Abstract
Objectives
Many individuals who smoke relapse due to weight gain. Mindfulness training has been shown to help smokers quit smoking, and, in other populations, has been used to help people lose weight. This study was designed to assess the effect of one week of mindfulness practice on food cravings in smokers during 12-hour smoking abstinence.
Methods
We assessed daily smokers with a history of smoking lapse after weight gain. Participants were provided with brief training in mindfulness meditation and mindful eating and were asked to practice each skill daily for one week. Before and after this week of mindfulness practice, participants completed surveys to rate their nicotine dependence and food cravings and underwent testing via functional magnetic resonance imaging.
Results
Study results included pre-post intervention reduction in brain activity in dorsomedial prefrontal cortex, visual areas, and pre-motor areas, regions potentially associated with response to food images.
Conclusions
The study was small; however, it suggests the possibility that mindfulness training might be used to decrease food cravings after smoking cessation.
Keywords: mindfulness, functional magnetic resonance imaging, weight, food cravings, smoking cessation
An estimated 15% of Americans smoke cigarettes (Jamal 2016). Although tobacco use is the leading cause of preventable morbidity and mortality in the United States, smoking is notoriously difficult to treat (Fiore 2008). For those who are able to quit, weight gain is common and predicts relapse (Aubin et al. 2012; Borrelli et al. 2001; Mukhopadhyay and Wendel 2011). People who quit smoking gain an average of 5–10 pounds; however, one third of all smokers gain over 20 pounds (Aubin et al. 2012; Eisenberg and Quinn 2006; O’Hara et al. 1998; Pistelli et al. 2016; Williamson et al. 1991). Some studies suggest that a key mechanism of post-quit weight gain is increased caloric consumption due to hedonic eating, or eating for pleasure (Epel et al. 2011; Filozof et al. 2004). Nicotine has been shown to suppress appetite via activation of pro-opiomelanocortin neurons in the hypothalamus (Mineur et al. 2011). Thus far, studies have not yet identified pharmacologic or cognitive behavioral smoking cessation treatments that are effective at attenuating post-quit weight gain (Farley et al. 2012; Filozof et al. 2004; Spring et al. 2009).
A new approach worth investigation is the use of mindfulness training, which is has been found to be an effective therapy for smoking cessation (Davis et al. 2013; Davis, Goldberg, et al. 2014; Davis, Manley, et al. 2014) and also for treating binge eating disorders (Kristeller and Hallett 1999; Spring et al. 2009). Mindfulness training has demonstrated effects in the medial cortex, insula, amygdala, lateral frontal regions, and basal ganglia with postulated impacts on automatic thinking and emotion regulation (Marchand 2014). One study of 19 subjects assessed a 7-week manualized program on mindful acceptance of eating and found significant reduction in food cravings, and numeric but non-significant difference in weight change compared to controls (Alberts et al. 2010). Thus, it seems possible that mindfulness training could be an effective therapy for post-quit weight gain by reducing food cravings. This pilot study was designed to assess the effect of a brief mindfulness training on food craving in daily smokers during 12-hour abstinence, as measured by functional magnetic resonance imaging (fMRI) of neural activity during exposure to visual food cues. To our knowledge, mindfulness training has never been studied for its effect on food craving during the post-quit period.
Method
Participants
Participants (N=5) were recruited from a metropolitan area. Inclusion criteria included age of at least 18 years, smoking at least 10 cigarettes per day, a history of prior smoking lapse caused by weight gain, and a body mass index between 19 and 35. Exclusion criteria included use of other tobacco products, use of smoking cessation medication, chance of pregnancy, and medical contraindications to fMRI.
Procedures
The study was designed to assess the impact in smokers of one week of mindfulness practice on food craving and brain reactivity to images of food during 12-hour smoking abstinence. Primary outcome was change in blood oxygen level-dependent activation assessed through functional magnetic resonance imaging in response to food images relative to scrambled images, before and after the week of mindfulness practice.
All participants received one hour of training in mindfulness meditation and mindful eating provided by a senior mindfulness instructor. Participants were then asked to practice meditation for 30 minutes per day and mindful eating once per day for one week. Aside from mindfulness practice, participants were instructed to eat and smoke as usual for the week. Practice compliance was recorded daily through phone-based assessments.
Measures
Nicotine dependence and food cravings were assessed as traits at baseline through the following commonly used standardized scales: six-item Fagerstrom Test for Nicotine Dependence (FTND) (Pomerleau et al. 1994) and 15-item Food Cravings Questionnaire-Trait Reduced (FCQ-TR) to assess for correlation between nicotine dependence and food craving (Meule et al. 2014). Stress and mindfulness were measured using the 10-item Perceived Stress Scale (PSS) (Cohen et al. 1994) and 39-item Five Facet Mindfulness Questionnaire (FFMQ) (Baer et al. 2008) before and after the intervention to assess impact of mindfulness training on stress and mindfulness. All measures used in this study were self-report measures. Likert-type scales were used in FCQ-TR, PSS, and FFMQ.
Imaging Procedures
Participants were scanned using a GE MR 75– 3.0 Tesla scanner using sense-spiral sequence with a 24cm field of view, 34 4mm slices, TR=1.5 seconds. An anatomic scan preceded functional imaging. Participants were asked to refrain from smoking and eating for 12 hours prior to fMRI on each of the two testing days; this was confirmed by carbon monoxide (CO) breath test with CO < 7 ppm considered smoking abstinence (Deveci et al. 2004). Pre-and post-intervention fMRIs recorded an anatomical scan for pre-processing and neural reactivity during visual presentation of food images categorized by nutritional content (high or low fat; high or low protein, carbohydrate, sugar) and control images, with all images scrambled and matched (Figure 1). The imaging protocol utilized a block design with four runs and eight blocks per run. Each block contained images randomized from one category of nutritional content or control images. Each run included six blocks of food images (six images per set for 3.5 to 7 seconds each with variable inter-stimulus interval) and two blocks of control images comprised of non-food images matched for light. Blocks were followed by a 20-second pause for craving rating. During the post-intervention fMRI, participants were asked to practice mindfulness during the image exposure tasks.
Figure 1.
Control images and food images as categorized by nutrient content
Data Analyses
Standard preprocessing was performed in FMRIB Software Library (FSL), a comprehensive library of imaging analysis tools. First-level models created contrasts for all food images vs. control images within each subject; second-level models combined data across runs. Group models comparing pre- to post-intervention changes in reactivity for food vs. control images were evaluated across the whole brain using paired sample t-tests within an FSL fixed effects model. Cluster-correction across the whole brain search volume was used to control for multiple comparisons. Thresholding for significant voxels was defined as Z=3.1, with cluster correction of P<0.05.
Results
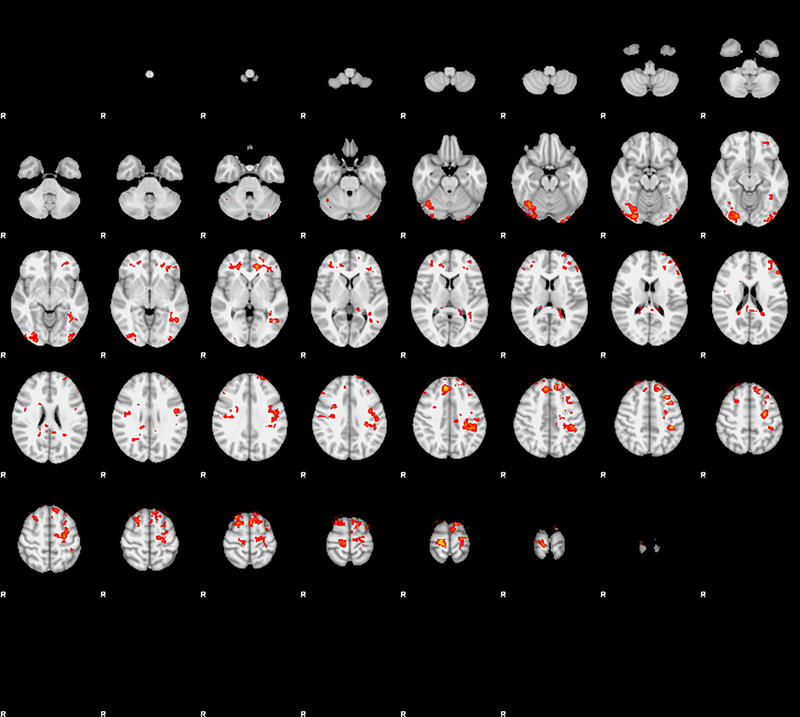
The study sample was daily smokers, 40% women, 60% African American, mean pack years = 17.1. All participants reported practicing mindfulness for at least 30 minutes per day for one week. There were no significant pre- to post-intervention changes on the Perceived Stress Scale and Five Facet Mindfulness Questionnaire. FTND scores were correlated with FCQ-TR at baseline (R = 0.8, P < 0.05). Functional imaging data showed significant pre-post intervention reduction on brain activity in the dorsomedial prefrontal cortex, visual areas, and premotor cortex for food greater than control (Figure 2 and Table 1). In all regions there was a large estimated effect size with Z-scores greater than 4 (Table 1).
Figure 2.
Pre-post mindfulness training comparison of all food images vs. control images across the whole-brain, thresholded at Z-3.1, cluster correction of P<.05.
Table 1.
Anatomical structures corresponding with coordinates of significantly decreased activity
| Coordinates |
|||||
|---|---|---|---|---|---|
| Region | x | y | z | Voxels | Z-Max |
| Right occipital fusiform gyrus | 24 | −84 | −14 | 288 | 4.58 |
| Left post-central gyrus/superior parietal lobule | −38 | −34 | 48 | 205 | 5.06 |
| Right precentral gyrus | 18 | −26 | 80 | 162 | 5.93 |
| Right dorsomedial prefrontal cortex | 26 | 22 | 66 | 126 | 4.72 |
| Left dorsomedial prefrontal cortex | −12 | 34 | 52 | 111 | 4.09 |
| Left premotor cortex | −26 | −10 | 52 | 101 | 4.75 |
| Right superior frontal gyrus/paracingulate gyrus | 8 | 38 | 40 | 99 | 5.57 |
Discussion
Major findings in this study included an association between nicotine dependence and food craving during 12-hour smoking abstinence and significant pre-post intervention reduction on brain activity in specific areas of the brain in response to food cues, including left and right dorsomedial prefrontal cortex (dMPFC), visual areas, and premotor cortex. These changes could be attributed to down-regulation of sensory processing of food images due to mindfulness practice. For example, the reduction in dMPFC activity could be a result of mindfulness decreasing internal negotiation or implementation of willpower to manage cravings, and the reduction in visual areas could be attributed to reduced cue salience as a result of mindfulness training. Our findings suggest that mindfulness training may impact brain activity related to food cues during the post-quit smoking period.
Limitations and Future Research
This study is small and did not provide a control group. The small sample size reduces power to demonstrate significant results, and the fixed effects analysis limits generalizability beyond the current sample. The lack of a control group means that changes observed could be the result of repetition of fMRI tasks rather than self-regulation. In order to determine the actual effect of mindfulness training on post-quit weight gain, further research is required, including a larger comparative trial with outcomes that include weight changes and longer smoking abstinence.
Supplementary Material
Acknowledgments
Jennifer Greyber provided editorial assistance in preparing the manuscript for submission.
Funding
This study was funded through discretionary funds.
Conflict of Interest
EK has received no research grant funding, no significant honorarium funding, and declares no conflict of interest. MS has received research grant funding from NIH and Brain and Behavior Research. MS received no significant honorarium funding and declares no conflict of interest. JD has received research grant funding from Pfizer Inc. and Axsome Therapeutics Inc. JD has received no significant honorarium funding and declares no conflict of interest.
Footnotes
Compliance with Ethical Standards
Ethical approval
This study was approved by the Duke University Institutional Review Board (PRO00079233).
Informed Consent
Informed consent was obtained from all individual participants in the study.
Additional File Information
• File name: Additional file 1.pdf
• File format: PDF
• Title: Cluster Index Table
• Description of data: Data collected for this study included self-report data on demographics, smoking history, food urges, mindfulness, stress, and biochemical assessment of smoking through carbon monoxide breath testing. Pregnancy tests were conducted for all female participants prior to fMRI. Additionally, the study collected structural and functional activity of the brain through functional imaging with BOLD signal (showing blood flow to specified brain regions) within each imaging voxel over time and coordinated in time with images presented through the functional imaging protocol.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Contributor Information
Emily A Kragel, Brody School of Medicine, East Carolina University, 600 Moye Blvd, Greenville, NC 27858..
Maggie M Sweitzer, Department of Psychiatry and Behavioral Sciences, Duke University School of Medicine, 2608 Erwin Road, Durham NC, 27705..
James M Davis, Duke Center for Smoking Cessation, Duke University School of Medicine, 2424 Erwin Road, Suite 201, Durham, NC 27705, USA..
References
- Alberts HJEM, Mulkens S, Smeets M, & Thewissen R (2010). Coping with food cravings. Investigating the potential of a mindfulness-based intervention. Appetite, 55(1), 160–163. [DOI] [PubMed] [Google Scholar]
- Aubin H-J, Farley A, Lycett D, Lahmek P, & Aveyard P (2012). Weight gain in smokers after quitting cigarettes: meta-analysis. BMJ, 345, e4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baer RA, Smith GT, Lykins E, Button D, Krietemeyer J, Sauer S, et al. (2008). Construct validity of the five facet mindfulness questionnaire in meditating and nonmeditating samples. Assessment, 15(3), 329–342. [DOI] [PubMed] [Google Scholar]
- Borrelli B, Spring B, Niaura R, Hitsman B, & Papandonatos G (2001). Influences of gender and weight gain on short-term relapse to smoking in a cessation trial. Journal of Consulting and Clinical Psychology, 69(3), 511. [DOI] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, & Mermelstein R (1994). Perceived stress scale. In Cohen S, Kessler RC, Gordon LU (Ed.), Measuring Stress: A Guide for Health and Social Scientists, 235–283. [Google Scholar]
- Davis JM, Goldberg SB, Anderson MC, Manley AR, Smith SS, & Baker TB (2014). Randomized trial on mindfulness training for smokers targeted to a disadvantaged population. Substance Use & Misuse, 49(5), 571–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JM, Manley AR, Goldberg SB, Smith SS, & Jorenby DE (2014). Randomized trial comparing mindfulness training for smokers to a matched control. Journal of Substance Abuse Treatment, 47(3), 213–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JM, Mills DM, Stankevitz KA, Manley AR, Majeskie MR, & Smith SS (2013). Pilot randomized trial on mindfulness training for smokers in young adult binge drinkers. BMC Complementary and Alternative Medicine, 13(1), 215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deveci SE, Deveci F, Açik Y, & Ozan AT (2004). The measurement of exhaled carbon monoxide in healthy smokers and non-smokers. Respiratory Medicine, 98(6), 551–556. [DOI] [PubMed] [Google Scholar]
- Eisenberg D, & Quinn BC (2006). Estimating the effect of smoking cessation on weight gain: an instrumental variable approach. Health Services Research, 41(6), 2255–2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epel E, Tomiyama A, & Dallman M (2011). Stress and Reward Neural Networks, Eating, and Obesity: Handbook of Food Addiction In Brownell KD, Gold MS (Ed.), Food and Addiction: A Comprehensive Handbook. Oxford: Oxford University Press. [Google Scholar]
- Farley AC, Hajek P, Lycett D, & Aveyard P (2012). Interventions for preventing weight gain after smoking cessation. Cochrane Database of Systematic Reviews. 10.1002/14651858.CD006219.pub3 [DOI] [PubMed] [Google Scholar]
- Filozof C, Fernandez Pinilla M, & Fernández-Cruz A (2004). Smoking cessation and weight gain. Obesity Reviews, 5(2), 95–103. [DOI] [PubMed] [Google Scholar]
- Fiore M (2008). Treating Tobacco Use and Dependence: 2008 Update: Clinical Practice Guideline. Darby, PA: Diane Publishing. [Google Scholar]
- Jamal A (2016). Current cigarette smoking among adults—United States, 2005–2015. MMWR. Morbidity and Mortality Weekly Report, 65. [DOI] [PubMed] [Google Scholar]
- Kristeller JL, & Hallett CB (1999). An exploratory study of a meditation-based intervention for binge eating disorder. Journal of Health Psychology, 4(3), 357–363. [DOI] [PubMed] [Google Scholar]
- Marchand WR (2014). Neural mechanisms of mindfulness and meditation: evidence from neuroimaging studies. World Journal of Radiology, 6(7), 471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meule A, Hermann T, & Kübler A (2014). A short version of the Food Cravings Questionnaire—Trait: the FCQT-reduced. Frontiers in Psychology, 5, 190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineur YS, Abizaid A, Rao Y, Salas R, DiLeone RJ, Gündisch D, et al. (2011). Nicotine decreases food intake through activation of POMC neurons. Science, 332(6035), 1330–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay S, & Wendel J (2011). Is post-smoking-cessation weight-gain a significant trigger for relapse? Applied Economics, 43(24), 3449–3457. [Google Scholar]
- O’Hara P, Connett JE, Lee WW, Nides M, Murray R, & Wise R (1998). Early and late weight gain following smoking cessation in the Lung Health Study. American Journal of Epidemiology, 148(9), 821–830. [DOI] [PubMed] [Google Scholar]
- Pistelli F, Aquilini F, & Carrozzi L (2016). Weight gain after smoking cessation. Monaldi Archives for Chest Disease, 71(2). [DOI] [PubMed] [Google Scholar]
- Pomerleau CS, Carton SM, Lutzke ML, Flessland KA, & Pomerleau OF (1994). Reliability of the Fagerstrom tolerance questionnaire and the Fagerstrom test for nicotine dependence. Addictive Behaviors, 19(1), 33–39. [DOI] [PubMed] [Google Scholar]
- Spring B, Howe D, Berendsen M, McFadden HG, Hitchcock K, Rademaker AW, & Hitsman B (2009). Behavioral intervention to promote smoking cessation and prevent weight gain: a systematic review and meta analysis. Addiction, 104(9), 1472–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson DF, Madans J, Anda RF, Kleinman JC, Giovino GA, & Byers T (1991). Smoking cessation and severity of weight gain in a national cohort. New England Journal of Medicine, 324(11), 739–745. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.