Abstract
Introduction
Smoking is a major risk factor for cardiovascular diseases (CVDs) and for many types of cancers. Despite recent policies, 1.1 billion people are active smokers and tobacco is the leading cause of mortality and illness throughout the world. The aim of this work was to identify smoking cessation interventions which could be implemented in primary care and/or at a community level.
Methods
A systematic review of CVDs prevention guidelines was realized using the ADAPTE Process. These were identified on G-I-N and TRIP databases. Additionally, a purposive search for national guidelines was successfully undertaken. Guidelines focusing on non-pharmacological lifestyle interventions, published or updated after 2011, were included. Exclusion criteria were specific populations, management of acute disease and exclusive focus on pharmacological or surgical interventions. After appraisal with the AGREE II tool, high-quality guidelines were included for analysis. High-grade recommendations and the supporting bibliographic references were extracted. References had to be checked in detail where sufficient information was not available in the guidelines.
Results
Nine hundred and ten guidelines were identified, 47 evaluated with AGREE II and 26 included. Guidelines recommended that patients quit smoking and that health care professionals provided advice to smokers but failed to propose precise implementation strategies for such recommendations. Only two guidelines provided specific recommendations. In the guideline bibliographic references, brief advice (BA) and multiple session strategies were identified as effective interventions. These interventions used Prochaska theory, motivational interviewing or cognitive-behavioral therapies. Self-help documentation alone was less effective than face-to-face counseling. Community-based or workplace public interventions alone did not seem effective.
Discussion
Behavioral change strategies were effective in helping patients to give up smoking. BA alone was less effective than multiple session strategies although it required fewer resources. Evidence for community-based interventions effectiveness was weak, mainly due to the lack of robust studies.
Keywords: cardiovascular diseases, primary prevention, smoking cessation, primary health care
Video abstract
Point your SmartPhone at the code above. If you have a QR code reader the video abstract will appear. Or use:
Introduction
Cardiovascular diseases (CVDs) are the leading cause of mortality worldwide. In 2016, they were responsible of 17.9 million of deaths, 31% of global mortality. Over 75% of deaths due to CVDs take place in low- and middle-income countries.1 In the last 20 years, mortality in low- and middle-income countries shifted from infectious diseases to CVDs. Current age standardized mortality rates in low-income countries are higher than those in developed countries.2 Additionally, global mortality by CVDs is expected to grow. By the year 2030, non-communicable diseases are projected to account for more than 75% of deaths worldwide, the majority being the result of CVDs.2
CVDs can be prevented by acting on modifiable risk factors. Tobacco consumption is a major risk factor for CVDs.1,3 The benefits of stopping smoking have a broad evidence base. Smoking cessation has been proved to be effective in reducing CVD-related morbidity and mortality.4,5,6,7 Smoking cessation is one of the most effective preventive measures: in secondary prevention it could reduce the risk of a new cardiovascular event by almost half.4
Considerable efforts have been made in recent years to control the epidemic in tobacco use. In 2003, the WHO adopted the WHO Framework Convention on Tobacco Control. It targeted national and regional organizations, providing the guiding principles required to reduce the prevalence of tobacco use. In 2008, the WHO proposed the MPOWER, a practical tool to implement this framework: monitor tobacco use and prevention policies, protect people from tobacco, offer help to quit, warn about the dangers, enforce bans on advertising, promotion and sponsorship, and raise taxes. Practical actions were price and tax measures, packaging and labelling regulations, smoking restriction in public places, advertising restrictions, provision of educational campaigns and cessation services.8,9 In 2019, 136 countries have implemented at least one of the key interventions to reduce tobacco demand.10
Despite recent policies, smoking is still a concern worldwide. 1.1 billion people are active smokers in 2019, 80% of them living in low- and middle-income countries. Total tobacco-attributable deaths increased from 5.4 million in 2004 to more than 8 million in 2019, representing the leading cause of death, illness and impoverishment.10,11 Tobacco use contributes to poverty via the direct cost of purchasing tobacco, the health care costs for tobacco-related diseases, as well as the lost human capital that results from morbidity and mortality.10,11 In high-income countries, the number of smokers is still high. In Europe, in 2016, 28.7% of the overall population smoked and tobacco was the leading cause of preventable death and disease in the UK.12,13
SPICES (Scaling-up Packages of Interventions for Cardiovascular disease prevention in selected sites in Europe and Sub-Saharan Africa) is an international implementation research project on CVDs non-pharmacological primary prevention. Different settings are involved in high and low- middle-income countries: France, United Kingdom, Belgium, Uganda and South Africa.
SPICES interventions will target the individual and the community level. Concerning smoking, this is the less implemented measure of the MPOWER: in 2019, only 23 countries were providing cessation services at the best-practice level.10
First step was to identify non-pharmacological effective interventions for CVDs primary prevention.14 The aim of this work was to identify evidence-based non-pharmacological effective smoking cessation interventions and their implementation strategies for use in a primary care setting and/or at a community level.
Materials And Methods
A systematic review of international CVD prevention guidelines and national guidelines of each participating country was carried out between September 2017 and January 2018 following the PRISMA statements criteria and using the ADAPTE procedure.15,16 The ADAPTE procedure provides a systematic approach to ensure quality standards for guideline development, evaluation and implementation.17 TRIP (Turning Research Into Practice) and G-I-N (Guidelines International Network) databases were searched for international guidelines. A purposive search for national guidelines was carried out using national health authority websites: the Haute Autorité de Santé for France; the Tijdschrift Huisarts and Nederland Huisartsen Genootschap guidelines for the Flemish part of Belgium; the National Institute for Care and Excellence (NICE) for United Kingdom; the European Society of Cardiology (ESC) within European countries and the WHO for Uganda. The South African researchers ensured that ESC guidelines were used in South Africa. Databases were searched for guidelines focusing on CVDs prevention and/or management of CVD risk factors, such as diabetes, hypertension, smoking, sedentary lifestyle, unhealthy diet, excess weight and obesity. Detailed research equations for each database are available in Annexure 1.
Guideline titles and summaries were analyzed for eligibility. Inclusion criteria were guidelines focusing on CVD primary prevention and/or management of CVD risk factors in an adult general population and to be applied in primary care or in a community setting. Only those published after 01/01/2012 were included. If different versions of the same guideline were found, only the most recent revision was included.
Exclusion criteria included; exclusive focus on specific populations (geriatric, infantile, socially deprived, etc.) or on secondary or tertiary prevention, exclusive focus on cardiovascular risk assessment, on pharmacological or surgical interventions, or on management of acute disease. Guidelines on specific conditions (eg, type 1 diabetes and familial hypercholesterolemia) were excluded as were those published before 01/01/2012 and not updated. The consortium agreed on this date in order to focus on recent guidelines with the most recently updated data. The research team considered that guideline authors would have reviewed and included literature published before 2012, so that no relevant data would have been missed.
Only guidelines published in English, French or Dutch were included. The lack of free full-text availability was an exclusion criterion as the research team assumed that a clinical practice guideline should be freely available. Two researchers completed this search independently, merging at the end.
Following the ADAPTE procedure, screened guidelines were eligible for quality evaluation with the AGREE II instrument (Appraisal of Guidelines for Research & Evaluation).18 AGREE II is a tool that assesses the quality of guidelines by evaluating their methodological rigor and transparency. It consists of 23 key items organized within 6 domains followed by a global rating (overall assessment [OA]). Each item is rated on a 7-point scale (7 being the best possible quality).
Full-text screened guidelines were evaluated independently by at least two researchers working blind and merging at the end. After discussion within the SPICES consortium, final inclusion was based on OA scores. We included guidelines with all OA scores superior or equal to 5 were excluded those with all OA scores below 5 or with OA scores around cut-off (one score of 4 and one of 5). Discrepant OA scores (more than 1-point difference and one score above 4) were discussed between appraisers and inclusion was based on a consensual decision taken with the researchers and a scientific committee of three senior researchers.
Data Extraction And Analysis
The included guidelines were analyzed, following the ADAPTE procedure, and a matrix of the extracted recommendations was created in order to facilitate the analysis.
Recommendations were included if they had an A or B level of evidence or 1++, 1+, 2++, 2+ for the NICE grading system and/or “Class 1” or “Strong” (regardless of the level of evidence). Bibliographic references which supported the included recommendations were listed to make further details on implementation strategies accessible. For guidelines without recommendation grades, references were included if they referred to randomized or non-randomized controlled trials, interventional cohort studies, or systematic reviews of such studies. Where few strategies had been described in the guidelines or where the description was too concise, the analysis of the full-text articles, from which the guidelines had been issued, was added. The aim was to fully describe the interventions used in such articles.
For these articles, inclusion and exclusion criteria were defined. Studies with a non-pharmacological lifestyle intervention for the primary prevention of CVDs in a general, adult population were included. Main outcomes had to be pertinent to primary care or commonly used for CVD risk assessment. Interventions had to be implemented in a primary care or community setting and had to be effective with a significant statistical difference. The objective of this work was the identification of effective interventions. Non-interventional studies and those on specific populations and on secondary or tertiary prevention population were excluded, as were studies in a hospital/clinic or university context or where patients were constantly monitored or supervised because that was deemed not feasible in a primary care context. Publication date was not an exclusion criterion. When different articles dealt with the same intervention they were identified, and duplicates were removed. Full-text articles were obtained on PubMed or via the University Web Library. If not available, the article in paper format was ordered via the library of the Université de Bretagne Occidentale.
Interventions and implementation strategies used in the included references were searched through the articles, especially in the method section, and described in detail and then summarized. Information was collected on the frequency and duration of the intervention, on the setting (eg, workplace or public event, individual or group sessions), on the material used (informative material, leaflets or videos), on the psychological model used and if mass media were involved. The status and background training (if provided) of those carrying out the interventions were noted. All data were collected in an excel matrix file.
Results
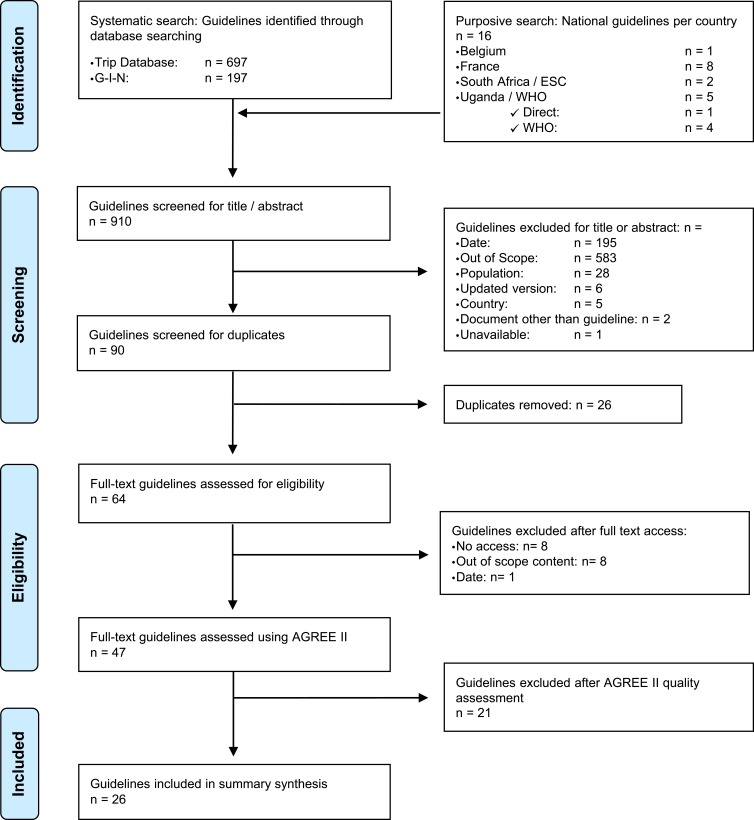
Guidelines selection process is shown in the PRISMA Flowchart (Figure 1).
Figure 1.
PRISMA Flowchart showing the guidelines selection process.
Forty-seven guidelines were screened for quality evaluation with AGREE II tool. Full results are available in Annexure 2. Twenty-six guidelines on CVD primary prevention were included based on their AGREE II overall score (Table 1). They were to be considered among the best quality and the most pertinent for primary CVD prevention in a primary care or community setting.
Table 1.
Included Guidelines For CVD Prevention
| Guideline Title | Organization | Year | Country |
|---|---|---|---|
| 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk19 | AHA/ACC | 2014 | USA |
| 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults20 | AHA/ACC/TOS | 2014 | USA |
| 2013 Clinical practice guidelines for the prevention and management of diabetes in Canada21 | Canadian Diabetes Association | 2013 | Canada |
| 2016 European Guidelines on cardiovascular disease prevention in clinical practice5 | ESC | 2016 | Europe |
| Arrêt de la consommation de tabac: du dépistage individuel au maintien de l’abstinence en premier recours22 | HAS | 2017 | France |
| Behavioral counseling to promote a healthful diet and physical activity for cardiovascular disease prevention in adults with cardiovascular risk factors23 | US Preventive Services Task Force | 2014 | USA |
| Behaviour change: individual approaches (PH49)24 | NICE | 2014 | UK |
| Cardiovascular disease prevention (PH 25)25 | NICE | Update 2014 | UK |
| Clinical practice guidelines for the management of overweight and obesity in adults, adolescents and children in Australia26 | National Health and Medical Research Council | 2014 | Australia |
| Guidelines for the management of absolute cardiovascular disease risk27 | NVDPA | 2014 | Australia |
| Hypertension evidence-based nutrition practice guideline28 | Academy of Nutrition and Dietetics | 2016 | USA |
| Lipid modification: cardiovascular risk assessment and the modification of blood lipids for the primary and secondary prevention of cardiovascular disease29 | NICE | 2014 | UK |
| Maintaining a healthy weight and preventing excess weight gain among adults and children30 | NICE | 2015 | UK |
| Obesity prevention (CG43)31 | NICE | 2014 | UK |
| Physical activity and the environment (PH8)32 | NICE | Update 2014 | UK |
| Physical activity: brief advice for adults in primary care (PH44)33 | NICE | 2013 | UK |
| Preventing type 2 diabetes – population and community interventions (PH35)34 | NICE | Update 2014 | UK |
| Prevention and Control of Noncommunicable Diseases. Guidelines for primary health care in low-resource settings35 | World Health Organization | 2012 | World |
| Recommendations for prevention of weight gain and use of behavioral and pharmacological interventions to manage overweight and obesity in adults in primary care36 | Canadian Task Force on Preventive Health Care | 2015 | Canada |
| Risk estimation and the prevention of cardiovascular disease37 | SIGN | 2017 | UK |
| Screening for and management of obesity in adults38 | US preventive services task force | 2012 | USA |
| Team-based care to improve blood pressure control: recommendation of the Community Preventive Services Task Force39 | Community Preventive Services Task Force | 2014 | USA |
| Tobacco harm reduction (PH45)40 | NICE | 2013 | UK |
| VA/DoD clinical practice guideline for the diagnosis and management of hypertension in the primary care setting41 | VA/DoD | 2015 | USA |
| VA/DoD clinical practice guideline for the management of dyslipidemia for cardiovascular risk reduction42 | VA/DoD | 2015 | USA |
| VA/DoD clinical practice guideline for screening and management of overweight and obesity43 | VA/DoD | 2015 | USA |
Abbreviations: AHA, American Heart Association; ACC, American College of Cardiology, TOS, The Obesity Society; ESC, European Society of Cardiology; HAS, Haute Autorité de Santé; NICE, National Institute for Health and Care Excellence; NVDPA, National Vascular Disease Prevention Alliance; SIGN, Scottish Intercollegiate Guidelines Network; VA/DoD, Department of Veterans Affairs/Department of Defense.
Guideline Analysis
Sixteen guidelines provided no recommendations on smoking. They focused on other risk factors, such as excess weight, sedentary lifestyle and unhealthy diet.19,20,23,26,28,30,35,36,38,39,41,43,31,32,33,34
Other guidelines recommended smoking cessation to prevent CVDs. It was recommended for all health care providers to advise smokers to quit, to provide information about methods to aid smoking cessation, including counselling services. Physicians were recommended to prescribe nicotine replacement therapy when indicated. No details, or very few, were found in the guidelines on how to advise smokers.21,25,27,29,37,42 Two guidelines focusing on smoking provided more detailed recommendations.22,40 The following recommendations were identified:
It was recommended to assess smoking status in all patients and their families and to use the Fagerstrom test (2 or 6 questions) to assess tobacco addiction (grade A).
If smoking cessation is not attainable, smoking reduction should be the target, as an intermediate step towards quitting completely (grade B).
Health care professionals were recommended to provide consultations dedicated to giving up smoking. The use of different techniques was recommended, such as counselling, psychological support, cognitive-behavioral therapy or motivational interviewing. Goals should be agreed with the patient and self-help documents should be provided. Past experiences, individual preferences and bio-psycho-social status should be taken into consideration (grade A).
Intervention should be matched to the behavioral stage of change of the smoker concerned (according to the Prochaska and DiClemente model44) and have an initial, intensive phase (weekly consultations) followed by a maintenance phase lasting 3 to 6 months (monthly sessions) (grade B).
Health care professionals should appraise themselves of the level of knowledge found within the community regarding beliefs held about smoking and other CVD risk factors.
Phone and/or internet follow-up and self-help, using eHealth technologies, could be alternatives even though they may be slightly less effective than face-to-face interventions (grade B).
It was recommended to involve media and public educational campaigns (grade A) and schools (grade B) as part of multicomponent strategies to promote healthy lifestyles. School personnel and health care providers were recommended to set the example and to avoid smoking at work (class 1, grade A).
ESC guidelines provided public health recommendations which dealt with law making (eg, ban smoking in public places and set higher taxes on tobacco) and were consequently excluded because they were considered infeasible on a primary care level.5 NICE PH49 provided general recommendations on behavioral approaches (such as social support) and on health care system organization but no detailed interventions for stopping smoking.24
References Analysis
One thousand eighty-one articles were identified of all 26 included guidelines for CVD prevention. After inclusion and exclusion criteria had been applied, 310 articles were included. Sixty-three included targeting smoking in their intervention. Seventeen studies were excluded from this analysis either because they presented no smoking-related outcomes (N=5) or they showed no significant differences for such outcomes (N=12). They were first included because the intervention was effective for other outcomes. Finally, 46 studies providing an effective smoking cessation intervention were included for analysis.
Interventions Description
Interventions and implementation strategies were described in detail and then summarized.
Fifteen studies provided a multicomponent intervention (targeting multiple risk factors) including an effective smoking cessation component and 31 studies targeted smoking only.
All interventions targeted a modification of the risk behavior related to smoking. They can be classified as behavioral change communication strategies.
Although interventions were heterogenous among included articles, some types of strategies were identified: self-help strategies alone, brief advice (BA), multiple sessions behavioral change strategies and community-based interventions.
Self-help strategies are presented in Table 2. These articles studied self-help strategies alone without face-to-face counseling.
Table 2.
Included Articles (Alphabetical Order) With A Self-Help Intervention
| Reference Study Type | Context | Strategy | Outcomes |
|---|---|---|---|
| Hollis et al 199145 RCT |
Netherlands. Primary care. 3161 smokers. | Nurse assisted self-help quit program. A trained nurse provided counseling and self-help material (video and printed) based on motivational interviewing. Follow-up phone calls. | Point prevalence abstinence 3 mo: 12.9% vs 7% (c) Continuous abstinence 3 mo: 10.6% vs 6% (c) |
| Slama et al 199546 RCT |
France. Primary care, general population. 2199 smokers | GP providing a self-help cessation guide. | Point prevalence abstinence 1 mo: 6.8% vs 4.1% (c) 12 mo: NS Continuous abstinence 12 mo: 1.9% vs 0.5% (c) |
Abbreviations: RCT, randomized controlled trial; GP, general practitioner; mo, months; vs, versus; (c), control group; NS, no significant difference.
Self-help documentation focused on the need to make a personal decision, the steps to successful quitting, the frequent need for repeated efforts, and the importance of setting a specific quit date and using substitutes for smoking. Self-help approaches were less effective compared with face-to-face counseling.47
BA was defined as one or two short individual consultations to provide information on the potential harm caused by smoking, advice on how to quit and on where to search for help. BA strategies are presented in Table 3.
Table 3.
Included Articles (Alphabetical Order) With A Brief Advice Intervention
| Reference Study Type |
Context | Strategy | Outcomes |
|---|---|---|---|
| Cohen et al 198948 Cluster RCT |
USA. Primary care, General population. 1420 adult smokers | Brief advice by GP (general practitioners) with reminders to talk about smoking and use the AAAA protocol: Ask/Advise/Agree/Arrange. | Point prevalence abstinence 6 mo: 4.2% vs 0.9% (c) 12 mo: 7.9% vs 1.5% (c) |
| Jamrozik et al 198449 Cluster RCT |
UK. Primary care, General population. 2110 adult smokers. | GP brief advice and self-help documentation. Neither the addition of the measure of exhaled CO nor the follow-up by health counselor improved outcomes. | Point prevalence abstinence 12 mo: 15% vs 10% (c) |
| Kadowaki et al 200050 RCT |
Japan. Workplace. 263 male smokers. | Individual brief counseling by a doctor with exhaled CO measure and periodic follow-up by doctor or nurse and booklet distribution and group discussions. One smoking cessation event organized in the workplace. | Point prevalence abstinence 5 mo: 12.9% vs 3.1% (c) |
| Lang et al 200051 Cluster RCT |
France. Workplace. 1095 employees. | Brief advice by occupational physicians and nurses and self-help documentation. Follow up: phone call and a 2-month visit. | Point prevalence abstinence 12 mo: 18.4% vs 13.5% (c) Continuous abstinence: NS |
| Louwagie et al 201452 RCT |
South Africa. Township primary care. 409 adult smokers diagnosed with tuberculosis. | One brief individual motivational interviewing session (15–20 mins) from the lay health care workers, and then referred to the nurse. Self-help booklet provided. | Point prevalence abstinence 3 mo: 39.5% vs 27% (c) 6 mo: NS Continuous abstinence 6 mo: 21.5% vs 9.3% (c) |
| Maguire et al 200153 RCT |
UK. Pharmacies, general population. 484 adult smokers. | Individual 10 to 30 mins brief advice by a trained pharmacist. Signs and posters at the pharmacy. Information on NRT. | Point prevalence abstinence 6 mo: 18.5% vs 8.2% (c) Continuous abstinence 12 mo: 14.3% vs 2.7% (c) |
| Morgan et al 1996 Cluster RCT |
USA. Primary care. 659 smokers aged 50–74. | GP brief advice based on AAAA and the transtheoretical model and self-help documentation. One follow-up phone call at 4 weeks. NRT by physicians if indicated. | Point prevalence abstinence 6 mo: 15.4% vs 8.2% (c) |
| Ojedokun et al.201354 RCT |
Ireland. Primary care, general population. 402 adult smokers. | Brief advice by GP and “lung age” evaluation with a spirometer, self-help documentation. | Point prevalence abstinence 1 mo: 22.1% vs 12% (c) |
| Pieterse et al 200155 RCT |
Netherlands. Primary care, general population. 530 smokers | Brief 10 mins motivational interviewing by trained GPs, self-help documentation and one follow-up meeting. Using the transtheoretical model. Information about NRT. | Point prevalence abstinence 6 mo: 11.9% vs 3.8% (c) 12 mo: 13.4% vs 7.3% (c) Continuous abstinence 12 mo: 8.2 vs 3.1% (c) |
| Russell et al 197956 RCT |
UK. Primary care, general population. 2138 smokers. |
GP brief advice plus information leaflet | Point prevalence abstinence 1 mo: 7.5% vs 3% (c) 12 mo: 19.1% vs 10.3% (c) Continuous abstinence 12 mo: 5.1% vs 0.3% (c) |
| Severson et al 200957 RCT |
USA. Dental clinics, military personnel. 785 smokers using smokeless tobacco. | Three 15 mins phone counseling sessions and self-help documentation material. Based on the transtheoretical model. | Point prevalence abstinence 3 mo: 25% vs 8% (c) 6 mo: 24% vs 12% (c) Continuous abstinence 3 mo: 20.4% vs 9.2% (c) 6 mo: 13.5% vs 5.6% (c) |
| Vetter et al 199058 RCT |
UK. Primary care, general population. 471 older smokers aged 60 and over | GP and practice nurse brief advice. | Point prevalence abstinence (per protocol) 6 mo: 14% vs 9% (c) |
| Wilson et al 199059 RCT |
Australia. Primary care, general population. 1238 adult smokers. | General practitioner brief advice and self-help documentation provision. | Continuous abstinence 12 mo: 7.5% vs 3.2% (c) |
| Windsor et al 198860 RCT |
USA. Workplace. 387 adult smokers. |
Brief advice with a health educator: cessation skills, self-efficacy and goals agreement. Using social support: the quit smoking “Buddy” system. | Continuous abstinence 12 mo: 19% vs 5% (c) Monetary incentive had no effect. |
Abbreviations: RCT, randomized controlled trial; NRT, nicotine replacement therapy; GP, general practitioner; mo, months; vs, versus; (c), control group; NS, no significant difference.
BA was more effective than usual care (including very brief advice): results for RCT with intention to treat analysis (ITT) showed 4% to 11% more continuous abstinence at 12 months of follow-up. The measure of exhaled carbon monoxide showed it to be slightly, but non-significantly, more effective than BA alone, while BA plus lung age estimation with a portable spirometer was more effective than BA alone.49,54
Multiple session behavioral change strategies (Table 4) were implemented as a series of meetings with participants to raise awareness of behavioral risk, to help them change and prevent relapse.
Table 4.
Included Articles (Alphabetical Order) With A Multiple Session Intervention
| Reference Study Type |
Context | Strategy | Outcomes |
|---|---|---|---|
| Barbarin O.A. 197861 RCT |
USA. Primary care. 60 adults smoking more than 1 pack/day | Self-control strategies focusing on negative consequences and side effects of smoking. Ten, 1 hr, group sessions over 1 month. Overt aversion (O), forced smoking to experience side effects, symbolic aversion (S), imagining negative consequences of smoking, or combined (Cb). | Point prevalence abstinence (per protocol) 3 mo: 72% (O) vs 41% (S) vs 47 (Cb) vs 8% (c) 12 mo: 44% (O) vs 16% (S) vs 25% (Cb) vs 0% (c) |
| Canga et al 200062 RCT |
Spain. Primary care, Nurse. 280 diabetic smokers. | An initial 40-min visit adapted to the patient’s smoking history. Follow-up by phone calls or visits: 5 contacts over 6 months. By a trained nurse, based on motivational interviewing and the transtheoretical (Prochaska) model. NRT when indicated. | Point prevalence abstinence 6 mo: 17% vs 2.3% (c) |
| Cinciripini et al.199463 Non-randomized controlled trial |
USA. Primary care, General population. 34 smokers. |
Eight 90 mins weekly motivational interviewing and cognitive-behavioral sessions and a relapse prevention program. Scheduled progressive reduction of the number of cigarettes over 5 weeks with a set date for quitting. | Point prevalence abstinence 6 mo: 53% vs 6% (c) 12 mo: 41% vs 6% (c) |
| Cinciripini et al.199564 RCT |
USA. Primary care, general population. 128 smokers |
Scheduled gradual reduction with a quit date set at week 5. Nine weekly meetings based on motivational interviewing. | Point prevalence abstinence 6 mo: 41% vs 13% (c) 12 mo: 44% vs 22%(c) |
| Hilberink et al 200565 RCT |
Netherlands. Primary care. 392 COPD patients. |
GPs individual motivational interviewing using the transtheoretical model. 5 consultations and 3 follow-up phone calls by the practice nurse. Information about NRT. Education booklet and videotape were provided. | Point prevalence abstinence 6 mo: 16% vs 8.8% (c) |
| Hollis et al 199145 RCT |
Netherlands. Primary care. 3161 smokers. | A professional group program: intensive nine group meetings over 2 months. | Point prevalence abstinence 3 mo: 14.1% vs 7% (c) Continuous abstinence 3 mo: 12% vs 6% (c) |
| Hollis et al 200766 RCT |
USA. Community, Telephone Quit-line. 4600 smokers, planning to quit. | Initial 40‐min session of phone counseling followed by two interventions. The moderate intervention (M): 1 follow-up call. Or the intensive intervention (I) by experienced tobacco counsellors (more effective): 4 additional phone calls over 3 months and personalized self-help material. Based on motivational interviewing techniques and transtheoretical model. | Continuous abstinence with NRT 6 mo: 24.3% (I) vs 21.3% (M) vs 16.8% (c) 12 mo: 21.2% (I) vs 20.1% (M) vs 17% (c) no NRT 6 mo: 13.1% (I) vs 10.7% (M) vs 10.2% (c) 12 mo: 17.1% (I) vs 13.8% (M) vs 11.7% (c) |
| Marcus et al 199967 RCT |
USA. General population. 281 sedentary female smokers aged 18 to 65. |
12 weekly sessions of a cognitive-behavioral program (self-monitoring, stimulus control, coping with cravings) associated with vigorous exercise (3 sessions per week) to reduce weight gain after smoking cessation. Provided by therapists and exercise specialists. | Continuous abstinence 3 mo: 16.4% vs 8.2% (c) 12 mo: 11.9% vs 5.4% (c) |
| Marks et al 200268 RCT |
UK, economically deprived area of north London. 260 smokers. |
Program using a spectrum of 30 cognitive- behavioral techniques and self-help material (written, audio). Initial 60-min session with therapists (3 to 12 people), one follow-up session and a phone call at 3 months. | Point prevalence abstinence 12 mo: 19.8% vs 5.8% (c) |
| Meyer et al 198069 Non randomized controlled trial |
USA. High CVD risk smokers in 3 towns with 500 people recruited in each town, aged 35 to 59. | Intervention on multiple risk behaviors: dietary, smoking and exercise behavior. Mass media campaign: radio and television, weekly newspaper columns, posters, and printed material sent by mail. Followed by 9 face-to-face counseling sessions (1 to 3.5 hrs) over 3 months for the subject and spouse. Led by a group leader and trained counselors. Based on the social learning theory and behavioral self-control principles. | Point prevalence abstinence 12 mo: 32.5% vs 6.4% (c) 24 mo: 47.1% vs 10.6% (c) 36 mo: 50% vs 14.9% (c) |
| Neaton et al 198170 RCT |
USA. Community recruitment. 12,866 CVD high-risk middle-aged men. |
10 weekly group meetings of 1 to 2 hrs led by counselors, combining nutrition, smoking and hypertension programs. Using social support (family) and skills development. Followed by the extended intervention which was individualized, based on results at 4 months. | Point prevalence abstinence 12 mo: 28% vs 8% (c) 24 mo: 28% vs 11% (c) 36 mo: 28.7% vs 13% (c) 48 mo: 29.9% vs 15.3% (c) |
| Nohlert et al 200971 RCT |
Sweden. General population. 300 smokers attending dental or primary care. | Eight 40-min individual sessions by a trained dental hygienist over a 4-month period. Based on a mixture of behavior therapy, coaching and pharmacological advices. |
Continuous abstinence 12 mo: 18% vs 9% (c) |
| Perkins et al 200172 RCT |
USA. 219 women smokers concerned about weight gain after smoking cessation. | Cognitive-behavioral therapy by a woman therapist: acceptance of modest weight gain, benefits of quitting superseding the health risks of weight gain. 90-min sessions, twice per week for 3 weeks then weekly sessions for 4 weeks. | Continuous abstinence 6 mo: 28% vs 12% (c) 12 mo: 21% vs 12% (c) |
| Soria et al 200673 RCT |
Spain. Primary care, general population. 200 smokers. |
Three individual 20-min sessions by a GP, based on motivational interviewing and the transtheoretical model. NRT when appropriate. | Point prevalence abstinence 6 and 12 mo: 18.4% vs 3.5% (c) |
| Steptoe et al 199974 Cluster RCT |
UK. Primary care. 883 people with one or more modifiable risk factors | Two to three counseling sessions with a practice nurse trained in behavioral change techniques followed by two phone calls. Based on the transtheoretical model. NRT when appropriate. | Cigarettes/day 3 mo: 7.1% vs 18 (c) 12 mo: 8.0% vs 2.7 (c) Continuous abstinence: NS |
| Wood et al 199475 RCT |
UK. Primary care, Nurses. Family recruitment: 12,472 men and their partners. | Family-centered nurse led counseling. Subjects were told their CVD risk in relative to other people of the same age. The frequency of follow-up visits was determined by both the CVD risk score and individual risk factors: the higher the risk score, the more frequent the visits. | Smoking prevalence 12 mo: Men 19% vs 23% (c) Women 17.7% vs 21.5% (c) |
| Wu et al 200976 RCT |
USA, Chinese community in New York. 122 smokers | Four 60-min individual sessions, in Chinese and based on motivational interviewing. Self-help materials. Phone calls follow-up. NRT provided if indicated. | Point prevalence abstinence 3 mo: 66.1% vs 32% (c) 6 mo: 66.7% vs 31.7% (c) |
| Cornuz et al 200277 Cluster RCT |
Switzerland. 35 residents in general practice. 251 smokers. | Training program for residents in general practice focusing on the medical issues of smoking. Based on the transtheoretical model. | Point prevalence abstinence 12 mo: 13% vs 5% (c) |
Abbreviations: RCT, randomized controlled trial; NRT, nicotine replacement therapy; GP, general practitioner; mo, months; vs, versus; (c), control group; NS, no significant difference.
The study from Hollis et al was included in both the self-help and the multiple session strategies as it implemented both interventions effectively.45
Based on RCTs with ITT results, multiple session behavioral change strategies were more effective than usual care (+3 to 9% on 12 months continuous abstinence) but also than BA (+4 to 5%).
Eight studies focused on workplace (Table 5) for the implementation of multiple session strategies. The workplace was specified in order to link individual strategies with interventions, targeting all workers, such as workplace information campaigns (posters), newsletters, smoking restricted areas, local media campaign and social support among employees.78,79,80,81,82 Trained employees were successfully involved in the organization and intervention.80,81
Table 5.
Included Articles (Alphabetical Order) With A Multiple Session Intervention At The Workplace
| Reference Study Type |
Context | Strategy | Outcomes |
|---|---|---|---|
| Bertera et al 199379 Cohort: before after comparative analysis. |
USA. Workplace health program. 7178 employees. | A personalized health risk assessment followed by a videotaped feedback or individual consultation. Group activities and on-site classes by trained medical personnel were offered for 10 weeks on how to quit smoking (and how to deal with other risk behaviors). Smoking restrictions and awards for achieving health objectives were implemented in the workplace, and management and employees were involved. | Point prevalence abstinence 24 mo (months): +18%. From 1621 to 1328 smokers. |
| Erfurt et al 199180 Cluster RCT |
USA. Workplaces. 7800 employees. |
After risk factor screening, employees could choose: 1. Guided self-help, 2. One-to-one formal consultation, and occasional phone contacts; 3. Mini-group interventions or 4. Full-group classes of eight or more participants. Led by wellness counselors. Informal health promotion and peer support groups and plant-wide health promotion activities. | Point prevalence abstinence 36 mo: 13.2% vs 7.8% (c) |
| Gomel et al 199383 Cluster RCT |
Australia. Workplaces. 431 employees. |
50-min behavioral standardized counseling followed by 6 individualized sessions with a psychologist over 10 weeks. Based on 4 stages model: preparation for change, action, maintenance, relapse prevention. Self-help documentation and economic incentives (lottery tickets and voucher) were provided. | Point prevalence abstinence 3 mo: 18% vs 3% c) 6 mo: NS. Continuous abstinence: NS |
| Groeneveld et al 201184 RCT |
Netherlands. Workplace, community. 816 male workers at high CVD risk. | Over 6 months, three 45- to 60-min face to face counseling sessions by an occupational physician or nurse and four 15- to 30-min telephone contacts. Based on motivational interviewing techniques such as open questions, summarizing, listening, supporting and raising awareness of ambivalence. | Point prevalence abstinence 6 mo: 31.1% vs 13.4% (c) 12 mo: NS |
| Jason et al 198781 Cluster RCT |
USA. Workplace. 425 smoker employees | During a television campaign for smoking cessation, six 45-min support group meetings were held twice a week in the workplace, led by employees trained on behavioral change techniques. Self-help manuals were provided, and posters displayed at the workplaces. | Point prevalence abstinence 3 mo: 22% vs 12% (c) Continuous abstinence 3 mo: NS |
| Jason et al 198982 Cluster RCT |
USA. Workplace. 850 smoker employees |
Six 45-min support group meetings, twice per week during a 3-week television program, focusing on techniques discussed in the television program and self-help manuals. Led by employees trained on behavioral change techniques. Then 12 monthly follow-up meetings, followed by lottery ticket incentives. | Point prevalence abstinence 6 mo: 29% vs 20% (c) 12 mo: 26% vs 16% (c) Continuous abstinence 6 mo: 12% vs 6% (c) 12 mo: 11% vs 3% (c) |
| Jason et al 199778 Cluster RCT |
USA. Workplaces (63 companies in Chicago). 840 adult smokers. | A community-wide media campaign (television and newspaper) and cognitive-behavioral support groups. Group meetings were held twice a week for the initial 3 weeks using a self-help manual. Maintenance phase: 14 meetings over 6 months, weekly, then biweekly and then monthly. The cognitive-behavioral support was the most effective intervention. | Point prevalence abstinence 6 mo: 24.6% vs 4.3% (c) 12 mo: 20.7% vs 7% (c) 18 mo: 15.8% vs 7% (c) 24 mo: 18% vs 10.3% (c) |
| Omenn et al 198847 RCT |
USA. Workplace. 402 smokers motivated to quit. | Two interventions. 1) Multiple component program (MCP): focus on initial cessation. Short time (3 weeks), intensive quit period. Behavior skills training, aversive imagery, stress management and audiovisual material. 2) Relapse prevention program (RPP): focus on relapse prevention, after smokers had quit. 2 hrs weekly meeting for 8 weeks. Self-help approach was not effective. |
Both interventions were effective vs control. Point prevalence abstinence 3 mo: 37.3% (MCP) vs 26.3% (RPP) vs 11.8% (c) 6 mo: 35.3% (MCP) vs 26.3% (RPP) vs 11.8% (c) 12 mo: NS |
Abbreviations: RCT, randomized controlled trial; NRT, nicotine replacement therapy; GP, general practitioner; mo, months; vs, versus; (c), control group; NS, no significant difference.
Three studies were linked in financial incentives but they did not seem very effective when compared with behavioral strategies.78,82,83 Three studies provided a BA intervention at the workplace, but with no or less involvement of the workplace structure and management.50,51,60
Community-Based Interventions
These were public interventions such as public campaigns carried out in the community and/or in workplaces (Table 6). They targeted the global CVD risk and were multi-component (diet, sedentary lifestyle, smoking).85,86,87,88,89
Table 6.
Included Articles (Alphabetical Order) With Community-Based Interventions
| Reference Study Type |
Context | Strategy | Outcomes |
|---|---|---|---|
| Giampaoli et al 199788 Non-randomized controlled trial |
Italy. Community, rural population. 1598 adults. | 10 years intervention. In the community, schools and workplaces: distribution of printed material, setting up of consulting rooms, organization of lectures and exhibitions, theoretical and practical courses for teachers and health care personnel. | Cigarettes/day, 10 years: Women: −0.27 cig/day Men: NS. Point prevalence abstinence: NS |
| Goodman et al 199585 Cohort study: cross sectional analysis with a matched comparison |
USA. Community, general population. 1642 people. | Community-wide campaigns to improve physical activity, diet and smoking. A health promotion program was distributed to local workplaces and media shared health information, and self-help kits for smoking cessation were distributed. | Smoking prevalence Men: - 2.1% vs −1.4% (c) Women: NS |
| Hoffmeister et al 199686 Cohort: cross sectional analysis, comparison to a reference population |
West Germany. Community. 8600 people. | A 7-year multifaceted prevention program to improve healthy behaviors. Non-smoking areas in public places, poster campaign and anti-smoking campaigns in the local media and seminars to help smokers quit. | Smoking prevalence 7 years: −6.7% |
| Malmgren, Andersson198689 Cohort: before-after analysis |
Sweden. Community. 2887 participants. | 1-year newspaper campaign to improve dietary, smoking and exercise habits. And 10 informational meetings with specialists. | Point prevalence abstinence 12 mo: +12% (62% respondents) |
| Nafziger et al 200187 Cohort: cross sectional analysis, comparison to a reference population |
USA. Community, rural population of 158,000 people. | 5 years intervention. Risk factor screening: workplaces, local health fairs, village festivals. Smoking cessation education and school-based smoking prevention programs. Media: radio, newspaper. Brochures and posters (worksites, grocery store, medical and dental clinics, schools). | Point prevalence abstinence 5 years: 10.3% vs 2.8% (c) |
Abbreviations: RCT, randomized controlled trial; mo, months; vs, versus; (c), control group; NS, no significant difference.
The efficacy of such interventions was unclear: none of the included studies was an RCT and none showed any statistical difference on continuous abstinence but only on point prevalence abstinence (last 7 days reported consumption).
Discussion
The objective of this work was to identify effective non-pharmacological interventions for smoking cessation to be used in a primary care or a community setting. A systematic review of guidelines, following the ADAPTE Process, included the best quality CVD prevention guidelines adapted to such settings. Health care professionals were recommended to screen for smoking, to give advice and support and to prescribe NRT when indicated. Guidelines globally failed to provide clear and detailed strategies to implement such recommended advice and support. Plus, they were centered on health care professionals. Recommendations for changes to law and to taxation were deemed infeasible in a primary care or community context and were excluded. Two guidelines only provided more detailed recommendations, such as the use of behavioral change strategies with an intensive and a maintenance phase, as well as the use of techniques such as motivational interviewing or the Prochaska stage of change theory.22,40
References analysis made it possible to specify implementation strategies for these recommendations. Although the included articles were seen to be heterogeneous in terms of the interventions, the settings and the outcomes, four main behavioral change strategies were identified.
Self-help materials showed a small positive effect in this review and in the literature, but they were less effective than face-to-face counseling.47,63,76,78,90 BA and multiple session behavioral change strategies were found to achieve better results than usual care or very BA (ie, 30 s to a couple of minutes).45,52,73 Relative risk (RR) for physician advice has been estimated to be 1.66 for smoking cessation.91 Such interventions seemed even more effective when a defined behavioral change technique was used (motivational interviewing, “Five A’s”, Prochaska model or cognitive-behavioral therapy).52,68,73 This was consistent with evidence in the literature summarized in two recent Cochrane reviews.92,93 More intensive multiple session strategies were more successful than BA.66 Similar results were found by other systematic reviews either for group or individual sessions: RR for abstinence was 1.22 to 1.57 for more intensive interventions compared with simple advice.91,94,95,96 It should also be taken into consideration that the more intensive the intervention it is, the more it impacts on time and resources.
Several effective workplace interventions were found in this review. Despite this, the role of the “workplace” itself in this effect was still unclear. Best quality studies, implementing effective workplace interventions in our review included multiple session behavioral change strategies. Implemented interventions might be as effective whether offered in the workplace or elsewhere.97 Spirometric lung age estimation was effective when added to BA in one study, but lung age did not differ among those who quit and those who did not. Smokers with poorer lung age were just as likely to quit as those with normal lung age. Other studies failed to show spirometry or lung age effective in promoting smoking cessation.98 Lung age biofeedback effect on smoking is still uncertain and concerns may be raised on the cost-effectiveness ratio.
Progressive and abrupt cessation showed similar long-term results.99 Evidence in the literature was insufficient to advocate aversive smoking techniques.100 Accordingly, only one article included in this review studied overt and symbolic aversion, but due to a small sample and a per protocol analysis, its results need to be interpreted with precaution.61 Money incentives were not very effective and their effect was time-limited.101 They did not seem a pertinent solution.
Diverse health care professionals were effective in leading such behavioral change interventions. This review showed evidence to indicate that trained community workers can successfully lead these interventions.52,66,78,82 This is important for settings where access to health care professionals is difficult.
Community-based public interventions, such as community events, mass media involvement and information campaigns, were identified in this review. They showed positive but small effects in non-controlled or non-randomized controlled trials which limited their strength. A systematic review found that community interventions had no effect, or a very limited effect, on quitting rates among adults. Among the 37 included studies, only 4 used random assignment of communities and largest and best conducted studies failed to detect a significant effect.102 Similarly, exclusive mass media interventions had an unclear effect mainly due to the lack of good quality studies.103 As smoking is also determined by the social context, the community approach still should be part of a smoking cessation project.
This review did not identify any intervention involving social media. The use of social media has been shown to be feasible and acceptable, although data are lacking regarding its effectiveness.104
Recent efforts to reduce smoking focused on anti-tobacco national policies, mass media communication and law-making. Less was done on the individual level. Cessation services were the least implemented component of MPOWER and physician interventions were not routinely provided, even in high-income countries.10,105,106 This review highlighted the efficacy of different behavioral change strategies that can be implemented in primary care and/or in the community. These approaches are highly cost-effective. BA seems more suitable for general practitioners or practice nurses because of the short duration of their consultation (5 to 20 mins in most countries).107 Pharmacists and dentist could play a major role as well.
Multiple session strategies, using a structured behavior change model (Prochaska Transtheoretical model, or motivational interviewing techniques) were seen to be more effective. Since they demand more time, they do not seem suitable for general practitioners worldwide.
This review showed that diverse health care professionals can successfully lead such interventions including lay health community workers.52,66,78,82 Training lay people to provide such behavioral change interventions could be a solution for public health authorities that deal with settings where access to care is difficult, such as low-income countries and regions in developed countries where physician availability is limited. Furthermore, involving lay people could strengthen the community approach of community-based smoking cessation projects.
Strengths And Limitations
International guidelines were reviewed following a validated and systematic protocol including their quality evaluation. Guidelines were selected, where pertinent for primary care or community context, by researchers who are primary care health care professionals (general practitioners and nurses).
This review might carry a selection bias. Studies were included if they showed a significant difference on primary outcomes. This could imply a bias concerning the effectiveness of these interventions. Since the references included arose from high-grade recommendations of the best quality international guidelines, these interventions were considered effective and this bias was therefore limited.
Multiple outcomes were found for smoking cessation interventions and the relevance of some outcomes is debatable and could involve a bias. Seven-day point prevalence abstinence was the most frequently recurring outcome in this review. Biochemical validation was frequent. An expert consensus stated that smoking cessation should be evaluated by 7-day point prevalence abstinence and prolonged abstinence at 6 and 12 months because shorter time intervals carry a very high probability of relapse.108 In this review, this bias concerned community interventions.
A publication bias was possible because this review protocol did not include a grey literature search. Nevertheless, different systematic reviews found similar results for the interventions described in this review.92,93,94
Conclusion
Multiple session behavioral change strategies including follow-up and self-help materials were the most effective interventions for smoking cessation in primary care or community context.
These were based on motivational interviewing, Prochaska and DiClemente stage of change theory or cognitive-behavioral therapies. BA was slightly less effective. Nonetheless, BA involves less time and fewer resources and it is more suitable for primary care. These interventions could be provided by physicians, nurses and other health care professionals. Adequately trained lay health workers were also shown to be successful in providing these interventions. Community-based and workplace public interventions without behavioral change strategies were not effective. Evidence for community-based interventions effectiveness was weak due to the lack of robust studies.
Acknowledgments
The authors would like to acknowledge the SPICES Team: Dr Geofrey Musinguzi, Pr Paul Van Royen, Pr Harm van Marwijk, Dr Linda Gibson, Dr Bowyer Mark, Pr Tholene Sodi, Pr Mbuyiselo Douglas. And the EA 7479 SPURBO team for the systematic review: Lucie Morvan, Anne Catherine Lecuyer, Christelle Le Gaffric, Hélène Roux, Marion Janyk, Nicolas Vimfles, Marion Le Bars, Pol-Maël Falhon, Louis Soulier, Cécile Guérin, Marine Réale, Hugo Vittori, Charlotte Donou, Eloïse Lissillour, Thomas Le Bras, Gabriel Eliot.
Author Contributions
All authors contributed toward data analysis, drafting and revising the paper, gave final approval of the version to be published and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.WHO - World Health Organisation. Cardiovascular diseases (CVDs). Available from: http://www.who.int/mediacentre/factsheets/fs317/en/ Published 2017. Accessed November13, 2018.
- 2.WHO - World Health Organisation. A Global Brief on Hypertension: Silent Killer. Global Public Health Crisis; 2013. Available from: http://chronicconditions.thehealthwell.info/search-results/global-brief-hypertension-silent-killer-global-public-health-crisis Accessed November13, 2018. [Google Scholar]
- 3.Teo KK, Ounpuu S, Hawken S, et al. Tobacco use and risk of myocardial infarction in 52 countries in the INTERHEART study: a case-control study. Lancet. 2006;368(9536):647–658. doi: 10.1016/S0140-6736(06)69249-0 [DOI] [PubMed] [Google Scholar]
- 4.Chow CK, Jolly S, Rao-Melacini P, Fox KAA, Anand SS, Yusuf S. Association of diet, exercise, and smoking modification with risk of early cardiovascular events after acute coronary syndromes. Circulation. 2010;121(6):750–758. doi: 10.1161/CIRCULATIONAHA.109.891523 [DOI] [PubMed] [Google Scholar]
- 5.Piepoli MF, Hoes AW, Agewall S, et al. European guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J. 2016;37(29):2315–2381. doi: 10.1093/eurheartj/ehw106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Unal B, Critchley JA, Capewell S. Modelling the decline in coronary heart disease deaths in England and Wales, 1981–2000: comparing contributions from primary prevention and secondary prevention. BMJ. 2005;331(7517):614. doi: 10.1136/bmj.38561.633345.8F [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qiao Q, Tervahauta M, Nissinen A, Tuomilehto J. Mortality from all causes and from coronary heart disease related to smoking and changes in smoking during a 35-year follow-up of middle-aged Finnish men. Eur Heart J. 2000;21(19):1621–1626. doi: 10.1053/euhj.2000.2151 [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization. WHO framework convention on tobacco control. Geneva; 2003. Available from: https://apps.who.int/iris/bitstream/handle/10665/78302/ea56r1.pdf?sequence=1&isAllowed=y Accessed August21, 2019. [Google Scholar]
- 9.World Health Organization. MPOWER; 2008. Available from: https://www.who.int/tobacco/mpower/mpower_report_six_policies_2008.pdf Accessed August21, 2019.
- 10.World Health Organization. WHO Report On Global Tobacco Epidemic, 2019; 2019. Available from: https://apps.who.int/iris/bitstream/handle/10665/326043/9789241516204-eng.pdf?ua=1 Accessed August21, 2019.
- 11.Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3(11):e442. doi: 10.1371/journal.pmed.0030442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization. World Health Statistics data visualizations dashboard. Tobacco smoking. WHO. Available from: http://apps.who.int/gho/data/node.sdg.3-a-viz?lang=en Published 2016. Accessed August21, 2019.
- 13.All Party Parliamentary Group on Smoking & Health. Fact sheets - action on smoking and health. Available from: https://ash.org.uk/information-and-resources/ash-fact-sheets/ Published 2019. Accessed September3, 2019.
- 14.Peters DH, Adam T, Alonge O, Agyepong IA, Tran N. Implementation research: what it is and how to do it. BMJ. 2013;347:f6753. doi: 10.1136/bmj.f6753 [DOI] [PubMed] [Google Scholar]
- 15.Moher D, Liberati A, Tetzlaff J, Altman DG, Altman D. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100. doi: 10.1371/journal.pmed.1000100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.The ADAPTE Collaboration. The ADAPTE process: resource toolkit for guideline adaptation. Version 2.0. 2009. Available from: http://www.g-i-n.net Published 2010 Accessed November27, 2017.
- 18.Brouwers MC, Kho ME, Browman GP, et al. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ. 2010;182(18):E839–E842. doi: 10.1503/cmaj.090449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eckel RH, Jakicic JM, Ard JD, et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk. Circulation. 2014;129(25suppl 2):S76–S99. doi: 10.1161/01.cir.0000437740.48606.d1 [DOI] [PubMed] [Google Scholar]
- 20.Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association task force on practice guidelines and the obesity society. Circulation. 2014;129(25Suppl 2):S102–S138. doi: 10.1161/01.cir.0000437739.71477.ee [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Canadian Diabetes Association. Clinical practice guidelines for the prevention and management of diabetes in Canada. 2013. doi: 10.1016/S1499-2671(13)00192-5 [DOI] [Google Scholar]
- 22.de Santé HA. Arrêt de La Consommation de Tabac : du Dépistage Individuel Au Maintien de l’abstinence En Premier Recours; 2014. Available from: https://www.has-sante.fr/portail/jcms/c_1718021/fr/arret-de-la-consommation-de-tabac-du-depistage-individuel-au-maintien-de-l-abstinence-en-premier-recours Accessed September26, 2017.
- 23.LeFevre ML. Behavioral counseling to promote a healthful diet and physical activity for cardiovascular disease prevention in adults with cardiovascular risk factors: u.s. preventive services task force recommendation statement. Ann Intern Med. 2014;161(8):587. doi: 10.7326/M14-1796 [DOI] [PubMed] [Google Scholar]
- 24.NICE. Behaviour change: individual approaches. Public health guideline [PH49]. Available from: https://www.nice.org.uk/guidance/ph49 Published 2014. Accessed November20, 2018.
- 25.NICE. Cardiovascular disease prevention | guidance and guidelines. 2010. Available from: https://www.nice.org.uk/guidance/ph25 Accessed November28, 2017.
- 26.National Health and Medical Research Council. Clinical practice guidelines for the management of overweight and obesity in adults, adolescents and children in Australia. Available from: https://www.nhmrc.gov.au/guidelines-publications/n57 Published 2013. Accessed November28, 2017.
- 27.National Vascular Disease Prevention Alliance. Guidelines for the management of absolute cardiovascular disease risk; 2012. Availabe from: https://www.heartfoundation.org.au/images/uploads/publications/Absolute-CVD-Risk-Full-Guidelines.pdf? Accessed November28, 2017.
- 28.Lennon SL, DellaValle DM, Rodder SG, et al. 2015 evidence analysis library evidence-based nutrition practice guideline for the management of hypertension in adults. J Acad Nutr Diet. 2017;117(9):1445–1458.e17. doi: 10.1016/j.jand.2017.04.008 [DOI] [PubMed] [Google Scholar]
- 29.NICE. Cardiovascular disease: risk assessment and reduction, including lipid modification | guidance and guidelines. Available from: https://www.nice.org.uk/guidance/cg181 Published 2014. Accessed November20, 2018.
- 30.NICE. Preventing excess weight gain. Available from: https://www.nice.org.uk/guidance/ng7 Published 2015. Accessed November20, 2018.
- 31.NICE. Obesity prevention. Clinical guideline [CG43]. Available from: https://www.nice.org.uk/guidance/cg43 Published 2015. Accessed November20, 2018.
- 32.NICE. Physical activity and the environment. Public health guideline [PH8]. Available from: https://www.nice.org.uk/guidance/ph8 Published 2008. Accessed November20, 2018.
- 33.NICE. Physical activity: brief advice for adults in primary care. Public health guideline [PH44]. 2013. Available from: https://www.nice.org.uk/guidance/ph44 Accessed November20, 2018.
- 34.NICE. Type 2 diabetes prevention: population and community-level interventions. Public health guideline [PH35]. 2011. Available from: https://www.nice.org.uk/guidance/ph35 Accessed November20, 2018.
- 35.WHO - World Health Organization. Prevention and control of noncommunicable diseases : guidelines for primary health care in low resource settings. Available from: https://apps.who.int/iris/handle/10665/76173 Published 2012. Accessed October6, 2018. [PubMed]
- 36.Brauer P, Connor Gorber S, Shaw E, et al. Recommendations for prevention of weight gain and use of behavioural and pharmacologic interventions to manage overweight and obesity in adults in primary care. CMAJ. 2015;187(3):184–195. doi: 10.1503/cmaj.140887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scottish Intercollegiate Guidelines Network. Risk estimation and the prevention of cardiovascular disease. SIGN 149. 2017. Available from: https://www.sign.ac.uk/sign-149-risk-estimation-and-the-prevention-of-cardiovascular-disease.html Accessed November20, 2018.
- 38.Moyer VA. Screening for and management of obesity in adults: U.S. preventive services task force recommendation statement. Ann Intern Med. 2012;157(5):373–378. doi: 10.7326/0003-4819-157-5-201209040-00475 [DOI] [PubMed] [Google Scholar]
- 39.Community Preventive Services Task Force CPST. Team-based care to improve blood pressure control: recommendation of the community preventive services task force. Am J Prev Med. 2014;47(1):100–102. doi: 10.1016/j.amepre.2014.03.003 [DOI] [PubMed] [Google Scholar]
- 40.NICE. Smoking: harm reduction. Public health guideline [PH45]. Available from: https://www.nice.org.uk/guidance/ph45 Published 2013. Accessed November20, 2018.
- 41.Department of Veterans Affairs D of D. VA/DoD clinical practice guidelines: diagnosis and management of hypertension in the primary care setting; 2014. Available from: https://www.healthquality.va.gov/guidelines/CD/htn/ Accessed November20, 2018.
- 42.Department of Veterans Affairs D of D. The management of dyslipidemia for cardiovascular risk reduction (Lipids). Available from: https://www.healthquality.va.gov/guidelines/CD/lipids/ Published 2014. Accessed November28, 2017.
- 43.Department of Veterans Affairs D of D. VA/DoD clinical practice guidelines: management of obesity and overweight. Available from: https://www.healthquality.va.gov/guidelines/cd/obesity/ Published 2014. Accessed November20, 2018.
- 44.Prochaska JO, DiClemente CC. Stages and processes of self-change of smoking: toward an integrative model of change. J Consult Clin Psychol. 1983;51(3):390–395. doi: 10.1037/0022-006X.51.3.390 [DOI] [PubMed] [Google Scholar]
- 45.Hollis JF, Lichtenstein E, Mount K, Vogt TM, Stevens VJ. Nurse-assisted smoking counseling and medical settings: minimizing demands on physicians. Prev Med (Baltim). 1991;20(4):497–507. doi: 10.1016/0091-7435(91)90047-8 [DOI] [PubMed] [Google Scholar]
- 46.Slama K, Karsenty S, Hirsch A. Effectiveness of minimal intervention by general practitioners with their smoking patients: a randomised, controlled trial in France. Tob Control. 1995;4(2):162–169. doi: 10.1136/tc.4.2.162 [DOI] [Google Scholar]
- 47.Omenn GS, Thompson B, Sexton M, et al. A randomized comparison of Worksite-sponsored smoking cessation programs. Am J Prev Med. 1988; 4(5):261–267. doi: 10.1016/S0749-3797(18)31159-0 [DOI] [PubMed] [Google Scholar]
- 48.Cohen SJ, Stookey GK, Katz BP, Drook CA, Smith DM. Encouraging primary care physicians to help smokers quit. A randomized, controlled trial. Ann Intern Med. 1989;110(8):648–652. doi: 10.7326/0003-4819-110-8-648 [DOI] [PubMed] [Google Scholar]
- 49.Jamrozik K, Vessey M, Fowler G, Wald N, Parker G, Van Vunakis H. Controlled trial of three different antismoking interventions in general practice. Br Med J (Clin Res Ed). 1984;288(6429):1499–1503. doi: 10.1136/bmj.288.6429.1499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kadowaki T, Watanabe M, Okayama A, Hishida K, Ueshima H. Effectiveness of smoking-cessation intervention in all of the smokers at a worksite in Japan. Ind Health. 2000;38(4):396–403. doi: 10.2486/indhealth.38.396 [DOI] [PubMed] [Google Scholar]
- 51.Lang T, Nicaud V, Slama K, et al. Smoking cessation at the workplace. Results of a randomised controlled intervention study. Worksite physicians from the AIREL group. J Epidemiol Community Health. 2000;54(5):349–354. doi: 10.1136/JECH.54.5.349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Louwagie GMC, Okuyemi KS, Ayo-Yusuf OA. Efficacy of brief motivational interviewing on smoking cessation at tuberculosis clinics in Tshwane, South Africa: a randomized controlled trial. Addiction. 2014;109(11):1942–1952. doi: 10.1111/add.12671 [DOI] [PubMed] [Google Scholar]
- 53.Maguire TA, McElnay JC, Drummond A. A randomized controlled trial of a smoking cessation intervention based in community pharmacies. Addiction. 2001;96(2):325–331. doi: 10.1080/09652140020021062 [DOI] [PubMed] [Google Scholar]
- 54.Ojedokun J, Keane S, O?Connor K. Lung age bio-feedback using a portable lung age meter with brief advice during routine consultations promote smoking cessation? Know2quit multicenter randomized control trial. J Gen Pract. 2013;01(03):1–5. doi: 10.4172/2329-9126.1000123 [DOI] [Google Scholar]
- 55.Pieterse ME, Seydel ER, DeVries H, Mudde AN, Kok GJ. Effectiveness of a minimal contact smoking cessation program for dutch general practitioners: a randomized controlled trial. Prev Med (Baltim). 2001;32(2):182–190. doi: 10.1006/pmed.2000.0791 [DOI] [PubMed] [Google Scholar]
- 56.Russell MA, Wilson C, Taylor C, Baker CD. Effect of general practitioners’ advice against smoking. Br Med J. 1979;2(6184):231–235. doi: 10.1136/bmj.2.6184.231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Severson HH, Peterson AL, Andrews JA, et al. Smokeless tobacco cessation in military personnel: A randomized controlled trial. Nicotine Tob Res. 2009;11(6):730–738. doi: 10.1093/ntr/ntp057 [DOI] [PubMed] [Google Scholar]
- 58.Vetter NJ, Ford D. Smoking prevention among people aged 60 and over: a randomized controlled trial. Age Ageing. 1990;19(3):164–168. doi: 10.1093/ageing/19.3.164 [DOI] [PubMed] [Google Scholar]
- 59.Wilson DH, Wakefield MA, Steven ID, Rohrsheim RA, Esterman AJ, Graham NM. Sick of Smoking”: evaluation of a targeted minimal smoking cessation intervention in general practice. Med J Aust. 1990;152(10):518–521. doi: 10.5694/j.1326-5377.1990.tb125351.x [DOI] [PubMed] [Google Scholar]
- 60.Windsor RA, Lowe JB, Bartlett EE. The effectiveness of a worksite self-help smoking cessation program: a randomized trial. J Behav Med. 1988;11(4):407–421. doi: 10.1007/BF00844939 [DOI] [PubMed] [Google Scholar]
- 61.Barbarin OA. Comparison of symbolic and overt aversion in the self-control of smoking. J Consult Clin Psychol. 1978;46(6):1569–1571. doi: 10.1037/0022-006X.46.6.1569 [DOI] [PubMed] [Google Scholar]
- 62.Canga N, De Irala J, Vara E, Duaso MJ, Ferrer A, Martínez-González MA. Intervention study for smoking cessation in diabetic patients: a randomized controlled trial in both clinical and primary care settings. Diabetes Care. 2000;23(10):1455–1460. doi: 10.2337/diacare.23.10.1455 [DOI] [PubMed] [Google Scholar]
- 63.Cinciripini PM, Lapitsky LG, Wallfisch A, Mace R, Nezami E, Van Vunakis H. An evaluation of a multicomponent treatment program involving scheduled smoking and relapse prevention procedures: initial findings. Addict Behav. 1994;19(1):13–22. doi: 10.1016/0306-4603(94)90047-7 [DOI] [PubMed] [Google Scholar]
- 64.Cinciripini PM, Lapitsky L, Seay S, Wallfisch A, Kitchens K, Van Vunakis H. The effects of smoking schedules on cessation outcome: can we improve on common methods of gradual and abrupt nicotine withdrawal? J Consult Clin Psychol. 1995;63(3):388–399. doi: 10.1037/0022-006X.63.3.388 [DOI] [PubMed] [Google Scholar]
- 65.Hilberink SR, Jacobs JE, Bottema BJAM, de Vries H, Grol RPTM. Smoking cessation in patients with COPD in daily general practice (SMOCC): six months’ results. Prev Med (Baltim). 2005;41(5–6):822–827. doi: 10.1016/j.ypmed.2005.08.003 [DOI] [PubMed] [Google Scholar]
- 66.Hollis JF, McAfee TA, Fellows JL, Zbikowski SM, Stark M, Riedlinger K. The effectiveness and cost effectiveness of telephone counselling and the nicotine patch in a state tobacco quitline. Tob Control. 2007;16:53–59. doi: 10.1136/tc.2006.019794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Marcus BH, Albrecht AE, King TK, et al. The efficacy of exercise as an aid for smoking cessation in women: a randomized controlled trial. Arch Intern Med. 1999;159(11):1229–1234. doi: 10.1001/archinte.159.11.1229 [DOI] [PubMed] [Google Scholar]
- 68.Marks DF, Sykes CM. Randomized controlled trial of cognitive behavioural therapy for smokers living in a deprived area of London: outcome at one-year follow-up. Psychol Health Med. 2002;7(1):17–24. doi: 10.1080/13548500120101513 [DOI] [Google Scholar]
- 69.Meyer AJ, Nash JD, McAlister AL, Maccoby N, Farquhar JW. Skills training in a cardiovascular health education campaign. J Consult Clin Psychol. 1980;48(2):129–142. doi: 10.1037/0022-006X.48.2.129 [DOI] [PubMed] [Google Scholar]
- 70.Neaton JD, Broste S, Cohen L, Fishman EL, Kjelsberg MO, Schoenberger J. The multiple risk factor intervention trial (MRFIT): VII. A comparison of risk factor changes between the two study groups. Prev Med (Baltim). 1981;10(4):519–543. doi: 10.1016/0091-7435(81)90063-3 [DOI] [PubMed] [Google Scholar]
- 71.Nohlert E, Å T, Tillgren P, Johansson P, Rosenblad A, Helgason ÁR. Comparison of a high and a low intensity smoking cessation intervention in a dentistry setting in Sweden – a randomized trial. BMC Public Health. 2009;9(1):121. doi: 10.1186/1471-2458-9-121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Perkins KA, Marcus MD, Levine MD, et al. Cognitive-behavioral therapy to reduce weight concerns improves smoking cessation outcome in weight-concerned women. J Consult Clin Psychol. 2001;69(4):604–613. [PubMed] [Google Scholar]
- 73.Soria R, Legido A, Escolano C, López Yeste A, Montoya J. A randomised controlled trial of motivational interviewing for smoking cessation. Br J Gen Pract. 2006;56(531):768–774. [PMC free article] [PubMed] [Google Scholar]
- 74.Steptoe A, Doherty S, Rink E, Kerry S, Kendrick T, Hilton S. Behavioural counselling in general practice for the promotion of healthy behaviour among adults at increased risk of coronary heart disease: randomised trial. BMJ. 1999;319(7215):943–947; discussion 947–8. doi: 10.1136/bmj.319.7215.943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wood DA, Kinmonth AL, Davies GA, et al. Randomised controlled trial evaluating cardiovascular screening and intervention in general practice: principal results of British family heart study. Family Heart Study Group. BMJ. 1994;308(6924):313–320. doi: 10.1136/BMJ.308.6924.313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wu D, Ma GX, Zhou K, Zhou D, Liu A, Poon AN. The effect of a culturally tailored smoking cessation for Chinese American smokers. Nicotine Tob Res. 2009;11(12):1448–1457. doi: 10.1093/ntr/ntp159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cornuz J, Humair J-P, Seematter L, et al. Efficacy of resident training in smoking cessation: a randomized, controlled trial of a program based on application of behavioral theory and practice with standardized patients. Ann Intern Med. 2002;136(6):429–437. doi: 10.7326/0003-4819-136-6-200203190-00006 [DOI] [PubMed] [Google Scholar]
- 78.Jason LA, Salina D, McMahon SD, Hedeker D, Stockton M. A worksite smoking intervention: a 2 year assessment of groups, incentives and self-help. Health Educ Res. 1997;12(1):129–138. doi: 10.1093/her/12.1.129 [DOI] [PubMed] [Google Scholar]
- 79.Bertera RL. Behavioral risk factor and illness day changes with workplace health promotion: two-year results. Am J Heal Promot. 1993;7(5):365–373. doi: 10.4278/0890-1171-7.5.365 [DOI] [PubMed] [Google Scholar]
- 80.Erfurt JC, Foote A, Heirich MA. Worksite wellness programs: incremental comparison of screening and referral alone, health education, follow-up counseling, and plant organization. Am J Heal Promot. 1991;5(6):438–448. doi: 10.4278/0890-1171-5.6.438 [DOI] [PubMed] [Google Scholar]
- 81.Jason LA, Gruder CL, Martino S, Flay BR, Warnecke R, Thomas N. Work site group meetings and the effectiveness of a televised smoking cessation intervention. Am J Community Psychol. 1987;15(1):57–72. doi: 10.1007/BF00919757 [DOI] [PubMed] [Google Scholar]
- 82.Jason LA, Lesowitz T, Michaels M, et al. A worksite smoking cessation intervention involving the media and incentives. Am J Community Psychol. 1989;17(6):785–799.doi: 10.1007/BF00922738 [DOI] [PubMed] [Google Scholar]
- 83.Gomel M, Oldenburg B, Simpson JM, Owen N. Work-site cardiovascular risk reduction: a randomized trial of health risk assessment, education, counseling, and incentives. Am J Public Health. 1993;83(9):1231–1238. doi: 10.2105/AJPH.83.9.1231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Groeneveld IF, Proper KI, van der Beek AJ, Hildebrandt VH, van Mechelen W. Short and long term effects of a lifestyle intervention for construction workers at risk for cardiovascular disease: a randomized controlled trial. BMC Public Health. 2011;11(1):836. doi: 10.1186/1471-2458-11-836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Goodman RM, Wheeler FC, Lee PR. Evaluation of the heart to heart project: lessons from a community-based chronic disease prevention project. Am J Heal Promot. 1995;9(6):443–455. doi: 10.4278/0890-1171-9.6.443 [DOI] [PubMed] [Google Scholar]
- 86.Hoffmeister H, Mensink GB, Stolzenberg H, et al. Reduction of coronary heart disease risk factors in the German cardiovascular prevention study. Prev Med (Baltim). 1996;25(2):135–145. [DOI] [PubMed] [Google Scholar]
- 87.Nafziger AN, Erb TA, Jenkins PL, Lewis C, Pearson TA. The Otsego-Schoharie healthy heart program: prevention of cardiovascular disease in the rural US. Scand J Public Health Suppl. 2001;56:21–32. [PubMed] [Google Scholar]
- 88.Giampaoli S, Poce A, Sciarra F, et al. Change in cardiovascular risk factors during a 10-year community intervention program. Acta Cardiol. 1997;52(5):411–422. [PubMed] [Google Scholar]
- 89.Malmgren S, Andersson G. Who were reached by and participated in a one year newspaper health information campaign? Scand J Soc Med. 1986;14(3):133–140. [DOI] [PubMed] [Google Scholar]
- 90.Hartmann-Boyce J, Lancaster T, Stead LF. Print-based self-help interventions for smoking cessation. Cochrane Database Syst Rev. 2014;(6). doi: 10.1002/14651858.CD001118.pub3 [DOI] [PubMed] [Google Scholar]
- 91.Stead LF, Buitrago D, Preciado N, Sanchez G, Hartmann-Boyce J, Lancaster T. Physician advice for smoking cessation. Cochrane Database Syst Rev. 2013;(5). doi: 10.1002/14651858.CD000165.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rice VH, Heath L, Livingstone-Banks J, Hartmann-Boyce J. Nursing interventions for smoking cessation. Cochrane Database Syst Rev. 2017;12:CD001188. doi: 10.1002/14651858.CD001188.pub5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lindson-Hawley N, Thompson TP, Begh R. Motivational interviewing for smoking cessation. Cochrane Database Syst Rev. 2015;(3). doi: 10.1002/14651858.CD006936.pub3 [DOI] [PubMed] [Google Scholar]
- 94.Stead LF, Carroll AJ, Lancaster T. Group behaviour therapy programmes for smoking cessation. Cochrane Database Syst Rev. 2017;(3). doi: 10.1002/14651858.CD001007.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lancaster T, Stead LF. Individual behavioural counselling for smoking cessation. Cochrane Database Syst Rev. 2017;(3). doi: 10.1002/14651858.CD001292.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Martín Cantera C, Puigdomènech E, Ballvé JL, et al. Effectiveness of multicomponent interventions in primary healthcare settings to promote continuous smoking cessation in adults: a systematic review. BMJ Open. 2015;5(10):e008807. doi: 10.1136/bmjopen-2015-008807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cahill K, Lancaster T. Workplace interventions for smoking cessation. Cochrane Database Syst Rev. 2014;(2). doi: 10.1002/14651858.CD003440.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bize R, Burnand B, Mueller Y, Rège-Walther M, Camain J-Y CJ. Biomedical risk assessment as an aid for smoking cessation. Cochrane Database Syst Rev. 2012;12:CD004705. doi: 10.1002/14651858.CD004705.pub4 [DOI] [PubMed] [Google Scholar]
- 99.Lindson-Hawley N, Aveyard P, Hughes JR. Reduction versus abrupt cessation in smokers who want to quit. Cochrane Database Syst Rev. 2012;(11). doi: 10.1002/14651858.CD008033.pub3 [DOI] [PubMed] [Google Scholar]
- 100.Hajek P, Stead LF. Aversive smoking for smoking cessation. Cochrane Database Syst Rev. 2001;(3). doi: 10.1002/14651858.CD000546.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cahill K, Hartmann-Boyce J, Perera R. Incentives for smoking cessation. Cochrane Database Syst Rev. 2015;(5). doi: 10.1002/14651858.CD004307.pub5 [DOI] [PubMed] [Google Scholar]
- 102.Secker-Walker R, Gnich W, Platt S, Lancaster T. Community interventions for reducing smoking among adults. Cochrane Database Syst Rev. 2002;(2). doi: 10.1002/14651858.CD001745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bala MM, Strzeszynski L, Topor-Madry R. Mass media interventions for smoking cessation in adults. Cochrane Database Syst Rev. 2017;(11). doi: 10.1002/14651858.CD004704.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Naslund JA, Kim SJ, Aschbrenner KA, et al. Systematic review of social media interventions for smoking cessation. Addict Behav. 2017;73:81–93. doi: 10.1016/j.addbeh.2017.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lindholm C, Adsit R, Bain P, et al. A demonstration project for using the electronic health record to identify and treat tobacco users. WMJ. 2010;109(6):335–340. [PMC free article] [PubMed] [Google Scholar]
- 106.Rosenberg G, Crawford C, Bullock S, Petty R, Vohra J Smoking cessation in primary care: a cross-sectional survey of primary care health practitioners in the UK and the use of very brief advice; 2019. Available from: http://www.cancerresearchuk.org/ Accessed September3, 2019.
- 107.Irving G, Neves AL, Dambha-Miller H, et al. International variations in primary care physician consultation time: A systematic review of 67 countries. BMJ Open. 2017;7(10):1–15. doi: 10.1136/bmjopen-2017-017902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cheung KL, de Ruijter D, Hiligsmann M, et al. Exploring consensus on how to measure smoking cessation. A Delphi study. BMC Public Health. 2017;17(1):890. doi: 10.1186/s12889-017-4902-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- WHO - World Health Organisation. Cardiovascular diseases (CVDs). Available from: http://www.who.int/mediacentre/factsheets/fs317/en/ Published 2017. Accessed November13, 2018.