Abstract
Impaired 11β-hydroxysteroid dehydrogenase type 2 (11β-HSD2)-dependent cortisol inactivation can lead to electrolyte dysbalance, hypertension and cardiometabolic disease. Furthermore, placental 11β-HSD2 essentially protects the fetus from high maternal glucocorticoid levels, and its impaired function has been associated with altered fetal growth and a higher risk for cardio-metabolic diseases in later life. Despite its important role, 11β-HSD2 is not included in current off-target screening approaches. To identify potential 11β-HSD inhibitors amongst approved drugs, a pharmacophore model was used for virtual screening, followed by biological assessment of selected hits. This led to the identification of several azole fungicides as 11β-HSD inhibitors, showing a significant structure-activity relationship between azole scaffold size, 11β-HSD enzyme selectivity and inhibitory potency. A hydrophobic linker connecting the azole ring to the other, more polar end of the molecule was observed to be favorable for 11β-HSD2 inhibition and selectivity over 11β-HSD1. The most potent 11β-HSD2 inhibition, using cell lysates expressing recombinant human 11β-HSD2, was obtained for itraconazole (IC50 139 ± 14 nM), its active metabolite hydroxyitraconazole (IC50 223 ± 31 nM) and posaconazole (IC50 460 ± 98 nM). Interestingly, experiments with mouse and rat kidney homogenates showed considerably lower inhibitory activity of these compounds toward 11β-HSD2, indicating important species-specific differences. Thus, 11β-HSD2 inhibition by these compounds are likely to be overlooked in preclinical rodent studies. Inhibition of placental 11β-HSD2 by these compounds, in addition to the known inhibition of cytochrome P450 enzymes and P-glycoprotein efflux transport, might contribute to elevated local cortisol levels, thereby affecting fetal programming.
Keywords: 11β-hydroxysteroid dehydrogenase, virtual screening, reproductive toxicity, itraconazole, posaconazole, glucocorticoid
1. Introduction
11β-hydroxysteroid dehydrogenase 2 (11β-HSD2) converts potent 11β-hydroxyglucocorticoids (cortisol, corticosterone) into their inactive 11-keto forms (cortisone, 11-dehydrocorticosterone), thereby controlling tissue-specific activities of mineralocorticoid receptors (MR) and glucocorticoid receptors (GR) [1]. 11β-HSD2 is essentially involved in the regulation of electrolyte balance, vascular function, and angiogenesis, as well as in the fetal-placental barrier to inactivate cortisol and protect the fetus from high maternal glucocorticoid levels [2, 3].
The consequences of impaired 11β-HSD2 function are manifested in patients with genetic loss-of-function mutations suffering from apparent mineralocorticoid excess (AME) [2, 4, 5]. In these patients the excessive cortisol-dependent MR activation in the kidney and colon results in hypokalemia, hypernatremia and water retention, leading to severe hypertension characterized by low renin, low aldosterone and increased plasma and urinary cortisol to cortisone ratios. 11β-HSD2 activity is essential since very early on in life, because birth weights of individuals homozygous/compound heterozygous for HSD11B2 mutations were found to be significantly lower than those of their unaffected siblings [6]. Milder acquired forms of AME can be caused by inhibition of 11β-HSD2, for instance upon consumption of considerable amounts of licorice, containing the potent 11β-HSD inhibitor glycyrrhetinic acid (GA) [7].
The exposure to 11β-HSD2 inhibitors is especially critical during pregnancy. 11β-HSD2 builds a placental barrier by protecting the fetus from the 5-10 times higher maternal glucocorticoid levels in the course of a normal pregnancy [8–11]. Nevertheless, this barrier is not entirely complete, as a minor proportion of maternal cortisol is able to cross the placenta [11]. Glucocorticoids are important mediators of fetal growth, development and organ maturation. Rising total plasma cortisol levels during pregnancy, peaking in the third trimester at three-fold non-pregnant levels [12], are in parallel with progressive maturation of fetal organs, most notably the stimulation of surfactant production by the lung. However, glucocorticoid administration, especially during late gestation, has been associated with reduced birth weight, elevated blood pressure, higher insulin, increased distractibility and attention deficit later in life [13–17]. The correlation between low birth weight, albeit in normal ranges, and subsequent diseases in adulthood was found to be largely independent of confounding life style factors such as smoking or obesity [15]. It has been hypothesized that a reduced placental 11β-HSD2 activity is responsible for the high glucocorticoid concentrations reaching the fetus and subsequent programming of disease susceptibility [18]. Importantly, low 11β-HSD2 expression was found to be associated with intrauterine growth restriction pregnancies in humans and in rodent models [19–22]. Determination of osteocalcin concentration in human cord blood samples, which is a sensitive marker of glucocorticoid exposure in adult humans, revealed a direct correlation with placental 11β-HSD2 activity [23].
Treatment of pregnant rats with dexamethasone, which cannot be inactivated by 11β-HSD2 [24], led to lower birth weights and caused HPA axis hyperactivity, hypertension, hyperglycemia and increased anxiety behavior [20, 25]. Similarly, administration of the unselective 11β-HSD inhibitor carbenoxolone to pregnant rats resulted in reduced birth weight, and adult offspring showed enhanced HPA activity with increased glucocorticoid and CRH levels as well as elevated blood pressure [26, 27]. Evidence for the importance of placental 11β-HSD2 was contributed by studies with placentas from 11β-HSD2-deficient mice showing increased amino acid and reduced glucose transport as well as lower expression levels of genes important for angiogenesis [28]. Furthermore, maternal stress and malnutrition in rats were reported to be able to down regulate placental 11β-HSD2 and program for diseases in adult life [29–31]. Factors including sex steroids, nitric oxide, prostaglandins, proinflammatory cytokines, infections and environmental pollutants were shown to have the potential to reduce 11β-HSD2 activity in studies using placental cell lines [32, 33].
Observational studies showed that pregnant Finnish women consuming large amounts of licorice (containing the unselective 11β-HSD inhibitor glycyrrhetinic acid) had shorter gestation times [34] and gave birth to children with behavioral disturbances and poorer cognitive functions coupled with increased HPA axis activity, in a dose-dependent manner [35, 36]. Compromised 11β-HSD2 function during pregnancy has also been implicated in preeclampsia, a major cause of maternal and perinatal mortality; however, the etiology is poorly understood [37, 38].
Due to its important physiological role and the adverse effects observed upon its inhibition, 11β-HSD2 can be considered as an anti-target for drug development (with a few specific exceptions where these effects are wanted); however, it is not included in current off-target screening approaches. The present study addressed possible inhibitory effects of approved drugs toward 11β-HSD2 by performing a virtual screening (VS) of the DrugBank database using an 11β-HSD pharmacophore model. This was followed by a biological evaluation of selected hits, with a focus on the azole fungicides itraconazole and posaconazole.
2. Materials and methods
2.1. Chemicals and reagents
[1,2,6,7-3H]-cortisol, [2,4,6,7-3H]-estrone and [2,4,6,7-3H]-estradiol were purchased from PerkinElmer (Boston, MA, USA), [1,2-3H]-cortisone from American Radiolabeled Chemicals (St. Louis, MO), hydroxyitraconazole (OHI) from Carbosynth (Berkshire, UK) and all other chemicals from Sigma Aldrich (Buchs, Switzerland) of the highest grade available. Cell culture media were obtained from Sigma Aldrich (Buchs, Switzerland).
2.2. Pharmacophore modeling and virtual screening
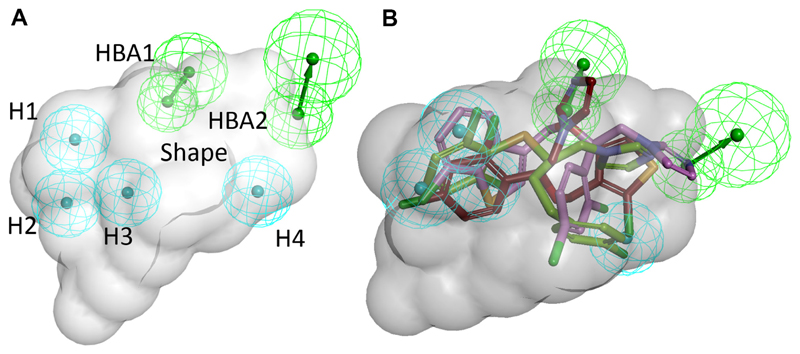
The 11β-HSD inhibitor pharmacophore model used for this study was previously reported and validated [39]. The model was initially based on three potent, structurally diverse 11β-HSD inhibitors [40] and refined with recent literature and novel screening data (model 4new in reference [39], Fig. 1A).
Fig. 1. Pharmacophore model used for virtual screening of the DrugBank database.
(A) The model consists of four hydrophobic (H) features (cyan), two hydrogen bond acceptors (HBA, green) and a sterical shape restriction (grey) [39]. (B) Virtual hits from the DrugBank screening fitted into the model. Compounds are color-coded: red – tioconazole; green – butoconazole; violet – sertaconazole.
To discover potential 11β-HSD inhibitors amongst FDA-approved drugs and nutraceuticals, the DrugBank version 3.0 was downloaded as sd file (1543 approved drugs and 84 nutraceuticals) and transformed into a 3D-multiconformational database using the “build database” protocol of Discovery Studio 4.0 (Discovery Studio, Version 4.0, Biovia Inc., San Diego, CA, 2014). For each compound, a maximum of 255 conformers was calculated using fast settings. For the VS, the “search 3D database” protocol with BEST flexible search was used. The DrugBank database was screened with the 11β-HSD inhibitors model using Discovery Studio 4.0.
2.3. Cell culture
Human Embryonic Kidney-293 cells (HEK-293) cells (used at passage number 15-30), human SW-620 colon carcinoma cells (passage number 11-15) and human MCF-7 breast cancer cells (passage number 19-27) were obtained from ATCC (Manassas, VA, USA) and were cultured in Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum, 4.5 g/L glucose, 100U/mL penicillin/streptomycin, 2 mM L-glutamine, 10 mM HEPES, pH 7.4, and 10% MEM non-essential amino acid solution.
2.4. Determination of 11β-HSD activity in cell lysates
Enzyme activities were determined as described earlier using lysates of HEK-293 cells stably expressing human 11β-HSD1 and hexose-6-phosphate dehydrogenase (H6PDH; HHH7 clone) or 11β-HSD2 (AT8 clone) [41]. 11β-HSD1 reductase activity was measured by incubating the lysates for 10 min at 37°C in a total volume of 22 μL containing 200 nM radiolabeled cortisone, 500 μM NADPH and test substance or vehicle (DMSO at a maximal concentration of 0.1%). 11β-HSD2-dependent oxidation was assessed in the presence of 200 nM radiolabeled cortisol and 500 μM NAD+. The reactions were stopped by adding an excess amount of unlabeled cortisone and cortisol (1:1, 2 mM in methanol). Approximately 20-25% of the substrate were converted to the corresponding product. Steroids were separated by thin layer chromatography (TLC) using chloroform and methanol (9:1). Conversion of radiolabeled substrate was measured by scintillation counting. The substrate conversion was determined and compared to the enzyme activity in the control sample.
To exclude irreversible 11β-HSD2 inhibition by the investigated compounds, HEK cell lysates were preincubated with the corresponding compounds for 0, 5 and 20 min at 4°C, followed by determination of enzyme activity. Alternatively, lysate were preincubated with the compounds for 10 min followed by subsequent dilution (1:2 or 1:4) and determination of enzyme activity [42]. Data (mean ± SD) were normalized to vehicle control (DMSO) and obtained from at least three independent experiments.
2.5. Determination of 11β-HSD2 activity in intact cells
11β-HSD2 activity measurement in intact SW-620 and MCF-7 cells was determined as described earlier [43]. Briefly, 100 000 SW-620 and 50 000 MCF-7 cells per well were seeded in 96-well plates. The medium was replaced after 24 h by 40 μL steroid- and serum-free DMEM (DMEMsf) containing either vehicle or inhibitor and 10 μL medium containing 10 nCi radiolabeled cortisol and unlabeled cortisol to reach a final concentration of 50 nM. SW-620 cells were incubated for 4 h and MCF-7 cells for 5 h at 37°C, followed by analysis of steroid conversion by TLC and scintillation counting
2.6. Determination of 17β-HSD1 and 17β-HSD2 activity in cell lysates
Determination of 17β-HSD1 and 17β-HSD2 activity was conducted as described earlier [44]. Briefly, lysates of HEK-293 cells transiently expressing human 17β-HSD1 and 17β-HSD2 were incubated for 10 min at 37°C in a total volume of 22 μL in the presence 200 nM estrone containing 50 nCi of [2,4,6,7-3H]-estrone and 500 μM NAPDH for 17β-HSD1, and with 200 nM estradiol containing 50 nCi of [2,4,6,7-3H]-estradiol and 500 μM NAD+ for 17β-HSD2 activity measurements. Steroids were separated using TLC and substrate conversion determined by scintillation counting. Data (mean ± SD) were normalized to vehicle control (DMSO) and obtained from at least three independent experiments.
2.7. Determination of 11β-HSD activity in mouse and rat kidney homogenates
Mouse (C57BL6) and rat (Wistar) kidney tissue from adult males were snap frozen in liquid nitrogen and stored at -80°C until further use. For homogenate preparation, frozen tissue was sonicated in homogenization buffer (250 mM sucrose, 10 mM HEPES, pH 7.4; 900 μL for 100 mg of kidney) and centrifuged at 2 000 × g, 4°C for 10 min to remove cell debris. The total protein concentration was determined by BCA assay [45]. The homogenates were prediluted to a protein concentration of 3.75 mg of protein/mL (mouse) or 10 mg of protein/mL (rat) in homogenization buffer. Reactions were performed in a total volume of 22 μL incubation buffer (300 mM NaCl, 20 mM Tris(hydroxymethyl)aminomethane-hydrocholoride, 1 mM EDTA, 10% glycerol, pH 7.7) containing 0.075 mg/mL of mouse and 0.2 mg/mL of rat total kidney protein, respectively, as well as 10 μM of test compound, 50 nM of corticosterone (containing 50 nCi [1,2,6,7-3H] corticosterone) and 500 μM NAD+ at 37°C for 20 min. Approximately 20-25% of the substrate were converted. The steroids were separated by TLC and conversion of radiolabeled substrate was measured by scintillation counting.
2.8. Docking
Docking was performed using GOLD 5.2 (The Cambridge Crystallographic Data Centre, Cambridge, UK, [46]) based on a crystal structure of 11β-HSD1 (co-crystallized with (2R)-4-[4-fluoro-2-(trifluoromethyl)phenyl]-2-methyl-1-([3-(1H-1,2,4-triazol-1-yl)phenyl]sulfonyl)piperazine, PDB code 3HFG [47]) and a homology model for human 11β-HSD2 [48] and murine 11β-HSD2 [49]. The PDB entry 3HFG was chosen for two reasons. First, it was the template for generating the homology model of human and murine 11β-HSD2. Second, the co-crystallized inhibitor is of similar size as some of the investigated antifungals and contains a triazole moiety. It therefore probably constitutes a binding site conformation suitable for docking this class of 11β-HSD1 inhibitors.
The ligand binding pocket was defined as a sphere with a 10Å radius around the coordinates X =-19.50 Y = 4.00, Z =14.25. ChemPLP was used as scoring function. As workflow validation, redocking of the original ligand was performed using default settings and the best ranked pose deviates from the crystal structure with an RMSD of 0.856 for 11β-HSD1. As both the homology models for human and murine 11β-HSD2 were initially based on PDB entry 3HFG, the same coordinates were used to define the binding site for this docking. For human 11β-HSD2, the docking was additionally repeated setting Arg212 as flexible amino acid. The binding poses were analyzed using LigandScout 4.1 [50] (Inte:Ligand GmbH, Vienna, Austria).
3. Results
3.1. Virtual screening of the DrugBank database using a 11β-HSD pharmacophore model and virtual hit selection for biological evaluation
Despite its role in the regulation of electrolyte balance and cardiovascular function and its importance during pregnancy to control the in utero environment and therefore fetal growth and development, 11β-HSD2 is not included in current drug off-target screenings. Thus, the present project aimed to evaluate some approved drugs for their ability to interfere with 11β-HSD2 activity. For this purpose, the FDA-approved small molecule drug entries of the DrugBank database were subjected to VS using a previously developed 11β-HSD pharmacophore model (Fig. 1A) [39]. This model is not expected to discriminate between 11β-HSD1 and 11β-HSD2 inhibitors because no crystal structure is available for 11β-HSD2 and the model is, at least in part, built based on available 11β-HSD1 ligands. Of the 1543 DrugBank entries, 101 approved drugs fitted into the model. Not surprisingly, several steroidal compounds (in total 18 hits) including mainly glucocorticoids, were among the hits. Besides, the virtual hit list contained several prostaglandin analogues as well as anti-infective agents, including antifungals, antibiotics and antiparasitic agents. Antifungals were especially represented by the class of azole fungicides, with sertaconazole, butoconazole and tioconazole as virtual hits (Fig. 1B). The majority of antibiotics comprised β-lactam antibiotics and lincosamides, with cloxacillin, flucloxacillin and nafcillin, as well as clindamycin and lyncomycin as representative structural classes. Further virtual hits included members of the classes of antihypertensives/antiarrhythmics, diuretics, lipid lowering drugs, antidiabetics, analgesics and antipsychotics. Several hits from the VS, belonging to the different structural classes mentioned above and available through an in-house chemical repository, were selected for biological assessment.
3.2. Effect of selected virtual hits and further structurally related compounds on 11β-HSD activity
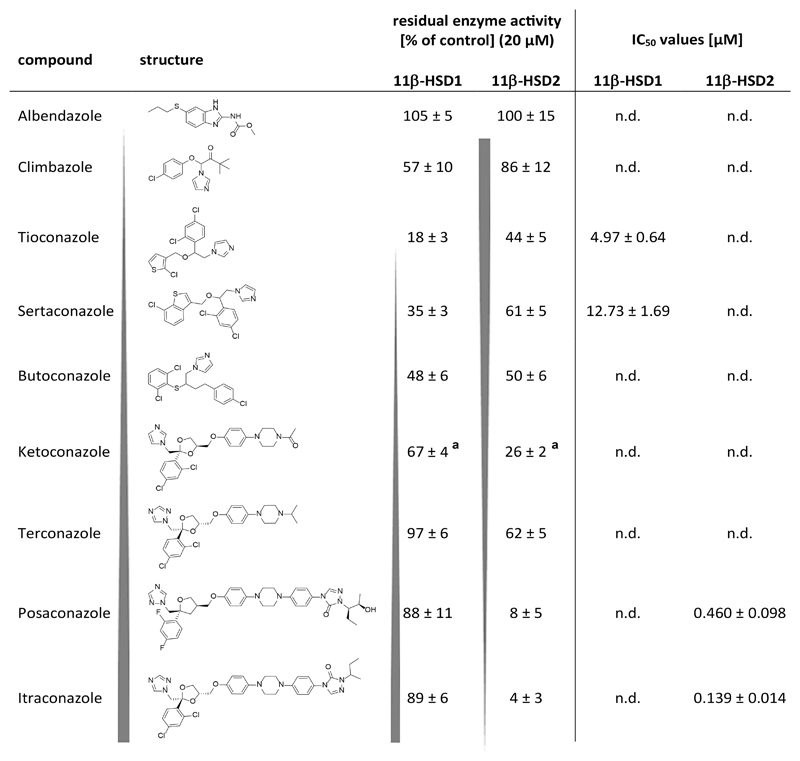
The selected compounds were first tested for their potential to inhibit cortisol to cortisone conversion in lysates of HEK-293 cells stably expressing human 11β-HSD2. The selectivity over the closely related 11β-HSD1 was then determined by measuring the effect of the chemicals on the conversion of cortisone to cortisol. The selected steroids (nandrolone), antiarrhythmics (amiodarone), lipid lowering drugs (atorvastatin, simvastatin), diabetics (rosiglitazone), diuretics (ethacrynic acid), analgesics (indomethacine, nabilone), antipsychotics/sedativa (chlorpromazine), the uricosuric probenecid, antiparasitic agents (amodiaquine, chloroquine and pentamidine), β-lactam antibiotics (cloxacillin, flucloxacillin and nafcillin), lincosamides (clindamycin and lincomycin) and the steroid-like antibiotic fusidic acid showed no or weak inhibition (less than 40% inhibition) of 11β-HSD1 and 11β-HSD2 enzyme activity at a concentration of 20 μM (data not shown). However, the azole fungicides sertaconazole, butoconazole and tioconazole showed moderate activity and inhibited 11β-HSD2 at a concentration of 20 μM, resulting in 61%, 44% and 50% residual activity, respectively. These azole fungicides were not selective, and they equally well or preferentially inhibited 11β-HSD1, showing residual enzyme activities of 35%, 48% and 18%, respectively.
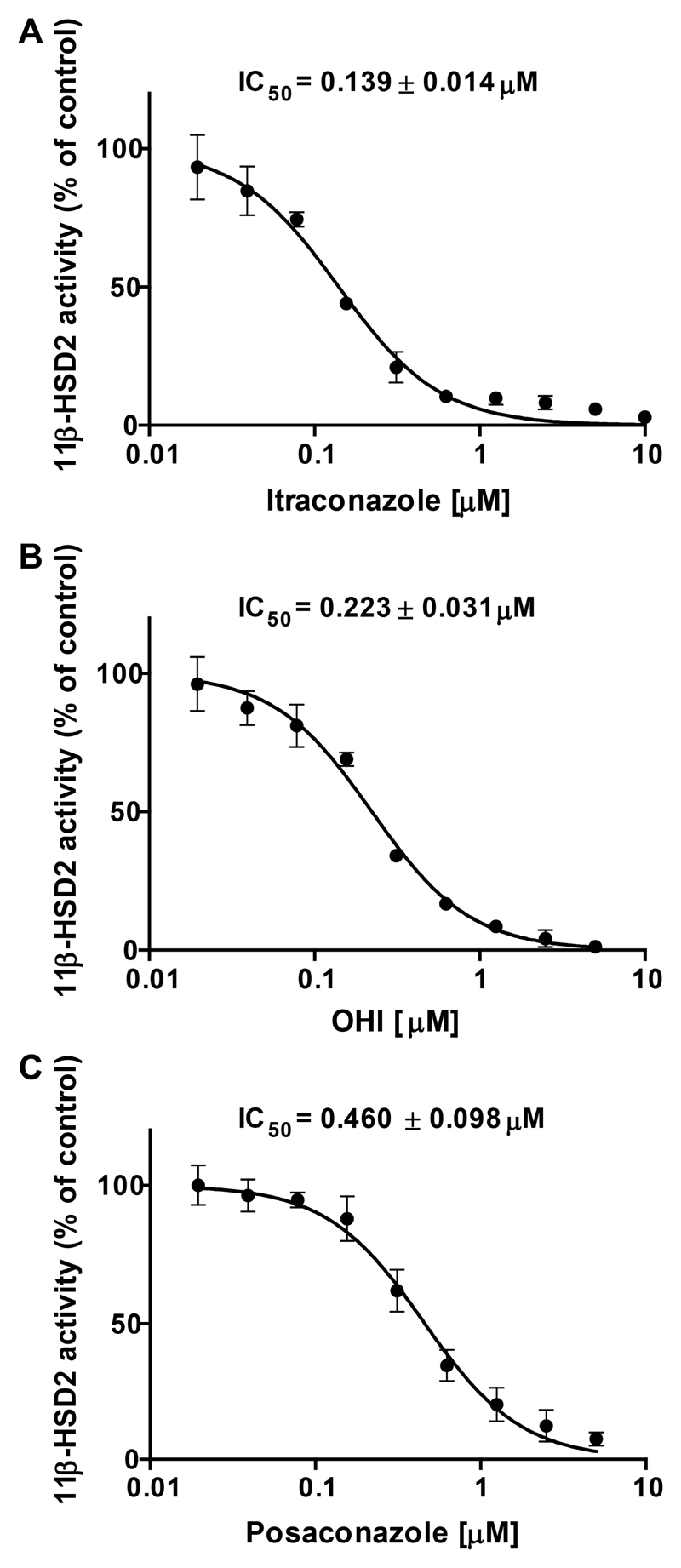
Due to their wide use and partial over-the-counter availability, and their previous association with 11β-HSDs (triadimefon as a substrate of 11β-HSD1 and ketoconazole as a weak inhibitor of 11β-HSD1 and 11β-HSD2 [39, 51]), additional, structurally related azole fungicides were selected for biological testing in order to provide proof of concept in applying pharmacophores as initial filter for the identification of hazardous compound classes [52]. The biological analyses using HEK-293 cell lysates expressing recombinant human enzymes revealed a clear structure-activity relationship between 11β-HSD selectivity and structural size and shape of the azoles: the larger the structural size the more potent its inhibitory activity against 11β-HSD2 and the higher the selectivity over 11β-HSD1 (Fig. 2). One requirement of high activity was a hydrophobic central region of the azole scaffold linked to a more polar end. The compounds with a smaller scaffold preferentially inhibited 11β-HSD1. In addition, the subdivision of azoles in imidazole and triazole derivatives revealed a further relationship. Imidazoles such as tioconazole, sertaconazole and butoconazole preferably inhibited 11β-HSD1 over 11β-HSD2, whereas triazoles such as terconazole, posaconazole and itraconazole more potently inhibited 11β-HSD2. Albendazole, possessing a small azole scaffold containing a benzimidazol structure, was inactive against both enzymes. The most potent 11β-HSD2 inhibition was found for itraconazole and posaconazole, with IC50 values of 139 ± 14 nM and 460 ± 98 nM (Fig. 3), respectively, and selectivity over 11β-HSD1 (Fig. 2). Furthermore, as itraconazole is mainly metabolized to the pharmacologically active OHI, this metabolite was also tested, yielding an IC50 value of 223 ± 31 nM against 11β-HSD2. Irreversible 11β-HSD2 inhibition by itraconazole, OHI and posaconazole was excluded by preincubation experiments; however, preincubation did not alter the inhibitory effect, suggesting a competitive mode of inhibition in line with competition for substrate binding.
Fig. 2. Structure-activity relationship of azole fungicides inhibiting 11β-HSDs.
11β-HSD1-dependent reduction of cortisone (200 nM) to cortisol and 11β-HSD2-dependent oxidation of cortisol (200 nM) to cortisone were measured using HEK-293 cell lysates in the presence of 500 μM NADPH or NAD+, respectively. Residual enzyme activity upon exposure to 20 μM test substance (mean ± SD) and IC50 values (mean ± SD) were obtained from three independent experiments. For compounds with residual enzyme activities (% of control) >40%, at a compound concentration of 20 μM, IC50 values were not determined (n.d.). a, values reported earlier by Vuorinen et al. [39].
Fig. 3. Inhibition of 11β-HSD2 enzyme activity determined in HEK-293 cell lysates.
Lysates of HEK-293 cells stably expressing recombinant human 11β-HSD2 were incubated for 10 min at 37°C with 200 nM radiolabeled cortisol, 500 μM NAD+ and increasing concentrations of itraconazole (A), hydroxyitraconazole (OHI) (B) and posaconazole (C). The substrate conversion was determined and compared to the enzyme activity in the control samples (0.1% DMSO). Data represent mean ± SD from three independent experiments.
3.3. Selectivity assessment of the azole fungicides over 17β-HSD1 and 17β-HSD2
Although 11β-HSD2 and 11β-HSD1 are responsible for the interconversion of the same substrate, i.e. glucocorticoids, they are distant homologs sharing only about 18% sequence identity [53]. In contrast, human 17β-HSD2 is more closely related to 11β-HSD2 with about 45% amino acid sequence identity. 17β-HSD2 is predominantly involved in the metabolism of sex steroid hormones, inactivating estradiol to estrone, testosterone into Δ4-androstene-3,17-dione (androstenedione), 5α-dihydrotestosterone (DHT) into 5α-androstanedione or 5-androstene-3β,17β-diol to dehydroepiandrosterone (DHEA). The reverse reaction of activating the weak estrogen estrone to the potent estradiol and to a minor extent DHEA to 5-androstene-3β,17β-diol is catalyzed by 17β-HSD1. Due to the high expression of 17β-HSD1 and 17β-HSD2 in placenta [54, 55], itraconazole, OHI and posaconazole were assessed for their potential to inhibit 17β-HSD1 and 17β-HSD2 enzyme activity in lysates of HEK-293 cells transiently expressing human 17β-HSD1 and 17β-HSD2. None of the compounds inhibited 17β-HSD1 or 17β-HSD2 activity (data not shown).
3.4. Species-specific 11β-HSD2 inhibition by the selected azole fungicides
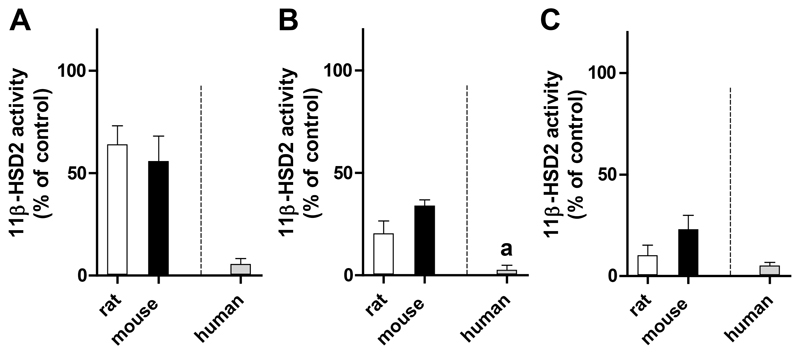
Earlier studies emphasized the importance to assess species-specific differences of 11β-HSD1 and 11β-HSD2 inhibitors, especially prior to conducting in vivo experiments [43, 51, 56]. Thus, prior to designing a rodent study to assess the effect of the selected azole fungicides on glucocorticoid inactivation, inhibition of 11β-HSD2 by itraconazole, OHI and posaconazole was further analyzed in rat and mouse kidney homogenates incubated with 50 nM of the rodent substrate corticosterone in the absence or presence of 10 μM test compound. In a qualitative comparison to the potent activity against recombinant human 11β-HSD2, which was expressed in HEK-293 cells and measured upon incubation with 200 nM of the human substrate cortisol, considerably lower inhibitory activity was detected against the rat and mouse enzymes. The different concentrations of corticosterone and cortisol used for rodent and human 11β-HSD2, respectively, reflect the approximately 5-10 fold affinity difference for these substrates [57, 58]. Itraconazole (Fig. 4A) and OHI (Fig. 4B) were at least 10-fold less potent toward rat and mouse 11β-HSD2 compared to the human enzyme, while posaconazole (Fig. 4C) tended to be 2 times less active against rat 11β-HSD2 and was about 4 times less active against mouse 11β-HSD2. Although the activities of the human and rodent enzymes were measured under different conditions, considering their different physiological substrates, the data suggest a higher inhibitory activity of these azole fungicides toward human 11β-HSD2 compared to the rodent enzymes.
Fig. 4. Inhibition of rat and mouse 11β-HSD2 activity measured in kidney homogenates, compared to human 11β-HSD2 in HEK-293 cell lysates.
Rat and mouse kidney homogenates were incubated for 20 min at 37°C with 50 nM radiolabeled corticosterone in the absence or presence of 10 μM itraconazole (A), 10 μM hydroxyitraconazole (OHI) (B) or 10 μM posaconazole (C). Human 11β-HSD2 activity was measured in HEK-293 cells stably expressing recombinant human 11β-HSD2 upon incubation for 10 min at 37°C with 200 nM radiolabeled cortisol, 500 μM NAD+ and the corresponding concentration of azole fungicide (10 μM itraconazole, 10 μM posaconazole or 5 μM OHI). The substrate conversion was calculated (in nmoles/min) and normalized to the enzyme activity in the control sample (0.1% DMSO). Data represent mean ± SD from at least three independent experiments. a, 5 μM was used (due to complete inhibition higher concentrations were not analyzed).
3.5. Inhibition of 11β-HSD2 in cell models with endogenous enzyme expression
To determine the inhibitory potential of itraconazole, OHI and posaconazole in intact cell systems, SW-620 and MCF-7 cells expressing relatively high endogenous 11β-HSD2 levels [43] were applied. Itraconazole inhibited 11β-HSD2 in a concentration-dependent manner with IC50 values of 1.07 ± 0.29 μM in SW-620 and 1.19 ± 0.24 μM in MCF-7 cells. In contrast to the inhibitory potency ranking observed in lysates of stably transfected HEK-293 cells, OHI was slightly more potent than itraconazole with IC50 values of 0.82 ± 0.18 μM in SW-620 and 0.68 ± 0.20 μM in MCF-7 cells, respectively. Posaconazole did not substantially inhibit 11β-HSD2 activity, resulting in approximately 60% and 40% residual 11β-HSD2 activity in SW-620 and MCF-7 cells at a concentration of 5 μM.
3.6. Predicted binding of selected fungicides to 11β-HSD1 and 11β-HSD2
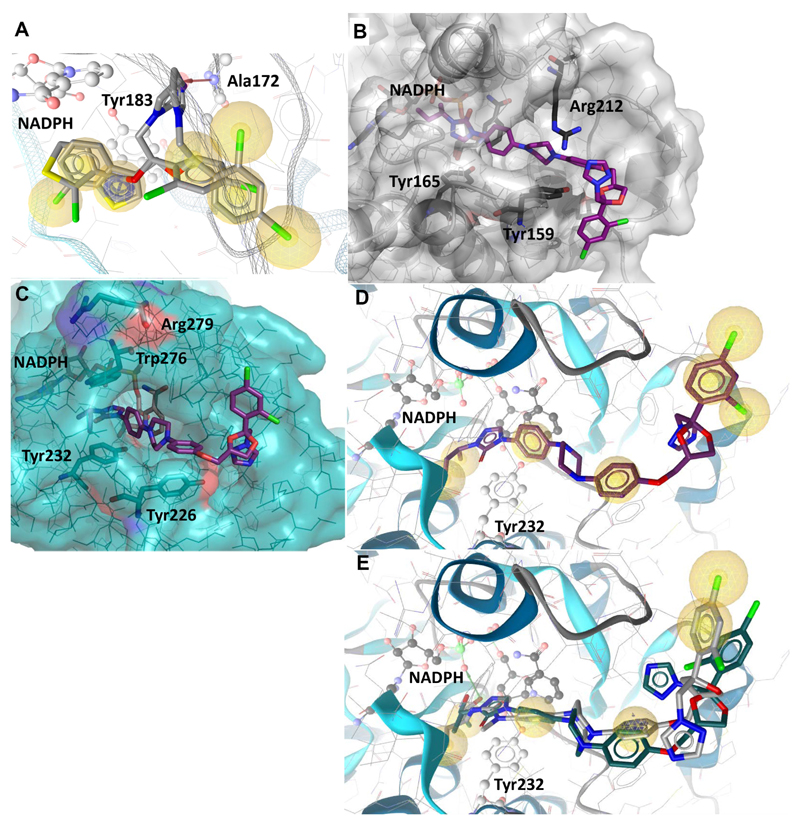
Docking studies with 11β-HSD1 showed two important protein-ligand interactions for azole fungicides such as sertaconazole and tioconazole: a hydrophobic contact with Tyr183 and a hydrogen bond of the azole ring with the backbone nitrogen of Ala172 (Fig. 5A). This hydrogen bond interaction was also the only ligand-coordinating hydrogen bond observed in the 11β-HSD1 crystal structure co-crystallized with a sulfonyl-piperazine inhibitor and thus, presumably essential for the inhibitory activity. In contrast, terconazole does not form any hydrogen bonds with the binding pocket of 11β-HSD1. Furthermore, docking of itraconazole and posaconazole revealed steric clashes with Thr124, Thr222 and Ala226 of the 11β-HSD1 substrate binding pocket, providing an explanation for their inactivity towards 11β-HSD1. However, a major difference between 11β-HSD1 and 11β-HSD2 is the shape of the binding site entry. While Met233 flanks the binding site entry of 11β-HSD1, Arg212 replaces this amino acid at the analogous position in 11β-HSD2. The difference in size and electrostatic properties of these amino acids likely influences the inhibitory activity. Indeed, all 11β-HSD2 active compounds analyzed formed an interaction with Arg212. Furthermore, the extended azole scaffolds were found to form additional interactions in their docking poses with 11β-HSD2, pointing towards tighter binding to the protein. Interestingly, itraconazole and posaconazole did not fit entirely into the binding site of 11β-HSD2 but rather lined the surface next to the binding site entry with their azole part (Fig. 5B). Moreover, the additional hydroxyl group of posaconazole was able to form several hydrogen bonds with the phosphate moiety of NADPH.
Fig. 5. Predicted binding of selected fungicides to 11β-HSD1 and 11β-HSD2.
(A) Representative binding poses of inhibitors in human 11β-HSD1 exemplified by tioconazole and sertaconazole. Important interactions for protein-ligand binding and the cofactor are shown in ball-and-stick style. Hydrophobic contacts between the ligand and the binding site are represented as yellow spheres, the HBA to Ala172 as red arrow and the aromatic stacking with Tyr183 as blue circle. (B) Binding mode of itraconazole docked into the homology model of human 11β-HSD2. (C) Itraconazole docked into the homology model of murine 11β-HSD2. Arg279 (equivalent to Arg212 in human 11β-HSD2) is pointing away from the binding pocket, while Trp276 blocks the binding pocket next to the cofactor NADPH. (D) Itraconazole docked into the homology model of murine 11β-HSD2. The ligand is not anchored via hydrogen bonds or charged interactions, only hydrophobic contacts (yellow spheres) are formed. (E) Posaconazole (grey) and OHI (green) docked into murine 11β-HSD2. Their hydroxyl groups form hydrogen bonds (green arrow) with the cofactor, which provides an explanation for their higher activity compared to itraconazole.
To rationalize the relative selectivity of the three azole compounds to inhibit human 11β-HSD2, they were also docked into the murine homology model [49]. The murine 11β-HSD2 homology model revealed some crucial differences in the amino acid sequence compared to the human 11β-HSD2. Unlike the equivalent Arg212 on human 11β-HSD2, Arg279 on mouse 11β-HSD2 was not flanking the binding pocket entrance but oriented away (Fig. 5C). Furthermore, part of the binding cavity near the cofactor was occupied by Trp276, a residue located outside the binding pocket in human 11β-HSD2, and this residue caused itraconazole to adopt a different angle within the binding cavity in the docking simulation. Additionally, the docking poses for itraconazole predicted no hydrogen bonds with the protein (Fig. 5D). This missing structural anchoring provides an explanation for the weak activity of this fungicide towards murine 11β-HSD2. Interestingly, regarding murine 11β-HSD2, posaconazole and OHI showed greater inhibitory activity than itraconazole. Compared to itraconazole these two fungicides formed interactions with the cofactor via their hydroxyl groups (Figure 5E).
One important aspect of in silico-driven screening studies is the analysis of the predictions and a possible refinement of the model that was used for screening. The screening model performance was very powerful, as all three azole fungicides identified by the model (tioconazole, sertaconazole and butoconazole) inhibited 11β-HSD1 enzyme activity at a concentration of 20 μM by at least 50%. However, although itraconazole and posaconazole were represented in the DrugBank, they were not found by the model as virtual hits. A previous refinement study on this model [39], addressed the shape restriction as one major restrictivity aspect. Shape deletion and subsequent VS of all tested compounds, including OHI, with this model retrieved all compounds except for climbazole as hits. For more thorough VS for potential 11β-HSD inhibitors, the shapeless model version may therefore be more suitable.
4. Discussion
Molecular modeling-based in silico approaches are important in drug development for the identification of bioactive molecules. Pharmacophore-based VS is a powerful strategy to enrich potentially active compounds among a large number of test compounds, thereby facilitating the identification of drug lead structures [59]. Such in silico tools are also applied in anti-target screenings; however, in contrast to lead compound identification this is more challenging since this approach aims to identify all potentially harmful substances. The present study applied a pharmacophore model based on 11β-HSD1 crystal structures for VS of the DrugBank database to find approved drugs that might inhibit 11β-HSD2, an enzyme not included in current off-target screenings during drug development. The goal was to identify, as a first step, structural compound classes inhibiting 11β-HSD2, followed by a more detailed analysis of a selected compound class including an in vitro validation of selected virtual hits.
The pharmacophore model used in this study demonstrated already earlier high predictive power [60], and azole fungicides have been previously associated with 11β-HSDs: The azole fungicide triadimefon was reported to be a substrate of 11β-HSD1 (Km 3.5 μM), thereby acting as a weak competitive inhibitor (IC50 15 μM) [51]. Ketoconazole was found to have weak inhibitory effects toward 11β-HSD1 and 11β-HSD2 [39, 61]. Thus, this compound class was chosen for further investigations. Biological evaluation revealed several azole fungicides as 11β-HSD inhibitors, showing a significant structure-activity relationship between azole scaffold size and 11β-HSD enzyme selectivity and potency of inhibition. The large scaffolds of itraconazole and posaconazole, which were not initial VS hits, potently inhibited 11β-HSD2 enzyme activity. Compared to terconazole, which was considerably less active on 11β-HSD2, itraconazole and posaconazole contain an extended hydrophobic central region and an additional triazolone side chain, which may form additional stabilizing interactions with the binding pocket, including Arg212, thereby providing an explanation for the potent inhibition. Thus, the VS approach has proven useful to identify azole fungicides as 11β-HSD2 inhibitors and to prioritize this compound class for further biological analyses, which were necessary to identify the most potent compounds itraconazole and posaconazole.
Itraconazole and posaconazole are clinically used for the prophylaxis and treatment of systemic mycotic infections and exert their mode of action by inhibiting the biosynthesis of ergosterol, an essential component of the fungal cell membrane [62, 63]. They can be applied for a prolonged period of time, up to years when used as prophylactic treatment, especially in immunosuppressed patients [64]. After oral exposure, itraconazole is extensively metabolized in the liver by cytochrome P450 3A4 (CYP3A4) to its main metabolite OHI, which retains potent 11β-HSD2 inhibitory activity. Thus, cortisol-dependent MR and GR activation due to prolonged 11β-HSD2 inhibition may contribute to the observed adverse effects of these fungicides. In contrast to itraconazole, hepatic metabolism of posaconazole plays a minor role and mainly involves conjugation by UDP-glucuronyltransferase UGT1A4 [65]. Although posaconazole is not a substrate of CYP3A4, it acts like itraconazole and OHI as a potent CYP3A4 inhibitor and it is therefore prone to considerable pharmacokinetic interactions with CYP3A4 substrates, including glucocorticoids [66, 67]. Inhibition of CYP3A4 by a single dose of itraconazole was shown to significantly decrease the formation clearance (CLf) of the metabolites 6β-hydroxycortisol and 6β-hydroxycortisone [68]. Plasma cortisol and cortisone concentrations were not altered, indicating negative feedback regulation by the HPA axis and suggesting that cortisol levels are locally increased in CYP3A4 expressing tissues, particularly in the liver. Itraconazole, OHI and posaconazole might further promote local glucocorticoid effects by inhibiting P-glycoprotein (P-gp) mediated cortisol efflux [69, 70]. Besides its important role in the liver, P-gp expression has been detected in the human placenta form the first trimester on towards full-term [71] and P-gp was shown in vitro to support the placental 11β-HSD2 glucocorticoid barrier [72].
Inhibition of 11β-HSD2 by itraconazole and posaconazole might impair glucocorticoid inactivation in the kidney, colon, vasculature and placenta. Indeed, Denolle et al. reported a case study of a patient on long-term itraconazole treatment developing hypokalemia, edema, hypertension, low plasma renin and aldosterone concentrations and normal serum cortisol, typical symptoms of AME as a result of cortisol-dependent MR activation [73]. Similar side effects including hypokalemia, edema, hypertension and mildly reduced aldosterone serum levels were described by Sharkey et al. for several patients during long-term itraconazole use [74]. Importantly, the drug safety sheet of Sporanox® (itraconazole) reports hypokalemia and edema as occasionally (≥1/1000, <1/100 patients) occurring, whereas the safety sheet for the i.v. solution of Noxafil® (posaconazole) notes hypokalemia as one of the most likely occurring adverse effect (22% of the reports) and a rise in blood pressure as frequent (1/100, <1/10) [75, 76].
Due to the high plasma protein binding capacity of itraconazole (99.8%), OHI (99.6%) and posaconazole (≥98%), predominately to albumin, the circulating fraction of unbound drug is low [75, 76]. However, in specific situations the plasma protein binding capacity can be reduced. Pregnancy leads to several metabolic and physiologic changes, including a decrease of serum albumin levels to about half of those in non-pregnant women. Considering this change, the unbound fraction of itraconazole/OHI and of posaconazole in plasma at steady state conditions during 200 mg b.d.i. itraconazole or 400 mg b.d.i posaconazole treatment [75, 77] can be roughly estimated to reach IC25 values of itraconazole (78 nM) and posaconazole (237 nM) for 11β-HSD2 as determined in HEK-293 cell lysates. Despite the high plasma protein binding ability, extensive extravascular distribution and accumulation in tissues are of considerable importance for these lipophilic compounds. Itraconazole was found to exceed plasma concentrations in fat tissue approximately 17-fold and in lung, liver, muscle and kidney about 2-3 fold [64]. Less data are available for posaconazole, although it was shown that posaconazole concentrations are 31-42 fold higher in pulmonary alveolar cells compared with plasma [78]. Thus, the concentration of these triazole fungicides might also be elevated in placental tissue compared with plasma and therefore may reach relevant concentrations to inhibit 11β-HSD2 enzyme activity.
Animal studies revealed embryotoxicity and teratogenicity with craniofacial and skeletal anomalies at concentrations exceeding the maximum recommended human dose by 5-20 fold during itraconazole treatment but at lower than therapeutic doses during posaconazole treatment [75, 76]. Therefore, it is recommended to use these drugs in pregnancy only if the benefit outweighs the potential risk. Extrapolation of findings from animal studies to human is often difficult due to species-specific differences. The present study provides evidence for a considerably weaker inhibition of mouse and rat 11β-HSD2 by itraconazole and OHI, and lower activity of posaconazole towards the rodent enzymes. Thus, 11β-HSD2-related effects on electrolyte balance, cardiovascular system and placental barrier function would be detected in rodent models only at much higher concentrations than in humans. Furthermore, regarding the latter, animal studies might be unreliable due to the highly variable 11β-HSD2 expression levels during gestation between different species. Mouse placental 11β-HSD2 mRNA drops towards late gestation, while rat placental 11β-HSD2 reduction occurs later and less pronounced. In contrast, human placental 11β-HSD2 levels are rising during gestation (reviewed by [13]).
In a prospective cohort study, de Santis et al. evaluated first trimester exposure to itraconazole in 206 pregnant women compared with 207 unexposed controls [79]. The mean duration of the therapy was 6.9 ± 6.4 days with a daily dose of 182 ± 63 mg. No statistical difference between the exposed and the control group was found in terms of major congenital anomalies, premature birth or birth weight, but rates of live births and spontaneous abortion were higher in the exposed group. Bar-Oz et al. reported in a retrospective cohort study four times higher congenital malformation rates after first trimester exposure to itraconazole; however, strongly suggesting reporting bias due to retrospective data analysis [80]. The same authors found no difference in the rate of malformations compared to the control in a prospective cohort study with 229 women exposed to 50-800 mg itraconazole daily throughout 8.5 ± 12.4 days during the first trimester of pregnancy [81]. However, the rate live birth and the mean birth weight were lower in the exposed group compared to the control. No human data for prenatal posaconazole exposure have been published so far.
Developmental programming implies that an environmental factor affects fetal development during a sensitive time window to predispose the fetus towards diseases permanently throughout life [15]. A critical parameter includes the duration of the exposure to an environmental factor. The mean prenatal itraconazole exposure reported by de Santis et al. and Bar-Oz et al. was 6.9-8.5 days, thus shorter than the 15 days of treatment needed to reach plasma steady-state levels of itraconazole. This is important regarding 11β-HSD2 inhibition and thus prenatal programming through glucocorticoids. Several investigations found a reduction in birth weight in infants exposed to multiple courses of antenatal glucocorticoid therapy when adjusting for gestational age [82–84]. In addition, only first trimester itraconazole exposures were examined in the above described studies, but the effects of dexamethasone exposure on birth weight in rats was reported to be more pronounced when administered during later stages of pregnancy [85]. Chronic glucocorticoid treatment in mice was observed to impair the development of the cerebellum through inhibition of Sonic hedgehog (Shh) induced proliferation, a pathway important during embryonic growth and postembryonic tissue homeostasis. By upregulation of 11β-HSD2 expression Shh signaling partly antagonized the glucocorticoid-dependent effects, thus representing a feedback mechanism [86]. Interestingly, itraconazole, OHI and posaconazole were found to inhibit Shh signaling [87–89], which may result in downregulation of 11β-HSD2 expression, thereby adding to the direct inhibitory effect of these azole fungicides.
Elevated glucocorticoid levels are known to inhibit angiogenesis, a crucial process during placental development that was shown to be inhibited by itraconazole [88, 90, 91]. Insufficient placental vascularization has been associated with intrauterine growth restriction, preeclampsia and fetal death, both in human and animal studies (reviewed by [92]).
In conclusion, itraconazole, OHI and posaconazole were identified as novel potent inhibitors of human 11β-HSD2 by VS of the DrugBank database using an 11β-HSD pharmacophore model. Inhibition of placental 11β-HSD2 by these azole fungicides might contribute to an elevated local increase of cortisol levels, in addition to the known inhibition of cytochrome P450 enzymes and P-gp, thereby affecting fetal programming. Due to the observed species-specific differences, the consequences of placental 11β-HSD2 inhibition may have been overlooked in preclinical studies using rodents, although similar biological responses might be detected in rodents, albeit at higher concentrations.
Acknowledgements
This work was supported by the Swiss National Science Foundation No 31003A-159454, the Austrian Science Fund (P26782), the Swiss Center for Applied Human Toxicology (SCAHT) and the Swiss Federal Office of Public Health (FOPH). A.O. was supported as Chair for Molecular and Systems Toxicology by the Novartis Research Foundation. D.S. is a Ingeborg Hochmair Professor at the University of Innsbruck. We thank Prof. Thierry Langer, University of Vienna, and Inte:Ligand GmbH for providing the LigandScout software.
Abbreviations
- 11β-HSD2
11β-hydroxysteroid dehydrogenase 2
- AME
apparent mineralocorticoid excess
- androstenedione
Δ4-androstene-3,17-dione
- CRH
corticotrophin releasing hormone
- DHEA
dehydroepiandrosterone
- DHT
5α-dihydrotestosterone
- DMEM
Dulbecco’s modified Eagle medium
- GR
glucocorticoid receptor
- H6PDH
hexose-6-phosphate dehydrogenase
- HEK-293
Human Embryonic Kidney-293 cells
- HPA
hypothalamic-pituitary-adrenal
- MR
mineralocorticoid receptor
- OHI
hydroxyitraconazole
- P-gp
P-glycoprotein
- TLC
thin layer chromatography
- VS
virtual screening
Footnotes
Conflicts of Interest
The authors declare no conflict of interest.
Chemical compounds studied in this article
Albendazole (PubChem CID: 2082); Butaconazole (PubChem CID: 47472); Climbazole (PubChem CID: 37907); Itraconazole (PubChem CID: 55283); Hydroxyitraconazole (PubChem CID: 108222); Ketoconazole (PubChem CID: 47576); Posaconazole (PubChem CID: 468595); Sertaconazole (PubChem CID: 65863); Terconazole (PubChem CID: 441383); Tioconazole (PubChem CID: 5482)
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Katharina R. Beck, Email: katharina.beck@unibas.ch.
Murielle Bächler, Email: murielle.baechler@stud.unibas.ch.
Anna Vuorinen, Email: anna.vuorinen1@gmail.com.
Sandra Wagner, Email: sandra.wagner@student.uibk.ac.at.
Muhammad Akram, Email: muhammad.akram@uibk.ac.at.
Ulrich Griesser, Email: ulrich.griesser@uibk.ac.at.
Veronika Temml, Email: veronika.temml@uibk.ac.at.
Petra Klusonova, Email: petra.klusonova@unibas.ch.
Hideaki Yamaguchi, Email: hyamagu@meijo-u.ac.jp.
Daniela Schuster, Email: daniela.schuster@uibk.ac.at.
References
- [1].Odermatt A, Kratschmar DV. Tissue-specific modulation of mineralocorticoid receptor function by 11β-hydroxysteroid dehydrogenases: An overview. Mol Cell Endocrinol. 2012;350(2):168–86. doi: 10.1016/j.mce.2011.07.020. [DOI] [PubMed] [Google Scholar]
- [2].Ferrari P. The role of 11β-hydroxysteroid dehydrogenase type 2 in human hypertension. Biochim Biophys Acta. 2010;1802:1178–87. doi: 10.1016/j.bbadis.2009.10.017. [DOI] [PubMed] [Google Scholar]
- [3].White PC, Mune T, Agarwal AK. 11β-Hydroxysteroid dehydrogenase and the syndrome of apparent mineralocorticoid excess. Endocr Rev. 1997;18(1):135–56. doi: 10.1210/edrv.18.1.0288. [DOI] [PubMed] [Google Scholar]
- [4].New MI, Levine LS, Biglieri EG, Pareira J, Ulick S. Evidence for an unidentified steroid in a child with apparent mineralocorticoid hypertension. J Clin Endocrinol Metab. 1977;44(5):924–33. doi: 10.1210/jcem-44-5-924. [DOI] [PubMed] [Google Scholar]
- [5].Mune T, Rogerson FM, Nikkila H, Agarwal AK, White PC. Human hypertension caused by mutations in the kidney isozyme of 11β-hydroxysteroid dehydrogenase. Nat Genet. 1995;10(4):394–9. doi: 10.1038/ng0895-394. [DOI] [PubMed] [Google Scholar]
- [6].Dave-Sharma S, Wilson RC, Harbison MD, Newfield R, Azar MR, Krozowski ZS, Funder JW, Shackleton CH, Bradlow HL, Wei JQ, Hertecant J, et al. Examination of genotype and phenotype relationships in 14 patients with apparent mineralocorticoid excess. J Clin Endocrinol Metab. 1998;83(7):2244–54. doi: 10.1210/jcem.83.7.4986. [DOI] [PubMed] [Google Scholar]
- [7].Gomez-Sanchez EP, Gomez-Sanchez CE. Central hypertensinogenic effects of glycyrrhizic acid and carbenoxolone. Am J Physiol. 1992;263(6 Pt 1):E1125–30. doi: 10.1152/ajpendo.2006.263.6.E1125. [DOI] [PubMed] [Google Scholar]
- [8].Beitins IZ, Bayard F, Ances IG, Kowarski A, Migeon CJ. The metabolic clearance rate, blood production, interconversion and transplacental passage of cortisol and cortisone in pregnancy near term. Pediatr Res. 1973;7(5):509–19. doi: 10.1203/00006450-197305000-00004. [DOI] [PubMed] [Google Scholar]
- [9].Gitau R, Cameron A, Fisk NM, Glover V. Fetal exposure to maternal cortisol. Lancet. 1998;352(9129):707–8. doi: 10.1016/S0140-6736(05)60824-0. [DOI] [PubMed] [Google Scholar]
- [10].Brown RW, Chapman KE, Kotelevtsev Y, Yau JL, Lindsay RS, Brett L, Leckie C, Murad P, Lyons V, Mullins JJ, Edwards CR, et al. Cloning and production of antisera to human placental 11β-hydroxysteroid dehydrogenase type 2. Biochem J. 1996;313(Pt 3):1007–17. doi: 10.1042/bj3131007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Benediktsson R, Calder AA, Edwards CR, Seckl JR. Placental 11β-hydroxysteroid dehydrogenase: a key regulator of fetal glucocorticoid exposure. Clin Endocrinol (Oxf) 1997;46(2):161–6. doi: 10.1046/j.1365-2265.1997.1230939.x. [DOI] [PubMed] [Google Scholar]
- [12].Jung C, Ho JT, Torpy DJ, Rogers A, Doogue M, Lewis JG, Czajko RJ, Inder WJ. A longitudinal study of plasma and urinary cortisol in pregnancy and postpartum. J Clin Endocrinol Metab. 2011;96(5):1533–40. doi: 10.1210/jc.2010-2395. [DOI] [PubMed] [Google Scholar]
- [13].Harris A, Seckl J. Glucocorticoids, prenatal stress and the programming of disease. Horm Behav. 2011;59(3):279–89. doi: 10.1016/j.yhbeh.2010.06.007. [DOI] [PubMed] [Google Scholar]
- [14].Cottrell EC, Seckl JR, Holmes MC, Wyrwoll CS. Foetal and placental 11β-HSD2: a hub for developmental programming. Acta Physiol (Oxf) 2014;210(2):288–95. doi: 10.1111/apha.12187. [DOI] [PubMed] [Google Scholar]
- [15].Barker DJ. The developmental origins of adult disease. J Am Coll Nutr. 2004;23(6 Suppl):588S–595S. doi: 10.1080/07315724.2004.10719428. [DOI] [PubMed] [Google Scholar]
- [16].Phillips DI, Barker DJ, Fall CH, Seckl JR, Whorwood CB, Wood PJ, Walker BR. Elevated plasma cortisol concentrations: a link between low birth weight and the insulin resistance syndrome? J Clin Endocrinol Metab. 1998;83(3):757–60. doi: 10.1210/jcem.83.3.4634. [DOI] [PubMed] [Google Scholar]
- [17].French NP, Hagan R, Evans SF, Godfrey M, Newnham JP. Repeated antenatal corticosteroids: size at birth and subsequent development. Am J Obstet Gynecol. 1999;180(1 Pt 1):114–21. doi: 10.1016/s0002-9378(99)70160-2. [DOI] [PubMed] [Google Scholar]
- [18].Seckl JR. Prenatal glucocorticoids and long-term programming. Eur J Endocrinol. 2004;151(Suppl 3):U49–62. doi: 10.1530/eje.0.151u049. [DOI] [PubMed] [Google Scholar]
- [19].Shams M, Kilby MD, Somerset DA, Howie AJ, Gupta A, Wood PJ, Afnan M, Stewart PM. 11β-hydroxysteroid dehydrogenase type 2 in human pregnancy and reduced expression in intrauterine growth restriction. Hum Reprod. 1998;13(4):799–804. doi: 10.1093/humrep/13.4.799. [DOI] [PubMed] [Google Scholar]
- [20].Benediktsson R, Lindsay RS, Noble J, Seckl JR, Edwards CR. Glucocorticoid exposure in utero: new model for adult hypertension. Lancet. 1993;341(8841):339–41. doi: 10.1016/0140-6736(93)90138-7. [DOI] [PubMed] [Google Scholar]
- [21].Dy J, Guan H, Sampath-Kumar R, Richardson BS, Yang K. Placental 11β-hydroxysteroid dehydrogenase type 2 is reduced in pregnancies complicated with idiopathic intrauterine growth Restriction: evidence that this is associated with an attenuated ratio of cortisone to cortisol in the umbilical artery. Placenta. 2008;29(2):193–200. doi: 10.1016/j.placenta.2007.10.010. [DOI] [PubMed] [Google Scholar]
- [22].McTernan CL, Draper N, Nicholson H, Chalder SM, Driver P, Hewison M, Kilby MD, Stewart PM. Reduced placental 11β-hydroxysteroid dehydrogenase type 2 mRNA levels in human pregnancies complicated by intrauterine growth restriction: an analysis of possible mechanisms. J Clin Endocrinol Metab. 2001;86(10):4979–83. doi: 10.1210/jcem.86.10.7893. [DOI] [PubMed] [Google Scholar]
- [23].Benediktsson R, Brennand J, Tibi L, Calder AA, Seckl JR, Edwards CR. Fetal osteocalcin levels are related to placental 11β-hydroxysteroid dehydrogenase activity in humans. Clin Endocrinol (Oxf) 1995;42(5):551–5. doi: 10.1111/j.1365-2265.1995.tb02676.x. [DOI] [PubMed] [Google Scholar]
- [24].Rebuffat AG, Tam S, Nawrocki AR, Baker ME, Frey BM, Frey FJ, Odermatt A. The 11-ketosteroid 11-ketodexamethasone is a glucocorticoid receptor agonist. Mol Cell Endocrinol. 2004;214(1–2):27–37. doi: 10.1016/j.mce.2003.11.027. [DOI] [PubMed] [Google Scholar]
- [25].Welberg LA, Seckl JR, Holmes MC. Prenatal glucocorticoid programming of brain corticosteroid receptors and corticotrophin-releasing hormone: possible implications for behaviour. Neuroscience. 2001;104(1):71–9. doi: 10.1016/s0306-4522(01)00065-3. [DOI] [PubMed] [Google Scholar]
- [26].Welberg LA, Seckl JR, Holmes MC. Inhibition of 11β-hydroxysteroid dehydrogenase, the foeto-placental barrier to maternal glucocorticoids, permanently programs amygdala GR mRNA expression and anxiety-like behaviour in the offspring. Eur J Neurosci. 2000;12(3):1047–54. doi: 10.1046/j.1460-9568.2000.00958.x. [DOI] [PubMed] [Google Scholar]
- [27].Lindsay RS, Lindsay RM, Edwards CR, Seckl JR. Inhibition of 11β-hydroxysteroid dehydrogenase in pregnant rats and the programming of blood pressure in the offspring. Hypertension. 1996;27(6):1200–4. doi: 10.1161/01.hyp.27.6.1200. [DOI] [PubMed] [Google Scholar]
- [28].Wyrwoll CS, Seckl JR, Holmes MC. Altered placental function of 11β-hydroxysteroid dehydrogenase 2 knockout mice. Endocrinology. 2009;150(3):1287–93. doi: 10.1210/en.2008-1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Mairesse J, Lesage J, Breton C, Breant B, Hahn T, Darnaudery M, Dickson SL, Seckl J, Blondeau B, Vieau D, Maccari S, et al. Maternal stress alters endocrine function of the feto-placental unit in rats. Am J Physiol Endocrinol Metab. 2007;292(6):E1526–33. doi: 10.1152/ajpendo.00574.2006. [DOI] [PubMed] [Google Scholar]
- [30].Langley-Evans SC, Phillips GJ, Benediktsson R, Gardner DS, Edwards CR, Jackson AA, Seckl JR. Protein intake in pregnancy, placental glucocorticoid metabolism and the programming of hypertension in the rat. Placenta. 1996;17(2–3):169–72. doi: 10.1016/s0143-4004(96)80010-5. [DOI] [PubMed] [Google Scholar]
- [31].Vieau D, Sebaai N, Leonhardt M, Dutriez-Casteloot I, Molendi-Coste O, Laborie C, Breton C, Deloof S, Lesage J. HPA axis programming by maternal undernutrition in the male rat offspring. Psychoneuroendocrinology. 2007;32(Suppl 1):S16–20. doi: 10.1016/j.psyneuen.2007.03.014. [DOI] [PubMed] [Google Scholar]
- [32].Seckl JR, Holmes MC. Mechanisms of disease: glucocorticoids, their placental metabolism and fetal 'programming' of adult pathophysiology. Nat Clin Pract Endocrinol Metab. 2007;3(6):479–88. doi: 10.1038/ncpendmet0515. [DOI] [PubMed] [Google Scholar]
- [33].Odermatt A, Gumy C. Glucocorticoid and mineralocorticoid action: why should we consider influences by environmental chemicals? Biochem Pharmacol. 2008;76(10):1184–93. doi: 10.1016/j.bcp.2008.07.019. [DOI] [PubMed] [Google Scholar]
- [34].Strandberg TE, Andersson S, Jarvenpaa AL, McKeigue PM. Preterm birth and licorice consumption during pregnancy. Am J Epidemiol. 2002;156(9):803–5. doi: 10.1093/aje/kwf130. [DOI] [PubMed] [Google Scholar]
- [35].Raikkonen K, Seckl JR, Heinonen K, Pyhala R, Feldt K, Jones A, Pesonen AK, Phillips DI, Lahti J, Jarvenpaa AL, Eriksson JG, et al. Maternal prenatal licorice consumption alters hypothalamic-pituitary-adrenocortical axis function in children. Psychoneuroendocrinology. 2010;35(10):1587–93. doi: 10.1016/j.psyneuen.2010.04.010. [DOI] [PubMed] [Google Scholar]
- [36].Raikkonen K, Pesonen AK, Heinonen K, Lahti J, Komsi N, Eriksson JG, Seckl JR, Jarvenpaa AL, Strandberg TE. Maternal licorice consumption and detrimental cognitive and psychiatric outcomes in children. Am J Epidemiol. 2009;170(9):1137–46. doi: 10.1093/aje/kwp272. [DOI] [PubMed] [Google Scholar]
- [37].Schoof E, Girstl M, Frobenius W, Kirschbaum M, Dorr HG, Rascher W, Dotsch J. Decreased gene expression of 11β-hydroxysteroid dehydrogenase type 2 and 15-hydroxyprostaglandin dehydrogenase in human placenta of patients with preeclampsia. J Clin Endocrinol Metab. 2001;86(3):1313–7. doi: 10.1210/jcem.86.3.7311. [DOI] [PubMed] [Google Scholar]
- [38].Aufdenblatten M, Baumann M, Raio L, Dick B, Frey BM, Schneider H, Surbek D, Hocher B, Mohaupt MG. Prematurity is related to high placental cortisol in preeclampsia. Pediatr Res. 2009;65(2):198–202. doi: 10.1203/PDR.0b013e31818d6c24. [DOI] [PubMed] [Google Scholar]
- [39].Vuorinen A, Nashev LG, Odermatt A, Rollinger JM, Schuster D. Pharmacophore Model Refinement for 11β-Hydroxysteroid Dehydrogenase Inhibitors: Search for Modulators of Intracellular Glucocorticoid Concentrations. Mol Inform. 2014;33(1):15–25. doi: 10.1002/minf.201300063. [DOI] [PubMed] [Google Scholar]
- [40].Schuster D, Maurer EM, Laggner C, Nashev LG, Wilckens T, Langer T, Odermatt A. The discovery of new 11β-hydroxysteroid dehydrogenase type 1 inhibitors by common feature pharmacophore modeling and virtual screening. J Med Chem. 2006;49(12):3454–66. doi: 10.1021/jm0600794. [DOI] [PubMed] [Google Scholar]
- [41].Kratschmar DV, Vuorinen A, Da Cunha T, Wolber G, Classen-Houben D, Doblhoff O, Schuster D, Odermatt A. Characterization of activity and binding mode of glycyrrhetinic acid derivatives inhibiting 11β-hydroxysteroid dehydrogenase type 2. J Steroid Biochem Mol Biol. 2011;125(1–2):129–42. doi: 10.1016/j.jsbmb.2010.12.019. [DOI] [PubMed] [Google Scholar]
- [42].Atanasov AG, Tam S, Rocken JM, Baker ME, Odermatt A. Inhibition of 11β-hydroxysteroid dehydrogenase type 2 by dithiocarbamates. Biochem Biophys Res Commun. 2003;308(2):257–62. doi: 10.1016/s0006-291x(03)01359-7. [DOI] [PubMed] [Google Scholar]
- [43].Furstenberger C, Vuorinen A, Da Cunha T, Kratschmar DV, Saugy M, Schuster D, Odermatt A. The anabolic androgenic steroid fluoxymesterone inhibits 11β-hydroxysteroid dehydrogenase 2-dependent glucocorticoid inactivation. Toxicol Sci. 2012;126(2):353–61. doi: 10.1093/toxsci/kfs022. [DOI] [PubMed] [Google Scholar]
- [44].Vuorinen A, Engeli R, Meyer A, Bachmann F, Griesser UJ, Schuster D, Odermatt A. Ligand-based pharmacophore modeling and virtual screening for the discovery of novel 17β-hydroxysteroid dehydrogenase 2 inhibitors. J Med Chem. 2014;57(14):5995–6007. doi: 10.1021/jm5004914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- [46].Jones G, Willett P, Glen RC, Leach AR, Taylor R. Development and validation of a genetic algorithm for flexible docking. J Mol Biol. 1997;267(3):727–48. doi: 10.1006/jmbi.1996.0897. [DOI] [PubMed] [Google Scholar]
- [47].Wan ZK, Chenail E, Xiang J, Li HQ, Ipek M, Bard J, Svenson K, Mansour TS, Xu X, Tian X, Suri V, et al. Efficacious 11β-hydroxysteroid dehydrogenase type I inhibitors in the diet-induced obesity mouse model. J Med Chem. 2009;52(17):5449–61. doi: 10.1021/jm900639u. [DOI] [PubMed] [Google Scholar]
- [48].Yamaguchi H, Akitaya T, Yu T, Kidachi Y, Kamiie K, Noshita T, Umetsu H, Ryoyama K. Homology modeling and structural analysis of 11β-hydroxysteroid dehydrogenase type 2. Eur J Med Chem. 2011;46(4):1325–30. doi: 10.1016/j.ejmech.2011.01.054. [DOI] [PubMed] [Google Scholar]
- [49].Yamaguchi H, Akitaya T, Kidachi Y, Kamiie K, Noshita T, Umetsu H, Ryoyama K. Mouse 11β-hydroxysteroid dehydrogenase type 2 for human application: homology modeling, structural analysis and ligand-receptor interaction. Cancer Inform. 2011;10:287–95. doi: 10.4137/CIN.S8725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Wolber G, Langer T. LigandScout: 3-D pharmacophores derived from protein-bound ligands and their use as virtual screening filters. J Chem Inf Model. 2005;45(1):160–9. doi: 10.1021/ci049885e. [DOI] [PubMed] [Google Scholar]
- [51].Meyer A, Vuorinen A, Zielinska AE, Da Cunha T, Strajhar P, Lavery GG, Schuster D, Odermatt A. Carbonyl reduction of triadimefon by human and rodent 11β-hydroxysteroid dehydrogenase 1. Biochem Pharmacol. 2013;85(9):1370–8. doi: 10.1016/j.bcp.2013.02.014. [DOI] [PubMed] [Google Scholar]
- [52].Kaserer T, Beck KR, Akram M, Odermatt A, Schuster D. Pharmacophore Models and Pharmacophore-Based Virtual Screening: Concepts and Applications Exemplified on Hydroxysteroid Dehydrogenases. Molecules. 2015;20(12):22799–832. doi: 10.3390/molecules201219880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Atanasov AG, Nashev LG, Tam S, Baker ME, Odermatt A. Organotins disrupt the 11β-hydroxysteroid dehydrogenase type 2-dependent local inactivation of glucocorticoids. Environ Health Perspect. 2005;113(11):1600–6. doi: 10.1289/ehp.8209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Mustonen MV, Isomaa VV, Vaskivuo T, Tapanainen J, Poutanen MH, Stenback F, Vihko RK, Vihko PT. Human 17β-hydroxysteroid dehydrogenase type 2 messenger ribonucleic acid expression and localization in term placenta and in endometrium during the menstrual cycle. J Clin Endocrinol Metab. 1998;83(4):1319–24. doi: 10.1210/jcem.83.4.4709. [DOI] [PubMed] [Google Scholar]
- [55].Takeyama J, Sasano H, Suzuki T, Iinuma K, Nagura H, Andersson S. 17β-hydroxysteroid dehydrogenase types 1 and 2 in human placenta: an immunohistochemical study with correlation to placental development. J Clin Endocrinol Metab. 1998;83(10):3710–5. doi: 10.1210/jcem.83.10.5212. [DOI] [PubMed] [Google Scholar]
- [56].Arampatzis S, Kadereit B, Schuster D, Balazs Z, Schweizer RA, Frey FJ, Langer T, Odermatt A. Comparative enzymology of 11β-hydroxysteroid dehydrogenase type 1 from six species. J Mol Endocrinol. 2005;35(1):89–101. doi: 10.1677/jme.1.01736. [DOI] [PubMed] [Google Scholar]
- [57].Atanasov AG, Ignatova ID, Nashev LG, Dick B, Ferrari P, Frey FJ, Odermatt A. Impaired protein stability of 11β-hydroxysteroid dehydrogenase type 2: a novel mechanism of apparent mineralocorticoid excess. J Am Soc Nephrol. 2007;18(4):1262–70. doi: 10.1681/ASN.2006111235. [DOI] [PubMed] [Google Scholar]
- [58].Ferrari P, Smith RE, Funder JW, Krozowski ZS. Substrate and inhibitor specificity of the cloned human 11β-hydroxysteroid dehydrogenase type 2 isoform. Am J Physiol. 1996;270(5 Pt 1):E900–4. doi: 10.1152/ajpendo.1996.270.5.E900. [DOI] [PubMed] [Google Scholar]
- [59].Schuster D. 3D pharmacophores as tools for activity profiling. Drug Discov Today Technol. 2010;7(4):205–211. doi: 10.1016/j.ddtec.2010.11.006. [DOI] [PubMed] [Google Scholar]
- [60].Nashev LG, Vuorinen A, Praxmarer L, Chantong B, Cereghetti D, Winiger R, Schuster D, Odermatt A. Virtual screening as a strategy for the identification of xenobiotics disrupting corticosteroid action. PLoS One. 2012;7(10):e46958. doi: 10.1371/journal.pone.0046958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Diederich S, Grossmann C, Hanke B, Quinkler M, Herrmann M, Bahr V, Oelkers W. In the search for specific inhibitors of human 11β-hydroxysteroid-dehydrogenases (11β-HSDs): chenodeoxycholic acid selectively inhibits 11β-HSD-I. Eur J Endocrinol. 2000;142(2):200–7. doi: 10.1530/eje.0.1420200. [DOI] [PubMed] [Google Scholar]
- [62].Morris MI. Posaconazole: a new oral antifungal agent with an expanded spectrum of activity. Am J Health Syst Pharm. 2009;66(3):225–36. doi: 10.2146/ajhp070532. [DOI] [PubMed] [Google Scholar]
- [63].Georgopapadakou NH, Walsh TJ. Antifungal agents: chemotherapeutic targets and immunologic strategies. Antimicrob Agents Chemother. 1996;40(2):279–91. doi: 10.1128/aac.40.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Willems L, van der Geest R, de Beule K. Itraconazole oral solution and intravenous formulations: a review of pharmacokinetics and pharmacodynamics. J Clin Pharm Ther. 2001;26(3):159–69. doi: 10.1046/j.1365-2710.2001.00338.x. [DOI] [PubMed] [Google Scholar]
- [65].Ghosal A, Hapangama N, Yuan Y, Achanfuo-Yeboah J, Iannucci R, Chowdhury S, Alton K, Patrick JE, Zbaida S. Identification of human UDP-glucuronosyltransferase enzyme(s) responsible for the glucuronidation of posaconazole (Noxafil) Drug Metab Dispos. 2004;32(2):267–71. doi: 10.1124/dmd.32.2.267. [DOI] [PubMed] [Google Scholar]
- [66].Isoherranen N, Kunze KL, Allen KE, Nelson WL, Thummel KE. Role of itraconazole metabolites in CYP3A4 inhibition. Drug Metab Dispos. 2004;32(10):1121–31. doi: 10.1124/dmd.104.000315. [DOI] [PubMed] [Google Scholar]
- [67].Wexler D, Courtney R, Richards W, Banfield C, Lim J, Laughlin M. Effect of posaconazole on cytochrome P450 enzymes: a randomized, open-label, two-way crossover study. Eur J Pharm Sci. 2004;21(5):645–53. doi: 10.1016/j.ejps.2004.01.005. [DOI] [PubMed] [Google Scholar]
- [68].Peng CC, Templeton I, Thummel KE, Davis C, Kunze KL, Isoherranen N. Evaluation of 6β-hydroxycortisol, 6β-hydroxycortisone, and a combination of the two as endogenous probes for inhibition of CYP3A4 in vivo. Clin Pharmacol Ther. 2011;89(6):888–95. doi: 10.1038/clpt.2011.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Vermeer LM, Isringhausen CD, Ogilvie BW, Buckley DB. Evaluation of Ketoconazole and Its Alternative Clinical CYP3A4/5 Inhibitors as Inhibitors of Drug Transporters: The In Vitro Effects of Ketoconazole, Ritonavir, Clarithromycin, and Itraconazole on 13 Clinically-Relevant Drug Transporters. Drug Metab Dispos. 2016;44(3):453–9. doi: 10.1124/dmd.115.067744. [DOI] [PubMed] [Google Scholar]
- [70].Saad AH, De Pestel DD, Carver PL. Factors influencing the magnitude and clinical significance of drug interactions between azole antifungals and select immunosuppressants. Pharmacotherapy. 2006;26(12):1730–44. doi: 10.1592/phco.26.12.1730. [DOI] [PubMed] [Google Scholar]
- [71].Ceckova-Novotna M, Pavek P, Staud F. P-glycoprotein in the placenta: expression, localization, regulation and function. Reprod Toxicol. 2006;22(3):400–10. doi: 10.1016/j.reprotox.2006.01.007. [DOI] [PubMed] [Google Scholar]
- [72].Mark PJ, Waddell BJ. P-glycoprotein restricts access of cortisol and dexamethasone to the glucocorticoid receptor in placental BeWo cells. Endocrinology. 2006;147(11):5147–52. doi: 10.1210/en.2006-0633. [DOI] [PubMed] [Google Scholar]
- [73].Denolle T, Azizi M, Massart C, Zennaro MC. Itraconazole: a new drug-related cause of hypertension. Ann Cardiol Angeiol (Paris) 2014;63(3):213–5. doi: 10.1016/j.ancard.2014.05.007. [DOI] [PubMed] [Google Scholar]
- [74].Sharkey PK, Rinaldi MG, Dunn JF, Hardin TC, Fetchick RJ, Graybill JR. High-dose itraconazole in the treatment of severe mycoses. Antimicrob Agents Chemother. 1991;35(4):707–13. doi: 10.1128/aac.35.4.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Janssen-Cilag-AG. Sporanox drug safety sheet. 2015 [Google Scholar]
- [76].MSD-MERCK-SHARP&DOHME-AG. NOXAFIL Inf Konz 18 mg/ml drug safety sheet. 2014 [Google Scholar]
- [77].Courtney R, Pai S, Laughlin M, Lim J, Batra V. Pharmacokinetics, safety, and tolerability of oral posaconazole administered in single and multiple doses in healthy adults. Antimicrob Agents Chemother. 2003;47(9):2788–95. doi: 10.1128/AAC.47.9.2788-2795.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Conte JE, Jr, Golden JA, Krishna G, McIver M, Little E, Zurlinden E. Intrapulmonary pharmacokinetics and pharmacodynamics of posaconazole at steady state in healthy subjects. Antimicrob Agents Chemother. 2009;53(2):703–7. doi: 10.1128/AAC.00663-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].De Santis M, Di Gianantonio E, Cesari E, Ambrosini G, Straface G, Clementi M. First-trimester itraconazole exposure and pregnancy outcome: a prospective cohort study of women contacting teratology information services in Italy. Drug Saf. 2009;32(3):239–44. doi: 10.2165/00002018-200932030-00006. [DOI] [PubMed] [Google Scholar]
- [80].Bar-Oz B, Moretti ME, Mareels G, Van Tittelboom T, Koren G. Reporting bias in retrospective ascertainment of drug-induced embryopathy. Lancet. 1999;354(9191):1700–1. doi: 10.1016/s0140-6736(99)04411-6. [DOI] [PubMed] [Google Scholar]
- [81].Bar-Oz B, Moretti ME, Bishai R, Mareels G, Van Tittelboom T, Verspeelt J, Koren G. Pregnancy outcome after in utero exposure to itraconazole: a prospective cohort study. Am J Obstet Gynecol. 2000;183(3):617–20. doi: 10.1067/mob.2000.105962. [DOI] [PubMed] [Google Scholar]
- [82].Murphy KE, Hannah ME, Willan AR, Hewson SA, Ohlsson A, Kelly EN, Matthews SG, Saigal S, Asztalos E, Ross S, Delisle MF, et al. Multiple courses of antenatal corticosteroids for preterm birth (MACS): a randomised controlled trial. Lancet. 2008;372(9656):2143–51. doi: 10.1016/S0140-6736(08)61929-7. [DOI] [PubMed] [Google Scholar]
- [83].Wapner RJ, Sorokin Y, Thom EA, Johnson F, Dudley DJ, Spong CY, Peaceman AM, Leveno KJ, Harper M, Caritis SN, Miodovnik M, et al. Single versus weekly courses of antenatal corticosteroids: evaluation of safety and efficacy. Am J Obstet Gynecol. 2006;195(3):633–42. doi: 10.1016/j.ajog.2006.03.087. [DOI] [PubMed] [Google Scholar]
- [84].Bevilacqua E, Brunelli R, Anceschi MM. Review and meta-analysis: Benefits and risks of multiple courses of antenatal corticosteroids. J Matern Fetal Neona. 2010;23(4):244–60. doi: 10.1080/14767050903165222. [DOI] [PubMed] [Google Scholar]
- [85].Nyirenda MJ, Lindsay RS, Kenyon CJ, Burchell A, Seckl JR. Glucocorticoid exposure in late gestation permanently programs rat hepatic phosphoenolpyruvate carboxykinase and glucocorticoid receptor expression and causes glucose intolerance in adult offspring. J Clin Invest. 1998;101(10):2174–81. doi: 10.1172/JCI1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Heine VM, Rowitch DH. Hedgehog signaling has a protective effect in glucocorticoid-induced mouse neonatal brain injury through an 11β-HSD2-dependent mechanism. J Clin Invest. 2009;119(2):267–77. doi: 10.1172/JCI36376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Kim J, Tang JY, Gong R, Lee JJ, Clemons KV, Chong CR, Chang KS, Fereshteh M, Gardner D, Reya T, Liu JO, et al. Itraconazole, a commonly used antifungal that inhibits Hedgehog pathway activity and cancer growth. Cancer Cell. 2010;17(4):388–99. doi: 10.1016/j.ccr.2010.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Pace JR, DeBerardinis AM, Sail V, Tacheva-Grigorova SK, Chan KA, Tran R, Raccuia DS, Wechsler-Reya RJ, Hadden MK. Repurposing the Clinically Efficacious Antifungal Agent Itraconazole as an Anticancer Chemotherapeutic. J Med Chem. 2016;59(8):3635–49. doi: 10.1021/acs.jmedchem.5b01718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Chen B, Trang V, Lee A, Williams NS, Wilson AN, Epstein EH, Jr, Tang JY, Kim J. Posaconazole, a Second-Generation Triazole Antifungal Drug, Inhibits the Hedgehog Signaling Pathway and Progression of Basal Cell Carcinoma. Mol Cancer Ther. 2016;15(5):866–76. doi: 10.1158/1535-7163.MCT-15-0729-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Chong CR, Xu J, Lu J, Bhat S, Sullivanc DJ, Jr, Liu JO. Inhibition of angiogenesis by the antifungal drug itraconazole. ACS Chem Biol. 2007;2(4):263–70. doi: 10.1021/cb600362d. [DOI] [PubMed] [Google Scholar]
- [91].Del Carratore R, Carpi A, Beffy P, Lubrano V, Giorgetti L, Maserti BE, Carluccio MA, Simili M, Iervasi G, Balzan S. Itraconazole inhibits HMEC-1 angiogenesis. Biomed Pharmacother. 2012;66(4):312–7. doi: 10.1016/j.biopha.2011.11.004. [DOI] [PubMed] [Google Scholar]
- [92].Khankin EV, Royle C, Karumanchi SA. Placental vasculature in health and disease. Semin Thromb Hemost. 2010;36(3):309–20. doi: 10.1055/s-0030-1253453. [DOI] [PubMed] [Google Scholar]