Abstract
Background:
Congenital muscular dystrophy (CMD) is a rare, inherited neuromuscular disease characterized by progressive muscle weakness, thoracic insufficiency and ultimately respiratory failure. Adherence to respiratory therapies in children with neuromuscular disorders is unknown. This study examined the multimodal assessment of adherence and barriers to 15 minutes, twice daily hyperinsufflation in children with CMD. Adherence was hypothesized to be greater than 50% and discomfort, embarrassment, and difficulty finding time were hypothesized to be barriers.
Methods:
Participants included 18 children with CMD. Personalized hyperinsufflation settings were determined based on pressure-volume measurements at each study visit. Adherence was measured by a daily phone diary (DPD) and by electronic data download from the hyperinsufflation device. The DPD was conducted twice over a 48-hour period to capture a weekend and weekday, with the goal being 60 minutes of hyperinsufflation over the 48 hours (100% adherence). The hyperinsufflation objective electronicdata reflected daily use of hyperinsufflation for the same 48 hour period. Data from DPD and the corresponding hyperinsufflation device data were used for analyses.
Results:
Adherence to hyperinsufflation was 40% via DPD and 44% for electronic data, with strong convergence between methods (r=0.75, p<.001). Surprisingly, 53% of participants reported no barriers despite low adherence Social distractions and family obligations were identified as barriers. There were no differences in adherence between those who did and did not endorse barriers to hyperinsufflation (DPD: t(13)=0.44, p=n.s.; hyperinsufflation device: t(13)=−0.23, p=n.s.)
Conclusion:
Adherence to hyperinsufflation is a significant problem in children with CMD and families have difficulty identifying adherence barriers. An important next step is to encourage open dialogue around adherence barriers and promote adherence behaviors via intervention.
Keywords: CMD, hyperinsufflation, cough assist, pulmonary function, restrictive lung disease, children, facilitators
Introduction
Congenital muscular dystrophy (CMD) is a rare, inherited neuromuscular disease that comprises heterogeneous subgroups. CMD manifests clinically by early onset progressive muscle weakness that presents from birth to up to two years of age1. Prevalence estimates indicate CMD affects about one in 100,000 children1. Of the multiple subtypes of CMD, collagen VI-deficiency and mutations in the laminin-2 (LAMA2) gene leading to merosen deficiency are the most common subtypes in countries with populations of European descent1. CMD secondary to collagen VI-deficiency consists of three clinical phenotypes; Ullrich, intermediate and Bethlem myopathies. The Ullrich type presents early in life and follows a rapidly progressive course while the Bethlem type presents in adults, progressing at a much slower rate. Fifty percent of ambulating children with Ullrich CMD become wheelchair dependent and lose more than 50% of their vital capacity by 10 years of age2,3. Similarly, recent data on the natural history of Ullrich CMD reported that by age 11 years, 50% require nocturnal noninvasive ventilation3. The subtype of CMD due to LAMA2 follows the same clinical course as Ullrich CMD4. These children also have progressive respiratory failure that begins in the first decade of life. The rapid decline in lung function in both subtypes is believed to be in part due to joint contractures of the chest wall and thoracic cage deformity, which develop over time as a result of inherent muscle weakness.
Therapies that inflate the lungs to near total lung capacity, or lung recruitment, are routinely used5 for neuromuscular disease (NMD), such as Duchenne muscular dystrophy6,7. Personalized hyperinsufflation protocols customize inflation pressures based on objective criteria, such as achieving a specific, targeted increase in the patient’s lung volume. While hyperinsufflation may be a promising treatment approach for children with CMD, the treatment can only work if patients are adherent. For comparable treatments in other pediatric pulmonary conditions (e.g. high frequency chest wall oscillation), adherence rates are suboptimal8–15. There are multiple measures of adherence, including self-report, diary, pharmacy refill histories, and electronic monitors16. Two of the most objective measures include electronic monitoring and diary methods15,17. The Daily Phone Diary (DPD) is a phone-based diary that provides information on both adherence and barriers. It is a reliable and valid tool that has been used in CF, asthma, HIV, and epilepsy15–22. Importantly, the DPD is highly correlated with electronic monitoring15,17. Assessing adherence to hyperinsufflation is novel in this population.
Identifying the unique barriers that children and families with CMD face when engaging in hyperinsufflation is critical to improving adherence in the future. Barriers and adherence to therapies in children with NMD are poorly described, with the exception of a recent study by Ennis and colleagues that reported adherence to NIV in five obese children and four children with NMD8. Potential barriers identified in the general adherence literature related to similar devices, include the following issues: physical mask discomfort, not using while away from home, forgetting, the child not feeling well, the child needing help with the device, and embarrassment8,11.
The primary aim of this study was to identify rates of adherence and barriers to hyperinsufflation treatment in children with CMD. Based on anecdotal experience, it was hypothesized that hyperinsufflation use, referred to as adherence, would be greater than 50% of the prescribed regimen. We also hypothesized that discomfort, embarrassment and difficulty incorporating the treatment into a busy family schedule would be the primary obstacles to adherence. Finally, given the novelty of the treatment and lack of literature on adherence in children with NMD, we wanted to compare the adherence measurement tools. It was hypothesized that the electronic monitoring data and DPD would be highly correlated.
Methods
Participants were part of a multi-site randomized controlled clinical trial (RCT) aimed at testing the efficacy of individualized hyperinsufflation therapy in children with CMD (National Institutes of Health- National Heart, Lung, and Blood Institute; R34HL113390; ). Participants were recruited using two different methods: 1) neurology and pulmonary clinics at Cincinnati Children’s Hospital (CCHMC) and Children’s Hospital of Philadelphia (CHOP) and 2) an international registry from the Cure Congenital Muscular Dystrophy Foundation (Cure CMD-IR) of patients. Inclusion and exclusion criteria included: 1) youth between the ages of five and 21 years, 2) forced vital capacity of ≥ 30 and ≤ 80% predicted within the past 18 months (highest vital capacity within the past 12 months was used) based on acceptable pulmonary function according to the ATS criteria23, 3) confirmed collagen type VI-related CMD or LAMA2-related muscular dystrophy by clinical history and muscle/skin biopsy or gene mutation, 4) absence of other chronic medical conditions, 5) no tracheostomy, and 6) no daytime ventilator support. Patients were excluded from the study if they 1) had a tracheostomy, 2) were on continuous ventilation, 3) did not comply with study protocols, 4) had difficulty tolerating the intervention or 5) voluntarily withdrew.
Procedure:
The study was approved by the Institutional Review Board at each institution and monitored by a data safety monitoring board with a safety officer. The recruitment of patients came largely from Cure CMD-IR. IRB approved postings were also placed in printed and electronic publications frequently reviewed by the muscular dystrophy communities such as MDA Quest magazine, ClinicalTrials.gov (), and CureCMD websites. Patients meeting acceptability criteria were initially consented by telephone and then recruited to any of the two participating centers by their preference. Formal consent and assent was performed at the first study visit.
Measures
DPD:
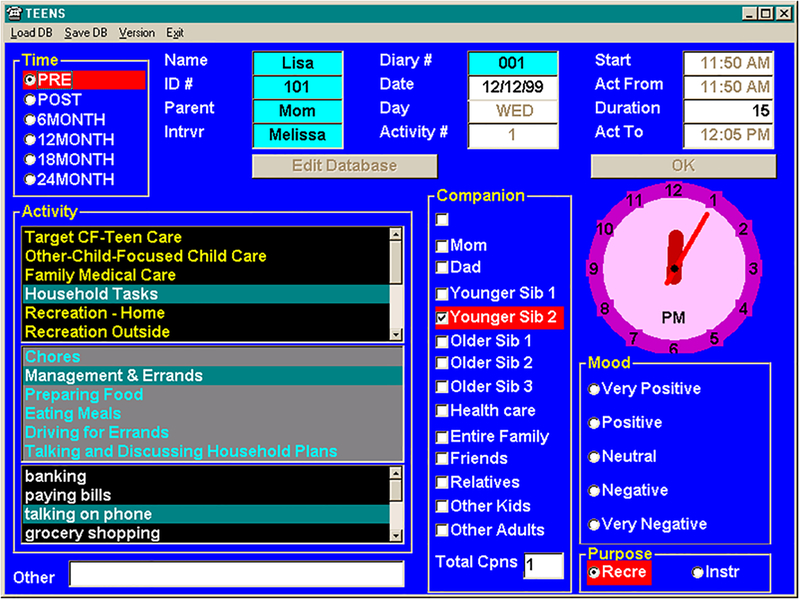
The DPD is an evidence-based measure of adherence that has been used in chronic pulmonary diseases, including asthma and cystic fibrosis15,16,18,22, and has high correlations with electronic adherence measures15–17 The DPD is a telephone-based diary that utilizes cued recall to record all activities over the prior 24 hours by a trained interviewer. Interviewers are able to reconstruct the previous 24 hours in a nonbiased manner by asking, ‘after you ate breakfast, what did you do next?’, for example. Only activities lasting five minutes or longer were included. As shown in figure 1, the DPD software allows interviewers to record the type of activity (i.e. grocery shopping, paying bills, preparing food, etc.), duration, number of companions, and rating of mood (Likert scale ranging from 1= extremely negative to 5=extremely positive). The DPD minimizes bias, social desirability, and reduces memory recall problems, which in turn increases accuracy of the data24. While many activities were coded for the DPD, only the hyperinsufflation codes were pulled for data analyses.
Figure 1.
Screenshot of the daily phone diary.
Barriers and Treatment Follow-Up Questions:
Hyperinsufflation:
Hyperinsufflation treatment was provided using the CoughAssist®T70 (CA) that is traditionally used to support the cough effort for patients unable to adequately clear airway secretions. The machine delivers a positive inhalation pressure followed by a rapid negative exhalation pressure to augment the patient’s spontaneous cough effort, thereby facilitating clearance of secretions from lower airways into the oral cavity. For the purposes of the study, only inspiratory pressure was used to administer hyperinsufflation. Personalized settings were determined at the first study visit. The CA was connected inline to a pneumotachometer (Series 4700, Hans Rudolph Inc., Shawnee, KS) and full face mask. The mask was applied tightly to the subject’s face thus allowing the measurement of insufflation pressures and volumes. The insufflation was performed using an initial insufflation pressure of 10 cm H2O for 3 seconds with a passive exhalation. Five acceptable insufflations without obvious leaks were performed. Pressure was increased incrementally by 5 cm H2O pressure as long as the exhaled volume increased by ≥ 10% at each step. The final pressure was identified when the volume change was < 10%, of the volume measured at the previous pressure and was well tolerated by the patient. In case the pressure was uncomfortable to the patient, the highest tolerated lower pressure was chosen. Ultimately, the individualized settings consisted of the determined pressure with inspiratory time 3 seconds, expiratory time 0 seconds, and a total of 5 to 9 maneuvers per minute. Cough-Trak, a function that synchronizes the machine with a patient’s breath, was turned off to ensure a standard number of maneuvers were administered.
Outcome Measures
Demographics and medical variables were obtained by parent report and chart review, including age, gender, diagnosis, race, and forced vital capacity. All participants were trained in hyperinsufflation therapy and were asked to use the hyperinsufflation device twice daily for 15 minutes per session. This regimen was suggested by an affected family, taking into consideration the potential for adding to the existing burden of care. The DPD was conducted four weeks following initiation of hyperinsufflation treatment. The DPD was performed by a single trained interviewer from CCHMC for all study participants. The DPD was conducted on two consecutive days, with one being a weekend and one a weekday. Perfect (100%) adherence was defined as 60 minutes of hyperinsufflation (15 minute sessions twice daily for two days equals 60 total minutes). Due to the inherent constraints of the DPD, the date and time of the DPD phone call was agreed upon by the interviewer and the family in advance to optimize successful data acquisition.
Participants were asked to bring back their hyperinsufflation device for electronic adherence data download at each clinic visit. Our primary outcome measure, being adherence rate, was obtained by analyzing electronic data download as well as the DPD. Our secondary outcome measure was to assess the convergence between adherence modalities; electronic download and the DPD.
Following the DPD, caregivers were asked a series of questions related to the hyperinsufflation device including the burden of using it, how difficult it was to remember to do the treatment, and how difficult it was to find time to do the treatment. A Likert scale was used to assess these constructs (1=Not at all, 2=A bit, 3=Moderately, 4=Quite, 5=Extremely). Regarding specific barriers to hyperinsufflation, caregivers were asked the following: “There are a lot of things that can get in the way of doing treatment. What most got in the way of your child doing hyperinsufflation treatment in the last 24 hours?” Caregivers then provided open-ended responses to this question. If no barriers were identified, the interviewer entered “none” into the program.
Statistical Analysis:
Adherence was calculated based on the total duration of hyperinsufflation performed over the two-day period that encompassed the DPD. Percent adherence was calculated using the number of minutes of hyperinsufflation performed divided by 60 minutes (15 minutes, twice daily over two days). The electronic adherence data was acquired from the hyperinsufflation device that has a memory card that stores data including pressure, volume, flow and duration of use. These data were analyzed to determine adherence to hyperinsufflation treatment over the corresponding 48 hours recorded by the DPD.
Descriptive data were obtained for all demographic, medical, and adherence variables. Chi-square and t-tests were used to examine differences between the control and treatment groups for race, age, gender, and PFT data. A paired t-test and paired correlation tested convergence between the DPD and electronically-monitored hyperinsufflation device data to elicit any potential differences between measurement modalities. Frequencies of barriers and the mean number of barriers identified were also calculated. Finally, a t-test was used to compare adherence rates between participants who reported one or more barriers, and those who did not. SPSS (version 23) was used for statistical analysis with significance considered as p<0.05.
RESULTS
Participants
Eighteen patients were prescribed hyperinsufflation; however, two participants were withdrawn prior to completion of the DPD due to inability to comply with study requirements and one participant was unable to be reached for the DPD. Participants were 11.2 years of age on average (SD=3.7 years), 53% were males, and 73% were Caucasian (13% Asian, 13% biracial). Mean forced vita capacity (% reference) was 54.3 (SD=14.6) and they were categorized with the following restrictive lung disease severity: 20% mild, 13% moderate, 20% moderate-severe, 47% severe, and 0% very severe.
Adherence
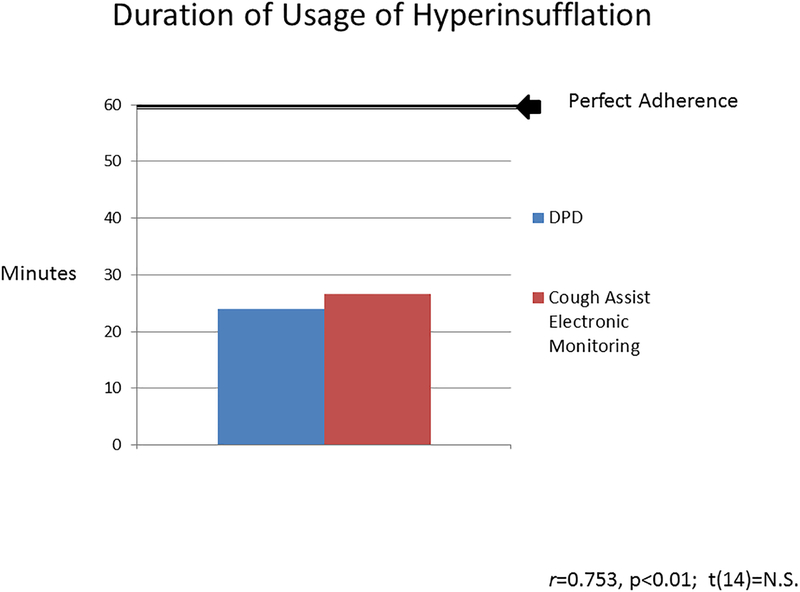
As shown in Figure 2, hyperinsufflation adherence as measured by the DPD was approximately 24.0 ± 20.6 minutes, which significantly positively correlated (r = 0.75, p ≤ 0.01) with hyperinsufflation electronic monitoring data of 26.6 ± 23.3 minutes. Specifically, adherence rates via the DPD were 40.0% and 44.3% for the hyperinsufflation device and were not statistically different (t(14) = −0.64, p = n.s.). The range of adherence by hyperinsufflation device was 20% of patients with no use, 13% with about 50% use, and 27% with full use. Adherence among those who experienced at least one barrier was statistically similar to those who endorsed no barriers by both the DPD (t(13) = 0.44; p=n.s) and the hyperinsufflation device (t(13) = −0.23; p=n.s).
Figure 2.
Duration of usage of hyperinsufflation as measured by modality: daily phone diary and hyperinsufflation device.
Barriers
Fifty-three percent (n=8) of families reported no barriers, while 33% (n=5) of families reported experiencing one barrier, and 13% of (n=2) of families reported experiencing two barriers over the 48 hour DPD period. If a family reported the same barrier on both days, it was only counted once. The average number of barriers reported was 0.6 ± 0.74. Of the barriers that were identified, families reported social distractions (22.2%), family obligations (22.2%), forgetting (11.1%), embarrassment (11.1%), side effect of treatment – makes patient tired (11.1%), travelling (11.1%), and acute illness (11.1%).
Other Treatment-Related Factors
The average mood of caregivers while performing hyperinsufflation was 3.5 ± 0.59 with three and four representing ‘neutral’ and ‘positive,’ respectively. The average burden was 1.81 ± 1.1, average difficulty remembering was 1.6 ± 1.1, and the average difficulty finding time was 1.5 ± 1.1, suggesting minimal difficulties/burden. Most commonly, 60% of participants had their parent deliver the treatment, 26.7% of participants did it themselves, and 13.3% had another caregiver deliver the intervention.
Discussion
Overall, this study demonstrated a high concordance between the DPD and electronic monitoring, which suggests they can both be used, depending on the purpose. For example, if the hyperinsufflation device data can be easily extracted and interpreted during a clinic visit, these data can be used for clinical decision-making. In contrast, if a researcher wants to understand how children with CMD fit treatments into their daily lives and barriers to adherence, the DPD may be a better adherence measure. Contrary to our hypothesis, adherence was less than 50% with mean adherence rates between 40–44%.
The measured adherence rate is consistent with therapies focused on the airways, including nebulized treatments, airway clearance, and CPAP for other pulmonary conditions25. In children with cystic fibrosis, adherence to a passive airway clearance therapy and nebulized medications is quite variable but similar to our study results12–15. Even a novel treatment with a significant impact on disease, such as Ivacaftor in cystic fibrosis, has low adherence at 61%26 despite marked improvement in lung function, quality of life, and nutritional status for those who qualify for the medication27–29. Thus, it is not surprising that patients and their families have difficulty adhering to complex treatment regimens15. Although it was hypothesized that hyperinsufflation could slow the progression of disease for children with CMD, our findings suggest that adherence is difficult and that there may be various barriers that interfere with families’ abilities to reliably implement this treatment at home.
Caregivers appeared to have difficulty identifying barriers to adherence soon after treatment initiation, with 53% reporting none. This could be for several potential reasons. First, caregiver’s may have wanted to appear as if they were doing well and reluctant to disclose barriers (i.e, social desirability) or they may have had difficulty articulating barriers. Alternatively, the parent may have lacked insight about barriers or been unaware of patient-specific barriers that could have influenced adherence, such as mask discomfort. Finally, the way barriers were elicited from the interview was open-ended and perhaps giving the parents potential barriers to choose from instead of free-response may have elicited more barriers. Interestingly, the degree of burden experienced by families was not reported to be substantial and they had little difficulty finding time to perform hyperinsufflation. Although barriers were not endorsed by half of the families, this did not appear to influence adherence rates. This is inconsistent with the pediatric adherence literature in which a higher number of barriers is typically associated with lower adherence30,31. Overall, these data suggest that families may benefit from adherence promotion.
Meta-analyses have demonstrated several effective strategies to improve adherence in pediatric populations32,33 (e.g., problem-solving, organizational and behavioral strategies), with health care provider-delivered adherence interventions resulting in higher effect sizes for adherence rates and in turn positive health benefits32,34 compared to other adherence interventions. This highlights the critical role of healthcare teams in adherence promotion during routine clinical practice. One strategy that healthcare providers can use to better identify barriers is the use of a barriers checklist with the most commonly reported adherence barriers experienced in pediatric diseases (e.g., forgetting, inconvenience, embarrassment). Identifying family-specific barriers would allow the clinician to provide more targeted adherence interventions. For example, if a patient endorsed forgetting, strategies around medication reminders could be suggested (e.g. cell phone alarms, text reminders, calendars).
This study has some limitations. The sample size was small. The quantity and duration of hyperinsufflation needed to show clinical efficacy is unknown. As such, whether or not the prescribed regimen in this study is excessive, or insufficient is also unknown. Despite the assistance of an affected family determining the hyperinsufflation regimen, 15 minutes twice daily could have been daunting for some families and therefore could have resulted in a low adherence rate. If a lesser duration would prove efficacious, families may view the therapy as less burdensome and adherence rates may subsequently improve. Thus, adherence to our regimen was likely inherently impacted by our inability to inform families of a specific amount of hyperinsufflation that would produce clinical efficacy. Another limitation is the open-ended question format utilized for barrier assessment. Caregivers may have been more forthcoming if a checklist of commonly encountered barriers to hyperinsufflation were provided so they did not need to use a free memory recall process. Barriers checklists are used in the pediatric renal transplant population35 and can be modified for this population in future studies. While there was high concordance between adherence measures, neither one of them can be used in clinical practice as significant time is required to either conduct the DPD or to extract and analyze the electronic data. One important area for future work would be to work with manufacturers of hyperinsuffliaton devices to facilitate the generation of fast and reliable adherence reports for the outpatient setting.
In summary, our study reveals that adherence to twice daily hyperinsufflation is difficult for children with CMD and lower than we hypothesized. As with other disease processes, a medication or therapy can only benefit patients if they adhere to the treatment. Furthermore, families had difficulty identifying barriers to adherence despite poor adherence. Further studies should be done to better identify barriers to hyperinsufflation which could then guide the development of adherence interventions in children with CMD. This study sheds light on our inherent misperceptions as clinicians and the utility of objectively assessing adherence and barriers to recommended therapies as a routine part of clinical practice.
Acknowledgements
This study could not have been performed without the dedication and resiliency of the participants and their families. We recognize a new regimen can be difficult to incorporate into already busy lives, and traveling, sometimes by plane, is a sizeable request. We thank the Cure CMD Foundation for assisting in patient recruitment. We would finally like to thank Julie Field for conducting the DPDs. We would also like to thank our coordinators, Jennifer Jeffries, Suzie Hicks, Jennifer Swope, as well as Rachel Alvarez from the Cure CMD Foundation.
Funding:
This study was funded by the National Institutes of Health - National Heart, Lung, and Blood Institute (R34HL113390) and CureCMD Foundation.
References
- 1.Kang PB, Morrison L, Iannaccone ST, Graham RJ, Bönnemann CG, Rutkowski A, Hornyak J, Wang CH, North K, Oskoui M, Getchius TS, Cox JA, Hagen EE, Gronseth G, Griggs RC, Medicine GDSotAAoNatPIRPotAAoNE. Evidence-based guideline summary: evaluation, diagnosis, and management of congenital muscular dystrophy: Report of the Guideline Development Subcommittee of the American Academy of Neurology and the Practice Issues Review Panel of the American Association of Neuromuscular & Electrodiagnostic Medicine. Neurology 2015;84(13):1369–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nadeau A, Kinali M, Main M, Jimenez-Mallebrera C, Aloysius A, Clement E, North B, Manzur AY, Robb SA, Mercuri E, Muntoni F. Natural history of Ullrich congenital muscular dystrophy. Neurology 2009;73(1):25–31. [DOI] [PubMed] [Google Scholar]
- 3.Foley AR, Quijano-Roy S, Collins J, Straub V, McCallum M, Deconinck N, Mercuri E, Pane M, D’Amico A, Bertini E, North K, Ryan MM, Richard P, Allamand V, Hicks D, Lamandé S, Hu Y, Gualandi F, Auh S, Muntoni F, Bönnemann CG. Natural history of pulmonary function in collagen VI-related myopathies. Brain 2013;136(Pt 12):3625–3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lisi MT, Cohn RD. Congenital muscular dystrophies: new aspects of an expanding group of disorders. Biochim Biophys Acta 2007;1772(2):159–172. [DOI] [PubMed] [Google Scholar]
- 5.Wang CH, Bonnemann CG, Rutkowski A, Sejersen T, Bellini J, Battista V, Florence JM, Schara U, Schuler PM, Wahbi K, Aloysius A, Bash RO, Béroud C, Bertini E, Bushby K, Cohn RD, Connolly AM, Deconinck N, Desguerre I, Eagle M, Estournet-Mathiaud B, Ferreiro A, Fujak A, Goemans N, Iannaccone ST, Jouinot P, Main M, Melacini P, Mueller-Felber W, Muntoni F, Nelson LL, Rahbek J, Quijano-Roy S, Sewry C, Storhaug K, Simonds A, Tseng B, Vajsar J, Vianello A, Zeller R, Dystrophy ISoCCfCM. Consensus statement on standard of care for congenital muscular dystrophies. J Child Neurol 2010;25(12):1559–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McKim DA, Katz SL, Barrowman N, Ni A, LeBlanc C. Lung volume recruitment slows pulmonary function decline in Duchenne muscular dystrophy. Arch Phys Med Rehabil 2012;93(7):1117–1122. [DOI] [PubMed] [Google Scholar]
- 7.Katz SL, Barrowman N, Monsour A, Su S, Hoey L, McKim D. Long-Term Effects of Lung Volume Recruitment on Maximal Inspiratory Capacity and Vital Capacity in Duchenne Muscular Dystrophy. Ann Am Thorac Soc 2016;13(2):217–222. [DOI] [PubMed] [Google Scholar]
- 8.Ennis J, Rohde K, Chaput JP, Buchholz A, Katz SL. Facilitators and Barriers to Noninvasive Ventilation Adherence in Youth with Nocturnal Hypoventilation Secondary to Obesity or Neuromuscular Disease. J Clin Sleep Med 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Donnell AR, Bjornson CL, Bohn SG, Kirk VG. Compliance rates in children using noninvasive continuous positive airway pressure. Sleep 2006;29(5):651–658. [PubMed] [Google Scholar]
- 10.Marcus CL, Rosen G, Ward SL, Halbower AC, Sterni L, Lutz J, Stading PJ, Bolduc D, Gordon N. Adherence to and effectiveness of positive airway pressure therapy in children with obstructive sleep apnea. Pediatrics 2006;117(3):e442–451. [DOI] [PubMed] [Google Scholar]
- 11.Simon SL, Duncan CL, Janicke DM, Wagner MH. Barriers to treatment of paediatric obstructive sleep apnoea: Development of the adherence barriers to continuous positive airway pressure (CPAP) questionnaire. Sleep Med 2012;13(2):172–177. [DOI] [PubMed] [Google Scholar]
- 12.Oates GR, Stepanikova I, Gamble S, Gutierrez HH, Harris WT. Adherence to airway clearance therapy in pediatric cystic fibrosis: Socioeconomic factors and respiratory outcomes. Pediatr Pulmonol 2015;50(12):1244–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Briesacher BA, Quittner AL, Saiman L, Sacco P, Fouayzi H, Quittell LM. Adherence with tobramycin inhaled solution and health care utilization. BMC Pulm Med 2011;11:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ball R, Southern KW, McCormack P, Duff AJ, Brownlee KG, McNamara PS. Adherence to nebulised therapies in adolescents with cystic fibrosis is best on week-days during school term-time. J Cyst Fibros 2013;12(5):440–444. [DOI] [PubMed] [Google Scholar]
- 15.Modi AC, Lim CS, Yu N, Geller D, Wagner MH, Quittner AL. A multi-method assessment of treatment adherence for children with cystic fibrosis. J Cyst Fibros 2006;5(3):177–185. [DOI] [PubMed] [Google Scholar]
- 16.Quittner AL, Espelage DL, levers-Landis C, Drotar D. Measuring Adherence to Medical Treatments in Childhood Chronic Illness: Considering Multiple Methods and Sources of Information. Journal of Clinical Psychology in Medical Settings 2000;7(1):41–54. [Google Scholar]
- 17.Modi A, Quittner A. Utilizing Computerized Phone Diary Procedures to Assess Health Behaviors in Family and Social Contexts. Children’s Health Care 2006;35(1). [Google Scholar]
- 18.Modi A, Marciel KK, Slater SK, Drotar D, Quittner A. The Influence of parental supervision on medical adherence in adolescents with cystic fibrosis: develpmental shifts from pre to late adolescence. Children’s Health Care 2008;37(1). [Google Scholar]
- 19.Modi AC. The impact of a new pediatric epilepsy diagnosis on parents: parenting stress and activity patterns. Epilepsy & behavior: E&B 2009;14(1):237–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wiener L, Riekert K, Ryder C, Wood LV. Assessing medication adherence in adolescents with HIV when electronic monitoring is not feasible. AIDS Patient Care STDS 2004;18(9):527–538. [DOI] [PubMed] [Google Scholar]
- 21.McClellan CB, Schatz JC, Puffer E, Sanchez CE, Stancil MT, Roberts CW. Use of handheld wireless technology for a home-based sickle cell pain management protocol. Journal of pediatric psychology 2009;34(5):564–573. [DOI] [PubMed] [Google Scholar]
- 22.Quittner AL, Opipari LC. Differential treatment of siblings: interview and diary analyses comparing two family contexts. Child Dev 1994;65(3):800–814. [DOI] [PubMed] [Google Scholar]
- 23.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CP, Gustafsson P, Jensen R, Johnson DC, MacIntyre N, McKay R, Navajas D, Pedersen OF, Pellegrino R, Viegi G, Wanger J, Force AET. Standardisation of spirometry. Eur Respir J 2005;26(2):319–338. [DOI] [PubMed] [Google Scholar]
- 24.Quittner A, Drotar D, Ievers-Landis C, Slocum N, Seidner D, Jacobsen J. Adherence to Medical Treatments in Adolescents with Cystic Fibrosis: The Development and Evaluation of Family-Based Interventions In: Drotar D, editor. Promoting Adherence to Medical Treatment in Chronic Childhood Illness: Concepts, Methods, and Interventions. New York, NY: Psychology Press; 2000. [Google Scholar]
- 25.Rapoff M Adherence to Pediatric Medical Regimens: Issues in Clinical Child Psychology. New York, NY: Springer Science+Business Media, LLC; 2010. [Google Scholar]
- 26.Siracusa CM, Ryan J, Burns L, Wang Y, Zhang N, Clancy JP, Drotar D. Electronic monitoring reveals highly variable adherence patterns in patients prescribed ivacaftor. J Cyst Fibros 2015;14(5):621–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heltshe SL, Mayer-Hamblett N, Burns JL, Khan U, Baines A, Ramsey BW, Rowe SM, Network GtGDO-AIotCFFTD. Pseudomonas aeruginosa in cystic fibrosis patients with G551D-CFTR treated with ivacaftor. Clin Infect Dis 2015;60(5):703–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramsey BW, Davies J, McElvaney NG, Tullis E, Bell SC, Dřevínek P, Griese M, McKone EF, Wainwright CE, Konstan MW, Moss R, Ratjen F, Sermet-Gaudelus I, Rowe SM, Dong Q, Rodriguez S, Yen K, Ordoñez C, Elborn JS, Group V--S. A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N Engl J Med 2011;365(18):1663–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rowe SM, Heltshe SL, Gonska T, Donaldson SH, Borowitz D, Gelfond D, Sagel SD, Khan U, Mayer-Hamblett N, Van Dalfsen JM, Joseloff E, Ramsey BW, Network GIotCFFTD. Clinical mechanism of the cystic fibrosis transmembrane conductance regulator potentiator ivacaftor in G551D-mediated cystic fibrosis. Am J Respir Crit Care Med 2014;190(2):175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Simons LE, McCormick ML, Devine K, Blount RL. Medication barriers predict adolescent transplant recipients’ adherence and clinical outcomes at 18-month follow-up. J Pediatr Psychol 2010;35(9):1038–1048. [DOI] [PubMed] [Google Scholar]
- 31.Modi AC, Monahan S, Daniels D, Glauser TA. Development and validation of the Pediatric Epilepsy Medication Self-Management Questionnaire. Epilepsy Behav 2010;18(1–2):94–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Graves MM, Roberts MC, Rapoff M, Boyer A. The efficacy of adherence interventions for chronically ill children: a meta-analytic review. Journal of pediatric psychology 2010;35(4):368–382. [DOI] [PubMed] [Google Scholar]
- 33.Kahana S, Drotar D, Frazier T. Meta-Analysis of Psychological Interventions to Promote Adherence to Treatment in Pediatric Chronic Health Conditions. Journal of Pediatric Psychology 2008;33(6):590–611. [DOI] [PubMed] [Google Scholar]
- 34.Wu YP, Pai AL. Health care provider-delivered adherence promotion interventions: a meta-analysis. Pediatrics 2014;133(6):e1698–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Varnell Charles, Loiselle K, Modi A, Hooper D. Assessing adherence barriers in pediatric kidney transplant recipients. American Society of Nephrology Kidney Week; San Diego, California: 2015. [Google Scholar]