Abstract
Purpose:
To evaluate predictors of cardiac events in esophageal cancer patients treated with neoadjuvant chemoradiotherapy followed by surgery (NA CRT) compared with surgery alone.
Methods and materials:
We retrospectively identified patients treated for esophageal cancer between 2006 and 2016. 123 patients were identified; 70 were treated with surgery alone, and 53 were treated with NA CRT. Cardiac events were scored based on Common Terminology Criteria for Adverse Events (CTCAE v4.03), and dosimetric data was compiled for all patients who received radiation. Univariate (UVA) and multivariable (MVA) analyses were performed to identify predictors of cardiac events. A competing risk of death regression was performed to model cumulative incidence of cardiac events.
Results:
The overall rates of grade ≥3 cardiac events were 24.5% in the NA CRT group versus 10% in the surgery group (p = 0.04). On MVA, use of NA CRT (p < 0.01, HR 3.45, 95% CI 1.35 – 9.09) predicted for grade ≥3 cardiac events, though no dosimetric variable predicted for grade ≥3 cardiac events or overall survival. On MVA, NA CRT predicted for pericardial effusions of any grade (p < 0.01, HR 3.70, 95% CI 1.67 – 8.33). The V45 Gy was the most significant predictor of pericardial effusions (p = 0.012, HR 1.03, 95% CI 1.01 – 1.06)
Conclusions:
NA CRT significantly increased the rate of grade ≥3 cardiac events compared with patients treated with surgery alone. While no dosimetric parameter predicted for grade ≥3 cardiac events or survival, the V45 Gy predicted for pericardial effusions.
Introduction
Neoadjuvant chemoradiotherapy (NA CRT) is the standard of care for patients with locally advanced esophageal or gastroesophageal junction (GEJ) cancers. The landmark CROSS trial demonstrated a substantial survival benefit for patients treated with NA CRT compared with surgery alone [1]. As patients have longer lifespans as a result of improving therapies, there is an increasing concern for treatment-related late toxicities. The CROSS trial reported cardiac outcomes only in the perioperative setting. Radiation-induced cardiac toxicity has previously been studied in other disease sites, with the resultant data demonstrating a relationship between radiation dose to the heart, survival, and cardiac events [2–3]. In locally advanced lung cancer, heart dose has been identified as a significant predictor of cardiac events, and in some studies, overall survival as well [4–5]. Not all patients with esophageal cancer receive radiation as part of their care, and in light of these data, it is important to fully understand the long term cardiac implications of radiation in this population.
Limited data exists cataloguing cardiac toxicities associated with NA CRT for esophageal cancer. Cardiac toxicity is a particularly relevant concern for esophageal and GEJ cancers given the location of the heart with respect to the target anatomy. Prior studies aiming to elucidate the impact of cardiac irradiation in this patient population have investigated variable outcomes, including echo-based cardiac function, FDG PET changes, and cardiac events, with variable associations with radiotherapy reported [6, 8–10]. In the present study, we attempt to identify potential cardiac risks posed by NA CRT in patients with esophageal cancer by comparing patients treated with NA CRT followed by surgery with a cohort of patients treated with surgery alone.
Methods and Materials
After IRB approval, we retrospectively identified patients with biopsy proven esophageal cancer treated at a single institution between January of 2006 and March of 2016. Cohorts of patients treated with NA CRT and surgery alone were identified. Patients with cervical esophageal tumors, those who did not have surgery, and those without evaluable follow up were excluded. For patients who received neoadjuvant treatment, those who did not receive concurrent chemotherapy or whose radiation treatment plans were unavailable for analysis were all excluded.
All patients that received neoadjuvant treatment were treated with concurrent chemotherapy. Regimens included cisplatin/5-FU based (35.8%), carboplatin/paclitaxel (34.0%), cisplatin/irinotecan (22.6%), and other chemotherapy protocols (7.5%). Seven patients (13.2%) either required dose reductions or needed to discontinue chemotherapy during treatment.
All patients were treated with either 3D conformal radiation therapy, or intensity modulated radiation therapy (IMRT) to a dose of 41.4 to 54 Gy in 23 to 30 fractions (range, 1.8–2 Gy/fraction) on a 5 day per week basis.
Gross tumor volume (GTV) was contoured on the basis of EGD, PET/CT, or CT scans (when PET was not obtained). The clinical target volume was defined as the GTV with 3–4 cm superior-inferior margins, and 0.5–1 cm lateral and anterior-posterior margins. An additional nodal CTV was typically added containing positive nodal regions plus a 1 cm expansion, and an elective nodal region which varied based on primary tumor location. 3D conformal radiation was typically delivered with a four-field technique employing opposed anterior/posterior fields with additional off-cord oblique fields. IMRT included step-and-shoot, helical tomotherapy, or volume modulated techniques. Prescribed dose and organ at risk constraints were at the discretion of the treating physicians. Typically, the heart was constrained such that 1/3 of the heart received <40 Gy, mean lung dose <20 Gy with a V20 Gy < 30%, and spinal cord dose Dmax < 45 Gy.
Surgery was performed at a median of 7 weeks (range, 4–14) after the completion of radiation. The most common esophagectomy type was Ivor Lewis (50.4%), followed by minimally invasive esophagectomy (30%), and transhiatal esophagectomy (19.5%).
Radiation treatment plans were reviewed and dosimetry was retrieved. Pericardial contours were reviewed and adjusted by a single author (J.S.W.) per the atlas prepared by Feng et al [7]. Plans for each patient were contoured and/or evaluated using the MIM software planning system (MIM Software Inc., Cleveland, Ohio). Cardiac dosimetry was extracted from the resultant dose-volume histograms (DVH) for each patient, and DVH data were recorded in discrete 5-Gy dose levels. Mean and maximum cardiac dose levels were also recorded on a per patient basis.
All available patient records were reviewed via the electronic health record to assess for cardiac events. Relevant imaging and laboratory data were also evaluated to assess for pericardial effusions, cardiac function, markers of cardiac injury, and arrhythmias. Pre-existing cardiac disease was defined as a known diagnosis of coronary artery disease or congestive heart failure prior to treatment.
All cardiac events were assessed and graded after therapy regardless of whether events were symptomatic or asymptomatic. Time 0 was defined as the time of surgical resection for both groups. Cumulative cardiac events were recorded for each individual patient, and the highest-grade event was reported on a per patient basis. When individual patients had multiple grade ≥3 cardiac events, the event with the shortest time interval from completion of therapy was recorded. Cardiac toxicity was evaluated retrospectively using the Common Terminology Criteria for Adverse Events (CTCAE, version 4.03).
Cumulative incidence curves for the development of first grade ≥3 cardiac event competing with death were generated using the Fine and Gray competing risk regression. Univariate (UVA) and multivariable (MVA) proportional sub-distribution hazard regression models (Fine and Gray) were employed to analyze the impact of each individual covariate on the development of grade ≥3 cardiac events. This included performance status, the presence of pre-existing cardiac disease, smoking history, diabetes mellitus, and other factors related to cardiovascular outcomes. MVA analyses were limited to the 2–3 most significant covariates per analysis given the small number of statistically significant factors identified, and the relatively small number of cardiac events. A two sided p value of < 0.05 was considered statistically significant. Difference in cardiac dosimetry was calculated using a two-tailed student’s t test.
Results
Patient characteristics are listed by cohort in Table 1. Patients in the surgery alone arm were older (p = 0.01) and had lower clinical stage (p = 0.01) than patients treated with NA CRT. Pathologic nodal positivity was significantly higher in the NA CRT group compared with the surgery alone group (37.7% versus 20%, p = 0.03). There was no statistically significant difference in pre-existing cardiac disease between the surgery alone group and the NA CRT group (p = 0.13). The majority of patients (89.4%) across both groups had tumors located in the lower third of the esophagus and GEJ.
Table 1:
Patient, Tumor and Treatment Characteristics
| Surgery Alone (%) | Chemoradiation + Surgery (%) | p-value | |
|---|---|---|---|
| Patient Number | 70 | 53 | |
| Median Age, years (range) | 71 (40–86) | 65 (45–75) | 0.01 |
| Sex | |||
| Male | 57 (81.4) | 44 (83.0) | 1 |
| Female | 13 (18.5) | 9 (17.0) | |
| Race | |||
| White | 68 (97) | 52 (98) | 1 |
| Black | 2 (3) | 1 (2) | |
| ECOG | |||
| 0–1 | 67 (95.8) | 51 (96.2) | 0.89 |
| 2 | 3 (4.2) | 2 (3.8) | |
| Smoking History | |||
| Current | 7 (10) | 12 (22.6) | 0.12 |
| Former | 50 (71.4) | 30 (56.6) | |
| Never | 13 (18.6) | 11 (20.8) | |
| BMI, Median (Range) | 28.02 (16.48–48.22) | 27.27 (16.32–42.10) | 0.06 |
| Diabetes | |||
| Yes | 19 (27.1) | 9 (17.0) | 0.27 |
| No | 51 (72.9) | 44 (83.0) | |
| HTN | |||
| Yes | 44 (62.9) | 29 (54.7) | 0.47 |
| No | 26 (37.1) | 24 (45.3) | |
| Clinical Stage | |||
| I | 40 (57.1) | 0 | 0.01 |
| II | 22 (31.4) | 12 (22.6) | |
| III | 8 (11.4) | 41 (77.4) |
Dosimetric data for patients who underwent NA CRT followed by surgery is reported in Table 2. The median prescription dose was 50.4 Gy (range, 41.4 – 54 Gy), with the majority of NA CRT patients (83%) receiving 50.4 Gy. The majority of patients (67.9%) were treated with 3D conformal radiation, with the remainder (32.1%) treated with IMRT. The median mean heart dose was 28.8 Gy (range, 10–43 Gy); those treated with 3D conformal technique had a median mean of 30.5 Gy, while those treated with IMRT had a median mean dose of 27.6 Gy (p = 0.25). There was a significant difference in the median cardiac volumes receiving doses ≥35 Gy between patients treated with IMRT and 3D technique. For example, V35 was 53.8% versus 30.0% (p = 0.03), V40 was 44.9% versus 18.4% (p < 0.01), and V45 was 20.0% versus 11.0% (p < 0.01) all in favor of IMRT (Table 2).
Table 2:
Median Dosimetric Data (Range)
| All Patients | 3D | IMRT | |
|---|---|---|---|
| Prescription Dose, Gy | 50.4 (41.4–54) | ||
| Technique | |||
| 3D | 36 (67.9%) | ||
| IMRT | 17 (32.1%) | ||
| Gross Tumor Volume (cc) | 65.9 (4.9–347) | 65.9 | 59.7 |
| Heart Volume (cc) | 664.1 (282–1270) | 655.3 | 688.4 |
| Heart Dmax (Gy) | 54.2 (33–61) | 55.0 | 53.3 |
| Heart Mean (Gy) | 28.8 (10–43) | 30.5 | 27.6 |
| V5 Gy (%) | 95.9(47–100) | 90.8 | 99.8 |
| V10 Gy (%) | 83.4 (29–100) | 81.2 | 92.3 |
| V15 Gy (%) | 74.9 (22–99) | 71.2 | 81.4 |
| V20 Gy (%) | 66.0 (18–98) | 66.5 | 66.0 |
| V25 Gy (%) | 59.3 (14–97) | 63.6 | 53.1 |
| V30 Gy (%) | 48.7 (7–95) | 58.9 | 41.7 |
| V35 Gy (%) | 37.9 (0–94) | 53.8 | 30.0 |
| V40 Gy (%) | 26.0 (0–86) | 44.9 | 18.4 |
| V45 Gy (%) | 15.5 (0–77) | 20.0 | 11.0 |
| V50 Gy (%) | 7.0 (0–36) | 9.3 | 3.8 |
Out of 123 patients, 20 (16.3%) experienced grade ≥3 cardiac events. Only 4 patients (3%) experienced a grade 4 event, while no patients had a grade 5 event. Median follow up from treatment was 37.3 months. The median time to grade ≥3 event was 3.7 months in the NA CRT group compared with 1.7 months with surgery alone. The maximum ≥3 grade cardiac events included 5 acute coronary syndromes, 2 new diagnoses of congestive heart failure, 8 arrhythmias, 1 cardiac arrest, 3 pericardial effusions, and 1 episode of pericarditis (Table 3). The overall rates of grade ≥3 cardiac toxicity were 24.5% in the NA CRT group compared with 10% in the surgery group (p = 0.04).
Table 3:
Highest grade cardiac event experienced by patient.
| Cardiac Event | None | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 | Grade 3–5 |
| Acute Coronary Syndrome | 0 | 0 | 5 | 0 | 0 | 5 | |
| New Congestive Heart Failure | 0 | 1 | 0 | 0 | 0 | 0 | |
| Arrhythmia | 3 | 15 | 2 | 0 | 0 | 2 | |
| Cardiac Arrest | 0 | 0 | 0 | 0 | 0 | 0 | |
| Pericardial Effusion | 0 | 5 | 0 | 0 | 0 | 0 | |
| Pericarditis | 0 | 0 | 0 | 0 | 0 | 0 | |
| Total (n=70) | 39 | 3 | 21 | 7 | 0 | 0 | 7 |
| Percent | 56 | 4 | 30 | 10 | 0 | 0 | 10 |
| Cardiac Event | None | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 | Grade 3–5 |
| Acute Coronary Syndrome | 0 | 2 | 0 | 0 | 0 | 0 | |
| New Congestive Heart Failure | 0 | 2 | 2 | 0 | 0 | 2 | |
| Arrhythmia | 4 | 8 | 4 | 2 | 0 | 6 | |
| Cardiac Arrest | 0 | 0 | 0 | 1 | 0 | 1 | |
| Pericardial Effusion | 0 | 11 | 2 | 1 | 0 | 3 | |
| Pericarditis | 0 | 0 | 1 | 0 | 0 | 1 | |
| Total (n=53) | 13 | 4 | 23 | 9 | 4 | 0 | 13 |
| Percent | 25 | 8 | 43 | 17 | 8 | 0 | 25 |
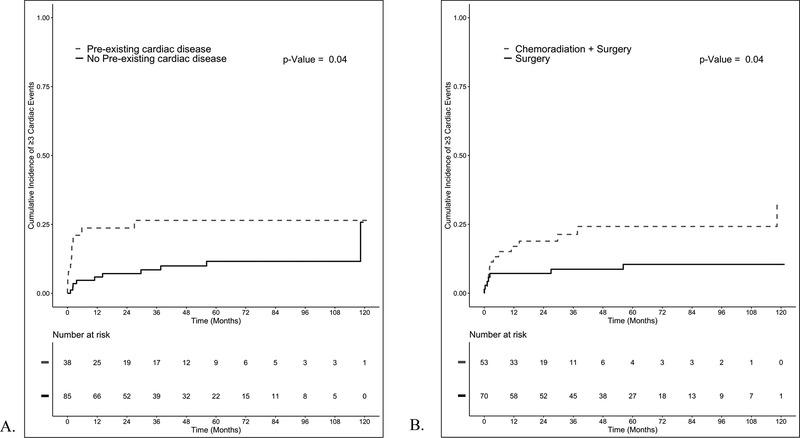
UVA and MVA analyses adjusted for the competing risk of death are listed in Table 4. Patients with pre-existing cardiac disease had higher rates of cardiac events on UVA analysis (p = 0.04, HR 2.56, 95% CI 1.06 – 6.25) (Figure 1A). NA CRT also predicted for grade ≥3 cardiac events after adjusting for the competing risk of death on UVA analysis (p = 0.04, HR 2.63, 95% CI 1.08 – 6.67) (Figure 1B). Both pre-existing cardiac disease (p < 0.01, HR 3.45, 95% CI 1.41 – 8.32) and use of NA CRT (p < 0.01, HR 3.45, 95% CI 1.35 – 9.09) persisted on MVA analysis after pairing them together. Among patients treated with NA CRT, no chemotherapy regimen predicted for grade ≥3 cardiac events (Supplemental Table 2).
Table 4:
Univariate and multivariable analyses for grade ≥3 cardiac events for all patients.
| Variable | Univariate Analysis | Multivariable Analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | |
| Age at Diagnosis | 1 | 0.96 – 1.04 | 0.99 | |||
| Sex (Male) | 1.46 | 0.48 – 4.45 | 0.51 | |||
| ECOG Score | 0.41 | 0.05 – 3.2 | 0.38 | |||
| BMI | 1.02 | 0.96 – 1.07 | 0.56 | |||
| Diabetes (No) | 2.79 | 0.66 – 11.76 | 0.16 | |||
| HTN (No) | 0.58 | 0.23 – 1.49 | 0.26 | |||
| Smoking (Never) | 0.73 | 0.22 – 2.48 | 0.62 | |||
| Pre-existing Cardiac Disease (Yes) | 2.56 | 1.06 – 6.25 | 0.04 | 3.45 | 1.41 – 8.33 | <0.01 |
| Location (GE Junction) | 0.69 | 0.27 – 1.81 | 0.45 | |||
| Radiation (Yes) | 2.63 | 1.08 – 6.67 | 0.04 | 3.45 | 1.35 – 9.09 | < 0.01 |
Abbreviations: BMI, body mass index; HTN, hypertension; GE, gastro-esophageal
Figure 1:
A. Cumulative incidence of ≥3 cardiac events for patients with and without pre-existing cardiac disease. B. Cumulative incidence of ≥3 cardiac events comparing patients treated with surgery alone to patients treated with neoadjuvant chemoradiation (NA CRT) followed by surgery adjusted for competing risk of death.
UVA analysis of the NA CRT group showed no significant predictors, dosimetric or otherwise, of grade ≥3 cardiac events (Supplemental Table 3). Though cardiac doses were lower with IMRT compared with 3D conformal technique, there was no statistically significant reduction in high-grade cardiac events when comparing the two modalities (p = 0.12). Additionally, there was no identifiable association between overall survival and cardiac dose (Supplemental Table 4).
Pericardial effusions of any grade were significantly increased in patients treated with NA CRT (26.4%) compared with surgery alone (7.1%) (p < 0.01, HR 1.75, 95% CI 1.06 – 2.94) (Supplemental Figure 1). The minority of the pericardial effusions were grade 3 or higher (21.6%) in the NA CRT group, while in the surgery alone group no patient had a symptomatic pericardial effusion.
On UVA analysis of patients treated with NA CRT, GTV (p = 0.01), as well as the heart volumes receiving 35 Gy (p = 0.03), 45 Gy (p = 0.001), and 50 Gy (p = 0.04) were all identified as predictors of developing pericardial effusions. No other baseline or treatment factors predicted for pericardial effusion, including both pre-existing cardiac disease and hypertension. A full listing of factors assessed is displayed in Table 5. Pairing each dosimetric factor with GTV for MVA analysis resulted in persistence of V45 being associated with pericardial effusion (p = 0.012, HR 1.03, 95% CI 1.01–1.06). After adjusting for the competing risk of death, we identified that a V45 of 33% represented the most statistically significant cutoff (p < 0.001) for pericardial effusions (Supplemental Figure 2).
Table 5:
Univariate and multivariable analyses for pericardial effusion (any grade) for patients treated with NA CRT followed by surgery.
| Variable | Univariate Analysis | Multivariable Analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | |
| Age at Diagnosis | 0.97 | 0.9 – 1.04 | 0.33 | |||
| Sex (Male) | 2.24 | 0.49 – 10.13 | 0.30 | |||
| ECOG Score | 0.69 | 0.28 – 1.68 | 0.41 | |||
| BMI | 1.05 | 0.96 – 1.14 | 0.32 | |||
| Diabetes (No) | 0.86 | 0.29 – 2.55 | 0.79 | |||
| HTN (No) | 1.99 | 0.87 – 4.6 | 0.11 | |||
| Smoking (Never) | 2.04 | 0.88 – 4.71 | 0.09 | |||
| Pre-Existing Cardiac Disease (Yes) | 1.16 | 0.40 – 3.53 | 0.83 | |||
| Clinical Stage (I) | 1.05 | 0.39 – 2.85 | 0.92 | |||
| Location (GE Junction) | 1.2 | 0.49 – 2.91 | 0.69 | |||
| Radiation Modality (IMRT) | 0.42 | 0.14 – 1.26 | 0.12 | |||
| Gross Tumor Volume | 1.01 | 1 – 1.01 | 0.01 | 1.01 | 1 – 1.01 | 0.016 |
| Heart Mean Dose | 1.06 | 0.99 – 1.14 | 0.10 | |||
| Heart Max | 0.98 | 0.9 – 1.07 | 0.70 | |||
| V5 | 1.01 | 0.97 – 1.04 | 0.73 | |||
| V10 | 1.01 | 0.98 – 1.04 | 0.74 | |||
| V15 | 1 | 1 – 1 | 0.06 | |||
| V20 | 1.01 | 0.99 – 1.04 | 0.31 | |||
| V25 | 1.02 | 0.99 – 1.04 | 0.17 | |||
| V30 | 1.02 | 1 – 1.04 | 0.08 | |||
| V35 | 1.02 | 1 – 1.04 | 0.03 | |||
| V40 | 1 | 1 – 1 | 0.09 | |||
| V45 | 1.03 | 1.01 – 1.05 | 0.001 | 1.03 | 1.01 – 1.06 | 0.012 |
| V50 | 1.04 | 1 – 1.08 | 0.04 | |||
Abbreviations: BMI, body mass index; HTN, hypertension; GE, gastro-esophageal; IMRT, intensity-modulated radiation therapy; NA CRT, neoadjuvant chemoradiotherapy
Discussion
In the present study, we retrospectively analyzed separate cohorts of patients treated for esophageal cancer with either surgery alone, or surgery in combination with NA CRT. After adjusting for the competing risk of death, our results showed that grade ≥3 cardiac events occurred more often in those with pre-existing cardiac conditions, as well as those treated with NA CRT. Though we did not identify any specific dosimetric variable that predicted for grade ≥3 cardiac events, there was a dosimetric relationship between heart dose and pericardial effusion.
Prior studies have attempted to analyze the risk of cardiac toxicity in patients treated with radiation for esophageal cancer. A SEER analysis showed an increased risk of heart disease-related death in patients with esophageal cancer treated with radiotherapy [8]. Other studies have used varying definitions of cardiac outcomes, including standardized toxicity reporting scales like the CTCAE, decreases in ejection fraction, FDG uptake on PET, and others [6,9–11]. Two prior studies evaluated decreases in ejection fraction in small cohorts of patients treated with neoadjuvant chemo-radiation, and concluded that there was no short-term dosimetric relationship on cardiac function [9–10]. Conversely, in a prior cohort of patients with locally advanced esophageal cancer treated either definitively or neoadjuvantly with chemo-radiation, Konski et al reported a dosimetric relationship between cardiac V20, V30, and V40 with all cardiac events [11]. In that study, cardiac outcomes were assessed based on the CTCAE v3.0, and they identified a total of 12 events over the period of follow up. However, they captured only 5 grade 3 events, and a single grade 4 event in their follow up period, with the remaining 6 events being grade 1. They did not further stratify by higher grade events given the relatively few number observed. They concluded that patients with events had higher V20–40, but did not attempt to identify any dosimetric cutoff. In comparison, our study identified a higher number of cardiac events, including a higher number of grade ≥3 cardiac events.
In comparison to the esophageal data, studies examining the effects of radiation on the heart in lung cancer patients have revealed a dosimetric relationship between cardiac dose and patient survival [12]. An analysis of RTOG 0617 identified cardiac V5 and V30 as predictors of death in this population, a finding that has changed the evaluation of radiation plans for locally advanced lung cancer. Further work by Dess et al and Wang et al analyzed treatment plans from a number of prospective lung radiotherapy protocols, including RTOG 0617, and found a statistically significant association between mean heart dose and clinically significant cardiac events, but no relationship with overall survival [4, 5]. In comparison with the lower doses delivered for neoadjuvant treatment in esophageal cancer, the higher doses delivered for definitive treatment of lung cancer may have a greater relative cardiac impact.
In our study, pericardial effusions were more prominent in the NA CRT group, and this was correlated with heart dose. Prior work by Wei et al also demonstrated a dosimetric relationship between pericardial dose and pericardial effusions in patients treated definitively for esophageal cancer with chemoradiation [13]. They noted a crude rate of 27.7% among their 101 patients, with statistically significant associations identified between both V30 and mean heart dose and the development of pericardial effusion. Cutoffs were identified including a mean heart dose of 26.1 Gy, and a V30 of 46%. Similar to our study, no other clinical factors were significantly associated with pericardial effusion aside from radiation dose. Though the majority of the observed pericardial effusions in our study were asymptomatic, there were 2 grade 3 and 1 grade 4 pericardial effusions recorded in the NA CRT group. Given the association between pericardial effusion and cardiac dose found here and in other studies, minimizing cardiac dose may reduce incidence of clinically significant events.
In our series, we found that IMRT reduced the heart V35, V40, and V45 compared to 3D conformal techniques. IMRT is one possible means of reducing cardiac dose, and ultimately improving cardiac outcomes. A propensity-matched analysis of a large cohort of patients treated either neoadjuvantly or definitively for esophageal cancer revealed a statistically significant decrease in cardiac death for patients treated with IMRT [14]. In the study, patients who received IMRT had a worse performance status, though were observed to have better overall survival and locoregional control compared with patients treated with 3D conformal technique. In particular, they identified a statistically significant decrease in cardiac and indeterminate deaths for patients treated with IMRT. Notably, there was no difference in cancer specific outcomes between these two groups. In a second publication by the same group, a SEER analysis investigating the role of IMRT for esophageal cancer in the elderly population demonstrated improved all-cause mortality and cardiac mortality in patients older than 65 years [15]. Proton radiotherapy has also been shown to reduce cardiac dose in esophageal cancer [16]. Taken together, these data suggest that techniques that limit heart dose may lead to an improved long-term toxicity profile that impacts survival. In the present study, there were significant differences in V35, V40, and V45 observed between plans employing IMRT versus 3D CRT. However, this did not translate into reduced cardiac events or an effect on survival. In our cohort, the majority (67.9%) of patients were treated with 3D CRT, limiting our evaluation of differences between the two techniques and raising the median cardiac dose of the overall cohort.
There are several limitations with the present study given its retrospective nature. As expected, the two cohorts varied significantly in stage and age, with surgical patients having more limited disease. However, baseline pre-existing cardiac disease was not statistically different between the two groups. All patients treated with radiation also received various concurrent chemotherapy regimens, though no specific chemotherapy regimen predicted for cardiac events. This is relevant, as common chemotherapy regimens are known to have cardiotoxic effects [17]. A strength of our data is the inclusion of a surgical comparison group. Our data show that there is a 10% baseline risk of developing clinically significant cardiac events after surgery, even without the use of NA CRT.
In conclusion, in this study comparing patients treated with neoadjuvant chemoradiation to patients treated with surgery alone, there was an increased incidence of grade ≥3 cardiac events associated with neoadjuvant treatment. While no dosimetric variable predicted for high-grade events, V45 predicted for pericardial effusions of any grade. Given the overwhelming evidence from the CROSS trial in favor of neoadjuvant treatment, NA CRT will continue to be the standard of care in patients with locally advanced esophageal cancer. The risk of cardiac toxicity should always be considered in relation to the probability of long-term survival and pre-existing cardiac conditions. Techniques to minimize heart dose, including IMRT, should be considered. Further work will be needed to understand the complex relationship between heart dose and cardiac events in this population.
Supplementary Material
References
- 1.Van Hagen P, Hulshof MC, Van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366(22):2074–84. doi: 10.1056/NEJMoa1112088 [DOI] [PubMed] [Google Scholar]
- 2.Speirs CK, Dewees TA, Rehman S, et al. Heart Dose Is an Independent Dosimetric Predictor of Overall Survival in Locally Advanced Non-Small Cell Lung Cancer. J Thorac Oncol. 2017;12(2):293–301. doi: 10.1016/j.jtho.2016.09.134 [DOI] [PubMed] [Google Scholar]
- 3.Darby SC, Ewertz M, Mcgale P, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368(11):987–98. doi: 10.1056/NEJMoa1209825 [DOI] [PubMed] [Google Scholar]
- 4.Wang K, Eblan MJ, Deal AM, et al. Cardiac Toxicity After Radiotherapy for Stage III Non-Small-Cell Lung Cancer: Pooled Analysis of Dose-Escalation Trials Delivering 70 to 90 Gy. J Clin Oncol. 2017;35(13):1387–94. doi: 10.1200/JCO.2016.70.0229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dess RT, Sun Y, Matuszak MM, et al. Cardiac Events After Radiation Therapy: Combined Analysis of Prospective Multicenter Trials for Locally Advanced Non-Small-Cell Lung Cancer. J Clin Oncol. 2017;35(13):1395–1402. doi: 10.1200/JCO.2016.71.6142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gayed IW, Liu HH, Yusuf SW, et al. The prevalence of myocardial ischemia after concurrent chemoradiation therapy as detected by gated myocardial perfusion imaging in patients with esophageal cancer. J Nucl Med. 2006;47(11):1756–62. [PubMed] [Google Scholar]
- 7.Feng M, Moran JM, Koelling T, et al. : Development and validation of a heart atlas to study cardiac exposure to radiation following treatment for breast cancer. Int J Radiat Oncol Biol Phys. 2011;79:10–8. doi: 10.1016/j.ijrobp.2009.10.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frandsen J, Boothe D, Gaffney DK, Wilson BD, Lloyd S. Increased risk of death due to heart disease after radiotherapy for esophageal cancer. J Gastrointest Oncol. 2015;6(5):516–23. doi: 10.3978/j.issn.2078-6891.2015.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mukherjee S, Aston D, Minett M, Brewster A, Crosby TD. The significance of cardiac doses received during chemoradiation of oesophageal and gastro-esophageal junctional cancers. Clin Oncol. 2003;15:115–20. [DOI] [PubMed] [Google Scholar]
- 10.Tripp P, Malhotra HK, Javle M, et al. Cardiac function after chemoradiation for esophageal cancer: comparison of heart dose–volume histogram parameters to multiple gated acquisition scan changes. Dis Esophagus. 2005;18:400–5. doi: 10.1111/j.1442-2050.2005.00523.x [DOI] [PubMed] [Google Scholar]
- 11.Konski A, Li T, Christensen M, et al. Symptomatic cardiac toxicity is predicted by dosimetric and patient factors rather than changes in 18F-FDG PET determination of myocardial activity after chemoradiotherapy for esophageal cancer. Radiother Oncol. 2012;104:72–7. doi: 10.1016/j.radonc.2012.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bradley JD, Paulus R, Komaki R, et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol. 2015;16(2):187–99. doi: 10.1016/S1470-2045(14)71207-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wei X, Liu HH, Tucker SL, et al. Risk factors for pericardial effusion in inoperable esophageal cancer patients treated with definitive chemoradiation therapy. Int J Radiat Oncol Biol Phys. 2008;70(3):707–14. doi: 10.1016/j.ijrobp.2007.10.056 [DOI] [PubMed] [Google Scholar]
- 14.Lin SH, Wang L, Bevan M, et al. Propensity Score-based Comparison of Long-term Outcomes With 3-Dimentional Conformal Radiotherapy vs Intensity-Modulated Radiotherapy for Esophageal Cancer. Int J Radiat Oncol Biol Phys. 2012; 84(5):1078–85. doi: 10.1016/j.ijrobp.2012.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin SH, Zhang N, Godby J, et al. Radiation modality use and cardiopulmonary mortality risk in elderly patients with esophageal cancer. Cancer. 2016;122(6):917–28. doi: 10.1002/cncr.29857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shiraishi Y, Xu C, Yang J, Komaki R, Lin SH. Dosimetric comparison to the heart and cardiac substructure in a large cohort of esophageal cancer patients treated with proton beam therapy or Intensity-modulated radiation therapy. Radiother Oncol. 2017;125(1):48–54. [DOI] [PubMed] [Google Scholar]
- 17.Saif MW, Shah MM, Shah AR. Fluoropyrimidine-associated cardiotoxicity: revisited. Expert Opin Drug Saf. 2009;8:191. doi: 10.1517/14740330902733961 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.