Abstract
In Bacillus subtilis, the extracytoplasmic function σ factor σM regulates cell wall synthesis and is critical for intrinsic resistance to cell wall targeting antibiotics. The anti-σ factors YhdL and YhdK form a complex that restricts the basal activity of σM, and the absence of YhdL leads to runaway expression of the σM regulon and cell death. Here, we report that this lethality can be suppressed by gain-of-function mutations in yidC1 (spoIIIJ), which encodes the major YidC membrane protein insertase in B. subtilis. B. subtilis PY79 YidC1 (SpoIIIJ) contains a single amino acid substitution in a functionally important hydrophilic groove (Q140K), and this allele suppresses the lethality of high σM. Analysis of a library of YidC1 variants reveals that increased charge (+2 or +3) in the hydrophilic groove can compensate for high expression of the σM regulon. Derepression of the σM regulon induces secretion stress, oxidative stress and DNA damage responses, all of which can be alleviated by the YidC1Q140K substitution. We further show that the fitness defect caused by high σM activity is exacerbated in the absence of the SecDF protein translocase or σM-dependent induction of the Spx oxidative stress regulon. Conversely, cell growth is improved by mutation of specific σM-dependent promoters controlling operons encoding integral membrane proteins. Collectively, these results reveal how the σM regulon has evolved to up-regulate membrane-localized complexes involved in cell wall synthesis, and to simultaneously counter the resulting stresses imposed by regulon induction.
Author summary
Bacteria frequently produce antibiotics that inhibit the growth of competitors, and many naturally occurring antibiotics target cell wall synthesis. In Bacillus subtilis, the alternative σ factor σM is induced by cell wall antibiotics, and upregulates genes for peptidoglycan and cell envelope synthesis. However, dysregulation of the σM regulon, resulting from loss of the YhdL anti-σM protein, is lethal. We here identify charge variants of the YidC1 (SpoIIIJ) membrane protein insertase that suppress the lethal effects of high σM activity. Further analyses reveal that induction of the σM regulon leads to high level expression of membrane proteins that trigger envelope stress, and this stress is countered by specific genes in the σM regulon.
Introduction
The ability of cells to adapt to changing conditions relies in large part on the expression of specific stress responses controlled by transcription regulators. The extracytoplasmic function (ECF) subfamily of σ factors are frequently involved in bacterial responses to stresses affecting the cell envelope [1, 2]. In Bacillus subtilis, there are seven ECF σ factors, with the σM regulon playing a particularly important role in modulating pathways involved in peptidoglycan synthesis, cell envelope stress responses, and intrinsic resistance to antibiotics [3]. Cells lacking σM (sigM null mutants) grow normally in unstressed conditions, but have greatly increased sensitivity to high salt and to cell wall active antibiotics, including ß-lactams and moenomycin [4–6].
Like many other ECF σ factors, the activity of σM is regulated by an anti-σ factor. For σM, the anti-σ factor comprises two membrane-localized proteins encoded as part of the sigM operon, sigM-yhdL-yhdK [7, 8]. The major anti-σ factor is YhdL, a transmembrane protein that directly binds σM [9]. However, full YhdL activity requires a second transmembrane protein, YhdK. Although a lack of σM is well tolerated under unstressed conditions, the lack of the anti-σM factors leads to a runaway activation of the autoregulated sigM operon, and overexpression of the σM regulon [10]. A null mutation of yhdK leads to an ~100-fold elevation of σM activity, morphological abnormalities, and slow growth [10]. A null mutation in yhdL is lethal, but suppressors arise readily that have inactivated sigM and grow normally in unstressed conditions [4, 10]. These findings imply that high level expression of σM is toxic to cells, but the basis for this toxicity has not been defined.
Previously, we explored the basis for σM toxicity by selecting for suppression of yhdL lethality in a sigM merodiploid strain to reduce the frequency of suppressors that had inactivated σM. These studies led to the recovery of mutations in rpoB and rpoC, encoding the ß and ß' subunits of RNA polymerase, that led to a reduction of σM activity sufficient to restore viability [10]. These mutations, which acted selectively on σM, affected a region of core RNA polymerase involved in σ factor binding. In the course of these studies we also demonstrated that the toxicity from high σM could be alleviated by mutation of the autoregulatory promoter for the sigM operon, or by overexpression of the housekeeping σ factor, σA [10]. These results suggest that the lack of a functional anti-σM factor (yhdL null mutant) leads to runaway activation of the sigM operon and a high level of σM activity that is incompatible with growth. However, it is unclear whether σM toxicity results from a decrease in activity of the essential housekeeping σA, overexpression of one or more σM-regulated genes, or both.
To further explore the impact of overexpression of specific σM-regulated genes on cell physiology we generated a library of strains in which specific σM-dependent promoters (PM) are inactivated by point mutations. This approach, which removes σM-dependent activation while leaving other promoters and regulatory inputs intact, is important since many σM-regulated genes have multiple promoters and encode essential genes, including several involved in peptidoglycan synthesis and cell division [3]. In the course of developing this library we received a previously described strain with a mutation inactivating the PM of rodA, encoding a SEDS family transglycosylase important for peptidoglycan synthesis [6]. We unexpectedly discovered that in this strain yhdL could be inactivated, and the same was true for the parent strain (B. subtilis PY79). These serendipitous observations led us to hypothesize that B. subtilis PY79 differs from other B. subtilis 168 strains in its ability to tolerate high level expression of the σM regulon.
Here, we report that the ability of B. subtilis PY79 to tolerate σM regulon overexpression results from a single amino acid substitution in the yidC1 (spoIIIJ) gene, which encodes the major YidC1 membrane insertase in B. subtilis [11, 12]. This finding motivated a detailed structure-function analysis of YidC1, which led to the discovery of mutations that increase the positive charge within the hydrophilic groove of YidC1 from +1 (wild-type in B.subtilis 168) to +2 or +3 (in specific combinations) and increase tolerance to overexpression of σM-regulated membrane proteins. Moreover, high level activity of σM leads to induction of genes associated with secretion stress, oxidative stress, and DNA damage responses, and the σM regulon includes functions that help compensate for stresses associated with membrane protein overexpression.
Results
A single amino acid change in YidC1 (SpoIIIJ) is necessary and sufficient for tolerance of high σM
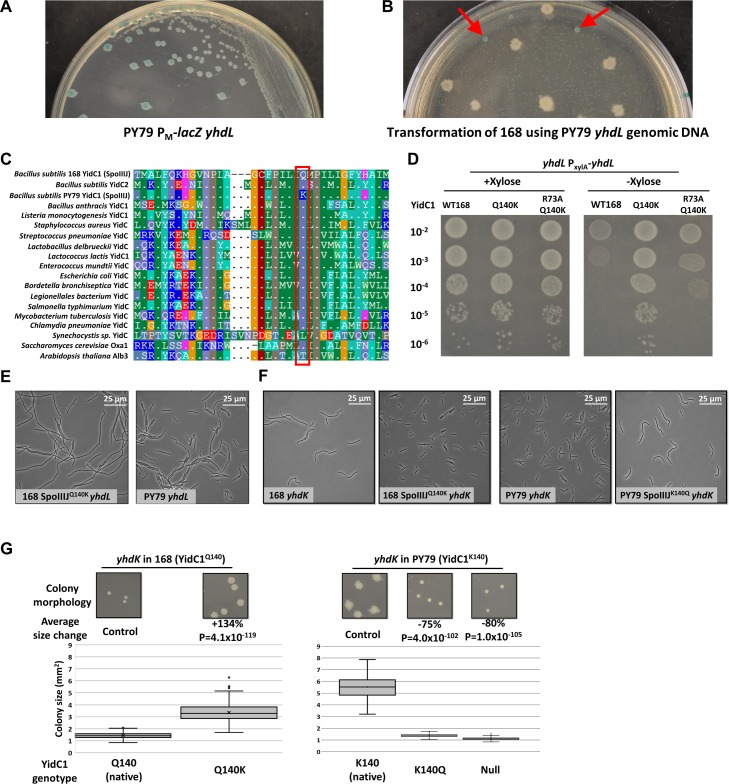
The anti-σ factors YhdL and YhdK regulate σM, and the absence of yhdL is lethal in B. subtilis strain 168 due to toxic levels of σM [4, 10]. Since σM controls a large regulon, including many essential genes involved in cell wall synthesis [1, 3], we sought to construct a library of strains in which specific σM-dependent promoters are inactivated by point mutations. One such promoter precedes rodA, which encodes a peptidoglycan transglycosylase [6]. We thus acquired a ΔPM-rodA strain (BAM1077 [6]), and tested whether yhdL is essential in that strain background, with its parent wild type strain PY79 as a control. Surprisingly, we found that yhdL is not essential in either PY79 strain. A yhdL mutant in PY79 exhibits reduced colony size compared to WT and high σM activity as indicated by the blue color on LB plates containing X-Gal (Fig 1A). This mutant is relatively stable, with the occasional appearance of suppressors that have a large white colony morphology (likely containing mutations in sigM). In contrast, introduction of a yhdL null mutation into other B. subtilis 168 strains results in tiny, pinpoint colonies that do not grow when re-streaked onto fresh plates, consistent with prior work [4, 10].
Fig 1. Identification of a YidC1 (SpoIIIJ) variant that is necessary and sufficient to abolish the essentiality of the anti-σM factor YhdL.
A) Growth of PY79 PM-lacZ yhdL strain on LB plate with 60 μg/ml X-Gal. B) Transformation plate using 168 PM-lacZ as recipient and PY79 PM-lacZ yhdL::kan as DNA donor, with LB medium supplemented with kanamycin and X-Gal. Red arrows are pointed at intermediate sized blue colonies that were further analyzed by whole genome re-sequencing. C) Alignment of YidC homologs from bacterial and eukaryotic species. Red box highlights the aligned position corresponding to Q140 of B. subtilis YidC1. Residues identical to B. subtilis YidC1 are shown as “.” . D) Spot dilution of yhdL PxylA-yhdL depletion strain with different YidC1 alleles. WT168 has YidC1Q140 allele. E) Representative phase contrast microscopy image of yhdL mutants in different strain backgrounds. PY79 WT has a yidC1K140 allele. F) Representative phase contrast microscopy photo of yhdK mutants in different strain backgrounds. G) Colony morphology and size of yhdK mutants in different backgrounds. Colony size data were plotted using Box and Whisker chart and the bottom and top of the box are the first and third quartiles, the band inside the box is the second quartile (the median), and the X inside the box is the mean. Whiskers are one standard deviation above or below the mean. Outliers are shown as single dots. Average colony size change was calculated using formulation “change = (sample—control) / control x 100%”. P value was calculated using student’s t test, two tails, assuming unequal variances. Sample number n is at least 100 for each sample.
To identify the genetic differences in PY79 that confer tolerance of high σM, we compared the genome between B. subtilis strain 168 and PY79. There are over a hundred single nucleotide polymorphisms (SNPs) between the 168 reference sequence and PY79, as well as four large deletions in the genome of PY79 (including the SPβ prophage), causing a reduction in size of 180 kb for the PY79 genome compared with 168 [13, 14]. To identify differences that might correlate with tolerance of high σM activity, we compared the sequences of genes encoding RNA polymerase subunits, and genes in the σM and Spx regulons (Spx is a transcription regulator that is regulated by σM). However, no differences were noted in these 103 genes between the PY79 and 168 reference genomes (S1 Table).
Next, we used an unbiased, forward genetics approach to identity the mutation in PY79 that suppresses the lethality of a yhdL null mutation. We used genomic DNA from a PY79 yhdL::kan strain to transform 168 to kanamycin resistance, reasoning that the only viable transformants will likely also acquire the suppressing mutation. Because each competent cell of B. subtilis contains about 50 binding sites for DNA uptake, a competent cell can import multiple fragments of DNA during transformation in a process known as congression [15]. When a 168 strain containing a PM-lacZ reporter was transformed with chromosomal DNA from the viable PY79 yhdL::kan strain and selected on an LB plate supplemented with kanamycin and X-gal, we recovered numerous tiny blue colonies that did not grow when re-streaked onto fresh plates (consistent with the essentiality of YhdL in the 168 background), a few large white colonies (likely sigM mutants), and intermediate sized blue colonies (Fig 1B). The intermediate blue colonies grew to a similar size as a yhdL null mutant in PY79, consistent with acquisition of both the yhdL::kan allele and a second locus that suppresses the toxicity of high σM. Whole genome sequencing was performed on these transformants and the reads were mapped to the reference genome of 168. Out of 15 sequenced 168 transformants, 14 contained the same SNP imported from PY79 that generates a missense mutation in yidC1 (encoding YidC1Q140K) (S1A Fig, S2 Table). We therefore hypothesized that the yidC1Q140K allele was the suppressor needed for cells to tolerate the yhdL mutation.
YidC1 belongs to the OXA1/ALB3/YidC family of protein insertases responsible for inserting membrane proteins into the lipid bilayer, independently or in association with the Sec secretion system [16–18]. Escherichia coli encodes one essential homolog of YidC, while some bacteria such as B. subtilis encode two homologs, YidC1 (SpoIIIJ) and YidC2 (YqjG) [18, 19]. The gene encoding YidC1 was originally named spoIIIJ because mutations at this locus lead to a block at stage III of sporulation [19, 20]. However, spoIIIJ is constitutively expressed and functional in vegetative cells. The expression of the paralog YidC2 is regulated by an upstream gene mifM, which monitors the total membrane protein insertase activity and only allows expression of YidC2 when MifM is not efficiently inserted into the membrane by YidC1 [21]. Both YidC1 and YidC2 can fulfill the essential function of YidC insertase, with YidC1 essential for sporulation [22] and YidC2 important for the development of competence (S2A Fig) [23]. Interestingly, an alignment of YidC homologs revealed that the Gln140 residue is highly conserved among bacteria, and only B. subtilis PY79 YidC1 contains Lys at this position (Fig 1C).
To test if this YidC1 Gln to Lys variant (YidC1Q140K) is necessary and sufficient for tolerance of high σM, we introduced the yidC1Q140K mutation at the native locus of strain 168 using CRISPR-based mutagenesis, and found that yhdL was no longer essential (Fig 1D, S1B Fig). Conversely, changing the Lys140 into Gln in PY79 abolished the ability of PY79 to tolerate loss of yhdL (S1B Fig), suggesting that yidC1Q140K is necessary and sufficient for tolerance of a yhdL deletion mutation. To test if the yidC1K140 allele is dominant over the yidC1Q140 allele, we constructed merodiploid strains expressing both alleles of yidC1 (using a vector with xylose-inducible promoter PxylA that integrates into ganA and when induced produces about 70% of the amount of the native protein; Fig 2B). Both merodiploid strains (168 expressing the PY79 allele, PxylA-yidC1K140, or PY79 expressing the 168 allele, PxylA- yidC1Q140) could still tolerate the loss of yhdL (S1B Fig). This dominance suggests that YidC1Q140K leads to a gain of function that enables cells to tolerate high σM activity. Phase contrast microscopy revealed that a 168 yidC1Q140K yhdL mutant had a similar but slightly more elongated cell morphology compared with a PY79 yhdL mutant (Fig 1E), confirming the major role of YidC1Q140K in tolerance of a yhdL null mutation.
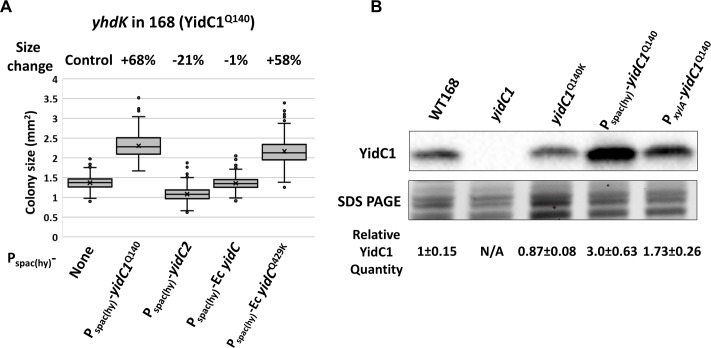
Fig 2. Overexpression of YidC1 increases tolerance of high σM activity.
A) Colony size of yhdK mutants in 168 background with overexpression of YidC homologs using an IPTG inducible Pspac(hy) promoter. Cells were grown on LB plate supplemented with 1 mM IPTG for 24 hours at 37°C. Box and Whisker chart was plotted as in Fig 1G. B) Representative Western blot of YidC1 protein in different strain backgrounds. Pspac(hy) and PxylA induced YidC1 strains contain the native YidC1 and were induced with 1 mM IPTG or 1% (w/v) xylose, respectively. Part of the SDS PAGE gel was shown as a loading control. Relative YidC1 quantity was calculated using band intensity values from three biological replicates and normalized using total lane intensity from the SDS PAGE gel. Data is presented as mean ± SEM.
As a general strategy to monitor cell fitness, we compared the impact of yidC1 alleles on morphology and colony size in the PY79 and 168 backgrounds for yhdK mutants. YhdK functions together with YhdL as an anti-σ complex, but unlike yhdL a yhdK null mutant is tolerated in 168 strains, although it does lead to an ~100-fold increase in σM regulon expression and severe growth defects [10]. Five independent trials with a 168 yhdK mutant, known to generate small colonies, revealed up to a ~10% change in average colony area. This variation likely results from small differences in the plating conditions. Although these small differences were in some cases judged to be statistically significant (based on P value in a t test, two tails, assuming unequal variances) (S1C Fig), this reflects the high sample number in each measurement (100–1000 colonies per measurement). Considering this level of variation between genetically identical strains, we only regard as significant those changes of >10% in colony size. Using this assay, we found that the small colony size, as well as the filamentous cell morphology of the 168 yhdK mutant, can be largely rescued by the yidC1Q140K allele (Fig 1F and 1G). Conversely, a yidC1K140Q mutation in PY79 yhdK converted the large colonies of the parent strain into the small round morphology of the 168 yhdK strain, with increased cell filamentation (Fig 1F and 1G). Deletion of yidC1 in a PY79 yhdK mutant mimicked a yidC1K140Q mutation (Fig 1G), likely because the cells now rely on the other YidC paralog, YidC2, which contains a glutamine residue in the equivalent position (Fig 1C) [19]. Overall, our results show that yidC1Q140K mutation is necessary and sufficient for B. subtilis to tolerate high σM activity caused by the absence of the anti-σ factor YhdL or its partner protein YhdK.
Overexpression of YidC1 increases tolerance of high σM activity
We hypothesized that the YidC1Q140K protein may simply be more active or abundant in cells than the native protein. To test if increasing insertase activity is sufficient to alleviate toxicity associated with high σM, we overexpressed the wild-type 168 YidCQ140 protein using a strong IPTG inducible promoter, Pspac(hy) [24]. Induction of the Pspac(hy)-yidC1Q140 allele led to a three-fold increase in the amount of YidC1 protein compared with WT (Fig 2B), and increased the colony size of a yhdK mutant by 68% (Fig 2A). This increase is less than the effect of the yidC1Q140K allele at the native locus (which increased yhdK colony size by 134%; Fig 1G), and consistently it only marginally increased the growth of a yhdL depletion strain under depletion condition (S2C Fig). The increase in the fitness of the yhdK mutant supports the hypothesis that higher insertase activity is beneficial for cells with elevated σM activity, but overexpression alone does not phenocopy the effect of the altered function allele.
We next tested whether overexpression of YidC2, the other YidC homolog in Bacillus, could benefit cells with high σM expression. Interestingly, when YidC2 was overexpressed from the Pspac(hy) promoter, the growth defect of either the yhdK mutant or the yhdL depletion strain was exacerbated (Fig 2A, S2C Fig). Furthermore, when the equivalent glutamine residue of YidC1Q140 was mutated to lysine, the YidC2Q148K mutant protein was toxic when overexpressed in a yhdK mutant (S2B Fig). Thus, YidC2 is unable to compensate for YidC1 in alleviating stress associated with high σM activity, even when the corresponding Gln to Lys substitution is present. Similarly, overexpression of E. coli YidC did not provide any benefit to a yhdK or yhdL mutant (Fig 2A). However, when the equivalent Gln to Lys substitution was present, overexpression of the E. coli YidCQ429K mutant was modestly beneficial, and colony size of the yhdK mutant increased by 58% (Fig 2A). These results suggest that different YidC homologs vary in their substrate preferences, and the Gln to Lys mutation may enhance the ability of the B. subtilis YidC1 and E. coli YidC insertases to facilitate membrane insertion of at least some σM-dependent proteins.
YidC1Q140K increases the positive charge inside the substrate binding groove
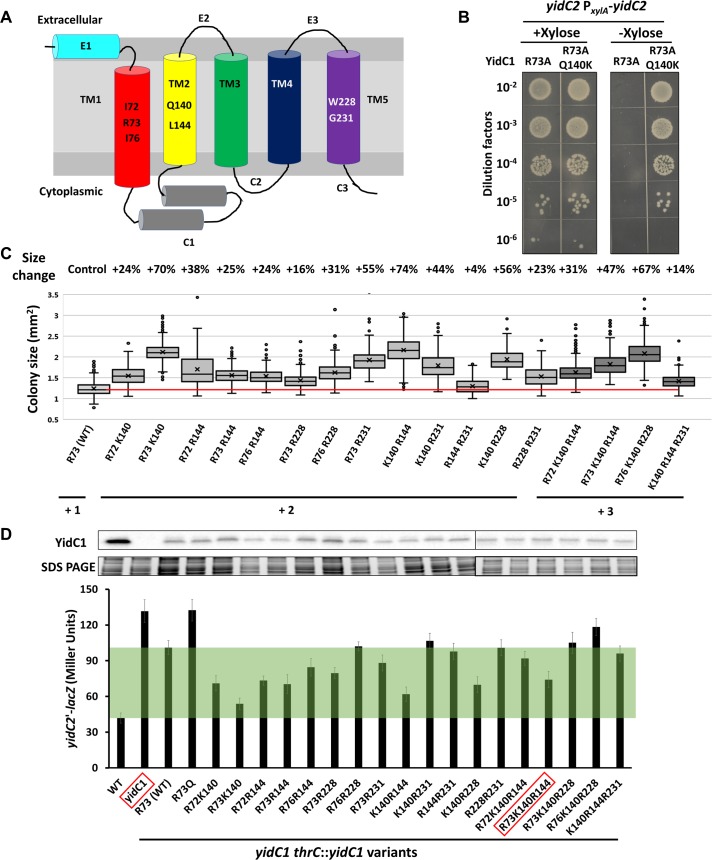
The structure of YidC2 from Bacillus halodurans revealed a positively charged hydrophilic groove formed by five transmembrane segments [16]. A positively charged residue in the substrate-binding groove is essential for the function of the insertase, as a R73A substitution in B. subtilis YidC1 completely abolished the essential function of YidC1 in vivo, while an R73K substitution retained function [16]. Although the positive charge is essential, the R73 residue is not, as the positive charge can be provided by substitution of any of six other residues inside the hydrophilic groove with Arg. These six positions include Ile72 and Ile76 in transmembrane region 1 (TM1), Gln140 and Leu144 in TM2, and Trp228 and Gly231 in TM5 [25] (Fig 3A, S3B Fig). Since both Arg and Lys are positively charged, we hypothesized that the key feature of the PY79 YidC1K140 protein is the +2 charge inside its substrate binding chamber. To test this hypothesis, we engineered a YidC1R73AQ140K double substitution protein with a net charge of +1 in the groove, where R73 is functionally replaced by K140. Expression of this protein is sufficient to support viability, as judged in a strain with depletion of yidC2 (Fig 3B), but is not sufficient to allow depletion of yhdL (Fig 1D). This supports the idea that the key effect of the Q140K substitution is to increase the positive charge in the substrate binding groove.
Fig 3. Effects of different charge variants inside the YidC1 hydrophilic groove.
A) Schematic drawing showing the extracellular, transmembrane and cytoplasmic domains of YidC1. The native sequence in strain 168 of the seven residues for the positive charge library are shown. B) Spot dilution of yidC2 PxylA-yidC2 depletion strain with different YidC1 variants in strain 168. C) Colony size of yhdK yidC1 mutants with different yidC1 alleles integrated in the thrC site. Residues that provide positive charge are labelled, and the total positive charge provided by these seven variable positions is indicated. The red line shows the median of colony size with a WT YidC1 from strain 168. Box and Whisker chart was plotted as in Fig 1G. D) Top, representative Western blots of YidC1 protein in different strain backgrounds. Part of the SDS PAGE gels were shown as loading control. Bottom, activity of yidC2’-lacZ translational fusion in the same strain backgrounds of the western blots. “WT” has a native copy of yidC1 while “yidC1” is a markerless deletion mutant. All other strains have the native yidC1 deleted and contain variants of yidC1 at thrC site. Among the seven variable positions, residues that provide positive charge(s) are labelled. YidC1 variants inside the red boxes cannot support growth of a yidC2 PxylA-yidC2 depletion strain in the absence of inducer xylose. Green shading highlights the difference between the activity of YidC1 at the native and thrC loci. Variants with yidC2’-lacZ activity inside the green shading exhibit increased MifM insertion activity compared with WT.
Increased charge inside the substrate binding groove is key for tolerance of high σM activity
Since it is the increased positive charge from +1 to +2, rather than the Q140K substitution per se, that rescues cells from high σM toxicity, we next set out to test if other combinations of positively charged residues can also rescue cells from high σM activity. To this end, we generated a library of YidC1 variants with five of the six positions mentioned above substituted (or not) with Arg, as shown previously to support function in the absence of Arg73 [25], and Gln140 substituted (or not) with Lys, as seen in PY79. In addition, we mutated Arg73 to Gln (or not) (Fig 3A). This leads to 27 = 128 possible charge combinations, ranging from 0 to a maximum of +7, with most containing a nominal positive charge of +2 to +5 in the substrate binding groove (S3A and S3B Fig). Note that, for the sake of simplicity, we assume that the K140 residue is positively charged since the epsilon-amino group of free Lys has a pKa of ~10.5, but the protonation state will likely depend on the local charge environment.
To identify YidC1 variants that can support growth of a yhdL depletion strain, we transformed a yidC1 null yhdL depletion strain with a library of yidC1 variants and selected for transformants that grew in the absence of yhdL induction. After sequencing and validation, we identified 103 yidC1 mutants that supported growth in the absence of yhdL induction (S3 Table). Among them, 88 mutants contained a nominal double positive charge in 13 unique combinations (S3A Fig). Five of these 13 combinations include K140, including the combination present in the PY79 YidC1 protein: R73 K140. The remaining 15 mutants contained a nominal triple positive charge, with 4 unique combinations. Interestingly, all four of these variants contained K140, and in each case K140 was present together with a pair Arg residues that also supported growth with Q140. It is possible that the presence of nearby Arg residues lowers the pKa of K140, and the protonation state of this residue may also vary depending on the nature of the bound substrate. Among the 17 unique combinations of double and triple positive charges, none has more than one positive charge in TM1, whereas TM2 and TM5 can each harbor double positive charges (S3A Fig, S3 Table). We conclude that all 17 functional YidC1 variants have an effective charge of between +2 and +3 in the hydrophilic groove. This strongly suggests that a modest increase of charge inside this groove facilitates the recruitment and insertion of σM-regulated proteins overproduced under YhdL depletion conditions, whereas a further increase may be detrimental to the activity or the stability of the insertase.
Each of these 17 YidC1 variants can alleviate the stress imposed by high σM activity and thereby support growth of the YhdL depletion strain (S4A Fig), and each increases the colony size of the yhdK mutant by up to 74% (Fig 3C). We also found 16 of these 17 variants can support growth of a YidC2 depletion strain (S4B Fig). Only the YidC1 R72 K140 R144 variant was unable to support cell growth under these conditions, suggesting that it is compromised in the ability to insert proteins essential for cell growth, despite its ability to modestly increase colony size of the yhdK mutant (31%; Fig 3C).
YidC1 inserts MifM into the membrane, which serves as a sensor of YidC1 function to regulate expression of yidC2 [21, 26]. If the ability of YidC1 to insert MifM is reduced, then the yidC2'-lacZ fusion is induced. Therefore, we used a yidC2’-lacZ translational fusion reporter to measure the ability of each YidC1 variant to insert MifM. A WT 168 strain exhibited very low level of yidC2’-lacZ activity in the presence of native yidC1 expression (~42 Miller Units (MU), Fig 3D), and deletion of yidC1 increased the reporter activity by about 3-fold (~132 MU, Fig 3D). Complementation of a yidC1 null mutant with the 168 version of yidC1 (single positive charge at R73) at the thrC locus reduced the reporter activity to ~101 MU (Fig 3D). The lack of complete complementation may be caused by the location of the gene, as the native locus is close to the origin of the chromosome and thus has a higher copy number than thrC in fast growing cells. Indeed, under our growth condition (late exponential phase in LB at 37°C), less YidC1 protein was detected in the thrC::spoIIIJ complementation strain than the WT (Fig 3D).
Using this strain background, we found that most of the YidC1 variants with double positive charge appear to have higher MifM insertion ability as they exhibited lower yidC2’-lacZ activity than the WT protein. Interestingly, the three mutants with double positive charge that have the largest colony size in a yhdK mutant background (R73 K140, K140 R144, and K140 R228), also showed the lowest yidC2’-lacZ activity, consistent with the hypothesis that they have higher insertase activity for both MifM and for membrane proteins that contribute to toxicity in strains with high σM-activity. Conversely, one mutant with a nominal triple positive charge (R76 K140 R228) exhibited a strong increase in colony size in the yhdK mutant background (Fig 3C), but appeared to have low MifM insertion activity (high yidC2'-lacZ expression; Fig 3D). Overall these results suggest that increasing the positive charge inside the substrate binding groove affects the efficiency of inserting membrane proteins, with several variants that appear to enhance the insertion efficiency for both MifM and σM-regulated proteins, while also retaining the ability to insert essential membrane proteins.
High σM activity causes a cascade of stresses that can be partially compensated by its regulon
YidC1 functions as a membrane insertase, by either independently inserting some single pass membrane proteins or by functioning as part of the Sec translocon to facilitate the folding of the translocated proteins [18, 27]. The finding that YidC1Q140K can suppress growth defects caused by high σM leads us to reason that the toxicity of high σM is caused by overexpression of membrane proteins that overwhelm the secretion system, and that YidC1Q140K suppresses this stress by functioning as a more efficient insertase/foldase or chaperone. In B. subtilis, membrane secretion stress is sensed by the CssRS (control of secretion stress regulator/sensor) two component system. In the presence of membrane secretion stress, caused either by overproduction of secreted proteins or high temperature, CssR upregulates expression of membrane proteases HtrA (high temperature requirement A) and HtrB to facilitate the re-folding or degradation of misfolded proteins [28, 29].
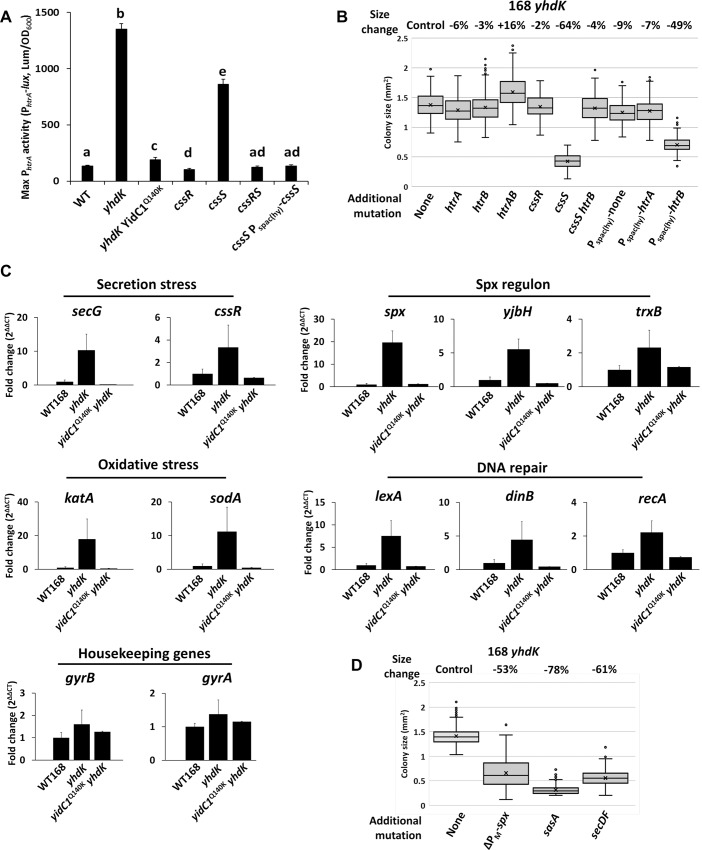
To test whether high σM causes secretion stress, we constructed a PhtrA-lux reporter to monitor induction of the CssR regulon. Indeed, maximum PhtrA activity was increased ten-fold in a yhdK mutant, and this induction was greatly reduced in the presence of the yidC1Q140K allele (Fig 4A, S5 Fig). As expected, deletion of the response regulator CssR abolished the induction of the regulon [29]. In contrast to a previous report [29], the absence of CssS increased transcription of PhtrA-lux reporter by ~six-fold (Fig 4A, S5 Fig). This increased expression could be complemented by ectopic expression of CssS, and this depends on CssR as judged by analysis of a cssS cssR double deletion (Fig 4A). This suggests that CssS may act both as a sensor kinase to activate CssR under inducing conditions, and also as a phosphatase to deactivate CssR in the absence of induction, as also noted for sensor kinases in other two component systems [30].
Fig 4. High σM activity causes a cascade of stresses that can be partially compensated by its own regulon.
A) Maximum promoter activity of htrA measured using a PhtrA-lux transcriptional fusion reporter in different strain backgrounds. Data is presented as mean ± SEM, and statistically significant different samples (Student’s t test, two-tailed P<0.05) are labelled with different letters. Sample number n is at least 4. B) Colony size of yhdK mutants in strain 168 with different additional mutations. Box and Whisker chart was plotted as in Fig 1G. C) Fold change of transcription levels of representative genes for stress response or housekeeping genes. Data is presented as mean ± SEM. Sample number n is at least 4. D) Colony size of yhdK mutants in strain 168 with different additional mutations. ΔPM-spx strain has the native spx deleted and an ectopic copy without the PM promoter [43].
To evaluate the role of the CssRS regulon in alleviating the stress associated with membrane protein overproduction, we constructed mutants lacking CssR-induced genes and used yhdK mutant colony size as a measure of fitness (Fig 4B). Surprisingly, we found that neither CssR-regulated membrane protease (HtrA and HtrB) is important for fitness of the yhdK mutant as judged by the effect of their deletion on colony size. Deletion of cssS reduced yhdK mutant colony size by over 60% (Fig 4B), and this reduction of colony size is reversed in a cssS htrB double mutant. This suggests that high HtrB activity is detrimental for the yhdK mutant. Consistently, when htrB, but not htrA, was overexpressed using the Pspac(hy) promoter, the colony size of the yhdK mutant was reduced by about 50% (Fig 4B). Deletion of these genes in the WT 168 background did not have much effect on the colony size (S6A Fig). These results suggest that the yhdK mutant is sensitive to the overproduction of membrane proteases. Single deletion of other proteases, including SipT, SipS, HtpX, PrsW and GlpG in a yhdK null mutant was also not detrimental, and in some cases led to a small beneficial effect in colony size (S6B Fig), again indicating that no single membrane protease is critical for yhdK mutant fitness. We speculate that under high σM conditions, the overproduced proteins may cause a backlog of membrane proteins that require a longer time to correctly insert and fold in the membrane. Some of these proteins may be essential for cell growth and are vulnerable to degradation by HtrB, and perhaps other proteases.
Misfolding of membrane proteins, as well as jamming the Sec translocon by hybrid proteins, leads to the generation of reactive oxygen species (ROS) and ultimately DNA damage [31, 32]. To test whether high σM also triggers a similar cascade of stresses, we performed quantitative RT-PCR to measure induction of representative stress genes. Indeed, the high σM activity in the yhdK null mutant was correlated with strong induction of genes involved in secretion stress (secG, cssR), oxidative stress (katA, sodA), and DNA repair (lexA, dinB and recA) (Fig 4C). The induction of these stress response genes was largely suppressed in the presence of the yidC1Q140K allele, suggesting that their induction is a downstream effect resulting from inefficient insertion of membrane proteins (Fig 4C).
Interestingly, while many genes in the σM regulon are involved in the synthesis and maintenance of the cell wall, there are also genes involved in regulation of redox balance (spx and the Spx regulon), DNA repair and recombination (for example, radA, radC and recU), and ppGpp synthesis and stringent response (sasA) [3]. There is also a candidate σM promoter associated with the secDF operon [3]. This suggests that a subset of genes in the σM regulon are involved in compensating for stresses associated with upregulation of σM. Indeed, deletion of secDF, sasA or the PM of spx dramatically reduced the colony size of the yhdK mutant (Fig 4D), while there was very little effect noted in the WT background (S6C Fig). Thus, these genes seem to play an important role in cell fitness specifically under conditions of high σM expression. Deleting single genes inside the Spx regulon in the yhdK mutant did not lead to noticeable reduction of colony size (S6D Fig), suggesting functional redundancy within the Spx regulon.
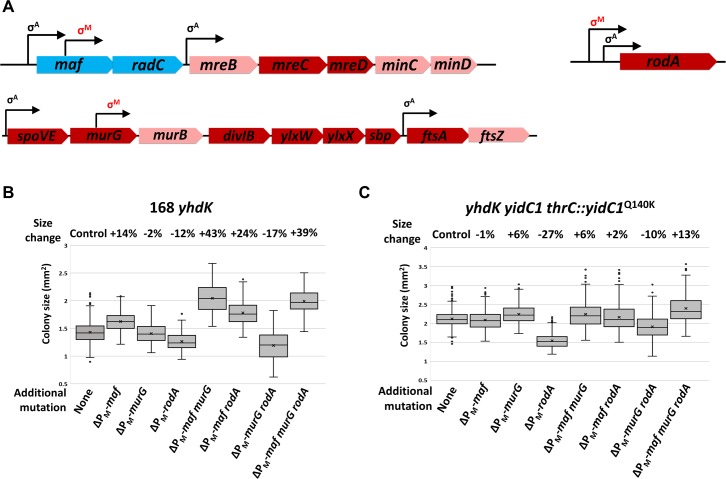
Among the 69 genes currently assigned to the σM regulon according to SubtiWiki [33], 38 code for membrane-associated or secreted proteins (S4 Table). To demonstrate the burden these membrane proteins may cause when overexpressed under high σM condition, we mutated the σM-dependent promoters (PM) for genes important for the elongasome and the divisome and tested the consequence on fitness measured by colony size of the yhdK mutant. We focused on a PM inside the maf gene that transcribes the radC-mreB-mreC-mreD-minC-minD operon, a PM upstream of rodA gene, and a PM inside murG that contributes to transcription of the murB-divIB-ylxW-ylxX-sbp-ftsA-ftsZ operon (Fig 5A). Mutation of these three promoters individually or in combination had little effect on the colony size of the WT strain (S6E Fig), since these genes are also expressed from σA-dependent promoters. In a yhdK mutant, however, mutation of PM(maf) and PM(murG) led to an additive increase of colony size, while mutation of PM(rodA) led to a small detrimental effect (Fig 5B). We conclude that overexpression of genes downstream of PM(maf) and PM(murG) may overwhelm the membrane-protein insertion pathway, and thereby contribute to a net negative effect on cell fitness. Consistent with this idea, in cells expressing YidC1Q140K, the beneficial effect of mutating PM(maf) and PM(murG) was largely abolished (Fig 5C).
Fig 5. Preventing induction of specific σM-dependent membrane proteins alleviates stresses caused by high σM activity.
A) Schematic drawing of the maf and murG operons and the rodA gene. σA and σM controlled promoters are shown with arrows. Cytoplasmic proteins are labelled in blue boxes, peripheral membrane proteins in pink boxes, and integral membrane proteins in red boxes. B,C) Colony size of yhdK mutants with different PM promoter(s) mutated in (B) WT strain 168 or (C) strain 168 with the native yidC1 deleted and a yidC1Q140K allele integrated at the thrC site. Box and Whisker chart was plotted as in Fig 1G.
Discussion
The YidC membrane protein insertase is the bacterial representative of the YidC/Oxa1/Alb3 protein family of evolutionary conserved integral membrane proteins [18, 27]. In E. coli, YidC is required for the assembly of one or more essential protein complexes that reside in the inner membrane, including subunits of the energy generating F1FO ATPase and NADH dehydrogenase I [34]. Although YidC can function in concert with the SecYEG translocon as a foldase/chaperone, YidC can also function independently for the insertion of small membrane proteins with one or two transmembrane segments [35].
Bacillus subtilis encodes two YidC paralogs, YidC1 (SpoIIIJ) and YidC2 (formerly YqjG). Either protein can support growth of B. subtilis, but a double mutant is inviable. Under most conditions YidC1 is the functional YidC homolog and is responsible for insertion of transmembrane proteins, including the F1FO ATPase [19]. MifM, a membrane protein encoded upstream of yidC2, is a monitoring substrate of YidC1 and serves as a sensor for YidC1 activity [21]. In the absence of YidC1 activity, a prolonged arrest during MifM translation occurs that alters the mifM-yidC2 mRNA structure to expose the ribosome-binding site of yidC2, thereby activated YidC2 expression [26, 36]. Although either YidC1 or YidC2 can support growth, they appear to differ in the efficacy of inserting specific proteins: cells lacking YidC1 are impaired in sporulation [20], whereas those lacking YidC2 have decreased competence [23]. The relationship between YidC protein sequence and the selection of specific client proteins is poorly understood. In cells depleted for both YidC1 and YidC2 there was a substantial upregulation in expression of the Clp protease system and the LiaIH membrane-stress proteins that are regulated by the LiaRS two-component system [23]. This suggests that impairment of membrane protein insertion leads to an accumulation of misfolded proteins and disruption of the cell membrane.
One critical feature required for YidC function, as first visualized in the structure of the B. halodurans YidC protein [16], is a hydrophilic groove postulated to interact transiently with transmembrane segments of nascent integral membrane proteins. In YidC proteins the first transmembrane region (TM1) contains a conserved Arg residue that is essential for function in many, but not all, YidC orthologs [37]. This positively charged residue is postulated to form a salt-bridge with single-pass transmembrane client proteins with acidic residues in their amino-terminal region [16]. Remarkably, the function of this conserved Arg residue (R73 in Bacillus YidC1) can be replaced by Arg residues introduced at any of six other positions in transmembrane segments of B. halodurans YidC [25].
In this work, we have described an unusual variant of YidC1 found in B. subtilis strain PY79 which contains the conserved R73 residue and additionally a second positively charged residue (K140) at a position that can functionally replace R73. This variant YidC1 protein (YidC1Q140K) is necessary and sufficient for B. subtilis to survive high level expression of the σM regulon. By screening a library of YidC1 variants, we revealed that all YidC1 proteins that enable cells to tolerate loss of yhdL contain at least two, and in some cases three, positively charged residues in this groove. We postulated that these YidC1 variants may be more capable of accommodating the increased expression of membrane proteins under σM control. Support for this hypothesis derives from experiments in which the deletion of individual σM-dependent promoters that control operons encoding multiple integral membrane proteins was found to improve fitness of strains with high σM activity. Thus we reason that the relevant feature of YidC1Q140K is the presence of increased positive charge in the presumed substrate-binding groove, which probably results in an increased insertase/foldase activity for specific σM-regulated membrane proteins.
A similar selective pressure may also account for the reproducible recovery of a missense mutation encoding a YidC1 Trp138Arg substitution (YidC1SmW138R) in a Streptococcus mutans strain lacking YidC2 [38]. In this case, the lack of YidC2 leads to a variety of phenotypes including poor growth, acid and osmotic stress sensitivity, and a lack of competence. The YidC1SmW138R protein retains the positive charge of Arg66 (equivalent to Arg73 in B. subtilis YidC1) and additionally has a second positive charge contributed by Arg138 (equivalent to Ile145 in B. subtilis YidC1 and adjacent to position 144, where an Arg substitution can functionally replace Arg73 [25]). A similar functional variant (R73, R144) was recovered three times from our B. subtilis YidC1 library (#40, 65, and 74; S3 Table).
Our results have provided further insight into the nature of the lethality associated with a yhdL deletion that unleashes high level σM activity [10]. In strains with elevated σM activity there is an increased flux of proteins targeted to the membrane and a subset of these may be inefficiently inserted by the native YidC1 protein. This can result in a jamming of YidC1-dependent protein translocation and a disruption of membrane function. The downstream sequelae associated with this disruption include misfolding of proteins and induction of the secretion stress response (CssR), as well as induction of genes associated with oxidative stress and DNA damage responses. These types of stresses may explain, in part, the inclusion of appropriate compensatory functions (including Spx, some DNA repair functions, and SecDF) as part of the σM regulon. It is presently unclear why the yidC1Q140K allele arose in the PY79 strain, or what conditions may have led to its selection. However, this strain was derived from strains treated with chemical mutagens to cure the endogenous prophage SPß [14] and this or other selection conditions may have contributed to emergence of this mutation. Further studies will be required to better understand how variations in YidC structure can fine-tune the substrate selectivity of this essential membrane protein insertase, and the stresses that arise when this system is challenged by the induction of highly expressed membrane proteins.
Materials and methods
Strains, plasmids and growth condition
All strains used in this work are listed in S5 Table, and all DNA primers are listed in S6 Table. Bacteria were routinely grown in liquid lysogeny broth (LB) with vigorous shaking, or on plates (1.5% agar; Difco) at 37°C unless otherwise stated. LB medium contains 10 g tryptone, 5 g yeast extract, and 5 g NaCl per liter. Plasmids were constructed using standard methods [39], and amplified in E. coli DH5α or TG1 before transforming into B. subtilis. For selection of transformants, 100 μg ml-1 ampicillin or 30 μg ml-1 kanamycin was used for E. coli. Antibiotics used for selection of B. subtilis transformants include: kanamycin 15 μg ml-1, spectinomycin 100 μg ml-1, macrolide-lincosamide-streptogramin B (MLS, contains 1 μg ml-1 erythromycin and 25 μg ml-1 lincomycin), and chloramphenicol 10 μg ml-1. To reduce the likelihood of suppressors in yhdK mutants, fresh transformants were isolated and grown for assays using yhdK mutant strains. For spot dilution assays, cells were first grown in liquid culture at 37°C with shaking to mid-exponential phase (OD600 ~0.3–0.4), washed twice in LB medium without inducer, then serial diluted in LB medium without inducer. 10 μl of each diluted culture was then spotted onto plates and allowed to dry before incubation at 37°C for 12–24 hours.
Genetic techniques
Chromosomal and plasmid DNA transformation was performed as previously stated [40]. The pPL82 plasmid-based Pspac(hy) overexpression constructs were sequencing confirmed before linearized and integrated into the amyE locus [24]. The pAX01 plasmid-based PxylA overexpression constructs were sequencing confirmed before linearized and integrated into the ganA locus [41]. The ganA::PxylA-yhdL-cat and thrC::PM-spoVG-lacZ-spec constructs were made with LFH PCR to avoid antibiotic marker conflicts [10]. Markerless in-frame deletion mutants were constructed from BKE or BKK strains as described [42]. Briefly, BKE or BKK strains were acquired from the Bacillus Genetics Stock Center (http://www.bgsc.org), chromosomal DNA was extracted, and the mutation containing an ermR (for BKE strains) or kanR (for BKK strains) cassette was transformed into our WT 168 strain. The antibiotic cassette was subsequently removed by introduction of the Cre recombinase carried on plasmid pDR244, which was later cured by growing at the non-permissive temperature of 42°C. Gene deletions were confirmed by PCR screening using flanking primers. Unless otherwise described, all PCR products were generated using B. subtilis 168 strain chromosomal DNA as template. DNA fragments used for gene over-expression were verified by sequencing. Null mutant constructions were verified by PCR.
Mutations to selectively inactivate σM-dependent promoters were generated by either promoter deletion (rodA) or by inactivating point mutations (murG, maf, spx). To inactivate PM(rodA), a 91 bp region containing the PM (located upstream of a σA-dependent promoter) was deleted using CRISPR. The resulting deletion had a junction sequence of: CACATTATCGC/TTTCGTGTAGC. The point mutations inactivating murG and maf both changed the -10 region sequence from consensus, CGTC, to TGTT. The PM* mutation inactivating the promoter regulating the yjbC-spx operon is a 3 bp substitution changing the -10 region from consensus, CGTC, to AAGT, as previously described [43].
Mutations of yidC1 at native locus, as well as ΔPM-rodA were constructed using a clustered regularly interspaced short palindromic repeats (CRISPR)-based mutagenesis method as previously described [10]. Briefly, possible protospacer adjacent motifs (PAM) site, which is NGG for Streptococcus pyogenes Cas9, was identified and off-target sites were checked against B. subtilis genome using BLAST. If no off-target site was identified, the PAM site was chosen and 20 bps upstream of the site were used as sgRNA and cloned into vector pJOE8999 [44]. The repair template was generated by joining two or more PCR products, with intended mutation (with additional mutation to abolish gRNA recognition if necessary) introduced by PCR primers, and cloned into the pJOE8999-sgRNA vector. DNA sequence for amino acid substitution was chosen according to the preferred codons of B. subtilis [45]. The pJOE8999 derivative containing both the sgRNA and repair template was then cloned into competent cells of E. coli strain TG1 to produce concatemer plasmids, which were transformed into B. subtilis at 30°C. Transformants were then grown at 42°C to cure the plasmid, and intended mutations were confirmed by sequencing. ΔPM-rodA was constructed with repair template amplified using primers 7426, 7427, 7428 and 7429, and the gRNA constructed using 7430 and 7431. YidC1Q140K mutation in 168 was constructed with repair template amplified using primers 7868, 7869, 7870 and 7871, and the gRNA constructed using 7866 and 7867. YidC1K140Q mutation in PY79 was constructed with repair template amplified using primers 7866, 8247, 8246 and 7871, and the gRNA constructed using 8248 and 8249. YidC1R73A mutation was constructed with repair template amplified using strain 168 genomic DNA as template with primers 7868, 8280, 8281 and 7871, and the gRNA constructed using 8278 and 8279. YidC1R73AQ140K mutation was constructed with the same primer sets for YidC1R73A mutation, expect the repair template was amplified using strain PY79 genomic DNA. All constructs were sequencing confirmed.
A YidC1 library of varying positive charge composition was constructed using degenerate primers and LFH PCR. Four DNA fragments were amplified and joined using LFH PCR. The fragments include one with part of gene hom and thrC (amplified with primers 8766 and 8767), one with the yidC1 gene from 168 (amplified with primers 8768 and 8681, containing the native promoter and ribosomal binding site), one with a specR cassette (amplified with primers 8682 and 8769), and one with part of thrC and full length of thrB (amplified with primers 8770 and 8771). The joined PCR product was first transformed into strain 168 to generate HB23976. Then using genomic DNA of HB23976 as template, four new DNA fragments were amplified using degenerate primer pairs 8766 and 8689, 8686 and 8690, 8687 and 8691, and 8688 and 8771. These fragments were joined together using LFH PCR and the joined PCR product (more than 10 μg DNA) was expected to contain all 128 possible combinations of YidC1 charge from 0 to +7. The PCR product library was used to transform strain 168, and more than ten-thousand transformants (from 20 plates, each contains more than 500 colonies) were pooled together to extract genomic DNA to form a virtually equivalent DNA library of the yidC1 variants. This genomic DNA library provides high transformation efficiency and was used to transform a yhdL depletion strain (HB23953) and transformants were selected on LB plate supplemented with X-gal but no xylose for yhdL induction. More than 200 transformants were re-streaked onto fresh LB plate with X-gal but no xylose to confirm robust growth under high σM condition, and 103 of them were Sanger sequenced for the thrC-yidC1-spec region to identify the yidC1 variants.
Whole genome re-sequencing and sequence analysis
Chromosomal DNA of suppressor strains was extracted using Qiagen DNeasy Blood & Tissue Kit. DNA was then sent to Cornell University Institute of Biotechnology for sequencing using Illumina HiSeq2500 with Single-end 100 bp reads. Sequencing results were analyzed using CLC workbench version 8.5.1 and mapped to the genome of strain 168 (reference accession number NC_000964.3). Note that our working stock of B. subtilis 168 has 21 SNPs compared to the cited reference sequence, and these common SNPs were not considered, and only newly introduced SNPs from the PY79 strain were tabulated (S2 Table). The unmapped reads were de novo assembled and contigs larger than 1 kb were aligned using the BLAST algorithm against the genome of strain PY79 (reference accession number NC_022898.1). Single nucleotide variants (SNVs) were detected using default settings, and gene deletions larger than 300 bps were identified by manually scanning regions of low coverage.
Colony size measurement
Colony size was measured using Fiji Image J [46]. Briefly, bacterial cells were grown in liquid LB medium at 37°C with vigorous shaking to mid-exponential phase (OD600~0.3–0.4), then serial diluted to desired concentrations. Diluted cells were plated onto fresh LB plates (15 ml medium per plate, the diameter of the plate is 10 cm and the height 15 mm, VWR, US, Catalog number 25384–342), and multiple dilutions were used. Plates were incubated at 37°C for 24 hours. Plates containing less than 100 separate single colonies were used for size measurement, because this number of colonies per plate ensures sufficient sample size and does not cause reduced colony size due to crowdedness and nutrient limitation. Pictures of plates were taken with a ruler as a length reference, and colony size was measured using Fiji Image J per software’s instruction. For each strain, at least 100 colonies were measured, and box and whisker plots were used.
Luciferase reporter construction and measurement
Luciferase reporter construction and measurement was performed as previous described [47]. The luciferase reporters were constructed by inserting the tested promoters into the multicloning site of pBS3Clux [48]. The promoter PhtrA was amplified using primers 8403 and 8404. The original chloramphenicol resistant cassette was replaced by an erythromycin resistance cassette when necessary to avoid antibiotic resistance conflicts. For luciferase measurements, 1 μl of exponentially growing cells were inoculated into 99 μl of fresh medium in a 96 well plate, incubated at 37°C with shaking using a SpectraMax i3x or a Synergy H1 (BioTek Instruments, Inc. VT) plate reader, and OD600 and luminescence were measured every 12 min. The data was analyzed using SoftMax Pro 7.0 software. Promoter activity was normalized by dividing the relative light units (RLU) by OD600.
Phase contrast microscopy
Cells were grown in liquid LB medium to exponential phase (OD600 ~0.4) and loaded on saline (0.90% NaCl, w/v) agarose pads (0.8% final concentration) on a glass slide. Phase contrast images were taken using a Leica DMi8 microscope equipped with a 100x immersion objective and Leica Application Suite X software.
Western blot
Western blot was performed as described previously [43]. Briefly, cells were grown in 5 ml LB medium in a 20 ml test tube a 37°C with vigorous shaking. Inducer IPTG or xylose were added when required by the construct to induce YidC1. After reaching exponential phase (OD600~0.3–0.4), 1 ml cells were pelleted by centrifugation at 4°C, resuspended in 100 μl pre-chilled buffer (containing 25 μl 4X Laemmli Sample buffer (Bio-Rad, USA), 10 μl 1M DTT, 65 μl H2O) and kept on ice. Cells were then lysed and crude cell lysate was loaded to a 4–20% SDS-PAGE stain-free gel for electrophoresis. The gel was visualized using ImageLab with stain-free gel protocol. Proteins were then transferred onto a PVDF membrane using the TransBlot Turbo Transfer System (Bio-Rad, USA), and immune blotting was performed using anti-YidC1 antiserum. The blot was visualized using the Clarity Western ECL substrate (Bio-Rad) and ImageLab software. Band intensity was calculated using the ImageLab software and normalized using total protein amount according to SDS-PAGE gel image.
β-galactosidase assay for yidC2’-lacZ
Bacterial cells containing the yidC2’-lacZ translational fusion were grown to late exponential phase (OD600 0.6–0.8) in 96-well plates with 200 μl LB medium per well at 37°C with vigorous shaking. Cells were pelleted by centrifugation, resuspended in Z buffer supplemented with DTT (dithiothreitol, 400 nM final concentration), and lysed by lysozyme. OD600 was measured before lysozyme treatment. After lysis, ONPG (ortho-nitrophenyl-β-galactoside) was added and OD420 and OD550 were measured every 2 minutes. Product accumulation was calculated using formula product = 1000×[OD420-(1.75×OD550)] and plotted against time. The slope of the linear part of the product accumulation curve was calculated using Excel and Miller Units (MU) were calculated using formula MU = Slope/OD600/V, where V is the volume of cells used for the reaction (200 μl).
RNA extraction and qRT-PCR
Cells were grown to mid-exponential phase (OD600 ~0.4) and RNA was extracted using RNeasy Mini Kit (Qiagen). The extracted was then treated with DNase I (Invitrogen) and the quality of RNA was checked with electrophoresis. RNA was then reverse transcribed into cDNA using High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems). Quantitative real-time PCR (qRT-PCR) was performed using SYBR Green (BioRad) and the topA gene was used for reference of data normalization.
Supporting information
A) Map of SNPs from strain PY79 to 168 and distribution of SNPs contained in each congression suppressors. Genome coordinates were based on the 168 reference genome with NCBI accession number NC_000964.3. B) Transformation plates of yhdL::kan allele transformed into different strain backgrounds, selected on LB plates supplemented with kanamycin, X-gal and 1% xylose. C) Variation of yhdK colony size measurement between trials on different days with different batches of LB plates. P value was calculated using Student’s t test, and percentage changes of average colony size were shown.
(TIF)
A) Venn diagram of function overlap and distinction of YidC1 and YidC2 of Bacillus subtilis. B) Streaking of yhdK Pspac(hy)-yidC2Q148K on plates of LB or LB supplemented with 1 mM IPTG (final concentration). C) Spot dilution of yhdL depletion strains with Pspac(hy) based overexpression of different YidC homologs, on LB plates supplemented with a final concentration of 1% xylose (+Xylose), 1 mM IPTG (+IPTG) or nothing (None).
(TIF)
A) Distribution of 128 possible charge variants of YidC1 in the tested library. Theoretically, the majority of YidC1 variants in the input library have a charge of +2 to +5 in the hydrophilic groove, while experimental data from 103 samples suggests that the ones capable of providing high σM tolerance contain a charge of +2 or +3. Among the 103 samples with charge of +2 or +3, the positive charge can be located in one, two or three transmembrane segments, with the exception that no sample contains more than one positive charge in TM1 alone. B) Crystal structure of YidC from Bacillus halodurans (PDB ID 3WO6), showing the seven variable amino acid providing positive charges in the hydrophilic substrate binding chamber of the enzyme. Gly231 is not visible due to the lack of side chain of this residue. TM1-5, transmembrane region 1–5; E1, extracytoplasmic region 1; C1, cytoplasmic region 1.This figure was generated using UCSF Chimera 1.13[49].
(TIF)
A) yhdL depletion strains with YidC1 variants were streaked on LB plates with or without xylose inducer for yhdL. The positive charge of each variant was labelled next to the streaking, with a negative control “None” meaning no yidC1 variant at thrC locus (weak growth due to the depletion conditions, and cannot be restreaked), and a positive control “YidC1PY79” meaning the native YidC1 mutated into the PY79 Q140K version (HB23719). B) Spot dilution of YidC depletion strains with yidC1 variants at thrC locus. The depletion strain has its native yidC1 and yidC2 deleted, and a xylose inducible copy of PxylA-yidC2. Diluted cultures were spotted on LB without xylose (-Xyl) or with 1% final concentration of xylose (+Xyl). The negative control (None) has no yidC1 at thrC locus, while the positive control has the 168 version of yidC1 that contains a single positive charge at R73. Some YidC1 variants exhibited reduced growth ability, and the variant containing R72 K140 R144 failed to grow, although the emergence of suppressors was noted.
(TIF)
A) Growth curves of WT strain 168, yhdK null, and yhdK with YidC1Q140K single amino acid substitution. B) PhtrA activity of strains in panel A during growth. C) Growth curves of WT strain 168, cssR null, cssS null, cssRS double null, and cssS null with an ectopic IPTG inducible copy under induced or uninduced conditions. D) PhtrA activity of strains in panel C during growth. The OD600 and luminescence were measured every 12 minutes. At least four biological replicates were used for each strain, and the results are shown as mean ± SEM.
(TIF)
A) WT B. subtilis 168 vs. strains with the indicated mutations. B) B. subtilis yhdK null mutant vs. strains additionally mutant for the indicated gene. C) WT B. subtilis 168 vs. strains lacking either the σM-regulated promoter for spx (σPM-spx) or the sasA or secDF genes. D) B. subtilis yhdK null mutant vs. strains additionally mutant for the indicated gene. E) WT B. subtilis 168 vs. strains lacking the σM-regulated promoter (PM) for the indicated gene(s). The percentage change relative to the average colony size is shown above each Box and Whisker plot.
(TIF)
No mutations were found in these genes.
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
Acknowledgments
We thank Dr. Shinobu Chiba for providing the yidC2’-lacZ translational fusion reporter and antiserum for YidC1(SpoIIIJ), Dr. David Rudner and Alexander Meeske for strain BAM1077, Dr. Vaidehi Patel and Dr. Wen-Wen Zhou for providing the PM* mutations for the murG and maf operons, and Dr. Tobias Doerr for valuable discussions and sharing equipment. We also thank Dr. Pete Chandrangsu and Ahmed Gaballa for help with experimental design and interpretation, and Daniel Roistacher for helping with experiments.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This work was funded by a grant from the National Institutes of Health (to JDH) under award number R35GM122461. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Helmann JD. Bacillus subtilis extracytoplasmic function (ECF) sigma factors and defense of the cell envelope. Curr Opin Microbiol. 2016;30:122–32. Epub 2016/02/24. 10.1016/j.mib.2016.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sineva E, Savkina M, Ades SE. Themes and variations in gene regulation by extracytoplasmic function (ECF) sigma factors. Curr Opin Microbiol. 2017;36:128–37. 10.1016/j.mib.2017.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eiamphungporn W, Helmann JD. The Bacillus subtilis σM regulon and its contribution to cell envelope stress responses. Mol Microbiol. 2008;67(4):830–48. Epub 2008/01/09. 10.1111/j.1365-2958.2007.06090.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Horsburgh MJ, Moir A. σM, an ECF RNA polymerase sigma factor of Bacillus subtilis 168, is essential for growth and survival in high concentrations of salt. Mol Microbiol. 1999;32(1):41–50. Epub 1999/04/27. 10.1046/j.1365-2958.1999.01323.x . [DOI] [PubMed] [Google Scholar]
- 5.Luo Y, Helmann JD. Analysis of the role of Bacillus subtilis σM in beta-lactam resistance reveals an essential role for c-di-AMP in peptidoglycan homeostasis. Mol Microbiol. 2012;83(3):623–39. Epub 2012/01/04. 10.1111/j.1365-2958.2011.07953.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meeske AJ, Riley EP, Robins WP, Uehara T, Mekalanos JJ, Kahne D, et al. SEDS proteins are a widespread family of bacterial cell wall polymerases. Nature. 2016;537(7622):634–8. Epub 2016/08/16. 10.1038/nature19331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Asai K. Anti-sigma factor-mediated cell surface stress responses in Bacillus subtilis. Genes Genet Syst. 2018;92(5):223–34. Epub 2018/01/19. 10.1266/ggs.17-00046 . [DOI] [PubMed] [Google Scholar]
- 8.Woods EC, McBride SM. Regulation of antimicrobial resistance by extracytoplasmic function (ECF) sigma factors. Microbes Infect. 2017;19(4–5):238–48. 10.1016/j.micinf.2017.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yoshimura M, Asai K, Sadaie Y, Yoshikawa H. Interaction of Bacillus subtilis extracytoplasmic function (ECF) sigma factors with the N-terminal regions of their potential anti-sigma factors. Microbiology. 2004;150(Pt 3):591–9. 10.1099/mic.0.26712-0 [DOI] [PubMed] [Google Scholar]
- 10.Zhao H, Roistacher DM, Helmann JD. Deciphering the essentiality and function of the anti-σM factors in Bacillus subtilis. Mol Microbiol. 2019. 10.1111/mmi.14216 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murakami T, Haga K, Takeuchi M, Sato T. Analysis of the Bacillus subtilis spoIIIJ gene and its paralogue gene, yqjG. J Bacteriol. 2002;184(7):1998–2004. 10.1128/JB.184.7.1998-2004.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tjalsma H, Bron S, van Dijl JM. Complementary impact of paralogous Oxa1-like proteins of Bacillus subtilis on post-translocational stages in protein secretion. J Biol Chem. 2003;278(18):15622–32. 10.1074/jbc.M301205200 . [DOI] [PubMed] [Google Scholar]
- 13.Zeigler DR, Pragai Z, Rodriguez S, Chevreux B, Muffler A, Albert T, et al. The origins of 168, W23, and other Bacillus subtilis legacy strains. J Bacteriol. 2008;190(21):6983–95. Epub 2008/08/30. 10.1128/JB.00722-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schroeder JW, Simmons LA. Complete Genome Sequence of Bacillus subtilis Strain PY79. Genome Announc. 2013;1(6). Epub 2013/12/21. 10.1128/genomeA.01085-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dubnau D, Cirigliano C. Fate of transforming DNA following uptake by competent Bacillus subtilis. Formation and properties of products isolated from transformed cells which are derived entirely from donor DNA. J Mol Biol. 1972;64(1):9–29. Epub 1972/02/28. 10.1016/0022-2836(72)90318-x . [DOI] [PubMed] [Google Scholar]
- 16.Kumazaki K, Chiba S, Takemoto M, Furukawa A, Nishiyama K, Sugano Y, et al. Structural basis of Sec-independent membrane protein insertion by YidC. Nature. 2014;509(7501):516–20. Epub 2014/04/18. 10.1038/nature13167 . [DOI] [PubMed] [Google Scholar]
- 17.Tsirigotaki A, De Geyter J, Sostaric N, Economou A, Karamanou S. Protein export through the bacterial Sec pathway. Nat Rev Microbiol. 2017;15(1):21–36. Epub 2016/11/29. 10.1038/nrmicro.2016.161 . [DOI] [PubMed] [Google Scholar]
- 18.Hennon SW, Soman R, Zhu L, Dalbey RE. YidC/Alb3/Oxa1 Family of Insertases. J Biol Chem. 2015;290(24):14866–74. Epub 2015/05/08. 10.1074/jbc.R115.638171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saller MJ, Fusetti F, Driessen AJ. Bacillus subtilis SpoIIIJ and YqjG function in membrane protein biogenesis. J Bacteriol. 2009;191(21):6749–57. Epub 2009/09/01. 10.1128/JB.00853-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Errington J, Appleby L, Daniel RA, Goodfellow H, Partridge SR, Yudkin MD. Structure and function of the spoIIIJ gene of Bacillus subtilis: a vegetatively expressed gene that is essential for sigma G activity at an intermediate stage of sporulation. J Gen Microbiol. 1992;138(12):2609–18. Epub 1992/12/01. 10.1099/00221287-138-12-2609 . [DOI] [PubMed] [Google Scholar]
- 21.Chiba S, Ito K. MifM monitors total YidC activities of Bacillus subtilis, including that of YidC2, the target of regulation. J Bacteriol. 2015;197(1):99–107. Epub 2014/10/15. 10.1128/JB.02074-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Corte L, Valente F, Serrano M, Gomes CM, Moran CP Jr., Henriques AO. A conserved cysteine residue of Bacillus subtilis SpoIIIJ is important for endospore development. PLoS One. 2014;9(8):e99811 Epub 2014/08/19. 10.1371/journal.pone.0099811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saller MJ, Otto A, Berrelkamp-Lahpor GA, Becher D, Hecker M, Driessen AJ. Bacillus subtilis YqjG is required for genetic competence development. Proteomics. 2011;11(2):270–82. Epub 2011/01/05. 10.1002/pmic.201000435 . [DOI] [PubMed] [Google Scholar]
- 24.Quisel JD, Burkholder WF, Grossman AD. In vivo effects of sporulation kinases on mutant Spo0A proteins in Bacillus subtilis. J Bacteriol. 2001;183(22):6573–8. 10.1128/JB.183.22.6573-6578.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shimokawa-Chiba N, Kumazaki K, Tsukazaki T, Nureki O, Ito K, Chiba S. Hydrophilic microenvironment required for the channel-independent insertase function of YidC protein. Proc Natl Acad Sci U S A. 2015;112(16):5063–8. Epub 2015/04/10. 10.1073/pnas.1423817112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chiba S, Ito K. Multisite ribosomal stalling: a unique mode of regulatory nascent chain action revealed for MifM. Mol Cell. 2012;47(6):863–72. Epub 2012/08/07. 10.1016/j.molcel.2012.06.034 . [DOI] [PubMed] [Google Scholar]
- 27.Kuhn A, Kiefer D. Membrane protein insertase YidC in bacteria and archaea. Mol Microbiol. 2017;103(4):590–4. Epub 2016/11/24. 10.1111/mmi.13586 . [DOI] [PubMed] [Google Scholar]
- 28.Hyyrylainen HL, Bolhuis A, Darmon E, Muukkonen L, Koski P, Vitikainen M, et al. A novel two-component regulatory system in Bacillus subtilis for the survival of severe secretion stress. Mol Microbiol. 2001;41(5):1159–72. 10.1046/j.1365-2958.2001.02576.x . [DOI] [PubMed] [Google Scholar]
- 29.Darmon E, Noone D, Masson A, Bron S, Kuipers OP, Devine KM, et al. A novel class of heat and secretion stress-responsive genes is controlled by the autoregulated CssRS two-component system of Bacillus subtilis. J Bacteriol. 2002;184(20):5661–71. Epub 2002/09/25. 10.1128/JB.184.20.5661-5671.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Podgornaia AI, Laub MT. Determinants of specificity in two-component signal transduction. Curr Opin Microbiol. 2013;16(2):156–62. Epub 2013/01/29. 10.1016/j.mib.2013.01.004 . [DOI] [PubMed] [Google Scholar]
- 31.Takahashi N, Gruber CC, Yang JH, Liu X, Braff D, Yashaswini CN, et al. Lethality of MalE-LacZ hybrid protein shares mechanistic attributes with oxidative component of antibiotic lethality. Proc Natl Acad Sci U S A. 2017. Epub 2017/08/11. 10.1073/pnas.1707466114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kohanski MA, Dwyer DJ, Wierzbowski J, Cottarel G, Collins JJ. Mistranslation of membrane proteins and two-component system activation trigger antibiotic-mediated cell death. Cell. 2008;135(4):679–90. Epub 2008/11/18. 10.1016/j.cell.2008.09.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhu B, Stulke J. SubtiWiki in 2018: from genes and proteins to functional network annotation of the model organism Bacillus subtilis. Nucleic Acids Res. 2018;46(D1):D743–D8. Epub 2018/05/23. 10.1093/nar/gkx908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Price CE, Driessen AJ. YidC is involved in the biogenesis of anaerobic respiratory complexes in the inner membrane of Escherichia coli. J Biol Chem. 2008;283(40):26921–7. 10.1074/jbc.M804490200 . [DOI] [PubMed] [Google Scholar]
- 35.Dalbey RE, Kuhn A, Zhu L, Kiefer D. The membrane insertase YidC. Biochim Biophys Acta. 2014;1843(8):1489–96. 10.1016/j.bbamcr.2013.12.022 . [DOI] [PubMed] [Google Scholar]
- 36.Chiba S, Lamsa A, Pogliano K. A ribosome-nascent chain sensor of membrane protein biogenesis in Bacillus subtilis. EMBO J. 2009;28(22):3461–75. Epub 2009/09/26. 10.1038/emboj.2009.280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen Y, Soman R, Shanmugam SK, Kuhn A, Dalbey RE. The role of the strictly conserved positively charged residue differs among the Gram-positive, Gram-negative, and chloroplast YidC homologs. J Biol Chem. 2014;289(51):35656–67. Epub 2014/11/02. 10.1074/jbc.M114.595082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mishra S, Crowley PJ, Wright KR, Palmer SR, Walker AR, Datta S, et al. Membrane proteomic analysis reveals overlapping and independent functions of Streptococcus mutans Ffh, YidC1, and YidC2. Mol Oral Microbiol. 2019;34(4):131–52. 10.1111/omi.12261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Luo Y, Asai K, Sadaie Y, Helmann JD. Transcriptomic and phenotypic characterization of a Bacillus subtilis strain without extracytoplasmic function sigma factors. J Bacteriol. 2010;192(21):5736–45. Epub 2010/09/08. 10.1128/JB.00826-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao H, Sun Y, Peters JM, Gross CA, Garner EC, Helmann JD. Depletion of Undecaprenyl Pyrophosphate Phosphatases Disrupts Cell Envelope Biogenesis in Bacillus subtilis. J Bacteriol. 2016;198(21):2925–35. 10.1128/JB.00507-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hartl B, Wehrl W, Wiegert T, Homuth G, Schumann W. Development of a new integration site within the Bacillus subtilis chromosome and construction of compatible expression cassettes. J Bacteriol. 2001;183(8):2696–9. Epub 2001/03/29. 10.1128/JB.183.8.2696-2699.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Koo BM, Kritikos G, Farelli JD, Todor H, Tong K, Kimsey H, et al. Construction and Analysis of Two Genome-Scale Deletion Libraries for Bacillus subtilis. Cell Syst. 2017;4(3):291–305 e7. Epub 2017/02/13. 10.1016/j.cels.2016.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rojas-Tapias DF, Helmann JD. Induction of the Spx regulon by cell wall stress reveals novel regulatory mechanisms in Bacillus subtilis. Mol Microbiol. 2018;107(5):659–74. Epub 2017/12/23. 10.1111/mmi.13906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Altenbuchner J. Editing of the Bacillus subtilis Genome by the CRISPR-Cas9 System. Appl Environ Microbiol. 2016;82(17):5421–7. Epub 2016/06/28. 10.1128/AEM.01453-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moszer I, Rocha EP, Danchin A. Codon usage and lateral gene transfer in Bacillus subtilis. Curr Opin Microbiol. 1999;2(5):524–8. Epub 1999/10/06. . [DOI] [PubMed] [Google Scholar]
- 46.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9(7):676–82. Epub 2012/06/30. 10.1038/nmeth.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhao H, Roistacher DM, Helmann JD. Aspartate deficiency limits peptidoglycan synthesis and sensitizes cells to antibiotics targeting cell wall synthesis in Bacillus subtilis. Mol Microbiol. 2018;109(6):826–44. Epub 2018/07/12. 10.1111/mmi.14078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Radeck J, Kraft K, Bartels J, Cikovic T, Durr F, Emenegger J, et al. The Bacillus BioBrick Box: generation and evaluation of essential genetic building blocks for standardized work with Bacillus subtilis. J Biol Eng. 2013;7(1):29 Epub 2013/12/04. 10.1186/1754-1611-7-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, et al. UCSF Chimera—a visualization system for exploratory research and analysis. J Comput Chem. 2004;25(13):1605–12. Epub 2004/07/21. 10.1002/jcc.20084 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A) Map of SNPs from strain PY79 to 168 and distribution of SNPs contained in each congression suppressors. Genome coordinates were based on the 168 reference genome with NCBI accession number NC_000964.3. B) Transformation plates of yhdL::kan allele transformed into different strain backgrounds, selected on LB plates supplemented with kanamycin, X-gal and 1% xylose. C) Variation of yhdK colony size measurement between trials on different days with different batches of LB plates. P value was calculated using Student’s t test, and percentage changes of average colony size were shown.
(TIF)
A) Venn diagram of function overlap and distinction of YidC1 and YidC2 of Bacillus subtilis. B) Streaking of yhdK Pspac(hy)-yidC2Q148K on plates of LB or LB supplemented with 1 mM IPTG (final concentration). C) Spot dilution of yhdL depletion strains with Pspac(hy) based overexpression of different YidC homologs, on LB plates supplemented with a final concentration of 1% xylose (+Xylose), 1 mM IPTG (+IPTG) or nothing (None).
(TIF)
A) Distribution of 128 possible charge variants of YidC1 in the tested library. Theoretically, the majority of YidC1 variants in the input library have a charge of +2 to +5 in the hydrophilic groove, while experimental data from 103 samples suggests that the ones capable of providing high σM tolerance contain a charge of +2 or +3. Among the 103 samples with charge of +2 or +3, the positive charge can be located in one, two or three transmembrane segments, with the exception that no sample contains more than one positive charge in TM1 alone. B) Crystal structure of YidC from Bacillus halodurans (PDB ID 3WO6), showing the seven variable amino acid providing positive charges in the hydrophilic substrate binding chamber of the enzyme. Gly231 is not visible due to the lack of side chain of this residue. TM1-5, transmembrane region 1–5; E1, extracytoplasmic region 1; C1, cytoplasmic region 1.This figure was generated using UCSF Chimera 1.13[49].
(TIF)
A) yhdL depletion strains with YidC1 variants were streaked on LB plates with or without xylose inducer for yhdL. The positive charge of each variant was labelled next to the streaking, with a negative control “None” meaning no yidC1 variant at thrC locus (weak growth due to the depletion conditions, and cannot be restreaked), and a positive control “YidC1PY79” meaning the native YidC1 mutated into the PY79 Q140K version (HB23719). B) Spot dilution of YidC depletion strains with yidC1 variants at thrC locus. The depletion strain has its native yidC1 and yidC2 deleted, and a xylose inducible copy of PxylA-yidC2. Diluted cultures were spotted on LB without xylose (-Xyl) or with 1% final concentration of xylose (+Xyl). The negative control (None) has no yidC1 at thrC locus, while the positive control has the 168 version of yidC1 that contains a single positive charge at R73. Some YidC1 variants exhibited reduced growth ability, and the variant containing R72 K140 R144 failed to grow, although the emergence of suppressors was noted.
(TIF)
A) Growth curves of WT strain 168, yhdK null, and yhdK with YidC1Q140K single amino acid substitution. B) PhtrA activity of strains in panel A during growth. C) Growth curves of WT strain 168, cssR null, cssS null, cssRS double null, and cssS null with an ectopic IPTG inducible copy under induced or uninduced conditions. D) PhtrA activity of strains in panel C during growth. The OD600 and luminescence were measured every 12 minutes. At least four biological replicates were used for each strain, and the results are shown as mean ± SEM.
(TIF)
A) WT B. subtilis 168 vs. strains with the indicated mutations. B) B. subtilis yhdK null mutant vs. strains additionally mutant for the indicated gene. C) WT B. subtilis 168 vs. strains lacking either the σM-regulated promoter for spx (σPM-spx) or the sasA or secDF genes. D) B. subtilis yhdK null mutant vs. strains additionally mutant for the indicated gene. E) WT B. subtilis 168 vs. strains lacking the σM-regulated promoter (PM) for the indicated gene(s). The percentage change relative to the average colony size is shown above each Box and Whisker plot.
(TIF)
No mutations were found in these genes.
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.