Abstract
Background
Previous work has demonstrated that anastomotic leak can be caused by collagenolytic bacteria such as Enterococcus faecalis via an effect on wound collagen. In humans, E. faecalis is the organism cultured most commonly from a leaking anastomosis, and is not routinely eliminated by standard oral or intravenous antibiotics. Novel strategies are needed to contain the virulence of this pathogen when present on anastomotic tissues.
Methods
Polyphosphorylated polymer ABA-PEG20k-Pi20 was tested in mice for its ability to prevent anastomotic leak caused by collagenolytic E. faecalis. The study design included a distal colonic resection and anastomosis followed by introduction of E. faecalis to anastomotic tissues via enema. Mice were assigned randomly to receive either ABA-PEG20-Pi20 or its unphosphorylated precursor ABA-PEG20k in their drinking water. The development of anastomotic leak was determined after the animals had been killed.
Results
Overnight incubation of two different E. faecalis collagenolytic strains with 2 mmol/l of ABA-PEG20k-Pi20 led to near complete inhibition of collagenase production (from 21 000 to 1000 and from 68 000 to 5000 units; P < 0·001; 6 samples per group) without suppressing bacterial growth. In mice drinking 1 per cent ABA-PEG20k-Pi20, the phosphate concentration in the distal colonic mucosa increased twofold and leak rates decreased from eight of 15 to three of 15 animals (P < 0·001). In mice drinking ABA-PEG20k-Pi20, the percentage of collagenolytic colonies among E. faecalis populations present at anastomotic tissue sites was decreased by 6–4800-fold (P = 0·008; 5 animals).
Conclusion
These data indicate that oral intake of ABA-PEG20k-Pi20 may be an effective agent to contain the virulence of E. faecalis and may prevent anastomotic leak caused by this organism.
Clinical relevance
Progress in understanding the pathogenesis of anastomotic leak continues to point to intestinal bacteria as key causative agents. The presence of pathogens such as Enterococcus faecalis that predominate on anastomotic tissues despite antibiotic use, coupled with their ability to produce collagenase, appears to alter the process of healing that leads to leakage. Further antibiotic administration may seem logical, but carries the unwanted risk of eliminating the normal microbiome, which functions competitively to exclude and suppress the virulence of pathogens such as E. faecalis. Therefore, non-antibiotic strategies that can suppress the production of collagenase by E. faecalis without affecting its growth, or potentially normal beneficial microbiota, may have unique advantages. The findings of this study demonstrate that drinking a phosphate-based polymer can achieve the goal of preventing anastomotic leak by suppressing collagenase production in E. faecalis without affecting its growth.
Further evidence the microbiome may be important
Introduction
Anastomotic leak is among the most feared complications of intestinal surgery. Despite decades of research, the aetiology and pathogenesis of anastomotic leak remains largely unknown. That microbes may be key causative agents was first proposed over 60 years ago1, and has been confirmed in multiple subsequent animal studies2. Recently, the mechanism by which intestinal microbes may cause anastomotic leak has been linked to a direct effect of bacterial collagenases3,4. Several pathogens that can express this collagenolytic phenotype, including Pseudomonas aeruginosa, Serratia marcescens and Enterococcus faecalis, have been associated with the development of leak3–6.
In rats, a 500-fold increase of Enterococcus has been observed at anastomotic tissues following a low colorectal anastomosis7. Clinical studies demonstrate that E. faecalis is the most common organism present at sites of anastomotic leak8. Importantly, current methods to prepare the bowel for surgery that include both intravenous and oral non-absorbable antibiotics demonstrate that E. faecalis persists in stool for up to 7 days after surgery8. Therefore, non-antibiotic strategies may need to be considered to ‘contain’ the virulence of E. faecalis by preventing its production of collagenase at the time of anastomotic surgery. In this regard, a phosphate-based approach that exploits the known action of extracellular phosphate to suppress quorum sensing9 may be considered. Quorum sensing is a cell-to-cell communication system of bacterial virulence activation. The bacteria secrete and take up small signalling molecules when they reach a high cell density, hence the term ‘quorum’10. Quorum sensing ostensibly evolved so that bacteria could ‘sense’ their population density, presumably that amount needed to overcome the host. However, at this point of growth nutrients often become limited, triggering bacteria to express virulence in order to acquire nutrients from host tissues and neighbouring microflora11. Importantly, E. faecalis uses quorum sensing to trigger the expression of its gelE gene, which encodes gelatinase (collagenase)12.
A major mechanism by which bacteria colonize intestinal tissues involves their ability to feed off intestinal mucus13, a rich source of organic phosphate14. Mucus production has been shown to be important for colonic anastomosis healing15. Mucus depletion and exposure of collagen at anastomotic tissue sites may create opportunism for microbes such as E. faecalis to adhere and become ‘cued’ to express collagenases. Therefore, maintaining local phosphate concentrations at the site of an anastomosis has the potential to suppress quorum sensing, given the interconnectedness between pathways of quorum sensing and phosphosensory/phosphoregulatory pathways of virulence expression9.
A high phosphate-containing food additive, hexametaphosphate (PPi-6), approved by the Food and Drug Administration, has been shown recently5 to be highly effective in suppressing collagenase production in two collagenolytic strains of Gram-negative bacteria, S. marcescens and P. aeruginosa. Oral delivery of PPi-6 to mice was highly efficacious in preventing anastomotic leak5. However, PPi-6 had a minimal effect on collagenase production in E. faecalis, perhaps owing to its classification as a Gram-positive organism. Given the high prevalence of E. faecalis as a ‘leak’ pathogen, it was hypothesized that ABA-PEG20k-Pi20, whose de novo synthesis has been described recently16, would more efficiently deliver phosphate to E. faecalis, attenuate its production of collagenase, and prevent anastomotic leak in mice. Thus, the aim of this study was to test the ability of ABA-PEG20k-Pi20 to suppress collagenase production in E. faecalis and prevent anastomotic leak in mice.
Methods
Animals
Fifty male C57BL/6 mice aged 10–12 weeks (Charles River Laboratories, Wilmington, Delaware, USA) were used in all experiments. All animals were maintained in accordance with the guidelines prepared by the Institutional Animal Care and Use Committee (IACUC) at the University of Chicago (IACUC protocol 72417). All mice were housed in filter-top cages under standard laboratory conditions and allowed to acclimatize for 48 h before all experiments in a temperature-controlled room (22–24 °C) with a 12-h light–dark diurnal cycle. Mice were allowed free access to food and water.
Mice were anaesthetized with an intraperitoneal injection of ketamine (100 mg/kg) and xylazine (10 mg/kg). All procedures were performed under sterile conditions by a single surgeon highly proficient in the surgery. Once unresponsive to light stimuli, mice were placed supine on a warming blanket, secured in a supine position using adhesive tape and covered by sterile towel drape (Dynarex, Orangeburg, New York, USA). Aseptic technique was used in accordance with guidelines of the Animal Resource Center at the University of Chicago.
Bacterial strains
E. faecalis E2 and E. faecalis E27 were obtained from a library of collagenolytic strains isolated from a previously described rat model of anastomotic leak3. As E. faecalis E2 has been highly characterized and its entire genome sequenced and annotated (S. Christley et al., unpublished results), this strain was used in all animal experiments.
ABA-PEG20k-Pi20
A polyphosphate-containing polyethylene glycol-based triblock co-polymer with defined ABA (hydrophilic–hydrophobic–hydrophilic) structure was recently synthesized de novo16. The molecular weight and antivirulence properties of this phosphorylated polymer, ABA-PEG20k-Pi20, and its unphosphorylated precursor polymer, ABA-PEG20k, have been characterized16.
Collagenolytic activity of E. faecalis
The collagenolytic activity of E. faecalis was measured using fluorescein-labelled gelatine or fluorescein-labelled collagen (EnzChek® Gelatinase/Collagenase assay kit; Thermo Fisher Scientific, Waltham, Massachusetts, USA) in 96-well plates as described previously3. The polymers were suspended in TY medium (10 g/l tryptone and 5 g/l yeast extract) at a final concentration of 2 mmol/l, and E. faecalis suspension was added to a final concentration of optical density at 600 nm (OD600 nm) = 0·05. Control plates were presented by E. faecalis grown in TY medium. Fluorescence (485/20 excitation, 528/20 emission) and OD600 nm were measured at 0- and 12-h time points, and 0-h values were subtracted from overnight time point values.
Fluorescence readings were normalized to the optical density corresponding to bacterial growth.
Mouse model of anastomotic leak caused by E. faecalis
A recently developed novel mouse model of spontaneous anastomotic leak was used by performing a low colorectal anastomosis followed by bacterial inoculation of the anastomosis (via enema) with pathogenic microbes (P. aeruginosa or S. marcescens)5. This model predictably results in anastomotic leaks similar to those that are clinically significant in humans (dense adhesion to the anastomosis, abscess formation or peritonitis)5.
Study design
All mice were assigned randomly to the following treatment groups: ad libitum access to water, colorectal anastomosis and administration of a saline enema (control; 10 animals); ad libitum access to water, colorectal anastomosis, administration of an E. faecalis enema (15 animals); ad libitum access to water containing 1 per cent ABA-PEG20k-Pi20, colorectal anastomosis, administration of an E. faecalis enema (15 animals); and ad libitum access to water containing 1 per cent ABA-PEG20k, colorectal anastomosis, administration of an E. faecalis enema (10 animals). In all groups, the oral intake of water or polymers began the week before surgery and continued until the mice were killed on postoperative day (POD) 7.
All 50 mice received oral clindamycin (100 mg/kg, approximately 50 μl oral gavage of 50 mg/ml) followed by a subcutaneous injection of cefoxitin (40 mg/kg, approximately 100 μl of 10 mg/ml) on the morning and evening before surgery. Administration of antibiotics was given to mimic the practice of oral and intravenous prophylactic antibiotic use in humans before surgery (Fig. 1). In a separate experiment, mice were allowed to drink either 1 per cent hexametaphosphate (PPi-6; Sigma, St Louis, Missouri, USA) in water or water (control group), followed by the same procedure of antibiotic treatment, colorectal anastomosis and E. faecalis enema as described above (5 animals per group).
Fig. 1.
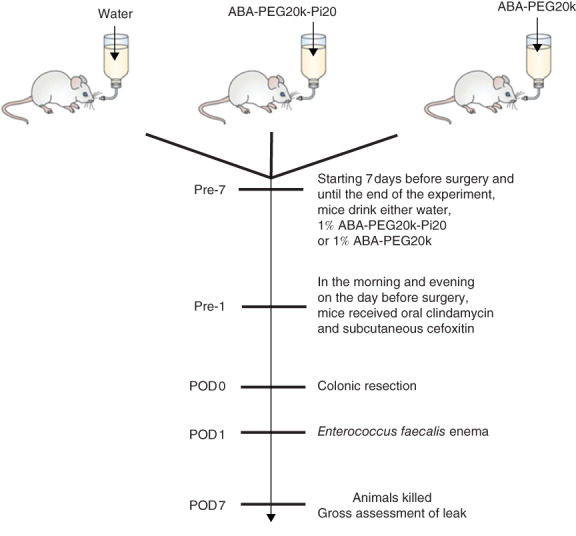
Mouse model of colonic anastomosis. Mice were allocated randomly to water (n = 15), 1 per cent ABA-PEG20k-Pi20 (n = 15) or 1 per cent ABA-PEG20k (n = 10), starting 7 days before surgery until they were killed on postoperative day (POD) 7
All operations were performed by a single surgeon. On the day of surgery (POD 0), a midline abdominal incision was made and the colon was transected at the peritoneal reflection. A single-layer anastomosis was created at the colorectal junction using interrupted sutures incorporating the full thickness of the colon wall using seven interrupted stitches of 8-0 polypropylene suture. The integrity of the anastomosis was tested by slowly distending the distal colon with normal saline via enema using a 22-G blunt-tipped gavage needle. Finally, the abdomen was closed in two layers with 5-0 polyglactin 910 and 5-0 nylon for the skin. All animals were volume-resuscitated with 1 ml 0·9 per cent normal saline administered as a subcutaneous injection. On POD 1, 100 μl of a freshly prepared E. faecalis suspension (optical density at 600 nm = 0·5 in 10 per cent glycerol) was administered gently to the anastomosis site via rectal enema using a 22-G blunt-tipped gavage needle, as described recently5. Mice were held upside down for 1 min after receiving the enema to promote its retention. Extensive experience with the administration of E. faecalis by enema demonstrated its safety, as there were no anastomotic disruptions or deaths due to the technique. All mice were killed on POD 7 using carbon dioxide euthanasia according to Animal Care and Use Protocol 72417.
Anastomotic healing score
The anastomotic healing score (AHS) was applied as described previously5: score 0, normal healing; 1, flimsy adhesions; 2, dense adhesions without abscess or intraperitoneal contamination; 3, dense adhesions with gross abscess at the anastomotic site; 4, gross leak with peritoneal contamination and a visible anastomotic dehiscence. A working group of three people including the operating surgeon evaluated all anastomoses at the time of killing and, by consensus, applied an AHS to each mouse. In addition, images of anastomoses were captured, presented and discussed at the weekly laboratory meeting to confirm, by consensus, that the AHS had been calculated correctly.
Anastomotic tissue sample collection
A long segment of colon was removed above and below the anastomotic line for analysis. The colonic contents (faecal material within the lumen), full-thickness anastomotic tissues, and the anastomotic mucosa were harvested from mice at the time of killing for analysis. All samples were homogenized. A portion of the harvested anastomotic tissue and the scrapped mucosa samples were placed in 1 ml sterile water for assay of phosphate content. At the same time, the anastomotic colonic contents and portions of the anastomotic tissue samples were placed in 10 per cent glycerol for bacterial culture and determination of bacterial collagenase activity. All samples were stored at −80 °C.
Tissue and luminal bacterial culture analysis
Anastomotic tissues were homogenized, serially diluted in normal saline (0·9 per cent sodium chloride), and 50 μl of each dilution was plated on Enterococcus selective agar (BBL™ Enterococcosel™ Agar; BD Diagnostics, Sparks, Maryland, USA). The colony-forming unit (CFU) count was then normalized to the sample weight. For identification of collagenolytic E. faecalis, special skim milk plates were made by adding 15 per cent skim milk to the Enterococcus agar. Collagenolytic species produced classical black colonies surrounded by a clearing halo, whereas non-collagenolytic species failed to produce this effect. The CFU count was normalized to the sample weight.
Phosphate assay
To determine the amount of phosphate that remained on the surface of the anastomosis following oral administration, and to avoid measuring submucosal intracellular phosphate, the mucosa was gently scraped off with a glass slide in a 1-cm2 area of the anastomotic segment. Samples were homogenized on FastPrep-24™ 5G homogenizer (MP Biomedicals, Santa Ana, California, USA) with lysing matrix D beads using the Animal Intestinal Tissue protocol provided by the manufacturer (speed 6 m/s for 40 s) followed by centrifugation at 3220 g for 10 min. The supernatants were used for phosphate analysis. The phosphate concentration was assayed using the PiPer™ phosphate assay kit (P22061) (Thermo Fisher Scientific). Briefly, 50 μl of the sample and 50 μl of the working solution were added to a 96-well plate and incubated for 30 min in the dark at 37 °C. Phosphate was measured as a fluorescence at excitation of 545 ± 15 nm and emission of 590 ± 15 nm using an M5 SpectraMax® plate reader (Molecular Devices, San Jose, California, USA).
Statistical analysis
A power calculation was performed based on an anticipated incidence of anastomosis leak (AHS 3 or more) between control and treatment groups. Anticipating a 60 per cent leak rate in the control group and a 10 per cent leak rate in the treatment group, and accepting an α error of 0·05 at a power of 80 per cent, the sample size required was 13 animals in each group. Considering a 10 per cent attrition rate due to possible technical error, the final sample size was determined to be 15. Results are represented as mean(s.d.). χ2 analysis and non-parametric t tests were used to test the significance of differences in AHS and phosphate, using GraphPad Prism® (GraphPad Software, San Diego, California, USA). The non-parametric Mann–Whitney U test was used for statistical analysis of all bacterial culture data. P < 0·050 was considered statistically significant.
Results
ABA-PEG20-Pi20 markedly suppresses the collagenolytic activity of E. faecalis
At baseline in TY medium, E. faecalis displayed significant collagenolytic activity as judged by its ability to degrade fluorescein-labelled gelatine (Fig. 2a). The addition of ABA-PEG20k-Pi20 to the TY medium to a final concentration of 2 mmol/l led to a significant decrease of collagenase production from approximately 21 000 to 1000 units in the E2 strain and from 68 000 to 5000 units in the E27 strain (Fig. 2a). The unphosphorylated parent polymer ABA-PEG20k at the same concentration displayed a diminished but significant collagenase suppressive effect (2-fold for the E2 strain and approximately 4-fold for the E27 strain). ABA-PEG20k-Pi20 enhanced the growth of both E. faecalis strains, whereas the unphosphorylated compound did not, suggesting that strains can consume phosphate from the phosphorylated polymer (Fig. 2b).
Fig. 2.
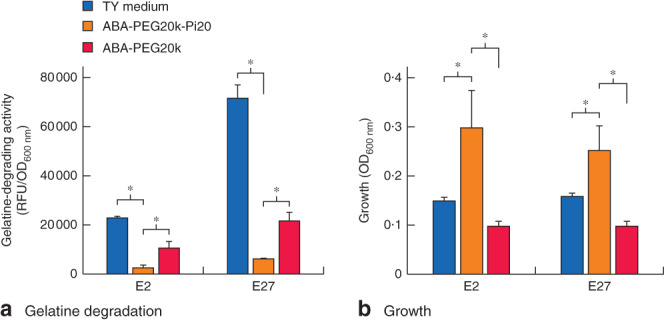
ABA-PEG20-Pi20 significantly suppresses collagenolytic activity in Enterococcus faecalis. a Degradation of fluorescein-labelled gelatine by E. faecalis is suppressed by both phosphorylated ABA-PEG20k-Pi20 and unphosphorylated ABA-PEG20k polymers, with ABA-PEG20k-Pi20 displaying greater suppressive effect. b ABA-PEG20k-Pi20 promotes the growth of E. faecalis. Values are mean(s.d.); fluorescence (485/20 excitation, 528/20 emission) and optical density at 600 nm (OD600 nm) were measured at 0- and 12-h time points, and 0-h values were subtracted from overnight time point values. Fluorescence readings (RFU) were normalized to OD600 nm, corresponding to bacterial growth. Six samples for TY medium and ABA-PEG20k-Pi20 groups, and three samples for the ABA-PEG20k group. *P < 0·001 (Student's t test)
ABA-PEG20k-Pi20 prevents healing complications caused by collagenolytic E. faecalis
In all mouse experiments, E. faecalis strain E2 was used. All polymers in the drinking water were tolerated well by mice with no signs of weight loss or change in behaviour (such as oral intake, stool quantity, grooming). No mice died or appeared moribund in the immediate postoperative period (POD 1–4). After this time, however, some mice with anastomoses exposed to E. faecalis developed signs of health deterioration (weight loss, lack of mobility, hunched posture, ruffled fur or distended abdomen) and were killed. Among them, five mice were in the group drinking water (killed on POD 5–6), one was in the group drinking ABA-PEG20k-Pi20 (killed on POD 5) and two were in the group drinking ABA-PEG20k (1 was killed on POD 5, the other on POD 6). After being killed, impaired anastomotic healing with an AHS of 3 or above was found in all these animals. All remaining mice were killed on POD 7.
Anastomotic healing was impaired when anastomotic tissues were exposed to E. faecalis (mean(s.d.) AHS 1·333(0·159) for control versus 2·600(0·214) for E. faecalis; P < 0·001, unpaired 2-tailed t test), with about eight of 15 mice in the E. faecalis group developing an AHS of 3 or more, a score considered to represent a clinical leak (Fig. 3a,b). In mice uninfected by E. faecalis (control group), no clinical-like leaks were observed, as indicated by an AHS of 2 or less. In mice randomly assigned to drink 1 per cent ABA-PEG20k-Pi20, healing was significantly improved (AHS 2·600(0·214) for E. faecalis alone versus 1·933(0·182) for E. faecalis + ABA-PEG20k-Pi20; P = 0·025, unpaired 2-tailed t test). In this group, only three of 15 mice developed an anastomotic leak (AHS 3), a significant decrease compared with the rate in mice receiving E. faecalis alone (8 of 15) (P < 0·001). There was less improvement of healing by the unphosphorylated polymer ABA-PEG20k (AHS 2·500(0·224)). A χ2 test was used to compare leak events (AHS 3 or more) between the control, E. faecalis, and E. faecalis + ABA-PEG20k-Pi20 groups (χ2 = 6·508, P = 0·039).
Fig. 3.
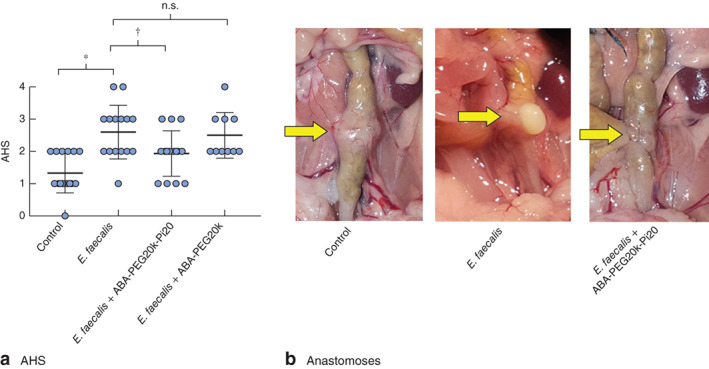
ABA-PEG20-Pi20 enhances anastomotic healing. a Anastomotic healing score (AHS) in control (n = 10) and Enterococcus faecalis enema groups (n = 15). Mean(s.d.) values from three separate experiments are shown, as well as individual AHA estimations for each mouse. b Representative images of anastomoses in control, E. faecalis and E. faecalis + 1 per cent ABA-PEG20k-Pi20 groups. Arrows indicate anastomotic healing lines, demonstrating adequate healing in the control group, dense adhesions with gross abscess in the E. faecalis group, and flimsy adhesions without abscess in the E. faecalis + 1 per cent ABA-PEG20k-Pi20 group. n.s., P not significant. *P < 0·001, †P = 0·025 (unpaired 2-tailed t test)
Collagenolytic Enterococcus is decreased at anastomotic tissue sites in mice drinking ABA-PEG20k-Pi20
The total Enterococcus population was not significantly different between luminal samples harvested at anastomotic sites and anastomotic tissue samples (Fig. 4a,b). However, collagenolytic colonies of E. faecalis were significantly reduced in both tissue (6–4800-fold decrease, P = 0·008; 5 animals) and luminal contents (0·1–1588-fold decrease, P = 0·016; 5 animals) in mice drinking ABA-PEG20k-Pi20 compared with those drinking water (Fig. 4a,c).
Fig. 4.
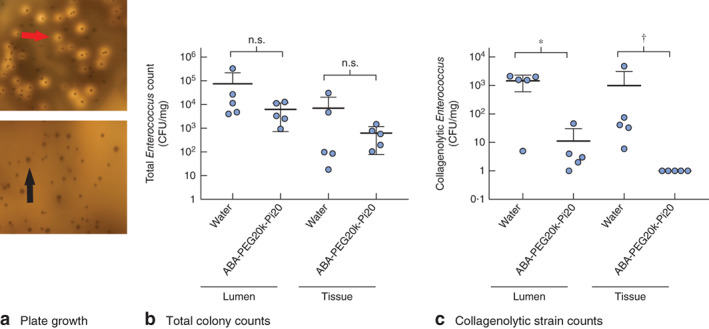
Collagenolytic populations of Enterococcus faecalis are decreased in mice drinking ABA-PEG20k-Pi20. aEnterococcus selective agar plate covered with skim milk to assess the total and collagenolytic counts of E. faecalis. The upper panel represents the E. faecalis group and bottom panel represents the E. faecalis + ABA-PEG20k-Pi20 group. The red arrow indicates collagenolytic colonies identified by the appearance of a halo surrounding the colonies. The black arrow indicates non-collagenolytic colonies. b Total colony counts of E. faecalis and c counts of collagenolytic strains (5 mice per group). Values are mean(s.d.). CFU, colony-forming units; n.s., P not significant. *P = 0·016, †P = 0·008 (non-parametric Mann–Whitney U test)
ABA-PEG20k-Pi20 enriches the anastomotic mucosal layer with phosphate
Phosphate concentration was measured in mucosa scraped off a 1-cm2 area of anastomotic tissue at the time of killing on POD 7. The concentration was significantly increased in mice drinking ABA-PEG20k-Pi20 compared with that in animals drinking water only (mean(s.d.) 48·42(7·63) μmol/l for E. faecalis + ABA-PEG20k-Pi20 versus 23·22(5·73) μmol/l for E. faecalis + water; P = 0·030; 5 animals per group) (Fig. 5).
Fig. 5.
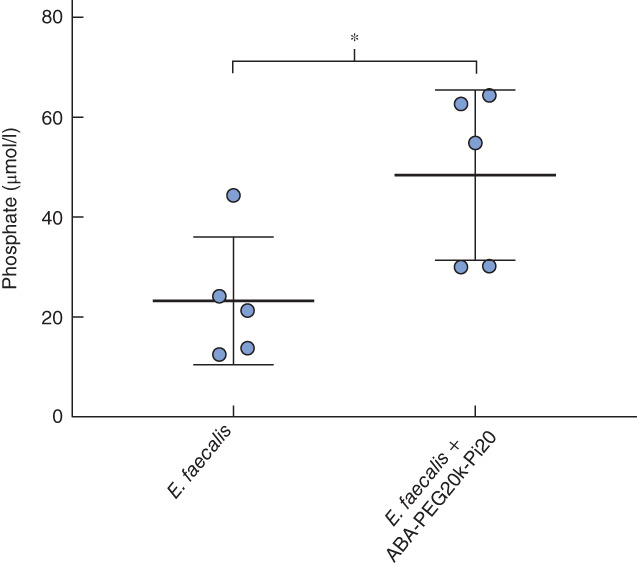
Oral delivery of ABA-PE20k-Pi20 leads to an increase in phosphate in the mucosa of anastomotic tissues. Phosphate concentration was measured in the mucosa from a 1-cm2 area of anastomotic tissues. Values are mean(s.d.) (5 mice per group). *P = 0·030 (2-tailed t test)
Hexametaphosphate does not suppress collagenolytic activity in E. faecalis or prevent anastomotic leak
Reiterative studies were performed in vitro with E. faecalis and in mice to determine the efficacy of PPi-6 in suppressing collagenase and preventing anastomotic leak. PPi-6 did not suppress collagenolytic activity in E. faecalis (Fig. S1a, supporting information) or prevent anastomotic leak in mice drinking PPi-6 ad libitum (Fig. S1b, supporting information).
Discussion
The role of intestinal microbes as key and causative agents in anastomotic leak is becoming increasingly evident3–5,17. Although there may be a role for ischaemia in this process, here the authors posit that without the presence of a collagenolytic strain of bacteria, and without its in vivo expression of the collagenolytic phenotype, leaks do not occur. Loss of the colonization resistance of the normal microbiota that protects intestinal tissues from invasion from collagenolytic microbes is a prerequisite for strains such as E. faecalis to predominate at anastomotic sites. This process is facilitated by the use of prophylactic antibiotics3,7. However, indiscriminate use of broad-spectrum antibiotics carries the unintended consequence of disabling the normal microbiome's ability to contain collagenolytic bacteria from proliferating. This practice may also promote the emergence of antibiotic-resistant strains. It is therefore important to explore alternative approaches such as phosphate-mediated suppression of bacterial virulence to avoid these potential untoward effects.
Organic phosphates in intestinal mucus are replenished continuously by local intestinal tissues under homeostatic conditions. However, during physiological stress, such as surgical injury, the mucous layer becomes depleted, limiting bacterial access to this rich source of phosphate. Oral dietary phosphate will not replete phosphate under these conditions as it is rapidly digested and absorbed proximally in the human intestine, leaving little to no free inorganic phosphate in the distal gut. Interestingly Lactobacillus species are known to be high producers of polyphosphate18. Unfortunately, surgical injury itself leads to a rapid depletion of lactobacilli7, as does the administration of standard antibiotics for prophylaxis. Finally, the precise form and dose of oral phosphate needed to suppress the virulence of a given species of bacterium is likely to be highly variable because of the great diversity of bacteria that can produce collagenase. It may be for this reason that PPi-6 functioned well against S. marcescens and P. aeruginosa, but not against E. faecalis. The findings in the present study suggest that a polydisperse solution of phosphate may be needed to deliver phosphate to anastomotic tissues in order to reach distal sites and remain bioactive against common pathogens that disrupt healing anastomotic tissues.
The ABA-PEG20k-Pi20 polymer in this study was synthesized de novo and verified for its ability to coat bacteria and make its phosphate available for delivery and virulence suppression19. In the present study, ABA-PEG20k-Pi20 was observed to deliver phosphate to the site of the anastomosis, significantly attenuate collagenolytic populations of E. faecalis at anastomotic sites, and enhance anastomotic healing. The ability of ABA-PEG20k-Pi20 to suppress collagenolytic activity is likely to be critical for its in vivo protective effect. Its unphosphorylated parent compound, ABA-PEG20k, and inorganic polyphosphate, PPi-6, were less effective in suppressing the collagenolytic activity of E. faecalis and were therefore less protective in preventing anastomotic leak caused by this pathogen. These findings suggests that, although the phosphate concentration at the site of the host–pathogen interaction is critical, not all forms of phosphate can be applied universally to all pathogens that cause impairment of anastomotic healing. Taken together, these findings indicate that future formulations to prepare the bowel for surgery may need to be effective against a broad range of collagenolytic pathogens. Employment of a phosphorylated polymer that can suppress the collagenolytic activity of E. faecalis, and hence its disruption of anastomotic healing, represents a novel approach to contain rather than eliminate unwanted pathogens present in the intestine during surgery. Although this study provides compelling evidence that collagenolytic microbes such as E. faecalis can complicate anastomotic healing, and their collagenolytic activity can be suppressed by ABA-PEG20k-Pi20, further study is required to determine whether these findings are applicable to human anastomotic leak pathogenesis and its prevention.
ABA-PEG20k-Pi20 has a potent suppressive effect on collagenase production in E. faecalis, a common bacterium associated with anastomotic leak and a commensal organism that is highly resistant to antibiotic elimination in the human gut following colonic surgery. Its in vivo efficacy in mice suggests that it might be an important adjunct to current formulations to prepare the bowel for surgery. Future studies are planned to determine the optimal dose for this and related compounds as part of bowel preparation. Given that polyethylene glycol polymers are used clinically both as drug carriers for intravenous use and as oral cleansing solutions for the intestinal tract, they are generally regarded as safe. Phase I trials to determine bioavailability, polymer distribution and systemic phosphate absorption of the various polymers will be needed before assessing their effect on anastomotic healing.
Supplementary Material
Fig. S1. Hexametaphosphate (PPi-6) does not suppress the collagenolytic activity of Enterococcus faecalis or protect against anastomotic leak. A Gelatine-degrading (collagenolytic activity) of E. faecalis E2 at various concentrations of PPi-6. Readings were determined following overnight growth in 96-well plates (n = 3 per group). *P = 0.48 (Student's t test). B Anastomotic healing score (AHS) on postoperative day 7 (n = 4 per group)
Acknowledgements
M.W. and S.K.H. contributed equally to this publication; O.Z., H.v.G. and J.C.A. are senior co-authors. This work was funded by National Institutes of Health (grant 5R01GM062344-15 to J.C.A.).
The patent for ABA_PEG20k-Pi20, applied for by the University of Chicago, is pending.
Disclosure: The authors declare no other conflict of interest.
References
- 1. Cohn I Jr, Rives JD. Antibiotic protection of colon anastomoses. Ann Surg 1955; 141: 707–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schardey HM, Kamps T, Rau HG, Gatermann S, Baretton G, Schildberg FW. Bacteria: a major pathogenic factor for anastomotic insufficiency. Antimicrob Agents Chemother 1994; 38: 2564–2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shogan BD, Belogortseva N, Luong PM, Zaborin A, Lax S, Bethel Cet al. . Collagen degradation and MMP9 activation by Enterococcus faecalis contribute to intestinal anastomotic leak. Sci Transl Med 2015; 7: 286ra68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Olivas AD, Shogan BD, Valuckaite V, Zaborin A, Belogortseva N, Musch Met al. . Intestinal tissues induce an SNP mutation in Pseudomonas aeruginosa that enhances its virulence: possible role in anastomotic leak. PLoS One 2012; 7: e44326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hyoju SK, Klabbers RE, Aaron M, Krezalek MA, Zaborin A, Wiegerinck Met al. . Oral polyphosphate suppresses bacterial collagenase production and prevents anastomotic leak due to Serratia marcescens and Pseudomonas aeruginosa. Ann Surg 2017; [Epub ahead of print]: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lee DS, Ryu JA, Chung CR, Yang J, Jeon K, Suh GYet al. . Risk factors for acquisition of multidrug-resistant bacteria in patients with anastomotic leakage after colorectal cancer surgery. Int J Colorectal Dis 2015; 30: 497–504. [DOI] [PubMed] [Google Scholar]
- 7. Shogan BD, Smith DP, Christley S, Gilbert JA, Zaborina O, Alverdy JC. Intestinal anastomotic injury alters spatially defined microbiome composition and function. Microbiome 2014; 2: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ohigashi S, Sudo K, Kobayashi D, Takahashi T, Nomoto K, Onodera H. Significant changes in the intestinal environment after surgery in patients with colorectal cancer. J Gastrointest Surg 2013; 17: 1657–1664. [DOI] [PubMed] [Google Scholar]
- 9. Zaborin A, Romanowski K, Gerdes S, Holbrook C, Lepine F, Long Jet al. . Red death in Caenorhabditis elegans caused by Pseudomonas aeruginosa PAO1. Proc Natl Acad Sci U S A 2009; 106: 6327–6332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fuqua WC, Winans SC, Greenberg EP. Quorum sensing in bacteria: the LuxR–LuxI family of cell density-responsive transcriptional regulators. J Bacteriol 1994; 176: 269–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Boyle KE, Monaco H, van Ditmarsch D, Deforet M, Xavier JB. Integration of metabolic and quorum sensing signals governing the decision to cooperate in a bacterial social trait. PLoS Comput Biol 2015; 11: e1004279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pinkston KL, Gao P, Diaz-Garcia D, Sillanpää J, Nallapareddy SR, Murray BEet al. . The Fsr quorum-sensing system of Enterococcus faecalis modulates surface display of the collagen-binding MSCRAMM Ace through regulation of gelE. J Bacteriol 2011; 193: 4317–4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tailford LE, Crost EH, Kavanaugh D, Juge N. Mucin glycan foraging in the human gut microbiome. Front Genet 2015; 6: 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stremmel W, Ehehalt R, Staffer S, Stoffels S, Mohr A, Karner Met al. . Mucosal protection by phosphatidylcholine. Dig Dis 2012; 30(Suppl 3): 85–91. [DOI] [PubMed] [Google Scholar]
- 15. Bosmans JW, Jongen AC, Birchenough GM, Nyström EE, Gijbels MJ, Derikx JPet al. . Functional mucous layer and healing of proximal colonic anastomoses in an experimental model. Br J Surg 2017; 104: 619–630. [DOI] [PubMed] [Google Scholar]
- 16. Mao J, Zaborin A, Poroyko V, Goldfeld D, Lynd NA, Chen Wet al. . De novo synthesis of phosphorylated triblock copolymers with pathogen virulence-suppressing properties that prevent infection-related mortality. ACS Biomater Sci Eng 2017; 3: 2076–2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shakhsheer BA, Versten LA, Luo JN, Defazio JR, Klabbers R, Christley Set al. . Morphine promotes colonization of anastomotic tissues with collagenase-producing Enterococcus faecalis and causes leak. J Gastrointest Surg 2016; 20: 1744–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kashima S, Fujiya M, Konishi H, Ueno N, Inaba Y, Moriichi Ket al. . Polyphosphate, an active molecule derived from probiotic Lactobacillus brevis, improves the fibrosis in murine colitis. Transl Res 2015; 166: 163–175. [DOI] [PubMed] [Google Scholar]
- 19. Mao J, Li C, Park HJ, Rouabhia M, Zhang Z. Conductive polymer waving in liquid nitrogen. ACS Nano 2017; 11: 10 409–10 416. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Hexametaphosphate (PPi-6) does not suppress the collagenolytic activity of Enterococcus faecalis or protect against anastomotic leak. A Gelatine-degrading (collagenolytic activity) of E. faecalis E2 at various concentrations of PPi-6. Readings were determined following overnight growth in 96-well plates (n = 3 per group). *P = 0.48 (Student's t test). B Anastomotic healing score (AHS) on postoperative day 7 (n = 4 per group)


