Abstract
The retinoid X receptors (RXR), peroxisome proliferator activated receptor gamma (PPARγ) and liver X receptors (LXR) all have been shown to regulate bone homeostasis. Tributyltin (TBT) is an environmental contaminant that is a dual RXRα/β and PPARγ agonist. TBT induces RXR, PPARγ and LXR-mediated gene transcription and suppresses osteoblast differentiation in vitro. Bone marrow multipotent mesenchymal stromal cells derived from female C57BL/6J mice were more sensitive to suppression of osteogenesis by TBT than those derived from male mice. In vivo, oral gavage of 12 week old female, C57Bl/6J mice with 10 mg/kg TBT for 10 weeks resulted in femurs with a smaller cross-sectional area and thinner cortex. Surprisingly, TBT induced significant increases in trabecular thickness, number, and bone volume fraction. TBT treatment did not change the Rankl:Opg RNA ratio in whole bone, and histological analyses showed that osteoclasts in the trabecular space were minimally reduced. In contrast, expression of cardiotrophin-1, an osteoblastogenic cytokine secreted by osteoclasts, increased. In primary bone marrow macrophage cultures, TBT marginally inhibited the number of osteoclasts that differentiated, in spite of significantly suppressing expression of osteoclast markers Nfatc1, Acp5 and Ctsk and resorptive activity. TBT induced expression of RXR- and LXR-dependent genes in whole bone and in vitro osteoclast cultures. However, only an RXR antagonist, but not an LXR antagonist, significantly inhibited TBTs ability to suppress osteoclast differentiation. These results suggest that TBT has distinct effects on cortical versus trabecular bone, likely resulting from independent effects on osteoblast and osteoclast differentiation that are mediated through RXR.
Keywords: tributyltin, retinoid X receptor, osteoblast, osteoclast, cortical bone, trabecular bone
1. Introduction
Bone remodeling is a coordinated sequence of bone resorption followed by bone formation, orchestrated by the respective activities of osteoclasts and osteoblasts. Maintenance of bone and mineral homeostasis requires tight regulation of this coupled process. Reciprocal communication between osteoblasts and osteoclasts occurs through generation of secreted factors (e.g. macrophage colony stimulating factor (M-CSF), receptor activator of nuclear factor kappa B ligand (RANKL), cardiotrophini-1 (Ct-1), and semaphoring 4D (Sema4D))(Lacey et al., 1998; Negishi-Koga et al., 2011; Walker et al., 2008; Yasuda et al., 1998). Where an imbalance favors the activity of osteoclasts, resorption prevails over osteoid deposition and overall bone mass decreases, potentially leading to pathologic states of osteopenia and osteoporosis. Conversely, inherited mutations causing impairments of osteoclast differentiation or function can result in osteopetrotic states of overly dense and, at times, brittle bone.
Multiple aspects of osteoblast and osteoclast differentiation and function are influenced by a family of interacting nuclear receptors. The peroxisome proliferator activated receptor γ (PPARγ) and liver X receptors (LXRα, LXRβ) are members of the nuclear receptor superfamily that form permissive heterodimers with the retinoid X receptors (RXRα, RXRβ, RXRγ), which can also homodimerize (Tontonoz et al., 1994; Willy et al., 1995; Zhang et al., 1992). All four receptor types are expressed in osteoblasts and osteoclasts (Imai et al., 2013). Transactivation of PPARγ in bone marrow mesenchymal osteoblast precursors by synthetic agonists, such as rosiglitazone, induces differentiation along the adipocyte lineage and suppresses osteoblast differentiation (Akune et al., 2004; Lecka-Czernik et al., 1999). In osteoclasts, PPARγ is essential for differentiation via support of RANKL signaling (Wan et al., 2007; Wei et al., 2010). LXR agonism in vivo has been shown to attenuate osteoclast-mediated bone loss in ovariectomized and inflammation-induced bone loss mouse models (Kim et al., 2013; Kleyer et al., 2012). RXR activation plays maturation-dependent roles in osteoclasts. In osteoclast precursors, RXR homodimer activity maintains a high level of Mafb expression, which is necessary for a proper proliferative response to M-CSF (Menendez-Gutierrez et al., 2015). In differentiating osteoclasts, RXRα:LXR activation suppresses osteoclast function and differentiation by interfering with RANKL signaling (Menendez-Gutierrez et al., 2015; Remen et al., 2011; Robertson et al., 2006).
Tributyltin (TBT) is a dual PPARγ and RXRα/β agonist shown to activate RXR homodimers, as well as PPARγ:RXR and LXR:RXR heterodimers and is a recognized bone toxicant (Baker et al., 2015; Cui et al., 2010; Kanayama et al., 2005; le Maire et al., 2009). In utero exposure to TBT prevents ossification in mouse fetuses and predisposes multipotent stromal cells to favor adipogenesis over osteogenesis by epigenetic modifications at PPARγ targeted promoters (Kirchner et al., 2010; Tsukamoto et al., 2004). In vitro, TBT suppresses the osteoblastogenic transcription factors Runx2 and osterix while activating PPARγ:RXR. This suppression promotes commitment of bone marrow mesenchymal stromal cells (BM-MSCs) towards an adipocyte lineage at the expense of the osteoblast lineage in a manner similar to rosiglitazone (Baker et al., 2015; Carfi et al., 2008; Kirchner et al., 2010; Watt and Schlezinger, 2015; Yanik et al., 2011). In contrast to rosiglitazone, TBT was shown to suppress osteoclast differentiation and resorptive capacity at nanomolar concentrations in Raw264.7 cells (Yonezawa et al., 2007); however, these cells do not express significant levels of PPARγ, RXRs or LXRs (Menendez-Gutierrez et al., 2015). It has not been established as to which of these effects predominates to determine a bone phenotype in adult mice exposed to TBT.
Organotins are environmental contaminants. While TBT has been banned for use as a marine antifouling agent, organotins continue to be used in food crop fungicides, wood preservatives and plastics manufacturing (Cornelissen et al., 2008). Organotins are measurable in house dust (Fromme et al., 2005; Kannan et al., 2010) and human exposure is indicated by the presence of organotins in liver, milk and blood (0.05–400 nM)(Kannan et al., 1999; Lo et al., 2003; Mino et al., 2008; Nielsen and Strand, 2002; Takahashi et al., 1999). Thus, it is of human health relevance to understand the biological activities of TBT. The studies reported here were designed to determine the overall effect of in vivo TBT exposure on long bones of skeletally mature, adult, female C57Bl/6J mice, and to characterize the role of TBT, as an activator of multiple nuclear receptor pathways, in the balance of osteoblast and osteoclast function.
2. Methods
2.1. Materials
Rosiglitazone was from Cayman Chemical (Ann Arbor, MI). DMSO was from American Bioanalytical (Natick, MA). GSK2033, LG100268, T0901317, p-nitrophenyl phosphate (pNPP) reagent, TBT chloride, Gil’s Hematoxilin and sodium tartrate were from Sigma-Aldrich (St. Louis, MO). HX531 was from Tocris Bioscience (Bristol, United Kingdom). All other reagents were from Thermo Fisher Scientific (Suwanee, GA) unless noted.
2.2. Primary osteoblast cell culture
Bone marrow was isolated from 9 and/or 12-week-old male and female, C57BL/6J mice (Jackson Laboratories). Bone marrow was flushed from the femur, tibia, and humerus, strained through a 70-μm cell strainer, and diluted in MSC media (α-MEM +10% FBS, 100 U/mL penicillin, 100 μg/mL streptomycin, 0.25 μg/mL amphotericin B). Cells were seeded at 12 × 106 per well in a 6-well plate in 2 ml of media. At 7 days, the medium was changed to include ascorbate (12.5 μg/mL; Sigma), β-glycerol phosphate (8 μM; Sigma), dexamethasone (10 nM; Sigma), and insulin (500 ng/mL; Sigma), and the cultures were dosed with vehicle (Vh, DMSO, 0.1% final concentration) or TBT (10−9 – 10−7 M). Naïve cells were maintained in MSC medium, received no treatments, and were harvested at day 7 in culture. Following initiation of differentiation, cells were cultured for 7 days (mRNA expression) or 10–12 days (lipid accumulation, alkaline phosphatase, bone mineralization and nodule formation). During these periods, medium was changed and the cultures were re-dosed 2 times for mRNA expression or 4 times for phenotype analysis.
Lipid accumulation was quantified by Nile Red staining (Yanik et al., 2011). Following Nile Red staining, cells were fixed in 2% paraformaldehyde. To quantify alkaline phosphatase activity, cells were incubated in pNPP solution (Sigma). After quenching with NaOH (final concentration: 0.75M), absorbance (405 nM) was measured using a Synergy2 multifunction plate reader (Biotek Inc., Winooski, WT). The absorbance was normalized by dividing by the absorbance measured in wells that received osteogenic medium but were not treated and reported as “Fold Difference.” Following the pNPP assay, cells were stained with Alizarin Red (Osteogenesis Quantitation Kit, Millipore, Billerica, MA), washed and then photographed using the UVP Bioimaging System (UVP, Inc., Upland, CA). The resulting images were analyzed for bone nodule count using Image-Pro Plus (MediaCybernetics, Bethesda, MD), and the number of nodules per square cm is reported. Following image capture, Alizarin Red staining was quantified as indicated in the manufacturer’s instructions. Absorbance (405 nM) was measured and normalized as described above.
Total RNA was extracted and genomic DNA was removed using the RNeasy Plus Mini Kit (Qiagen, Valencia, CA). cDNA was prepared from total RNA using the GoScript™ Reverse Transcription System (Promega), with a 1:1 mixture of random and Oligo (dT)15 primers. All qPCR reactions were performed using the GoTaq® qPCR Master Mix System (Promega). Validated primers were purchased from Qiagen or synthesized by Integrated DNA Technologies (Coralville, IA) (see Table S1). qPCR reactions were performed using a 7300 Fast Real-Time PCR System (Applied Biosystems, Carlsbad, CA): Hot-Start activation at 95°C for 10 min, 40 cycles of denaturation (95°C for 15 sec) and annealing/extension (55°C or 60°C for 60 sec). Relative gene expression was determined using the Pfaffl method to account for differential primer efficiencies (Pfaffl, 2001). The average Cq value for 18s ribosomal RNA (Rn18s) and beta-2-microglobulin (B2m) was used for normalization. The Cq value for naïve, undifferentiated cultures was used as the reference point, and the data are reported as “Fold Change from Naive.”
2.3. In vivo studies
All animal studies were approved by the Institutional Animal Care and Use Committee at Boston University and performed in an American Association for the Accreditation of Laboratory Animal Care accredited facility (Animal Welfare Assurance Number: A3316–01). Female C57BL/6J mice (stock #:000664, 12 weeks of age) (Jackson Laboratories, Bar Harbor, ME) were gavaged 3x per week for 10 weeks with vehicle (sesame oil, 10 μl/g) or TBT (10 mg/kg dissolved in sesame oil). Mice were euthanized four days after the last dosing. Serum was collected at euthanasia and analyzed by ELISA for PINP (Rat/Mouse PINP EIA (AC-33F1), Immunodiagnostic Systems, Tyne & Wear, UK) and Trap5b (Mouse Trap Assay (SB-TR103), Immunodiagnostic Systems). Humeri were collected and flash frozen for RNA analyses. Total RNA was extracted and genomic DNA removed by double TRIzol® extractions (Life Technologies, Grand Island, NY). RNA integrity was confirmed by gel electrophoresis. cDNA preparation and RT-qPCR were performed as above. The Cq value for naïve, 22 week old, male, C57BL/6J-derived humerus samples (n = 5) was used as a reference point, and expression levels were normalized to the sample-specific Cq average of Rn18s, B2m and hypoxanthine-guanine phosphoribosyltransferase (Hprt). The data are reported as “Fold Difference.”
2.4. Micro-Computed Tomography (micro-CT)
At euthanasia, the right femur was collected and fixed in 4% paraformaldehyde for micro-computed tomography (micro-CT) and histological analyses. Sample analyses were performed according to guidelines outlined in (Bouxsein et al., 2010). Samples were scanned using a Scanco micro-CT 40 system (Scanco Medical; Brütisellen, Switzerland) using power, current, and integration time of 70 kVP, 113 μA, and 200 ms, respectively. Gaussian filtering (sigma = 0.8, support = 1) was used for reducing background noise. To analyze trabecular bone in the distal femur, the distal metaphysis was scanned at a nominal resolution of 6 μm/voxel, and the region of interest analyzed began at 0.03 mm proximal to the growth plate and extended 0.9 mm proximally. The trabecular compartment was manually segmented from the cortical shell. Cortical bone was analyzed from 12 μm/voxel scans of a 0.6 mm-tall region extending proximally from the mid-diaphysis. For trabecular bone, treatment-specific thresholding was used (Vh = 438.5 mg HA/cm3; TBT = 455.2 mg HA/cm3) with thresholds determined by an iterative method (Scanco Medical); cortical bone was evaluated at a global threshold of 502 mg HA/cm3. Tissue mineral density was calculated with the aid of a standard curve obtained from a scan of a hydroxyapatite phantom consisting of five different mineral densities.
2.6. Histology
Following micro-CT scans, femurs were decalcified in 14% w/v EDTA at 4°C and embedded in paraffin. 5μm slices were stained with hematoxylin and eosin or with tartrate resistant acid phosphatase, as previously described (Gerstenfeld et al., 2003). Micrographs (20x) were visualized on an Olympus BX51 light microscope (Olympus America Inc.; Center Valley, PA). Osteoclast surface, osteoclast number, and adipocyte number were determined manually within a 1.5-mm selection proximal to the growth plate.
2.7. Primary osteoclast culture
Wildtype bone cells were isolated from 12-week-old female, C57BL/6J mice (Jackson Laboratories). Bone marrow cells from LXRα−/− mice (C57BL/6 background) and their LXR expressing littermates were kindly provided by Dr. Koren Mann (Lady David Institute, McGill University, Montreal, QC, Canada)(Lemaire et al., 2014). Following overnight incubation in MSC media with 25 ng/ml recombinant human M-CSF (BioLegend, San Diego, CA), non-adherent cells from whole bone marrow were collected and seeded at 3 × 106 per well in a 6-well plate with 2 ml media (gene expression) or 2.5 × 104 per well in a 24-well plate with 1 ml media (TRAP+ MuNC counts) with 50 ng/ml M-CSF. On day 1, recombinant murine RANKL (BioLegend) was added at 50 ng/ml. On days 2 and 4, media and additives were replaced and treatments (Vh, TBT (20, 50, 80 nM), rosiglitazone (500 nM), LG100268 (1 μM), or T0901317 (1 μM)) were added. Vh or inhibitors (HX531; 1 μM or GSK2033; 0.8 μM) were added to cultures 30 min prior to dosing with TBT. Cells were harvested on day 6 for gene expression analyses resorption assays or Trap staining. mRNA isolation, cDNA preparation and qPCR were carried out as described for osteoblast cultures. For resorption assays, cells were collected from the differentiating osteoclast cultures, counted, plated (40,000 per well in 800 μl osteoclast differentiation medium) in 24-well Corning Osteo-Assay plates (CLS3987, Sigma Aldrich) and re-treated with Vh or TBT. After 3 days incubation, the cells were removed, and the mineral surface was stained with a modified von Kossa stain (Kartner et al., 2010). For Trap staining, cultures were rinsed with PBS and fixed in 4% paraformaldehyde. Membranes were permeabilized with ethanol/acetone (1:1) and stained with sodium tartrate (50 mM). Cultures were counterstained with Gil’s Hematoxilin solution. Multi-nucleated (3 or more nuclei), TRAP-positive cells were counted manually. Micrographs (20x) were visualized on an Olympus BX51 light microscope.
2.9. Statistical analyses
Statistical analyses were performed with Prism 6 (GraphPad Software Inc., La Jolla, CA). Data are presented as means ± standard error (SE). In vivo data are from 11–12 individual mice. In vitro experiments were performed with a minimum of 4 independently prepared pools of bone marrow. In vitro gene expression data were log-transformed prior to analysis. One-way or two-way ANOVAs with the Dunnett’s or Sidak’s post hoc test and Student’s T tests were performed where noted. The non-parametric Mann-Whitney test was used for in vivo analyses. All analyses were performed using α = 0.05.
3. Results
3.1. Effect of TBT on osteogenic differentiation in vitro
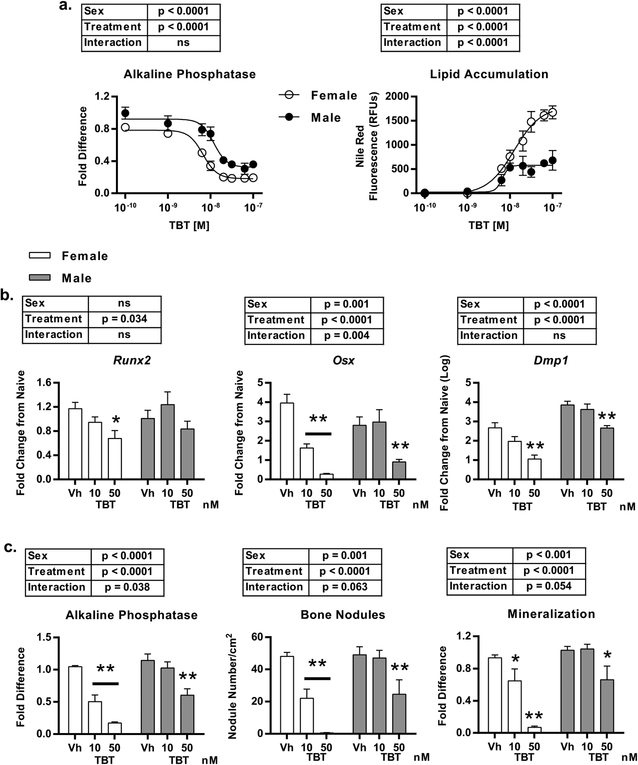
We and others have previously documented TBT’s capability to suppress the in vitro osteogenic differentiation of mesenchymal stromal cells (Kirchner et al., 2010; Tsukamoto et al., 2004; Watt and Schlezinger, 2015; Yanik et al., 2011). Initial studies herein were designed to compare TBT’s in vitro efficacy and potency between male and female C57Bl/6J mice. Primary bone marrow mesenchymal stromal cells (BM-MSC) were harvested from 9-week old female and male C57Bl/6J mice and cultured in the presence of osteogenic medium, and treated with either Vh (DMSO) or TBT (1 – 100 nM). Lipid accumulation, indicative of adipogenesis, and alkaline phosphatase activity, indicative of osteogenesis, were analyzed sequentially after 10 days of culture. Lipid accumulated to a significantly greater extent in female-derived cultures versus in male-derived cultures, accompanied be a greater suppression of osteogenesis (Figure 1a).
Figure 1. TBT suppresses osteogenic differentiation and mineralization more efficaciously in female-derived compared to male-derived cultures.
BM-MSCs were harvested from 9-week old female and male, C57Bl/6J mice, cultured in the presence of osteogenic media and treated with Vh (DMSO) or TBT (1–100 nM) for 7 (gene expression) or 10 (phenotype) days. a.) Dose response analysis of lipid accumulation and alkaline phosphatase activity. b.) Relative mRNA expression of osteoblast/osteocyte differentiation markers Runx2, Osx, and Dmp1. c.) Alkaline phosphatase activity (left), nodule count (center) and mineralization (right). Data are presented as mean ± SE. n=4–5 independent cultures. *p < 0.05, **p<0.01, Two-way ANOVA (Sidak’s multiple comparison test).
Differences in responses between female- and male-derived cultures were most significant between 10–50 nM TBT, therefore we examined differences in osteogenesis more thoroughly at these concentrations. As expected, TBT suppressed the expression of pro-osteogenic genes at nanomolar concentrations in both male- and female-derived cultures (Figure 1b). Levels of Runx2 expression in Vh-treated cultures were comparable between sexes, although TBT only decreased its expression in female-derived cultures (Figure 1b). Osx was expressed at a higher level in female-derived cultures. TBT (10 nM) was sufficient to suppress Osx expression in female-derived cultures, while 50 nM TBT was required to suppress expression in male-derived cultures (Figure 1b). Levels of Dmp1 were lower overall in female-derived cultures, and TBT significantly suppressed Dmp1 expression only in female-derived cultures. It should be noted that the relative expression level of Dmp1 in cultures treated with 50 nM TBT was 30x lower in female-derived cultures compared to male-derived cultures (Fold Difference: 16.4 ± 6.2 vs. 558 ± 195, respectively) (Figure 1b). In contrast, TBT had a minimal effect on gene expression in osteocytes, when treatment was initiated at 10 days of differentiation (Figure S1).
The dimorphic response was more pronounced in the bone mineralization assays. TBT significantly inhibited alkaline phosphatase activity at both 10 and 50 nM in female-derived cultures, but only at 50 nM in male cultures (Figure 1c, images can be found in Figure S2). This pattern also was evident in the number of bone nodules and mineral deposition (Figure 1c), with there being a significant interaction between sex and treatment for each aspect of bone mineralization. From these data, we conclude that, in vitro, TBT disproportionately suppresses the osteogenic differentiation of BM-MSCs in female-derived cultures.
3.2. Effect of TBT on bone homeostasis in vivo
Based on the suppression of osteogenesis in vitro, we hypothesized that in vivo exposure to TBT would produce an overall deficit of bone tissue in the long bones of female C57Bl/6J mice. To test this hypothesis, 12 week old female mice were treated 3x/week for 10 weeks with either vehicle (sesame oil) or TBT (10 mg/kg bw) by oral gavage. TBT treatment induced slightly larger increases in body weight relative to initial weight, but thymus weight relative to body weight (a measure of systemic TBT toxicity) at euthanasia was not affected (Figure S3).
At the end of the treatment period, micro-CT was used to assess TBT’s impact on the long-bone structural phenotype. A femur was harvested from each mouse (n = 12 control, 11 TBT), and the cortical and trabecular compartments were analyzed at the diaphysis and metaphysis, respectively (Figure 2). Overall, a decrease in cortical cross-sectional area was evident from visual inspection of the 3D reconstructions (Figure 2a). Cortical tissue mineral density was not affected by treatment (Figure 2b). As expected from the in vitro results, cortical thickness (Ct.Th) and cortical area (Ct.Ar) were significantly decreased by TBT treatment (Figure 2b). In TBT-treated mice, medullary area (Ma.Ar) decreased in parallel with total area (Tt.Ar), resulting in a ratio of bone area to total area that was indistinguishable from Vh-treated mice (Ct.Ar/Tt.Ar) (Figure 2b).
Figure 2. TBT reduces diaphysis cross-sectional area and cortical thickness while increasing trabecular structure in female C57Bl/6J femurs.
12-week old female wild type C57Bl/6J mice were treated with sesame oil (Vh) (n=12) or 10 mg/kg TBT (n=11) via oral gavage for 10 weeks. a.) Representative micro-CT images of mid-diaphysis. Scale bar = 400 μm. b.) Cortical bone parameters. tissue mineral density; Ct.Ar: cortical bone area; Ma.Ar: medullary (marrow) area; Tt.Ar: total cross-sectional area; Ct.Ar/Tt.Ar: cortical area fraction; Ct.Th: cortical bone thickness. c.) Representative micro-CT images of distal metaphysis. Scale bar = 400 μm. d.) Trabecular bone parameters. BV/TV: bone volume fraction; tissue mineral density; Conn.D: connectivity density; Tb.N: trabecular number; Tb.Sp: mean trabecular spacing; Tb.Th: mean trabecular thickness. Data are presented from individual mice, and the mean is indicated by a line. n=11–12 individual mice. **p < 0.01, *** p < 0.0001 Student’s T test.
In the trabecular compartment, TBT treatment unexpectedly caused a robust increase in the amount of bone, which was clearly discernable in the 3-D renderings (Figure 2c). The bone volume fraction (BV/TV) was increased in the TBT-treated mice, as was the tissue mineral density (Figure 2d). The connectivity density (Conn.D) and number of trabeculae (Tb.N) increased with TBT treatment, as trabecular separation (Tb.Sp) decreased (Figure 2d). Trabecular thickness was not affected (Tb.Th) (Figure 2d).
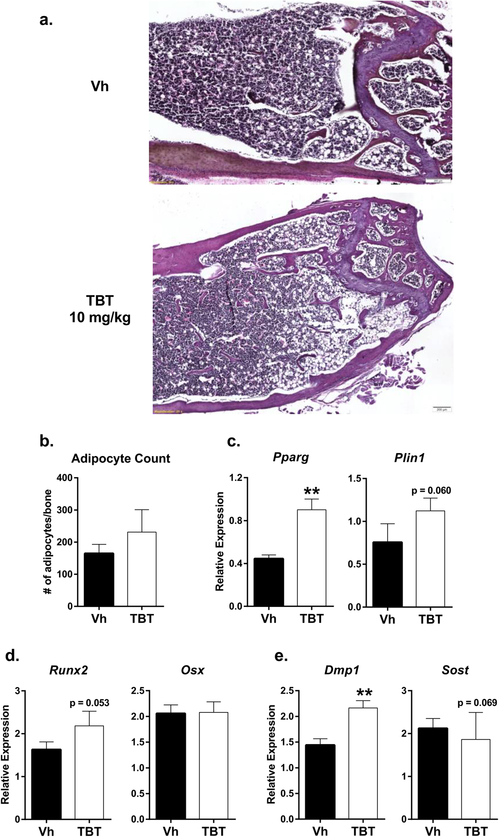
Histological analyses were carried out to probe the differences observed in the micro-CT data (Figure 3). An increased number of trabeculae was evident in femur sections of TBT-treated mice (Figure 3a). Adipocyte number was highly variable in the TBT-treated bone sections (Figure 3b). However, RNA expression of the adipocyte markers PPARγ (Pparg) and perilipin 1 (Plin1) in whole bone was higher in TBT-treated mice (Figure 3c). With regard to osteoblast differentiation and function, TBT treatment was associated with a slight but non-significant increase in Runx2 and no change in Osx (Figure 3d). In accordance with the structural data, TBT significantly increased the expression of Dmp1 (Figure 3e) and slightly decreased expression of Sost, an osteocyte-derived bone formation inhibitor (Figure 3e). However, serum PINP was not significantly changed (Vh: 31.9±4.4, TBT: 29.3±5.0 U/L, Mann-Whitney).
Figure 3. In vivo TBT exposure increases Dmp1 expression in the presence of increased Pparg expression.
Mice were treated as described in Figure 2. a.) Representative images of 5 μm slices of distal femur stained with hematoxylin and eosin (20x magnification) (n=4 Vh, 5 TBT). b.) Adipocyte count per bone section (1.5 mm from growth plate). Whole humerus bone mRNA expression (n = 12 Vh, 11 TBT 10 mg/kg) of c.) adipocyte markers (Pparg, Plin1), d.) osteoblast (Runx2, Osx) osteoblast markers and e.) osteocyte differentiation (Dmp1) and signaling (Sost) markers. Data are presented as mean ± SE. **p < 0.01, Mann-Whitney.
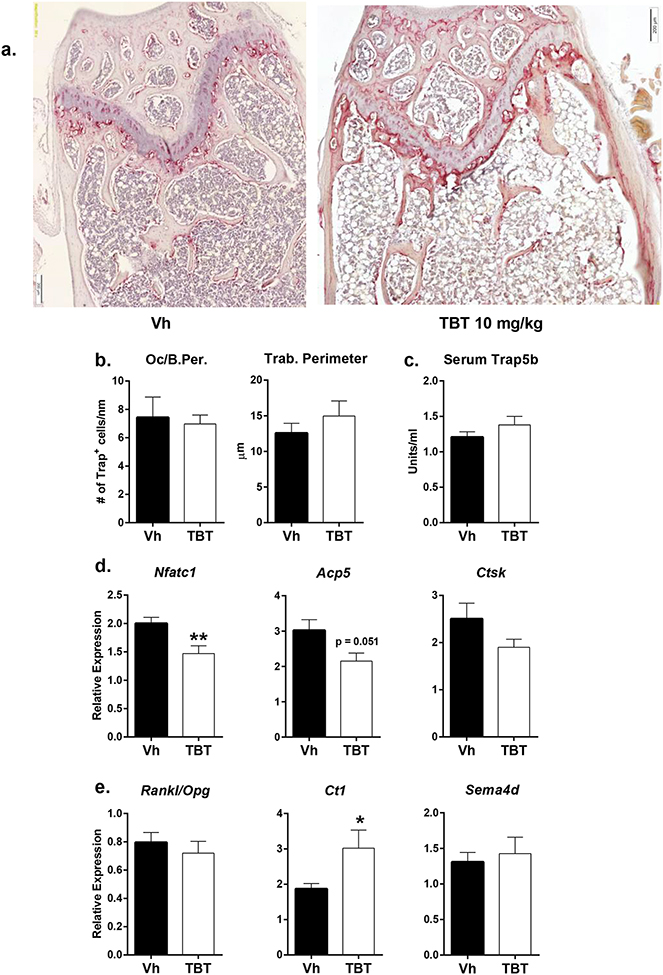
Increases in bone can result from an increase in osteoblast activity, a decrease in osteoclast number, and/or a decrease in osteoclast resorptive function. Therefore, we investigated the potential contribution of osteoclasts to the observed phenotype. Histological sections of the distal femur were tartrate-resistant acid phosphatase (TRAP) stained to visualize and quantify the osteoclast population (Figure 4a). TBT treatment did not cause a decrease in osteoclast numbers (Figure 4b). Additionally, there was no change in serum TRAP levels between Vh- and TBT-treated mice (Figure 4c), suggesting that osteoclast number had minimally decreased with TBT treatment.
Figure 4. In vivo TBT exposure modifies osteoclast gene expression without influencing osteoclast cell number.
Mice were treated as described in Figure 2. a.) Representative images of 5 μm slices of distal femur stained for TRAP (20x magnification)(red shading; n=4 Vh, 5 TBT). b.) Oc/B.Per.: number of TRAP-positive osteoclasts per nm of trabecular perimeter, excluding cortex. Trab. Perimeter: total perimeter of trabecular bone (um). c.) Quantification of serum TRAP by ELISA (n = 12 Vh, 11 TBT 10 mg/kg). Whole humerus bone mRNA expression (n = 12 Vh, 11 TBT 10 mg/kg) of d.) Osteoclast differentiation markers Nfatc1, Apc5 and Ctsk, and e.) Intercellular communication proteins Rankl/Opg (osteoblast to osteoclast), Ct1 and Sema4d (osteoclast to osteoblast). Data are presented as mean ± SE. *p < 0.05 **p < 0.01, Mann-Whitney.
Transcript levels of Nfatc1, Acp5, and Ctsk were measured to assess TBT’s effect on osteoclast differentiation and resorptive capacity. TBT treatment caused significant decreases in Nfatc1 and Acp5, along with a slight but non-significant decrease in Ctsk expression (Figure 4d). Osteoblast stimulation of osteoclast differentiation was assessed by measuring relative levels of Rankl and Opg expression. TBT increased mRNA expression levels of both genes (data not shown), but the ratio of Rankl:Opg was not altered from Vh-treated samples (Figure 4e). TBT was associated with a significant increase in the expression of the pro-osteogenic cytokine cardiotrophin 1 (Ct1) (Walker et al., 2008) (Figure 4e), suggesting a mechanism of increased osteoblast stimulation by osteoclasts. The expression of the anti-osteogenic semaphorin-4D (Sema4D) mRNA was unchanged (Negishi-Koga et al., 2011) (Figure 4e).
Together, the difference in cortical area (Ct.Ar) between Vh- and TBT-treated femurs without a change in Ct.Ar/Tt.Ar suggest diminished periosteal modeling and potentially diminished endosteal resorption along the diaphysis associated with TBT treatment. Measures of whole bone RNA transcripts suggest that TBT treatment may perturb the balance between osteoblasts and osteoclasts to favor osteoblast activity and suppress osteoclast activity, effecting a gain in mineralized tissue in the trabecular compartment.
3.3. Effects of TBT on osteoclast differentiation in vitro
To determine whether TBT treatment resulted in dose-dependent transcriptional and/or functional changes in osteoclasts specifically, primary bone marrow macrophages from C57Bl/6J females were cultured in the presence of M-CSF and RANKL to induce osteoclast differentiation. After 24 hours of RANKL-induced differentiation, cells were treated with either Vh (DMSO) or TBT (20, 50, or 80 nM), and total RNA was harvested after 6 days. Consistent with whole bone mRNA expression (Figure 4d), expression of Nfatc1 and both Acp5 and Ctsk were significantly decreased by 50 and 80 nM TBT relative to Vh-treated cultures (Figure 5a). TBT’s effect on osteoclast gene expression mirrored that of the RXR ligand, LG100268, but not that of the PPARγ ligand, rosiglitazone, or the LXR ligand, T0901317 (Figure 5a). However, the expression osteoclast-derived signaling molecules, Ct1 and Sema4d, was unaffected by TBT treatment in vitro. (Figure S4). Like the in vivo results, TBT marginally decreased the number of osteoclasts that differentiated (Figure 5b, Vh: 43 ± 4, TBT 20 nM: 41 ± 8, TBT 50 nM: 32 ± 10, TBT 80 nM: 34 ± 11 Trap+, multinucleated cells per well). Cell viability, as assessed by MTT activity, was not affected by TBT at 20 nM or 50 nM, though 80 nM showed a small but significant decrease; cell death (release of lactate dehydrogenase) was not altered by TBT (Figure S5). To assess the relative resorptive function of osteoclasts differentiated in the presence of TBT, osteoclasts were differentiated under the same conditions as the TRAP-positive cell count assay, collected, and counted. Equal numbers of cells then were plated on a synthetic inorganic surface. After 3 days, the cells were removed, and the surface von Kossa stained. The 50 and 80 nM TBT concentrations reduced the total resorbed surface (Figure 5c).
Figure 5. TBT suppresses differentiation of osteoclasts in vitro.
Primary bone marrow macrophages were isolated from female C57BL/6J mice, induced to differentiate to osteoclasts with M-CSF and RANKL (see Methods), and after 24 hrs treated with Vh (DMSO), TBT (20, 50, or 80 nM), rosiglitazone (Rosi, 500 nM, PPARγ agonist), LG100268 (LG268, RXR agonist 1 μM), or T0101317 (T317, LXRα/β agonist, 1 μM) for a total of 6 days of differentiation. a.) mRNA expression of osteoclast differentiation markers. n=10–17 independent cultures. Data are presented as mean ± SE. *p < 0.05, **p < 0.01, ***p < 0.001 compared to Vh, one-way ANOVA (Dunnett’s). TBT treatments were compared to Vh separately from control comparisons. b.) Representative images of TRAP-positive, multinucleated (3 or more nuclei) cells (Scale bar = 400 μm) n=10 independent cultures. c.) Representative images of von Kossa stained mineral surface after 3 days of incubation with differentiated osteoclasts. n=5 independent cultures.
We conclude that TBT exposure does not significantly attenuate the population of osteoclasts in vitro or in vivo. The gene expression data suggest that expression of the differentiation program is impacted by TBT, but with marginal changes in osteoclast cell number. An interesting possibility may be that TBT modifies the type of osteoclast that differentiates (Thudium et al., 2014), altering expression of genes related to osteoclast-osteoblast communication to favor osteoblastogenesis.
3.4. Activation of LXR- and RXR-dependent pathways by TBT
Recent literature has highlighted the role of RXRs and LXRs in the osteoclast (Kim et al., 2013; Kleyer et al., 2012; Menendez-Gutierrez et al., 2015; Remen et al., 2011). Because TBT is a known activator of RXRs (le Maire et al., 2009), and based on previous results demonstrating TBT activation of RXR and LXR-dependent genes in bone marrow stromal cells (Figure S3) (Baker et al., 2015), we hypothesized that TBT would activate RXR and/or LXR-dependent pathways in vivo and in in vitro osteoclast cultures.
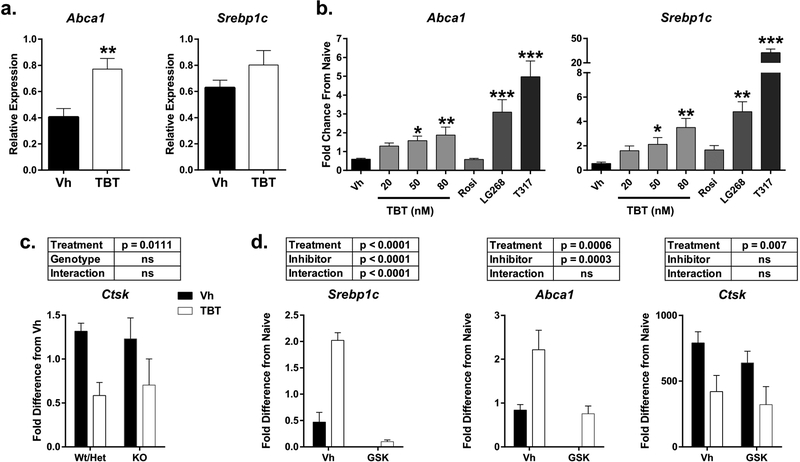
In the whole bone RNA, TBT exposure did not change the expression of Lxra or Lxrb (data not shown). TBT exposure was associated with a significant, 2-fold increase in the expression of Abca1, an LXRβ gene target, whereas Srebp1c, an LXRα target gene, was not upregulated (Figure 6a). In the differentiating osteoclast cultures, TBT induced a dose-dependent increase in Abca1 expression (Figure 6b). The RXR-specific agonist LG100268 also induced an increase in Abca1 expression, implying activation of the RXRα:LXRβ permissive heterodimer. This pattern was also seen in the expression of Srebp1c (Figure 6b). As expected, the LXR-specific agonist T0901317 efficaciously induced expression of both LXR target genes. By comparison, the PPARγ-specific agonist rosiglitazone did not induce expression of Abca1 or Srebp1c above the Vh control baseline. To test the necessity of LXRs for TBT’s effects on osteoclast differentiation, osteoclasts were prepared from LXRα knockout mice. Lack of expression of LXRα did not inhibit TBT’s ability to suppress osteoclast differentiation, as indicated by Ctsk expression (Figure 6c). Similarly, the LXR antagonist GSK2033 did not inhibit TBT’s ability to suppress osteoclast differentiation, even though it suppressed TBT’s ability to upregulate expression of Abca1 and Srebp1c (Figure 6d).
Figure 6. TBT activates LXR-dependent pathways in whole bone and in vitro osteoclast culture.
a.) Mice were treated as described in Figure 2. Whole humerus bone mRNA expression of LXR-dependent genes Abca1 and Srebp1c. Data are presented as mean ± SE. n = 11–12 individual mice. **p<0.01, Mann-Whitney b.) Osteoclast cultures were prepared as described in Figure 5 and treated with Vh (DMSO), TBT (20, 50, or 80 nM), rosiglitazone (Rosi, 500 nM, PPARγ agonist), LG100268 (LG268, RXR agonist 1 μM), or T0101317 (T317, LXRα/β agonist, 1 μM) and analyzed for mRNA expression Abca1 and Srebp1c. n=10–17 independent cultures. Data are presented as mean ± SE. *p<0.05, **p<0.01, ***p<0.001 compared to Vh, one-way ANOVA (Dunnett’s). TBT treatments were compared to Vh separately from control comparisons. c.) Primary bone marrow macrophages were isolated from male and female LXRα +/+, +/− and −/− mice, induced to differentiate into osteoclasts with M-CSF and RANKL, treated after 24 hrs with Vh (DMSO) or TBT (50 nM) for 4 days, and analyzed for mRNA expression of Ctsk. n=4 independent cultures. Data are presented as mean ± SE. Two-way ANOVA. d.) Osteoclast cultures were prepared as described in b and treated with Vh (DMSO) or TBT (50 nM) with or without GSK2033 (GSK, LXR antagonist, 0.8 μM) and analyzed for mRNA expression Abca1, Srebp1c and Ctsk. n=5 independent cultures. Data are presented as mean ± SE. Two-way ANOVA.
Mafb, a target of either RXR homodimers or SREBP1c, was strongly upregulated in whole bone from TBT-treated animals (Figure 7a). In differentiating osteoclasts, Mafb was induced significantly by TBT, LG100268, and T0901317, with no induction by rosiglitazone (Figure 7b). The RXR antagonist HX531 significantly inhibited TBT’s ability to suppress osteoclast differentiation, as indicated by Ctsk expression (Figure 7c, 47% decrease vs 22% decrease in Vh vs HX531 co-treated cultures).
Figure 7. TBT activates RXR-dependent pathways in whole bone and in vitro osteoclast culture.
a.) Mice were treated as described in Figure 2. Whole humerus bone mRNA expression the RXR-dependent gene Mafb. Data are presented as mean ± SE. n = 11–12 individual mice. **p<0.01, Mann-Whitney b.) Osteoclast cultures were prepared as described in Figure 5 and treated with Vh (DMSO), TBT (20, 50, or 80 nM), rosiglitazone (500 nM), LG100268 (LG268, 1 μM), or T0101317 (T317, LXRα/β agonist, 1 μM) and analyzed for mRNA expression of Mafb. Data are presented as mean ± SE. n=10–17 independent cultures. *p<0.05, **p<0.01, *** p<0.001 compared to Vh, one-way ANOVA (Dunnett’s). TBT treatments were compared to Vh separately from control comparisons. Osteoclast cultures were prepared as described in b and treated with Vh (DMSO) or TBT (50 nM) with or without HX531 (RXR antagonist, 1 μM) and analyzed for mRNA expression Mafb and Ctsk. n=5 independent cultures. Data are presented as mean ± SE. Two-way ANOVA.
These results indicate that TBT induces activation of multiple nuclear receptors in osteoblasts and osteoclasts. However, it appears that TBT’s ability to interact with RXR plays a more important role in TBT’s ability to suppress osteoclast differentiation than its ability to activate the LXR pathway.
4. Discussion
Nuclear receptors play an important role in determining the differentiation and function of both osteoblasts and osteoclasts. Consequently, the balance of bone resorption and formation can be perturbed by activation of multiple nuclear receptors. The goal of this study was to use tributyltin (TBT), which can directly activate RXRα and PPARγ (le Maire et al., 2009) and indirectly activate LXR (Baker et al., 2015; Cui et al., 2010), to examine the in vivo outcomes and in vitro mechanisms associated with RXR dimer-related signaling in bone.
As a PPARγ agonist, the ability of TBT to induce adipogenesis and suppress osteogenesis in differentiating osteoblasts has been characterized (Carfi et al., 2008; Tsukamoto et al., 2004; Watt and Schlezinger, 2015; Yanik et al., 2011). We began by re-evaluating this effect in primary bone marrow MSCs and found that cells derived from female mice are more sensitive to TBT’s ability to suppress osteogenesis than those from male mice. It is well known that mature female mice have lower trabecular density and thinner bones compared to males, and that female-derived osteoblasts have lower potential for differentiation (Callewaert et al., 2010; Somerville et al., 2004; Zanotti et al., 2014). We have previously shown that female-derived BM-MSCs were more sensitive to rosiglitazone-induced suppression of osteogenesis (Bragdon et al., 2015). However, this is the first report of TBT displaying a sexually dimorphic effect at the transcriptional level.
It has been reported that TBT suppresses ossification in fetal mice (Tsukamoto et al., 2004). Here, we assessed the in vivo effects of TBT exposure in post-natally. The thinner cortex at mid-diaphysis associated with TBT treatment was expected, as this phenotype is consistent with the suppression of osteogenesis seen in vitro. Cortical bone is built by periosteal bone deposition, and the smaller cortical diameter suggests that the thinner cortex resulted from a failure of periosteal bone deposition. Importantly, thinner (i.e. smaller cross-sectional area) bones are less structurally sound (Bouxsein and Karasik, 2006). The medullary space also was smaller in the TBT-treated mice. Osteoclast activity on the endosteal surface typically thins cortical bone. The smaller medullary space suggests that either osteoclast activity at the endosteal surface is being suppressed by treatment with TBT or that intramedullary osteoblast activity is increased.
Surprisingly, there was an observed increase in trabecular bone volume (increase in trabecular number without an increase in average trabecular thickness). This was corroborated by the increased expression of Dmp1, which is highly expressed in osteocytes (Rios et al., 2005). Together, these results suggest that TBT may increase trabecular osteoblast activity. This increase in bone occurred despite the fact that TBT activates PPARγ. In vivo studies with the PPARγ agonist rosiglitazone show clear reduction of trabecular bone in femur of treated animals that is accompanied by increased marrow adipocytes (Ali et al., 2005; Lazarenko et al., 2007; Rzonca et al., 2004). In contrast to rosiglitazone and the paradigm that adipocyte and osteoblast differentiation are mutually exclusive, TBT-exposed bones also showed increased adipogenesis. Selective PPARγ activation has been shown to increase marrow adiposity without affecting bone density (Lazarenko et al., 2006), though TBT treatment also induced a clear increase in mineralization. These results suggest that TBT may induce an uncoupling of the pro-adipogenic and anti-osteogenic roles of the RXR:PPARγ heterodimer (also suggested by (Rahman et al., 2012)).
Osteoclasts play a dual role in bone, both physically resorbing bone and signaling to osteoblasts to initiate bone formation. Nuclear receptors have been shown to regulate osteoclast differentiation. In vivo, osteoclast number can be increased by a PPARγ agonist, though this effect is controversial (Wan et al., 2007; Wei et al., 2010; Zhao et al., 2014). LXR agonists can protect against ovariectomy-induced bone loss by reducing osteoclast number (Kim et al., 2013; Kleyer et al., 2012). RXR homodimers maintain the response to M-CSF in pre-osteoclasts, but as a heterodimer with LXR can suppress RANKL signaling in differentiating osteoclasts (Menendez-Gutierrez et al., 2015). The histological counts of osteoclasts and serum TRAP measurements indicated that osteoclast number was minimally altered by TBT in vivo, and TBT also marginally reduced osteoclast numbers in vitro. Therefore, a reduction in osteoclast number likely plays a small part in the increased amount of trabecular bone. However, this lack of change in osteoclast number is inconsistent with the observed changes in gene expression from whole bone (humerus) or osteoclast culture, where TBT suppressed osteoclast differentiation, function markers, and resorptive activity. Notably, expression of the Il-6 family cytokine cardiotrophin-1 (Ct-1) was increased by TBT in vivo and in vitro. Ct-1 is essential for osteoclast function, but also acts as a stimulatory signal for osteogenesis (Walker et al., 2008). On the other hand, TBT treatment did not impact Sema4d expression in vivo or in vitro, which inhibits osteoblastogenesis under normal conditions (Negishi-Koga et al., 2011). Together, these expression profiles suggest a change in the function of osteoclasts at the transcriptional level rather than a large change to the osteoclast numbers.
Distinct responses of cortical and trabecular bone have been reported for several other genetic manipulations and treatments. Loss of BMP receptor 1a leads to an increase in trabecular bone due to hyperproliferation of Sp7+ pre-osteoblasts, while it also leads to decreased trabecular bone due to low osteoblast activity on the periosteal surface (Lim et al., 2016). Trabecular bone volume is only mildy reduced in serum IGF1 deficient mice, while cortical bone thickness and polar momentum of inertia are strongly decreased (Yakar et al., 2009). The non-nuclear actions of estrogen receptor α prevent cortical bone loss caused by estrogen deficiency, but have no effect on trabecular bone loss caused by estrogen deficiency (Bartell et al., 2013). Thus, it also is possible that distinct traits of cortical and trabecular osteoblasts, as well as effects on osteoclasts, contribute to TBT’s disparate effects in the two bone compartments.
TBT’s effect on bone has been primarily investigated in the context of PPARγ agonism (Grun et al., 2006). Although TBT also binds and activates RXRs with greater affinity than it does PPARγ (le Maire et al., 2009), the role of TBT as an RXR agonist in bone that is capable of activating RXR:RXR homodimers and other RXR heterodimers (e.g. RXR:LXR) has received less attention (Baker et al., 2015; Cui et al., 2010; Yonezawa et al., 2007). TBT engages the PPARγ pathway to skew MSC differentiation to adipocytes. However, in osteoclasts, TBT acted distinctly from rosiglitazone, which increased expression of Nfatc1 but did not change the expression of Abca1, Srebp1c or Mafb. We confirmed TBT’s activation of RXR and LXR target genes (previously shown in osteoblast (Baker et al., 2015) and osteoclast (Cui et al., 2010) cell lines) in whole bone and primary osteoclast cultures, and show that it activates a pathway relevant to osteoclastogenesis, namely the expression of Mafb. This gene has been shown to be a target of RXR:RXR homodimers in osteoclast precursors and required for osteoclast differentiation (Menendez-Gutierrez et al., 2015). In maturing osteoclasts, Mafb is indirectly upregulated by RXRα:LXRα activation via increased expression of SREBP1c, which suppresses osteoclast maturation (Menendez-Gutierrez et al., 2015). The TBT-induced upregulation of Mafb is consistent with the decreases in Nfatc1 transcript levels. TBT could be increasing Mafb via RXRα homodimers, RXRα:LXR heterodimers, or both. It is notable that the increase in Mafb induced by TBT in vitro more closely reflects that of the RXR homodimer agonist LG100268 rather than the LXR agonist T0901317 and that the RXR antagonist, but not the LXR antagonist significantly inhibited the ability of TBT to suppress Ctsk expression.
Both the LXR and RXR have been proposed as targets for interventions to combat low bone density, given their roles in moderating osteoclast differentiation (Kleyer et al., 2012; Menendez-Gutierrez et al., 2015; Remen et al., 2011). To our knowledge, these are the first results to demonstrate an association between RXR or LXR agonism and increased bone density in intact, adult mice rather than ovariectomy (Kleyer et al., 2012) or inflammation-induced (Kim et al., 2013) models of bone loss. The results also show for the first time that TBT’s role as a bone suppressive agent is dependent upon the level of coupled remodeling that occurs at a given bone site. Use of TBT as a multi-functional model compound in conjunction with the appropriate Cre-lox knockout models may provide important insights into the relative contributions of different nuclear receptors in determining the balance of bone resorption and formation in future studies.
Supplementary Material
Acknowledgements
The authors thank Cassie Huang and Zack Webster for their technical expertise.
This work was supported by the Boston University micro-CT imaging core facility [NIH S10 RR021072].
Grant Information:
Superfund Research Program [P42 ES007381]
Footnotes
The authors have no conflict of interests.
References
- Akune T, Ohba S, Kamekura S, Yamaguchi M, Chung UI, Kubota N, Terauchi Y, Harada Y, Azuma Y, Nakamura K, Kadowaki T, Kawaguchi H. 2004. PPARgamma insufficiency enhances osteogenesis through osteoblast formation from bone marrow progenitors. J Clin Invest 113(6):846–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali AA, Weinstein RS, Stewart SA, Parfitt AM, Manolagas SC, Jilka RL. 2005. Rosiglitazone causes bone loss in mice by suppressing osteoblast differentiation and bone formation. Endocrinology 146(3):1226–1235. [DOI] [PubMed] [Google Scholar]
- Baker AH, Watt J, Huang CK, Gerstenfeld LC, Schlezinger JJ. 2015. Tributyltin engages multiple nuclear receptor pathways and suppresses osteogenesis in bone marrow multipotent stromal cells. Chem Res Toxicol 28(6):1156–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartell SM, Han L, Kim HN, Kim SH, Katzenellenbogen JA, Katzenellenbogen BS, Chambliss KL, Shaul PW, Roberson PK, Weinstein RS, Jilka RL, Almeida M, Manolagas SC. 2013. Non-nuclear-initiated actions of the estrogen receptor protect cortical bone mass. Mol Endocrinol 27(4):649–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, Jepsen KJ, Muller R. 2010. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J Bone Miner Res 25(7):1468–1486. [DOI] [PubMed] [Google Scholar]
- Bouxsein ML, Karasik D. 2006. Bone geometry and skeletal fragility. Current osteoporosis reports 4(2):49–56. [DOI] [PubMed] [Google Scholar]
- Bragdon B, Burns R, Baker AH, Belkina AC, Morgan EF, Denis GV, Gerstenfeld LC, Schlezinger JJ. 2015. Intrinsic sex-linked variations in osteogenic and adipogenic differentiation potential of bone marrow multipotent stromal cells. J Cell Physiol 230(2):296–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callewaert F, Sinnesael M, Gielen E, Boonen S, Vanderschueren D. 2010. Skeletal sexual dimorphism: relative contribution of sex steroids, GH-IGF1, and mechanical loading. The Journal of endocrinology 207(2):127–134. [DOI] [PubMed] [Google Scholar]
- Carfi M, Croera C, Ferrario D, Campi V, Bowe G, Pieters R, Gribaldo L. 2008. TBTC induces adipocyte differentiation in human bone marrow long term culture. Toxicology 249(1):11–18. [DOI] [PubMed] [Google Scholar]
- Cornelissen G, Pettersen A, Nesse E, Eek E, Helland A, Breedveld GD. 2008. The contribution of urban runoff to organic contaminant levels in harbour sediments near two Norwegian cities. Mar Pollut Bull 56(3):565–573. [DOI] [PubMed] [Google Scholar]
- Cui H, Okuhira K, Ohoka N, Naito M, Kagechika H, Hirose A, Nishimaki-Mogami T. 2010. Tributyltin chloride induces ABCA1 expression and apolipoprotein A-I-mediated cellular cholesterol efflux by activating LXRalpha/RXR. Biochem Pharmacol 81(6):819–824. [DOI] [PubMed] [Google Scholar]
- Fromme H, Mattulat A, Lahrz T, Ruden H. 2005. Occurrence of organotin compounds in house dust in Berlin (Germany). Chemosphere 58(10):1377–1383. [DOI] [PubMed] [Google Scholar]
- Gerstenfeld LC, Cho TJ, Kon T, Aizawa T, Tsay A, Fitch J, Barnes GL, Graves DT, Einhorn TA. 2003. Impaired fracture healing in the absence of TNF-alpha signaling: the role of TNF-alpha in endochondral cartilage resorption. J Bone Miner Res 18(9):1584–1592. [DOI] [PubMed] [Google Scholar]
- Grun F, Watanabe H, Zamanian Z, Maeda L, Arima K, Cubacha R, Gardiner DM, Kanno J, Iguchi T, Blumberg B. 2006. Endocrine-disrupting organotin compounds are potent inducers of adipogenesis in vertebrates. Mol Endocrinol 20(9):2141–2155. [DOI] [PubMed] [Google Scholar]
- Imai Y, Youn MY, Inoue K, Takada I, Kouzmenko A, Kato S. 2013. Nuclear receptors in bone physiology and diseases. Physiol Rev 93(2):481–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanayama T, Kobayashi N, Mamiya S, Nakanishi T, Nishikawa J. 2005. Organotin compounds promote adipocyte differentiation as agonists of the peroxisome proliferator-activated receptor gamma/retinoid X receptor pathway. Mol Pharmacol 67(3):766–774. [DOI] [PubMed] [Google Scholar]
- Kannan K, Senthilkumar K, Giesy JP. 1999. Occurence of butyltin compounds in human blood. Environ Sci Technol 33:1776–1779. [Google Scholar]
- Kannan K, Takahashi S, Fujiwara N, Mizukawa H, Tanabe S. 2010. Organotin compounds, including butyltins and octyltins, in house dust from Albany, New York, USA. Arch Environ Contam Toxicol 58(4):901–907. [DOI] [PubMed] [Google Scholar]
- Kartner N, Yao Y, Li K, Crasto GJ, Datti A, Manolson MF. 2010. Inhibition of osteoclast bone resorption by disrupting vacuolar H+-ATPase a3-B2 subunit interaction. J Biol Chem 285(48):37476–37490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Yoon KA, Yoon HJ, Hong JM, Lee MJ, Lee IK, Kim SY. 2013. Liver X receptor activation inhibits osteoclastogenesis by suppressing NF-kappaB activity and c-Fos induction and prevents inflammatory bone loss in mice. J Leukoc Biol 94(1):99–107. [DOI] [PubMed] [Google Scholar]
- Kirchner S, Kieu T, Chow C, Casey S, Blumberg B. 2010. Prenatal exposure to the environmental obesogen tributyltin predisposes multipotent stem cells to become adipocytes. Mol Endocrinol 24(3):526–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleyer A, Scholtysek C, Bottesch E, Hillienhof U, Beyer C, Distler JH, Tuckermann JP, Schett G, Kronke G. 2012. Liver X receptors orchestrate osteoblast/osteoclast crosstalk and counteract pathologic bone loss. J Bone Miner Res 27(12):2442–2451. [DOI] [PubMed] [Google Scholar]
- Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S, Hsu H, Sullivan J, Hawkins N, Davy E, Capparelli C, Eli A, Qian YX, Kaufman S, Sarosi I, Shalhoub V, Senaldi G, Guo J, Delaney J, Boyle WJ. 1998. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 93(2):165–176. [DOI] [PubMed] [Google Scholar]
- Lazarenko OP, Rzonca SO, Hogue WR, Swain FL, Suva LJ, Lecka-Czernik B. 2007. Rosiglitazone induces decreases in bone mass and strength that are reminiscent of aged bone. Endocrinology 148(6):2669–2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarenko OP, Rzonca SO, Suva LJ, Lecka-Czernik B. 2006. Netoglitazone is a PPAR-gamma ligand with selective effects on bone and fat. Bone 38(1):74–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- le Maire A, Grimaldi M, Roecklin D, Dagnino S, Vivat-Hannah V, Balaguer P, Bourguet W. 2009. Activation of RXR-PPAR heterodimers by organotin environmental endocrine disruptors. EMBO Rep 10(4):367–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecka-Czernik B, Gubrij I, Moerman EJ, Kajkenova O, Lipschitz DA, Manolagas SC, Jilka RL. 1999. Inhibition of Osf2/Cbfa1 expression and terminal osteoblast differentiation by PPARgamma2. J Cell Biochem 74(3):357–371. [PubMed] [Google Scholar]
- Lemaire M, Lemarie CA, Flores Molina M, Guilbert C, Lehoux S, Mann KK. 2014. Genetic deletion of LXRalpha prevents arsenic-enhanced atherosclerosis, but not arsenic-altered plaque composition. Toxicol Sci 142(2):477–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim J, Shi Y, Karner CM, Lee SY, Lee WC, He G, Long F. 2016. Dual function of Bmpr1a signaling in restricting preosteoblast proliferation and stimulating osteoblast activity in mouse. Development 143(2):339–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo S, Allera A, Albers P, Heimbrecht J, Jantzen E, Klingmuller D, Steckelbroeck S. 2003. Dithioerythritol (DTE) prevents inhibitory effects of triphenyltin (TPT) on the key enzymes of the human sex steroid hormone metabolism. The Journal of steroid biochemistry and molecular biology 84(5):569–576. [DOI] [PubMed] [Google Scholar]
- Menendez-Gutierrez MP, Roszer T, Fuentes L, Nunez V, Escolano A, Redondo JM, De Clerck N, Metzger D, Valledor AF, Ricote M. 2015. Retinoid X receptors orchestrate osteoclast differentiation and postnatal bone remodeling. J Clin Invest 125(2):809–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mino Y, Amano F, Yoshioka T, Konishi Y. 2008. Determination of organotins in human breast milk by gas chromatography with flame photometric detection. J Health Sci 54(2):224–228. [Google Scholar]
- Negishi-Koga T, Shinohara M, Komatsu N, Bito H, Kodama T, Friedel RH, Takayanagi H. 2011. Suppression of bone formation by osteoclastic expression of semaphorin 4D. Nature medicine 17(11):1473–1480. [DOI] [PubMed] [Google Scholar]
- Nielsen JB, Strand J. 2002. Butyltin compounds in human liver. Environ Res 88(2):129–133. [DOI] [PubMed] [Google Scholar]
- Pfaffl MW. 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic acids research 29(9):e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman S, Czernik PJ, Lu Y, Lecka-Czernik B. 2012. beta-catenin directly sequesters adipocytic and insulin sensitizing activities but not osteoblastic activity of PPARgamma2 in marrow mesenchymal stem cells. PloS one 7(12):e51746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remen KM, Henning P, Lerner UH, Gustafsson JA, Andersson G. 2011. Activation of liver X receptor (LXR) inhibits receptor activator of nuclear factor kappaB ligand (RANKL)-induced osteoclast differentiation in an LXRbeta-dependent mechanism. J Biol Chem 286(38):33084–33094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios HF, Ye L, Dusevich V, Eick D, Bonewald LF, Feng JQ. 2005. DMP1 is essential for osteocyte formation and function. J Musculoskelet Neuronal Interact 5(4):325–327. [PubMed] [Google Scholar]
- Robertson KM, Norgard M, Windahl SH, Hultenby K, Ohlsson C, Andersson G, Gustafsson JA. 2006. Cholesterol-sensing receptors, liver X receptor alpha and beta, have novel and distinct roles in osteoclast differentiation and activation. J Bone Miner Res 21(8):1276–1287. [DOI] [PubMed] [Google Scholar]
- Rzonca SO, Suva LJ, Gaddy D, Montague DC, Lecka-Czernik B. 2004. Bone is a target for the antidiabetic compound rosiglitazone. Endocrinology 145(1):401–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville JM, Aspden RM, Armour KE, Armour KJ, Reid DM. 2004. Growth of C57BL/6 mice and the material and mechanical properties of cortical bone from the tibia. Calcif Tissue Int 74(5):469–475. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Mukai H, Tanabe S, Sakayama K, Miyazaki T, Masuno H. 1999. Butyltin residues in livers of humans and wild terrestrial mammals and in plastic products. Environ Pollut 106(2):213–218. [DOI] [PubMed] [Google Scholar]
- Thudium CS, Moscatelli I, Flores C, Thomsen JS, Bruel A, Gudmann NS, Hauge EM, Karsdal MA, Richter J, Henriksen K. 2014. A comparison of osteoclast-rich and osteoclast-poor osteopetrosis in adult mice sheds light on the role of the osteoclast in coupling bone resorption and bone formation. Calcif Tissue Int 95(1):83–93. [DOI] [PubMed] [Google Scholar]
- Tontonoz P, Graves RA, Budavari AI, Erdjument-Bromage H, Lui M, Hu E, Tempst P, Spiegelman BM. 1994. Adipocyte-specific transcription factor ARF6 is a heterodimeric complex of two nuclear hormone receptors, PPAR gamma and RXR alpha. Nucleic acids research 22(25):5628–5634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukamoto Y, Ishihara Y, Miyagawa-Tomita S, Hagiwara H. 2004. Inhibition of ossification in vivo and differentiation of osteoblasts in vitro by tributyltin. Biochem Pharmacol 68(4):739–746. [DOI] [PubMed] [Google Scholar]
- Walker EC, McGregor NE, Poulton IJ, Pompolo S, Allan EH, Quinn JM, Gillespie MT, Martin TJ, Sims NA. 2008. Cardiotrophin-1 is an osteoclast-derived stimulus of bone formation required for normal bone remodeling. J Bone Miner Res 23(12):2025–2032. [DOI] [PubMed] [Google Scholar]
- Wan Y, Chong LW, Evans RM. 2007. PPAR-gamma regulates osteoclastogenesis in mice. Nature medicine 13(12):1496–1503. [DOI] [PubMed] [Google Scholar]
- Watt J, Schlezinger JJ. 2015. Structurally-diverse, PPARgamma-activating environmental toxicants induce adipogenesis and suppress osteogenesis in bone marrow mesenchymal stromal cells. Toxicology 331:66–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W, Wang X, Yang M, Smith LC, Dechow PC, Sonoda J, Evans RM, Wan Y. 2010. PGC1beta mediates PPARgamma activation of osteoclastogenesis and rosiglitazone-induced bone loss. Cell metabolism 11(6):503–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willy PJ, Umesono K, Ong ES, Evans RM, Heyman RA, Mangelsdorf DJ. 1995. LXR, a nuclear receptor that defines a distinct retinoid response pathway. Genes Dev 9(9):1033–1045. [DOI] [PubMed] [Google Scholar]
- Yakar S, Rosen CJ, Bouxsein ML, Sun H, Mejia W, Kawashima Y, Wu Y, Emerton K, Williams V, Jepsen K, Schaffler MB, Majeska RJ, Gavrilova O, Gutierrez M, Hwang D, Pennisi P, Frystyk J, Boisclair Y, Pintar J, Jasper H, Domene H, Cohen P, Clemmons D, LeRoith D. 2009. Serum complexes of insulin-like growth factor-1 modulate skeletal integrity and carbohydrate metabolism. FASEB J 23(3):709–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanik SC, Baker AH, Mann KK, Schlezinger JJ. 2011. Organotins are potent activators of PPAR{gamma} and adipocyte differentiation in bone marrow multipotent mesenchymal stromal cells. Toxicol Sci 122(2):476–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki S, Tomoyasu A, Yano K, Goto M, Murakami A, Tsuda E, Morinaga T, Higashio K, Udagawa N, Takahashi N, Suda T. 1998. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci U S A 95(7):3597–3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonezawa T, Hasegawa S, Ahn JY, Cha BY, Teruya T, Hagiwara H, Nagai K, Woo JT. 2007. Tributyltin and triphenyltin inhibit osteoclast differentiation through a retinoic acid receptor-dependent signaling pathway. Biochem Biophys Res Commun 355(1):10–15. [DOI] [PubMed] [Google Scholar]
- Zanotti S, Kalajzic I, Aguila HL, Canalis E. 2014. Sex and genetic factors determine osteoblastic differentiation potential of murine bone marrow stromal cells. PloS one 9(1):e86757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XK, Lehmann J, Hoffmann B, Dawson MI, Cameron J, Graupner G, Hermann T, Tran P, Pfahl M. 1992. Homodimer formation of retinoid X receptor induced by 9-cis retinoic acid. Nature 358(6387):587–591. [DOI] [PubMed] [Google Scholar]
- Zhao D, Shi Z, Warriner AH, Qiao P, Hong H, Wang Y, Feng X. 2014. Molecular mechanism of thiazolidinedione-mediated inhibitory effects on osteoclastogenesis. PloS one 9(7):e102706. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.