Abstract
Primary brain tumors account for ~1% of new cancer cases and ~2% of cancer deaths in the United States; however, they are the most commonly occurring solid tumors in children. These tumors are very heterogeneous and can be broadly classified into malignant and benign (or non-malignant), and specific histologies vary in frequency by age, sex, and race/ethnicity. Epidemiological studies have explored numerous potential risk factors, and thus far the only validated associations for brain tumors are ionizing radiation (which increases risk in both adults and children) and history of allergies (which decreases risk in adults). Studies of genetic risk factors have identified 32 germline variants associated with increased risk for these tumors in adults (25 in glioma, 2 in meningioma, 3 in pituitary adenoma, and 2 in primary CNS lymphoma), and further studies are currently under way for other histologic subtypes, as well as for various childhood brain tumors. While identifying risk factors for these tumors is difficult due to their rarity, many existing datasets can be leveraged for future discoveries in multi-institutional collaborations. Many institutions are continuing to develop large clinical databases including pre-diagnostic risk factor data, and developments in molecular characterization of tumor subtypes continue to allow for investigation of more refined phenotypes.
Key Point
1. Brain tumors are a heterogeneous group of tumors that vary significantly in incidence by age, sex, and race/ethnicity.2. The only well-validated risk factors for brain tumors are ionizing radiation (which increases risk in adults and children) and history of allergies (which decreases risk).3. Genome-wide association studies have identified 32 histology-specific inherited genetic variants associated with increased risk of these tumors.
Keywords: epidemiology, glioma, meningioma, pediatric brain tumors, risk factors
Primary brain tumors (BTs) account for approximately 1% of all newly diagnosed cancers in the United States, and about 2% of cancer deaths.1,2 BTs are the most common pediatric solid tumors and represent a substantial burden in terms of morbidity and mortality in children.3 Central nervous system (CNS) tumors are heterogeneous, including tumors of the brain, cranial nerves, spinal nerves, and meninges, and comprise over 100 histologic types based on cell of origin and other histopathological features.4 BTs can be broadly classified as malignant or non-malignant (benign) tumors, and graded from I to IV using a classification scheme specified by the World Health Organization (WHO) (see Table 1 for an overview of the most common BT histologies discussed in this review). The majority of BTs diagnosed in the US are non-malignant (WHO grades I and II), of which the majority are meningiomas.1 The most common malignant BTs (WHO grades III and IV) are gliomas, of which glioblastoma (GBM) is the most common histologic subtype.1 The goal of this review is to serve as an update to Ostrom et al5 and Johnson et al.3
Table 1.
Overview of most commonly occurring brain tumor histologies
| Major Histology | Histologic Subtypes | Percent of All CNS Tumors in the USa | Incidence per 100 000 Population in the USa | Behavior (WHO grade) | Most Commonly Affected Population |
|---|---|---|---|---|---|
| Glioma | GBM | 14.7 | 3.21 | Malignant (grade IV) | Older adults, more common in males than females |
| Pilocytic astrocytoma | 1.3 | 0.35 | Non-malignant (grade I) | Children | |
| Diffuse astrocytoma | 1.9 | 0.46 | Malignant (grade II) | Children and older adults | |
| Anaplastic astrocytoma | 1.7 | 0.41 | Malignant (grade III) | Adults | |
| Oligodendroglioma | 1.3 | 0.34 | Malignant (grades II‒III) | Adults | |
| Ependymoma | 1.7 | 0.43 | Non-malignant and malignant (grades I‒III) | All ages | |
| Meningioma | Benign meningioma | 34.9 | 7.82 | Non-malignant (grade I) | Adults, more common in females than males |
| Atypical meningioma | 1.8 | 0.40 | Malignant (grade II) | Adults | |
| Malignant meningioma | 0.5 | 0.10 | Malignant (grade III) | Adults | |
| Embryonal tumors | Medulloblastoma | 0.6 | 0.15 | Malignant (grade IV) | Children |
| Primary neuroectodermal tumors | 0.1 | 0.04 | Malignant (grade IV) | Children | |
| Atypical teratoid/rhabdoid tumors | 0.1 | 0.03 | Malignant (grade IV) | Children | |
| Nerve sheath tumors | Vestibular schwannoma (also known as acoustic neuroma) | 8.2 | 1.90 | Mostly non-malignant (grade I) | Adults |
| Pituitary tumors | Pituitary adenoma | 16.5 | 3.94 | Non-malignant (not graded) | Adults |
| Germ cell tumors | Germ cell tumors | 0.4 | 0.10 | Not graded | Children |
| Lymphomas and hematopoietic neoplasms | Primary central nervous system lymphoma | 1.9 | 0.43 | Malignant | Older adults |
Genetic Risk Factors
Mendelian Cancer Syndromes and Rare Variants
The majority of glioma cases occur in individuals without a family history of glioma, but approximately 5% of gliomas are familial.6 An even smaller proportion of gliomas are due to known Mendelian disorders or inherited syndromes, approximately 1–2% of adult and 4% of pediatric cases.3,5 Most of these syndromes are characterized by loss-of-function mutations in tumor suppressor genes, which may arise de novo or may be inherited, most commonly in either autosomal dominant or autosomal recessive fashion7,8; a summary of these is included in Table 2.
Table 2.
Inherited syndromes associated with brain and other CNS tumors
| Gene (chromosome location) | Disorder/Syndrome (OMIM ID) | Mode of Inheritance | Phenotypic Features | Associated Brain Tumors |
|---|---|---|---|---|
| AIP (11q13.2) | Pituitary adenoma predisposition (102200) | Dominant | Familial aggregation of pituitary adenoma; acromegaly or Cushing disease secondary to hormone-secreting tumors | Pituitary adenomas |
| APC, MMR (5q21) | Familial adenomatous polyposis (FAP, 175100), Turcot’s syndrome type 2 | Dominant | Development of multiple adenomatous colon polyps (>100), predisposition to colorectal cancer, and brain tumors | Medulloblastoma, glioma |
| ATM (11q22.3) | Ataxia-telangiectasia (208900) | Autosomal recessive trait | Progressive cerebellar ataxia, susceptibility to infections, predisposition to lymphoma and lymphocytic leukemia | Astrocytoma and medulloblastoma |
| BAP1 (3p21.1) | BAP1 tumor predisposition syndrome (614327) | Dominant | Predisposition to melanoma, mesothelioma, and other cancers. | Meningioma |
| CDKN2A (9p21.3) | Melanoma-neural system tumor syndrome (155755) | Dominant | Predisposition to malignant melanoma and malignant brain tumors | Glioma |
| CREBBP, EP300 (16p13.3, 22q13.2) | Rubinstein–Taybi syndrome (180849) | Dominant | Dysmorphic facial features, intellectual disability, and predisposition to cancers including CBTs, neuroblastoma, rhabdomyosarcoma, leukemia, lymphoma, and others | Medulloblastoma, oligodendroglioma, and meningioma |
| DICER1, DROSHA (14q32.13, 5p13.3) | DICER1 syndrome | Dominant | Impaired DNA damage response; embryonal tumors; kidney, ovary, and thyroid tumors; goiter | Pineoblastoma, pituitary blastoma |
| FANCA (16q24.3) | Fanconi anemia (227650) | Autosomal recessive trait | Heterogeneous congenital malformations, bone marrow failure, and acute myeloid leukemia | Medulloblastoma |
| IDH1/IDH2 (2q33.3/15q26.1) | Ollier disease | Acquired post-zygotic mosaicism, dominant with reduced penetrance | Development of intraosseous benign cartilaginous tumors, cancer predisposition | Glioma |
| MEN1 (11q13.1) | Multiple endocrine neoplasia, type 1 (131100) | Dominant | Hyperparathyroidism; predisposition to tumors at multiple sites including thyroid, adrenal gland, pancreas, duodenum | Pituitary prolactinoma, meningioma |
| MLH1, PMS2 | Turcot’s syndrome type 1 | Autosomal recessive trait | Development of multiple adenomatous colon polyps (<100), predisposition to colorectal cancer, and brain tumors | Medulloblastoma, glioma, |
| MSH2,MLH1,MSH6,PMS2 | Lynch syndrome (120435), biallelic mismatch repair deficiency, constitutional MMR deficiency | Dominant | Predisposition to gastrointestinal, endometrial, and other cancers | Glioblastoma, other gliomas |
| MSH2,MLH1,MSH6,PMS2 | Mismatch repair deficiency syndrome (276300) | Recessive | Pediatric cancer predisposition; café-au-lait spots; colon polyps | Glioma |
| NF1 (17q11.2) | Neurofibromatosis 1 (NF1) (162200) | Dominant | Neurofibromas, schwannomas, café-au-lait macules | Astrocytoma, schwannomas, optic nerve glioma |
| NF2 (22q12.2) | Neurofibromatosis 2 (NF2) (101000) | Dominant | Acoustic neuromas, meningiomas, neurofibromas, eye lesions | Acoustic neuromas, meningiomas, Ependymoma |
| PRKAR1A (17q24.2) | Carney complex (160980) | Dominant | Pigmented nevi; myxomas of the skin, breast, and other sites | Pituitary adenomas |
| PTCH1 (9q22.3) | Gorlin’s syndrome (nevoid basal cell carcinoma) | Dominant | Development of basal cell carcinomas, benign jaw tumors, fibromas of the heart or ovaries, and medulloblastoma | Medulloblastoma, meningioma |
| PTEN (10q23.31) | Cowden syndrome 1 (158350) | Dominant | Macrocephaly; breast, endometrial and thyroid cancer predisposition | Cerebellar gangliocytoma, meningioma |
| RB1 (13q14) | Retinoblastoma | Dominant | Development of multiple tumors of the eye, increased risk of some brain tumors | Retinoblastoma, Pineoblastoma, Malignant glioma |
| RECQL2 (8p12) | Werner syndrome (277700) | Recessive | Diabetes mellitus; early-onset cataract; premature graying of the hair and an aged appearance | Meningioma |
| SMARCB1 (22q11.23), SMARCE1 (17q21.2), SUFU (10q24.32), MN1 (22q12.1), PDGFB (22q13.1) | Familial meningiomatoses (607174) | Dominant | Familial meningioma aggregation | Meningioma |
| TP53 (17p13.1) | Li–Fraumeni syndrome (151623) | Dominant | Predisposition to numerous cancers, especially breast, brain, and soft-tissue sarcoma | Glioblastoma, other gliomas |
| TSC1,TSC2 (9q34.14,16p13.3) | Tuberous sclerosis (TSC) (191100, 613254) | Dominant | Development of multisystem non-malignant tumors | Giant cell astrocytoma |
| VHL (3p25) | Von Hippel–Lindau syndrome (193300) | Dominant | Predisposition to kidney cysts, renal cell carcinoma, pancreatic neuroendocrine tumors; pheochromocytomas, endolymphatic sac tumors, and brain tumors | Hemangioblastoma |
For more information, see Supplementary Note 1:
Abbreviations: AIP: aryl hydrocarbon receptor interacting protein; APC: adenomatous polyposis coli; BAP1: BRCA1 associated protein 1; CBT: childhood brain tumor; CDKN2A: cyclin-dependent kinase inhibitor 2A; DICER1: dicer 1, ribonuclease III; DROSHA: drosha ribonuclease III; IDH1: isocitrate dehydrogenase 1; IDH2: isocitrate dehydrogenase 2; MEN1: menin 1; MLH1: mutL homolog 1, colon cancer, nonpolyposis type 2; MN1: MN1 proto-oncogene, transcriptional regulator; MSH2: mutS protein homolog 2; MSH6: mutS protein homolog 6; NF1: neurofibromin 1; NF2: neurofibromin 2; PDGFB: platelet derived growth factor subunit B; PMS2: PMS1 homolog 2, mismatch repair system component; PTCH1: Patched 1; PTEN: phosphatase and tensin homolog; PRKAR1A: protein kinase cAMP-dependent type I regulatory subunit alpha; RB1: RB transcriptional corepressor 1; RECQL2 (WRN ): Werner syndrome RecQ like helicase; SMARCB1: SWI/SNF related, matrix associated, actin dependent regulator of chromatin, subfamily b, member 1; SMARCE1: SWI/SNF related, matrix associated, actin dependent regulator of chromatin, subfamily e, member 1; TP53: tumor protein p53; TSC1: TSC complex subunit 1; TSC2: TSC complex subunit 1; SUFU: SUFU negative regulator of hedgehog signaling; VHL: von Hippel–Lindau tumor suppressor
Sporadic Brain Tumors and Common Variants
Common genetic variants in adult brain tumors.
—Since the development of rapid whole genome genotyping, 8 genome-wide association studies (GWAS) of glioma,9–16 2 GWAS in meningioma,17,18 1 GWAS in pituitary adenoma19, and 1 in primary CNS lymphoma20 have been conducted. Together, these studies identified 30 genomic variants associated with increased BT risk (25 variants for adult glioma, 2 for meningioma, 3 for pituitary adenoma and 2 for primary CNS lymphoma Table 3). The proportion in incidence variance of glioma estimated as being attributable to genetic factors is 25%, and ~30% of this is explained by currently identified variants, with 70% of the genetic risk unexplained.13,21 Many of these factors have stronger associations with specific grades and histologies of glioma, though some confer increased risk for all types. Eleven of 25 single nucleotide polymorphisms (SNPs) were identified as having a significant association with GBM, and 18 were significantly associated with non-GBM glioma (or lower-grade glioma). The strongest association identified to date is CCDC26 (rs55705857), which increases risk for lower-grade glioma. This SNP is most strongly associated with oligodendroglioma, where it confers an odds ratio (OR) >4.22
Table 3.
Previously reported brain tumor risk loci identified by GWAS, by histology including allele frequencies, P-values, OR, and 95% CI
| Histology | SNP rs IDa (locus) | Associated Gene | Population | RAF in Studied Populationb | P-value | Odds Ratio (95% CI) | Associated Brain Tumor Type |
|---|---|---|---|---|---|---|---|
| All glioma | rs1920116 (3q26.2) | TERC | European/East Asian | Eur: 0.720 EA: 0.389 | Eur: 7.36 × 10−5 EA: 0.0060 | Eur: 1.08 (1.04–1.13) EA: 1.20 (1.05–1.36) | All glioma subtypes |
| rs10069690 (5p15.33) | TERT | European | 0.276 | 2.71 × 10−66 | 1.45 (1.39–1.51) | All glioma subtypes | |
| rs2853676 (5p15.33) | TERT | European/East Asian | Eur: 0.290 EA: 0.611 | Eur: 4.0 × 10−14 EA: 0.0001 | Eur: 1.26 (1.20–1.32) EA: 1.53 (1.21–1.94) | Astrocytoma | |
| rs2252586 (7p11.2) | EGFR | European | 0.281 | 1.38 × 10−13 | 1.16 (1.11–1.20) | All glioma subtypes | |
| rs648044 (11q23.2) | PHLDB1 | European | 0.390 | 4.66 × 10−12 | 1.19 (1.13–1.25) | All glioma subtypes | |
| rs17748 (11q23.2) | PHLDB1 | East Asian | 0.227 | 2.36 × 10−5 | 1.36(1.17–1.59) | All glioma subtypes | |
| rs2236661 (11q23.2) | PHLDB1 | East Asian | 0.231 | 1.0 × 10−5 | 1.46 (1.23–1.72) | All glioma subtypes | |
| rs494560 (11q23.2) | PHLDB1 | East Asian | 0.326 | 4.23 × 10−5 | 0.71 (0.60–0.85) | All glioma subtypes | |
| rs10842893 (12p11.23) | STK38L | East Asian | 0.035 | 2.33 × 10−12 | 2.07 (1.71–2.50) | All glioma subtypes | |
| rs4774756 (15q21.3) | RAB27A | East Asian | 0.297 | 6.12 × 10−8 | 1.24 (1.15–1.33) | All glioma subtypes | |
| rs78378222 (17p13.1) | TP53 | European | 0.013 | 8.64 × 10−38 | 2.53 (2.19–2.91) | All glioma subtypes | |
| rs6010620 (20q13.33) | RTEL1 | European/ East Asian | Eur: 0.794 EA: 0.293 | Eur: 2.81 × 10−40 EA: 0.021 | Eur: 1.34 (1.29–1.40) EA: 1.28 (1.04–1.57) | All glioma subtypes | |
| Glioblastoma | rs12752552 (1p31.3) | RAVER2 | European | 0.870 | 2.04 × 10−9 | 1.22 (1.15–1.31) | Glioblastoma |
| rs11979158 (7p11.2) | EGFR | European | 0.831 | 1.94 × 10−19 | 1.31 (1.24–1.39) | Glioblastoma | |
| rs730437 (7p11.2) | EGFR | East Asian | 0.0160 | 1.32 (1.05–1.66) | Glioblastoma | ||
| rs1468727 (7p11.2) | EGFR | East Asian | 0.0080 | 1.31 (1.04–1.65) | Glioblastoma | ||
| rs11233250 (11q14.1) | Intergenic | European | 0.868 | 9.95 × 10−10 | 1.24 (1.16–1.33) | Glioblastoma | |
| rs2562152 (16p13.3) | RHBDF1 | European | 0.850 | 1.93 × 10−8 | 1.21 (1.13–1.29) | Glioblastoma | |
| rs10852606 (16q12.1) | HEATR3 | European | 0.713 | 1.29 × 10−11 | 1.18 (1.13–1.24) | Glioblastoma | |
| rs2235573 (22q13.1) | SLC16A8 | European | 0.507 | 1.76 × 10−10 | 1.15 (1.10–1.20) | Glioblastoma | |
| Lower grade glioma | rs4252707 (1q32.1) | MDM4 | European | 0.220 | 3.34 × 10−9 | 1.19 (1.12–1.26) | Lower grade glioma |
| rs12076373 (1q44) | AKT3 | European | 0.837 | 2.63 × 10−10 | 1.23 (1.16–1.32) | Lower grade glioma | |
| rs7572263 (2q33.3) | C2orf80 | European | 0.756 | 2.18 × 10−10 | 1.20 (1.13–1.26) | Lower grade glioma | |
| rs11706832 (3p14.1) | LRIG1 | European | 0.456 | 7.66 × 10−9 | 1.15 (1.09–1.20) | Lower grade glioma | |
| rs55705857 (8q24.21) | CCDC26 | European | 0.057 | 7.28 × 10−149 | 3.39 (3.09–3.71) | Lower grade glioma, in particular IDH-mutant tumors | |
| rs4977756 (9p21.3) | CDKN2B-AS1 | European | 0.400 | 2.28 × 10−14 | 1.20 (1.15–1.26) | Lower grade glioma, in particular WHO grade II-IV astrocytic tumors | |
| rs11598018 (10q24.33) | OBFC1 | European | 0.462 | 3.39 × 10−8 | 1.14 (1.09–1.20) | Lower grade glioma | |
| rs11599775 (10q25.2) | VTI1A | European | 0.620 | 3.44 × 10−9 | 1.16 (1.10–1.22) | Lower grade glioma | |
| rs7107785 (11q21) | MAML2 | European | 0.479 | 3.87 × 10−10 | 1.16 (1.11–1.21) | Lower grade glioma | |
| rs498872 (11q23.3) | PHLDB1 | European | 0.307 | 8.46 × 10−33 | 1.35 (1.28–1.41) | Lower grade glioma, in particular IDH-mutant gliomas | |
| rs1275600 (12q21.2) | Intergenic | European | 0.595 | 3.72 × 10−9 | 1.16 (1.10–1.21) | Lower grade glioma | |
| rs10131032 (14q12) | AKAP6 | European | 0.916 | 5.07 × 10−11 | 1.33 (1.22–1.44) | Lower grade glioma | |
| rs1801591 (15q24.2) | ETFA | European | 0.088 | 6.36 × 10−13 | 1.33 (1.23–1.44) | Lower grade glioma | |
| rs3751667 (16p13.3) | RHBDF1 | European | 0.208 | 2.61 × 10−9 | 1.18 (1.12–1.25) | Lower grade glioma | |
| Meningioma | rs11012732 (10p12.31) | MLLT10 | European | 0.320 | 1.88 × 10−14 | 1.46 (1.32–1.61) | Meningioma |
| rs2686876 (11p15.5) | Possibly RIC8A | European | 0.906 | 9.86 × 10–9 | 1.44 (1.27–1.63) | Meningioma | |
| Pituitary adenoma | rs2359536 (10p12.31) | MIR4675, NEBL | East Asian | 0.082 | 2.25 × 10−10 | 1.44 (1.29–1.62) | Pituitary adenoma |
| rs10763170 (10q21.1) | PCDH15 | East Asian | 0.201 | 6.27 × 10−10 | 1.28 (1.18–1.39) | Pituitary adenoma | |
| rs17083838 (13q12.13) | CDK8 | East Asian | 0.104 | 1.89 × 10−8 | 1.37 (1.23–1.53) | Pituitary adenoma | |
| Primary central nervous system lymphoma | rs41289586 (3p22.1) | ANO10 | European | 0.021 | 1.87 × 10−8 | 3.82 (2.39–6.09) | Primary central nervous system lymphoma |
| rs116446171 (6q25.3) | EXOC2, IRF4 | European | 0.021 | 1.53 × 10−13 | 4.99 (3.26–7.65) | Primary central nervous system lymphoma |
For more information, see Supplementary Note 2:
Abbreviations: AKAP6: A-kinase anchoring protein 6; AKT3: AKT serine/threonine kinase 3; ANO10: anoctamin 10; C2orf80: chromosome 2 open reading frame 80; CCDC26: CCDC26 long non-coding RNA; CDKN2B-AS1: cyclin dependent kinase inhibitor 2B antisense RNA 1; CDK8: cyclin-dependent kinase 8; EGFR: epidermal growth factor receptor; ETFA: electron transfer flavoprotein subunit alpha; EXOC2: exocyst complex component 2; HEATR: HEAT repeat containing 3; IRF4: interferon regulatory factor 4; LRIG1: leucine rich repeats and immunoglobulin like domains 1; MAML2: mastermind like transcriptional coactivator 2; MDM4: MDM4 regulator of p53; MIR4672: microRNA 4672; MLLT10: MLLT10 histone lysine methyltransferase DOT1L cofactor; NEB: Nebulin; OBFC1: STN1 subunit of CST complex; PCDH15: protocadherin related 15; PHLDB1: pleckstrin homology like domain family B member 1; RAF: risk allele frequency; RAVER2: ribonucleoprotein, PTB binding 2; RHBDF1: rhomboid 5 homolog 1; RIC8A: RIC8 guanine nucleotide exchange factor A; RTEL: regulator of telomere elongation helicase 1; SLC16A8: solute carrier family 16 member 8; SNP: single nucleotide polymorphism; TERT: telomerase reverse transcriptase; TP53: tumor protein p53; VTI1A: vesicle transport through interaction with t-SNAREs 1A
a.Included SNPs are those identified within cited publications, and generally represent the strongest independent association within a linkage block.
b.Allele frequencies from: 1000 genomes project phase 3 (The 1000 Genomes Project Consortium, Auton A, Brooks LD, et al. A global reference for human genetic variation. Nature. 2015;526(7571):68–74.), and gnomAD (Lek M, Karczewski KJ, Minikel EV, et al. Analysis of protein-coding genetic variation in 60,706 humans. Nature. 2016;536(7616):285–291.)
Though BTs are known to be heterogeneous, most analyses attempting to discover germline risk variants have been conducted on pooled histologies or with classification based on histologically assigned type and grade. Due to their rarity, BT case cohorts are usually ascertained at multiple centers over extended periods. Results from molecular tests may not be available on all cases, though many groups are attempting to reclassify these cases as technologies and classifications evolve, which is now part of standard of care for glioma diagnosis.
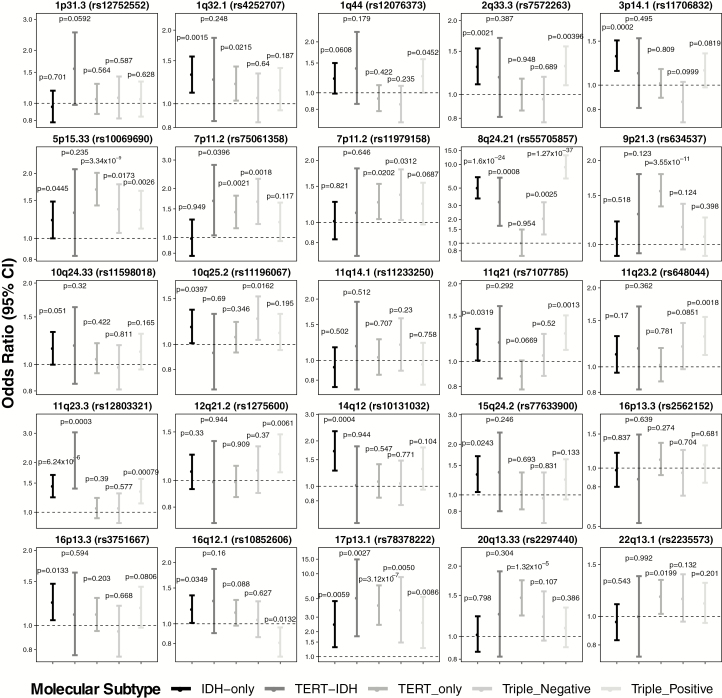
In a subset of molecularly characterized glioma cases with matching data from GWAS, Labreche et al estimated the OR associated with the 25 previously identified risk loci by molecular subtypes (Fig. 1).23 While some single SNPs were significantly associated with all molecular subtypes (eg, 17p13.1/TP53), most varied in their association. SNPs previously associated with non-GBM glioma were significantly associated with isocitrate dehydrogenase 1 and 2 (IDH1/2) mutant subtypes, including associations at 8q24.21/CCDC26 and 11q23.3/PHLDB1. SNPs previously found to have strong association with GBM, such as those at 5p15.33/TERT and 20q13.33/RTEL1, showed the strongest association with TERT-mutant tumors. Due to limitations in their sample size, further research is necessary in order to characterize the association between glioma risk SNPs and somatic variation.
Fig. 1.
ORs, 95% CIs, and P-values for previously identified glioma risk SNPs by molecular subtype (data from Labreche et al23).
Germline genetic factors associated with risk for BTs other than glioma have not been well studied. A recent meta-analysis GWAS for meningioma showed genome-wide significant associations in regions 10p12.31 (associated with MLLT10) and 11p15.5.17 The identified SNP in 11p15.5 (rs2686876) is in strong linkage disequilibrium (LD) with RIC8A, which is involved in development of the meninges.17 A GWAS of sporadic pituitary adenoma conducted in a Chinese population identified 3 genome-wide significant associations in regions 10p21.31 (near NEBL), 10q21.1 (PCDH15), and 13q12.13 (CDK8).19 A recent meta-analysis of 2 studies of primary CNS lymphoma identified 2 genome-wide significant associations in regions 3p22.1 (ANO10) and 6q25.3 (between EXOC2 and IRF4), as well as associations in 6p25.3 (in the IRF4 promoter) and 8q24.21 (MYC) that did not reach genome-wide significance.20
Common genetic variants in childhood brain tumors
To date, few genetic association studies have been conducted in childhood brain tumors (CBTs), and there are no published GWAS in CBTs. As a result, the contribution of common genetic variants to CBT risk is largely unknown. There have been several candidate gene studies conducted, many of which have been conducted in pooled datasets including multiple subtypes. These studies have identified risk variants associated with CBTs in CYP1A1, GSTT1, GSTM1 (genes of xenobiotic detoxification), 24,25NOS1 (involved in several pathways including inflammation), XPD (involved in DNA repair pathway), 24AICDA, CASP1 (genes involved in cell cycle pathway),26 as well as genes involved in folate metabolism. Dahlin et al found that of the 10 genes known to be somatically altered in medulloblastoma, 8 variants in 3 genes (CCND2, PTCH1, and GLI2) are nominally associated with risk of medulloblastoma.27 Associations have also been identified between SNPs in IRS2 and CDKN2A/B, involved in cell cycle pathway, and medulloblastoma.28,29 Adel Fahmideh et al investigated whether genetic variants identified in adult glioma are related to CBT risk and found that variants in EGFR, ERCC1, CHAF1A, XRCC1, EME1, ATM, GLTSCR1, XRCC4, CDKN2BAS, telomerase reverse transcriptase (TERT), regulator of telomere elongation helicase 1 (RTEL1), and CCDC26, involved in either DNA repair or cell cycle pathways, may also be associated with CBTs.30,31 These findings may suggest that CBTs and adult BTs share common genetic risk factors. These candidate gene studies are based on small samples and have not been replicated. These were issues which plagued candidate gene studies in adult glioma.32 As most validated genetic associations for adult BTs have been identified by GWAS, it is necessary to conduct GWAS in CBTs, and such efforts are currently under way.
Telomere Length
GWAS of leukocyte telomere length (LTL) have identified multiple germline variants which can be used to build a polygenic risk score (PRS) to estimate individual telomere length.33 Longer LTL estimated using this PRS has been associated with increased risk of glioma and meningioma (reviewed by Walsh et al34).35,36 Similarly, longer relative LTL (measured by quantitative PCR) has been associated with increased glioma risk in a Swedish case-control study.37 Multiple common variants associated with glioma through GWAS are located near telomere-associated genes, including TERT and RTEL1.35 Analysis of glioma tumor samples has demonstrated longer telomere length compared with other cancers.38
Genetic Ancestry
Malignant brain tumor incidence is highest in countries with primarily European ancestry populations.39 To date, most GWAS in glioma and all in meningioma have been conducted in primarily individuals of European ancestry, while 1 GWAS of pituitary adenoma has been conducted in an East Asian population. 19 One recent GWAS of glioma in a Chinese population confirmed associations near TERT, PHLDB1, and RTEL1, and identified 2 new variants, but strong statistical signal in the human leukocyte antigen region suggests this analysis may be biased by population stratification.40 Previous analyses have attempted to compare allele frequencies of previous GWAS hits within reference datasets by ancestry groups, but these have failed to identify new risk variants in non–European ancestry populations.41 Several candidate SNP studies have been conducted in East Asian populations, which have found novel association loci as well as validated associations previously discovered in European ancestry populations, including loci in TERC, TERT, EGFR, and PHLDB1.42,43 Analysis of patterns of continental ancestry in African Americans and Hispanics with glioma has identified increased overall European ancestry in glioma cases compared with controls.44
Demographic Factors Associated with Brain Tumor Incidence
Age
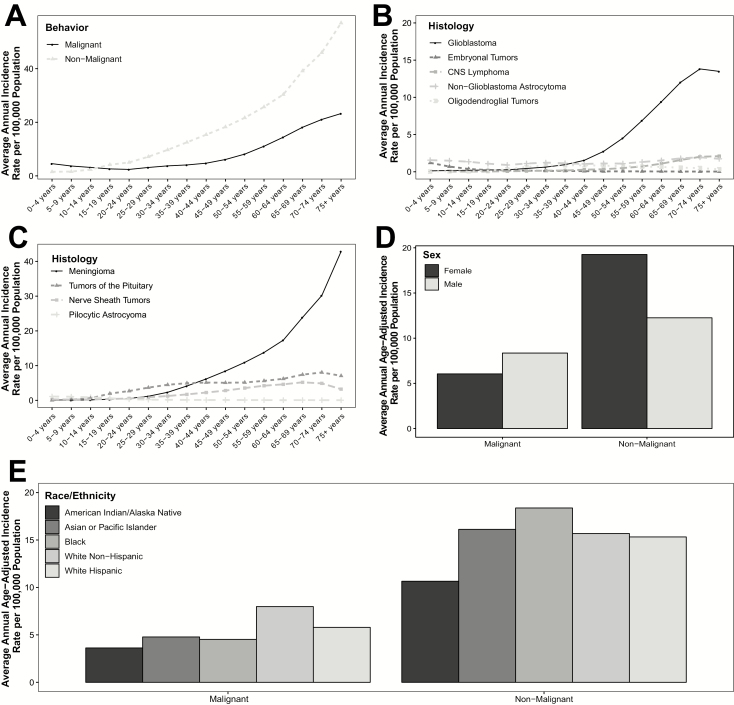
Incidence of BTs overall increases with age (Fig. 2A).1 For malignant glioma in particular, the incidence is bimodal, with highest incidence in the youngest and oldest ages. The age distribution also varies by histologic type. Embryonal tumors, a group that includes medulloblastoma and primitive neuroectodermal tumors, occur most frequently in children <10 years old (Fig. 2B). Incidence of pituitary tumors is also bimodal, with peaks in adolescence/young adulthood, and again in older adulthood (Fig. 2C). The most common BTs, such as GBM, vestibular schwannoma, and meningioma, have the highest incidence in individuals in their late 60s and early 70s, with a decreasing incidence thereafter. This may be due to competing causes of mortality, different diagnostic patterns in the oldest age group, or true differences in BT incidence.
Fig. 2.
Average annual incidence of primary brain and CNS tumors in the United States by age and (a) behavior, (b) selected malignant histologies, and (c) selected non-malignant histologies, and average annual age-adjusted incidence by behavior and (d) sex and (e) race/ethnicity (Central Brain Tumors Registry of the United States [CBTRUS], 2011–2015).1
Sex
Incidence of BTs varies by sex, with malignant tumors occurring much more frequently in males and non-malignant tumors generally occurring more frequently in females (Fig. 2D).1 This sex difference varies significantly by histology, with some histologies showing little or no variation in incidence by sex. The largest sex difference is observed in non-malignant meningioma (which is nearly twice as common in females), and high-grade gliomas (particularly GBM, which is ~60% more common in males). Sex differences are smaller or nonexistent in many tumors that are common in children, such as pilocytic astrocytoma and medulloblastoma.
Race/Ethnicity
Incidence of different histologies of BTs varies by race/ethnicity (Fig. 2E).1 Neuroepithelial tumors, including gliomas, are much more common in individuals of European ancestry (white non-Hispanics) compared with other groups. Meningiomas and pituitary adenomas occur more frequently in black non-Hispanics compared with other groups. Nerve sheath tumors and germ cell tumors have the highest incidence in Asian/Pacific Islanders.
Socioeconomic Status
Individuals of higher socioeconomic status (SES) are at higher risk of developing malignant BTs compared with those of low SES.45–51 SES is associated with many cancers, often due to its correlation with causal risk factors or increased case ascertainment associated with better access to health care and screening.52–58 For BTs, the causal factors underlying differential incidence in SES strata have not yet been determined. Although some of the trends of higher incidence in areas of high SES are explained by racial differences, studies that adjusted for race also demonstrated a higher incidence in areas of higher SES.59 Using census tract level data, analyses have shown a 45% higher incidence rate of GBM comparing highest versus lowest SES after adjustment for self-reported race,45 with a dose-response relationship for increasing quartiles of SES.49 A 2016 cohort study showed higher incidence of BT among the highly educated (≥3 y university education) as well as an association with higher income in males only.46 Highly educated women also had a higher risk of meningioma. Other non-malignant BTs, such as vestibular schwannoma, showed similar trends. For malignant BTs, these findings are unlikely to be the result of ascertainment bias alone, as these BTs have an aggressive clinical course and are often fatal and likely to be universally diagnosed in countries with high-functioning medical systems.5,45,47 Given that studies adjusted for race show higher incidence among those in high SES areas, there appears to be some independent effect of SES beyond its association with race that may cause higher incidence of malignant BT. For other tumors, such as meningioma, acoustic neuroma, and pituitary tumors, however, improved access to medical care may lead to higher rates of incidental imaging, leading to ascertainment bias.60 Future studies should assess these associations further, with stratification by possible confounders (eg, rural/urban status and health care–seeking behavior).
Validated and Potential Non-Genetic Risk Factors
Allergies
Studies of large and diverse groups of cases and controls have consistently shown that history of atopic conditions (including asthma, hay fever, eczema, and allergies) leads to reduced glioma risk (Supplementary Table 1).61 History of allergies has been shown to decrease risk of glioma by ~30%, and histology-specific analyses have suggested that the protective effect conferred by allergy may vary by glioma histology.62 Allergies and atopic disease, as well as early life exposure to infections, have also been associated with reduced risk of CBT.3,63 The underlying mechanism through which allergy protects against development of BTs is unknown, but the primary hypothesis is that allergic conditions may lead to a heightened state of immune-surveillance, discouraging abnormal cell growth, leading to development of a brain tumor.61,64,65
Recently, a Mendelian randomization (MR) analysis was published using GWAS summary statistics to assess whether a causal association exists between allergy and glioma. These studies identified no relationship between germline variants associated with asthma, hay fever, immunoglobulin E levels, and/or self-reported allergy, but did identify a weak association between genetic predisposition to atopic dermatitis and reduced risk of glioma.66
Medications
Aspirin and non-steroidal anti-inflammatory drugs
Non-steroidal anti-inflammatory drugs (NSAIDs) act by inhibiting prostaglandin production via suppression of the cyclooxygenase enzymes. The association of these drugs, including aspirin, has been investigated in multiple solid cancers, including glioma and meningioma (Supplementary Table 2).67,68 A recent meta-analysis found that regular use of aspirin was associated with significantly decreased risk of all glioma (WHO grades II/III glioma) and GBM.68 There was no significant association between use of non-aspirin NSAIDs and glioma risk. Further studies are necessary to elucidate the mechanisms through which aspirin may confer decreased risk of glioma.
Statins
Hydroxy-methylglutaryl-coenyzme A reductase inhibitors, or statins, are widely used to treat hypercholesterolemia. Much research has investigated the possibility that statins may lower the incidence of neurological disease.69,70 Some statins can penetrate the blood–brain barrier, and may specifically reduce brain inflammation, which may result in lower risk of malignant transformation.71–73 Statin use and subsequent risk of glioma has been investigated in 3 major case-control studies: 2 using self-report data and 1 using pharmacy linkage (Supplementary Table 2). All 3 of these studies reported similar point estimates, suggesting that regular statin use may be associated with a 25% reduction in glioma risk.74–77
Endogenous and Exogenous Hormone Exposure
Previous analyses have examined the impact of exogenous and endogenous sex hormone exposure as a potential environmental risk factor for BTs (Supplementary Table 3).78 The lower incidence of glioma in females has led some to hypothesize that increased lifetime estrogen exposure may act as a protective factor against developing these tumors, while increased incidence of meningioma among females suggests that this hormone exposure may be a risk factor. Lifetime hormone exposure can be difficult to accurately measure, and analyses have focused on surrogates for endogenous exposure (eg, age at menarche, parity, age at menopause) and use of supplemental estrogen or progesterone (eg, hormone replacement therapy, oral contraceptives). While some studies have found negative associations between glioma and estrogen exposure, results have generally been conflicting or null.79 Positive associations between meningioma and estrogen exposure have also been identified, and hormone exposure remains one of the strongest risk associations identified to date in these tumors.80 Due to the difficulty in accurately assessing lifetime hormone exposure, it is not possible to definitively state if these exposures are associated with BT risk.
Anthropometric Factors
Anthropometric factors, including height and body mass index (BMI), have been repeatedly studied in relation to BT risk (Supplementary Table 4). As shown for other cancers in several prospective studies, taller adult height has been identified as a glioma risk factor, with ~20% increase in risk for every 10 cm increase in height.81,82 In the National Institutes of Health–AARP cohort, Moore et al found significant associations between taller height and increased risk of glioma (relative risk = 2.12 comparing those >1.9 m with those <1.6 m).83
While taller adult height appears to be a consistent risk factor for glioma, there is less evidence for an effect of adult BMI on glioma incidence. Studies into the association between BMI and glioma risk have mostly been null.81,84–89 A recent meta-analysis did not identify an increased risk of glioma for overweight and obese individuals,86 although a separate meta-analysis reported an association in women only.85 Higher BMI has been associated with increased meningioma risk in multiple large prospective cohort studies, while associations between meningioma, and height, daily activity, and waist circumference were less consistent across these studies.88,90–92
Recently, GWAS summary statistics have been leveraged to perform MR analyses to assess whether a causal association exists between obesity and BT. These analyses found no association between germline variants associated with BMI, waist-to-hip ratio, lipids, type 2 diabetes, hyperglycemia, and/or insulin resistance and glioma.93 When the same analysis was performed for meningioma, significant associations were found for BMI (OR = 1.27; 95% CI = 1.03–1.56; P = 0.028) and body fat percentage (OR = 1.28; 95% CI = 1.01–1.63; P = 0.042).94 These analyses support the results of epidemiological studies that have repeatedly identified associations between BMI and meningioma. There are many mechanisms through which excess body fat is thought to increase predisposition to cancer,95 and for meningioma in particular it is hypothesized that the increased levels of circulating estrogen created by adipose tissue may be one pathway through which risk is increased.96,97
Diet
Studies of diet and BT risk are limited, in part due to the rarity of BTs and the well-known limitations of case-control studies in assessing diet. Although cohort investigations may be generally more unbiased, the number of accumulated BT cases in prospective cohorts tends to be small. Nevertheless, a recent investigation using 3 large prospective cohorts included 2313 glioma cases and did not demonstrate an association between major food groups, nutrients, or healthy dietary patterns and glioma incidence, particularly after excluding the first 5 years of follow-up. This study suggests that diet may not play a role in glioma incidence.98 Several other studies have examined diet in relation to glioma risk, with publications on a variety of dietary exposures that have been previously reviewed by Kyritsis et al and are included in Supplementary Table 5.99 The associations between processed meats, nitrites, and glioma risk have been previously examined, with largely null results, while an inverse association has been suggested for both meningioma and glioma with higher intake of vegetables.100–103 Recent meta-analyses have shown inverse associations between glioma risk and dietary vitamins C and A.104,105 A recent MR study of vitamin D and glioma showed no evidence for a causal association.106 Coffee and tea intake have been associated with lower risk of several cancers, and has also been studied in relation to glioma risk, but associations have largely been null or borderline inverse.107–111
Ionizing Radiation
Moderate-to-high doses of radiation are the strongest and most consistently documented environmental risk factor for BTs, having been independently observed in atomic bomb survivor studies, therapeutic radiation cohorts (both for treatment of prior cancer and for benign conditions), and occupational and environmental studies (Supplementary Table 6). The carcinogenic effects of ionizing radiation are stronger in children, as they are more radiosensitive and have more years of potential life to express the risk. Some studies have shown that radiation therapy for childhood cancers is associated with development of BTs later in life, particularly those being treated for acute lymphoblastic leukemia (reviewed by Davis et al62). Estimated risk was higher for younger children, and the latency between irradiation and BT occurrence has been estimated at 7–9 years,112 with meningiomas and gliomas being the predominant induced tumor types.113
Maternal diagnostic radiation during pregnancy has been found to be related to an increased risk of BT,114 and increasing use of diagnostic techniques such as CT and PET have raised health concerns. A recent article reviewed the research to date and concluded that for children exposed to one or more head CT scans, the excess relative risk of developing a BT was 1.29.115 However, these findings might be confounded by reverse causation, as children with higher cancer susceptibility and preexisting cancer are more likely to undergo head CT scans.115 These studies indicate an elevated risk of CBTs related to radiation dose, with increased risk with a younger age at exposure.116
Non-Ionizing Radiation
Cellular phones
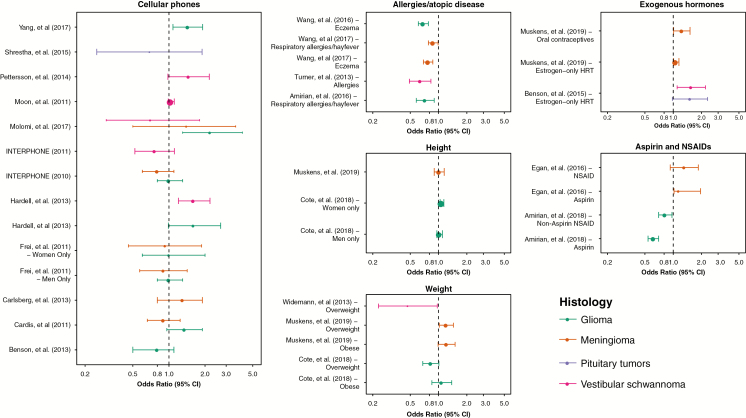
Cellular phone technology was introduced in the 1980s and has rapidly increased so that the vast majority of people globally now use cellular phones (Supplementary Fig. 1). Cellular phones emit radiofrequency fields, and, when used against the head, the brain absorbs the largest dose. Due to public health concerns, the association between risk of BTs and cellular phone use has been investigated extensively. In 2011, the International Agency for Research on Cancer (IARC) classified radiofrequency fields as a possible carcinogen, based largely on preliminary epidemiological findings of an increased risk of glioma and vestibular schwannoma in heavy cellular phone users.117 Since publication of the results of the INTERPHONE consortium case-control study118,119 and the IARC Monograph in 2010–2011, there have been at least 13 published epidemiological studies reporting on BT risk in relation to cellular phone use (Fig. 3). Case-control studies of cellular phone use and BTs have suffered from previously described methodological issues120,121 and have often included the same population across multiple analyses. The majority of these have found no significant association between cellular phone use and risk of any type of BT.
Fig. 3.
ORs and 95% CIs selected potential risk factors for primary brain tumors for glioma, meningioma, vestibular schwannoma, and pituitary tumors (See Supplementary Note 3 for data sources).
Analyses of time trends of age-standardized BT incidence rates from high-quality registration data are an important source of data to examine the possible association between cellular phone use and BTs. Since 2011, there have been at least 16 studies examining incidence trends during the period of increasing cellular phone use (Supplementary Table 7). The majority of these analyses have found little to no change in incidence of specific BT histologies, with the exception of some analyses of Israeli and UK data that found increased incidence of GBM.122,123 Two studies assessed the US and Australian data and found that small observed increases in incidence were not compatible with the magnitude of risks reported by case-control studies.124,125 An analysis by de Vocht comparing actual incidence to models based on earlier incidence data before widespread cellular phone use indicated that site-specific incidence of GBM did exceed these but was most significantly associated in the oldest populations: those least likely to have been heavy cellular phone users.126
The epidemiological evidence published since the IARC Monograph in 2011 does not support an association between cellular phone use and risk of BT. If an association exists, the latency period for this exposure is unknown, and monitoring of incidence trends data is advisable.
Low frequency electromagnetic fields
A number of studies have also examined the association between extremely low frequency magnetic fields (ELFs) and BT risk (Supplementary Table 8).5 In 2002, despite some observed positive associations, particularly in case-control studies, the IARC concluded that the summation of existing data was insufficient to classify ELFs as a risk factor for BT.127 Recently, published results from the INTEROCC consortium did not find an association with lifetime cumulative occupational exposure to ELF.128
Non-Radiation Occupational Exposures
Possible associations between specific occupations and/or non-radiation occupational exposures and BTs have been studied extensively (Supplementary Table 9).5 However, to date, no occupational exposures have been consistently associated with risk of BTs.
Smoking
Although smoking remains one of the most common and well-understood causes of cancer worldwide, its association with BTs is mixed. Smoking has consistently been found to have a null association with incidence of malignant BTs. A 2016 meta-analysis reviewed 19 case-control and 6 cohort studies, reporting a summary risk ratio of 0.98 (95% CI = 0.92–1.05) for ever smokers compared with never smokers.129 Across multiple cohorts, as well as studies from geographically diverse regions, cigarette smoking has consistently been shown to be unrelated to glioma risk.129–133 For non-malignant BTs, smoking appears to have a protective effect. Claus et al reported significantly decreased risk of meningioma among female smokers compared with non-smokers (OR = 0.8; 95% CI = 0.7–0.9), but no significant differences for men.134 Other studies did not identify a significant association between smoking and meningioma, even when women were analyzed separately.82,90,91 Similarly, the Million Women Study demonstrated reduced risk of acoustic neuroma (vestibular schwannoma) among women who were ever smokers compared with non-smokers (OR = 0.41; 95% CI = 0.24–0.70).135,136 The reasons for these unexpected inverse associations have yet to be explored.
Birth Weight and Other Birth Characteristics
Relatively consistent evidence has been provided by large studies and meta-analyses revealing positive associations between CBTs and both advanced parental age3,137 and birth characteristics.138,139A recent meta-analysis including 41 articles concluded that high birth weight (>4000 g) is associated with increased risk of CBT, particularly for astrocytoma and embryonal tumors.138 These findings were supported by another meta-analysis indicating an elevated risk of astrocytoma and embryonal tumors related to high birth weight.139 In contrast, a relatively smaller study based on pooled data from 2 French case-control studies could not detect any association between birth weight and risk of CBTs.140 Despite the potential important role of maternal genetics in the initiation and progression of CBT, knowledge on the role of maternal genetic variants in CNS etiology is limited.
Structural Birth Defects
Non-chromosomal structural birth defects (excluding trisomy and other chromosomal disorders) are among the strongest and most consistent risk factors for childhood cancer, and the percentage of CBTs attributable to birth defects is about 7%.141,142 Several large, population-based studies have recently assessed the association of any major non-chromosomal birth defect with CBT risk. These studies suggest that diagnosis of a birth defect is associated with ~2-fold increased risk of CBT.143–146
While considerable work remains to be done in characterizing specific birth defect–CBT associations, some patterns have emerged. Specifically, greater increases in CBT risk are seen among children with a birth defect of the CNS or with a neurological anomaly.145–151 In addition, multiple studies reported increased prevalence of rib anomalies among CBT patients compared with controls or population norms, with only astrocytoma patients demonstrating a statistically significant excess of these anomalies,152–154 as well as increased risk associated with oral clefts, or defects of the ear, face, and neck.146,150,155 Studies which have investigated childhood cancer risk as a function of age suggest that hazard functions for cancer overall and CBTs specifically converge to those of unaffected controls by ~5 years of age.143,144,147 Ascertainment biases are unlikely to fully explain these associations. Cancer surveillance is not routine in children with structural birth defects.143 To address the possibility that birth defects may be identified incidentally during evaluation of children with cancer, one study restricted analyses to children diagnosed with cancer >1 year of age, and the maximum age at which participating registries recorded birth defects. Associations between CNS defects and CBTs remained statistically significant.151
This field has benefited immensely from the propagation of population-based birth defects and cancer registries; however, the identification of birth defect–childhood cancer associations remains challenging owing to the rarity of co-occurring cases. Consequently, studies have had limited power to investigate the associations of specific birth defects with CBTs or the major subgroups, and further identification of specific birth defect–BT associations will require extremely large sample sizes, such as by meta-analysis or by pooling data across multiple registries. In a recent systematic review, Johnson et al stressed the need for future studies to use standardized systems when classifying birth defects in order to facilitate such research.141
In addition to the previously discussed factors, many other environmental exposures have been investigated in relation to CBT development with conflicting results, including: non-ionizing radiation, maternal medications, N-nitroso compounds, maternal nutrition and vitamins, parental smoking and alcohol use, pesticides, infectious agents, allergic conditions, parental occupational exposures, parental age, and other birth characteristics.3
Where Do We Go from Here in Brain Tumor Epidemiology?
Significant progress has been made in identifying and confirming potential risk factors for BTs, including numerous heritable genetic factors, allergic/atopic diseases, and ionizing radiation exposures. Numerous other exposures have yielded promising results—including aspirin use for glioma, BMI for meningioma, and birth characteristics for CBTs—and many others are currently under study. Large, well-annotated datasets have now been collected for many BT subtypes, and continued analysis of these in multi-institutional collaborations will potentially lead to further understanding of the interaction of genes and environment in development of primary BTs. Several developments are critical to the future of understanding risk factors for BTs, particularly: (i) refining risk factor measurements, (ii) “omic” approaches for both germline risk and tumor phenotyping, (iii) expanding risk factor and genomics research to broader and more diverse populations, and (iv) applying novel approaches to both existing and new datasets.
Risk Factor Measurement
Johansen et al recently reviewed the state of the literature in glioma risk factor research and found substantial variability in the results derived from case-control studies as opposed to cohort study designs.120 In evaluating the evidence present by case-control studies of medical ionizing radiation exposure and exogenous hormone exposure (relying on retrospective self-report) as opposed to those from cohort studies with prospective self-report (or prescribing records where available), they concluded that studies based on self-report resulted in estimates further from the null compared with associations reported by analyses using prescription data. The inability to replicate these case-control findings in cohort designs suggests that these case-control studies may be affected by bias and emphasizes the importance of using validated and tested instruments or pre-diagnostic records for collecting risk factor data. In comparison, glioma GWAS using cohort (Rajaraman et al10) or case-control designs (all others to date) have identified the same genetic loci associated with glioma risk, demonstrating that these associations should be concordant when reliable instruments (eg, germline genotyping) are used. BT epidemiology has benefited significantly from the data available within large databases, including but not limited to national cancer registry systems, institutional or national prescribing databases, birth defect registries, and administratively collected risk factor data. As these data are often collected for non-research purposes, it is critical to approach them systematically and with understanding of their limitations.
Molecular Classification
Accurate classification of disease and exposure status is critical in case-control studies, as misclassification of these factors causes poorly estimated or incorrect associations and significant decreases in power. This unmeasured phenotyping error likely contributes to both “missing heritability” and difficulties in replicating associations. Historically, most BT epidemiology studies have classified phenotype based on histologic criteria only. The advancement of high-throughput technologies that have allowed for full molecular classification of BTs, particularly glioma and medulloblastoma, has resulted in refinement of histologic classification and the creation and elimination of histologies with the 2016 WHO revision.4 While many of these molecular markers have been used consistently at large academic medical centers for years, they did not become components of the WHO criteria until the 2016 revision, and therefore are absent from many datasets.4 BT subtypes have distinct lineages of acquisition of malignant behavior, even when they arise within the same tissue site, for example: IDH1/2 mutation is a precipitating event in gliomagenesis, and delineates between 2 distinct glioma phenotypes. In addition to the development of medulloblastoma subclassification based on gene expression,156–158 incorporation of molecular characteristics into classification of other pediatric tumors is ongoing, including rare histologies such as diffuse intrinsic pontine glioma.159 The development of molecular classification is even more critical in these pediatric phenotypes, as precise phenotype will increase the power to detect both genetic and non-genetic risk factors for these extremely rare tumors.
In order to improve risk prediction models, it is essential to fully understand associations between exposures, germline variants, and somatic characteristics of tumors. Replicating previously discovered findings within these subsets of individuals with molecular classification is an important step for more precisely targeted discovery as well as beginning to refine these associations. In addition, development of molecular classification, by integrating germline and somatic variations, leads to a better understanding of brain tumorigenesis.
Risk Factor Research in Diverse Populations
BT research to date has largely been conducted in pooled sex and primarily white/European ancestry populations. Recent research has begun to focus on differences by sex and has preliminarily identified sex-specific differences in germline risk factors as well as age at onset, somatic features, and tumor behavior.160–162 Due to the rarity of BTs overall, there have been limited analyses done to identify genetic variants associated with BT risk in non-European populations as it is often difficult to obtain sample sizes that are appropriately powered to identify associations that reach genome-wide significance. Candidate gene analyses in glioma have demonstrated that tagged risk variants may vary by ancestry population,42–44 and studies of non-genetic risk factors in diverse populations have demonstrated that these associations may vary by race/ethnicity,90,163 perhaps pointing to the importance of examining gene–environment interactions in epidemiologic studies. Limited analysis of somatic alterations in tumors also suggests that initiating mutations and pathways of gliomagenesis may vary by race/ethnicity or ancestry.164 Future research should incorporate these known population-based differences, as research both within and between specific populations has the potential to reveal information about BT risk and etiology applicable to the population at large.
Novel Computational Approaches
Decades of BT epidemiological research has generated large amounts of existing data, including both “omic” and risk factor data that can be leveraged using newly developed statistical and computational techniques that may not have been available at the time these data were collected. Techniques that use publicly available summary statistics generated from GWAS, such as MR, which uses genetic predictors of risk factors to assess whether a causal association exists between these and an outcome, can be used to further interrogate associations identified by case-control or other epidemiological studies.66,94,106,165,166 These methods can also be used to assess relationships between risk factors that may have not been included on questionnaires but for which there may be suggestive evidence, such as age at menarche or menopause. Machine learning and other artificial intelligence approaches can also be applied to combinations of risk factor and genetic data, and can be used to assess not only direct relationships but also the extent to which these factors interact to contribute to BT risk. Artificial intelligence methodologies such as natural language processing could also be used to further enhance existing datasets through extraction of additional risk factor or other clinical data from electronic health records.
Supplementary Material
Funding
Q.T.O. and J.S. are supported by a Research Training Grant from the Cancer Prevention and Research Institute of Texas (RP160097T). D.J.C. is supported by National Institutes of Health (NIH) Training Grant T32 CA 009001. M.A.F. is supported by the EU/EFPIA Innovative Medicines Initiative 1 Joint Undertaking (ULTRA-DD grant 115766) and Takeda Pharmaceutical Company Limited. I.S.M. is supported by NIH grant R01CA194189.
Conflict of interest statement.
There are no conflicts of interest to disclose.
References
- 1. Ostrom QT, Gittleman H, Truitt G, Boscia A, Kruchko C, Barnholtz-Sloan JS. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2011–2015. Neuro Oncol. 2018;20(suppl 4):iv1–iv86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30. [DOI] [PubMed] [Google Scholar]
- 3. Johnson KJ, Cullen J, Barnholtz-Sloan JS, et al. Childhood brain tumor epidemiology: a brain tumor epidemiology consortium review. Cancer Epidemiol Biomarkers Prev. 2014;23(12):2716–2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803–820. [DOI] [PubMed] [Google Scholar]
- 5. Ostrom QT, Bauchet L, Davis F, et al. The epidemiology of glioma in adults: a “state of the science” review. Neuro Oncol. 2014;16(7):896–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wrensch M, Lee M, Miike R, et al. Familial and personal medical history of cancer and nervous system conditions among adults with glioma and controls. Am J Epidemiol. 1997;145(7):581–593. [DOI] [PubMed] [Google Scholar]
- 7. Ranger AM, Patel YK, Chaudhary N, Anantha RV. Familial syndromes associated with intracranial tumours: a review. Childs Nerv Syst. 2014;30(1):47–64. [DOI] [PubMed] [Google Scholar]
- 8. Vijapura C, Saad Aldin E, Capizzano AA, Policeni B, Sato Y, Moritani T. Genetic syndromes associated with central nervous system tumors. Radiographics. 2017;37(1):258–280. [DOI] [PubMed] [Google Scholar]
- 9. Chen H, Chen G, Li G, et al. Two novel genetic variants in the STK38L and RAB27A genes are associated with glioma susceptibility. Int J Cancer. 2019. doi: 10.1002/ijc.32179 [DOI] [PubMed] [Google Scholar]
- 10. Rajaraman P, Melin BS, Wang Z, et al. Genome-wide association study of glioma and meta-analysis. Hum Genet. 2012;131(12):1877–1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shete S, Hosking FJ, Robertson LB, et al. Genome-wide association study identifies five susceptibility loci for glioma. Nat Genet. 2009;41(8):899–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Enciso-Mora V, Hosking FJ, Kinnersley B, et al. Deciphering the 8q24.21 association for glioma. Hum Mol Genet. 2013;22(11):2293–2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Melin BS, Barnholtz-Sloan JS, Wrensch MR, et al. Genome-wide association study of glioma subtypes identifies specific differences in genetic susceptibility to glioblastoma and non-glioblastoma tumors. Nat Genet. 2017;49(5):789–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wrensch M, Jenkins RB, Chang JS, et al. Variants in the CDKN2B and RTEL1 regions are associated with high-grade glioma susceptibility. Nat Genet. 2009;41(8):905–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Walsh KM, Codd V, Smirnov IV, et al. Variants near TERT and TERC influencing telomere length are associated with high-grade glioma risk. Nat Genet. 2014;46(7):731–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kinnersley B, Labussiere M, Holroyd A, et al. Genome-wide association study identifies multiple susceptibility loci for glioma. Nat Commun. 2015;6:8559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Claus EB, Cornish AJ, Broderick P, et al. Genome-wide association analysis identifies a meningioma risk locus at 11p15.5. Neuro Oncol. 2018;20(11):1485–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dobbins SE, Broderick P, Melin B, et al. Common variation at 10p12.31 near MLLT10 influences meningioma risk. Nat Genet. 2011;43(9):825–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ye Z, Li Z, Wang Y, et al. Common variants at 10p12.31, 10q21.1 and 13q12.13 are associated with sporadic pituitary adenoma. Nat Genet. 2015;47(7):793–797. [DOI] [PubMed] [Google Scholar]
- 20. Labreche K, Daniau M, Sud A, et al. A genome-wide association study identifies susceptibility loci for primary central nervous system lymphoma at 6p25.3 and 3p22.1: a LOC network study. Neuro Oncol. 2019;21(8):1039–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kinnersley B, Mitchell JS, Gousias K, et al. Quantifying the heritability of glioma using genome-wide complex trait analysis. Sci Rep. 2015;5:17267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jenkins RB, Xiao Y, Sicotte H, et al. A low-frequency variant at 8q24.21 is strongly associated with risk of oligodendroglial tumors and astrocytomas with IDH1 or IDH2 mutation. Nat Genet. 2012;44(10):1122–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Labreche K, Kinnersley B, Berzero G, et al. Diffuse gliomas classified by 1p/19q co-deletion, TERT promoter and IDH mutation status are associated with specific genetic risk loci. Acta Neuropathol. 2018;135(5):743–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Salnikova LE, Zelinskaya NI, Belopolskaya OB, Aslanyan MM, Rubanovich AV. Association study of xenobiotic detoxication and repair genes with malignant brain tumors in children. Acta Naturae. 2010;2(4):58–65. [PMC free article] [PubMed] [Google Scholar]
- 25. Salnikova LE, Belopolskaya OB, Zelinskaya NI, Rubanovich AV. The potential effect of gender in CYP1A1 and GSTM1 genotype-specific associations with pediatric brain tumor. Tumour Biol. 2013;34(5):2709–2719. [DOI] [PubMed] [Google Scholar]
- 26. Jeon S, Han S, Lee K, et al. Genetic variants of AICDA/CASP14 associated with childhood brain tumor. Genet Mol Res. 2013;12(2):2024–2031. [DOI] [PubMed] [Google Scholar]
- 27. Dahlin AM, Hollegaard MV, Wibom C, et al. CCND2, CTNNB1, DDX3X, GLI2, SMARCA4, MYC, MYCN, PTCH1, TP53, and MLL2 gene variants and risk of childhood medulloblastoma. J Neurooncol. 2015;125(1):75–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chen YD, Zhang N, Qiu XG, Yuan J, Yang M. LncRNA CDKN2BAS rs2157719 genetic variant contributes to medulloblastoma predisposition. J Gene Med. 2018;20(1):e3000. [DOI] [PubMed] [Google Scholar]
- 29. Baocheng W, Zhao Y, Meng W, et al. Polymorphisms of insulin receptor substrate 2 are putative biomarkers for pediatric medulloblastoma: considering the genetic susceptibility and pathological diagnoses. Nagoya J Med Sci. 2017;79(1):47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Adel Fahmideh M, Lavebratt C, Schuz J, et al. CCDC26, CDKN2BAS, RTEL1 and TERT Polymorphisms in pediatric brain tumor susceptibility. Carcinogenesis. 2015;36(8):876–882. [DOI] [PubMed] [Google Scholar]
- 31. Adel Fahmideh M, Lavebratt C, Schuz J, et al. Common genetic variations in cell cycle and DNA repair pathways associated with pediatric brain tumor susceptibility. Oncotarget. 2016;7(39):63640–63650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Walsh KM, Anderson E, Hansen HM, et al. Analysis of 60 reported glioma risk SNPs replicates published GWAS findings but fails to replicate associations from published candidate-gene studies. Genet Epidemiol. 2013;37(2):222–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Codd V, Nelson CP, Albrecht E, et al. Identification of seven loci affecting mean telomere length and their association with disease. Nat Genet. 2013;45(4):422–427, 427e421–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Walsh KM, Wiencke JK, Lachance DH, et al. Telomere maintenance and the etiology of adult glioma. Neuro Oncol. 2015;17(11):1445–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Walsh KM, Codd V, Rice T, et al. Longer genotypically-estimated leukocyte telomere length is associated with increased adult glioma risk. Oncotarget. 2015;6(40):42468–42477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Muskens IS, Hansen HM, Smirnov IV, et al. Longer genotypically-estimated leukocyte telomere length is associated with increased meningioma risk. J. Neurooncol. 2019. doi: 10.1007/s11060-019-03119-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Andersson U, Degerman S, Dahlin AM, et al. The association between longer relative leukocyte telomere length and risk of glioma is independent of the potentially confounding factors allergy, BMI, and smoking. Cancer Causes Control. 2019;30(2):177–185. [DOI] [PubMed] [Google Scholar]
- 38. Barthel FP, Wei W, Tang M, et al. Systematic analysis of telomere length and somatic alterations in 31 cancer types. Nat Genet. 2017;49(3):349–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Leece R, Xu J, Ostrom QT, Chen Y, Kruchko C, Barnholtz-Sloan JS. Global incidence of malignant brain and other central nervous system tumors by histology, 2003–2007. Neuro Oncol. 2017;19(11):1553–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chen H, Chen G, Li G, et al. Two novel genetic variants in the STK38L and RAB27A genes are associated with glioma susceptibility. Int J Cancer. 2019. doi: 10.1002/ijc.32179 [DOI] [PubMed] [Google Scholar]
- 41. Jacobs DI, Walsh KM, Wrensch M, et al. Leveraging ethnic group incidence variation to investigate genetic susceptibility to glioma: a novel candidate SNP approach. Front Genet. 2012;3:203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Li G, Jin T, Liang H, et al. RTEL1 tagging SNPs and haplotypes were associated with glioma development. Diagn Pathol. 2013;8:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Liu HB, Peng YP, Dou CW, et al. Comprehensive study on associations between nine SNPs and glioma risk. Asian Pac J Cancer Prev. 2012;13(10):4905–4908. [DOI] [PubMed] [Google Scholar]
- 44. Ostrom QT, Egan KM, Nabors LB, et al. Glioma risk associated with extent of estimated European genetic ancestry in African-Americans and Hispanics. Int J Cancer. 2019. doi: 10.1002/ijc.32318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Porter AB, Lachance DH, Johnson DR. Socioeconomic status and glioblastoma risk: a population-based analysis. Cancer Causes Control. 2015;26(2):179–185. [DOI] [PubMed] [Google Scholar]
- 46. Khanolkar AR, Ljung R, Talback M, et al. Socioeconomic position and the risk of brain tumour: a Swedish national population-based cohort study. J. Epidemiol Community Health. 2016;70(12):1222–1228. [DOI] [PubMed] [Google Scholar]
- 47. Porter AB, Lachance DH, Johnson DR. Socioeconomic status and glioblastoma risk: a population-based analysis. Cancer Causes Control. 2015;26(2):179–185. [DOI] [PubMed] [Google Scholar]
- 48. Khanolkar AR, Ljung R, Talback M, et al. Socioeconomic position and the risk of brain tumour: a Swedish national population-based cohort study. J Epidemiol Community Health. 2016. doi:10.1136/jech-2015–207002 [DOI] [PubMed] [Google Scholar]
- 49. Plascak JJ, Fisher JL. Area-based socioeconomic position and adult glioma: a hierarchical analysis of surveillance epidemiology and end results data. PLoS One. 2013;8(4):e60910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wigertz A, Lonn S, Hall P, Feychting M. Non-participant characteristics and the association between socioeconomic factors and brain tumour risk. J Epidemiol Community Health. 2010;64(8):736–743. [DOI] [PubMed] [Google Scholar]
- 51. Deb S, Pendharkar AV, Schoen MK, Altekruse S, Ratliff J, Desai A. The effect of socioeconomic status on gross total resection, radiation therapy and overall survival in patients with gliomas. J Neurooncol. 2017;132(3):447–453. [DOI] [PubMed] [Google Scholar]
- 52. Clegg LX, Reichman ME, Miller BA, et al. Impact of socioeconomic status on cancer incidence and stage at diagnosis: selected findings from the surveillance, epidemiology, and end results: National Longitudinal Mortality Study. Cancer Causes Control. 2009;20(4):417–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mackenbach JP, Stirbu I, Roskam AJ, et al. Socioeconomic inequalities in health in 22 European countries. N Engl J Med. 2008;358(23):2468–2481. [DOI] [PubMed] [Google Scholar]
- 54. Baquet CR, Horm JW, Gibbs T, Greenwald P. Socioeconomic factors and cancer incidence among blacks and whites. J Natl Cancer Inst. 1991;83(8):551–557. [DOI] [PubMed] [Google Scholar]
- 55. Clegg LX, Reichman ME, Miller BA, et al. Impact of socioeconomic status on cancer incidence and stage at diagnosis: selected findings from the surveillance, epidemiology, and end results: national longitudinal mortality study. Cancer Causes Control. 2009;20(4):417–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Megwalu UC. Impact of county-level socioeconomic status on oropharyngeal cancer survival in the United States. Otolaryngol Head Neck Surg. 2017;156(4):665–670. [DOI] [PubMed] [Google Scholar]
- 57. Diez-Roux AV, Kiefe CI, Jacobs DR Jr., et al. Area characteristics and individual-level socioeconomic position indicators in three population-based epidemiologic studies. Ann Epidemiol. 2001;11(6):395–405. [DOI] [PubMed] [Google Scholar]
- 58. Uphoff E, Cabieses B, Pinart M, Valdes M, Anto JM, Wright J. A systematic review of socioeconomic position in relation to asthma and allergic diseases. Eur Respir J. 2015;46(2):364–374. [DOI] [PubMed] [Google Scholar]
- 59. Cote DJ, Ostrom QT, Gittleman H, et al. Glioma incidence and survival variation by county-level socioeconomic measures. Cancer. 2019. doi:10.1002/cncr.32328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Cote DJ, Laws ER Jr.. The ethics of “choosing wisely”: the use of neuroimaging for uncomplicated headache. Neurosurgery. 2017;80(5):816–819. [DOI] [PubMed] [Google Scholar]
- 61. Amirian ES, Zhou R, Wrensch MR, et al. Approaching a scientific consensus on the association between allergies and glioma risk: a report from the glioma international case-control study. Cancer Epidemiol Biomarkers Prev. 2016;25(2):282–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Davis F, Il’yasova D, Rankin K, McCarthy B, Bigner DD. Medical diagnostic radiation exposures and risk of gliomas. Radiat Res. 2011;175(6):790–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Lupatsch JE, Bailey HD, Lacour B, et al. Childhood brain tumours, early infections and immune stimulation: a pooled analysis of the ESCALE and ESTELLE case-control studies (SFCE, France). Cancer Epidemiol. 2018;52:1–9. [DOI] [PubMed] [Google Scholar]
- 64. Turner MC. Epidemiology: allergy history, IgE, and cancer. Cancer Immunol Immunother. 2012;61(9):1493–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Cui Y, Hill AW. Atopy and specific cancer sites: a review of epidemiological studies. Clin Rev Allergy Immunol. 2016;51(3):338–352. [DOI] [PubMed] [Google Scholar]
- 66. Disney-Hogg L, Cornish AJ, Sud A, et al. Impact of atopy on risk of glioma: a Mendelian randomisation study. BMC Med. 2018;16(1):42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Cuzick J, Thorat MA, Bosetti C, et al. Estimates of benefits and harms of prophylactic use of aspirin in the general population. Ann Oncol. 2015;26(1):47–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Amirian ES, Ostrom QT, Armstrong GN, et al. Aspirin, non-steroidal anti-inflammatory drugs (NSAIDs), and glioma risk: original data from the glioma international case-control study and a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2018. doi:10.1158/1055–9965.epi-18–0702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Sierra S, Ramos MC, Molina P, Esteo C, Vazquez JA, Burgos JS. Statins as neuroprotectants: a comparative in vitro study of lipophilicity, blood-brain-barrier penetration, lowering of brain cholesterol, and decrease of neuron cell death. J Alzheimers Dis. 2011;23(2):307–318. [DOI] [PubMed] [Google Scholar]
- 70. Fong CW. Statins in therapy: understanding their hydrophilicity, lipophilicity, binding to 3-hydroxy-3-methylglutaryl-CoA reductase, ability to cross the blood brain barrier and metabolic stability based on electrostatic molecular orbital studies. Eur J Med Chem. 2014;85:661–674. [DOI] [PubMed] [Google Scholar]
- 71. Wang Q, Yan J, Chen X, et al. Statins: multiple neuroprotective mechanisms in neurodegenerative diseases. Exp Neurol. 2011;230(1):27–34. [DOI] [PubMed] [Google Scholar]
- 72. Farooqui AA, Ong WY, Horrocks LA, Chen P, Farooqui T. Comparison of biochemical effects of statins and fish oil in brain: the battle of the titans. Brain Res Rev. 2007;56(2):443–471. [DOI] [PubMed] [Google Scholar]
- 73. Li Q, Zhuang QK, Yang JN, Zhang YY. Statins excert neuroprotection on cerebral ischemia independent of their lipid-lowering action: the potential molecular mechanisms. Eur Rev Med Pharmacol Sci. 2014;18(8):1113–1126. [PubMed] [Google Scholar]
- 74. Greenland S. A serious misinterpretation of a consistent inverse association of statin use with glioma across 3 case-control studies. Eur J Epidemiol. 2017;32(1):87–88. [DOI] [PubMed] [Google Scholar]
- 75. Ferris JS, McCoy L, Neugut AI, Wrensch M, Lai R. HMG CoA reductase inhibitors, NSAIDs and risk of glioma. Int J Cancer. 2012;131(6):E1031-1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Seliger C, Meier CR, Becker C, et al. Statin use and risk of glioma: population-based case-control analysis. Eur J Epidemiol. 2016;31(9):947–952. [DOI] [PubMed] [Google Scholar]
- 77. Gaist D, Andersen L, Hallas J, Sorensen HT, Schroder HD, Friis S. Use of statins and risk of glioma: a nationwide case-control study in Denmark. Br J Cancer 2013;108(3):715–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Cowppli-Bony A, Bouvier G, Rue M, et al. Brain tumors and hormonal factors: review of the epidemiological literature. Cancer Causes Control. 2011;22(5):697–714. [DOI] [PubMed] [Google Scholar]
- 79. Zong H, Xu H, Geng Z, et al. Reproductive factors in relation to risk of brain tumors in women: an updated meta-analysis of 27 independent studies. Tumour Biol. 2014;35(11):11579–11586. [DOI] [PubMed] [Google Scholar]
- 80. Fan ZX, Shen J, Wu YY, Yu H, Zhu Y, Zhan RY. Hormone replacement therapy and risk of meningioma in women: a meta-analysis. Cancer Causes Control. 2013;24(8):1517–1525. [DOI] [PubMed] [Google Scholar]
- 81. Wiedmann MKH, Brunborg C, Di Ieva A, et al. The impact of body mass index and height on the risk for glioblastoma and other glioma subgroups: a large prospective cohort study. Neuro Oncol. 2017;19(7):976–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Benson VS, Pirie K, Green J, Casabonne D, Beral V. Lifestyle factors and primary glioma and meningioma tumours in the Million Women Study cohort. Br J Cancer. 2008;99(1):185–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Moore SC, Rajaraman P, Dubrow R, et al. Height, body mass index, and physical activity in relation to glioma risk. Cancer Res. 2009;69(21):8349–8355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Kitahara CM, Gamborg M, Rajaraman P, Sorensen TI, Baker JL. A prospective study of height and body mass index in childhood, birth weight, and risk of adult glioma over 40 years of follow-up. Am J Epidemiol. 2014;180(8):821–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Sergentanis TN, Tsivgoulis G, Perlepe C, et al. Obesity and risk for brain/CNS tumors, gliomas and meningiomas: a meta-analysis. PLoS One. 2015;10(9):e0136974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Niedermaier T, Behrens G, Schmid D, Schlecht I, Fischer B, Leitzmann MF. Body mass index, physical activity, and risk of adult meningioma and glioma: A meta-analysis. Neurology. 2015;85(15):1342–1350. [DOI] [PubMed] [Google Scholar]
- 87. Cote DJ, Downer MK, Smith TR, Smith-Warner SA, Egan KM, Stampfer MJ. Height, waist circumference, body mass index, and body somatotype across the life course and risk of glioma. Cancer Causes Control. 2018;29(8):707–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Michaud DS, Bove G, Gallo V, et al. Anthropometric measures, physical activity, and risk of glioma and meningioma in a large prospective cohort study. Cancer Prev Res. 2011;4(9):1385–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Little RB, Madden MH, Thompson RC, et al. Anthropometric factors in relation to risk of glioma. Cancer Causes Control. 2013;24(5):1025–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Muskens IS, Wu AH, Porcel J, et al. Body mass index, comorbidities, and hormonal factors in relation to meningioma in an ethnically diverse population: the Multiethnic Cohort. Neuro Oncol. 2019;21(4):498–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Johnson DR, Olson JE, Vierkant RA, et al. Risk factors for meningioma in postmenopausal women: results from the Iowa Women’s Health Study. Neuro Oncol. 2011;13(9):1011–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Schildkraut JM, Calvocoressi L, Wang F, et al. Endogenous and exogenous hormone exposure and the risk of meningioma in men. J Neurosurg. 2014;120(4):820–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Disney-Hogg L, Sud A, Law PJ, et al. Influence of obesity-related risk factors in the aetiology of glioma. Br J Cancer. 2018;118(7):1020–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Takahashi H, Cornish AJ, Sud A, et al. Mendelian randomization provides support for obesity as a risk factor for meningioma. Sci Rep. 2019;9(1):309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Avgerinos KI, Spyrou N, Mantzoros CS, Dalamaga M. Obesity and cancer risk: Emerging biological mechanisms and perspectives. Metabolism. 2019;92:121–135. [DOI] [PubMed] [Google Scholar]
- 96. Jay JR, MacLaughlin DT, Riley KR, Martuza RL. Modulation of meningioma cell growth by sex steroid hormones in vitro. J Neurosurg. 1985;62(5):757–762. [DOI] [PubMed] [Google Scholar]
- 97. Bezemer ID, Rinaldi S, Dossus L, et al. C-peptide, IGF-I, sex-steroid hormones and adiposity: a cross-sectional study in healthy women within the European Prospective Investigation into Cancer and Nutrition (EPIC). Cancer Causes Control. 2005;16(5):561–572. [DOI] [PubMed] [Google Scholar]
- 98. Kuan AS, Green J, Kitahara CM, et al. Diet and risk of glioma: combined analysis of three large prospective studies in the UK and USA. Neuro Oncol. 2019;21(7):944–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Kyritsis AP, Bondy ML, Levin VA. Modulation of glioma risk and progression by dietary nutrients and antiinflammatory agents. Nutr Cancer. 2011;63(2):174–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Ward HA, Gayle A, Jakszyn P, et al. Meat and haem iron intake in relation to glioma in the European prospective investigation into cancer and nutrition study. Eur J Cancer Prev. 2018;27(4):379–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Michaud DS, Holick CN, Batchelor TT, Giovannucci E, Hunter DJ. Prospective study of meat intake and dietary nitrates, nitrites, and nitrosamines and risk of adult glioma. Am J Clin Nutr. 2009;90(3):570–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Li Y. Association between fruit and vegetable intake and risk for glioma: a meta-analysis. Nutrition. 2014;30(11–12):1272–1278. [DOI] [PubMed] [Google Scholar]
- 103. Alles B, Pouchieu C, Gruber A, et al. Dietary and alcohol intake and central nervous system tumors in adults: results of the CERENAT multicenter case-control study. Neuroepidemiology. 2016;47(3–4):145–154. [DOI] [PubMed] [Google Scholar]
- 104. Zhou Q, Luo ML, Li H, Li M, Zhou JG. Coffee consumption and risk of endometrial cancer: a dose-response meta-analysis of prospective cohort studies. Sci Rep. 2015;5:13410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Lv W, Zhong X, Xu L, Han W. Association between dietary vitamin a intake and the risk of glioma: evidence from a meta-analysis. Nutrients. 2015;7(11):8897–8904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Takahashi H, Cornish AJ, Sud A, et al. Mendelian randomisation study of the relationship between vitamin D and risk of glioma. Sci Rep. 2018;8(1):2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Malmir H, Shayanfar M, Mohammad-Shirazi M, Tabibi H, Sharifi G, Esmaillzadeh A. Tea and coffee consumption in relation to glioma: a case-control study. Eur J Nutr. 2017. doi: 10.1007/s00394-017-1575-z [DOI] [PubMed] [Google Scholar]
- 108. Malerba S, Galeone C, Pelucchi C, et al. A meta-analysis of coffee and tea consumption and the risk of glioma in adults. Cancer Causes Control. 2013;24(2):267–276. [DOI] [PubMed] [Google Scholar]
- 109. Dubrow R, Darefsky AS, Freedman ND, Hollenbeck AR, Sinha R. Coffee, tea, soda, and caffeine intake in relation to risk of adult glioma in the NIH-AARP diet and health study. Cancer Causes Control. 2012;23(5):757–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Holick CN, Smith SG, Giovannucci E, Michaud DS. Coffee, tea, caffeine intake, and risk of adult glioma in three prospective cohort studies. Cancer Epidemiol Biomarkers Prev. 2010;19(1):39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Ogawa T, Sawada N, Iwasaki M, et al. Coffee and green tea consumption in relation to brain tumor risk in a Japanese population. Int J Cancer. 2016;139(12):2714–2721. [DOI] [PubMed] [Google Scholar]
- 112. Ohgaki H, Kleihues P. Epidemiology and etiology of gliomas. Acta Neuropathol. 2005;109(1):93–108. [DOI] [PubMed] [Google Scholar]
- 113. Braganza MZ, Kitahara CM, Berrington de Gonzalez A, Inskip PD, Johnson KJ, Rajaraman P. Ionizing radiation and the risk of brain and central nervous system tumors: a systematic review. Neuro Oncol. 2012;14(11):1316–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Linet MS, Kim KP, Rajaraman P. Children’s exposure to diagnostic medical radiation and cancer risk: epidemiologic and dosimetric considerations. Pediatr Radiol. 2009;39(Suppl 1):S4–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Sheppard JP, Nguyen T, Alkhalid Y, Beckett JS, Salamon N, Yang I. Risk of brain tumor induction from pediatric head CT procedures: a systematic literature review. Brain Tumor Res Treat. 2018;6(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Kleinerman RA. Cancer risks following diagnostic and therapeutic radiation exposure in children. Pediatr Radiol. 2006;36(Suppl 2):121–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Baan R, Grosse Y, Lauby-Secretan B, et al. Carcinogenicity of radiofrequency electromagnetic fields. Lancet Oncol. 2011;12(7):624–626. [DOI] [PubMed] [Google Scholar]
- 118. Interphone Study Group. Brain tumour risk in relation to mobile telephone use: results of the INTERPHONE international case-control study. Int J Epidemiol. 2010;39(3):675–694. [DOI] [PubMed] [Google Scholar]
- 119. Interphone Study Group. Acoustic neuroma risk in relation to mobile telephone use: results of the INTERPHONE international case-control study. Cancer Epidemiol. 2011;35(5):453–464. [DOI] [PubMed] [Google Scholar]
- 120. Johansen C, Schuz J, Andreasen AS, Dalton SO. Study designs may influence results: the problems with questionnaire-based case-control studies on the epidemiology of glioma. Br J Cancer. 2017;116(7):841–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Swerdlow AJ, Feychting M, Green AC, Leeka Kheifets LK, Savitz DA. International commission for non-ionizing radiation protection standing committee on E. Mobile phones, brain tumors, and the Interphone study: where are we now? Environ Health Perspect. 2011;119(11):1534–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Philips A, Henshaw DL, Lamburn G, O’Carroll MJ. Brain tumours: rise in glioblastoma multiforme incidence in England 1995–2015 suggests an adverse environmental or lifestyle factor. J Environ Public Health. 2018;2018:7910754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Barchana M, Margaliot M, Liphshitz I. Changes in brain glioma incidence and laterality correlates with use of mobile phones--a nationwide population based study in Israel. Asian Pac J Cancer Prev. 2012;13(11):5857–5863. [DOI] [PubMed] [Google Scholar]
- 124. Little MP, Rajaraman P, Curtis RE, et al. Mobile phone use and glioma risk: comparison of epidemiological study results with incidence trends in the United States. BMJ. 2012;344:e1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Chapman S, Azizi L, Luo Q, Sitas F. Has the incidence of brain cancer risen in Australia since the introduction of mobile phones 29 years ago? Cancer Epidemiol. 2016;42:199–205. [DOI] [PubMed] [Google Scholar]
- 126. de Vocht F. Analyses of temporal and spatial patterns of glioblastoma multiforme and other brain cancer subtypes in relation to mobile phones using synthetic counterfactuals. Environ Res. 2019;168:329–335. [DOI] [PubMed] [Google Scholar]
- 127. IARC Monographs on the Identification of Carcinogenic Hazards to Humans. Non-Ionizing Radiation, Part 1: Static and Extremely Low-Frequency (ELF) Electric and Magnetic Fields. Lyon, France: International Agency for Research on Cancer; 2002:80. [PMC free article] [PubMed] [Google Scholar]
- 128. Turner MC, Benke G, Bowman JD, et al. Occupational exposure to extremely low-frequency magnetic fields and brain tumor risks in the INTEROCC study. Cancer Epidemiol Biomarkers Prev. 2014;23(9):1863–1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Shao C, Zhao W, Qi Z, He J. Smoking and glioma risk: evidence from a meta-analysis of 25 observational studies. Medicine. 2016;95(2):e2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Braganza MZ, Rajaraman P, Park Y, et al. Cigarette smoking, alcohol intake, and risk of glioma in the NIH-AARP diet and health study. Br J Cancer. 2014;110(1):242–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Zheng T, Cantor KP, Zhang Y, Chiu BC, Lynch CF. Risk of brain glioma not associated with cigarette smoking or use of other tobacco products in Iowa. Cancer Epidemiol Biomarkers Prev. 2001;10(4):413–414. [PubMed] [Google Scholar]
- 132. Efird JT, Friedman GD, Sidney S, et al. The risk for malignant primary adult-onset glioma in a large, multiethnic, managed-care cohort: cigarette smoking and other lifestyle behaviors. J Neurooncol. 2004;68(1):57–69. [DOI] [PubMed] [Google Scholar]
- 133. Holick CN, Giovannucci EL, Rosner B, Stampfer MJ, Michaud DS. Prospective study of cigarette smoking and adult glioma: dosage, duration, and latency. Neuro Oncol. 2007;9(3):326–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Claus EB, Walsh KM, Calvocoressi L, et al. Cigarette smoking and risk of meningioma: the effect of gender. Cancer Epidemiol Biomarkers Prev. 2012;21(6):943–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Benson VS, Green J, Pirie K, Beral V. Cigarette smoking and risk of acoustic neuromas and pituitary tumours in the million women study. Br J Cancer. 2010;102(11):1654–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Schoemaker MJ, Swerdlow AJ, Auvinen A, et al. Medical history, cigarette smoking and risk of acoustic neuroma: an international case-control study. Int J Cancer. 2007;120(1):103–110. [DOI] [PubMed] [Google Scholar]
- 137. Wang R, Metayer C, Morimoto L, et al. Parental age and risk of pediatric cancer in the offspring: a population-based record-linkage study in California. Am J Epidemiol. 2017;186(7):843–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Georgakis MK, Kalogirou EI, Liaskas A, et al. Anthropometrics at birth and risk of a primary central nervous system tumour: a systematic review and meta-analysis. Eur J Cancer. 2017;75:117–131. [DOI] [PubMed] [Google Scholar]
- 139. Dahlhaus A, Prengel P, Spector L, Pieper D. Birth weight and subsequent risk of childhood primary brain tumors: an updated meta-analysis. Pediatr Blood Cancer. 2017;64(5): e26299. [DOI] [PubMed] [Google Scholar]
- 140. Bailey HD, Rios P, Lacour B, et al. Factors related to pregnancy and birth and the risk of childhood brain tumours: the ESTELLE and ESCALE studies (SFCE, France). Int J Cancer. 2017;140(8):1757–1769. [DOI] [PubMed] [Google Scholar]
- 141. Johnson KJ, Lee JM, Ahsan K, et al. Pediatric cancer risk in association with birth defects: a systematic review. PLoS One. 2017;12(7):e0181246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Mili F, Khoury MJ, Flanders WD, Greenberg RS. Risk of childhood cancer for infants with birth defects. I. A record-linkage study, Atlanta, Georgia, 1968–1988. Am J Epidemiol. 1993;137(6):629–638. [DOI] [PubMed] [Google Scholar]
- 143. Botto LD, Flood T, Little J, et al. Cancer risk in children and adolescents with birth defects: a population-based cohort study. PLoS One. 2013;8(7):e69077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Janitz AE, Neas BR, Campbell JE, et al. Childhood cancer in children with congenital anomalies in Oklahoma, 1997 to 2009. Birth Defects Res A Clin Mol Teratol. 2016;106(7):633–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Fisher PG, Reynolds P, Von Behren J, Carmichael SL, Rasmussen SA, Shaw GM. Cancer in children with nonchromosomal birth defects. J Pediatr. 2012;160(6):978–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Bjorge T, Cnattingius S, RT Lie, Tretli S, Engeland A. Cancer risk in children with birth defects and in their families: a population based cohort study of 5.2 million children from Norway and Sweden. Cancer Epidemiol Biomarkers Prev. 2008;17(3):500–506. [DOI] [PubMed] [Google Scholar]
- 147. Norwood MS, Lupo PJ, Chow EJ, et al. Childhood cancer risk in those with chromosomal and non-chromosomal congenital anomalies in Washington State: 1984–2013. PLoS One. 2017;12(6):e0179006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Del Risco Kollerud R, Blaasaas KG, Claussen B, et al. Family history of cancer and the risk of childhood solid tumours: a Norwegian nationwide register-based cohort study. Br J Cancer. 2018;118(6):905–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Wong-Siegel JR, Johnson KJ, Gettinger K, et al. Congenital neurodevelopmental anomalies in pediatric and young adult cancer. Am J Med Genet A. 2017;173(10):2670–2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Sun Y, Overvad K, Olsen J. Cancer risks in children with congenital malformations in the nervous and circulatory system—a population based cohort study. Cancer Epidemiol. 2014;38(4):393–400. [DOI] [PubMed] [Google Scholar]
- 151.Lupo PJ, Schraw JM, Desrosiers TA, et al. Association between birth defects and cancer risk among children and adolescents in a population-based assessment of 10 million live births. JAMA Oncol. 2019. doi:10.1001/jamaoncol.2019.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Loder RT, Huffman G, Toney E, Wurtz LD, Fallon R. Abnormal rib number in childhood malignancy: implications for the scoliosis surgeon. Spine. 2007;32(8):904–910. [DOI] [PubMed] [Google Scholar]
- 153. Merks JH, Smets AM, Van Rijn RR, et al. Prevalence of rib anomalies in normal Caucasian children and childhood cancer patients. Eur J Med Genet. 2005;48(2):113–129. [DOI] [PubMed] [Google Scholar]
- 154. Narod SA, Hawkins MM, Robertson CM, Stiller CA. Congenital anomalies and childhood cancer in Great Britain. Am J Hum Genet. 1997;60(3):474–485. [PMC free article] [PubMed] [Google Scholar]
- 155. Durmaz A, Durmaz B, Kadioglu B, et al. The Association of minor congenital anomalies and childhood cancer. Pediatr Blood Cancer. 2011;56(7):1098–1102. [DOI] [PubMed] [Google Scholar]
- 156. Northcott PA, Dubuc AM, Pfister S, Taylor MD. Molecular subgroups of medulloblastoma. Expert Rev Neurother. 2012;12(7):871–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Northcott PA, Jones DT, Kool M, et al. Medulloblastomics: the end of the beginning. Nat Rev Cancer. 2012;12(12):818–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Northcott PA, Buchhalter I, Morrissy AS, et al. The whole-genome landscape of medulloblastoma subtypes. Nature. 2017;547(7663):311–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Lapin DH, Tsoli M, Ziegler DS. Genomic insights into diffuse intrinsic pontine glioma. Front Oncol. 2017;7:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Ostrom QT, Kinnersley B, Wrensch MR, et al. Sex-specific glioma genome-wide association study identifies new risk locus at 3p21.31 in females, and finds sex-differences in risk at 8q24.21. Sci Rep. 2018;8(1):7352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Yang W, Warrington NM, Taylor SJ, et al. Sex differences in GBM revealed by analysis of patient imaging, transcriptome, and survival data. Sci Transl Med. 2019;11(473):pii:eaao5253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Ostrom QT, Kinnersley B, Armstrong G, et al. Age-specific genome-wide association study in glioblastoma identifies increased proportion of ‘lower grade glioma’-like features associated with younger age. Int J Cancer. 2018;143(10):2359–2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Krishnamachari B, Il’yasova D, Scheurer ME, et al. A pooled multisite analysis of the effects of atopic medical conditions in glioma risk in different ethnic groups. Ann Epidemiol. 2015;25(4):270–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Wiencke JK, Aldape K, McMillan A, et al. Molecular features of adult glioma associated with patient race/ethnicity, age, and a polymorphism in O6-methylguanine-DNA-methyltransferase. Cancer Epidemiol Biomarkers Prev. 2005;14(7):1774–1783. [DOI] [PubMed] [Google Scholar]
- 165. Howell AE, Zheng J, Haycock PC, et al. Use of Mendelian randomization for identifying risk factors for brain tumors. Front Genet. 2018;9:525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Disney-Hogg L, Sud A, Law PJ, et al. Influence of obesity-related risk factors in the aetiology of glioma. Br J Cancer. 2018;118(7):1020–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.