Abstract
Background
It remains unclear whether or which prediagnostic lifestyle and dietary factors influence colorectal cancer (CRC) survival following diagnosis. This study used competing mortality risks analysis to evaluate the association between these factors and CRC survival.
Methods
A total of 96 889 cancer-free participants of the Norwegian Women and Cancer Study completed the study’s baseline questionnaire on lifestyle and dietary factors between 1996 and 2004. Of the 1861 women who subsequently developed CRC, 550 had CRC as the cause of death, while 110 had a non-CRC cause of death. We used multiple imputation to handle missing data. We performed multivariable competing mortality risks analyses to determine the associations between prediagnostic lifestyle and dietary factors and CRC survival. Cause-specific HRs were estimated by Cox regression and subdistribution HRs were estimated by the Fine-Gray regression with corresponding 95% CIs.
Results
Following multivariable adjustment, a prediagnostic vitamin D intake of >10 μg/day compared with ≤10 μg/day was associated with better CRC survival (HR=0.75, 95% CI 0.61 to 0.92). Other prediagnostic lifestyle and dietary factors showed no association with CRC survival. The corresponding results obtained from cause-specific Cox and Fine-Gray regressions were similar.
Conclusion
Our study shows that prediagnostic vitamin D intake could improve CRC survival.
Keywords: prediagnosis, lifestyle, diet, vitamin D, colorectal cancer, survival
Summary box.
What is already known about this subject?
Colorectal cancer (CRC) survival is a function of the stage of the disease at diagnosis.
Prediagnostic and postdiagnostic lifestyle and dietary factors may influence survival.
What are the new findings?
This study evaluates several lifestyle and dietary factors in the presence of competing mortality risks.
Prediagnostic vitamin D intake could improve CRC survival.
How might it impact on clinical practice in the foreseeable future?
Ensuring adequate daily intake of vitamin D could become an essential clinical and nutritional goal.
Introduction
Colorectal cancer (CRC) is the fourth leading cause of cancer-related death worldwide1 2 and the second leading cause in high-income countries.3 CRC is an important public health concern in that it imposes a considerable medical and economic burden, and temporal and demographic projections predict that this burden will increase by about 60% by 2030.1–4 CRC incidence is increasing globally,2 4 and the combination of this high incidence and improved CRC management is giving rise to a relatively large population of CRC survivors, especially in countries such as Norway, where incidence is still on the rise and mortality continues to decrease.1
Primarily, CRC is considered both a genetic and lifestyle disease. The relationship between CRC incidence and factors like adult-attained height, physical activity, obesity, socioeconomic status, alcohol consumption, smoking status, and certain dietary factors has been investigated extensively, with some clearly established associations.2 However, the relationship between CRC survival and these same factors has yet to be expansively researched.5 This knowledge gap has been attributed to a comparative lack of relevant data.6
The primary predictor of CRC survival is the stage of the disease at the time of diagnosis.2 7 However, there is still variability in the survival among people with similar stages of CRC and similar access to healthcare,7 which may be due to variations in lifestyle and dietary habits before and/or after CRC diagnosis.6–9 For instance, vitamin D status as much as three decades prior to diagnosis has been shown to be related to survival among patients with some organ-specific cancers.10 A recent review of the literature concluded that both prediagnostic and postdiagnostic lifestyle factors, including physical activity, obesity, and dietary habits, may play a critical role in improving CRC survival.8
The Norwegian Women and Cancer (NOWAC) Study offers the opportunity to study prediagnostic lifestyle and dietary factors and subsequent CRC survival following diagnosis. This study used competing mortality risks analysis to evaluate the association between these factors and CRC survival.
Methods
The NOWAC Study is a prospective population-based cohort study that was initiated in 1991 and has been described in detail elsewhere.11 12 In brief, Norwegian women between the ages of 30 and 70 years were randomly selected from the Norwegian Central Population Register (Statistics Norway) and invited to participate. More than 172 000 women, recruited at different time periods, gave written informed consent and completed a questionnaire that collected information on lifestyle factors, health status, and dietary habits.
Study sample
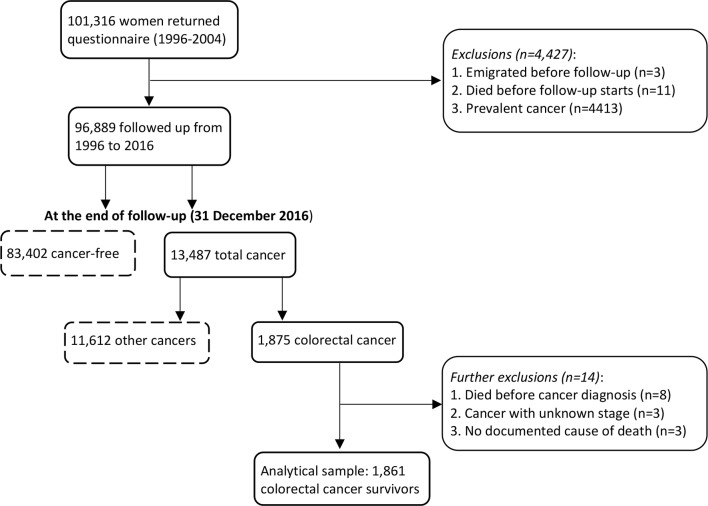
In this study, we included 101 316 eligible participants who completed a baseline questionnaire, which included a food frequency questionnaire (FFQ), between 1996 and 2004. We excluded those who emigrated, died, or had prevalent cancer (n=4427) before the start of study recruitment. Of the 96 889 remaining participants, 13 487 developed cancer during follow-up, of whom 1875 were diagnosed with CRC. Women diagnosed at autopsy (n=8), as well as those with unknown cancer stage (n=3) or an undocumented cause of death (n=3), were excluded, leaving a final analytical sample of 1861 women with a CRC diagnosis (figure 1).
Figure 1.
Flowchart of the study sample of colorectal cancer survivors in the Norwegian Women and Cancer Study.
Ascertainment of cancer diagnosis in the study sample
The 1861 women included in our study sample had primary incident CRC (International Classification of Diseases, 10th Revision (ICD-10) codes C18–C20) diagnosed between study recruitment and 31 December 2016. CRC diagnosis, dates of diagnosis, and cancer stage were obtained through record linkage to the Cancer Registry of Norway (CRN), which has been acknowledged to be more than 98% complete.13 The CRN uses the pathological tumour, node, and metastasis staging system, which is considered the most accurate and reliable staging system.14 15
Assessment of emigration, death, and cause of death
Information on dates of emigration and death was obtained through record linkage to the Norwegian Population Registry, while information on cause of death was obtained from the Cause of Death Registry. Primary causes of death were then categorised into death due to CRC (ICD-10 codes C18–C20), hereafter referred to as CRC death, and death due to any other causes, hereafter referred to as non-CRC death. Follow-up time was defined as the period in days between the date of CRC diagnosis and the date of emigration, death, or the end of follow-up (31 December 2016), whichever occurred first.
Assessment of prediagnostic lifestyle and dietary factors
The choice of prediagnostic lifestyle and dietary factors considered in this analysis was based on the literature, previous similar studies,6–9 16 and availability in the NOWAC Study database. Information on prediagnostic physical activity, height, weight, duration of education, annual household income, alcohol intake, smoking habits, dietary habits, and self-reported medical conditions was extracted from the NOWAC questionnaire.
Physical activity was reported on a 10-point scale, where 1 was ‘very low’ and 10 was ‘very high’. This is a validated scale,17 which implicitly included recreational, occupational, transportation, and domestic physical activities in a global format. We categorised this into 1–2 (least active), 3–4, 5–6, 7–8, and 9–10 (most active). Height and body weight were self-reported and were used to compute the body mass index (BMI) as the weight in kilogram divided by the square of the height in metre. We categorised BMI into underweight (<20.0 kg/m2), normal weight (20.0–24.9 kg/m2), overweight (25.0–29.9 kg/m2), and obese (≥30.0 kg/m2). We used <20.0 kg/m2 as the cut-off point for the underweight category because few women had a BMI of <18.5 kg/m2. Duration of education was categorised into low (0–9 years), medium (10–12 years), and high (>12 years). These categories correspond to primary and lower secondary schools, upper secondary school, and higher education, respectively. Annual household income in Norwegian kroner (NOK) was categorised into low (<300 000 NOK), medium (300–600 000 NOK), and high (>600 000 NOK). Alcohol intake was categorised as none, ≤3.0 g/day, >3.0–10.0 g/day, and >10.0 g/day; and smoking status was categorised as never, former, or current.
The validated FFQ in the baseline questionnaire included foods that are common in Norway.18 We used either hypothesis-driven or data-driven percentiles to categorise average daily dietary intake into groups. Hypothesis-driven cut-offs were based on nutritional recommendations and/or knowledge of diet–disease associations; while data-driven cut-offs were based on the 50th or 75th percentile values of the study sample. We combined red meat and processed meat and created two categories of consumption: ≤70 and >70 g/day. The World Cancer Research Fund/American Institute for Cancer Research recommend a red meat intake of not more than 50–70 g/day, and little or no processed meat intake.19 Fish intake was categorised into ≤130 and >130 g/day, fruit and vegetable intake into ≤300 and >300 g/day, and vitamin D intake into ≤10.0 and >10 µg/day, as 10 µg/day is the Nordic nutrition recommendation.20 Participants were categorised as having or not having (yes or no) the prediagnostic comorbidities of diabetes mellitus and cardiovascular disease (CVD). CVD included self-reported medical conditions such as hypertension, angina pectoris, infarction, and stroke.
Statistical methods
Competing mortality risks analysis
CRC survivors are also at risk of dying from causes other than CRC. Indeed, CRC is predominantly a disease of the middle-aged and the elderly, bearing in mind that mortality increases exponentially with age after the age of 35 years, especially in high-income countries.21 Thus, we chose to use competing risks analysis.
We extended the standard Cox proportional hazard model, normally used when there are no competing events, to model cause-specific hazards as proposed by Prentice et al.22 We applied the model to cause-specific hazards of (1) CRC death and (2) non-CRC death, respectively. In each model, we censored the competing event while estimating the effects of lifestyle and dietary factors on the risk of death. This is the method of choice when focusing on epidemiological questions of aetiology (such as factors associated with CRC death), rather than the probability of CRC death, both in the presence of competing risks.23–26
In addition, we used the subdistribution hazard model approach proposed by Fine and Gray.27 This is because of the inherent hypothetical setting of a cause-specific hazard model in which the ‘competing event is removed’ (censored). The estimations from the Fine and Gray approach is in the ‘presence of competing events’, thereby removing the hypothetical setting by modelling hazards on the basis of the cumulative incidence function.23 We used these two statistical methods to gain complete understanding of the effects of lifestyle and dietary factors on competing risk endpoints, as recommended by Latouche et al.28 We used these methods to estimate HRs and subdistribution HRs (SHRs), with 95% CIs for the associations between lifestyle and dietary factors, and CRC death and non-CRC death, respectively. CRC death was the event of interest, while non-CRC death was the competing event.
We used Schoenfeld residuals to check the proportional hazards assumption in the two approaches and with the two competing events, respectively. In order to keep the proportional hazards assumption, we had to run all models stratified by CRC stage. We used the Breslow approximation method to handle tied failures. We adjusted for prediagnostic follow-up duration, which is the period between the date of NOWAC recruitment and CRC diagnosis, in all models. We tested for linear trend by using variables originally in continuous scale as continuous variables in the model. We assessed collinearity between the prediagnostic variables fish intake and vitamin D intake, and predefined interactions between physical activity and BMI, physical activity and vitamin D intake, duration of education and annual household income, and fish intake and vitamin D intake, respectively. The final prediagnostic variables included in all analyses were age at diagnosis of CRC; physical activity; BMI; duration of education; annual household income; alcohol intake; smoking status; red and processed meat intake, fish intake, and fruit and vegetable intake; and vitamin D intake. We also included diabetes mellitus status and CVD status.
Multiple imputation
We used multiple imputation to handle missing data under the assumption that data were missing at random.29 We replaced missing values by computed estimates of 50 replicate datasets from multiple imputations using chained equations. We created 50 replicates to minimise variability30 and used Rubin’s rules to coalesce the values from the 50 imputed replicates to estimate HRs and SHRs with corresponding 95% CIs.31 32
In order to rule out the effects of latent disease conditions, we conducted sensitivity analyses excluding those who died less than 1 year after recruitment and those who died less than 1 year after CRC diagnosis, respectively. We also assessed reverse causation by excluding women who had CRC diagnosis less than 1 year after recruitment. To minimise the impact of changes in lifestyle and dietary habits during follow-up, we conducted further analysis restricted to women who received a CRC diagnosis within 10 years of recruitment. Finally, we ran a sensitivity analysis in which we did not include CRC cases diagnosed after 31 December 2014 to allow for a longer follow-up time.
Analyses were performed using STATA V.15.0 and R V.3.5.3 (R Foundation for Statistical Computing 2019). All statistical analyses were two-sided, and p values were considered statistically significant at a level of <0.05.
Results
Of the 1861 women with CRC in our study sample, 65% (1201/1861) were alive, and 35% (660/1861) had died by the end of follow-up (31 December 2016). Of these deaths, 83% (550/660) were CRC deaths and 17% (110/660) were non-CRC deaths. The mean age was 67.6 years at CRC death and 75.9 years at non-CRC death. The average duration of follow-up was 5 years. This was lower among CRC deaths (2.1 years) compared with non-CRC deaths (5.3 years) (table 1). Other cancer types (41.8%) and CVD (30.0%) were the most common causes of non-CRC death.
Table 1.
Prediagnostic demographic, lifestyle, and dietary characteristics of the study sample at recruitment and during follow-up: the Norwegian Women and Cancer Study (N=1861)
| Characteristics | Categories or parameters | Numbers (%) | Alive | Died of CRC |
Died of other causes |
| Total cohort, n (%) | 1861 | 1201 (64.5) | 550 (29.6) | 110 (5.9) | |
| Mean age at enrolment | Years (SD) (range) |
55.8 (7.3) (41–75) |
55.0 (6.9) (41–75) |
56.6 (7.5) (41–74) |
61.2 (7.4) (41–75) |
| Mean age at diagnosis | Years (SD) (range) |
66.4 (8.7) (43–89) |
66.4 (8.3) (43–89) |
65.5 (9.4) (43–87) |
70.6 (8.6) (50–86) |
| Mean age at death | Years (SD) (range) |
69.0 (47–89) |
67.6 (9.1) (47–88) |
75.9 (8.6) (50–89) |
|
| Mean prediagnostic follow-up duration | Years (SD) | 10.5 (5.4) | 11.3 (5.3) | 8.8 (5.2) | 9.3 (4.7) |
| Mean postdiagnosis survival duration | Years (SD) | 5.0 (4.7) | 6.4 (5.0) | 2.1 (2.3) | 5.3 (4.3) |
| CRC stage, n (%) | Localised | 452 (24.3) | 377 (31.4) | 32 (5.8) | 43 (39.1) |
| Regional spread | 972 (52.2) | 730 (60.8) | 188 (34.2) | 54 (49.1) | |
| Remote metastases | 437 (23.5) | 94 (7.8) | 330 (60.0) | 13 (11.8) | |
| Physical activity | 1–2 (least active) | 83 (5.2) | 46 (4.4) | 26 (5.4) | 11 (12.4) |
| 3–4 | 366 (22.7) | 236 (22.7) | 112 (23.3) | 18 (20.2) | |
| 5–6 | 687 (42.6) | 458 (43.9) | 198 (41.3) | 31 (34.8) | |
| 7–8 | 385 (23.9) | 248 (23.8) | 116 (24.2) | 21 (23.6) | |
| 9–10 (most active) | 90 (5.6) | 54 (5.2) | 28 (5.8) | 8 (9.0) | |
| Body mass index, n (%) | Underweight (<20.0) | 100 (5.5) | 52 (4.4) | 41 (7.7) | 7 (6.6) |
| Normal (20.0–24.9) | 883 (48.7) | 583 (49.6) | 253 (47.6) | 47 (44.4) | |
| Overweight (25.0–29.9) | 640 (35.3) | 417 (35.5) | 188 (35.3) | 35 (3.0) | |
| Obese ≥30.0 | 190 (10.5) | 123 (10.5) | 50 (9.4) | 17 (16.0) | |
| Duration of education | <10 years | 658 (38.2) | 413 (36.9) | 197 (39.2) | 48 (48.0) |
| 10–12 years | 583 (33.9) | 383 (34.2) | 171 (34.1) | 29 (29.0) | |
| >12 years | 480 (27.9) | 323 (28.9) | 134 (26.7) | 23 (23.0) | |
| Annual household income | <300 000 NOK | 857 (50.7) | 515 (46.7) | 274 (55.8) | 68 (70.1) |
| 301 000–600 000 NOK | 688 (40.6) | 483 (43.7) | 178 (36.3) | 27 (27.8) | |
| >600 000 NOK | 147 (8.7) | 106 (9.6) | 39 (7.9) | 2 (2.1) | |
| Alcohol intake (g/day) | None | 472 (27.5) | 315 (28.3) | 119 (23.5) | 38 (38.4) |
| ≤3.0 g | 720 (41.9) | 447 (40.2) | 231 (45.5) | 42 (42.4) | |
| >3.0–10.0 g | 395 (23.0) | 259 (23.3) | 121 (23.9) | 15 (15.2) | |
| >10.0 g | 131 (7.6) | 91 (8.2) | 36 (7.1) | 4 (4.0) | |
| Smoking status | Never smoker | 627 (34.2) | 410 (34.7) | 173 (31.9) | 44 (40.7) |
| Former smoker | 666 (36.4) | 437 (37.0) | 202 (37.3) | 27 (25.0) | |
| Current smoker | 538 (29.4) | 334 (28.3) | 167 (30.8) | 37 (34.3) | |
| Red and processed meat intake combined (g/day) | ≤70.0 g | 1523 (81.8) | 986 (82.1) | 444 (80.7) | 93 (84.6) |
| >70.0 g | 338 (18.2) | 215 (17.9) | 106 (19.3) | 17 (15.4) | |
| Fish intake (g/day) | ≤130 g | 1364 (73.3) | 890 (74.1) | 401 (72.9) | 73 (66.4) |
| >130 g | 497 (26.7) | 311 (25.9) | 149 (27.1) | 37 (33.6) | |
| Fruit and vegetable intake (g/day) | ≤300 g | 1002 (53.8) | 629 (52.4) | 296 (53.8) | 77 (70.0) |
| >300 g | 859 (46.2) | 572 (47.6) | 254 (46.2) | 33 (30.0) | |
| Vitamin D intake (μg/day) | ≤10.0 µg | 1340 (72.0) | 851 (70.9) | 411 (74.7) | 78 (70.9) |
| >10.0 µg | 521 (28.0) | 350 (29.1) | 139 (25.3) | 32 (29.1) | |
| Diabetes mellitus, n (%) | No | 1813 (97.4) | 1175 (97.8) | 532 (96.7) | 106 (96.4) |
| Yes | 48 (2.6) | 26 (2.2) | 18 (3.3) | 4 (3.6) | |
| Cardiovascular diseases | No | 1503 (80.8) | 981 (81.7) | 450 (81.8) | 72 (65.4) |
| Yes | 358 (19.2) | 220 (18.3) | 100 (18.2) | 38 (34.6) |
CRC, colorectal cancer; NOK, Norwegian kroner; SD, standard deviation.
Most CRC survivors (92%, 1107/1201) who were alive at the end of follow-up had been diagnosed with early-stage CRC (localised or regional spread), whereas more than half (60%, 330/550) of CRC deaths had been diagnosed with advanced-stage CRC (remote metastases). Non-CRC death (88%, 97/110) was more common in those who were diagnosed with early-stage CRC (localised or regional spread). Few CRC survivors reported comorbidities at recruitment, with less than 3% (48/1861) having diabetes mellitus and less than 20% (358/1861) having CVD (table 1).
The prediagnostic variables with the highest proportion of missing values were physical activity (13.4%), annual household income (9.1%), alcohol intake (7.7%), and duration of education (7.5%) (online supplementary table 1). After multiple imputation, there was no substantial change in the characteristic features of the study sample between the complete-case and the imputed datasets (online supplementary table 2).
bmjgast-2019-000338supp001.pdf (44.3KB, pdf)
Competing risks mortality analyses
We present the multivariable competing risk regressions of the imputed datasets in table 2, with the estimated HRs, SHRs, and the corresponding 95% CIs. We observed a 5% increase in the cause-specific hazard of CRC death (HR=1.05, 95% CI 1.03 to 1.06) for each 1-year increase in age at diagnosis; the corresponding increase for non-CRC death was 12% (HR=1.12, 95% CI 1.08 to 1.16). Similarly, each 1-year increase in age at diagnosis raised the cumulative incidence of CRC death by 4% (SHR=1.04, 95% CI 1.03 to 1.06) and that of non-CRC death by 9% (SHR=1.09, 95% CI 1.06 to 1.13) (table 2).
Table 2.
Multivariable cause-specific and subdistribution HRs, and 95% CIs of CRC and non-CRC deaths, with chained multiple imputation (N=1861)
| Prediagnostic variables | Categories | Cause-specific hazard model* | Subdistribution hazard model (Fine and Gray regression)* | ||||||
| HR (95% CI) | HR (95% CI) | SHR (95% CI) | SHR (95% CI) | ||||||
| CRC death | P trend | Non-CRC death | P trend | CRC death | P trend | Non-CRC death | P trend | ||
| Age at diagnosis of CRC | Per year increase | 1.05 (1.03 to 1.06 ) | <0.01 | 1.12 (1.08 to 1.16 ) | <0.01 | 1.04 (1.03 to 1.06 ) | <0.01 | 1.09 (1.06 to 1.13 ) | <0.01 |
| Physical activity | 1–2 (least active) | 0.95 (0.62 to 1.44) | 0.75 | 2.14 (1.05 to 4.37 ) | 0.85 | 0.96 (0.62 to 1.50) | 0.77 | 2.05 (1.03 to 4.04 ) | 0.94 |
| 3–4 | 0.92 (0.73 to 1.18) | 0.98 (0.54 to 1.78) | 0.94 (0.73 to 1.22) | 0.96 (0.54 to 1.72) | |||||
| 5–6 | 1.00 | 1.00 | 1.00 | 1.00 | |||||
| 7–8 | 0.97 (0.77 to 1.23) | 1.19 (0.67 to 2.11) | 0.98 (0.77 to 1.24) | 1.17 (0.66 to 2.06) | |||||
| 9–10 (most active) | 1.01 (0.67 to 1.53) | 2.00 (0.89 to 4.48) | 1.05 (0.70 to 1.57) | 1.82 (0.81 to 4.12) | |||||
| Body mass index (kg/m2) | Underweight (<20.0) | 1.09 (0.77 to 1.53) | 0.44 | 1.56 (0.68 to 3.57) | 0.40 | 1.12 (0.76 to 1.64) | 0.40 | 1.15 (0.52 to 2.57) | 0.25 |
| Normal (20.0–24.9) | 1.00 | 1.00 | 1.00 | 1.00 | |||||
| Overweight (25.0–29.9) | 0.97 (0.80 to 1.18) | 0.84 (0.53 to 1.32) | 0.95 (0.77 to 1.15) | 0.87 (0.56 to 1.35) | |||||
| Obese (≥30.0) | 0.91 (0.66 to 1.26) | 1.44 (0.77 to 2.68) | 0.92 (0.66 to 1.28) | 1.48 (0.80 to 2.75) | |||||
| Duration of education (years) | <10 | 1.00 | 0.52 | 1.00 | 0.07 | 1.00 | 0.37 | 1.00 | 0.06 |
| 10–12 | 0.99 (0.79 to 1.24) | 1.21 (0.74 to 1.98) | 1.00 (0.79 to 1.26) | 1.19 (0.73 to 1.96) | |||||
| >12 | 0.96 (0.74 to 1.24) | 1.79 (1.02 to 3.15 ) | 0.94 (0.73 to 1.20) | 1.81 (1.02 to 3.24 ) | |||||
| Annual household income | Low (<300 000 NOK) | 1.00 | 0.21 | 1.00 | 0.45 | 1.00 | 0.26 | 1.00 | 0.28 |
| Middle (300–600 000 NOK) | 0.93 (0.75 to 1.14) | 0.97 (0.58 to 1.64) | 0.92 (0.75 to 1.13) | 0.98 (0.57 to 1.67) | |||||
| High (>600 000 NOK) | 0.93 (0.64 to 1.35) | 0.53 (0.12 to 2.32) | 0.92 (0.65 to 1.30) | 0.43 (0.10 to 1.88) | |||||
| Alcohol intake (g/day) | None | 1.00 | 0.90 | 1.00 | 0.57 | 1.00 | 0.98 | 1.00 | 0.67 |
| ≤3.0 g | 1.17 (0.92 to 1.47) | 0.89 (0.57 to 1.40) | 1.18 (0.92 to 1.53) | 0.90 (0.58 to 1.39) | |||||
| >3.0–10.0 g | 1.02 (0.78 to 1.34) | 0.75 (0.39 to 1.42) | 1.06 (0.80 to 1.41) | 0.71 (0.39 to 1.31) | |||||
| >10.0 g | 1.01 (0.68 to 1.48) | 0.55 (0.19 to 1.62) | 1.03 (0.68 to 1.56) | 0.61 (0.20 to 1.80) | |||||
| Smoking status | Never | 1.00 | – | 1.00 | – | 1.00 | – | 1.00 | – |
| Former | 1.09 (0.88 to 1.34) | 0.89 (0.54 to 1.47) | 1.10 (0.89 to 1.36) | 0.76 (0.47 to 1.23) | |||||
| Current | 1.16 (0.92 to 1.46) | 1.98 (1.21 to 3.23 ) | 1.18 (0.93 to 1.49) | 1.60 (0.98 to 2.60) | |||||
| Red and processed meat (g/day) | ≤70 g | 1.00 | 0.69 | 1.00 | 0.31 | 1.00 | 0.77 | 1.00 | 0.46 |
| >70 g | 1.04 (0.83 to 1.30) | 0.96 (0.56 to 1.64) | 1.04 (0.83 to 1.29) | 0.90 (0.53 to 1.55) | |||||
| Fish intake (g/day) | ≤130 g | 1.00 | 0.04 | 1.00 | 0.75 | 1.00 | 0.03 | 1.00 | 0.70 |
| >130 g | 1.01 (0.82 to 1.23) | 1.05 (0.68 to 1.62) | 1.02 (0.82 to 1.26) | 1.06 (0.68 to 1.64) | |||||
| Vegetable and fruit intake (g/day) | ≤300 g | 1.00 | 0.53 | 1.00 | 0.06 | 1.00 | 0.32 | 1.00 | 0.04 |
| >300 g | 0.89 (0.74 to 1.07) | 0.61 (0.39 to 0.93 ) | 0.95 (0.79 to 1.14) | 0.61 (0.40 to 0.94 ) | |||||
| Vitamin D intake (μg/day) | ≤10.0 μg | 1.00 | 0.001 | 1.00 | 0.80 | 1.00 | 0.001 | 1.00 | 0.80 |
| >10.0 μg | 0.75 (0.61 to 0.92 ) | 0.91 (0.58 to 1.43) | 0.77 (0.62 to 0.96 ) | 1.03 (0.66 to 1.60) | |||||
| Diabetes mellitus | No | 1.00 | – | 1.00 | – | 1.00 | – | 1.00 | – |
| Yes | 1.19 (0.73 to 1.94) | 1.36 (0.48 to 3.84) | 1.13 (0.66 to 1.92) | 0.99 (0.32 to 3.02) | |||||
| Cardiovascular diseases | No | 1.00 | – | 1.00 | – | 1.00 | – | 1.00 | – |
| Yes | 0.98 (0.77 to 1.23) | 1.80 (1.17 to 2.77 ) | 0.90 (0.71 to 1.14) | 1.74 (1.13 to 2.68 ) | |||||
Boldfaced values are statistically significant.
*Stratified by CRC stages and adjusted for prediagnostic follow-up duration.
CRC, colorectal cancer; NOK, Norwegian kroner; SHR, subdistribution HR.
Participants with a prediagnostic physical activity level of 1–2 (compared with 5–6) had a small and non-significant lower cause-specific hazard of CRC death (HR=0.95, 95% CI 0.62 to 1.44), whereas the corresponding cause-specific hazard of non-CRC death was more than 100% higher (HR=2.14, 95% CI 1.05 to 4.37). The cause-specific HRs for the two competing events went in opposite directions. This same phenomenon was also demonstrated by prediagnostic CVD (table 2). Similar results were observed in corresponding cumulative incidence estimates. Prediagnostic current smoking was important only in non-CRC deaths (HR=1.98, 95% CI 1.21 to 3.23).
Our results revealed that participants with a prediagnostic vitamin D intake of >10 μg/day, compared with those with an intake of ≤10.0 µg/day, had a 25% lower cause-specific hazard of CRC death (HR=0.75, 95% CI 0.61 to 0.92, p trend=0.001) and a 23% lower cumulative incidence of CRC death (SHR=0.77, 95% CI 0.62 to 0.96, p trend=0.001). In both cause-specific and cumulative incidence approaches, our data did not show any association between prediagnostic BMI and CRC death. Similarly, no association was observed between other prediagnostic variables, such as duration of education, annual household income, alcohol intake, fish intake, and diabetes mellitus status, and CRC death.
Sensitivity analyses excluding those who died less than 1 year after recruitment and another excluding those who died less than 1 year after diagnosis did not change our findings in either of the competing risks approaches. When women diagnosed with CRC less than 1 year after recruitment were excluded to test for reverse causation, the analysis yielded similar estimates. We also conducted analyses that considered only incident CRC diagnosed before 31 December 2014, in order to allow for more follow-up time. However, for both of the aforementioned analyses, the associations and estimates of other variables remained essentially the same. None of the predefined interaction terms tested were statistically significant in any of the outcomes investigated.
The Arctic Circle divides Norway into approximately two equal parts. As a complementary analysis for vitamin D status through sunlight exposure, we conducted a parallel multivariable-adjusted analysis in which we compared CRC survival in participants living above and below the Arctic Circle. Those living above the Arctic Circle (North Norway) were at a non-significant higher risk of CRC death (HR=1.10, 95% CI 0.90 to 1.35) compared with the rest of Norway.
Discussion
We found a lower risk of CRC death associated with a prediagnostic vitamin D intake of >10 μg/day, with evidence of a monotonic relationship between the intake and the risk of CRC death, using competing mortality risks approach and chained multiple imputation. The results were consistent with those of the complete-case analysis. The lower risk of death associated with prediagnostic fruit and vegetable intake and the increased risk of death associated with prediagnostic current smoking were both more pronounced and statistically significant only for non-CRC death. The apparent reduction in the risk of CRC death in those with CVD that we observed could be explained via the effects of the variable on the competing cause of death (non-CRC death). The same phenomenon was also seen among participants with the lowest prediagnostic physical activity level. We did not find any evidence of association between prediagnostic BMI, annual household income, alcohol intake, red and processed meat intake, fish intake, and diabetes mellitus status, and CRC survival.
Our results regarding prediagnostic vitamin D intake and decreased risk of CRC death are consistent with findings from the European Prospective Investigation into Cancer and Nutrition (EPIC) study, in which prediagnostic vitamin D level was estimated directly by measuring circulating 25-hydroxyvitamin D (25(OH)D) levels in the blood. The EPIC study reported a 31% lowered risk of death in those within the highest quintile compared with the lowest quintile of 25(OH)D (adjusted HR=0.69, 95% CI 0.50 to 0.93).33 Some other studies found similar results,34–36 while others found no association.10 37 However, a recent updated systematic review and meta-analysis compared the highest and lowest categories of blood 25(OH)D and concluded that sufficient vitamin D offers better survival in patients with CRC (pooled HR=0.67, 95% CI 0.57 to 0.78).38 Physiologically, the most active molecular form of vitamin D, 1α,25-dihydroxyvitamin D3, has the capacity to inhibit cell proliferation, angiogenesis, and metastatic potential. It also induces differentiation and apoptosis in the cells of organs such as the large intestine.39 40
Few studies have investigated the association between prediagnostic fruit and vegetable intake and CRC-specific mortality, but the comparable studies that do exist found results similar to ours. A study using data from the Cancer Prevention Study-II (CPS-II) Nutrition Cohort did not find any association.41 That study used prediagnostic dietary patterns, characterised mainly by a high intake of fruits and vegetables (termed the prudent dietary pattern),41 and the American Cancer Society (ACS) Guidelines on Nutrition and Physical Activity for Cancer Prevention42 to score participants.41 43 The ACS score is based on the intake of at least five servings per day of a variety of mainly fruits and vegetables. Neither the prudent dietary pattern nor the ACS score-based dietary pattern was associated with CRC-specific mortality (HR=0.85, 95% CI 0.64 to 1.13, and HR=0.74, 95% CI 0.54 to 1.03, respectively).41 In contrast to CRC incidence, we did not find an association between prediagnostic combined red and processed meat intake and CRC survival. This is consistent with results from the EPIC study44 and the Western dietary pattern described in the CPS-II Nutrition Cohort study, which was characterised by a high intake of red and processed meats.41 A recent, large, pooled analysis of CRC survivors also did not find any association between the highest prediagnostic red or processed meat intake and CRC survival when compared with the lowest intake.45 However, consistently high prediagnosis and postdiagnosis red and processed meat intake has been associated with an increased risk of CRC death (HR=1.79, 95% CI 1.11 to 2.89).46 Similar to our findings, most previous studies found no evidence of an association between prediagnostic alcohol intake and CRC death.16 47 48 Interestingly, some studies posited that prediagnostic wine intake may favour CRC survival.48–50
Smoking is a well-known risk factor for many cancers, including CRC,51–53 and it has also been associated with overall mortality.54–56 Our study did not find any association between prediagnostic smoking status and CRC survival, although we observed an almost 100% increased risk of non-CRC death among participants who were current smokers prior to CRC diagnosis compared with never smokers. The lack of an association between prediagnostic smoking status and CRC death, and the presence of an association between this variable and non-CRC death could be attributed to the fact that smoking increases the incidence of several diseases and thus could indirectly increase the risk of non-CRC death. Nevertheless, a study using data from the CPS-II Nutrition Cohort found an association with prediagnostic current smoking but not former smoking.55 However, our findings are in agreement with the results of a recent meta-analysis of 14 prospective cohort studies on prediagnostic smoking status and CRC survival.56 The authors found no association between prediagnostic former smoking (pooled HR=1.00, 95% CI 0.91 to 1.09) or current smoking (pooled HR=1.15, 95% CI 0.95 to 1.41) and CRC survival, but they did find an association with overall survival.56 While higher education has been noted as a predictor of healthy lifestyle57 58 and is inversely related to CRC incidence in the NOWAC cohort,59 we found no association between duration of education and CRC survival.
The cause-specific HRs for the two competing events (CRC death and non-CRC death) in our study apparently went in opposite directions in the least physically active and those with CVD, which is consistent with the SHRs of cumulative incidence. This ‘opposite directions’ phenomenon was previously reported by Latouche et al 28 and Austin et al.60 This demonstrates that a variable could reduce the occurrence of the event of interest (CRC death) by increasing the occurrence of the competing event (non-CRC death). However, the variable does not necessarily affect the causal mechanism that produces the event of interest (CRC death). Nonetheless, patients with comorbidities such as diabetes mellitus and CVD are known to have lower odds of receiving treatment with a curative intent and to be at a greater risk of death than those without any comorbidity.61
Competing risks imply that a subject can experience a competing event that prevents the occurrence of the outcome of interest.60 The two approaches we used for handling the competing mortality risk data rendered similar results. These approaches could give different results because the composition of the risk sets in the two approaches differs,24 and especially if the competing event occurs early in follow-up and is frequent.24 62 63 In our study, the corresponding HRs and SHRs were similar numerically because the competing event (non-CRC death) was relatively infrequent.
The interpretation of these findings is subject to some limitations. One main limitation is that the prediagnostic lifestyle and dietary information we used was collected at recruitment, and only once before CRC diagnosis. Lifestyle and dietary habits could have changed before or after diagnosis and may have affected CRC survival. A repeat measurement of prediagnostic lifestyle and dietary factors could mitigate the impact of such changes. To minimise the impact of such changes during follow-up, we conducted sensitivity analyses restricted to CRC diagnosed within 10 years of recruitment. Even though we observed some changes, the estimates and associations (or lack thereof) remained essentially the same. Nonetheless, in a previous study of the NOWAC cohort, where prediagnosis and postdiagnosis assessments in CRC survivors were made, results showed only substantial changes in vegetable intake, BMI, and smoking status.64 Notably, over 50% of the participants quit smoking after their CRC diagnosis, compared with 20% in the cancer-free women.64 This may create a healthy ripple effect, leading to fewer comorbidities and an improved quality of life among CRC survivors. Second, we do not have access to the details of CRC treatment, and thus we were unable to evaluate treatment as an outcome modifier. CRC stage at diagnosis correlates with treatment options, but this will not completely assuage the limitation.65 Third, measurement errors and misclassification of variables are inherent in self-reported assessments of lifestyle and dietary habits (including overestimation or underestimation of social desirable behaviours), and unmeasured potential confounding factors may have influenced our estimates. For instance, vitamin D intake estimation was based on dietary intake and cod liver oil supplement intake; thus, intake of other vitamin D supplements or outdoor exposure to the solar radiation may have confounded these estimates. Moreover, we did not have data on family history of CRC and its precursors (such as colonic adenomas). Fourth, the relatively small size of some of the subgroups in our sample (for instance, in the most physically active participants) may have limited our analysis from detecting valid associations. Finally, we obtained information on cause of death from the Cause of Death Registry, and misclassification of the primary cause of death is a possibility we cannot completely rule out.66
The strengths of this study include its prospective nature, the large sample size, prediagnostic information on several important lifestyle and dietary factors, and the high quality of data in the CRN that was used to identify CRC cases. The use of chained multiple imputation to handle missing data maximises the number of CRC survivors in the analyses. Most lifestyle and dietary factors in the NOWAC Study have been validated previously.17 18 67 68
Conclusion
While we found no evidence of an association between CRC survival and prediagnostic physical activity, BMI, education, alcohol, or red and processed meat intake, our study showed that prediagnostic vitamin D intake could improve CRC survival. However, prediagnostic repeat measurements and/or postdiagnostic measurements would be desirable to draw a firmer conclusion.
Acknowledgments
We are grateful to the participants in the Norwegian Women and Cancer Study for their time and efforts in providing the data. Many thanks to Therese Haugdahl Nøst and Jan Håkon Rudolfsen for good discussions regarding R. We acknowledge Trudy Perdrix-Thoma of Professional Standards Editing for language review and editing. Some of the data in this article came from the Cancer Registry of Norway and Cause of Death Registry, but they are not responsible for the analysis or interpretation of the data presented.
Footnotes
Contributors: SOO and KBB conceived the initial study idea. SOO carried out the statistical analyses and drafted the manuscript. TB prepared the data and participated in the statistical analyses and critical revision of the manuscript. GS contributed to the statistical analyses and critical revision of the manuscript. KBB contributed to the statistical analyses, drafting, and critical revision of the manuscript. All authors approved the final manuscript version to be published and agreed to be accountable for all aspects of the work.
Funding: The publication charges for this article were funded from the publication fund of UiT The Arctic University of Norway. SOO, TB, SG, and KBB were supported by the Faculty of Health of UiT The Arctic University of Norway. The Faculty of Health, UiT The Arctic University of Norway did not contribute to the study design or data analysis nor did it influence the decision to submit the manuscript for publication.
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: The Norwegian Women and Cancer Study obtained approval from the Regional Committee for Medical Research Ethics and the Norwegian Data Inspectorate.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available upon reasonable request.
References
- 1. Arnold M, Sierra MS, Laversanne M, et al. . Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017;66:683–91. 10.1136/gutjnl-2015-310912 [DOI] [PubMed] [Google Scholar]
- 2. WCRF/AICR World cancer research fund International/American Institute for cancer research. How diet, nutrition and physical activity affect colorectal (bowel) cancer risk. continuous update project expert report; 2017.: WCRF international, 2017. Available: https://www.wcrf.org/dietandcancer/colorectal-cancer [Accessed 21 May 2019].
- 3. WHO World health organisation 2018. top 10 causes of deaths in high-income countries in 2016, 2018. Available: https://www.who.int/en/news-room/fact-sheets/detail/the-top-10-causes-of-death [Accessed 14 Dec 2018].
- 4. GLOBOCAN Estimated cancer incidence, mortality and prevalence worldwide in 2018, 2018. Available: http://globocan.iarc.fr/ [Accessed 07 Jun 2019].
- 5. Wu W, Guo F, Ye J, et al. . Pre- and post-diagnosis physical activity is associated with survival benefits of colorectal cancer patients: a systematic review and meta-analysis. Oncotarget 2016;7:52095–103. 10.18632/oncotarget.10603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vrieling A, Kampman E. The role of body mass index, physical activity, and diet in colorectal cancer recurrence and survival: a review of the literature. Am J Clin Nutr 2010;92:471–90. 10.3945/ajcn.2009.29005 [DOI] [PubMed] [Google Scholar]
- 7. Romaguera D, Ward H, Wark PA, et al. . Pre-Diagnostic concordance with the WCRF/AICR guidelines and survival in European colorectal cancer patients: a cohort study. BMC Med 2015;13:107 10.1186/s12916-015-0332-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lee J, Jeon J, Meyerhardt JA. Diet and lifestyle in colorectal cancer survivors. Hematol Oncol Clin North Am 2015;29:1–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Boyle T, Fritschi L, Platell C, et al. . Lifestyle factors associated with survival after colorectal cancer diagnosis. Br J Cancer 2013;109:814–22. 10.1038/bjc.2013.310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Weinstein SJ, Mondul AM, Yu K, et al. . Circulating 25-hydroxyvitamin D up to 3 decades prior to diagnosis in relation to overall and organ-specific cancer survival. Eur J Epidemiol 2018;33:1087–99. 10.1007/s10654-018-0428-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lund E, Kumle M, Braaten T, et al. . External validity in a population-based national prospective study--the Norwegian Women and Cancer Study (NOWAC). Cancer Causes Control 2003;14:1001–8. 10.1023/B:CACO.0000007982.18311.2e [DOI] [PubMed] [Google Scholar]
- 12. Lund E, Dumeaux V, Braaten T, et al. . Cohort profile: The Norwegian Women and Cancer Study--NOWAC--Kvinner og kreft. Int J Epidemiol 2008;37:36–41. 10.1093/ije/dym137 [DOI] [PubMed] [Google Scholar]
- 13. Larsen IK, Småstuen M, Johannesen TB, et al. . Data quality at the cancer registry of Norway: an overview of comparability, completeness, validity and timeliness. Eur J Cancer 2009;45:1218–31. 10.1016/j.ejca.2008.10.037 [DOI] [PubMed] [Google Scholar]
- 14. UICC International Union Against Cancer : Sobin LH, Gospodarowicz MK, TNM classification of malignant tumours. Ch. Wittekind: John Wiley & Sons, Ltd., Publication, 2009. [Google Scholar]
- 15. Amin MB, Greene FL, Edge SB, et al. . The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more "personalized" approach to cancer staging. CA Cancer J Clin 2017;67:93–9. 10.3322/caac.21388 [DOI] [PubMed] [Google Scholar]
- 16. Pelser C, Arem H, Pfeiffer RM, et al. . Prediagnostic lifestyle factors and survival after colon and rectal cancer diagnosis in the National Institutes of Health (NIH)-AARP Diet and Health Study. Cancer 2014;120:1540–7. 10.1002/cncr.28573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Borch KB, Ekelund U, Brage S, et al. . Criterion validity of a 10-category scale for ranking physical activity in Norwegian women. Int J Behav Nutr Phys Act 2012;9 10.1186/1479-5868-9-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hjartåker A, Andersen LF, Lund E. Comparison of diet measures from a food-frequency questionnaire with measures from repeated 24-hour dietary recalls. The Norwegian women and cancer study. Public Health Nutr 2007;10:1094–103. 10.1017/S1368980007702872 [DOI] [PubMed] [Google Scholar]
- 19. WCRF/AICR How diet, nutrition and physical activity affect colorectal (bowel) cancer risk. continuous update project expert report; 2017. limit red and processed meat 2018, 2018. Available: https://www.wcrf.org/dietandcancer/recommendations/limit-red-processed-meat
- 20. NordicCouncilofMinisters Nordic nutrition recommendations 2012. integrating nutrition and physical activity 2014. Available: http://norden.diva-portal.org/smash/get/diva2:704251/FULLTEXT01.pdf [Accessed 21 May 2019].
- 21. Dolejs J, Marešová P. Onset of mortality increase with age and age trajectories of mortality from all diseases in the four Nordic countries. Clin Interv Aging 2017;12:161–73. 10.2147/CIA.S119327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Prentice RL, Kalbfleisch JD, Peterson AV, et al. . The analysis of failure times in the presence of competing risks. Biometrics 1978;34:541–54. 10.2307/2530374 [DOI] [PubMed] [Google Scholar]
- 23. Hsu JY, Roy JA, Xie D, et al. . Statistical methods for cohort studies of CKD: survival analysis in the setting of competing risks. Clin J Am Soc Nephrol 2017;12:1181–9. 10.2215/CJN.10301016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Noordzij M, Leffondré K, van Stralen KJ, et al. . When do we need competing risks methods for survival analysis in nephrology? Nephrol Dial Transplant 2013;28:2670–7. 10.1093/ndt/gft355 [DOI] [PubMed] [Google Scholar]
- 25. Putter H, Fiocco M, Geskus RB. Tutorial in biostatistics: competing risks and multi-state models. Stat Med 2007;26:2389–430. 10.1002/sim.2712 [DOI] [PubMed] [Google Scholar]
- 26. Andersen PK, Geskus RB, de Witte T, et al. . Competing risks in epidemiology: possibilities and pitfalls. Int J Epidemiol 2012;41:861–70. 10.1093/ije/dyr213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fine JP, Gray RJ. A proportional hazards model for the Subdistribution of a competing risk. J Am Stat Assoc 1999;94:496–509. 10.1080/01621459.1999.10474144 [DOI] [Google Scholar]
- 28. Latouche A, Allignol A, Beyersmann J, et al. . A competing risks analysis should report results on all cause-specific hazards and cumulative incidence functions. J Clin Epidemiol 2013;66:648–53. 10.1016/j.jclinepi.2012.09.017 [DOI] [PubMed] [Google Scholar]
- 29. White IR, Carlin JB. Bias and efficiency of multiple imputation compared with complete-case analysis for missing covariate values. Stat Med 2010;29:2920–31. 10.1002/sim.3944 [DOI] [PubMed] [Google Scholar]
- 30. Sterne JAC, White IR, Carlin JB, et al. . Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ 2009;338:b2393–b93. 10.1136/bmj.b2393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rubin DB. Multiple imputation after 18+ years. J Am Stat Assoc 1996;91:473–89. 10.1080/01621459.1996.10476908 [DOI] [Google Scholar]
- 32. White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med 2011;30:377–99. 10.1002/sim.4067 [DOI] [PubMed] [Google Scholar]
- 33. Fedirko V, Riboli E, Tjønneland A, et al. . Prediagnostic 25-hydroxyvitamin D, VDR and CaSR polymorphisms, and survival in patients with colorectal cancer in Western European ppulations. Cancer Epidemiol Biomarkers Prev 2012;21:582–93. 10.1158/1055-9965.EPI-11-1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mezawa H, Sugiura T, Watanabe M, et al. . Serum vitamin D levels and survival of patients with colorectal cancer: Post-hoc analysis of a prospective cohort study. BMC Cancer 2010;10:347–47. 10.1186/1471-2407-10-347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ng K, Meyerhardt JA, Wu K, et al. . Circulating 25-hydroxyvitamin D levels and survival in patients with colorectal cancer. JCO 2008;26:2984–91. 10.1200/JCO.2007.15.1027 [DOI] [PubMed] [Google Scholar]
- 36. Ng K, Venook AP, Sato K, et al. . Vitamin D status and survival of metastatic colorectal cancer patients: results from CALGB/SWOG 80405 (Alliance). J Clin Oncol 2015;33:3503–03. 10.1200/jco.2015.33.15_suppl.3503 [DOI] [Google Scholar]
- 37. Jeffreys M, Redaniel MT, Martin RM. The effect of pre-diagnostic vitamin D supplementation on cancer survival in women: a cohort study within the UK clinical practice research Datalink. BMC Cancer 2015;15:670–70. 10.1186/s12885-015-1684-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Maalmi H, Walter V, Jansen L, et al. . Association between blood 25-hydroxyvitamin D levels and survival in colorectal cancer patients: an updated systematic review and meta-analysis. Nutrients 2018;10:896 10.3390/nu10070896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fuchs MA, Yuan C, Sato K, et al. . Predicted vitamin D status and colon cancer recurrence and mortality in CALGB 89803 (Alliance). Ann Oncol 2017;28:1359–67. 10.1093/annonc/mdx109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lamprecht SA, Lipkin M. Chemoprevention of colon cancer by calcium, vitamin D and folate: molecular mechanisms. Nat Rev Cancer 2003;3:601–14. 10.1038/nrc1144 [DOI] [PubMed] [Google Scholar]
- 41. Guinter MA, McCullough ML, Gapstur SM, et al. . Associations of pre- and postdiagnosis diet quality with risk of mortality among men and women with colorectal cancer. JCO 2018;36:3404–10. 10.1200/JCO.18.00714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kushi LH, Doyle C, McCullough M, et al. . American cancer Society guidelines on nutrition and physical activity for cancer prevention. CA Cancer J Clin 2012;62:30–67. 10.3322/caac.20140 [DOI] [PubMed] [Google Scholar]
- 43. McCullough ML, Gapstur SM, Shah R, et al. . Pre- and postdiagnostic diet in relation to mortality among breast cancer survivors in the CPS-II nutrition cohort. Cancer Causes Control 2016;27:1303–14. 10.1007/s10552-016-0802-x [DOI] [PubMed] [Google Scholar]
- 44. Ward HA, Norat T, Overvad K, et al. . Pre-Diagnostic meat and fibre intakes in relation to colorectal cancer survival in the European prospective investigation into cancer and nutrition. Br J Nutr 2016;116:316–25. 10.1017/S0007114516001859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Carr PR, Banbury BL, Berndt SI, et al. . Association Between Intake of Red and Processed Meat and Survival in Patients With Colorectal Cancer in a Pooled Analysis. Clin Gastroenterol Hepatol 10.1016/j.cgh.2018.11.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. McCullough ML, Gapstur SM, Shah R, et al. . Association between red and processed meat intake and mortality among colorectal cancer survivors. J Clin Oncol 2013;31:2773–82. 10.1200/JCO.2013.49.1126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Phipps AI, Baron J, Newcomb PA. Prediagnostic smoking history, alcohol consumption, and colorectal cancer survival: the Seattle colon cancer family registry. Cancer 2011;117:4948–57. 10.1002/cncr.26114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Phipps AI, Robinson JR, Campbell PT, et al. . Prediagnostic alcohol consumption and colorectal cancer survival: the colon cancer family registry. Cancer 2017;123:1035–43. 10.1002/cncr.30446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zell JA, McEligot AJ, Ziogas A, et al. . Differential effects of wine consumption on colorectal cancer outcomes based on family history of the disease. Nutr Cancer 2007;59:36–45. 10.1080/01635580701413926 [DOI] [PubMed] [Google Scholar]
- 50. Phipps AI, Shi Q, Limburg PJ, et al. . Alcohol consumption and colon cancer prognosis among participants in North central cancer treatment group phase III trial N0147. Int J Cancer 2016;139:986–95. 10.1002/ijc.30135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Botteri E, Iodice S, Bagnardi V, et al. . Smoking and colorectal cancer: a meta-analysis. JAMA 2008;300:2765–78. [DOI] [PubMed] [Google Scholar]
- 52. Parajuli R, Bjerkaas E, Tverdal A, et al. . The increased risk of colon cancer due to cigarette smoking may be greater in women than men. Cancer Epidemiology Biomarkers & Prevention 2013;22:862–71. 10.1158/1055-9965.EPI-12-1351 [DOI] [PubMed] [Google Scholar]
- 53. Leufkens AM, Van Duijnhoven FJB, Siersema PD, et al. . Cigarette smoking and colorectal cancer risk in the European prospective investigation into cancer and nutrition study. Clin Gastroenterol Hepatol 2011;9:137–44. 10.1016/j.cgh.2010.10.012 [DOI] [PubMed] [Google Scholar]
- 54. Baer HJ, Glynn RJ, Hu FB, et al. . Risk factors for mortality in the nurses' health study: a competing risks analysis. Am J Epidemiol 2011;173:319–29. 10.1093/aje/kwq368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yang B, Jacobs EJ, Gapstur SM, et al. . Active smoking and mortality among colorectal cancer survivors: the cancer prevention study II nutrition cohort. J Clin Oncol 2015;33:885–93. 10.1200/JCO.2014.58.3831 [DOI] [PubMed] [Google Scholar]
- 56. Ordóñez-Mena JM, Walter V, Schöttker B, et al. . Impact of prediagnostic smoking and smoking cessation on colorectal cancer prognosis: a meta-analysis of individual patient data from cohorts within the chances Consortium. Ann Oncol 2018;29:472–83. 10.1093/annonc/mdx761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lutz W, Kebede E. Education and health: redrawing the Preston curve. Popul Dev Rev 2018;44:343–61. 10.1111/padr.12141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hahn RA, Truman BI. Education improves public health and promotes health equity. Int J Health Serv 2015;45:657–78. 10.1177/0020731415585986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Oyeyemi SO, Braaten T, Botteri E, et al. . Exploring geographical differences in the incidence of colorectal cancer in the Norwegian women and cancer study: a population-based prospective study. Clin Epidemiol 2019;11:669–82. 10.2147/CLEP.S207413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Austin PC, Lee DS, Fine JP. Introduction to the analysis of survival data in the presence of competing risks. Circulation 2016;133:601–9. 10.1161/CIRCULATIONAHA.115.017719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Cuthbert CA, Hemmelgarn BR, Xu Y, et al. . The effect of comorbidities on outcomes in colorectal cancer survivors: a population-based cohort study. J Cancer Surviv 2018;12:733–43. 10.1007/s11764-018-0710-z [DOI] [PubMed] [Google Scholar]
- 62. Dignam JJ, Zhang Q, Kocherginsky M. The use and interpretation of competing risks regression models. Clin Cancer Res 2012;18:2301–8. 10.1158/1078-0432.CCR-11-2097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Van Der Pas S, Nelissen R, Fiocco M. Different competing risks models for different questions may give similar results in arthroplasty registers in the presence of few events. Acta Orthop 2018;89:145–51. 10.1080/17453674.2018.1427314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Skeie G, Hjartåker A, Braaten T, et al. . Dietary change among breast and colorectal cancer survivors and cancer-free women in the Norwegian women and cancer cohort study. Cancer Causes Control 2009;20:1955–66. 10.1007/s10552-009-9390-3 [DOI] [PubMed] [Google Scholar]
- 65. Jayasekara H, English DR, Haydon A, et al. . Associations of alcohol intake, smoking, physical activity and obesity with survival following colorectal cancer diagnosis by stage, anatomic site and tumor molecular subtype. Int J Cancer 2018;142:238–50. 10.1002/ijc.31049 [DOI] [PubMed] [Google Scholar]
- 66. Alfsen GC, Mæhlen J. The value of autopsies for determining the cause of death. Tidsskr Nor Laegeforen 2012;132:147–51. 10.4045/tidsskr.11.0427 [DOI] [PubMed] [Google Scholar]
- 67. Skeie G, Mode N, Henningsen M, et al. . Validity of self-reported body mass index among middle-aged participants in the Norwegian women and cancer study. Clin Epidemiol 2015;7:313–23. 10.2147/CLEP.S83839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Parr CL, Veierød MB, Laake P, et al. . Test-Retest reproducibility of a food frequency questionnaire (FFQ) and estimated effects on disease risk in the Norwegian women and cancer study (NOWAC). Nutr J 2006;5:4 10.1186/1475-2891-5-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjgast-2019-000338supp001.pdf (44.3KB, pdf)