Abstract
Canine seminal plasma contains antioxidant enzymes to protect sperm against internally generated ROS. These enzymes are removed from seminal plasma during the process of cryopreservation. The freezing/thawing process can cause some morphological and functional changes via ice crystallization and osmolality imbalance. The present study was conducted to evaluate the effects of curcumin supplementation on sperm total count, motility, progressive motility, viability, morphology, total antioxidant capacity (TAC), DNA integrity and NOX5 gene expression of dog frozen semen. The pooled semen was allocated to fresh (Group 1) and frozen (Group 2) controls, curcumin (2.50 mM) (Group 3) and curcumin (5.00 mM), (Group 4). Sperm parameters including total sperm count, morphology, motility, progressive motility, sperm concentration and DNA integrity in addition to TAC were evaluated in fresh and frozen-thawed semen samples. Real-time RT-PCR was used to investigate NOX5 and GADPH (reference gene) genes expressions. Curcumin at 2.50 mM provided a greater protective effect on the DNA integrity compared to 5.00 mM and control groups. TAC was significantly higher in 2.50 mM group than other groups. NOX5 gene expression in curcumin 2.50 mM was higher than 5.00 mM group. In conclusion, curcumin seems to emolliate sperm parameters and to protect sperm against sperm reactive oxygen stress and increases NOX5 gene expression.
Key Words: Cryopreservation, Curcumin, Dog, NOX5, Total antioxidant capacity
Introduction
The success of canine artificial insemination by frozen semen depends on sperm post-thaw quality which is itself influenced by the contents of semen extender and the freezing rate.1 Cryopreservation processes cause some morphological and functional changes to the sperm.2 Reactive oxygen species (ROS) such as hydrogen peroxide, superoxide anion and hydroxyl radical are small molecules generated from oxygen and produced by sperm cells, granulocytes and macrophages in male genital tract and can exert lipid peroxidation damage to sperm cell membrane. They play a significant role in male infertility by either their over production or decrease in sperm antioxidant defense system. Dog semen rich in polyunsaturated fatty acids is potentially vulnerable to lipid peroxidation. All natural antioxidants effective in the protection of sperm cells are removed from dog seminal plasma during the process of cryopreservation techniques.3 Canine sperm antioxidant capability is also limited by sperm cell mid-piece sparse cytoplasmic volume.4
Curcumin, a yellow pigment from curcuma is used as spice, food coloring, cosmetic agents and in some medical preparations. The potential preventive or therapeutic characteristics of curcumin are believed to be correlated to its antioxidant, anti-inflammatory, antitumor, antiviral, immune modulating and antiallergenic properties.5,6 The role of curcumin have been studied in a number of diseases in human.7 Curcumin bears protective effects on ram frozen-thawed sperm parameters at different doses.8 It has also improved the histopathological alterations induced by monosodium glutamate in testis and epididymis in rats and increased the sperm count9 while suppressed downstream gene products.7 The NADPH oxidase 5 (NOX5) is the fifth member of enzymes called NOX family and is located on chromosome 15 (17 exons) in canidae. For the first time, extensively abundant NOX5 transcripts were identified in human spermatocytes and spermatids.10 NOX5 proteins were also localized in maturing spermatids and ejaculated equine spermatozoa that were highly homolog with human counterpart.11 Orthologs of NOX5 have been identified in the genome of monkey, bovine, zebra fish and sea urchins.12 It was also recently identified in human spleen, testes and kidney as a new protein which was highly similar to Nox1 and NOX2. There are five interrelated variants of NOX5 including α, β, γ, δ and a shortened variant called ε or NOX5-S. Considering the high expression of NOX5 mRNA in testes and sperms and the deteriorative effect of ROS on male reproductive function, NOX5 may play an important role in male reproduction.10 Binding Ca2 to NOX5 cytosolic N-terminal EF-hand domain activates NOX5 and alters cell arrangement leading to produce oxidative stress.13 Their findings further support the idea that ROS is mostly generated by NOX5 in human sperm.
Therefore, whether NOX5 expression in spermatozoa is associated with sperm parameters has yet been studied. The aim of this study was to evaluate the effects of curcumin supplementation to dog sperm cryoprotectant on sperm parameters such as total sperm count, viability, motility, progressive motility, abnormalities, TAC, DNA integrity and the expression of NOX5 gene after freezing-thawing process.
Materials and Methods
Chemicals. All chemicals were obtained from Sigma-Aldrich (Taufkirchen, Germany) unless otherwise stated.
Experimental animals . Six healthy and sexually matured mixed-breed dogs aged from 2 to 5 years were used in this study. This experimental study was approved by the Institutional Animal Care and Use Committee (No. D30/2985). The animals were fed with commercially formulated dog food and kept in individual pens with free access to water. Second-fraction ejaculates were collected by digital manipulation.14 Ejaculates with good sperm motility (> 90.00%) were pooled to obtain reasonable volume and to eliminate individual differences between animals and ejaculates. Pooled samples were divided into 4 groups, each group containing five replicates. The groups were as follow: group 1) fresh semen control (no supplementation), group 2) frozen-thawed control (no supplementation), groups 3 and 4 supplemented with 2.50 mM and 5.00 mM curcumin, respectively. Seminal plasma was removed by centrifuging semen at 720 g for 6 min. The sperm pellet was diluted in Tris-egg yolk-citric acid (6.05 g Tris, 3.40 g citric acid, 2.50 g fructose, 2.00 × 105 IU penicillin, 0.20 g dihydro-streptomycin, 12.00 mL glycerol, 2.00 mL egg yolk, 184 mL double-distilled water) as a standard extender in dog15 at concentration of 200 × 106 sperm per mL. The diluted semen was allowed to equilibrate for 30 min at room temperature, after which it was cooled to 5 ˚C. After 75 min, semen samples were drawn into the required number of pre-cooled 0.50 mL straws and were placed 4.00 cm above liquid nitrogen for 10 min before plunging into liquid nitrogen for freezing. The frozen straws were stored in liquid nitrogen (– 196 ˚C) and then assessed for sperm parameters.
Assessment of sperm parameters. The parameters including total sperm count, morphology, motility, progressive motility, sperm concentration and DNA integrity in addition to TAC were evaluated in fresh and frozen-thawed semen samples. The sperm motility was assessed based on World Health Organization protocol.16 A volume of 10.00 μL of the diluted sperm inserted in the semen analysis chamber and at least five microscopic fields including a minimum of 200 sperm cells were observed. Diff-Quik and eosin-nigrosin (1.00% eosin-Y and 5.00% nigrosin) staining methods16 were used to evaluate sperm morphology and viability, respectively, as percentages.
DNA integrity. Acridine orange (AO) staining was used to test DNA integrity2,17 A thin smeared slide was immersed in methanol/acetic acid (3:1) at 4 ˚C for 14 hr after which stained with AO solution (0.19% in phosphate citrate buffer, pH = 2.50) for 10 min. The stained slide was washed with distilled water for 5 min and air-dried then was evaluated under fluorescent microscope (GS7; Nikon Co., Tokyo, Japan) at 1000× magnification. Sperms with green and yellow-red colors in their head were considered as double- and single-stranded DNA, respectively.
Ferric reducing antioxidant power (FRAP) assay. The FRAP assay was used to evaluate total antioxidant capacity of seminal plasma.18 In brief, 300 mmol L-1 acetate buffer (pH 3.6); 10 mmol L-1 2, 4, 6‐tri‐(2‐pyridyl)‐1, 3, 5‐triazine 98.00% (Sigma‐Aldrich) and 20 mmol L-1 FeCl3.6H2O were mixed in a ratio of 10:1:1, respectively, to get FRAP working solution. A volume of 50.00 µL seminal plasma was added to 1 mL FRAPS working solution and its absorbance was measured by a spectrophotometer (UV-1700; Shimadzu, Kyoto, Japan) at 593 nm (A593) wavelength 10 min later. A mixture of 50.00 µL of FeSO4.7H2O (1 mmol L-1) and 1.00 mL FRAP solution was used as standard.
Sperm cell separation. Previously described standard swim-up method was used for collecting motile sperm cells.19 The desired semen aliquot was centrifuged at 400 g for 15 min at room temperature, then the supernatant was discarded while the pellet was re-suspended in Ham's F-10 culture medium enriched with human serum albumin and centrifuged once again. The supernatant was discarded and the pellet was carefully overlaid with the same culture medium and incubated (at 37 ˚C and 5.00% CO2) on 45 angle position.
RNA isolation, cDNA synthesis and real-time RT- PCR. Total RNA was extracted from fresh and curcumin supplemented frozen-thawed sperms as described by others.20 Homogenization, isolation, precipitation and purification of RNA were performed following the manufacturer’s instructions with an extra step of RNase-free DNase I (Invitrogen by Life Technologies, Carlsbad, USA) treatment to eliminate residual genomic DNA. The concentration of extracted total RNA was determined by spectrophotometry. The RNA (1.00 µg) was reverse transcribed into cDNA using a commercial cDNA synthesis kit (Vivantis, Selangor, Malaysia). The RT-PCR was performed on Step One Plus instrument (Applied Biosystems Inc., Foster City, USA) cycler using HotTag Eva Green qPCR mixture (CinnaGen, Tehran, Iran) containing MgCl2, ultrapure dNTPs and HotTaq DNA polymerase. Primer sequences for the gene of interest NOX5 and reference gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) are presented in Table 1. A total reaction volume of 20.00 µL containing 4.00 µL Eva Green mixtue and 1.00 µL (1.00 µM) from each of forward and reverse primers, 2.00 µL of DNase I treated cDNA, adjusted with RNAase free water was used in RT- qPCR. The program used consisted of a denaturing cycle of 15 min at 95 ˚C; 40 cycles of PCR (95 ˚C for 10 sec, 57 ˚C for 5 sec, and 72 ˚C for 10 sec); a melting cycle consisting of 95 ˚C for 20 sec, 70 ˚C for 15 sec, and a step cycle starting at 70 ˚C until 95 ˚C with a 0.10 ˚C per sec transition rate; and finally, a cooling cycle of 40 ˚C for 30 sec. Three replicate experiments were carried out for all genes. Melt curve analysis was performed to confirm the specificity of each product. As negative controls, 1 tube was always prepared in which RNA was omitted and another tube lacking reverse transcriptase was used during RT-reaction. ROX as an internal passive reference dye was included in qPCR Mix by the manufacturer. A standard curve was obtained using five dilution series of pooled cDNA for each set of primer in order to verify amplification efficiency. The following formula was utilized for the calculation of qPCR amplification efficiency.
Table 1.
Sequences, accession numbers, product sizes and references (if applicable) of the primers used in this study
| Gene | Primer sequences (5' to 3') | Accession number | Product size (bp) | Reference |
|---|---|---|---|---|
| NOX5 | F: TCACCCCCTTTGCTTCCATC R: TATCTCGGCCTGGTCCATC |
M_001103218.1 | 215 | Designed by the authors |
| GAPDH | F: GTGATGCTGGTGCTGAGTATGTT R: ATGGATGACTTTGGCTAGAGGA |
NM_001003142.1 | 233 | Salavati et al.21 |
E= (10 –1/slope – 1) × 100
Statistical analysis. The data was analyzed by analysis of variance (ANOVA) using SPSS (version 22.0; SPSS, IBM Corp., Armonk, USA). The Duncan test was used to perform multiple comparisons where ANOVA was significant. The significant level of p < 0.05 was used to show significance. The results of qPCR were normalized using GAPDH as reference gene. Efficiency of qPCR for the genes of interest and reference gene was similar and the data were analyzed by the ΔΔCT method.22
Results
Sperm parameters. The results of total sperm count, motility, progressive motility, sperm viability and the percentage of normal sperm are presented in Table 2. There was a significant difference between the mean ± SD total sperm count in fresh and frozen-thawed control semen. Mean ± SD total sperm count in 2.50 mM curcumin group did not significantly differ from the frozen control group. Mean ± SD total sperm count in 5.00 mM curcumin group was significantly lower than frozen-thawed control group (p < 0.05). Mean ± SD percent motility of sperms in fresh semen was significantly higher than the frozen-thawed control semen (p < 0.05). The percentage of sperm motility in frozen-thawed semen supplemented with 2.50 mM curcumin was not significantly different from the frozen-thawed control semen (p < 0.05). It means that there was no significant reduction in sperm motility in this group in relation to the control group. The percentage of sperm motility in frozen-thawed semen supplemented with 2.50 mM curcumin was significantly higher than that of frozen-thawed semen supplemented with 5.00 mM curcumin (p < 0.05).
Table 2.
Mean ± SD of sperm morphological parameters, DNA integrity, total antioxidant capacity (TAC) in dog fresh semen (Group 1), frozen-thawed semen (Group 2), frozen semen supplemented with 2.50 mM (Group 3) and 5.00 mM of curcumin (Group 4), respectively
| Groups |
Total count
(× 10 6 mL -1 ) |
Motility (%) | Progressive motility (%) | Live sperm (%) | Abnormal Sperm (%) | DNA integrity | TAC (mmol L -1 ) |
|---|---|---|---|---|---|---|---|
| 1 | 1263.34 ± 462.54a | 82.00 ± 9.19a | 81.20 ± 8.58a | 88.20 ± 2.68a | 15.60 ± 5.22a | 99.98 ± 0.01a | 80.21 ± 0.40a |
| 2 | 904.86 ± 183.90b | 68.20 ± 17.62b | 68.40 ± 14.18b | 65.40 ± 10.70b | 34.20 ± 6.74b | 80.40 ± 0.83b | 62.16 ± 1.55b |
| 3 | 866.38 ± 121.57b | 66.80 ± 17.85b | 66.20 ± 13.18b | 64.80 ± 9.23c | 34.60 ± 10.26b | 87.32 ± 6.30c | 78.38 ± 3.00c |
| 4 | 486.80 ± 115.62c | 54.00 ± 8.15c | 52.40 ± 17.45c | 54.00 ± 9.32d | 52.40 ± 8.56c | 78.63 ± 6.50d | 65.77 ± 2.96bd |
abcd Different superscript letters within columns indicate significant difference (p < 0.05).
Mean ± SD percent progressive motility of sperm in fresh semen significantly differed from frozen-thawed control semen (p < 0.05). Mean ± SD percent progressive motility of sperm in frozen-thawed semen supplemented with 2.50 mM curcumin was not significantly different from the frozen-thawed control semen (p < 0.05). Also, Mean ± SD percent progressive motility of sperm in frozen-thawed semen supplemented with 2.50 mM curcumin was significantly higher than frozen-thawed semen supplemented with 5.00 mM curcumin (p < 0.05).
Mean ± SD percent of live sperm in fresh semen was significantly higher than all other groups (p < 0.05). There was a significant difference in the viability of sperm between both curcumin concentrations (2.50 and 5.00 mM) and frozen-thawed control group (p < 0.05). The percent of live sperm in frozen-thawed control group was significantly higher than 5.00 mM curcumin (p < 0.05). The sperms were significantly more viable in semen samples cryopreserved in extender supplemented with 2.50 mM curcumin than 5.00 mM curcumin group (p < 0.05).
Sperm abnormalities found in this study included detached head, double head, macrocephalic head, bent midpiece, cytoplasmic droplets, coiled tail and kinked tail. The statistical analysis revealed a significant difference in mean ± SD percent abnormal sperm among the experimental groups (p < 0.05). The mean ± SD percent of abnormal sperm in fresh semen was significantly less than that of all other groups (p < 0.05). Mean ± SD percent of abnormal sperm in 2.50 mM curcumin group was significantly lower than 5.00 mM curcumin group (p < 0.05).
Relative mRNA expression. NOX5 gene relative expression in curcumin 2.50 mM group (6.92 ± 1.05 fold change) was not significantly (p < 0.05) different from that of the control group (6.74 ± 0.99 fold change), in other words its expression level was remained unchanged (Fig. 1). Its expression was reduced in 5.00 mM group (0.51 ± 0.17).
Fig. 1.
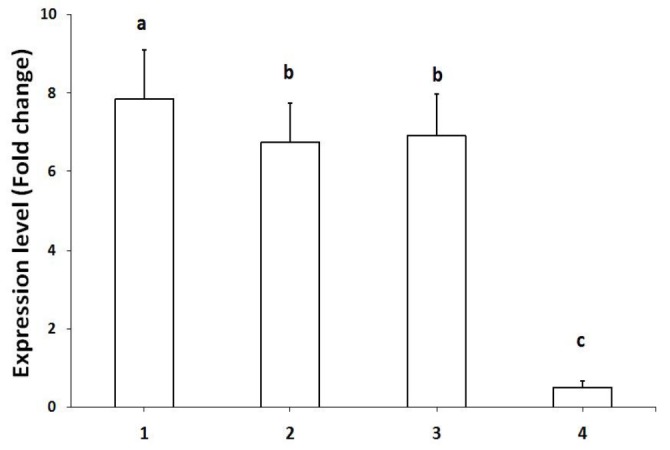
The expression levels of NOX5 gene mRNA in dog fresh semen (Group 1), frozen-thawed control semen (Group 2), frozen semen supplemented with 2.50 mM (Group 3) and frozen semen supplemented with 5.00 mM (Group 4). * = p < 0.05, ** = p < 0.01
DNA integrity. DNA integrity was significantly higher in unsupplemented fresh semen group compared to all other groups (p < 0.05). The DNA integrity in 2.50 mM curcumin group was significantly higher than 5.00 mM curcumin and frozen-thawed control groups (p < 0.05).
FRAP assay. The TAC in all groups was significantly less than that of fresh semen group. Frozen-thawed group supplemented with 2.50 mM curcumin showed significantly higher TAC than the frozen-thawed control group (p < 0.05). Other groups did not differ significantly with the frozen-thawed control group in TAC. There was not significant difference between frozen-thawed semen supplemented with 5.00 mM curcumin and frozen-thawed control group.
Discussion
In this study, 2.50 mM concentration of curcumin was able to prevent reduction of sperm motility and progressive motility caused by the cryopreservation process. This was in accordance with the work conducted on rat sperm23 in which motility, viability, DNA integrity and TAC in cryopreserved sperm supplemented with 2.50 mM curcumin were significantly higher than the control group. Similarly, 50.00 and 10.00 µM L-1 in vitro supplementation of curcumin on bull semen extender was able to improve sperm motility and progressive motility and has protected the sperm from damages induced by oxidative stress.24 Sperm viability and abnormalities were also ameliorated by 2.50 mM supplementation in the present study. The effect of curcumin on sperm parameters is controversial. While considerable number of studies has strongly revealed an energy-promoting and protective effects of curcumin on sperm parameters23-27other researchers showed conflicting findings.28 Higher sperm counts, more viable and motile sperms were shown in varicocelized rats orally treated with 100 mg kg-1 curcumin than in control group.23
The molecular mechanism by which curcumin affect sperm function is not well known. One of the suggested possible ameliorative mechanisms of curcumin on sperm parameters is to scavenge the free radicals as an anti-oxidant through its phenolic, β-diketone and methoxy functional groups.26,29 Curcumin inhibits superoxide anion and dydroxyl radical generation by preventing oxidation of Fe2+ and Fe3+ through Fenton reaction. However, the increasing concentrations of ROS-scavenging molecules due to supplementation of curcumin may be another reason for the enhancement of sperm motility.3,24,25 Curcumin might also exert its inhibitory effects on oxidative stress through the suppression of NF-κB DNA-binding activity and the expression of genes that need activator protein (AP1) and NF-κB for their activation.30 The role of sperm intracellular pH has been reported to be important in mammalian sperm motility. Curcumin inhibits sperm motility by acidifying sperm intracellular PH and hyperpolarization of sperm cell membrane through sodium-proton membrane exchange.31 Curcumin may also affect sperm motility, capacitation and function by inhibiting tyrosine phosphorylation of sperm surface proteins and Ca2+ channels.
The NOX5 gene expression in 2.50 mM curcumin group did not differ from the control group in the present study, indicating that its expression levels were remained unchanged. Meanwhile, NOX5 gene expression levels were reduced in 5.00 mM group. The NOX5 gene is found in varied type of cells and is evolutionarily conserved in wide range of species except for rodent.10,32 The mechanism in which curcumin affects NOX5 NADPH oxidase function is not clear, however, it might be associated with cell membrane Ca2+ entry changes induced by curcumin. NOX5 activation is triggered to produce ROS by binding of Ca2+ to its cytosolic N-terminal EF-hand domains inducing a conformational change that leads to the interaction of the N terminus of NOX5 with its C-terminal substrate-binding and flavin-containing domains. The presence of calcium-dependent NADPH oxidase encoded by NOX5 gene was shown in human sperm acrosome and midpiece.11 Redox status of human spermatozoa, indicative of its motility and ovum-fertilizing capability, is well determined by the expression levels of NOX5, c-Abl, and HV1. In a human-based study, teratozoospermic men expressed more NOX5 transcripts in their semen samples than normal men.33 Although ROS production by spermatozoa has been correlated with poor fertility, it is now clear that redox activity is physiologically necessary for sperm normal functions such as capacitation, acrosome reaction and the final maturation steps associated with hyperactive motility.34 Both concentrations of curcumin (2.50 and 5.00 mM) maintained DNA stability. A relationship between ejaculate quality and chromatin integrity was evident in some researches. Neither of short- and long-term preservation of sperm resulted in adverse effects on sperm chromatin integrity. In addition, poor quality sperm had lower chromatin integrity in comparison with good quality ejaculates. The dilution of some mammalian sperm, particularly with egg yolk-based extenders can affect sperm antioxidant defense system exposing sperm cells to a super physiological concentration of ROS which causes DNA integrity damage. However, this is argued by lack of any significant differences in DNA integrity between canine fresh and 10 days incubated semen in egg yolk-based extender.2 Sperm processing, particularly centrifugation and incubation as well as the type of extenders have been reported to be the most common influencing factors on the quality of sperm chromatin.2,35 In the present study, 2.50 mM curcumin concentration group had significantly higher TAC than the frozen-thawed control group. This was not in agreement with reports of the literature. A positive correlation was found between TAC and, normal morphology and progressive motility in human sperms.36 Variation in curcumin effect on some sperm parameters in this study may be due to its apoptotic cell death effect by DNA damage and prevention of cell growth in higher dose.
In conclusion, although some sperm characteristics were ameliorated on the supplementation of 2.50 and 5.00 mM curcumin in dog cryopreservation media, it was only 2.50 mM concentrations that ameliorated sperm total count, motility, progressive motility, abnormality, DNA integrity, TAC and mRNA expression of NOX5 gene. Based on these results, it can be stated that curcumin in 2.50 mM concentration may be useful in protecting dog sperm from damages caused by cryopreservation procedures.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Szasz F, Gabor G, Solti L. Comparative study of different methods for dog semen cryopreservation and testing under clinical conditions. Acta Vet Hung. 2000;48(3):325–333. doi: 10.1556/AVet.48.2000.3.9. [DOI] [PubMed] [Google Scholar]
- 2.Prinosilova P, Rybar R, Zajicova A, et al. DNA integrity in fresh, chilled and frozen-thawed canine sperm. Vet Med. 2012;57(3):133–142. [Google Scholar]
- 3.Gavazza M, Gutierrez AM, Marmunti M, et al. Lipid peroxidation assays in canine fresh semen. Rev Vet. 2009;20(2):97–102. [Google Scholar]
- 4.Strzezek R, Koziorowska-Gilun M, Kowalowka M, et al. Characteristics of antioxidant system in dog semen. Pol J Vet Sci. 2009;12(1):55–60. [PubMed] [Google Scholar]
- 5.Lin JK, Pan MH, Lin-Shiau SY. Recent studies on the biofunctions and biotransformations of curcumin. Biofactors. 2000;13(1-4):153–158. doi: 10.1002/biof.5520130125. [DOI] [PubMed] [Google Scholar]
- 6.Sharaf HA, Morsy FA, Shaffie NM, et al. Histological and histochemical study on the protective effect of curcumin on ultraviolet irradiation induced testicular damage in albino rats. J Cytol Histol. 2012;3(6):159–166. [Google Scholar]
- 7.LoTempio MM, Veena MS, Steele HL, et al. Curcumin suppresses growth of head and neck squamous cell carcinoma. Clin Cancer Res. 2005;11(19):6994–7002. doi: 10.1158/1078-0432.CCR-05-0301. [DOI] [PubMed] [Google Scholar]
- 8.Omur AD, Coyan K. Protective effects of the antioxidants curcumin, ellagic acid and methionine on motility, mitochondrial transmembrane potential, plasma membrane and acrosome integrity in freeze-thawed Merino ram sperm. Vet Med. 2016;61(1):10–16. [Google Scholar]
- 9.Sarkar FH, Li Y. Cell signaling pathways altered by natural chemopreventive agents. Mutat Res. 2004;555(1-2):53–64. doi: 10.1016/j.mrfmmm.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 10.Banfi B, Molnar G, Maturana A, et al. A Ca (2+)-activated NADPH oxidase in testis, spleen, and lymph nodes. J Biol Chem. 2001;276(40):37594–37601. doi: 10.1074/jbc.M103034200. [DOI] [PubMed] [Google Scholar]
- 11.Sabeur K, Ball BA. Characterization of NADPH oxidase 5 in equine testis and spermatozoa. Reproduction. 2007;134(2):263–270. doi: 10.1530/REP-06-0120. [DOI] [PubMed] [Google Scholar]
- 12.Fulton DJ. Nox5 and the regulation of cellular function. Antioxid Redox Signal. 2009;11(10):2443–2452. doi: 10.1089/ars.2009.2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen SJ, Allam JP, Duan YG, et al. Influence of reactive oxygen species on human sperm functions and fertilizing capacity including therapeutical approaches. Arch Gynecol Obstet. 2013;288(1):191–199. doi: 10.1007/s00404-013-2801-4. [DOI] [PubMed] [Google Scholar]
- 14.Kutzler MA. Semen collection in the dog. Theriogenology. 2005;64(3):747–754. doi: 10.1016/j.theriogenology.2005.05.023. [DOI] [PubMed] [Google Scholar]
- 15.Andersen K. Fertility of frozen dog semen. Acta Vet Scand. 1972;13(1):128–130. [PubMed] [Google Scholar]
- 16.World Health Organization. WHO laboratory manual for the examination of human semen and sperm-cervical mucus interaction. 4th ed. Cambridge, UK: Cambridge University Press, 1999:14–22. [Google Scholar]
- 17.Tejada RI, Mitchell JC, Norman A, et al. A test for the practical evaluation of male fertility by acridine orange (AO) fluorescence. Fertil Steril. 1984;42(1):87–91. doi: 10.1016/s0015-0282(16)47963-x. [DOI] [PubMed] [Google Scholar]
- 18.Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of "antioxidant power: the FRAP assay. Anal Biochem. 1996;239(1):70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 19.Jameel T. Sperm swim-up: A simple and effective technique of semen processing for intrauterine insemination. J Pak Med Assoc. 2008;58(2):71–74. [PubMed] [Google Scholar]
- 20.Almeida IL, Durfey CL, Gastal GDA, et al. 134 relaxin and its receptors in mature canine spermatozoa. Reprod Fertil Dev. 2016;29(1):175–175. [Google Scholar]
- 21.Salavati M, Ghafari F, Zhang T, et al. Effects of oxygen concentration on in vitro maturation of canine oocytes in a chemically defined serum-free medium. Reproduction. 2012;144(5):547–556. doi: 10.1530/REP-12-0176. [DOI] [PubMed] [Google Scholar]
- 22.Livak JK, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta CT) method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 23.Izadpanah M, Alizadeh R, Minaee MB, et al. The effects of curcumin on sperm parameters and nitric oxide production in varicocelized rats. Int J Morphol. 2015;33(4):1530–1535. [Google Scholar]
- 24.Tvrda E, Lukac N, Jambor T, et al. Curcumin in male fertility: Effects on spermatozoa vitality and oxidative balance. J Mircobiol Biotech Food Sci. 2015;4(2):120–124. [Google Scholar]
- 25.Soleimanzadeh A, Saberivand A. Effect of curcumin on rat sperm morphology after the freeze-thawing process. Vet Res Forum. 2013;4(3):185–189. [PMC free article] [PubMed] [Google Scholar]
- 26.Cohly HH, Taylor A, Angel MF, et al. Effect of turmeric, turmerin and curcumin on H2O2-induced renal epithelial (LLC-PK1) cell injury. Free Radic Biol Med. 1998;24(1):49–54. doi: 10.1016/s0891-5849(97)00140-8. [DOI] [PubMed] [Google Scholar]
- 27.Salahshoor MR, Jalili C, Khazaei M, et al. Effects of curcumin on reproductive parameters in male mice. J Clin Res Paramed Sci. 2012;1(3):1–3. [Google Scholar]
- 28.Xia X, Cai H, Qin S, et al. Histone acetylase inhibitor curcumin impairs mouse spermiogenesis – an in vitro study. PLoS One. 2012;7(11):e48673. doi: 10.1371/journal.pone.0048673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sreejayan N, Rao MNA. Curcuminoids as potent inhibitors of lipid-peroxidation. J Pharmacol. 1994;46(12):1013–1016. doi: 10.1111/j.2042-7158.1994.tb03258.x. [DOI] [PubMed] [Google Scholar]
- 30.Singh S, Aggarwal BB. Activation of transcription factor NF-kB is suppressed by curcumin (Diferuloylmethane) J Biol Chem. 1995;270(42):24995–25000. doi: 10.1074/jbc.270.42.24995. [DOI] [PubMed] [Google Scholar]
- 31.Naz RK. The effect of corcumin on intracellular pH (pHi), membrane hyperpolarization and sperm motility. J Reprod Infertil. 2014;15(2):62–70. [PMC free article] [PubMed] [Google Scholar]
- 32.Salles N, Szanto I, Herrmann F, et al. Expression of mRNA for ROS generating NADPH oxidases in the aging stomach. Exp Gerontol. 2005;40(4):353–357. doi: 10.1016/j.exger.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 33.Ghani E, Keshtgar S, Habibagahi M, et al. Expression of NOX5 in human teratozoospermia compared tonormozoospermia. Andrologia. 2013;45(5):351–356. doi: 10.1111/and.12023. [DOI] [PubMed] [Google Scholar]
- 34.Aitken RJ, Ryan AL, Baker MA, et al. Redox activity associated with the maturation and capacitation of mammalian spermatozoa. Free Radic Biol Med. 2004;36(8):994–1010. doi: 10.1016/j.freeradbiomed.2004.01.017. [DOI] [PubMed] [Google Scholar]
- 35.Matsuura R, Takeuchi T, Yoshida A. Preparation and incubation conditions affect the DNA integrity of ejaculated human spermatozoa. Asian J Androl. 2010;12(5):753–759. doi: 10.1038/aja.2010.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hong J, Behar J, Wands J, et al. Bile acid reflux contributes to development of esophageal adeno-carcinoma via activation of phosphatidylinositol-specific phospholipase Ca2+ and NADPH oxidase NOX5-S. Cancer Res. 2010;70(3):1247–1255. doi: 10.1158/0008-5472.CAN-09-2774. [DOI] [PMC free article] [PubMed] [Google Scholar]


