Abstract
We describe a term female infant who presented with multiple seizures early in infancy. The clinical and electrical seizures were refractory to traditional antiepileptic medications. After extensive workup, seizure panel testing revealed KCNT1 gene mutation, which is associated with nocturnal frontal lobe epilepsy and epilepsy of infancy with migrating focal seizures. The infant’s condition improved with the combination of traditional as well non-traditional antiepileptic therapy.
Keywords: neurology (drugs and medicines), epilepsy and seizures, neuro genetics
Background
Neonatal seizures are common in both term and preterm infants with most presentations occurring in the first week after birth. While ischaemic injury appears to be the most common cause in term infants, other aetiologies such as haemorrhage, infection, metabolic abnormalities, congenital abnormalities, and genetic and epileptic syndromes could also present as neonatal seizures. Evaluation and treatment of these seizures are often based on the underlying pathology, and it is pertinent that common aetiologies of seizures be ruled out before pursuing rare causes. After an extensive workup for assessing the aetiology of the neonatal seizure, we present an infant with refractory seizures due to a rare de novo KCNT1 mutation. The combined management approach of diet and medications followed in this case led to significant decrease in the severity and duration of seizures.
Case presentation
A 4-day-old full-term female infant was transferred to our tertiary hospital for concerns of seizures. The antenatal course was uncomplicated with multivitamins being the only medication that was taken during pregnancy. The parents were unrelated. There was no family history of seizure disorder or significant inherited hearing or heart defects. The infant was born at 40 weeks of gestation by spontaneous normal vaginal delivery. Immediate postnatal history was unremarkable until day 3 of life when she was noted to have brief tonic-clonic movements of all extremities. On admission to our hospital, the infant continued to have clinical seizures that correlated with the seizure activity seen on the electroencephalogram (EEG).
Initial physical examination revealed an appropriate for gestational age female neonate. The overall appearance of the neonate was within normal limits. Neurological examination revealed an infant that awakens with light tactile stimuli. The face was symmetrical without dysmorphism. Eye examination showed that pupils were equal, round and reactive to light without gaze deviation. The palate was seen to elevate symmetrically along with symmetrical anterior tongue protrusion. Motor examination showed mild diffuse hypotonia, with normal muscle bulk and strength. The infant moved all four extremities against gravity and resistance. Sensory examination was intact to noxious stimuli bilaterally. Deep tendon reflexes were two+symmetrical bilaterally. The toes were down-going bilaterally. Neonatal reflexes revealed normal suck, Moro, rooting and grasp reflexes. Exam during seizure episode showed tonic and clonic activity involving the upper extremities followed by postictal behavioural pattern. These episodes were also followed by apnoea, bradycardia and desaturations that had variable durations. Her seizure episodes were progressively worsening and the duration of these seizures ranged from 20 s to 5 min.
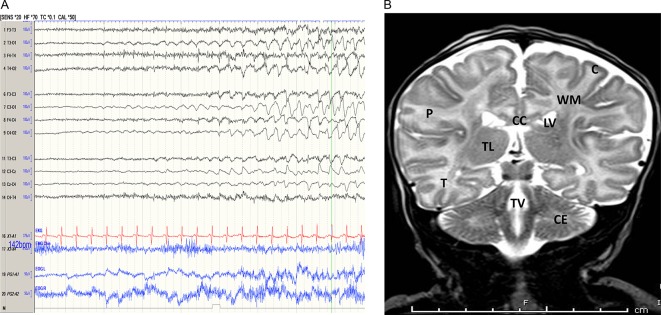
Continuous video EEG monitoring showed frequent sharp waves seen independently at the left and right central and temporal regions. The EEG background revealed poor wake-sleep transitions. Numerous EEG and clinical seizures were recorded during the study. The seizures onset was seen independently from the left and right hemispheres, and consisted of evolving rhythmic θ and δ patterns. Seizure semiology consisted of clonic and/or tonic activity of the extremities. Other seizures had subtle clinical manifestations. Seizures typically lasted 20 s to 3 min and occurred many times per recording (figure 1A). While the differential diagnosis for neonatal seizures is broad, including ischaemic injury, infection (early onset neonatal sepsis/herpes simplex virus), metabolic abnormalities and genetic/epileptic syndrome, the clinical diagnosis of severe epilepsy of infancy with migrating focal seizures due to KCNT1 gene mutation was suspected based on the refractory seizures and typical EEG features.
Figure 1.
(A) Neonatal bipolar montage revealing an ictal pattern of left more than the right hemisphere. The EEG seizure lasted 3 min on EEG. Neonatal bipolar montage revealing an ictal pattern of left more than the right hemisphere. The EEG seizure lasted 3 min on EEG. (B) MRI brain coronal section T2 image: normal MRI exam. C, cerebral cortex; CC, corpus callosum; CE, cerebellum; LV, lateral ventricle; M, medulla; P, parietal lobe; T, temporal lobe; TL, thalamus; TV, third ventricle; WM, white matter.
Investigations
Infectious aetiology workup including, blood, urine and cerebrospinal fluid (CSF) cultures were negative. Herpes simplex virus culture and PCR tests were negative. To exclude the possibility of inborn errors of metabolism, extensive metabolic workup which included basic metabolic panel, serum ammonia, lactate, liver function tests, urine and CSF amino acids, urine organic acids, carnitine profile, CSF glycine level, and state newborn screens were performed and resulting values were within normal limits. Administration of 100 mg pyridoxine intravenously did not improve the seizure frequency and severity. Imaging studies including cranial ultrasound, head CT scan and MRI of the brain (figure 1B) did not show areas of ischaemia or congenital abnormalities. Genetic workup included a microarray and comprehensive epilepsy gene panel to potentially detect any associated syndromic and non-syndromic gene mutations. The seizure panel was positive for a missense mutation related to a pathogenic variant identified in KCNT1 regions (associated with epilepsy of infancy with migrating focal seizures). Two other variants of unknown significance were identified as well (KCTD7 and SZT2) as seen in table 1. The KCNT1 mutation has been identified earlier as an aetiology for infantile refractory seizures.1 Following these results, parental genetic testing was performed, which was negative for KCNT1 mutations.
Table 1.
Seizure panel genetic testing result confirming the diagnosis
| Gene | DNA sequence | Amino acid change | Significance |
| KCNT1 | c.1283G>A | p.Arg428Gln | Pathogenic variant |
| KCTD7 | c.400 A>G | p.ile134Val | Uncertain significance |
| SZT2 | c.2787C>G | p.Asp929Glu | Uncertain significance |
Treatment
The infant continued to have frequent seizures with multiple events. She was initially started on the traditional neonatal antiepileptic medications (phenobarbital, phenytoin, levetiracetam, midazolam drip), without improvement. While on multiple medications, she developed severe respiratory depression, which required mechanical ventilation briefly.
She was later weaned to a nasal cannula to cope up with desaturations that accompanied her seizures. Trials of combination therapy with clonazepam, clobazam, lacosamide2 and oxcarbazepine had variable effects on her intractable seizures. Non-traditional antiepileptic interventions such as pyridoxine, pyridoxine-5-phosphate and biotin had no significant seizure reduction. The infant was gradually transitioned to a ketogenic diet. Cannabidiol (CBD, Epidiolex) oil was trialled but she had multiple episodes of diarrhoea and vomiting resulting in moderate dehydration. These symptoms resolved after CBD oil was discontinued. Baseline frequency of 5–10 subclinical brief seizures in a day was achieved with clobazam, phenobarbital, levetiracetam, zonisamide and quinidine
Outcome and follow-up
The infant continued to have generalised hypotonia. A gastrostomy tube was placed due to the high risk for aspiration and inadequate oral intake. The ketogenic diet was gradually advanced to the ratio of 4:1. After decreasing the seizure severity and frequency, the infant was discharged home at 3 months of age on a weight-adjusted dose of clobazam, phenobarbital, levetiracetam, zonisamide and quinidine. For the episodes of mild desaturation, the infant was discharged on home oxygen, which was discontinued 2 months later. Follow-up at 6 months of age revealed four to five baseline brief clinical seizures with no electrographic activity. Physical examination was remarkable for global developmental delay.
Discussion
Neonatal epilepsy is a frequent problem that can present within the first days of life.3 Early recognition and diagnosis can be challenging due to the subtle and wide variety of clinical presentations that can mimic other common neonatal conditions. EEG studies can be a helpful additional tool to confirm, characterise or localise the epileptogenic activity in the brain. While the most common aetiology of neonatal seizures is ischaemic brain injury because of perinatal asphyxia, other causes should be considered, especially if the clinical scenario points to an alternate underlying pathology.4
Epilepsy of infancy with migrating focal seizure and autosomal dominant nocturnal frontal lobe epilepsy are both rare conditions characterised by multiple different seizure types originating from different areas in the brain. This entity was first described in 1995.5 The KCNT1 gene, which is highly expressed in the brain (mainly in the frontal and piriform areas), encodes for sodium-activated potassium channel. KCNT1 results in gain of function of the potassium channels located in the neurons.5 Mutation in this gene carries autosomal dominant inheritance pattern. While it can be associated with many epileptic conditions (eg, West and Ohtahara syndromes), it has been found to play a significant role in the pathophysiology of both migrating focal seizure and nocturnal frontal lobe epilepsy syndromes. While the former condition typically presents as a severe catastrophic neonatal epilepsy, the latter typically presents in older children and adolescents.1
Epilepsy of infancy with migrating focal seizures usually manifests very early in age within days to weeks after birth. EEG features are quite characteristic for this condition revealing a migrating seizure focus with different ictal patterns in different cortical regions during the EEG study. EEG suppression and burst suppression were reported in other studies.1 Infants could sometimes develop hypsarrhythmia later on.
The intractable seizures associated with this syndrome vary significantly in type (focal to generalised) and duration (5 seconds to 5 minutes). Most cases fail to respond to traditional antiepileptic medications or other forms of therapy. Developmental delays, as well as autonomic instability, are present in the majority of cases. Due to these associations, most of these patients require home nursing visit/support secondary to the need for respiratory support in the form of home oxygen therapy and a gastrostomy tube to help with feeding.
Readmissions to the hospital may also occur secondary to worsening of the condition. Non-traditional antiepileptic management strategies are often trialled due to the inadequate response to the traditional antiepileptic therapies. Quinidine, which is a Food and Drug Administration approved (class 1A) antiarrhythmic drug, that antagonises the KCNT1 coded channel, did show conflicting results as previously shown by Mikati et al.6 They reported a significant improvement with 80% reduction in seizure frequency while another case did not. In our infant, quinidine did result in decreased seizure frequency and severity in conjunction with the other antiepileptic medications and the ketogenic diet. It is imperative to monitor the patients closely for cardiac arrhythmias and drug-drug interactions when they are placed on quinidine. Routine electrocardiogram (EKGs) are needed prior to adjusting the dose of quinidine.
Another medication that was used in our case was zonisamide, which is a sulfonamide antiepilepsy drug with sodium and calcium channel-blocking actions. A randomised controlled trial showed significant improvement when zonisamide was used as an adjunctive for refractory seizures.7
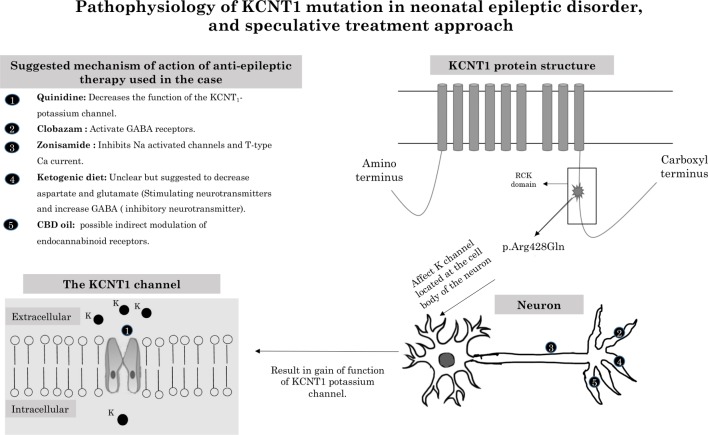
The ketogenic diet did result in decreasing seizure severity in a few children with seizure syndromes related to KCNT1 mutation.8 9 Despite the limited literature about the use of the ketogenic diet in infants younger than 2 months,10 our patient did tolerate the diet with no complications. She was also started on CBD oil, given the mild inconsistent improvement reported in the observational study done by Hausman-Kedem et al.11 Similar to 46% of the cases reported in this observational study, our patient did not tolerate the therapy with severe gastrointestinal symptoms, which necessitated cessation of CBD oil therapy. The pathophysiology, cellular effect and suggested mechanism of action for these non-traditional antiepileptic therapies are summarised in figure 2.
Figure 2.
Pathophysiology of KCNT1 mutation in neonatal epileptic disorder, and speculative treatment approach. CBD, cannabidiol; GABA, gamma-aminobutyric acid.
Our patient did show improvement in the frequency, severity as well as the duration of the seizure episodes associated with her condition. At the follow-up visits, developmental assessments showed the global developmental delay in all domains. This association with KCNT1 mutation is common and well reported in the literature with long-term association with intellectual disabilities.1
Learning points.
The presentation of an infant with refractory seizures unresponsvie to the traditional antiepileptic medications should alert the clinicians to the possibility of seizure disorders related to KCNT1 mutation.
Genetic testing for KCNT1 mutations can help guide the management and prognosis counselling for refractory seizures due to this mutation
There are no proven treatment guidelines for KCNT1 mutation associated with intractable seizures. Combined therapy with traditional and non-traditional antiepileptics for KCNT1 mutation refractory seizures may help decrease the frequency and severity of the associated seizures
Footnotes
Twitter: @chandrpk
Contributors: MA, PC and VC contributed to the design, draft and revision for this report. AW extensively revised this draft and contributed to the design of the revised paper. All authors approved the final version of this draft and agreed to be accountable for all aspects of the work; ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Parental/guardian consent obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Lim CX, Ricos MG, Dibbens LM, et al. . KCNT1 mutations in seizure disorders: the phenotypic spectrum and functional effects. J Med Genet 2016;53:217–25. 10.1136/jmedgenet-2015-103508 [DOI] [PubMed] [Google Scholar]
- 2. Flor-Hirsch H, Heyman E, Livneh A, et al. . Lacosamide for SCN2A-related intractable neonatal and infantile seizures. Epileptic Disord 2018;20:440–6. 10.1684/epd.2018.1001 [DOI] [PubMed] [Google Scholar]
- 3. Volpe JJ. Neonatal Seizures : Volpe JJ, Neurology of the newborn. Philadelphia: WB Saunders, 2008: 203–44. [Google Scholar]
- 4. Silverstein FS, Jensen FE. Neonatal seizures. Ann Neurol 2007;62:112–20. 10.1002/ana.21167 [DOI] [PubMed] [Google Scholar]
- 5. Heron SE, Smith KR, Bahlo M, et al. . Missense mutations in the sodium-gated potassium channel gene KCNT1 cause severe autosomal dominant nocturnal frontal lobe epilepsy. Nat Genet 2012;44:1188–90. 10.1038/ng.2440 [DOI] [PubMed] [Google Scholar]
- 6. Mikati MA, Jiang Y-hui, Carboni M, et al. . Quinidine in the treatment of KCNT1-positive epilepsies. Ann Neurol 2015;78:995–9. 10.1002/ana.24520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Faught E, Ayala R, Montouris GG, et al. . Randomized controlled trial of zonisamide for the treatment of refractory partial-onset seizures. Neurology 2001;57:1774–9. 10.1212/WNL.57.10.1774 [DOI] [PubMed] [Google Scholar]
- 8. Mori T, Imai K, Oboshi T, et al. . Usefulness of ketogenic diet in a girl with migrating partial seizures in infancy. Brain Dev 2016;38:601–4. 10.1016/j.braindev.2015.12.012 [DOI] [PubMed] [Google Scholar]
- 9. McTague A, Appleton R, Avula S, et al. . Migrating partial seizures of infancy: expansion of the electroclinical, radiological and pathological disease spectrum. Brain 2013;136:1578–91. 10.1093/brain/awt073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dressler A, Trimmel-Schwahofer P, Reithofer E, et al. . The ketogenic diet in infants – advantages of early use. Epilepsy Res 2015;116:53–8. 10.1016/j.eplepsyres.2015.06.015 [DOI] [PubMed] [Google Scholar]
- 11. Hausman-Kedem M, Menascu S, Kramer U. Efficacy of CBD-enriched medical cannabis for treatment of refractory epilepsy in children and adolescents - An observational, longitudinal study. Brain Dev 2018;40:544–51. 10.1016/j.braindev.2018.03.013 [DOI] [PubMed] [Google Scholar]