Abstract
Background
We identify cognitive impairment and MRI structural brain changes in long-term oligodendroglial tumor survivors treated with radiation therapy (RT) alone (21%) or with chemotherapy (CT) (79%).
Methods
Oligodendroglial tumor patients (based on the World Health Organization [WHO] 2007 classification) who completed RT ± CT at least 2 years before the study initiation, were classified into 3 groups according to the time treatment was completed: Group 1 = 2–5 years (n = 22), Group 2 = 6–10 years (n = 13), and Group 3 >10 years (n = 13). All patients had a cross-sectional neuropsychological evaluation (n = 48) and a longitudinal volumetric analysis (gray matter [GM; n = 34]) between postsurgical and last follow-up MRI. White matter (WM) changes on MRI were assessed using a qualitative scale.
Results
There were no differences regarding tumor or treatment-related characteristics between groups. Six of 22 patients (27.3%) in Group 1; 5/13 (38.5%) in Group 2; and 9/13 (69.2%) in Group 3 had cognitive impairment that was considered severe in 3/22 patients (13.6%) in Group 1; 4/13 (30.8%) in Group 2; and 6/13 (46.2%) in Group 3. Patients in Groups 2 and 3 showed significant GM atrophy and more leukoencephalopathy than Group 1. Cognitive deficits were associated with brain atrophy and WM changes.
Conclusions
Long-term oligodendroglial tumor survivors who underwent standard RT ± CT treatment, mainly >5 years of its completion, present cognitive impairment, especially on memory and executive functions, associated with late GM and WM damage, thus highlighting the need of developing future strategies in patients with oligodendroglial tumor and long expected survival.
Keywords: cognition, long-term survivors, neurotoxicity, oligodendroglioma, radiotherapy
Key Points.
1. Long-term oligodendroglial tumor survivors are at risk of developing cognitive impairment.
2. Late cognitive toxicity is associated with brain atrophy and white matter damage.
Importance of the Study.
Oncological treatment significantly prolongs survival in oligodendroglial tumor patients. However its potential toxicity on cognition in long-term survivors is still unknown. Our study showed that WHO grades II and III oligodendroglial tumor patients after >5 years of treatment completion, present cognitive impairment, that was considered severe in up to 38% of patients, accompanied by structural GM and WM damage, highlighting the need of developing future strategies in patients with long expected survival.
The recent standard treatment including chemotherapy (CT) (procarbazine, lomustine, and vincristine [PCV]) and cranial radiotherapy (RT) significantly improves overall survival and progression-free survival in World Health Organization (WHO) grades II and III oligodendrogliomas.1–3 Specifically, the 10-year overall survival rate with the standard treatment is nearly 80% for grade II and 60% for 1p/19q codeleted grade III oligodendrogliomas, a figure never observed before in malignant gliomas.2–4 Hence, the impact of long-term effects of oncological treatment, especially on cognitive functioning, in oligodendroglial tumor patients has become increasingly relevant. To date, only a few studies have focused on cognitive and structural brain changes in long-term glioma survivors.5–7 Overall, these studies showed that low grade glioma (LGG) survivors who received RT (mean follow-up of 12 y) in comparison with those who did not, exhibited cognitive deficits, mainly focused in executive functioning and visual memory, accompanied by global cortical atrophy and white matter (WM) changes in MRI. Still, these studies had small sample sizes and included a reduced number of oligodendroglial tumor patients, and none of them used a volumetric neuroimaging analysis to quantify brain changes over time.5–7
The aim of our study was to examine cognitive deficits together with brain structural changes in a series of WHO grades II and III oligodendroglial tumor long-term survivors who were treated with RT ± CT.
Materials and Methods
Patients
Medical records were retrospectively reviewed from histologically confirmed grades II and III oligodendroglial tumors based on the WHO 2007 classification,8 diagnosed between 1994 and 2014 at the Bellvitge University Hospital-Catalan Institute of Oncology and Hospital Clinic of Barcelona. Patients were included if they were older than 18 years and had completed RT ± CT treatment ≥2 years before study initiation.
Exclusion criteria included the presence of cerebrovascular disease, gliomatosis cerebri, infratentorial location, previous history of other primary malignancies, previous exposure to RT and/or CT, previous or concurrent neurologic disorder, aphasia, or marked visual compromise. All patients were fluent Spanish speakers and provided written informed consent. This study was approved by the review board of both hospitals. Patients were split into 3 subgroups according to the length of follow-up since treatment was completed: Group 1 = 2–5 years, Group 2 = 6–10 years, and Group 3 >10 years.
Neuropsychological and Quality of Life Assessment
A cross-sectional neuropsychological assessment was done for all patients between November and December 2016 (≥2 y from treatment completion). Patients were evaluated using a verbal memory test (Hopkins Verbal Learning Test–Revised [HVLT-R]),9 a visuospatial abilities and visual memory test (Rey–Osterrieth Complex Figure Test [ROCF] Copy and Delayed Recall),10 a verbal fluency test (Controlled Oral Word Association), and a processing speed test (Trail Making Test [TMT] A-B).11 If inadequate completion was noted due to moderate cognitive compromise, the Mini Mental State Examination (MMSE) was completed. Also used was the Beck Depression Inventory, a 21-item, self-report rating tool that measures characteristic attitudes and symptoms of depression.12 Raw cognitive test scores were compared with the validated Spanish normative values, corrected for age and education, and converted into z-scores. Cognitive impairment was defined as mild if: (i) a single test z-score of all of the aforementioned was ≥2 standard deviations (SD) or (ii) 2 tests had z-scores ≥1.5 SD below the sample mean. Cognitive impairment was defined as severe if z-score was below 2 SD in at least 2 tests or an adjusted MMSE score was less than or equal to percentile 5.11,13,14
Quality of life (QoL) was measured by a validated core QoL questionnaire (QLQ-C30 version 3.0)15 and a QoL questionnaire specifically for patients with brain tumors (QLQ-BN-20) of the European Organisation for Research and Treatment of Cancer (EORTC).16 The patients’ individual raw score on each subscale of the EORTC QLQ-C30 and BN20 was calculated and responses linearly transformed to a scale ranging from 0 to 100 per patient in accordance with the official EORTC scoring manual (http://groups.eortc.be/qol/manuals). Reference and normative data adjustments were completed and scored using the standardized recommended approach of the EORTC.17,18 A comparison between global health status/QoL from the EORTC QLQ-C30 questionnaire19 and cognitive impairment (yes/no) were calculated through a chi-square test.
MRI Data and Image Processing for Volumetric Gray Matter Analysis
Longitudinal MRI (1.5 Tesla) analysis was performed from first postsurgical (baseline) to last follow-up MRI. First, tumor remnant and/or surgical cavity were identified and drawn in native space over the gadolinium enhanced (Gd-enhanced) T1-weighted imaging for each patient using MRIcron (http://www.mccauslandcenter.sc.edu/mricro/mricron). Contrast-enhanced areas were identified as tumor masks. When there was no contrast-enhanced area, location of the tumor was determined using fluid-attenuated inversion recovery (FLAIR) images.
Then, a morphometric analysis was carried out using the SPM8 software package (Welcome Department of Imaging Neuroscience Group) running on MATLAB v7 (Mathworks). Specifically, first, unified segmentation20 with medium regularization and cost function masking was applied, in order to segment gray matter (GM) images and normalize the Gd-enhanced T1 image and the tumor mask for each patient into the Montreal Neurological Institute space.
GM, WM, and cerebrospinal fluid (CSF) were calculated after segmentation. Then, GM normalized images were modulated by total intracranial volume (GM + WM + CSF) at both evaluations (baseline and last MRI) in order to take into account potential differences in brain volume between patients. Additionally, and in order to avoid tumor-related confounding effects, T1-weighted native images were segmented using a similar described protocol into GM, WM, and CSF only from the tumor-free contralateral hemisphere. After applying an absolute threshold for masking around 0.5 for GM and correcting each MRI for each voxel size, GM volume in milliliters was extracted.
Statistical analysis was performed using SPSS 18.0 software. Repeated measures analysis of variance (ANOVA) for comparison among Groups (3) × Time (2) was used, using Bonferroni correction for post hoc analysis. Additionally, to study the association between cognitive deficits and GM volume changes, we carried out a Spearman’s correlation analysis between the individual z-scores of the statistical significant neuropsychological tests and the individual GM volume loss (last MRI − baseline).
MRI Data and Image Processing for White Matter Analysis
We qualitatively evaluated the presence of WM hyperintensities on FLAIR images using a 4-point rating scale.21 Specifically, the assigned values for this rating scale were: 0 for no lesions, 1 for focal lesions, 2 for beginning confluence of lesions, and 3 for diffuse involvement of the entire regions with or without involvement of U fibers. A Mann–Whitney U-test was used for comparison between groups. Kendall’s tau was calculated to correlate individual z-scores of the statistical significant neuropsychological tests with WM rating scores.
Results
Demographic and Clinical Characteristics
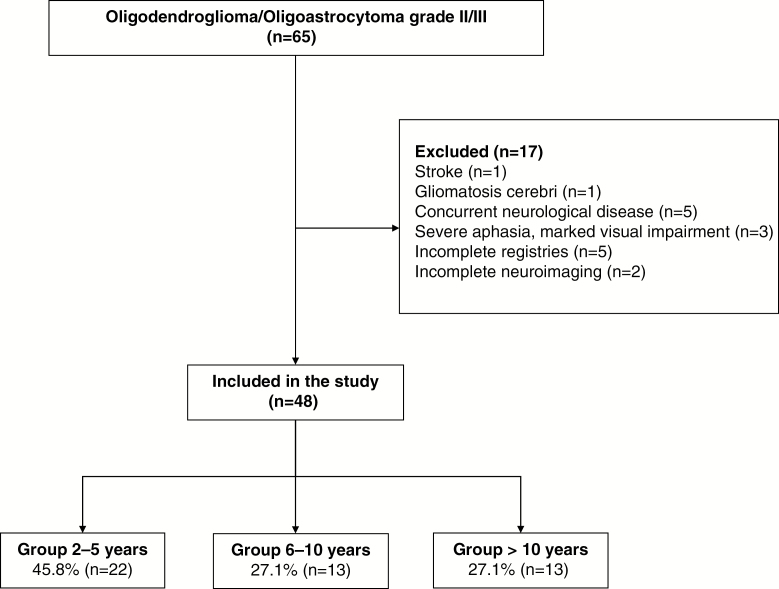
In a total of 242 oligodendroglial tumors diagnosed between 1994 and 2014, sixty-five patients were initially included; they were alive and older than 18 years and had completed RT ± CT treatment ≥2 years before the study initiation. After our exclusion criteria, 48 oligodendroglial tumor patients who completed RT alone (21%) or with CT (79%) were analyzed: 22 patients (45.8%) had completed treatment between 2 and 5 years (Group 1), 13 patients (27.1%) between 6 and 10 years (Group 2), and 13 patients (27.1%) >10 years (Group 3) (Fig. 1). There were no relevant differences regarding demographic, clinical, or treatment characteristics between groups (Table 1). Level of education and previous history of vascular risk factors were similar between groups. Median age at diagnosis was 39 years (range, 20–71) and 30 patients (62%) were male. Nine (19%) oligodendroglial tumors were grade II and 39 (81%) grade III. Status of 1p/19q codeletion and isocitrate dehydrogenase (IDH) was determined in 37 patients. Twenty-eight (75.6%) showed a 1p/19q codeletion and IDH mutant. Only 1 patient showed an IDH-mutant and 1p/19q non-codeleted tumor and the other 8 patients showed an IDH-wildtype and 1p/19q non-codeleted tumor. In the other 11 patients (23%), 1p/19q codeletion and IDH status could not be retrospectively performed because of insufficient tumor material available. All patients underwent 3D conformal RT. The number of patients undergoing CT was similar between groups but there were differences concerning the schedule received. All patients in Group 3 received PCV regimen (6 weekly cycles of 60 mg/m2 procarbazine [days 8–21], 110 mg/m2 lomustine [day 1], and 1.4 mg/m2 vincristine [days 8 and 29]), while in Groups 1 and 2 nearly half of patients received PCV and the other half adjuvant temozolomide (TMZ) (150–200 mg/m2 × 5 days every 4 wk). Four patients (8.3%) had tumor progression, all of them ≥2 years before the study initiation and had clinical and radiological tumor stability at the time that neuropsychological assessment was done (n = 3 in Group 1 and n = 1 in Group 3). All of them received a second-line CT. Three underwent sequential first-line CT and RT and 1 was first treated with RT alone and received first-line CT at tumor progression. Three patients (2 in Group 1 and 1 in Group 2) who were previously treated with TMZ received PCV, and 1 patient (Group 3) initially treated with PCV received TMZ as second-line CT. This patient (Group 3) treated with PCV and TMZ received fotemustine as third-line CT. There were significantly more patients in Group 1 (95%) on anti-epileptic drugs compared with Group 2 (38%) and Group 3 (61%) (P < 0.01). Levetiracetam was the most common prescribed anti-epileptic drug (80%).
Fig. 1.
CONSORT flow chart diagram.
Table 1.
Clinical and treatment variables of patients with grade II or III oligodendroglioma according to length of follow-up
| Group 1 = 2–5 y (n = 22) |
Group 2 = 6–10 y (n = 13) |
Group 3 >10 y (n = 13) |
P-value | |
|---|---|---|---|---|
| Age, years at diagnosis (mean ± SD) | 38.27 ± 9.73 | 43.08 ± 12.38 | 37.77 ± 11.68 | ns |
| Sex | 0.01a | |||
| Male | 18 (81.8) | 8 (61.5) | 4 (30.8) | |
| Female | 4 (18.2) | 5 (38.5) | 9 (69.2) | |
| Employed, n | 7 (31.8) | 3 (23.1) | 2 (15.4) | ns |
| KPS at study entry (median, range) | 90 (50–100) | 80 (50–100) | 80 (50–100) | ns |
| First symptom | ns | |||
| Seizures | 15 (68.2) | 8 (61.5) | 7 (53.8) | |
| Focal symptoms | 3 (13.6) | 2 (15.4) | 1 (7.7) | |
| Nonfocal symptomsc | 4 (18.2) | 3 (23.1) | 5 (38.5) | |
| WHO 2007 grade | ns | |||
| II | 3 (13.6) | 1 (7.7) | 5 (38.5) | |
| III | 19 (86.4) | 12 (92.3) | 8 (61.5) | |
| Tumor histology (WHO 2007) | ns | |||
| Oligodendroglioma | 11 (50) | 9 (69.2) | 7 (53.8) | |
| Oligoastrocytoma | 11 (50) | 4 (30.8) | 6 (46.2) | |
| Hemisphere location | ns | |||
| Right | 12 (54.4) | 8 (61.5) | 8 (61.5) | |
| Left | 10 (45.5) | 5 (38.5) | 5 (38.5) | |
| Lesion location | ns | |||
| Frontal | 14 (63.6) | 7 (53.8) | 7 (53.8) | |
| Other lobes | 4 (18.2) | 4 (30.8) | 3 (23.1) | |
| Two lobes | 4 (18.2) | 2 (15.4) | 3 (23.1) | |
| 1p/19q (n = 37), codeletion | 15 (71.4) | 9 (90) | 4 (66.7) | ns |
| Surgery | ns | |||
| Gross total resection | 7 (31.8) | 7 (53.8) | 5 (38.5) | |
| Partial resection | 15 (68.2)d | 6 (46.2) | 8 (61.5) | |
| Total RT dose, Gy, median (range) | 60 (50–60) | 60 (50–60) | 60 (48–60) | ns |
| First-line chemotherapy | 18 (81.8) | 11 (84.6) | 9 (69.2) | ns |
| PCV | 8 (44.4) | 6 (54.5) | 9 (100) | 0.01a/b |
| TMZ | 10 (55.6) | 5 (45.5) | 0 (0) | |
| Total number of first-line CT cycles, median (range) | ||||
| PCV | 4 (4–6) | 4 (4–6) | 5 (4–6) | 0.04a |
| TMZ | 6 (4–12) | 6 (6) | - | ns |
One-way ANOVA test and Bonferroni post hoc test were used to compare group means. Kruskal–Wallis and chi-square were used to compare group medians and percentages.
a Statistical differences between Group 1 and Group 3.
b Statistical differences between Group 2 and Group 3.
c Nonfocal symptoms: cognitive dysfunction (n = 1 in Group 3), vertigo (n = 2, one in Group 1 and one in Group 3), headache (n = 9, three patients in each group).
d Biopsy in one patient. KPS, Karnofsky performance score; ns, no significant differences (P > 0.05).
Neuropsychological Assessment and Quality of Life Measures
Table 2 and Figure 2 show the results of neuropsychological evaluation. Of the 48 patients included, 39 completed all tests in the neuropsychological battery: 19/22 (86.4%) in Group 1; 11/13 (84.6%) in Group 2; and 9/13 (69.2%) in Group 3. Four patients (2 in Group 1 and 2 in Group 3) did not complete the entire neuropsychological battery because of patients’ refusal or fatigue. The remaining 5 patients (1 in Group 1; 2 in Group 2; and 2 in Group 3) completed only the MMSE because of severe cognitive impairment.
Table 2.
Neuropsychological results
| Group 1 = 2–5 ye | Group 2 = 6–10 y | Group 3 >10 yf | P value* | |
|---|---|---|---|---|
| Cognitive impairmenta,b | 6 (27.3) | 5 (38.5) | 9 (69.2) | 0.06g |
| Mild | 3 (13.64) | 1 (7.69) | 3 (23.08) | 0.67 |
| Severe | 3 (13.64) | 4 (30.77) | 6 (46.15) | 0.12 |
| BDI ≥ 13a,c | 5 (22.7) | 2 (16.7) | 5 (45.5) | 0.31 |
| Phonemic fluencyd | ||||
| COWA | 0.31 (1.12) | 0.19 (1.23) | −0.56 (1.44) | 0.16 |
| Visuospatial abilitiesd | ||||
| ROCF First Copy | 1.4 (1.37) | 1.4 (1.40) | 1.3 (1.51) | 0.98 |
| Visual memoryd | ||||
| ROCF Delayed Copy | 0.95 (0.82) | −0.15 (0.52) | −1.06 (0.73) | 0.001g,h |
| Verbal memoryd | ||||
| HVLT total recall | 0.82 (1.05) | 0.98 (1.34) | 0.09 (1.70) | 0.25 |
| HVLT delayed recall | 0.91 (0.97) | 0.79 (1.74) | −0.53 (2.34) | 0.06 |
| HVLT delayed recognition | 0.22 (0.85) | 0.22 (0.66) | −0.43 (1.24) | 0.15 |
| Processing speed/executive functionsd | ||||
| Trail Making Test A | −0.50 (1.14) | −0.70 (1.20) | −1.73 (1.04) | 0.02g |
| Trail Making Test B | −0.52 (1.31) | −0.81 (0.93) | −1.46 (0.98) | 0.12 |
All results are z-score.
*Between-group ANOVA P-value with Bonferroni as post hoc test, except for a(chi-square).
a n (%);
b5 patients (1 in Group 1, 2 in Group 2, and 2 in Group 3) completed only MMSE due to severe cognitive impairment;
c 3 patients were excluded from the analysis due to incomplete registries;
d mean (SD);
e 2 patients in Group 1 did not complete TMT B.
f One patient of Group 3 did not complete HVLT-R and 1 patient in Group 3 did not complete ROCF (first Copy and Delayed Copy) and TMT A-B.
g Statistical differences between the Group 1 and Group 3.
h Statistical differences between Group 2 and Group 3. BDI, Beck Depression Inventory test; COWA, Controlled Oral Word Association Test; ROCF, Rey–Osterrieth Complex Figure; HVLT, Hopkins Verbal Learning Test.
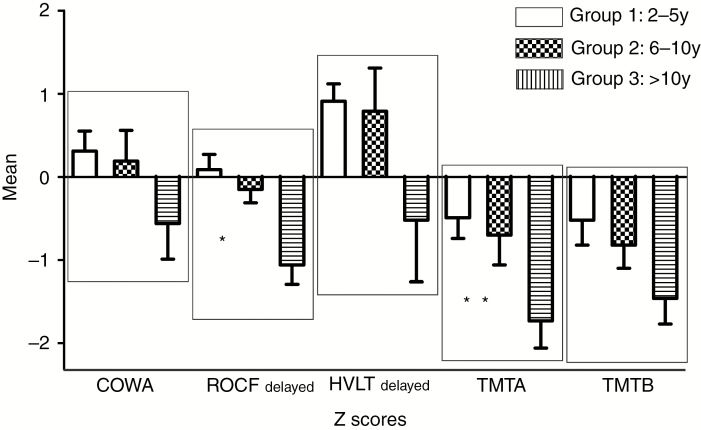
Fig. 2.
Summary of neuropsychological results (mean ± SEM): *Statistically differences between the Group 1 vs Group 3 and Group 2 vs Group 3; ** Statistically differences between the Group 1 vs Group 3.
Six of 22 patients (27.3%) in Group 1; 5/13 (38.5%) in Group 2; and 9/13 (69.2%) in Group 3 met criteria for cognitive impairment. Group 3 showed significant cognitive impairment compared with Group 1 (P = 0.015). Specifically, Group 3 did worse in measure of visual memory (ROCF Delayed Copy) compared with Groups 1 and 2 (P = 0.001 and P = 0.02, respectively) and executive functioning (TMT A) compared with Group 1 (P = 0.02). Of the patients with cognitive impairment (n = 20), 3 in Group 1 (15%), 4 in Group 2 (20%), and 6 in Group 3 (30%) met criteria for severe cognitive impairment.
In QoL measures, clinical relevant and significant differences were found regarding hair loss and dyspnea between groups. Those in Group 3 had more dyspnea and were more bothered about hair loss than Group 1 (P = 0.03 and 0.01, respectively). The other EORTC QLQ-C30 and QLQ-BN20 measures did not show significant differences between groups (see Supplementary Table 1). The presence of cognitive impairment was not associated with worse QoL score (P = 0.52).
Neuroimaging Data
Median time between the end of treatment (RT ± CT) and the last follow-up MRI was 3 years (range, 2–5 y) for Group 1; 7 years (range, 6–9 y) for Group 2; and 13 years (range, 10–14 y) for Group 3.
GM Volumetric Analysis
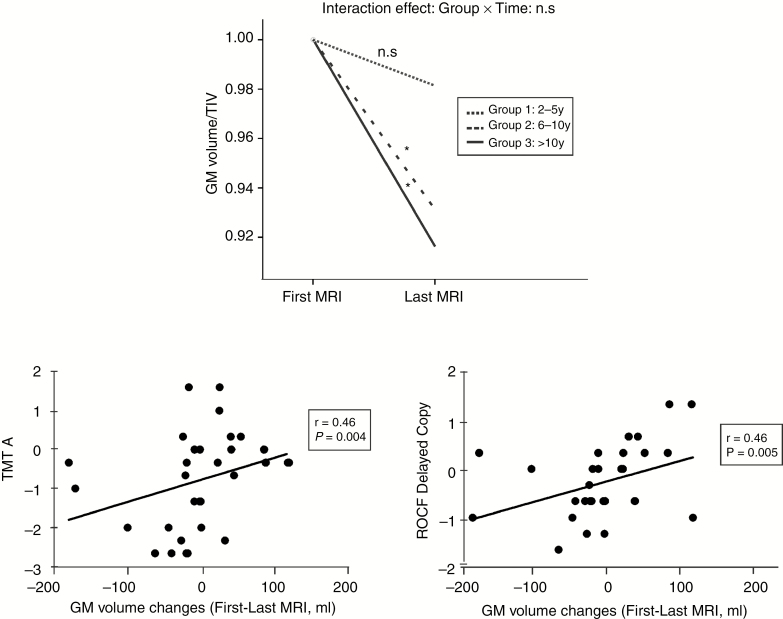
Concerning whole-brain analysis, Groups 2 and 3 exhibited a significant loss of absolute GM volume relative to baseline imaging (F = 10.89, P = 0.003; Fig. 3A) with a mean percent GM volume loss of 5.20% and 6.12% for Group 2 and Group 3, respectively. See Supplementary Table 2. When only the tumor-free contralateral hemisphere was analyzed, similar results were obtained. There was a positive correlation between GM absolute volume loss and performance on neuropsychological tests (for TMT A, r = 0.45, P < 0.005 and for ROCF Delayed Copy, r = 0.46, P < 0.004) (Fig. 3B and C).
Fig. 3.
(A) Longitudinal GM volume changes: GM median volume corrected by total intracranial volume (TIV) from first to last follow-up MRI is represented for the 3 groups of analysis. * Statistical significant changes. (B) Correlation between individual GM volume changes (first to last MRI, in mL) and individual Trail Making Test A scores (z score). (C) Correlation between individual GM volume changes (first to last MRI, in mL) and individual Rey–Osterrieth Complex Figure Delayed Copy scores (z score).
White Matter Analysis
Median rating scale of WM hyperintensities using last posttreatment FLAIR images was 1 (0–2) for Group 1; 2 (1–3) for Group 2; and 2.5 (1–3) for Group 3, showing significantly more WM hyperintensities in Groups 2 and 3 than in Group 1 (Mann‒Whitney U-test; P = 0.026 and P = 0.008, respectively). There was a negative correlation between WM scale score and performance on neuropsychological tests (for TMT A, r = −0.28, P < 0.07 and for ROCF delayed copy, r = −0.36, P < 0.02). Patients who scored worse in neuropsychological testing exhibited more leukoencephalopathy (higher rate in WM score). See Supplementary Figure 1.
Discussion
Our study revealed that 38% of patients with WHO grades II and III oligodendroglial tumors and long-term survival (>5 y since treatment completion) met criteria for severe cognitive impairment. Although cognitive deficits were also reported in patients at <5 years of follow-up, no structural brain abnormalities were observed. Following 5 years since treatment completion, nearly 40% of patients presented cognitive deficits (being severe in most of them) accompanied by an absolute GM volume loss (brain atrophy) and increased WM damage. After >10 years, more than two thirds of patients (69%) presented cognitive impairment especially focused on visual memory and executive functioning associated with GM atrophy and leukoencephalopathy. More important, cognitive deterioration was strongly associated with GM volume loss and increased leukoencephalopathy in this population with expected long survival.
Previous longitudinal studies in long-term survivors of glioma, mostly LGG (20–45% oligodendroglioma), treated only with RT, showed no evidence of cognitive deterioration during the first 5–6 years following treatment.5,7,22–25 However, our study showed that oligodendroglial tumor patients suffered from cognitive impairment as soon as 2 years following treatment. This difference between previous and our results may respond to the heterogeneity in the core set of neuropsychological tests and criteria for defining cognitive impairment used,11 as well as to the fact that nearly all of our patients underwent RT (median dose 60 Gy vs 55 Gy in previous studies, because we included grades II and III oligodendroglial tumors) but also CT. Conversely, in line with our findings, following >5 years since treatment completion previous studies in LGG reported a 53% rate of cognitive deterioration (12 y after diagnosis).5 In this sense, our work not only supports these findings showing a rate of 40–69% of cognitive deterioration at >5 and >10 years following treatment, but also reports for the first time in an oligodendroglial tumor population a 38% rate of severe cognitive impairment in long-term survivors.
Concerning neuroimaging findings, our study showed that oligodendroglial tumor patients with >5 years of follow-up present significant brain atrophy at last MRI compared with postsurgical evaluation (done at a median of 8.5 mo since surgery when possible confounding effects of postoperative edema are no longer present). Moreover, these longitudinal GM changes showed a positive correlation with executive functioning and visual memory scores. Previous studies addressing this issue have the limitations that the follow-up was short (≤2 y) and no neuropsychological evaluation was done.26 In contrast, several recent longitudinal studies conducted in glioblastoma populations, found GM volume loss during concomitant RT and TMZ treatment and nearly 1 year after cessation of treatment. Unfortunately, no neuropsychological evaluation and no longer follow-up were reported.27–29 Compared with oligodendroglioma, glioblastoma patients underwent concomitant CT treatment that might contribute to the early atrophy observed in these patients. Actually, concomitant therapy is being evaluated for patients with WHO grade III astrocytoma (CATNON trial), a group of glioma tumors with longer expected survival than glioblastoma, thus highlighting the relevance of delineating the potential toxic effects of RT and CT on cognition.
In regard to WM changes, patients with >5 years of follow-up showed more leukoencephalopathy than patients at shorter follow-up. In line with our findings, one previous study conducted in an LGG population treated with RT alone showed that at 12 years after diagnosis, patients exhibited more WM hyperintensities than at 6 years.5 Our study not only corroborates these previous findings but also found that cognitive deficits (especially visual memory) correlate with leukoencephalopathy. Recently, the use of a more sensitive method to study WM microstructure, such as diffusion tensor imaging, evidenced early (<2 y) WM damage following RT in LGG.30,31 Although advance neuroimaging techniques are able to detect earlier microstructure brain changes, no study with long-term follow-up has been published in the glioma population. Despite our findings of brain structural changes and cognitive impairment in long-term oligodendroglial tumor survivors, no differences between groups in most QoL measures or depression were observed. Although little data exist on QoL in oligodendroglial tumor survivors,25,32,33 our results highlight the fact that QoL measures might not be appropriate to detect subtle changes in the cognitively impaired population.
The mechanisms involved in CT and RT cognitive toxicity have not been clearly elucidated. Preclinical studies demonstrated that RT has a detrimental effect on neuronal precursor cells, vascular endothelial cells, neuronal functioning, and WM integrity.34,35 On the other hand, CT-induced cognitive toxicity or what is also called “chemobrain” has been mostly described in non-CNS tumor patients.36–38 In the glioma population, 2 interesting studies, one focused on the LGG population and the other on mixed anaplastic oligodendrogliomas according to WHO 2007 criteria, showed that the addition of PCV to RT did not result in significantly higher rates of MMSE score decline. However, no more extended cognitive assessment was performed.25,39 Although less studied, it has been suggested that CT may provoke diverse cellular toxicity due to DNA damage, thus contributing to the activation of neurotoxic cytokines and hormonal dysregulation, promoting the inhibition of hippocampal neurogenesis, damaging oligodendrocytes and reducing brain vascularization and blood flow.40,41
Our study presents some limitations. Due to its retrospective nature, neuropsychological evaluation at the time of tumor diagnosis was not done, thus it cannot be inferred whether there were longitudinal cognitive changes due to CT and/or RT. Additionally, the heterogeneity of our MRI data (different sites and imaging parameters) made the segmentation analysis difficult. In order to overcome the potential confounding effect of GM–WM boundaries, we decided to focus the volumetric analysis in only GM tissue.26 On the other hand, in 11 patients (23%) 1p/19q and IDH status could not be analyzed because of insufficient tumor available. We are aware of the weakness of this missing molecular data, especially taken into account the imperative need of this parameter for the current diagnostic criteria (WHO 2016). As a result, we carefully reviewed the histologic samples of these patients, all showing morphological characteristics of pure oligodendroglial tumor. Therefore, they would be classified according to the current diagnostic criteria as oligodendrogliomas not otherwise specified.42 Finally, the particular contribution of RT and PCV on cognitive deficits cannot be ruled out. Interestingly, time to neurocognitive progression associated with RT plus CT (PCV or TMZ) is actually being addressed in 2 ongoing phase III trials on newly diagnosed 1p/19q codeleted anaplastic glioma (NCT00887146, NCT02444000). In addition, it would be of interest to validate our results with the incorporation of modern RT techniques that offer better dose distribution and selective avoidance of structures at risk.43,44
In conclusion, our study shows that long-term oligodendroglial tumor survivors who underwent standard RT ± CT treatment are at risk of developing late cognitive toxicity, especially at >5 years following therapy, accompanied by brain atrophy and leukoencephalopathy. These findings highlight the need to improve and develop future treatment strategies for brain tumor patients with expected long survival.
Funding
This study has been funded by Instituto de Salud Carlos III through the project PI18/01253 (Co-funded by European Regional Development Fund. ERDF, a way to build Europe). We thank CERCA Programme / Generalitat de Catalunya for institutional support.
Conflict of interest statement.
Miguel Gil-Gil declares honoraria from Novartis, Pfizer, and Roche and participation on an advisory board for Daiichi. Dr Graus declares honoraria from MedLink Neurology for his work as associate editor. Dr Bruna declares honoraria from Bohringer.
Authorship statement.
All authors take full responsibility for the data, analyses, interpretation, and conduct of the research and approved the final version of the manuscript. Specifically: N.C.: writing the draft manuscript; acquisition of data; analysis or interpretation of data; statistical analysis; drafting/revising the manuscript for content, including medical writing for content. E.J.: writing the draft of the manuscript; acquisition of data; analysis or interpretation of data; statistical analysis; drafting/revising the manuscript for content, including medical writing for content. E.C.: analysis or interpretation of data; drafting/revising the manuscript for content, including medical writing for content. C.M., N.V., A.L., M.G., F.G.: acquisition of data; drafting/revising the manuscript for content, including medical writing for content. J.B.: analysis or interpretation of data; study concept or design; drafting/revising the manuscript for content, including medical writing for content; statistical analysis; study supervision or coordination. M.S.: analysis or interpretation of data; study concept or design; drafting/revising the manuscript for content, including medical writing for content; statistical analysis; study supervision or coordination.
Supplementary Material
References
- 1. Ostrom QT, Gittleman H, Truitt G, Boscia A, Kruchko C, Barnholtz-Sloan JS. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2011–2015. Neuro Oncol. 2018;20(suppl_4):iv1–iv86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. van den Bent MJ, Brandes AA, Taphoorn MJ, et al. Adjuvant procarbazine, lomustine, and vincristine chemotherapy in newly diagnosed anaplastic oligodendroglioma: long-term follow-up of EORTC brain tumor group study 26951. J Clin Oncol. 2013;31(3):344–350. [DOI] [PubMed] [Google Scholar]
- 3. Cairncross G, Wang M, Shaw E, et al. Phase III trial of chemoradiotherapy for anaplastic oligodendroglioma: long-term results of RTOG 9402. J Clin Oncol. 2013;31(3):337–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Buckner JC, Shaw EG, Pugh SL, et al. Radiation plus procarbazine, CCNU, and vincristine in low-grade glioma. N Engl J Med. 2016;374(14):1344–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Douw L, Klein M, Fagel SS, et al. Cognitive and radiological effects of radiotherapy in patients with low-grade glioma: long-term follow-up. Lancet Neurol. 2009;8(9):810–818. [DOI] [PubMed] [Google Scholar]
- 6. Correa DD, DeAngelis LM, Shi W, Thaler HT, Lin M, Abrey LE. Cognitive functions in low-grade gliomas: disease and treatment effects. J Neurooncol. 2007;81(2):175–184. [DOI] [PubMed] [Google Scholar]
- 7. Armstrong CL, Hunter JV, Ledakis GE, et al. Late cognitive and radiographic changes related to radiotherapy: initial prospective findings. Neurology. 2002;59(1):40–48. [DOI] [PubMed] [Google Scholar]
- 8. Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114(2):97–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. González-Palau F, Franco M, Jiménez F, Parra E, Bernate M, Solis A. Clinical utility of the Hopkins Verbal Test–Revised for detecting Alzheimer’s disease and mild cognitive impairment in Spanish population. Arch Clin Neuropsychol. 2013;28(3):245–253. [DOI] [PubMed] [Google Scholar]
- 10. Palomo R, Casals-Coll M, Sánchez-Benavides G, et al. Spanish normative studies in young adults (NEURONORMA young adults project): norms for the Rey-Osterrieth complex Figure (copy and memory) and free and cued selective reminding test. Neurologia. 2013;28(4):226–235. [DOI] [PubMed] [Google Scholar]
- 11. Wefel JS, Vardy J, Ahles T, Schagen SB. International Cognition and Cancer Task Force recommendations to harmonise studies of cognitive function in patients with cancer. Lancet Oncol. 2011;12(7):703–708. [DOI] [PubMed] [Google Scholar]
- 12. Lasa L, Ayuso-Mateos JL, Vázquez-Barquero JL, Díez-Manrique FJ, Dowrick CF. The use of the Beck Depression Inventory to screen for depression in the general population: a preliminary analysis. J Affect Disord. 2000;57(1–3):261–265. [DOI] [PubMed] [Google Scholar]
- 13. Crum RM, Anthony JC, Bassett SS, Folstein MF. Population-based norms for the Mini-Mental State Examination by age and educational level. JAMA. 1993;269(18):2386–2391. [PubMed] [Google Scholar]
- 14. Ingraham LJ, Aiken CB. An empirical approach to determining criteria for abnormality in test batteries with multiple measures. Neuropsychology. 1996;10(1):120–124. [Google Scholar]
- 15. Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85(5):365–376. [DOI] [PubMed] [Google Scholar]
- 16. Osoba D, Aaronson NK, Muller M, et al. The development and psychometric validation of a brain cancer quality-of-life questionnaire for use in combination with general cancer-specific questionnaires. Qual Life Res. 1996;5(1):139–150. [DOI] [PubMed] [Google Scholar]
- 17. Scott NW, Fayers PM, Aaronson NK, et al. ; EORTC and the Quality of Life Cross-Cultural Meta-Analysis Group The use of differential item functioning analyses to identify cultural differences in responses to the EORTC QLQ-C30. Qual Life Res. 2007;16(1):115–129. [DOI] [PubMed] [Google Scholar]
- 18.Fayers P, Aaronson NK, Bjordal K, Groenvold M, Curran D, Bottomley A. EORTC QLQ-C30 Scoring Manual. 3rd ed. Brussels: European Organisation for Research and Treatment of Cancer, 2001. [Google Scholar]
- 19. Hinz A, Singer S, Brähler E. European reference values for the quality of life questionnaire EORTC QLQ-C30: results of a German investigation and a summarizing analysis of six European general population normative studies. Acta Oncol. 2014;53(7):958–965. [DOI] [PubMed] [Google Scholar]
- 20. Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26(3):839–851. [DOI] [PubMed] [Google Scholar]
- 21. Wahlund LO, Barkhof F, Fazekas F, et al. ; European Task Force on Age-Related White Matter Changes A new rating scale for age-related white matter changes applicable to MRI and CT. Stroke. 2001;32(6):1318–1322. [DOI] [PubMed] [Google Scholar]
- 22. Laack NN, Brown PD, Ivnik RJ, et al. ; North Central Cancer Treatment Group Cognitive function after radiotherapy for supratentorial low-grade glioma: a North Central Cancer Treatment Group prospective study. Int J Radiat Oncol Biol Phys. 2005;63(4):1175–1183. [DOI] [PubMed] [Google Scholar]
- 23. Torres IJ, Mundt AJ, Sweeney PJ, et al. A longitudinal neuropsychological study of partial brain radiation in adults with brain tumors. Neurology. 2003;60(7):1113–1118. [DOI] [PubMed] [Google Scholar]
- 24. Vigliani MC, Sichez N, Poisson M, Delattre JY. A prospective study of cognitive functions following conventional radiotherapy for supratentorial gliomas in young adults: 4-year results. Int J Radiat Oncol Biol Phys. 1996;35(3):527–533. [DOI] [PubMed] [Google Scholar]
- 25. Wang M, Cairncross G, Shaw E, et al. Cognition and quality of life after chemotherapy plus radiotherapy (RT) vs. RT for pure and mixed anaplastic oligodendrogliomas: radiation therapy oncology group trial 9402. Int J Radiat Oncol Biol Phys. 2010;77(3):662–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gommlich A, Raschke F, Wahl H, Troost EGC. Retrospective assessment of MRI-based volumetric changes of normal tissues in glioma patients following radio(chemo)therapy. Clin Transl Radiat Oncol. 2018;8:17–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Petr J, Platzek I, Hofheinz F, et al. Photon vs. proton radiochemotherapy: effects on brain tissue volume and perfusion. Radiother Oncol. 2018;128(1):121–127. [DOI] [PubMed] [Google Scholar]
- 28. Prust MJ, Jafari-Khouzani K, Kalpathy-Cramer J, et al. Standard chemoradiation for glioblastoma results in progressive brain volume loss. Neurology. 2015;85(8):683–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Prust ML, Jafari-Khouzani K, Kalpathy-Cramer J, et al. Standard chemoradiation in combination with VEGF targeted therapy for glioblastoma results in progressive gray and white matter volume loss. Neuro Oncol. 2018;20(2):289–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chapman CH, Zhu T, Nazem-Zadeh M, et al. Diffusion tensor imaging predicts cognitive function change following partial brain radiotherapy for low-grade and benign tumors. Radiother Oncol. 2016;120(2):234–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chapman CH, Nagesh V, Sundgren PC, et al. Diffusion tensor imaging of normal-appearing white matter as biomarker for radiation-induced late delayed cognitive decline. Int J Radiat Oncol Biol Phys. 2012;82(5):2033–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Habets EJ, Taphoorn MJ, Nederend S, et al. Health-related quality of life and cognitive functioning in long-term anaplastic oligodendroglioma and oligoastrocytoma survivors. J Neurooncol. 2014;116(1):161–168. [DOI] [PubMed] [Google Scholar]
- 33. Boele FW, Douw L, Reijneveld JC, et al. Health-related quality of life in stable, long-term survivors of low-grade glioma. J Clin Oncol. 2015;33(9):1023–1029. [DOI] [PubMed] [Google Scholar]
- 34. Greene-Schloesser D, Robbins ME, Peiffer AM, Shaw EG, Wheeler KT, Chan MD. Radiation-induced brain injury: a review. Front Oncol. 2012;2:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Makale MT, McDonald CR, Hattangadi-Gluth JA, Kesari S. Mechanisms of radiotherapy-associated cognitive disability in patients with brain tumours. Nat Rev Neurol. 2017;13(1):52–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ahles TA, Saykin AJ. Candidate mechanisms for chemotherapy-induced cognitive changes. Nat Rev Cancer. 2007;7(3):192–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Anderson-Hanley C, Sherman ML, Riggs R, Agocha VB, Compas BE. Neuropsychological effects of treatments for adults with cancer: a meta-analysis and review of the literature. J Int Neuropsychol Soc. 2003;9(7):967–982. [DOI] [PubMed] [Google Scholar]
- 38. Matsuda T, Takayama T, Tashiro M, Nakamura Y, Ohashi Y, Shimozuma K. Mild cognitive impairment after adjuvant chemotherapy in breast cancer patients—evaluation of appropriate research design and methodology to measure symptoms. Breast Cancer. 2005;12(4):279–287. [DOI] [PubMed] [Google Scholar]
- 39. Prabhu RS, Won M, Shaw EG, et al. Effect of the addition of chemotherapy to radiotherapy on cognitive function in patients with low-grade glioma: secondary analysis of RTOG 98-02. J Clin Oncol. 2014;32(6):535–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Seigers R, Fardell JE. Neurobiological basis of chemotherapy-induced cognitive impairment: a review of rodent research. Neurosci Biobehav Rev. 2011;35(3):729–741. [DOI] [PubMed] [Google Scholar]
- 41. Hyrien O, Dietrich J, Noble M. Mathematical and experimental approaches to identify and predict the effects of chemotherapy on neuroglial precursors. Cancer Res. 2010;70(24):10051–10059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016;131(6):803–820. [DOI] [PubMed] [Google Scholar]
- 43. Yang Z, Zhang Z, Wang X, et al. Intensity-modulated radiotherapy for gliomas:dosimetric effects of changes in gross tumor volume on organs at risk and healthy brain tissue. Onco Targets Ther. 2016;9:3545–3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chung C, Laperriere N. Radiation therapy and grade II/III oligodendroglial tumors. CNS Oncol. 2015;4(5):325–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.