Abstract
Immune checkpoint inhibitors are novel oncological medications, current classes of which include monoclonal antibodies that target inhibitory receptors cytotoxic T lymphocyte-associated antigen 4 (CTLA-4), programmed death 1 protein (PD-1) and programmed death-ligand 1. While they are novel in their ability to treat cancer, they also have a unique spectrum of immune-related adverse events. Renal-related immune adverse events, though rare, are an increasingly recognised clinical entity. We present the case of a 67-year-old man with acute kidney injury (AKI) after the second cycle of combination anti-CTLA-4 and anti-PD-1 antibodies for metastatic cutaneous melanoma. He presented with vomiting and diarrhoea, and AKI secondary to dehydration was treated with aggressive rehydration. After failing to recover biochemically, a renal biopsy was performed, which demonstrated severe acute interstitial nephritis. The culprit medications were held and he was treated with steroids. With immunosuppression, creatinine improved to pretreatment values.
Keywords: renal system, chemotherapy, unwanted effects / adverse reactions, acute renal failure
Background
Immune checkpoint inhibitors (CPIs) have revolutionised oncological treatment in many tumour groups. Melanoma in particular shows limited response to chemotherapy, with survival from traditional chemotherapeutic agents being limited to months.1 CPIs release the immune system from specific inhibitory ‘check points’ meant to allow for self-tolerance, and to prevent an excessive inflammatory response.2 This causes the patient’s own immune system to act against the malignancy, as opposed to conventional chemotherapeutic agents, which are directly cytotoxic.3 With the innovation of immunotherapy also comes a unique spectrum of side effects, immune-related adverse events (irAEs). Renal irAEs, though rare, are being increasingly recognised. The increased use of these medications in treating chemorefractory cancers will lead to patients presenting to internists, emergency room (ER) physicians and nephrologists, who will be increasingly tasked to treat these unavoidable, but often clinically manageable, side effects.
Case presentation
A 67-year-old man presented to the ER with 3 days of acute onset of diarrhoea, nausea and vomiting. He had received cycle 2 of combination ipilimumab with nivolumab 3 days prior for treatment of metastatic melanoma. On arrival, he was clinically dehydrated (decreased skin turgor, dry mucous membranes) and hypotensive (blood pressure (BP) 89/58 mm Hg). Oxygen saturation was 98% on room air, respiratory rate 18/min and heart rate 110 beats/min. He appeared chronically ill but was not in any acute distress. He gave a history of feeling generally unwell, with ~10 loose watery stools per day, nausea and vomiting for 3 days prior to arrival. There was a preceding history of weight loss as a result of oesophageal stricture from prior radiation, which had resolved with balloon dilatation.
Comorbidities included hypertension, dyslipidemia and gastroesophageal reflux disease. Medications included valsartan-hydrochlorothiazide 80/25 mg/day, rosuvastatin 10 mg/day, pantoprazole 40 mg/day, diclofenac for malignancy-associated pain and multivitamins. He occasionally used CBD oil for side effects of treatment. There was no family history of malignancy, and no relevant contributory social factors.
Eleven months prior to presentation, he had been diagnosed with primary malignant melanoma of the right upper back, and he underwent primary resection of the lesion. Staging CT was indeterminate for metastatic lesions, and positron emission tomography (PET) scan showed involvement to the right axillary lymph nodes, and he subsequently underwent wide excision at the primary site to obtain a 2 cm margin, as well as axillary lymph node dissection. Final stage was IIIc, pT3a pN3 (10/30) cM0. Postaxillary dissection, he underwent radiotherapy (48 Gy in 20 fractions) to his postoperative right axilla and supraclavicular fossa for local control of the disease. Postradiation treatment was complicated by development of high-grade stricture of the proximal oesophagus requiring balloon dilation, which led to improvement in nausea and return of his appetite.
Dysphagia prompted follow-up imaging, revealing multiple nodules on CT scan that had not been previously PET avid, but had increased in size and number. Follow-up imaging with PET revealed diffuse metastatic disease, with multiple skeletal, splenic, gastric, adrenal, subcutaneous and nodal metastases. The patient was started on nivolumab and ipilimumab combination therapy. The patient had presented to the ER 3 days postcycle 2 of combination immunotherapy—24 days postinitiation of treatment. At that time, serum creatinine was 237 μmol/L (baseline 72 μmol/L) with urea 18.0 mmol/L. Urinalysis revealed moderate blood and leucocytes with no proteinuria. Despite intravenous fluids, and discontinuation of valsartan-hydrochlorothiazide, pantoprazole and diclofenac, there was no biochemical recovery. Three days later, the creatinine increased further to 383 μmol/L.
Acting on case reports of acute kidney injury (AKI) from CPI use, he underwent a kidney biopsy. He was also simultaneously introduced to steroids with intravenous methylprednisolone 2 mg/kg for 3 days per European Society for Medical Oncology guidelines, then transitioned to 1 mg/kg of prednisone.4 Introduction of steroids led to an impressive reduction in his serum creatinine and improvement to the diarrhoea, and within a week creatinine was near pre-CPI initiation value at 85 μmol/L. (table 1).
Table 1.
Serial creatinine values in micromole/L
| Baseline | On arrival | Posthydration | 2 weeks poststeroids | 4 weeks poststeroids | |
| Creatinine (μmol/L) | 72 | 237 | 383 | 189 | 85 |
On l ight microscopy of the biopsy, there were 17 glomeruli in each representative sample. Three of the glomeruli were globally sclerotic. Glomeruli were normocellular and completely within normal limits. There were multiple aggregates of chronic inflammatory infiltrate in the interstitium pushing apart the tubules. The inflammation was mostly composed of histiocytes, lymphocytes and a few plasma cells. Immunohistochemical reveals that the aggregate was composed of a mixture of CD8+ve/CD4+ve T cells with rare B cells. CD8 T cells predominant and intracytoplasmic cytotoxic markers. Mild tubulitis with 1–2 lymphocytes per tubule is associated with the inflammation. Tubules involved by the inflammation show features of severe injury (figures 1 and 2). On immunofluorescence , there were nine glomeruli and they stained negative for immunoglobulins, complements fragments and light chains. Electron microscopy was unremarkable and did not reveal any glomerular involvement.
Figure 1.

(A) Multiple foci of interstitial inflammation marked by black arrows (H&E—100x). There are also scattered lymphoplasmacytic infiltration in between cortical tubules (B) (H&E—400×). (C,D) Dense interstitial inflammation with accompanying lymphocytic tubulitis (black arrows) causing serious damage in tubular epithelial cells (PAS—100x and 400x). PAS, Periodic acid-Schiff.
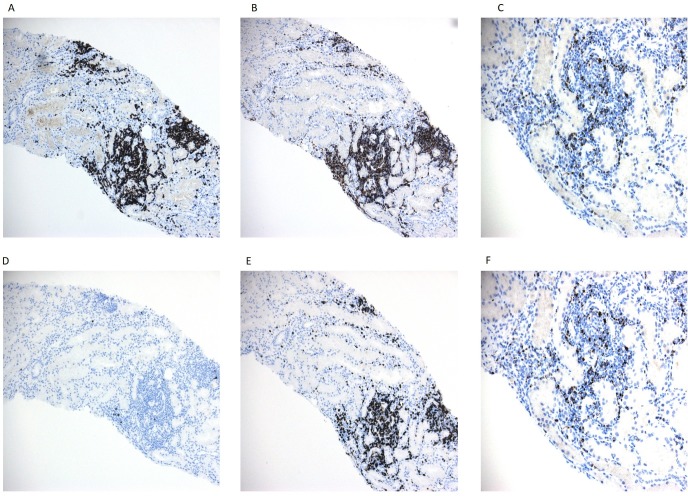
Figure 2.
Immunohistochemical staining of lymphoid markers is used to characterise the infiltrating lymphocytic population. The inflammatory lymphocytes are composed of exclusively T cells expressing CD3 (A), are negative for CD20, B cell marker, (B) and are the admixture of CD4+ and CD8+ (C,D) cells. Most of the infiltrating cells are cytotoxic and express cytotoxic molecules, TIA-1 and perforin (E,F). Light microscopy—200x magnification.
Differential diagnosis
Though the patient was clinically dehydrated on arrival to our institution, he was well hydrated by the time he underwent a kidney biopsy and unlikely to have resulted in the pattern of injury seen on biopsy. The patient was on non-steroidal anti-inflammatory and proton pump inhibitor (PPI), both of which can lead to tubular inflammation and can cause tubulointerstitial nephritis. However, he was taking the two medications for close to 2 years, and the drugs had been held and reintroduced on multiple occasions in the past (based on clinical need) without evidence of renal injury. Furthermore, the interstitial inflammation provoked by classic and common medications such as PPI or nonsteroidal anti-inflammatory drug (NSAID) is commonly comprised of T lymphocytes along with a prominent population of plasma cells and/or B cells. In many instances, particularly in case of PPI, a dominant eosinophil population is also present, which is suggestive of an allergic component. Typically, CD4+ T cells is the most abundant type of lymphocyte.4 In contrast, there were rare B cells or plasma cells and no polymorphonuclear cells among the infiltrate. CD8+ T cells with strong positivity for cytotoxic markers comprise the majority of the infiltrating lymphocytes in our case. These factors taken together these factors point to acute interstitial nephritis (AIN) due to CPIs.5
Discussion
This case highlights the need for maintaining a high index of clinical suspicion for irAE in patients with a history of CPI use. Conventional chemotherapeutic agents may cause AKI via a variety of mechanisms which can result in direct cellular toxicity.5 6 CPIs are a relatively new class of agents that treat a variety of malignancies by releasing the immune system from specific inhibitory check points which allow for self-tolerance, and prevent an excessive inflammatory response.7 8 Cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and programmed death-1 protein (PD-1) are both examples of checkpoint receptors that negatively regulate T-cell activation and blunt T-cell function.9 CPIs are antibodies designed to block these negative regulators and help stimulate the immune system to control and kill tumour cells. Ipilimumab, a CTLA-4 receptor antagonist, and nivolumab, a PD-1 receptor antagonist, both improve overall survival in patients with metastatic melanoma.10 11 Nivolumab plus ipilimumab yields greater objective response rates, progression-free survival and overall survival compared with ipilimumab alone.12 Unleashing the immune system from negative regulation comes with the potential for immune side effects, through a loss of self-tolerance and excessive inflammatory activity. The most common organ systems affected by adverse autoimmune effects include skin, colon, endocrine organs, liver and lungs.5 CPI-induced AKI is being increasingly observed as the use of CPIs in oncology continues to expand. ER physicians, internists and nephrologists will be increasingly tasked with diagnosing and managing AKI that may be related to these agents.
In a combined analysis of 3695 patients treated with a CPI, the overall incidence of AKI was 2.2%, and the incidence of grade III or IV AKI, defined as an increase in creatinine >3-fold above baseline, an increase in creatinine to a level >350 μmol/L, or the need for renal replacement therapy, was 0.6%.3 Cortazar et al after analysing phase II and III data observed that AKI occurred more frequently in patients who received combination therapy with ipilimumab and nivolumab (4.9%) than in patients who received monotherapy with ipilimumab (2.0%), nivolumab (1.9%) or pembrolizumab (1.4%).13 14 The cumulative data thus far suggest that incidence of AKI is low. Manohar et al conducted a meta-analysis of 48 trials of 11 482 patients receiving PD-1 inhibitors and concluded that the incidence of AKI was 2.2%.15 There is often a period of latency between CPI initiation and renal irAE that makes it clinically difficult to detect association. The onset of AKI with PD-1 inhibitors is usually late (3–10 months), compared with CTLA-4 antagonist-related injury that occurs earlier (2–3 months).16 17 The most common biopsy finding was acute interstitial nephritis, with glomerular involvement being a distant second.18–20 The delayed presentation is in stark contrast to irAE’s in other target organs, such as skin lesions, which occur within the first few weeks, hypophysitis and hepatic involvement which occurs at 6 weeks post-treatment, and colitis which tends to occur at 5–10 weeks post-treatment. 20 The heterogeneity of the time course and delayed response are suggestive of a mechanism distinct from typical drug-induced AIN. Thus, CPI-induced AIN may be due to ‘reprogramming’ of the immune system, leading to loss of tolerance against endogenous kidney antigens, as opposed to the delayed-type hypersensitivity response characteristic of other drugs.21 The mechanisms leading to CPI-induced AKI were elucidated in an elegantly written, recently published article by Perazella et al, in which they speculate that CPIs may favour development of autoantibodies pathogenic to the kidney. Tissues may normally express checkpoint receptors, and once they bind to anti-CPI antibodies with resulting immune reaction, causing the formation of new or reactivated T cells against tumour antigens that cross-react with off target kidney tissues.22 The National Comprehensive Cancer Network guidelines based on the severity of the kidney injury were recently published (table 2).23
Table 2.
Proposed CPI nephrotoxicity management, National Comprehensive Cancer Network guidelines
| Grade | Acute kidney injury | Intervention |
| Grade 1 | Creatinine 1.5-2 times above baseline, increase of 27 μmol/L. |
|
| Grade 2 | Creatinine 2-3 times above baseline. |
|
| Grade 3 (severe) Grade 4 |
Creatinine >3 times above baseline or > 355 μmol/L On dialysis. |
|
Outcome and follow-up
The creatinine fell to pre-CPI treatment values 6 weeks postintroduction of steroids. He improved from a renal perspective, but declined without immunotherapy from his underlying malignancy. Two months later, he chose to transition to comfort care
Learning points.
Immune-related adverse renal events are rare compared with side effects in other organ systems.
The period of latency between checkpoint inhibitor (CPI) initiation and renal immune-related adverse event may cause difficulty in clinically detecting the association.
CPI-induced acute kidney injury (AKI) is being increasingly observed as the use of CPIs in oncology continues to expand. Emergency room physicians, internists and nephrologists will be increasingly tasked with diagnosing and managing AKI that may be related to these agents.
Footnotes
Contributors: LG created the initial draft. PD contributed to the slides. KH contributed to the drafts and BP assisted LG and coordinated between authors for multiple versions of the draft. All the authors have read the final version and agree with the content.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Next of kin consent obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Middleton MR, Grob JJ, Aaronson N, et al. Randomized phase III study of temozolomide versus dacarbazine in the treatment of patients with advanced metastatic malignant melanoma. J Clin Oncol 2000;18:158–66. 10.1200/JCO.2000.18.1.158 [DOI] [PubMed] [Google Scholar]
- 2. Ashour T, Nakhoul G, Patil P, et al. Immune check point inhibitor-associated glomerulonephritis. Kidney Int Rep 2019;4:355–9. 10.1016/j.ekir.2018.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Postow MA, Callahan MK, Wolchok JD. Immune checkpoint blockade in cancer therapy. J Clin Oncol 2015;33:1974–82. 10.1200/JCO.2014.59.4358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Praga M, Sevillano A, Auñón P, et al. Changes in the aetiology, clinical presentation and management of acute interstitial nephritis, an increasingly common cause of acute kidney injury. Nephrol Dial Transplant 2015;30:1472–9. 10.1093/ndt/gfu326 [DOI] [PubMed] [Google Scholar]
- 5. Bender WL, Whelton A, Beschorner WE, et al. Interstitial nephritis, proteinuria, and renal failure caused by nonsteroidal anti-inflammatory drugs. immunologic characterization of the inflammatory infiltrate. Am J Med 1984;76:1006–12. 10.1016/0002-9343(84)90849-0 [DOI] [PubMed] [Google Scholar]
- 6. Haanen JBAG, Carbonnel F, Robert C, et al. Management of toxicities from immunotherapy: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2017;28:iv119–42. 10.1093/annonc/mdx225 [DOI] [PubMed] [Google Scholar]
- 7. Rosner MH, Perazella MA. Acute kidney injury in patients with cancer. N Engl J Med 2017;376:1770–81. 10.1056/NEJMra1613984 [DOI] [PubMed] [Google Scholar]
- 8. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012;12:252–64. 10.1038/nrc3239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dong H, Strome SE, Salomao DR, et al. Tumor-Associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med 2002;8:793–800. 10.1038/nm730 [DOI] [PubMed] [Google Scholar]
- 10. Keir ME, Butte MJ, Freeman GJ, et al. Pd-1 and its ligands in tolerance and immunity. Annu Rev Immunol 2008;26:677–704. 10.1146/annurev.immunol.26.021607.090331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363:711–23. 10.1056/NEJMoa1003466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 2015;372:320–30. 10.1056/NEJMoa1412082 [DOI] [PubMed] [Google Scholar]
- 13. Wolchok JD, Chiarion-Sileni V, Gonzalez R, et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med 2017;377:1345–56. 10.1056/NEJMoa1709684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Champiat S, Lambotte O, Barreau E, et al. Management of immune checkpoint blockade dysimmune toxicities: a collaborative position paper. Ann Oncol 2016;27:559–74. 10.1093/annonc/mdv623 [DOI] [PubMed] [Google Scholar]
- 15. Cortazar FB, Marrone KA, Troxell ML, et al. Clinicopathological features of acute kidney injury associated with immune checkpoint inhibitors. Kidney Int 2016;90:638–47. 10.1016/j.kint.2016.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Manohar S, Kompotiatis P, Thongprayoon C, et al. Programmed cell death protein 1 inhibitor treatment is associated with acute kidney injury and hypocalcemia: meta-analysis. Nephrol Dial Transplant 2019;34:108–17. 10.1093/ndt/gfy105 [DOI] [PubMed] [Google Scholar]
- 17. Wanchoo R, Karam S, Uppal NN, et al. Adverse renal effects of immune checkpoint inhibitors: a narrative review. Am J Nephrol 2017;45:160–9. 10.1159/000455014 [DOI] [PubMed] [Google Scholar]
- 18. Shirali AC, Perazella MA, Gettinger S. Association of acute interstitial nephritis with programmed cell death 1 inhibitor therapy in lung cancer patients. Am J Kidney Dis 2016;68:287–91. 10.1053/j.ajkd.2016.02.057 [DOI] [PubMed] [Google Scholar]
- 19. Fadel F, El Karoui K, Knebelmann B. Anti-Ctla4 antibody-induced lupus nephritis. N Engl J Med 2009;361:211–2. 10.1056/NEJMc0904283 [DOI] [PubMed] [Google Scholar]
- 20. Weber JS, Dummer R, de Pril V, et al. Patterns of onset and resolution of immune-related adverse events of special interest with ipilimumab: detailed safety analysis from a phase 3 trial in patients with advanced melanoma. Cancer 2013;119:1675–82. 10.1002/cncr.27969 [DOI] [PubMed] [Google Scholar]
- 21. Spanou Z, Keller M, Britschgi M, et al. Involvement of drug-specific T cells in acute drug-induced interstitial nephritis. J Am Soc Nephrol 2006;17:2919–27. 10.1681/ASN.2006050418 [DOI] [PubMed] [Google Scholar]
- 22. Perazella MA, Shirali AC. Nephrotoxicity of cancer immunotherapies: past, present and future. J Am Soc Nephrol 2018;29:2039–52. 10.1681/ASN.2018050488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. NCCN Clinical Practice Guidelines in Oncology Management of immunotherapy-related toxicities. Version1.2018 1⁄4 February 14, 2018, 2018. Available: http://www.omedit-idf.fr/wp- content/uploads/2018/05/NCCN-2018-Reco-EI-immunotherapies. pdf. [Accessed Feb 22, 2019].