Abstract
Oral delivery of proteins and peptides is a challenge due to their degradation in the stomach. To overcome this challenge, ragweed (Ambrosia elatior) pollen grains were engineered to serve as protective microcapsules. A matrix comprising of Eudragit L100-55, an enteric polymer was deposited on the inner surfaces of ragweed pollens to protect the encapsulated protein from gastric degradation and to achieve pH-dependent release in the intestine. The Eudragit L100-55 matrix was formed without use of organic solvents so that solvent-induced damage to protein molecules could be prevented. To demonstrate the concept, bovine serum albumin (BSA) a model protein was used. A matrix of Eudragit L100-55 embedded with BSA was prepared in ragweed pollens by optimizing their respective concentrations for maximizing BSA loading in the matrix. The ability of this optimized formulation to protect BSA in simulated gastric acid fluid was evaluated. Release studies in simulated gastric fluid (pH 1.2) showed minimal BSA release from the ragweed-Eudragit L100-55 formulation. Analysis of BSA retained in the formulation after its exposure to gastric fluid confirmed that the residual BSA had not denatured. Release studies in the simulated intestinal fluid (pH 6.8) showed that ragweed pollen offered additional controlled release mechanism within the first few hours of release by virtue of their solid wall. In conclusion, upon use of a protein-friendly solvent for Eudragit L100-55, proteins could be encapsulated in ragweed pollen without denaturing them, and the resulting formulation exhibited selective release of the proteins at intestinal pH suggesting that the ragweed pollen grain-based formulation could be promising for oral delivery of proteins.
Keywords: Eudragit L100-55 matrix, Glycofurol, Oral protein delivery, Pollen drug delivery, Protein stability, Sporopollenin
Graphical Abstract

1. Introduction
Proteins and peptides are rapidly growing as promising therapeutic entities for a number of medical ailments. There are many protein and peptide products including peptide hormones (NovoRapid®, Procrit®), vaccines (Twinrix®, RabAvert®), therapeutic enzymes (Activase®, Retavase®), cytokines (Betaseron®, Neupogen®), blood factors (Fibrogammin®, Idelvion®), and monoclonal antibodies (Herceptin®, Erbitux®) that are currently available in the market (Kinch, 2015; Lagasse et al., 2017; Moorkens et al., 2017; Usmani et al., 2017). Most of these products are administered by the parenteral route, which is painful, has low patient compliance, and often requires a visit by the patient to the physician’s office. As an alternative, the oral route can offer a number of advantages such as simplicity, convenience, and painless administration, all of which can promote better patient compliance. Oral formulations also lead to cost savings to the healthcare industry since they do not require expensive sterile manufacturing facilities (Shakya et al., 2016). However, multiple challenges exist in the development of oral protein pharmaceuticals. The main challenges include their labile nature, which makes them susceptible to degradation in the acid and enzyme-rich environment of the stomach, and poor permeability across the intestinal mucosal barriers (Maher et al., 2016; Moroz et al., 2016). In order to overcome these challenges, pH responsive materials and particulate delivery systems such as polymeric nanoparticles, micelles, solid lipid nanoparticles and liposomes have been extensively studied (Fan et al., 2014; Liau et al., 2015; Liu et al., 2017; Liu et al., 2016).
Pollen grains have recently emerged as novel microcarriers for oral drug and vaccine delivery. A natural pollen grain consists of a rigid walled microcapsule, which encloses a male gamete and associated cellular material and proteins (Atwe et al., 2014). Before pollens can be used for oral delivery, the biomolecules and cellular material dwelling in them must be removed. This is to ensure that they can be rid of the allergy causing material. Clean pollens have been used in vivo for delivery of eicosapentaenoic acid from fish oil (Wakil et al., 2010), gadolinium (III) based contrast agents for magnetic resonance imaging (MRI) (Lorch et al., 2009), and 3,4-diaminopyridine for the treatment of botulinum neurotoxin A intoxication (Harris et al., 2016). Our group has developed vaccine formulations based on Lycopodium clavatum (club moss) (Atwe et al., 2014) and Ambrosia elatior (ragweed) (Uddin and Gill, 2017), each containing ovalbumin as a model antigen, and have shown successful oral vaccine delivery in a mouse model.
Pollen grains are generating interest because their rigid wall can withstand harsh environmental conditions such as high temperature, strong acids, strong bases and most organic solvents (Mackenzie et al., 2015). Thus, in the gastric environment, pollens grains remain unaffected by the degradative environment and maintain their structure. Pollen grains are also mucoadhesive (Diego-Taboada et al., 2014). This can result in their longer retention on the intestinal wall and can lead to prolonged release and higher bioavailability. To illustrate safety of pollens, we have recently shown using Caco-2 cells in vitro that ragweed pollen grains are not cytotoxic and do not compromise intestinal epithelial monolayer integrity (Uddin and Gill, 2017).
As a consequence of chemical treatment for removing allergy-causing materials from pollens, apertures in the pollen wall, which are normally closed, get opened up (Gonzalez Cruz et al., 2018). These open apertures function as orifices in the pollen wall and can be used to fill the cavity of clean pollens with drugs and vaccines. However, these open apertures act as a double-edged sword, and can also allow the gastric fluids to enter the cavity, which can then denature encapsulated proteins. To address this limitation, different approaches such as a calcium alginate coating (Mundargi et al., 2015), an enteric coating (Potroz et al., 2017), and a chitosan coating crosslinked with glutaraldehyde (Alshehri et al., 2017) have been used. The major drawback of the alginate-based system is the use of high concentration of calcium chloride salt for complexing alginate which can denature labile proteins (Guo et al., 2003; Lovrien and Matulis, 2001). In addition, the use of organic solvents such as isopropyl alcohol and acetone for enteric-coating with Eudragit L100 polymer (Potroz et al., 2017), or use of cross-linking agents (Alshehri et al., 2017) can result in protein denaturation or damage.
In this study, our objective was to develop a strategy to protect the pollen-encapsulated protein from gastric degradation using benign processing conditions that are protein friendly. We report successful formulation of protein loaded pollen grains wherein the proteins are entrapped in a matrix made of an enteric polymer called Eudragit L100-55. Importantly we have used a protein-friendly and a biocompatible solvent called glycofurol (tetraglycol) to dissolve Eudragit L100-55 rather than protein-denaturing organic solvents. Glycofurol is a polyethylene glycol (PEG) derivative. PEG and PEG derivatives, unlike most organic solvents, act as stabilizing agents for various proteins (Rawat et al., 2010). Glycofurol is an approved pharmaceutical excipient (Mottu et al., 2000). In this study we demonstrate the feasibility of this approach by using bovine serum albumin (BSA) as a model protein in conjunction with ragweed pollens. Eudragit L100-55 is expected to protect the loaded proteins from denaturation at gastric pH but should allow its selective release at intestinal pH for systemic absorption or to elicit gut immunity in case of antigens for vaccine applications. We have optimized concentrations of Eudragit and BSA so as to maximize BSA loading efficiency. Protein release and stability experiments were conducted in simulated gastric and intestinal fluids. Overall, this study demonstrates the potential of using pollen grains for oral delivery of proteins and peptides with an Eudragit matrix that is created using a protein-friendly solvent.
2. Materials & Methods
2.1. Materials
Raw ragweed pollens were purchased from Pharmallerga (Lisov, Czech Republic). BSA, glycofurol (tetraglycol), simulated gastric fluid (pH 1.2), simulated intestinal fluid (pH 6.8) were obtained from Sigma-Aldrich (MO, USA). Eudragit L100-55 was obtained from Evonik (NJ, USA). Pierce™ bicinchoninic acid (BCA) protein assay kit was purchased from Thermo Fisher Scientific (MA, USA). Acetone, potassium hydroxide, ortho-phosphoric acid, ethanol, sodium chloride (NaCl) were purchased from Fisher Scientific (PA, USA). Milli-Q water with a resistivity of 18.2 MΩ.cm was used in all the experiments.
2.2. Chemical cleaning of ragweed pollens
Ragweed pollens were cleaned by chemical treatment as reported earlier (Gonzalez Cruz et al., 2018). Briefly, ragweed pollens (20 g) were refluxed with acetone (350 mL) overnight. After filtration and drying, they were treated with ortho-phosphoric acid (400 mL) under reflux for 7 days. The filtered pollens were washed sequentially with water (5 × 250 mL), acetone (250 mL), 2 M hydrochloric acid (250 mL), 2 M sodium hydroxide (250 mL), water (5 × 250 mL), acetone (250 mL) and ethanol (250 mL). Next, pollens were refluxed in 2 M potassium hydroxide (KOH) solution (600 mL) for a total of 12 h with a change to fresh KOH solution after 6 h. The pollens were then filtered, washed sequentially with water (5 × 250 mL) and ethanol (5 × 250 mL), and dried overnight at 60 °C.
2.3. Characterization of ragweed pollens
Pollens were visualized using a scanning electron microscope (SEM) (Hitachi S-4300 E/N FESEM, NY, USA). For SEM imaging, pollens were first coated with gold and palladium (Technics Hummer V sputter coater, Anatech USA, CA, USA). To observe the internal morphology of pollens, they were broken using a mortar and pestle, and then observed under the SEM. Pollens were also characterized using a CHN analyzer (PerkinElmer 2400 Series II CHNS/O Analyzer, MA, USA) to determine their protein content. A multiplication factor of 6.25 was used to convert percent nitrogen to percent protein in pollens (Atwe et al., 2014; Vanderplanck et al., 2014).
2.4. Optimization of Eudragit L100-55 concentration
Processed ragweed pollens (0.2 g) were added into 3 mL Eudragit L100-55 solution in glycofurol (10, 25 and 50 mg/ml), mixed thoroughly and incubated overnight under vacuum (25 inches of Hg) at room temperature. The mixture was centrifuged at 3000× g to remove excess Eudragit L100-55 polymer solution. Saline solution (0.9% NaCl) (12 mL) was added to precipitate Eudragit L100-55, the tube was vortexed, and supernatant was removed after centrifugation at 3000×g. Addition of saline solution (12 mL) was repeated once more to help remove residual glycofurol. The pollens were next separated from the solution using filtration. Isolated pollens were kept on a filter paper, dried overnight under vacuum (25 inches of Hg) at room temperature, and characterized using SEM.
2.5. Optimization of protein loading in pollens
Dry and processed ragweed pollens (0.2 g) were added to 2 mL aqueous BSA solutions to obtain mixtures containing 20-100% w/w of BSA with respect to pollens. The mixtures were incubated overnight at room temperature under vacuum (25 inches of Hg), centrifuged at 3000× g, and the supernatant was discarded to remove excess aqueous BSA solution. Glycofurol solution containing Eudragit L100-55 (25 mg/ml) (3 mL) was then added to the BSA loaded pollens. The tubes were vortexed, incubated for 3 hours at room temperature under vacuum (25 inches of Hg), and then centrifuged at 3000× g to remove excess Eudragit L100-55 solution. Saline solution (0.9% NaCl) (12 mL) was added to precipitate Eudragit L100-55, the tube was vortexed, and supernatant was removed after centrifugation at 3000×g. Addition of saline solution (12 mL) was repeated once more to help remove residual glycofurol. The pollens were next separated from the solution using filtration. Isolated pollens were kept on a filter paper, and dried overnight at 4 °C.
2.6. Preparation of pollen-Eudragit formulations for release studies
Chemically treated ragweed pollens (0.2 g) were added to aqueous BSA solution (2 mL, 20 mg/mL) to obtain a mixture containing 20% w/w of BSA with respect to pollens. Subsequently, the procedure described under ‘Optimization of protein loading in pollens’ was followed to obtain a dry product containing pollens loaded with BSA+Eudragit (‘Pollens + Eudragit Matrix’). Similarly, the pollen control without Eudragit (‘Only Pollens’) was prepared by following the above procedure except the addition of Eudragit L100-55 solution. Eudragit control without pollens (‘Only Eudragit’) was prepared by following the above procedure except the addition of pollens. The coding system for the prepared formulations and their description is shown in Table 1.
Table 1.
Composition of prepared formulations
| Code | Description |
|---|---|
| Only Pollens | BSA loaded pollens (no Eudragit L100-55 matrix) |
| Pollens + Eudragit Matrix | BSA loaded pollens with Eudragit L100-55 matrix |
| Only Eudragit | BSA loaded Eudragit L100-55 particles (no pollens) |
Amount of BSA loaded in a formulation was determined by extracting BSA from the formulation after incubating it in phosphate buffer saline (pH 7.4) (PBS) under stirring for 48 h. The sample was then centrifuged at 20000 × g for 10 min to separate the supernatant containing BSA. BSA amount was calculated using BCA assay as per manufacturer’s protocol. BSA loading and encapsulation efficiency was determined using the following formulas:
2.7. Measurement of BSA conformational stability
Intrinsic fluorescence spectroscopy was used to determine conformational changes in BSA. Different dry formulations of ‘Only Pollens’, ‘Only Eudragit’, and ‘Pollens + Eudragit Matrix’ containing BSA (50 mg) were incubated in simulated gastric fluid (pH 1.2) (2 mL) for 2 hours. The formulations were then recovered from the gastric fluid by filtration and incubated in PBS (2 mL) to extract the remaining BSA. BSA amount in the extract was calculated using BCA assay as per manufacturer’s protocol, and subsequently BSA solutions at a concentration of 100 μg/ml were prepared and analyzed using a fluorescence spectrophotometer (Cary Eclipse, Agilent, CA, USA) at excitation wavelength of 295 nm with emission spectra collected from 305 - 400 nm at a scanning rate of 120 nm/min in high sensitivity setting. Untreated BSA protein (Native BSA), and BSA incubated with simulated gastric fluid (pH 1.2) for 2 hours were used as controls.
2.8. BSA release kinetics
Formulations (20 mg)were incubated in simulated gastric fluid (pH 1.2) (5 mL) for 2 hours or in simulated intestinal fluid (pH 6.8) (5 mL) at 37 °C for 48 h at 120 rpm. Samples (1 mL) were periodically taken while replacing it with 1 mL of respective media. Samples were analyzed for the released BSA from pollens using BCA assay kit as per manufacturer’s instructions.
2.9. Statistical analysis
All the experiments were carried out in triplicate. Data are presented as the mean ± standard deviation. Statistical analysis was performed using unpaired t-test with GraphPad Prism 7.0 (GraphPad Software, Inc., CA, USA) A p-value of <0.05 was considered as statistically significant.
3. Results
3.1. Concept of using ragweed pollen containing Eudragit L100-55 matrix for oral delivery of proteins
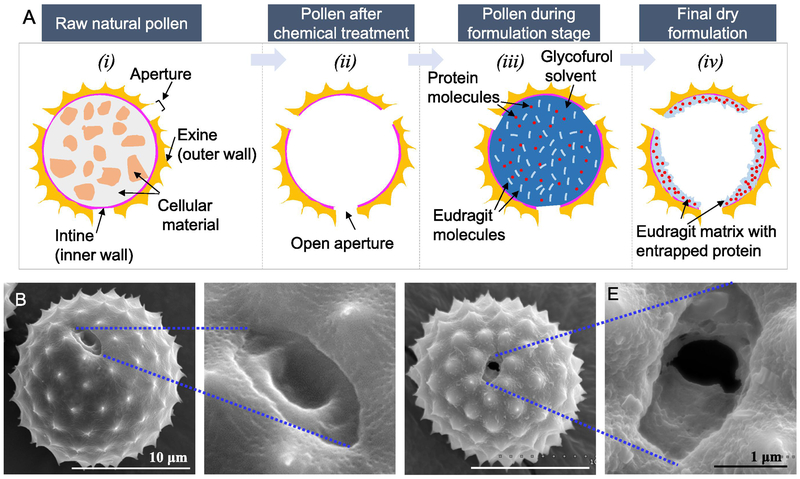
The schematic representation of the pollen grain-based system for oral protein delivery is shown in Figure 1A. Pollen grains are natural microparticles, which contain the male gametophyte. To protect the gametophytes, pollen grains possess a bilayered shell, the inner layer is called the intine and the outer layer is called the exine (Figure 1A-i). The intine is made mostly of cellulose, while the exine is composed of a material known as sporopollonin. Sporopollonin is resistant to most known chemicals including harsh gastric conditions, and this toughness of pollen shells has prompted their use as oral transporters of drugs and proteins (Diego-Taboada et al., 2014; Mackenzie et al., 2015). In some regions of the pollen wall, the exine is thin or absent, and only the intine layer is present as a casing. These regions are called apertures (Figure 1A-i). The pollen wall at the apertures is naturally weak so as to allow the pollen tube to emerge from the pollen during fertilization (Parre and Geitmann, 2005). Before pollens can be used, the cellular organelles, proteins and lipids that are naturally present in them must be removed since they can be a potential source of allergies (Diego-Taboada et al., 2007). Chemical treatment of pollen grains can allow the removal of these materials to produce an empty pollen shell (Figure 1A-ii). The apertures naturally stay closed, but as a consequence of chemical treatment they get opened, creating orifices in the pollen wall (Figure 1A-ii). These orifices can be used to load drugs and proteins into the pollen shell. However, through the same orifices, entrapped drug can also leak out of pollens. Thus, to better entrap and protect the protein molecules in the pollens, we have mixed the proteins with Eudragit L100-55 solubilized in glycofurol (Figure 1A-iii), and subsequently precipitated the Eudragit L100-55 to create a solid matrix containing the protein (Figure 1A-iv). Since Eudragit L100-55 is insoluble at pH lower than 5.5, it can prevent release of the protein in the stomach but can initiate its release in the small intestine where the pH is higher than 5.5.
Figure 1.
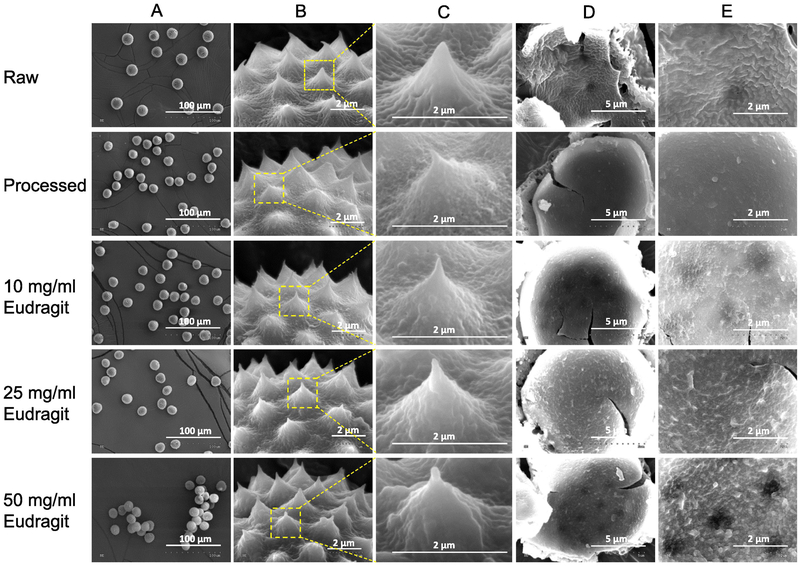
Pollen grains for oral delivery of proteins. A) Schematic representation of the pollen grain-based formulation. Scanning electron micrographs of B & C) Raw pollen grain showing closed aperture, D & E) Processed pollen grain showing open aperture
3.2. Chemical treatment of ragweed pollens
Ragweed pollens were chemically treated to remove their cellular contents and biomolecules. The apertures in ragweed pollens are naturally closed (Figure 1B and 1C), but after treatment they got opened (Figure 1D and 1E). The apertures in ragweed measure about 1-2 μm in length. Pollens remained unbroken and maintained their morphological and spiky topographical features after treatment (Figure 2: ‘Raw’ vs ‘Processed’). Furthermore, the interior of processed pollens was observed to be significantly cleaner as compared to that of raw pollens (Figure 2: ‘Raw’ vs ‘Processed’). The effectiveness of pollen processing was confirmed using CHN analysis. As shown in Table 2, the protein concentration in pollens after treatment significantly reduced to 0.49 ± 0.16% as compared to 21.06 ± 1.07% in raw pollens before chemical treatment. The effectiveness of pollen processing was confirmed using CHN analysis. As shown in Table 2, the protein concentration in pollens after treatment significantly reduced to 0.49 ± 0.16% as compared to 21.06 ± 1.07% in raw pollens before chemical treatment. It should be noted that pollen wall may also contain nitrogen, but since molecular structure of building blocks of pollen wall is unknown, its contribution to elemental analysis results is unclear (Uddin and Gill, 2017). Hence, 0.49% protein estimation based on nitrogen content from CHN analysis is a cautious overestimate. Indeed, Potroz et al. (Potroz et al., 2017), demonstrated in their study using sunflower pollens that even though CHN analysis of the processed sunflower pollens indicated that a small amount of proteins remained, a more sensitive matrix assisted laser desorption/ionization- time of flight mass spectrometry (MALDI-TOF) method confirmed absence of any proteins.
Figure 2.
Optimization of Eudragit L100-55 concentration. Scanning electron micrographs of pollens showing: A) low magnification view to emphasize non-aggregated versus aggregated state of pollen particles, B) view of the spiky topography of ragweed pollens to emphasize Eudragit L100-55 coating on the outer surface, C) zoomed in view of a single spike to emphasize Eudragit L100-55 coating, D) inside surface of a manually cracked pollen, E) zoomed in view of the inside surface of a manually cracked pollen. Ruffles and rough features are indicative of Eudragit L100-55 coating on the inside for treated pollens, and of presence of native cellular material for raw pollens.
Table 2.
Protein content of raw and processed pollens
| Pollens | Nitrogen Content (%) | Protein Content (%) |
|---|---|---|
| Raw ragweed | 3.37 ± 0.17 | 21.06 ± 1.07 |
| Processed ragweed | 0.08 ± 0.03 | 0.49 ± 0.16 |
3.3. Optimization of Eudragit L100-55 concentration
Next, we optimized the concentration of Eudragit L100-55 polymer for forming the matrix. Our criterion for optimization was to maximize Eudragit L100-55 in the formulation without causing clumping or aggregation of pollens. Processed pollens were incubated in glycofurol containing different concentrations of Eudragit L100-55 (10 mg/ml, 25 mg/ml and 50 mg/ml). As shown in Figure 2 (‘Processed’ vs ‘10 mg/ml Eudragit’ vs ‘25 mg/ml Eudragit’ vs ‘50 mg/ml Eudragit’), pollens remained intact after exposure to glycofurol. Pollens incubated in 10 mg/ml Eudragit L100-55 were not aggregated and showed a polymer coating on both the outer and inner surfaces. Similar observations were noted when pollens were incubated in glycofurol with 25 mg/ml Eudragit L100-55 but notably, greater coating of Eudragit was formed on the outer and inner surfaces as compared to use of 10 mg/ml Eudragit L100-55. At 50 mg/ml Eudragit L100-55 many pollens were observed to be clumped together. This can be attributed to the excess Eudragit polymer on their surface, which potentially acted as a glue to bind adjacent pollen particles together. Significant deposition of Eudragit L100-55 was seen on the inner pollen surface so much so that the surface morphology became similar in appearance to that of raw untreated pollens. Since Eudragit at a concentration of 25 mg/ml produced pollens that were unaggregated and showed higher deposition of Eudragit, it was determined to be the most optimal condition from amongst the concentrations that were studied and was selected for further studies.
3.4. Optimization of BSA loading in pollens
After optimizing concentration of Eudragit L100-55, the BSA concentration was optimized. The criterion for optimization was to maximize protein encapsulation efficiency such that wastage of the drug could be minimized. Pollens were incubated in aqueous BSA solutions at different concentrations ranging from 20% w/w (1:5 ratio of BSA to pollens) to 100% w/w (1:1 ratio of BSA to pollens). Glycofurol with 25 mg/ml Eudragit L100-55 was subsequently used to create the matrix as described in the method section. BSA percent loading and encapsulation efficiency was determined. Table 3 shows that as the amount of BSA was increased from 20% w/w to 100% w/w, the percent loading of BSA in ‘Pollens + Eudragit Matrix’ system increased, but at the same time, percent of BSA encapsulated decreased. At 20% w/w BSA concentration, the percent encapsulation in ‘Pollens + Eudragit Matrix’ system was highest (about 12%) while it was in the range of 5-7% for higher concentrations of BSA, indicating excessive loss of BSA during formulation steps. Based on these results, 20% w/w BSA concentration with respect to pollen amount was selected for preparing formulations for further studies.
Table 3.
Optimization of protein loading
| % w/w of BSA with respect to pollens |
% Loading | % Encapsulation |
|---|---|---|
| 20% | 2.45 ± 0.29 | 12.23 ± 1.44 |
| 40% | 2.87 ± 0.17 | 7.17 ± 0.44 |
| 60% | 3.07 ± 0.15 | 5.11 ± 0.25 |
| 80% | 5.37 ± 0.17 | 6.71 ± 0.22 |
| 100% | 5.46 ± 0.73 | 6.10 ± 0.82 |
As a control, when 20% w/w BSA was formulated with just the pollens without Eudragit (‘Only Pollens’ formulation), BSA loading was observed to be about 1% with an encapsulation efficiency of about 7%. This lower protein loading and encapsulation efficiency in the ‘Only Pollens’ case as compared to ‘Pollens + Eudragit Matrix’ case highlights the advantage of using Eudragit because it can entrap BSA and prevent it from being removed during wash steps. As another control, when 20% w/w BSA was formulated with only Eudragit and without pollens (‘Only Eudragit’ formulation), BSA loading was observed to be about 2% with an encapsulation efficiency of about 10%.
3.5. Stability evaluation of encapsulated BSA
During formulation, BSA is exposed to glycofurol, Eudragit, vacuum loading, and a drying step. To assess if these steps can harm BSA, and whether Eudragit can protect encapsulated BSA after exposure to simulated gastric fluid (pH 1.2), we characterized BSA stability by analyzing its tertiary structure with fluorescence spectroscopy.
Tertiary structure is an important aspect of protein stability. Any change in the tertiary structure of the protein can result in its loss of activity. Intrinsic fluorescence of tyrosine and tryptophan residues is widely used to determine tertiary structure of proteins. Fluorescence properties of both tyrosine and tryptophan are sensitive to protein folding/unfolding. In native state of a protein molecule, both tyrosine and tryptophan are in the core of the protein molecule whereas after protein unfolding, they are exposed to the outer environment. In the hydrophobic core of a protein in its native state, tyrosine and tryptophan both have high quantum yield and therefore exhibit high fluorescence intensity. However, in an unfolded state, they get exposed to the outer hydrophilic environment and their quantum yield decreases leading to low fluorescence intensity (Lakowicz, 2006 pp. 530-573; Royer, 1995). Thus, intrinsic fluorescence of tyrosine and tryptophan is dependent on tertiary structure and folding/unfolding state of a protein. Any decrease in tyrosine and tryptophan fluorescence indicates loss of tertiary structure of a protein. As shown in Figure 3, BSA that was extracted from the as prepared ‘Pollens + Eudragit Matrix’ formulation into PBS showed a fluorescence spectra and intensity similar to that of native BSA solution in PBS. This shows that the formulation conditions did not adversely affect the tertiary structure of BSA.
Figure 3.
Intrinsic fluorescence of tyrosine and tryptophan through fluorescence spectroscopy to assess BSA stability.
When the ‘Pollens + Eudragit Matrix’ system was exposed to simulated gastric fluid (pH 1.2) for 2 hours and the BSA that remained in the ‘Pollens + Eudragit Matrix’ was extracted into PBS, its fluorescence spectra and intensity was similar to that of native BSA. This demonstrates the effectiveness of the formulation (‘Pollens + Eudragit Matrix’) in protecting the encapsulated BSA from gastric degradation. A similar effect was observed with ‘Only Eudragit’ formulation where the fluorescence spectra and intensity of BSA was similar to that of native BSA, indicating protection of BSA by Eudragit. However, in the case of ‘Only Pollens’ the spectra and intensity of BSA was unlike that of native BSA but similar to that of native BSA incubated at pH 1.2 for 2 h. This indicates loss of BSA tertiary structure and suggests that ragweed pollen grain by itself is not able to provide total protection to the encapsulated BSA protein. Overall, this demonstrates that the enteric polymer matrix of Eudragit L100-55 can offer protection to the encapsulated protein against gastric degradation.
3.6. BSA release in gastric and intestinal fluids
Eudragit L100-55 is soluble at pH greater than 5.5 (Jeganathan and Prakya, 2015). It should thus remain insoluble at acidic pH but dissolve at basic pH. We thus studied in vitro release of BSA encapsulated in the different formulations in simulated gastric fluid (pH 1.2) and simulated intestinal fluid (pH 6.8).
As shown in Figure 4A, in simulated gastric fluid (pH 1.2), in the first 2 hours, ‘Only Pollens’ showed about 64% BSA release, whereas ‘Pollens + Eudragit Matrix’ and ‘Only Eudragit’ formulations showed about 24% BSA release. The lower release from ‘Pollens + Eudragit Matrix’ and ‘Only Eudragit’ can be attributed to Eudragit L100-55 polymer matrix that stays insoluble and traps the BSA molecules, and thus inhibits the action of the acidic fluid to extract BSA out. Since ‘Only Pollens’ do not have a Eudragit matrix, a significant amount of BSA was released from the pollens after incubation with the simulated gastric fluid (pH 1.2).
Figure 4.
Cumulative release of BSA from different formulations in A) simulated gastric fluid (pH 1.2) **** p<0.0001, and B) simulated intestinal fluid (pH 6.8). Arrows in B) indicate time points with statistical differences between different groups.
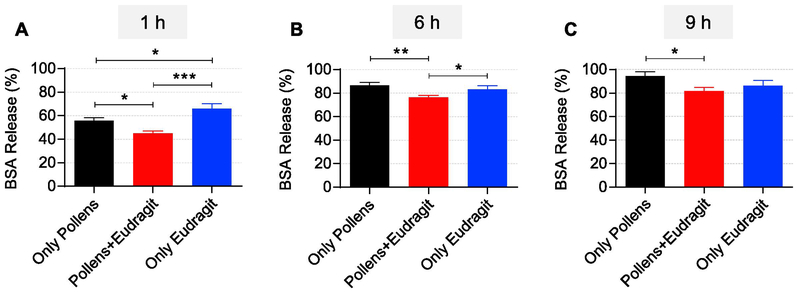
Next, we assessed the release profile of BSA in simulated intestinal fluid (pH 6.8) for 48 hours. As shown in Figure 4B, in simulated intestinal fluid (pH 6.8), release was quickest in the ‘Only Pollens’ (about 87% in 6 h) as compared to the other two groups. In case of ‘Pollens + Eudragit Matrix’, the release of BSA was found to be about 76% in 6 h whereas it was about 83% in the ‘Only Eudragit’ formulation. A comparison of release amount at early time points showed that at 1h, the ‘Pollens + Eudragit Matrix’ released just 45 ± 1.9 % BSA, while ‘Only Eudragit’ released a significantly (p < 0.001) higher amount of BSA (65.8 ± 4.4 %) (Figure 5A). Interestingly, at the 1h time point, the ‘Only Pollens’ formulation also released a significantly (p<0.05) lower amount (55.6 ± 2.6 %) of BSA than the ‘Only Eudragit’ formulation (Figure 5A). This effect is likely because of the barrier confinement property of the pollen wall, which impedes outward diffusion of BSA. This phenomenon also explains the lower release from the ‘Pollens + Eudragit Matrix’ formulation. The trend of lower BSA release from ‘Pollens + Eudragit Matrix’ formulation continued up to 9 h, although statistically significant differences were noted only at the 6 h (Figure 5B) and the 9 h (Figure 5C) time points. At 24 h and 48 h time points all formulations had released similar amounts (about 95%) of the encapsulated BSA.
Figure 5.
Comparison of BSA release from different formulations in simulated intestinal fluid (pH 6.8) at different time points: A) after 1h, B) after 6h, and C) after 9h. * p<0.05, ** p<0.01, *** p<0.001.
4. Discussion
In this study our objective was to develop a formulation based on pollen grains that can be used to deliver biologics orally. The chemical treatment not only removes allergy-causing materials from pollens but also opens the apertures in the pollen wall creating orifices. These orifices can cause the encapsulated proteins to leak out and can allow the gastric fluid to readily enter the pollens, which can result in protein loss and denaturation. We wanted to overcome this challenge by creating a pH-sensitive matrix in the pollens that is insoluble at acidic pH but can solubilize at basic pH to release the proteins. Our prime constraints in achieving this objective were to use FDA-approved excipients, and to not use organic solvents that are detrimental to protein stability. We selected ragweed pollens as the model system since we have previously shown that they are safe and do not impair the integrity of the intestinal epithelial monolayer (Uddin and Gill, 2017). Using our published protocol (Gonzalez Cruz et al., 2018), we removed the lipids, proteins, and other cellular and nuclear material naturally present in the ragweed pollens. BSA was used as a model protein. To load BSA into the pollen shells, dry and clean ragweed pollens were added to an aqueous BSA solution and mild vacuum was applied. Vacuum application pulls the air from inside the pollens, and creates partial vacuum inside them, which drives the aqueous BSA solution into the pollens.
To minimize the loss of encapsulated BSA through the orifices, a pH sensitive polymer Eudragit L100-55 was selected from the Eudragit family known for its enteric properties. Eudragit L100-55 precipitates at pH less than 5.5. We postulated that Eudragit L100-55 could be used to entrap BSA in a solid matrix inside the pollens to protect it against the acidic gastric fluid but would rapidly solubilize at pH greater than 5.5 to selectively release BSA in the duodenum (pH 5-6) (Lundquist and Artursson, 2016). Various researchers have tried oral delivery of peptides/proteins using Eudragit polymers (Banerjee et al., 2016; Marais et al., 2013; Xu et al., 2018; Zhang et al., 2015). Generally, Eudragit polymers are soluble in organic solvents such as ethanol, acetone, methylene chloride, and ethyl acetate. The problem with use of organic solvents is that they can denature proteins resulting in loss of function. Also, in some of these reported systems, protein is loaded after the matrix is formed resulting in adsorption of proteins rather than encapsulation in the polymer matrix. Consequently, the polymer matrix is unable to effectively protect the adsorbed protein when placed in the acidic gastric environment.
Herein, we avoided use of organic solvents for Eudragit solubilization. Instead we used glycofurol (tetraglycol, tetrahydrofurfuryl alcohol polyethylene glycol ether), which is amongst the many polyethylene glycol (PEG) based solvents that can also solubilize Eudragit polymers. We selected glycofurol from amongst the many PEGs such as PEG 200 and PEG 400 since it has the lowest viscosity amongst PEGs that can solubilize Eudragit polymers. We postulated that the lower viscosity of glycofurol can help to better fill the pollens as compared to the higher viscosity solvents since lower viscosity solutions can more readily enter pollens through their small orifices. In addition, glycofurol is safe, non-toxic and is an approved pharmaceutical excipient (Rowe et al., 2006). Glycofurol has been previously used in parenteral products (intravenous and intramuscular injections) as well as in polymer implant systems (Eliaz and Kost, 2000; Nasongkla et al., 2012). Glycofurol has also been used in oral delivery of insulin and has shown to increase its oral bioavailability (Viehof et al., 2013). PEG solvents are also good stabilizing agents for proteins. They inhibit protein aggregation and enhance their stability (Rawat et al., 2010). In this study glycofurol showed no destabilizing effects towards encapsulated BSA. As observed in the fluorescence study, the tertiary structure of BSA remained stable after exposure to glycofurol.
To create the Eudragit L100-55 + BSA matrix, BSA solution was first filled in ragweed pollens. Upon removal of excess BSA solution, Eudragit L100-55 dissolved in glycofurol was added. After exposure to vacuum, excess Eudragit L100-55 outside the pollens was removed. To precipitate the Eudragit that was left behind in the pollen grains, saline solution (0.9% w/w sodium chloride) was added. Eudragit L100-55 is insoluble in water and precipitates slowly in water whereas it precipitates rapidly in the presence of sodium chloride. Rapid precipitation of Eudragit is necessary to minimize leakage of proteins in to the saline solution and maximize their entrapment in the matrix. The amount of Eudragit L100-55 used to prepare the matrix was optimized with the goal of minimizing pollen aggregation. Pollen aggregation not only affects downstream processability of the formulation, but it also implies that there is an excess of Eudragit L100-55 on the outside of the pollens. This Eudragit outside of the pollens can entrap BSA but it escapes the beneficial effect offered by the pollen wall. The mechanism for slower release of BSA from ‘Only Pollens’ can be attributed to resistance offered by the pollen wall. The pollen wall offers a controlled release mechanism because it creates physical confinement and only permits the release of BSA through the open orifices or nano-channels found in the pollen wall (Rowley et al., 2003). Pollens of different species have different number of apertures and their dimensions after chemical treatment vary (Uddin et al., 2018). Furthermore, not all apertures in a pollen get opened after chemical treatment (Uddin et al., 2018).
The observed release rate of BSA from ragweed pollens in simulated gastric and intestinal fluid is similar to or greater than the release rates shown previously by other investigators. Sargin et al reported similar slower release rates for anticancer drug imatinib mesylate from Betula pendula pollens with ~55% in 2 h in SGF and 65% in 24 h in SIF (Sargin et al., 2017). Similarly, Akyuz et al reported release rates of ~80% in 24 h in SIF for freely water soluble drug pantoprazole sodium from Corylus avellana pollens (Akyuz et al., 2017). However, few others have reported faster release rates for encapsulated drug molecules from pollens. Mundargi et al. have shown faster release rates of ~100% release in 30 min for BSA and ~95% release in 30 min for 5-flurouracil from Lycopodium clavatum (Mundargi et al., 2016a, Mundargi et al., 2016b). The difference in release rates observed in different articles can be attributed to use of different pollens.
Drug release from pollens is dependent on pollen type. This is because different pollen types will have different shapes, sizes, morphologies, and number and dimensions of apertures/openings. It has also been reported in the literature that drug release from pollens is dependent on size and form of the nano-sized channels that are present in the pollen walls, and since every pollen type will have unique nano-sized channels in the pollen wall, drug release will differ from pollen to pollen (Diego-Taboada et al., 2014) (Rowley et al., 2003).
Consistent with this expectation of slower release from use of pollens, the ‘Pollens + Eudragit Matrix’ group indeed showed a statistically lower release of BSA than the ‘Only Eudragit’ group up to 6 h in simulated intestinal fluid. As expected, the Eudragit L100-55 matrix in the pollens also protected BSA from the acidic pH (1.2) of the simulated gastric fluid. Notably, BSA that was extracted from the ‘Pollens + Eudragit Matrix’ after exposure to gastric fluid for 2 h was intact while BSA from the ‘Only Pollens’ group was damaged.
Another important trait that makes pollens attractive for oral delivery is their mucoadhesive nature (Uddin and Gill, 2017). By sticking to the mucus, pollens can offer improved residence time and can thus increase bioavailability of proteins and peptides. To enhance bioavailability of proteins and peptides carried by the pollens, intestinal permeability enhancers could be co-loaded in the pollens.
Pollens are commercially available in large scale and high purity to cater to the allergen pharmaceutical industry. Thus, from a manufacturing perspective, pollen-based formulations have potential to be scaled up. Overall, the data presented here support future studies of ragweed pollen-Eudragit delivery system for oral protein delivery.
5. Conclusion
A ragweed pollen grain and an enteric polymer-based formulation was developed for oral delivery of proteins and peptides. A matrix of the enteric polymer, Eudragit L100-55, encapsulating BSA was formed in ragweed pollens. Instead of the usual protein denaturing organic solvents, a protein friendly solvent, namely, glycofurol was used to solubilize Eudragit. BSA was not denatured by the formulation procedure, and the Eudragit L100-55 matrix conferred protection to encapsulated BSA in simulated gastric fluid. Release studies showed minimal release of BSA in simulated gastric fluid (pH 1.2) whereas rapid release was observed in simulated intestinal fluid (pH 6.8). Ragweed pollen also offered additional controlled release by virtue of its wall that acts as a physical barrier. Thus, ragweed pollen and Eudragit L100-55 delivery system shows promise for oral delivery of acid labile protein pharmaceuticals.
Acknowledgments
This research was supported by the National Institutes of Health (NIH) [Grant number DP2HD075691] and the Defense Advanced Research Projects Agency (DARPA) [Grant number N66001-12-1-4251].
Footnotes
Conflict of interest
HSG is a co-inventor on a patent related to the development of pollen grains for oral vaccines. This potential conflict of interest has been disclosed and is managed by Texas Tech University.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akyuz L, Sargin I, Kaya M, Ceter T, Akata I, 2017. A new pollen-derived microcarrier for pantoprazole delivery. Mater. Sci. Eng. C 71, 937–942. 10.1016/j.msec.2016.11.009 [DOI] [PubMed] [Google Scholar]
- Alshehri SM, Al-Lohedan HA, Chaudhary AA, Al-Farraj E, Alhokbany N, Issa Z, Alhousine S, Ahamad T, 2017. Delivery of ibuprofen by natural macroporous sporopollenin exine capsules extracted from Phoenix dactylifera L. Eur. J. Pharm. Sci 88, 158–165. 10.1016/j.ejps.2016.02.004 [DOI] [PubMed] [Google Scholar]
- Atwe SU, Ma Y, Gill HS, 2014. Pollen grains for oral vaccination. J. Control. Release 194, 45–52. 10.1016/j.jconrel.2014.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee A, Lee J, Mitragotri S, 2016. Intestinal mucoadhesive devices for oral delivery of insulin. Bioeng. Transl. Med 1, 338–346. 10.1002/btm2.10015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diego-Taboada A, Barrier S, Thomasson M, Atkin S, Mackenzie G, 2007. Pollen: A novel encapsulation vehicle for drug delivery. Innovations in Pharmaceutical Technology 63–68. [Google Scholar]
- Diego-Taboada A, Beckett ST, Atkin SL, Mackenzie G, 2014. Hollow pollen shells to enhance drug delivery. Pharmaceutics 6, 80–96. 10.3390/pharmaceutics6010080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliaz RE, Kost J, 2000. Characterization of a polymeric PLGA-injectable implant delivery system for the controlled release of proteins. J. Biomed. Mater. Res 50, 388–396. [DOI] [PubMed] [Google Scholar]
- Fan T, Chen C, Guo H, Xu J, Zhang J, Zhu X, Yang Y, Zhou Z, Li L, Huang Y, 2014. Design and evaluation of solid lipid nanoparticles modified with peptide ligand for oral delivery of protein drugs. Eur. J. Pharm. Biopharm 88, 518–528. 10.1016/j.ejpb.2014.06.011 [DOI] [PubMed] [Google Scholar]
- Gonzalez Cruz P, Uddin MJ, Atwe SU, Abidi N, Gill HS, 2018. A chemical treatment method for obtaining clean and intact pollen shells of different species. ACS Biomater. Sci. Eng 4, 2319–2329. 10.1021/acsbiomaterials.8b00304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo C, Campbell BE, Chen K, Lenhoff AM, Velev OD, 2003. Casein precipitation equilibria in the presence of calcium ions and phosphates. Colloids Surf. B 29, 297–307. 10.1016/S0927-7765(03)00018-3 [DOI] [Google Scholar]
- Harris TL, Wenthur CJ, Diego-Taboada A, Mackenzie G, Corbitt TS, Janda KD, 2016. Lycopodium clavatum exine microcapsules enable safe oral delivery of 3,4-diaminopyridine for treatment of botulinum neurotoxin A intoxication. Chem. Commun 52, 4187–4190. 10.1039/c6cc00615a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeganathan B, Prakya V, 2015. Interpolyelectrolyte complexes of Eudragit EPO with hypromellose acetate succinate and Eudragit EPO with hypromellose phthalate as potential carriers for oral controlled drug delivery. AAPS PharmSciTech 16, 878–888. 10.1208/s12249-014-0252-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinch MS, 2015. An overview of FDA-approved biologics medicines. Drug Discovery Today 20, 393–398. 10.1016/j.drudis.2014.09.003 [DOI] [PubMed] [Google Scholar]
- Lagasse HAD, Alexaki A, Simhadri VL, Katagiri NH, Jankowski W, Sauna ZE, Kimchi-Sarfaty C, 2017. Recent advances in (therapeutic protein) drug development. F1000Res. 6, 113 10.12688/f1000research.9970.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakowicz JR, 2006. Principles of fluorescence spectroscopy, Third ed. Springer US, Boston, MA: pp. 530–573. [Google Scholar]
- Liau JJ, Hook S, Prestidge CA, Barnes TJ, 2015. A lipid based multi-compartmental system: Liposomes-in-double emulsion for oral vaccine delivery. Eur. J. Pharm. Biopharm 97, 15–21. 10.1016/j.ejpb.2015.09.018 [DOI] [PubMed] [Google Scholar]
- Liu C, Kou Y, Zhang X, Cheng H, Chen X, Mao S, 2017. Strategies and industrial perspectives to improve oral absorption of biological macromolecules. Expert Opin. Drug Deliv 1–11. 10.1080/17425247.2017.1395853 [DOI] [PubMed] [Google Scholar]
- Liu L, Yao W, Rao Y, Lu X, Gao J, 2016. pH-Responsive carriers for oral drug delivery: Challenges and opportunities of current platforms. Drug Deliv. 24, 569–581. 10.1080/10717544.2017.1279238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorch M, Thomasson MJ, Diego-Taboada A, Barrier S, Atkin SL, Mackenzie G, Archibald SJ, 2009. MRI contrast agent delivery using spore capsules: controlled release in blood plasma. Chem. Commun 6442–6444. 10.1039/B909551A [DOI] [PubMed] [Google Scholar]
- Lovrien RE, Matulis D, 2001. Selective precipitation of proteins. Curr. Protoc. Protein. Sci Chapter 4, Unit 4.5. 10.1002/0471140864.ps0405s07 [DOI] [PubMed] [Google Scholar]
- Lundquist P, Artursson P, 2016. Oral absorption of peptides and nanoparticles across the human intestine: Opportunities, limitations and studies in human tissues. Adv. Drug Deliv. Rev 106, 256–276. 10.1016/j.addr.2016.07.007 [DOI] [PubMed] [Google Scholar]
- Mackenzie G, Boa AN, Diego-Taboada A, Atkin SL, Sathyapalan T, 2015. Sporopollenin, the least known yet toughest natural biopolymer. Front. Mater. 2, 66 10.3389/fmats.2015.00066 [DOI] [Google Scholar]
- Maher S, Mrsny RJ, Brayden DJ, 2016. Intestinal permeation enhancers for oral peptide delivery. Adv. Drug Deliv. Rev 106, 277–319. 10.1016/j.addr.2016.06.005 [DOI] [PubMed] [Google Scholar]
- Marais E, Hamman J, Plessis L, Lemmer R, Steenekamp J, 2013. Eudragit L100/N-trimethylchitosan chloride microspheres for oral insulin delivery. Molecules 18, 6734–6747. 10.3390/molecules18066734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorkens E, Meuwissen N, Huys I, Declerck P, Vulto AG, Simoens S, 2017. The market of biopharmaceutical medicines: A snapshot of a diverse industrial landscape. Front. Pharmacol 8, 314 10.3389/fphar.2017.00314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroz E, Matoori S, Leroux JC, 2016. Oral delivery of macromolecular drugs: Where we are after almost 100 years of attempts. Adv. Drug Deliv. Rev 101, 108–121. 10.1016/j.addr.2016.01.010 [DOI] [PubMed] [Google Scholar]
- Mottu F, Laurent A, Rufenacht DA, Doelker E, 2000. Organic Solvents for Pharmaceutical Parenterals and Embolic Liquids: A Review of Toxicity Data. PDA J. Pharm. Sci. Technol 54, 456–469. [PubMed] [Google Scholar]
- Mundargi RC, Potroz MG, Park S, Park JH, Shirahama H, Lee JH, Seo J, Cho NJ, 2015. Lycopodium spores: A naturally manufactured, superrobust biomaterial for drug delivery. Adv. Funct. Mater 26, 487–497. 10.1002/adfm.201502322 [DOI] [Google Scholar]
- Mundargi RC, Tan EL, Seo J, & Cho NJ 2016. Encapsulation and controlled release formulations of 5-fluorouracil from natural Lycopodium clavatum spores. J. Ind. Eng. Chem 36, 102–108. 10.1016/j.jiec.2016.01.022 [DOI] [Google Scholar]
- Nasongkla N, Boongird A, Hongeng S, Manaspon C, Larbcharoensub N, 2012. Preparation and biocompatibility study of in situ forming polymer implants in rat brains. J. Mater. Sci. Mater. Med. 23, 497–505. 10.1007/s10856-011-4520-3 [DOI] [PubMed] [Google Scholar]
- Parre E, Geitmann A, 2005. More Than a Leak Sealant. The Mechanical Properties of Callose in Pollen Tubes. Plant Physiol. 137, 274–286. 10.1104/pp.104.050773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potroz MG, Mundargi RC, Gillissen JJ, Tan EL, Meker S, Park JH, Jung H, Park S, Cho D, Bang SI, Cho NJC, 2017. Plant-based hollow microcapsules for oral delivery applications: Toward optimized loading and controlled release. Adv. Funct. Mater 27, 1700270 10.1002/adfm.201700270 [DOI] [Google Scholar]
- Rawat S, Raman Suri C, Sahoo DK, 2010. Molecular mechanism of polyethylene glycol mediated stabilization of protein. Biochem. Biophys. Res. Commun 392, 561–566. 10.1016/j.bbrc.2010.01.067 [DOI] [PubMed] [Google Scholar]
- Rowe R, Sheskey P, Owen S, 2006. Handbook of Pharmaceutical Excipients, Fifth ed. Pharmaceutical Press, London, UK: pp. 313–314. [Google Scholar]
- Rowley JR, Skvarla JJ and El-Ghazaly G, 2003. Transfer of material through the microspore exine from the loculus into the cytoplasm, Can. J. Bot 81, 1070–1082. 10.1139/b03-095 [DOI] [Google Scholar]
- Royer CA, 1995. Fluorescence Spectroscopy, Protein stability and folding: Theory and practice. Humana Press, Totowa, NJ, pp. 65–89. [Google Scholar]
- Sargin I, Akyuz L, Kaya M, Tan G, Ceter T, Yildirim K, Ertosun S, Aydin GH, Topal M, 2017. Controlled release and anti-proliferative effect of imatinib mesylate loaded sporopollenin microcapsules extracted from pollens of Betula pendula, Int. J. Biol. Macromol 105,749–756. 10.1016/j.ijbiomac.2017.07.093 [DOI] [PubMed] [Google Scholar]
- Shakya AK, Chowdhury MYE, Tao W, Gill HS, 2016. Mucosal vaccine delivery: Current state and a pediatric perspective. J. Control. Release, 240, 394–413. 10.1016/j.jconrel.2016.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin MJ, Liyanage S, Abidi N, Gill HS, 2018. Physical and biochemical characterization of chemically-treated pollen shells for potential use in oral delivery of therapeutics. J. Pharm. Sci 10.1016/j.xphs.2018.07.028 [DOI] [PubMed] [Google Scholar]
- Uddin MJ, Gill HS, 2017. Ragweed pollen as an oral vaccine delivery system: Mechanistic insights. J. Control. Release 268, 416–426. 10.1016/j.jconrel.2017.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usmani SS, Bedi G, Samuel JS, Singh S, Kalra S, Kumar P, Ahuja AA, Sharma M, Gautam A, Raghava GPS, 2017. THPdb: Database of FDA-approved peptide and protein therapeutics. PLoS ONE 12, e0181748 10.1371/journal.pone.0181748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderplanck M, Leroy B, Wathelet B, Wattiez R, Michez D, 2014. Standardized protocol to evaluate pollen polypeptides as bee food source. Apidologie 45, 192–204. 10.1007/s13592-013-0239-0 [DOI] [Google Scholar]
- Viehof A, Javot L, Beduneau A, Pellequer Y, Lamprecht A, 2013. Oral insulin delivery in rats by nanoparticles prepared with non-toxic solvents. Int. J. Pharm 443, 169–174. 10.1016/j.ijpharm.2013.01.017 [DOI] [PubMed] [Google Scholar]
- Wakil A, Mackenzie G, Diego-Taboada A, Bell JG, Atkin SL, 2010. Enhanced bioavailability of eicosapentaenoic acid from fish oil after encapsulation within plant spore exines as microcapsules. Lipids 45, 645–649. 10.1007/s11745-010-3427-y [DOI] [PubMed] [Google Scholar]
- Xu B, Zhang W, Chen Y, Xu Y, Wang B, Zong L, 2018. Eudragit L100-coated mannosylated chitosan nanoparticles for oral protein vaccine delivery. Int. J. Biol. Macromol 113:534–542. 10.1016/j.ijbiomac.2018.02.016 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Yang Z, Hu X, Zhang L, Li F, Li M, Tang X, Xiao W, 2015. Development and evaluation of mucoadhesive nanoparticles based on thiolated Eudragit for oral delivery of protein drugs. J. Nanopart. Res 17, 98 10.1007/s11051-015-2909-5 [DOI] [Google Scholar]