Abstract
Alzheimer’s disease (AD) is the leading cause of dementia in the United States and afflicts greater than 5.7 million Americans in 2018. Therapeutic options remain extremely limited to those that are symptom targeting, while no drugs have been approved for the modification or reversal of the disease itself. Risk factors for AD including aging, the female sex, as well as carrying an APOE4 genotype. These risk factors have been extensively examined in the literature, while less attention has been paid to modifiable risk factors, including lifestyle, and environmental risk factors such as exposures to air pollution and pesticides. This review highlights the most recent data on risk factors in AD and identifies gene by environment interactions that have been investigated. It also provides a suggested framework for a personalized therapeutic approach to AD, by combining genetic, environmental and lifestyle risk factors. Understanding modifiable risk factors and their interaction with non-modifiable factors (age, susceptibility alleles, and sex) is paramount for designing personalized therapeutic interventions.
Keywords: Alzheimer’s disease, dementia, APOE4 genotype, personalized medicine
1.0. Introduction
1.1. Prevalence and Incidence
Alzheimer’s disease (AD), is the most common progressive neurodegenerative disease worldwide and accounts for 60 to 80% of dementia cases. Individuals who suffer from AD experience memory loss and a decline in reasoning and planning abilities. As the disease progresses, patients ultimately experience a loss of executive function and in many cases become entirely dependent on caregivers. According to the 2018 report by the Alzheimer’s Association, an estimated 5.7 million Americans are diagnosed with AD (“2018 Alzheimer’s disease facts and figures,” 2018). The current estimated cost of caring for these individuals is expected to reach 277 billion dollars in 2018 (“2018 Alzheimer’s disease facts and figures,” 2018). By 2050 it is estimated that the number of AD cases will grow to 13.8 million, and the cost of care will be greater than 1.1 trillion dollars (“2018 Alzheimer’s disease facts and figures,” 2018; Hebert, Weuve, Scherr, & Evans, 2013). While AD presents very differently in individual patients, it is often described as two forms based on the age of onset. Early onset AD (EOAD) sometimes termed familial AD occurs early in life (<65 years of age) and accounts for 5% of all AD cases (Zhu, Tan, et al., 2015). Late onset AD (LOAD) or sporadic AD is more complex and occurs relatively later in life (onset >65 years of age) comprising about 95% of cases (Rosenthal & Kamboh, 2014).
1.2. Pathology
AD is a multifactorial neurodegenerative disease, which involves a combination of aggregated proteins, chronic neuroinflammation and neuronal cell loss. It is pathologically defined by the presence of two hallmarks, amyloid beta (Aβ) plaques and neurofibrillary tangles (NFTs). Proteolytic cleavage of the amyloid precursor protein (APP) by β-secretase-1 enzyme (BACE1), generates Aβ peptides of varying length (Aβ40 and Aβ42) which further aggregate to form insoluble plaques. In addition, soluble peptides are thought to initiate changes in tau and destabilize the cell’s skeletal network (Andreeva, Lukiw, & Rogaev, 2017; O’Brien & Wong, 2011; Simic et al., 2016). NFTs are formed due to aberrant phosphorylation of the microtubule associated protein tau (De-Paula, Radanovic, Diniz, & Forlenza, 2012; Kumar et al., 2011). This hyperphosphorylation causes tau to become detached from microtubules, leads to their destabilization therefore contributing to a disruption in axonal transport (Ballatore, Lee, & Trojanowski, 2007). This disruption ultimately leads to damage and degeneration of neurons (Ballatore et al., 2007; Iqbal, Liu, Gong, & Grundke-Iqbal, 2010). Chronic neuroinflammation is also heavily involved in AD, and both microglia and astrocytes play significant roles (Avila-Munoz & Arias, 2014; Heneka et al., 2015). This inflammation is not only increased directly by the presence of Aβ, tau, and neuronal injury, but also greatly exacerbates these pathologies leading to further damage (Akiyama et al., 2000).
1.3. Difficulty of Diagnosis
The primary goal of the Alzheimer’s Disease Neuroimaging Initiative (ADNI) is to gather information from imaging studies, such as magnetic resonance imaging (MRI) and positron emission topography (PET), biomarker data, in combination with cognitive assessments to predict progression. Since its inception in 2004, data from the ADNI have contributed to over 1500 publications, and these data have profiled progression of both amyloid beta and hyperphosphorylated tau in individuals (Weiner & Veitch, 2015; Weiner et al., 2012; Weiner, Veitch, et al., 2017). Eventually, these data coupled with other major risk factors such as family history, genetic risks, lifestyle factors, and environmental exposures could contribute to identifying at-risk individuals who are more likely to develop the disease and allow for earlier intervention. When most patients present to a physician, they may be past effective therapeutic windows (Jack et al., 2010; Sperling et al., 2011). Identifying patients that are at risk earlier could greatly increase the success of therapeutics. For example, mild cognitive impairment (MCI) is an earlier development in AD, and it is evident that individuals who are identified as having MCI end up progressing to AD (Petersen, 2009). Identifying these individuals early on may allow the administration of a potential therapies aimed at limiting conversion to AD.
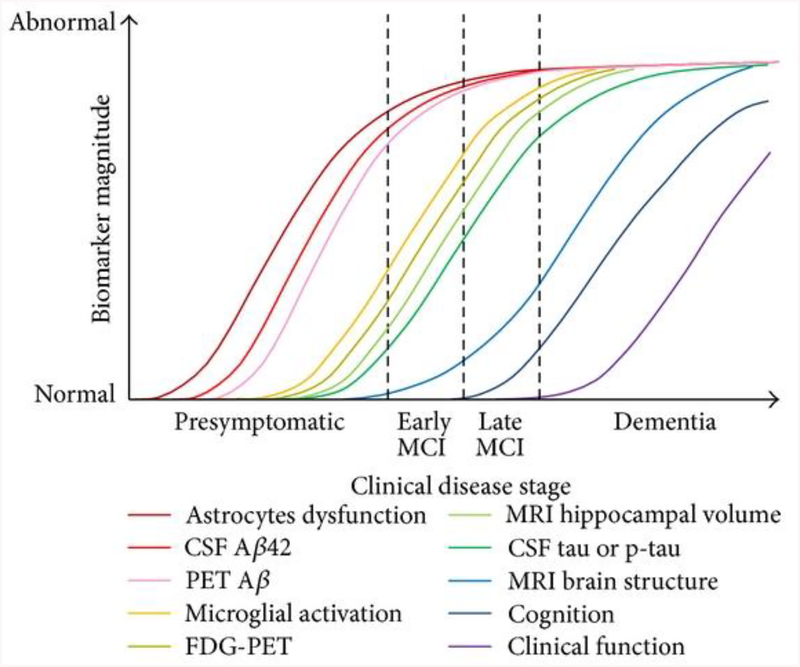
AD progression has been characterized extensively by examining biomarkers in patients, both in cerebrospinal fluid (CSF) levels and by PET amyloid imaging, or fluorodeoxyglucose-PET (tau) (Jack et al., 2010; Sperling et al., 2011). Aβ levels begin to rise during preclinical AD and increase dramatically with age, even before the onset of MCI (Jack et al., 2010; Sperling et al., 2011). During MCI and before the onset of dementia, Aβ have reached maximum levels. However, tau mediated neuronal injury in the CSF increases much later than Aβ in patients, and begins to reach high levels at the onset of MCI, reaching maximum levels at the onset of dementia (Figure 1) (Jack et al., 2010; Sperling et al., 2011). Importantly, these changes occur in patients well before the onset of any clinical dysfunction and make it exceedingly difficult to identify individuals (Figure 1) (Jack et al., 2010; Sperling et al., 2011). Due to the broad range of disease presentation and the unknown nature of disease development, better diagnostics are needed to identify patients earlier in the progression of the disease.
Figure 1:
Biomarker progression in patients by clinical disease stage. This image is directly reproduced from an open access article (Leclerc & Abulrob, 2013) distributed under the Creative Commons Attribution License.
To date, therapeutic development aimed at disease modification has primarily focused on the two major pathways, amyloid and tau, as contributors to AD. Researchers have devoted years of resources, in both basic science research funding, and in therapeutics that target mostly Aβ and in some instances tau (Anand, Gill, & Mahdi, 2014). These targets have been the basis for development of animal models with these hallmarks as driving factors. Clinically, biomarker tests identify these components and allow assessment for individuals at risk for the disease. However, these strategies have not produced a therapeutic drug with safety and efficacy that can be used universally in AD patients. The limited understanding of the disease etiology is magnified when considering the lack of pharmaceutical options available to AD patients. Current research strategies have focused on a number of targets that are involved in AD, such as the pathological aggregates NFTs and Aβ, inflammation, cholesterol and the cholinergic system (J. L. Cummings, Morstorf, & Zhong, 2014). Of the 210 drugs that have either entered into a clinical trial, or are currently in a trial, less than 2% of them have obtained FDA approval (J. L. Cummings et al., 2014). There are currently only five clinically approved AD drugs, but these are not disease modifying therapies (DMT), and only target the symptoms of AD by acting directly on the cholinergic system, with the exception of memantine which is an antagonist of NMDA receptors (Farlow, Graham, & Alva, 2008).
2.0. Nonmodifiable Risk Factors in AD
Nonmodifiable factors refer to those risk that increase the likelihood of AD diagnosis and cannot be modified at the present. These are factors to which an individual is born with and in the case of AD, these include aging, gene variants, family history of disease and others discussed in the subsequent sections. These factors have consistently shown an association with increased AD risk. Specifically, increased age is considering the leading risk factor, and the best predictor for the development of late-onset AD. Similarly, genetic variants have also been shown to greatly contribute to AD risk. Understanding the common pathways, or the actions that these risks confer is paramount for understanding the disease. For instance, how do these nonmodifiable factors interact with one another, and how do they interact with those factors that an individual is able to modify? Ultimately by identifying these risks, and understanding the mechanisms by which they act, there may be an opportunity to minimize their contributions to the disease.
2.1. Aging
The greatest risk factor for late-onset AD is age. The positive correlation between age and AD risk has been consistently reported, with a significant increase in incidence after 60 years (Hebert et al., 2010; Hebert et al., 2013; Kawas, Gray, Brookmeyer, Fozard, & Zonderman, 2000), with 3% of people between ages 65–74, 17% of people aged 75–84 and 32% of people that are aged 85 years or older diagnosed with AD, respectively (“2018 Alzheimer’s disease facts and figures,” 2018). While the reason behind this remains largely unclear, literature suggests that it is due to dysregulation and disruption of the critical pathways of the inflammatory system, lipid homeostasis, as well protein degradation and synthesis (Spittau, 2017). However, it is unlikely that AD occurs as a result of normal aging, as there are distinctive differences in those aged individuals who develop AD compared to those who do not (Toepper, 2017). Most notably, while AD and aging share some commonalities, such as atrophy of specific brain regions and changes in cognition, these occur much more rapidly in AD compared to normal aging (Toepper, 2017). Therefore, while aging is a necessary and significant risk factor for LOAD, it alone is not sufficient to cause disease.
2.2. Genetic Contributors to AD
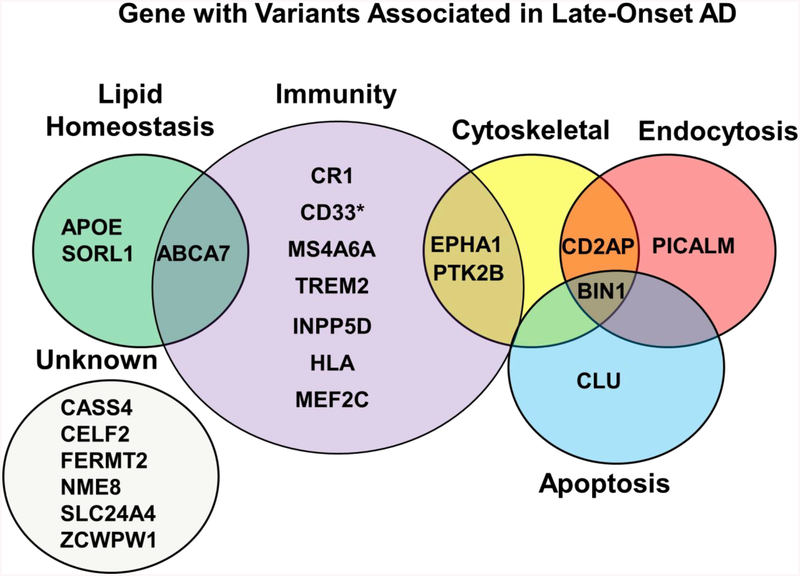
Genetic variations have been associated with an increased risk of developing both EOAD and LOAD. Three of these genes (APP, PSEN1, PSEN2) are autosomal dominant for developing EOAD (Dai, Zheng, Zeng, & Zhang, 2018). As previously mentioned, the APP protein is sequentially cleaved into peptides which then aggregate and form Aβ plaques, and PSEN1 and PSEN2 encode subunits of one of the cleavage enzymes, γ-secretase. Mutations in these genes are associated with the inheritance of familial AD, and have been extensively examined (Bertram & Tanzi, 2012; Bird, 1993), however they do not increase risk for late-onset sporadic AD and are therefore beyond the scope of this review. The remaining variants are either splice variants, or single nucleotide polymorphisms (SNPs), and while not causative, increase risk for the development of sporadic late-onset forms of AD. As of 2018, researchers have identified and confirmed 21 susceptibility loci: ABCA7, BIN1, CD2AP, CLU, CR1, EPHA1, MS4A6A, PICALM, APOE, TREM2, CD33, CASS4, CELF2, FERMT2, HLA, INPP5D, MEF2C, NME8, PTK2B, SLC24A4, SORL1, and ZCWPW1 that confer risk for AD (Dos Santos et al., 2017; Hartl et al., 2018; Hollingworth et al., 2011; Lacour et al., 2017; Lambert et al., 2013; Medway & Morgan, 2014; Naj et al., 2011; Nordestgaard, Tybjaerg-Hansen, Rasmussen, Nordestgaard, & Frikke-Schmidt, 2018; Ruiz et al., 2014). Of these genes, all have been confirmed by the International Genomics Alzheimer’s Project (IGAP) by a meta-analysis of multiple genome wide association studies (GWAS) (Lambert et al., 2013), with the exception of CD33 (Figure 2)
Figure 2: Genes with Variants Associated with Late-Onset AD.
Genes confirmed by IGAP, with the exception of CD33 identified by*. These genes cluster in specific categories based on function. The vast majority are involved in immunity, others involve lipid homeostasis, cytoskeletal interactions, endocytosis and apoptosis. A fair number of genes still have either unknown functions, or it is unknown how they relate to AD disease mechanisms.
The IGAP genes are involved in a number of different processes linked to AD, some of which are known, including lipid homeostasis/cholesterol metabolism, immunity, endocytosis, cytoskeletal and apoptosis (Medway & Morgan, 2014). The actions of ABCA7 and CD33 have been implicated in phagocytosis and clearance of Aβ plaques, variants in these genes have been shown to alter these mechanisms (Fu, Hsiao, Paxinos, Halliday, & Kim, 2016; Griciuc et al., 2013). In addition to its role in lipid homeostasis, SORL1 has also been shown to be regulate aspects of APP trafficking and processing (Yin, Yu, & Tan, 2015). The validated genes involved in inflammation and immunity, including CR1, MS4A6A, INPP5D, HLA and MEF2C have all shown to have roles in neuroinflammation and altered expression following microglial activation (Efthymiou & Goate, 2017; Rosenthal & Kamboh, 2014; Zhu, Yu, et al., 2015). Genes involved in endocytosis, such as PICALM participates in production and clearance Aβ (Xu, Tan, & Yu, 2015) whereas BIN1 has shown to modulate tau pathology in addition to its role in endocytosis (M. S. Tan, Yu, & Tan, 2013). The protein products of these validated genes span multiple functions and are not limited to these categories, new studies are consistently emerging to suggest their actions on multiple pathways involved in AD. While many of these actions have been elucidated the actions of other genes identified by IGAP and their biological relationship to LOAD pathways remain unclear (Figure 2) (Medway & Morgan, 2014).
The genes presented in this review have been validated by IGAP (Medway & Morgan, 2014) if indicated, or have been included because they have been identified by multiple GWAS studies (CD33). Evidence from other studies have emerged implicating new gene variants of interest, however these will need to be verified. SNPs in the PLCG2 and ABI3 and genes have been associated with increased risk of AD and specifically microglial immunity, which further implicates the role of neuroinflammation in the development of late-onset AD (Sims et al., 2017). Similarly, The Interleukin-1 receptor accessory protein (IL1RAP) gene is also involved in microglial activation with identified SNPs potentially increasing risk (Mun, Kim, Choi, & Jang, 2016). Individuals who are IL1RAP rs12053868-G carriers are more likely to progress from MCI to AD and have greater temporal cortex atrophy. This same polymorphism is associated with more rapid cognitive decline, a decrease in microglial activation and higher levels of amyloid accumulation (Ramanan et al., 2015). It is also clinically vital to examine individuals with many of the essential AD risk factors, yet appear to be protected from the disease as in a subset of patients with a variant of RAB10 which is shown to drastically protect against AD in those individuals over 75 years (Ridge et al., 2017).
2.2.1. TREM2
Variants of the triggering receptor expressed on myeloid cells 2 (TREM2) have gained significant recent attention as they increase the risk of developing LOAD by 2–4-fold (Guerreiro et al., 2013; S. C. Jin et al., 2014; Jonsson et al., 2013). TREM2 is a transmembrane protein that is expressed on microglia, to which ligands bind and thus activate the TREM2 pathway, this activation leads to numerous downstream signaling events. A host of ligands have been shown to bind to and activate TREM2, these ligands include the apolipoprotein E (APOE), clusterin (CLU), and Aβ have shown affinity for this receptor (Yeh, Wang, Tom, Gonzalez, & Sheng, 2016; Zhong et al., 2018). This is of interest for AD risk, as variants in TREM2, APOE and CLU genes have been identified as significant risk factors for the development of LOAD (Lambert et al., 2013). The activation of TREM2 has been shown to inhibit proinflammatory signaling, increase proliferation of microglia and myeloid cells, as well as increase phagocytotic processes (Colonna & Wang, 2016; Yeh, Hansen, & Sheng, 2017). This pathway has been shown to regulate microglial function and homeostasis in neurodegenerative disease (Krasemann et al., 2017). Variants of TREM2 are associated with loss or decrease function of the receptor, which in turn disrupts its functions (Ulland et al., 2017). This disruption of microglial function by a TREM2 variant would have tremendous impact on pathways involved in AD. Potentially by reducing the response of microglia to phagocytose Aβ plaques and by impairing the anti-inflammatory properties associated with TREM2 activation (Ulrich, Ulland, Colonna, & Holtzman, 2017). The role of TREM2 in AD is still being elucidated, and likely includes other functions contributing to disease pathology and progression (Colonna & Wang, 2016; Ulrich et al., 2017).
2.2.2. APOE
Apolipoprotein (APOE) is a lipid carrier and is significantly involved in cholesterol metabolism. It is located on chromosome 19 and found in the human population as either E2, E3 or E4 variants, which differ from one another by the presence of either arginine or cysteine at specific residues (Utermann, Hees, & Steinmetz, 1977; Utermann, Langenbeck, Beisiegel, & Weber, 1980). This structural difference confers changes in the physiological function of the protein (Mahley, Weisgraber, & Huang, 2006); specifically, the APOE4 protein has a much lower affinity for lipoproteins compared to the E2 and E3 forms (Rall, Weisgraber, & Mahley, 1982), and also poorly binds Aβ which may explain its poor clearance (LaDu et al., 1994). APOE is most highly expressed in liver, but it is also abundant in the CNS, predominately in astrocytes (Boyles, Pitas, Wilson, Mahley, & Taylor, 1985).
The APOE4 allele remains the strongest genetic risk factor across multiple GWAS studies in different populations, it is gene dose dependent and allele homozygosity confers an 8–12-fold increased risk (C. C. Liu, Liu, Kanekiyo, Xu, & Bu, 2013; Pimenova, Raj, & Goate, 2018). In discussion of gene-gene, gene-environment, and gene-sex interactions in this review, it is almost always in relationship with APOE4 and AD risk, as it is by far the most widely studied risk gene in AD. APOE has repeatedly been shown to play important roles in Aβ transport and metabolism (Kim, Basak, & Holtzman, 2009; C. C. Liu et al., 2013) and recent evidence has suggested that APOE regulates APP transcription and Aβ production in neurons, with APOE4 driving higher expression (Y. A. Huang, Zhou, Wernig, & Sudhof, 2017). Further, research suggests that APOE genotype plays a prominent role in regulation of neuroinflammation in AD and regulation of microglial and astrocyte function (Tai et al., 2015). Despite the attention that has been paid by the scientific community, much is left to understand and uncover with regard to its contribution to AD risk.
2.3. Family History
Family history confers a significant increased risk for AD. Individuals who have a first-degree relative, such as a parent or sibling diagnosed with AD are predisposed to develop the disease with a 4–10 times increased risk, compared to individuals who do not (Cupples et al., 2004; Farrer, O’Sullivan, Cupples, Growdon, & Myers, 1989; Lautenschlager et al., 1996). Individuals with a family history of AD exhibited lower recognition accuracy and deficits in executive functioning (Aschenbrenner, Balota, Gordon, Ratcliff, & Morris, 2016; Hazlett, Figueroa, & Nielson, 2015). In a recent cohort study examining family history of AD diagnosis on AD risk and dementia, the authors reported a relationship of family history negatively impacting certain sub-cognitive domains, which is stronger as individuals age (Zeng et al., 2013).
The role of family history in screening AD biomarkers has indicated a positive correlation between family history and susceptibility to AD (Xiong et al., 2011). Studies on datasets from the ADNI database have associated maternal family history with AD biomarkers (Honea, Vidoni, Swerdlow, Burns, & Alzheimer’s Disease Neuroimaging, 2012). Honea and colleagues have shown that normal adults with a maternal family history of AD have progressive reductions in gray matter volume in regions that are vulnerable to AD (Honea, Swerdlow, Vidoni, & Burns, 2011; Honea, Swerdlow, Vidoni, Goodwin, & Burns, 2010). A cross sectional study of cerebrospinal fluid (CSF) biomarkers in subjects with or without family history of AD suggested that an individual with maternal family history is at a higher risk of developing AD (Z. Liu, Chen, Li, Xu, & Du, 2013). This familial risk also appears to affect AD related pathology. Subjects with a family history of AD have shown accelerated thinning of the cortex as compared to age matched control individuals (Donix et al., 2010; Ganske et al., 2016). However, family history is not completely due to genetic risk factors, but rather represents a combination of both genetic factors and shared environments among family members.
2.4. Race and Ethnicity
Based on data for Medicare beneficiaries over the age of 65, AD and other dementias have been diagnosed in 6.9% of Caucasians, 9.4% of African-Americans and 11.5% of Hispanics (“2018 Alzheimer’s disease facts and figures,” 2018). Several studies have attributed these differences to socio-economic status, lifestyle and health conditions, including cardiovascular disease and diabetes (Glymour & Manly, 2008; Yaffe et al., 2013). The Aging, Demographics, and Memory Study reported that older African-Americans are about twice as likely to develop AD and other dementias compared to older non-Hispanic whites (Potter et al., 2009). Hispanics (Mexican-Americans or Caribbean-Americans) are also about 1.5 times more likely to develop AD than non-Hispanic whites (Gurland et al., 1999; Mehta & Yeo, 2017). Several attempts have been made to recognize biomarkers for the onset of preclinical and clinical AD in these populations (Brookmeyer & Abdalla, 2018; Brookmeyer, Abdalla, Kawas, & Corrada, 2018).
The role of genetic factors in contributing to the racial disparity in AD has been debated. Initially it was thought that genetic factors did not account for large prevalence differences among racial/ethnic groups, however, more recent evidence suggests that the genetic architecture of an individual plays a role in developing both EOAD and LOAD (Cruchaga et al., 2018). A GWAS meta-analysis of data from African-American participants suggested a significantly strong association between ABCA7, with an OR= 1.79, and APOE4 with an OR= 2.31 and the increased risk of LOAD (Reitz et al., 2013). Using multiple independent data sets of individuals with MCI, variants in the CLU gene have emerged as players in the risk of the conversion of patients from MCI to AD (Lacour et al., 2017). Variants in the CLU gene have been identified as potential risk factors in late-onset AD, specifically in Caucasian populations (Han, Qu, Zhao, & Zou, 2018; Lu et al., 2014; Nordestgaard et al., 2018; Szymanski, Wang, Bassett, & Avramopoulos, 2011; Zhang et al., 2016).
2.5. Sex as a Risk Factor
Women are an exceptionally susceptible population for the development of AD. A staggering 3.6 million women in the US (over 2/3 of patients) are currently living with AD, and this number is expected to continue to rise (“2018 Alzheimer’s disease facts and figures,” 2018). Furthermore, women at 45 years of age have a 19.5% lifetime risk of developing AD compared to 10.3% in men (“2018 Alzheimer’s disease facts and figures,” 2018). These vulnerabilities may occur for a variety of reasons. Evidence also suggests that these sex differences may also be rooted in inflammatory mechanisms (Uchoa, Moser, & Pike, 2016) and/or mitochondrial dynamics (Lejri, Grimm, & Eckert, 2018).Hormonal differences, specifically loss of protection by estrogen (R. Li, Cui, & Shen, 2014), which can be partially mitigated by beginning hormone therapy within 5 years of after menopause (Shao et al., 2012). Other studies have also implicated the estrogen receptor α (ESR1) gene, and six different SNPs of ESR1 are shown to significantly delay the onset of AD in females but not in males (Janicki et al., 2014). Hormone therapy clinical trials with estrogen have failed for AD, which partly may be due to the timing of treatment and progression of women in these studies (Henderson, 2014). The role of estrogens in the brain has been a topic of wide investigation, with many reviews highlighting mechanisms involved in neuroprotection (Brann, Dhandapani, Wakade, Mahesh, & Khan, 2007; Gillies & McArthur, 2010; Green & Simpkins, 2000). Some of these factors, maybe extremely relevant to AD due to the progressive neurodegeneration in the disease. Specifically, estrogen has been associated with the maintenance of cholinergic neurons (Gibbs, 2010; Gibbs & Aggarwal, 1998), a target in late-onset AD and loss of estrogen may lead to deleterious effects on these cells.
Studies from the ADNI cohort indicate differences in disease manifestations between men and women, as well as in disease progression. Women progress faster from MCI to AD compared to men, and this is even more pronounced in APOE4 carriers (Lin et al., 2015). Other large-scale epidemiological studies from ADNI also support that APOE4 confers a greater risk for women than men (Altmann, Tian, Henderson, Greicius, & Alzheimer’s Disease Neuroimaging Initiative, 2014). Women with probable AD and MCI also have higher atrophy rates (1–1.5% faster than their male counterparts) (Hua et al., 2010). Furthermore, in almost all aspects of the disease, studies from the ADNI cohort identify that women tend to accumulate more Aβ42, total tau levels, and worse and more rapid atrophy in brain regions, specifically the hippocampus as well as worsened cognitive abilities, which again appear to be modulated by other risk factors, such as APOE4 (Koran, Wagener, Hohman, & Alzheimer’s Neuroimaging, 2017). A recent study indicates that this increased risk for women is only at earlier stages of the disease, and that older individuals of both sexes have the same risk of developing AD (Neu et al., 2017).
2.6. Traumatic Brain Injury (TBI)
Epidemiological and laboratory studies support a role of TBI in AD etiology. Overwhelmingly in human epidemiological studies, this risk is thought to occur because the age at onset (AAO) of individuals with a history of TBI is lower compared to those without this history (Mendez, 2017). Notably, human patients and animal models of TBI show increased aggregation of misfolded proteins in the brain, and increased amounts of their precursors, Aβ and hyperphosphorylated tau (Edwards, Moreno-Gonzalez, & Soto, 2017; W. Hu, Tung, Zhang, Liu, & Iqbal, 2018). However, most earlier epidemiological studies support a role for TBI history in increasing risk of AD (Guo et al., 2000; Mortimer et al., 1991; Nicoll, Roberts, & Graham, 1995, 1996; Plassman et al., 2000; Schofield et al., 1997) and in some cases have associated a history of TBI with AD pathology (Jellinger, Paulus, Wrocklage, & Litvan, 2001). In contrast, more recent large-scale studies have produced mixed results, which has made the characterization of TBI as a major risk factor for AD more difficult. Studies that identify TBI as a factor in decreasing the AAO of AD or contributing to a more rapid decline in cognitive function and faster conversion from MCI to AD (Gilbert et al., 2014; W. Li, Risacher, McAllister, & Saykin, 2016; LoBue et al., 2017), suggest that the timing of TBI occurrence is significant, as well as age, in that adults who experience a TBI have worse cognitive outcomes than children (W. Li, Risacher, McAllister, Saykin, & Alzheimer’s Disease Neuroimaging, 2017). In some instances the earlier AAO was also associated with pathological hallmarks and confirmed by examining Braak NFTS staging and plaque score in postmortem samples (Schaffert et al., 2018).
Large-scale epidemiological studies have convincingly argued for a role of TBI in increasing risk of dementia and AD. In Denmark, a nationwide population cohort study revealed an HR of 1.24 for dementia, and 1.16 for AD (Fann et al., 2018). A recent meta-analysis pooled 32 different studies and showed an RR=1.63 for dementia and RR=1.51 for AD (Y. Li et al., 2017). Although these data are compelling, there are other studies that do not support a role for TBI in disease etiology (Crane et al., 2016; Dams-O’Connor et al., 2013; LoBue et al., 2018; Mehta et al., 1999; Tripodis et al., 2017; Weiner, Harvey, et al., 2017). Overall, research in this field has produced mixed results on the exact nature of TBI risk in AD. These data from both animal models and human studies, suggest that TBI history lowers AAO, and increase formation of pathological proteins. The decrease in AAO appears to be modified by APOE4, but remains equivocal. More rigorous research strategies should be implemented going forward, and information where clinical self-reports are utilized should be approached with caution, as they have large potential for bias and variation (Kokiko-Cochran & Godbout, 2018).
3.0. Modifiable Risk Factors
Producing a therapy for AD presents as an incredible challenge, and although research has contributed a great deal of data with regard to patients diagnosed with the disease, there remains a lack of understanding of the disease pathogenesis, particularly at the individual level. The difficulty lies with the variability in presentation, and the wide host of factors that may be involved in its etiology and progression. As such, modifiable factors at the individual level are highly likely to be involved. Identifying how these risks, which are alterable are of great interest. These may include an individual’s education level, lifestyle habits, and the exposures that an individual may encounter, as they all may influence health. Furthermore, many nonmodifiable factors may play a role in which of how these risks may contribute. For instance, there are groups of individuals, such as the elderly, or women which may be more susceptible to air pollutants (Boezen et al., 2005). Similarly, there is evidence for environmental disparities and increased exposures to environmental toxicants amongst minority groups (Gee & Payne-Sturges, 2004). Lastly, it is important to consider how genetic variants may interact with lifestyle factors, for example genetic variants which regulate cholesterol metabolism, and individuals who may develop type 2 diabetes. In the following sections, we will present evidence for these modifiable factors as risk factors for AD, and the likely pathways they may act upon.
3.1. Education
Having higher education experience has been shown repeatedly to decrease risk for AD in several epidemiological studies. A recent meta-analysis examining published studies through July 2014 reported a significant association of low education attainment (less than or equivalent to primary school) and increased AD risk, relative risk (RR) =1.41–1.60. Similarly, in a more recent large-scale (>1700 individuals with AD) study as part of IGAP, Larrson and colleagues evaluated the relationship among various risk factors in AD in relationship to genetic variants. Higher degree attainment was associated with a significantly lower odds ratio (OR) of AD, OR=0.89 per year of completed education (Larsson et al., 2017). This study is convincing, however the biological mechanism by which this factor may confer risk is unknown. There is some evidence that AD patients with a higher education also have greater hippocampal volume, suggesting a lower atrophy in those individuals (Shpanskaya et al., 2014).
3.2. Cardiovascular Risk Factors
Aberrant lipid profiles and high serum/plasma cholesterol levels are associated with AD along with several metabolic syndromes metabolic syndromes such as Type 2 diabetes mellitus (T2DM), obesity, and hyperinsulinemia. Sparks and colleagues in the 1990s observed plaques in the brains of patients with coronary artery disease (Sparks et al., 1990), and reported a link between high cholesterol levels and AD (Sparks, 1997; Sparks et al., 1994). Cholesterol in the brain, which accounts for the highest content of all the organs in the body, is involved in a series of interdependent Aβ processing including synthesis, aggregation, neurotoxicity and elimination (Sun, Yu, & Tan, 2015). Given this role, numerous studies support the hypothesis that midlife cholesterol or hypercholesterolemia is a risk factor for developing dementia and AD. An increase in total cholesterol from 181 mg/dL to 200 mg/dL almost triples the odds of developing AD (OR=2.96) (Pappolla et al., 2003; Whitmer, Sidney, Selby, Johnston, & Yaffe, 2005). High serum cholesterol during middle age can be predictive of developing AD and evidence in vivo suggests significant association between serum cholesterol and Aβ levels with HR=1.23 for borderline cholesterol (200–239 mg/dL) and 1.57 for high cholesterol (≥240 mg/dl) (Solomon, Kivipelto, Wolozin, Zhou, & Whitmer, 2009). Studies in mice show that accumulation of neuronal cholesterol caused a respective 3.4 ± 0.7-fold and a 1.6 ± 0.14-fold increase in Aβ-C terminal fragment and Aβ42 in the hippocampus (Djelti et al., 2015). Similarly, rabbits fed a 2% cholesterol diet displayed a significant increase Aβ42 levels in both cortex and hippocampus (P. Jin et al., 2018). However, epidemiological studies and human data from serum/plasma or brain indicating an association between high cholesterol and AD have been highly inconsistent (Wood, Li, Muller, & Eckert, 2014). While some research supports that high cholesterol may contribute to the progression of AD, others suggest that cholesterol is not a causative factor, but rather the result of AD (Rantanen et al., 2014; Sun et al., 2015).
Conflicting results in human studies have also been reported regarding whether AD patients have altered cholesterol levels, with some data supporting no differences between plasma cholesterol levels of AD and control subjects, and others more recently identifying a significant difference in plasma cholesterol levels (Popp et al., 2012; Popp et al., 2013). A longitudinal study revealed significant differences in the serum cholesterol levels of AD patients compared with control subjects in the first exam, but this association was lost in a follow up examination 11–26 years later (Kivipelto et al., 2001). Other community-based studies reported that total serum cholesterol levels at midlife were not associated with developing dementia or AD (Stewart, White, Xue, & Launer, 2007; Z. S. Tan et al., 2003). Several pre-clinical studies investigated the therapeutic role of statins, inhibitors of 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) reductase, which is a rate-limiting enzyme in cholesterol synthesis, in alleviating AD pathology (Austen, Christodoulou, & Terry, 2002). Because statins cross the blood brain barrier, these drugs have a direct effect on cholesterol in the brain (Cibickova, 2011). Clinical studies show that simvastatin reduced levels of Aβ42 and Aβ40 significantly both in vivo and in vitro (Fassbender et al., 2001), and furthermore, there are protective effects of statins in Aβ-induced inflammation, both preclinically and clinically (Kurata et al., 2012; Kurata et al., 2011). However, other prospective studies, both preclinical and clinical, have failed to show efficacy in treating symptoms of mild to moderate AD despite significant reduction in total cholesterol levels, which could be attributed to pleiotropic effects of the drugs (Liang, Li, & Cheng, 2015; Sano et al., 2011).
Over the past three decades, a growing body of evidence shows strong epidemiological, clinical and molecular associations among Type 2 diabetes mellitus (T2DM), obesity, and hyperinsulinemia with an increased risk of developing AD. By 2015, over 415 million people worldwide were affected by diabetes and by 2040 this number is predicted to exceed nearly 640 million (International Diabetes Federation, 2015). Because of increasing diabetic and geriatric populations, several studies have focused on investigating the underlying links between T2DM and AD, which is the most common form of dementia in the elderly. Initial evidence from the Rotterdam Study conducted in the 1990s linked diabetes to AD, as data revealed that T2DM almost doubled the risk of AD (Ott et al., 1999). Similarly, in a nationwide population-based study Taiwan spanning 11 years, a higher incidence of dementia was reported in diabetic patients (C. C. Huang et al., 2014). The Cache County study evaluated cardiovascular risk factors including T2DM, hypertension, obesity, stroke and the subsequent risk of AD and vascular dementia by sex in 3308 subjects aged 65 or older. In this cohort, obesity and diabetes increased the risk of AD and vascular dementia in elderly females but not males (Hayden et al., 2006). Several epidemiological studies and meta-analyses have documented diabetes as a risk factor in development of AD (relative risk 1.5) after assessing the underlying mechanisms, such as lipid metabolism and insulin signaling which interconnect diabetes and AD (Cheng, Huang, Deng, & Wang, 2012; Gudala, Bansal, Schifano, & Bhansali, 2013).
Studies in humans and animal models have also suggested bi-directional interactions between diabetes and AD where AD pathology exacerbates the risk of developing T2DM and vice versa (Matsuzaki et al., 2010; Shinohara & Sato, 2017). Defective insulin signaling has been cited as one of the relevant mechanisms that modifies AD pathology by modulating APP processing, increased production and release of Aβ, and higher tau phosphorylation levels (Macauley et al., 2015; Matsuzaki et al., 2010; Moloney et al., 2010; Nuzzo et al., 2015). Growing evidence supports the concept that insulin resistance and reduced insulin receptor expression are important mediators of cognitive deficits, brain atrophy, and cerebrovascular changes including reduced hippocampal volume and subsequent inflammation (Kerti et al., 2013; Moran et al., 2013; Steen et al., 2005). Contribution of inflammatory mediators such as tumor necrosis factor α (TNFα), interleukin-1β (IL-1β) and interleukin 6 (IL-6) to the progression of AD has been established (Akiyama et al., 2000). Taken together, results from preclinical studies in AD and diabetes models suggest a crucial role of inflammatory pathways in pathogenesis of both diseases thus elucidating an interaction through the immune system (Baglietto-Vargas, Shi, Yaeger, Ager, & LaFerla, 2016; Takeda et al., 2010; Valente, Gella, Fernandez-Busquets, Unzeta, & Durany, 2010). Recently, scientists have proposed the concept of AD representing a metabolic disease due to the overlapping molecular mechanisms underlying diabetes and AD. Based on the role of insulin in AD pathology, De la Monte proposed that AD could be referred as ‘Type-3-Diabetes’ as it has composite features of both Type 1 diabetes (insulin deficiency) and Type 2 diabetes (insulin resistance) (de la Monte, 2014; Hoyer, 2004; Rivera et al., 2005; Talbot et al., 2012).
3.3. Lifestyle
It is exceedingly common for lifestyle habits to present as either positive or negative risk factors in disease. In fact, healthy diets and lifestyle habits (abstaining from excessive alcohol and tobacco use) protect against cardiovascular disease (Willett et al., 2006). Conversely, a poor or inadequate diet promotes metabolic syndrome, a condition that includes low levels of high-density lipoprotein (HDL), high levels of triglycerides and glucose intolerance, which consequently increases the risk of diabetes (Willett et al., 2006). In cohorts of individuals living in New York City, adherence to a Mediterranean diet and participating in physical activity decreased AD risk, with individuals exhibiting high adherence to diet, and increased participation in physical exercise had a combined HR=0.65 compared to individuals who did not (Scarmeas et al., 2009).
Studies regarding nutrition and diet have predominantly examined antioxidants, vitamins, and the effect of different diets on AD risk. However, despite the overwhelming amount of research, roles for specific dietary habits, and more mechanistic data are needed to understand the relationship between diet and AD (N. Hu et al., 2013).
3.4. Tobacco and Alcohol Consumption
In many epidemiological studies, alcohol consumption and smoking tend to be evaluated within the same cohort or using the same questionnaire, primarily due to their effects on cardiovascular health, as well as assessment of a patient’s lifestyle patterns. The data for both cigarette smoke and alcohol consumption are relatively inconsistent in the field of AD (Graves et al., 1991). One potential reason for this inconsistency, in the case of smoking, is the competing risk of cigarette smokers with AD and death. In age-matched studies, cigarette smokers may represent a biased population pool and could account for the “healthiest” of smokers. Other individuals who were smokers may have had other risk factors associated with AD, and would have ultimately developed AD, but died from other factors before AD diagnosis was confirmed (Chang, Zhao, Lee, & Ganguli, 2012). An early meta-analysis suggested a potential protection of smoking, in which individuals with higher cigarette usage exhibited decreased AD (Graves et al., 1991), but reported no effect of alcohol consumption. Similar findings were reported in China, where second-hand smoke or otherwise passive smoking was associated with a RR=1.78 for dementia, and RR=2.28 for AD (Chen, 2012). Numerous epidemiological studies, as well as meta-analyses with pooled data sets, reported either a decrease or no change in risk of AD for cigarette smokers, and no change associated with alcohol consumption (Almeida, Hulse, Lawrence, & Flicker, 2002; Garcia, Ramon-Bou, & Porta, 2010; Hebert et al., 1992; Ilomaki, Jokanovic, Tan, & Lonnroos, 2015; Tyas, Koval, & Pederson, 2000; Tyas, Pederson, & Koval, 2000).
For alcohol consumption, it is considered that low to moderate consumption of alcohol (1–4 drinks in 2 weeks), may be beneficial (Panza et al., 2012; Piazza-Gardner, Gaffud, & Barry, 2013), but there is conflicting evidence on the potential beneficial effects of low consumption. Data from the Framingham offspring cohort suggest that consumption is inversely related to brain volume, and that even mild alcohol consumption may decrease brain volume (Paul et al., 2008; Solfrizzi et al., 2009). The type of alcohol being consumed also appears to be relevant (Panza et al., 2012; Piazza-Gardner et al., 2013). In several epidemiological studies, wine is shown to have antioxidant properties, thus reducing proinflammatory pathways and allowing a reduction of reactive oxygen species (W. J. Huang, Zhang, & Chen, 2016). In a large population study in Norway, individuals who drank more frequently, or abstained completely had higher HR ratios (1.30 and 1.45, respectively) for dementia and AD than those who drank infrequently (Langballe et al., 2015). Similar reports in another cohort were provided which showed that individuals who were heavy drinkers and who tended to drink hard liquor had a more rapid cognitive decline than those who drank wine or drank less frequently (Heymann et al., 2016).
Recent studies have attempted to provide mechanistic insights as to why these changes may occur. Specifically, by examining alcohol consumption or cigarette smoking and alteration of specific pathways involved in AD. Transcriptome analysis of microglia treated with ethanol revealed dysregulation in a number of genes in the phagosome Kyoto Encyclopedia of Genes and Genomes pathway; suggesting reduced uptake of Aβ42 by primary microglia (Kalinin et al., 2018). Inflammatory mechanisms suggest a similarity of cytokine pathways between alcoholism and AD, specifically that patients suffering from alcoholism have an increase in proinflammatory cytokines (Venkataraman, Kalk, Sewell, C, & Lingford-Hughes, 2017). Different components of cigarette smoke have been shown to increase Aβ aggregation, these components include polycyclic aromatic hydrocarbons such as naphthalene, and metal ions such as lead and cadmium (Wallin et al., 2017).
3.5. Air pollution
Air pollution is a complex mixture made up of sources which are considered natural occurring, as well as anthropogenic in origin. The environmental protection agency defines its composition and regulation based on essential elements in the National Ambient Air Quality Standards (NAAQS). These major components are considered to be sulfur dioxide, carbon monoxide, nitrogen dioxide, ozone, particular matter (PM 10 and PM 2.5) and lead (Bachmann, 2007; Daniel, 2013). While these are considered essential elements, they may vary in levels and composition depending on geographical region (Strosnider, Kennedy, Monti, & Yip, 2017). Despite this variation, epidemiological studies have associated a number of adverse health effects across multiple biological systems (Brunekreef & Holgate, 2002), with the primary targets being cardiovascular, respiratory and the nervous system (Brunekreef & Holgate, 2002; Curtis, Rea, Smith-Willis, Fenyves, & Pan, 2006). The actions of these air pollutants on the CNS are suggested to occur via an increase in neuroinflammation and the upregulation of proinflammatory pathways. These mechanisms have recently been reviewed in experimental models, and involve the activation of microglia, the subsequent increase of cytokines and reactive oxygen species in the brain, which ultimately is associated with death and damage to neurons (Jayaraj, Rodriguez, Wang, & Block, 2017).
Epidemiological studies examining the risk of air pollution consistently found a contribution to cognitive dysfunction in both children and adults (Power, Adar, Yanosky, & Weuve, 2016; Tonne, Elbaz, Beevers, & Singh-Manoux, 2014; Weuve et al., 2012). Connections between air pollution and AD biomarkers were first made in early studies of individuals living in Mexico City, Mexico, with postmortem analyses demonstrating significantly lower levels of Aβ42 and brain-derived neurotrophic factor (BDNF) in the CSF of highly exposed individuals relative to controls (Calderon-Garciduenas et al., 2016), as well as increased levels of proinflammatory markers (COX2) and Aβ42 in the hippocampus and the cortex (Calderon-Garciduenas et al., 2004). Recent epidemiological studies also support a role for air pollution and PM in increased risk of developing AD. In a case-control study in Taiwan, individuals with PM10 and ozone exposure had an increased risk of developing AD, with the highest tertile of PM10 exposure having an AOR =4.17 for AD, as well as the highest tertile of ozone exposure, AOR= 2.0 for dementia risk (Wu et al., 2015). Using data from the Women’s Health Initiative Memory Study (WHIMS) cohort, an additional study reported a significant association with PM2.5 exposure and cognitive dysfunction (Cacciottolo et al., 2017).
3.6. Pesticide Exposure
Pesticides are agents that are commonly used to control organisms that maybe harmful to the growth of crops, or in some cases to prevent the transmission of vector borne diseases (Aktar, Sengupta, & Chowdhury, 2009). There are many different types of pesticides, among them are insecticides (targeting insects), herbicides (targeting plants) and fungicides (targeting fungi) (Casida, 2009). In the case of insecticides, many of these compounds act on the nervous system, at the nerve synapse or axons, others have been found to act on the mitochondria, primary by complex inhibition (Casida, 2009). Due to their known actions on the nervous system, these pesticides have received attention as risk factors for neurological and neurodegenerative disease.
Epidemiological studies in vineyard workers with past exposure to insecticides and fungicides have associated exposure with deficits in cognitive performance (Baldi et al., 2001; Baldi et al., 2011; Baldi et al., 2003). One of the major challenges for most pesticide epidemiological studies is that typically the specific pesticide is not identified, with more general categories are used to define pesticides as a class. Furthermore, it is important to consider many of the confounders that are frequently presented with epidemiological studies of environmental exposures, which largely depend on self-reported incidences of exposure, are non-specific, and many are longitudinal studies, with potential data bias from inclusion of surviving or returning individuals.
In a longitudinal study in Manitoba Canada authors reported a RR= 4.35 for the development of AD following exposure to fumigants and defoliants (Tyas, Manfreda, Strain, & Montgomery, 2001). Similarly, residents of the agricultural community of Cache County, UT who resided in a neighborhood with exposure to both organophosphate and organochlorine insecticides, had an increased hazard ratio (HR) of 1.53 for AD in individuals with a history of exposure to organophosphates, and an increase HR of 1.49 for AD in those with organochlorine exposure (Hayden et al., 2010).
The organochlorine insecticide dichlorodiphenyltrichloroethane (DDT) has been implicated as a potential risk factor in AD, with a significant increase of p’p dichlorodiphenyldichloroethylene (DDE), the metabolite of DDT, first reported in postmortem brain samples from individuals with AD (Fleming, Mann, Bean, Briggle, & Sanchez-Ramos, 1994). These findings were expanded upon by Richardson and collaborators, which showed significantly higher levels of DDE in the serum of patients compared to their age-matched controls (Richardson et al., 2014; Richardson et al., 2009). Individuals within the highest tertile of serum DDE levels also had an OR= 4.18 for increased risk of AD (Richardson et al., 2014). Mechanistic data exploring the role of DDT on the amyloid pathway have also shown DDT exposure in vitro and in vivo increases APP expression and Aβ levels in a number of animal and cell models (D.C. German, 2018; Richardson et al., 2014). DDT has been identified to interact with voltage gated sodium channels, by delaying their closure and leading to a state of hyperexcitability of the cell (Casida, 2009; Costa, 2015), this mechanism of action of DDT may be involved in the increase of amyloidogenic proteins.
4.0. Gene Environment Interactions
The risk factors highlighted in this manuscript are not supported by enough evidence or data to independently be considered as causative for AD. Although APOE4 significantly increases risk, it cannot explain all cases nor why some individuals who are carriers never develop AD. The RR, HR and OR presented by the epidemiological evidence for the presented risk factors range from an HR of 1.45 (alcohol) to a RR of 2.96 (cholesterol). Data for general pesticide exposure and general air pollution is higher (RR 4.35 and 4.17, respectively), but these are based on only a few studies and require further validation. These factors alone cannot explain AD risk or susceptibility, however, a combination of lifestyle, environmental, and genetic factors provide stronger evidence.
Gene-environment interactions are classified as different effects of environmental exposures or lifestyle factors on disease risk in individuals with different genotypes (Baye, Abebe, & Wilke, 2011; Ottman, 1996). These phenomena are exceedingly relevant, yet understudied in a disease as complex as AD, as the disease presents in many complex forms, with arguably a multitude of different etiologies. In complex diseases such as late-onset AD, it is rare to identify single genetic risks, and most genetic findings present as modest responses (Bookman et al., 2011), with the exception of APOE. These observations point to other risk factors that are more likely involved or interact with this genotype (Baye et al., 2011). To study and understand the mechanisms by which the environment may interact with an individual’s genetic profile, one must have a foundational understanding of what these environmental risks are, as well as the predominant genetic risk factors. It has become even more evident that other risk factors also must be considered when examining an individual’s unique risk signature, which specifically include age, sex, and family history of AD.
There are two unique environmental factors that can modulate risk, the first is individual lifestyle factors, such as diet, exercise, smoking and alcohol consumption. Secondly, the environmental exposure component significantly contributes to AD risk. Together this defines the exposome concept, which states that a general external environment (climate, traffic, etc.), and a specific external environment (diet, physical activity) directly influence our internal environment to have an adverse or positive effect on our health (Siroux, Agier, & Slama, 2016). It is evident that AD is not a homogeneous disease that can be treated with one specific drug, but rather AD presents as a spectrum, with complex interactions between genetic, lifestyle and environmental factors (Au, Piers, & Lancashire, 2015; Neugroschl & Wang, 2011).
Gene-environment interactions have been identified for many lifestyle factors in AD. In a longitudinal study, examining physical activity in APOE4 carriers in older patients (75 years of age), individuals were assessed for dementia, and re-examined 4.5 years later. Individuals with higher physical activity had a decrease in dementia and AD. If individuals had both an APOEε4 allele and low physical activity, they were at a significantly higher risk of dementia, than with either factor alone (Luck et al., 2014). The Honolulu-Asia aging study is a longitudinal epidemiological study that examined risk factors and their relationship to dementia and cognitive decline in Japanese-American men (Gelber, Launer, & White, 2012). Here, the authors report that smoking increased the risk of dementia by an OR= 2.18 compared to non-smokers, which was not modified by APOE genotype (Tyas et al., 2003). In another study, the rs7179008 polymorphism of CHRNA7 (α7 nicotinic acetylcholine receptor) decreased risk in LOAD (AOR=0.29) and protect against increased risk of smoking in LOAD (Weng et al., 2016).
A relationship between APOE4 genotype and low to moderate alcohol consumption is reported by some groups. In a cohort study of individuals from the Washington Heights-Inwood Columbia Aging Study, researchers originally reported no association of alcohol consumption and AD risk (Luchsinger, Tang, Siddiqui, Shea, & Mayeux, 2004). However, when the results were stratified by APOE4 genotype, low to moderate consumption was associated lower risk of AD, but this was restricted to individuals without the APOEε4 allele, whereas individuals who were homozygous or heterozygous for the APOEε4 allele were not protected (Luchsinger et al., 2004). Similar findings were reported in the Framingham Heart Study Offspring Cohort, as low to moderate consumption of alcohol was associated with a greater decline in learning in memory in APOE4 carriers, and the inverse was associated with non-carriers (Downer, Zanjani, & Fardo, 2014). At least one study associated the combined risks of smoking, alcohol consumption and APOE4 genotype. In a cross-sectional study, authors examined the effects of heavy alcohol consumption and a history of heavy smoking (>1 pack a day) on AD risk; all three risk factors independently lowered AAO by 2–3 years, with one or more of these risks in conjunction with one another also significantly reduced age of onset by approximately 5 years. Furthermore, having all three risk factors decreased the age of onset by 10 years (mean age 67 years), compared to individuals without any of these risks (76.6 years) (Harwood et al., 2010). The type of alcohol consumed is also relevant and whether individuals are carriers of the APOE4 genotype, with each playing a significant role in determining risk (Panza et al., 2012; Piazza-Gardner et al., 2013).
Exposure to the pesticide DDT and air pollution, specifically exposure to PM, are also identified as potential risk factors in AD. Richardson et al. reported 3.8-fold higher levels of DDE in the serum of AD patients compared to controls. Furthermore, serum levels of DDE were correlated highly with brain levels of DDE in the same individuals. Individuals were then stratified into tertiles by serum DDE levels, and individuals in the highest DDE tertile had the lowest cognitive scores, and this association was modified by APOE genotype (Richardson et al., 2014). These findings have been replicated in a different cohort of individuals residing in Deli and regions of North India. In that study, individuals with AD had significantly higher levels of β-HCH, dieldrin, and p’p DDE, but this was not modified by APOE genotype (N. Singh et al., 2013; N. K. Singh et al., 2012), suggesting race/ethnicity or other factors may limit generalizability of this association. In the case of air pollution, Cacciottolo and colleagues examined a sex by gene by environment interaction and report an increased risk of females to the development of AD by air pollution that was most pronounced in APOE4 carriers (Cacciottolo et al., 2017). Furthermore, using the 5xFAD mice that carry a mutation in the five confirmed AD risk genes mice crossed with the target replacement APOE4 mice, the group provided mechanistic data demonstrating an increase in Aβ oligomers following exposure to PM, which was significantly increased by the APOE4 genotype (Cacciottolo et al., 2017).
Many studies have evaluated a role for gene-environment interactions for APOE, yet there are still avenues to be explored, especially with other gene variants, and modifiable risk factors. While investigators have examined metal exposure (aluminum, lead, and copper) or solvent exposure as potential risk factors for AD, most epidemiological studies produced mixed results (reviewed by (Santibanez, Bolumar, & Garcia, 2007)). However, these agents may act in conjunction with other risk factors mentioned in this review, and although may not significantly contribute to AD alone, could in the presence of specific gene variants, aging or other factors. As previously described, modifiable risk factors modulate pathways that have been linked with AD. For example, PM has been associated with an increase of proinflammatory mechanisms, yet the opportunity to investigate it with genes associated with immunity/inflammation remain understudied. Candidate genes for such studies may be the TREM2 variants which have been implicated in microglial dysfunction. Similarly, cardiovascular risk factors or lifestyle factors such as diet may interact with genes involved in cholesterol metabolism and cholesterol homeostasis as outlined in Figure 2.
Both modifiable and non-modifiable factors present in individuals in combination, and it is highly likely that an individual may have multiple risk factors that predispose them to disease. By taking a gene by environment approach and examining these risks in conjunction, we may reveal associations with AD that are far greater than if any of these present alone and could provide greater translational relevance for patients.
In 2018, there are 26 drugs that are in Phase III clinical trials for AD, and of these 17 are DMTs (J. Cummings, Lee, Ritter, & Zhong, 2018). The mode of action of these drugs is primarily aimed at a reduction in amyloid, with a mixture of immunotherapies, Bace-1 inhibitors, and anti-aggregation drugs (J. Cummings et al., 2018). Only one DMT targets tau (TRx0237) and two others act on alternative pathways (Hung & Fu, 2017). Many more drugs currently in Phase I and Phase II clinical trials, and many in Phase III that are not DMTs are reviewed extensively elsewhere (J. Cummings et al., 2018). From 2017 to 2018, 9 drugs have failed and exited Phase III clinical trials: including idalopirdine and intepirdine, (antagonists of the 5HT6 receptor), pioglitazone, AC-1204, aripiprazole, MK-8931, nilvadipine, and azreliragon. Most surprising was the failure of solanezumab which had been completing its third clinical trial (Murphy, 2018). Solanezumab is an immunotherapy targeting soluble Aβ, and while the drug succeeded in its mode of action, it produced no clinical benefit in patients (Honig et al., 2018). The failure of a promising immunotherapy points to the importance of exploring alternative therapeutic avenues, or other DMT options that can be used in conjunction with amyloid targeting drugs (Murphy, 2018).
One reason for the failure of these therapeutics may be due to the advanced stage of the disease before patients enter clinical trials. In 2017, 46.7 million Americans were diagnosed with “preclinical AD” (Brookmeyer et al., 2018). This presents an enormous challenge, but also a great opportunity to intervene at earlier timepoints of disease progression. Having a better understanding of those individuals at risk, could allow researchers to intervene with a much more appropriate therapeutic. A promising opportunity of therapeutic approach is the inclusion of patients or individuals into specific trials based on genetic information. One specific strategy utilizes an algorithm based on age, and genetic factors (APOE4, TOMM40) to categorize patients into risk severity, and the subsequent enrollment of those individuals in specific trials (Lutz et al., 2016). These approaches led to the recruitment for specific trials, such as the TOMORROW trials, which are aimed at preventing AD in a vulnerable population. This attempt to deliver a more personalized preventative approach to at-risk individuals is novel. Similarly, the Dominantly Inherited Alzheimer Network Trials Unit (DIAN-TU) are trials focus on slowing the progression of AD, but specifically target the autosomal dominant form of the disease (Bateman et al., 2017). DIAN-TU trials aim to do by genetic testing and incorporating multiple drugs based on their ability to slow the progression or prevent AD in the genetically vulnerable population (Bateman et al., 2017; Panza et al., 2018). However, lifestyle and environmental factors have not yet been incorporated into these emerging models.
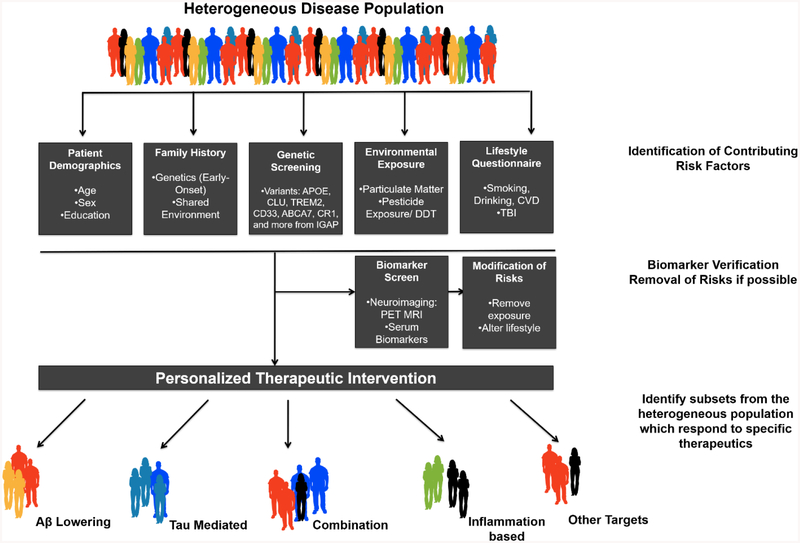
Figure 3 depicts a proposed personalized therapy approach for individuals with AD. In this model, we highlight the importance identifying multiple risk factors, and that they may contribute individually, and/or in some cases act in combination to increase susceptibility for AD (Carlsten et al., 2014). For instance, the APOE gene is consistently identified as a major risk factor, and presents itself as a distinct therapeutic target (Villeneuve, Brisson, Marchant, & Gaudet, 2014). It is likely that lifestyle or other environmental factors likely work in combination with the APOE gene, and may enhance these effects. Similarly, in a subset of individuals the ESR1 gene has been implicated with AD risk. SNPS in this gene are shown to significantly delay the onset of AD in females but not males (Janicki et al., 2014). As such the importance of identifying sex by gene interactions was demonstrated in a clinical trial, during which women with ESR1 gene polymorphisms were far more responsive to treatment with cholinesterase inhibitors (donepezil or rivastigmine) (Scacchi, Gambina, Broggio, & Corbo, 2014).
Figure 3: Proposed scenario for providing a personalized therapeutic approach to AD.
Patients would undergo a series of assessments to identify which factors increase risk of AD. These assessments include demographics, family history of disease, genetic screening for common variants and other, more rare polymorphisms, environmental exposure assessment, and lastly a lifestyle questionnaire would be administered. Following confirmation of risk factors, patients would be tested for disease biomarkers, via neuroimaging or serum/CSF markers. If possible, the risks that are modifiable such as environmental exposures and lifestyle habits would be modified to reduce contribution to disease progression. Based on these data patients would then be provided with a therapeutic option with a mode of action tailored to the mechanism most relevant to their individual risk profile.
By building and validating models as presented in Figure 3, targeted or personalized therapeutic strategies for individuals at risk for AD may be developed. This would include establishing a comprehensive patient history, which would include patient demographics (sex, age, education level), and identification of known genetic variants (sequencing analysis), as well as any family history of disease. Finally, a detailed description of other modifiable factors that may contribute to an individual’s risk or disease progression, such as alcohol consumption or CVD, and in the case of the environmental exposure this would include air pollution and pesticide exposure. Depending on the outcomes of these assessments, this would subsequently be followed up by disease biomarker analyses, which would include serum or neuroimaging analysis. In individuals who are at risk for specific exposures, such as pesticides may also be monitored for pesticide levels in urine, or serum levels as a measurement of current or past exposure. By utilizing these approaches and taking a personalized history of an individual’s risk history (exposure, lifestyle, genetic factors) this could allow for monitoring of these susceptible individuals before the onset of clinical symptoms, and potentially allow for earlier therapeutic intervention. In the case of individuals who may be exceedingly vulnerable due to some of these non-modifiable factors (i.e, age and genetic variants), the identification and removal of these factors may provide more favorable outcomes. It would also provide researchers and clinicians greater opportunity to monitor how these factors may contribute uniquely to the individual.
Examples of this procedure could be theoretically proposed for those individuals with a previous history of pesticide exposure who develop AD. By implementing this personalized approach, researchers could incorporate data from a multitude of risk factors outlined in Figure 3. In the pesticide example, an individual with the APOE4 gene variant may be more susceptible to the cognitive disruption produced by pesticide exposure, based on the evidence provided by previous work in the case of DDT (Richardson et al., 2014). Furthermore, the environmental exposure history, and serum measurements for pesticides would be particularly important for acquiring proper data and relevant information about patient exposure history. It should be noted that this process is not without challenges, as self-reported exposures would likely be necessary for newer, less persistent pesticides whose levels would indicate only recent exposure. In this scenario, patients would subsequently be monitored for buildup of amyloid using neuroimaging or other sources of biomarkers (i.e. serum and/or CSF), as pesticides have been implication in the upregulation of APP. The most appropriate option for these individuals would be therapeutic with a mode of action of decreasing amyloid, such as a β-secretase inhibitor or an amyloid beta immunotherapy.
6.0. Conclusion
The challenges that face researchers with a disease of such complexity as AD are in part due to the inability to identify specific groups of patients that exhibit similar needs for therapy. While the focus has largely remained on ameliorating pathological hallmarks, it also would be beneficial to understand differences among individuals in development of these pathologies. An understanding of converging pathways of disease would greatly aid in providing better therapeutic options for patients as well as increasing desired outcomes. AD is a disease which manifests as many intertwined factors, including genetic risk, environmental, and lifestyle which contribute to its different risk levels. Understanding these risks, and modifying them is the first step, and the second is better understanding how they converge on known pathways involved in AD pathology. The inclusion of both sexes and a comprehensive analyses of multiple risk factors (genetic or environmental, lifestyle) should become standard practice for diagnostics. To provide a more personalized therapeutic option for patients, all possible avenues of risk must be fully considered, as this may provide better understanding and allow for patients to receive drugs with a specific mode of action, which may be more efficacious.
Table 1:
Gene Variants associated with AD
| APP | Amyloid precursor protein |
| PSEN1 | Presenilin 1 |
| PSEN2 | Presenilin-2 |
| MAPT | Microtubule associated protein tau |
| TREM2 | Triggering receptor expressed on myeloid cells 2 |
| ABCA7 | ATP binding cassette subfamily A member 7 |
| BIN1 | Bridging integrator 1 |
| CD2AP | CD2 associated protein |
| CLU | Clusterin |
| CR1 | Complement receptor type 1 |
| EPHA1 | Ephrin type-A receptor 1 |
| MS4A6A | Membrane-spanning 4-domains, subfamily A, member 6A |
| PICALM | Phosphatidylinositol binding clathrin assembly protein |
| APOE | Apolipoprotein E |
| CD33 | CD33 molecule or sialic acid-binding Ig-like lectin 3 |
| CASS4 | Cas scaffold protein family member 4 |
| CELF2 | CUGBP elav-like family member 2 |
| FERMT2 | Fermitin family member 2 |
| HLA | Human leukocyte antigen |
| INPP5D | Inositol polyphosphate-5-phosphatase D |
| MEF2C | Myocyte enhancer factor 2C |
| NME8 | NME/NM23 family member 8 |
| PTK2B | Protein tyrosine kinase 2 beta |
| SLC24A4 | Solute carrier family 24 member 4 |
| SORL1 | Sortilin related receptor 1 |
| ZWCPW1 | Zinc finger CW-type and PWWP domain containing 1 |
Acknowledgements:
The authors gratefully acknowledge the support of NIH grants R01ES021800, R01ES026057, R01ES026067-S2, U01NS108956 and the Alan and Janice Woll Research Fund.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement: The authors declare that there are no conflicts of interest.
References
- 2018 Alzheimer’s disease facts and figures. (2018). Alzheimer’s & Dementia, 14(3), 367–429. doi: 10.1016/j.jalz.2018.02.001 [DOI] [Google Scholar]
- Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, … Wyss-Coray T (2000). Inflammation and Alzheimer’s disease. Neurobiol Aging, 21(3), 383–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aktar MW, Sengupta D, & Chowdhury A (2009). Impact of pesticides use in agriculture: their benefits and hazards. Interdiscip Toxicol, 2(1), 1–12. doi: 10.2478/v10102-009-0001-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida OP, Hulse GK, Lawrence D, & Flicker L (2002). Smoking as a risk factor for Alzheimer’s disease: contrasting evidence from a systematic review of case-control and cohort studies. Addiction, 97(1), 15–28. [DOI] [PubMed] [Google Scholar]
- Altmann A, Tian L, Henderson VW, Greicius MD, & Alzheimer’s Disease Neuroimaging Initiative, I. (2014). Sex modifies the APOE-related risk of developing Alzheimer disease. Ann Neurol, 75(4), 563–573. doi: 10.1002/ana.24135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand R, Gill KD, & Mahdi AA (2014). Therapeutics of Alzheimer’s disease: Past, present and future. Neuropharmacology, 76 Pt A, 27–50. doi: 10.1016/j.neuropharm.2013.07.004 [DOI] [PubMed] [Google Scholar]
- Andreeva TV, Lukiw WJ, & Rogaev EI (2017). Biological Basis for Amyloidogenesis in Alzheimer’s Disease. Biochemistry (Mosc), 82(2), 122–139. doi: 10.1134/S0006297917020043 [DOI] [PubMed] [Google Scholar]
- Aschenbrenner AJ, Balota DA, Gordon BA, Ratcliff R, & Morris JC (2016). A diffusion model analysis of episodic recognition in preclinical individuals with a family history for Alzheimer’s disease: The adult children study. Neuropsychology, 30(2), 225–238. doi: 10.1037/neu0000222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Au R, Piers RJ, & Lancashire L (2015). Back to the future: Alzheimer’s disease heterogeneity revisited. Alzheimers Dement (Amst), 1(3), 368–370. doi: 10.1016/j.dadm.2015.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austen B, Christodoulou G, & Terry JE (2002). Relation between cholesterol levels, statins and Alzheimer’s disease in the human population. J Nutr Health Aging, 6(6), 377–382. [PubMed] [Google Scholar]
- Avila-Munoz E, & Arias C (2014). When astrocytes become harmful: functional and inflammatory responses that contribute to Alzheimer’s disease. Ageing Res Rev, 18, 29–40. doi: 10.1016/j.arr.2014.07.004 [DOI] [PubMed] [Google Scholar]
- Bachmann J (2007). Will the circle be unbroken: a history of the U.S. National Ambient Air Quality Standards. J Air Waste Manag Assoc, 57(6), 652–697. [DOI] [PubMed] [Google Scholar]
- Baglietto-Vargas D, Shi J, Yaeger DM, Ager R, & LaFerla FM (2016). Diabetes and Alzheimer’s disease crosstalk. Neurosci Biobehav Rev, 64, 272–287. doi: 10.1016/j.neubiorev.2016.03.005 [DOI] [PubMed] [Google Scholar]
- Baldi I, Filleul L, Mohammed-Brahim B, Fabrigoule C, Dartigues JF, Schwall S, … Brochard P (2001). Neuropsychologic effects of long-term exposure to pesticides: results from the French Phytoner study. Environ Health Perspect, 109(8), 839–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldi I, Gruber A, Rondeau V, Lebailly P, Brochard P, & Fabrigoule C (2011). Neurobehavioral effects of long-term exposure to pesticides: results from the 4-year follow-up of the PHYTONER study. Occup Environ Med, 68(2), 108–115. doi: 10.1136/oem.2009.047811 [DOI] [PubMed] [Google Scholar]
- Baldi I, Lebailly P, Mohammed-Brahim B, Letenneur L, Dartigues JF, & Brochard P (2003). Neurodegenerative diseases and exposure to pesticides in the elderly. Am J Epidemiol, 157(5), 409–414. [DOI] [PubMed] [Google Scholar]
- Ballatore C, Lee VM, & Trojanowski JQ (2007). Tau-mediated neurodegeneration in Alzheimer’s disease and related disorders. Nat Rev Neurosci, 8(9), 663–672. doi: 10.1038/nrn2194 [DOI] [PubMed] [Google Scholar]
- Bateman RJ, Benzinger TL, Berry S, Clifford DB, Duggan C, Fagan AM, … Network, D.-T. P. C. f. t. D. I. A. (2017). The DIAN-TU Next Generation Alzheimer’s prevention trial: Adaptive design and disease progression model. Alzheimers Dement, 13(1), 8–19. doi: 10.1016/j.jalz.2016.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baye TM, Abebe T, & Wilke RA (2011). Genotype-environment interactions and their translational implications. Per Med, 8(1), 59–70. doi: 10.2217/pme.10.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertram L, & Tanzi RE (2012). The genetics of Alzheimer’s disease. Prog Mol Biol Transl Sci, 107, 79–100. doi: 10.1016/B978-0-12-385883-2.00008-4 [DOI] [PubMed] [Google Scholar]
- Bird TD (1993). Early-Onset Familial Alzheimer Disease. In Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Stephens K, & Amemiya A (Eds.), GeneReviews((R)) Seattle (WA). [Google Scholar]
- Boezen HM, Vonk JM, van der Zee SC, Gerritsen J, Hoek G, Brunekreef B, … Postma DS (2005). Susceptibility to air pollution in elderly males and females. Eur Respir J, 25(6), 1018–1024. doi: 10.1183/09031936.05.00076104 [DOI] [PubMed] [Google Scholar]
- Bookman EB, McAllister K, Gillanders E, Wanke K, Balshaw D, Rutter J, … participants, N. I. H. G. I. W. (2011). Gene-environment interplay in common complex diseases: forging an integrative model-recommendations from an NIH workshop. Genet Epidemiol, 35(4), 217–225. doi: 10.1002/gepi.20571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyles JK, Pitas RE, Wilson E, Mahley RW, & Taylor JM (1985). Apolipoprotein E associated with astrocytic glia of the central nervous system and with nonmyelinating glia of the peripheral nervous system. J Clin Invest, 76(4), 1501–1513. doi: 10.1172/JCI112130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brann DW, Dhandapani K, Wakade C, Mahesh VB, & Khan MM (2007). Neurotrophic and neuroprotective actions of estrogen: basic mechanisms and clinical implications. Steroids, 72(5), 381–405. doi: 10.1016/j.steroids.2007.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookmeyer R, & Abdalla N (2018). Estimation of lifetime risks of Alzheimer’s disease dementia using biomarkers for preclinical disease. Alzheimers Dement, 14(8), 981–988. doi: 10.1016/j.jalz.2018.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookmeyer R, Abdalla N, Kawas CH, & Corrada MM (2018). Forecasting the prevalence of preclinical and clinical Alzheimer’s disease in the United States. Alzheimers Dement, 14(2), 121–129. doi: 10.1016/j.jalz.2017.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunekreef B, & Holgate ST (2002). Air pollution and health. Lancet, 360(9341), 1233–1242. doi: 10.1016/S0140-6736(02)11274-8 [DOI] [PubMed] [Google Scholar]
- Cacciottolo M, Wang X, Driscoll I, Woodward N, Saffari A, Reyes J, … Chen JC (2017). Particulate air pollutants, APOE alleles and their contributions to cognitive impairment in older women and to amyloidogenesis in experimental models. Transl Psychiatry, 7(1), e1022. doi: 10.1038/tp.2016.280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderon-Garciduenas L, Avila-Ramirez J, Calderon-Garciduenas A, Gonzalez-Heredia T, Acuna-Ayala H, Chao CK, … Lachmann I (2016). Cerebrospinal Fluid Biomarkers in Highly Exposed PM2.5 Urbanites: The Risk of Alzheimer’s and Parkinson’s Diseases in Young Mexico City Residents. J Alzheimers Dis, 54(2), 597–613. doi: 10.3233/JAD-160472 [DOI] [PubMed] [Google Scholar]
- Calderon-Garciduenas L, Reed W, Maronpot RR, Henriquez-Roldan C, Delgado-Chavez R, Calderon-Garciduenas A, … Swenberg JA (2004). Brain inflammation and Alzheimer’s-like pathology in individuals exposed to severe air pollution. Toxicol Pathol, 32(6), 650–658. doi: 10.1080/01926230490520232 [DOI] [PubMed] [Google Scholar]
- Carlsten C, Brauer M, Brinkman F, Brook J, Daley D, McNagny K, … Denburg J (2014). Genes, the environment and personalized medicine: We need to harness both environmental and genetic data to maximize personal and population health. EMBO Rep, 15(7), 736–739. doi: 10.15252/embr.201438480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casida JE (2009). Pest toxicology: the primary mechanisms of pesticide action. Chem Res Toxicol, 22(4), 609–619. doi: 10.1021/tx8004949 [DOI] [PubMed] [Google Scholar]
- Chang CC, Zhao Y, Lee CW, & Ganguli M (2012). Smoking, death, and Alzheimer disease: a case of competing risks. Alzheimer Dis Assoc Disord, 26(4), 300–306. doi: 10.1097/WAD.0b013e3182420b6e [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R (2012). Association of environmental tobacco smoke with dementia and Alzheimer’s disease among never smokers. Alzheimers Dement, 8(6), 590–595. doi: 10.1016/j.jalz.2011.09.231 [DOI] [PubMed] [Google Scholar]
- Cheng G, Huang C, Deng H, & Wang H (2012). Diabetes as a risk factor for dementia and mild cognitive impairment: a meta-analysis of longitudinal studies. Intern Med J, 42(5), 484–491. doi: 10.1111/j.1445-5994.2012.02758.x [DOI] [PubMed] [Google Scholar]
- Cibickova L (2011). Statins and their influence on brain cholesterol. J Clin Lipidol, 5(5), 373–379. doi: 10.1016/j.jacl.2011.06.007 [DOI] [PubMed] [Google Scholar]
- Colonna M, & Wang Y (2016). TREM2 variants: new keys to decipher Alzheimer disease pathogenesis. Nat Rev Neurosci, 17(4), 201–207. doi: 10.1038/nrn.2016.7 [DOI] [PubMed] [Google Scholar]
- Costa LG (2015). The neurotoxicity of organochlorine and pyrethroid pesticides. Handb Clin Neurol, 131, 135–148. doi: 10.1016/B978-0-444-62627-1.00009-3 [DOI] [PubMed] [Google Scholar]
- Crane PK, Gibbons LE, Dams-O’Connor K, Trittschuh E, Leverenz JB, Keene CD, … Larson EB (2016). Association of Traumatic Brain Injury With Late-Life Neurodegenerative Conditions and Neuropathologic Findings. JAMA Neurol, 73(9), 1062–1069. doi: 10.1001/jamaneurol.2016.1948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruchaga C, Del-Aguila JL, Saef B, Black K, Fernandez MV, Budde J, … Harari O (2018). Polygenic risk score of sporadic late-onset Alzheimer’s disease reveals a shared architecture with the familial and early-onset forms. Alzheimers Dement, 14(2), 205–214. doi: 10.1016/j.jalz.2017.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings J, Lee G, Ritter A, & Zhong K (2018). Alzheimer’s disease drug development pipeline: 2018. Alzheimers Dement (N Y), 4, 195–214. doi: 10.1016/j.trci.2018.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings JL, Morstorf T, & Zhong K (2014). Alzheimer’s disease drug-development pipeline: few candidates, frequent failures. Alzheimers Res Ther, 6(4), 37. doi: 10.1186/alzrt269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cupples LA, Farrer LA, Sadovnick AD, Relkin N, Whitehouse P, & Green RC (2004). Estimating risk curves for first-degree relatives of patients with Alzheimer’s disease: the REVEAL study. Genet Med, 6(4), 192–196. doi:10.109701.GIM.0000132679.92238.58 [DOI] [PubMed] [Google Scholar]
- Curtis L, Rea W, Smith-Willis P, Fenyves E, & Pan Y (2006). Adverse health effects of outdoor air pollutants. Environ Int, 32(6), 815–830. doi: 10.1016/j.envint.2006.03.012 [DOI] [PubMed] [Google Scholar]
- German DC, R. JR, Eid A, Buckley B, White CL. (2018, 2018). DT exposure increases amyloid precursor protein levels and amyloid-beta pathology: Mechanistic links to Alzheimer’s disease risk.. Paper presented at the Neuroscience Meeting 2018, San Diego, CA. [Google Scholar]
- Dai MH, Zheng H, Zeng LD, & Zhang Y (2018). The genes associated with early-onset Alzheimer’s disease. Oncotarget, 9(19), 15132–15143. doi: 10.18632/oncotarget.23738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dams-O’Connor K, Gibbons LE, Bowen JD, McCurry SM, Larson EB, & Crane PK (2013). Risk for late-life re-injury, dementia and death among individuals with traumatic brain injury: a population-based study. J Neurol Neurosurg Psychiatry, 84(2), 177–182. doi: 10.1136/jnnp-2012-303938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel LC, Terry Gordon. (2013). Air Pollution In Curtis P Klassen D (Ed.), Casarett and Doull’s Toxicology, The Basic Science of Poisons (8th ed.): McGraw Hill. [Google Scholar]
- De-Paula VJ, Radanovic M, Diniz BS, & Forlenza OV (2012). Alzheimer’s Disease In Harris JR (Ed.), Protein Aggregation and Fibrillogenesis in Cerebral and Systemic Amyloid Disease (pp. 329–352). Dordrecht: Springer Netherlands. [Google Scholar]
- de la Monte SM (2014). Type 3 diabetes is sporadic Alzheimers disease: mini-review. Eur Neuropsychopharmacol, 24(12), 1954–1960. doi: 10.1016/j.euroneuro.2014.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djelti F, Braudeau J, Hudry E, Dhenain M, Varin J, Bieche I, … Cartier N (2015). CYP46A1 inhibition, brain cholesterol accumulation and neurodegeneration pave the way for Alzheimer’s disease. Brain, 138(Pt 8), 2383–2398. doi: 10.1093/brain/awv166 [DOI] [PubMed] [Google Scholar]
- Donix M, Burggren AC, Suthana NA, Siddarth P, Ekstrom AD, Krupa AK, … Bookheimer SY (2010). Family history of Alzheimer’s disease and hippocampal structure in healthy people. Am J Psychiatry, 167(11), 1399–1406. doi: 10.1176/appi.ajp.2010.09111575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dos Santos LR, Pimassoni LHS, Sena GGS, Camporez D, Belcavello L, Trancozo M, … de Paula F (2017). Validating GWAS Variants from Microglial Genes Implicated in Alzheimer’s Disease. J Mol Neurosci, 62(2), 215–221. doi: 10.1007/s12031-017-0928-7 [DOI] [PubMed] [Google Scholar]
- Downer B, Zanjani F, & Fardo DW (2014). The relationship between midlife and late life alcohol consumption, APOE e4 and the decline in learning and memory among older adults. Alcohol Alcohol, 49(1), 17–22. doi: 10.1093/alcalc/agt144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards G 3rd, Moreno-Gonzalez I, & Soto C (2017). Amyloid-beta and tau pathology following repetitive mild traumatic brain injury. Biochem Biophys Res Commun, 483(4), 1137–1142. doi: 10.1016/j.bbrc.2016.07.123 [DOI] [PubMed] [Google Scholar]
- Efthymiou AG, & Goate AM (2017). Late onset Alzheimer’s disease genetics implicates microglial pathways in disease risk. Mol Neurodegener, 12(1), 43. doi: 10.1186/s13024-017-0184-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fann JR, Ribe AR, Pedersen HS, Fenger-Gron M, Christensen J, Benros ME, & Vestergaard M (2018). Long-term risk of dementia among people with traumatic brain injury in Denmark: a population-based observational cohort study. Lancet Psychiatry, 5(5), 424–431. doi: 10.1016/S2215-0366(18)30065-8 [DOI] [PubMed] [Google Scholar]
- Farlow MR, Graham SM, & Alva G (2008). Memantine for the treatment of Alzheimer’s disease: tolerability and safety data from clinical trials. Drug Saf, 31(7), 577–585. [DOI] [PubMed] [Google Scholar]
- Farrer LA, O’Sullivan DM, Cupples LA, Growdon JH, & Myers RH (1989). Assessment of genetic risk for Alzheimer’s disease among first-degree relatives. Ann Neurol, 25(5), 485–493. doi: 10.1002/ana.410250511 [DOI] [PubMed] [Google Scholar]
- Fassbender K, Simons M, Bergmann C, Stroick M, Lutjohann D, Keller P, … Hartmann T (2001). Simvastatin strongly reduces levels of Alzheimer’s disease beta-amyloid peptides Abeta 42 and Abeta 40 in vitro and in vivo. Proc Natl Acad Sci U S A, 98(10), 5856–5861. doi: 10.1073/pnas.081620098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming L, Mann JB, Bean J, Briggle T, & Sanchez-Ramos JR (1994). Parkinson’s disease and brain levels of organochlorine pesticides. Ann Neurol, 36(1), 100–103. doi: 10.1002/ana.410360119 [DOI] [PubMed] [Google Scholar]
- Fu Y, Hsiao JH, Paxinos G, Halliday GM, & Kim WS (2016). ABCA7 Mediates Phagocytic Clearance of Amyloid-beta in the Brain. J Alzheimers Dis, 54(2), 569–584. doi: 10.3233/JAD-160456 [DOI] [PubMed] [Google Scholar]
- Ganske S, Haussmann R, Gruschwitz A, Werner A, Osterrath A, Baumgaertel J, … Donix M (2016). Family History of Alzheimer’s Disease and Cortical Thickness in Patients With Dementia. Am J Alzheimers Dis Other Demen, 31(5), 450–456. doi: 10.1177/1533317516653827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia AM, Ramon-Bou N, & Porta M (2010). Isolated and joint effects of tobacco and alcohol consumption on risk of Alzheimer’s disease. J Alzheimers Dis, 20(2), 577–586. doi: 10.3233/JAD-2010-1399 [DOI] [PubMed] [Google Scholar]
- Gee GC, & Payne-Sturges DC (2004). Environmental health disparities: a framework integrating psychosocial and environmental concepts. Environ Health Perspect, 112(17), 1645–1653. doi: 10.1289/ehp.7074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelber RP, Launer LJ, & White LR (2012). The Honolulu-Asia Aging Study: epidemiologic and neuropathologic research on cognitive impairment. Curr Alzheimer Res, 9(6), 664–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs RB (2010). Estrogen therapy and cognition: a review of the cholinergic hypothesis. Endocr Rev, 31(2), 224–253. doi: 10.1210/er.2009-0036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs RB, & Aggarwal P (1998). Estrogen and basal forebrain cholinergic neurons: implications for brain aging and Alzheimer’s disease-related cognitive decline. Horm Behav, 34(2), 98–111. doi: 10.1006/hbeh.1998.1451 [DOI] [PubMed] [Google Scholar]
- Gilbert M, Snyder C, Corcoran C, Norton MC, Lyketsos CG, & Tschanz JT (2014). The association of traumatic brain injury with rate of progression of cognitive and functional impairment in a population-based cohort of Alzheimer’s disease: the Cache County Dementia Progression Study. Int Psychogeriatr, 26(10), 1593–1601. doi: 10.1017/S1041610214000842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillies GE, & McArthur S (2010). Estrogen actions in the brain and the basis for differential action in men and women: a case for sex-specific medicines. Pharmacol Rev, 62(2), 155–198. doi: 10.1124/pr.109.002071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glymour MM, & Manly JJ (2008). Lifecourse social conditions and racial and ethnic patterns of cognitive aging. Neuropsychol Rev, 18(3), 223–254. doi: 10.1007/s11065-008-9064-z [DOI] [PubMed] [Google Scholar]
- Graves AB, van Duijn CM, Chandra V, Fratiglioni L, Heyman A, Jorm AF, … et al. (1991). Alcohol and tobacco consumption as risk factors for Alzheimer’s disease: a collaborative re-analysis of case-control studies. EURODEM Risk Factors Research Group. Int J Epidemiol, 20 Suppl 2, S48–57. [DOI] [PubMed] [Google Scholar]
- Green PS, & Simpkins JW (2000). Neuroprotective effects of estrogens: potential mechanisms of action. Int J Dev Neurosci, 18(4–5), 347–358. [DOI] [PubMed] [Google Scholar]
- Griciuc A, Serrano-Pozo A, Parrado AR, Lesinski AN, Asselin CN, Mullin K, … Tanzi RE (2013). Alzheimer’s disease risk gene CD33 inhibits microglial uptake of amyloid beta. Neuron, 78(4), 631–643. doi: 10.1016/j.neuron.2013.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudala K, Bansal D, Schifano F, & Bhansali A (2013). Diabetes mellitus and risk of dementia: A meta-analysis of prospective observational studies. J Diabetes Investig, 4(6), 640–650. doi: 10.1111/jdi.12087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerreiro R, Wojtas A, Bras J, Carrasquillo M, Rogaeva E, Majounie E, … Alzheimer Genetic Analysis, G. (2013). TREM2 variants in Alzheimer’s disease. N Engl J Med, 368(2), 117–127. doi: 10.1056/NEJMoa1211851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z, Cupples LA, Kurz A, Auerbach SH, Volicer L, Chui H, … Farrer LA (2000). Head injury and the risk of AD in the MIRAGE study. Neurology, 54(6), 1316–1323. [DOI] [PubMed] [Google Scholar]
- Gurland BJ, Wilder DE, Lantigua R, Stern Y, Chen J, Killeffer EH, & Mayeux R (1999). Rates of dementia in three ethnoracial groups. Int J Geriatr Psychiatry, 14(6), 481–493. [PubMed] [Google Scholar]
- Han Z, Qu J, Zhao J, & Zou X (2018). Analyzing 74,248 Samples Confirms the Association Between CLU rs11136000 Polymorphism and Alzheimer’s Disease in Caucasian But Not Chinese population. Sci Rep, 8(1), 11062. doi: 10.1038/s41598-018-29450-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartl D, May P, Gu W, Mayhaus M, Pichler S, Spaniol C, … Aesg. (2018). A rare loss-of-function variant of ADAM17 is associated with late-onset familial Alzheimer disease. Mol Psychiatry. doi: 10.1038/s41380-018-0091-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwood DG, Kalechstein A, Barker WW, Strauman S, George-Hyslop P St, Iglesias C, … Duara R (2010). The effect of alcohol and tobacco consumption, and apolipoprotein E genotype, on the age of onset in Alzheimer’s disease. Int J Geriatr Psychiatry, 25(5), 511–518. doi: 10.1002/gps.2372 [DOI] [PubMed] [Google Scholar]
- Hayden KM, Norton MC, Darcey D, Ostbye T, Zandi PP, Breitner JC, … Cache County Study, I. (2010). Occupational exposure to pesticides increases the risk of incident AD: the Cache County study. Neurology, 74(19), 1524–1530. doi: 10.1212/WNL.0b013e3181dd4423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden KM, Zandi PP, Lyketsos CG, Khachaturian AS, Bastian LA, Charoonruk G, … Cache County, I. (2006). Vascular risk factors for incident Alzheimer disease and vascular dementia: the Cache County study. Alzheimer Dis Assoc Disord, 20(2), 93–100. doi: 10.1097/01.wad.0000213814.43047.86 [DOI] [PubMed] [Google Scholar]
- Hazlett KE, Figueroa CM, & Nielson KA (2015). Executive functioning and risk for Alzheimer’s disease in the cognitively intact: Family history predicts Wisconsin Card Sorting Test performance. Neuropsychology, 29(4), 582–591. doi: 10.1037/neu0000181 [DOI] [PubMed] [Google Scholar]
- Hebert LE, Bienias JL, Aggarwal NT, Wilson RS, Bennett DA, Shah RC, & Evans DA (2010). Change in risk of Alzheimer disease over time. Neurology, 75(9), 786–791. doi: 10.1212/WNL.0b013e3181f0754f [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert LE, Scherr PA, Beckett LA, Funkenstein HH, Albert MS, Chown MJ, & Evans DA (1992). Relation of smoking and alcohol consumption to incident Alzheimer’s disease. Am J Epidemiol, 135(4), 347–355. [DOI] [PubMed] [Google Scholar]
- Hebert LE, Weuve J, Scherr PA, & Evans DA (2013). Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology, 80(19), 1778–1783. doi: 10.1212/WNL.0b013e31828726f5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson VW (2014). Alzheimer’s disease: review of hormone therapy trials and implications for treatment and prevention after menopause. J Steroid Biochem Mol Biol, 142, 99–106. doi: 10.1016/j.jsbmb.2013.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heneka MT, Carson MJ, El Khoury J, Landreth GE, Brosseron F, Feinstein DL, … Kummer MP (2015). Neuroinflammation in Alzheimer’s disease. Lancet Neurol, 14(4), 388–405. doi: 10.1016/S1474-4422(15)70016-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heymann D, Stern Y, Cosentino S, Tatarina-Nulman O, Dorrejo JN, & Gu Y (2016). The Association Between Alcohol Use and the Progression of Alzheimer’s Disease. Curr Alzheimer Res, 13(12), 1356–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingworth P, Harold D, Sims R, Gerrish A, Lambert JC, Carrasquillo MM, … Williams J (2011). Common variants at ABCA7, MS4A6A/MS4A4E, EPHA1, CD33 and CD2AP are associated with Alzheimer’s disease. Nat Genet, 43(5), 429–435. doi: 10.1038/ng.803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honea RA, Swerdlow RH, Vidoni ED, & Burns JM (2011). Progressive regional atrophy in normal adults with a maternal history of Alzheimer disease. Neurology, 76(9), 822–829. doi: 10.1212/WNL.0b013e31820e7b74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honea RA, Swerdlow RH, Vidoni ED, Goodwin J, & Burns JM (2010). Reduced gray matter volume in normal adults with a maternal family history of Alzheimer disease. Neurology, 74(2), 113–120. doi: 10.1212/WNL.0b013e3181c918cb [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honea RA, Vidoni ED, Swerdlow RH, Burns JM, & Alzheimer’s Disease Neuroimaging, I. (2012). Maternal family history is associated with Alzheimer’s disease biomarkers. J Alzheimers Dis, 31(3), 659–668. doi: 10.3233/JAD-2012-120676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honig LS, Vellas B, Woodward M, Boada M, Bullock R, Borrie M, … Siemers E (2018). Trial of Solanezumab for Mild Dementia Due to Alzheimer’s Disease. N Engl J Med, 378(4), 321–330. doi: 10.1056/NEJMoa1705971 [DOI] [PubMed] [Google Scholar]
- Hoyer S (2004). Glucose metabolism and insulin receptor signal transduction in Alzheimer disease. Eur J Pharmacol, 490(1–3), 115–125. doi: 10.1016/j.ejphar.2004.02.049 [DOI] [PubMed] [Google Scholar]
- Hu N, Yu JT, Tan L, Wang YL, Sun L, & Tan L (2013). Nutrition and the risk of Alzheimer’s disease. Biomed Res Int, 2013, 524820. doi: 10.1155/2013/524820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W, Tung YC, Zhang Y, Liu F, & Iqbal K (2018). Involvement of Activation of Asparaginyl Endopeptidase in Tau Hyperphosphorylation in Repetitive Mild Traumatic Brain Injury. J Alzheimers Dis, 64(3), 709–722. doi: 10.3233/JAD-180177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua X, Hibar DP, Lee S, Toga AW, Jack CR Jr., Weiner MW, … Alzheimer’s Disease Neuroimaging, I. (2010). Sex and age differences in atrophic rates: an ADNI study with n=1368 MRI scans. Neurobiol Aging, 31(8), 1463–1480. doi: 10.1016/j.neurobiolaging.2010.04.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CC, Chung CM, Leu HB, Lin LY, Chiu CC, Hsu CY, … Chan WL (2014). Diabetes mellitus and the risk of Alzheimer’s disease: a nationwide population-based study. PLoS One, 9(1), e87095. doi: 10.1371/journal.pone.0087095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang WJ, Zhang X, & Chen WW (2016). Association between alcohol and Alzheimer’s disease. Exp Ther Med, 12(3), 1247–1250. doi: 10.3892/etm.2016.3455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YA, Zhou B, Wernig M, & Sudhof TC (2017). ApoE2, ApoE3, and ApoE4 Differentially Stimulate APP Transcription and Abeta Secretion. Cell, 168(3), 427–441 e421. doi: 10.1016/j.cell.2016.12.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung SY, & Fu WM (2017). Drug candidates in clinical trials for Alzheimer’s disease. J Biomed Sci, 24(1), 47. doi: 10.1186/s12929-017-0355-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilomaki J, Jokanovic N, Tan EC, & Lonnroos E (2015). Alcohol Consumption, Dementia and Cognitive Decline: An Overview of Systematic Reviews. Curr Clin Pharmacol, 10(3), 204–212. [DOI] [PubMed] [Google Scholar]
- International Diabetes Federation, B., Belgium: (2015). International Diabetes Federation IDF diabetes atlas, Seventh edition. [Google Scholar]
- Iqbal K, Liu F, Gong CX, & Grundke-Iqbal I (2010). Tau in Alzheimer disease and related tauopathies. Curr Alzheimer Res, 7(8), 656–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR Jr., Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, … Trojanowski JQ (2010). Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol, 9(1), 119–128. doi: 10.1016/S1474-4422(09)70299-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janicki SC, Park N, Cheng R, Clark LN, Lee JH, & Schupf N (2014). Estrogen receptor alpha variants affect age at onset of Alzheimer’s disease in a multiethnic female cohort. Dement Geriatr Cogn Disord, 38(3–4), 200–213. doi: 10.1159/000355559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaraj RL, Rodriguez EA, Wang Y, & Block ML (2017). Outdoor Ambient Air Pollution and Neurodegenerative Diseases: the Neuroinflammation Hypothesis. Curr Environ Health Rep, 4(2), 166–179. doi: 10.1007/s40572-017-0142-3 [DOI] [PubMed] [Google Scholar]
- Jellinger KA, Paulus W, Wrocklage C, & Litvan I (2001). Traumatic brain injury as a risk factor for Alzheimer disease. Comparison of two retrospective autopsy cohorts with evaluation of ApoE genotype. BMC Neurol, 1, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin P, Pan Y, Pan Z, Xu J, Lin M, Sun Z, … Xu M (2018). Alzheimer-like brain metabolic and structural features in cholesterol-fed rabbit detected by magnetic resonance imaging. Lipids Health Dis, 17(1), 61. doi: 10.1186/s12944-018-0705-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin SC, Benitez BA, Karch CM, Cooper B, Skorupa T, Carrell D, … Cruchaga C (2014). Coding variants in TREM2 increase risk for Alzheimer’s disease. Hum Mol Genet, 23(21), 5838–5846. doi: 10.1093/hmg/ddu277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson T, Stefansson H, Steinberg S, Jonsdottir I, Jonsson PV, Snaedal J, … Stefansson K (2013). Variant of TREM2 associated with the risk of Alzheimer’s disease. N Engl J Med, 368(2), 107–116. doi: 10.1056/NEJMoa1211103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalinin S, Gonzalez-Prieto M, Scheiblich H, Lisi L, Kusumo H, Heneka MT, … Feinstein DL (2018). Transcriptome analysis of alcohol-treated microglia reveals downregulation of beta amyloid phagocytosis. J Neuroinflammation, 15(1), 141. doi: 10.1186/s12974-018-1184-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawas C, Gray S, Brookmeyer R, Fozard J, & Zonderman A (2000). Age-specific incidence rates of Alzheimer’s disease: the Baltimore Longitudinal Study of Aging. Neurology, 54(11), 2072–2077. [DOI] [PubMed] [Google Scholar]
- Kerti L, Witte AV, Winkler A, Grittner U, Rujescu D, & Floel A (2013). Higher glucose levels associated with lower memory and reduced hippocampal microstructure. Neurology, 81(20), 1746–1752. doi: 10.1212/01.wnl.0000435561.00234.ee [DOI] [PubMed] [Google Scholar]
- Kim J, Basak JM, & Holtzman DM (2009). The role of apolipoprotein E in Alzheimer’s disease. Neuron, 63(3), 287–303. doi: 10.1016/j.neuron.2009.06.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kivipelto M, Helkala EL, Laakso MP, Hanninen T, Hallikainen M, Alhainen K, … Nissinen A (2001). Midlife vascular risk factors and Alzheimer’s disease in later life: longitudinal, population based study. BMJ, 322(7300), 1447–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokiko-Cochran ON, & Godbout JP (2018). The Inflammatory Continuum of Traumatic Brain Injury and Alzheimer’s Disease. Front Immunol, 9, 672. doi: 10.3389/fimmu.2018.00672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koran MEI, Wagener M, Hohman TJ, & Alzheimer’s Neuroimaging, I. (2017). Sex differences in the association between AD biomarkers and cognitive decline. Brain Imaging Behav, 11(1), 205–213. doi: 10.1007/s11682-016-9523-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasemann S, Madore C, Cialic R, Baufeld C, Calcagno N, El Fatimy R, … Butovsky O (2017). The TREM2-APOE Pathway Drives the Transcriptional Phenotype of Dysfunctional Microglia in Neurodegenerative Diseases. Immunity, 47(3), 566–581 e569. doi: 10.1016/j.immuni.2017.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Rezaei-Ghaleh N, Terwel D, Thal DR, Richard M, Hoch M, … Walter J (2011). Extracellular phosphorylation of the amyloid beta-peptide promotes formation of toxic aggregates during the pathogenesis of Alzheimer’s disease. EMBO J, 30(11), 2255–2265. doi: 10.1038/emboj.2011.138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurata T, Miyazaki K, Kozuki M, Morimoto N, Ohta Y, Ikeda Y, & Abe K (2012). Atorvastatin and pitavastatin reduce senile plaques and inflammatory responses in a mouse model of Alzheimer’s disease. Neurol Res, 34(6), 601–610. doi: 10.1179/1743132812Y.0000000054 [DOI] [PubMed] [Google Scholar]
- Kurata T, Miyazaki K, Kozuki M, Panin VL, Morimoto N, Ohta Y, … Abe K (2011). Atorvastatin and pitavastatin improve cognitive function and reduce senile plaque and phosphorylated tau in aged APP mice. Brain Res, 1371, 161–170. doi: 10.1016/j.brainres.2010.11.067 [DOI] [PubMed] [Google Scholar]
- Lacour A, Espinosa A, Louwersheimer E, Heilmann S, Hernandez I, Wolfsgruber S, … Ruiz A (2017). Genome-wide significant risk factors for Alzheimer’s disease: role in progression to dementia due to Alzheimer’s disease among subjects with mild cognitive impairment. Mol Psychiatry, 22(1), 153–160. doi: 10.1038/mp.2016.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaDu MJ, Falduto MT, Manelli AM, Reardon CA, Getz GS, & Frail DE (1994). Isoform-specific binding of apolipoprotein E to beta-amyloid. J Biol Chem, 269(38), 23403–23406. [PubMed] [Google Scholar]
- Lambert JC, Ibrahim-Verbaas CA, Harold D, Naj AC, Sims R, Bellenguez C, … Amouyel P (2013). Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer’s disease. Nat Genet, 45(12), 1452–1458. doi: 10.1038/ng.2802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langballe EM, Ask H, Holmen J, Stordal E, Saltvedt I, Selbaek G, … Tambs K (2015). Alcohol consumption and risk of dementia up to 27 years later in a large, population-based sample: the HUNT study, Norway. Eur J Epidemiol, 30(9), 1049–1056. doi: 10.1007/s10654-015-0029-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson SC, Traylor M, Malik R, Dichgans M, Burgess S, Markus HS, & CoStream Consortium, o. b. o. t. I. G. o. A. s. P. (2017). Modifiable pathways in Alzheimer’s disease: Mendelian randomisation analysis. BMJ, 359, j5375. doi: 10.1136/bmj.j5375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lautenschlager NT, Cupples LA, Rao VS, Auerbach SA, Becker R, Burke J, … Farrer LA (1996). Risk of dementia among relatives of Alzheimer’s disease patients in the MIRAGE study: What is in store for the oldest old? Neurology, 46(3), 641–650. [DOI] [PubMed] [Google Scholar]
- Leclerc B, & Abulrob A (2013). Perspectives in molecular imaging using staging biomarkers and immunotherapies in Alzheimer’s disease. ScientificWorldJournal, 2013, 589308. doi: 10.1155/2013/589308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lejri I, Grimm A, & Eckert A (2018). Mitochondria, Estrogen and Female Brain Aging. Front Aging Neurosci, 10, 124. doi: 10.3389/fnagi.2018.00124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Cui J, & Shen Y (2014). Brain sex matters: estrogen in cognition and Alzheimer’s disease. Mol Cell Endocrinol, 389(1–2), 13–21. doi: 10.1016/j.mce.2013.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Risacher SL, McAllister TW, & Saykin AJ (2016). Traumatic brain injury and age at onset of cognitive impairment in older adults. J Neurol, 263(7), 1280–1285. doi: 10.1007/s00415-016-8093-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Risacher SL, McAllister TW, Saykin AJ, & Alzheimer’s Disease Neuroimaging, I. (2017). Age at injury is associated with the long-term cognitive outcome of traumatic brain injuries. Alzheimers Dement (Amst), 6, 196–200. doi: 10.1016/j.dadm.2017.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Li Y, Li X, Zhang S, Zhao J, Zhu X, & Tian G (2017). Head Injury as a Risk Factor for Dementia and Alzheimer’s Disease: A Systematic Review and Meta-Analysis of 32 Observational Studies. PLoS One, 12(1), e0169650. doi: 10.1371/journal.pone.0169650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang T, Li R, & Cheng O (2015). Statins for Treating Alzheimer’s Disease: Truly Ineffective? Eur Neurol, 73(5–6), 360–366. doi: 10.1159/000382128 [DOI] [PubMed] [Google Scholar]
- Lin KA, Choudhury KR, Rathakrishnan BG, Marks DM, Petrella JR, Doraiswamy PM, & Alzheimer’s Disease Neuroimaging, I. (2015). Marked gender differences in progression of mild cognitive impairment over 8 years. Alzheimers Dement (N Y), 1(2), 103–110. doi: 10.1016/j.trci.2015.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CC, Liu CC, Kanekiyo T, Xu H, & Bu G (2013). Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat Rev Neurol, 9(2), 106–118. doi: 10.1038/nrneurol.2012.263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Chen HH, Li TL, Xu L, & Du HQ (2013). A cross-sectional study on cerebrospinal fluid biomarker levels in cognitively normal elderly subjects with or without a family history of Alzheimer’s disease. CNS Neurosci Ther, 19(1), 38–42. doi: 10.1111/cns.12028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LoBue C, Wadsworth H, Wilmoth K, Clem M, Hart J Jr., Womack KB, … Cullum CM (2017). Traumatic brain injury history is associated with earlier age of onset of Alzheimer disease. Clin Neuropsychol, 31(1), 85–98. doi: 10.1080/13854046.2016.1257069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LoBue C, Woon FL, Rossetti HC, Hynan LS, Hart J, & Cullum CM (2018). Traumatic brain injury history and progression from mild cognitive impairment to Alzheimer disease. Neuropsychology, 32(4), 401–409. doi: 10.1037/neu0000431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu SJ, Li HL, Sun YM, Liu ZJ, Yang P, & Wu ZY (2014). Clusterin variants are not associated with southern Chinese patients with Alzheimer’s disease. Neurobiol Aging, 35(11), 2656 e2659–2656 e2611. doi: 10.1016/j.neurobiolaging.2014.05.015 [DOI] [PubMed] [Google Scholar]
- Luchsinger JA, Tang MX, Siddiqui M, Shea S, & Mayeux R (2004). Alcohol intake and risk of dementia. J Am Geriatr Soc, 52(4), 540–546. doi: 10.1111/j.1532-5415.2004.52159.x [DOI] [PubMed] [Google Scholar]
- Luck T, Riedel-Heller SG, Luppa M, Wiese B, Kohler M, Jessen F, … Maier W (2014). Apolipoprotein E epsilon 4 genotype and a physically active lifestyle in late life: analysis of gene-environment interaction for the risk of dementia and Alzheimer’s disease dementia. Psychol Med, 44(6), 1319–1329. doi: 10.1017/S0033291713001918 [DOI] [PubMed] [Google Scholar]
- Lutz MW, Sundseth SS, Burns DK, Saunders AM, Hayden KM, Burke JR, … Roses AD (2016). A Genetics-based Biomarker Risk Algorithm for Predicting Risk of Alzheimer’s Disease. Alzheimers Dement (N Y), 2(1), 30–44. doi: 10.1016/j.trci.2015.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macauley SL, Stanley M, Caesar EE, Yamada SA, Raichle ME, Perez R, … Holtzman DM (2015). Hyperglycemia modulates extracellular amyloid-beta concentrations and neuronal activity in vivo. J Clin Invest, 125(6), 2463–2467. doi: 10.1172/JCI79742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahley RW, Weisgraber KH, & Huang Y (2006). Apolipoprotein E4: a causative factor and therapeutic target in neuropathology, including Alzheimer’s disease. Proc Natl Acad Sci U S A, 103(15), 5644–5651. doi: 10.1073/pnas.0600549103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki T, Sasaki K, Tanizaki Y, Hata J, Fujimi K, Matsui Y, … Iwaki T (2010). Insulin resistance is associated with the pathology of Alzheimer disease: the Hisayama study. Neurology, 75(9), 764–770. doi: 10.1212/WNL.0b013e3181eee25f [DOI] [PubMed] [Google Scholar]
- Medway C, & Morgan K (2014). Review: The genetics of Alzheimer’s disease; putting flesh on the bones. Neuropathol Appl Neurobiol, 40(2), 97–105. doi: 10.1111/nan.12101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta KM, Ott A, Kalmijn S, Slooter AJ, van Duijn CM, Hofman A, & Breteler MM (1999). Head trauma and risk of dementia and Alzheimer’s disease: The Rotterdam Study. Neurology, 53(9), 1959–1962. [DOI] [PubMed] [Google Scholar]
- Mehta KM, & Yeo GW (2017). Systematic review of dementia prevalence and incidence in United States race/ethnic populations. Alzheimers Dement, 13(1), 72–83. doi: 10.1016/j.jalz.2016.06.2360 [DOI] [PubMed] [Google Scholar]
- Mendez MF (2017). What is the Relationship of Traumatic Brain Injury to Dementia? J Alzheimers Dis, 57(3), 667–681. doi: 10.3233/JAD-161002 [DOI] [PubMed] [Google Scholar]
- Moloney AM, Griffin RJ, Timmons S, O’Connor R, Ravid R, & O’Neill C (2010). Defects in IGF-1 receptor, insulin receptor and IRS-1/2 in Alzheimer’s disease indicate possible resistance to IGF-1 and insulin signalling. Neurobiol Aging, 31(2), 224–243. doi: 10.1016/j.neurobiolaging.2008.04.002 [DOI] [PubMed] [Google Scholar]
- Moran C, Phan TG, Chen J, Blizzard L, Beare R, Venn A, … Srikanth V (2013). Brain atrophy in type 2 diabetes: regional distribution and influence on cognition. Diabetes Care, 36(12), 4036–4042. doi: 10.2337/dc13-0143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortimer JA, van Duijn CM, Chandra V, Fratiglioni L, Graves AB, Heyman A, … et al. (1991). Head trauma as a risk factor for Alzheimer’s disease: a collaborative re-analysis of case-control studies. EURODEM Risk Factors Research Group. Int J Epidemiol, 20 Suppl 2, S28–35. [DOI] [PubMed] [Google Scholar]
- Mun MJ, Kim JH, Choi JY, & Jang WC (2016). Genetic polymorphisms of interleukin genes and the risk of Alzheimer’s disease: An update meta-analysis. Meta Gene, 8, 1–10. doi: 10.1016/j.mgene.2016.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy MP (2018). Amyloid-Beta Solubility in the Treatment of Alzheimer’s Disease. N Engl J Med, 378(4), 391–392. doi: 10.1056/NEJMe1714638 [DOI] [PubMed] [Google Scholar]
- Naj AC, Jun G, Beecham GW, Wang LS, Vardarajan BN, Buros J, … Schellenberg GD (2011). Common variants at MS4A4/MS4A6E, CD2AP, CD33 and EPHA1 are associated with late-onset Alzheimer’s disease. Nat Genet, 43(5), 436–441. doi: 10.1038/ng.801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu SC, Pa J, Kukull W, Beekly D, Kuzma A, Gangadharan P, … Toga AW (2017). Apolipoprotein E Genotype and Sex Risk Factors for Alzheimer Disease: A Meta-analysis. JAMA Neurol, 74(10), 1178–1189. doi: 10.1001/jamaneurol.2017.2188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neugroschl J, & Wang S (2011). Alzheimer’s disease: diagnosis and treatment across the spectrum of disease severity. Mt Sinai J Med, 78(4), 596–612. doi: 10.1002/msj.20279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll JA, Roberts GW, & Graham DI (1995). Apolipoprotein E epsilon 4 allele is associated with deposition of amyloid beta-protein following head injury. Nat Med, 1(2), 135–137. [DOI] [PubMed] [Google Scholar]
- Nicoll JA, Roberts GW, & Graham DI (1996). Amyloid beta-protein, APOE genotype and head injury. Ann N Y Acad Sci, 777, 271–275. [DOI] [PubMed] [Google Scholar]
- Nordestgaard LT, Tybjaerg-Hansen A, Rasmussen KL, Nordestgaard BG, & Frikke-Schmidt R (2018). Genetic variation in clusterin and risk of dementia and ischemic vascular disease in the general population: cohort studies and meta-analyses of 362,338 individuals. BMC Med, 16(1), 39. doi: 10.1186/s12916-018-1029-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuzzo D, Picone P, Baldassano S, Caruana L, Messina E, Marino Gammazza A, … Di Carlo M (2015). Insulin Resistance as Common Molecular Denominator Linking Obesity to Alzheimer’s Disease. Curr Alzheimer Res, 12(8), 723–735. [DOI] [PubMed] [Google Scholar]
- O’Brien RJ, & Wong PC (2011). Amyloid precursor protein processing and Alzheimer’s disease. Annu Rev Neurosci, 34, 185–204. doi: 10.1146/annurev-neuro-061010-113613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott A, Stolk RP, van Harskamp F, Pols HA, Hofman A, & Breteler MM (1999). Diabetes mellitus and the risk of dementia: The Rotterdam Study. Neurology, 53(9), 1937–1942. [DOI] [PubMed] [Google Scholar]
- Ottman R (1996). Gene-environment interaction: definitions and study designs. Prev Med, 25(6), 764–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panza F, Frisardi V, Seripa D, Logroscino G, Santamato A, Imbimbo BP, … Solfrizzi V (2012). Alcohol consumption in mild cognitive impairment and dementia: harmful or neuroprotective? Int J Geriatr Psychiatry, 27(12), 1218–1238. doi: 10.1002/gps.3772 [DOI] [PubMed] [Google Scholar]
- Panza F, Seripa D, Lozupone M, Solfrizzi V, Imbimbo BP, Barulli MR, … Logroscino G (2018). The potential of solanezumab and gantenerumab to prevent Alzheimer’s disease in people with inherited mutations that cause its early onset. Expert Opin Biol Ther, 18(1), 25–35. doi: 10.1080/14712598.2018.1389885 [DOI] [PubMed] [Google Scholar]
- Pappolla MA, Bryant-Thomas TK, Herbert D, Pacheco J, Fabra Garcia M, Manjon M, … Refolo LM (2003). Mild hypercholesterolemia is an early risk factor for the development of Alzheimer amyloid pathology. Neurology, 61(2), 199–205. [DOI] [PubMed] [Google Scholar]
- Paul CA, Au R, Fredman L, Massaro JM, Seshadri S, Decarli C, & Wolf PA (2008). Association of alcohol consumption with brain volume in the Framingham study. Arch Neurol, 65(10), 1363–1367. doi: 10.1001/archneur.65.10.1363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen RC (2009). Early diagnosis of Alzheimer’s disease: is MCI too late? Curr Alzheimer Res, 6(4), 324–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza-Gardner AK, Gaffud TJ, & Barry AE (2013). The impact of alcohol on Alzheimer’s disease: a systematic review. Aging Ment Health, 17(2), 133–146. doi: 10.1080/13607863.2012.742488 [DOI] [PubMed] [Google Scholar]
- Pimenova AA, Raj T, & Goate AM (2018). Untangling Genetic Risk for Alzheimer’s Disease. Biol Psychiatry, 83(4), 300–310. doi: 10.1016/j.biopsych.2017.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plassman BL, Havlik RJ, Steffens DC, Helms MJ, Newman TN, Drosdick D, … Breitner JC (2000). Documented head injury in early adulthood and risk of Alzheimer’s disease and other dementias. Neurology, 55(8), 1158–1166. [DOI] [PubMed] [Google Scholar]
- Popp J, Lewczuk P, Kolsch H, Meichsner S, Maier W, Kornhuber J, … Lutjohann D (2012). Cholesterol metabolism is associated with soluble amyloid precursor protein production in Alzheimer’s disease. J Neurochem, 123(2), 310–316. doi: 10.1111/j.1471-4159.2012.07893.x [DOI] [PubMed] [Google Scholar]
- Popp J, Meichsner S, Kolsch H, Lewczuk P, Maier W, Kornhuber J, … Lutjohann D (2013). Cerebral and extracerebral cholesterol metabolism and CSF markers of Alzheimer’s disease. Biochem Pharmacol, 86(1), 37–42. doi: 10.1016/j.bcp.2012.12.007 [DOI] [PubMed] [Google Scholar]
- Potter GG, Plassman BL, Burke JR, Kabeto MU, Langa KM, Llewellyn DJ, … Steffens DC (2009). Cognitive performance and informant reports in the diagnosis of cognitive impairment and dementia in African Americans and whites. Alzheimers Dement, 5(6), 445–453. doi: 10.1016/j.jalz.2009.04.1234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power MC, Adar SD, Yanosky JD, & Weuve J (2016). Exposure to air pollution as a potential contributor to cognitive function, cognitive decline, brain imaging, and dementia: A systematic review of epidemiologic research. Neurotoxicology, 56, 235–253. doi: 10.1016/j.neuro.2016.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rall SC Jr., Weisgraber KH, & Mahley RW (1982). Human apolipoprotein E. The complete amino acid sequence. J Biol Chem, 257(8), 4171–4178. [PubMed] [Google Scholar]
- Ramanan VK, Risacher SL, Nho K, Kim S, Shen L, McDonald BC, … Alzheimer’s Disease Neuroimaging, I. (2015). GWAS of longitudinal amyloid accumulation on 18F-florbetapir PET in Alzheimer’s disease implicates microglial activation gene IL1RAP. Brain, 138(Pt 10), 3076–3088. doi: 10.1093/brain/awv231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rantanen KK, Strandberg AY, Pitkala K, Tilvis R, Salomaa V, & Strandberg TE (2014). Cholesterol in midlife increases the risk of Alzheimer’s disease during an up to 43-year follow-up. European Geriatric Medicine, 5(6), 390–393. doi: 10.1016/j.eurger.2014.05.002 [DOI] [Google Scholar]
- Reitz C, Jun G, Naj A, Rajbhandary R, Vardarajan BN, Wang LS, … Alzheimer Disease Genetics, C. (2013). Variants in the ATP-binding cassette transporter (ABCA7), apolipoprotein E 4,and the risk of late-onset Alzheimer disease in African Americans. JAMA, 309(14), 1483–1492. doi: 10.1001/jama.2013.2973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson JR, Roy A, Shalat SL, von Stein RT, Hossain MM, Buckley B, … German DC (2014). Elevated serum pesticide levels and risk for Alzheimer disease. JAMA Neurol, 71(3), 284–290. doi: 10.1001/jamaneurol.2013.6030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson JR, Shalat SL, Buckley B, Winnik B, O’Suilleabhain P, Diaz-Arrastia R, … German DC (2009). Elevated serum pesticide levels and risk of Parkinson disease. Arch Neurol, 66(7), 870–875. doi: 10.1001/archneurol.2009.89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridge PG, Karch CM, Hsu S, Arano I, Teerlink CC, Ebbert MTW, … Alzheimer’s Disease Neuroimaging, I. (2017). Linkage, whole genome sequence, and biological data implicate variants in RAB10 in Alzheimer’s disease resilience. Genome Med, 9(1), 100. doi: 10.1186/s13073-017-0486-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera EJ, Goldin A, Fulmer N, Tavares R, Wands JR, & de la Monte SM (2005). Insulin and insulin-like growth factor expression and function deteriorate with progression of Alzheimer’s disease: link to brain reductions in acetylcholine. J Alzheimers Dis, 8(3), 247–268. [DOI] [PubMed] [Google Scholar]
- Rosenthal SL, & Kamboh MI (2014). Late-Onset Alzheimer’s Disease Genes and the Potentially Implicated Pathways. Curr Genet Med Rep, 2, 85–101. doi: 10.1007/s40142-014-0034-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz A, Heilmann S, Becker T, Hernandez I, Wagner H, Thelen M, … Ramirez A (2014). Follow-up of loci from the International Genomics of Alzheimer’s Disease Project identifies TRIP4 as a novel susceptibility gene. Transl Psychiatry, 4, e358. doi: 10.1038/tp.2014.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano M, Bell KL, Galasko D, Galvin JE, Thomas RG, van Dyck CH, & Aisen PS (2011). A randomized, double-blind, placebo-controlled trial of simvastatin to treat Alzheimer disease. Neurology, 77(6), 556–563. doi: 10.1212/WNL.0b013e318228bf11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santibanez M, Bolumar F, & Garcia AM (2007). Occupational risk factors in Alzheimer’s disease: a review assessing the quality of published epidemiological studies. Occup Environ Med, 64(11), 723–732. doi: 10.1136/oem.2006.028209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scacchi R, Gambina G, Broggio E, & Corbo RM (2014). Sex and ESR1 genotype may influence the response to treatment with donepezil and rivastigmine in patients with Alzheimer’s disease. Int J Geriatr Psychiatry, 29(6), 610–615. doi: 10.1002/gps.4043 [DOI] [PubMed] [Google Scholar]
- Scarmeas N, Luchsinger JA, Schupf N, Brickman AM, Cosentino S, Tang MX, & Stern Y (2009). Physical activity, diet, and risk of Alzheimer disease. JAMA, 302(6), 627–637. doi: 10.1001/jama.2009.1144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffert J, LoBue C, White CL, Chiang HS, Didehbani N, Lacritz L, … Cullum CM (2018). Traumatic brain injury history is associated with an earlier age of dementia onset in autopsy-confirmed Alzheimer’s disease. Neuropsychology, 32(4), 410–416. doi: 10.1037/neu0000423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield PW, Tang M, Marder K, Bell K, Dooneief G, Chun M, … Mayeux R (1997). Alzheimer’s disease after remote head injury: an incidence study. J Neurol Neurosurg Psychiatry, 62(2), 119–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao H, Breitner JC, Whitmer RA, Wang J, Hayden K, Wengreen H, … Cache County, I. (2012). Hormone therapy and Alzheimer disease dementia: new findings from the Cache County Study. Neurology, 79(18), 1846–1852. doi: 10.1212/WNL.0b013e318271f823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara M, & Sato N (2017). Bidirectional interactions between diabetes and Alzheimer’s disease. Neurochem Int, 108, 296–302. doi: 10.1016/j.neuint.2017.04.020 [DOI] [PubMed] [Google Scholar]
- Shpanskaya KS, Choudhury KR, Hostage C Jr., Murphy KR, Petrella JR, Doraiswamy PM, & Alzheimer’s Disease Neuroimaging, I. (2014). Educational attainment and hippocampal atrophy in the Alzheimer’s disease neuroimaging initiative cohort. J Neuroradiol, 41(5), 350–357. doi: 10.1016/j.neurad.2013.11.004 [DOI] [PubMed] [Google Scholar]
- Simic G, Babic Leko M, Wray S, Harrington C, Delalle I, Jovanov-Milosevic N, … Hof PR (2016). Tau Protein Hyperphosphorylation and Aggregation in Alzheimer’s Disease and Other Tauopathies, and Possible Neuroprotective Strategies. Biomolecules, 6(1), 6. doi: 10.3390/biom6010006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims R, van der Lee SJ, Naj AC, Bellenguez C, Badarinarayan N, Jakobsdottir J, … Schellenberg GD (2017). Rare coding variants in PLCG2, ABI3, and TREM2 implicate microglial-mediated innate immunity in Alzheimer’s disease. Nat Genet, 49(9), 1373–1384. doi: 10.1038/ng.3916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh N, Chhillar N, Banerjee B, Bala K, Basu M, & Mustafa M (2013). Organochlorine pesticide levels and risk of Alzheimer’s disease in north Indian population. Hum Exp Toxicol, 32(1), 24–30. doi: 10.1177/0960327112456315 [DOI] [PubMed] [Google Scholar]
- Singh NK, Chhillar N, Banerjee BD, Bala K, Mukherjee AK, Mustafa MD, & Mitrabasu. (2012). Gene-environment interaction in Alzheimer’s disease. Am J Alzheimers Dis Other Demen, 27(7), 496–503. doi: 10.1177/1533317512456067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siroux V, Agier L, & Slama R (2016). The exposome concept: a challenge and a potential driver for environmental health research. Eur Respir Rev, 25(140), 124–129. doi: 10.1183/16000617.0034-2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solfrizzi V, Frisardi V, Capurso C, D’Introno A, Colacicco AM, Vendemmiale G, … Panza F (2009). Moderate alcohol consumption, apolipoprotein E, and neuroprotection. Arch Neurol, 66(4), 541–542. doi: 10.1001/archneurol.2009.35 [DOI] [PubMed] [Google Scholar]
- Solomon A, Kivipelto M, Wolozin B, Zhou J, & Whitmer RA (2009). Midlife serum cholesterol and increased risk of Alzheimer’s and vascular dementia three decades later. Dement Geriatr Cogn Disord, 28(1), 75–80. doi: 10.1159/000231980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparks DL (1997). Coronary artery disease, hypertension, ApoE, and cholesterol: a link to Alzheimer’s disease? Ann N Y Acad Sci, 826, 128–146. [DOI] [PubMed] [Google Scholar]
- Sparks DL, Hunsaker JC 3rd, Scheff SW, Kryscio RJ, Henson JL, & Markesbery WR (1990). Cortical senile plaques in coronary artery disease, aging and Alzheimer’s disease. Neurobiol Aging, 11(6), 601–607. [DOI] [PubMed] [Google Scholar]
- Sparks DL, Scheff SW, Hunsaker JC 3rd, Liu H, Landers T, & Gross DR (1994). Induction of Alzheimer-like beta-amyloid immunoreactivity in the brains of rabbits with dietary cholesterol. Exp Neurol, 126(1), 88–94. doi: 10.1006/exnr.1994.1044 [DOI] [PubMed] [Google Scholar]
- Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, … Phelps CH (2011). Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement, 7(3), 280–292. doi: 10.1016/j.jalz.2011.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spittau B (2017). Aging Microglia-Phenotypes, Functions and Implications for Age-Related Neurodegenerative Diseases. Front Aging Neurosci, 9, 194. doi: 10.3389/fnagi.2017.00194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steen E, Terry BM, Rivera EJ, Cannon JL, Neely TR, Tavares R, … de la Monte SM (2005). Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer’s disease--is this type 3 diabetes? J Alzheimers Dis, 7(1), 63–80. [DOI] [PubMed] [Google Scholar]
- Stewart R, White LR, Xue QL, & Launer LJ (2007). Twenty-six-year change in total cholesterol levels and incident dementia: the Honolulu-Asia Aging Study. Arch Neurol, 64(1), 103–107. doi: 10.1001/archneur.64.1.103 [DOI] [PubMed] [Google Scholar]
- Strosnider H, Kennedy C, Monti M, & Yip F (2017). Rural and Urban Differences in Air Quality, 2008–2012, and Community Drinking Water Quality, 2010–2015 - United States. MMWR Surveill Summ, 66(13), 1–10. doi: 10.15585/mmwr.ss6613a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun JH, Yu JT, & Tan L (2015). The role of cholesterol metabolism in Alzheimer’s disease. Mol Neurobiol, 51(3), 947–965. doi: 10.1007/s12035-014-8749-y [DOI] [PubMed] [Google Scholar]
- Szymanski M, Wang R, Bassett SS, & Avramopoulos D (2011). Alzheimer’s risk variants in the clusterin gene are associated with alternative splicing. Transl Psychiatry, 1. doi: 10.1038/tp.2011.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai LM, Ghura S, Koster KP, Liakaite V, Maienschein-Cline M, Kanabar P, … LaDu MJ (2015). APOE-modulated Abeta-induced neuroinflammation in Alzheimer’s disease: current landscape, novel data, and future perspective. J Neurochem, 133(4), 465–488. doi: 10.1111/jnc.13072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda S, Sato N, Uchio-Yamada K, Sawada K, Kunieda T, Takeuchi D, … Morishita R (2010). Diabetes-accelerated memory dysfunction via cerebrovascular inflammation and Abeta deposition in an Alzheimer mouse model with diabetes. Proc Natl Acad Sci U S A, 107(15), 7036–7041. doi: 10.1073/pnas.1000645107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot K, Wang HY, Kazi H, Han LY, Bakshi KP, Stucky A, … Arnold SE (2012). Demonstrated brain insulin resistance in Alzheimer’s disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. J Clin Invest, 122(4), 1316–1338. doi: 10.1172/JCI59903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan MS, Yu JT, & Tan L (2013). Bridging integrator 1 (BIN1): form, function, and Alzheimer’s disease. Trends Mol Med, 19(10), 594–603. doi: 10.1016/j.molmed.2013.06.004 [DOI] [PubMed] [Google Scholar]
- Tan ZS, Seshadri S, Beiser A, Wilson PW, Kiel DP, Tocco M, … Wolf PA (2003). Plasma total cholesterol level as a risk factor for Alzheimer disease: the Framingham Study. Arch Intern Med, 163(9), 1053–1057. doi: 10.1001/archinte.163.9.1053 [DOI] [PubMed] [Google Scholar]
- Toepper M (2017). Dissociating Normal Aging from Alzheimer’s Disease: A View from Cognitive Neuroscience. J Alzheimers Dis, 57(2), 331–352. doi: 10.3233/JAD-161099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonne C, Elbaz A, Beevers S, & Singh-Manoux A (2014). Traffic-related air pollution in relation to cognitive function in older adults. Epidemiology, 25(5), 674–681. doi: 10.1097/EDE.0000000000000144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripodis Y, Alosco ML, Zirogiannis N, Gavett BE, Chaisson C, Martin B, … Stern RA (2017). The Effect of Traumatic Brain Injury History with Loss of Consciousness on Rate of Cognitive Decline Among Older Adults with Normal Cognition and Alzheimer’s Disease Dementia. J Alzheimers Dis, 59(1), 251–263. doi: 10.3233/JAD-160585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyas SL, Koval JJ, & Pederson LL (2000). Does an interaction between smoking and drinking influence the risk of Alzheimer’s disease? Results from three Canadian data sets. Stat Med, 19(11–12), 1685–1696. [DOI] [PubMed] [Google Scholar]
- Tyas SL, Manfreda J, Strain LA, & Montgomery PR (2001). Risk factors for Alzheimer’s disease: a population-based, longitudinal study in Manitoba, Canada. Int J Epidemiol, 30(3), 590–597. [DOI] [PubMed] [Google Scholar]
- Tyas SL, Pederson LL, & Koval JJ (2000). Is smoking associated with the risk of developing Alzheimer’s disease? Results from three Canadian data sets. Ann Epidemiol, 10(7), 409–416. [DOI] [PubMed] [Google Scholar]
- Tyas SL, White LR, Petrovitch H, Webster Ross G, Foley DJ, Heimovitz HK, & Launer LJ (2003). Mid-life smoking and late-life dementia: the Honolulu-Asia Aging Study. Neurobiol Aging, 24(4), 589–596. [DOI] [PubMed] [Google Scholar]
- Uchoa MF, Moser VA, & Pike CJ (2016). Interactions between inflammation, sex steroids, and Alzheimer’s disease risk factors. Front Neuroendocrinol, 43, 60–82. doi: 10.1016/j.yfrne.2016.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulland TK, Song WM, Huang SC, Ulrich JD, Sergushichev A, Beatty WL, … Colonna M (2017). TREM2 Maintains Microglial Metabolic Fitness in Alzheimer’s Disease. Cell, 170(4), 649–663 e613. doi: 10.1016/j.cell.2017.07.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich JD, Ulland TK, Colonna M, & Holtzman DM (2017). Elucidating the Role of TREM2 in Alzheimer’s Disease. Neuron, 94(2), 237–248. doi: 10.1016/j.neuron.2017.02.042 [DOI] [PubMed] [Google Scholar]
- Utermann G, Hees M, & Steinmetz A (1977). Polymorphism of apolipoprotein E and occurrence of dysbetalipoproteinaemia in man. Nature, 269(5629), 604–607. [DOI] [PubMed] [Google Scholar]
- Utermann G, Langenbeck U, Beisiegel U, & Weber W (1980). Genetics of the apolipoprotein E system in man. Am J Hum Genet, 32(3), 339–347. [PMC free article] [PubMed] [Google Scholar]
- Valente T, Gella A, Fernandez-Busquets X, Unzeta M, & Durany N (2010). Immunohistochemical analysis of human brain suggests pathological synergism of Alzheimer’s disease and diabetes mellitus. Neurobiol Dis, 37(1), 67–76. doi: 10.1016/j.nbd.2009.09.008 [DOI] [PubMed] [Google Scholar]
- Venkataraman A, Kalk N, Sewell G, C WR, & Lingford-Hughes A (2017). Alcohol and Alzheimer’s Disease-Does Alcohol Dependence Contribute to Beta-Amyloid Deposition, Neuroinflammation and Neurodegeneration in Alzheimer’s Disease? Alcohol Alcohol, 52(2), 158. doi: 10.1093/alcalc/agw101 [DOI] [PubMed] [Google Scholar]
- Villeneuve S, Brisson D, Marchant NL, & Gaudet D (2014). The potential applications of Apolipoprotein E in personalized medicine. Front Aging Neurosci, 6, 154. doi: 10.3389/fnagi.2014.00154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallin C, Sholts SB, Osterlund N, Luo J, Jarvet J, Roos PM, … Warmlander S (2017). Alzheimer’s disease and cigarette smoke components: effects of nicotine, PAHs, and Cd(II), Cr(III), Pb(II), Pb(IV) ions on amyloid-beta peptide aggregation. Sci Rep, 7(1), 14423. doi: 10.1038/s41598-017-13759-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner MW, Harvey D, Hayes J, Landau SM, Aisen PS, Petersen RC, … Department of Defense Alzheimer’s Disease Neuroimaging, I. (2017). Effects of traumatic brain injury and posttraumatic stress disorder on development of Alzheimer’s disease in Vietnam Veterans using the Alzheimer’s Disease Neuroimaging Initiative: Preliminary Report. Alzheimers Dement (N Y), 3(2), 177–188. doi: 10.1016/j.trci.2017.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner MW, & Veitch DP (2015). Introduction to special issue: Overview of Alzheimer’s Disease Neuroimaging Initiative. Alzheimers Dement, 11(7), 730–733. doi: 10.1016/j.jalz.2015.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner MW, Veitch DP, Aisen PS, Beckett LA, Cairns NJ, Green RC, … Alzheimer’s Disease Neuroimaging, I. (2012). The Alzheimer’s Disease Neuroimaging Initiative: a review of papers published since its inception. Alzheimers Dement, 8(1 Suppl), S1–68. doi: 10.1016/j.jalz.2011.09.172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner MW, Veitch DP, Aisen PS, Beckett LA, Cairns NJ, Green RC, … Alzheimer’s Disease Neuroimaging, I. (2017). The Alzheimer’s Disease Neuroimaging Initiative 3: Continued innovation for clinical trial improvement. Alzheimers Dement, 13(5), 561–571. doi: 10.1016/j.jalz.2016.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng PH, Chen JH, Chen TF, Sun Y, Wen LL, Yip PK, … Chen YC (2016). CHRNA7 Polymorphisms and Dementia Risk: Interactions with Apolipoprotein epsilon4 and Cigarette Smoking. Sci Rep, 6, 27231. doi: 10.1038/srep27231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weuve J, Puett RC, Schwartz J, Yanosky JD, Laden F, & Grodstein F (2012). Exposure to particulate air pollution and cognitive decline in older women. Arch Intern Med, 172(3), 219–227. doi: 10.1001/archinternmed.2011.683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitmer RA, Sidney S, Selby J, Johnston SC, & Yaffe K (2005). Midlife cardiovascular risk factors and risk of dementia in late life. Neurology, 64(2), 277–281. doi: 10.1212/01.WNL.0000149519.47454.F2 [DOI] [PubMed] [Google Scholar]
- Willett WC, Koplan JP, Nugent R, Dusenbury C, Puska P, & Gaziano TA (2006). Prevention of Chronic Disease by Means of Diet and Lifestyle Changes In nd, Jamison DT, Breman JG, Measham AR, Alleyne G, Claeson M, Evans DB, Jha P, Mills A, & Musgrove P (Eds.), Disease Control Priorities in Developing Countries. Washington (DC). [PubMed] [Google Scholar]
- Wood WG, Li L, Muller WE, & Eckert GP (2014). Cholesterol as a causative factor in Alzheimer’s disease: a debatable hypothesis. J Neurochem, 129(4), 559–572. doi: 10.1111/jnc.12637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu YC, Lin YC, Yu HL, Chen JH, Chen TF, Sun Y, … Chen YC (2015). Association between air pollutants and dementia risk in the elderly. Alzheimers Dement (Amst), 1(2), 220–228. doi: 10.1016/j.dadm.2014.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong C, Roe CM, Buckles V, Fagan A, Holtzman D, Balota D, … Morris JC (2011). Role of family history for Alzheimer biomarker abnormalities in the adult children study. Arch Neurol, 68(10), 1313–1319. doi: 10.1001/archneurol.2011.208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Tan L, & Yu JT (2015). The Role of PICALM in Alzheimer’s Disease. Mol Neurobiol, 52(1), 399–413. doi: 10.1007/s12035-014-8878-3 [DOI] [PubMed] [Google Scholar]
- Yaffe K, Falvey C, Harris TB, Newman A, Satterfield S, Koster A, … Health A. B. C. S. (2013). Effect of socioeconomic disparities on incidence of dementia among biracial older adults: prospective study. BMJ, 347, f7051. doi: 10.1136/bmj.f7051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh FL, Hansen DV, & Sheng M (2017). TREM2, Microglia, and Neurodegenerative Diseases. Trends Mol Med, 23(6), 512–533. doi: 10.1016/j.molmed.2017.03.008 [DOI] [PubMed] [Google Scholar]
- Yeh FL, Wang Y, Tom I, Gonzalez LC, & Sheng M (2016). TREM2 Binds to Apolipoproteins, Including APOE and CLU/APOJ, and Thereby Facilitates Uptake of Amyloid-Beta by Microglia. Neuron, 91(2), 328–340. doi: 10.1016/j.neuron.2016.06.015 [DOI] [PubMed] [Google Scholar]
- Yin RH, Yu JT, & Tan L (2015). The Role of SORL1 in Alzheimer’s Disease. Mol Neurobiol, 51(3), 909–918. doi: 10.1007/s12035-014-8742-5 [DOI] [PubMed] [Google Scholar]
- Zeng Y, Chang W, Shu C, Ma L, Huang Y, Wang R, … McClintock SM (2013). Decreased cognitive function in extended family members from the single late-onset-Alzheimer’s-disease pedigree. J Int Neuropsychol Soc, 19(7), 809–819. doi: 10.1017/S1355617713000581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Li X, Ma G, Jiang Y, Liao M, Feng R, … Liu G (2016). CLU rs9331888 Polymorphism Contributes to Alzheimer’s Disease Susceptibility in Caucasian But Not East Asian Populations. Mol Neurobiol, 53(3), 1446–1451. doi: 10.1007/s12035-015-9098-1 [DOI] [PubMed] [Google Scholar]
- Zhong L, Wang Z, Wang D, Wang Z, Martens YA, Wu L, … Chen XF (2018). Amyloid-beta modulates microglial responses by binding to the triggering receptor expressed on myeloid cells 2 (TREM2). Mol Neurodegener, 13(1), 15. doi: 10.1186/s13024-018-0247-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu XC, Tan L, Wang HF, Jiang T, Cao L, Wang C, … Yu JT (2015). Rate of early onset Alzheimer’s disease: a systematic review and meta-analysis. Ann Transl Med, 3(3), 38. doi: 10.3978/j.issn.2305-5839.2015.01.19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu XC, Yu JT, Jiang T, Wang P, Cao L, & Tan L (2015). CR1 in Alzheimer’s disease. Mol Neurobiol, 51(2), 753–765. doi: 10.1007/s12035-014-8723-8 [DOI] [PubMed] [Google Scholar]