Abstract
A young man with neuropsychiatric problems has a small 22q13.33 duplication. We suggest this causes his condition. His disorder may represent a 22q13.33 behavioural phenotype. In childhood, he was diagnosed with mild intellectual disability. He was later diagnosed with Tourette syndrome, atypical autism spectrum disorder and bipolar disorder. Lithium seems effective in treating his affective symptoms. He has mild dysmorphic features, full lips and protruding ears. An array comparative genomic hybridisation showed a 300 kb duplication. The duplication harbours several genes, notably SH3 and multiple ankyrin repeat domain 3 (SHANK 3). The small size helps focus on a critical region for a 22q13.33 duplication syndrome. Mutations, deletions and duplications should be kept in mind as causes of neuropsychiatric disorders, especially in a patient with dysmorphic traits.
Keywords: child and adolescent psychiatry, mood disorders (including depression), genetic screening / counselling
Background
Array comparative genomic hybridisation (aCGH) has become an accessible diagnostic tool, and a microdeletion or microduplication of part of a chromosome is recognised as a cause in more cases of autism and other neuropsychiatric disorders.1 Cases caused by a deletion or duplication tend to be syndromic, with physical phenotypic traits.
We present a patient with mild intellectual disability (ID), autism spectrum disorder (ASD), Tourette syndrome and bipolar disease. An aCGH demonstrated a 300 kb (kilobases/300 000 bases) duplication of part of the long arm of chromosome 22, in band 22q13.33, involving the gene SH3 and multiple ankyrin repeat domain 3 (SHANK3). The duplication we describe is small, and this may represent a critical region of a 22q13.33 duplication syndrome.
A common denominator for clinically relevant 22q13 duplications is that the duplicated region most often involves the SHANK3 gene. SHANK3 encodes a scaffolding protein that is enriched in postsynaptic densities of excitatory synapses and plays an important role in neuronal development.2 SHANK3 is highly expressed in the striatum and medial prefrontal cortex of humans, which involves regulation of emotional, social and cognitive behaviour, and brain motor and reward systems, respectively.3 4 The 22q13.3 deletion syndrome—Phelan-McDermid syndrome—can also be caused by SHANK3 mutations.5 SHANK3 is also implicated in a type of schizophrenia (SCZD15).6
Symptoms reported in patients with 22q13 duplication syndrome include mild dysmorphic features,7–10 ID,10–12 developmental delay,7 8 10 11 behavioural problems,7 9 10 epilepsy/seizures,7 attention deficit hyperactivity disorder (ADHD),7 11 and attention deficit,9 bipolar disorder,7 schizophrenia9 and ASD.11 12 These case studies include altogether nine patients. The patients all had difficulties coping with everyday life, and had mental health problems as listed above. It has been suggested that the 22q13 microduplication is a recognisable syndrome.8 13 14 Within this region, the duplicated sequences vary in size.
Case presentation
The patient is a young man adopted from South East Asia. As an infant he was tranquil, but was severely distressed when left alone. He was sensitive to sudden sounds. He had a narrow field of interests, and was drawn to more violent content in his early teens. He got extra support in school, but his difficulties with learning and social interaction got worse, especially in the transition from primary to secondary school. He did not finish secondary school because of his increasing symptoms of anxiety and social withdrawing.
He was diagnosed with mild ID in childhood. He was later diagnosed with Tourette syndrome based on vocal and motor tics (grimaces), and obsessive compulsive disorder (OCD) involving fear of contamination, germs and dirt. In his late teens, he was diagnosed with atypical ASD. He had social problems, anxiety, narrow field of interests, rigidity and poor communication skills. He was also assessed for ADHD due to concentration problems, but the diagnosis could not be supported. He was shortly after this hospitalised in a psychiatric ward for the first time, because of behavioural outbursts. There was suspicion of psychosis, but this could not be substantiated. He was later admitted to our unit, a specialised psychiatric unit for adults with intellectual disabilities, autism and psychiatric comorbidity. The patient was admitted for evaluation of diagnoses and for treatment.
Investigations
As part of the neuropsychiatric evaluation, he had a blood test workup. An aCGH detected a duplication of part of the long arm of chromosome 22, reported as arr[GRCh37]22q13.33 (50883397_51186249) x3, that is, with a size of 300 kb (302 852 base pairs). This region encompasses the SHANK3 gene. The lab reported no other duplications or deletions (180 k array, expected to find duplications or deletions larger than 50 kb).
Plasma ceruloplasmin was below the cut-off (0.15 g/L), but Wilson disease was excluded with further investigations. Other blood tests, for example, haematological, liver, kidney, thyroid gland function, B12 levels, iron and inflammatory markers, were normal. MRI scan and EEG were normal. Metabolic screening of both urine and blood was normal.
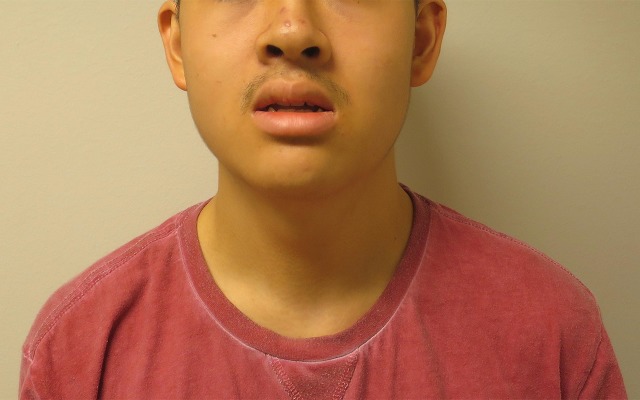
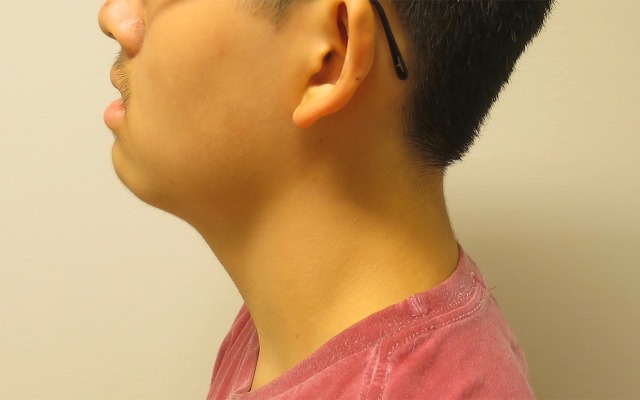
He had mild dysmorphic features with full lips, a slightly upturned nose/anteverted nares, and protruding ears, most pronounced on the left side (figures 1 and 2).
Figure 1.
Front view of the patient. Notice the mild dysmorphic features with full lips, a slightly upturned nose/anteverted nares and protruding ears, most pronounced on the left side.
Figure 2.
Side view of the patient.
Differential diagnosis
Symptoms were evaluated through 24 hours observation, and also through the screening instrument psychopathology in autism checklist,15 the MINI international neuropsychiatric interview16 and behavioural problems assessed using the aberrant behaviour checklist.17 The combination of observations and psychometric data concluded with a bipolar disorder, in addition to mild ID, atypical ASD and Tourette syndrome.
Mild intellectual disability
The intellectual level was retested with the Wechsler Adult Intelligence Scale-IV and mild ID was supported.
Bipolar disease, mixed type
We observed manic phases during the hospitalisation, and he had similar phases prior to admission. In the manic state, he had several behavioural outbursts. He was agitated, restless, physically aggressive towards the staff and emotionally unstable. He had severe hang-ups involving violent content on the internet, which we found to be far beyond the restricted patterns of interest as part of ASD. He could not stop watching or talking about this. He was near the threshold of psychosis in this period, as he got paranoid ideas. Somewhat atypically, he slept well during these periods. After a manic episode, he had a phase with depressed mood, reduced appetite, withdrawal and indifference. He lost interest in things he cared about previously. The parents also described a depressive episode in his teens. The shifts between manic and depressive episodes were rapid, hence according to the International Classification of Diseases and related health problems 10, he got the diagnosis of bipolar disease, mixed type.
Atypical ASD
The former diagnosis of atypical ASD was supported as he struggled with deficits in social communication and interactions, especially interpretation of emotions and intentions of others, and pragmatic communication.
Tourette syndrome
The former diagnosis of Tourette syndrome was supported as he had both vocal (hawking) and motor tics (facial grimacing).
Prior diagnoses excluded
The former diagnosis of OCD was not supported. He still had thoughts involving fear of contamination, but not to an extent that interfered with his daily routines or function, and these thoughts were considered part of his ASD. Anxiety symptoms were limited to sudden changes, the way patients with ASD often experience, but did not fulfil a diagnosis of anxiety.
Treatment
Sertraline (100 mg/day) had effect on the obsessive and anxiety symptoms. Risperidone was initially tried out to treat the tics, as antipsychotic treatment can reduce symptoms of Tourette syndrome in young adults.18 Additionally, risperidone (or aripiprazole) could have effect on his behavioural outbursts and irritability, as behavioural disturbances and hyperactivity associated with ASD often respond to this medication clinically.19 20 Risperidone severely reduced his function as he got sedated. A new attempt with aripiprazole (5 mg) was successful, mainly on his tics. He was diagnosed with bipolar disease, and was treated with lithium. Both lithium and valproate were treatment options. The dose was slowly increased to 83+125 mg/day, corresponding to a serum level of 0.6 mmol/L (recommended interval is 0.4–0.7).
Outcome and follow-up
The psychiatric outpatient clinic that followed the patient for 10 months after the hospitalisation at our unit reported fewer emotional outbursts with lithium treatment. He had some periods of manic behaviour, for example, he still watched internet videos of violent content in periods, but did not act on them or get agitated as prior to the hospitalisation at our unit. He had no depressive periods. He was not hospitalised in the observation period. He did not report side effects of lithium.
He lives in residential care, which helps him structure his everyday life better, and he feels secure. His function level is better, for example, he is less stressed, is out in the public, often on his own initiative, and he attends a language class.
Discussion
Table 1 presents similar patients found through searches in PubMed using the terms ((22q13.3[All Fields] OR 22q13.33[All Fields]) AND duplication [All Fields]) and searches in the websites omim.org, gene.sfari.org/database/cnv and decipher.sanger.ac.uk with the search term ‘22q13.33’. In addition, an abstract presented at the European Society of Human Genetics Conference 2011 is included in the table.10
Table 1.
Similar reports describing patients with a 22q13.33 duplication and their symptoms and dysmorphic traits
| Base pair span | (Neuro-)psychiatric symptoms and main dysmorphic traits | |
| Han et al
7
Patient 1 |
49461602–49525130/49451468–49691432 | ADHD, developmental delay, learning problems, poor impulse control, destructive behaviour, seizures, upslanted palpebral fissures, epicanthus inversus, pinched nose with anteverted nares. |
| Han et al
7
Patient 2 |
51063071–51178264/51002394–51304566 | Bipolar disease, learning problems, anger management problems, suicide attempts, seizures. No dysmorphic features described. |
| Chen et al 11 | 49388701–51188494 | ADHD, Autism, ID (mild), delayed speech development. The patient had no dysmorphic features. |
| Okamoto et al
8
Patient 1 |
Unknown | Mental development moderately delayed, round face, hypertelorism, downslanting palpebral fissures, broad/flat nasal bridge, shallow philtrum, prominent forehead, arched eyebrows, low-set deformed ears and high-arched palate. |
| Okamoto et al 8 Patient 2 | Unknown (There was also a deletion on chromosome 17 in addition to the 22q13.33 duplication) |
Mental development moderately delayed. Round face, hypertelorism, epicanthal folds, arch-shaped eyebrows, downslanting palpebral fissures, broad/flat nasal bridge, low-set deformed ears and high-arched palate. |
| Failla et al 9 | Unknown | Schizophrenia, aggressive acting out, Intelligence Quotient 73, psychomotor restlessness, attention deficit, irritability, microbrachycephaly, hypertelorism, downslanting palpebral fissures, deviation of nasal septum, helix hyperplasia, thick lips, joint hyperlaxity, splayfoot. |
| Destrée et al
10
Patient 1 |
Unknown | ID (mild), developmental delay, especially in language sphere, behavioural disturbance, microcephaly, growth retardation and mild dysmorphic features (not further specified). |
| Patient 2 | Unknown | ID (mild), developmental delay, especially in language sphere, behavioural disturbance, microcephaly, growth retardation and mild dysmorphic features (not further specified). |
| Pinto et al 12 | 49452422–49567307 | Autism, ID (severe), no language, no seizures. Normal physical exam. |
| Decipher ID.*; 865 | 47857356–51174581 | Autism, ID, macrocephaly, obesity, abnormally low-pitched voice. |
| ID 248262 | 43948554–51219009 | ID, delayed speech and language development. |
| ID 250383 | 48164501–51222324 | Autism, ID, abnormality of dentition. |
| ID 250853 | 37369198–51218950 | ID, self-mutilation, seizures, microcephaly, short stature, upslanted palpebral fissure, thin upper lip vermilion, brachy- and camptodachtyly. |
| ID 251542 | 51072161–51169045 | ID. |
| ID 253900 | 51132603–51219150 | ID, delayed speech and language development, microcephaly, brachycephaly, hypoplasia of corpus callosum, short stature. |
| ID 270554 | 50733976–51219009 | ID. |
| ID 280492 | 48976867–51219009 | Global developmental delay. |
| ID 289492 | 51168854–51170255 | ID, psychosis. |
| ID 296391 | 50515240–51178205 | Cognitive impairment, dolichocephaly. |
| ID 296424 | 50706851–51178264 | 3–4 finger cutaneous syndactyly, hypospadias. |
| ID 327568 | 32539375–51169045 | ID, cleft lip- and palate, microcephaly, growth retardation. |
| ID 338358 | 39481187–51169045 | Seizures, hypertelorism, hypoplasia of the corpus callosum, median cleft palate, hypospadias, sandal gap, sacral dimple. |
| ID 339605 | 47285486–51169045 | Neurodevelopmental delay. |
| ID 349743 | 49995123–51178264 | ID, global developmental delay, hearing impairment. |
| ID 350097 | 51104116–51165386 | 2–3 toe syndactyly, abnormal facial shape. |
| ID 353765 | 51163647–51218980 | Coarctation of aorta, renal dysplasia, ventricular septal defect. |
| ID 355297 | 50821612–51178264 | Behavioural abnormality. |
| ID 382958 | 51123491–51224252 | Autism, anxiety, dysgraphia, dyscalculia. |
| Our patient | 50883397–51186249 | ID (mild), autism, Tourette syndrome, bipolar disease, full lips, slightly upturned nose/anteverted nares, protruding ears. |
*Decipher ID is the ID number of patients described in the decipher.sanger.ac.uk database.
ADHD, attention deficit hyperactivity disorder; ID, intellectual disability.
In total, 28 patient phenotypes, with 22q13.33 duplications involving SHANK3 were included, in addition to our patient. Fourteen had ID, 15 had mild dysmorphic features, 7 had behavioural problems, 5 had ASD and 4 had delayed speech and language development, 2 had ADHD, 1 had attention deficit, 1 had bipolar disease and 2 had psychosis/ schizophrenia.
ASD is present in nearly all patients with 22q13.3 deletion syndrome. In contrast, only 5 out of the above-mentioned 28 patients with a duplication had ASD. Our patient first got the diagnosis of ASD in his late teens. Seen retrospectively, he did exhibit symptoms of ASD in early childhood, but it was at that time interpreted as anxiety and obsessions. His trouble in his early teens is typical for people with ASD. His bipolar disease was somewhat atypical as the mood swings were sudden and he untypically slept well during the manic like periods. Our patient was treated with aripiprazole during the whole stay because of his Tourette syndrome. This may have made the symptoms of mania less severe. His anxiety symptoms overlapped with symptoms of ASD. Symptoms of anxiety in patients with ID often bear resemblance to age appropriate anxiety seen in children.21 There is an increased risk of developing psychiatric symptoms among those who have ID or ASD.15 Our patient got the diagnosis of bipolar disease, which might initially have been interpreted as behavioural problems due to his ID/ASD.22 Our patient also had symptoms of psychosis in periods. It is well known that the psychotic threshold is lower in patients with ASD.17 However, his episodes were brief, and he did not get diagnosed with a psychotic disorder.
A 35-year-old man described by Han et al had bipolar disease, like our patient.7 Han et al also used a mouse model to illustrate the phenotype of Shank3 overexpression. They found that mice with overexpression of Shank3 had manic like behaviour, which could support a hypothesis of manic like symptoms associated with SHANK3 overexpression in humans. They found that valproate, but not lithium, alleviated the manic like behaviour in mice. In contrast, lithium seems to be an effective therapy for our patient. His everyday life is improved, he is emotionally more stable, and he had no hospitalisations during the observation period. Although lithium seems effective, a secure environment and structure in his residential care seems to be a contributing factor to his improvement. Case reports of patients with Phelan-McDermid syndrome and behavioural instability state that patients responded to lithium but not valproate.23 24 Thus, it appears that both those with a deletion and those with a duplication of this gene respond to lithium therapy. Since he has effect of lithium and no side effects, we have not tried valproate, although it would be interesting to see if it had the effect it had in the mouse model.
Our patient exhibited hyperactivity, but to a degree that did not fulfil the diagnostic criteria of ADHD. The patient described by Chen et al resembles our patient.11 He was diagnosed with ADHD at the age of 6 years, based on symptoms such as hyperactivity and inattention. At the age of 18 years though, he only had subthreshold inattention symptoms and a moderate degree of functional impairment, which did not fulfil the full Diagnostic and Statistical Manual of Mental Disorder IV
diagnostic criteria. In addition, like our patient, the patient represented by Chen et al had ASD and mild ID.
Our patient showed different neuropsychiatric disorders, for example, Tourette syndrome, ASD, bipolar disease and mild ID. A limitation of our study is that it is not possible to conclude if all of these disorders could be associated with the 22q13.33 duplication, or whether the genetic variant makes a patient more vulnerable to neuropsychiatric disease. Although there are some resemblances between the cases described, for example, ID is a recurrent finding, more patients with a 22q13.33 duplication need to be evaluated. The finding of a behavioural phenotype and a small duplication points to a 22q13.33 duplication syndrome.
Learning points.
A microdeletion or microduplication should be considered in syndromic autism.
One such duplication may be the 22q13.33 duplication.
A microdeletion or microduplication should be considered in all patients with psychiatric disorders, especially if dysmorphic traits are present.
Lithium seems to be an effective therapy on bipolar disorder in patients with a 22q13.33 duplication.
Footnotes
Contributors: MJ authored the case reports and did the litterature search. IBH was involved in managing the patient when he was admitted. ØB was the attending clinical geneticist for the patient. TLB contributed with background literature and the design of the report. Critical review of the manuscript: MJ, IBH and ØB.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Malhotra D, Sebat J. Cnvs: harbingers of a rare variant revolution in psychiatric genetics. Cell 2012;148:1223–41. 10.1016/j.cell.2012.02.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Naisbitt S, Kim E, Tu JC, et al. . Shank, a novel family of postsynaptic density proteins that binds to the NMDA receptor/PSD-95/GKAP complex and cortactin. Neuron 1999;23:569–82. 10.1016/S0896-6273(00)80809-0 [DOI] [PubMed] [Google Scholar]
- 3. Herbert MR. Shank3, the synapse, and autism. N Engl J Med 2011;365:173–5. 10.1056/NEJMcibr1104261 [DOI] [PubMed] [Google Scholar]
- 4. Jin C, Kang H, Ryu JR, et al. . Integrative Brain Transcriptome Analysis Reveals Region-Specific and Broad Molecular Changes in Shank3-Overexpressing Mice. Front Mol Neurosci 2018;11:250 10.3389/fnmol.2018.00250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wilson HL, Wong ACC, Shaw SR, et al. . Molecular characterisation of the 22q13 deletion syndrome supports the role of haploinsufficiency of SHANK3/PROSAP2 in the major neurological symptoms. J Med Genet 2003;40:575–84. 10.1136/jmg.40.8.575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gauthier J, Champagne N, Lafrenière RG, et al. . De novo mutations in the gene encoding the synaptic scaffolding protein Shank3 in patients ascertained for schizophrenia. Proc Natl Acad Sci U S A 2010;107:7863–8. 10.1073/pnas.0906232107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Han K, Holder JL, Schaaf CP, et al. . Shank3 overexpression causes manic-like behaviour with unique pharmacogenetic properties. Nature 2013;503:72–7. 10.1038/nature12630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Okamoto N, Kubota T, Nakamura Y, et al. . 22q13 microduplication in two patients with common clinical manifestations: a recognizable syndrome? Am J Med Genet A 2007;143A:2804–9. 10.1002/ajmg.a.31771 [DOI] [PubMed] [Google Scholar]
- 9. Failla P, Romano C, Alberti A, et al. . Schizophrenia in a patient with subtelomeric duplication of chromosome 22q. Clin Genet 2007;71:599–601. 10.1111/j.1399-0004.2007.00819.x [DOI] [PubMed] [Google Scholar]
- 10. Destrée A, Hilbert P, Boulanger S, et al. . A 273-kb duplication at 22q13.33 encompassing the Shank3 gene in 2 sibs with microcephaly, behavioral disorder and learning disabilities. European Society of human genetics conference 2011, P02.034. Eur J Hum Genet 2011;19(suppl 2). [Google Scholar]
- 11. Chen C-H, Chen H-I, Liao H-M, et al. . Clinical and molecular characterization of three genomic rearrangements at chromosome 22q13.3 associated with autism spectrum disorder. Psychiatr Genet 2017;27:23–33. 10.1097/YPG.0000000000000151 [DOI] [PubMed] [Google Scholar]
- 12. Pinto D, Pagnamenta AT, Klei L, et al. . Functional impact of global rare copy number variation in autism spectrum disorders. Nature 2010;466:368–72. 10.1038/nature09146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. O-OMIi M. 22q13 duplication syndrome, 2019. Available: omim.org/entry/615538
- 14. Bacino CA. Microduplication syndromes: UpToDate, 2018. Available: uptodate.com/contents/microduplication-syndromes
- 15. Bakken TL, Helverschou SB, Eilertsen DE, et al. . Psychiatric disorders in adolescents and adults with autism and intellectual disability: a representative study in one County in Norway. Res Dev Disabil 2010;31:1669–77. 10.1016/j.ridd.2010.04.009 [DOI] [PubMed] [Google Scholar]
- 16. Sheehan DV, Lecrubier Y, Sheehan KH, et al. . The Mini-International neuropsychiatric interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 1998;59(Suppl 20):22–33. [PubMed] [Google Scholar]
- 17. Myrbakk E, von Tetzchner S. Psychiatric disorders and behavior problems in people with intellectual disability. Res Dev Disabil 2008;29:316–32. 10.1016/j.ridd.2007.06.002 [DOI] [PubMed] [Google Scholar]
- 18. Hollis C, Pennant M, Cuenca J, et al. . Clinical effectiveness and patient perspectives of different treatment strategies for tics in children and adolescents with Tourette syndrome: a systematic review and qualitative analysis. Health Technol Assess 2016;20:1–450. 10.3310/hta20040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fung LK, Mahajan R, Nozzolillo A, et al. . Pharmacologic treatment of severe irritability and problem behaviors in autism: a systematic review and meta-analysis. Pediatrics 2016;137(Suppl 2):S124–35. 10.1542/peds.2015-2851K [DOI] [PubMed] [Google Scholar]
- 20. Accordino RE, Kidd C, Politte LC, et al. . Psychopharmacological interventions in autism spectrum disorder. Expert Opin Pharmacother 2016;17:937–52. 10.1517/14656566.2016.1154536 [DOI] [PubMed] [Google Scholar]
- 21. Cooray S, Andrews T, Baley NM : Fletcher RJ, Barnhill J, Cooper S-A, Diagnostic manual – intellectual disability. DM-ID 2. New York: The NADD Press, 2016. [Google Scholar]
- 22. Helverschou SB, Bakken TL, Martinsen H. Psychiatric disorders in people with autism spectrum disorders: phenomenology and recognition. International Handbook of autism and pervasive developmental disorders. Springer, 2011. [Google Scholar]
- 23. Serret S, Thümmler S, Dor E, et al. . Lithium as a rescue therapy for regression and catatonia features in two Shank3 patients with autism spectrum disorder: case reports. BMC Psychiatry 2015;15:107 10.1186/s12888-015-0490-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Egger JIM, Verhoeven WMA, Groenendijk-Reijenga R, et al. . Phelan-McDermid syndrome due to SHANK3 mutation in an intellectually disabled adult male: successful treatment with lithium. BMJ Case Rep 2017;2017. doi: 10.1136/bcr-2017-220778. [Epub ahead of print: 28 Sep 2017]. [DOI] [PMC free article] [PubMed] [Google Scholar]