Abstract
This review covers the role of ultrasonography as an essential non-invasive diagnostic approach when facing patients with anaemia, a common clinical problem. Abdomen ultrasound is well recognised as a first-line examination in the setting of blood loss, both acute and chronic. Less is clear about the additional opportunities, given by ultrasound in anaemia, due to the many other possible causes.
Here we provide information on the utility of ultrasound in different contexts and a practical guide for clinicians facing anaemic patients.
Keywords: Anaemia, Ultrasound
Introduction
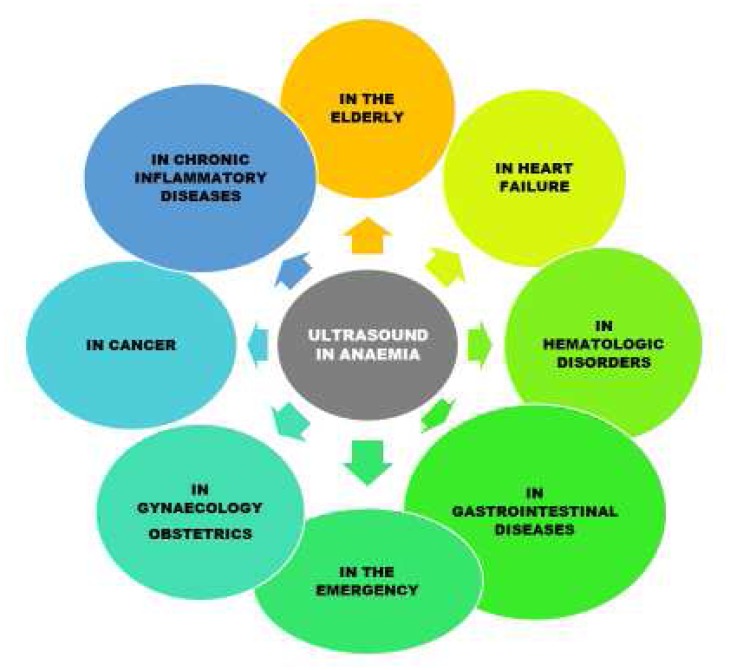
Anaemia is the most common haematological disorder, affecting more than two billions people worldwide, with iron deficiency being the prevalent cause.1 According to the World Health Organization, anaemia is defined by haemoglobin levels less than 13 g/dL in adult males, and less than 12 g/dL in adult females.1 Anaemia is classified in several ways, e.g., acute versus chronic and/or according to the leading cause, as detailed elsewhere.2–5 Ultrasonography, a widely and increasingly used noninvasive diagnostic approach, can be very useful also in patients with anaemia. The aim of this paper is to review the role of ultrasound in different conditions, eventually providing a practical guide for clinicians facing anaemic patients. A large emphasis is given to the elderly, as well as to patients with cancer or other chronic diseases, in whom anaemia is an independent predictor of adverse outcomes, and non-invasive approaches are often preferred because of their fragile conditions. The possible applications of ultrasonography in anaemic patients are depicted in Figure 1.
Figure 1.
Ultrasound in the anaemic patients: fields of application.
Abdomen Ultrasound: the First Line Examination in Different Clinical Settings
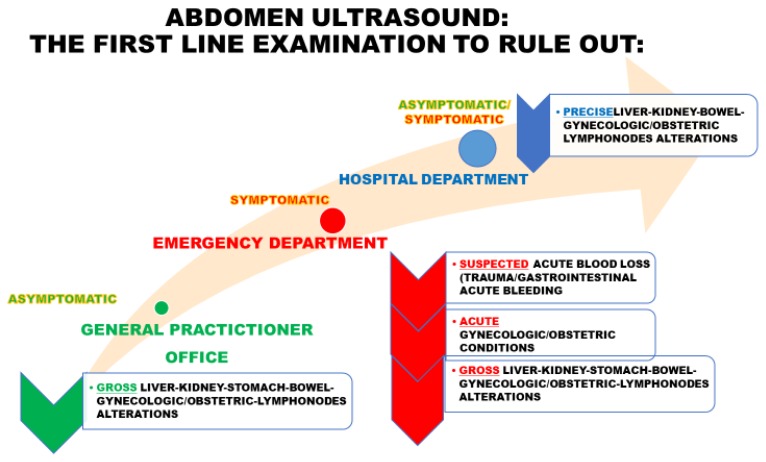
Abdomen ultrasound may be the first-line examination in different settings, as summarised in Figure 2.
Figure 2.
The role of abdomen ultrasound as a first-line examination in different settings and with different patients.
General Practitioners (GPs) usually represent the first contact with the healthcare system for patients with chronic anaemia, especially when it is due to iron-deficiency, which in turn tends to be underdiagnosed and/or under-coded.6–8 In this setting, an appropriate first-line examination aimed not only at the diagnosis of iron deficiency per se but also addressing its possible aetiology, is crucial. Indeed, point-of-care abdomen ultrasound could detect gross alterations (e.g. abdominal masses) guiding further investigation.
Anaemia due to acute blood loss in the Emergency Department
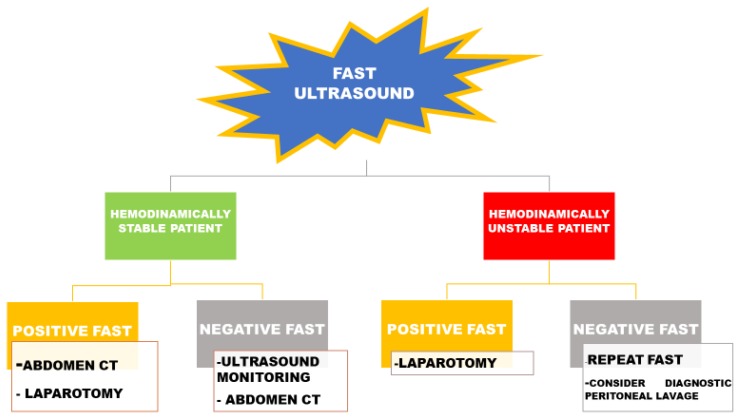
In the setting of acute bleeding, ultrasound has a prominent role. For example, in patients referring because of trauma, ultrasound represents a useful complement to basic clinical evaluation, influencing bedside decision making, and determining whether or not the patient requires further procedural intervention. Of note, in as many as 50% of patients with severe abdominal trauma, the initial physical examination can appear normal, leading to dangerous reassurance. Similarly, unconscious patients or those unable to provide a clear history of the trauma whatever the reason, can be particularly difficult to manage. Thus, physicians largely depend on diagnostic imaging, so that ultrasound represents an essential tool in the trauma resuscitation area. In this setting, the Focused Assessment with Sonography for Trauma (FAST) evaluation can be of crucial help for the rapid identification of the presence of free fluid suggestive for hemoperitoneum, hemothorax, and/or hemopericardium.9 The overarching assumption of FAST is that all clinically significant abdominal injuries are associated with hemoperitoneum. The traditional FAST approach includes four basic sonographic views: pericardial, perihepatic, perisplenic and pelvic. The detailed procedure is well described.10 Ultrasound can easily detect as little as 200 mL of fluid in the Morrison pouch. This technique can be completed in less than one minute. Nevertheless, FAST has a number of limitations, especially in penetrating trauma, as well as in detecting small retroperitoneal bleeding. Thus, computed tomography (CT) remains the gold-standard technique.
Understanding the strengths and limitations of FAST is essential to recognise when further testing is indicated. It has been reported that FAST contributes to a decrease in abdominal CT use by about 50%.11
The algorithm for FAST-oriented further investigations is described in Figure 3 and is influenced by patient’s hemodynamic status.
Figure 3.
FAST-oriented algorithm in the abdominal trauma. FAST: Focused Assessment with Sonography for Trauma; CT: computed tomography.
Ultrasound has been suggested as a useful noninvasive tool for the early detection of bleeding also in non-traumatic settings. Two well recognised sonographic markers of hypovolemia are the diameter of the inferior cave vein (DICV) and the thickness of the left ventricle. Several studies12,13 have highlighted the correlation between DICV and the need for blood transfusions in patients with acute bleeding.14
Moreover, serial changes in DICV reliably predict ongoing hemorrhagic shock, even better than arterial pressure or heart rate.15 Pseudo-hypertrophy of the left ventricle has been reported as another possible noninvasive early marker for hemorrhagic shock in experimental animal models.13,16
Gastrointestinal (GI) bleeding
GI bleeding is a common problem in the Emergency Department. Hemodynamic monitoring by ultrasound of the inferior cave vein (inspiratory collapse) and the so-called “kissing sign” of the left ventricle are useful markers of high risk in emergency GI bleeding.17
Acute or overt GI bleeding can be easily recognised when hematemesis, melena, or hematochezia are present, while chronic or occult GI bleeding often leading to iron-deficiency anaemia can be detected by a positive faecal occult blood test. Upper endoscopy and colonoscopy are the mainstays of the investigations. Angiography, radionuclide imaging, capsule endoscopy, and deep enteroscopy are further options to investigate acute GI bleeding and obscure GI bleeding, respectively.18
In the elderly, nutrient deficiency (in particular of iron, folate, and B12 vitamin) accounts for at least one-third of all cases of anaemia. Within this group, more than half is related to absolute iron deficiency.3,4 The so-called anaemia of chronic diseases (in particular cardiovascular, kidney, and inflammatory diseases including cancer), is present in near another third of the anaemic elderly.19,20 Nevertheless, a substantial proportion of anaemia in the elderly remains apparently unexplained. In hospitalised elderly patients, anaemia is present in up to near 50% and is independently associated with increased length of in-hospital stay, in-hospital readmission, and mortality.21–23
Ultrasound has a minimal role in the diagnosis of gastric bleeding, where endoscopy represents the gold standard. On the other hand, ultrasound can be helpful in intestinal diseases. Conventional ultrasound can provide quick information about bowel status and helps in the choice of adequate further examinations. However, it is worthy of note that negative findings do not exclude the presence of bowel disease. Two types of probes with different ultrasound frequencies may be used (3.5–5 MHz and 5–17 MHz) to obtain a panoramic view of the abdomen. The five layers of the colonic wall may be clearly distinguishable as concentric rings of alternating echogenicity. The measurement of wall thickness (normal value < 3 mm) is essential.24
More recent ultrasound techniques, as elastography, contrast-enhanced, and Doppler ultrasound, rectal and trans-perineal ultrasonography allow further examinations, in particular, to evaluate bowel vascularisation abnormalities.25
Inflammatory bowel disease (IBD)
Anaemia is among the most frequent manifestations of IBD. Its prevalence is around 24% and can be due to chronic inflammation, chronic blood loss, or both.26 The European Crohn’s and Colitis Organisation (ECCO) guidelines indicate intestinal ultrasonography as the imaging technique of choice for screening patients with clinically suspected Crohn’s disease.26 Nevertheless, intestinal ultrasound is particularly important in the follow-up of patients after the initial diagnosis, where fluctuations in disease course require repeated examinations.27 The ultrasonographic signs usually detected in Crohn’s disease are well characterised,27 and include thickening, decreased compressibility, and increased vascularisation of the bowel wall. Pericolic fluid and lymph node enlargement may be detected with high sensitivity and specificity.28 Also abscesses narrowing the bowel lumen, fistulas in the intestinal loops, and cutaneous fistulas may be detected.28 The assessment of the disease activity and the precise overview of Crohn’s extraluminal complications is beyond the scope of conventional abdomen ultrasound. Contrast-enhanced magnetic resonance enterography is the method of choice.29,30 The use of colour Doppler, contrast ultrasound and elastography increases the accuracy of the conventional ultrasound, when magnetic resonance is not available.31 In particular, Doppler ultrasound may show bowel wall increased vascularisation due to inflammation. Elastography techniques, such as strain and shear wave elastography, have shown promising results, because of their ability to differentiate active inflammation from fibrosis. A comprehensive review of the current evidence supporting the use of elastography techniques in intestinal disorders is reported elsewhere.32
Cross-sectional imaging should be considered where conventional ultrasound is inconclusive or non-diagnostic or when it appears normal, but there is still high clinical suspicion of disease.
Cancer: focus on colorectal cancer and gastrointestinal lymphoma
Anaemia is frequent in cancer patients and often characterised by multifactorial pathophysiology.20 Blood losses (either by tumour mass or associated with surgery), and inadequate nutrient intake due to cachexia or malnutrition are often present, along with inflammation mainly due to the release of cancer-associated pro-inflammatory cytokines. Such cytokines, especially interleukin-6, increase in turn hepcidin synthesis in the liver, eventually leading to iron sequestration into macrophages and functional iron deficiency.34,35 Anaemia in cancer patients can also be due to decreased red cell survival, erythropoiesis disorders, low erythropoietin levels and progressive erythropoietin resistance of erythroid progenitors.33
Bone marrow infiltration by neoplastic cells, myelosuppression due to chemo- or radiotherapy, and possibly concomitant kidney disease also contribute to anaemia in individual cancer patients.
The role of conventional abdomen ultrasound is negligible as compared to endoscopy. Nevertheless, it often represents the first-line examination, when the disease is suspected, or endoscopy is not unfeasible.
Hypoechoic bowel wall thickening with irregular contour, loss of stratification of the wall layers, and the absence of normal peristalsis can be suggestive of malignancy (the so-called “pseudo-kidney sign”), as well as the detection of liver lesions suggesting metastasis from colorectal cancer.36 Martinez-Ares and colleagues36 evaluated the diagnostic performance of abdomen ultrasonography in 145 patients with suspected colorectal carcinoma who were admitted for colonoscopy. They concluded that abdominal ultrasound is a technique with high sensibility and specificity to detect colon cancer, in accordance with other authors.37 High specificity and sensibility have been reported in the diagnosis of tumours located above the recto-sigmoid junction, but small polypoid lesions can be overlooked. Due to its non-invasiveness, abdominal ultrasound should be considered as an alternative to the conventional radiological and endoscopic examinations, especially in patients for which no therapeutic option would be possible because of comorbidities or advanced age. For other patients with suspected cancer, abdomen ultrasound should be the first diagnostic examination that may justify further endoscopic examinations also in the Emergency Department.
Gastrointestinal lymphoma is the second more frequent extra-nodal lymphoproliferative disorder. The clinical presentation is non-specific, including weight loss, dyspepsia, abdominal pain, and also anaemia. With the exception of relatively indolent gastric localisation, it usually has a high degree of malignancy.38
Over 77% of GI lymphoma exceeds 5 cm in diameter, and the average length of the affected bowel is 12 cm.38 Abdominal ultrasound is the first-line examination to address the right diagnosis, followed by CT and endoscopy. In small bowel lymphoma, ultrasonography usually shows a bulky, lobulated, predominantly hypoechoic mass with a central echogenic component. Enlarged mesenteric and periaortic lymph nodes may also be detected.38 In IBD, especially in patients with Crohn’s diseases on immunosuppressive treatment, the worsening of anaemia may herald the evolution towards a lymphoproliferative disease.39 In such cases, changes in the size, morphology, echogenicity or the appearance of abdominal lymph nodes may be useful in confirming/ruling out the suspected diagnosis.
Other causes of anaemia in the gastrointestinal tract
In addition to acute/overt bleeding, anaemia in gastrointestinal diseases may result from other causes: obscure bleeding, malabsorption, and maldigestion (e.g. celiac disease, chronic pancreatitis), or autoimmune disorder (e.g. pernicious anaemia).40 The role of intestinal ultrasound in this clinical setting is not entirely defined. Recent guidelines issued by the Italian Association of Hospital Gastroenterologists and Endoscopists (AIGO), and by the Italian Society of Pediatric Gastroenterology Hepatology and Nutrition (SIGENP) do not recommend intestinal ultrasound, suggesting other imaging techniques like CT, enterography, magnetic resonance enterography, capsule enteroscopy, and endoscopy.40
However, abdominal ultrasound can still play a role in diseases of the small bowel, as an initial and low-cost procedure to guide the successive diagnostic approaches. It should be underlined that one of the reasons for the controversies in including ultrasonography among the recommended techniques for exploring the intestinal/abdominal causes of anaemia may be related to its poor reproducibility. To overcome this limitation, the European Federation of Societies for Ultrasound in Medicine and Biology (EFSUMB) has recently published new guidelines intending to standardise the examination technique and to facilitate the correct training education of operators.41
Malabsorption and Maldigestion Syndromes
One of the most frequent signs of malabsorption syndromes is anaemia, in particular, iron deficiency anaemia.40 Indeed anaemia can be present in 12–69% of patients with celiac disease (CD). CD diagnosis is usually pursued by serology including IgA anti-transglutaminase (anti-tTG IgA) and IgG-antideamidated gliadin peptides (anti-DGP IgG), and confirmed by histological documentation of flattening of the villi.40 In CD patients, intestinal ultrasound examinations can reveal small bowel loops thickening, enlarged mesenteric lymph nodes, and free fluid within the bowel loops in 50–60% of cases.42 The absence of dilated and/or thickened loops has a negative predictive value of 98% in excluding the diagnosis of CD by intestinal ultrasound.43
In maldigestion due to pancreatic insufficiency, e.g., due to chronic pancreatitis, anaemia can be related to decreased iron absorption, chronic inflammation, and vitamin B12 deficiency. In patients with anaemia and clinical signs of maldigestion, ultrasound may detect a reduction in the size of the pancreas, irregular profiles, parenchymal calcifications, or dilated Wirsung duct with stones. All these signs lead to the diagnosis of chronic pancreatitis.44
Gynaecology/Obstetrics
Anaemia is commonly encountered in gynaecology/obstetrics practice.45 Bleeding caused by adenomyosis, uterine fibroids, or endometrial hyperplasia frequently results in even severe iron-deficiency anaemia. Ultrasound is the main diagnostic tool also to assess the location and status of early pregnancy. Transvaginal ultrasound is the method of choice. Nevertheless, in several situations, as for women who decline transvaginal ultrasound, the transabdomen scan is the alternative option. In particular, uterine disorders causing acute bleeding include retained products of conception, uterine arterio-venous malformations, and fibroids. Adnexal disorders may also cause bleeding, including hemorrhagic ovarian cysts, and ectopic pregnancies.46 A detailed description of such diseases is beyond the scope of this work. Comprehensive reviews on these topics can be found elsewhere.47,48
Focus on: Ultrasound for the Haematologist
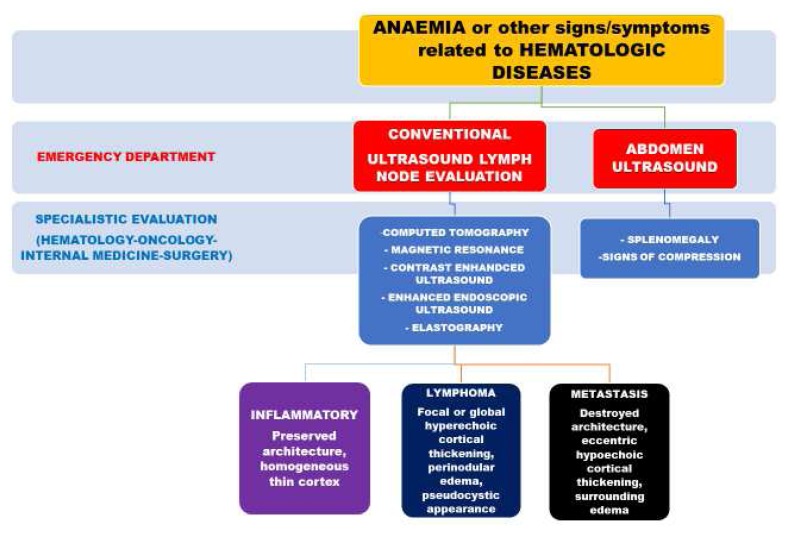
Conventional ultrasound is the recommended imaging method for lymph node evaluation, with the advantages of high resolution, real-time evaluation, safety, and low costs.49 Nevertheless, recent advances in ultrasound techniques, as contrast-enhanced ultrasound (CEUS), contrast-enhanced endoscopic ultrasound (CE-EUS), and real-time elastography, improves the evaluation accuracy for the differential diagnosis between benign and malignant lymph nodes. CE-EUS is also used for guiding fine needle aspiration. The differentiation of malignant versus benign lymph nodes by ultrasound traditionally relies on size and topographic distribution.50 However, malignant lymph node infiltration can occur in up to 30% lymph nodes of less than 5 mm. The evaluation of shape and borders does not allow a definitive classification.51 New ultrasound techniques provide additional information; for example, CEUS can give information about vascularisation and perfusion pattern. This technique identifies changes in vascular architecture and avascular areas of malignant infiltration. In summary, perfusion defects and centripetal non-homogeneous enhancement suggest lymph node infiltration by malignancy.52 In lymphoma, CEUS patterns are highly variable, particularly regarding the vascular features.52 Elastography is a non-invasive method in which the stiffness of the tissue is viewed as a colour map or shear wave velocity.53 The details of these techniques are reported elsewhere.53,54
Abdomen ultrasound and lymph nodes conventional ultrasound usually constitute the initial diagnostic workup in haematological diseases as recently reviewed.55 Figure 4 represents the possible instrumental examination flow chart for suspected hematologic diseases.
Figure 4.
The instrumental examination flow chart for suspected haematologic diseases.
Focus on: Anaemia and Cardiac Ultrasound (Heart Failure and the Oncologic Patient)
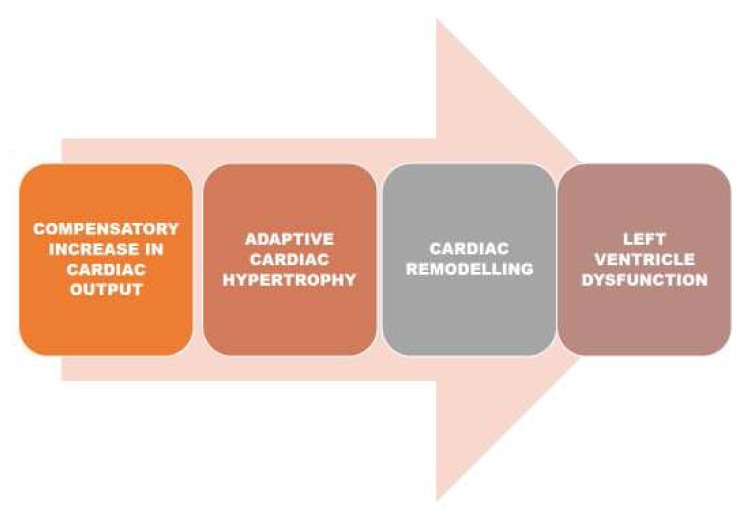
HF is a clinical syndrome characterised by typical symptoms and signs caused by structural or functional cardiac abnormalities, resulting in a reduced cardiac output or elevated intra-cardiac pressures at rest or during stress.56 The prevalence of HF is approximately 1–2% of the adult population in developed countries, rising to 10% among people >70 years of age, where it represents the leading cause of hospitalisation.57 Anaemia is a common co-morbidity in HF patients, and it is associated with worse long-term outcomes.58 In HF clinical trials and registries, the prevalence of anaemia ranges from 15% to 70% among hospitalised patients.56,59 The physiologic response to anaemia is a compensatory increase in cardiac output in order to maintain adequate oxygen delivery, with a decrease in myocardial contractility when the haemoglobin level is below 7 g/dL.60 Left ventricle hypertrophy and dilation have been observed in animal and human models of severe anaemia.61 The mechanisms by which anaemia worsens HF outcome are not fully understood. They may be related to increased myocardial workload due to hemodynamic, neurohormonal, and pro-inflammatory alterations finally leading to left ventricle remodelling.62 Absolute or functional iron deficiency, inappropriate erythropoietin production, and depressed bone marrow function are common cofactors leading to anaemia in HF patients. Dysregulation of molecules involved in these pathways is critical for the transition from adaptive cardiac hypertrophy to cardiac remodelling, as represented in Figure 5.
Figure 5.
The mechanisms that lead to HF in anaemic patient.
Echocardiographic alterations in anaemic patients have been studied, in particular, related to the left ventricle function.63–66 The presence of anaemia is associated with diastolic dysfunction (alteration in peak mitral early diastolic, E velocity, and peak mitral late diastolic, A velocity, E/A ratio, which reflects the increase of left ventricular filling pressure), increased left ventricle mass index and diameter, increased left atrium volume index, and higher systolic pulmonary artery pressure estimated by tricuspid Doppler. The correction of anaemia often results in the improvement of echocardiographic parameters.63–66
Cardiac imaging, particularly trans-thoracic echocardiography, plays an essential role in the baseline assessment and serial follow- up of oncologic patients, in which anaemia is a common feature. This ultrasound technique is part of the relatively new discipline of cardio-oncology, intending to prevent and monitor cardiovascular complications resulting from cancer treatment.67 Cardiotoxicity is defined as a decrease in left ventricle ejection fraction greater than 10% to a value of less than 53%, confirmed on repeated imaging at 2–3 weeks from the initial evaluation.68 Current guidelines68 recommend a standardised cardio-oncology echocardiographic protocol with the description of traditional approach and the introduction of more advanced modalities, like the assessment of global longitudinal strain as an early a marker of left ventricle dysfunction.
The role of ultrasound in HF has been well established also in the routine assessment of non-oncologic patients.
Echocardiography has a prominent role, but other ultrasound techniques have been proposed for a complete evaluation, as recently proposed.69 A five-step ultrasound examination (“ABCDE”) to evaluate and monitor HF patients may include the evaluations of the Ankle-brachial index (A), B-lines (B), the Carotid intima-media thickness (C), the Diameters of the abdominal aorta and of the inferior cave vein (D), and the Echocardiographic assessment of the ejection fraction (E). All these parameters may be helpful in bedside monitoring of recovery after acute heart decompensation, as well as for a global cardiovascular assessment tool in HF patients.69
Conclusions
Ultrasonography is a safe and effective imaging tool that has to be considered when facing patients with anaemia. Anaemia, especially in the elderly, is often multifactorial,22 and defining the prominent cause(s) can be difficult. However, ultrasound may be a useful diagnostic method to address the right diagnosis.
Future challenges include standardisation of the training process for physicians (other than Radiologists) to ensure the appropriate use of this technology, and a structuring policy to promote its effectiveness.
Educational strategies for increasing competency on the point of care ultrasound (that is ultrasonography performed and interpreted by the clinician at the bedside) represent the goal of several ultrasound societies worldwide. Indeed, both EFSUMB and WFUMB (World Federation of Ultrasound in Medicine and Biology) have developed periodically up-dated guidelines on the minimum training requirements that should be achieved at each level of practice in order to perform examinations according to the operator’s capacity (from point of care/focused ultrasound to conventional ultrasound and specialized application of the technique).70
Footnotes
Competing interests: The authors declare no conflict of Interest.
References
- 1.World Health Organization (WHO) Vitamin and mineral nutrition system. Geneva: WHO; 2011. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. [Google Scholar]
- 2.Platt A, Eckman J. Diagnosing anaemia. Rev Clin. 2006;16(2):44–50. [Google Scholar]
- 3.Camaschella C. Iron deficiency anaemia. N Engl J Med. 2015;372:1832–1843. doi: 10.1056/NEJMra1401038. [DOI] [PubMed] [Google Scholar]
- 4.Camaschella C. Iron deficiency Blood. 2019;133(1):30–39. doi: 10.1182/blood-2018-05-815944. [DOI] [PubMed] [Google Scholar]
- 5.Long B, Koyhman A. Emergency medicine: evaluation and management of anaemia. Emerg Med Clin Am. 2018;36:609–630. doi: 10.1016/j.emc.2018.04.009. [DOI] [PubMed] [Google Scholar]
- 6.Gikas A, Triantafillidis JK. The role of primary care physicians in early diagnosis and treatment of chronic gastrointestinal diseases. Int J Gen Med. 2014;7:159–173. doi: 10.2147/IJGM.S58888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gandhi SJ, Hagens I, Nathan K, Hunter K, Roy S. Prevalence, comorbidity and investigation of anaemia in the primary care office. J Clin Med Res. 2017;9:970–980. doi: 10.14740/jocmr3221w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levi M, Rosselli M, Simonetti M, Brignoli O, Cancian M, et al. Epidemiology of iron deficiency anaemia in four European countries: a population-based study in primary care. Eur J Haematol. 2016;97:583–593. doi: 10.1111/ejh.12776. [DOI] [PubMed] [Google Scholar]
- 9.Scalea TM, Rodriguez A, Chiu YC, Brenneman FD, Fallon WF, et al. Focused assessment with sonography for trauma (FAST): results from an international consensus conference. J Trauma. 1999;46:466–472. doi: 10.1097/00005373-199903000-00022. [DOI] [PubMed] [Google Scholar]
- 10.Rozycki GS, Newman PG. Surgeon-performed ultrasound for the assessment of abdominal injuries. Adv Surg. 1999;33:243–259. [PubMed] [Google Scholar]
- 11.Stengel D, Bauwens K, Rademaker G, Ekkernkamp A, Guthoff G. Emergency ultrasound-based algorithms for diagnosing blunt abdominal trauma. Cochrane Database Sys Rev. 2013;7:CD004446. doi: 10.1002/14651858.CD004446.pub3. [DOI] [PubMed] [Google Scholar]
- 12.Lyon M, Blaivas M, Brannam L. Sonographic measurement of the inferior vena cava as a marker of blood loss. Am J Emerg Med. 2005;23:45–50. doi: 10.1016/j.ajem.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 13.Segni Di E, Preisman S, Ohad DG. Echocardiographic left ventricular remodelling and pseudohypertrophy as markers of hypovolemia. An experimental study on bleeding and volume repletion. J Am Soc Echocardiogr. 1997;10:926–936. doi: 10.1016/S0894-7317(97)80009-0. [DOI] [PubMed] [Google Scholar]
- 14.Yanagawa Y, Nishi K, Sakamoto T, Okada Y. Early diagnosis of hypovolemic shock by sonographic measurement of inferior vena cava in trauma patients. J Trauma. 2005;58:825–829. doi: 10.1097/01.TA.0000145085.42116.A7. [DOI] [PubMed] [Google Scholar]
- 15.Yanagawa Y, Sakamoto T, Okada Y. Hypovolemic shock evaluated by sonographic measurement of the inferior vena cava during resuscitation in trauma patients. J Trauma. 2007;63:1245–1248. doi: 10.1097/TA.0b013e318068d72b. [DOI] [PubMed] [Google Scholar]
- 16.Carr BG, Dean AJ, Everett WW. intensive bedside ultrasound (INBU) for volume assessment in the intensive care unit: a pilot study. J Trauma. 2007;63:495–500. doi: 10.1097/TA.0b013e31812e51e5. [DOI] [PubMed] [Google Scholar]
- 17.Tung Chen Y, Blancas Gomez-Casero R, Quintana Diaz M, Diaz HB. Inspiratory collapse of the inferior vena cava and the kissing ventricle sign: markers of poor prognosis in emergency gastrointestinal bleeding. Emergencias. 2019;31:79–85. [PubMed] [Google Scholar]
- 18.Kim BSM, Li BT, Engel A, Samza JS, Clarke S. Diagnosis of gastrointestinal bleeding: a practical guide for clinicians. World J Gastrointest Pathophysiol. 2014;15:467–478. doi: 10.4291/wjgp.v5.i4.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guralnik JM, Eisenstaedt RS, Ferucci L, Klein HG, Woodman RC. Prevalence of anaemia in persons 65 years older in the United States: evidence for a high rate of unexplained anaemia. Blood. 2004;104:2263–2268. doi: 10.1182/blood-2004-05-1812. [DOI] [PubMed] [Google Scholar]
- 20.Busti F, Marchi G, Ugolini S, Castagna A, Girelli D. Anaemia and iron deficiency in cancer patients: role of iron replacement therapy. Pharmaceuticals. 2018;11:94–108. doi: 10.3390/ph11040094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nathavithrana RL. Anaemia is highly prevalent among unselected internal medicine inpatients and is associated with increased mortality, earlier readmission and more prolonged hospital stay: an observational retrospective cohort study. Intern Med J. 2014;42:683–691. doi: 10.1111/j.1445-5994.2011.02566.x. [DOI] [PubMed] [Google Scholar]
- 22.Girelli D, Marchi G, Camaschella C. Anemia in the elderly. HemaSphere. 2018;2:pe40. doi: 10.1097/HS9.0000000000000040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Riva E, Colombo R, Moreo G, Mandelli S, Franchi C, et al. Prognostic value of degree and types of anaemia on clinical outcomes for hopitalized patients. Arch Gerontol Geriatr. 2017;69:21–30. doi: 10.1016/j.archger.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 24.Muradali D, Goldberg DR. US of gastrointestinal tract disease. Radiographics. 2015;35:50–68. doi: 10.1148/rg.351140003. [DOI] [PubMed] [Google Scholar]
- 25.Havre R, Giljs OH. Elastography and strain rate imaging of the gastrointestinal tract. Eur J Radiol. 2014;83:438–441. doi: 10.1016/j.ejrad.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 26.Gomollón F, Dignass A, Annese V., 3rd European Evidence-based Consensus on the Diagnosis and Management of Crohn’s Disease 2016: Part 1: Diagnosis and Medical Management. J Crohn Colitis. 2017;11:3–25. doi: 10.1093/ecco-jcc/jjw168. [DOI] [PubMed] [Google Scholar]
- 27.Conti CB, Giunta M, Gridavilla D, Conte D, Fraquelli M. Role of bowel ultrasound in the diagnosis and follow-up of patients with Crohn’s disease. Ultrasound in Med and Biol. 2017;43:725–734. doi: 10.1016/j.ultrasmedbio.2016.12.014. [DOI] [PubMed] [Google Scholar]
- 28.Migaleddu V, Quaia E, Scano D, Virgilio G. Inflammatory activity in Crohn’s disease: ultrasound findings. Abdom Imaging. 2008;33:589–597. doi: 10.1007/s00261-007-9340-z. [DOI] [PubMed] [Google Scholar]
- 29.Biernacka CB, Baranska D, Grzelak P, Czkwianaianc E, Szabelska K. Up-to-date overview of imaging techniques in the diagnosis and management of inflammatory bowel diseases. Gastroenterology Rev. 2019;14:19–25. doi: 10.5114/pg.2019.83423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Imsirovic B, Zarem E, Gusa E, Djedovic M, Cengic A, et al. Comparison of conventional ultrasound and contrast enhanced magnetic resonance (MR) enterography in evaluation of patients with Cronh’s disease. Acta Inform Med. 2018;26:93–97. doi: 10.5455/aim.2018.26.93-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Biernacka CB, Baranska D, Grzelak P, Czkwianaianc E, Szabelska K. Up-to-date overview of imaging techniques in the diagnosis and management of inflammatory bowel diseases. Gastroenterology Rev. 2019;14:19–25. doi: 10.5114/pg.2019.83423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Branchi F, Caprioli F, Orlando S, Conte D, Fraquelli M. Non-invasive evaluation of intestinal disorders: the role of elastographic techniques. World J Gastroenterol. 2017;23:2832–2840. doi: 10.3748/wjg.v23.i16.2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roy CN. Anaemia of inflammation. Haematology Am Soc Hematol Educ Program. 2010:276–280. doi: 10.1182/asheducation-2010.1.276. [DOI] [PubMed] [Google Scholar]
- 34.Kario K, Matsuo T, Nakao K. Serum erythropoietin levels in the elderly. Gerontology. 1991;37:345–348. doi: 10.1159/000213283. [DOI] [PubMed] [Google Scholar]
- 35.Bruunsgaard H, Pedersen BK. Age-related inflammatory cytokines and disease. Immunol Allergy Clin North Am. 2003;23:15–39. doi: 10.1159/000213283. [DOI] [PubMed] [Google Scholar]
- 36.Martinez-Ares DS, Barrenechea I, Souto-Rouzo J, Yanez-Lopez J, Pallares Peral A, Vazquez-Iglesias JL. The value of abdominal ultrasound in the diagnosis of colon cancer. Rev Exp Enferm Dig. 2005;97:877–886. doi: 10.4321/S1130-01082005001200004. [DOI] [PubMed] [Google Scholar]
- 37.Rutgeerts LJ, Vernbanck JJ, Carpe AW, Buyse BM, Ghillebert GL. Detection of colorectal cancer by routine ultrasound. J Belge Radiol. 1991;74:11–13. [PubMed] [Google Scholar]
- 38.Chang ST, Menias CO. Imaging of primary gastrointestinal lymphoma. Semin Ultrasound. 2013;34:558–65. doi: 10.1053/j.sult.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 39.Annese V, Beaugerie L, Egan L, et al. European Evidence-based Consensus: Inflammatory Bowel Disease and Malignancies. J Crohns Colitis. 2015;9:945–65. doi: 10.1093/ecco-jcc/jjv141. [DOI] [PubMed] [Google Scholar]
- 40.Elli L, Norsa L, Zullo A, et al. Diagnosis of chronic anaemia in gastrointestinal disorders: A guideline by the Italian Association of Hospital Gastroenterologists and Endoscopists (AIGO) and the Italian Society of Paediatric Gastroenterology Hepatology and Nutrition (SIGENP) Dig Liver Dis. 2019;51:471–483. doi: 10.1016/j.dld.2019.01.022. [DOI] [PubMed] [Google Scholar]
- 41.Nylund K, Maconi G, Hollerweger A, et al. EFSUMB Recommendations and Guidelines for Gastrointestinal Ultrasound - Part 1: Examination Techniques and Normal Findings (Long version) Ultraschall in Med. 2017;38:e1–e15. doi: 10.1055/s-0042-115853. [DOI] [PubMed] [Google Scholar]
- 42.Soresi M, Mansueto P, Terranova A. Abdominal Ultrasound Does Not Reveal Significant Alterations in Patients With Nonceliac Wheat Sensitivity. J Clin Gastroenterol. 2019;53:e31–e36. doi: 10.1097/MCG.0000000000000969. [DOI] [PubMed] [Google Scholar]
- 43.Soresi M, Pirrone G, Giannitrapani L. A key role for abdominal ultrasound examination in “difficult” diagnoses of celiac disease. Ultraschall Med. 2011;32:S53–61. doi: 10.1055/s-0028-1110009. [DOI] [PubMed] [Google Scholar]
- 44.Frulloni L, Falconi M, Gabbrielli A. Italian consensus guidelines for chronic pancreatitis. Dig Liver Dis. 2010;42:S381–406. doi: 10.1016/S1590-8658(10)60682-2. [DOI] [PubMed] [Google Scholar]
- 45.Breymann C, Auerbarch M. Iron deficiency in gynaecology and obstetrics: clinical implications and management. Hematology. 2017;1:152–159. doi: 10.1182/asheducation-2017.1.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Murugandoss N, Coyle N, Dotta S. Ultrasound in obstetrics and gynaecology. Obstetrics, Gynaecology and Reproductive Medicine. 2018;29:42–50. doi: 10.1016/j.ogrm.2018.12.009. [DOI] [Google Scholar]
- 47.Woodfield CA. The usefulness of ultrasound imaging in gynaecologic oncology. PET Clin. 2018;13:143–163. doi: 10.1016/j.cpet.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 48.Iraha Y, Okada M, Iraha R, Azama K, Yamashiro T. CT and MR imaging of gynaecologic emergencies. Radiographics. 2017;37:1569–1586. doi: 10.1148/rg.2017160170. [DOI] [PubMed] [Google Scholar]
- 49.Chiorean L, Cui XW, Kleen SA. Clinical value of imaging for lymph nodes evaluation with particular emphasis on ultrasonography. Z Gastroenterol. 2016;54:774–790. doi: 10.1055/s-0042-108656. [DOI] [PubMed] [Google Scholar]
- 50.Sharma A, Fidias P, Hayman LA, Loonis SL, Taber KH, et al. Patterns of lymphoadenopathy in thoracic malignancies. Radiographics. 2004;24:419–434. doi: 10.1148/rg.242035075. [DOI] [PubMed] [Google Scholar]
- 51.Vassallo P, Wernecke K, Roos N, Peters PE. Differentiation of benign from malignant superficial lymphadenopathy: the role of high resolution US. Radiology. 1992;183:215–220. doi: 10.1148/radiology.183.1.1549675. [DOI] [PubMed] [Google Scholar]
- 52.Yu M, Liu Q, Song HP, Han ZH, Su HL, et al. Clinical application of contrast enhanced ultrasonography in diagnosis of superficial lymphadenopathy. J Ultrasound Med. 2010;29:735–740. doi: 10.7863/jum.2010.29.5.735. [DOI] [PubMed] [Google Scholar]
- 53.Dietrich CF. Elastography applications. Endo Heute. 2011;24:177–212. doi: 10.1055/s-0030-1262579. [DOI] [Google Scholar]
- 54.Bachmann-Nielsen M, Saftoiou A. Elastography, true or false? Ultraschall Med. 2011;32:5–7. doi: 10.1055/s-0029-1246008. [DOI] [PubMed] [Google Scholar]
- 55.Trenker C, Gorg C, Jenssen C, Klein S, Neubauer A, et al. The role of abdominal ultrasound in haematological diseases. Z Gastroenterol. 2018;56:1063–1076. doi: 10.1055/a-0603-4240. [DOI] [PubMed] [Google Scholar]
- 56.The task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2016;37:2129–2200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 57.Maggioni AP. EUR observational Research Programme. The heart failure pilot survey (ESH-HF Pilot) Eur J Heart Fail. 2010;12(10):1076–1084. doi: 10.1093/eurjhf/hfq154. [DOI] [PubMed] [Google Scholar]
- 58.Tang YD, Katz SD. The prevalence of anemia in chronic heart failure and its impact on clinical outcomes. Heart Fail Rev. 2008;13:387–392. doi: 10.1007/s10741-008-9089-7. [DOI] [PubMed] [Google Scholar]
- 59.Amand IS. Anaemia and chronic heart failure. J Am Coll Cardiol. 2008;52:501–511. doi: 10.1016/j.jacc.2008.04.044. [DOI] [PubMed] [Google Scholar]
- 60.Hegde N, Rich MW, Gayomali C. The cardiomiopathy of iron deficiency. Tex Heart Inst J. 2006;33:340–344. [PMC free article] [PubMed] [Google Scholar]
- 61.Datta BN, Silver MD. Cardiomegaly in chronic anaemia in rats and man experimental study including ultrastructural histometric and stereologic observations. Lab Invest. 1975;32:503–514. doi: 10.1080/00033797500200421. [DOI] [PubMed] [Google Scholar]
- 62.Yoo RK, Wook BP, Gil JS. Relation of anaemia to echocardiographically estimated left ventricular filling pressure in hypertensive patients over 50 years old. J Cardiovasc Ultrasound. 2010;18:56–90. doi: 10.4250/jcu.2010.18.3.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tobili JE, Di Gennaro F, Rivas C. Changes in echocardiographic parameters in iron deficiency patients with heart failure and chronic kidney disease treated with intravenous iron. Heart Lung Circ. 2015;24:686–695. doi: 10.1016/j.hlc.2014.12.161. [DOI] [PubMed] [Google Scholar]
- 64.Sulti-Vega M, Rizzo M, Martinez-Pablo A. Anaemia and iron deficiency in heart failure: a review of echocardiographic features. Echocardiography. 2019 doi: 10.1111/echo.14271. [DOI] [PubMed] [Google Scholar]
- 65.Shen J, Zhou Q, Liu Y, Runlan L, Li T. Evaluation of left atrial function in patients with iron-deficiency anaemia by two-dimensional speckle tracking echocardiography. Cardiovasc Ultrasound. 2016;14:34–43. doi: 10.1186/s12947-016-0078-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.In-Jeong C, Yeung CM, Ki HK, Gil JS. Effect of anaemia correction on left ventricular structure and filling pressure in anaemic patients without overt heart disease. Korean J Intern Med. 2014;29:445–453. doi: 10.3904/kjim.2014.29.4.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Venneri L, Zoppellaro G, Khattar RS. Cardio-oncology: the role of advanced echocardiography in cancer patients. Expert Rev of Cardiovasc Ther. 2018;16:249–258. doi: 10.1080/14779072.2018.1443394. [DOI] [PubMed] [Google Scholar]
- 68.Plana JC, Galderisi M, Barac A. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy. A report form the American Society of Echocardiography and the European association of Cardiovascular imaging. Eur Heart J Cardiovasc Imaging. 2014;15:1063–1093. doi: 10.1093/ehjci/jeu192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mozzini C, Cominacini L, Casadei A, Schiavone C, Soresi M. Ultrasonography in heart failure: a story that matters. Curr Probl Cardiol. 2019;44:116–136. doi: 10.1016/j.cpcardiol.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 70.Dietrich CF, Hoffmann B, Cantisani V, et al. Medical student ultrasound education: A WFUMB position paper, Part I. Ultrasound Med Biol. 2019;45:271–281. doi: 10.1016/j.ultrasmedbio.2018.09.017. [DOI] [PubMed] [Google Scholar]