Abstract
Background
Symptom management in glioma patients remains challenging, as patients suffer from various concurrently occurring symptoms. This study aimed to identify symptom clusters and examine the association between these symptom clusters and patients’ functioning.
Methods
Data of the CODAGLIO project was used, including individual patient data from previously published international randomized controlled trials (RCTs) in glioma patients. Symptom prevalence and level of functioning were assessed with European Organisation for Research and Treatment of Cancer (EORTC) quality of life QLQ-C30 and QLQ-BN20 self-report questionnaires. Associations between symptoms were examined with Spearman correlation coefficients and partial correlation networks. Hierarchical cluster analyses were performed to identify symptom clusters. Multivariable regression analyses were performed to determine independent associations between the symptom clusters and functioning, adjusted for possible confounders.
Results
Included in the analysis were 4307 newly diagnosed glioma patients from 11 RCTs who completed the EORTC questionnaires before randomization. Many patients (44%) suffered from 5–10 symptoms simultaneously. Four symptom clusters were identified: a motor cluster, a fatigue cluster, a pain cluster, and a gastrointestinal/seizures/bladder control cluster. Having symptoms in the motor cluster was associated with decreased (≥10 points difference) physical, role, and social functioning (betas ranged from −11.3 to −15.9, all P < 0.001), independent of other factors. Similarly, having symptoms in the fatigue cluster was found to negatively influence role functioning (beta of −12.3, P < 0.001), independent of other factors.
Conclusions
Two symptom clusters, the fatigue and motor cluster, were frequently affected in glioma patients and were found to independently have a negative association with certain aspects of patients’ functioning as measured with a self-report questionnaire.
Keywords: EORTC QLQ-C30, glioma, health-related quality of life, QLQ-BN20, symptom, symptom cluster
Key Points.
1. Four symptom clusters were identified in newly diagnosed glioma patients.
2. The motor and fatigue cluster were associated with decreased functioning.
3. Comprehensive symptom assessment is important to address symptoms in a timely manner.
Importance of the Study.
This study identified 4 symptom clusters in a large group of newly diagnosed glioma patients, assessed with the EORTC self-report health-related quality of life questionnaires. The motor cluster was found to negatively influence patients’ physical, role, and social functioning to a significant and clinically relevant extent, independently of other sociodemographic and clinical variables. Similarly, the fatigue cluster negatively impacted role functioning. Therefore, relieving symptoms in the fatigue and motor clusters may guide symptom management in newly diagnosed glioma patients.
Patients with a glioma, the most prevalent malignant primary brain tumor,1 suffer from a variety of symptoms during the course of disease, including fatigue, cognitive problems, behavioral problems, and motor dysfunction.2 Many patients experience more than one symptom simultaneously,3 and typically more symptoms are experienced than are reported to or detected by clinicians.4,5 Depending on the definition, 2 or more symptoms that are related to each other and occur together are referred to as a symptom cluster, and associations between symptoms within a symptom cluster are stronger than associations among different symptom clusters and/or separate symptoms.6,7 Identification of these symptom clusters may aid symptom management, because the co-occurrence of symptoms may have a larger impact on patients’ functioning and overall health-related quality of life (HRQoL) than each symptom alone.8 If management is aimed at improvement of patients’ functioning, targeting these specific symptom clusters may provide an opportunity.
In other cancer populations, several symptom clusters have been identified9,10 which were found to be associated with patients’ functioning. In glioma patients, however, symptom clusters have not been studied sufficiently. The few studies that were conducted have limitations, including limited sample sizes or the lack of inclusion of glioma-specific symptoms.9,11,12 Patients with a glioma may suffer from generic cancer symptoms such as fatigue and mood disorders, but also from disease-specific symptoms such as seizures, headaches, motor deficits, or cognitive deficits.13,14 Both these generic and disease-specific symptoms may be associated with a patients’ well-being and functioning, including physical, role, emotional, cognitive, and social functioning.
The aim of this study was to identify symptom clusters in a large sample of newly diagnosed glioma patients, and to investigate the associations between the identified symptom clusters and patients’ functioning and global health status/quality of life.
Methods
Study Population
Patients included in this study participated in previously published phase II and III randomized controlled trials (RCTs) including adult patients with both recurrent and newly diagnosed glioma (Supplementary Table 1). Over 6000 patients are included in the CODAGLIO (ie, COmbining clinical trial DAtasets in GLIOma) project.15 For the purpose of the current analysis, focusing on identifying symptoms clusters at the time of diagnosis, only RCTs involving newly diagnosed glioma patients and using the European Organisation for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire C30 (QLQ-C30) and QLQ–Brain Neoplasm (BN)20 module were included. All RCTs were approved by the ethical committees of all participating centers and all patients gave their informed consent to participate in the respective RCT. Moreover, all principal investigators of these RCTs gave permission for use of the collected data within the CODAGLIO project.
Measurements
Generic cancer-specific and brain tumor–specific symptoms, as well as levels of functioning, were measured with the EORTC QLQ-C30 and the QLQ-BN20 questionnaire. The EORTC QLQ-C30 version 3.016 and the brain-cancer specific QLQ-BN2017 were administered at baseline, ie, before the start of the allocated treatment (after surgery and irrespective of supportive treatment), and at prespecified timepoints during follow-up. The EORTC QLQ-C30 is the core EORTC questionnaire that includes 30 items, comprising 5 functioning scales (physical, role, emotional, cognitive, and social functioning), 3 symptom scales (fatigue, pain, and nausea/vomiting), a global health status/quality of life scale, and 6 single items (dyspnea, appetite loss, insomnia, constipation, diarrhea, and financial difficulties). The QLQ-BN20 is specifically designed for brain tumor patients and consists of 20 items in 4 symptom scales (future uncertainty, visual disorder, motor dysfunction, and communication deficit) and 7 single items (headaches, seizures, drowsiness, hair loss, itchy skin, weakness of legs, and bladder control). Responses for all items are on a 4-point Likert scale (ie, not at all, a little, quite a bit, and very much), except for the global health status/quality of life scale, which is scored on a 7-point Likert scale ranging from very poor to excellent. For both questionnaires, raw scores were linearly transformed to a scale from 0 to 100 according to the standard EORTC procedures.18 For the functioning scales and the global health status/QoL scale, a higher score indicates a better HRQoL. For the symptom scales and items, higher scores indicate more symptoms and worse functioning, respectively.
Other sociodemographic and clinical variables collected included age, sex, type of tumor (World Health Organization [WHO] grade II or III astrocytoma, oligodendroglioma, and oligoastrocytoma, or grade IV glioblastoma), WHO performance status (PS) (0 vs 1 vs 2), and type of surgery (resection vs biopsy).
Statistical Analysis
Descriptive methods were used to summarize baseline sociodemographic, clinical, and HRQoL data, including the prevalence and severity of symptoms. For this study, only fully completed baseline HRQoL forms were considered. To evaluate differences between patients with and without a completed HRQoL baseline form (ie, possible selection bias), several clinical characteristics were compared using the chi-square test for categorical data and an independent Student’s t-test for continuous variables. Mean scores on the functioning scales of the included patients were compared with a healthy normgroup to have an indication of the level of functioning of the included patients.19
Clustering of the symptoms was carried out in 3 steps, and we chose to define a symptom cluster as having a minimum of 2 symptoms. First, to explore symptom clustering, Spearman correlational analyses were carried out on all symptom scales and single items of the EORTC QLQ-C30 and QLQ-BN20, except financial difficulties and future uncertainty, which we did not classify as symptoms (ie, defined as “a physical or mental feature which is regarded as indicating a condition of disease”). The magnitudes of the correlations were interpreted as follows: between 0 and ±0.3 as “little if any”; between ±0.3 and ±0.5 as “low”; between ±0.5 and ±0.7 as “moderate”; and above ±0.7 as “high.” 20 Next, the associations among the symptoms were presented in an unregularized partial correlation network based on Spearman correlations, which was used to examine whether the associations between the symptoms were still present when adjusting for the other symptoms. The network model was estimated using the Gaussian graphical model, which estimates a network of partial correlation coefficients.21,22 Network models provide an alternative method to visualize associations and consist of nodes (circles = the symptoms) and edges (lines = the relation between the symptoms). Each link in the network represents a partial correlation coefficient between 2 symptoms after controlling for the other symptoms. We included at least 2 symptoms in the symptom clusters.
Thereafter, hierarchical cluster analysis (HCA) was performed as a last step to assess how the symptom scales/items cluster.23 HCA is an exploratory technique that identifies groups of symptoms based on similarity between them: symptoms within the same cluster resemble each other but differ from those in another symptom cluster.24 The symptoms were included as continuous variables in the HCA and the similarity between the different clusters was assessed with the average-linkage-between-groups method, using the Euclidean distance. A dendrogram for the symptom clusters was plotted to illustrate the arrangement of the variables produced by clustering. A stronger similarity between the symptoms is reflected by a smaller distance between the branches. In order to determine the optimal number of clusters, a range of clusters from one (all symptoms clustered together) to 18 (all symptoms as separate single symptoms) was produced in the cluster membership analysis. The optimal number of clusters was based on the results of all 3 steps: the correlation analysis, the partial Spearman matrix, and the HCA. Subanalyses in predefined subgroups based on sex, age (<55 vs ≥55 y), WHO performance status (PS = 0/1 vs PS = 2), resection (biopsy vs resected), and type of tumor (glioblastoma vs non-glioblastoma) were performed to investigate whether the symptom clusters were invariant across subpopulations. Also, a subanalysis for tumor location was carried for patients with such information available.
After the identification of the clusters, patients were classified as having “symptoms” or “no symptoms” for both the symptom clusters and the single symptoms. Patients were classified as having symptoms when they reported mild to severe symptoms on at least one item in a symptom cluster, or on the single symptoms. Thereafter, univariable linear regression analyses were performed to determine the association between each symptom cluster and the 5 functioning scales (physical, cognitive, emotional, role, and social functioning) and the global health status/QoL scale. Subsequently, 6 multivariable linear regression analyses were performed for each functioning scale and the global health status/QoL scale, including the symptom cluster, single symptoms as well as relevant clinical/sociodemographic variables (sex, age, WHO PS, type of tumor. and type of surgery), to determine the independent association between the symptom clusters and the functioning scales and the global health status/QoL scale. All variables were included simultaneously, allowing adjustment for confounders for the associations between the symptom clusters and functioning. In each multivariable regression model, a two-tailed P-value < 0.05 was considered statistically significant. In terms of clinical relevance, beta coefficients ≥10 were considered clinically relevant and beta coefficients ≥20 were considered a large effect, corresponding with, respectively, a 10 and 20 point change in HRQoL scores.25 Analyses were performed using IBM SPSS v23.026 and R27 with the qgraph package.22
Results
Patient Population
A total of 11 RCTs (Supplementary Table 1)28–38 were analyzed, comprising 5287 patients with newly diagnosed glioma, of whom 4307 patients (81%) completed a full HRQoL baseline form. When comparing patients who completed an HRQoL form with patients who did not, a selection bias toward a healthier population was observed. Patients with an HRQoL form were younger (mean of 54 vs 57 y, P < 0.001), had a better WHO PS (percentage of patients with score 0–1 was 88% vs 81%, P = 0.001), more often had a resection rather than biopsy (82% vs 79%, P = 0.025), and were less often diagnosed with glioblastoma (69% vs 73%, P = 0.007).
Level of Functioning and Symptom Prevalence and Severity
As a group, the included patients scored lower on all functioning scales and the global health status/QoL scale compared with the general European population19 (≥10 points difference between the groups), representing an impairment in functioning. On the individual patient level, impaired functioning was observed ranging from 38% of patients for physical functioning to 69% of patients for cognitive functioning (Table 2).
Table 2.
Number of patients with impaired functioning and with symptoms
| Functioning Scales | Mean (SD) | Patients with Impaired Functioning,an (%) |
|---|---|---|
| Global health status | 63.9 (22.6) | 1828 (42) |
| Physical functioning | 81.5 (22.1) | 1648 (38) |
| Role functioning | 65.2 (33.3) | 2351 (55) |
| Emotional functioning | 71.7 (23.8) | 1824 (42) |
| Cognitive functioning | 72.5 (27.3) | 2961 (69) |
| Social functioning | 69.3 (30.3) | 2865 (67) |
| Symptoms | mean (SD) | Patients with symptoms, b n (%) |
| Fatigue (scale) | 34.9 (25.3) | 3706 (86) |
| Nausea and vomiting (scale) | 4.6 (12.1) | 759 (18) |
| Pain (scale) | 14.7 (21.7) | 1882 (44) |
| Dyspnea (item) | 10.9 (21.3) | 1063 (25) |
| Insomnia (item) | 24.9 (29.9) | 2135 (50) |
| Appetite loss (item) | 10.8 (22.1) | 1008 (23) |
| Constipation (item) | 12.7 (24.2) | 1135 (26) |
| Diarrhea (item) | 5.6 (15.5) | 588 (14) |
| Visual disorder (scale) | 13.2 (20.3) | 1924 (45) |
| Motor dysfunction (scale) | 17.4 (22.8) | 2363 (55) |
| Communication deficit (scale) | 19.1 (25.6) | 2304 (54) |
| Headache (item) | 19.9 (26.3) | 1894 (44) |
| Seizures (item) | 6.1 (18.6) | 519 (12) |
| Drowsiness (item) | 27.5 (27.4) | 2593 (60) |
| Hair loss (item) | 9.8 (22.8) | 833 (19) |
| Itchy skin (item) | 9.8 (20.8) | 949 (22) |
| Weakness of legs (item) | 14.8 (26.0) | 1288 (30) |
| Bladder control (item) | 8.1 (20.3) | 726 (17) |
aPatients who reported a ≥10 points lower score compared with the normgroup18; bpatients who reported any symptoms (mild to severe)
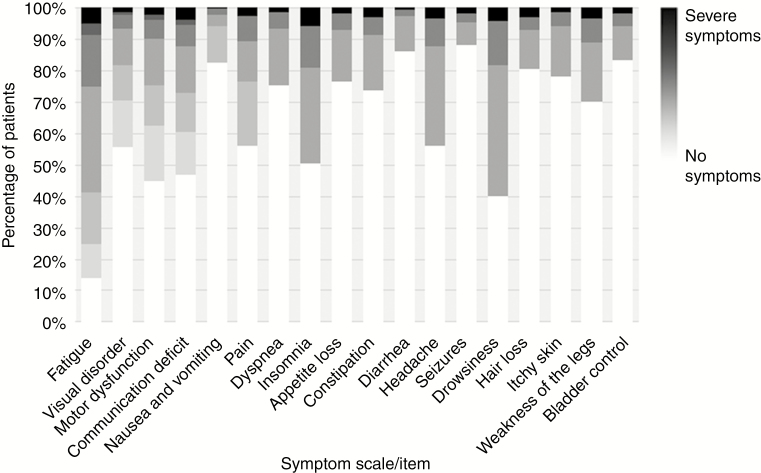
On the individual patient level, 4183 of 4307 included patients (97%) who self-reported at least one symptom. Most patients tallied between 1 and 4 (40%) or between 5 and 10 concurrent symptoms (44%), while 562 patients (13%) reported more than 10 concurrent symptoms (Table 1). Among the 18 reported symptoms, fatigue was the most prevalent, experienced by 86% of patients, followed by drowsiness (60%) and motor dysfunction (55%) (Table 2). In terms of severity of the symptoms, the majority of symptoms were experienced as mild, and less often as moderate or severe (Fig. 1).
Table 1.
Baseline sociodemographic and clinical characteristics of all patients participating in the RCTs and separately for those who have a valid baseline HRQoL form
| All Patients (n = 5287), n (%) | Patients Who Completed an HRQOL Form (n = 4307), n (%) | Patients Who Did Not Complete an HRQOL Baseline Form (n = 980), n (%) | P-value | |
|---|---|---|---|---|
| Male | 3191 (60) | 2659 (62) | 532 (54) | <0.001 |
| Female | 2096 (40) | 1648 (38) | 448 (46) | |
| Age (mean, SD) | 54 (14) | 54 (14) | 57 (13) | <0.001 |
| Gr II/III, A/O/OA | 1594 (30) | 1331 (31) | 263 (27) | 0.007 |
| Gr IV glioblastoma | 3693 (70) | 2976 (69) | 717 (73) | |
| WHO PS 0–1 | 4597 (87) | 3805 (88) | 792 (81) | 0.001 |
| WHO PS 2 | 659 (13) | 487 (11) | 172 (18) | |
| Missing | 31 (1) | 15 (0) | 16 (2) | |
| Biopsy | 985 (19) | 780 (18) | 205 (21) | 0.025 |
| Resection | 4287 (81) | 3514 (82) | 773 (79) | |
| Missing | 15 (0) | 13 (0) | 2 (0) | |
| 0 symptoms | - | 124 (3) | - | - |
| 1–4 concurrent symptoms | - | 1725 (40) | - | |
| 5–10 concurrent symptoms | - | 1896 (44) | - | |
| >10 concurrent symptoms | - | 562 (13) | - |
Gr, grade of the tumor; A, astrocytoma; O, oligondendroglioma; OA, oligoastrocytoma; SD, standard deviation.
Fig. 1.
Severity of symptoms for the selected symptoms scales/items measured with the EORTC QLQ-C30 and QLQ-BN20 questionnaires. A darker color indicates more severe symptoms. The single items (dyspnea, insomnia, appetite loss, constipation, diarrhea, headache, seizures, drowsiness, hair loss, itchy skin, weakness of the legs, and bladder control) were rated as: no, mild, moderate, and severe. For the symptom scales (fatigue, visual disorder, motor dysfunction, communication deficit, nausea and vomiting, and pain), the symptoms consisted of multiple items. The Fig. represents the severity on a 0–100 scale, where 0 (white) indicates no symptoms and 100 (black) indicates severe symptoms.
Symptom Clusters
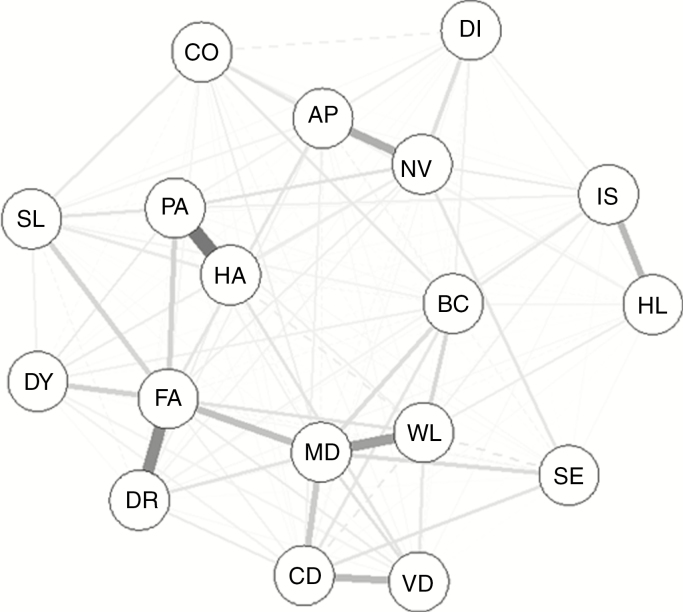
The strength of the correlations between symptoms was low to moderate, ranging between 0.01 and 0.59, with the strongest correlations found for fatigue with drowsiness (.59) and motor dysfunction (.52), for pain and headache (.57), and for motor dysfunction with weakness of the legs (.52) (Supplementary Table 2). A graphical representation of the Spearman correlations between symptoms is presented in Fig. 2, based on the partial correlation matrix (Supplementary Table 3). Fatigue and motor dysfunction were the symptoms that showed the largest centrality in terms of closeness, betweenness, and strength (ie, measures indicating the importance of the symptoms in the network, of the correlation with the other symptoms) (Supplementary Fig. 1).
Fig. 2.
Spearman correlation matrix of selected symptoms measured with the EORTC QLQ-C30 and QLQ-BN20 questionnaires. Thicker and darker lines represent stronger partial correlations. Continued lines represent positive partial correlations, dotted lines represent negative partial correlations. The position of the variables represent the closeness, node strength, and betweenness of the variables. Central variables with more connections and thicker lines are most strongly correlated with other variables.
AP, appetite loss; BC, bladder control; CD, communication deficit; CO, constipation; DI, diarrhea; DY, dyspnea; DR; drowsiness; FA, fatigue; HA, headache; HL, hair loss; SL, insomnia; IS, itchy skin; MD, motor dysfunction; NV, nausea and vomiting; PA, pain; SE, seizures; VD, visual disorder; WL, weakness of the legs.
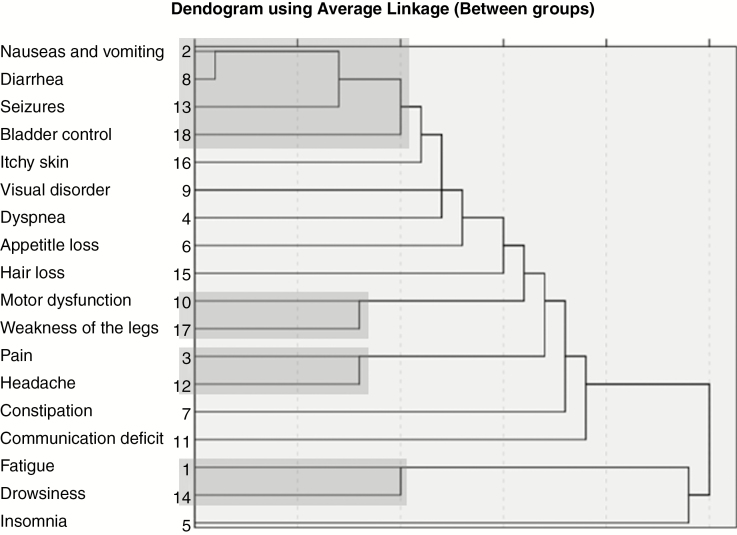
Thereafter, HCA was performed to identify clusters based on the similarities between them, illustrated by the dendrogram (Fig. 3). Based on the correlation analyses, the partial correlation matrix, and the cluster membership analysis/dendrogram, the clustering step consisting of 4 symptom clusters and 8 single symptoms was found most suitable based on both clinical considerations and the Spearman correlation matrix. The 4 symptom clusters were as follows: “pain cluster” (consisting of pain and headache), “motor cluster” (consisting of motor dysfunction and weakness of the legs), “fatigue cluster” (consisting of fatigue and drowsiness), and “gastrointestinal/seizures/bladder control cluster” (consisting of nausea and vomiting, diarrhea, seizures, and bladder control). The pain cluster, the motor cluster, and the fatigue cluster were consistently found across the subgroups—age, sex, WHO PS, tumor type, and surgery—while the gastrointestinal/seizures/bladder control cluster was not observed in patients with a low PS and non-glioblastoma patients (data not shown). Data on tumor location were available for 2283 of 4307 of patients (53%). The motor cluster and fatigue cluster were consistently found in patients with different tumor locations, whereas the pain cluster and the GI/seizures/bladder control cluster were not found across all tumor locations (data not shown).
Fig. 3.
Dendrogram illustrating the results of the hierarchical cluster analysis (HCA). The distance at which the branches join indicates the similarity between the symptoms (shorter branches represent greater similarity). Symptoms with greater similarity were clustered first, presented on the left side of the figure. This cluster analysis shows that nausea and vomiting were clustered as a first step, followed by seizures (step 2). Next, pain and headache (step 3) and motor dysfunction and weakness of the legs were clustered (step 4), and so on. The optimal number of clusters was determined at step 6, resulting in the 4 clusters indicated in this Fig. (indicated in gray).
Prevalence of the Symptom Clusters
Most patients (88%) experienced symptoms in the fatigue cluster, followed by the motor cluster (59%), the pain cluster (56%), and the GI/seizures/bladder control cluster (43%). The majority of patients experienced symptoms in several symptom clusters: 79% of the patients experienced symptoms in at least 2 clusters, 51% in at least 3 clusters, and 22% in all 4 clusters. The symptom clusters that occurred most frequently together were the fatigue and the motor cluster, which was experienced by 56% of the patients, and the fatigue and pain cluster, experienced by 53% of the patients.
Association Between Symptom Clusters and Functioning and Global Health Status/Quality of Life
Results of the univariable regression analyses showed that the 4 symptom clusters negatively influenced all functioning scales and the global health status/QoL scale, except for the association between the pain cluster and physical functioning (betas ranged from −9.25 to −30.94, all P < 0.001). Results of the multivariable regression analyses indicated that only the motor cluster and the fatigue cluster negatively influenced functioning in the presence of other factors (Table 3). The motor cluster had a clinically relevant negative impact on patients’ physical, role, and social functioning (betas ranged from −11.3 to −15.9, all P < 0.001), whereas the fatigue cluster had a clinically relevant negative impact on the patient’s role functioning (beta −12.3, P < 0.001). With respect to the single symptoms, visual disorder and communication deficit negatively influenced cognitive functioning in the presence of other factors (Table 3).
Table 3.
Multivariable linear regression analysis showing the association between the four symptom clusters and the functional scales and the global health status/quality of life scale, adjusted for important confounding variables
| Cluster | HRQoL Functioning Scales beta, P-value | |||||
|---|---|---|---|---|---|---|
| Global Health Status/QoL Scale | Physical Functioning | Role Functioning | Emotional Functioning | Cognitive Functioning | Social Functioning | |
| Symptom clusters a | ||||||
| Pain | −5.7 | −2.9 | −4.9 | −5.4 | −3.5 | −3.8 |
| <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Motor | −8.8 | −11.6* | −15.9* | −4.5 | −7.3 | −11.3* |
| <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Fatigue | −5.7 | −3.8 | −12.3* | −4.9 | −3.7 | −8.3 |
| <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| GI/seizures/bladder control | −3.7 | −3.6 | −3.4 | −3.6 | −1.3 | −2.8 |
| <0.001 | <0.001 | <0.001 | <0.001 | 0.067 | 0.001 | |
| Single symptoms a | ||||||
| Dyspnea | −5.1 | −6.8 | −9.4 | −5.5 | −3.1 | −6.6 |
| <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Insomnia | −1.3 | −1.3 | −2.9 | −7.3 | −0.46 | −5.8 |
| 0.032 | 0.020 | 0.001 | <0.001 | 0.498 | <0.001 | |
| Appetite loss | −3.8 | −3.6 | −3.4 | −5.3 | −5.2 | −4.5 |
| <0.001 | <0.001 | 0.001 | <0.001 | <0.001 | <0.001 | |
| Constipation | −2.0 | −0.07 | −2.0 | −2.5 | −3.3 | −1.8 |
| 0.003 | 0.905 | 0.039 | 0.001 | <0.001 | 0.063 | |
| Visual disorder | −4.9 | −2.9 | −6.9 | −4.5 | −14.6* | −5.7 |
| <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Communication deficit | −2.6 | −.36 | −3.3 | −4.0 | −16.4* | −4.3 |
| <0.001 | 0.535 | <0.001 | <0.001 | <0.001 | 0.87 | |
| Hair loss | −1.0 | −.82 | 1.7 | −2.8 | 0.11 | −2.5 |
| 0.172 | 0.247 | 0.130 | 0.001 | 0.894 | 0.018 | |
| Itchy skin | 0.33 | −0.42 | 0.30 | −0.48 | 0.63 | −2.5 |
| 0.655 | 0.535 | 0.778 | 0.555 | 0.448 | 0.013 | |
| Clinical/sociodemographic variables | ||||||
| Age | −0.12 | −0.13 | −0.05 | −0.01 | −0.05 | 0.06 |
| <0.001 | <0.001 | 0.139 | 0.861 | 0.064 | 0.076 | |
| Female sex (ref: male) | −0.24 | −4.62 | −0.00 | −2.1 | −0.96 | −0.36 |
| 0.689 | <0.001 | 00.998 | 0.001 | 0.157 | 0.668 | |
| Surgery (ref: biopsy only) | 2.26 | 1.34 | −0.27 | 1.78 | 1.79 | 0.50 |
| 0.003 | 0.06 | 0.809 | 0.034 | 0.037 | 0.64 | |
| Gr IV glioblastoma (ref: Gr II/III, A/O/OA) | −0.15 | 3.2 | −0.84 | −1.64 | −0.28 | 0.51 |
| 0.831 | <0.001 | 0.425 | 0.037 | 0.731 | 0.61 | |
| WHO PS 2 (ref: WHO PS 0–1) | −3.3 | −7.8 | −9.7 | −1.27 | −3.18 | −5.4 |
| <0.001 | <0.001 | <0.001 | 0.015 | <0.001 | <0.001 | |
Gr, grade of the tumor; A, astrocytoma; O, oligondendroglioma; OA, oligoastrocytoma; aseverity of symptoms ranging from mild to severe; *clinically relevant difference (≥10 points).
In addition, results of subanalyses showed that the impact of functioning was larger, and entailed more functioning scales in patients with symptoms in ≥3 symptom clusters compared with patients with symptoms in only 1 or 2 clusters (data not shown). For example, when selecting only those patients with symptoms in 1 symptom cluster (13% of the patients), there was no clinical impact on patients’ functioning scales. When selecting patients who experienced symptoms in ≥3 clusters (51% of the patients), the motor cluster had a clinically relevant negative impact on the same functioning scales, but with a larger impact (betas ranged from −13.4 to −16.6), and the fatigue cluster had a clinically relevant negative impact on global health, physical functioning, and social functioning, in addition to role functioning. Also, the impact was larger (betas ranged from −13.3 for the global health status to −27.8 for role functioning). Consequently, patients who experience symptoms in more symptom clusters are likely to experience a larger impact on functioning.
Discussion
The results of this study show that glioma patients experience multiple symptoms simultaneously shortly after surgery, but before initiation of further antitumor treatment. This suggests the need for comprehensive symptom assessment at baseline, in order to address symptoms in a timely manner. Consistent with the literature, overall quality of life and functioning was impaired at randomization (ie, before the start of the allocated treatment, reflecting the impact of the disease and possible surgical and supportive treatment side effects such as fatigue, insomnia, and nausea/vomiting). The most frequently reported symptoms, occurring in more than 40% of the patients, were fatigue, drowsiness, motor dysfunction, communication deficits, insomnia, visual disorders, and headache/pain, corresponding with the core symptoms in glioma patients.8,39–42 Results of the correlation analyses (revealing low to moderate correlations), partial correlation matrix, and HCA together identified 4 symptom clusters: a pain cluster, a motor cluster, a fatigue cluster, and a GI/seizures/bladder cluster.
The fatigue cluster, comprising both fatigue and drowsiness, was most often prevalent (88%). This result indicates fatigue already is an important symptom in early stages of disease, as patients included in this study were newly diagnosed, assessed after surgery but before further antitumor treatment. In a previous study in primary brain tumor patients, fatigue clustered with pain, insomnia, motor problems, and depression.8 Although these results were not replicated in the current study, fatigue was more strongly associated with pain, insomnia, and motor problems compared with the other symptoms in terms of correlations and position in the network matrix (Fig. 2). Mood disorders/complaints were not assessed in the current study as a single symptom. Nevertheless, the emotional functioning scale, which entails questions on mood, was not found to be independently associated with the fatigue cluster in our study.
The second most prevalent cluster was the motor cluster, experienced by 59% of patients. Motor dysfunction and muscle weakness can both be caused by the presence of a tumor in the motor brain region, or even when the tumor is located outside the motor cortex, due to diminished functional connectivity.43 Also, it can be a side effect of corticosteroids. Indeed, patients who used corticosteroids reported more problems in the motor cluster (67% vs 52%). We found that pain and headache clustered together, and one reason may be that pain has many dimensions and patients may have interpreted the item “Have you had pain” as both bodily pain and headache. Indeed, headache is a known presenting symptom in brain tumor patients.44 Similar to what has been reported in previous studies, headache and pain were present in almost half of the patients (both 44%).45,46 Most patients who experienced pain also experienced headache (74%), and vice versa (73%). However, although the correlation between pain and headache was the second highest found in our study (.57), it can still be interpreted as moderate, indicating that they do not measure the same concept. This is also true for fatigue and drowsiness, with a correlation of .59.
One unexpected finding is the clustering of nausea and vomiting, diarrhea, seizures, and bladder control. Clustering of GI symptoms was found in earlier studies; however, in our study the symptoms of appetite loss and constipation did not cluster with nausea and vomiting and diarrhea. An explanation for the clustering of these symptoms may be statistical, as each of these symptoms showed floor effects.41 These symptoms are the 4 least reported, all experienced by less than 25% of the patients (Table 2), and clustering of these symptoms in the HCA may be the result of numerical similarities rather than clinical similarities.
Although almost all symptom clusters showed a statistically significant association with the level of functioning and the global health status/QoL, only the motor and fatigue clusters were independently associated—ie, adjusted for important clinical characteristics, with role functioning (both clusters), physical functioning, and social functioning (the motor cluster) at a level that can be considered clinically relevant. Post hoc analyses showed that patients who experience symptoms in more symptom clusters experience impaired functioning to a larger extent and on more functioning scales. Although not surprising, this study is the first to observe an association between symptom clusters and functioning in glioma patients. Similar results were found in other cancer populations: a pain/fatigue/cognitive cluster impacted physical, role, and social functioning in advanced cancer patients,10 and an emotional cluster was found to negatively influence role and social functioning in patients with lung, breast, colorectal, and stomach cancer undergoing palliative chemotherapy.47 The results of our study suggest that a clinically relevant improvement in functioning could be achieved by relieving motor and fatigue symptoms in glioma patients. As the fatigue and motor clusters were also the most frequently affected clusters, and since most patients experienced symptoms in both, reducing the burden of these symptoms may benefit the majority of glioma patients in terms of improved functioning. Also, fatigue and motor problems were found to be most central to other symptoms (Fig. 2, Supplementary Fig. 3), suggesting that alleviating these symptoms most likely will positively influence the other symptoms as well. Fatigue and motor problems are, however, not easily treated. There is little evidence for pharmacological and non-pharmacological interventions for fatigue in glioma patients.48,49 The literature on interventions targeting motor problems is scarce, although mobility was improved in patients undergoing multidisciplinary treatment including physical exercise.50,51
One important limitation of this study is the selection bias toward a healthier population, as the patients included in our analyses were those deemed fit enough to participate in an RCT and who also completed HRQoL questionnaires. This could potentially limit the generalizability of the results. Another limitation is that only baseline data were used in the analyses, and we do not know if the clusters identified at pretreatment are stable during follow-up. Moreover, only symptoms were included that were measured with the QLQ-C30 and the QLQ-BN20 questionnaires. Some relevant symptoms, such as mood disorders or cognitive complaints, were not covered, and therefore the use of instruments that specifically and extensively measure symptoms may be more useful. Furthermore, we included glioma patients with different tumor types in the analyses and, besides the subgroups glioblastoma/non-glioblastoma, could not look further into different molecular subtypes. One implication could be that the results of our study may not be generalizable to all glioma subtypes, as we saw for the GI/seizures/bladder control cluster, which was not found in non-glioblastoma patients. Another limitation is that, in the regression analyses, the severity of the symptoms was not taken into account, as patients were classified as having “no symptoms” or “symptoms.” One could hypothesize that patients with more severe symptoms in the symptom clusters experience more impaired functioning than patients with mild symptoms only. Another limitation concerns the choices made regarding the definition of “symptom clusters” and the method used to identify them. First, different definitions of a symptom cluster exist, and there is no consensus on the minimum number of symptoms required to form a symptom cluster. We chose to define a symptom cluster as consisting of at least 2 symptoms. Of course, this choice impacted our results, as for example the fatigue cluster consists of only two symptoms. Further, the identified symptom clusters, for which we have combined 3 frequently used methods (ie, correlation analysis, partial correlation analysis, and HCA), might have been different when other methods were used—for example, factor analyses. Which is the best method remains a matter of debate.52
Even though the mentioned selection bias may hamper generalizability of the study results and is limited because of the overrepresentation of patients with a better health status, these results may have potential clinical implications. As most patients experience between 5 and 10 symptoms simultaneously, many symptoms may remain unnoticed as only the most severe symptoms are likely to be discussed during a consultation, and subsequently treated. Awareness of patients experiencing multiple concurrent symptoms and of the existence of symptom clusters and their association with functioning as measured with a self-report questionnaire might help clinicians to identify and treat patients with these symptoms in a more timely manner. Also, the awareness of the presence of these co-occurring symptoms could help clinicians to develop interventions with the intention to treat or prevent problems that appear together. Multimodal rehabilitation programs, for example, can be effective in treating multiple symptoms53 and may subsequently improve functioning. Furthermore, the identified symptom clusters may provide insight into the underlying mechanisms that caused these symptoms. It should be kept in mind, however, that the current study identified symptom clusters before the initiation of antitumor treatment including radiotherapy and/or chemotherapy. Further research should aim at investigating symptom clusters over time, to determine whether the identified symptom clusters are stable during the treatment and follow-up phases. Ideally, a prospective study investigating symptom clusters at baseline and during follow-up phases would allow us to examine the impact of a specific treatment regimen and the stability of symptom clusters over time. Moreover, future studies could also examine the (added) predictive value of symptom clusters for survival. This would be helpful in initiating interventions at the time patients benefit most from these treatment strategies.
Supplementary Material
Funding
This study was funded by a grant from the EORTC Quality of Life Group.
Conflict of interest statement.
ABo received research grants from Roche, Genentech, and Boeringher-Ingelheim, outside the submitted work; AAB received travel grant to ASCO from Roche and Celgene, outside the submitted work; OC has received research grants from Roche, and honoraria for lectures or advisory board from Celldex, Immatics, Abbvie, and Servier, outside the submitted work; UH reports grants and personal fees from Roche, personal fees and nonfinancial support from Medac and Bristol-Myers Squibb, and personal fees from Novocure, Novartis, Daichii-Sankyo, Riemser, and Noxxon, outside the submitted work; WW received study drug from Apogenix, Pfizer, and Roche as well as compensation for advisory activities to Abbvie and Roche, outside the submitted work; all other authors reported no conflict of interest.
Authorship statement.
BB, MB, AAB, OC, UH, FKG, AM, RS, MW, and WW were the principal investigators of the RCTs for which the data were originally collected, and were involved in data collection. In addition, JR and MT were involved in data collection in several RCTs. All authors were involved in the conceptualization of this study. MC and LD performed the statistical analysis and wrote the first draft of the manuscript. All authors were involved in the revision of the manuscript and have read and approved the final version.
References
- 1. Zhang AS, Ostrom QT, Kruchko C, Rogers L, Peereboom DM, Barnholtz-Sloan JS. Complete prevalence of malignant primary brain tumors registry data in the United States compared with other common cancers, 2010. Neuro Oncol. 2016;19(5):726–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Boele FW, Klein M, Reijneveld JC, Verdonck-de Leeuw IM, Heimans JJ. Symptom management and quality of life in glioma patients. CNS Oncol. 2014;3(1):37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mukand JA, Blackinton DD, Crincoli MG, Lee JJ, Santos BB. Incidence of neurologic deficits and rehabilitation of patients with brain tumors. Am J Phys Med Rehabil. 2001;80(5):346–350. [DOI] [PubMed] [Google Scholar]
- 4. Homsi J, Walsh D, Rivera N, et al. . Symptom evaluation in palliative medicine: patient report vs systematic assessment. Support Care Cancer. 2006;14(5):444–453. [DOI] [PubMed] [Google Scholar]
- 5. Esther Kim JE, Dodd MJ, Aouizerat BE, Jahan T, Miaskowski C. A review of the prevalence and impact of multiple symptoms in oncology patients. J Pain Symptom Manage. 2009;37(4):715–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kim HJ, McGuire DB, Tulman L, Barsevick AM. Symptom clusters: concept analysis and clinical implications for cancer nursing. Cancer Nurs. 2005;28(4):270–282; quiz 283. [DOI] [PubMed] [Google Scholar]
- 7. Barsevick A. Defining the symptom cluster: how far have we come? Semin Oncol Nurs. 2016;32(4):334–350. [DOI] [PubMed] [Google Scholar]
- 8. Armstrong TS, Cohen MZ, Eriksen LR, Hickey JV. Symptom clusters in oncology patients and implications for symptom research in people with primary brain tumors. J Nurs Scholarsh. 2004;36(3):197–206. [DOI] [PubMed] [Google Scholar]
- 9. Martinelli F, Quinten C, Maringwa JT, et al. ; European Organisation for Research and Treatment of Cancer Clinical Groups Examining the relationships among health-related quality-of-life indicators in cancer patients participating in clinical trials: a pooled study of baseline EORTC QLQ-C30 data. Expert Rev Pharmacoecon Outcomes Res. 2011;11(5):587–599. [DOI] [PubMed] [Google Scholar]
- 10. Dong ST, Costa DS, Butow PN, et al. . Symptom clusters in advanced cancer patients: an empirical comparison of statistical methods and the impact on quality of life. J Pain Symptom Manage. 2016;51(1):88–98. [DOI] [PubMed] [Google Scholar]
- 11. Fox SW, Lyon D, Farace E. Symptom clusters in patients with high-grade glioma. J Nurs Scholarsh. 2007;39(1):61–67. [DOI] [PubMed] [Google Scholar]
- 12. Kim SR, Shin YS, Kim JH, Choi M, Yoo SH. Differences in type composition of symptom clusters as predictors of quality of life in patients with meningioma and glioma. World Neurosurg. 2017;98:50–59. [DOI] [PubMed] [Google Scholar]
- 13. Ricard D, Idbaih A, Ducray F, Lahutte M, Hoang-Xuan K, Delattre JY. Primary brain tumours in adults. Lancet. 2012;379(9830):1984–1996. [DOI] [PubMed] [Google Scholar]
- 14. Armstrong TS, Vera-Bolanos E, Acquaye AA, Gilbert MR, Ladha H, Mendoza T. The symptom burden of primary brain tumors: evidence for a core set of tumor- and treatment-related symptoms. Neuro Oncol. 2016;18(2):252–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Johnson BE, Patronas N, Hayes W, et al. . Neurologic, computed cranial tomographic, and magnetic resonance imaging abnormalities in patients with small-cell lung cancer: further follow-up of 6- to 13-year survivors. J Clin Oncol. 1990;8(1):48–56. [DOI] [PubMed] [Google Scholar]
- 16. Aaronson NK, Ahmedzai S, Bergman B, et al. . The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85(5):365–376. [DOI] [PubMed] [Google Scholar]
- 17. Taphoorn MJ, Claassens L, Aaronson NK, et al. ; EORTC Quality of Life Group, and Brain Cancer, NCIC and Radiotherapy Groups An international validation study of the EORTC brain cancer module (EORTC QLQ-BN20) for assessing health-related quality of life and symptoms in brain cancer patients. Eur J Cancer. 2010;46(6):1033–1040. [DOI] [PubMed] [Google Scholar]
- 18. Fayers PM, Aaronson NK, Bjordal K, Grønvold M, Curran D, Bottomley A. EORTC QLQ-C30 scoring manual. 2001.
- 19. Hinz A, Singer S, Brähler E. European reference values for the quality of life questionnaire EORTC QLQ-C30: Results of a German investigation and a summarizing analysis of six European general population normative studies. Acta Oncol. 2014;53(7):958–965. [DOI] [PubMed] [Google Scholar]
- 20. Hinkle DE, Wiersma W, Jurs SG.. Applied Statistics for the Behavioral Sciences. 5th ed. Boston: Houghton Mifflin; 2003. [Google Scholar]
- 21. Borsboom D, Cramer AO. Network analysis: an integrative approach to the structure of psychopathology. Annu Rev Clin Psychol. 2013;9:91–121. [DOI] [PubMed] [Google Scholar]
- 22. Epskamp S, Cramer AO, Waldorp LJ, Schmittmann VD, Borsboom D. qgraph: network visualizations of relationships in psychometric data. J Stat Softw. 2012;48(4):1–18. [Google Scholar]
- 23. Everitt BS, Landau S, Leese M, Stahl D. Hierarchical clustering. In: Cluster Analysis. 5th ed. 2011:71–110. [Google Scholar]
- 24. Aktas A, Walsh D, Hu B. Cancer symptom clusters: an exploratory analysis of eight statistical techniques. J Pain Symptom Manage. 2014;48(6):1254–1266. [DOI] [PubMed] [Google Scholar]
- 25. Osoba D, Rodrigues G, Myles J, Zee B, Pater J. Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol. 1998;16(1):139–144. [DOI] [PubMed] [Google Scholar]
- 26. Released I. IBM SPSS Statistics for Windows. 20. Armonk, NY: IBM Corp; 2013. [Google Scholar]
- 27. Team RC. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2014. [Google Scholar]
- 28. Stupp R, Hegi ME, Gorlia T, et al. ; European Organisation for Research and Treatment of Cancer (EORTC); Canadian Brain Tumor Consortium; CENTRIC study team Cilengitide combined with standard treatment for patients with newly diagnosed glioblastoma with methylated MGMT promoter (CENTRIC EORTC 26071-22072 study): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15(10):1100–1108. [DOI] [PubMed] [Google Scholar]
- 29. Stupp R, Mason WP, van den Bent MJ, et al. ; European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. [DOI] [PubMed] [Google Scholar]
- 30. Baumert BG, Hegi ME, van den Bent MJ, et al. . Temozolomide chemotherapy versus radiotherapy in high-risk low-grade glioma (EORTC 22033-26033): a randomised, open-label, phase 3 intergroup study. Lancet Oncol. 2016;17(11):1521–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. van den Bent MJ, Baumert B, Erridge SC, et al. . Interim results from the CATNON trial (EORTC study 26053-22054) of treatment with concurrent and adjuvant temozolomide for 1p/19q non-co-deleted anaplastic glioma: a phase 3, randomised, open-label intergroup study. Lancet. 2017;390(10103):1645–1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Malmström A, Grønberg BH, Marosi C, et al. ; Nordic Clinical Brain Tumour Study Group (NCBTSG) Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: the Nordic randomised, phase 3 trial. Lancet Oncol. 2012;13(9):916–926. [DOI] [PubMed] [Google Scholar]
- 33. Wick W, Platten M, Meisner C, et al. ; NOA-08 Study Group of Neuro-Oncology Working Group (NOA) of German Cancer Society Temozolomide chemotherapy alone versus radiotherapy alone for malignant astrocytoma in the elderly: the NOA-08 randomised, phase 3 trial. Lancet Oncol. 2012;13(7):707–715. [DOI] [PubMed] [Google Scholar]
- 34. Keime-Guibert F, Chinot O, Taillandier L, et al. ; Association of French-Speaking Neuro-Oncologists Radiotherapy for glioblastoma in the elderly. N Engl J Med. 2007;356(15):1527–1535. [DOI] [PubMed] [Google Scholar]
- 35. Chinot OL, Wick W, Mason W, et al. . Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):709–722. [DOI] [PubMed] [Google Scholar]
- 36. Herrlinger U, Schäfer N, Steinbach JP, et al. . Bevacizumab plus irinotecan versus temozolomide in newly diagnosed O6-methylguanine-DNA methyltransferase nonmethylated glioblastoma: the randomized GLARIUS trial. J Clin Oncol. 2016;34(14):1611–1619. [DOI] [PubMed] [Google Scholar]
- 37. Stupp R, Taillibert S, Kanner A, et al. . Effect of tumor-treating fields plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma: a randomized clinical trial. JAMA. 2017;318(23):2306–2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. van den Bent MJ, Carpentier AF, Brandes AA, et al. . Adjuvant procarbazine, lomustine, and vincristine improves progression-free survival but not overall survival in newly diagnosed anaplastic oligodendrogliomas and oligoastrocytomas: a randomized European Organisation for Research and Treatment of Cancer phase III trial. J Clin Oncol. 2006;24(18):2715–2722. [DOI] [PubMed] [Google Scholar]
- 39. Mukand JA, Blackinton DD, Crincoli MG, Lee JJ, Santos BB. Incidence of neurologic deficits and rehabilitation of patients with brain tumors. Am J Phys Med Rehabil. 2001;80(5):346–350. [DOI] [PubMed] [Google Scholar]
- 40. Robertson ME, McSherry F, Herndon JE, Peters KB. Insomnia and its associations in patients with recurrent glial neoplasms. Springerplus. 2016;5(1):823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Taphoorn MJ, Claassens L, Aaronson NK, et al. . An international validation study of the EORTC brain cancer module (EORTC QLQ-BN20) for assessing health-related quality of life and symptoms in brain cancer patients. Eur J Cancer. 2010;46(6):1033–1040. [DOI] [PubMed] [Google Scholar]
- 42. Armstrong TS, Bishof AM, Brown PD, Klein M, Taphoorn MJ, Theodore-Oklota C. Determining priority signs and symptoms for use as clinical outcomes assessments in trials including patients with malignant gliomas: Panel 1 Report. Neuro Oncol. 2016;18(Suppl 2):ii1–ii12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Otten ML, Mikell CB, Youngerman BE, et al. . Motor deficits correlate with resting state motor network connectivity in patients with brain tumours. Brain. 2012;135(Pt 4):1017–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kirby S, Purdy RA. Headaches and brain tumors. Neurol Clin. 2014;32(2):423–432. [DOI] [PubMed] [Google Scholar]
- 45. Mahalakshmi P, Vanisree AJ. Quality of life measures in glioma patients with different grades: a preliminary study. Indian J Cancer. 2015;52(4):580–585. [DOI] [PubMed] [Google Scholar]
- 46. Osoba D, Brada M, Prados MD, Yung WK. Effect of disease burden on health-related quality of life in patients with malignant gliomas. Neuro Oncol. 2000;2(4):221–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rha SY, Lee J. Symptom clusters during palliative chemotherapy and their influence on functioning and quality of life. Support Care Cancer. 2017;25(5):1519–1527. [DOI] [PubMed] [Google Scholar]
- 48. Day J, Yust-Katz S, Cachia D, et al. . Interventions for the management of fatigue in adults with a primary brain tumour. Cochrane Database Syst Rev. 2016;4:Cd011376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Pace A, Dirven L, Koekkoek JAF, et al. ; European Association of Neuro-Oncology palliative care task force European Association for Neuro-Oncology (EANO) guidelines for palliative care in adults with glioma. Lancet Oncol. 2017;18(6):e330–e340. [DOI] [PubMed] [Google Scholar]
- 50. Khan F, Amatya B, Drummond K, Galea M. Effectiveness of integrated multidisciplinary rehabilitation in primary brain cancer survivors in an Australian community cohort: a controlled clinical trial. J Rehabil Med. 2014;46(8):754–760. [DOI] [PubMed] [Google Scholar]
- 51. Han EY, Chun MH, Kim BR, Kim HJ. Functional improvement after 4-week rehabilitation therapy and effects of attention deficit in brain tumor patients: comparison with subacute stroke patients. Ann Rehabil Med. 2015;39(4):560–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kim HJ, Abraham I, Malone PS. Analytical methods and issues for symptom cluster research in oncology. Curr Opin Support Palliat Care. 2013;7(1):45–53. [DOI] [PubMed] [Google Scholar]
- 53. Roberts PS, Nuño M, Sherman D, et al. . The impact of inpatient rehabilitation on function and survival of newly diagnosed patients with glioblastoma. PM R. 2014;6(6):514–521. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.