Abstract
At first look, cell division and neurite formation seem to be two very different, essential biological processes. However, both processes require extensive reorganization of the cytoskeleton, and especially microtubules. Remarkably, in recent years, independent work from several groups has shown that multiple cytoskeletal components previously considered specific for the mitotic machinery play important roles in neurite initiation and extension. In this review, we describe how several cytoplasmic and “mitotic” microtubule motors, components of mitotic kinetochores and cortical actin participate in reorganization of the microtubule network required to form and maintain axons and dendrites. The emerging similarities between these two biological processes will certainly generate new insights into the mechanisms generating the unique morphology of neurons.
Keywords: neuron, neurites, cytoskeleton, cell division, microtubule, motor, sliding
Introduction: Microtubules are key players in neurite formation
Each individual neuron is developed from a precursor that has to extend neurites, called axons and dendrites, to form connections for communication. Axons are responsible for sending signals from cell bodies to other neurons or non-neuronal cells. In multipolar neurons, generally the rest of the neurites are dendrites, responsible for receiving and integrating signals. Both axons and dendrites are filled with microtubules, actin microfilaments and, in the case of vertebrate neurons, neurofilaments [reviewed in [1]]. Forces generated by cytoskeletal components are responsible for neurite formation, mainly through the dynamics of microtubule and actin networks. Experiments in primary neuronal cultures show that the microtubule network plays a major role in neurite formation (see Box 1 for strategies of imaging microtubule behavior in neurites). In the search for mechanisms responsible for microtubule reorganization in neurons, initial studies in the early 90s demonstrated that several “mitotic” motors, well-known for their role in mitotic spindle formation, surprisingly control neurite length of post-mitotic neurons [reviewed in [2]]. In agreement with these early observations, there has been an explosion of recent data showing that not only mitotic motors, but other important mitotic components are involved in generating the mechanical forces required for proper neurite outgrowth and maintenance. In this review, we discuss how “mitotic” machinery is repurposed in neurodevelopment.
BOX 1. Strategies for imaging and quantify microtubule transport and dynamics in neurons.
In the search for mechanisms responsible for microtubule reorganization in neurons, several labs have taken advantage of different microscopy approaches that allow visualization and quantification of microtubule motility (Fig.I). Three common strategies to image and quantify microtubule motility in neurites include: i. Photobleaching of GFP-tubulin (FRAP) to visualize movement of non-photobleached microtubules through the photobleached region. This technique also enables scoring of frequencies for anterograde and retrograde direction of microtubule movement [6, 7] (Fig.I, left panel); ii.
Fusion of photoconvertible proteins to tubulin, e.g. EOS-tub provides specific marks on microtubules using UV light so the motility of photoconverted microtubules can be easily tracked and quantified [14, 64] (Fig.I, middle panel); and iii. Indirect measurement of microtubule motility by tracking the movement of mitochondria docked on motile microtubules [17] (Fig.I, right panel).
Figure I for Text Box 1. Strategies for imaging and quantify microtubule transport and dynamics in neurons.
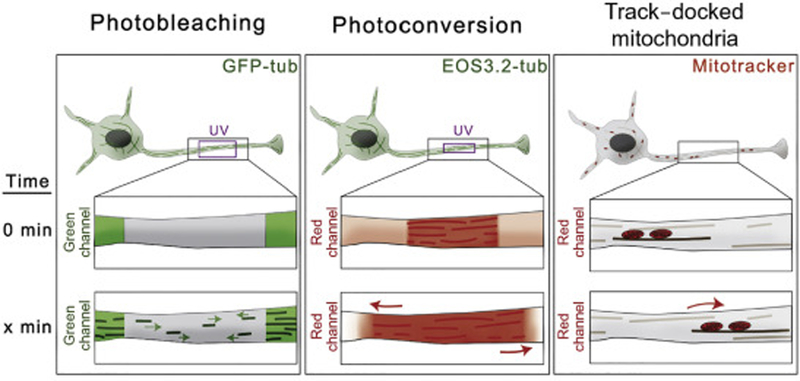
Left Panel. Neurons expressing GFP-tubulin. Photobleaching of a small region in the axons allows to visualize movement of non-bleached microtubules through the photobleached region.
Middle Panel. Neurons expressing tubulin fused to a photoconvertible protein EOS3.2 display microtubules in green. Photoconversion of a small region of EOS-labeled microtubules with UV light creates red marks on microtubules. Photoconverted microtubules can be easily tracked and quantified in the red channel.
Right Panel. Neurons are labeled with a mitochondria-specific dye. Movement of microtubules can be tracked indirectly by observing the position of mitochondria docked on microtubules.
Conserved mechanisms of microtubule reorganization in cell division and neuronal development
Despite obvious morphological differences between these two processes, the forces that act on microtubules in the spindle are equally important in neurodevelopment. Indeed, microtubules, regardless of the motors involved, can undergo sliding against each other and this sliding is required both for cell division and neurite extension. Furthermore, in both cases cytoplasmic dynein, anchored at the actin cortex, pulls on microtubules through similar mechanisms. Here we describe how microtubule rearrangements can be mechanistically similar while performing different functions in mitotic and postmitotic cells.
Sliding of microtubules against each other drives both formation of bipolar spindle and neurite outgrowth
Microtubule-microtubule sliding is the ability of microtubule motors to move microtubule against one another. A common feature of microtubule sliding is that, independently of the identity of the microtubule motor, a microtubule can function as both the cargo and track of the microtubule motor. Motors moving microtubules generate forces that are used either to reorganize microtubule network, i.e. mitotic spindle formation, or to change cell shape, i.e. neurite formation and outgrowth.
During mitosis, chromosome segregation requires a massive reorganization of the microtubule network from radial to bipolar array. Although bipolar spindle formation requires the cooperation of several mitotic motors, it is microtubule-microtubule sliding by the kinesin-5 motor that provides the main force that establishes spindle bipolarity and drives spindle elongation [3]. Kinesin-5 forms a homotetramer complex with pairs of motors at opposite ends (see Fig.1, left panel). As a result, kinesin-5 can crosslink two antiparallel microtubules and move them against each other [4]. Inactivation of kinesin-5 leads collapse of the bipolar spindle to monopolar asters and chromosome segregation failure [3]. Like mitosis, neurite initiation requires the generation of cytoplasmic forces that drive changes in cell shape. The microtubule network in cultured Drosophila neurons is continuously reorganized on a time scale of seconds. Rapidly moving microtubules push the plasma membrane at the tips of growing neurites [5]. Similar movement is also seen in developing mammalian neurons [6, 7]. Based on its role in mitosis, kinesin-5 seemed like a good candidate motor that drives microtubule sliding in neurons. However, depletion of kinesin-5 in neurons promotes rather than inhibits neurite outgrowth [8]. Additionally, the reported speed for kinesin-5 is too slow for the microtubule transport rates observed in primary cultures [9]. Instead microtubule movement in the cytoplasm is mainly driven by kinesin-1. Kinesin-1 knockdown impaired microtubule sliding and neurite outgrowth in cultured Drosophila neurons [5]. Kinesin heavy chain contains two microtubules microtubule-binding domains (MBDs); the N-terminal motor domain and C-terminal ATP-independent MBD [10–12]. As a result, a microtubule that binds to the C-terminus of the kinesin heavy chain becomes a “cargo” that is moved by the motor along another “track” microtubule (Fig.1, right panel). Because kinesin-1 is a plus-end directed motor, the only possible symmetrical model of microtubule-microtubule sliding is the movement of microtubules against each other with minus-ends leading and plus-ends trailing (Fig. 1, right panel). Mutations in the C-terminal ATP-independent MBD create a motor with impaired sliding but intact ability to move other cargoes. Replacement of the wild-type kinesin with a sliding-deficient mutant in Drosophila causes severe neurological defects. Detailed examination of the nervous system in these animals reveals that neurons develop very short axons (e.g. in optic lobes) and dendrites (e.g. in sensory neurons) [13].
Figure 1. Roles of microtubule-microtubule sliding in neurodevelopment and cell division.
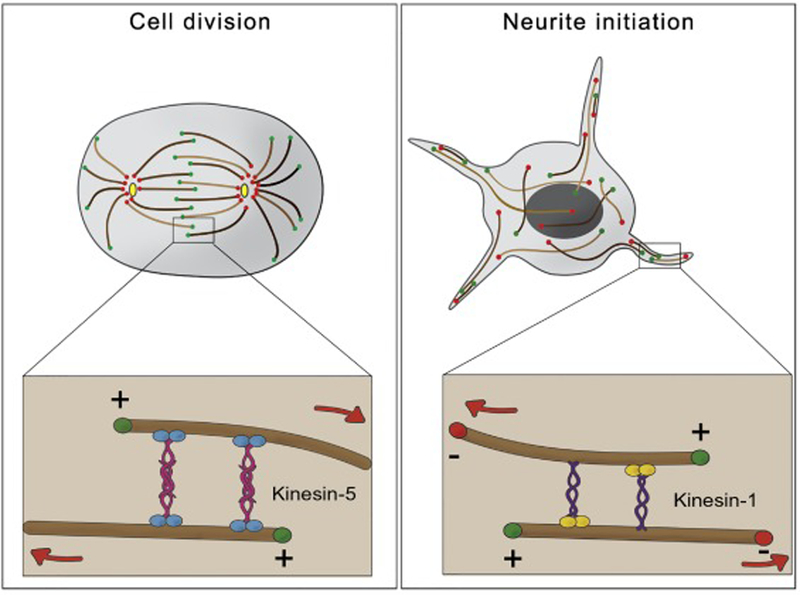
Left Panel. In cell division, microtubule-microtubule sliding, driven by the mitotic kinesin-5 family motors, is responsible for pole segregation and maintaining spindle bipolarity. Kinesin-5 motors form homotetramers with two pairs of plus-end directed motor heads at opposite ends that cross-link and slide antiparallel microtubules.
Right Panel. Neurite initiation requires the forces generated by microtubule-microtubule sliding of antiparallel microtubules by kinesin-1. Kinesin-1 links two microtubules through the kinesin heavy chain and slides microtubules with their minus-ends leading and plus-ends trailing.
Moreover, microtubule sliding is stimulated during axon regeneration. Axonal injury causes a fast spike of calcium influx that destabilizes microtubules near the axotomy site and creates new microtubule arrays with mixed orientation. This microtubule configuration leads to reactivation of kinesin-1 driven microtubule sliding and subsequently axon regeneration [14].
Role of cortical dynein in neuronal development
Cytoplasmic dynein, together with the dynactin complex, is the major minus-end directed motor involved in many interphase processes such as organelle positioning and cargo trafficking. During cell division, dynein activity is responsible for centrosome separation and chromosome segregation by pulling kinetochores, but it is also involved in proper mitotic spindle positioning and orientation by pulling on astral microtubules. The latter activity requires enrichment of dynein/dynactin complex at the cortex distal to the spindle poles [15]. For example, this cortical association in asymmetrical cell division is mediated by Gαi/LGN/NuMA-dependent pathway [reviewed in [16]] (Fig 2, top panel).
Figure 2. Roles of cortical dynein in neurodevelopment and cell division.
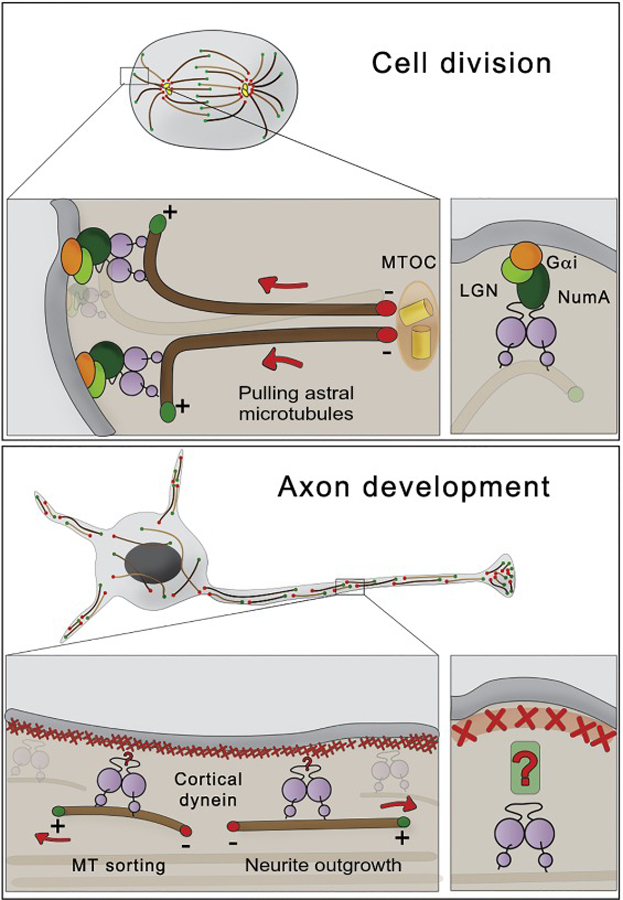
Top Panel. During cell division, dynein is enriched at the cortex distal to the spindle poles. Cortical dynein generates pulling forces on astral microtubules required for centrosome separation, positioning and orientation of the mitotic spindle. The recruitment of dynein to the cortex in mitotic cells is mediated by Gαi/LGN/NuMA.
Bottom Panel. Cytoplasmic dynein is recruited to the cortical actin in axons. This recruitment is required for removing microtubules with “wrong” (minus-ends out) orientation from axons. On the other hand, microtubules with “right” (plus-ends out) orientation are transported towards the tip of the axons, inducing neurite outgrowth. Currently, it is unknown how dynein is targeted to the cortical actin in the axons.
Dynein has been shown to have important roles on microtubule reorganization in neurites.
Photobleaching of GFP-tagged microtubules showed that short fragments of microtubules are moving in the axons of developed cultured neurons [6]. The motion of these microtubules is rapid, highly asynchronous and bidirectional. The transport of short microtubules (both anterograde and retrograde) is diminished after inactivation of cytoplasmic dynein [7]. Recent work has shown that dynein is responsible not only for transport of short fragments of microtubules, but also the bulk cytoskeletal network that powers axonal elongation [17].
At earlier developmental stages, neurons develop neurites that contain microtubules with mixed orientation [18, 19]. Later in development, only one of these neurites will become an axon and its microtubule orientation will gradually switch to uniformly, plus-end out [19] [20]. The uniform orientation of axonal microtubules in Drosophila neurons requires the activity of cytoplasmic dynein [21]. In agreement with these data, microtubule polarity defects were found in axons of cultured Drosophila and mammalian neurons after knocking down dynein heavy chain or dynactin components [19] or pharmacological inhibition of dynein [22]. Together, these data suggest that the dynein/dynactin complex is universally required for sorting microtubules in axons. Cytoplasmic dynein can cross-link and slide microtubules in vitro. It was suggested that the two motor domains of dynein can bind and move along two separate microtubules [23]. An alternative possibility, based on imaging fluorescently labeled components of the dynein/dynactin complex, is that dynein, immobilized at the actin cell cortex, captures and pulls microtubules [24] similar to cortical dynein pulling on astral microtubules during cell division [25]. In agreement with the cortical recruitment model, treatment of Drosophila cultured neurons with actin-depolymerizing drugs prevents dynein-driven microtubule sorting in the axons [19] (Fig.2 bottom panel). Furthermore, direct artificial recruitment of dynein to the plasma membrane bypassed the actin requirement for dynein-driven sorting of microtubules [19] confirming the role of the actin cortex in anchoring dynein pulling on axonal microtubules.
As dynein inactivation only affects microtubule polarity in axons but not in dendrites [21] it is likely that the mechanism of dynein recruitment to the cortex is axon-specific. There is some evidence that NDEL1 (a dynein cofactor) is enriched to the axonal initial segment via Ankyrin-G in mouse hippocampal neurons. Cultured neurons isolated from NDEL1 conditional knockout animals displayed defects in axon morphology as well as cargo sorting defects suggesting that recruitment of NDEL1 to the axon initial segment locally activates dynein [18]. We favor a scenario where dynein/dynactin cofactors are anchored at the cortical actin specifically in the axons but not in dendrites. It would be very interesting to identify the cortical recruitment mechanism of dynein in axons required for its microtubule sorting activity.
Role of spindle-reorganizing kinesins in neurite outgrowth
Kinesin-5 is the main motor responsible for sliding the overlapping sets of antiparallel microtubules that is required for bipolar spindle formation. Its microtubule sliding forces are counter-acted by other sets of microtubule motors such as kinesin-6 and kinesin-12, and the minus-end directed motor kinesin-14. Kinesin-6, kinesin-12 and kinesin-14 bind to mitotic microtubules in the metaphase spindle and act as an antagonist to kinesin-5-driven microtubule sliding [reviewed in [26, 27]]. These “mitotic” motors responsible for building the mitotic spindle are expressed in developing neurons, and post-mitotic depletion of these motors have implications in neurite outgrowth (Table 1). Here, we discuss the post-mitotic role of four mitotic kinesins, kinesin-5, kinesin-6, kinesin-12 and kinesin-14 in neurodevelopment.
Table 1.
Cytoskeletal proteins in neurite outgrowth
| Protein | Role in mitosis | Experimental approaches | Role in neuronal development | Reference |
|---|---|---|---|---|
| Kinesin-5 | Bipolar spindle formation | siRNA in cultured rat neurons. Chemical inhibition in cultured rat neurons. |
Axon outgrowth, Brake on microtubule transport, Growth cone turning | [8, 29, 30] |
| Kinesin-1 | RNAi and mutation in cultured Drosophila neurons. Mutation in Drosophila in vivo |
Neurite outgrowth. Microtubule sliding |
[5, 13] | |
| Dynein | Centrosome separation, mitotic spindle positioning | Dynein heavy chain RNAi in cultured Drosophila neurons Dynein complex component mutation in Drosophila in vivo siRNA/Chemical inhibition in rat cultured neurons |
Microtubule polarity Microtubule transport | [7, 19, 21, 22] |
| Kinesin-6 | Bundle spindle microtubules | RNAi in Drosophila in vitro and in vivo RNAi in mouse hippocampal cultures |
Brake on sliding Brake on neurite overextension Microtubule polarity |
[39, 41] |
| Kinesin-12 | Microtubule crosslinker. Maintain spindle. |
siRNA in rat hippocampal cultures Knock out in zebrafish in vivo |
Axon outgrowth Bidirectional microtubule transport Regulate microtubule invasion into growth cones Axon outgrowth Axon regeneration |
[48–50] |
| Kinesin-14 | Spindle organization | siRNA and chemical inhibition in rat hippocampal or sympathyetic cultures | Axon outgrowth Axon branching Microtubule polarity |
[46] |
| Kinesin-13 | Microtubule depolymerizer | Inhibition and Conditional knockout in mouse culture. Mutant Kinesin-13 in C. elegans in vivo |
Correct branching. Axon specification |
[57–59] |
| Mis-12 | Kinetochore component | Drosophila RNAi and mutant in vivo | Neurite extension. NMJ formation. |
[62] |
| Ndc80 | Kinetochore-microtubule attachment | Drosophila RNAi in vivo C. elegans depletion and mutation in vivo | Neurite extension. NMJ formation. |
[62, 63] |
Kinesin-5
Mammalian Eg5 (a kinesin-5 family member) is highly expressed in developing neurons that have already lost their ability to divide [28]. In post-mitotic neurons, a fraction of cytoplasmic Eg5 can be found in axons, especially in growth cones [29]. Knockdown of Eg5, or its chemical inhibition, accelerates the growth rate of axons of cultured sympathetic neurons and prevents proper microtubule polarization within growth cones, suggesting that Eg5 generates forces that oppose axonal growth, most likely suppressing retrograde transport of microtubules [8, 30]. Parallels can also be drawn between regulation of kinesin-5 itself in mitosis and neuronal development.
Kinesin-5 activity during cell division is regulated by several proteins such as NEK7 and TPX2. NEK7 is a mitotic kinase that is required for recruitment of kinesin-5 around centrosomes to promote spindle assembly and mitotic progression [31]. TPX2 is a Ran-regulated microtubule associated protein (MAP) that plays several roles in spindle assembly [32]. TPX2 can simultaneously bind to spindle microtubules and to kinesin-5. It is believed that TPX2 works as a mechanical brake that slows down microtubule sliding driven by kinesin-5 [33]. NEK7 and TPX2 also affect neurite outgrowth. NEK7 kinase activity regulates dendrite growth and branching, as well as formation of dendritic spines [34]. Depletion of TPX2 in neurons resulted in faster neurite outgrowth with rates similar to those observed after inactivation of Kinesin-5. Normal neurite extension rates can be rescued with the full-length TPX2 but not with the variant missing its kinesin-5 interacting domain [35]. It would be of interest to further examine the roles of other kinesin adaptor/modifier proteins between mitosis and neurodevelopment.
Kinesin-6
Kinesin-6 is a major constituent of the Centralspindlin complex. Centralspindlin accumulates at the spindle midzone in anaphase and initiates cleavage furrow formation via Rho signaling [36]. Here, kinesin-6 stabilizes the mitotic spindle by bundling antiparallel microtubules (Fig.3, left panel). Kinesin-6 depletion induces defects in morphology of the mitotic spindle and failure to recruit contractile ring components [37].
Figure 3. Roles of kinesin-6 in neurodevelopment and cell division.
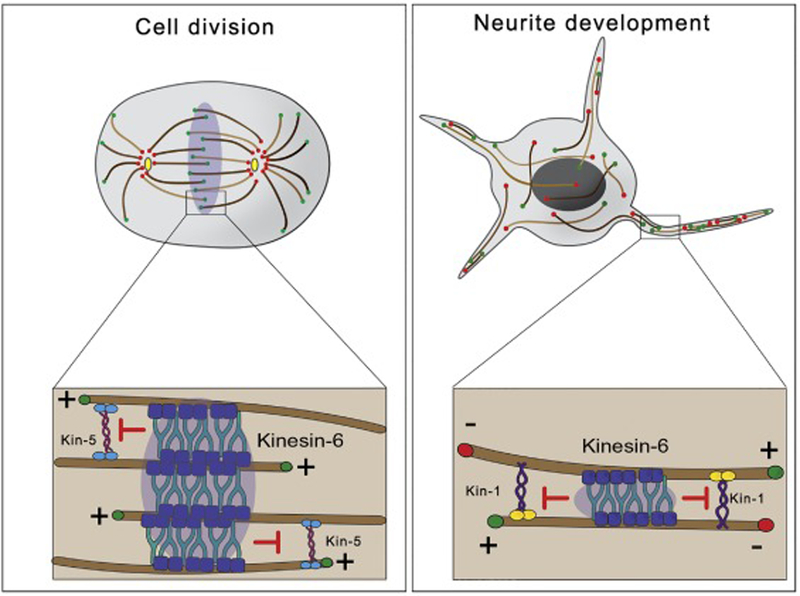
Left panel. In anaphase B, kinesin-6 motors concentrate at the midzone of the mitotic spindle. There, microtubule bundling activity of kinesin-6 counteracts microtubule sliding forces driven by kinesin-5 and stabilizes the mitotic spindle.
Right panel. In developing neurons, neurite outgrowth is mainly driven by kinesin-1-powered microtubule sliding. Sliding and neurite outgrowth are downregulated in mature neurons by the microtubule bundling activity of kinesin-6.
The kinesin-6 proteins continue to be expressed in post-mitotic neurons [38]. Mammals express three different kinesin-6 family members: Kif23 (MKLP1), Kif20a (MKLP2), and Kif20b. MKLP1 was the first kinesin-6 reported to regulate microtubule polarity in mammalian dendrites [39]. In addition, Kif20b plays an important role in neuronal development during corticogenesis. Loss of function mutations of Kif20b in cortical mouse neurons disrupts polarization, neurite outgrowth and branching [40]. In a screen looking for microtubule-microtubule sliding regulators, it was found that depletion of Pav-KLP (Pavarotti, the Drosophila ortholog of MKLP1) induced a dramatic increase in microtubule sliding and neurite length in Drosophila neurons [41]. Inversely, ectopic expression of Pav-KLP variants that cross-link, or bundle, microtubules, block microtubule sliding and neurite outgrowth, confirming that Pav-KLP is a negative regulator of microtubule sliding driven by other motors (kinesin-1 and cortical dynein) (Fig 3, right panel). This is consistent with the role of Pav-KLP as a microtubule bundler in mitosis [36]. Pav-KLP expression levels are transcriptionally controlled via Toll-6-FoxO signaling, and that regulates microtubule rearrangements required during remodeling of synaptic terminals [42]. Taken together, these works show the important role of kinesin-6 family members in neuronal polarization and neurite outgrowth through microtubule rearrangement. This activity requires a tight spatial and temporal regulation, most likely through controlling microtubule bundling properties of kinesin-6, and so contributes to the developmental downregulation observed for microtubule sliding in neurons.
Kinesin-14
The minus-end directed KIFC1 (kinesin-14 family member) is another “mitotic” motor expressed in mature neurons [43]. During cell division, Kinesin-14 regulates spindle organization and assembly through its microtubule crosslinking and sliding activity [44, 45]. Post-mitotic inhibition of KIFC1 activity resulted in cultured neurons that displayed shorter axons and lower number of axonal branches, as well as microtubule polarity defects [46]. Rescue assays with KIFC1 mutants that can slide but not cross-link, and vice versa, showed that microtubule sliding activity is important for axon growth, microtubule orientation and normal spacing between neighboring microtubules. In contrast, its cross-linking activity seems to be important for opposing normal microtubule sliding driven by other motors and for preventing axonal retraction. [46].
Kinesin-12
Kinesin-12 (KIF15) concentrates in the midzone of the mitotic spindle where microtubules from opposite poles overlap. It is thought that kinesin-12 microtubule crosslinking activity is important for maintaining the mitotic spindle [47]. KIF15 is expressed in developing vertebrate neurons; its expression is downregulated at later stages. Depletion of KIF15 in developing neurons enhances axonal extension and bidirectional microtubule transport in the axons [48]. Inversely, its overexpression results in shorter axons [49]. Growth cones in KIF15-depleted cultured neurons display increased invasion of filopodia by microtubules resulting in inhibition of proper growth cone turning and guidance. The ability of KIF15 to bind to actin, a major component of growth cones, might be responsible for the phenotypes observed in these structures [48]. More recently, it has been shown that depletion of KIF15 increases the regeneration velocities of injured axons in zebrafish neurons [50].
Role of microtubule dynamic instability in neurite outgrowth
Simultaneously to microtubule transport, microtubule reorganization is influenced by polymerization/depolymerization events. Microtubules are dynamic polymers that undergo states of growth, catastrophe, shortening and rescue, together termed microtubule dynamic instability [51]. Dynamic instability is regulated by local concentration of tubulin dimers, microtubule-associated proteins, and several kinesins. Microtubule dynamic instability is essential in mitosis and for process extension [52]. During mitosis, dynamic instability allows spatial exploration for spindle microtubules in their search for kinetochores. This activity prevents spontaneous microtubule assembly, controls proper spindle assembly and kinetochore-microtubule attachments. Kinetochore-microtubule attachments are regulated by the microtubule-depolymerization activity of several kinesins, including kinesin-13 [reviewed in [53]] and kinesin-8 [54]. Several mitotic kinesins (kinesin-13 and kinesin-8) [55, 56] that control dynamics of spindle microtubules also regulate neurite outgrowth.
The kinesin-13 family is a group of four subfamilies (KIF2A, KIF2B, KIF2C and KIF24) that catalyze microtubule depolymerization. KIF2A is enriched in developing mouse neurons and its inhibition induces formation of neurons with extended branches [56]. A Kif2a conditional knockout mouse develops hippocampal neurons with multiple axons [57]. KIF2A microtubule depolymerization activity is regulated by several kinases. Phosphorylation of KIF2A by CDK5 and PAK1 kinases decrease its depolymerizing activity and stimulate neurite outgrowth. Inversely, upregulation of KIF2A activity by ROCK2 inhibits outgrowth [58]. Kinesin-13 activity is locally downregulated after axonal injury, leading to upregulation of growing microtubules required for axon regeneration [59].
The role of kinetochore proteins in neurodevelopment
In addition to “mitotic” molecular motors, multiple components of the kinetochore-microtubule attachment machinery (termed KMN network for constituent proteins of the three subcomplexes) are required for nervous system development.
The KMN complex is assembled on inner components in contact with the centromere, e.g. CENP-A [60]. Intermediate complexes Mis12 and KNL-1 act as platforms for signaling and kinetochore assembly. The Ndc80 complex is the major site of microtubule attachment [61]. The KMN complex is necessary for proper alignment and subsequent segregation of chromosomes [61]. To ensure correct segregation, the KMN network must sense tension between sister chromatids and kinetochore microtubules. In order for progression into anaphase, the kinetochore must inactivate the spindle assembly checkpoint. This is achieved via a concerted action of kinases including Plk1 and Aurora B, and subsequent dephosphorylation [60].
In neurons, decreased neuronal expression of proteins from different KMN subcomplexes, (Mis12 and Ndc80) results in defects in NMJ formation and central nervous system (CNS) development in Drosophila [62]. Knl1 and Ndc80 are expressed in post-mitotic neurons in C. elegans and their depletion led to sensory neuron disorganization in C. elegans [63]. Nuf2, a component of the Ndc80 subcomplex is concentrated at microtubule rich dendrites, supportive of microtubule binding roles. Importantly, via sensory neuron-specific degradation, the authors were able to eliminate expression of endogenously Knl1 and Ndc80 in these cells. Under such conditions, a decrease in dendrite extension rate was noted, as was abnormal positioning of cell bodies of these head sensory neurons [63]. The disorganization/elongation of these neurons mirrors a phenotype observed in the Drosophila CNS in a Mis12 mutant, where the ventral nerve cord was narrower and longer than that of wild type embryos. Finer analysis of defects induced by loss of Mis12 revealed hyperextension of neurites at the NMJ and failure to form a normal pre-synapse, although neuronal differentiation itself was unaffected [62]. Notably, this hyperextension may be due to a similar mechanism by which kinesin-6 depletion leads to axon overextension in Drosophila [41]. Furthermore, cultured rat hippocampal neurons show an increase in filopodia-like dendritic extensions and expression of Mis12 at the protein level was confirmed in human iPSC derived cortical and motor neurons [62]. These findings suggest that repurposing of kinetochore machinery in cytoskeletal organization may be conserved from flies and worms to human.
Importantly, the Mis12 hyperextension phenotype was observed with multiple components of the KMN network in Drosophila, including each of the Ndc80 and Knl1 subcomplexes, as well as other Mis12 subunits [63]. These findings suggest that the KMN network functions as a comparable system in mitosis and in neurons. In contrast, both Ndc80 MBDs are required in nervous system development, while only one of these two MBDs is needed for correct chromosome segregation [63]. Therefore, divergence between mitosis and neuronal development, and the precise mechanisms by which KMN proteins contribute to neuronal and synaptic maturation require further investigation.
Concluding Remarks
In recent years, an explosion of experimental data has shown that neurons repurpose the machinery involved in bipolar spindle formation to generate microtubule-based neurites.
Here we have discussed the conserved and contrasting roles of the cytoskeleton in cell cycle progression and neuronal development. Multiple studies have shed light on expression of many “mitotic” proteins in non-dividing neurons; and the functions of these proteins have been tailored to meet the demands of the morphologically complex cytoskeletal rearrangements in neurons. Common themes emerging from these studies include the remodeling of the cytoskeleton by microtubule sliding and polymerization/depolymerization events. These parallels are extended when we note the factors involved in kinesin-5 regulation are common between mitosis and neurodevelopment. In contrast, it is interesting to note that, in the case of microtubule sliding, the motors responsible are different. In the case of dynein mediated microtubule reorganization, both cell division and microtubule sorting in axons require cortical recruitment of dynein, but the recruiting cortical anchors likely differ (see Outstanding Questions).
Outstanding Questions Box.
How do the “mitotic” kinesins regulate sliding during neurite development? Do signaling pathways identified in cell division play a role in neurodevelopment?
Why do microtubules get sorted in axons but not in dendrites?
What are the components that recruit dynein to the cortical actin in axons?
What is the mechanism of action of “kinetochore” proteins in nervous system development?
This review was written from a microtubule-centric point of view. Obviously, there are other mitotic components, such as the anaphase promoting complex that also regulates synapse formation through transcription regulation and protein turnover. Based on the similarities found between mitosis and neurodevelopment, it would be interesting to investigate if signaling that controls the function of “mitotic” motors in mitosis is also conserved in neurodevelopment. We expect that additional mitotic proteins involved in neuronal development will be identified soon, followed by a more general picture that integrates these numerous components in a logical pathway.
Highlights.
Many components of the cell division machinery are repurposed in post-mitotic neurons to control neurite outgrowth.
The mechanical forces generated by motor-driven reorganization of the microtubule network play a major role in neurite outgrowth
Microtubule transport driven by kinesin-1 and cortical dynein generates forces responsible for neurite formation
Several mitotic kinesins regulate neurite outgrowth by counteracting microtubule transport driven by kinesin-1 and dynein
Cortical dynein is responsible for sorting of microtubules in axons
Kinetochore proteins are required for nervous system development
Acknowledgements.
Supported by NIH Grants R01GM052111 and R35GM131752.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kapitein LC and Hoogenraad CC (2011) Which way to go? Cytoskeletal organization and polarized transport in neurons. Mol Cell Neurosci 46 (1), 9–20. [DOI] [PubMed] [Google Scholar]
- 2.Baas PW (1999) Microtubules and neuronal polarity: lessons from mitosis. Neuron 22 (1), 23–31. [DOI] [PubMed] [Google Scholar]
- 3.Sawin KE et al. (1992) Mitotic spindle organization by a plus-end-directed microtubule motor. Nature 359 (6395), 540–3. [DOI] [PubMed] [Google Scholar]
- 4.Kapitein LC et al. (2005) The bipolar mitotic kinesin Eg5 moves on both microtubules that it crosslinks. Nature 435 (7038), 114–8. [DOI] [PubMed] [Google Scholar]
- 5.Lu W et al. (2013) Initial neurite outgrowth in Drosophila neurons is driven by Kinesin-powered microtubule sliding. Curr Biol 23 (11), 1018–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang L and Brown A (2002) Rapid movement of microtubules in axons. Curr Biol 12 (17), 1496–1501. [DOI] [PubMed] [Google Scholar]
- 7.He Y et al. (2005) Role of cytoplasmic dynein in the axonal transport of microtubules and neurofilaments. J Cell Biol 168 (5), 697–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Myers KA and Baas PW (2007) Kinesin-5 regulates the growth of the axon by acting as a brake on its microtubule array. J Cell Biol 178 (6), 1081–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roostalu J et al. (2018) Determinants of Polar versus Nematic Organization in Networks of Dynamic Microtubules and Mitotic Motors. Cell 175 (3), 796–808.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Straube A et al. (2006) Conventional kinesin mediates microtubule-microtubule interactions in vivo. Mol Biol Cell 17 (2), 907–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Navone F et al. (1992) Cloning and expression of a human kinesin heavy chain gene: interaction of the COOH-terminal domain with cytoplasmic microtubules in transfected CV-1 cells. J Cell Biol 117 (6), 1263–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hackney DD and Stock MF (2000) Kinesin’s IAK tail domain inhibits initial microtubule-stimulated ADP release. Nat Cell Biol 2 (5), 257–60. [DOI] [PubMed] [Google Scholar]
- 13.Winding M et al. (2016) Role of kinesin-1-based microtubule sliding in Drosophila nervous system development. Proc Natl Acad Sci U S A 113 (34), E4985–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu W et al. (2015) Kinesin-1-powered microtubule sliding initiates axonal regeneration in Drosophila cultured neurons. Mol Biol Cell 26 (7), 1296–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Collins ES et al. (2012) Cell cycle-regulated cortical dynein/dynactin promotes symmetric cell division by differential pole motion in anaphase. Mol Biol Cell 23 (17), 3380–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kiyomitsu T (2019) The cortical force-generating machinery: how cortical spindle-pulling forces are generated. Curr Opin Cell Biol 60, 1–8. [DOI] [PubMed] [Google Scholar]
- 17.Roossien DH et al. (2014) Cytoplasmic dynein pushes the cytoskeletal meshwork forward during axonal elongation. J Cell Sci 127 (Pt 16), 3593–602. [DOI] [PubMed] [Google Scholar]
- 18.Kuijpers M et al. (2016) Dynein Regulator NDEL1 Controls Polarized Cargo Transport at the Axon Initial Segment. Neuron 89 (3), 461–71. [DOI] [PubMed] [Google Scholar]
- 19.del Castillo U et al. (2015) Interplay between kinesin-1 and cortical dynein during axonal outgrowth and microtubule organization in Drosophila neurons. Elife 4, e10140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baas PW et al. (1988) Polarity orientation of microtubules in hippocampal neurons: uniformity in the axon and nonuniformity in the dendrite. Proc Natl Acad Sci U S A 85 (21), 8335–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zheng Y et al. (2008) Dynein is required for polarized dendritic transport and uniform microtubule orientation in axons. Nat Cell Biol 10 (10), 1172–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rao AN et al. (2017) Cytoplasmic Dynein Transports Axonal Microtubules in a Polarity-Sorting Manner. Cell Rep 19 (11), 2210–2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tanenbaum ME et al. (2013) Cytoplasmic dynein crosslinks and slides anti-parallel microtubules using its two motor domains. Elife 2, e00943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mazel T et al. (2014) Direct observation of microtubule pushing by cortical dynein in living cells. Mol Biol Cell 25 (1), 95–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Okumura M et al. (2018) Dynein-Dynactin-NuMA clusters generate cortical spindle-pulling forces as a multi-arm ensemble. Elife 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu WD et al. (2019) Roles and mechanisms of Kinesin-6 KIF20A in spindle organization during cell division. Eur J Cell Biol 98 (2–4), 74–80. [DOI] [PubMed] [Google Scholar]
- 27.She ZY and Yang WX (2017) Molecular mechanisms of kinesin-14 motors in spindle assembly and chromosome segregation. J Cell Sci 130 (13), 2097–2110. [DOI] [PubMed] [Google Scholar]
- 28.Ferhat L et al. (1998) Expression of the mitotic motor protein Eg5 in postmitotic neurons: implications for neuronal development. J Neurosci 18 (19), 7822–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nadar VC et al. (2008) Kinesin-5 is essential for growth-cone turning. Curr Biol 18 (24), 1972–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haque SA et al. (2004) Monastrol, a prototype anti-cancer drug that inhibits a mitotic kinesin, induces rapid bursts of axonal outgrowth from cultured postmitotic neurons. Cell Motil Cytoskeleton 58 (1), 10–6. [DOI] [PubMed] [Google Scholar]
- 31.Yissachar N et al. (2006) Nek7 kinase is enriched at the centrosome, and is required for proper spindle assembly and mitotic progression. FEBS Lett 580 (27), 6489–95. [DOI] [PubMed] [Google Scholar]
- 32.Trieselmann N et al. (2003) Ran modulates spindle assembly by regulating a subset of TPX2 and Kid activities including Aurora A activation. J Cell Sci 116 (Pt 23), 4791–8. [DOI] [PubMed] [Google Scholar]
- 33.Ma N et al. (2011) TPX2 regulates the localization and activity of Eg5 in the mammalian mitotic spindle. J Cell Biol 195 (1), 87–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Freixo F et al. (2018) NEK7 regulates dendrite morphogenesis in neurons via Eg5-dependent microtubule stabilization. Nat Commun 9 (1), 2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kahn OI et al. (2015) TPX2 regulates neuronal morphology through kinesin-5 interaction. Cytoskeleton (Hoboken) 72 (7), 340–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mishima M et al. (2002) Central spindle assembly and cytokinesis require a kinesin-like protein/RhoGAP complex with microtubule bundling activity. Dev Cell 2 (1), 41–54. [DOI] [PubMed] [Google Scholar]
- 37.Adams RR et al. (1998) pavarotti encodes a kinesin-like protein required to organize the central spindle and contractile ring for cytokinesis. Genes Dev 12 (10), 1483–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ferhat L et al. (1998) Expression of the mitotic motor protein CHO1/MKLP1 in postmitotic neurons. Eur J Neurosci 10 (4), 1383–93. [DOI] [PubMed] [Google Scholar]
- 39.Lin S et al. (2012) Mitotic motors coregulate microtubule patterns in axons and dendrites. J Neurosci 32 (40), 14033–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McNeely KC et al. (2017) Mutation of Kinesin-6 Kif20b causes defects in cortical neuron polarization and morphogenesis. Neural Dev 12 (1), 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.del Castillo U et al. (2015) Pavarotti/MKLP1 regulates microtubule sliding and neurite outgrowth in Drosophila neurons. Curr Biol 25 (2), 200–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McLaughlin CN et al. (2016) A Toll receptor-FoxO pathway represses Pavarotti/MKLP1 to promote microtubule dynamics in motoneurons. J Cell Biol 214 (4), 459–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Silverman MA et al. (2010) Expression of kinesin superfamily genes in cultured hippocampal neurons. Cytoskeleton (Hoboken) 67 (12), 784–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mountain V et al. (1999) The kinesin-related protein, HSET, opposes the activity of Eg5 and cross-links microtubules in the mammalian mitotic spindle. J Cell Biol 147 (2), 351–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fink G et al. (2009) The mitotic kinesin-14 Ncd drives directional microtubule-microtubule sliding. Nat Cell Biol 11 (6), 717–23. [DOI] [PubMed] [Google Scholar]
- 46.Muralidharan H and Baas PW (2019) Mitotic Motor KIFC1 is an organizer of microtubules in the axon. J Neurosci 39 (20), 3792–3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rogers GC et al. (2000) A kinesin-related protein, KRP(180), positions prometaphase spindle poles during early sea urchin embryonic cell division. J Cell Biol 150 (3), 499–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu M et al. (2010) Kinesin-12, a mitotic microtubule-associated motor protein, impacts axonal growth, navigation, and branching. J Neurosci 30 (44), 14896–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu M et al. (2014) Kinesin-12 influences axonal growth during zebrafish neural development. Cytoskeleton (Hoboken) 71 (10), 555–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dong Z et al. (2019) Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR)/Cas9-mediated kif15 mutations accelerate axonal outgrowth during neuronal development and regeneration in zebrafish. Traffic 20 (1), 71–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mitchison T and Kirschner M (1984) Dynamic instability of microtubule growth. Nature 312 (5991), 237–42. [DOI] [PubMed] [Google Scholar]
- 52.Oelz DB et al. (2018) Microtubule Dynamics, Kinesin-1 Sliding, and Dynein Action Drive Growth of Cell Processes. Biophys J 115 (8), 1614–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moores CA and Milligan RA (2006) Lucky 13-microtubule depolymerisation by kinesin-13 motors. J Cell Sci 119 (Pt 19), 3905–13. [DOI] [PubMed] [Google Scholar]
- 54.Stumpff J et al. (2008) The kinesin-8 motor Kif18A suppresses kinetochore movements to control mitotic chromosome alignment. Dev Cell 14 (2), 252–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kevenaar JT et al. (2016) Kinesin-Binding Protein Controls Microtubule Dynamics and Cargo Trafficking by Regulating Kinesin Motor Activity. Curr Biol 26 (7), 849–61. [DOI] [PubMed] [Google Scholar]
- 56.Homma N et al. (2003) Kinesin superfamily protein 2A (KIF2A) functions in suppression of collateral branch extension. Cell 114 (2), 229–39. [DOI] [PubMed] [Google Scholar]
- 57.Homma N et al. (2018) KIF2A regulates the development of dentate granule cells and postnatal hippocampal wiring. Elife 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ogawa T and Hirokawa N (2015) Microtubule Destabilizer KIF2A Undergoes Distinct Site-Specific Phosphorylation Cascades that Differentially Affect Neuronal Morphogenesis. Cell Rep 12 (11), 1774–88. [DOI] [PubMed] [Google Scholar]
- 59.Ghosh-Roy A et al. (2012) Kinesin-13 and tubulin posttranslational modifications regulate microtubule growth in axon regeneration. Dev Cell 23 (4), 716–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hinshaw SM and Harrison SC (2018) Kinetochore Function from the Bottom Up. Trends Cell Biol 28 (1), 22–33. [DOI] [PubMed] [Google Scholar]
- 61.Varma D and Salmon ED (2012) The KMN protein network--chief conductors of the kinetochore orchestra. J Cell Sci 125 (Pt 24), 5927–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhao G et al. (2019) Kinetochore Proteins Have a Post-Mitotic Function in Neurodevelopment. Dev Cell 48 (6), 873–882 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cheerambathur DK et al. (2019) The Kinetochore-Microtubule Coupling Machinery Is Repurposed in Sensory Nervous System Morphogenesis. Dev Cell 48 (6), 864–872 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jolly AL et al. (2010) Kinesin-1 heavy chain mediates microtubule sliding to drive changes in cell shape. Proc Natl Acad Sci U S A 107 (27), 12151–6. [DOI] [PMC free article] [PubMed] [Google Scholar]


