Abstract
Natural compounds including essential oils (EOs) are used to inhibit the pathogenic bacterial growth in foods. The objective of this study was to investigate the combined effect of Trachyspermum ammi EO and propolis ethanolic extract (PEE) on some food pathogenic bacteria. In this study, the effect of T. ammi EO and PEE at different concentrations, individually and in combination, on Escherichia coli, Salmonella typhimurium, Listeria monocytogenes, Bacillus cereus, and Staphylococcus aureus were investigated using minimum inhibitory concentration and fractional inhibitory concentration (FIC) indices. Moreover, the effect of sub-inhibitory concentrations of EO and extract on E. coli and S. aureus growth curve was determined. The results revealed the synergistic effect of T. ammi EO and PEE on S. aureus, B. cereus, E. coli, S. typhimurium, and L. monocytogenes. The effects of EO and extract sub-inhibitory concentrations on bacterial growth curve resulted in an increased lag phase of the culture and decelerated bacterial growth, especially S. aureus. The results showed that T. ammi EO and PEE, especially when combined, are effective in bacterial growth inhibition and their potential use in food systems is recommended.
Key Words: Combined effect, Food pathogenic bacteria, Propolis, Trachyspermum ammi
Introduction
Antimicrobials and additives are used to control pathogenic bacteria growth in the food products. With respect to the antibacterial function of natural compounds as flavoring agents and the growing concerns of inter-national organizations responsible for food hygiene and safety about using chemical preservatives, studies have increased to substitute chemical flavoring agents with natural ones.1
Trachyspermum ammi essential oil (EO) and propolis ethanolic extract (PEE) are among these natural compounds that could be readily used as flavorings and additives. The T. ammi commonly known as Ajowan is one of the aromatic seed spices originated from the Middle East, India, Iran, Afghanistan, and Egypt. In these regions, it is traditionally used as a medicinal plant for its antiseptic, appetizer and carminative properties. Thymol, the major phenolic compound of Ajowan, has been reported to be a germicide, antispasmodic and antifungal agent.2 Propolis, a honeybee product, is used to cover hive walls and fill gaps. Bees collect the resin-like product from cracks in the bark of trees and leaf buds. Thereby, propolis does not only act as a structural compound, but it is mainly responsible as a chemical agent for the honeycombs safety, especially against microorganisms.3 Antibacterial, antifungal, anti-viral, anti-inflammatory and anti-tumor effects of propolis have been previously demonstrated.4 Also, it is used for infections treatment in oriental medicine and as an antiseptic and anti-inflammatory agent in European pharmacology.4
Application of high concentrations of EOs and other antimicrobial substances negatively affects the flavor of the food. Together with this, the high cost of using an additive in high amounts may limit the use of plant EO or any natural additive individually. Thus, recently, growing interest has focused on the combined use of two or more natural additives to achieve synergistic or additive effects.5
This study aimed to investigate the individual and combined activity of T. ammi EO and PEE on Escherichia coli O157:H7, Salmonella typhimurium, Listeria mono-cytogenes, Bacillus cereus and Staphylococcus aureus as the most important food pathogenic bacteria, and the effect of their inhibitory concentrations on E. coli O157:H7 and S. aureus growth curves.
Materials and Methods
Preparation of T. ammi EO. The T. ammi plants were obtained from Sistan and Baluchestan province, Iran in spring and the scientific name was confirmed at the Department of Pharmacognosy, Faculty of Pharmacy, Tehran University of Medical Sciences, Tehran, Iran. The herbarium voucher number was 15125. The EO was extracted from the top branches through hydro-distillation using Clevenger system and dried by sodium sulphate (Merck, Darmstadt, Germany). The extracted EO was then stored in dark glass containers at 4 ˚C.2
Preparation of PEE. Propolis was obtained from an apiary in Hamedan province, Iran. Initially, 50 g of bee propolis was extracted using 80.00% ethanol and a shaker for 24 hr. The extract was concentrated by a rotary evaporator (RC 600; KNF, Freiburg, Germany), dried and finally lyophilized with a freeze-dryer-cryodo (Telstar, Barcelona, Spain).3
Gas chromatography (GC) and gas chromatography /mass spectroscopy (GC/MS) analyses of T. ammi EO. The compounds of T. ammi EO were characterized by GC and GC/MS. The GC-MS (model 6890; Agilent, Santa Clara, USA) was equipped with a capillary column (30 m, inner diameter 250 µm, thickness 0.25 µm) with the temperature-programmed at 50 to 265 ˚C (2.50 ˚C per min) and kept at 265 ˚C for 30 min. The temperature of the injection room was 250 ˚C and the carrier gas was helium (1.50 mL min-1). The ionization energy of 70.00 ev and a source temperature of 250 ˚C were used. The components were determined using inhibition index as compared to the injected series of alkanes (Sigma, Dorset, UK) with DB5 column as well as standard compounds spectra and the reflection pattern was reported in valid references and mass data with the help of databases.6 Relative percentage of each component was determined by under-curve area.
Studied bacteria. First, strains of E. coli 0157:H7 (ATCC 10536), S. typhimurium II (ATCC 14028), L. mono-cytogenes (ATCC 19118), B. cereus (ATCC 11778) and S. aureus (ATCC 6538) were provided from previous studies in Department of Food Hygiene, Faculty of Veterinary Medicine, University of Tehran were cultured in brain heart infusion (BHI; Merck) at 37 ˚C for 18 hr and then re-cultured and stored at 37 ˚C for 18 hr. The second culture was mixed with sterile glycerine (Bracco, Milan, Italy; 1:5) and stored in 100 µL sterile glass tubes at – 20 ˚C.
Experimental design. The effect of T. ammi EO (0, 62.50, 125, 250, 500, 1000, 2000 and 4000 µg mL-1) and PEE (0, 62.50, 125, 250, 500, 1000, 2000, 4000 and 6000µg mL-1), individually or in combination, was investigated in order to determine minimum inhibitory concentration (MIC) via microdilution method. Also, the effect of subinhibitory concentrations of T. ammi EO and PEE on E. coli and S. aureus growth curves were studied.
Bacterial inoculum preparation. The stock was introduced into BHI broth and incubated at 37 ˚C for 18 hr. The first culture was re-cultured and incubated at 37 ˚C for 18 hr. Different amounts of the second culture were transferred to a cuvette containing 4.00 mL sterile BHI to obtain 0.10 optical density (OD) at 600 nm. Then, 1.00 mL of the bacterial suspension was transferred into a tube containing 9.00 mL of 0.10% peptone water to prepare successive dilutions of up to 10-6. Then, 100 µL from each dilution was transferred to plates containing BHI agar. The plates were incubated at 37.00 ˚C for 24 hr and then, the bacteria were counted twice to determine the mean bacterial count in the cuvette containing bacterial suspension with 0.10 OD. Thus, the bacteria count in a bacterial suspension with 0.10 OD was equal to the calculated number. The exact number of inoculated bacteria was calculated through co-culture and colony count.2
Determination of MIC by microdilution method. In the present study, a 96-well plate with 300 µL round–botton wells was used for MIC determination. Different concentrations of T. ammi EO (0, 62.50, 125, 250, 500, 1000, 2000 and 4000 µg mL-1) and PEE (0, 62.50, 125, 250, 500, 1000, 2000, 4000 and 6000 µg mL-1) in 5.00% dimethyl sulfoxide (Merck) containing BHI were prepared. Then, 100 µL of EO and extract were transferred to each well to which, 10.00 µL of each bacterial suspension (with 0.10 OD) was added (maximum concentration of 5 × 105 CFU mL-1 bacteria per well) and confirmed by successive dilutions of bacterial suspension in plate culture and colony count. The well contents were mixed with a plate reader (M201; Emperor Electronic Technology Co., Ltd., Shenzhen, China) equipped with a shaker for 2 min. The plates were then incubated at 37.00 ˚C for 24 hr, the turbidity if any was observed with eye and the absorbance was read using the plate reader at 630 nm. Increase in absorbance ( ≥ 0.10) suggested bacterial growth and turbidity and the first single or combined concentration of the EO and extract without turbidity was MIC.7
Interaction of T. ammi EO and PEE. Antibacterial activity of T. ammi EO and PEE, individually and in combination, was evaluated and their interactive effect was measured using the following relation and fractional inhibitory concentration (FIC) index as below:
If FIC index was < 1, then, the interactive effect of EO and extract was synergistic and if FIC was = 1, 1 < FIC < 2 or FIC > 2, their effects were enhancing, neutral and antagonistic, respectively.8
Effect of T. ammi EO and PEE on the bacterial growth curve. The effect of sub-inhibitory concentrations of T. ammi EO and PEE, individually or in combination, on the growth of E. coli and S. aureus within 24 hr was evaluated. The concentrations were as follows: T. ammi EO: 0 and 250 µg mL-1 for both bacteria and PEE: 0 and 500 µg mL-1 for S. aureus and 0 and 3000 µg mL-1 for E. coli. 10 mL of the solutions (at different concentrations) were distributed into the tubes to which, 100 µL of the bacterial suspension was added (maximum bacterial concentration of 5 × 105 CFU mL-1). The tubes were incubated at 35 ˚C, the dilutions of interest were made from each tube at 0, 2, 4, 8, 10, 12 and 24 hr and triple-cultured in BHI agar at 35 ˚C for 24 hr, colony count and bacteria number (log CFU mL-1) were determined.9
Statistical analysis. Bacterial growth curves were drawn by Graphpad Prism Software (version 4.0; GraphPad Software Inc., La Jolla, USA) and all statistical analyses for bacteria number log at different times as well as the growth curves gradient were performed using SPSS software (version 16.0; SPSS Inc., Chicago, USA). One-way analysis of variance (ANOVA) and Tukey posthoc test were used for means comparison. Difference among samples was considered significant at 5.00% level.
Results
Analysis of T. ammi EO components. The results from the analysis of T. ammi EO components by GC/MS are given in Table 1. As shown in Table 1, the most predominant compounds in T. ammi EO were thymol, paracimen and γ–terpinen, respectively.
Table 1.
Trachyspermum ammi chemical compositions
| Component | Inhibition index | Percentage |
|---|---|---|
| Total | - | 98.09 |
| α-thujene | 931 | 0.07 |
| α-pinene | 939 | 0.09 |
| Sabinen | 976 | 0.44 |
| β-pinene | 980 | 0.84 |
| ρ-cymene | 1018 | 18.01 |
| γ-terpinen | 1061 | 15.89 |
| Thymol | 1278 | 62.42 |
| Carvacrol | 1292 | 0.33 |
MIC determination. The results of MIC determination for T. ammi EO and PEE and their combination against the five aforementioned bacteria using the microdilution method are presented in Table 2. As shown, the MIC of T. ammi EO alone against E. coli, S. typhimurium, L. monocytogenes, B. cereus, and S. aureus was 500 µg mL-1, whereas it was 6000, 6000, 500, 500 and 1000 µg mL-1 for PEE individually against the above bacteria, respectively.
Table 2.
The minimum inhibitory concentration (MIC) of Trachyspermum ammi and propolis ethanolic extract on Escherichia coli, Salmonella typhimurium, Listeria monocytogenes, Bacillus cereus and Staphylococcus aureus (µg mL-1) separately and combinatorial approaches determined using microdilution method
| Microorganism | MIC T. ammi essential oil | MIC PEE | T. ammi essential oil/PEE | Combination effect | FIC index |
|---|---|---|---|---|---|
| Escherichia coli | 500 | 6000 | 125 + 2000/250 + 1000 | Synergistic | 0.63 |
| Salmonella typhimurium | 500 | 6000 | 62.50 + 4000/125 + 2000 | Synergistic | 0.65 |
| Listeria monocytogenes | 500 | 500 | 250 + 500/250 + 62.50/125 + 250 | Synergistic | 0.69 |
| Bacillus cereus | 500 | 500 | 250 + 62.50/62.50 + 125 | Synergistic | 0.50 |
| Staphylococcus aureus | 500 | 1000 | 250 + 125/125 + 250 | Synergistic | 0.56 |
MIC: minimum inhibitory concentration; FIC: Fractional inhibitory concentration; PEE: propolis ethanolic extract.
The results showed that the combination of T. ammi EO and PEE could inhibit the growth of all mentioned bacteria and the FIC indexes for this combined effect were, respectively, 0.63, 0.65, 0.69, 0.50 and 0.56 for E. coli, S. typhimurium, L. monocytogenes, B. cereus, and S. aureus, suggesting a synergistic inhibitory effect of EO and extract on all studied bacteria.
Effect of T. ammi EO and PEE on E. coli and S. aureus growth curves. Figure 1 shows the growth curves and linear gradient (increasing trend) of the curves for S. aureus in the presence of T. ammi EO and PEE. The statistical analysis showed that from the second hour of incubation, the bacterial count in the control was greater than other treatments (p < 0.001) and within 8 and 24 hr, the bacteria count in the mixed treatment was lower than T. ammi EO treatment. There was also fewer bacterial count in T. ammi EO treatment than PEE individually (p < 0.001). Comparison of the bacterial count and its significant increase (p < 0.001) revealed that lag phase periods for the control, PEE, T. ammi EO and T. ammi EO plus PEE treatments were 1, 4, 6 and 8 hr, respectively. Comparison of linear gradient of growth curves for S. aureus in the presence of T. ammi EO and PEE revealed that there was no significant difference in increasing trend of bacteria count for S. aureus between the control (gradient of 0.17 ± 0.02) and PEE alone (0.16 ± 0.02) and also between T. ammi EO alone (0.12 ± 0.01) and T. ammi EO plus PEE (0.11 ± 0.02; p > 0.05) but increasing trend of bacterial count of S. aureus in T. ammi EO alone and T. ammi EO plus PEE was significantly lower than control (p < 0.05).
Fig. 1.
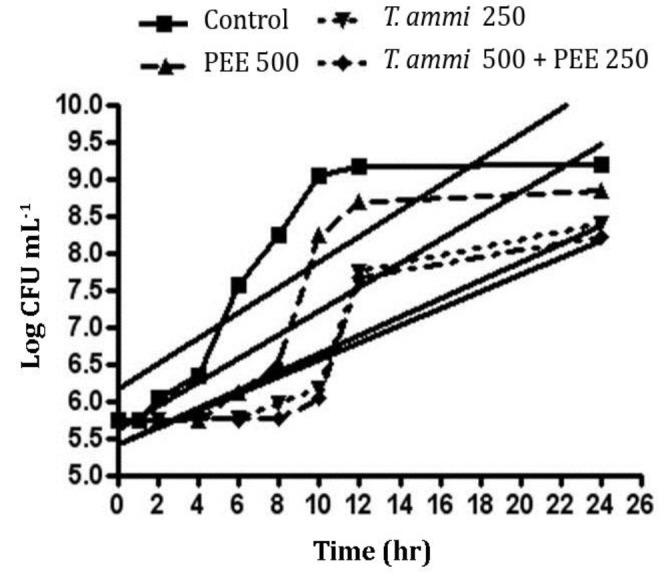
Staphylococcus aureus growth curve and linear gradient (additive growth rate) in the presence of different treatments including control, Trachyspermum ammi essential oil (T.ammi EO) and propolis ethanolic extract (PEE) each one exclusively and also in combination simultaneously
Figure 2 shows the growth curves and linear gradient (rising trend) of the curves for E. coli in the presence of T. ammi EO and PEE. The statistical analysis showed that from the second hour of incubation, the bacterial count in the control and propolis extract groups was greater than T. ammi EO and T. ammi EO plus PEE treatments (p < 0.001) and there was no significant difference between the control and PEE treatments (p > 0.05). Comparison of T. ammi EO and T. aami EO plus PEE treatments revealed that there was no significant difference between these two groups over 6 hr of incubation (p > 0.05).
Fig. 2.
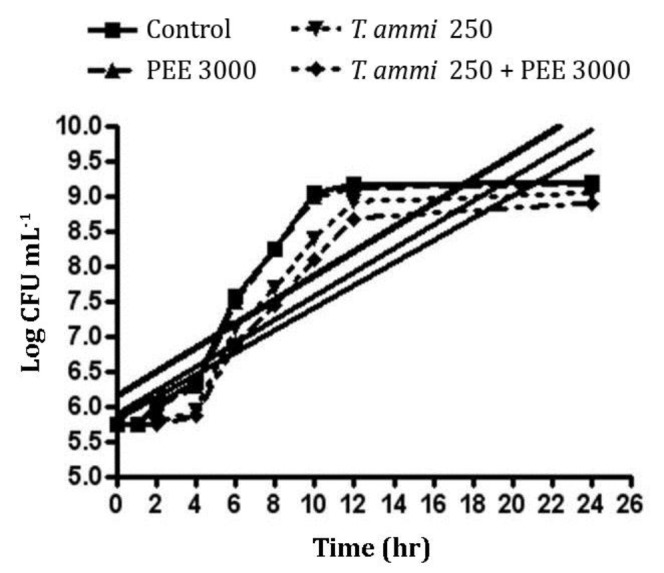
Escherichia coli growth curve and linear gradient (additive growth rate) in the presence of different treatments including control, Trachyspermum ammi essential oil (T.ammi EO) and propolis ethanolic extract (PEE) each one exclusively and also in combination simultaneously
The comparison of bacterial count as well as significant increase examination in bacterial count showed that lag phase periods for the control (p = 0.003), PEE alone (p = 0.01), T. ammi EO alone (p = 0.001) and T. ammi EO plus PEE (p < 0.001) were 1, 1, 2 and 2 hr, respectively. Comparison of linear gradient of growth curves for E. coli in the presence of T. ammi (EO) plus PEE revealed that there was no significant difference in increasing trend of bacteria count for E. coli among the control (gradient of 0.17 ± 0.02), PEE alone (0.17 ± 0.02), T. ammi EO alone (0.17 ± 0.01) and T. ammi EO plus PEE (0.16 ± 0.01; p > 0.05).
Discussion
Herbs have long been used as medicines or flavoring agent; however, since the 19th century, plant compounds have been replaced with chemicals. Today, there is a new attitude towards the use of natural preservatives, especially herbs, in foods due to growing knowledge and concerns about side-effects of chemicals as well as medicinal resistance. Interest in preservative-free products and/or products containing natural preservatives has been increased, among them, plant Eos are noted. The EOs and their constitutes have well-known antibacterial effects.10 In general, a higher concentration of EOs is required compared to in vitro conditions to enhance their anti-bacterial activity. Higher concentrations of EO negatively
affect the taste of the food. Also, creating bacterial resistance is another problem of using an antimicrobial agent in high concentration.11 Thus, the use of two or more combined preservatives is considered.
In the present study, the effect of T. ammi EO and PEE on E. coli S. typhimurium, L. monocytogenes, B. cereus, and S. aureus was investigated. The MIC of these two compounds as well as MIC of their combination was determined using the microdilution method. The antioxidant activity of T. ammi EO and its antimicrobial properties have been studied previously.12,13 Its antibacterial effect using dilution method has also been investigated by several researchers. Kumar et al. have evaluated the antibacterial activity of T. ammi EO against water pathogens. They used microdilution method and determined MIC of T. ammi EO for two different strains including E. coli and S. typhimurium as 87 and 128 (two strains of E. coli) and 109 µg mL-1, respectively.14 Goodarzi et al. have studied the antibacterial effect of T. ammi EO on some bacteria including E. coli (MIC of 310 µg mL-1 ), S. aureus (150 µg mL-1) and S. typhimurium (310 µg mL-1 ).15 Gandomi et al. have investigated the antibacterial effect of T. ammi EO on E. coli, B. cereus, S. aureus, S. typhimurium and L. monocytogenes using broth microdilution. Their results showed MIC of 500 µg mL-1 for all the bacteria.2 In the present study, the antimicrobial effect of T. ammi EO on the studied bacteria was demonstrated and MIC of T. ammi EO against E. coli, S. typhimurium, L. monocytogenes, B. cereus and S. aureus was 500 µg mL-1.
Different studies have shown the antibacterial effect of propolis due to its flavonoids. Also, it has been shown that propolis produced in different parts of world has different composition resulting in different effects.16 However, Iranian propolis has not been comprehensively studied yet. Choi et al. have investigated two Korean propolis, Yeosu and Cheorwon, and a Brazilian sample against S. aureus, B. subtilis, S. typhimurium and Candida albicans.17 Yaghoubi et al. have examined the antibacterial activity of ethanolic extract of Iranian propolis by disc diffusion and showed the activity just against Gram-positive bacteria and fungi, while no activity was observed against Gram-negative bacteria. Among microorganisms, B. cereus showed the highest sensitivity at 67000 µg mL-1.18
Rahman et al. have studied the antibacterial activity of propolis on E. coli and S. aureus by disc diffusion method. The suggested concentration of propolis for both species to be inhibited was 3500 µg mL-1 and propolis showed better inhibitory effect on S. aureus compared to E. coli.19 Siripatrawan et al. have examined the antimicrobial activity of ethanolic extract of Thai propolis using disc diffusion method against Gram-positive and Gram-negative bacteria and showed that it had a greater effect on Gram-positive bacteria.20
In the present study, MIC of PEE was 6000 µg mL-1 for E. coli, 6000 µg mL-1 for S. typhimurium, 500 µg mL-1 for L. monocytogenes, 500 µg mL-1for B. cereus and 1000 µg mL-1 for S. aureus. Propolis had the greatest inhibitory effect on Gram-positive bacteria. This is due to the presence of outer membrane over the cell wall in Gram-negative bacteria limiting the diffusion of hydrophobic compound into the lipopolysaccharide coating of the bacteria.21
The difference in MIC among the present and other studies for both T. ammi EO and PEE may be attributed to the difference in chemical composition due to the origin, harvesting season and extraction method as well as the strain and procedure of antimicrobial effect determination. In this study, the combined treatment effect on different bacteria was different, confirming previous study; in this regard, Fou et al. have investigated the effect of clove and rosemary EO and reported that these two EOs could have enhancing synergistic and/or antagonistic effect depending on the species.22 Also, Gutierrez et al. have studied the combined effect of marjoram and thyme on different bacteria and reported the enhancing effect.23 It should be noted that there is no research on the combined antimicrobial effect of T. ammi EO and PEE. This study showed that the combined use required lower concentrations, indicating the synergistic effect.
With regards to the effect of these two preservatives on the growth curves and linear gradient of the curves for S. aureus and E. coli, their combination resulted in an increase in lag phase significantly reducing bacterial growth. The T. ammi EO had greater effect compared to PRR and S. aureus showed more sensitivity.
It is known that thymol and carvacrol have the greatest antimicrobial effect due to their hydrophobic nature and free hydroxyl groups.24 The greater antibacterial effect of T. ammi EO on the growth curve seems to be due to the presence of a high amount of thymol in the EOs.
This study suggests the antimicrobial effect of T. ammi EO and propolis ethanolic extract on E. coli, S. thyphimurium, L. monocytogenes, B. cereus and S. aureus and application of these two agents is recommended due to their synergistic effect. However, their practical use is subjected to further studies using food models.
Acknowledgements
The authors would like to acknowledge Semnan University Research Offices for the financial support.
Conflict of interest
The authors declare that there is no conflict of interest.
References
- 1.Jebelli-Javan A, Ghazvinian K, Mahdavi A, et al. The effect of dietary Zataria multiflora Boiss essential oil supplementation on microbial growth and lipid peroxidation of broiler breast fillets during refrigerated storage. J Food Process Preserv. 2013;37(5):881–888. [Google Scholar]
- 2.Gandomi H, Abbaszadeh S, Jebelli-Javan A, et al. Chemical constituents, antimicrobial and antioxidative effects of Trachyspermum ammi essential oil. J Food Process Preserv. 2014;38(4):1690–1695. [Google Scholar]
- 3.Lubbe J, Sanchez‐Politta S. Propolis, beeswax, and the sensitization potential of topical calcineurin inhibitors. Clin Exp Dermatol. 2006;31(1):147–148. doi: 10.1111/j.1365-2230.2005.01964.x. [DOI] [PubMed] [Google Scholar]
- 4.De Vecchi E, Drago L. Propolis' antimicrobial activity: What’s new? Infez Med. 2007;15(1):7–15. [PubMed] [Google Scholar]
- 5.Hamouda A, Elbanna H. Combined antimicrobial effect against some isolated bacteria from chickens. J Phys Pharm Adv. 2013;3(12):272–276. [Google Scholar]
- 6.Adams RP. Identification of essential oil components by gas chromatography/mass spectroscopy. 4th ed. New York, USA: Allured Publishing Corporation; 1997. pp. 9–31. [Google Scholar]
- 7.Schwalbe R, Steele-Moore L, Goodwin AC. Anti-microbial susceptibility testing protocols. USA: Taylor and Francis Group CRC Press ; 2007. pp. 75–81. [Google Scholar]
- 8.Santiesteban‐Lopez A, Palou E, Lopez‐Malo A. Susceptibility of food‐borne bacteria to binary combinations of antimicrobials at selected aw and pH. J Appl Microbiol. 2007;102(2):486–497. doi: 10.1111/j.1365-2672.2006.03092.x. [DOI] [PubMed] [Google Scholar]
- 9.Ataee M, Hosseini H, Noori N, et al. Effect of Zataria multiflora Boiss essential oil on growth curve and shigatoxin 2 production of enterohemorrhagic E coli O157: H7. J Med Plants. 2013;4(48):62–71. [Google Scholar]
- 10.Alpsoy L. Inhibitory effect of essential oil on aflatoxin activities. Afr J Biotechnol. 2010;9(17):2474–2481. [Google Scholar]
- 11.Gill AO, Holley RA. Interactive inhibition of meat spoilage and pathogenic bacteria by lysozyme, nisin and EDTA in the presence of nitrite and sodium chloride at 24 ˚C. Int J Food Microbiol. 2003;80(3):251–259. doi: 10.1016/s0168-1605(02)00171-x. [DOI] [PubMed] [Google Scholar]
- 12.Rota C, Carraminana J, Burillo J, et al. In vitro antimicrobial activity of essential oils from aromatic plants against selected foodborne pathogens. J Food Prot. 2004;67(6):1252–1256. doi: 10.4315/0362-028x-67.6.1252. [DOI] [PubMed] [Google Scholar]
- 13.Selim S. Antimicrobial activity of essential oils against vancomycin-resistant enterococci (VRE) and Escherichia coli O157: H7 in feta soft cheese and minced beef meat. Braz J Microbiol. 2011;42(1):187–196. doi: 10.1590/S1517-83822011000100023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kumar A, Mishra RK, Srivastava S, et al. Role of phylo-genetic analysis for anti-bacterial activity of essential oil of Trachyspermum ammi L against water borne pathogens. Adv Environ Biol. 2011;5(6):1271–1279. [Google Scholar]
- 15.Goudarzi GR, Saharkhiz M, Sattari M, et al. Anti-bacterial activity and chemical composition of Ajowan (Carum copticum Benth & Hook) essential oil. J Agr Sci Tech. 2010;13:203–208. [Google Scholar]
- 16.Khanizadeh AM, Zaringhalam-moghadam J, Sonboli A, et al. Effects of hydroalcholic and chloroformic extracts of Salvia candidissima on hyperalgesia and edema during adjuvant-induced arthritis. Koomesh. 2015;16(2):239–245. [Google Scholar]
- 17.Choi Y, Noh D, Cho S, et al. Antioxidant and anti-microbial activities of propolis from several regions of Korea. LWT-Food Sci Technol. 2006;39(7):756–761. [Google Scholar]
- 18.Yaghoubi SMJ, Ghorbani GR, Soleimani Zad S, et al. Antimicrobial activity of Iranian propolis and its chemical composition. Daru. 2007;15(1):45–48. [Google Scholar]
- 19.Rahman MM, Richardson A, Sofian-Azirun M. Antibacterial activity of propolis and honey against Staphylococcus aureus and Escherichia coli. Afr J Microbiol Res. 2010;4(18):1872–1878. [Google Scholar]
- 20.Siripatrawan U, Vitchayakitti W, Sanguandeekul R. Antioxidant and antimicrobial properties of Thai propolis extracted using ethanol aqueous solution. Int J Food Sci Tech. 2013;48(1):22–27. [Google Scholar]
- 21.Singh G, Maurya S, Marimuthu P, et al. Antioxidant and antibacterial investigations on essential oils and acetone extracts of some spices. Nat Prod Rad. 2007;6(2):114–121. [Google Scholar]
- 22.Fu Y, Zu Y, Chen L, et al. Antimicrobial activity of clove and rosemary essential oils alone and in combination. Phytother Res. 2007;21(10):989–994. doi: 10.1002/ptr.2179. [DOI] [PubMed] [Google Scholar]
- 23.Gutierrez J, Rodriguez G, Barry-Ryan C, et al. Efficacy of plant essential oils against foodborne pathogens and spoilage bacteria associated with ready-to-eat vegetables: Antimicrobial and sensory screening. J Food Prot. 2008;71(9):1846–1854. doi: 10.4315/0362-028x-71.9.1846. [DOI] [PubMed] [Google Scholar]
- 24.Ben Arfa A, Combes S, Preziosi‐Belloy L, et al. Anti-microbial activity of carvacrol related to its chemical structure. Lett Appl Microbiol. 2006;43(2):149–154. doi: 10.1111/j.1472-765X.2006.01938.x. [DOI] [PubMed] [Google Scholar]


