Erratum to: Acta Neuropathol (2016) 131:281–298 DOI 10.1007/s00401-015-1521-1
The original article was published with the incorrect author group. The correct author group is provided below:
Yasir A. Syed · Chao Zhao · Don Mahad · Wiebke Möbius · Friedrich Altmann · Franziska Foss · G. A. González · Aycan Sentürk · Amparo Acker-Palmer · Gert Lubec · Kathryn Lilley · Robin J. M. Franklin · Klaus-A. Nave · Mark R. N. Kotter
The affiliation for the new author, G. A. González is as follows:
Anne McLaren Laboratory for Regenerative Medicine, and Department of Clinical Neuroscience, University of Cambridge, Cambridge, UK
It has also been noticed that Fig. 3c, d included erroneously duplicated images in the colour channels of the immunocytochemical stainings.
The correct image is given below.
Fig. 3.
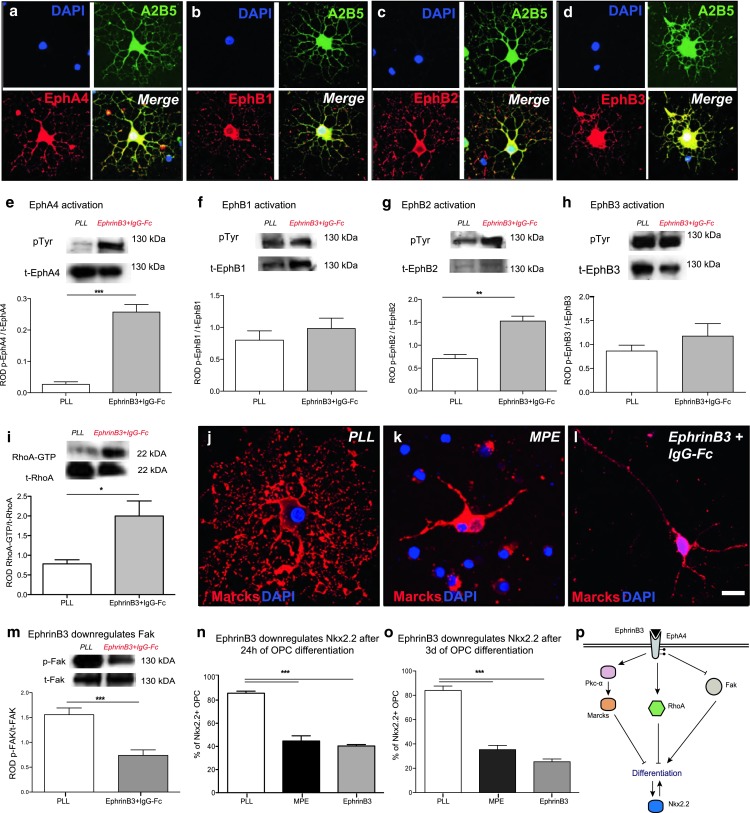
EphrinB3 activates RhoA and Pkc-α, inhibits Fak, and decreases Nkx2.2 expression. a–d OPCs in culture for 48 h expressed EphA4, EphB1, EphB2 and EphB3 receptor tyrosine kinases (RTKs). e–h EphrinB3-induced phosphorylation of EphA4 and EphB2 RTKs in OPCs [immunoprecipitation followed by WB, pTyr phosphorylated Eph-RTK, t-Eph total Eph-RTK, quantification of phosphorylated Eph-RTK relative to total Eph-RTK (ROD), n = 3: t test; EphA4 ***P < 0.05; EphB1 P > 0.1; EphB2 **P < 0.01; EphB3 P > 0.1]. i Furthermore, EphrinB3-induced RhoA activity in OPCs (24-h differentiation; quantification of RhoA-GTP relative to RhoA-GDP (ROD), n = 3: ANOVA; *P < 0.05). j–l Whilst Marcks is localized at the membrane in differentiating OPCs cultured on control substrates, EphrinB3-mediated activation of Pkc-α resulted in phosphorylation and membrane-to-cytosol translocation of Marcks. m EphrinB3 also inhibited the activation of Fak [quantification of phosphorylated (p-)Fak relative to total (t-)Fak: (ROD), n = 3; t test; **P < 0.001]. n, o Similar to MPE, EphrinB3 inhibited Nkx2.2 expression in OPCs (24-h differentiation, n = 3; ANOVA; ****P < 0.0001; Dunnett’s post hoc test PLL vs. MPE: P < 0.001; PLL vs. EphrinB3: P < 0.001; 3-day differentiation, n = 3; ANOVA; ****P < 0.0001; Dunnett’s post hoc test PLL vs. MPE: P < 0.001; PLL vs. EphrinB3: P < 0.001). p Proposed model of EphrinB3 signalling regulating OPC differentiation: the presence of EphrinB3 activates EphA4 and EphB2-RTKs. This results in activation of RhoA and Pkc-α signalling, both known to inhibit OPC differentiation. EphrinB3 also inhibits Fak signalling, a positive regulator of OPC differentiation. Failed differentiation is associated with reduced expression of Nkx2.2. Error bars ± SEM. Scale bar in a–d = 40 μm, j–l = 20 μm
Footnotes
The online version of the original article can be found under doi:10.1007/s00401-015-1521-1.


