Abstract
Diffuse idiopathic skeletal hyperostosis (DISH) is a common degenerative enthesopathy seen in the elderly with male preponderance. It is uncommon in patients before 50 years of age and is extremely rare in patients younger than 40 years. We report a case of 33-year-old unmarried woman who presented with inflammatory spinal pain and stiffness, limited chest expansion, decreased range of spinal motion and postural abnormalities, all of which suggested the diagnosis of ankylosing spondylitis, considering the patient’s age. But, further evaluation led us to the final diagnosis of DISH with associated metabolic syndrome and polycystic ovarian syndrome (PCOS). To the best of our knowledge, our patient is the first reported case of DISH in a woman less than 40 years of age, and also the first case of DISH associated with PCOS and metabolic syndrome.
Keywords: diffuse idiopathic skeletal hyperostosis, DISH, ankylosing spondylitis, metabolic syndrome, polycystic ovarian syndrome, PCOS
Background
In 1950, Forestier and Rotes-Querol described an ankylosing disease of the spine developing in elderly men.1 2 In 1970, Resnick termed this condition as diffuse idiopathic skeletal hyperostosis (DISH).3 4 It is considered a disease of the elderly and has been reported in 12% of random autopsy series in a veterans administration hospital population.3 5
DISH is a common non-inflammatory disorder, characterised by excessive ligamentous calcification and ossification at spinal and extraspinal locations. There is paucity of data on the aetiopathogenesis of the condition. There are few controlled clinical studies of DISH which have addressed this aspect.6 7 Mechanical factors (such as the location of the aorta), genetic factors (human leucocyte antigen (HLA) alleles), environmental exposures (fluoride, vitamin A/retinol), drugs (isotretinoin), endocrine factors and metabolic syndrome are speculated to be associated with DISH.8 But these facts are not supported by all the investigators, and causal relationship is not definitively established as of now.
Polycystic ovarian syndrome (PCOS) is one of the most common endocrine disorders in women of the reproductive age group, with a prevalence of 4%–12%.9 10 It is associated with metabolic syndrome with a prevalence of 43%–47%, which is twice as high as the prevalence in the general population of comparable age, even after adjusting for BMI.10–12 Metabolic syndrome is known to be associated with DISH and PCOS separately, but PCOS has not been reported in association with DISH.
DISH in a young woman younger than 40 years old is an extremely rare scenario and also, to best of our knowledge, there has been no reported case of DISH in association with PCOS.
Case presentation
A 33-year-old unmarried woman, presented with history of neck pain, low backache and bilateral shoulder pain for the past 3 years. The pain was worse after exercise and subsided with analgesics. She also had progressive stiffness of her neck and lower back in the past 8 months, but there was no significant morning stiffness. She did not have any constitutional symptoms. There was no history suggestive of inflammatory bowel disease or psoriasis.
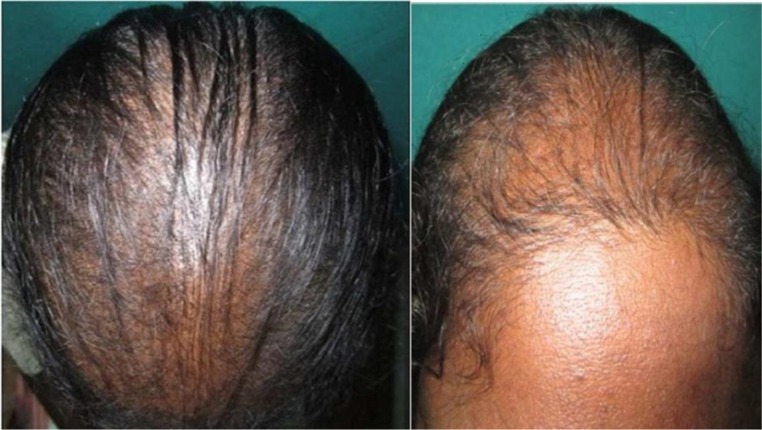
At the age of 27 years, she was diagnosed with PCOS on the basis of being overweight and having oligomenorrhea and hyperandrogenism (androgenic alopecia, hirsuitism, seborrhoea, acne, elevated serum total testosterone levels) (figure 1). Serum leutinising hormone (LH) levels were elevated and follicle stimulating hormone (FSH) was found to be normal. Her thyroid function tests and serum prolactin levels were normal. The pelvic ultrasound showed normal ovaries and uterus. She was advised weight reduction and life style changes. The alopecia was treated with topical minoxidil and platelet rich plasma injections, with no favourable results. Acne and seborrhea were treated with topical retinoids. Her body mass index (BMI) reduced to normal level and 2 years and 6 months later she had regular menstrual cycles.
Figure 1.
Androgenic alopecia.
At presentation to our hospital, her BMI was 24.5 and clinical examination revealed severe limitation of mobility at the cervical, thoracic and lumbar spine with mild forward stoop. Her chest expansion was restricted. There was tenderness at the sacroiliac joints and minimal restriction of range of movements at bilateral shoulder joints.
Investigations
Plain radiographs revealed extensive bony spinal bridges anteriorly at the cervical, thoracic and lumbar levels, with four or more contiguous vertebral bodies involved at each level. There was no facet joint involvement and the intervertebral disc heights were maintained.
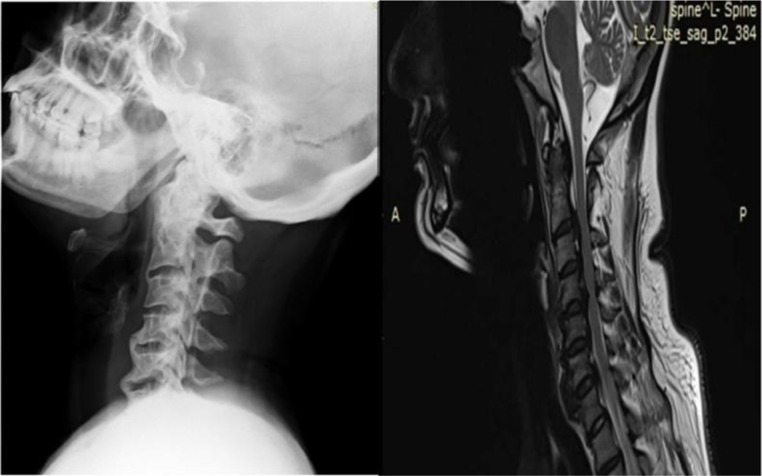
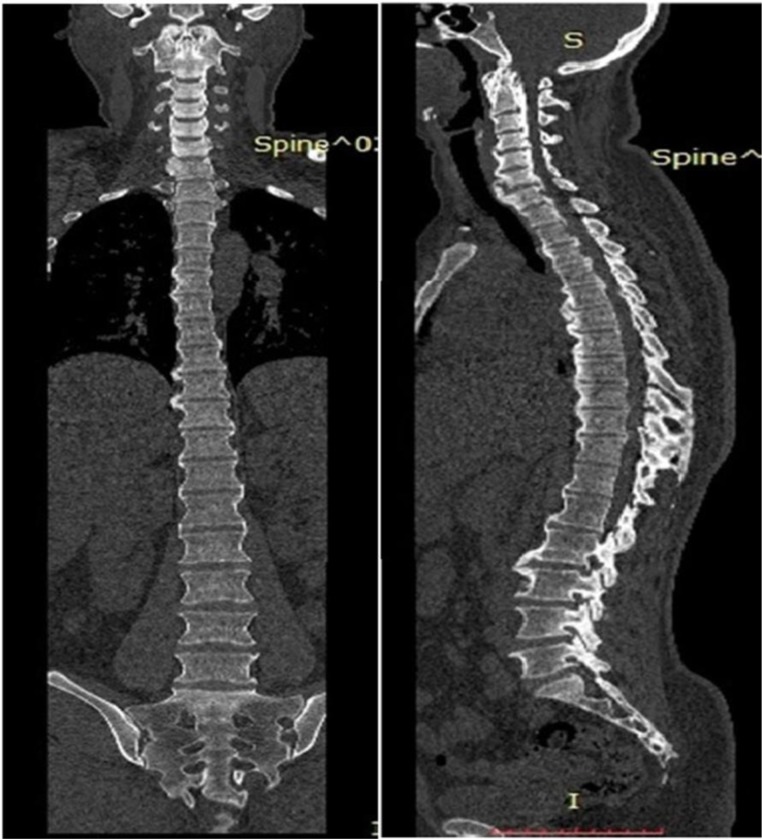
CT and MRI spine confirmed bridging ossification of the anterior longitudinal ligament, which was more horizontal in orientation and distributed mainly on the right side. It also revealed continuous type of ossification of posterior longitudinal ligament at the cervical spine level (figures 2–5). There was no spinal canal stenosis. There were bony bridges and some changes of enthesopathy in the upper ligamentous region of bilateral sacroiliac joints, but no erosions or sclerosis (figure 6).
Figure 2.
C-spine X-ray and MRI revealing flowing mantles of ossified anterior longitudinal ligament.
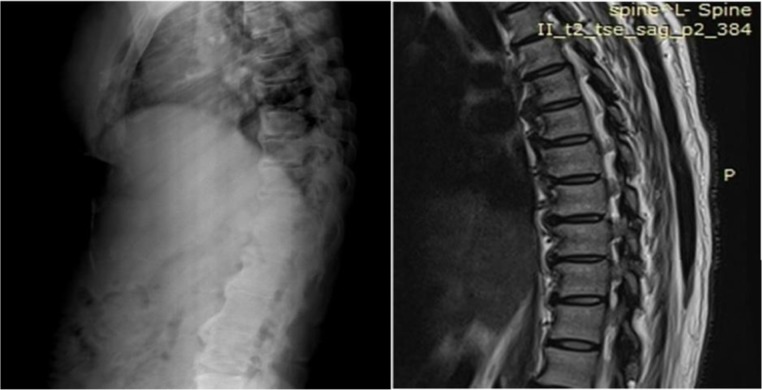
Figure 3.
Thoracic spine X-ray and MRI revealing the flowing mantles of ossified anterior longitudinal ligament.
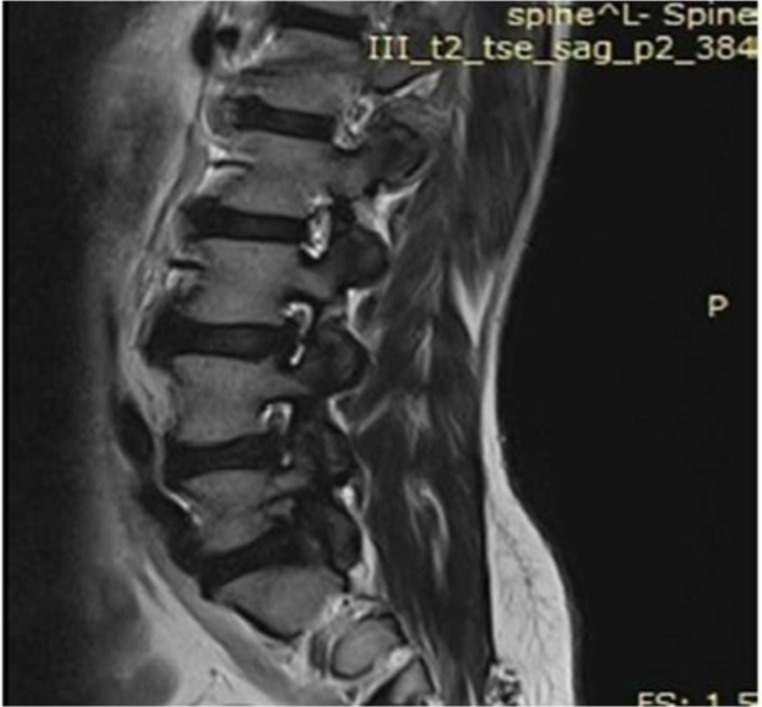
Figure 4.
T-2 weighted sagittal MRI of lumbar spine, revealing flowing mantles of ossified anterior longitudinal ligament.
Figure 5.
Coronal and sagittal CT images of the whole spine, revealing horizontally oriented thick bridging ossification of anterior longitudinal ligament in cervical, thoracic and lumbar spine levels with more involvement at the right side and continous ossification of posterior longitudinal ligament at the cervical spine.
Figure 6.
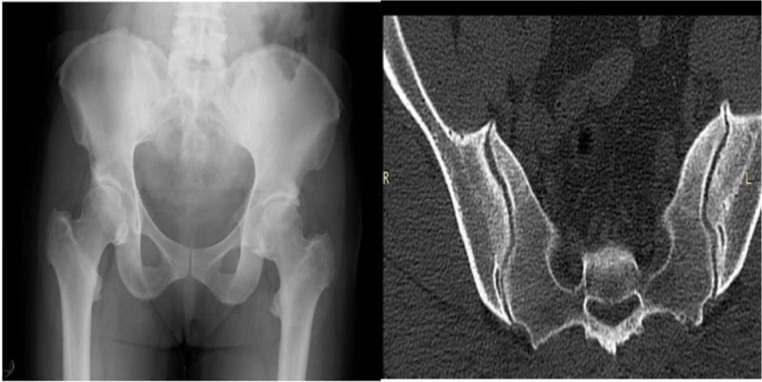
X-ray of pelvis with hips and CT of sacroiliac joints showing symmetrical bilateral hyperostotic spurs at the iliac crests, greater trochanter, lesser trochanter and bridging ossification at the upper part of sacroiliac joints.
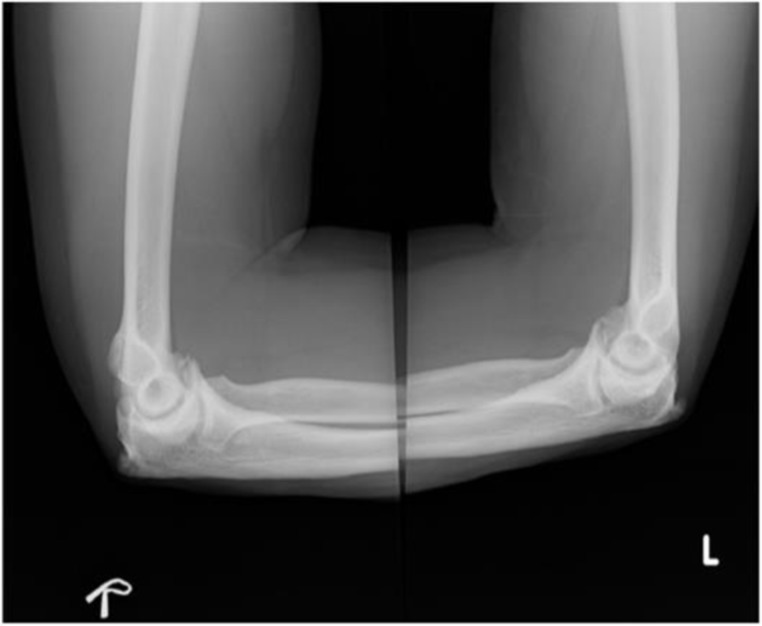
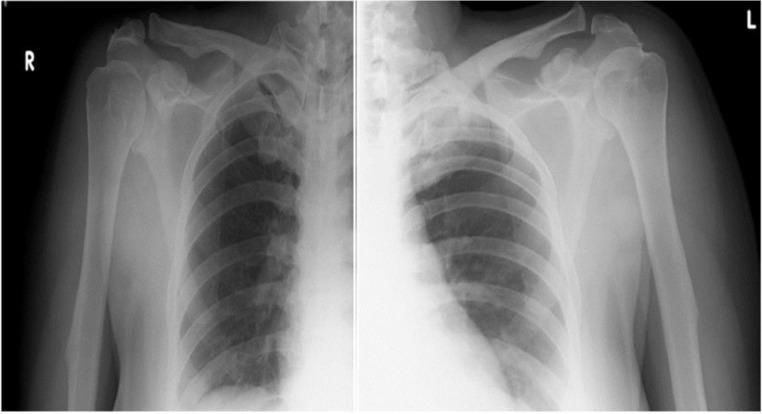
In the upper limbs, the radiographs revealed bilateral symmetrical hyperostotic spurs at the acromia, greater tuberosities, humeral medial and lateral epicondyles, bilateral coronoid and olecranon (figures 7 and 8).
Figure 7.
Symmetrical hyperostotic spurs at bilateral olecranon and coronoid.
Figure 8.
Symmetrical hyperostotic spurs at bilateral acromion and coracoid processes.
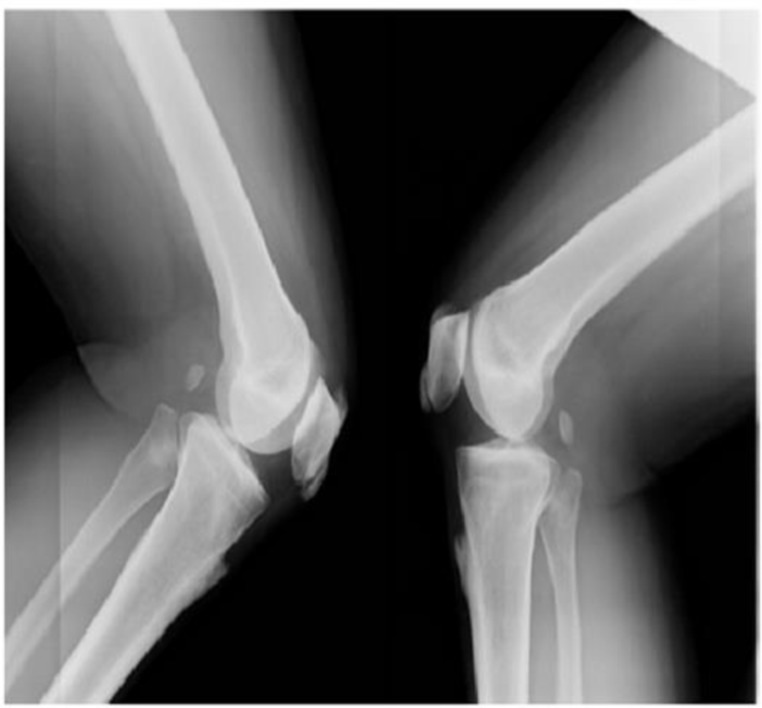
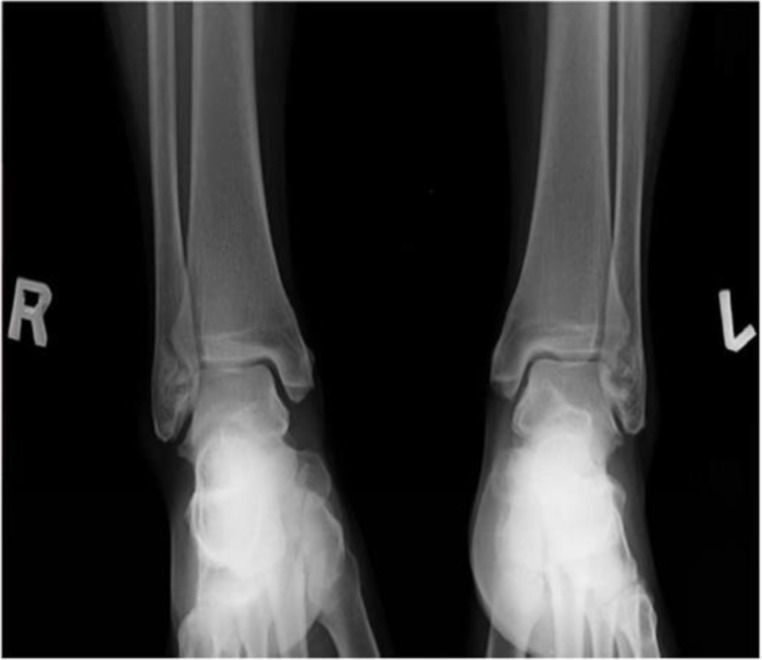
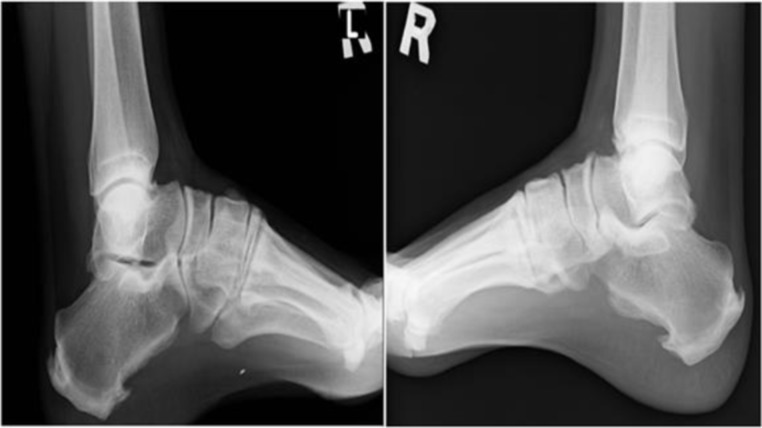
In the lower limbs, radiographs revealed bilateral symmetrical hyperostotic spurs on the iliac crests, the ischial tuberosities, the pubis, lateral acetabulum, and the greater and lesser trochanters, the attachment of quadriceps femoris to the base of the patellae, the insertions of the ligamentum patellae at the patellar apex, tibial tuberosities, medial malleoli, along the posterior and inferior aspects of calcanea and dorsal aspects of tali (figures 6 and 9–11).
Figure 9.
Symmetrical bilateral hyperostotic spurs at the tibial tuberosities and superior and inferior aspect of patellae.
Figure 10.
Symmetrical bilateral hyperostotic spurs at the malleoli.
Figure 11.
Symmetrical hyperostotic spurs at posterior and inferior aspects of bilateral calcanea and dorsal aspects of tali.
Laboratory investigations revealed diabetes mellitus with hyperinsulinemia, elevated triglycerides, elevated total cholesterol, reduced high-density lipoprotein (HDL) cholesterol and elevated low density lipoprotein (LDL). HLA B-27 was found to be negative. The levels of serum uric acid, thyroid and parathyroid hormone, prolactin, vitamin D, calcium, phosphorous, alkaline phosphatase, creatinine, erythrocyte sedimentation rate (ESR) and C reactive protein (CRP) were within normal limits. Her serum testosterone, leutinising hormone (LH) and follicle stimulating hormone (FSH) levels were also normal at presentation.
Differential diagnosis
Considering the age of the patient, ankylosing spondylitis (AS) should be first in the list of differential diagnoses. Also, there is a chance of both AS and DISH occurring simultaneously in a patient.13 But the absence of characteristic inflammatory pain, negative laboratory markers as well as lack of typical radiological findings rule out the possibility of AS in this patient. The other differential diagnosis is skeletal fluorosis as there is cervical continuous ossified posterior longitudinal ligament (OPLL). But the patient is not from an endemic area for fluorosis and also there are no typical features of osteoscleroris of bones or any calcification of interosseous membranes. In addition, there were no associated conditions like hyperparathyroidism, hypovitaminosis-D or renal failure. Furthermore, this patient has typical features of DISH as per radiological criteria and also, OPLL with DISH is reported in the literature and even suspected to have genomic association.14 15
Treatment
She was advised strict diet control and was treated with NSAIDs, bisphosphonates, oral hypoglycaemic agents and statins. She also recieved regular sessions of physiotherapy.
Outcome and follow-up
She is on regular follow-up for the past 1 year. There is only mild improvement in her spinalmobility though her pain has subsided to a great extent.
Discussion
DISH is estimated to be present in approximately 19% of men and 4% of women older than 50 years. It is uncommon in patients before 50 years of age and is considered extremely rare in patients younger than 40 years.16
In a major population study of 6167 persons aged over 30 years, carried out in nine population groups in Southern Finland, the frequency of men affected with DISH was 0.3%, 2.7%, 8.4% and 11.2% and women was 0.2%,1.7%,4.3% and 6.9% in the age groups of 40–49 years, 50–59 years, 60–69 years and 70 years or older, respectively. As observed in this study, the prevalence of DISH was lowest in the age group of 40–49 years, and highest in the age group more than 70 years. Also women had less prevalence than men in all the age groups. More importantly, none of their patients below 40 years of age were found to be affected with DISH.17
AS is entirely a different disease and unlike in DISH, symptoms usually begin in the second and third decades of life and rarely after age of 40 years.18 DISH and AS can have similar clinical features, since in both the conditions there is involvement of the axial skeleton and peripheral entheses and both produce bony proliferations, similar restriction of spinal mobility and postural abnormalities in advanced stages.19
Unlike any cases of DISH reported so far in literature, our patient is affected with DISH at a very young age of 33 years and has clinical features similar to advanced AS.
In our patient, from the clinical features, it was difficult to differentiate AS from DISH, and taking age into consideration, our initial working diagnosis was AS. The patient even had clinical features suggestive of sacroilitis which was again in favour of AS, but was later explained by enthesopathy and osteophytes in the upper ligamentous region, which has been demonstrated in DISH.19 Similarly, the bilateral shoulder pain of this patient is explained by the hyperostosis of the shoulders,20 and restriction of chest expansion is explained by the involvement of costochondral and sternochondral junctions.14
The presence of metabolic syndrome also favours the diagnosis of DISH. Again the radiological findings are quite different and based on these, we could differentiate DISH from AS.
The spinal column is most often affected in DISH, with thoracic spine being most commonly involved (95% of the patients), followed by the lumbar and cervical spine regions.21 22 In our patient, cervical spine, thoracic spine and lumbar spine regions were all involved.
The commonly accepted Resnick and Niwayama diagnostic criteria for DISH, require flowing ossification over four contiguous vertebral bodies, relative preservation of the intervertebral disc space without apparent degenerative disc disease as well as the absence of apophyseal or sacroiliac joints’ erosions, sclerosis or ankylosis.3
Plain radiographs of our patient confirm the diagnosis as per Resnick’s criteria, which is supported by the findings in CT and MRI. Our patient had coarse and thick bony spinal bridges along the anterior longitudinal ligament in a more horizontal orientation and was mainly on the right side which all are typical of DISH. These distinguish the condition from AS, in which case there are thin and delicate vertically oriented syndesmophytes.14
She was also noted to have OPLL. Similar to DISH, OPLL is also twice as common in men compared with women and symptoms usually manifest in the fourth to sixth decades of life.17 23 24 It usually affects the cervical spine, as in our case. Some of the pathogenic pathways of DISH have been adopted from that of OPLL. The main concept is the excess of growth factors such as insulin, insulin-like growth factor-1, transforming growth factor-1, platelet-derived growth factor-BB, prostaglandin I2 and endothelin-1.25–28 The growth factor excess may induce transformation of mesenchymal cells into fibroblasts and osteoblasts. High levels of testosterone and hyperinsulinemia in our patient support the above hypothesis.
The relationship between DISH and hyperinsulinemia, dyslipidemia and diabetes mellitus in our patient is best approached from a hypothesis incorporating metabolic syndrome. According to NCEP ATP III definition, our patient was diagnosed to have metabolic syndrome, as she had a waist circumference of 36.5 inches, fasting triglyceride level of 283 mg/dL, fasting HDL cholesterol level of 38 mg/dL and fasting blood sugar of 268 mg/dL.29
DISH is considered a multisystem hormonal disorder with protean presentations.30 PCOS is the most common hormonal disorder in the reproductive age group and has strong association with metabolic syndrome.31–34 Also, American Association of Clinical Endocrinologists and the American College of Endocrinology recommend screening for diabetes by age of 30 years in all patients with PCOS, regardless of BMI.35 It is estimated that more than 80% of the women presenting with symptoms of androgen excess have PCOS and approximately 15%–30% of them present with acne.36 Hirsutism is a common clinical presentation of hyperandrogenism occurring in up to 70% of women with PCOS.37 And 85%–90% of women with oligomenorrhea in the reproductive age group are found to have PCOS.38 Our patient had all these clinical features and was investigated further to confirm PCOS, with laboratory tests and ultrasonography of abdomen.
This patient fulfils the criteria for the diagnosis of PCOS as per the European Society for Human Reproduction and Embryology and the American Society for Reproductive Medicine as well as Hamburg criteria.39 40 PCOS is a clinical diagnosis of exclusion and other endocrinopathies need to be excluded. Our patient did not have any cushingoid or acromegalic features . She had normal thyroid function tests as well as serum prolactin levels. Also, ultrasound abdomen showed normal adrenals. Total testosterone level was assessed since it is regarded as more reliable option than a free testosterone for the diagnosis of hyperandrogenism.41
In a very recently published review by Mader et al, concluded that, the diagnosis of DISH as per Resnick’s criteria can be made only late in its course, as in our case. They suggested that for early detection of the condition, patients with associated conditions like metabolic syndrome and/or increased BMI, should be considered for MRI scanning of spine.14 It has been demonstrated that, it could take upto approximately 10 years from the initial ossification process to completion of the ossified bridges on vertebrae in DISH.42 Taking this into the context, our patient should have got screened for DISH earlier, if PCOS is considered an associated condition. But there has been no reports yet, to prove this fact though DISH is considered a multisystemic hormonal disorder. Also, Milner et al proved that early-onset DISH in white American women is associated with an increased mortality rate and hence it could be a potential component of health-monitoring programme for middle-aged women.43 To the best of our knowledge, our patient is the first case report in the literature to have this association and further studies are needed to support and confirm this in the future.
Learning points.
Though ankylosing spondylitis is more common in young patients with back pain, spine stiffness and restricted chest expansion, diffuse idiopathic skeletal hyperostosis (DISH) may be a possiblility in young women with associated metabolic syndrome and polycystic ovarian syndrome.
DISH may occur even in young patients with metabolic syndrome and it has to be correlated with future studies.
This case report supports the consideration of DISH as a multisystemic hormonal disorder.
Acknowledgments
The authors would like to thank Dr Jerene Mathews.
Footnotes
Contributors: JJC and BJ have contributed to the planning, conduct, acquisition of data and interpretation of dataand reporting of the work described in this article.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Forestier J, Rotes-Querol J. Senile ankylosing hyperostosis of the spine. Ann Rheum Dis 1950;9:321–30. 10.1136/ard.9.4.321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Resnick D, Niwayama G. Diffuse idiopathic skeletal hyperostosis (DISH), in diagnosis of bone and joint disorders. 3rd edn Philadelphia: WB Saunders, 2013: 1463–95. [Google Scholar]
- 3. Resnick D, Niwayama G. Radiographic and pathologic features of spinal involvement in diffuse idiopathic skeletal hyperostosis (DISH). Radiology 1976;119:559–68. 10.1148/119.3.559 [DOI] [PubMed] [Google Scholar]
- 4. Mader R, Novofestovski I, Adawi M, et al. Metabolic syndrome and cardiovascular risk in patients with diffuse idiopathic skeletal hyperostosis. Semin Arthritis Rheum 2009;38:361–5. 10.1016/j.semarthrit.2008.01.010 [DOI] [PubMed] [Google Scholar]
- 5. Resnick D, Shaul SR, Robins JM. Diffuse idiopathic skeletal hyperostosis (DISH): Forestier's disease with Extraspinal manifestations. Radiology 1975;115:513–24. 10.1148/15.3.513 [DOI] [PubMed] [Google Scholar]
- 6. Troillet N, Gerster JC. Maladie de Forestieret troubles Du metabolism. Etude prospective controle’e de vingt-cinqcas. Rev Rhum 1993;60:274–9. [PubMed] [Google Scholar]
- 7. Mata S, Fortin PR, Fitzcharles MA, et al. A controlled study of diffuse idiopathic skeletal hyperostosis. Clinical features and functional status. Medicine 1997;76:104–17. 10.1097/00005792-199703000-00003 [DOI] [PubMed] [Google Scholar]
- 8. Nascimento F, Neto H, Gatto LM, et al. Diffuse idiopathic skeletal hyperostosis: a review. Surg Neurol Int 2014;5:122–5. 10.4103/2152-7806.130675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Azziz R, Woods KS, Reyna R, et al. The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab 2004;89:2745–9. 10.1210/jc.2003-032046 [DOI] [PubMed] [Google Scholar]
- 10. Knochenhauer ES, Key TJ, Kahsar-Miller M, et al. Prevalence of the polycystic ovary syndrome in unselected black and white women of the southeastern United States: a prospective study. J Clin Endocrinol Metab 1998;83:3078–82. 10.1210/jc.83.9.3078 [DOI] [PubMed] [Google Scholar]
- 11. Essah PA, Nestler JE. Metabolic syndrome in women with polycystic ovary syndrome. Fertil Steril 2006;86 Suppl 1:S18–19. 10.1016/j.fertnstert.2006.04.013 [DOI] [PubMed] [Google Scholar]
- 12. Pasquali R, Casimirri F. The impact of obesity on hyperandrogenism and polycystic ovary syndrome in premenopausal women. Clin Endocrinol 1993;39:1–16. 10.1111/j.1365-2265.1993.tb01744.x [DOI] [PubMed] [Google Scholar]
- 13. Kuperus JS, Waalwijk JF, Regan EA, et al. Simultaneous occurrence of ankylosing spondylitis and diffuse idiopathic skeletal hyperostosis: a systematic review. Rheumatology 2018;57:2120–8. 10.1093/rheumatology/key211 [DOI] [PubMed] [Google Scholar]
- 14. Mader R, Verlaan J-J, Eshed I, et al. Diffuse idiopathic skeletal hyperostosis (DISH): where we are now and where to go next. RMD Open 2017;3:e000472 10.1136/rmdopen-2017-000472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ehara S, Shimamura T, Nakamura R, et al. Paravertebral ligamentous ossification: dish, OPLL and olf. Eur J Radiol 1998;27:196–205. 10.1016/S0720-048X(97)00164-2 [DOI] [PubMed] [Google Scholar]
- 16. Rothschild BM. Diffuse idiopathic skeletal hyperostosis (DISH): misconceptions and reality. Clin Rheumatol 1985;4:207–12. [Google Scholar]
- 17. Julkunen H, Knekt P, Aromaa A. Spondylosis deformans and diffuse idiopathic skeletal hyperostosis (DISH) in Finland. Scand J Rheumatol 1981;10:193–203. [DOI] [PubMed] [Google Scholar]
- 18. Olivieri I, D’Angelo S, Palazzi C, et al. Diffuse idiopathic skeletal hyperostosis: differentiation from ankylosing spondylitis. Curr Rheumatol Rep 2009;11:321–8. 10.1007/s11926-009-0046-9 [DOI] [PubMed] [Google Scholar]
- 19. Leibushor N, Slonimsky E, Aharoni D, et al. Ct abnormalities in the sacroiliac joints of patients with diffuse idiopathic skeletal hyperostosis. AJR Am J Roentgenol 2017;208:834–7. 10.2214/AJR.16.16994 [DOI] [PubMed] [Google Scholar]
- 20. Beyeler CH, Schlapbach P, Gerber NJ, et al. Diffuse idiopathic skeletal hyperostosis (DISH) of the shoulder: a cause of shoulder pain? Rheumatology 1990;29:349–53. 10.1093/rheumatology/29.5.349 [DOI] [PubMed] [Google Scholar]
- 21. Utsinger PD. Diffuse idiopathic skeletal hyperostosis. Clin Rheum Dis 1985;11:325–51. [PubMed] [Google Scholar]
- 22. Cammisa M, De Serio A, Guglielmi G. Diffuse idiopathic skeletal hyperostosis. Eur J Radiol 1998;27:S7–11. 10.1016/S0720-048X(98)00036-9 [DOI] [PubMed] [Google Scholar]
- 23. Choi B-W, Song K-J, Chang H. Ossification of the posterior longitudinal ligament: a review of literature. Asian Spine J 2011;5:267–76. 10.4184/asj.2011.5.4.267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Smith ZA, Buchanan CC, Raphael D, et al. Ossification of the posterior longitudinal ligament: pathogenesis, management, and current surgical approaches. Neurosurg Focus 2011;30:E10 10.3171/2011.1.FOCUS10256 [DOI] [PubMed] [Google Scholar]
- 25. Eckertova M, Krskova K, Penesova A, et al. Impaired insulin secretion and uptake in patients with diffuse idiopathic skeletal hyperostosis. Endocr Regul 2009;43:149–55. [PubMed] [Google Scholar]
- 26. Denko CW, Malemud CJ. Body mass index and blood glucose: correlations with serum insulin, growth hormone, and insulin-like growth factor-1 levels in patients with diffuse idiopathic skeletal hyperostosis (DISH). Rheumatol Int 2006;26:292–7. 10.1007/s00296-005-0588-8 [DOI] [PubMed] [Google Scholar]
- 27. Littlejohn GO, Smythe HA. Marked hyperinsulinemia after glucose challenge in patients with diffuse idiopathic skeletal hyperostosis. J Rheumatol 1981;8:965–8. [PubMed] [Google Scholar]
- 28. Mueller MB, Blunk T, Appel B, et al. Insulin is essential for in vitro chondrogenesis of mesenchymal progenitor cells and influences chondrogenesis in a dose-dependent manner. Int Orthop 2013;37:153–8. 10.1007/s00264-012-1726-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American heart Association/National heart, lung, and blood Institute scientific statement. Circulation 2005;112:2735–52. 10.1161/CIRCULATIONAHA.105.169404 [DOI] [PubMed] [Google Scholar]
- 30. Denko CW, Boja B, Moskowitz RW. Growth promoting peptides in osteoarthritis and diffuse idiopathic skeletal hyperostosis--insulin, insulin-like growth factor-I, growth hormone. J Rheumatol 1994;21:1725–30. [PubMed] [Google Scholar]
- 31. McGowan MP. Polycystic ovary syndrome: a common endocrine disorder and risk factor for vascular disease. Curr Treat Options Cardiovasc Med 2011;13:289–301. 10.1007/s11936-011-0130-0 [DOI] [PubMed] [Google Scholar]
- 32. Dunaif A. Insulin resistance and the polycystic ovary syndrome: mechanism and implications for pathogenesis. Endocr Rev 1997;18:774–800. 10.1210/edrv.18.6.0318 [DOI] [PubMed] [Google Scholar]
- 33. Legro RS. Polycystic ovary syndrome and cardiovascular disease: a premature association? Endocr Rev 2003;24:302–12. 10.1210/er.2003-0004 [DOI] [PubMed] [Google Scholar]
- 34. Korhonen S, Hippeläinen M, Niskanen L, et al. Relationship of the metabolic syndrome and obesity to polycystic ovary syndrome: a controlled, population-based study. Am J Obstet Gynecol 2001;184:289–96. 10.1067/mob.2001.109596 [DOI] [PubMed] [Google Scholar]
- 35. American Association of Clinical Endocrinologists American association of clinical endocrinologists position statement on metabolic and cardiovascular consequences of polycystic ovary syndrome. National guideline Clearinghouse, 2009. [DOI] [PubMed] [Google Scholar]
- 36. Azziz R, Sanchez LA, Knochenhauer ES, et al. Androgen excess in women: experience with over 1000 consecutive patients. J Clin Endocrinol Metab 2004;89:453–62. 10.1210/jc.2003-031122 [DOI] [PubMed] [Google Scholar]
- 37. Fauser BCJM, Tarlatzis BC, Rebar RW, et al. Consensus on women’s health aspects of polycystic ovary syndrome (PCOS): the Amsterdam ESHRE/ASRM-Sponsored 3rd PCOS Consensus Workshop Group. Fertil Steril 2012;97:28–38. e25 10.1016/j.fertnstert.2011.09.024 [DOI] [PubMed] [Google Scholar]
- 38. Hart R, Hickey M, Franks S. Definitions, prevalence and symptoms of polycystic ovaries and polycystic ovary syndrome. Best Pract Res Clin Obstet Gynaecol 2004;18:671–83. 10.1016/j.bpobgyn.2004.05.001 [DOI] [PubMed] [Google Scholar]
- 39. PCOS Consensus Workshop Group Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril 2004;81:19–25. 10.1016/j.fertnstert.2003.10.004 [DOI] [PubMed] [Google Scholar]
- 40. Homburg R. What is polycystic ovarian syndrome?: a proposal for a consensus on the definition and diagnosis of polycystic ovarian syndrome. HumReprod 2002;17:2495–9. 10.1093/humrep/17.10.2495 [DOI] [PubMed] [Google Scholar]
- 41. Rosner W. Letter to the editor: an extraordinarily inaccurate assay for free testosterone is still with us. J Clin Endocrinol Metab 2001;86 10.1210/jcem.86.6.7643 [DOI] [PubMed] [Google Scholar]
- 42. Yaniv G, Bader S, Lidar M, et al. The natural course of bridging osteophyte formation in diffuse idiopathic skeletal hyperostosis: retrospective analysis of consecutive CT examinations over 10 years. Rheumatology 2014;53:1951–7. 10.1093/rheumatology/ket335 [DOI] [PubMed] [Google Scholar]
- 43. Milner GR, Boldsen JL, Ousley SD, et al. Selective mortality in middle-aged American women with diffuse idiopathic skeletal hyperostosis (DISH). PLoS One 2018;13:e0202283 10.1371/journal.pone.0202283 [DOI] [PMC free article] [PubMed] [Google Scholar]