Abstract
Objective
To study the prevalence and trends of lower extremity complications of diabetes over an 8-year period in a single nation.
Research design and methods
Nationwide data for people with type 2 diabetes (T2D) and diabetic foot complications (DFCs) were analyzed over an 8-year period (2007–2014) from National Health Insurance Research Database using the International Classification of Diseases, Ninth Revision disease coding. The DFCs were defined as ulcers, infections, gangrene, and hospitalization for peripheral arterial disease (PAD). Trends of patient characteristics, foot presentation, and the execution of major procedures were studied, including lower-extremity amputations (LEAs).
Results
Along with the T2D population increasing over time, the absolute number of people with DFCs increased by 33.4%, but retained a prevalence of around 2% per year. The annual incident of LEAs decreased from 2.85 to 2.06 per 1000 T2D population (p=0.001) with the major LEA proportion decreasing from 56.2% to 47.4% (p<0.001).
The mean age of patients increased from 65.3 to 66.3 years and most of the associated comorbidities of diabetes were increased. For example, end-stage renal disease increased from 4.9% to 7.7% (p=0.008). The incidence of gangrene on presentation decreased from 14.7% to 11.3% (p<0.001) with a concomitant increase in vascular interventions (6.2% to 19.5%, p<0.001).
Conclusions
DFCs remain a sustained major medical problem. These nationwide long-term data suggest trends toward older people with greater comorbidities such as PAD and renal disease. Nevertheless, promising trends of reducing gangrene on presentation paired with increases in vascular interventions support continued vigilance and rapid, coordinated interdisciplinary diabetic foot care.
Keywords: Foot Complications, Limb Ischemia, Revascularization, National Health Surveys, Lower Limb Amputation
Significance of this study.
What is already known about this subject?
Diabetic foot complications are major sequelae of diabetes that often end in end-stage complications including lower-extremity amputations (LEAs), shortened lifespan and the commensurate increased burden of social care.
What are the new findings?
Diabetic foot complications continue to remain a major medical and public health issue as we face patients in increased numbers, age, and comorbidities.
Over 8 years, the annual incidence of LEAs decreased from 2.85 to 2.06 per 1000 type 2 diabetic population whereas the proportion of people receiving peripheral artery intervention increased from 6.2% to 19.5%.
How might these results change the focus of research or clinical practice?
Promising trends of reducing gangrene on presentation paired with increases in vascular interventions support continued vigilance and rapid, coordinated interdisciplinary team of diabetic foot care across hospitals, hospital networks, and national health services.
Introduction
Diabetic foot complications (DFCs) are major sequelae of diabetes and contribute to most causes of non-traumatic lower-extremity amputations (LEAs) worldwide.1 2 In addition, patients with DFCs have been known to have higher recurrence rate of ulcers3 and worse survival than that of many common cancers.4 The medical expense of patients with these complications is even higher than the most costly cancers5 6; moreover, patients who received LEAs usually had worsened consequences including lower self-esteem,7 shortened lifespan,8 9 and the burden of social care.5 10
The US has previously reported a decreasing trend in LEAs but a disturbing reversal has been noted in recent years in patients with DFCs.11 Similar trends have been reported in people with end-stage renal diseases.12 Nevertheless, any explanation of the trend of LEAs is challenging because of scant information available. Information regarding risk factors for LEAs in treating patients with DFCs such as age,13 degree of peripheral arterial diseases (PADs)14 and infection,15 renal function,16 and nutritional status17 is required for further investigations.
Because of the robust and comprehensive nature of the Taiwan National Health System database, we were able to report the in-hospital foot diseases characteristics between years 2000 and 2009.18 The annual incidence of diabetes-related LEA in Taiwan between 1998 and 2007 was 3.79 and 2.27/1000/year, respectively.19
The present study was conducted to better understand the prevalence and time trends of patients with DFCs between 2007 and 2014, including demographics of patients, affected foot and major procedures introduced to treat these patients, and LEAs.
Research design and methods
Study population and source
The present study was based on data collected over an 8-year period (2007–2014) derived from the Taiwan National Health Insurance Research Database (NHIRD). The Taiwanese government implemented its National Health Insurance (NHI) system in 1995. This provided coverage for 95% of the population in 2000, which increased to 98% in 2005 and 99.6% of the total 23 million people in Taiwan in 2009.18 Large computerized administrative and claims data sets derived from this programme have provided diagnoses, procedures, and prescriptions of inpatient and outpatient records. Patients with diabetes mellitus were identified through the International Classification of Diseases, Ninth Revision diagnostic code of 250 (except 250.01, 250.03 for type 1 diabetes) at least once during hospital admission or three or more times for ambulatory clinic patients in each calendar year.
Definition of diabetic foot complications and comorbidities
DFCs include diabetic foot infection, severe PADs, and foot ulcers. The diabetic foot infections were identified by foot cellulitis or abscess (680.6, 680.7, 681.10, 682.6, 682.7); osteomyelitis (730.06, 730.07, 730.16, 730.17, 730.26, 730.27, 730.96, 730.97); and necrotizing fasciitis (728.86, 040.0). Severe PADs were identified by the presentation of gangrene (785.4, 440.24) or hospitalization for PAD (440.2, 440.3, 440.4, 443.81, 443.89, 443.9, 444.22, 445.02, 785.4). Foot ulcers included the diagnostic codes of 707.06, 707.07, 707.1, 892, and 893.
LEAs were identified by diagnostic codes of V49.70, V49.71, V49.72, V49.73, V49.74 and procedure codes of 84.11, 84.12 for minor LEA and by V49.75, V49.76, V49.77 and 84.14, 84.15, 84.17 for major LEA, respectively in each calendar year. Only the highest-level amputation was enrolled for per-patient-per-calendar-year measure to prevent overestimation of the amputation amount because of possible multistep procedures for one person.
The comorbidities of patients with DFCs were identified based on the records of coding for hypertension (401, 402, 403, 404, 405), dyslipidemia (272.0, 272.1, 272.2, 272.3, 272.4), diabetic retinopathy (362, 250.5), diabetic neuropathy (250.6), diabetic nephropathy (250.4, 585, 586, V42.0, V45.1, V56, 403, 404), end-stage renal disease (585.5, 585.6, 586, V45.11, V45.1, V56.X, 403.01, 403.11, 403.91, 404.02, 404.03, 404.12, 404.13, 404.92, 404.93), coronary heart diseases (410, 411, 412, 414.00–414.07, 414.2–414.9), heart failure (428), and cerebral vascular accident (433, 434, 435, 436, 437.1, 430, 431, 432) before the diagnosis of DFCs.
Vascular interventions and medical expenditure
The endovascular treatments were defined by the procedure codes for percutaneous transluminal angioplasty (PTAs), either simple PTA (33074B) or complicated PTA (33115B). The bypass graft surgery was defined by code of 69004B. These specific procedure codes of NHIRD were provided in the online supplementary file 1. To avoid the noise of coding for arterial-venous shunt treatment, the patients with end-stage renal disease who received procedure of simple PTA were excluded.
bmjdrc-2019-000795supp001.pdf (26.4KB, pdf)
The annual medical expenditure of each patient was provided from NHI. All dollar values were adjusted and presented in 2019 US dollars.
Statistical analysis
The demographic characteristics including age, gender, comorbidities, and medical cost among patients with diabetes having foot diseases and/or all patients with diabetes were summarized by year using frequencies (proportions) and means (SD) as appropriate. The prevalence was described as a percentage. Within the population of people coded with DFCs, the prevalence and the proportion of the comorbidities, LEAs, phenotypes of foot diseases including ischemic foot, foot infections and foot ulcers, and vascular interventions were analyzed annually. In addition, 95% CIs of diabetic foot prevalence were obtained. Changes in the prevalence and proportion from 2007 to 2014 were analyzed using a Joinpoint regression model. All statistical analyses were performed using the SAS V.9.4 (SAS Institute, Cary, North Carolina, USA) and the Join-point Trend Analysis Software V.4.5.0.1.20
Results
As the T2D population rose from 1 115 556 to 1 556 988 from year 2007 to 2014, the number of people with T2D and DFCs increased by 33.4% (table 1). Nevertheless, the prevalence of DFCs in patients with T2D was 2.07% in 2007 and 1.98% in 2014, which showed a slight decrease in trend (p for trend=0.024).
Table 1.
Trends of diabetic foot complications among patients with T2D
| Year | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | P for trend |
| Patients with DFC | 23 068 | 26 039 | 27 368 | 28 729 | 29 770 | 30 007 | 30 332 | 30 783 | – |
| T2D population | 1 115 556 | 1 192 004 | 1 269 496 | 1 339 223 | 1 404 474 | 1 472 448 | 1 535 613 | 1 556 988 | – |
| DFCs prevalence, % (95% CI) |
2.07 (2.04 to 2.09) |
2.18 (2.16 to 2.21) |
2.16 (2.13 to 2.18) |
2.15 (2.12 to 2.17) |
2.12 (2.10 to 2.14) |
2.04 (2.02 to 2.06) |
1.98 (1.95 to 2.00) |
1.98 (1.96 to 2.00) |
0.024 |
| Cost*: DFCs/T2D ratio (%) |
8.8/2.7 330 |
8.8/2.7 325 |
9.1/2.7 335 |
9.1/2.7 338 |
9.2/2.7 336 |
9.0/2.7 337 |
8.9/2.7 328 |
8.5/2.6 320 |
0.659 |
| LEAs (per 1000 subjects with T2D) | 2.85 | 2.91 | 2.72 | 2.59 | 2.68 | 2.29 | 2.07 | 2.06 | 0.001 |
| Minor LEA | 1.25 | 1.33 | 1.29 | 1.24 | 1.33 | 1.14 | 1.09 | 1.09 | 0.019 |
| Major LEA | 1.60 | 1.57 | 1.43 | 1.35 | 1.35 | 1.15 | 0.98 | 0.98 | <0.001 |
| Major/total LEAs (%) | 56.2 | 54.1 | 52.7 | 52.3 | 50.5 | 50.3 | 47.3 | 47.4 | <0.001 |
*Cost unit=US$1000 per one person-years.
DFC, diabetic foot complication; LEA, lower extremity amputations; T2D, type 2 diabetes.
The annual expenditure for any medical performance per person with DFCs was constantly higher than that of the T2D population, in a 3.2-fold to 3.4-fold range.
The absolute number of the T2D population suffering from LEAs was 3181 in year 2007–3213 in 2014, with the annual LEAs rate decreasing from 2.85 to 2.06 (p for trend=0.001) per 1000 T2D persons per year. The trends for both major LEA and minor LEA rates were noted to be decreased (p<0.001 for major LEAs and p=0.019 for minor LEAs). Of note, the ratio of major LEA in LEAs decreased from 56.2% to 47.4% (p<0.001).
In table 2, characteristics of patients with T2D and DFCs are described. The mean age was 65.3±12.8 years in 2007 and 66.3±13.6 years in 2014. The trend was increased over time (p=0.003). We also noted an increasing trend in the percentage of male gender (from 54.2% to 57.1%, p<0.001). The increases of trends were noted in the comorbidities of hypertension (from 68.8% to 73.0%, p=0.001), dyslipidemia (from 34.5% to 42.5%, p<0.001), and diabetic nephropathy (from 14.2% to 17.1%, p=0.001). The prevalence of end-stage renal disease in patients with DFCs increased from 4.9 in 2007 to 7.7 in 2014 (p=0.008). No significant difference was found in coronary artery diseases, hemorrhagic stroke, or major adverse cardiovascular events (MACEs).
Table 2.
Descriptive characteristics of patients with DFCs
| Year | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | P for trend | ||||||||
| Age (mean, SD) | 65.33±12.84 | 65.84±13.05 | 65.83±13.19 | 65.92±13.34 | 66.27±13.42 | 66.33±13.47 | 66.06±13.58 | 66.30±13.63 | |||||||||
| Age≥60 years | 15 305 (66.4) | 17 483 (67.1) | 18 352 (67.1) | 19 356 (67.4) | 20 431 (68.6) | 20 762 (69.2) | 20 713 (68.3) | 21 276 (69.1) | 0.003 | ||||||||
| Male gender (N, %) | 12 511 | (54.2) | 14 387 | (55.3) | 15 271 | (55.8) | 16 055 | (55.9) | 16 616 | (55.8) | 16 991 | (56.6) | 17 320 | (57.1) | 17 584 | (57.1) | <0.001 |
| Comorbidities (N, %) | |||||||||||||||||
| Hypertension | 15 873 | (68.8) | 18 232 | (70.0) | 19 494 | (71.2) | 20 409 | (71.0) | 21 673 | (72.8) | 21 874 | (72.9) | 22 076 | (72.8) | 22 476 | (73.0) | 0.001 |
| Dyslipidemia | 7960 | (34.5) | 9025 | (34.7) | 9781 | (35.7) | 10 570 | (36.8) | 11 371 | (38.2) | 11 593 | (38.6) | 12 419 | (40.9) | 13 067 | (42.5) | <0.001 |
| Diabetic nephropathy | 3286 | (14.2) | 3812 | (14.6) | 4118 | (15.1) | 4667 | (16.2) | 4963 | (16.7) | 5187 | (17.3) | 5157 | (17.0) | 5258 | (17.1) | 0.001 |
| End-stage renal disease | 1128 | (4.9) | 1435 | (5.5) | 1735 | (6.3) | 2103 | (7.3) | 2376 | (8.0) | 2421 | (8.1) | 2375 | (7.8) | 2382 | (7.7) | 0.008 |
| Diabetic retinopathy | 452 | (2.0) | 520 | (2.0) | 502 | (1.8) | 540 | (1.9) | 507 | (1.7) | 497 | (1.7) | 403 | (1.3) | 377 | (1.2) | 0.001 |
| Diabetic neuropathy | 1005 | (4.4) | 991 | (3.8) | 1035 | (3.8) | 1064 | (3.7) | 1045 | (3.5) | 1045 | (3.5) | 949 | (3.1) | 960 | (3.1) | <0.001 |
| Coronary heart disease | 1309 | (5.7) | 1492 | (5.7) | 1510 | (5.5) | 1551 | (5.4) | 1739 | (5.8) | 1669 | (5.6) | 1784 | (5.9) | 1728 | (5.6) | 0.667 |
| Heart failure | 740 | (3.2) | 824 | (3.2) | 920 | (3.4) | 1034 | (3.6) | 1065 | (3.6) | 1075 | (3.6) | 969 | (3.2) | 924 | (3.0) | 0.763 |
| Ischemic stroke | 397 | (1.7) | 493 | (1.9) | 457 | (1.7) | 490 | (1.7) | 495 | (1.7) | 462 | (1.5) | 450 | (1.5) | 417 | (1.4) | 0.003 |
| Hemorrhagic stroke | 21 | (0.1) | 36 | (0.1) | 35 | (0.1) | 44 | (0.2) | 31 | (0.1) | 49 | (0.2) | 29 | (0.1) | 31 | (0.1) | 0.740 |
| MACEs* | 2467 | (10.7) | 2845 | (10.9) | 2922 | (10.7) | 3119 | (10.9) | 3330 | (11.2) | 3255 | (10.9) | 3232 | (10.7) | 3100 | (10.1) | 0.250 |
*MACEs: including coronary heart disease, heart failure, ischemic stroke and hemorrhagic stroke.
DFC, diabetic foot complication; MACE, major adverse cardiac event; N, number of patients.
Table 3 demonstrates the major presentations of DFCs over time. The rate for foot infection increased from 59.0% in 2007 to 63.1% in 2014, with an increased trend (p<0.001). Although the absolute number increased, the trend of foot ulcers presentation decreased (38.4% to 33.8%, p<0.001). The trend for the prevalence of PAD had no significant difference (from 28.8% to 26.8%, p=0.064). The major presentations of interest on DFCs were also listed, and an increasing trend of osteomyelitis (6.8% to 7.4%, p=0.039) and necrotizing fasciitis (7.0% to 8.2%, p<0.001) were noted. Nevertheless, the presence of foot gangrene decreased significantly, from 14.7% to 11.3% (p<0.001). According to the selection criteria of this study, >60% of patients with DFCs required in-hospital treatment. The trend for utility with in-hospital management was slightly decreased, from 64.6% to 61.6% (p=0.023). The continual increase of endovascular interventions was noted from 6.2% in 2007 to 19.5% in 2014 (p<0.001). This was primarily due to a fourfold increase in PTAs (p<0.001) rather than bypass surgery (p=0.995).
Table 3.
Trends of disease characteristics and treatment in patients with type 2 diabetes and DFCs
| Year | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | P for trend | ||||||||
| Foot infections (N, %) | 13 618 | (59.0) | 15 410 | (59.2) | 16 599 | (60.7) | 17 589 | (61.2) | 18 262 | (61.3) | 18 588 | (62.0) | 19 200 | (63.3) | 19 437 | (63.1) | <0.001 |
| PADs (N, %) | 6646 | (28.8) | 7710 | (29.6) | 7793 | (28.5) | 8361 | (29.1) | 8924 | (30.0) | 8511 | (28.4) | 8322 | (27.4) | 8241 | (26.8) | 0.064 |
| Foot ulcers (N, %) | 8846 | (38.4) | 9758 | (37.5) | 9815 | (35.9) | 10 040 | (35.0) | 10 402 | (34.9) | 10 121 | (33.7) | 9947 | (32.8) | 10 415 | (33.8) | <0.001 |
| Major phenotypes | |||||||||||||||||
| Cellulitis or abscess (N, %) | 11 595 | (50.3) | 13 171 | (50.6) | 14 140 | (51.7) | 14 971 | (52.1) | 15 461 | (51.9) | 15 739 | (52.5) | 16 320 | (53.8) | 16 565 | (53.8) | <0.001 |
| Osteomyelitis (N, %) | 1575 | (6.8) | 1723 | (6.6) | 1914 | (7.0) | 2074 | (7.2) | 2283 | (7.7) | 2213 | (7.4) | 2208 | (7.3) | 2273 | (7.4) | 0.039 |
| Necrotizing fasciitis (N, %) | 1616 | (7.0) | 1910 | (7.3) | 2072 | (7.6) | 2253 | (7.8) | 2360 | (7.9) | 2490 | (8.3) | 2467 | (8.1) | 2538 | (8.2) | <0.001 |
| Gangrene (N, %) | 3389 | (14.7) | 3760 | (14.4) | 3638 | (13.3) | 3760 | (13.1) | 3959 | (13.3) | 3652 | (12.2) | 3486 | (11.5) | 3477 | (11.3) | <0.001 |
| Hospitalization (N, %) | 14 901 | (64.6) | 17 313 | (66.5) | 17 791 | (65.0) | 18 861 | (65.7) | 19 464 | (65.4) | 19 339 | (64.5) | 19 075 | (62.9) | 18 966 | (61.6) | 0.023 |
| Vascular interventions (N, %) | 1423 | (6.2) | 2202 | (8.5) | 2929 | (10.7) | 3720 | (13.0) | 4711 | (15.8) | 5020 | (16.7) | 5448 | (18.0) | 5988 | (19.5) | <0.001 |
| PTAs (N, %) | 1059 | (4.6) | 1710 | (6.6) | 2379 | (8.7) | 3229 | (11.2) | 4258 | (14.3) | 4676 | (15.6) | 5199 | (17.1) | 5745 | (18.7) | <0.001 |
| Bypass surgery (N, %) | 478 | (2.1) | 741 | (2.9) | 908 | (3.3) | 912 | (3.2) | 1005 | (3.4) | 885 | (3.0) | 792 | (2.6) | 818 | (2.7) | 0.995 |
N, number of patients;PAD, peripheral arterial disease; PTA, percutaneous transluminal angioplasty.
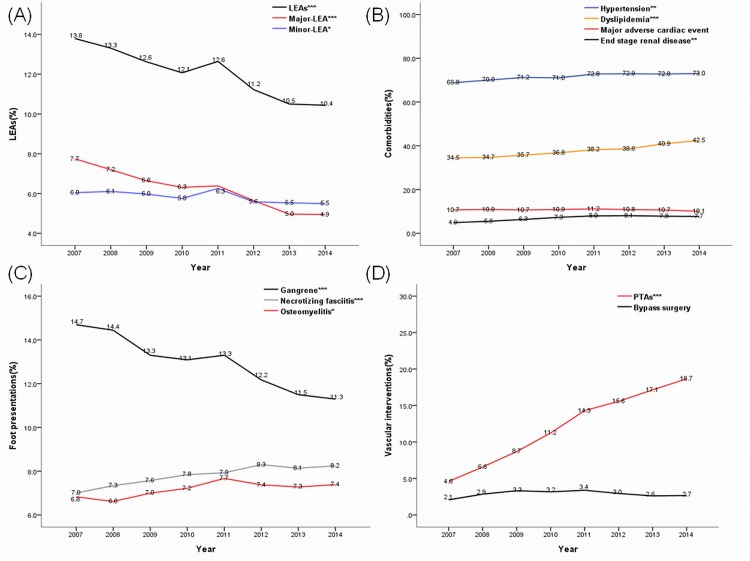
Figure 1 summarizes the major findings of patients with T2D and DFCs. Figure 1A shows the decrement of LEAs over time, with a more significant reduction in major rather than minor LEA. Figure 1B demonstrates the increase of trends of hypertension, dyslipidemia, and end-stage renal disease prevalence. The prevalence of associated MACEs, however, had no statistical change. Figure 1C shows the significant reduction of foot gangrene, and the increased trend of osteomyelitis and necrotizing fasciitis. Figure 1D panel reveals a fourfold increase in the performance of PTAs while bypass surgeries remained at the same rate.
Figure 1.
Summary of trends of limb outcome (A) patient comorbidities, (B) foot presentation, (C) and vascular intervention, (D) in patients with type 2 diabetes and diabetic foot complications. *P for trend<0.05; **p for trend<0.01; ***p for trend<0.001. LEA, lower extremity amputation; PTA, percutaneous transluminal angioplasty.
Discussion
The results of this large, national, 8-year study suggest that DFCs remain common. The updated nationwide trends in a high-income country might provide more objective information for future management of this complex disease.
The incidence of type 1 diabetes has been reported between 2 and 5 per 100 000 person-years,21 and the prevalence is much lower than those in Western countries.22–24 In this survey, the prevalence of type 1 diabetes was found to be <1% (data not provided). To simplify the epidemiological information, this study excluded patients with type 1 diabetes. The observation of the increase of patients with T2D in this study affirms the soaring rate of diabetic prevalence in Asian countries.23 25 Between years 2007 and 2014, increases of 39.6% in patients with T2D and 33.4% in patients with T2D and DFCs were found. The prevalence of DFCs in the T2D population was therefore unchanged at around 2% a year and had declining trend.
It is notable that the rates of LEAs were significantly reduced from 2.85 to 2.06 per 1000 T2D population per year, and our data showed this was mainly attributable to the reduction of major LEA. These trends are similar to the reports of Geiss et al in the USA.11 Nevertheless, the rates of LEAs reported by Geiss et al was higher (5.38–3.07 per 1000 adults of diabetes) and the trend of LEAs in Taiwan consistently decreased without change in more recent years. It is difficult to analyze the difference of LEAs between individual health systems or entire national health services. The type of diabetes, ethnicities, age distribution, comorbidities and the utility of medical resource may all attribute to the difference. Sheen et al reported a similar decrease of LEAs in patients with diabetes between 1998 and 2007 (3.79 and 2.27/1000/year).19 Although the authors suggested the impact of pay-for-performance programme in Taiwan, this conclusion may be over-reaching because only 12% coverage rate of that programme for patients with diabetes at that time.26 Understanding the underlying factors might provide beneficial strategies for future foot care.
Along with the lifespan increase in patients with T2D,27 we are now facing a trend of more aged patients with higher associated comorbidities. This study demonstrates similar trends in patient with DFCs. Of note, in contrast to the trend of patients associated with MACE remaining stable, the association with chronic kidney disease or end-stage renal disease has increased significantly. We know that chronic kidney diseases28 and especially end-stage renal disease29 themselves are the major risk factors for LEAs. This association between kidney disease and amputation has induced mandatory foot care education in this subgroup of the T2D population.
From studies reported, it follows that the risk factors reported for LEAs while treating patients with DFCs are age,13 PAD14 especially gangrene,16 severity of infection,15 nutritional status,17 and kidney function.16 For the study period, the trend of increasing age and associated comorbidities should not be the cause of the decreasing trend of LEAs. Although we found decreased trends of patients with associated peripheral neuropathy and retinopathy, the role that neuropathy30 or microvascular circulation31 has on LEAs in patients with DFCs still remains controversial.
Regarding the affected foot presentation, the trends showed an increase of foot infections, but a decrease of foot ulcers. More significantly, we noticed a drop of gangrene presentations. Foot gangrene usually suggests critical ischemia with or without infection, and in many situations, is inevitable for certain degrees of LEA.32 Although direct evidence was not obtained, the continual decrease of gangrene presentations in DFCs over time was mostly likely due to clinical awareness of early management.
The performance of vascular interventions increased dramatically nationwide, from 6.2% of patients in 2007 to 19.5% in 2014. This may result from an increased awareness of the benefit of interdisciplinary treatments for local ulcer or infection and the underlying PADs. As no specific analysis was performed associating this dramatic increase in peripheral artery intervention and reduction in amputation, drawing conclusions should be made with caution. We plan future efforts to specifically assess this potential association.
Any appropriate rate of vascular intervention could not be determined from this study; nevertheless, the PAD prevalence rate ranging from 26.8% to 28.8%, and among the patients with PAD, a gangrene prevalence of 11.3%–14.7% might indicate that 19.5% of vascular procedures were still within reasonable range. This study also shows that along with the increase of vascular procedures, the utility of annual hospitalization or medical cost for patients with DFCs was not increased. In Taiwan, the performance of PTAs increased at a much greater rate than that of bypass surgery. Similar outcomes have been reported.33–35
In this large population database study, several limitations should be considered. The diagnostic codes usually provide limited information; more detailed information, for example, wound classification or the degree of PAD and the successful rate of vascular interventions were lacking. Furthermore, as mentioned above, no specific relationship between increased peripheral artery intervention and amputation was made using these current data. The statistical estimations of the correlation between the clinical characteristics or DFC phenotypes and the LEAs were weak, and their causal relationship requires further prospective study design to be validated.
In conclusion, the DFCs continue to remain a major medical and public health issue as we face patients in increased numbers, age, and comorbidities. Nevertheless, increasing medical attention including early intervention for ulcers to avoid gangrene, and proper medical treatments (in this study, vascular intervention) could provide a decrease in LEA outcomes.
Footnotes
Contributors: C-WL reached data and wrote the manuscript. DGA contributed to the discussion and reviewed/edited the manuscript. C-HL contributed to the study concept and design and interpretation of data. P-HL researched data and contributed to discussion. S-YH reviewed/edited the manuscript. S-RL researched data. C-HH interpreted data and contributed to discussion. Y-YH contributed to the study design, discussion and wrote the manuscript.
Funding: This study was supported by project grant from the CMRPD1H0531and CORPG3J0021.
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: This study was approved by the Chang Gung Medical Foundation Institutional Review Board (No. 107–2041 C1).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: All data relevant to the study are included in the article or uploaded as supplementary information.
References
- 1. Morbach S, Furchert H, Gröblinghoff U, et al. . Long-Term prognosis of diabetic foot patients and their limbs: amputation and death over the course of a decade. Diabetes Care 2012;35:2021–7. 10.2337/dc12-0200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wukich DK, Raspovic KM. Assessing health-related quality of life in patients with diabetic foot disease: why is it important and how can we improve? the 2017 Roger E. Pecoraro Award Lecture. Diabetes Care 2018;41:391–7. 10.2337/dci17-0029 [DOI] [PubMed] [Google Scholar]
- 3. Armstrong DG, Boulton AJM, Bus SA. Diabetic foot ulcers and their recurrence. N Engl J Med Overseas Ed 2017;376:2367–75. 10.1056/NEJMra1615439 [DOI] [PubMed] [Google Scholar]
- 4. Armstrong DG, Wrobel J, Robbins JM. Guest editorial: are diabetes-related wounds and amputations worse than cancer? Int Wound J 2007;4:286–7. 10.1111/j.1742-481X.2007.00392.x [DOI] [PubMed] [Google Scholar]
- 5. Skrepnek GH, Mills JL, Lavery LA, et al. . Health care service and outcomes among an estimated 6.7 million ambulatory care diabetic foot cases in the U.S. Diabetes Care 2017;40:936–42. 10.2337/dc16-2189 [DOI] [PubMed] [Google Scholar]
- 6. Barshes NR, Sigireddi M, Wrobel JS, et al. . The system of care for the diabetic foot: objectives, outcomes, and opportunities. Diabet Foot Ankle 2013;4. doi: 10.3402/dfa.v4i0.21847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Holzer LA, Sevelda F, Fraberger G, et al. . Body image and self-esteem in lower-limb amputees. PLoS One 2014;9:e92943 10.1371/journal.pone.0092943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gurney JK, Stanley J, Rumball-Smith J, et al. . Postoperative death after lower-limb amputation in a national prevalent cohort of patients with diabetes. Diabetes Care 2018;41:1204–11. 10.2337/dc17-2557 [DOI] [PubMed] [Google Scholar]
- 9. Lin C-W, Hsu BR-S, Tsai J-S, et al. . Effect of limb preservation status and body mass index on the survival of patients with limb-threatening diabetic foot ulcers. J Diabetes Complications 2017;31:180–5. 10.1016/j.jdiacomp.2016.09.011 [DOI] [PubMed] [Google Scholar]
- 10. Bommer C, Heesemann E, Sagalova V, et al. . The global economic burden of diabetes in adults aged 20–79 years: a cost-of-illness study. The Lancet Diabetes & Endocrinology 2017;5:423–30. 10.1016/S2213-8587(17)30097-9 [DOI] [PubMed] [Google Scholar]
- 11. Geiss LS, Li Y, Hora I, et al. . Resurgence of diabetes-related nontraumatic lower-extremity amputation in the young and middle-aged adult U.S. population. Diabetes Care 2019;42:50–4. 10.2337/dc18-1380 [DOI] [PubMed] [Google Scholar]
- 12. Harding JL, Pavkov ME, Gregg EW, et al. . Trends of nontraumatic lower-extremity amputation in end-stage renal disease and diabetes: United States, 2000–2015. Diabetes Care 2019;42:1430–5. 10.2337/dc19-0296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Boyko EJ, Seelig AD, Ahroni JH. Limb- and person-level risk factors for lower-limb amputation in the prospective Seattle diabetic foot study. Diabetes Care 2018;41:891–8. 10.2337/dc17-2210 [DOI] [PubMed] [Google Scholar]
- 14. American Diabetes Association Peripheral arterial disease in people with diabetes. Diabetes Care 2003;26:3333–41. 10.2337/diacare.26.12.3333 [DOI] [PubMed] [Google Scholar]
- 15. Lin C-W, Hsu L-A, Chen C-C, et al. . C-Reactive protein as an outcome predictor for percutaneous transluminal angioplasty in diabetic patients with peripheral arterial disease and infected foot ulcers. Diabetes Res Clin Pract 2010;90:167–72. 10.1016/j.diabres.2010.08.002 [DOI] [PubMed] [Google Scholar]
- 16. Sun J-H, Tsai J-S, Huang C-H, et al. . Risk factors for lower extremity amputation in diabetic foot disease categorized by wagner classification. Diabetes Res Clin Pract 2012;95:358–63. 10.1016/j.diabres.2011.10.034 [DOI] [PubMed] [Google Scholar]
- 17. Gau B-R, Chen H-Y, Hung S-Y, et al. . The impact of nutritional status on treatment outcomes of patients with limb-threatening diabetic foot ulcers. J Diabetes Complications 2016;30:138–42. 10.1016/j.jdiacomp.2015.09.011 [DOI] [PubMed] [Google Scholar]
- 18. Huang Y-Y, Lin K-D, Jiang Y-D, et al. . Diabetes-Related kidney, eye, and foot disease in Taiwan: an analysis of the nationwide data for 2000–2009. J Formos Med Assoc 2012;111:637–44. 10.1016/j.jfma.2012.09.006 [DOI] [PubMed] [Google Scholar]
- 19. Sheen Y-J, Kung P-T, Kuo W-Y, et al. . Impact of the pay-for-performance program on lower extremity amputations in patients with diabetes in Taiwan. Medicine 2018;97:e12759 10.1097/MD.0000000000012759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Division of Cancer Control & Population Sciences, National Cancer Institute Joinpoint trend analysis software [Internet]. Available: https://surveillance.cancer.gov/joinpoint/ [Accessed 18 June 2019].
- 21. Mayer-Davis EJ, Kahkoska AR, Jefferies C, et al. . ISPAD clinical practice consensus guidelines 2018: definition, epidemiology, and classification of diabetes in children and adolescents. Pediatr Diabetes 2018;19(6):7–19. 10.1111/pedi.12773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chan JCN, Cho NH, Tajima N, et al. . Diabetes in the Western Pacific Region—Past, present and future. Diabetes Res Clin Pract 2014;103:244–55. 10.1016/j.diabres.2013.11.012 [DOI] [PubMed] [Google Scholar]
- 23. International Diabetes Federation IDF diabetes atlas. 8th edn Brussels, Belgium: International Diabetes Federation, 2017. https://diabetesatlas.org/ [Google Scholar]
- 24. Won JC, Lee JH, Kim JH, et al. . Diabetes fact sheet in Korea, 2016: an appraisal of current status. Diabetes Metab J 2018;42:415–24. 10.4093/dmj.2018.0017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. World Health Organization Country and regional data on diabetes: prevalence of diabetes in the WHO South-East Asia Region [Internet], 2019. Available: https://www.who.int/diabetes/facts/world_figures/en/index5.html [Accessed 18 June 2019].
- 26. Lee I-T, Hsu C-C, Sheu WH-H, et al. . Pay-For-Performance for shared care of diabetes in Taiwan. J Formos Med Assoc 2019;6646 30350-X 10.1016/j.jfma.2019.08.011 [DOI] [PubMed] [Google Scholar]
- 27. Kalyani RR, Golden SH, Cefalu WT. Diabetes and aging: unique considerations and goals of care. Diabetes Care 2017;40:440–3. 10.2337/dci17-0005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Patel VI, Mukhopadhyay S, Guest JM, et al. . Impact of severe chronic kidney disease on outcomes of infrainguinal peripheral arterial intervention. J Vasc Surg 2014;59:368–75. 10.1016/j.jvs.2013.09.006 [DOI] [PubMed] [Google Scholar]
- 29. Franz D, Zheng Y, Leeper NJ, et al. . Trends in rates of lower extremity amputation among patients with end-stage renal disease who receive dialysis. JAMA Intern Med 2018;178:1025–32. 10.1001/jamainternmed.2018.2436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ugwu E, Adeleye O, Gezawa I, et al. . Predictors of lower extremity amputation in patients with diabetic foot ulcer: findings from MEDFUN, a multi-center observational study. J Foot Ankle Res 2019;12:34 10.1186/s13047-019-0345-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schaper NC, Andros G, Apelqvist J, et al. . Diagnosis and treatment of peripheral arterial disease in diabetic patients with a foot ulcer. A progress report of the International Working group on the diabetic foot. Diabetes Metab Res Rev 2012;28(Suppl 1):218–24. 10.1002/dmrr.2255 [DOI] [PubMed] [Google Scholar]
- 32. Shatnawi NJ, Al-Zoubi NA, Hawamdeh HM, et al. . Predictors of major lower limb amputation in type 2 diabetic patients referred for hospital care with diabetic foot syndrome. Diabetes Metab Syndr Obes 2018;11:313–9. 10.2147/DMSO.S165967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lepäntalo MJA, Houbballah R, Raux M, et al. . Lower extremity bypass vs endovascular therapy for young patients with symptomatic peripheral arterial disease. J Vasc Surg 2012;56:545–54. 10.1016/j.jvs.2012.06.053 [DOI] [PubMed] [Google Scholar]
- 34. Iida O, Nakamura M, Yamauchi Y, et al. . 3-Year outcomes of the olive registry, a prospective multicenter study of patients with critical limb ischemia: a prospective, multi-center, three-year follow-up study on endovascular treatment for infra-inguinal vessel in patients with critical limb ischemia. JACC Cardiovasc Interv 2015;8:1493–502. [DOI] [PubMed] [Google Scholar]
- 35. Wiseman JT, Fernandes-Taylor S, Saha S, et al. . Endovascular versus open revascularization for peripheral arterial disease. Ann Surg 2017;265:424–30. 10.1097/SLA.0000000000001676 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjdrc-2019-000795supp001.pdf (26.4KB, pdf)