To today’s experimental biologists, the best known nematode is Caenorhabditis elegans, one of the major model organisms for genetic and biomedical research, the first metazoan with a sequenced genome and a key partner in the winning of three Nobel prizes — for the discoveries of programmed cell death and RNA interference, and for the development of green fluorescent protein (GFP) as a marker for gene expression. Approximately a thousand labs work on C. elegans worldwide; thus, this nematode is surely of great importance.
But when asked about nematodes, experts in tropical medicine, epidemiology or agriculture may think of other species. After all, the phylum Nematoda also contains plant and animal parasites that inflict substantial damage on humans and their agricultural products. Approximately half of the world’s human population is infected with gastrointestinal nematodes: about 3.5 billion cases, of which 450 million, mostly children, are seriously ill. Approximately 8–15% of crop losses are attributed to nematodes worldwide, with a cost of at least $80 billion. This Primer will take a broad look at the diverse groups that make up the phylum Nematoda.
What is a nematode?
True to their name, nematodes generally have a body that is long, narrow and threadlike (‘nema’ is Greek for thread), but not segmented like that of earthworms. Their body plan is basically a tube within a tube: the intestine and gonad are surrounded by the body wall with its dorsal and ventral longitudinal muscles, epidermis and a cuticle. In between the inner and outer tubes is a pressurized, fluid-filled cavity that acts as a hydrostatic skeleton. This organization allows nematodes to move elegantly in sinusoidal waves while lying on one side. It likely also puts a powerful constraint on evolutionary change of this simple body plan. Most likely because nematodes rely on their tough body wall and pressurized body cavity as an antagonist for muscle action, they never evolved any appendages, such as legs or wings. As a consequence, morphological diversity in this group is restricted and much less than that of other successful phyla like arthropods, or vertebrates.
All nematodes go through four larval stages. At the end of each larval stage, a new cuticle is synthesized and the old one is molted off. In the stem species of Rhabditida (for phylogenetic relationships see Figure 1), a clade comprising C. elegans and its relatives as well as most parasitic nematode species, a specialized survival and dispersal stage evolved, the dauer larva. This alternative third larval stage does not feed or age and can withstand adverse conditions much better than other stages. The dauer larva is the evolutionary precursor of the infective juveniles in all parasitic Rhabditida.
Figure 1.
Our current understanding of the phylogenetic relationships of nematodes.
The main panel shows the phylogeny of the major nematode groups, information about ecological range and presence of parasites (black bars indicate definitive hosts, grey bars intermediate hosts), and some representative genera. Phylogenetic relationships within Nematoda currently rely mostly on small-subunit rRNA sequences, but this gene provides surprisingly good resolution even when hundreds of species are included. There are three large monophyletic groups: the mostly marine Enoplia, Dorylaimia that include the parasitic trichinellids and mermithids, and Chromadoria with nematodes of all habitats and life styles. Most animal parasites belong to Spirurina, but hookworms (Strongylida) are found in Rhabditina along with Caenorhabditis elegans. Recent analyses with whole genomes for a smaller number of species as well as features of early embryonic development support Enoplia as the first branch of the nematode tree. (Adapted with permission from De Ley, P.A. (2006).) (Inset) Nematodes in the tree of life. All recent phylogenetic studies, using sequence data from a variety of genes and species, place Nematoda together with its sistergroup Nematomorpha (Gordian worms) as the closest relatives of Panarthropoda (arthropods, onychophorans and tardigrades) in a clade that is sometimes called Ecdysozoa.
Nematode diversity
Despite their invariant body plan, nematodes show an astounding biodiversity. Only around 30,000 species have been described, but the number of extant nematode species is estimated to be a million or more. These species are by no means all alike. The most obvious difference is seen in body size, which ranges from fractions of a millimeter to several meters, but nematodes also differ in cuticular decorations and especially in feeding structures (Figure 2). A nematode mouth may be a simple tube, or it may be adorned with a piercing stylet (in plant parasites and fungal feeders) or with frightening teeth that can slice, rip or bite (in predatory species like Mononchus and in some gut parasites like strongylids).
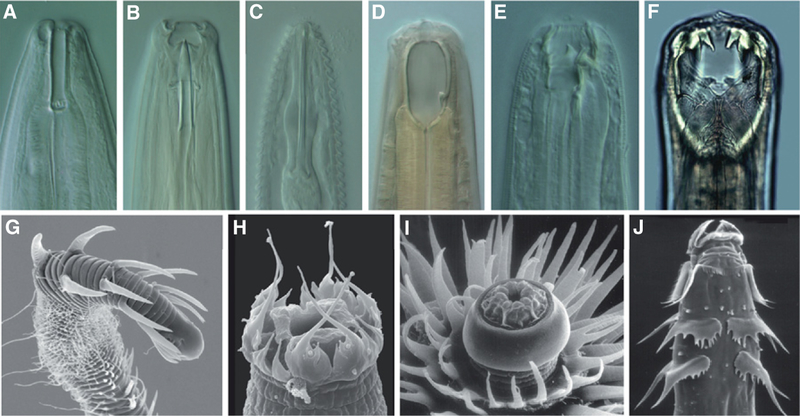
Figure 2.
Nematodes do not all look alike.
Portraits of the bacteriovorous Rhabditomorpha Rhabditoides inermis (A); two plant-feeding nematodes that use a hypodermic needle-like stylet to puncture plant cell walls and ingest food: Paractinolaimus sp. (Dorylaimida) (B) and Mesocriconema sp. (Tylenchomorpha) (C); two predatory nematodes that use large teeth to slice open their prey: Coomansus zschokkei (Mononchida) (D) and Monochoides sp. (Diplogasteromorpha) (E); the gut-parasitic dog hookworm Ancylostoma caninum (Rhabditomorpha, Strongylida) (F). Nematodes with cuticular decorations: the marine nematode Glochinema spinithorni (Desmodorida) (G), its head bent to the right; Scottnema lindsayae (Cephalobomorpha) (H); Carnoya stobilina (I) and a Heth species (J) (Rhigonematomorpha) from the gut of millipedes. Photos: (A,E) K. Kiontke; (B-D) courtesy of collective efforts of the De Ley Lab; (F) J. Eisenback, Nemapix Vol. 1, Mactode Publications; (G) C. Neira; (H) O. Holovachov; (I,J) D. Hunt.
The largest and most fascinating diversity, however, is found in physiological adaptations and in the interactions that nematodes have with other organisms. Nematodes live literally everywhere: in your backyard, in tree canopies, in desert soil, arctic ice, hot springs, the deep sea and in marine sediments that lack any trace of oxygen. Many are present outside or inside of plants and animals or in close contact with them and have evolved fascinating interactions, from adverse to advantageous.
Parasites and pathogens
Phylogenetic analyses of nematodes have shown that plant parasitism evolved at least three times and animal parasitism at least six times (Figure 1). The animal hosts are mostly arthropods or vertebrates of all kinds. Some animal parasitic nematodes have complex life cycles that may involve alternating generations of selfing and outcrossing animals (for example, Strongyloides species), a switch between hosts (for example, filariids, the intermediate host of which is an insect and the definitive host a vertebrate), or an essential migration through different tissues of a vertebrate host (for example, Ascaris). Like other parasites, some nematodes alter the behavior of their host in a way that facilitates transmission: An ant-parasitic tetradonematid turns the abdomen of its host bright red, such that it is attractive to birds; birds that eat the red ant will spread nematode eggs with their feces, which in turn are eaten by ants. Mosquitoes that are infected with filariids eat only small blood meals, so that they have to bite soon again and thus spread the parasites to more hosts.
Nematodes have their own parasites and pathogens. Recently, natural pathogens of C. elegans have been isolated, among them bacteria (for example, Microbacterium nematophilum), a virus related to nodaviruses, and Microsporidia. Investigating the immune response of infected nematodes to these and other pathogens is an active and exciting area of C. elegans research.
Predators and prey
Most nematodes are microbial feeders and play an important role in decomposition. There are, however, also predators, such as all species in the clade Monochida (Figure 3). Some species in the taxon Diplogasteromorpha (for example, Pristionchus spp.) have two mouth morphs: a narrow one more suited for eating bacteria, and a wider one with large teeth for slicing open their prey. Which morph is expressed depends on food availability. The victims of these fierce predators seem to be mostly other nematodes and, at least in the lab, they do not hesitate to eat members of their own species.
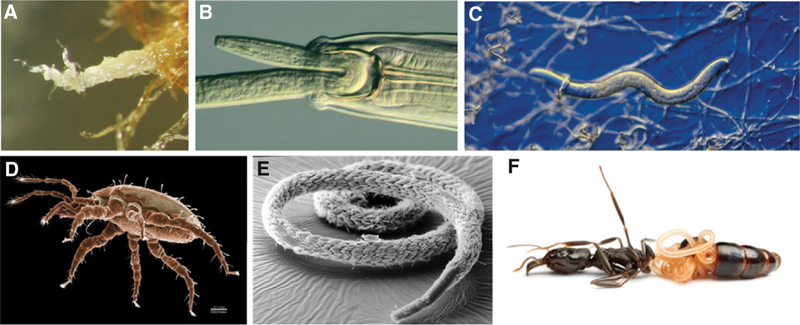
Figure 3.
Nematode interactions.
(A) Dauer larvae of a Caenorhabditis species from rotten fruit congregate in bundles and wave. This behavior is thought to facilitate contact with a larger animal that can carry the nematodes to a new habitat. The dauer larva is the evolutionary precursor of the infective larva of parasitic species in the clade Rhabditida. (B-D) Predators of nematodes: (B) the mononchid nematode Anatonchus tridentatus feeds on another nematode (photo by R. Neilson, The James Hutton Institute, UK), (C) the nematode-trapping fungus Arthrobotrys conoides has ensnared a nematode with adhesive rings (photo by W.R. West, ©Carolina Biological Supply Company, used by permission) and (D) Macrocheles sp., a nematophagous mesostigmatid mite (photo D.E. Walter). (E) Mutualistic bacteria cover the surface of the stilbonematid Eubostrichus parasitiferus (reproduced with permission from J. Ott and Wiley, from the cover of Volume 23, Issue 2 of Biologie in unserer Zeit). (F) A parasitic mermithid nematode in the abdomen of a trap-jaw ant from Belize (photo from ©Alex Wild, used by permission).
Nematodes can fall prey not only to other nematodes but also to some insects (such as diplurans and beetle and fly larvae), tardigrades, centipedes, symphylans and mites. Symphylans and mites are the most important arthropod predators of nematodes in grassland ecosystems. Mesostigmatid mites are also being explored for their potential to control plant parasitic nematodes. The sneakiest predators of nematodes are fungi, one of which is the oyster mushroom. These nematophagous fungi catch their prey with an arsenal of clever devices, including adhesive knobs, sticky nets and constrictive rings. Nematodes are attracted to these devices and, once they touch them, they are trapped, invaded by hyphae, and consumed. Some fungi specialize in eating nematode eggs. Work in Arthrobotrys oligospora has shown that traps are formed only when the fungus senses the presence of nematodes, using their victim’s own pheromones as a cue. Recently, a variety of nematophagous fungi have been tested for their ability to control gut parasites of grazing animals by trapping the infective larvae in dung.
Mutualists
Not all interactions between nematodes and other organisms are antagonistic. Some nematodes have a mutualistic relationship with the bacteria they eat. Nematodes of the genera Heterorhabditis and Steinernema have independently evolved close relationships with bacteria of the genera Photorhabdus and Xenorhabdus, respectively. These nematodes are insect-pathogenic and are used for biological pest control. Their infective juveniles invade the body cavity of insects and release symbiotic bacteria from a specialized region of their guts. These bacteria multiply quickly and kill the insect within 24 hours. They are the food source for two to three generations of nematodes until the insect cadaver is used up; then, infective juveniles develop, package some bacteria into their guts, and are ready for finding and infecting the next host. The partners in this mutualism depend on each other; under natural conditions, the bacteria develop exclusively on insect cadavers and the nematodes can only survive on a diet of their specific bacteria.
In anoxic marine sediments rich in toxic hydrogen sulfide, some nematodes live in symbiosis with sulfur-oxidizing bacteria; these can be endosymbionts, and the nematode hosts (for example, Astomonema species) then have no mouths and the cells of their lumen-less rudimentary guts are filled with bacteria. In members of the nematode taxon Stilbonematinae (Chromadorida), the worm cuticle is covered with ectosymbiotic bacteria in intriguing regular patterns (Figure 3). These nematodes are thought to feed by grazing on their bacterial coat. The sulfide-oxidizing bacteria benefit from the nematodes as a shuttle between sulfidic sediment layers where they can pick up sulfide or thiosulfide, and layers where oxygen is present as an electron acceptor.
Only recently, a mutualistic relationship has been discovered between animal parasitic filariid nematodes, which cause human diseases such as river blindness, elephantiasis and heartworm, and the endosymbiotic bacteria of the genus Wolbachia, better known for their role as sexual parasites of arthropods. Wolbachia provide essential nutrients to the nematodes, and when the bacteria are removed by antibiotic treatment, larval growth and development arrest and adult females become sterile. Filariasis can therefore be successfully treated with antibiotics such as Doxycyclin.
Most nematodes that live in temporary habitats like cow dung or rotting fruit need the help of larger invertebrates for dispersal. Developmentally arrested dauer larvae will hitch a ride, for example under the elytra of a beetle or in the intersegmental furrows of an isopod. Once the ‘taxi’ has arrived in a suitable place, the dauer larvae disembark and resume development. This phoretic relationship can be quite specific and does not seem to harm the carrier, while it is essential for the nematodes.
Because they eat bacteria and fungi, some microbivorous nematodes adopt the role of hygiene police for nest-building insects. Sweat bees and sand bees, bark beetles, carrion beetles, dung beetles, burrower bugs and even fig wasps are specifically associated with one or a few nematode species (of the groups Rhabditina and Aphelenchina) that live with them in their nests or burrows. There, the nematodes curb bacterial and fungal growth that may develop on food-provisions, feces and shed skins, or on dead eggs and larvae. When the next generation of the hosts leaves the nest, they take along some larvae of their nematode roommates. These larvae wait until a new nest or burrow is built, dismount and develop once microorganisms are available.
Their ability to influence bacterial growth and the composition of the microbe community appears to turn gut-dwelling nematodes into mutualists rather than parasites as one might expect at first. For instance, frog tadpoles grow faster when their gut harbors oxyurid nematodes. Here, it was shown that the nematodes alter gut fermentation in a positive way. Other nematodes traditionally viewed as harmful gut parasites appear to have similar effects on the intestinal flora even in humans. Recently, attempts to treat autoimmune diseases of the gut in humans via a controlled infection with gut nematodes showed promising results. Aside from a direct effect on T cells, the nematodes altered the composition of the gut flora, promoting bacteria that are generally viewed as ‘probiotic’.
Thus, in contradistinction to their superficially unexciting appearance, nematodes not only provide important model systems for biomedical research and significant challenges to agriculture and medicine, but occupy an enormous ‘ecospace’ of adaptations to habitats and organisms in the economy of nature. Perhaps most astonishingly, the depth of this diversity has barely been penetrated, as tens of thousands more new species are estimated to lie undiscovered. Clearly, much more explorative work is needed to truly understand this diversity, the functions of nematodes in ecosystems and the beneficial and detrimental roles they play for human economy and health.
Further reading
- Anderson RC (2000). Nematode Parasites of Vertebrates, Their Development and Transmission (Wallingford, UK: CABI Publishing; ). [Google Scholar]
- Blaxter M. (2011). Nematodes: the worm and its relatives. PLoS Biol 9, e1001050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Ley PA (2006). A quick tour of nematode diversity and the backbone of nematode phylogeny In WormBook, The C. elegans Research Community, ed. (http://www.wormbook.org). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones J, Gheysen G, and Fenoll C. (2011). Genomics and Molecular Genetics of Plant-Nematode Interactions (Springer; ). [Google Scholar]
- Lee DL (2002). The Biology of Nematodes (London, UK: Taylor & Francis; ) [Google Scholar]
- WormBook, http://www.wormbook.org
- WormAtlas, http://www.wormatlas.org