Abstract
In the present study, the effect of different concentrations of cell-free supernatant (CFS; 10.00 and 35.00 mg g-1) of Lactobacillus salivarius (Ls-BU2) on chemical, microbial and sensorial specifications of ground beef stored under the refrigerated condition was investigated. The antibacterial activity of CFS on Escherichia coli was also assessed. According to agar-disk diffusion method, CFS of Ls-BU2 revealed a promising antibacterial activity against E. coli in culture media compared to CFS of a well-known probiotic (L. acidophilus LA-5). In meat, CFS of Ls-BU2 showed a minimal effective concentration (MEC) of 35.00 mg g-1 on E. coli, while CFS of L. acidophilus represented a MEC of 45.00 mg g-1. The CFS of Ls-BU2 at 35.00 mg g-1 concentration retained psychrophilic counts of meat at a lower value than maximum accepted level (7 log10 CFU g-1). In a similar trend, CFS of Ls-BU2 at 35.00 mg g-1 concentration was also displayed high sensorial scores compared to other CFS-treated samples. In conclusion, we demonstrated that CFS of Ls-BU2 and to some extent CFS of L. acidophilus could act as a safe food additive for the control of bacterial pathogens and to extend the shelf life of ground beef.
Key Words: Antimicrobial activity, Lactic acid bacteria, Minced meat, Minimal effective concentration, Shelf life
Introduction
The food industry faces challenges requiring specific approaches to overcome or diminish them. One of the challenges is the packaging food with a short shelf life (i.e., ground meat).1 Ground beef is a highly perishable food due to its high contact surface and high protein and moisture composition, requiring careful handling and strict preservation.2 Currently, various preservation techniques including traditional and innovative methods are used by the industry to protect meat products. A novel method is the use of naturally occurring preservatives (bio-preservatives) widely used to control pathogens and to ensure the microbial and chemical quality of meat.3 There are a number of ways to classify bio-preservatives, nevertheless, the classification based on the origin of preservatives is the most popular approach. Therefore, based on the origin, bio-preservatives fall into three categories, those originating from plant, animal and microbial sources.4
Lactic acid bacteria (LAB), a heterogeneous group of Gram-positive bacteria, comprise a diverse group of bacteria with similar morphological, metabolic and physio-logical characteristics.5 Lactobacillus is one of the most important genera in this group and the bacterial species in this genus are found in the flora of the mouth, intestines and female reproductive organs. 6 The antimicrobial activity of these bacteria is due to secretion of various substances including the organic acids (mainly lactic and acetic acids), bacteriocins, hydrogen peroxide, di-acetyslene, acetaldehyde and ammonia.5-7 Most of Lactobacillus salivarius are commonly used probiotic micro-organism with multiple biological benefits. The L. salivarius secretes unmodified bacteriocins of sub-classes IIa, IIb and IId, organic acids and bacteriocins-like substance and exhibits antimicrobial activity against different pathogens.8-10
During the growth of LAB, these compounds are excreted into the cell-free supernatant (CFS) of the bacterial suspension.11 Nowadays, due to the rise of antibiotic resistance and the side effects of chemical preservatives in food, the use of alternative natural compounds is highly emphasized. Despite well-defined characteristics of LAB, their growth and survival in food are influenced strongly by many food internal and external factors such as pH, temperature, salt contents, etc and their functional metabolites such as bacteriocins are narrow-spectrum antibacterial substances as well.12 An alternative method explored in this work is the preparation of CFS of L. salivarius containing almost all the functional compounds (i.e., antimicrobial and anti-oxidants) acting as a novel substitute for using whole bacteria or metabolites.
Currently, the use of LAB and their antimicrobial metabolites is very common to improve the food safety and to increase food shelf life, while, some studies have been carried out on the application of CFS, LAB cellular fractions such as pure exopolysaccharides-a and intracellular cell-free extracts on foodborne pathogens inactivation in the food models.12-16 The aims of the present work were to evaluate the effects of Ls-BU2 CFS on chemical, microbial and sensorial specifications of ground beef stored under the refrigerated condition and to investigate the antibacterial activity of CFS on Escherichia coli.
Materials and Methods
Materials. Fresh ground beef was directly purchased from a meat packaging company and transferred to the microbiology laboratory aseptically at 4.00 ˚C. Sorbitol MacConkey (SMAC) agar was purchased from Merck (Darmstadt, Germany). Peptone water (PW), plate count agar (PCA), de Mann, Rogosa and Sharpe (MRS) agar and broth and Luria Bertani (LB) agar and broth were provided by Quelab Laboratories (Quebec, Canada), while all other chemicals were purchased from Sigma-Aldrich (St. Louis, USA). The L. acidophilus LA-5 and E. coli ATTC 11303 were obtained from Department of Food Hygiene and Quality Control, Faculty of Veterinary Medicine, Urmia University, Urmia, Iran.
Preparation of CFS from L. salivarius and L. acidophilus. The Ls-BU2 was originally isolated from buffalo raw milk and identified based on the 16S rRNA gene sequencing. The isolate showed promising antibacterial activity.17 The L. salivarius was grown in MRS broth and incubated for 48 hr at 37.00 ± 1.00 ˚C in a CO2 incubator (Sina Lab., Tehran, Iran) and then the culture was centrifuged (Farzaneh Arman Co., Isfahan, Iran) at 4000 g for 10 min. The supernatant was removed, freeze-dried (Zist Farayand Tajhiz Sahand, Tabriz, Iran; freezing temperature: – 40 ˚C, pump pressure: 100 mTorr and shelf temperature: – 60 ˚C) and used as a CFS powder (CFSLs; Fig. 1). The CFS of a well-known probiotic (L. acidophilus LA-5; CFSLa) was also prepared and used as a control in all experiments.
Fig. 1.

The cell-free supernatant image of L. salivarius after lyophilization process
Antibacterial activity of prepared CFSs. The E. coli suspension was prepared by sub-culturing bacteria in LB broth and the bacterial dose was adjusted using visible-ultraviolet spectrophotometer (Amersham Pharmacia Biotech Inc., Buckinghamshire, UK) at 600 nm to ∼8 log10 CFU mL-1 (optical density: ∼ 0.10). Agar-disk diffusion method was selected to investigate the antibacterial efficacy of CFSs on an inoculated LB agar with E. coli.18 A 6.00 mm sterile blank disk was coated in 10.00 mg mL-1 of CFS solution for 5 min and after drying at room temperature, the disk was placed on an inoculated LB agar (~ 6 log10 CFU mL-1). Plates were incubated at 37.00 ± 1.00 ˚C for 24 hr and the zone diameter of inhibition was measured in triplicate.
Antimicrobial activity of CFSs against E. coli in ground beef. A challenge test was carried out to estimate the minimal effective concentration (MEC) of CFSs in the ground beef.12 Required amount of E. coli suspension was added into 100 g meat in plastic bags to reach a final bacterial population of 4.30 log10 CFU g-1.1 The CFSs of Ls-BU2 and L. acidophilus at concentrations between 10.00 and 45.00 mg mL-1 were added into the meat samples separately and the samples were completely homogenized using stomacher (Seward Medical Ltd, London, UK) at 200 rpm for 2 min. The meat samples were refrigerated at 4.00 ˚C for 6 days. The SMAC agar was used to culture and count E. coli in meat samples. The MEC was defined as CFS concentration reducing the initial bacterial count under culture limit of 100 bacteria for meat during three days of storage at 4.00 ˚C. A similar experiment was also performed in LB broth instead of the food matrix. Our initial experiment showed that the original meat was not contaminated with E. coli (limit of detection of 10 CFU g-1).
Application of CFS on ground beef shelf life. One hundred gram of meat was transferred into a sterile stomaching bag and CFS of both lactobacillus strains were added separately into meat to reach final concentrations of 10.00 and 35.00 mg g-1 of CFS in meat. The groups included control, CFS of Ls-BU2 (CFSLs 10.00 mg g-1 and CFSLs 35.00 mg g-1) and CFS of L. acidophilus (CFSLa 10.00 mg g-1 and CFSLa 35.00 mg g-1). Samples were properly homogenized using stomacher at 200 rpm for 2 min, packaged in a polyethylene container and stored at 4.00 ± 1.00 ˚C for 9 days for microbiological, chemical and sensorial evaluations.4
Microbiological analysis. At each sampling time (day 0, 3, 6 and 9), 10.00 g of each specimen was aseptically transferred into sterile stomaching bag containing 90 mL sterile 0.10% PW and homogenized using a stomacher at 200 rpm for 2 min. After preparation of decimal dilutions (1:10) in 0.10% PW, appropriate dilutions were cultured on PCA for total psychrophiles counts (TPC) and incubated at 10.00 ˚C for 5 to 7 days. Bacterial counts were represented as log10 CFU g-1. 1
Chemical analysis. At each sampling intervals, 10.00 g from each sample was thoroughly mixed in 90.00 mL sterile 0.10% PW in a stomacher and pH of samples was measured at room temperature using pH meter (Metrohm, Zofingen, Switzerland). Thiobarbituric acid (TBA) was determined in terms of milligrams of malondialdehyde per kilogram of the sample by an extraction procedure according to the method explained by Tajik et al.1 Cold (4.00 ˚C) extraction solution composed of perchloric acid (4.00%) and 1.00 mL of 1.00 mg mL-1 butylated hydroxyanisole (BHA) was added to 10.00 g sample, blended at 13500 rpm for 1 min and then filtered. The filtrate was adjusted to 50 mL with 4.00% perchloric acid and 5.00 mL aliquot of the filtrate was added to 5.00 mL of TBA (0.02 M). The mixture was mixed, maintained at 100 ˚C for 60 min and then cooled. The absorbance was measured at 532 nm. The TBA values were expressed as mg malondialdehyde kg-1 sample.
Sensorial analysis. Sensorial specifications (color, odor and overall acceptability) of the samples were evaluated at day 6 of storage by 21 sensorial members on the basis of nine-point hedonic scale (9 = like extremely; 8 = like very much; 7 = like moderately; 6 = like slightly; 5 = neither like nor dislike; 4 = dislike slightly; 3 = dislike moderately; 2 = dislike very much; 1 = dislike extremely).1
Statistical analysis. The data (three replicate for each test) were statistically analyzed using analysis of variance (ANOVA) in GraphPad Prism (version 5.00; GraphPad Software Inc., San Diego, USA). Duncan's multiple range test was used to assess significant differences (p < 0.05) between the groups.
Results
Antimicrobial activity of CFS. The inhibitory zones of CFSLs and CFSLa against E. coli were 12.98 ± 0.70 and 5.20 ± 0.50 mm in LB culture medium, respectively. Moreover, MEC of CFS against E. coli in meat and the culture media was estimated. The MEC of CFSLs in ground meat and LB broth was respectively 35.00 and 25.00 mg g-1, while CFSLa showed MEC of 45 and 45.00 mg g-1, in order.
The effect of CFSs on the shelf life of ground beef. The effectiveness of prepared CFSs on TPC of meat is shown in Figure 2. The initial TPC of samples were 4.70 log10 CFU g-1. Control and treatment containing CFSLa at 10.00 mg mL-1 concentration were crossed the maximum accepted level (7.00 log10 CFU g-1) of TPC before day 6. Treatments containing 10.00 mg g-1 CFSLs passed the limit after day 9. The CFSLs at 35.00 mg g-1 concentration retained TPC at a lower value than maximum level during 9 days of storage.
Fig. 2.
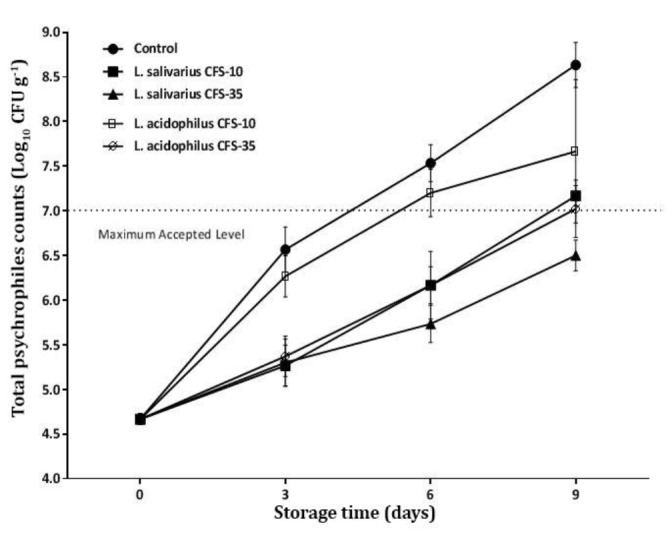
The effects of different concentrations of Lactobacilli cell-free supernatant (CFS) (10.00 and 35.00 mg g-1) on total psychrophiles counts (Log10 CFU g-1) of ground meat during storage at 4.00 ˚C for 9 days
The TBA (mg malondialdehyde kg-1 sample) values of ground meat with CFS are shown in Table 1. Except for 10.00 mg g-1 CFSLa, the TBA values of all the other treatment groups were significantly lower than those of the control group (p < 0.05) at all time points. In the control and CFSLa at 10.00 mg g-1 groups, TBA values were rapidly increased throughout the refrigerated storage, whereas in the group with 35 mg g-1 CFS, TBA value was recorded 0.67 - 0.75 mg malondialdehyde kg-1 at the end of storage time. There was a rapid increase in pH in the control and 10.00 mg mL-1 CFSLa groups compared to the other treatment groups (Table 1).
Table 2.
The effects of different concentrations (10.00 and 35.00 mg g-1) of Lactobacillus salivarius cell-free supernatant (CFS) on pH and thiobarbituric acid (TBA) values of ground beef during storage at 4.00 C for 9 days
| Treatment |
pH
|
TBA (
mg of malondialdehyde per kg of sample)
|
||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 3 | 6 | 9 | 0 | 3 | 6 | 9 | |
| Control | 6.10±0.10a | 6.70±0.10e | 7.10±0.10d | 7.40±0.10d | 0.29±0.04a | 0.53±0.04c | 0.76±0.01d | 0.96±0.03cd |
| L. salivarius CFS-10 | 6.10±0.10a | 6.50±0.10c | 6.70±0.10b | 7.00± 0.00b | 0.29±0.04a | 0.33±0.15ab | 0.45±0.12b | 0.74±0.11b |
| L. salivarius CFS-35 | 6.10±0.10a | 6.20±0.10a | 6.50±0.10a | 6.70±0.10a | 0.29±0.04a | 0.30±0.12a | 0.39±0.07a | 0.67±0.09a |
| L. acidophilus CFS-10 | 6.10±0.10a | 6.60±0.10d | 7.10±0.10d | 7.10±0.10c | 0.29±0.04a | 0.53±0.14c | 0.75±0.09d | 0.93±0.15c |
| L. acidophilus CFS-35 | 6.10±0.10a | 6.30±0.10b | 6.90±0.10c | 6.90±0.00b | 0.29±0.04a | 0.39±0.05b | 0.68±0.08c | 0.75±0.11b |
abcd Values in the same column with different lowercase superscripts mean that the values are significantly different (p < 0.05).
Sensorial analysis. The scores of sensorial evaluations of the treatment groups which carried out at day 6 of storage are shown in Figure 3. The treatment group of CFSLs at 35.00 mg g-1 showed the highest scores in color (8), odor (7) and overall acceptability (8), while the control group showed the lowest scores ( 5) for all checked attributes among all the treatment groups. The overall acceptability scores of the other groups were 6.
Fig. 3.
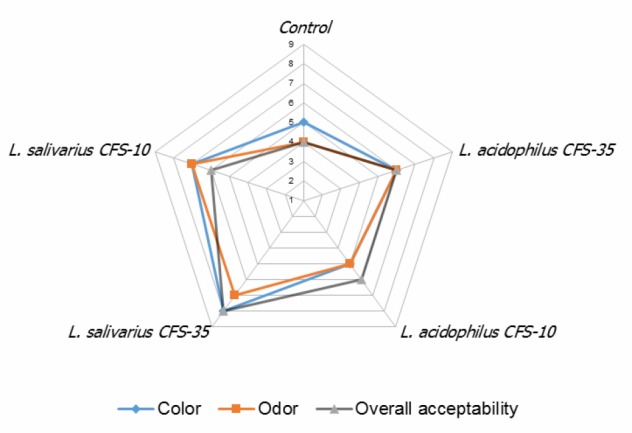
The effects of different concentrations of Lactobacillus cell-free supernatant (CFS) (10.00 and 35.00 mg g-1) on sensorial properties of ground meat during storage at 4.00 ˚C on day 6
Discussion
Bio-preservation of meat using microorganisms such as LAB is a new-emerged technology to preserve meat from spoilage.19 There are two different approaches to use LAB in meat including direct addition of live bacteria and addition of purified antimicrobial agents of LAB.12 Both methods have their own disadvantages to use as a substance to extend the shelf life of food.20,21 In the present study, CFSs were prepared from two well-known probiotic bacteria to investigate their possible bio-preservative activities in the ground beef. The CFSs of both lactobacilli represented antibacterial activity against E. coli in culture media and ground beef meat, nevertheless, CFSLs was more potent antibacterial than CFSLa in both matrices. Both CFSs were more effective in culture media than meat matrix. Similar to the results presented by de Barros et al., the presence of inhibitory zones on agar media confirmed the antibacterial activity of all tested CFSs on pathogenic bacteria.22 In a study by Mirnejad et al., among different examined CFSs, CFS of L. casei 431 showed the lowest antilisterial activity suggesting a weak antibacterial activity associated with the presence of organic acids in CFS and bacteriocins did not involve.23 Hamad et al. have demonstrated the combined antimicrobial activity of CFSs from L. acidophilus, Bifidobacterium bifidum, and L. plantarum at 1.00% concentration against E. coli in milk and cheese and concluded that this activity could be a result of the organic acids composition of CFS.14
As noted in this study, it is clear that the efficacy of CFS in culture media is much higher than meat due to the complexity of commodity, solubility, and adsorption of CFS to meat as well as interactions of CFS components with meat ingredients.24 The LAB exhibit their antimicrobial activity by secreting compounds like bacteriocins, organic acids and hydrogen peroxide against pathogenic and spoilage-related bacteria.25 The CFSLa showed weak antimicrobial activity in comparison with CFSLs. The anti-microbial activity of L. acidophilus LA-5 does not correlate with the production of bacteriocins,26 whereas L. salivarius produces bacteriocins, organic acids, and bacteriocin-like substances being responsible for its strong antimicrobial activity.9 We have demonstrated that CFS of Ls-BU2 contains an antimicrobial/antioxidant agent known as pyrrolo[1,2-a] pyrazine-1, 4-dione (unpublished data).
The shelf life of food products is a matter, which needs to be considered from three different aspects including microbial, chemical and sensorial qualities. The results demonstrated that the supplementing ground beef with 35.00 mg g-1 CFSLs significantly decreased the growth of the psychrophilic population in ground beef (Fig. 2). The results revealed that higher concentrations of CFSs could control microbial growth in meat more effectively than lower concentrations. According to Tajik et al., meat psychrotrophic bacteria are the most sensitive spoilage agents to the antimicrobial compounds.1
This is the first report on the application of CFS for extending the shelf life of food commodity. Ground beef is known as a sensitive commodity to lipid oxidation due to its large surface area being easily reached by oxygen.27 Therefore, the control of oxidative off-flavors (rancidity) is an important procedure to postpone the spoilage of meat. It has been previously demonstrated that CFS of LAB represents higher antioxidant capacity than intact bacteria and intracellular extracts.28 The anti-oxidative activity of CFS would be related to the production of compounds such as superoxidase dismutase, nicotinamide adenine dinucleotide, nicotinamide adenine dinucleotide phosphate, glutathione and peptides.29 As reported in the previous studies, the antioxidant capacity of CFS is related to the bacterial strain and different genera of bacteria exhibit different antioxidant activities.28,30 In the present study, it was revealed that not only the antioxidant activity is genus dependent, but also dose-dependent. A limit of 1.00 mg of malondialdehyde per kg of meat has been proposed for sensory perceived rancidity of meat.31 No samples passed the limit during the storage, nevertheless control and samples treated with 10.00 mg g-1 CFSLa expressed close TBA contents to the mentioned limits during the 9 days storage. The CFSLs at 35.00 mg g-1 showed high antioxidant activity (TBA value of 0.70 mg of malondialdehyde per kg of meat) in ground meat during refrigerated storage (Table 1).
Addition of CFS of both lactobacilli in both 10.00 and 35.00 mg g-1 to ground meat did not significantly affect consumer preference and overall acceptability of the examined meat. In CFS treated groups, sensorial scores were more than average ( 5). Despite a mild brown color of CFS (Fig. 1), the color scores were higher than five in all the groups supplemented with CFS. The similarity of meat and CFS color masks the color of CFS and the sensorial members could not differentiate the incorporated additive. Due to the color appearance of CFS of LAB, it is not preferred for food with white and opaque features (i.e., milk and dairy products). Kamble et al. have reported that CFS of Pediococcus acidilactici NCDC252 with ethylene-diamine-tetraacetic acid (EDTA) represents high sensorial scores in chicken carcasses stored at 4.00 ˚C.32 It has also been reported that the addition of intracellular extract of L. salivarius H strain in dry-cured ham keeps the color stability of fresh pork.33 In meat products, the scores higher than 6 are acceptable for odor attributes.34 Considering the results of the sensorial evaluation, concentrations of CFSLs at 10.00 and 35.00 mg g-1 and CFSLa at 35.00 mg g-1 (Fig. 1) could be considered as the acceptable doses to be added in ground beef.
The results of this study demonstrated that CFS could control microbial and oxidative deteriorations of ground beef in a concentration-dependent trend during the storage at the refrigerated condition. The CFSLs at 35.00 mg g-1 concentration revealed acceptable antimicrobial and antioxidant activities and extended the overall shelf life of ground meat. As an alternative, CFSLa could also serve certainly like a safe food additive with lower antimicrobial/antioxidant activity than CFSLs. Further analytical investigations will be required to elucidate the possible main antimicrobial/antioxidant agents of CFS involving in the meat shelf life extension.
Acknowledgments
The authors appreciate Iran National Science Foundation (INSF) for funding the project (grant No. 95848925). The technical assistance of Ms. Elham Alipour is also greatly acknowledged.
Conflict of interest
None.
References
- 1.Tajik H, Farhangfar A, Moradi M, et al. Effectiveness of clove essential oil and grape seed extract combination on microbial and lipid oxidation characteristics of raw buffalo patty during storage at abuse refrigeration temperature. J Food Process Preserv. 2014;38(1):1–38. [Google Scholar]
- 2.Antoniewski MN, Barringer SA, Knipe CL, et al. Effect of a gelatin coating on the shelf life of fresh meat. J Food Sci. 2007;72(6):E382–E387. doi: 10.1111/j.1750-3841.2007.00430.x. [DOI] [PubMed] [Google Scholar]
- 3.Zhou GH, Xu XL, Liu Y. Preservation technologies for fresh meat – A review. Meat Sci. 2010;86(1):119–128. doi: 10.1016/j.meatsci.2010.04.033. [DOI] [PubMed] [Google Scholar]
- 4.Irkin R, Esmer OK. Novel food packaging systems with natural antimicrobial agents. J Food Sci Technol. 2015;52(10):6095–6111. doi: 10.1007/s13197-015-1780-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Almeida Júnior WLG, da Silva Ferrari Í, de Souza JV, et al. Characterization and evaluation of lactic acid bacteria isolated from goat milk. Food Control. 2015;53:96–103. [Google Scholar]
- 6.Bernardeau M, Vernoux JP, Henri-Dubernet S, et al. Safety assessment of dairy micro-organisms: The Lactobacillus genus. Int J Food Microbiol. 2008;126(3):278–285. doi: 10.1016/j.ijfoodmicro.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 7.Suskovic J, Kos B, Beganovic J, et al. Antimicrobial activity – The most important property of probiotic and starter lactic acid bacteria. Food Technol Biotechnol. 2010;48(3):296–307. [Google Scholar]
- 8.O’ Shea EF, O’ Connor PM, Raftis EJ, et al. Subspecies diversity in bacteriocin production by intestinal Lactobacillussalivarius strains. Gut Microbes. 2012;3(5):468–473. doi: 10.4161/gmic.21417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Messaoudi S, Manai M, Kergourlay G, et al. Lactobacillussalivarius: Bacteriocin and probiotic activity. Food Microbiol. 2013;36(2):296–304. doi: 10.1016/j.fm.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 10.Therdtatha P, Tandumrongpong C, Pilasombut K, et al. Characterization of antimicrobial substance from Lactobacillussalivarius KL-D4 and its application as biopreservative for creamy filling. SpringerPlus. 2016;5(1):1060. doi: 10.1186/s40064-016-2693-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koohestani M, Moradi M, Tajik H, et al. Effects of cell-free supernatant of Lactobacillusacidophilus LA5 and Lactobacilluscasei 431 against planktonic form and biofilm of Staphylococcusaureus. Vet Res Forum. 2018;9(4):301–306. doi: 10.30466/vrf.2018.33086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hartmann HA, Wilke T, Erdmann R. Efficacy of bacteriocin-containing cell-free culture supernatants from lactic acid bacteria to control Listeriamonocytogenes in food. Int J Food Microbiol. 2011;146(2):192–199. doi: 10.1016/j.ijfoodmicro.2011.02.031. [DOI] [PubMed] [Google Scholar]
- 13.Abdallah M, Benoliel C, Drider D, et al. Biofilm formation and persistence on abiotic surfaces in the context of food and medical environments. Arch Microbiol. 2014;196(7):453–472. doi: 10.1007/s00203-014-0983-1. [DOI] [PubMed] [Google Scholar]
- 14.Hamad G, Botros W, Hafez E. Combination of probiotic filtrates as antibacterial agent against selected some pathogenic bacteria in milk and cheese. Int J Dairy Sci. 2017;12:368–376. [Google Scholar]
- 15.Koo OK, Kim SM, Kang SH. Antimicrobial potential of Leuconostoc species against E coli O157:H7 in ground meat. J Korean Soc Appl Biol Chem. 2015;58(6):831–838. [Google Scholar]
- 16.Lee KJ, Park HW, Choi EJ, et al. Effects of CFSs produced by lactic acid bacteria in combination with grape seed extract on the microbial quality of ready-to-eat baby leaf vegetables. Cogent Food Agric. 2016;2(1):1268742. [Google Scholar]
- 17.Hossein-Alipour E, Mardani K, Moradi M. Isolation and identification of Lactobacillus salivarius from buffalo milk and evaluation of its antimicrobial activity. Iran J Med Microbiol. 2018;12(2):96–106. [Google Scholar]
- 18.Buncic S, Avery SM, Moorhead SM. Insufficient antilisterial capacity of low inoculum Lactobacillus cultures on long-term stored meats at 4 ˚C. Int J Food Microbiol. 1997;34(2):157–170. doi: 10.1016/s0168-1605(96)01181-6. [DOI] [PubMed] [Google Scholar]
- 19.Mani-López E, Palou E, López-Malo A. Bio-preservatives as agents to prevent food spoilage. In: Grumezescu A, Massachusetts, USA, Holban AM, editors. Handbook of food bioengineering. Academic Press; 2018. pp. 235–270. [Google Scholar]
- 20.Ghanbari M, Jami M, Domig KJ, et al. Seafood biopreservation by lactic acid bacteria- A review. LWT - Food Sci Technol. 2013;54(2):315–324. [Google Scholar]
- 21.Landete JM. A review of food-grade vectors in lactic acid bacteria: From the laboratory to their application. Crit Rev Biotechnol. 2017;37(3):296–308. doi: 10.3109/07388551.2016.1144044. [DOI] [PubMed] [Google Scholar]
- 22.de Barros JC, da Conceicao ML, Neto NJG, et al. Combination of Origanum vulgare L essential oil and lactic acid to inhibit Staphylococcus aureus in meat broth and meat model. Brazilian J Microbiol. 2012;43(3):1120–1127. doi: 10.1590/S1517-838220120003000039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mirnejad R, Vahdati AR, Rashidiani J, et al. The antimicrobial effect of lactobacillus casei culture supernatant against multiple drug resistant clinical isolates of Shigella sonnei and Shigella flexneriin vitro. Iran Red Crescent Med J. 2013;15(2):122–126. doi: 10.5812/ircmj.7454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Galvez A, Abriouel H, Lopez RL, et al. Bacteriocin-based strategies for food biopreservation. Int J Food Microbiol. 2007;120(1):51–70. doi: 10.1016/j.ijfoodmicro.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 25.Sahoo TK, Jena PK, Nagar N, et al. In vitro evaluation of probiotic properties of lactic acid bacteria from the gut of Labeo rohita and Catla catla. Probiotics Antimicrob Proteins. 2015;7(2):126–136. doi: 10.1007/s12602-015-9184-8. [DOI] [PubMed] [Google Scholar]
- 26.Abedi D, Feizizadeh S, Akbari V, et al. In vitro anti-bacterial and anti-adherence effects of Lactobacillus delbrueckii subsp bulgaricus on Escherichia coli. Res Pharm Sci. 2013;8(4):260–268. [PMC free article] [PubMed] [Google Scholar]
- 27.Ahn J, Grun IU, Mustapha A. Antimicrobial and antioxidant activities of natural extracts in vitro and in ground beef. J Food Prot. 2004;67(1):148–155. doi: 10.4315/0362-028x-67.1.148. [DOI] [PubMed] [Google Scholar]
- 28.Wang YQ, Wu YY, Li LH, et al. Comparative study of eight strains of lactic acid bacteria in vitro antioxidant activity. Adv Mater Res. 2015;1073–1076:183–188. [Google Scholar]
- 29.Lin MY, Yen CL. Antioxidative ability of lactic acid bacteria. J Agric Food Chem. 1999;47(4):1460–1466. doi: 10.1021/jf981149l. [DOI] [PubMed] [Google Scholar]
- 30.Xing J, Wang G, Zhang Q, et al. Determining antioxidant activities of lactobacilli cell-free supernatants by cellular antioxidant assay: A comparison with traditional methods. PLoS One. 2015;10(3):e0119058. doi: 10.1371/journal.pone.0119058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Limbo S, Torri L, Sinelli N, et al. Evaluation and predictive modeling of shelf life of minced beef stored in high-oxygen modified atmosphere packaging at different temperatures. Meat Sci. 2010;84(1):129–136. doi: 10.1016/j.meatsci.2009.08.035. [DOI] [PubMed] [Google Scholar]
- 32.Kamble P, Appa Rao V, Abraham RJ, et al. Effect of Pediocin NCDC 252 as cell free supernatant produced from Pediococus acidilactici NCDC 252 with EDTA on total viable count and sensory evaluation of chicken carcasses stored at refrigeration temperature. Int J Curr Microbiol App Sci. 2017;6(7):2269–2276. [Google Scholar]
- 33.Luo Z, Gasasira V, Huang Y, et al. Effect of Lactobacillus salivarius H strain isolated from Chinese dry-cured ham on the color stability of fresh pork. Food Sci Hum Wellness. 2013;2(3-4):139–145. [Google Scholar]
- 34.Economou T, Pournis N, Ntzimani A, et al. Nisin–EDTA treatments and modified atmosphere packaging to increase fresh chicken meat shelf-life. Food Chem. 2009;114(4):1470–1476. [Google Scholar]


