Abstract
Objective
While extensive research revealed that interleukin (IL)-1β contributes to insulin resistance (IR) development, the role of IL-1α in obesity and IR was scarcely studied. Using control, whole body IL-1α knockout (KO) or myeloid-cell-specific IL-1α-deficient mice, we tested the hypothesis that IL-1α deficiency would protect against high-fat diet (HFD)-induced obesity and its metabolic consequences.
Research design and methods
To induce obesity and IR, control and IL-1α KO mice were given either chow or HFD for 16 weeks. Glucose tolerance test was performed at 10 and 15 weeks, representing early and progressive stages of glucose intolerance, respectively. Liver and epididymal white adipose tissue (eWAT) samples were analyzed for general morphology and adipocyte size. Plasma levels of adiponectin, insulin, total cholesterol and triglyceride (TG), lipoprotein profile as well as hepatic lipids were analyzed. Expression of lipid and inflammation-related genes in liver and eWAT was analyzed. Primary mouse hepatocytes isolated from control mice were treated either with dimethyl sulfoxide (DMSO) (control) or 20 ng/mL recombinant IL-1α for 24 hours and subjected to gene expression analysis.
Results
Although total body weight gain was similar, IL-1α KO mice showed reduced adiposity and were completely protected from HFD-induced glucose intolerance. In addition, plasma total cholesterol and TG levels were lower and HFD-induced accumulation of liver TGs was completely inhibited in IL-1α KO compared with control mice. Expression of stearoyl-CoA desaturase1 (SCD1), fatty acid synthase (FASN), elongation of long-chain fatty acids family member 6 (ELOVL6), acetyl-CoA carboxylase (ACC), key enzymes that promote de-novo lipogenesis, was lower in livers of IL-1α KO mice. Treatment with recombinant IL-1α elevated the expression of ELOVL6 and FASN in mouse primary hepatocytes. Finally, mice with myeloid-cell-specific deletion of IL-1α did not show reduced adiposity and improved glucose tolerance.
Conclusions
We demonstrate a novel role of IL-1α in promoting adiposity, obesity-induced glucose intolerance and liver TG accumulation and suggest that IL-1α blockade could be used for treatment of obesity and its metabolic consequences.
Keywords: obesity, glucose intolerance, de novo lipogenesis, interleukin-1
Significance of this study.
What is already known about this subject?
Interleukin (IL)-1β contributes to insulin resistance development. A functional polymorphism of IL-1α in humans suggested its potential role in obesity.
What are the new findings?
Gene deletion of IL-1α in mice, reduced adiposity and completely protected from HFD-induced glucose intolerance.
IL-1α deficiency reduced plasma TG and cholesterol, lowered the expression of genes that promote hepatic de-novo lipogenesis and completely inhibited hepatic TG accumulation.
How might these results change the focus of research or clinical practice?
IL-1α blockade could be used for treatment of obesity and its metabolic consequences.
Introduction
The incidence of obesity and associated insulin resistance (IR) has risen dramatically in the past two decades and this pathophysiological defect is an important predictor for progression to type 2 diabetes.1 Adipose tissue inflammation, particularly when involving visceral fat, is now a well-recognized manifestation of obesity, promoting both IR and fatty liver formation.2
Interleukin (IL)-1α and IL-1β, the main agonists of the IL-1 family of cytokines, stimulate the expression of a range of inflammatory genes. IL-1α, IL-1β and the specific receptor antagonist (IL-1Ra) exert their effects on binding to IL-1 receptor type 1 (IL-1R1).3 4 IL-1α and IL-1β are synthesized as precursors and their processing to mature forms requires specific cellular proteases. In contrast to IL-1β, which is only active as a mature secreted molecule after cleavage by caspase-1, IL-1α exerts its effects both in the mature and the precursor forms when binding to IL-1R1. IL-1α belongs to a newly recognized group of dual-function cytokines that play a role in sterile inflammation and tissue remodeling.3 5 Non-immune cells constitutively express low levels of IL-1α protein.3 6–8 Following an apoptotic stimuli, the IL-1α precursor translocates to the nucleus, binds to chromatin and is retained in the cells.9 Necrosis of cells releases IL-1α, which serves as a “danger signal” by binding to IL-1R1 on neighboring cells to induce sterile inflammation manifested by recruitment of inflammatory cells.7 10 11 The secretion of IL-1α can also be a regulated process and is either inflammasome/caspase-1 dependent or independent.11–14 While the role of IL-1β, IL-1R1 and IL-1Ra in obesity and IR was investigated, the role of IL-1α was scarcely studied.15 Gene deletion of the inflammasome components, which regulate the activity of IL-1β, either prevented obesity and/or reduced obesity-induced IR and hepatic steatosis.16–19 In addition, IL-1β deficiency increased adipose tissue expandability yet with reduced inflammation and reduced fat accumulation in the liver.20 Lack of IL-1R1 itself also protected against high-fat diet (HFD)-induced IR despite immune cell recruitment but with reduced local adipose tissue inflammation.21 Administration of IL-1Ra to obese mice markedly reduced steatosis and hepatic lipogenic gene expression demonstrating that IL-1β signaling upregulates hepatic lipogenesis in obesity.22 Moreover, IL-1Ra seems to contribute to the development of IR in a mechanism independent of IL-1Ra binding to IL-1R1.23 The pro-atherogenic role of IL-1α and specifically bone marrow-derived IL-1α was reported by us and confirmed by work from another laboratory.4 7 12 Recently, a few reports suggested a possible role of IL-1α in obesity-associated comorbidities. A functional polymorphism of IL-1α and its potential role in obesity in humans and mice was reported.24 In addition, IL-1α was shown to be important in immune cell recruitment following the injection of endogenous oils isolated from human adipocytes into mouse peritoneum.25 Although these reports suggest the involvement of IL-1α in obesity, there are no studies that directly addressed its role in diet-induced obesity. Therefore, in this work, we studied the effect of IL-1α deficiency on diet-induced obesity in mice.
Research design and methods
Animals and diets
IL-1α-Loxp mice were generated by the Taconic Artemis Company (Cologne, Germany) by introducing flanked Loxp sites between the coding exons 2–5.26 27 IL-1α knockout (KO) mice that were generated by Iwakura only lack exon 5 of the IL-1α gene. Therefore, we generated mice with complete deficiency of the IL-1α gene by crossing IL-1α-Loxp mice with a General-Cre-deleter mouse (Taconic Artemis Company, Cologne, Germany), resulting in the deletion of exons 2–5 in all body cells.27 To generate mice with a myeloid-cell-specific deletion of IL-1α, mice expressing a Cre recombinase transgene from the Lysozyme M locus (LyzM-Cre), which were purchased from Jackson, were mated with IL-1α-Loxp mice, to generate LyzM-Cre-IL-1α mice as described.27 Mice were bred and kept at the animal facility of the Sheba Medical Center, Tel Hashomer, Israel. The Animal Care Committee of the Sheba Medical Center approved animal studies and the animals received human care. To induce obesity and IR, 6-week old, male control IL-1α-Loxp (n=12) and IL-1α KO (n=10) mice were given 45% HFD (45% kcal from fat, 19% kcal from protein, 36.2% kcal from carbohydrate, TD06415, Harlan). The control chow diet used was Teklad Global 18% protein rodent diet (18% kcal from fat, 24% kcal from protein, 58% kcal from carbohydrates, 2018SC, Harlan). The use of a control diet, which does not completely match the HFD (especially with regard to fiber source), is a study limitation.28 Chow-fed mice from both genotypes (n=7–9 in each group) were used as control. Body weight was recorded weekly throughout the experiment. Blood was collected after a 16-hour fast, by a puncture of the inferior vena cava or the retro orbital sinus. At the end of the experiment, animals were sacrificed by inhaling CO2.
Tissue preparation and histology
Livers and epididymal white adipose tissue (eWAT) were fixed in 4% paraformaldehyde and embedded in paraffin. All samples were routinely stained for general morphology with hematoxylin and eosin (H&E).
Adipocyte size
Morphometry of individual fat cells was assessed using digital image analysis. Microscopic images were digitized in 24-bit red/green/blue (specimen level pixel size 1.28×1.28 μm2). Recognition of fat cells was initially performed by applying a region-growing algorithm on manually indicated seed points. If required, results of region growing were interactively corrected. From the resulting regions, the area and minimum Feret diameter were calculated. The minimum Feret diameter is useful because fat cells are generally (near) convex objects. For each specimen, all fat cells in 3–5 microscopic fields of view were measured. On average, 465 fat cells were measured per specimen (range 335–591). Differences were evaluated using Student’s t-test.
Glucose tolerance test
Glucose tolerance test (GTT) was performed on 4-hour fasted mice by giving an intraperitoneal injection of D-glucose (2 g/kg body weight). Glucose levels in tail blood were measured at indicated time points after glucose administration using a glucometer system and the glucose area under curve (AUC) was calculated.
Analysis of plasma insulin and adiponectin levels
Plasma adiponectin concentrations were determined using a RIA kit (Linco Research, USA). Insulin levels were assessed using a mouse Insulin ELISA kit (Mercodia, Sweden).
Analysis of plasma lipoproteins
Analysis of total plasma cholesterol and triglyceride (TG) levels was performed using an automated enzymatic technique (Boehringer Mannheim, Germany). Plasma lipoproteins were separated by size exclusion chromatography using a Superose-6 column (1×30 cm) on an FPLC system (Pharmacia).4
Hepatic lipids
Hepatic lipids were extracted according to the method of Folch et al 29 and total liver lipids distribution was analyzed by thin-layer chromatography (TLC) as described in Shomonov-Wagner et al and Gilat et al.30 31
Primary hepatocytes isolation
Primary mouse hepatocytes were isolated from IL-1α-Loxp male mice by collagen perfusion and percoll gradient purification as described.32 Isolated hepatocytes were treated either with dimethyl sulfoxide (DMSO) (control) or 20 ng/mL recombinant IL-1α (PeproTech) for 24 hours. At the end of the experiment, cells were harvested for analysis of gene expression (as described below).
RNA isolation and cDNA synthesis
Total RNA from eWAT, livers or isolated hepatocytes was isolated using Rneasy Mini Kit (Qiagen) or Nucleospin RNA II Kit, respectively, and treated with DNase (Machery-Nagel) to eliminate genomic DNA contamination. First-strand cDNA was synthesized using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems).
Analysis of gene expression by quantitative real-time PCR
Real-time PCR for individual genes was carried out using a 7500 sequence detection System (Applied Biosystems). cDNA was amplified using Faststart Universal Probe Master (Rox) with the specific primers and probes designed with the Probe Library Assay Design Center (http://www.roche-applied-science.com/sis/rtpcr/upl/adc.jsp) (Roche). Target gene expression levels were normalized to mRNA levels of HPRT (∆Ctsample = Cttarget gene − Ctreference gene) and relative quantification was analyzed using the 2−(∆∆Ct) method.
Statistical analysis
We conducted a two-way analysis of variance (ANOVA) and to examine the origin of the significance, we have conducted a simple effect analysis. Student’s t-test was performed when applicable. Three-way mixed design ANOVA (two between-subject variables: diet and mice and one within-subject variable: time) was applied to compare changes in body weight over the study period and in blood glucose levels over the GTT. Values were reported as the mean±SE. The cut-off for statistical significance was set at a p value of 0.05 or below. All statistical analyses were performed with SPSS statistical analysis software.
Results
IL-1α deficiency reduced eWAT weight and adipocyte size without affecting total body weight
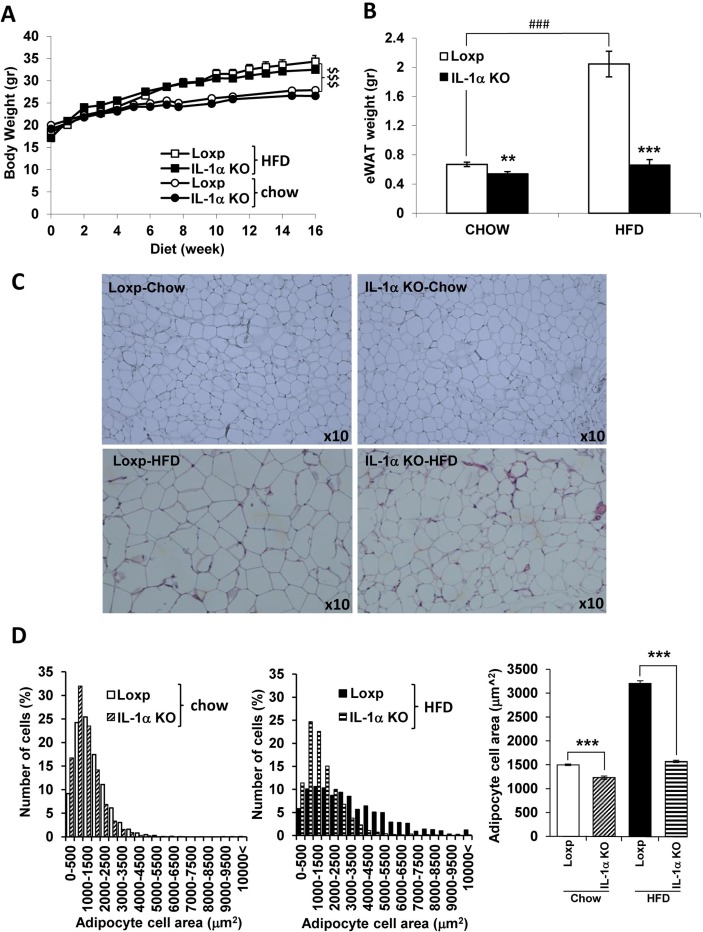
At the age of 6 weeks, male Loxp and IL-1α KO mice were fed regular chow or HFD providing 45% energy in the form of fat, for 16 weeks. There was no difference in food consumption between Loxp and IL-1α KO mice (data not shown). Although both Loxp and IL-1α KO mice gained more weight compared with chow diet-fed mice, there was no significant difference in body weight between the genotypes (figure 1A). Despite their similar total body weight, eWAT weight of IL-1α KO was fourfold lower compared with HFD-fed Loxp mice and similar to eWAT weight of chow-fed Loxp mice (figure 1B). Interestingly, eWAT weight was slightly lower in chow-fed IL-1α KO compared with chow-fed Loxp mice (figure 1B). Histology of eWAT with H&E and adipocyte size quantification (figure 1C,D, respectively) demonstrated an increase in adipocyte size (hypertrophy) in HFD-fed Loxp compared with chow-fed Loxp mice. The adipocyte size was significantly lower in HFD-fed and chow-fed IL-1α KO compared with Loxp mice (figure 1D).
Figure 1.
IL-1α deficiency reduced eWAT weight and adipocyte size without affecting total body weight. (A) Body weight, (B) eWAT weight, (C) eWAT histology with H&E and (D) adipocyte size quantification in male Loxp and IL-1α KO mice (6 weeks of age at start of dietary intervention) fed either regular chow or HFD (n=7–12 per group) for 16 weeks. Data are presented as mean±SE. Asterisk/dollar/Hash marks depict statistically significant differences. **p≤0.01 ***p≤0.001 to Loxp. ###p≤0.001 to chow (two-way ANOVA). $$$p≤0.001 between chow to HFD (three-way mixed design ANOVA). ANOVA, analysis of variance; H&E, hematoxylin and eosin; HFD, high-fat diet; IL, interleukin; KO, knockout.
IL-1α deficiency prevented the onset of HFD-induced glucose intolerance and attenuated fasting plasma insulin and adiponectin levels
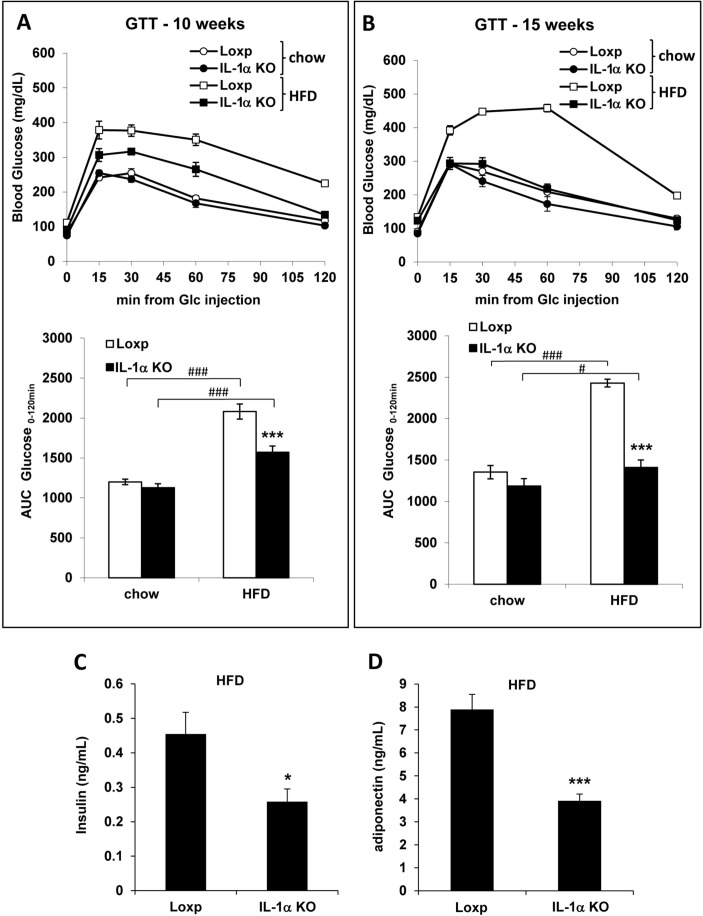
The HFD induced glucose intolerance in Loxp mice as shown in the GTT at 10 and 15 weeks (figure 2A and B, respectively). The glucose AUC was 25% and 42% lower in HFD-fed IL-1α KO compared with Loxp mice at 10 and 15 weeks, respectively. Moreover, after 15 weeks, the glucose AUC of HFD-fed IL-1α KO mice was similar to chow-fed Loxp mice. After 8 weeks of HFD, fasting plasma insulin and adiponectin levels were about twofold lower in IL-1α KO compared with Loxp mice (figure 2C and D).
Figure 2.
IL-1α deficiency prevented the onset of HFD-induced glucose intolerance and attenuated fasting plasma insulin and adiponectin levels. GTT and glucose AUC at 10 (A) and 15 (B) weeks of HFD. Fasting plasma levels of insulin (C) and adiponectin (D) after 8 weeks of HFD. Data are presented as mean±SE. Asterisks/Hash marks depict statistically significant differences. *p≤0.05; ***p≤0.001 to Loxp. #p≤0.05 ###p≤0.001 to chow (student’s t-test or two-way ANOVA). ANOVA, analysis of variance; AUC, area under curve; GTT, glucose tolerance test; HFD, high-fat diet; IL, interleukin; KO, knockout.
Fasting plasma cholesterol and TG levels were lower in IL-1α KO compared with Loxp mice
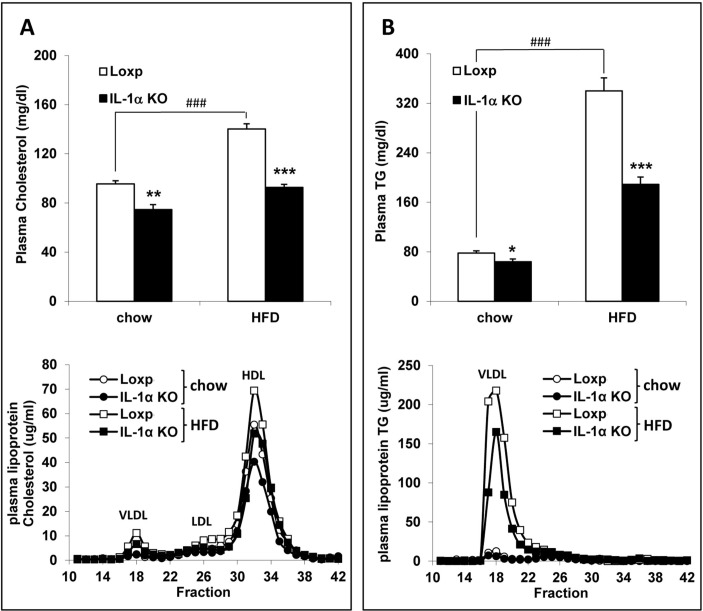
Analysis of fasting plasma lipids at 8 weeks showed that total plasma cholesterol and TG levels were significantly lower in chow-fed IL-1α KO mice compared with Loxp mice. Furthermore, this effect of IL-1α deficiency was more pronounced and very significant in the HFD-fed Loxp compared with IL-1α KO mice (figure 3A,B, upper panel). Further analysis of lipoprotein profile with FPLC revealed that the difference in total plasma cholesterol levels was due to lower cholesterol in the very low-density lipoprotein (VLDL), low-density lipoprotein (LDL) and high-density lipoprotein (HDL) fractions (figure 3A, lower panel) and TG levels were lower in the VLDL fraction (figure 3B, lower panel).
Figure 3.
Fasting plasma cholesterol and TG levels were lower in IL-1α KO compared with Loxp mice. Fasting total plasma cholesterol (A, upper panel) and TG (B, upper panel) levels in male Loxp and IL-1α KO mice fed either regular chow or HFD (n=7–12 per group) for 8 weeks. Analysis of the distribution of plasma lipoprotein cholesterol (A, lower panel) and TG (B, lower panel) was performed with FPLC. Blood was obtained from fasted animals and plasma samples were pooled in each group. Data are presented as mean±SE. Asterisks/Hash marks depict statistically significant differences. ***p≤0.001 to Loxp. ###p≤0.001 to chow (two-way ANOVA). ANOVA, analysis of variance; FPLC, fast protein liquid chromatography; HFD, high-fat diet; IL, interleukin; KO, knockout; TG, triglyceride.
IL-1α deficiency completely inhibited HFD-induced accumulation of liver TGs
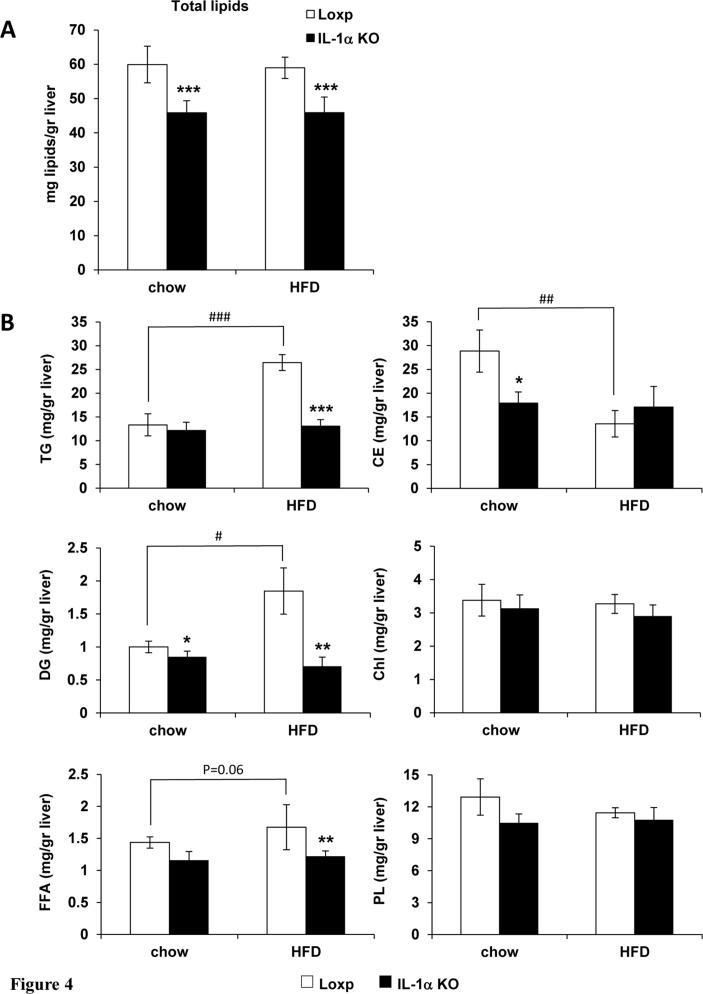
Liver histology revealed that the HFD induced a mild microvesicular fatty change, which was similar in Loxp as well as IL-1α KO mice. Importantly, there was no evidence of inflammation in livers of HFD-fed Loxp or IL-1α KO mice (data not shown). We performed a more detailed analysis of liver lipids and assessed total liver lipid weight normalized to liver weight and the lipid composition with TLC. The HFD did not elevate the normalized total liver lipid weight in both mouse strains (figure 4A). However, total liver lipid weight was 25% lower in IL-1α KO compared with Loxp mice both on chow and HFD (figure 4A). Despite no elevation of total lipid weight in Loxp mice, the HFD induced a redistribution of liver lipids with elevation of TGs, diglycerides and free fatty acids (FFA) on the expense of cholesterol esters, which was completely inhibited in IL-1α KO mice (figure 4B). In addition, liver cholesterol esters and diglycerides were lower on chow diet in IL-1α KO compared with Loxp mice (figure 4B).
Figure 4.
IL-1α deficiency completely inhibited HFD-induced accumulation of liver TGs. Liver specimens were obtained from male Loxp and IL-1α KO mice fed either regular chow or HFD (n=7–12 per group) for 16 weeks. (A) Total lipid weight in the liver normalized to liver weight. (B) Analysis of hepatic lipids with TLC. Data are presented as mean±SE. Asterisks/Hash marks depict statistically significant differences. *p≤0.05; **p≤0.01; ***p≤0.001 to Loxp. #p≤0.05; ##p≤0.01; ###p≤0.001 to chow (two-way ANOVA). ANOVA, analysis of variance; HFD, high-fat diet; IL, interleukin; KO, knockout; TG, triglyceride; TLC, thin-layer chromatography.
The HFD did not elevate the expression of inflammatory cytokines in adipose tissue or the liver
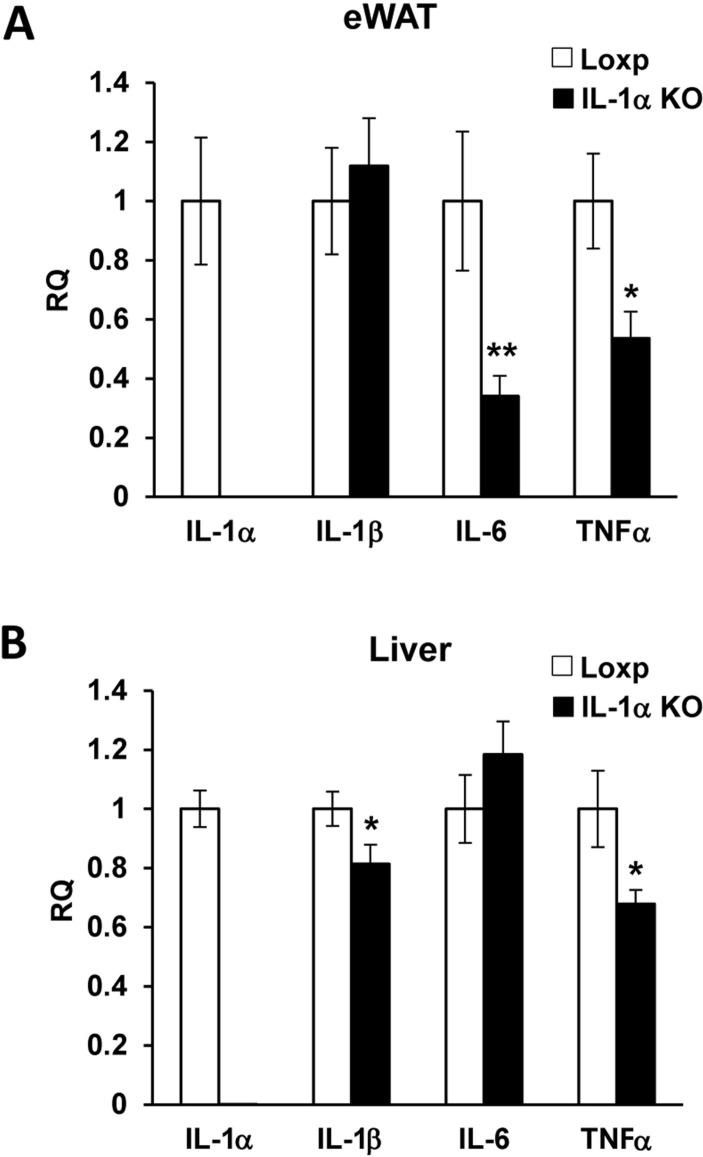
The HFD we used (45%) did not significantly elevate the mRNA levels of IL-6, tumor necrosis factor alpha (TNFα), IL-1β and IL-1α in adipose tissue or the liver (data not shown). The mRNA levels of IL-6 and TNFα in eWAT and of IL-1β and TNFα in the liver were lower in HFD-fed IL-1α KO compared with Loxp mice (figure 5A,B).
Figure 5.
The mRNA levels of IL-6 and TNFα in eWAT were lower in HFD-fed IL-1α KO compared with Loxp mice. The mRNA expression level of IL-1α, IL-1β, IL-6 and TNFα in eWAT (A) and liver (B) of Loxp and IL-1α KO mice-fed HFD for 16 weeks. Data are presented as mean±SE. Asterisks depict statistically significant differences. *p≤0.05; **p≤0.01 to Loxp (student’s t-test). ANOVA, analysis of variance; HFD, high-fat diet; IL, interleukin; KO. knockout; TG, triglyceride; TNF, tumor necrosis factor.
IL-1α deficiency lowered the expression of genes that promote hepatic de-novo lipogenesis
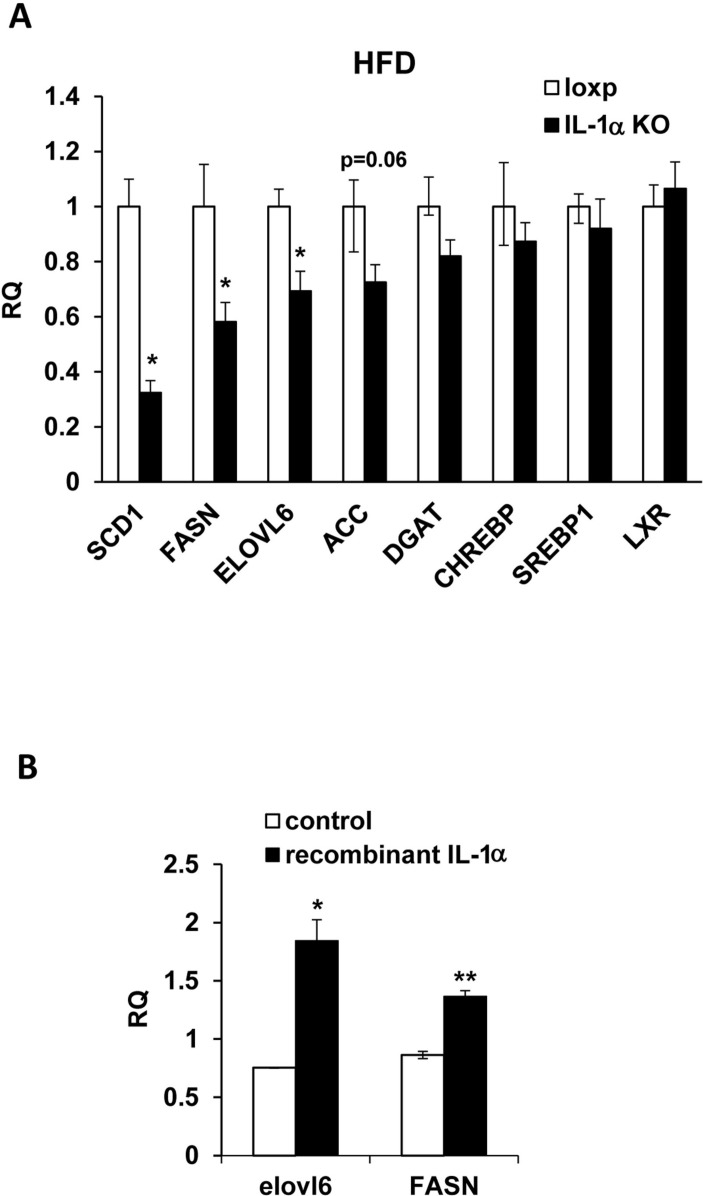
The marked reduction of eWAT weight, adipocyte size and hepatic TG levels, in IL-1α KO compared with Loxp mice, encouraged us to look at the expression levels of genes that promote de-novo lipogenesis (DNL) such as stearoyl-CoA desaturase1 (SCD1), fatty acid synthase (FASN), elongation of long-chain fatty acids family member 6 (ELOVL6), acetyl-CoA carboxylase α (ACCα), diacylglycerol acyltransferase (DGAT), the primary regulators of DNL including liver X receptors (LXR), sterol regulatory element-binding protein-1c (Srebpf-1c) and carbohydrate response element binding protein (ChREBP), and cholesterol metabolism related genes such as 3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMGCR), cholesterol 7 alpha-hydroxylase (Cyp7a) and LDL receptor (LDLR). The HFD did not induce an elevation in expression of these genes in the liver. Yet, the mRNA levels of SCD1, FASN, ELOVL6 and ACCα were significantly lower in IL-1α KO compared with Loxp mice (figure 6A), with similar levels of DGAT, LXR, Srebpf-1c, ChREBP, HMGCR, Cyp7a and LDLR (Fig. 6A and online supplementary fig. S1). In addition, we assessed the direct effect of IL-1α on the expression of genes that promote DNL in hepatocytes. Treatment with recombinant IL-1α significantly elevated the mRNA expression levels of FASN and ELOVL6 in primary mouse hepatocytes (figure 6B).
Figure 6.
IL-1α deficiency lowered the expression of genes that promote hepatic DNL. (A) The mRNA expression levels of SCD1, FASN, ELOVL6, ACCα, DGAT, ChREBP, Srebpf-1c and LXR in livers of Loxp (control) and IL-1α KO mice-fed HFD for 16 weeks. (B) mRNA expression levels of ELOVL6 and FASN in primary mouse hepatocytes treated either with DMSO (control) or recombinant IL-1α for 24 hours. Data are presented as mean±SE. Asterisks depict statistically significant differences. *p≤0.05; **p≤0.01 to control mice/cells (student’s t-test). ACCα, acetyl-CoA carboxylase α; ChREBP, carbohydrate response element binding protein; DGAT, diacylglycerol acyltransferase; DMSO, dimethyl sulfoxide; DNL, de-novo lipogenesis; ELOVL6, elongation of long-chain fatty acids family member 6; FASN, fatty acid synthase; IL, interleukin; KO, knockout; LXR, liver X receptor; SCD1, stearoyl-CoA desaturase1; Srebpf-1c, sterol regulatory element-binding protein 1.
bmjdrc-2019-000650supp001.pdf (1.1MB, pdf)
Myeloid-cell-specific deletion of IL-1α did not attenuate diet-induced adiposity and glucose intolerance
At the age of 6 weeks, Loxp and LysMcre-IL-1α male mice were fed HFD providing 45% energy in the form of fat, for 16 weeks. There was no significant difference between the genotypes in body weight, liver weight, and % liver to body weight, eWAT weight or ALT (online supplementary fig. S2 A-F, respectively). In addition, glucose intolerance was similar in both genotypes, as shown in the GTT and glucose AUC (online supplementary fig. S2 G-H, respectively) as well as similar insulin levels (online supplementary fig. S2 I). Moreover, analysis of fasting plasma lipids at 8 weeks of HFD showed that total plasma cholesterol and TG levels were similar in LysMcre-IL-1α compared with Loxp mice (online supplementary fig. S2 J-K, respectively) and further analysis of lipoprotein profile with FPLC revealed similar profile of VLDL, LDL and HDL cholesterol and TG (online supplementary fig. S2 L-M, respectively).
Discussion
While the role of other members of the IL-1 family was extensively investigated, the role of IL-1α in obesity and IR is yet to be elucidated. In this work, we studied the effect of IL-1α deficiency in obesity and IR at an early stage of fat accumulation using a diet-induced obesity mouse model with minimal adipose tissue or hepatic inflammation.
IL-1α deficiency had dramatic effects on adiposity, glucose intolerance and hepatic DNL. IL-1α deficiency (1) inhibited eWAT expansion and adipocyte hypertrophy. (2) Prevented the onset of HFD-induced glucose intolerance. (3) Inhibited the HFD-induced accumulation of liver TGs and lowered the expression of genes that promote hepatic DNL. (4) Attenuated the elevation of plasma cholesterol and TG levels. (5) Specific deficiency of IL-1α in myeloid cells did not improve adiposity and glucose intolerance.
IL-1α deficiency inhibited eWAT expansion and adipocyte hypertrophy
Despite similar body weight gain while consuming the HFD, IL-1α-deficient mice had a dramatically lower eWAT mass, smaller adipocyte size and improved glucose intolerance compared with wild-type (WT) mice. This observation of no effect of IL-1α deficiency on body weight is not in line with the report in humans that functional polymorphism of IL-1α is correlated with increased body weight.24 In contrast to our results, gene deletion of Caspase-1, ASC or NLRP3 in mice, which reduces the activity of IL-1β, protected from development of diet induced obesity and as a consequence improved insulin sensitivity.18 Similar to our results, IL-1β-deficient mice had a similar body weight gain and improved insulin sensitivity while fed HFD. Yet, IL-1β-deficient mice had a larger eWAT mass compared with WT mice.20 Similarly, despite increased adipose tissue depot weight, IL-1RI deficiency reduced adipose tissue inflammation and IR. These results suggest that IL-1β and IL-1RI have differential effects on adipose tissue expandability, inflammation and insulin sensitivity. The smaller eWAT mass and adipocyte size in IL-1α KO mice compared with the phenotype of IL-1β KO and IL-1RI KO mice, points toward a specific biological function of IL-1α in HFD-induced expansion of adipose tissue. Further work is required to study how IL-1α promotes adipocyte hypertrophy and/or adipogenesis under HFD.
IL-1α deficiency prevented the onset of HFD-induced glucose intolerance
Expansion of WAT and adipocyte hypertrophy is associated with tissue hypoxia and cell death and DAMP released from necrotic adipocytes promote adipose tissue macrophage infiltration and development of systemic IR.33–35 The expression or release of IL-1α is known to increase in adipose tissue in response to cell death, cell senescence or aging.36 The HFD used in our model did not elevate the mRNA expression levels of inflammatory cytokines such as IL-1b, IL-6, TNF and IL-1α in adipose tissue or the liver. This is similar to previous studies in which the HFD-induced obesity model did not change or even reduced the mRNA expression levels of IL-1α and IL-1β in the liver and adipose tissue, respectively.24 37 This is in contrast to the elevated serum IL-1α protein levels in obese compared with lean mice.24 These results raise the possibility that serum protein IL-1α levels are elevated due to its release from necrotic adipocytes and hepatocytes during obesity affecting neighboring adipocytes and hepatocyte in a paracrine and autocrine fashion. We suggest that expansion of adipose tissue and release of IL-1α from dying adipocytes promotes the development of glucose intolerance by interfering with insulin signaling in adipocytes. Indeed, IL-1α was shown to inhibit insulin signaling in 3T3-L1 adipocytes by phosphorylating insulin receptor substrate-1 on serine residues.15 In addition, long-term IL-1α treatment was shown to inhibit insulin signaling via IL-6 production and SOCS3 expression.38 Our results showing lack of effect on glucose intolerance in mice with deficiency of IL-1α in myeloid cells support the notion that indeed it is IL-1α from adipocytes, which is critical for the development of diet-induced adiposity and glucose intolerance in mice.
This is complementary to the fact that necrotic human adipocytes may function as an immunological “danger signal” which are capable of promoting IL-1α-dependent recruitment of neutrophils and macrophages into adipose tissue.25 We are currently generating mice with gene deletion of IL-1α in adipocytes to test our hypothesis that adipocyte-IL-1α indeed is critical to development of diet-induced adiposity and glucose intolerance.
IL-1α deficiency inhibited liver TG accumulation
The accumulation of TGs in obesity-associated fatty liver disease is attributed mainly to fatty acids derived from increased lipolysis of TG from adipose tissue due to IR as well as from hepatic DNL.39 Hepatic DNL, in turn, can be upregulated both by the hormonal signal of insulin as well as by hepatic-derived factors that locally act to promote DNL.40 Lower plasma insulin levels in HFD-fed IL-1α KO mice compared with WT mice is one contributing factor to lower hepatic DNL. In addition, we found that IL-1α treatment in primary mouse hepatocytes upregulates the expression of the DNL genes FASN and ELOVL6. Therefore, we propose that IL-1α released from necrotic hepatocytes due to fat accumulation could directly stimulate neighboring hepatocytes and upregulate the expression of genes that promote hepatic DNL. Indeed, the expression of hepatic FASN and ELOVL6 as well as liver TG levels was lower in IL-1α-deficient compared with WT mice. Similar to our results, Um et al showed that administration of IL-1α increased both liver and plasma TGs in mice.24 In addition, our results are in line with the effects shown by IL-1β which upregulated the expression of FASN and TG accumulation in primary mouse hepatocytes.22
Clinical implications
Recently, the Cantos study showed that lowering inflammation by targeting IL-1β signaling results in favorable cardiovascular outcome in patients with previous myocardial infarction without affecting plasma lipids.41 So far, there was no clinical trial testing the effect of IL-1α neutralization in the context of the metabolic syndrome. The first study, which tested the effect of IL-1α neutralization, was in adults with metastatic cancer using the MABp1 monoclonal antibody cloned from a human being to target IL-1α. The treatment was well tolerated, showed biological activity including positive effects on body composition and quality of life, and showed some anti-tumor activity.42 In previous preclinical studies of our group, later confirmed by another laboratory, we showed that IL-1α deficiency protected from atherosclerosis development and progression.4 7 12 In addition, we discovered that lack of IL-1α or IL-1β inhibits transformation of steatosis to steatohepatitis and liver fibrosis in hypercholesterolemic mice.5 The new data in the present study show that IL-1α deficiency also reduces adiposity, glucose intolerance and hepatic DNL in diet-induced obese mice. Altogether, these studies indicate that inhibiting IL-1α signaling could have beneficial effects on obesity-associated comorbidities including impaired glucose tolerance, dyslipidemia, fatty liver disease and atherosclerosis. Therefore, IL-1α could be an attractive therapeutic target for treating these deadly conditions.
Acknowledgments
The authors thank Dr Esfir Ulman for contributing to lipid analysis and Dr Edna Peleg for measuring plasma adiponectin levels.
Footnotes
TA and MKK contributed equally.
Contributors: TA and MKK directed the project, performed experiments and wrote the manuscript. AS directed the project, contributed to animal experiments, commented on the manuscript and approved the final version of the manuscript. HL contributed to animal experiments and gene expression analysis. GS contributed to gene expression analysis. IB analyzed liver histology. RS performed adipocyte size analysis. YL contributed to primary hepatocytes isolation. ALF contributed to hepatic lipid analysis and data interpretation. AH contributed to animal experiments and data interpretation. YB commented on the manuscript. RA created complete IL-1α and myeloid-cell-specific IL-1α knockout mice, DH contributed to data interpretation. YK conceived the project, directed the project and wrote the manuscript. YK is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Funding: The study was partly supported by the Sheba Talipot Medical Leadership programme (YK) and D-Cure grants (TA).
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: All data relevant to the study are included in the article or uploaded as supplementary information.
References
- 1. Miranda PJ, DeFronzo RA, Califf RM, et al. Pathophysiology, and mechanisms. Am Heart J 2005;149:33–45. [DOI] [PubMed] [Google Scholar]
- 2. Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu Rev Immunol 2011;29:415–45. 10.1146/annurev-immunol-031210-101322 [DOI] [PubMed] [Google Scholar]
- 3. Dinarello CA. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood 2011;117:3720–32. 10.1182/blood-2010-07-273417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kamari Y, Werman-Venkert R, Shaish A, et al. Differential role and tissue specificity of interleukin-1α gene expression in atherogenesis and lipid metabolism. Atherosclerosis 2007;195:31–8. 10.1016/j.atherosclerosis.2006.11.026 [DOI] [PubMed] [Google Scholar]
- 5. Kamari Y, Shaish A, Vax E, et al. Lack of interleukin-1α or interleukin-1β inhibits transformation of steatosis to steatohepatitis and liver fibrosis in hypercholesterolemic mice. J Hepatol 2011;55:1086–94. 10.1016/j.jhep.2011.01.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shemesh S, Kamari Y, Shaish A, et al. Interleukin-1 receptor type-1 in non-hematopoietic cells is the target for the pro-atherogenic effects of interleukin-1 in apoE-deficient mice. Atherosclerosis 2012;222:329–36. 10.1016/j.atherosclerosis.2011.12.010 [DOI] [PubMed] [Google Scholar]
- 7. Kamari Y, Shaish A, Shemesh S, et al. Reduced atherosclerosis and inflammatory cytokines in apolipoprotein-E-deficient mice lacking bone marrow-derived interleukin-1α. Biochem Biophys Res Commun 2011;405:197–203. 10.1016/j.bbrc.2011.01.008 [DOI] [PubMed] [Google Scholar]
- 8. Kandel-Kfir M, Almog T, Shaish A, et al. Interleukin-1α deficiency attenuates endoplasmic reticulum stress-induced liver damage and CHOP expression in mice. J Hepatol 2015;63:926–33. 10.1016/j.jhep.2015.05.012 [DOI] [PubMed] [Google Scholar]
- 9. Cohen I, Rider P, Carmi Y, et al. Differential release of chromatin-bound IL-1alpha discriminates between necrotic and apoptotic cell death by the ability to induce sterile inflammation. Proc Natl Acad Sci U S A 2010;107:2574–9. 10.1073/pnas.0915018107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rider P, Carmi Y, Guttman O, et al. IL-1α and IL-1β Recruit Different Myeloid Cells and Promote Different Stages of Sterile Inflammation. J.i. 2011;187:4835–43. 10.4049/jimmunol.1102048 [DOI] [PubMed] [Google Scholar]
- 11. Groß O, Yazdi AS, Thomas CJ, et al. Inflammasome activators induce interleukin-1α secretion via distinct pathways with differential requirement for the protease function of caspase-1. Immunity 2012;36:388–400. 10.1016/j.immuni.2012.01.018 [DOI] [PubMed] [Google Scholar]
- 12. Freigang S, Ampenberger F, Weiss A, et al. Fatty acid–induced mitochondrial uncoupling elicits inflammasome-independent IL-1α and sterile vascular inflammation in atherosclerosis. Nat Immunol 2013;14:1045–53. 10.1038/ni.2704 [DOI] [PubMed] [Google Scholar]
- 13. Zheng Y, Humphry M, Maguire JJ, et al. Intracellular interleukin-1 receptor 2 binding prevents cleavage and activity of interleukin-1α, controlling necrosis-induced sterile inflammation. Immunity 2013;38:285–95. 10.1016/j.immuni.2013.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yazdi AS, Drexler SK. Regulation of interleukin 1α secretion by inflammasomes: Table 1. Ann Rheum Dis 2013;72(Suppl 2):ii96–9. 10.1136/annrheumdis-2012-202252 [DOI] [PubMed] [Google Scholar]
- 15. He J, Usui I, Ishizuka K, et al. Interleukin-1α Inhibits Insulin Signaling with Phosphorylating Insulin Receptor Substrate-1 on Serine Residues in 3T3-L1 Adipocytes. Mol Endocrinol 2006;20:114–24. 10.1210/me.2005-0107 [DOI] [PubMed] [Google Scholar]
- 16. Vandanmagsar B, Youm Y-H, Ravussin A, et al. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med 2011;17:179–88. 10.1038/nm.2279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wen H, Gris D, Lei Y, et al. Fatty acid–induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat Immunol 2011;12:408–15. 10.1038/ni.2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Stienstra R, van Diepen JA, Tack CJ, et al. Inflammasome is a central player in the induction of obesity and insulin resistance. Proc Natl Acad Sci U S A 2011;108:15324–9. 10.1073/pnas.1100255108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stienstra R, Mandard S, Tan NS, et al. The interleukin-1 receptor antagonist is a direct target gene of PPARα in liver. J Hepatol 2007;46:869–77. 10.1016/j.jhep.2006.11.019 [DOI] [PubMed] [Google Scholar]
- 20. Nov O, Shapiro H, Ovadia H, et al. Interleukin-1β Regulates Fat-Liver Crosstalk in Obesity by Auto-Paracrine Modulation of Adipose Tissue Inflammation and Expandability. PLoS One 2013;8:e53626 10.1371/journal.pone.0053626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. McGillicuddy FC, Harford KA, Reynolds CM, et al. Lack of interleukin-1 receptor I (IL-1RI) protects mice from high-fat diet-induced adipose tissue inflammation coincident with improved glucose homeostasis. Diabetes 2011;60:1688–98. 10.2337/db10-1278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Negrin KA, Roth Flach RJ, DiStefano MT, et al. Il-1 signaling in obesity-induced hepatic lipogenesis and steatosis. PLoS One 2014;9:e107265 10.1371/journal.pone.0107265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Franck N, Maris M, Nalbandian S, et al. Knock-Down of IL-1ra in obese mice decreases liver inflammation and improves insulin sensitivity. PLoS One 2014;9:e107487 10.1371/journal.pone.0107487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Um J-Y, Rim H-K, Kim S-J, et al. Functional polymorphism of IL-1 alpha and its potential role in obesity in humans and mice. PLoS One 2011;6:e29524 10.1371/journal.pone.0029524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tynan GA, Hearnden CH, Oleszycka E, et al. Endogenous oils derived from human adipocytes are potent adjuvants that promote IL-1α–Dependent inflammation. Diabetes 2014;63:2037–50. 10.2337/db13-1476 [DOI] [PubMed] [Google Scholar]
- 26. Bersudsky M, Luski L, Fishman D, et al. Non-Redundant properties of IL-1α and IL-1β during acute colon inflammation in mice. Gut 2014;63:598–609. 10.1136/gutjnl-2012-303329 [DOI] [PubMed] [Google Scholar]
- 27. Almog T, Kandel-Kfir M, Shaish A, et al. Knockdown of interleukin-1α does not attenuate LPS-induced production of interleukin-1β in mouse macrophages. Cytokine 2015;73:138–43. 10.1016/j.cyto.2015.01.029 [DOI] [PubMed] [Google Scholar]
- 28. Pellizzon MA, Ricci MR. The common use of improper control diets in diet-induced metabolic disease research confounds data interpretation: the fiber factor. Nutr Metab 2018;15 10.1186/s12986-018-0243-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. The Journal of biological chemistry 1957;226:497–509. [PubMed] [Google Scholar]
- 30. Shomonov-Wagner L, Raz A, Leikin-Frenkel A. Alpha linolenic acid in maternal diet halts the lipid disarray due to saturated fatty acids in the liver of mice offspring at weaning. Lipids Health Dis 2015;14:14 10.1186/s12944-015-0012-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gilat T, Leikin-Frenkel A, Goldiner I. Prevention of diet-induced fatty liver in experimental animals by the oral administration of a fatty acid bile acid conjugate (FABAC). Hepatology 2003;38:436–42. 10.1053/jhep.2003.50348 [DOI] [PubMed] [Google Scholar]
- 32. Lustig Y, Ruas JL, Estall JL, et al. Separation of the gluconeogenic and mitochondrial functions of PGC-1{alpha} through S6 kinase. Genes Dev 2011;25:1232–44. 10.1101/gad.2054711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hosogai N, Fukuhara A, Oshima K, et al. Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes 2007;56:901–11. 10.2337/db06-0911 [DOI] [PubMed] [Google Scholar]
- 34. Alkhouri N, Gornicka A, Berk MP, et al. Adipocyte apoptosis, a link between obesity, insulin resistance, and hepatic steatosis. J Biol Chem 2010;285:3428–38. 10.1074/jbc.M109.074252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kuroda M, Sakaue H. Adipocyte death and chronic inflammation in obesity. J Med Invest 2017;64:193–6. 10.2152/jmi.64.193 [DOI] [PubMed] [Google Scholar]
- 36. Sun X, Zou T, Zuo C, et al. IL-1α inhibits proliferation and adipogenic differentiation of human adipose-derived mesenchymal stem cells through NF-κB- and ERK1/2-mediated proinflammatory cytokines. Cell Biol Int 2018;42:794–803. 10.1002/cbin.10932 [DOI] [PubMed] [Google Scholar]
- 37. Wang F, Zhao B. Uba6 and its bispecific pathways for ubiquitin and FAT10. Int J Mol Sci 2019;20:2250 10.3390/ijms20092250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Uno T, He J, Usui I, et al. Long-Term interleukin-1α treatment inhibits insulin signaling via IL-6 production and SOCS3 expression in 3T3-L1 adipocytes. Horm Metab Res 2008;40:8–12. 10.1055/s-2007-1004515 [DOI] [PubMed] [Google Scholar]
- 39. Kawano Y, Cohen DE. Mechanisms of hepatic triglyceride accumulation in non-alcoholic fatty liver disease. J Gastroenterol 2013;48:434–41. 10.1007/s00535-013-0758-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Czech MP, Tencerova M, Pedersen DJ, et al. Insulin signalling mechanisms for triacylglycerol storage. Diabetologia 2013;56:949–64. 10.1007/s00125-013-2869-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ridker PM, Everett BM, Thuren T, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 2017;377:1119–31. 10.1056/NEJMoa1707914 [DOI] [PubMed] [Google Scholar]
- 42. Hong DS, Hui D, Bruera E, et al. Mabp1, a first-in-class true human antibody targeting interleukin-1α in refractory cancers: an open-label, phase 1 dose-escalation and expansion study. Lancet Oncol 2014;15:656–66. 10.1016/S1470-2045(14)70155-X [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjdrc-2019-000650supp001.pdf (1.1MB, pdf)