men with prostate cancer, and bone metastases are the main contributor of prostate cancer-related morbidity and mortality.1 While antiresorptive agents (eg, denosumab and zoledronic acid) delay the onset and reduce the frequency of skeletal-related events (SRE) such as pain, pathological fractures, and cord compressions, they do not alter the overall survival (OS) of men with metastatic, castration-resistant prostate cancer (mCRPC).2,3
Radium-223 (Ra223) is the first, and thus far only, alpha-emitting radiopharmaceutical approved for the treatment of bone metastases in men with mCRPC.4 Due to the high linear energy transfer of alpha particles, combined with a low range of tissue penetration, Ra223 treatment allows for the safe administration of high levels of radiation to areas of bone metastases.5 In fact, the calcium-mimetic Ra223 is deposited in areas of high bone turnover such as the matrix of bone metastases, induces DNA double-strand breaks in prostate cancer cells, and decreases the number of osteoblasts as well as osteoclasts.6 In the randomized, double-blind and placebo-controlled ALSYMPCA Phase III registration trial, mCRPC patients with symptomatic bone metastases who had received, were not eligible for, or declined docetaxel chemotherapy were treated with Ra223 versus best supportive care.4 Ra223 improved OS by 3.6 months (14.9 versus 11.3 months, HR 0.7, P<0.001), delayed the median time to first symptomatic SRE by 5.8 months (15.6 versus 9.8 months, HR 0.66, P<0.001), and was well tolerated with typically low-grade treatment-related adverse events, such as myelosuppression, diarrhea, nausea, and fatigue. In fact, men undergoing Ra223 therapy experienced less adverse events overall than patients in the placebo arm. In the experimental arm, the median number of Ra223 cycles of 50 kBq per kilogram of body weight intravenously every 4 weeks was six (ie, the maximal number of cycles studied), whereas the median number of placebo treatments in the control arm was four. Based on these findings, Ra223 was made available to men with mCRPC to bones in Ontario through Cancer Care Ontario’s New Drug Funding Program (NDFP) in January 2015.
An analysis of mCRPC patients undergoing docetaxel chemotherapy in routine practice at the Princess Margaret Cancer Centre (Toronto/ON, Canada) showed that real-world patients were older, had a worse performance status, and received fewer treatment cycles compared to clinical trial patients, even though the reasons for docetaxel discontinuation were similar.7 The OS of patients seen in routine practice was shorter, whereas the rate of treatment-associated adverse events was higher.
Thus, we found it of great interest to study whether the real-world experience with Ra223 in Ontario showed similar trends, notably if there was a higher rate of early treatment discontinuation (ie, before completion of six cycles) compared to ALSYMPCA. In addition, we aimed to study patient and disease characteristics predicting early treatment discontinuation and outcome in men with mCRPC undergoing Ra223 therapy.
Patients And Methods
Study Design And Patient Population
In Ontario, the NDFP provides universal funding of new intravenous or subcutaneous systemic therapies for the treatment of cancer. To review Ra223 utilization in Ontario, we identified men with mCRPC who received at least one dose of Ra223 via NDFP funding in three participating centres from January 2015 to April 2016 (Sunnybrook Odette Cancer Centre [Toronto], Juravinski Hospital [Hamilton], and Lakeridge Health [Oshawa]), and at Princess Margaret Cancer Centre [Toronto] from January 2015 to October 2016. The provincial funding criteria are as follows: 1) Ra223 is for the treatment of patients with CRPC with symptomatic bone metastases and no known visceral metastatic disease; 2) Ra223 cannot be combined with cabazitaxel, abiraterone, or enzalutamide; 3) if Ra223 is funded in the pre-docetaxel setting, no subsequent funding will be considered in the post-docetaxel setting; and 4) there is a mandatory consult with a medical or radiation oncologist prior to starting Ra223 treatment. For our analyses, patients that received Ra223 as part of clinical trials or early access programs were excluded.
With approval from local research ethics boards (ie, Sunnybrook Research Institute, Toronto, Ontario, Canada; Juravinski Hospital, Hamilton, Ontario, Canada; Durham Regional Cancer Centre, Oshawa, Ontario, Canada; and Princess Margaret Cancer Centre, Toronto, Ontario, Canada), we retrospectively collected 1) patient and disease characteristics, 2) details of Ra223 treatment, 3) baseline as well as on-treatment hematological and biochemical parameters, 4) adverse events, and 5) Edmonton Symptom Assessment System (ESAS) scores (assessing 9 symptoms on a scale from 0 to 10, including pain).8 The study was performed in compliance with the Declaration of Helsinki. Based on the absence of any therapeutic or diagnostic interventions, and accounting for the fact that many patients had expired at the time of data collection we obtained permission to proceed without obtaining informed consent from patients. Data were de-identified immediately after collection to guarantee confidentiality.
Ra223 Treatment And Response Evaluation
We collected information on the number of Ra223 cycles per patient and the reasons for early Ra223 discontinuation, defined as having received <6 cycles. For prostate-specific antigen (PSA) and alkaline phosphatase (ALP)-based parameters we used definitions applied in ALSYMPCA and the International Ra223 Early Access Program (iEAP),4,9 as follows: 1) PSA30: ≥30% PSA decrease (whenever) during Ra223 therapy compared to baseline value, confirmed by a second PSA value approximately 4 or more weeks later 2) PSA50: ≥50% PSA decrease (whenever) during Ra223 therapy compared to baseline value, confirmed by a second PSA value approximately 4 or more weeks later, 3) ALP30: ≥30% ALP reduction from baseline value (whenever), confirmed 4 or more weeks later, 4) ALP normalization: ALP decrease to below upper limit of normal within 12 weeks of Ra233 therapy (in patients with elevated ALP at baseline), confirmed by two consecutive measurements at least 2 weeks apart. June 2017 was the cut-off for following up on the vital status of each patient.
Statistical Analyses
To compare patient and disease characteristics in men receiving 1–5 vs 6 cycles of Ra223, Wilcoxon rank-sum and Chi-squared/Fisher exact tests were applied for continuous and categorical variables, respectively. A two-sided p-value <0.05 was considered significant. The findings of time-to-event analyses were illustrated using Kaplan–Meier curves, and the log-rank test was applied for detecting statistical significance of differential OS (defined as the time from first dose of Ra223 to death or last follow-up date) seen between patients who completed Ra223 therapy and those that received 1–5 treatments only.
To identify predictive factors of receiving less than 6 cycles of Ra223, univariate logistic regression analysis was applied to create a logit prediction model of 1–5 vs 6 cycles of Ra223 using demographic/clinical factors as possible predictors. R2 was applied for measure of fit in the modeling. R2 equals (LO–LM)/LO, where LO and LM represent the maximized –2(log-likelihood) of the null model and the fitted model, respectively. R2 indicates the proportion of the overall response variation that can be explained by the predictive factors. The larger the R2, the better the model fit. Natural log-transformation was applied for some covariates for normalizing their distribution (eg, PSA, ALP, hemoglobin (Hb), and ESAS pain score subscale). P-values, OR, and 95% CIs were calculated for each predictive factor. A p-value of <0.05 was considered as statistically significant. All variables with a p-value of <0.10 obtained from univariate analysis were added in a backward stepwise selection procedure in the logistic regression analysis to find the most significant predictive factors for receiving less than 6 cycles of Ra223.
To identify predictive factors of OS, we applied the univariate Cox proportional hazards model. Natural log-transformation was used for some covariates to normalize their distribution (ie, PSA, ALP, Hb, and ESAS pain score subscale). A p-value <0.05 was considered statistically significant. HR and CI were calculated for each covariate. The generalized R2 statistic was calculated based on the likelihood ratio statistic (LRT) for testing the global null hypothesis using the formula R2=1–e–(LRT/n) (where LRT=−2logL(0)–[−2logL(p)]; n is the sample size used; logL(0) is the log-likelihood for a null model with no covariates; and logL(p) is the log-likelihood for the fitted model with p covariates).10 R2 (between 0 and 1) is larger when the covariates are more strongly associated with the outcome. Variables from univariate analysis with a p-value of <0.10 were included in the multivariable Cox proportional hazard model. Applying a stepwise backward selection procedure, we performed separated stepwise selection procedures due to co-linearity between continuous and categorical ALP and Hb variables, respectively. The following steps were used:
All variables with p<0.10+ALP continuous variable+Hb continuous variable
All variables with p<0.10+ALP ≥115 versus <115+Hb continuous variable
All variables with p<0.10+ALP continuous variable+Hb ≤100 versus >100
All variables with p<0.10+ALP ≥115 versus <115+Hb ≤100 versus >100
Comparing R2 values among all the above 4 selected models, we chose the model with the largest R2 value as the final model, including only significant covariates (p<0.05).
The statistical analyses were performed by Dr L. Zhang, PhD, biostatistician, using Statistical Analysis Software (SAS version 9.4, Cary, NC) and R package (version 3.5.2).
Results
Patients, Treatment Completion Rates, And Reasons For Early Ra223 Discontinuation
We identified 203 patients of which 198 were treated with at least one dose of Ra223 (Figure 1). Medical oncologists (100 patients, 50.5%) and radiation oncologists (94 patients, 47.5%) supervised the majority of Ra223 treatments. Uro-oncologists prescribed Ra223 in 4 patients (2%) only, likely due to the provincial funding guidelines mandating uro-oncologists in Ontario to obtain a medical or radiation oncology opinion before initiating Ra223 therapy. One hundred and ten patients (55.6%) were already undergoing CRPC therapy at the four participating centres (hereafter termed “local patients”) prior to the start of Ra223, whereas 88 patients (44.4%) were external referrals (named “referred patients”) sent from peripheral centres for treatment.
Figure 1.
Consort diagram and listing of reasons for early Ra223 discontinuation. One hundred and six of 198 patients (53.5%) did not receive 6 cycles of Ra223, and disease progression was the most common reason for early treatment discontinuation in those patients.
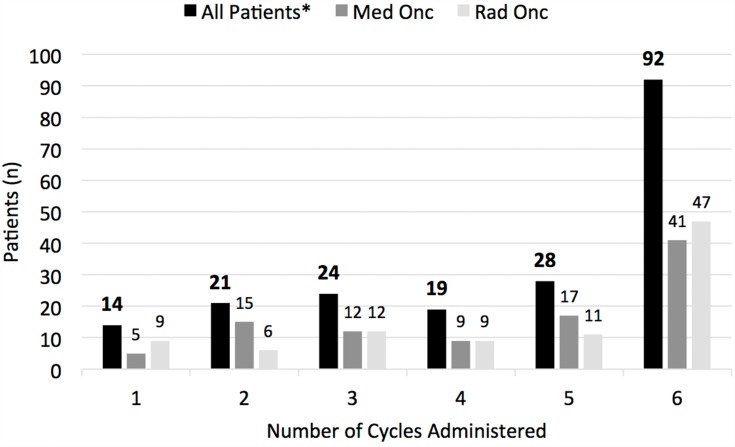
Ninety-two men (46.5%) received a full treatment course of 6 doses, and overall, the median number of Ra223 cycles was 5 (Figure 2). There was a trend for a higher Ra223 therapy completion rate over time, with 45.2% of men finishing 6 courses of Ra223 when treatment started during the period from May to August 2015, compared to 54.4% of men starting treatment from January to April 2016.
Figure 2.
Distribution of the number of Ra223 cycles administered overall, and when comparing the number of Ra223 cycles given in patients supervised by medical versus radiation oncologists. There was no significant difference in the distribution of Ra223 cycles administered under the supervision of medical oncologists (Med Onc) versus radiation oncologists (Rad Onc). *“All patients” includes patients administered Ra223 by medical oncologists (n=100), radiation oncologists (n=94), and uro-oncologists (n=4).
Amongst the 106 patients that did not complete 6 cycles of Ra223, the main reasons for treatment discontinuation as indicated by the prescribing physicians were “disease progression” (44.3%) and “symptomatic progression” (28.3%) (Figure 1). Another 12 patients (11.3%) stopped Ra223 therapy early because of low blood counts. Due to the retrospective nature of our analysis, we were not able to differentiate between Ra223-mediated myelotoxicity versus disease progression (notably progressive bone marrow infiltration) as the reason for low blood counts. “Disease progression” and “symptomatic progression” combined accounted for 69.6% of treatment discontinuations in men prescribed Ra223 from May to August 2015, compared to a higher rate of 84.6% for patients starting Ra223 from January to April 2016. In contrast, “low blood counts” was less frequently cited as reason for treatment discontinuation over time (29.1% versus 3.9% of patients that started Ra223 therapy from May to August 2015 compared to January to April 2016, respectively).
Baseline Patient And Disease Characteristics
Demographic and other patient characteristics are summarized in Table 1. The median age of the overall cohort was 74.1 years. The majority of patients were initially diagnosed with localized prostate cancer. Almost half of all patients featured Gleason Score 8–10 disease. Prior to Ra223, two-thirds of men had received at least two lines of systemic therapy for recurrent or metastatic disease (excluding androgen deprivation therapy), including 92.4% of men with exposure to abiraterone and/or enzalutamide, and 48.5% with prior docetaxel chemotherapy. The sequences of systemic therapies preceding Ra223 are detailed in Table S1. Thirty-one patients (15.7%) had experienced a SRE before starting Ra223, and 36 (18.2%) had been subjected to prior denosumab and/or zoledronic acid therapy.
Table 1.
Patient Characteristics
| TotalN = 198 | Received 1-5 Cycles n=106 | Received 6 Cycles n=92 | p-value | |
|---|---|---|---|---|
| Age (years) | 0.0574 | |||
| N | 198 | 106 | 92 | |
| Mean ± SD | 74.1 ± 9.5 | 73.0 ± 10.0 | 75.4 ± 8.8 | |
| Median (interquartiles) | 75.0 (68.0, 81.0) | 72.0 (66.0, 80.0) | 77.0 (69.5, 81.5) | |
| Range | 52, 93 | 52, 93 | 55, 92 | |
| Treatment centre | 0.0996 | |||
| Sunnybrook | 33 (16.67%) | 16 (15.09%) | 17 (18.48%) | |
| Princess Margaret | 77 (38.89%) | 42 (39.62%) | 35 (38.04%) | |
| Lakeridge | 28 (14.14%) | 10 (9.43%) | 18 (19.57%) | |
| Hamilton | 60 (30.30%) | 38 (35.85%) | 22 (23.91%) | |
| Specialty of treating physician | 0.3040 | |||
| Medical Oncologist | 100 (50.51%) | 58 (54.72%) | 42 (45.65%) | |
| Radiation Oncologist | 94 (47.47%) | 47 (44.34%) | 47 (51.09%) | |
| Urological Oncologist | 4 (2.02%) | 1 (0.94%) | 3 (3.26%) | |
| Local versus referred patients | 0.2648 | |||
| Local | 110 (55.56%) | 55 (51.89%) | 55 (59.78%) | |
| Referred | 88 (44.44%) | 51 (48.11%) | 37 (40.22%) | |
| Initial stage | 0.8334 | |||
| Localized | 116 (58.59%) | 64 (60.38%) | 52 (56.52%) | |
| Metastatic | 36 (18.18%) | 19 (17.92%) | 17 (18.48%) | |
| Unknown | 46 (23.23%) | 23 (21.70%) | 23 (25.00%) | |
| Local therapy | 0.0869 | |||
| Prostatectomy | 67 (33.84%) | 30 (28.30%) | 37 (40.22%) | |
| Radiation | 61 (30.81%) | 40 (37.74%) | 21 (22.83%) | |
| Prostatectomy/Radiation | 20 (10.10%) | 12 (11.32%) | 8 (8.70%) | |
| No local therapy | 50 (25.25%) | 24 (22.64%) | 26 (22.26%) | |
| Adjuvant androgen deprivation therapy | 0.3608 | |||
| No | 140 (70.71%) | 77 (72.64%) | 63 (68.48%) | |
| Yes | 56 (28.28%) | 29 (27.36%) | 27 (29.35%) | |
| Unknown | 2 (1.01%) | 0 (0.00%) | 2 (2.17%) | |
| Gleason score categories | 0.5814 | |||
| 6 | 5 (2.53%) | 2 (1.89%) | 3 (3.26%) | |
| 7 | 57 (28.79%) | 32 (30.19%) | 25 (27.17%) | |
| 8-10 | 94 (47.47%) | 53 (50.00%) | 41 (44.57%) | |
| Unknown | 42 (21.21%) | 19 (17.92%) | 23 (25.00%) | |
| Time from start of androgen deprivation therapy to castration resistance (months) | 0.3861 | |||
| N | 119 | 61 | 58 | |
| Mean ± SD | 34.67 ± 37.52 | 28.83 ± 25.04 | 40.82 ± 46.68 | |
| Median (interquartiles) | 18.0 (11.0, 49.0) | 19.0 (10.0, 44.4) | 18.0 (12.0, 49.0) | |
| Range | 1.0, 224.4 | 1.0, 100.0 | 1.0, 224.4 | |
| Number of lines of prior therapies | 0.0977 | |||
| N | 198 | 106 | 92 | |
| Mean ± SD | 2.0 ± 1.1 | 2.2 ± 1.1 | 1.9 ± 1.0 | |
| Median (interquartiles) | 2.0 (1.0, 3.0) | 2.0 (1.0, 3.0) | 2.0 (1.0, 3.0) | |
| Range | 0, 5 | 0, 5 | 0, 5 | |
| Distribution of number of lines of prior therapies | 0.6994 | |||
| 0 | 7 (3.54%) | 3 (2.83%) | 4 (4.35%) | |
| 1 | 64 (32.32%) | 30 (28.30%) | 34 (36.96%) | |
| 2 | 62 (31.31%) | 34 (32.08%) | 28 (30.43%) | |
| 3 | 50 (25.25%) | 29 (27.36%) | 21 (22.83%) | |
| 4 | 10 (5.05%) | 7 (6.60%) | 3 (3.26%) | |
| 5 | 5 (2.53%) | 3 (2.83%) | 2 (2.17%) | |
| Prior Abiraterone and/or Enzalutamide | 0.2743 | |||
| No | 15 (7.58%) | 6 (5.66%) | 9 (9.78%) | |
| Yes | 183 (92.42%) | 100 (94.34%) | 83 (90.22%) | |
| Prior Docetaxel | 0.0596 | |||
| No | 102 (51.52%) | 48 (45.28%) | 54 (58.70%) | |
| Yes | 96 (48.48%) | 58 (54.72%) | 38 (41.30%) | |
| Prior Denosumab and/or Zoledronic Acid | 0.1143 | |||
| No | 25 (12.63%) | 10 (9.43%) | 15 (16.30%) | |
| Yes | 36 (18.18%) | 16 (15.09%) | 20 (21.74%) | |
| Unknown | 137 (69.19%) | 80 (75.47%) | 57 (61.96%) | |
| Prior skeletal related events | 0.1735 | |||
| No | 166 (83.84%) | 86 (81.13%) | 80 (86.96%) | |
| Yes | 31 (15.66%) | 20 (18.87%) | 11 (11.96%) | |
| Unknown | 1 (0.51%) | 0 (0.00%) | 1 (1.09%) | |
| Vital status June 2017 | 0.0045 | |||
| Alive | 128 (64.65%) | 59 (55.66%) | 69 (75.00%) | |
| Dead | 70 (35.35%) | 47 (44.34%) | 23 (25.00%) | |
| Time to death/last follow-up (months) | <.0001 | |||
| N | 198 | 106 | 92 | |
| Mean ± SD | 8.31 ± 5.16 | 5.60 ± 3.87 | 11.43 ± 4.70 | |
| Median (interquartiles) | 7.5 (4.1, 12.0) | 4.4 (3.1, 7.8) | 11.0 (8.0, 15.1) | |
| Range | 0.0, 22.3 | 0.0, 18.4 | 1.3, 22.3 |
Notes: Patient characteristics significantly different in patients receiving 1–5 versus 6 cycles of Ra223 are highlighted (bold).
Median baseline PSA, ALP, and Hb values were 78 μg/L, 111 U/L, and 120 g/L, respectively (Table 2). The presence of nodal metastases was documented in 34 patients (17.2%), and although Ra223 use is not advised in case of visceral metastases we found evidence for visceral metastatic disease in 18 men (9.1%). The median ESAS pain score was 2 (range 0 to 10), indicating typically mild pain in the majority of patients.11 The median ESAS total score was found to be 6 (range 0 to 15) and thus was corresponding to an overall low self-reported symptom burden.
Table 2.
Baseline Laboratory And Radiological Findings, And ESAS Scores
| TotalN = 198 | Received 1-5 Cycles n=106 | Received 6 Cycles n=92 | p-value | |
|---|---|---|---|---|
| PSA (µg/L) | <.0001 | |||
| N | 164 | 89 | 75 | |
| Mean ± SD | 271.50 ± 692.83 | 354.70 ± 776.24 | 172.76 ± 567.95 | |
| Median (interquartiles) | 78.0 (28.3, 193.0) | 112.0 (48.9, 376.0) | 43.1 (12.8, 101.9) | |
| Range | 0.8, 6500.7 | 9.2, 6500.7 | 0.8, 4081.5 | |
| ALP (U/L) | 0.0322 | |||
| N | 151 | 79 | 72 | |
| Mean ± SD | 191.3 ± 254.0 | 230.2 ± 320.5 | 148.6 ± 141.1 | |
| Median (interquartiles) | 111.0 (72.0, 206.0) | 148.0 (76.0, 234.0) | 94.5 (71.0, 167.5) | |
| Range | 12, 2152 | 12, 2152 | 38, 739 | |
| ALP ≥115 U/L | 0.0052 | |||
| <115 | 76 (38.38%) | 30 (28.30%) | 46 (50.00%) | |
| ≥115 | 75 (37.88%) | 49 (46.23%) | 26 (28.26%) | |
| Unknown | 47 (23.74%) | 27 (25.47%) | 20 (21.74%) | |
| Hb (g/L) | <.0001 | |||
| N | 178 | 97 | 81 | |
| Mean ± SD | 119.3 ± 17.1 | 114.4 ± 16.8 | 125.1 ± 15.6 | |
| Median (interquartiles) | 120.0 (109.0, 132.0) | 115.0 (104.0, 126.0) | 125.0 (116.0, 134.0) | |
| Range | 75, 161 | 75, 160 | 85, 161 | |
| Hb ≤100 g/L | 0.0205 | |||
| >100 | 154 (77.78%) | 78 (73.58%) | 76 (82.61%) | |
| ≤100 | 24 (12.12%) | 19 (17.92%) | 5 (5.43%) | |
| Unknown | 20 (10.10%) | 9 (8.49%) | 11 (11.96%) | |
| Neutrophils (×109/L) | 0.1005 | |||
| Mean ± SD | 4.83 ± 2.28 | 4.96 ± 1.99 | 4.65 ± 2.63 | |
| Median (interquartiles) | 4.5 (3.3, 5.7) | 4.9 (3.3, 6.1) | 4.1 (3.2, 5.4) | |
| Range | 0.8, 20.4 | 0.8, 10.9 | 1.2, 20.4 | |
| Neutrophils >3×109/L | 0.0815 | |||
| ≤3 | 27 (13.64%) | 15 (14.15%) | 12 (13.04%) | |
| >3 | 121 (61.11%) | 71 (66.98%) | 50 (54.35%) | |
| Unknown | 50 (25.25%) | 20 (18.87%) | 30 (32.61%) | |
| Bone metastases | 0.1388 | |||
| No | 0 (0%) | 0 (0%) | 0 (0%) | |
| Yes | 172 (86.87%) | 96 (90.57%) | 76 (82.61%) | |
| Unknown | 26 (13.13%) | 10 (9.43%) | 16 (17.39%) | |
| Nodal metastases | 0.0067 | |||
| No | 138 (69.70%) | 70 (66.04%) | 68 (73.91%) | |
| Yes | 34 (17.17%) | 26 (24.53%) | 8 (8.70%) | |
| Unknown | 26 (13.13%) | 10 (9.43%) | 16 (17.39%) | |
| Visceral metastases | 0.2548 | |||
| No | 154 (77.78%) | 86 (81.13%) | 68 (73.91%) | |
| Yes | 18 (9.09%) | 10 (9.43%) | 8 (8.70%) | |
| Unknown | 26 (13.13%) | 10 (9.43%) | 16 (17.39%) | |
| ESAS Pain Score | 0.0516 | |||
| N | 116 | 55 | 61 | |
| Mean ± SD | 2.7 ± 2.7 | 3.1 ± 2.7 | 2.3 ± 2.8 | |
| Median (interquartiles) | 2.0 (0.0, 4.0) | 3.0 (1.0, 5.0) | 1.0 (0.0, 3.0) | |
| ESAS Total Score | 0.0282 | |||
| N | 117 | 55 | 62 | |
| Mean ± SD | 6.4 ± 3.4 | 7.1 ± 3.5 | 5.8 ± 3.3 | |
| Median (interquartiles) | 6.0 (4.0, 9.0) | 7.0 (4.0, 10.0) | 5.0 (4.0, 7.0) |
Notes: Patient findings significantly different in patients receiving 1–5 versus 6 cycles of Ra223 are highlighted (bold).
Patient Outcome
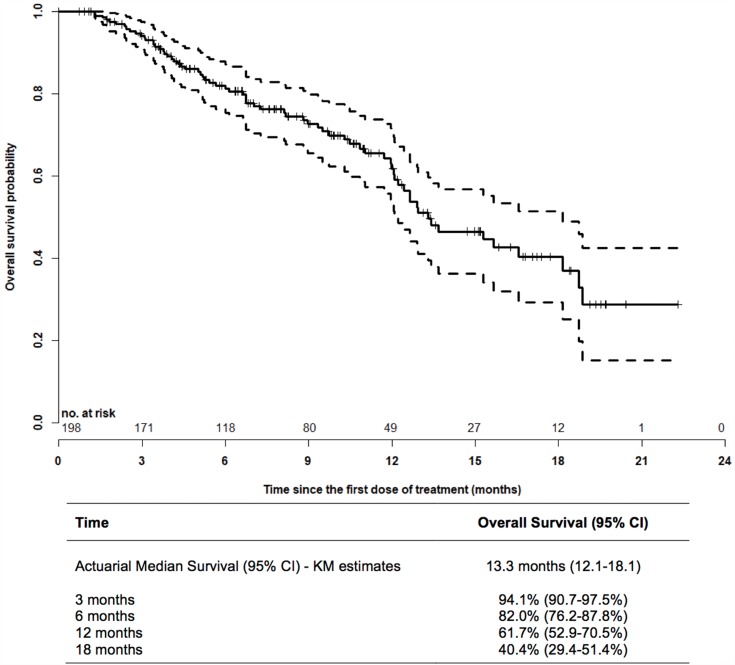
The PSA30 and PSA50 response rates were 16% and 8%, respectively, whereas an ALP30 response was observed in 44% of evaluable patients, and ALP normalization in 35% (Table 3). The median OS of the entire study population was 13.3 months (Figure 3). By comparison, the OS of men receiving 6 cycles of Ra223 was 18.7 months, and thus significantly longer than the survival of patients with early treatment discontinuation (8.1 months; log-rank test p<0.0001) (Figure 4).
Table 3.
Biochemical Response Rates
| Ontario Cohort | ALSYMPCA (n=614, Ra223 Treatment Arm) |
International Ra223 EAP (n=696) |
||
|---|---|---|---|---|
| n (%) | Evaluable Patients | |||
| PSA 30% response | 21 (16) | 134 | 98 (16) | 97 (14) |
| PSA 50% response | 10 (8) | 134 | N/A | 57 (8) |
| ALP 30% response | 54 (44) | 123 | 233 (47) | 327 (47) |
| ALP normalization | 18 (35) | 51 | 109 (34) | N/A |
Abbreviations: EAP, early access program; N/A, not available.
Figure 3.
Kaplan–Meier overall survival analysis from the first dose of Ra223. In the entire cohort of patients, the actuarial median overall survival from the first dose of Ra223 was 13.3 months. Seventy patients had died, 128 patients were censored. Dashed lines: 95% CI.
Figure 4.
Comparison of the overall survival of patients completing Ra223 therapy (6 cycles) versus patients with early treatment discontinuation (1–5 cycles). Log-rank testing revealed a significant (p<0.0001) overall survival benefit of patients completing Ra223 therapy compared to men receiving 1–5 treatments only, with an actuarial median survival time of 18.7 versus 8.1 months, respectively.
Predictors Of Early Treatment Discontinuation
When comparing men receiving 1–5 versus 6 cycles of Ra223, patients who completed Ra223 therapy tended to be older and less likely to have undergone docetaxel chemotherapy, but none of the patient characteristics detailed in Table 1 were significantly different in the two treatment cohorts. However, early Ra223 discontinuation was associated with higher total EASA scores (p=0.0282) (Table 2). Similarly, higher PSA and ALP values were significantly related to receiving less than 6 cycles of Ra223, whereas Hb values were found to be higher in patients completing Ra223 therapy. Nodal metastases were reported more frequently in men with early Ra223 discontinuation (p=0.0067).
In univariate logistic regression analysis, the same type of covariates were significantly associated with early Ra223 discontinuation (Table S2). However, only nodal metastasis (yes versus no) and PSA (log, continuous) remained significant in multivariable analysis (Table 4).
Table 4.
Multivariable Logistic Regression Analysis Of 1–5 Cycles Versus 6 Cycles Of Ra223
| Outcome: 1–5 Cycles Versus 6 Cycles Of Ra223 | Independent Covariate | R2 (%) In The Final Model | |||
|---|---|---|---|---|---|
| Multivariable Analysis | p-value | OR (95% CI) | |||
| Nodal metastases (yes versus no) | 0.0457 | 2.707 | 1.059 | 7.649 | 16.53% |
| PSA (log, continuous) | <0.0001 | 1.737 | 1.359 | 2.290 | |
Predictors Of OS
To find predictors of OS in men undergoing Ra223 therapy, we applied the Cox proportional hazards model. Univariate analysis revealed numerous characteristics associated with poor survival: prior SRE (yes versus no), number of lines of prior therapies (categorical ≥2 versus 0–1, and continuous), and history of abiraterone and/or enzalutamide exposure (yes versus no); 1–5 versus 6 cycles of Ra223; treatment centre, referred versus local patients, type of treating medical specialty supervising Ra223 administration; initial localized versus metastatic stage; visceral metastases (yes versus no); higher ESAS total score; and PSA, ALP, and Hb-related parameters (Table S3).
In multivariable analyses, receiving 1–5 versus 6 cycles of Ra223 was the strongest predictor of survival, but Hb (≤100 g/L versus >100 g/L), referred versus local patients, the presence of visceral metastases, and prior SRE remained significant factors in the final model (Table 5). In the model excluding the number of Ra223 cycles, Hb (≤100 g/L versus >100 g/L) was the strongest predictor of survival, aside from referred versus local patients, the presence of visceral metastases, and prior SRE. In addition, in this model PSA (log, continuous) was negatively associated with survival (Table 6).
Table 5.
Multivariable Cox Proportional Hazards Model Of Overall Survival (Including 1–5 Cycles Versus 6 Cycles Of Treatment)
| Including 1–5 Cycles Versus 6 Cycles Of Ra223 | Independent Covariate | R2 (%) In The Final Model | |||
|---|---|---|---|---|---|
| Final Model (No. Of Patients Used =166) | p-value | HR (95% CI) | |||
| Ra223 therapy (1–5 cycles versus 6 cycles) | <0.0001 | 6.050 | 3.348 | 10.932 | 35.09% |
| Hb ≤100 versus >100 g/L | 0.0104 | 2.463 | 1.237 | 4.906 | |
| Referred versus local patients | <0.0001 | 3.497 | 1.968 | 6.216 | |
| Visceral metastases (yes versus no) | 0.0001 | 4.088 | 1.981 | 8.434 | |
| Prior skeletal related event (yes versus no) | 0.0348 | 1.908 | 1.047 | 3.474 | |
Table 6.
Multivariable Cox Proportional Hazards Model Of Overall Survival (Excluding 1–5 Cycles Versus 6 Cycles Of Treatment)
| Excluding 1–5 Cycles Versus 6 Cycles Of Ra223 | Independent Covariate | R2 (%) In The Final Model | |||
|---|---|---|---|---|---|
| Final Model (No. Of Patients Used = 150) | p-value | HR (95% CI) | |||
| Hb ≤100 versus >100 g/L | 0.0045 | 2.853 | 1.383 | 5.884 | 22.00% |
| Referred versus local patients | 0.0064 | 2.295 | 1.264 | 4.167 | |
| PSA (log, continuous) | 0.0034 | 1.305 | 1.092 | 1.559 | |
| Visceral metastases (yes versus no) | 0.0047 | 3.052 | 1.407 | 6.622 | |
| Prior skeletal related event (yes versus no) | 0.0237 | 2.014 | 1.098 | 3.694 | |
Discussion
Ra223 is amongst six different treatment modalities that prolong the survival and improve the quality of life of men with mCRPC.3 While the use of different control arms and targeting of different disease stages preclude direct comparisons between the corresponding registration trials, the survival benefit provided by these agents is consistently in the order of a few months. Aside from life prolongation compared to best supportive care, Ra223 excels with a beneficial safety profile.4 On the other hand, Ra223 utilization comes with a number of unique challenges. First, the anti-mCRPC activity of Ra223 is restricted to bone metastases, an anatomical location that is difficult to monitor with conventional imaging techniques.12 Interestingly, Etchebehere et al describe skeletal tumor burden as assessed by baseline 18F-PET/CT as a predictor of OS in patients undergoing Ra223 therapy.13 However, the results await prospective validation, and 18F-PET/CT is not widely available and/or utilized. Furthermore, men treated within ALSYMPCA achieved a similar OS benefit across a wide range of disease burden as assessed by conventional bone scan (ie, 6–20 bone metastases vs >20 bone metastases vs superscan presentation).4 Second, Ra223-associated PSA responses are rare, and there are no validated biochemical surrogate markers of survival in patients undergoing Ra223 therapy.14 Third, Ra223 use is limited to centers with nuclear medicine capability, which could result in access disparities. In addition, the benefit provided by mCRPC therapies might be decreased in a population-based setting compared to study conditions, as has been shown for docetaxel chemotherapy.7
Herein we present the results of a retrospective chart analysis of 198 men with mCPRC to characterize the real-world utilization of Ra223. To the best of our knowledge this represents that largest study of its kind, comprising patients treated with Ra223 at four cancer centres in Ontario (Canada) outside of clinical trials or early access programs. Overall, our patients tended to be older, had lower baseline PSA and ALP levels, but featured similar Hb values compared to the ALSYMPCA Ra223 registration trial (n=614 in the Ra223 arm) or the iEAP (N=696).4,9 Our frequency of prior docetaxel chemotherapy was 48.5% and thus lower than the rate described in ALSYMPCA (57%) or in the iEAP (60%). However, prior to Ra223 therapy 92.4% of our patients had been exposed to abiraterone and/or enzalutamide, drugs that were not available to patients in ALSYMPCA. In the iEAP, previous exposure to abiraterone or enzalutamide took place in 40% and 8% of participants, respectively.
In our cohort, 46% of patients received 6 cycles of Ra223, with a median number of Ra223 treatments of 5. These numbers are lower than seen in ALSYMPCA (63%, median of 6 cycles) and in the iEAP (58%, median of 6 cycles). However, despite reduced Ra223 exposure we found a median OS similar to ALSYMPCA (13.3 versus 14.9 months), albeit shorter than in the iEAP (16 months)(Figure 3). PSA and ALP-based response parameters were also found to be comparable to both ALSYMPCA and the iEAP (Table 3). When further accounting for other population-based retrospective analyses reporting OS times between 10.5 and 13.0 months, including a population-based study from British Columbia (Canada), altogether our results document encouraging patient outcomes.15–18
Nonetheless, early treatment discontinuation was common, and the median survival of patients receiving less than 6 cycles of Ra223 was 8.1 months only (Figure 4). The median survival in our early discontinuation cohort is numerically superior to ALSYMPCA, the iEAP, and retrospective analyses revealing median survival times between 4.5 and 6.3 months for patients that did not complete Ra223 therapy (ie, that received ≤4–5 cycles of Ra223).9,15,16,18,19 Similar to other analyses, disease progression was found to be the main cause of early Ra223 discontinuation.
In order to help guiding Ra223 treatment initiation, we were interested to identify markers predicting Ra223 non-completion. While the presence of nodal metastases, and both presumed surrogate markers of high disease burden (high PSA, elevated ALP, anemia) as well as symptom burden (ESAS total score) were associated with early treatment discontinuation in univariate analysis (Table S1), only the association between high baseline PSA levels and the presence of nodal metastases remained significant predictors in multivariable logistic regression analysis (Table 4). Higher baseline PSA levels were associated with early treatment discontinuation in exploratory analyses of ALSYMPCA and the iEAP, but no such correlation was found in other retrospective studies.15,16,19,20 The predictive value of nodal metastases for Ra223 non-completion is a novel finding. Of note, ALSYMPCA allowed the enrollment of men with malignant lymphadenopathy of up to 3 cm in short-axis, whereas the upper limit was 6 cm in the iEAP.
Adding to a growing body of evidence, we found early treatment discontinuation to be a strong predictor of poor OS (Figure 4).15,16,18,19,21 Furthermore, in a multivariable Cox proportional hazards model of OS excluding the number of Ra223 cycles administered as covariate, surrogate markers of high disease burden (high PSA, anemia), the presence of visceral metastases, and a history of prior SRE were all significant predictors of poor survival. Intriguingly, patients referred for Ra223 therapy as opposed to local patients also did worse, which merits further investigation. The latter finding and the association of prior SRE with worse OS in men undergoing Ra223 therapy are novel. On the other hand, mCRPC patients with visceral metastases were purposely excluded from Ra223 trials, and our analysis supports this decision. Although available analyses have identified multiple demographic (age), patient (baseline pain, performance status), disease (biochemical markers of disease burden), and treatment-related (early Ra223 discontinuation, concurrent therapies) factors predicting survival in men subjected to Ra223 therapy, they all await prospective validation.9,15,17,18
In Ontario, Ra223 is not funded for use in combination with other treatment modalities (such as abiraterone, enzalutamide, or cabazitaxel). While there are ongoing efforts to define the role of Ra223 in combination with taxane chemotherapy or enzalutamide, the combination of Ra223 and abiraterone (plus prednisone) as first-line therapy for bone-predominant mCRPC resulted in a higher rate of bone fractures compared to Ra223 and placebo in the ERA 223 study.22
Our study has numerous strengths, including the sizeable number of patients included, and the strict focus on patients treated with Ra223 outside of clinical trials or access programs. Furthermore, our findings reflect the benefit of using Ra223 in a contemporary mCPRC patient population mainly pretreated with abiraterone and/or enzalutamide, the most frequently used first-line treatment options for mCRPC these days. There are also shortcomings, notably the retrospective nature of data collection. In addition, the study was limited to patients treated between 2015 and 2016, and thus may not account for changes in the utilization patterns of Ra223 thereafter. However, it is reassuring that there was a trend for increased treatment completion over time. Finally, our findings might not apply to constituencies with different Ra223 access criteria than in Ontario.23
Conclusions
Our study supports the notion that the benefit of Ra223 described in ALSYMPCA is maintained under real-world conditions, although the treatment completion rate is lower. We have identified novel factors associated with treatment completion and superior OS in men undergoing Ra223 therapy. Furthermore, there was an encouraging trend over time for an increasing Ra223 treatment completion rate. Given that Ra223 therapy is restricted to centres with nuclear medicine capability, further studies are needed to understand why patients referred for Ra223 therapy seem to do worse than patients already known to the centre where Ra223 is administered.
Acknowledgments
The study was made possible by the support to U Emmenegger from Bayer Inc., Canada. The research activities of U Emmenegger are also supported by the Joseph and Silvana Melara Cancer Fund.
Abbreviations
18F-PET/CT, 18-Fluoride positron emission tomography/computed tomography; ALP, alkaline phosphatase; ESAS, Edmonton Symptom Assessment System; Hb, hemoglobin; LRT, likelihood ratio (statistic); mCRPC, metastatic, castration-resistant prostate cancer; NDFP, New Drug Funding Program; OS, overall survival; PSA, prostate-specific antigen; Ra223, Radium-223; SRE, skeletal-related event.
Disclosure
Pawel Zalewski: Consulting or Advisory Role: Bayer Inc. Canada. Anil Kapoor: Consulting or Advisory Role: Bayer Inc. Canada; reports grants, personal fees from Bayer Oncology, during the conduct of the study. Neil E Fleshner: Consulting or Advisory Role: Bayer Inc. Canada; reports personal fees from Abbvie, personal fees from Amgen, grants, personal fees from Bayer, grants, personal fees from Ferring, grants, personal fees from Janssen, grants, personal fees from Sanofi, personal fees from Hybridyne Health, grants, personal fees from Astellas, grants from Bavarian Nordic, grants from Medivation, grants from Nucleix, grants from Progenics, grants from Spectracure AB, outside the submitted work. Research Support: Bayer Inc. Canada. Edward Chow: Consulting or Advisory Role: Bayer Inc. Canada. Urban Emmenegger: Consulting or Advisory Role, Research support: Bayer Inc. Canada; reports grants from Bayer, during the conduct of the study. Consulting or Advisory Role: Amgen Inc. Canada. Consulting or Advisory Role: Astellas Inc. Canada. Research Support: AstraZeneca Inc. Canada. Research Support: Endocyte Inc. (Novartis Inc.). Research Support: Exelixis Inc. Consulting or Advisory Role: Ferring Inc. Canada. Consulting or Advisory Role, Research Support: Janssen Inc. Canada. Research support: Merck Inc. Canada. Research support: Roche-Genentech Inc. Canada. Consulting or Advisory Role: Sanofi Genzyme Inc. Canada. The aforementioned authors report no other conflicts of interest in this work. The remaining authors report no conflicts of interest in this work.
References
- 1.Gartrell BA, Saad F. Managing bone metastases and reducing skeletal related events in prostate cancer. Nat Rev Clin Oncol. 2014;11(6):335–345. doi: 10.1038/nrclinonc.2014.70 [DOI] [PubMed] [Google Scholar]
- 2.Fizazi K, Carducci M, Smith M, et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet. 2011;377(9768):813–822. doi: 10.1016/S0140-6736(10)62344-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sartor O, de Bono JS. Metastatic prostate cancer. N Engl J Med. 2018;378(7):645–657. doi: 10.1056/NEJMra1701695 [DOI] [PubMed] [Google Scholar]
- 4.Parker C, Nilsson S, Heinrich D, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369(3):213–223. doi: 10.1056/NEJMoa1213755 [DOI] [PubMed] [Google Scholar]
- 5.Bruland OS, Nilsson S, Fisher DR, Larsen RH. High-linear energy transfer irradiation targeted to skeletal metastases by the alpha-emitter 223Ra: adjuvant or alternative to conventional modalities? Clin Cancer Res. 2006;12(20 Pt 2):6250s–6257s. doi: 10.1158/1078-0432.CCR-06-0841 [DOI] [PubMed] [Google Scholar]
- 6.Suominen MI, Fagerlund KM, Rissanen JP, et al. Radium-223 inhibits osseous prostate cancer growth by dual targeting of cancer cells and bone microenvironment in mouse models. Clin Cancer Res. 2017;23(15):4335–4346. doi: 10.1158/1078-0432.CCR-16-2955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Templeton AJ, Vera-Badillo FE, Wang L, et al. Translating clinical trials to clinical practice: outcomes of men with metastatic castration resistant prostate cancer treated with docetaxel and prednisone in and out of clinical trials. Ann Oncol. 2013;24(12):2972–2977. doi: 10.1093/annonc/mdt397 [DOI] [PubMed] [Google Scholar]
- 8.Bruera E, Kuehn N, Miller MJ, Selmser P, Macmillan K. The Edmonton Symptom Assessment System (ESAS): a simple method for the assessment of palliative care patients. J Palliat Care. 1991;7(2):6–9. [PubMed] [Google Scholar]
- 9.Saad F, Carles J, Gillessen S, et al. Radium-223 and concomitant therapies in patients with metastatic castration-resistant prostate cancer: an international, early access, open-label, single-arm phase 3b trial. Lancet Oncol. 2016;17(9):1306–1316. doi: 10.1016/S1470-2045(16)30173-5 [DOI] [PubMed] [Google Scholar]
- 10.Allison PDSAUtSSAPGC, NC: SAS Institute Inc. Second edition; 282–283.
- 11.Selby D, Cascella A, Gardiner K, et al. A single set of numerical cutpoints to define moderate and severe symptoms for the Edmonton Symptom Assessment System. J Pain Symptom Manage. 2010;39(2):241–249. doi: 10.1016/j.jpainsymman.2009.06.010 [DOI] [PubMed] [Google Scholar]
- 12.Armstrong AJ, Anand A, Edenbrandt L, et al. Phase 3 assessment of the automated bone scan index as a prognostic imaging biomarker of overall survival in men with metastatic castration-resistant prostate cancer: a secondary analysis of a randomized clinical trial. JAMA Oncol. 2018;4(7):944–951. doi: 10.1001/jamaoncol.2018.1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Etchebehere EC, Araujo JC, Fox PS, Swanston NM, Macapinlac HA, Rohren EM. Prognostic factors in patients treated with 223Ra: the role of skeletal tumor burden on baseline 18F-fluoride PET/CT in predicting overall survival. J Nucl Med. 2015;56(8):1177–1184. doi: 10.2967/jnumed.115.158626 [DOI] [PubMed] [Google Scholar]
- 14.Sartor O, Coleman RE, Nilsson S, et al. An exploratory analysis of alkaline phosphatase, lactate dehydrogenase, and prostate-specific antigen dynamics in the phase 3 ALSYMPCA trial with radium-223. Ann Oncol. 2017;28(5):1090–1097. doi: 10.1093/annonc/mdx044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parikh S, Murray L, Kenning L, et al. Real-world outcomes and factors predicting survival and completion of radium 223 in metastatic castrate-resistant prostate cancer. Clin Oncol (R Coll Radiol). 2018;30(9):548–555. doi: 10.1016/j.clon.2018.06.004 [DOI] [PubMed] [Google Scholar]
- 16.Parimi S, Tsang E, Alexander A, et al. A population-based study of the use of radium 223 in metastatic castration-resistant prostate cancer: factors associated with treatment completion. Can Urol Assoc J. 2017;11(10):350–355. doi: 10.5489/cuaj.4415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wong WW, Anderson EM, Mohammadi H, et al. Factors associated with survival following radium-223 treatment for metastatic castration-resistant prostate cancer. Clin Genitourin Cancer. 2017;15(6):e969–e975. doi: 10.1016/j.clgc.2017.04.016 [DOI] [PubMed] [Google Scholar]
- 18.van der Doelen MJ, Kuppen MCP, Jonker MA, et al. 223Ra therapy in patients with advanced castration-resistant prostate cancer with bone metastases: lessons from daily practice. Clin Nucl Med. 2018;43(1):9–16. doi: 10.1097/RLU.0000000000001904 [DOI] [PubMed] [Google Scholar]
- 19.Saad F, O’Sullivan JM, Carles J, et al. Analysis of overall survival by number of radium-223 injections received in an international expanded access program (iEAP). J Clin Oncol. 2016;34(15):suppl_abstract_5082. doi: 10.1200/JCO.2016.34.15_suppl.5082 [DOI] [Google Scholar]
- 20.McKay RR, Jacobus S, Fiorillo M, et al. Radium-223 use in clinical practice and variables associated with completion of therapy. Clin Genitourin Cancer. 2017;15(2):e289–e298. doi: 10.1016/j.clgc.2016.08.015 [DOI] [PubMed] [Google Scholar]
- 21.Sartor O, Vogelzang NJ, Sweeney C, et al. Radium-223 safety, efficacy, and concurrent use with abiraterone or enzalutamide: first U.S. experience from an expanded access program. Oncologist. 2018;23(2):193–202. doi: 10.1634/theoncologist.2017-0413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith M, Parker C, Saad F, et al. Addition of radium-223 to abiraterone acetate and prednisone or prednisolone in patients with castration-resistant prostate cancer and bone metastases (ERA 223): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20(3):408–419. doi: 10.1016/S1470-2045(18)30860-X [DOI] [PubMed] [Google Scholar]
- 23.Woon DTS, Chandrasekar T, Aaron L, et al. Disparity in public funding of therapies for metastatic castrate-resistant prostate cancer across Canadian provinces. Can Urol Assoc J. 2018;12(10):328–336. doi: 10.5489/cuaj.5378 [DOI] [PMC free article] [PubMed] [Google Scholar]