Abstract
Background
In low‐ and middle‐income countries (LMICs), health services are under‐utilised, and several studies have reported improvements in neonatal outcomes following health education imparted to mothers in homes, at health units, or in hospitals. However, evaluating health educational strategy to deliver newborn care, such as one‐to‐one counselling or group counselling via peer or support groups, or delivered by health professionals, requires rigorous assessment of methodological design and quality, as well as assessment of cost‐effectiveness, affordability, sustainability, and reproducibility in diverse health systems.
Objectives
To compare a community health educational strategy versus no strategy or the existing approach to health education on maternal and newborn care in LMICs, as imparted to mothers or their family members specifically in community settings during the antenatal and/or postnatal period, in terms of effectiveness for improving neonatal health and survival (i.e. neonatal mortality, neonatal morbidity, access to health care, and cost).
Search methods
We used the standard search strategy of Cochrane Neonatal to search the Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 4), in the Cochrane Library, MEDLINE via PubMed (1966 to 2 May 2017), Embase (1980 to 2 May 2017), and the Cumulative Index to Nursing and Allied Health Literature (CINAHL) (1982 to 2 May 2017). We also searched clinical trials databases, conference proceedings, and the reference lists of retrieved articles for randomised controlled trials and quasi‐randomised trials.
Selection criteria
Community‐based randomised controlled, cluster‐randomised, or quasi‐randomised controlled trials.
Data collection and analysis
Two review authors independently assessed trial quality and extracted the data. We assessed the quality of evidence using the GRADE method and prepared 'Summary of findings' tables.
Main results
We included in this review 33 original trials (reported in 62 separate articles), which were conducted across Africa and Central and South America, with most reported from Asia, specifically India, Pakistan, and Bangladesh. Of the 33 community educational interventions provided, 16 included family members in educational counselling, most frequently the mother‐in‐law or the expectant father. Most studies (n = 14) required one‐to‐one counselling between a healthcare worker and a mother, and 12 interventions involved group counselling for mothers and occasionally family members; the remaining seven incorporated components of both counselling methods.
Our analyses show that community health educational interventions had a significant impact on reducing overall neonatal mortality (risk ratio (RR) 0.87, 95% confidence interval (CI) 0.78 to 0.96; random‐effects model; 26 studies; n = 553,111; I² = 88%; very low‐quality evidence), early neonatal mortality (RR 0.74, 95% CI 0.66 to 0.84; random‐effects model; 15 studies that included 3 subsets from 3 studies; n = 321,588; I² = 86%; very low‐quality evidence), late neonatal mortality (RR 0.54, 95% CI 0.40 to 0.74; random‐effects model; 11 studies; n = 186,643; I² = 88%; very low‐quality evidence), and perinatal mortality (RR 0.83, 95% CI 0.75 to 0.91; random‐effects model; 15 studies; n = 262,613; I² = 81%; very low‐quality evidence). Moreover, community health educational interventions increased utilisation of any antenatal care (RR 1.16, 95% CI 1.11 to 1.22; random‐effects model; 18 studies; n = 307,528; I² = 96%) and initiation of breastfeeding (RR 1.56, 95% CI 1.37 to 1.77; random‐effects model; 19 studies; n = 126,375; I² = 99%). In contrast, community health educational interventions were found to have a non‐significant impact on use of modern contraceptives (RR 1.10, 95% CI 0.86 to 1.41; random‐effects model; 3 studies; n = 22,237; I² = 80%); presence of skilled birth attendance at birth (RR 1.09, 95% CI 0.94 to 1.25; random‐effects model; 10 studies; n = 117,870; I² = 97%); utilisation of clean delivery kits (RR 4.44, 95% CI 0.71 to 27.76; random‐effects model; 2 studies; n = 17,087; I² = 98%); and care‐seeking (RR 1.11, 95% CI 0.97 to 1.27; random‐effects model; 7 studies; n = 46,154; I² = 93%).
Cost‐effectiveness analysis conducted in seven studies demonstrated that the cost‐effectiveness for intervention packages ranged between USD 910 and USD 11,975 for newborn lives saved and newborn deaths averted. For averted disability‐adjusted life‐year, costs ranged from USD 79 to USD 146, depending on the intervention strategy; for cost per year of lost lives averted, the most effective strategy was peer counsellors, and the cost was USD 33.
Authors' conclusions
This review offers encouraging evidence on the value of integrating packages of interventions with educational components delivered by a range of community workers in group settings in LMICs, with groups consisting of mothers, and additional education for family members, for improved neonatal survival, especially early and late neonatal survival.
Plain language summary
Community‐based maternal and newborn educational care packages for improving neonatal health and survival in low‐ and middle‐income countries
Review question
Is community health educational intervention for newborn care effective in improving neonatal health and survival in low‐ and middle‐income countries?
Background
In low‐ and middle‐income countries (LMICs), health service utilisation is low and neonatal mortality and morbidity are high. However, improvements in neonatal outcomes have been documented in several studies with simple health educational interventions. This review assessed the effectiveness of health education strategies imparted to mothers or their family members in community settings of LMICs. It also assessed the impact of health education strategies on neonatal mortality, neonatal morbidity, access to health care, and cost.
Study characteristics
A total of 33 experimental studies were conducted across Africa and Central and South America, with most reported from Asia, specifically India, Pakistan, and Bangladesh. Of the 33 community educational interventions, 16 required involvement of family members, most frequently the mother‐in‐law or the expectant father. Most studies (n = 14) involved one‐to‐one counselling between a range of community healthcare workers and mothers, and 12 involved group counselling consisting predominantly of mothers, with family members included occasionally; the remaining seven had components of both one‐to‐one and group counselling.
Key results
This review found that community health educational interventions significantly reduced newborn death, early newborn mortality, and late newborn mortality, as well as perinatal mortality. These interventions also positively impacted utilisation of any before birth (antenatal), care during pregnancy, and initiation of breastfeeding within an hour after birth. The review shows that educational interventions delivered to both mothers and other family members in a group setting had a greater impact on these outcomes. Educational interventions delivered during antenatal care were more effective for reducing early neonatal deaths, and those delivered during both antenatal and postnatal (after birth) periods were effective for reducing late neonatal deaths and perinatal deaths. Educational interventions during the postnatal period were most effective for improving breastfeeding practices.
Quality of evidence
The quality of evidence is low for newborn mortality outcomes and very low for early, late, and perinatal mortality. This reflects concerns of bias, inconsistency (unexplained variability of results), and imprecision (variation in studies presenting both benefit and harm from the intervention) of the included randomised controlled trials.
Summary of findings
Background
Description of the condition
Globally 2.6 million children die in the first month of life and approximately 7000 newborn deaths occur every day, with about one million dying on the first day and close to one million dying within the next six days of life. Most of these deaths occur in low‐ and middle‐income countries (LMICs) (WHO 2018; UNICEF 2017). Deaths occurring in the neonatal period (aged 0 to 27 days) account for 46% (2.614 million) of all deaths among children younger than five years (UNICEF 2017). Extremely high neonatal mortality rates (over 28 per 1000 live births) are typical of several sub‐Saharan African and South Asian countries (UNICEF 2017); currently 1 in 36 newborns die in sub‐Saharan Africa during the first month of life (UNICEF 2017). The precise contributions of various causes of neonatal death are difficult to ascertain because a vast majority of births and deaths occur in homes and thus are poorly reported and categorised (Black 2010). However, birth asphyxia/intrapartum complications and complications due to preterm birth and infectious causes are recognised as major cause of neonatal death (Black 2010; Lawn 2004; Lawn 2005; UNICEF 2017).
Description of the intervention
In an effort to improve outcomes for both mothers and their newborn infants, the "Mother‐Baby Package" was introduced by the World Health Organization (WHO) in 1994 (WHO 2006a). The Mother‐Baby Package consists of a diverse set of interventions considered essential to maternal and newborn health. These interventions include antenatal registration and care, iron or folate supplementation, tetanus toxoid immunisation, and prevention and management of sexually transmitted infections (STIs) and human immunodeficiency virus (HIV) in endemic areas. They involve treatment for underlying medical conditions such as malaria and hookworm infestation, nutritional advice, ensuring clean delivery, presence of a trained birth attendant at delivery, recognition and management of maternal and neonatal complications, neonatal resuscitation, early and exclusive breastfeeding, and prevention and management of neonatal hypothermia and infections such as ophthalmia neonatorum and cord infection. Implementation and coverage of the "Mother‐Baby Package" vary and the services offered are poorly utilised. Community educational interventions targeting expectant mothers and their family members provide an opportunity to educate the mother and her support network on appropriate care during the antenatal and postnatal periods, especially in populations with minimal access to appropriate antenatal care. Providing mothers with skills and methods to access appropriate care can greatly benefit neonatal and maternal health outcomes. These interventions may be disseminated in homes, at health units, or in hospitals and consist of different counselling strategies from a range of healthcare workers.
In LMICs, almost half of mothers lack adequate antenatal care and home births are extremely common (Benova 2018); however only 13% of women who have birthed at home receive postnatal care within 24 hours (WHO 2018), and in 28 African countries, only 66% of births take place in the presence of a skilled birth attendant (Chukwuma 2017). In many settings, care for mother and baby during the critical first few days after delivery is provided entirely outside the formal healthcare sector. In the least developed regions, contraception prevalence is only 40% (UN 2015a), with the proportion of unmet need for contraception highest among women in sub‐Saharan Africa, at 24% (UN 2015a). Also in the least developed regions, fertility rates (average lifetime number of live births per woman used as current fertility rates) are as high as 4.3 live births per woman (UN 2015), which is especially prevalent in Middle and Western Africa (UN 2015). There is also an urban‐rural gap in contraceptive use in many developing regions. This gap is particularly large in sub‐Saharan Africa, where just 18% of rural women and 31% of urban women are using any method of contraception (UNICEF 2010).
Although reasons for high neonatal mortality rates are multi‐factorial and include shortcomings in supply (such as lack of manpower, poor quality, or dearth of medical supplies and equipment), poor health centre to community linkages, malfunctioning referral systems, non‐existent emergency transportation facilities, and inadequately trained service providers and birth attendants, a major factor is the lack of demand for services provided (Atuoye 2015; Ensor 2004; Lawn 2004; Lawn 2005; Nair 2010; Osrin 2003). This is the result of numerous socioeconomic and cultural factors operating at an individual level and at a collective community level, such as poverty, lack of awareness of services offered, aversion to hospitalisation and formal medical care, lack of awareness of when and how to seek help if desired, and lack of female participation in family decision‐making (Ahmed 2001; Bang 2001; Bhardwaj 1995; Bohren 2014; de Zoysa 1998; Ensor 2004; Riaz 2015).
How the intervention might work
In LMICs, health services may be under‐utilised. Several studies have reported improvements in neonatal outcomes following health education on maternal and newborn care imparted to mothers, in home, at a health unit, or in hospital (Baqui 2008 (a); Pasha 2013; Tripathy 2010). However, the evaluation of any health educational strategy, such as one‐to‐one counselling or group counselling via peer or support groups, through the organisation of men's or women's groups, or delivered by healthcare professionals, requires rigorous assessment of methodological design and quality, as well as assessment of cost‐effectiveness, affordability, sustainability, and reproducibility in diverse health systems. The "Warmi Project" in rural Bolivia achieved significant reductions in perinatal and neonatal mortality rates (from 11.7% pre‐intervention to 4.4% post‐intervention) through support of women's organisations and community health education (O'Rourke 1998). In three rural districts of Pakistan, local women were trained to deliver primary health care and health education and to facilitate community organisation for health improvement (Barzgar 1997). In a poor urban district of Brazil, significant improvements in maternal knowledge and health behaviour were documented following implementation of the "ProNatal Project" which, among other interventions, provided health education at newly established antenatal clinics and in homes (Emond 2002). One year after initiation, significant reductions in infant mortality and diarrhoea‐related mortality, as well as increased use of contraception, were reported. In Bangalore, India, a one‐to‐one educational session with mothers of children under five years resulted in significant improvements in most aspects of home management of diarrhoea (Mangala 2001). The "Newhints" trial is a cluster‐randomised controlled trial based in Ghana that utilises community‐based surveillance officers to deliver education to improve newborn care practices and care‐seeking during pregnancy and childbirth (Kirkwood 2013). This trial was shown to increase care‐seeking by mothers while reducing the neonatal mortality rate. The "UNEST" cluster‐randomised controlled trial based in Uganda utilised community health workers (CHWs) to provide pregnant women with one‐to‐one counselling to improve newborn practices and showed that the intervention group had a greater proportion of women who initiated breastfeeding within an hour after birth compared to the control group (Waiswa 2015). In Malawi, Africa, the MaiKhanda trial utilised a participatory women's group community intervention and facility quality improvement; 50% of the formed groups developed maternal and neonatal health task forces to enhance antenatal coverage and maternal and neonatal health knowledge, as well as facility delivery (Colbourn 2013). Through this women's group approach, a 22% reduction in neonatal mortality was observed and the facility quality improvement intervention appeared to be most effective in reducing late neonatal deaths.
Why it is important to do this review
This systematic review assessed the effectiveness of community health education on maternal and newborn care for improving neonatal survival in LMICs, and attempted to compare the costs of such strategies. The purpose of this review was to determine whether community health education on maternal and newborn care is an effective and cheap method for reducing neonatal mortality and morbidity, and to identify which strategies resulted in the best neonatal outcomes. A plethora of evidence suggests that community‐based interventions are important for improving healthcare delivery and related outcomes (Lassi 2015; Lassi 2016); however no systematic reviews are currently focusing on targeting mothers and their families with health education to improve neonatal and maternal health outcomes. CHWs and other community facilitators can be at the forefront of interventions that may involve education and health promotion, with some interventions demonstrating that CHWs are able to empower communities to change their health behaviours. Because of the increasing rise in healthcare costs, it has become crucial to focus on developing affordable ways to promote health in community settings. It is therefore important to review and compile recent evidence in the form of randomised controlled trials to determine whether community health education on maternal and newborn care is cost‐effective and has the ability to improve neonatal health and survival.
Objectives
To compare a community health education strategy versus no strategy or the existing approach to health education on maternal and newborn care in LMICs, as imparted to mothers or their family members specifically in community settings during the antenatal and/or postnatal period, in terms of effectiveness for improving neonatal health and survival (i.e. neonatal mortality, neonatal morbidity, access to health care, and cost).
Methods
Criteria for considering studies for this review
Types of studies
Community‐based (home, basic health unit (BHU)† or first‐level health facility‡) randomised controlled, cluster‐randomised, or quasi‐randomised controlled trials. We obtained disaggregated data for neonates from trials conducted on neonates as well as children in older age groups.
†A BHU providing primary level health care is either "a dispensary, health post or Maternal and Child health/Family planning (MCH/FP) clinic which provides basic health services, such as health education, simple laboratory tests and treatment".
‡A first‐level health facility or the first referral level is a "district hospital with around 20 beds, providing inpatient services with staff of one or more physicians and few medical specialists, and equipment necessary to carry out most life‐saving surgical and medical procedures".
Types of participants
Types of participants included the following groups.
Women of reproductive age.
Pregnant women at any period of gestation.
Mothers of neonates (up to 28 days of life).
Their spouses/partners.
Other family members (such as mothers‐in‐law).
All participants resided in LMICs.
Types of interventions
Intervention
Community health education on maternal and newborn care* imparted to mothers or their family members in community settings of LMICs (according to the World Bank list (World Bank 2018)) via:
one‐to one‐counselling;
group counselling (in the form of group sessions);
mass media (radio, television, cellular messages, newspaper, brochures, banners, etc.); we will include only studies in which mass media was introduced as a trial for a certain period or as a pilot before launching at a national level to see clear impacts of this intervention compared to control.
Any combination of the above.
The original protocol described a comparison between one‐to‐one counselling and group counselling; however no included studies compared one‐to‐one versus group counselling.
For this review, maternal and newborn care was defined as an intervention essential to maternal and newborn health such as antenatal care, iron/folic acid supplementation, tetanus toxoid immunisation, prevention and management of STI/HIV in endemic areas, nutritional advice, ensuring clean delivery, presence of a trained birth attendant at delivery, recognition and management of maternal and neonatal complications, neonatal resuscitation, early and exclusive breastfeeding, and prevention and management of neonatal hypothermia, neonatal infections, and immunisation.
We did not include studies with a single intervention such as promotion of breastfeeding or use of family planning methods.
Control
The control did not receive the additional educational intervention or received a conventional level of health education.
Types of outcome measures
Primary outcomes
Neonatal mortality
The number of neonatal deaths from any cause among all live births during the trial period.
Early neonatal mortality: from birth through six completed days of life.
Late neonatal mortality: from seven to 28 completed days of life.
Perinatal mortality
The number of stillbirths and the number of newborn deaths within one week of life among all stillbirths and live births during the trial period.
Secondary outcomes
-
Neonatal infections
-
Number of neonates diagnosed with infection (as defined by study authors) among all live births during the trial period. Infections included:
sepsis;
pneumonia;
meningitis;
gastroenteritis;
tetanus; or
any combination of the above.
-
-
Any antenatal care
Number of pregnant women among all pregnant women who were attended for reasons related to pregnancy by skilled health personnel (a doctor, or people with midwifery skills who can manage normal deliveries and diagnose or refer obstetrical complications, or both) at least once during pregnancy. Both trained traditional and untrained traditional birth attendants were excluded (WHO 2006).
-
Use of any method of contraception
Number of women of reproductive age (15 to 49 years) or their spouses who reported that they used any contraceptive method during the trial period (any contraceptive method such as female and male sterilisation, injectable and oral hormones, intrauterine devices, diaphragms, spermicides and condoms, natural family planning, and lactational amenorrhoea) (WHO 2006).
-
Skilled attendance at delivery
Number of births among all live births during the trial period that were attended by skilled health personnel (such as a doctor or a skilled attendant capable of managing normal deliveries and referring obstetrical complications, excluding trained or untrained traditional birth attendants) (WHO 2006).
-
Delivery attended by unskilled or semi‐skilled birth attendant
Number of births among all live births during the trial period that were attended by an unskilled (such as an untrained traditional birth attendant, or a relative) or semi‐skilled birth attendant (such as a trained traditional birth attendant).
-
Use of clean delivery kit
Number of deliveries occurring during the trial period for which a clean delivery kit was used by the birth attendant (typically containing a plastic sheet delivery surface, a clean cutting instrument (a new razor blade), clean ties for the cord, soap for ensuring clean hands of the birth attendant, and instructions) (Beun 2003; PATH 2005).
-
Care‐seeking
Number of mothers among all mothers of neonates who sought or were reported to have sought medical care for their neonate's illness during the trial period.
-
Use of colostrum
Number of women who used colostrum among all women who delivered live born babies during the trial period.
-
Timely initiation of breastfeeding
Number of women who initiated breastfeeding within one hour of birth among all women who delivered live born babies during the trial period.
-
Mothers' understanding of each of the following "healthy" behaviours, among all women interviewed
Ways to prevent neonatal infection (such as handwashing, cord care)
Signs of neonatal infection
Advantages of breastfeeding
Family planning methods
Willingness to seek formal medical care for neonatal illness
Knowledge of health services offered in the community
-
Total cost of intervention
In US dollars for intervention (including recruiting and training personnel to deliver interventions and conducting sessions) among all recipients of the intervention.
-
Cost per neonatal life saved
Cost in US dollars for each neonatal life saved among all live births during the trial period.
Search methods for identification of studies
We used the criteria and standard methods of Cochrane and Cochrane Neonatal.
Electronic searches
We conducted a comprehensive search including the Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 4) in the Cochrane Library; MEDLINE via PubMed (1 January 2012 to 2 May 2017); Embase (1 January 2012 to 2 May 2017); and the Cumulative Index to Nursing and Allied Health Literatue (CINAHL) (1 January 2012 to 2 May 2017), using the search detailed in Appendix 1. We did not apply language restrictions. This search was run to update searches previously run for the reviews published in October 2010 and October 2012, using the search detailed in Appendix 2.
We searched clinical trials registries for ongoing or recently completed trials (clinicaltrials.gov; the World Health Organization’s International Trials Registry and Platform www.whoint/ictrp/search/en/; and the ISRCTN Registry) on 2 May 2017.
Searching other resources
We also searched the reference lists of any articles selected for inclusion in this review to identify additional relevant articles.
Data collection and analysis
We used the standard review methods of Cochrane and Cochrane Neonatal (Cochrane Handbook for Systematic Reviews of Interventions) (Higgins 2011).
Selection of studies
Two review authors ‐ Zohra Lassi (ZL) and Sophie Kedzior (SK) ‐ independently assessed inclusion of all potential studies identified through the search. We resolved disagreement through discussion, and, if required, we consulted a third review author ‐ Zulfiqar Bhutta (ZB).
Data extraction and management
We designed a form on which to extract data. For eligible studies, two review authors (ZL and SK) independently extracted data using the agreed form. We resolved discrepancies through discussion, or, if required, we consulted a third review author. We entered data into Review Manager software and checked them for accuracy (RevMan 2011). We attempted to contact authors of the original reports to request further details when information regarding any of the above was unclear.
Assessment of risk of bias in included studies
Two review authors (ZL and SK) independently assessed the methodological quality of each included trial using the criteria displayed in Appendix 3.
Measures of treatment effect
We carried out statistical analysis using Review Manager software (RevMan 2011).
Dichotomous data
We presented results as summary risk ratio (RRs) with 95% confidence intervals (CI) for dichotomous data.
Continuous data
We planned to use the mean difference (MD) if outcomes were measured in the same way between trials for continuous data. We used the standardised mean difference (SMD) to combine trials that measure the same outcome but used different methods.
Unit of analysis issues
Cluster‐randomised trials
When trials used clustered‐randomisation, we anticipated that study investigators would have presented their results after appropriately controlling for clustering effects (e.g. variance inflated standard errors, hierarchical linear models). When appropriate controls for clustering were not used, we adjusted for cluster effect using the intracluster coefficient (ICC) from the study similar in context and nature. We included cluster‐randomised/quasi‐randomised trials in the analyses along with individually randomised/quasi‐randomised trials. We incorporated data from cluster‐randomised/quasi‐randomised trials using the generic inverse variance method, in which logarithms of RR estimates were used along with the standard error of the logarithms of RR estimates (Higgins 2011).
Dealing with missing data
We noted levels of attrition for included studies. For all outcomes, we carried out analyses, as far as possible, on an intention‐to‐treat basis, that is, we attempted to include in the analyses all participants randomised to each group. The denominator for each outcome in each trial was the number randomised minus the number of any participants whose outcomes are known to be missing.
Assessment of heterogeneity
We applied tests of heterogeneity between trials, if appropriate, using the I² statistic, and by visual inspection of forest plots. If we identified high levels of heterogeneity among trials and visual inspection of forest plots was suggestive, we explored this by performing pre‐specified subgroup analysis. We pre‐specified the following subgroup analysis to investigate heterogeneity in the primary outcome.
Counselling type: one‐to‐one compared to group counselling, or both.
Neonatal mortality rate at baseline.
Timing of intervention: pre‐conceptual, antenatal versus postnatal.
Who receives intervention: mothers, their spouses, or other family members (such as mothers‐in‐law).
Who provides counselling: support groups or peers, health professionals, traditional birth attendants, village health workers, and so forth.
Assessment of reporting biases
Refer to Appendix 3.
Data synthesis
We carried out statistical analysis using Review Manager software (RevMan 2011). We used fixed‐effect meta‐analysis for combining data when trials were examining the same intervention and the trials' populations and methods were judged sufficiently similar. We used random‐effects meta‐analyses when we suspected clinical or methodological heterogeneity between studies sufficient to suggest that treatment effects may differ between trials. If we identified substantial heterogeneity in a fixed‐effect meta‐analysis, we noted this and repeated the analysis using a random‐effects method.
Quality of evidence
We used the GRADE approach to assess the quality of evidence for the following outcomes: neonatal mortality, early neonatal mortality, late neonatal mortality, and perinatal mortality. Two review authors (SK and ZL) independently assessed the quality of evidence for each of the aforementioned outcomes. Primary outcome data were pooled for randomised controlled trials and evidence was downgraded from "high quality" by one level for serious (or by two for very serious) for study limitations (risk of bias), indirectness of evidence, serious inconsistency, imprecision of effect estimates, or potential publication bias. We used the GRADEpro GDT Guideline Development Tool to create a ‘Summary of findings’ table to report the quality of evidence.
The GRADE approach results in an assessment of the quality of a body of evidence according to one of four grades.
High: we are very confident that the true effect lies close to that of the estimate of the effect.
Moderate: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect.
Very low: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
Subgroup analysis and investigation of heterogeneity
We applied tests of heterogeneity between trials, if appropriate, by using the I² statistic and by visually inspecting forest plots. If we identified high levels of heterogeneity among the trials and visual inspection of forest plots was suggestive, we explored this by conducting pre‐specified subgroup analysis. We pre‐specified the following subgroup analysis to investigate heterogeneity in the primary outcome.
Counselling type: one‐to‐one versus group counselling, or both.
Neonatal mortality rate at baseline: 30 or more per 1000 live births versus fewer than 30 per 1000 live births.
Timing of intervention: pre‐conceptional, antenatal versus postnatal.
Who receives intervention: mothers, their spouses, or other family members (such as mothers‐in‐law).
Who provides counselling: support groups or peers, health professionals, traditional birth attendants, village health workers, and so forth.
Sensitivity analysis
We carried out sensitivity analyses to explore the effects of adequate allocation concealment and other 'Risk of bias' components on primary outcomes (Appendix 3).
Results
Description of studies
See Characteristics of included studies, Characteristics of excluded studies, Characteristics of studies awaiting classification, and Characteristics of ongoing studies tables.
Results of the search
We ran multiple comprehensive searches for the full review. The PRISMA diagram includes the completed searches and study selections for both 2012 and 2018 (Figure 1). A total of 178 full‐text papers were reviewed for the systematic review; we classified one as ongoing and eight as awaiting classification. Finally, 33 (reported in 62 separate reports) studies met the eligibility criteria for inclusion. All were published journal articles.
1.
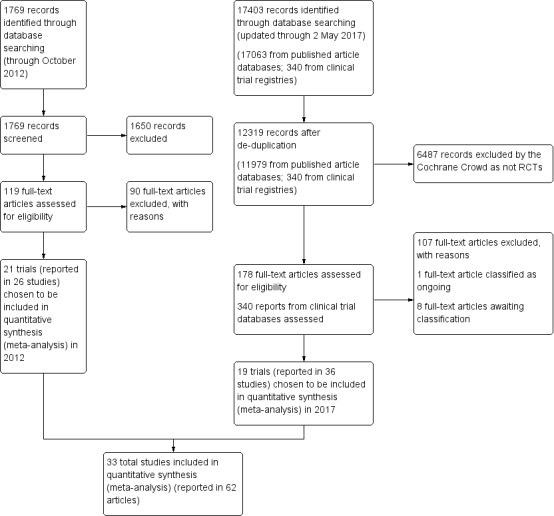
Study flow diagram.
Included studies
All 33 included studies were randomised or quasi‐randomised controlled trials.
Comparison 1
"ANY community health educational interventions versus control" (Analysis 1.1; Analysis 1.2; Analysis 1.3; Analysis 1.4; Analysis 1.5; Analysis 1.6; Analysis 1.7; Analysis 1.8; Analysis 1.9; Analysis 1.10; Analysis 1.11; Analysis 1.12; Analysis 1.13)
1.1. Analysis.
Comparison 1 Community health educational interventions versus control, Outcome 1 Neonatal mortality.
1.2. Analysis.
Comparison 1 Community health educational interventions versus control, Outcome 2 Early neonatal mortality.
1.3. Analysis.
Comparison 1 Community health educational interventions versus control, Outcome 3 Late neonatal mortality.
1.4. Analysis.
Comparison 1 Community health educational interventions versus control, Outcome 4 Perinatal mortality.
1.5. Analysis.
Comparison 1 Community health educational interventions versus control, Outcome 5 Neonatal infection.
1.6. Analysis.
Comparison 1 Community health educational interventions versus control, Outcome 6 Any antenatal care.
1.7. Analysis.
Comparison 1 Community health educational interventions versus control, Outcome 7 Use of any method of contraception.
1.8. Analysis.
Comparison 1 Community health educational interventions versus control, Outcome 8 Skilled attendance at delivery.
1.9. Analysis.
Comparison 1 Community health educational interventions versus control, Outcome 9 Delivery attended by unskilled or semi‐skilled birth attendant.
1.10. Analysis.
Comparison 1 Community health educational interventions versus control, Outcome 10 Use of clean delivery kit.
1.11. Analysis.
Comparison 1 Community health educational interventions versus control, Outcome 11 Care‐seeking for neonatal illness.
1.12. Analysis.
Comparison 1 Community health educational interventions versus control, Outcome 12 Use of colostrum.
1.13. Analysis.
Comparison 1 Community health educational interventions versus control, Outcome 13 Timely initiation of breastfeeding.
Comparison 2
"Community health educational one‐to‐one and group and both counselling (subgroup) versus control" (Analysis 2.1; Analysis 2.2; Analysis 2.3; Analysis 2.4; Analysis 2.5; Analysis 2.6; Analysis 2.7; Analysis 2.8; Analysis 2.9)
2.1. Analysis.
Comparison 2 Community health educational one‐to‐one and group and both counselling (subgroup) versus control, Outcome 1 Neonatal mortality.
2.2. Analysis.
Comparison 2 Community health educational one‐to‐one and group and both counselling (subgroup) versus control, Outcome 2 Early neonatal mortality.
2.3. Analysis.
Comparison 2 Community health educational one‐to‐one and group and both counselling (subgroup) versus control, Outcome 3 Late neonatal mortality.
2.4. Analysis.
Comparison 2 Community health educational one‐to‐one and group and both counselling (subgroup) versus control, Outcome 4 Perinatal mortality.
2.5. Analysis.
Comparison 2 Community health educational one‐to‐one and group and both counselling (subgroup) versus control, Outcome 5 Any antenatal care.
2.6. Analysis.
Comparison 2 Community health educational one‐to‐one and group and both counselling (subgroup) versus control, Outcome 6 Skilled attendance at delivery.
2.7. Analysis.
Comparison 2 Community health educational one‐to‐one and group and both counselling (subgroup) versus control, Outcome 7 Delivery attended by unskilled or semi‐skilled birth attendant.
2.8. Analysis.
Comparison 2 Community health educational one‐to‐one and group and both counselling (subgroup) versus control, Outcome 8 Care‐seeking for neonatal illness.
2.9. Analysis.
Comparison 2 Community health educational one‐to‐one and group and both counselling (subgroup) versus control, Outcome 9 Timely initiation of breastfeeding.
One‐to one‐counselling (14 studies; Ayiasi 2016; Bashour 2008; Darmstadt 2010; Degefie 2017; Ijumba 2015; Jokhio 2005; Kirkwood 2013; Magoma 2013; McConnell 2016; Mersal 2013; Penfold 2014; Srinivasan 1995; Waiswa 2015; Wu 2011)
Group counselling (in the form of group sessions) (12 studies; Azad 2010; Baqui 2008 (b); Bhandari 2012; Colbourn 2013; Fottrell 2013; Manandhar 2004; Memon 2015; Midhet 2010; More 2012; Persson 2013; Tripathy 2010; Tripathy 2016)
Any combination of the above (seven studies; Baqui 2008 (a); Bhutta 2008; Bhutta 2011; Kumar 2008; Lewycka 2013; Pasha 2013; Soofi 2017)
Comparison 3
"Community health educational [antenatal care] ANC period and [postnatal care] PNC period and both periods (subgroup) versus control" (Analysis 3.1; Analysis 3.2; Analysis 3.3; Analysis 3.4; Analysis 3.5; Analysis 3.6)
3.1. Analysis.
Comparison 3 Community health educational ANC period and PNC period and both periods (subgroup) versus control, Outcome 1 Neonatal mortality.
3.2. Analysis.
Comparison 3 Community health educational ANC period and PNC period and both periods (subgroup) versus control, Outcome 2 Early neonatal mortality.
3.3. Analysis.
Comparison 3 Community health educational ANC period and PNC period and both periods (subgroup) versus control, Outcome 3 Late neonatal mortality.
3.4. Analysis.
Comparison 3 Community health educational ANC period and PNC period and both periods (subgroup) versus control, Outcome 4 Perinatal mortality.
3.5. Analysis.
Comparison 3 Community health educational ANC period and PNC period and both periods (subgroup) versus control, Outcome 5 Care‐seeking for neonatal illness.
3.6. Analysis.
Comparison 3 Community health educational ANC period and PNC period and both periods (subgroup) versus control, Outcome 6 Timely initiation of breastfeeding.
ANC period: three studies (Azad 2010; Midhet 2010; More 2012)
PNC period: four studies (Bashour 2008; Bhandari 2012; McConnell 2016; Pasha 2013)
Both periods: 26 studies (Ayiasi 2016; Baqui 2008 (a); Baqui 2008 (b); Bhutta 2008; Bhutta 2011; Colbourn 2013; Darmstadt 2010; Degefie 2017; Fottrell 2013; Ijumba 2015; Jokhio 2005; Kirkwood 2013; Kumar 2008; Lewycka 2013; Magoma 2013; Manandhar 2004; Memon 2015; Mersal 2013; Penfold 2014; Persson 2013; Soofi 2017; Srinivasan 1995; Tripathy 2010; Tripathy 2016; Waiswa 2015; Wu 2011)
Comparison 4
"Community health educational intervention for family members and mothers and mothers only (subgroup) versus control" (Analysis 4.1; Analysis 4.2; Analysis 4.3; Analysis 4.4; Analysis 4.5; Analysis 4.6; Analysis 4.7; Analysis 4.8; Analysis 4.9)
4.1. Analysis.
Comparison 4 Community health educational intervention for family members and mothers and for mothers only (subgroup) versus control, Outcome 1 Neonatal mortality.
4.2. Analysis.
Comparison 4 Community health educational intervention for family members and mothers and for mothers only (subgroup) versus control, Outcome 2 Early neonatal mortality.
4.3. Analysis.
Comparison 4 Community health educational intervention for family members and mothers and for mothers only (subgroup) versus control, Outcome 3 Late neonatal mortality.
4.4. Analysis.
Comparison 4 Community health educational intervention for family members and mothers and for mothers only (subgroup) versus control, Outcome 4 Perinatal mortality.
4.5. Analysis.
Comparison 4 Community health educational intervention for family members and mothers and for mothers only (subgroup) versus control, Outcome 5 Any antenatal care.
4.6. Analysis.
Comparison 4 Community health educational intervention for family members and mothers and for mothers only (subgroup) versus control, Outcome 6 Skilled attendance at delivery.
4.7. Analysis.
Comparison 4 Community health educational intervention for family members and mothers and for mothers only (subgroup) versus control, Outcome 7 Care‐seeking for neonatal illness.
4.8. Analysis.
Comparison 4 Community health educational intervention for family members and mothers and for mothers only (subgroup) versus control, Outcome 8 Use of colostrum.
4.9. Analysis.
Comparison 4 Community health educational intervention for family members and mothers and for mothers only (subgroup) versus control, Outcome 9 Timely initiation of breastfeeding.
Family members and mothers: 16 studies (Ayiasi 2016; Azad 2010; Baqui 2008 (a); Bhandari 2012; Bhutta 2011; Darmstadt 2010; Degefie 2017; Fottrell 2013; Ijumba 2015; Kirkwood 2013; Kumar 2008; Magoma 2013; Memon 2015; Midhet 2010; More 2012; Penfold 2014)
Mothers only: 17 studies (Baqui 2008 (b); Bashour 2008; Bhutta 2008; Colbourn 2013; Jokhio 2005; Lewycka 2013; Manandhar 2004; McConnell 2016; Mersal 2013; Pasha 2013; Persson 2013; Soofi 2017; Srinivasan 1995; Tripathy 2010; Tripathy 2016; Waiswa 2015; Wu 2011)
Studies did not classify participants consistently, for example, some studies reported on their population as pregnancies, live births, or mothers; therefore participant numbers are not reported above.
No studies with mass media interventions were identified; therefore the proposed comparison was not performed.
Setting
The studies included in this review spanned across Asia, Africa, and Central/South America. From Asia, seven studies were conducted in India (Baqui 2008 (b); Bhandari 2012; Kumar 2008; More 2012; Srinivasan 1995; Tripathy 2010; Tripathy 2016), six in Pakistan (Bhutta 2008; Bhutta 2011; Jokhio 2005; Memon 2015; Midhet 2010; Soofi 2017), four in Bangladesh (Azad 2010; Baqui 2008 (a); Darmstadt 2010; Fottrell 2013), and one each in Nepal (Manandhar 2004), China (Wu 2011), Vietnam (Persson 2013), and Syria (Bashour 2008). A total of 11 studies were conducted in Africa; of those, two were conducted in each of Malawi (Colbourn 2013; Lewycka 2013), Tanzania (Magoma 2013; Penfold 2014), and Uganda (Ayiasi 2016; Waiswa 2015); and one each in Ethiopia (Degefie 2017), Egypt (Mersal 2013), Ghana (Kirkwood 2013), Kenya (McConnell 2016), and South Africa (Ijumba 2015). One study was a multi‐country trial with sites in India, Pakistan, Kenya, Zambia, Guatemala, and Argentina (Pasha 2013).
Sample size
The studies included in this review reported different measures of sample sizes, including number of pregnant women at enrolment and number of live births at the commencement of the study. Some studies reported both of these measures. A proportion of sample sizes were reported as women of reproductive age (15 to 49 years) (Azad 2010; Baqui 2008 (a); Darmstadt 2010; Degefie 2017; Kirkwood 2013; Manandhar 2004; Tripathy 2016; Waiswa 2015). Some studies utilised estimated population sizes at baseline as their sample size (e.g. unions ‐ Azad 2010).
Twenty one of the included studies reported the number of enrolled pregnant women at the start of the study period, and studied a total of 444,324 pregnancies (Ayiasi 2016; Baqui 2008 (a); Baqui 2008 (b); Bashour 2008; Bhandari 2012; Bhutta 2011; Darmstadt 2010; Ijumba 2015; Jokhio 2005; Kirkwood 2013; Kumar 2008; Lewycka 2013; Magoma 2013; Manandhar 2004; Mersal 2013; Midhet 2010; Pasha 2013; Penfold 2014; Soofi 2017; Srinivasan 1995; Tripathy 2010). Sample sizes ranged from 86 in Mersal 2013 to 134,688 in Pasha 2013. Twenty‐six of the included studies reported number of live births at the end of the study period, with a collected total of 563,562 live births (Azad 2010; Baqui 2008 (a); Bhandari 2012; Bhutta 2008; Bhutta 2011; Colbourn 2013; Darmstadt 2010; Degefie 2017; Fottrell 2013; Ijumba 2015; Jokhio 2005; Kirkwood 2013; Kumar 2008; Lewycka 2013; Manandhar 2004; Memon 2015; Midhet 2010; More 2012; Pasha 2013; Penfold 2014; Persson 2013; Soofi 2017; Tripathy 2010; Tripathy 2016; Waiswa 2015; Wu 2011). Live birth sample sizes ranged from 521 in Penfold 2014 to 109,270 in Pasha 2013.
Interventions
All included studies provided a combination of interventions to promote maternal and newborn care for improving neonatal health and survival. These interventions included promotion of routine antenatal care, tetanus toxoid immunisation, nutrition counselling including iron folic acid supplementation, maternal health education, promotion of institutional deliveries, birth and newborn care preparedness, provision of safe delivery kits, clean delivery practices, referrals for emergency obstetrics care, promotion of early and exclusive breastfeeding, kangaroo mother care, newborn resuscitation, management of neonatal infections, referrals for sick newborns, and postnatal visitation and recognition of neonatal danger signs. The studies described packages of interventions; therefore education often was only a component of the intervention and was used in conjunction with other interventions.
The characteristics of all included studies are provided in the Characteristics of included studies table. We identified some key contextual factors from each study and reported those in Table 6.
1. Key contextual factors in included studies.
| Study |
Family members included |
Women/support group |
Education given by |
ANC, PNC, or both |
Baseline NMR (> or < 30 per 1000) I, C |
Counselling type (one‐to‐one counselling/group counselling/both) |
| Ayiasi 2016 | Yes | No | CHWs (Village Health Team) | Yes (both) |
NA | One‐to‐one |
| Azad 2010 | Yes (mother‐in‐law) |
Yes | Local female peer facilitators | Yes (ANC) | 21.6, 26.8 | Group |
| Baqui 2008 (a) | Yes (husband) | No | CHWs | Yes (both) | (46.9, 46.7), 48 | Both |
| Baqui 2008 (b) | No | No | CHWs | Yes (both) | NA | Group |
| Bashour 2008 | No | No | Midwives | Yes (PNC) | NA | One‐to‐one |
| Bhandari 2012 | Yes | Yes | CHWs | Yes (PNC) | 32.6, 32.4 | Group |
| Bhutta 2008 | No | No | LHWs and TBAs | Yes (both) | 110.08, 94.64 | Both |
| Bhutta 2011 | Yes (additional family members, husband) | Yes | LHWs and TBAs | Yes (both) | 48, 51.3 | Both |
| Colbourn 2013 | No | Yes | Volunteer facilitators | Yes (both) |
(33.3, 29.4, 24) 31.8 | Group |
| Darmstadt 2010 | Yes | No | CHWs | Yes (both) | 27.9, 25.2 | One‐to‐one |
| Degefie 2017 | Yes | No | CHWs, volunteers | Yes (both) | During days 0 to 27: 35, 33.6 | One‐to‐one |
| Fottrell 2013 | Yes (men) | Yes | Facilitators | Yes (both) | 38.5 (CI 34.8 to 37.4) | Group |
| Jokhio 2005 | No | No | TBAs, LHWs | Yes (both) |
NA ‐ No baseline | One‐to‐one |
| Kirkwood 2013 | Yes | No | CBSVs | Yes (both) | 32.3, 32.7 | One‐to‐one |
| Kumar 2008 | Yes (mother and father‐in‐law, husband) | No | CHWs | Yes (both) | (64.1, 58.9) 54.2 | Both |
| Lewycka 2013 | No | Yes | Volunteer peer counsellors | Yes (both) | Assumed baseline MR (34, 27, 76) | Both |
| Magoma 2013 | Yes (male partner) | No | Care providers | Yes (both) | NA | One‐to‐one |
| Manandhar 2004 | No | Yes | Female facilitators | Yes (both) | 25.4, 25.1 | Group |
| McConnell 2016 | No | No | CHWs | Yes (PNC) | NA | One‐to‐one |
| Memon 2015 | Yes (father, father‐in‐law, mother‐in law) | Yes | CHWs, LHWs | Yes (both) | 26, 39.8 | Group |
| Mersal 2013 | No | No | NA | Yes (both) | NA | One‐to‐one |
| Midhet 2010 | Yes (husband) | Yes | Female volunteers | Yes (ANC) | NA | Group |
| More 2012 | Yes (other women in the family) | Yes | Female facilitators (Sakhi) | Yes (ANC) | 22.3, 18.6 | Group |
| Pasha 2013 | No | Yes | TBAs | Yes (PNC) | 23.8, 22.5 | Both |
| Penfold 2014 | Yes (father, mothers‐in‐law) | No | Community volunteers | Yes (both) | 35, 47 | One‐to‐one |
| Persson 2013 | No (only if details of neonatal death were required) | Yes | Volunteers from Women's Union, VHWs | Yes (both) | > 15 for both groups | Group |
| Soofi 2017 | No (male community mobilisers ‐ no specifics on relation) | Yes | LHWs | Yes (both) | 43.7, 44.6 | Both |
| Srinivasan 1995 | No | No | Female ANMs | Yes (both) | 18.7, 15.5, 18 | One‐to‐one |
| Ijumba 2015 | Yes (participants' mothers, grandmothers, sisters, husband/boyfriend) | No | CHWs | Yes (both) | NA | One‐to‐one |
| Tripathy 2010 | No | Yes | Facilitators | Yes (both) |
61.8, 53.6 | Group |
| Tripathy 2016 | No | Yes | ASHAs | Yes (both) | 63.4, 51.7 | Group |
| Waiswa 2015 | No | No | CHWs | Yes (both) | NA | One‐to‐one |
| Wu 2011 | No (no intervention; for survey, yes if mother was not available) | No | Midwives | Yes (both) | 37.9, 30.8 | One‐to‐one |
ANC: antenatal care.
ANM: auxiliary nurse midwife.
ASHA: accredited social health activist.
C: control.
CBSV: community‐based surveillance volunteer.
CHW: community health worker.
I: intervention.
LHW: Lady Health Worker; local health worker.
NA: not available.
NMR: neonatal mortality rate.
PNC: postnatal care.
TBA: traditional birth attendant.
VHW: village health worker.
Comparisons
The comparison groups in all respective studies received the usual health services provided by the government, non‐governmental organisations, and private providers.
Excluded studies
After reviewing the articles, we excluded 100 studies (consisting of 108 papers) and provided reasons for exclusion in the Characteristics of excluded studies table. We excluded 31 studies because they were not conducted in LMICs (Coombs 1998; Di Napoli 2004; Escobar 2001; Hannula 2008; Hoddinott 2006; Ingram 2004; Ingram 2009; Kafatos 1989; Kafatos 1991; Kools 2005; Lin 2008; MacArthur 2009; Martens 2002; Mottl‐Santiago 2008; Murihead 2006; Petrova 2009; Philipp 2001; Pobocik 2000; Rishel 2005; Rosen 2008; Rossiter 1994; Russell 1999; Ryser 1999; Ryser 2004; Sandy 2009; Schneider 2001; Shaw 1999; Stille 2001; Volpe 2000; Whitelaw 1988; Yun 2010); 22 studies because they were not conducted in community settings (Bolam 1998; Chapman 2004; Coskun 2009; Foreit 1993; Forster 2004; Froozani 1999; Gill 2007; Grossman 2009; Haider 1996; Ickovics 2007; Jang 2008; Johnson 2017; Kadam 2005; Merewood 2003; Nichols 2009; Seema 1997; Serwint 1996; Shinwell 2006; Shrestha 2016; Susin 2008; Svenson 2009; Wong 2007); 20 studies because they were not RCTs or quasi‐RCTs (Agboatwalla 1997; Ahluwalia 2000; Alexandre 2007; Bang 1990; Bang 1999; Bartington 2006; Bland 2008; Boulvain 2004; Carlo 2010; Castrucci 2007; Dennis 2001; Gross 2009; Guise 2003; Moran 2006; Quinn 2005; Raghupathy 1996; Rosato 2006; Syed 2006; Syed 2008; Warren 2010); 13 studies because the intervention was not directly related to maternal and newborn care to improve neonatal survival and health (Ayiasi 2015b; Baqui 2015; Bhandari 2004; Bhutta 2009; Davies‐Abetugbo 1996; Dearden 2002; Flax 2009; Haider 2000; Nabulsi 2014; Rahman 2008; Raj 2016; Rawat 2017; Tylleskär 2011); seven studies because the age of the infant was not appropriate for inclusion in the review (Bhandari 2003; Bhattacharya 1988; Hoare 1999; Hotz 2005; Nair 2017; Sachdeva 1994; Roy 2007); four studies because they had no educational component (Gill 2011; Tshefu 2015; Zaidi 2012; Zaidi 2013;); one study because study authors reported findings only in an abstract, resulting in insufficient information (Bhopal 2017); one study because it included no control arm (Sloan 2008); and one study because it did not report on any of the primary outcomes (Kimani‐Murage 2015).
Risk of bias in included studies
All 33 included studies were randomised (n = 5; Ayiasi 2016; Bashour 2008; Ijumba 2015; McConnell 2016; Mersal 2013), cluster‐randomised (n = 26; Azad 2010; Baqui 2008 (a); Bhandari 2012; Bhutta 2008; Bhutta 2011; Colbourn 2013; Darmstadt 2010; Degefie 2017; Fottrell 2013; Jokhio 2005; Kirkwood 2013; Kumar 2008; Lewycka 2013; Magoma 2013; Manandhar 2004; Midhet 2010; More 2012; Pasha 2013; Penfold 2014; Persson 2013; Soofi 2017; Srinivasan 1995; Tripathy 2010; Tripathy 2016; Waiswa 2015; Wu 2011), or quasi‐experimental studies (n = 2; Baqui 2008 (b); Memon 2015).
Please refer to Figure 2 and Figure 3 for details.
2.
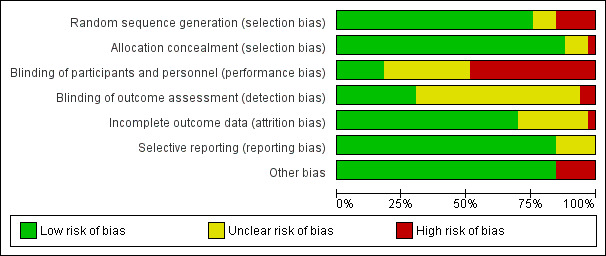
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
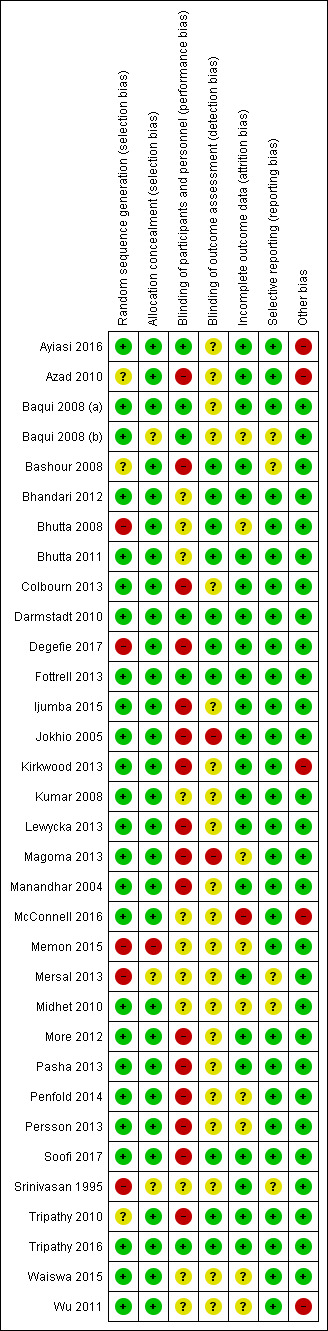
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
In this review, 29 studies had no issues with allocation concealment, as all clusters were randomised at the start, and studies were considered at low risk. One study had high risk of selection bias due to the quasi‐experimental design (Memon 2015). Three studies provided insufficient data on allocation concealment to permit judgement (Baqui 2008 (b); Mersal 2013; Srinivasan 1995).
Blinding
We noted mixed results for blinding of participants and personnel and considered the majority of studies (n = 17) to be at high risk (Azad 2010; Bashour 2008; Colbourn 2013; Degefie 2017; Ijumba 2015; Jokhio 2005; Kirkwood 2013; Lewycka 2013; Magoma 2013; Manandhar 2004; More 2012; Pasha 2013; Penfold 2014; Persson 2013; Soofi 2017; Tripathy 2010; Wu 2011), with the remainder at unclear or low risk, most often due to the study design (cluster‐randomised). Eight studies were deemed at low risk for performance bias (Ayiasi 2016; Baqui 2008 (a); Baqui 2008 (b); Bhutta 2008; Darmstadt 2010; Fottrell 2013; McConnell 2016; Tripathy 2016), and eight were considered to have unclear risk due to insufficient evidence (Bhandari 2012; Bhutta 2011; Kumar 2008; Memon 2015; Mersal 2013; Midhet 2010; Srinivasan 1995; Waiswa 2015).
Regarding detection bias, information in 19 studies was insufficient to permit any judgement for blinding of outcome assessment (Ayiasi 2016; Azad 2010; Baqui 2008 (a); Baqui 2008 (b); Colbourn 2013; Ijumba 2015; Kirkwood 2013; Lewycka 2013; Manandhar 2004; Memon 2015; Mersal 2013; Midhet 2010; More 2012; Pasha 2013; Penfold 2014; Persson 2013; Srinivasan 1995; Waiswa 2015; Wu 2011). Most remaining studies were considered at low risk (n = 12; Bashour 2008; Bhandari 2012; Bhutta 2008; Bhutta 2011; Darmstadt 2010; Degefie 2017; Fottrell 2013; Kumar 2008; McConnell 2016; Soofi 2017; Tripathy 2010; Tripathy 2016), and two were considered at high risk (Jokhio 2005; Magoma 2013).
Incomplete outcome data
Incomplete data were addressed in all studies except eight, which provided insufficient data to permit any judgment (Baqui 2008 (b); Bhutta 2008; Magoma 2013; Memon 2015; Midhet 2010; Penfold 2014; Persson 2013; Waiswa 2015). Of the remaining 25 studies, 24 were considered low risk (Ayiasi 2016; Azad 2010; Baqui 2008 (b); Bashour 2008; Bhandari 2012; Bhutta 2011; Colbourn 2013; Darmstadt 2010; Degefie 2017; Fottrell 2013; Ijumba 2015; Jokhio 2005; Kirkwood 2013; Kumar 2008; Lewycka 2013; Manandhar 2004; Mersal 2013; More 2012; Pasha 2013; Soofi 2017; Srinivasan 1995; Tripathy 2010; Tripathy 2016; Wu 2011), and one was considered high risk due to a high attrition rate (˜ 43%) (McConnell 2016).
Selective reporting
Twenty‐six studies mentioned results as per stated objectives and appeared to be free of selective reporting. Seven studies presented with unclear risk due to insufficient information to permit any judgement (Baqui 2008 (b); Bashour 2008; Ijumba 2015; Magoma 2013; Mersal 2013; Midhet 2010; Srinivasan 1995). For five of these studies, the insufficient information was related to the study not being a registered trial (Ijumba 2015; Magoma 2013; Mersal 2013; Midhet 2010; Srinivasan 1995).
Other potential sources of bias
Most included studies were deemed free from other biases and therefore at low risk (n = 28; Baqui 2008 (a); Baqui 2008 (b); Bashour 2008; Bhandari 2012; Bhutta 2008; Bhutta 2011; Colbourn 2013; Darmstadt 2010; Degefie 2017; Fottrell 2013; Ijumba 2015; Jokhio 2005; Kumar 2008; Lewycka 2013; Magoma 2013; Manandhar 2004; Memon 2015; Mersal 2013; Midhet 2010; More 2012; Pasha 2013; Penfold 2014; Persson 2013; Soofi 2017; Srinivasan 1995; Tripathy 2010; Tripathy 2016; Waiswa 2015). The five remaining studies were considered at high risk for other sources of bias (Ayiasi 2016; Azad 2010; Kirkwood 2013; McConnell 2016; Wu 2011), such as purposive selection, self‐reporting, and potential cross‐contamination.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5
Summary of findings for the main comparison. Community health educational interventions compared to control in LMICs.
| Community health educational interventions compared to control in developing countries | ||||
| Patient or population: developing countries Setting: low‐middle‐income countries, community Intervention: community health educational interventions Comparison: control | ||||
| Outcomes | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments |
| Neonatal mortality | RR 0.87 (0.78 to 0.96) | 553111 (26 RCTs) | ⊕⊕⊝⊝ Lowa,b | 25/26 studies have unsure risk or high risk for more than 1 type of bias, with the most common high risk being performance bias. However because the outcome is mortality, blinding is objective and therefore is unlikely to be affected by blinding of outcome assessment. Not all confidence intervals from the studies overlap; there is also inconsistency in direction across studies |
| Early neonatal mortality | RR 0.74 (0.66 to 0.84) | 321588 (15 RCTs) | ⊕⊝⊝⊝ Very lowa,b,c | There was a combination of high and unclear risk of bias regarding blinding; this can mainly be attributed to the cluster‐randomised design. The study with the greatest risk of bias concerns had the smallest weighting. Most of the studies overlap and show the same direction of effect; however there is 1 major outlier that is also in the opposite direction. The statistical measure for heterogeneity is high, suggesting inconsistency. The confidence interval is wide; however the sample size is sufficient |
| Late neonatal mortality | RR 0.54 (0.40 to 0.74) | 186643 (11 RCTs) | ⊕⊝⊝⊝ Very lowa,b,c | High risk of bias was present for blinding across studies; however studies were cluster‐randomised trials, and this is justifiable. I² (88%) was considerably large; however most of the confidence intervals overlap, and direction of effect is consistent. The confidence interval is wide; however the sample size is sufficient |
| Perinatal mortality | RR 0.83 (0.75 to 0.91) | 262613 (15 RCTs) | ⊕⊝⊝⊝ Very lowb,c | I² (81%) was considerably large; some studies (although with small weighting) support the control, and others support the intervention. Most of the confidence intervals overlap; however some CIs are large. The confidence interval is wide; however the sample size is sufficient |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; LMICs: low‐ and middle‐income countries; RCT: randomised controlled trial; RR: risk ratio. | ||||
| GRADE Working Group grades of evidence. High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||
aRisk of bias.
bInconsistency.
cImprecision.
Summary of findings 2. Community health educational one‐to‐one and group and both counselling (subgroup) compared to control in LMICs.
| Community health educational one‐to‐one and group and both counselling (subgroup) compared to control in LMICs | ||||
| Patient or population: developing countries Setting: low‐middle‐income countries, community Intervention: community health educational one‐to‐one and group and both counselling (subgroup) Comparison: control | ||||
| Outcomes | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments |
| Neonatal mortality ‐ One‐to‐one counselling | RR 0.92 (0.71 to 1.20) | 105,735 (8 RCTs) | ⊕⊝⊝⊝ Very lowa,b,c | Not all confidence intervals overlap, and there is some inconsistency in the direction of effect. However the study with the greatest confidence interval reported weights of only 0.2%. This was further downgraded by 1 as the confidence interval includes both important benefit or harm and no effect. Most studies had concerns in areas of bias that may have affected the outcome; however those with high risk of performance bias should not have impacted the outcome of neonatal mortality |
| Neonatal mortality ‐ Group counselling | RR 0.83 (0.74 to 0.92) | 211,164 (12 RCTs) | ⊕⊕⊝⊝ Lowa,b | Around half of the studies raised concerns in areas of bias that may impact the direction of effect; therefore this was downgraded by one level. Not all confidence intervals from the studies overlap; there is also inconsistency in direction across studies |
| Neonatal mortality ‐ Both group and one‐to‐one counselling | RR 0.90 (0.76 to 1.06) | 236,212 (6 RCTs) | ⊕⊕⊕⊝ Moderateb | Not all confidence intervals from the studies overlap; there is also inconsistency in direction across studies |
| Early neonatal mortality ‐ Group counselling | RR 0.70 (0.61 to 0.80) | 122,151 (9 RCTs) | ⊕⊕⊝⊝ Lowa,b | There was a combination of high and unclear risk of bias regarding blinding; this can be attributed mainly to the cluster‐randomised design. The study with the greatest risk of bias concerns had the smallest weighting. Most studies are consistent in the direction of effect; however not all confidence intervals overlap |
| Early neonatal mortality ‐ One‐to‐one counselling | RR 1.30 (1.01 to 1.67) | 18,747 (1 RCT) | ⊕⊕⊝⊝ Lowa,c | There was high risk of bias for selection; however this was a cluster‐randomised trial with a large confidence interval |
| Early neonatal mortality ‐ Both one‐to‐one and group counselling | RR 0.78 (0.65 to 0.93) | 180,690 (5 RCTs) | ⊕⊕⊕⊝ Moderateb | There was considerable heterogeneity (I² = 85%), and not all confidence intervals overlapped |
| Late neonatal mortality ‐ Group counselling | RR 0.50 (0.31 to 0.81) | 118,239 (7 RCTs) | ⊕⊝⊝⊝ Very lowa,b,c | High risk of bias was present for blinding across studies; however these were cluster‐randomised trials, and this is justifiable. There was considerable heterogeneity (I² = 91%), and all studies are consistent in their direction; however not all of them overlap. The confidence interval is wide; however the sample size is sufficient |
| Late neonatal mortality ‐ Both group and one‐to‐one counselling | RR 0.72 (0.57 to 0.91) | 68,404 (4 RCTs) | ⊕⊕⊕⊝ Moderatec | Confidence interval is wide |
| Perinatal mortality ‐ One‐to‐one counselling | RR 0.88 (0.57 to 1.34) | 23,829 (2 RCTs) | ⊕⊝⊝⊝ Very lowa,b,c | One of the studies that had greater weighting had considerable risk of bias that may impact the direction of effect Confidence intervals do not overlap, and the direction of effect is inconsistent Confidence interval include both important benefit or harm and no effect |
| Perinatal mortality ‐ Group counselling | RR 0.85 (0.77 to 0.94) | 156,505 (8 RCTs) | ⊕⊕⊝⊝ Lowa,b | The 2 heaviest weighted studies have concerns regarding selection bias Most confidence intervals overlap, and only 1 study (that has less weighting) is going in the opposite direction of effect |
| Perinatal mortality ‐ Both group and one‐to‐one counselling | RR 0.78 (0.67 to 0.90) | 82,279 (5 RCTs) | ⊕⊕⊝⊝ Lowb,c | The confidence interval is wide; however the sample size is sufficient. Most confidence intervals overlap; however 1 of the studies is inconsistent in its direction of effect (although it is weighted the least) |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; LMICs: low‐ and middle‐income countries; RCT: randomised controlled trial; RR: risk ratio. | ||||
| GRADE Working Group grades of evidence. High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||
aRisk of bias.
bInconsistency.
cImprecision.
Summary of findings 3. Community health educational ANC period and PNC period and both periods (subgroup) compared to control in LMICs.
| Community health educational ANC period and PNC period and both periods (subgroup) compared to control in LMICs | ||||
| Patient or population: developing countries Setting: low‐middle‐income countries, community Intervention: community health educational ANC period and PNC period and both periods (subgroup) Comparison: control | ||||
| Outcomes | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments |
| Neonatal mortality ‐ Education provided during ANC period only | RR 0.84 (0.64 to 1.09) | 47,849 (3 RCTs) | ⊕⊝⊝⊝ Very lowa,b | Studies were rated evenly, and the quality of evidence was downgraded as all studies had biases that were of unclear or high risk that may have affected the outcome. Not all confidence intervals from the studies overlap; there is also inconsistency in direction across studies |
| Neonatal mortality ‐ Education provided during PNC period only | RR 1.02 (0.84 to 1.24) | 172,882 (3 RCTs) | ⊕⊕⊝⊝ Lowb | There is inconsistency in the direction of effect |
| Neonatal mortality ‐ Education provided in both ANC and PNC periods | RR 0.85 (0.76 to 0.96) | 332,380 (20 RCTs) | ⊕⊝⊝⊝ Very lowa,b | Because the outcome is mortality, blinding is objective and therefore is unlikely to be affected by blinding of outcome assessment Quality of evidence was downgraded 2 points due to inconsistent direction of effect and because not all confidence intervals overlapped |
| Early neonatal mortality ‐ Education provided during ANC period only | RR 0.64 (0.43 to 0.95) | 33,209 (2 RCTs) | ⊕⊝⊝⊝ Very lowa,b,c | Quality assessment was downgraded both both studies had reasonable amounts of bias that may have impacted the effect Heterogeneity is high according to I², and there is consistency in the direction of effect, although the larger study's confidence intervals do not overlap the others Confidence interval shows large spread |
| Early neonatal mortality ‐ Education provided during PNC period only | RR 1.03 (0.94 to 1.12) | 111,529 (1 RCT) | ⊕⊕⊕⊝ Moderatec | The confidence interval includes benefit, harm, and no effect |
| Early neonatal mortality ‐ Education provided during both ANC and PNC periods | RR 0.76 (0.68 to 0.84) | 176,850 (12 RCTs) | ⊕⊝⊝⊝ Very lowa,b | There was a combination of high and unclear risk of bias regarding blinding; this can be attributed mainly to the cluster‐randomised design. The study with greatest risk of bias concerns had the smallest weighting. Quality of evidence was downgraded 2 points due to inconsistent direction of effect, and not all confidence intervals overlap |
| Late neonatal mortality ‐ Education provided during ANC period only | RR 0.87 (0.54 to 1.40) | 30,952 (1 RCT) | ⊕⊝⊝⊝ Very lowa | This was downgraded by 1 level due to possible selection bias |
| Late neonatal mortality ‐ Education provided during both ANC and PNC periods | RR 0.52 (0.38 to 0.72) | 155,691 (10 RCTs) | ⊕⊝⊝⊝ Very lowa,b,c | High risk of bias was present for blinding across studies; however studies were cluster‐randomised trials, and this is justifiable I² (88%) was considerably large; however most of the confidence intervals overlap and direction of effect is consistent This was downgraded by 1 level, as there was a sufficient number of events; however the confidence interval was wide |
| Perinatal mortality ‐ Education provided during PNC only | RR 0.89 (0.78 to 1.02) | 60,480 (1 RCT) | ⊕⊕⊕⊕ High | There were no concerns regarding certainty assessment; however this is for only 1 study |
| Perinatal mortality ‐ Education provided during ANC period only | RR 0.90 (0.59 to 1.39) | 33,513 (2 RCTs) | ⊕⊝⊝⊝ Very lowa,b | Quality assessment was downgraded because both studies had reasonable amounts of bias that may have impacted the effect. Heterogeneity is large; direction of effect shows inconsistency, and overlapping of confidence intervals is minimal |
| Perinatal mortality ‐ Education provided during both ANC and PNC periods | RR 0.81 (0.72 to 0.91) | 168,620 (12 RCTs) | ⊕⊕⊝⊝ Lowb | Direction of effect shows inconsistency; there is also inconsistency in confidence intervals overlapping each other |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ANC: antenatal care; CI: confidence interval; LMICs: low‐ to middle‐income countries; PNC: post‐natal care; RCT: randomised controlled trial; RR: risk ratio. | ||||
| GRADE Working Group grades of evidence. High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||
aRisk of bias.
bInconsistency.
cImprecision.
Summary of findings 4. Community health educational intervention for family members and mothers and for mothers only (subgroup) compared to control in LMICs.
| Community health educational intervention for family members and mothers and for mothers only (subgroup) compared to control in LMICs | ||||
| Patient or population: developing countries Setting: low‐middle‐income countries, community Intervention: community health educational intervention for family members and mothers and for mothers only (subgroup) Comparison: control | ||||
| Outcomes | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments |
| Neonatal mortality ‐ Intervention given to mothers and family members | RR 0.84 (0.74 to 0.95) | 282,817 (13 RCTs) | ⊕⊕⊝⊝ Lowa,b | Studies that were weighted the highest had the greatest concerns for risk of bias. Not all confidence intervals from these studies overlap, and the direction of effect shows inconsistency across studies |
| Neonatal mortality ‐ Intervention given to mothers only | RR 0.90 (0.77 to 1.05) | 270294 (13 RCTs) | ⊕⊝⊝⊝ Very lowa,b,c | An even split of studies presented with risk of bias that may have impacted the results. The confidence interval includes both important benefit or harm and no effect, but the total number of events appears to be sufficient |
| Early neonatal mortality ‐ Intervention given to mothers and family members | RR 0.70 (0.56 to 0.87) | 99,097 (7 RCTs) | ⊕⊝⊝⊝ Very lowa,b,c | There was a combination of high and unclear risk of bias regarding blinding; this can be attributed mainly to the cluster‐randomised design. The study with the greatest risk of bias concerns had the smallest weighting. Heterogeneity is high (I² = 88%) and not all confidence intervals overlap; one study is inconsistent in terms of direction of effect. The confidence interval is wide; however the sample size is sufficient |
| Early neonatal mortality ‐ Intervention given to mothers only | RR 0.78 (0.68 to 0.90) | 222,491 (8 RCTs) | ⊕⊝⊝⊝ Very lowa,b,c | There was a combination of high and unclear risk of bias regarding blinding; this can be attributed mainly to the cluster‐randomised design. The study with the greatest risk of bias concerns had the smallest weighting. Most of the confidence intervals overlap, a small amount of inconsistency regarding direction of effect is evident. The confidence interval is wide; however the sample size is sufficient |
| Late neonatal mortality ‐ Intervention given to mothers and family members | RR 0.69 (0.51 to 0.92) | 76,388 (4 RCTs) | ⊕⊕⊝⊝ Lowb,c | I² (49%) is low; all studies are consistent in direction; however some studies cross the line of no effect. The confidence interval is wide; however the sample size is sufficient |
| Late neonatal mortality ‐ Intervention given to mothers only | RR 0.50 (0.31 to 0.78) | 110,255 (7 RCTs) | ⊕⊝⊝⊝ Very lowa,b,c | High risk of bias was present for blinding across studies; however studies were cluster‐randomised trials, and this is justifiable I² (92%) is considerably large; not all confidence intervals of these studies overlap; however some consistency in the direction of effect is evident The confidence interval is wide; however the sample size is sufficient |
| Perinatal mortality ‐ Intervention given to mothers and family members | RR 0.83 (0.72 to 0.96) | 141,824 (7 RCTs) | ⊕⊕⊝⊝ Lowa,b | Several studies with greater weighting were at considerable risk of bias in areas (random sequence generation) that may have impacted the outcome Slight inconsistency in the direction of effect is evident between studies, and wide confidence intervals do not all overlap |
| Perinatal mortality ‐ Intervention given to mothers only | RR 0.83 (0.72 to 0.96) | 120,789 (8 RCTs) | ⊕⊕⊝⊝ Lowa,b | Several studies with greater weighting were at considerable risk of bias in areas (random sequence generation) that may have impacted the outcome There is slight inconsistency in the direction of effect between studies, and wide confidence intervals do not all overlap |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; LMICs: low‐ and middle‐income countries; RCT: randomised controlled trial; RR: risk ratio. | ||||
| GRADE Working Group grades of evidence. High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||
aRisk of bias.
bInconsistency.
cImprecision.
Summary of findings 5. Community health educational interventions compared to control in LMICs: Sensitivity analysis on primary outcomes.
| Community health educational interventions compared to control in developing countries in LMICs: sensitivity analysis | ||||
| Patient or population: developing countries Setting: low‐middle‐income countries, community Intervention: community health educational interventions Comparison: control | ||||
| Outcomes | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments |
| Neonatal mortality | RR 0.88 (0.79 to 0.98) | 497,258 (22 RCTs) | ⊕⊕⊝⊝ Moderatea | Not all confidence intervals from these studies overlap; there is also inconsistency in direction across studies |
| Early neonatal mortality | RR 0.71 (0.62 to 0.82) | 26,472 (11 RCTs) | ⊕⊝⊝⊝ Moderatea | Most studies overlap and are in the same direction of effect; however there is one major outlier that is also in the opposite direction. The statistical measure for heterogeneity is also high, suggesting inconsistency |
| Late neonatal mortality | RR 0.51 (0.36 to 0.72) | 150,867 (9 RCTs) | ⊕⊝⊝⊝ Moderatea | I² (88%) was considerably large; however most confidence intervals overlap, and there is consistent direction of effect |
| Perinatal mortality | RR 0.84 (0.75 to 0.94) | 262,613 (12 RCTs) | ⊕⊝⊝⊝ Moderatea | I² (81%) is considerably large; some studies (although with small weighting) support the control, and others support the intervention. Most confidence intervals overlap; however some CIs are large |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio. | ||||
| GRADE Working Group grades of evidence. High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||
aInconsistency.
Primary outcomes
Neonatal mortality (outcomes 1.1, 2.1, 3.1, and 4.1)
Community health education interventions showed a significant impact on reducing neonatal mortality by 13% (risk ratio (RR) 0.87, 95% confidence interval (CI) 0.78 to 0.96; random‐effects model; 26 studies; n = 553,111; Chi² P < 0.00001; I² = 88%; low‐quality evidence on GRADE) (Analysis 1.1; Figure 4; Table 1). It was determined that this finding was of low quality due to concerns of risk of bias and inconsistency across studies. On sensitivity analysis, community health education interventions showed a significant impact on reducing neonatal mortality by 12% (RR 0.88, 95% CI 0.79 to 0.98; random‐effects model; 22 studies; n = 497,258; Chi² P < 0.00001; I² = 89%; medium‐quality evidence on GRADE) (Analysis 5.1; Table 5). After studies with high risk of bias were removed, there was still some inconsistency across studies.
4.
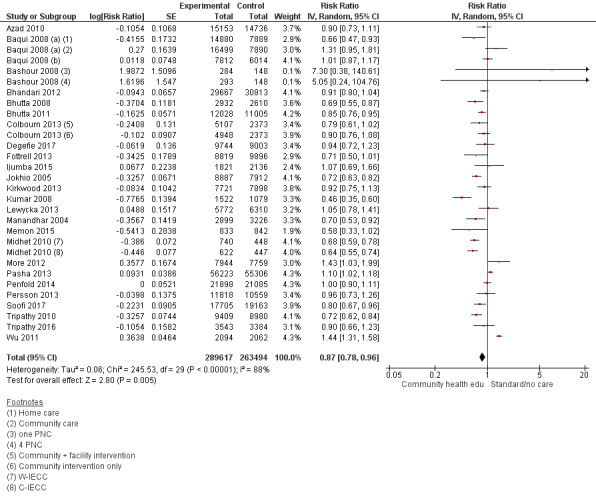
Forest plot of comparison: 1 Community health educational interventions versus control, outcome: 1.1 Neonatal mortality.
5.1. Analysis.
Comparison 5 Sensitivity analysis on primary outcomes, Outcome 1 Neonatal mortality.
On subgroup analysis, we found that studies that provided education on one‐to‐one contact had a non‐significant impact on neonatal mortality (RR 0.92, 95% CI 0.71 to 1.20; random‐effects model; 8 studies; n = 105,735; Chi² P < 0.00001; I² = 93%; very low‐quality evidence on GRADE). The quality of evidence was downgraded to very low due to concerns of risk of bias, inconsistency, and imprecision. Education through group counselling managed to reduce neonatal deaths by 17% (RR 0.83, 95% CI 0.74 to 0.92; random‐effects model; 12 studies; n = 211,164; Chi² P < 0.00001; I² = 93%; low‐quality evidence on GRADE); we considered the evidence to be of low quality due to concerns related to risk of bias and inconsistency. Studies that used both these methods during the trial period for each woman did not significantly reduce neonatal mortality (RR 0.90, 95% CI 0.76 to 1.06; random‐effects model; 6 studies; n = 236,212; Chi² P < 0.00001; I² = 84%; moderate‐quality evidence on GRADE) (Analysis 2.1; Figure 5; Table 2). This strategy was determined to have moderate‐quality evidence from six RCTs, and we downgraded it for inconsistency.
5.
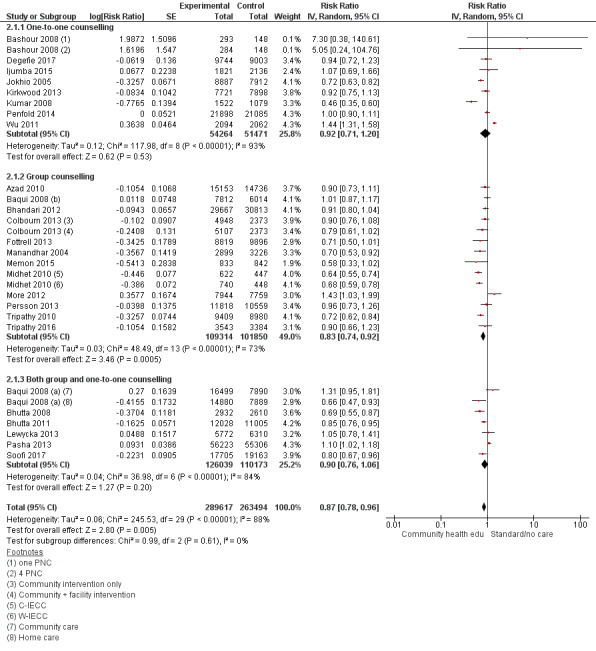
Forest plot of comparison: 2 Community health educational one‐to‐one and group and both counselling (subgroup) versus control, outcome: 2.1 Neonatal mortality.
Similarly, studies that delivered educational interventions during both antenatal and postnatal periods managed to reduce neonatal deaths by 15% (RR 0.85, 95% CI 0.76 to 0.96; random‐effects model; 20 studies; n = 332,380; Chi² = P < 0.0000; I² = 88%; very low‐quality evidence on GRADE) (Analysis 3.1; Figure 6; Table 3). However, this finding is of very low quality due to concerns of risk of bias and inconsistency. Education delivered during the antenatal period had no impact (RR 0.84, 95% CI 0.64 to 1.09; random‐effects model; 3 studies; n = 47,849; Chi² P < 0.0001; I² = 87%; very low‐quality evidence on GRADE), and those delivered during the postnatal period had no significant impact on neonatal mortality (RR 1.02, 95% CI 0.84 to 1.24; random‐effects model; 3 studies; n = 172,882; Chi² P = 0.03; I² = 66%; low‐quality evidence on GRADE). Evidence concerning these strategies and this outcome was downgraded due to risk of bias and inconsistency for the antenatal care period and inconsistency for interventions delivered during the postnatal period.
6.
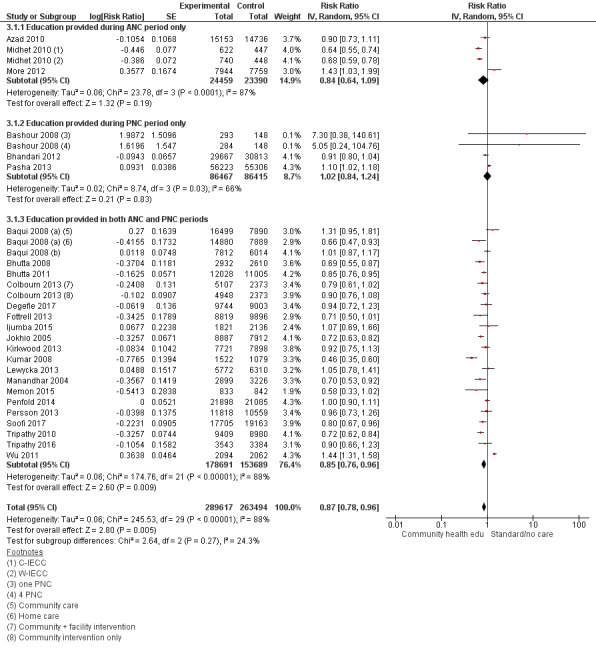
Forest plot of comparison: 3 Community health educational ANC period and PNC period and both periods (subgroup) versus control, outcome: 3.1 Neonatal mortality.
Education delivered to both family members and mothers managed to reduce neonatal mortality by 16% (RR 0.84, 95% CI 0.74 to 0.95; random‐effects model; 13 studies; n = 282,817; Chi² P < 0.00001; I² = 82%; low‐quality evidence on GRADE), and interventions provided only to mothers showed no impact on reducing neonatal mortality (RR 0.90, 95% CI 0.77 to 1.05; random‐effects model; 13 studies; n = 270,294; Chi² P < 0.00001; I² = 90%; very low‐quality evidence on GRADE) (Analysis 4.1; Figure 7; Table 4). Evidence was downgraded for interventions delivered to both family members and mothers due to concerns of risk of bias and inconsistency, and it was downgraded to very low quality for mothers only due to concerns of risk of bias, inconsistency, and imprecision.
7.
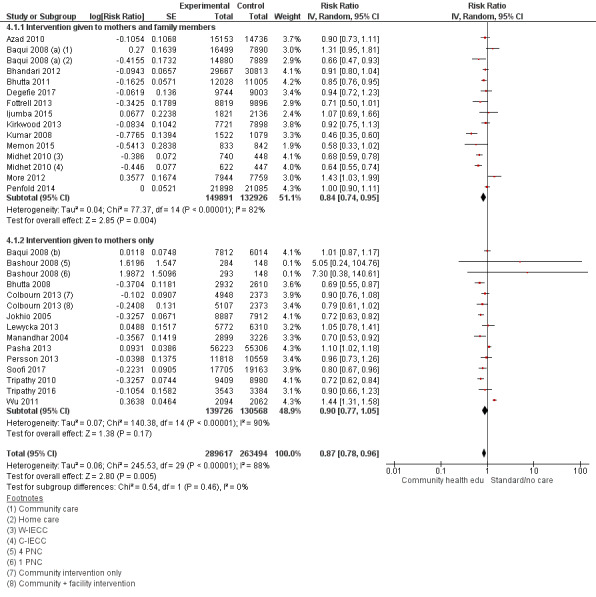
Forest plot of comparison: 4 Community health educational intervention for family members and mothers and for mothers only (subgroup) versus control, outcome: 4.1 Neonatal mortality.
Early and late neonatal mortality (outcomes 1.2, 2.2, 3.2, 4.2; 1.3, 2.3, 3.3, and 4.3)
Community health education interventions showed a significant impact on reducing early neonatal deaths by 26% (RR 0.74, 95% CI 0.66 to 0.84; random‐effects model; 15 studies that included 3 subsets from 3 studies; n = 321,588; Chi² P < 0.00001; I² = 86%; very low‐quality evidence on GRADE) (Analysis 1.2; Figure 8; Table 1). It was concluded that the evidence was of very low quality because of concerns for risk of bias, inconsistency, and imprecision. On sensitivity analysis, community health education interventions showed a significant impact on reducing early neonatal mortality by 29% (RR 0.71, 95% CI 0.62 to 0.82; random‐effects model; 11 studies; n = 264,72; Chi² P < 0.00001; I² = 87%; medium‐quality evidence on GRADE) (Analysis 5.2; Table 5). After studies with high risk of bias were removed, there was still some inconsistency across studies.
8.
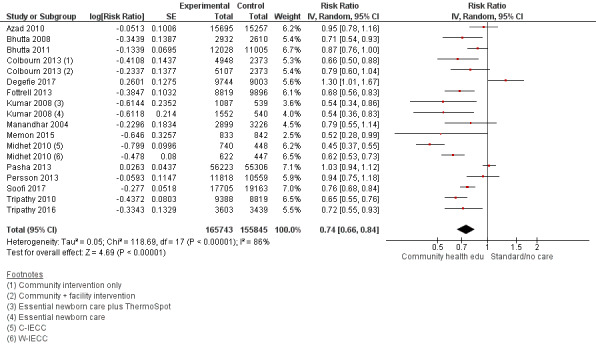
Forest plot of comparison: 1 Community health educational interventions versus control, outcome: 1.2 Early neonatal mortality.
5.2. Analysis.
Comparison 5 Sensitivity analysis on primary outcomes, Outcome 2 Early neonatal mortality.
Subgroup analysis showed that group counselling had the most significant effect on early neonatal mortality, with a reduction of 30% (RR 0.70, 95% CI 0.61 to 0.80; random‐effects model; 9 studies; n = 122,151; P < 0.0001; I² = 76%; low‐quality evidence on GRADE), which was considered to be of low quality due to risk of bias and inconsistency. Studies that used both group and one‐to‐one counselling reduced early neonatal mortality by 22% (RR 0.78, 95% CI 0.65 to 0.93; random‐effects model; 5 studies; n = 180,690; Chi² P < 0.00001; I² = 85%; moderate‐quality evidence on GRADE) (Analysis 2.2; Figure 9; Table 2), and this evidence was determined to be of moderate quality with some concern for inconsistency. Education delivered during the ANC period reduced early neonatal mortality by 36% (RR 0.64, 95% CI 0.43 to 0.95; random‐effects model; 2 studies; n = 33,209; Chi² P < 0.00001; I² = 93%; very low‐quality evidence on GRADE), and education delivered during both ANC and PNC periods reduced early neonatal mortality by 24% (RR 0.76, 95% CI 0.68 to 0.84; random‐effects model; 12 studies; n = 176,850; Chi² P = 0.0003; I² = 65%; very low‐quality evidence on GRADE) (Analysis 3.2; Figure 10; Table 3). However, evidence for interventions during the ANC period or both ANC and PNC periods was of very low quality. For ANC, this was due to risk of bias, inconsistency, and imprecision, whereas for both ANC and PNC, there were concerns about risk of bias and inconsistency. On subgroup analysis, we found that educational sessions that involved both mothers and family members had an impact on reducing early neonatal mortality by 30% (RR 0.70, 95% CI 0.56 to 0.87; random‐effects model; 7 studies; n = 99,097; Chi² P < 0.00001; I² = 88%; very low‐quality evidence on GRADE), whereas counselling involving only mothers reduced neonatal mortality by 22% (RR 0.78, 95% CI 0.68 to 0.90; random‐effects model; 8 studies; n = 222,491; Chi² P < 0.00001; I² = 81%; very low‐quality evidence on GRADE) (Analysis 4.2; Figure 11; Table 4). Both of these findings were based on very low‐quality evidence attributed to risk of bias, inconsistency, and imprecision.
9.
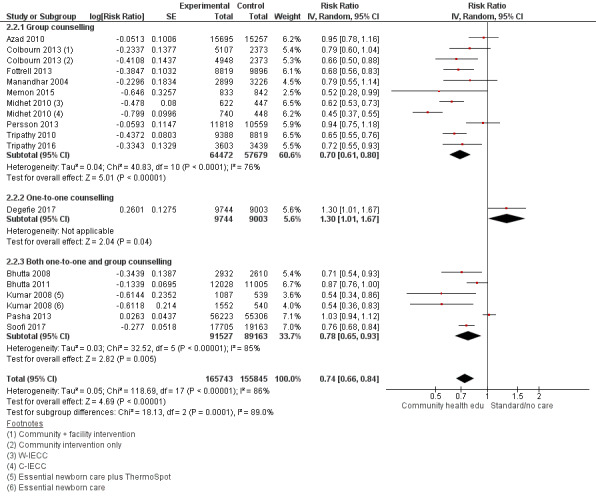
Forest plot of comparison: 2 Community health educational one‐to‐one and group and both counselling (subgroup) versus control, outcome: 2.2 Early neonatal mortality.
10.
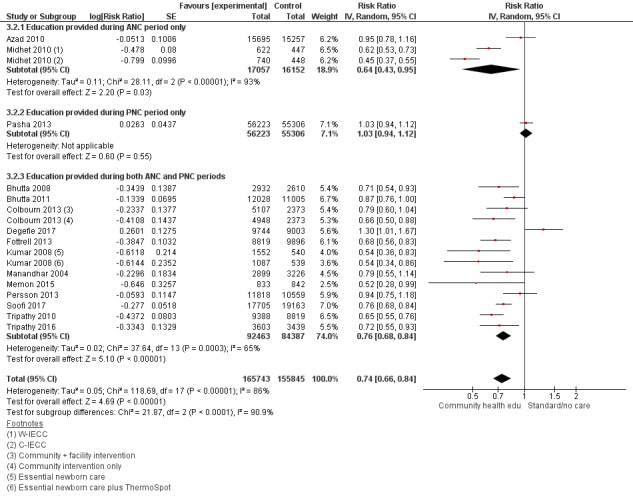
Forest plot of comparison: 3 Community health educational ANC period and PNC period and both periods (subgroup) versus control, outcome: 3.2 Early neonatal mortality.
11.
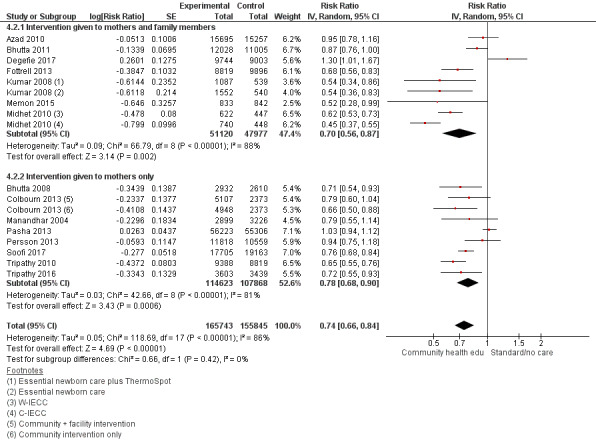
Forest plot of comparison: 4 Community health educational intervention for family members and mothers and for mothers only (subgroup) versus control, outcome: 4.2 Early neonatal mortality.
Community health education interventions showed a significant impact on reducing late neonatal mortality by 46% (RR 0.54, 95% CI 0.40 to 0.74; random‐effects model; 11 studies; n = 186,643; Chi² P < 0.00001; I² = 88%; very low‐quality evidence) (Analysis 1.3; Figure 12; Table 1), and it was determined that the evidence was of very low quality due to risk of bias, inconsistency, and imprecision. On sensitivity analysis, community health education interventions showed a significant impact on reducing late neonatal mortality by 49% (RR 0.51, 95% CI 0.36 to 0.72; random‐effects model; 9 studies; n = 150,876; Chi² P < 0.00001; I² = 89%; medium‐quality evidence on GRADE) (Analysis 5.3; Table 5). After studies with high risk of bias were removed, there was still some inconsistency across studies.
12.
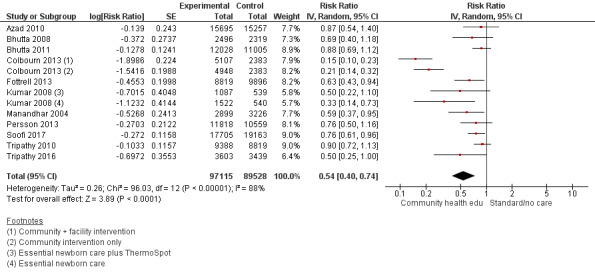
Forest plot of comparison: 1 Community health educational interventions versus control, outcome: 1.3 Late neonatal mortality.
5.3. Analysis.
Comparison 5 Sensitivity analysis on primary outcomes, Outcome 3 Late neonatal mortality.
Subgroup analysis showed that educational interventions delivered by group counselling significantly reduced late neonatal mortality by 50% (RR 0.50, 95% CI 0.31 to 0.81; random‐effects model; 7 studies; n = 118,239; Chi² P < 0.00001; I² = 91%; very low‐quality evidence on GRADE), although the evidence was of very low quality due to concerns of risk of bias, inconsistency, and imprecision. The combination of group and one‐to‐one counselling reduced neonatal mortality by 28% (RR 0.72, 95% CI 0.57 to 0.91; random‐effects model; 4 studies; n = 68,404; Chi² P = 0.15; I² = 41%; moderate‐quality evidence on GRADE) (Analysis 2.3; Figure 13; Table 2); this finding was based on moderate‐quality evidence, which was downgraded due to imprecision. Educational interventions delivered during both ANC and PNC periods had the most significant impact on reducing late neonatal mortality by 58% (RR 0.52, 95% CI 0.38 to 0.72; random‐effects model; 10 studies; n = 155,691; Chi² P < 0.00001; I² = 88%; very low‐quality evidence on GRADE), whereas education provided during only ANC showed no impact (RR 0.87, 95% CI 0.54 to 1.40; random‐effects model; 1 study; n = 30,952; very low‐quality evidence on GRADE) (Analysis 3.3; Figure 14; Table 3). Both interventions delivered during both ANC and PNC, and solely in ANC, yielded evidence that was of very low quality due to risk of bias, inconsistency, and imprecision, and large concerns for risk of bias, respectively. Educational sessions that involved both mothers and family members and only mothers had a significant impact on reducing late neonatal mortality by 31% (RR 0.69, 95% CI 0.51 to 0.92; random‐effects model; 4 studies; n = 76,388; Chi² P = 0.10; I² = 49%; low‐quality evidence on GRADE) and by 50% (RR 0.50, 95% CI 0.31 to 0.78; random‐effects model; 7 studies; n = 110,255; Chi² P < 0.00001; I² = 92%; very low‐quality evidence on GRADE), respectively (Analysis 4.3; Figure 15; Table 4). The evidence was considered to be of low and very low quality due to risk of bias, inconsistency, and imprecision.
13.
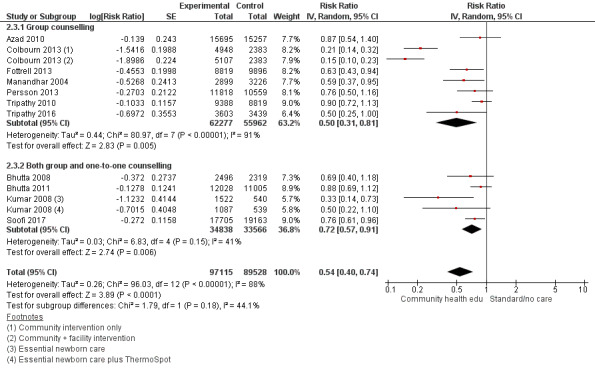
Forest plot of comparison: 2 Community health educational one‐to‐one and group and both counselling (subgroup) versus control, outcome: 2.3 Late neonatal mortality.
14.
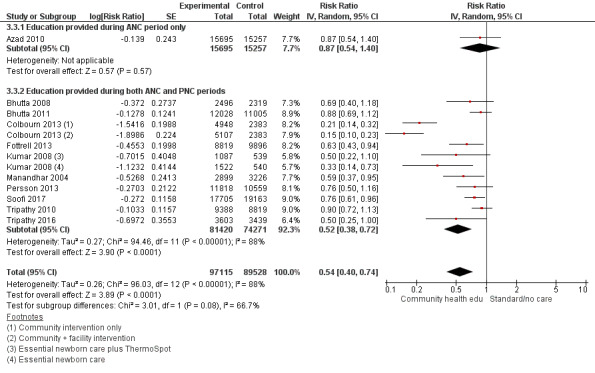
Forest plot of comparison: 3 Community health educational ANC period and PNC period and both periods (subgroup) versus control, outcome: 3.3 Late neonatal mortality.
15.
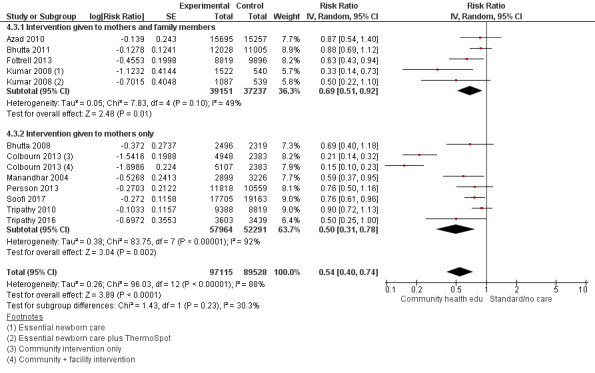
Forest plot of comparison: 4 Community health educational intervention for family members and mothers and for mothers only (subgroup) versus control, outcome: 4.3 Late neonatal mortality.
Perinatal mortality (outcomes 1.4, 2.4, 3.4, and 4.4)
Community health education interventions showed a significant impact on reducing perinatal mortality by 17% (RR 0.83, 95% CI 0.75 to 0.91; random‐effects model; 15 studies; n = 262,613; Chi² P < 0.00001; I² = 81%; very low‐quality evidence on GRADE) (Analysis 1.4; Figure 16; Table 1). This evidence was downgraded to very low quality due to strong concerns for inconsistency and imprecision. On sensitivity analysis, community health education interventions showed a significant impact on reducing perinatal neonatal mortality by 16% (RR 0.84, 95% CI 0.75 to 0.94; random‐effects model; 12 studies; n = 224,107; Chi² P < 0.00001; I² = 83%; medium‐quality evidence on GRADE) (Analysis 5.4; Table 5). After studies with high risk of bias were removed, there was still some inconsistency across studies.
16.
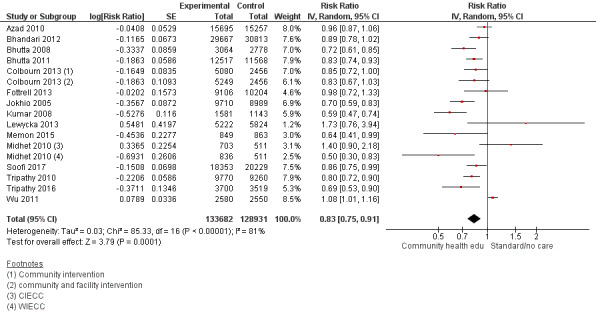
Forest plot of comparison: 1 Community health educational interventions versus control, outcome: 1.4 Perinatal mortality.
5.4. Analysis.
Comparison 5 Sensitivity analysis on primary outcomes, Outcome 4 Perinatal mortality.
We conducted a subgroup analysis on the mode of educational strategy. Studies that provided education in the form of group meetings had a significant impact on perinatal death reduction by 15% (RR 0.85, 95% CI 0.77 to 0.94; random‐effects model; 8 studies; n = 156,505; Chi² P = 0.02; I² = 55%; low‐quality evidence on GRADE), whereas educational interventions delivered via one‐to‐one counselling had no impact (RR 0.88, 95% CI 0.57 to 1.34; random‐effects model; 2 studies; n = 23,829; Chi² P < 0.00001; I² = 95%; very low‐quality evidence on GRADE), and those delivered by both group and one‐to‐one counselling yielded perinatal mortality reduction by 22% (RR 0.78, 95% CI 0.67 to 0.90; random‐effects model; 5 studies; n = 82,279; Chi² P = 0.010; I² = 70%; low‐quality evidence on GRADE) (Analysis 2.4; Figure 17; Table 2). The evidence for these strategies was determined to be of low or very low quality, which was attributed to concerns surrounding risk of bias, inconsistency, and imprecision.
17.
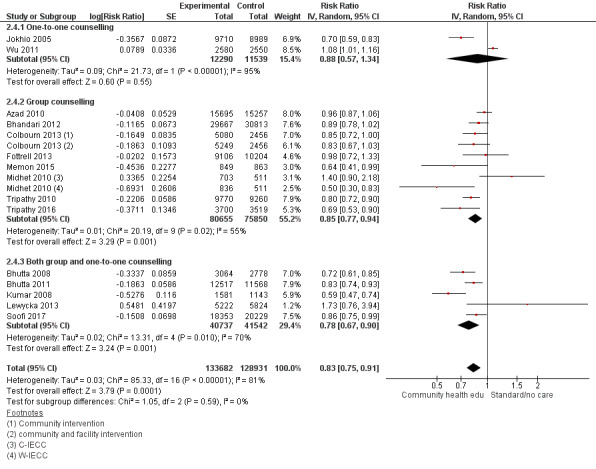
Forest plot of comparison: 2 Community health educational one‐to‐one and group and both counselling (subgroup) versus control, outcome: 2.4 Perinatal mortality.
On subgroup analysis, we found that studies that delivered educational intervention during the ANC period had no significant impact on reducing perinatal deaths (RR 0.90, 95% CI 0.59 to 1.39; random‐effects model; 1 study and 1 study with 2 subsets; n = 33,513; Chi² P = 0.01; I² = 78%; very low‐quality evidence on GRADE), and those that delivered interventions during both ANC and PNC periods managed to reduce 19% of the perinatal mortality (RR 0.81, 95% CI 0.72 to 0.91; random‐effects model; 12 studies; n = 168,620; Chi² P < 0.00001; I² = 84%; low‐quality evidence on GRADE) (Analysis 3.4; Figure 18; Table 3). The quality of evidence was very low and low due to concerns of risk of bias and inconsistency for ANC, and inconsistency for interventions delivered in both ANC and PNC.
18.
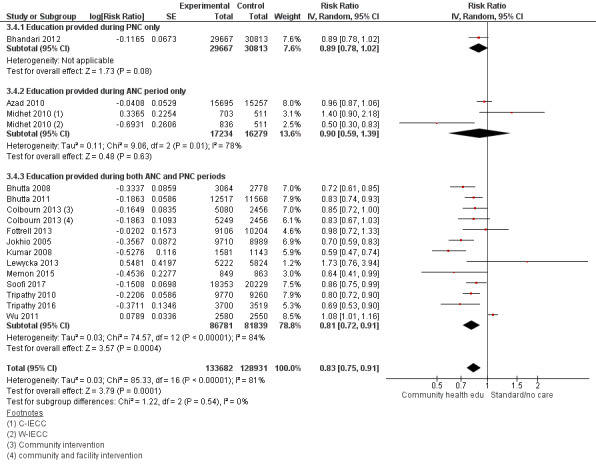
Forest plot of comparison: 3 Community health educational ANC period and PNC period and both periods (subgroup) versus control, outcome: 3.4 Perinatal mortality.
Another subgroup analysis was performed to determine the impact of recipients receiving the intervention (family and mothers vs only mothers) on perinatal mortality, with a significant reduction of 17% (RR 0.83, 95% CI 0.72 to 0.96; random‐effects model; 7 studies; n = 141,824; Chi² P = 0.0004; I² = 74%; low‐quality evidence on GRADE; and RR 0.83, 95% CI 0.72 to 0.96; random‐effects model; 8 studies; n = 120,789; Chi² P < 0.00001; I² = 85%; low‐quality evidence on GRADE), respectively (Analysis 4.4; Figure 19; Table 4). Both strategies were considered to be of low quality due to concerns with both risk of bias and inconsistency.
19.
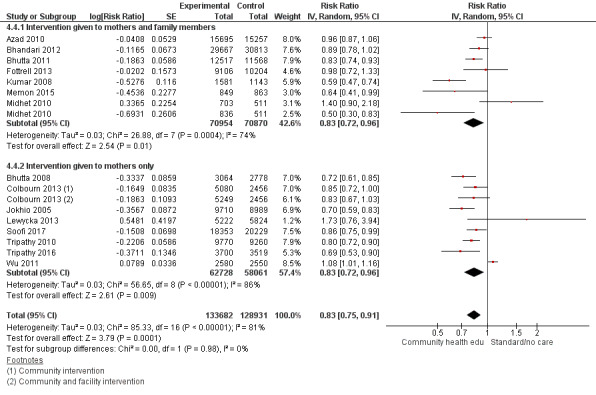
Forest plot of comparison: 4 Community health educational intervention for family members and mothers and for mothers only (subgroup) versus control, outcome: 4.4 Perinatal mortality.
Secondary outcomes
Any antenatal care, use of contraceptives, use of clean delivery kits (outcomes 1.6, 2.5, 4.5; 1.7; and 1.10)
Community health educational interventions increased utilisation of any antenatal care in pregnant women by 16% (RR 1.16, 95% CI 1.11 to 1.22; random‐effects model; 18 studies; n = 307,528; I² = 96%; Chi² P < 0.00001) (Analysis 1.6; Figure 20). Subgroup analysis comparing one‐to‐one versus group counselling showed a non‐significant impact when implemented separately; however when mothers received both one‐to‐one and group counselling, utilisation of any ANC increased by 21% (RR 1.21, 95% CI 1.07 to 1.37; random‐effects model; 5 studies; n = 51,352; I² = 97%; Chi² P < 0.00001) (Analysis 2.5; Figure 21). On subgroup analysis, no improvement was demonstrated for education provided during ANC (RR 0.99, 95% CI 0.98 to 1.00; random‐effects model; 2 studies; n = 51,305; I² = 0%; Chi² P = 0.38). Subgroup analysis comparing the recipient of education (family members and mothers vs only mothers) showed a significant impact for both methods of 20% (RR 1.20, 95% CI 1.06 to 1.36; random‐effects model; 9 studies; n = 102,886; I² = 98%; Chi² P < 0.00001) and 9% (RR 1.09, 95% CI 1.02 to 1.17; random‐effects model; 9 studies; n = 96,042; I² = 93%; Chi² P < 0.00001), respectively (Analysis 4.5; Figure 22).
Community health educational interventions had no impact on the use of contraceptives (RR 1.10, 95% CI 0.86 to 1.41; random‐effects model; 3 studies; n = 22,237; I² = 80%; Chi² P = 0.0004) (Analysis 1.7; Figure 23); no subgroup analysis was possible. Community health education interventions also did not impact the utilisation of clean delivery kits (RR 4.44, 95% CI 0.71 to 27.76; random‐effects model; 2 studies; n = 17,087; I² = 98%; Chi² P < 0.00001) (Analysis 1.10; Figure 24); no subgroup analysis was possible.
Birth attendance at delivery (outcomes 1.8, 1.9, 2.6, 2.7, and 4.6)
Community health educational interventions did not have any impact on utilisation of skilled birth attendance at delivery (RR 1.09, 95% CI 0.94 to 1.25; random‐effects model; 10 studies; n = 117,870; I² = 97%; Chi² P = 0.00001) (Analysis 1.8; Figure 25), nor did the intervention have an impact on delivery attended by an unskilled or semi‐skilled birth attendant (RR 1.02, 95% CI 0.70 to 1.49; random‐effects model; 3 studies; n = 40,456; I² = 50%; Chi² P = 0.13) (Analysis 1.9; Figure 26). When we performed a subgroup analysis, we found that education received through one‐to‐one counselling, group counselling, or both one‐to‐one and group counselling showed a non‐significant increase for the use of a skilled attendant at delivery (RR 1.09, 95% CI 0.94 to 1.25; random‐effects model; 10 studies; n = 117,870; I² = 97%; Chi² P < 0.00001) (Analysis 2.6; Figure 27). Educational interventions delivered in a group counselling setting and by a combination of one‐to‐one and group counselling did not demonstrate an impact on delivery attended by an unskilled or semi‐skilled birth attendant (RR 1.18, 95% CI 0.64 to 2.19; random‐effects model; 2 studies; n = 21,333; I² = 64%; Chi² P = 0.10; and RR 0.85, 95% CI 0.59 to 1.22; random‐effects model; 1 study; n = 19,123) (Analysis 2.7; Figure 28).
Whether the educational intervention was provided to family members and mothers or only to mothers showed a non‐significant increase for the utilisation of a skilled birth attendant at delivery (RR 1.05, 95% CI 0.93 to 1.18; random‐effects model; 4 studies; n = 58,584; I² = 33%; Chi² P = 0.22; and RR 1.11, 95% CI 0.92 to 1.34; random‐effects model; 6 studies; n = 59,286; I² = 98%; Chi² P < 0.00001), respectively (Analysis 4.6; Figure 29).
Neonatal health care‐seeking (outcomes 1.11, 2.8, 3.5, and 4.7)
Community health interventions had no impact on care‐seeking for neonatal illness (RR 1.11, 95% CI 0.97 to 1.27; random‐effects model; 7 studies; n = 46,154; I² = 93%; Chi² P < 0.00001) (Analysis 1.11; Figure 30). The three subgroup analyses performed (timing of intervention, counselling type, and recipient of intervention) did not show any significant impact of the health education intervention on care‐seeking for neonatal illness (Analysis 2.8; Analysis 3.5; Analysis 4.7; Figure 31; Figure 32; Figure 33).
Neonatal infections (outcome 1.5)
Community health educational interventions demonstrated no impact for reducing the amount of neonatal infection by 12% (RR 0.88, 95% CI 0.72 to 1.08; random‐effects model; 2 studies; n = 42,043; I² = 0%, Chi ² P = 0.46) (Analysis 1.5; Figure 34). No subgroup analysis was performed for this outcome.
Colostrum administration and timely initiation of breastfeeding (outcomes 1.12, 1.13, 2.9, 3.6, and 4.9)
Community health educational interventions managed to increase colostrum administration by 16% (RR 1.16, 95% CI 0.83 to 1.61; random‐effects model; 5 studies; n = 28,631; I² = 100%; Chi² P < 0.00001) (Analysis 1.12; Figure 35). When the educational intervention was received by both family members and mothers, there was a significant increase in colostrum administration by 34% (RR 1.34, 95% CI 1.26 to 1.43; random‐effects model; 2 studies; n = 28,631; I² = 44%; Chi² P = 0.18). The intervention did not show an impact of colostrum administration for the intervention received by only mothers (RR 1.06, 95% CI 0.57 to 1.98; random‐effects model; 3 studies; n = 23,534; I² = 100%; P < 0.00001) (Analysis 4.8; Figure 36).
Timely initiation of breastfeeding was improved by 56% (RR 1.56, 95% CI 1.37 to 1.77; random‐effects model; 19 studies; n = 126,375; I² = 99%, Chi² P < 0.00001) (Analysis 1.13; Figure 20). Subgroup analysis based on mode of delivery of education showed that one‐to‐one and combined one‐to‐one and group counselling increased the timely initiation of breastfeeding by 16% (RR 1.16, 95% CI 1.01 to 1.32; random‐effects model; 5 studies; n = 23,927; I² = 96%; Chi² P < 0.00001) and 63% (RR 1.63, 95% CI 1.39 to 1.92; random‐effects model; 6 studies; n = 44,437; I² = 99%; Chi² P < 0.00001), respectively. The mode of delivery that had the greatest impact was group counselling, with an 80% increase for timely initiation of breastfeeding (RR 1.80, 95% CI 1.25 to 2.58; random‐effects model; 8 studies; n = 58,011; I² = 100%; P < 0.00001) (Analysis 2.9; Figure 21). On subgroup analysis, we found that promotion given during the PNC period increased the timely initiation of breastfeeding three‐fold (RR 3.64, 95% CI 3.38 to 3.93; random‐effects model; 1 study; n = 12,367), whereas trials that promoted breastfeeding education in both ANC and PNC periods managed to increase rates by 47% (RR 1.47, 95% CI 1.32 to 1.65; random‐effects model; 18 studies; n = 114,008; I² = 99%; Chi² P < 0.00001) (Analysis 3.6; Figure 22). The subgroup analysis for recipient(s) of the intervention for family members and mothers and for only mothers showed an improvement in timely initiation of breastfeeding of 56% for both groups (Analysis 4.9; Figure 23).
20.
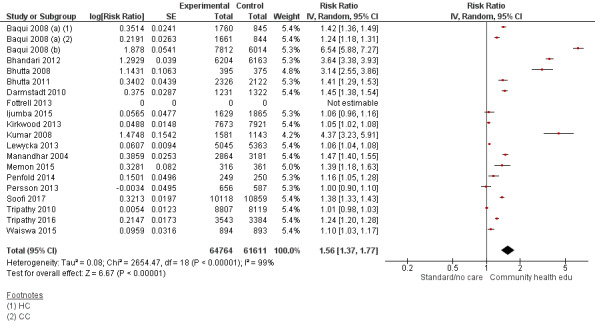
Forest plot of comparison: 1 Community health educational interventions versus control, outcome: 1.13 Timely initiation of breastfeeding.
21.
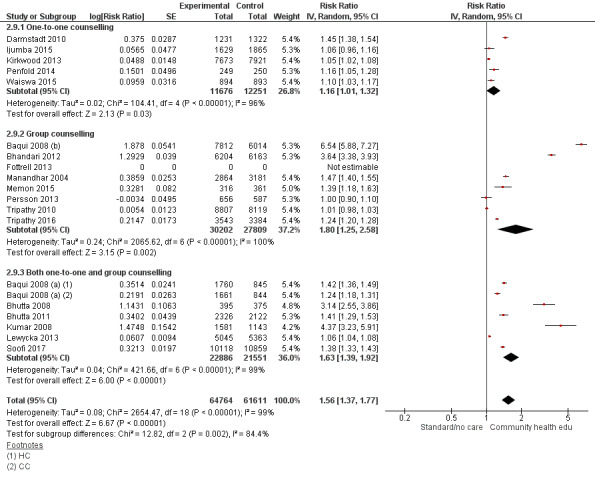
Forest plot of comparison: 2 Community health educational one‐to‐one and group and both counselling (subgroup) versus control, outcome: 2.9 Timely initiation of breastfeeding.
22.
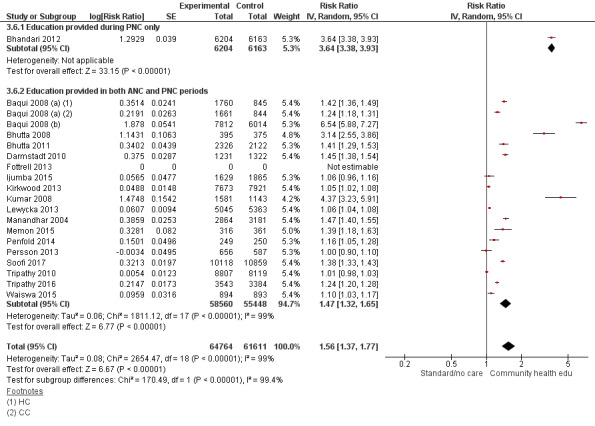
Forest plot of comparison: 3 Community health educational ANC period and PNC period and both periods (subgroup) versus control, outcome: 3.6 Timely initiation of breastfeeding.
23.
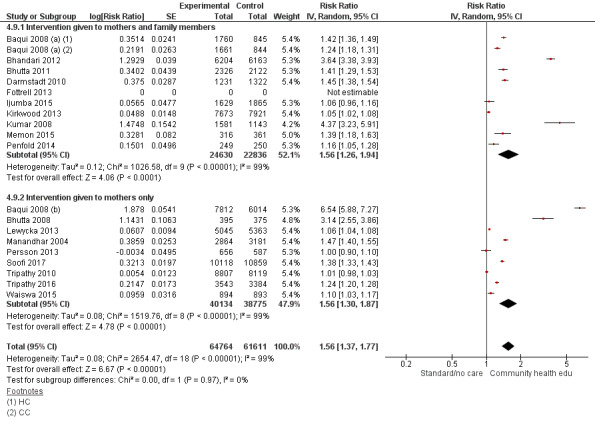
Forest plot of comparison: 4 Community health educational intervention for family members and mothers and for mothers only (subgroup) versus control, outcome: 4.9 Timely initiation of breastfeeding.
Infection‐related neonatal mortality
We found no studies in which association of community health educational interventions was observed for infection‐related neonatal mortality.
Mothers' understanding on "healthy" behaviours
We found no studies that reported mothers' understanding of neonatal danger signs.
Costs of interventions and one life saved
Cost data were reported in seven studies. In Manandhar 2004, cost‐effectiveness analysis was done, and the cost per newborn life saved was USD 3442 (USD 4397 including health‐service strengthening costs) and per life‐year saved was USD 111 (USD 142 including health‐service strengthening costs). The cost per neonatal death averted in Baqui 2008 (a) was USD 2995, including health‐systems strengthening costs. In Tripathy 2010, the incremental cost of the women's group intervention was USD 910 per newborn life saved, increasing to USD 1308 (in 2007 prices) when health‐service strengthening activities were included, and the incremental cost per life‐year saved was USD 33 for the women's group intervention (USD 48 inclusive of health‐service strengthening activities). In Fottrell 2013, the prospective cost per newborn death averted was USD 11974, and the cost per year of life lost averted was USD 394. The cost per year of life lost averted in Lewycka 2013 was USD 144 for women's groups and USD 33 for peer counsellors. Tripathy 2016 reported that the incremental cost per newborn death averted was USD 2545, and for averted disability‐adjusted life‐year (DALY), the cost of the intervention was USD 83. In Colbourn 2013, cost‐effectiveness analysis showed that the costs of community intervention, facility intervention, and combined community and facility interventions were $79, $281, and $146 per DALY averted, respectively.
Discussion
Summary of main results
Evidence from 33 studies contributing data to the primary outcomes of this review shows that intervention packages with community health educational components targeting women and their families provided both antenatally and postnatally reduced neonatal mortality (both early and late) and perinatal mortality, and improved several health behaviours, such as using antenatal care. The impact on contraceptive use and on use of clean delivery kits was less certain, and no studies reported on infection‐related neonatal mortality or mothers' understanding of "healthy" behaviours. The cluster‐randomised and quasi‐randomised controlled trials included in this systematic review provide convincing evidence of the effectiveness of community health educational interventions, particularly those that included both one‐to‐one and group counselling as a method of imparting knowledge and awareness for reducing neonatal and perinatal mortalities. These educational interventions also promoted better utilisation of antenatal care and timely initiation of breastfeeding.
We found a paucity of eligible studies that implemented interventions (generally as birth preparedness and antenatal care (ANC) and postnatal care (PNC) with emphasis on management of neonatal illness). Our meta‐analysis showed a significant reduction in neonatal mortality (13%) and perinatal mortality (17%), as well as a significant reduction in early (26%) and late neonatal mortality (46%), observed as a consequence of implementation of educational interventions. We noted a significant increase in use of any antenatal care (16%), use of colostrum (16%), and timely initiation of breastfeeding (56%).
Subgroup analysis investigating the most appropriate delivery method ‐ one‐to‐one counselling, group counselling, or both ‐ showed that when the educational strategy utilised only group counselling, neonatal mortality was reduced by 17%. Subgroup analysis also demonstrated that group counselling was the most effective method for reducing both early and late neonatal mortality, with reductions of 30% and 50% observed. It was also effective in reducing perinatal mortality by 15%.
In our subgroup analysis, we found that community health education interventions that focused on and promoted awareness related to neonatal health and birth preparedness based on both the antenatal period and the postnatal period reduced neonatal mortality by 15%. For reducing early neonatal mortality, educational interventions provided during the ANC period were most effective (36%), whereas late neonatal mortality interventions provided during both ANC and PNC were most effective (58%). The greatest reductions in perinatal mortality were observed when education was provided during both antenatal and postnatal periods (19%) as opposed to only the antenatal period, which had no significant effect. It was also observed that there were greater reductions in neonatal mortality when mothers were provided with educational interventions during both antenatal and postnatal periods (15%) as opposed to antenatal and postnatal periods separately, as both yielded a non‐significant impact. These results highlight the importance of receiving quality care during and after pregnancy and its impact on neonatal outcomes, and they suggest that the most effective educational interventions should be delivered in both the antenatal period and the postnatal period for the greatest reductions in neonatal and perinatal mortality.
The final subgroup analysis investigated whether the type of recipient who received the intervention ‐ mother, or both mother and family members ‐ impacted the outcomes. It was shown that educational interventions were most effective for reducing neonatal mortality (16%) when received by both mothers and family members. This was also demonstrated for early neonatal mortality with a reduction of 30%, late neonatal mortality with a reduction of 31%, and perinatal mortality with a reduction of 17% when education was provided to both mothers and family members.
Overall completeness and applicability of evidence
Notably, most of the reviewed studies when implemented neglected to document the complete description and characteristics of educators deployed, especially the level and amount of training provided, which could have helped in identifying the importance of these factors and their association with study outcomes. This information would be of great relevance, and additional information on the initial level of educators, their mode of training, and the balance of practical or theoretical sessions would have provided greater assistance to those seeking to understand effects of these factors on educators' performance in community settings. Although the crude impact of educational interventions can be interpreted further, information on contextual factors of educators would have provided the underpinnings needed to recommend the most effective type of training for successful programmes.
It is important to understand that most of the interventions studied in this review were provided in the form of 'packages', and it is therefore difficult to establish the temporality of intervention with outcomes. This means that protective effects cannot be attributed to an individual component of the intervention. For example, antibiotics for neonatal sepsis can be one component of an intervention package delivered by community health workers that has the potential to save lives; however, other components of the package, such as neonatal resuscitation, would have similar effects. Another indicator of expected differences in protective effects from different intervention packages was significant statistical heterogeneity in the pooled data. Statistical heterogeneity depends on difference in magnitude (small or large) and direction of effect (favouring and against). It is, therefore, important to interpret these results carefully.
The types of eligible participants for this review included (among others) pregnant women at any period of gestation and mothers of neonates up to 28 days of life. For the outcome 'neonatal mortality' among all births during the 'trial period', data may include deaths that could have occurred before the educational intervention was administered. These deaths cannot be interpreted as reflecting the effect of the intervention, which is a potential limitation of this intervention type to be studied.
Quality of the evidence
Results were analysed using a random‐effects model due to high heterogeneity between studies; the quality of evidence for neonatal mortality was downgraded by two levels to low quality due to high risk of bias and moderate inconsistency between studies (Table 1). For remaining outcomes (early, late, and perinatal mortality), the quality of evidence for community education interventions was downgraded to very low. Evidence for these outcomes was downgraded due to concerns surrounding risk of bias, inconsistency, and imprecision of included randomised controlled trials. Given the low and very low quality of evidence, the true effect of community‐based education on neonatal mortality (early, late, and perinatal) may be substantially different from the estimate of effect.
In the subgroup analysis for timing of educational interventions, evidence ranged from very low to high quality (Table 3). Delivery of education during the postnatal period yielded high‐quality evidence for reducing perinatal mortality; however this GRADE assessment was based on only one study (Bhandari 2012). This is also the case for education provided during the postnatal period, which reduced early neonatal mortality; the GRADE assessment resulted in a moderate‐quality rating, but this finding was based on the results of one study (Pasha 2013).
In the subgroup analysis examining the method of community educational interventions (one‐on‐one vs group counselling), evidence was of very low to moderate quality (Table 2). Evidence was downgraded due to high risk of bias, inconsistency of results across studies, and imprecision. For the final subgroup analysis examining recipients of the intervention (family and mother vs only mother), evidence was of very low or low quality (Table 4).
It should be noted that for all analyses, none of the evidence met GRADE criteria higher than moderate quality, besides the GRADE assessment for one study that made up the subgroup analysis of interventions during the postnatal period for perinatal mortality (Bhandari 2012), due to concerns related to risk of bias, inconsistency, or imprecision.
Potential biases in the review process
We undertook a systematic, thorough search of the literature to identify all studies that met the inclusion criteria for this review. We are confident that all trials that met the inclusion criteria were included in this review. Study selection and data extraction were done in duplicate and independently, and we reached consensus by discussing any discrepancies. A protocol was published for this review. However, it is important to acknowledge that the studies included in this review included education as one component of a package of interventions; therefore it is possible that the benefits of these interventions are attributed to other non‐educational components.
Agreements and disagreements with other studies or reviews
One systematic review ‐ Bryanton 2010 ‐ was conducted to assess the effects of structured postnatal education delivered by an educator on infant general health and parent‐infant relationships. Review authors concluded that the benefits of educational programmes for participants and their newborns remain unclear, whereas our review showed a significant decrease in neonatal and perinatal mortality with further decreases in early and late neonatal mortality observed, as well as improvement in other neonatal health outcomes with educational interventions. However, the studies included in our review included educational interventions as a component of a package of interventions; therefore this disagreement may be attributed to other components of the packages included in this review. No previous reviews have examined the impact of packages of interventions with an educational component.
Authors' conclusions
Implications for practice.
We believe that our review offers some encouraging evidence of the value of community health educational interventions in the form of a package of interventions for a significant decrease in early and late neonatal mortality. Providing educational intervention in both the antenatal and postnatal periods can reduce overall neonatal mortality and perinatal mortality, as well as late neonatal mortality, whereas educational interventions delivered during the antenatal period were most effective for reducing early neonatal mortality. Our review also provides evidence that an educational strategy that includes group counselling for participants was the most successful intervention for reducing neonatal mortality and promoting early initiation of breastfeeding. However, because educational interventions were part of a package of interventions, it is difficult to determine if purely educational interventions would be just as effective; this is an area that requires further investigation, which is a limitation of this review. It is also important to note that the quality of evidence for these findings was frequently of low or very low quality due to concerns of risk of bias, inconsistency, and imprecision. Therefore these findings need to be interpreted critically, and there is not sufficient evidence to determine which strategy (e.g. delivered during ANC or PNC or both) performed in these educational packages is most effective. Results from seven trials awaiting classification will contribute to the evidence base for this intervention in the future.
Implications for research.
Despite these findings, this analysis largely derives from trials that were conducted mainly in Asia and Africa, with limited evidence from Central and South America. The interventions utilised a combination of strategies, including promotion of routine antenatal care, counselling for iron folic acid supplementation, and ensuring awareness of mothers and access to appropriate health services throughout pregnancy, as well as home visits to provide education and support from a range of community health workers during and following pregnancy. A broad range of educators were utilised across the trials; most studies utilised community health workers or lady health workers, and educators were frequently working on a volunteer basis, with some trials offering incentives in the form of certificates and merchandise. There is thus a clear need for additional research at an appropriate scale and in the correct settings. Also needed are high‐quality randomised controlled trials that employ stringent methods to ensure quality. Although assessment of cost‐effectiveness was one of the objectives of this review, we found paucity of such data in our included trials and only seven studies that reported the actual cost incurred for providing interventions for saving one life, or for one disability‐adjusted life‐year (DALY) averted. Therefore, cost‐effectiveness is a priority area for research in the future, and researchers should facilitate a cost‐effectiveness meta‐analysis by collecting and reporting cost‐effectiveness data in a standardised format and by specifying which components of the intervention were most cost‐effective when provided in the form of packages. As previously mentioned, the major limitation of this review is that the educational intervention provided by most studies was part of a package of interventions; therefore interventions that are purely education based are required, to determine the true effect of community health education on maternal and neonatal outcomes.
What's new
| Date | Event | Description |
|---|---|---|
| 7 January 2019 | New search has been performed | Submitted for an editorial review |
Acknowledgements
We would like to acknowledge the contributions of Durrane Thaver, Aatekah Owais, Batool A Haider, and Dr Anita KM Zaidi, who helped in writing the protocol of this review.
The following members of the Cochrane Crowd helped screen results of the search to remove non‐RCTs and to identify RCTs: Ali Tafazoli, Hariklia Nguyen, RiccardoGuarise, Therese Dalsbø, Nikhil Sisodiya, Sydney Rebello, Fraser Old, Kit Byatt, Karen Ma, Silvia Jin, Fakher Rahim, Tunde Odugbemi, Erik Küng, Marta Rossignoli, Stefanie Rosumeck, JamesThomas, Ambrish Singh, Linda Hart, Anita Chasiotis, Tomislav Meštrović, Esther Pozzani, Jennifer Cartwright, Soon Eu Chong, Jo Thompson Coon, Beatrice Bouton, Ghaleb Mihyar, Naomi Julien, Louise Murphy, Jolene Carioni, Auxiliadora Fraiz Padin, Veincent Pepito, Kerstin Rolfe, Mersiha Mahmic‐Kaknjo, Luigi Ronga, Vanisree Staniforth, Aladdin Alshawa, Dorothy Halfhide, Dr. Sanjay Singh, Rocío Ayala Romero, Bernardo Costa, Alex Freeman, Mana Golesorkhi, Suzanne Duffin, Iris Hinneburg, Nikki Edworthy, Elena Lantsova, and Deirdre Beecher.
The methods section of this protocol is based on a standard template used by Cochrane Neonatal.
Appendices
Appendix 1. 2017 Search methods
PubMed: (Intervention* OR Package* OR Promotion* OR Participate* OR Support* OR Group* OR Discussion* OR Education* OR Worker* OR Services OR Program* OR Improve* OR Lower* OR Reduce* OR Utilization OR Health education[MeSH] OR Community Health Services[MeSH] OR Counseling[MeSH] OR Community Health Workers[MeSH]) AND (Basic health unit OR Community* OR Community‐based OR Domiciliary OR Developing OR Facility OR Home OR home‐based OR Peripheral OR Poor OR Rural OR Underdeveloped OR Unit* OR Village* OR Rural population[MeSH] OR Developing countries[MeSH]) AND ((infant, newborn[MeSH] OR newborn OR neonate OR neonatal OR infan* or neonat* OR Birth OR Mother OR Maternal OR Pregnancy* OR Antenatal OR Prenatal OR Postnatal OR Periconcept* Parturition[MeSH] OR Mothers[MeSH] OR Pregnant Women[MeSH] OR Pregnancy[MeSH) AND (randomised controlled trial [pt] OR controlled clinical trial [pt] OR randomised [tiab] OR placebo [tiab] OR randomly [tiab] OR trial [tiab]) NOT (animals [mh] NOT humans [mh])) CINAHL: (Intervention* OR Package* OR Promotion* OR Participate* OR Support* OR Group* OR Discussion* OR Education* OR Worker* OR Services OR Program* OR Improve* OR Lower* OR Reduce* OR Utilization OR Health education OR Community Health OR Counseling) AND (Basic health unit OR Community* OR Community‐based OR Domiciliary OR Developing OR Facility OR Home OR home‐based OR Peripheral OR Poor OR Rural OR Underdeveloped OR Unit* OR Village*) AND (infant, newborn OR newborn OR neonate OR neonatal OR birth OR mother OR maternal OR pregnancy OR prenatal OR postnatal OR periconcept* OR parturition OR Newborn or infan* or neonat*) AND (randomised controlled trial OR controlled clinical trial OR randomised OR placebo OR clinical trials as topic OR randomly OR trial OR PT clinical trial) Embase: (Intervention* OR Package* OR Promotion* OR Participate* OR Support* OR Group* OR Discussion* OR Education* OR Worker* OR Services OR Program* OR Improve* OR Lower* OR Reduce* OR Utilization OR Health education OR Community Health OR Counseling) AND (Basic health unit OR Community* OR Community‐based OR Domiciliary OR Developing OR Facility OR Home OR home‐based OR Peripheral OR Poor OR Rural OR Underdeveloped OR Unit* OR Village*) AND (infant, newborn OR newborn OR neonate OR neonatal OR birth OR mother OR maternal OR pregnancy OR prenatal OR postnatal OR periconcept* OR parturition OR Newborn or infan* or neonat*) AND (human not animal) AND (randomised controlled trial or controlled clinical trial or randomised or placebo or clinical trials as topic or randomly or trial or clinical trial) The Cochrane Library: (Intervention* OR Package* OR Promotion* OR Participate* OR Support* OR Group* OR Discussion* OR Education* OR Worker* OR Services OR Program* OR Improve* OR Lower* OR Reduce* OR Utilization OR Health education OR Community Health OR Counseling) AND (Basic health unit OR Community* OR Community‐based OR Domiciliary OR Developing OR Facility OR Home OR home‐based OR Peripheral OR Poor OR Rural OR Underdeveloped OR Unit* OR Village*) AND (infant or newborn or neonate or neonatal or birth OR mother OR maternal OR pregnancy OR prenatal OR postnatal OR periconcept* OR parturition)
Appendix 2. 2012 Search methods
The standard search methods of Cochrane and the Cochrane Neonatal Group were used (Cochrane Handbook for Systematic Reviews of Interventions). Trials of educational or mobilisation interventions in community settings were identified from MEDLINE (1966 to October 2012), EMBASE (1974 to October 2012), CENTRAL (The Cochrane Library, Issue 10, 2012), Cochrane Specialized Trials Register (Neonatal and Pregnancy and Childbirth Group; October 2012), and LILACS (1985 to October 2012).
We used the following search terms, which was adapted as necessary for each database listed above: Limited to: "Clinical Trials"; and "Randomized Controlled Trials".
We used combinations of the following words in the format: Participants AND Intervention AND Setting
Participants
Text words: Birth OR Delivery OR Infant* OR Neonate* OR Newborn* OR Mother OR Woman OR Maternal OR Pregnancy* OR Antenatal OR Prenatal OR Postnatal OR Periconcept* MeSH words: Parturition OR Infant, Newborn OR Mothers OR Women OR Pregnancy
Interventions
Text words: Intervention* OR Package* OR Promotion* OR Participate* OR Support* OR Group* OR Discussion* OR Education* OR Worker* OR Services OR Program* OR Improve* OR Lower* OR Reduce* OR Utilization OR Use MeSH words: Health education OR Community Health Services OR Counseling OR Intervention studies OR Community Health Aides
Settings
Text words: "Basic health unit" OR Community* OR Community‐based OR Domiciliary OR Developing OR Facility OR Home OR home‐based OR Peripheral OR Poor OR Rural OR Underdeveloped OR Unit* OR Village* MeSH words: Rural population OR Developing countries
We included relevant studies regardless of language or publication status (published, unpublished, in press, and in progress). We checked the reference lists of all trials identified by the above methods. We also searched the following conference proceedings for relevant abstracts: Federation of Asia and Oceania Perinatal Societies (FAOPS), World Congress on Perinatology, International Association for Maternal and Neonatal Health (IAMANEH), Indian Academy of Pediatrics (IAP), International Pediatric Association (IPA). We contacted organisations and researchers in the field for information on unpublished and ongoing trials, including all South Asian and African community‐based newborn care trials such as the Saving Newborn Lives (SNL) funded projects in Hala, Pakistan, Bangladesh (Projahnmo trials), India (Ankur and Shivgarh trials), Nepal and Mali, and trials in Makwanpur (Nepal) and of the Maternal and Infant Nutrition in Matlab (MINIMAT) project.
In addition to the above, trials registries, www.clinicaltrials.gov, and www.controlled‐trials.com were searched.
Appendix 3. 'Risk of bias' tool
(1) Sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups. We assessed the method as:
probably done (any truly random process, e.g. random number table; computer random number generator);
probably not done (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number); or
unclear.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal the allocation sequence in sufficient detail and to determine whether intervention allocation could have been foreseen in advance of, or during, recruitment, or changed after assignment. We assessed the methods as:
probably done (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
probably not done (e.g. open random allocation; unsealed or non‐opaque envelopes; alternation; date of birth); or
unclear.
(3) Blinding (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. Studies were judged at low risk of bias if they were blinded, or if we judged that lack of blinding could not have affected the results. Blinding was assessed separately for different outcomes or classes of outcomes. We assessed the methods as:
probably done, probably not done, or unclear for participants;
probably done, probably not done, or unclear for personnel; and
probably done, probably not done, or unclear for outcome assessors.
(4) Blinding of participants and personnel (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. Studies were judged at low risk of bias if they were blinded, or if we judged that lack of blinding could not have affected the results. We assessed the methods as:
probably done, probably not done, or unclear for participants; and
probably done, probably not done, or unclear for personnel,
(5) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study, for different outcomes or classes of outcomes, whether any methods were used to blind the outcome assessors. Blinding was assessed separately for different outcomes or classes of outcomes. We assessed the methods as:
probably done, probably not done, or unclear for outcome assessors.
(6) Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomised participants), and if reasons for attrition or exclusion are reported. We assessed methods as:
probably done;
probably not done; or
unclear.
(7) Selective reporting bias
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found. We assessed the methods as:
probably done (when it is clear that all of the study's pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
probably not done (when not all of the study's pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; or study fails to include results of a key outcome that would have been expected to have been reported); or
unclear.
(8) Other sources of bias
We described for each included study any important concerns we have about other possible sources of bias, such as the contribution of funding sources to the study method and analysis. We assessed whether each study was free of other problems that could put it at risk of bias.
Yes.
No.
Unclear.
(9) Overall risk of bias
We made explicit judgements about whether studies are at high risk of bias, according to the criteria given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). With reference to (1) to (6) above, we assessed the likely magnitude and direction of the bias, and whether we consider it likely to impact the findings. We explored the impact of the level of bias by undertaking sensitivity analyses.
Data and analyses
Comparison 1. Community health educational interventions versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Neonatal mortality | 26 | 553111 | Risk Ratio (Random, 95% CI) | 0.87 [0.78, 0.96] |
| 2 Early neonatal mortality | 15 | 321588 | Risk Ratio (Random, 95% CI) | 0.74 [0.66, 0.84] |
| 3 Late neonatal mortality | 11 | 186643 | Risk Ratio (Random, 95% CI) | 0.54 [0.40, 0.74] |
| 4 Perinatal mortality | 15 | 262613 | Risk Ratio (Random, 95% CI) | 0.83 [0.75, 0.91] |
| 5 Neonatal infection | 2 | 42043 | Risk Ratio (Random, 95% CI) | 0.88 [0.72, 1.08] |
| 6 Any antenatal care | 18 | 307528 | Risk Ratio (Random, 95% CI) | 1.16 [1.11, 1.22] |
| 6.1 Any ANC | 7 | 65811 | Risk Ratio (Random, 95% CI) | 1.19 [1.07, 1.33] |
| 6.2 ≥ 1 ANC | 5 | 67050 | Risk Ratio (Random, 95% CI) | 1.17 [1.04, 1.31] |
| 6.3 ≥ 3 ANCs | 4 | 48738 | Risk Ratio (Random, 95% CI) | 1.07 [0.85, 1.35] |
| 6.4 ≥ 4 ANCs | 8 | 124701 | Risk Ratio (Random, 95% CI) | 1.14 [1.01, 1.28] |
| 6.5 > 5 ANCs | 1 | 1228 | Risk Ratio (Random, 95% CI) | 1.31 [1.16, 1.46] |
| 7 Use of any method of contraception | 3 | 22237 | Risk Ratio (Random, 95% CI) | 1.10 [0.86, 1.41] |
| 8 Skilled attendance at delivery | 10 | 117870 | Risk Ratio (Random, 95% CI) | 1.09 [0.94, 1.25] |
| 9 Delivery attended by unskilled or semi‐skilled birth attendant | 3 | 40456 | Risk Ratio (Random, 95% CI) | 1.02 [0.70, 1.49] |
| 10 Use of clean delivery kit | 2 | 17087 | Risk Ratio (Random, 95% CI) | 4.44 [0.71, 27.76] |
| 11 Care‐seeking for neonatal illness | 7 | 46154 | Risk Ratio (Random, 95% CI) | 1.11 [0.97, 1.27] |
| 12 Use of colostrum | 5 | 28631 | Risk Ratio (Random, 95% CI) | 1.16 [0.83, 1.61] |
| 13 Timely initiation of breastfeeding | 19 | 126375 | Risk Ratio (Random, 95% CI) | 1.56 [1.37, 1.77] |
Comparison 2. Community health educational one‐to‐one and group and both counselling (subgroup) versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Neonatal mortality | 26 | 553111 | Risk Ratio (Random, 95% CI) | 0.87 [0.78, 0.96] |
| 1.1 One‐to‐one counselling | 8 | 105735 | Risk Ratio (Random, 95% CI) | 0.92 [0.71, 1.20] |
| 1.2 Group counselling | 12 | 211164 | Risk Ratio (Random, 95% CI) | 0.83 [0.74, 0.92] |
| 1.3 Both group and one‐to‐one counselling | 6 | 236212 | Risk Ratio (Random, 95% CI) | 0.90 [0.76, 1.06] |
| 2 Early neonatal mortality | 15 | 321588 | Risk Ratio (Random, 95% CI) | 0.74 [0.66, 0.84] |
| 2.1 Group counselling | 9 | 122151 | Risk Ratio (Random, 95% CI) | 0.70 [0.61, 0.80] |
| 2.2 One‐to‐one counselling | 1 | 18747 | Risk Ratio (Random, 95% CI) | 1.30 [1.01, 1.67] |
| 2.3 Both one‐to‐one and group counselling | 5 | 180690 | Risk Ratio (Random, 95% CI) | 0.78 [0.65, 0.93] |
| 3 Late neonatal mortality | 11 | 186643 | Risk Ratio (Random, 95% CI) | 0.54 [0.40, 0.74] |
| 3.1 Group counselling | 7 | 118239 | Risk Ratio (Random, 95% CI) | 0.50 [0.31, 0.81] |
| 3.2 Both group and one‐to‐one counselling | 4 | 68404 | Risk Ratio (Random, 95% CI) | 0.72 [0.57, 0.91] |
| 4 Perinatal mortality | 15 | 262613 | Risk Ratio (Random, 95% CI) | 0.83 [0.75, 0.91] |
| 4.1 One‐to‐one counselling | 2 | 23829 | Risk Ratio (Random, 95% CI) | 0.88 [0.57, 1.34] |
| 4.2 Group counselling | 8 | 156505 | Risk Ratio (Random, 95% CI) | 0.85 [0.77, 0.94] |
| 4.3 Both group and one‐to‐one counselling | 5 | 82279 | Risk Ratio (Random, 95% CI) | 0.78 [0.67, 0.90] |
| 5 Any antenatal care | 18 | 198928 | Risk Ratio (Random, 95% CI) | 1.15 [1.09, 1.21] |
| 5.1 One‐to‐one counselling | 5 | 29743 | Risk Ratio (Random, 95% CI) | 1.13 [0.94, 1.35] |
| 5.2 Group counselling | 8 | 117833 | Risk Ratio (Random, 95% CI) | 1.14 [0.99, 1.31] |
| 5.3 Both one‐to‐one and group counselling | 5 | 51352 | Risk Ratio (Random, 95% CI) | 1.21 [1.07, 1.37] |
| 6 Skilled attendance at delivery | 10 | 117870 | Risk Ratio (Random, 95% CI) | 1.09 [0.94, 1.25] |
| 6.1 One‐to‐one counselling | 2 | 2296 | Risk Ratio (Random, 95% CI) | 1.04 [0.96, 1.12] |
| 6.2 Group counselling | 5 | 93799 | Risk Ratio (Random, 95% CI) | 1.13 [0.81, 1.59] |
| 6.3 Both group and one‐to‐one counselling | 3 | 21775 | Risk Ratio (Random, 95% CI) | 1.10 [0.83, 1.44] |
| 7 Delivery attended by unskilled or semi‐skilled birth attendant | 3 | 40456 | Risk Ratio (Random, 95% CI) | 1.02 [0.70, 1.49] |
| 7.1 Group counselling | 2 | 21333 | Risk Ratio (Random, 95% CI) | 1.18 [0.64, 2.19] |
| 7.2 Both group and one‐to‐one counselling | 1 | 19123 | Risk Ratio (Random, 95% CI) | 0.85 [0.59, 1.22] |
| 8 Care‐seeking for neonatal illness | 7 | 46154 | Risk Ratio (Random, 95% CI) | 1.11 [0.97, 1.27] |
| 8.1 One‐to‐one counselling | 2 | 1006 | Risk Ratio (Random, 95% CI) | 1.15 [0.81, 1.64] |
| 8.2 Group counselling | 5 | 45148 | Risk Ratio (Random, 95% CI) | 1.11 [0.87, 1.41] |
| 9 Timely initiation of breastfeeding | 19 | 126375 | Risk Ratio (Random, 95% CI) | 1.56 [1.37, 1.77] |
| 9.1 One‐to‐one counselling | 5 | 23927 | Risk Ratio (Random, 95% CI) | 1.16 [1.01, 1.32] |
| 9.2 Group counselling | 8 | 58011 | Risk Ratio (Random, 95% CI) | 1.80 [1.25, 2.58] |
| 9.3 Both one‐to‐one and group counselling | 6 | 44437 | Risk Ratio (Random, 95% CI) | 1.63 [1.39, 1.92] |
Comparison 3. Community health educational ANC period and PNC period and both periods (subgroup) versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Neonatal mortality | 26 | 553111 | Risk Ratio (Random, 95% CI) | 0.87 [0.78, 0.96] |
| 1.1 Education provided during ANC period only | 3 | 47849 | Risk Ratio (Random, 95% CI) | 0.84 [0.64, 1.09] |
| 1.2 Education provided during PNC period only | 3 | 172882 | Risk Ratio (Random, 95% CI) | 1.02 [0.84, 1.24] |
| 1.3 Education provided in both ANC and PNC periods | 20 | 332380 | Risk Ratio (Random, 95% CI) | 0.85 [0.76, 0.96] |
| 2 Early neonatal mortality | 15 | 321588 | Risk Ratio (Random, 95% CI) | 0.74 [0.66, 0.84] |
| 2.1 Education provided during ANC period only | 2 | 33209 | Risk Ratio (Random, 95% CI) | 0.64 [0.43, 0.95] |
| 2.2 Education provided during PNC period only | 1 | 111529 | Risk Ratio (Random, 95% CI) | 1.03 [0.94, 1.12] |
| 2.3 Education provided during both ANC and PNC periods | 12 | 176850 | Risk Ratio (Random, 95% CI) | 0.76 [0.68, 0.84] |
| 3 Late neonatal mortality | 11 | 186643 | Risk Ratio (Random, 95% CI) | 0.54 [0.40, 0.74] |
| 3.1 Education provided during ANC period only | 1 | 30952 | Risk Ratio (Random, 95% CI) | 0.87 [0.54, 1.40] |
| 3.2 Education provided during both ANC and PNC periods | 10 | 155691 | Risk Ratio (Random, 95% CI) | 0.52 [0.38, 0.72] |
| 4 Perinatal mortality | 15 | 262613 | Risk Ratio (Random, 95% CI) | 0.83 [0.75, 0.91] |
| 4.1 Education provided during PNC only | 1 | 60480 | Risk Ratio (Random, 95% CI) | 0.89 [0.78, 1.02] |
| 4.2 Education provided during ANC period only | 2 | 33513 | Risk Ratio (Random, 95% CI) | 0.90 [0.59, 1.39] |
| 4.3 Education provided during both ANC and PNC periods | 12 | 168620 | Risk Ratio (Random, 95% CI) | 0.81 [0.72, 0.91] |
| 5 Care‐seeking for neonatal illness | 7 | 46154 | Risk Ratio (Random, 95% CI) | 1.11 [0.97, 1.27] |
| 5.1 Education provided during ANC period only | 2 | 15192 | Risk Ratio (Random, 95% CI) | 0.96 [0.85, 1.09] |
| 5.2 Education provided during both ANC and PNC periods | 5 | 30962 | Risk Ratio (Random, 95% CI) | 1.14 [0.97, 1.34] |
| 6 Timely initiation of breastfeeding | 19 | 126375 | Risk Ratio (Random, 95% CI) | 1.56 [1.37, 1.77] |
| 6.1 Education provided during PNC only | 1 | 12367 | Risk Ratio (Random, 95% CI) | 3.64 [3.38, 3.93] |
| 6.2 Education provided in both ANC and PNC periods | 18 | 114008 | Risk Ratio (Random, 95% CI) | 1.47 [1.32, 1.65] |
Comparison 4. Community health educational intervention for family members and mothers and for mothers only (subgroup) versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Neonatal mortality | 26 | 553111 | Risk Ratio (Random, 95% CI) | 0.87 [0.78, 0.96] |
| 1.1 Intervention given to mothers and family members | 13 | 282817 | Risk Ratio (Random, 95% CI) | 0.84 [0.74, 0.95] |
| 1.2 Intervention given to mothers only | 13 | 270294 | Risk Ratio (Random, 95% CI) | 0.90 [0.77, 1.05] |
| 2 Early neonatal mortality | 15 | 321588 | Risk Ratio (Random, 95% CI) | 0.74 [0.66, 0.84] |
| 2.1 Intervention given to mothers and family members | 7 | 99097 | Risk Ratio (Random, 95% CI) | 0.70 [0.56, 0.87] |
| 2.2 Intervention given to mothers only | 8 | 222491 | Risk Ratio (Random, 95% CI) | 0.78 [0.68, 0.90] |
| 3 Late neonatal mortality | 11 | 186643 | Risk Ratio (Random, 95% CI) | 0.54 [0.40, 0.74] |
| 3.1 Intervention given to mothers and family members | 4 | 76388 | Risk Ratio (Random, 95% CI) | 0.69 [0.51, 0.92] |
| 3.2 Intervention given to mothers only | 7 | 110255 | Risk Ratio (Random, 95% CI) | 0.50 [0.31, 0.78] |
| 4 Perinatal mortality | 15 | 262613 | Risk Ratio (Random, 95% CI) | 0.83 [0.75, 0.91] |
| 4.1 Intervention given to mothers and family members | 7 | 141824 | Risk Ratio (Random, 95% CI) | 0.83 [0.72, 0.96] |
| 4.2 Intervention given to mothers only | 8 | 120789 | Risk Ratio (Random, 95% CI) | 0.83 [0.72, 0.96] |
| 5 Any antenatal care | 18 | 198928 | Risk Ratio (Random, 95% CI) | 1.15 [1.09, 1.21] |
| 5.1 Intervention given to mothers and family members | 9 | 102886 | Risk Ratio (Random, 95% CI) | 1.20 [1.06, 1.36] |
| 5.2 Intervention given to mothers only | 9 | 96042 | Risk Ratio (Random, 95% CI) | 1.09 [1.02, 1.17] |
| 6 Skilled attendance at delivery | 10 | 117870 | Risk Ratio (Random, 95% CI) | 1.09 [0.94, 1.25] |
| 6.1 Intervention given to mothers and family members | 4 | 58584 | Risk Ratio (Random, 95% CI) | 1.05 [0.93, 1.18] |
| 6.2 Intervention given to mothers only | 6 | 59286 | Risk Ratio (Random, 95% CI) | 1.11 [0.92, 1.34] |
| 7 Care‐seeking for neonatal illness | 7 | 46154 | Risk Ratio (Random, 95% CI) | 1.11 [0.97, 1.27] |
| 7.1 Intervention given to mothers and family members | 4 | 16198 | Risk Ratio (Random, 95% CI) | 1.07 [0.90, 1.27] |
| 7.2 Intervention given to mothers only | 3 | 29956 | Risk Ratio (Random, 95% CI) | 1.17 [0.84, 1.62] |
| 8 Use of colostrum | 5 | 28631 | Risk Ratio (Random, 95% CI) | 1.16 [0.83, 1.61] |
| 8.1 Intervention given to mothers and family members | 2 | 5097 | Risk Ratio (Random, 95% CI) | 1.34 [1.26, 1.43] |
| 8.2 Intervention given to mothers only | 3 | 23534 | Risk Ratio (Random, 95% CI) | 1.06 [0.57, 1.98] |
| 9 Timely initiation of breastfeeding | 19 | 126375 | Risk Ratio (Random, 95% CI) | 1.56 [1.37, 1.77] |
| 9.1 Intervention given to mothers and family members | 10 | 47466 | Risk Ratio (Random, 95% CI) | 1.56 [1.26, 1.94] |
| 9.2 Intervention given to mothers only | 9 | 78909 | Risk Ratio (Random, 95% CI) | 1.56 [1.30, 1.87] |
Comparison 5. Sensitivity analysis on primary outcomes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Neonatal mortality | 22 | 497258 | Risk Ratio (Random, 95% CI) | 0.88 [0.79, 0.98] |
| 2 Early neonatal mortality | 11 | 264672 | Risk Ratio (Random, 95% CI) | 0.71 [0.62, 0.82] |
| 3 Late neonatal mortality | 9 | 150876 | Risk Ratio (Random, 95% CI) | 0.51 [0.36, 0.72] |
| 4 Perinatal mortality | 12 | 224107 | Risk Ratio (Random, 95% CI) | 0.84 [0.75, 0.94] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ayiasi 2016.
| Methods | Randomised community intervention trial conducted in Masindi and Kiryandongo districts, Uganda | |
| Participants | Pregnant women were recruited during their first antenatal visit; in total 1644 were enrolled (control = 893, intervention = 751) 16 health centres were randomly and equally assigned to 1 of 2 arms: the intervention arm or the control arm 48 Village Health Teams (VHTs) were selected for training |
|
| Interventions | Intervention arm: received a package consisting of (1) VHT making home visits to provide educational messages about maternal and newborn care, and (2) VHT members equipped with mobile phones to make unlimited phone call consultations with professional health workers for further clarification and advice Control arm: women in control group had access to group education that was routinely offered by health centres. The control group did not receive follow‐up visits by VHTs, and VHTs in control groups did not receive mobile devices |
|
| Outcomes | Primary outcomes: health facility delivery (compared to home delivery) Secondary outcomes: antenatal consultations, birth preparedness, newborn care practices (cord care, thermal care, initiation of exclusive breastfeeding, care‐seeking for newborn illness) |
|
| Notes | The funding agency (Directorate General for Development Cooperation of Belgium) had no role in study design, data management, or manuscript writing | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "random allocation was conducted by writing the names of HCs, VHTs or villages on small pieces of papers which were then folded to conceal the names. Two persons, each representing a study arm, but not associated to the study were asked to randomly pick folded papers from the pool of names. The process was repeated until allocation was completed for HCs, villages and VHTs" Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote: "random allocation was conducted by writing the names of HCs, VHTs or villages on small pieces of papers which were then folded to conceal the names" Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "blinding was not necessary since randomisation was at the level of Health Centres" Comment: blinding was not possible due to the nature of the intervention. Probably no |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: insufficient information to make any judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: attrition was 11.2% in the intervention arm and 9.7% in the control arm Reasons for attrition were similar across the 2 arms |
| Selective reporting (reporting bias) | Low risk | Comment: this is a registered trial, and the study has reported all outcomes mentioned in the protocol |
| Other bias | High risk | Quote: "the control arm had four HCs of level II and four HCs of level III. The intervention arm had two HCs of level II and six HCs of level III. Therefore, pre‐intervention differences between control and intervention arms can be explained by variation in the distribution of HC levels in each arm"; "the difference in utilisation between HCs of level II and III explains why there were significantly more women recruited in HCs of level II among the control group compared to HCs of level II in the intervention group. The same argument can be extended to explain significant difference in antenatal care attendance in control arm compared to the intervention arm. These differences could have affected our results" Comment: probably not done |
Azad 2010.
| Methods | Cluster‐randomised controlled trial conducted in Bangladesh | |
| Participants | Target population included women aged 15 to 49 years residing in the project area who gave birth during the study period, adolescents, and mothers‐in‐law. 18 clusters in 3 districts were randomly assigned to either intervention or control. There were 36,139 births in an overall population of 478,611 during 3 years | |
| Interventions | Intervention clusters: a facilitator convened 18 groups every month to support participatory action and learning for women, and to develop and implement strategies to address maternal and neonatal health problems. Other intervention involved training traditional birth attendants in bag‐valve‐mask resuscitation of neonates with symptoms of birth asphyxia Facilitators received 5 training sessions that covered participatory modes of communication and maternal and neonatal health issues There were 3162 live births in the intervention arm Control group: received the usual care provided in the area (n = 3227 total births, n = 3069 live births) |
|
| Outcomes | Primary outcomes: neonatal mortality rate, maternal deaths, stillbirths Secondary outcomes: uptake of antenatal and delivery services, home care practices during and after delivery, infant mortality, health care‐seeking behaviour, perinatal mortality |
|
| Notes | In all clusters (intervention and control), health services were strengthened and traditional birth attendants were trained Sponsors of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "unions were randomly allocated to either intervention or control groups by district in the presence of four project staff and two external individuals" Comment: insufficient information to permit judgement |
| Allocation concealment (selection bias) | Low risk | Comment: since this was a cluster‐randomised trial, allocation concealment should not be an issue, as in this design, all clusters are randomised at once Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "neither the study investigators nor the participants were masked to group allocation" Comment: probably not done |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: insufficient information to permit judgment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Exclusion (0%) and attrition (14.3%) were reported along with reasons |
| Selective reporting (reporting bias) | Low risk | Comment: this is a registered trial, and this study has reported all outcomes mentioned in the protocol |
| Other bias | High risk | Quote: "the purposive selection of districts and upazilas, and the stratification of sampling, together with the restricted number of clusters, might have limited the effectiveness of randomisation procedures" Comment: probably not done |
Baqui 2008 (a).
| Methods | Cluster‐randomised controlled trial conducted in 3 rural subdistricts (Beanibazar, Zakiganj, Kanaighat) of Sylhet district of Bangladesh | |
| Participants | All married women of reproductive age (15 to 49 years) were eligible to participate. Total of 24 clusters: 12 in the intervention group and 12 in the control group. Total number of participants in all study groups was 113,816 | |
| Interventions | The intervention was delivered through CHWs who identified pregnancies and provided the intervention package. Study had 3 arms (i.e. HC, CC, and control). Interim sample household surveys were done to measure intervention inputs, coverage, and changes in key newborn care practices in all 3 study arms Intervention (n = 2846 live births): HC model: in this study arm, CHWs identified pregnancies through routine surveillance during visits to each household once every 2 months; promoted birth and newborn care preparedness through 2 scheduled antenatal and 3 early postnatal home visits; and provided iron and folic acid supplements during birth and newborn care preparedness visits. Home screening/management/referral of sick newborns was also included Control (n = 2638 live births): families in the control arm received the usual health services provided by the government, non‐governmental organisations, and private providers |
|
| Outcomes | Primary outcomes: neonatal mortality, stillbirth, abortion Secondary outcomes: antenatal visits from trained providers, use of iron and folic acid supplements, use of clean cord cutting instruments, delays in newborn first bath, breastfeeding within 1 hour of birth, tetanus‐toxoid immunisation coverage |
|
| Notes | Refresher training sessions for management of maternal and newborn complications were provided for government health workers in all 3 study arms Two intervention arms were separately analysed with the control arm Funding: study sponsors had no role in study design, data collection, analysis, interpretation, or dissemination, nor in the decision to submit this paper for publication |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...computer‐generated pseudo‐random number sequence without stratification or matching. The computer‐generated randomisation was implemented by a study investigator who had no role in the implementation of the study" Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Comment: since this is a cluster‐randomised trial, allocation concealment should not be an issue, as in this design, all clusters are randomised at once |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: due to the nature of the trial, participants and personnel could not be blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition (12.3%) was described with reasons. Exclusion data were not reported, nor were reasons |
| Selective reporting (reporting bias) | Low risk | Comment: this is a registered trial, and this study has reported all outcomes mentioned in the protocol |
| Other bias | Low risk | Quote: "a potential limitation was that we relied on retrospective recall for information about changes in maternal and newborn‐care practices and neonatal mortality. We used standardised data collection methods, and any potential recall lapses were expected to be similar across the study arms" Comment: probably done |
Baqui 2008 (b).
| Methods | Quasi‐experimental study with 1 intervention district and 1 comparison (control) district of rural Uttar Pradesh, India | |
| Participants | Each district had 15 rural blocks; 9 of these made up the intervention group and 8 made up the comparison district. One sector from each block was selected for baseline and end of line surveys. Women who had experienced a live birth in the reference period were included in the analysis. At baseline, a total of 6196 women were included in the comparison group compared to 8756 in the intervention group. For the end line survey, 6014 women were included in the comparison and 7812 in the intervention district Community‐based workers: home visitation during neonatal and postnatal periods |
|
| Interventions | Intervention: community‐based workers (CBWs) conducted home visits during antenatal and postnatal periods as the main strategy for behaviour change communication about healthy maternal and newborn care practices, including recognition of danger signs and care‐seeking. CBWs also promoted recognition of maternal and newborn complications during pregnancy, during delivery, or postpartum Control: received the standard government programme Baseline and end line surveys were conducted to determine effects of the intervention regarding rates of programme coverage, maternal and newborn care practices, and healthcare utilisation |
|
| Outcomes | Antenatal care: antenatal home visit, birth preparation, emergency preparation, tetanus toxoid immunisation, iron/folic acid supplementation, antenatal care visit Delivery care: medically trained birth attendant Newborn care: clean cord care, newborn dried and wrapped, newborn bath delayed, immediate breastfeeding, postnatal home visit, newborn check‐up |
|
| Notes | Funding: project was funded by USAID, India Mission, through Global Research Activity Award # HRNA‐00‐96‐90006‐00 to the Johns Hopkins Bloomberg School of Public Health | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "each district had 15 rural blocks; 9 blocks in the intervention district and 8 in the comparison district were randomly selected using a computer program. WIthin each block, one sector,..., was randomly selected" Comment: probably done. However the intervention group had a significantly larger sample size (8756) compared to the control group (6196) |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: since this is a cluster‐randomised trial, allocation concealment should not be an issue, as in this design, all clusters are randomised at once |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: insufficient information to permit judgement |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient information to permit judgement |
| Other bias | Low risk | Study seems to be free from other biases |
Bashour 2008.
| Methods | Randomised controlled trial conducted in Damascus, Syria | |
| Participants | Women who delivered a healthy newborn whether by vaginal delivery or Caesarean section, who lived within 30 km from the hospital, and who were available for follow‐up for the coming 6 months were included (n = 876) | |
| Interventions | Intervention groups: the intervention consisted of home visits aimed to examine, follow up, educate, support, and counsel women who had recently given birth. Registered midwives undertook 5 days of special training and implemented postpartum home visits. Training included a review of postnatal care, the role of home visits, the content of each visit, including the physical examination as well as educational messages, and communication skills. Two intervention groups received 4 postnatal home visits (group A = 285) or 1 visit (group B = 294). Women in group B received 1 home visit on day 3, similar in content to visits to group A Control: women (group C = 297) received the current standard of care in Syria, that is, no visit following hospital discharge |
|
| Outcomes | Primary outcomes: maternal postpartum morbidities; postnatal care uptake; contraceptive uptake and type; infant morbidities; infant immunisation according to the national schedule at 3 months; infant feeding, namely, exclusive breastfeeding during the first 4 months of life Secondary outcomes: women's perceptions of their health and their impressions about the home visit and perceptions of its quality |
|
| Notes | We included data for group B vs C in this analysis Funding: study was supported by the American University of Beirut Award (Regional Changing Childbirth Research Program at Faculty of Health Sciences supported by Wellcome Trust) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomisation was in blocks of 7 where a caseload of 21 eligible deliveries per day was assumed" Comment: insufficient information to permit judgement |
| Allocation concealment (selection bias) | Low risk | Quote: "the method that was used to implement the random allocation relied on numbered opaque and sealed envelopes" Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "the midwives who carried out the home visits were not blinded to the group assignments, but the outcome assessors were" Comment: probably not done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "the midwives who carried out the home visits were not blinded to the group assignments, but the outcome assessors were" Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Exclusion (1.2%) was given and reasons mentioned |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient information to make an assessment, as study protocol is not available |
| Other bias | Low risk | Comment: study seems to be free from other biases |
Bhandari 2012.
| Methods | Cluster‐randomised trial conducted in catchment areas of 18 primary health centres in Haryana, India | |
| Participants | Women including pregnant women, neonates, and children (60,480 births) | |
| Interventions | Intervention arm (n = 29,667 births): IMNCI training for AWWs, ANMs, TBAs, and private practitioners These sessions covered neonatal conditions requiring referral, pre‐referral treatment, problems that can be managed at home, and components of essential newborn care. Management of diarrhoea, pneumonia, conditions requiring referral, and pre‐referral treatment, as well as appropriate complementary feeding practices for older children, was also covered. Improved availability of medicines was achieved by establishing medicine stores with the VLCs, which were replenished every 3 months by a study supply officer. Supervision of health workers and community health workers was strengthened in the intervention areas. The study recruited 2 supervisors per 30,000 population in consultation with the local government; they conducted monthly visits to ASHAs and AWWs, observed women's group meetings, and attended monthly government review meetings Control arm (n = 30,813): AWWs and ANMs were in place in control areas and continued to provide the usual services. However, they were not trained to conduct IMNCI activities such as home visits for newborns and community management of sick infants and children. Management of sick infants and children remained in the hands of private providers and physicians at government facilities |
|
| Outcomes | Primary outcomes: perinatal deaths, neonatal deaths, postneonatal deaths, infant deaths Secondary outcomes: danger signs of severe illness, local infections, diarrhoea, general danger signs, pneumonia |
|
| Notes | Funding: study was funded by the World Health Organization, Geneva (through an umbrella grant from USAID); the United Nations Children’s Fund, New Delhi; and the GLOBVAC Program of the Research Council of Norway through grant No. 183722. Individual scientists at WHO and Unicef contributed importantly to planning, analysis, and reporting of this study. However, the central bodies of these agencies and the Research Council of Norway had no influence on how data were collected, analysed, or presented | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "an independent epidemiologist generated 10 stratified randomisation schemes to allocate the clusters to intervention or control groups"; "We selected one of the remaining seven allocation schemes by a computer generated random number" Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Comment: since this is a cluster trial, allocation concealment should not be an issue, as in this design, as all clusters are randomised |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "the surveillance team was not told the intervention status of the community they were visiting" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 12.3% were lost to follow‐up in intervention clusters, and 14% were lost to follow‐up in control clusters |
| Selective reporting (reporting bias) | Low risk | Comment: this is a registered trial, and the study has reported all outcomes mentioned in the protocol |
| Other bias | Low risk | Study seems to be free from other biases |
Bhutta 2008.
| Methods | Pilot clustered‐randomised trial from Hala and Matiari subdistricts of Sind, Pakistan | |
| Participants | Target population consisted of pregnant women. 24 village clusters were identified at a primary care facility. Out of those, 8 clusters were randomly selected for this pilot study. 4 districts chosen to receive intervention were matched with 4 control clusters for population size and birth and neonatal mortalities rates (5134 births) | |
| Interventions | Intervention: promotion of maternal nutrition and rest, early breastfeeding (within first hour), colostrum administration (avoidance of pre‐lacteal feeds), thermoregulation, home care for low birth weight infants, treatment of pneumonia with oral trimethoprim‐sulphamethoxazole (TMP‐SMX), recognition of danger signs, training in group counselling and communication strategies (n = 2672 births) Control: routine care (n = 2462 births) The following activities were conducted in both intervention and control groups: promotion of antenatal care, iron and folate use during pregnancy, immediate newborn care, cord care, promotion of exclusive breastfeeding |
|
| Outcomes | Primary outcomes: stillbirths, early neonatal deaths, late neonatal deaths, total neonatal deaths, perinatal deaths | |
| Notes | Local health workers (LHWs) were encouraged to visit all pregnant women twice during pregnancy, within 24 hours of birth, and 4 times in the first postnatal month, and were encouraged to link up with local Dais (traditional birth attendants). LHWs were supported by the creation of voluntary community health committee, which helped in conducting community education group sessions Funding: the Hala project is supported by a collaborative grant from WHO and the Saving Newborn Lives (SNL) programme of Save the Children (USA), funded by the Bill & Melinda Gates Foundation |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "eight clusters were randomly selected for this pilot study"; "subsequently, the four clusters chosen to receive the intervention were matched with four control clusters" Comment: probably not done |
| Allocation concealment (selection bias) | Low risk | Comment: since this is a cluster‐randomised trial, allocation concealment should not be an issue, as in this design, all clusters are randomised at once |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "cross‐sectional surveys of all households were conducted by a separate team in both the middle (June–July 2004) and at the end (August –September 2005) of the pilot study to collect data on births, deaths and care‐seeking behaviour in the preceding 12 months" Comment: done |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: insufficient information to permit judgement |
| Selective reporting (reporting bias) | Low risk | Comment: study reported all outcomes outlined in the protocol |
| Other bias | Low risk | Comment: study seems to be free from other biases |
Bhutta 2011.
| Methods | Cluster‐randomised trial of community‐based interventions conducted in 2 Talukas (subdistricts) of Naushero Feroze, rural districts of Sind, Pakistan | |
| Participants | Pregnant women. 16 clusters were assigned to intervention and control groups. Total number of births in intervention group was 12,391, and in control group 11,443 (n = 23,834) | |
| Interventions | There were 2 study groups Intervention arm (n = 12,391): local health workers (LHWs) = along with basic training (for control group), they received additional training on recognition of high‐risk pregnancies and referral and management of birth asphyxia, serious bacterial infections, and low birth weight infants Traditional birth attendants (TBAs) = along with basic training (for control group), they received additional training on promotion of LHW attendance at births and resuscitation (mouth‐to‐mouth) of newborns Control arm (n = 11,443): trained LHWs on community mobilisation through building support groups, promotion of use of clean delivery kits, recognition of neonatal illness, and referral for care; TBAs were linked with LHWs; they were trained on promotion and use of clean delivery kits | |
| Outcomes | Primary outcomes: neonatal mortality rates, perinatal mortality rates Secondary outcomes: birth asphyxia‐related neonatal mortality rates, neonatal mortality rates in low birth weight infants, neonatal mortality rates due to sepsis | |
| Notes | To create awareness in the community and at the household level in control and intervention clusters, female and male support groups (health committees) were formed/strengthened. The LHW formed a female health committee, and male activists formed male health committees, in the LHW catchment area. Meetings of both groups were arranged with the assistance of the community health committee, and LHWs met on a monthly basis for dissemination of health messages and education related to maternal and newborn health and problems. Separate community group educational sessions for mothers, mothers‐in‐law, married women especially with pregnancy, fathers, fathers‐in‐law were conducted for health education of communities through support groups in the LHW catchment area using educational material as flip charts on antenatal care, identification of danger signs related to pregnancy, recognition of simple risk factors for high‐risk pregnancies and births (these include severe maternal malnutrition, illness, short stature, previous perinatal deaths, etc.), and birth preparedness (transport, money, skilled birth attendant, facility) Funding: WHO; Saving Newborn Lives Program of Save the Children USA, funded by the Bill & Melinda Gates Foundation |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "twenty‐six such clusters with available LHWs were identified in the district, 8 of which were involved in the pilot study. Two further clusters were excluded as they had very few LHWs. The full‐cluster RCT was thus implemented in the remaining 16 clusters"; "used restricted, stratified randomisation to allocate clusters to the intervention and control arms (21). Three strata (comprising 2, 6 and 8 clusters) were identified based on their size and the number of LHWs per 1000 population. We identified 126 random allocations which resulted in similar population sizes in the 2 arms.........From this list of "balanced" allocations we selected one scheme at random"; "From this list of balanced allocations, we selected one scheme using a computer generated random number" Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Comment: since this is a cluster‐randomised trial, allocation concealment should not be an issue, as in this design, all clusters are randomised at once |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "data collectors were masked to cluster allocation; those analysing data were not" Comment: data collectors were independent of implementers |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition was 12.4% in intervention clusters and 10.8% in control clusters, and reasons for exclusion were mentioned |
| Selective reporting (reporting bias) | Low risk | Comment: study reported all outcomes outlined in the protocol |
| Other bias | Low risk | Comment: study seems to be free from other biases |
Colbourn 2013.
| Methods | Two‐by‐two factorial cluster‐randomised controlled trial evaluating community‐ and facility‐based interventions to reduce deaths in 3 districts of Malawi | |
| Participants | Randomly sampled approximately 4000 people per cluster and set up community surveillance to track pregnancies, births, and deaths of consenting women Baseline (n = 14,576 births) Intervention (n = 20,576 births) |
|
| Interventions | Community mobilisation: 81 volunteer facilitators each formed 9 women's groups, which followed an "action cycle" to identify and prioritise maternal and neonatal health problems. Fifty per cent of groups had maternal and neonatal task forces added to them to enhance antenatal coverage, maternal/neonatal health knowledge, and facility delivery Quality improvement: facility level Control: 17 clusters (out of 62) were assigned to control, where these clusters underwent no interventions |
|
| Outcomes | Primary outcomes: maternal, neonatal and perinatal mortality Secondary outcomes: % of deliveries at a health facility, % of maternal deaths subjected to maternal death audit, case‐fatality rates, practice of signal obstetrical care at community level, number of women's groups mobilised annually, % of pregnant women attending women's groups |
|
| Notes | Funding: project was funded by The Health Foundation, London, UK. Project funders commissioned the randomised controlled trial to evaluate the 2 interventions but had no direct input into the design of the study, data collection, nor data analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "clusters were health centre catchment areas assigned using stratified computer generated randomisation" (random number sequence generated in Strata 7) Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote: "to ensure concealment of intervention allocation, identification numbers were assigned for each cluster and a random number generated for each" Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "neither participants nor those administering the interventions were blinded to group assignment" Comment: probably not done |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "during the baseline period, the recorded loss to follow‐up to birth outcomes was 19% and during the intervention it was 29%, with higher rates in later months. Given that observed birth rates in the study matched those expected from the crude birth rate 1.27 to within 3%, and that in‐migration probably broadly matched out‐migration, many of the pregnancies recorded by KIs as ‘lost to follow‐up’ may have been recorded as pregnancies by mistake and true loss‐to‐follow‐up was probably much lower; there was little difference in loss‐to‐follow‐up between arms" Comment: probably not |
| Selective reporting (reporting bias) | Low risk | Comment: this is a registered trial, and this study has reported all outcomes mentioned in the protocol |
| Other bias | Low risk | Study seems to be free from other biases |
Darmstadt 2010.
| Methods | Cluster‐randomised controlled trial conducted in Mirzapur, Bangladesh | |
| Participants | All married women of reproductive age (15 to 49 years). 12 unions were randomised to intervention or comparison arm. Total numbers of participants were 9987 and 11,153 in intervention and comparison arms, respectively | |
| Interventions | In the intervention arm (n = 9987), community health workers (CHWs) identified pregnant women; made 2 antenatal home visits to promote birth and newborn care preparedness; made 4 postnatal home visits to negotiate preventive care practices and to assess newborns for illness; and referred sick neonates to a hospital and facilitated compliance Control (n = 11,153): newborns in the control arm received the usual health services provided by the government, non‐governmental organisations, and private providers CHWs were trained for 36 days on pregnancy surveillance, counselling and negotiation skills, essential newborn care, neonatal illness surveillance, and management of illness based on a clinical algorithm adapted from Integrated Management of Childhood Illness. After initial training and evaluation, routine monitoring and refresher training were provided each fortnight |
|
| Outcomes | Primary outcomes: antenatal and immediate newborn care behaviours, knowledge of danger signs, care‐seeking for neonatal complications, neonatal mortality | |
| Notes | Funding: supported by the Wellcome Trust ‐ Burroughs Wellcome Fund Infectious Disease Initiative 2000 and the Office of Health, Infectious Diseases and Nutrition, Global Health Bureau, United States Agency for International Development (USAID) through the Global Research Activity Cooperative agreement with the Johns Hopkins Bloomberg School of Public Health (award HRN‐A‐00‐96‐90006‐00). Support for data analysis and manuscript preparation was provided by the Saving Newborn Lives programme through a grant by the Bill & Melinda Gates Foundation to Save the Children‐US. Sponsors had no role in study design, study implementation, or data analysis or interpretation, or in the decision to publish the paper | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "12 rural unions, which were randomly allocated to either comparison or intervention arm using a computer‐generated pseudo‐random number sequence without stratification or matching" Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Since this was a cluster‐randomised trial, allocation concealment should not be an issue, as in this design, all clusters are randomised at once |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "blinding was unachievable given the nature of the intervention" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "data, including sings and symptoms of illness leading to deaths, were collected by separate interviewers who were trained in verbal autopsy data collection for six days" Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition (7.8%) and reasons were mentioned |
| Selective reporting (reporting bias) | Low risk | Comment: this is a registered trial, and the study has reported all outcomes mentioned in the protocol |
| Other bias | Low risk | Study seems to be free from other biases |
Degefie 2017.
| Methods | Two‐arm cluster‐randomised trial in Rural Ethiopia, evaluating the impact of making newborn infection management available at health posts when referral to health centres were not possible | |
| Participants | 22 geographical clusters (11 in intervention and 11 in control) Number of women of reproductive age (15 to 49) interviewed: comparison (control), n = 58,497; intervention, n = 56,733 |
|
| Interventions | Trained 3500 female volunteers on what to do during house visits, counselling families about importance of antenatal care, danger signs for women and newborns that should prompt care‐seeking, birth preparedness, clean delivery, and healthy newborn practices that prevent infection. 270 HEWs were also trained on home visits, volunteer support, iCCM, and case management. HEWs in the intervention arm also received training on the treatment algorithm, administration of medicine, and injection safety Control: HEWs in the control group did not receive additional training related to the algorithm, administration of medicine, injection safety, or treatment of PSBI at health posts. However, home visits, referral of newborns to health centres, and project officers were available at health posts for both arms |
|
| Outcomes | Primary outcome: post day 1 neonatal mortality (2 to 27 days after birth) Secondary outcomes: socioeconomic status, knowledge, practices, and care‐seeking from women who had delivered in previous 60 days. Home visit coverage by volunteers and HEWs, PSBI cases, HEW performance, PSBI signs, mortality impact |
|
| Notes | Funding: funding agency had no role in study design, data collection, data analysis and interpretation, writing of the report, or the decision to submit for publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "clusters were randomised 1:1 stratified on region and using restriction to ensure arms were balanced in population size, annual number of births, baseline neonatal mortality rate, and proportion of HEWs resident in their village" Comment: probably not done |
| Allocation concealment (selection bias) | Low risk | Comment: since this is a cluster‐randomised trial, allocation concealment should not be an issue, as in this design, all clusters are randomised at once |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: "allocation was not masked"; "training was an element of the intervention, therefore blinding of female volunteers and Health Extension Workers was not possible" Comment: not done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "although survey teams were blinded to minimize interviewer bias" Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: attrition was similar across groups and reasons were provided |
| Selective reporting (reporting bias) | Low risk | Comment: this is a registered trial, and the study has reported all outcomes mentioned in the protocol |
| Other bias | Low risk | Study seems to be free from other biases |
Fottrell 2013.
| Methods | Cluster‐randomised controlled trial in Bangladesh to assess the effect of participatory women's group intervention with higher population coverage on neonatal mortality | |
| Participants | Women who were permanently residing in 18 unions, in 3 districts, and accounting for 19,301 births during the final 24 months of the intervention. In intervention areas, 648 new women's groups were formed in addition to 162 women's groups set up as a part of an earlier trial (combined women's groups: n = 810). All unions, regardless of allocation, received health system‐strengthening initiatives | |
| Interventions | Women's group utilising participatory learning and action cycle, where they prioritise issues that affected maternal and neonatal health, and design and implement strategies to address said issues Control: both intervention and control clusters received health system‐strengthening activities. A small number of women from control areas participated in the women's group meetings; however contamination is fairly limited due to the geographical location of the clusters |
|
| Outcomes | Primary outcome: neonatal mortality rates Secondary outcomes: facility deliveries, birth attendant home delivery practices (e.g. washed hands with soap), thermal care of newborns, early infant‐feeding practices, health service utilisation (antepartum, intrapartum, postpartum) |
|
| Notes | Funding: implementation and evaluation of women’s groups was funded by a Big Lottery Fund International Strategic Grant. This study was supported with funds from a Wellcome Trust Strategic Award (085417ma /Z/08/Z). Sponsors did not participate in design and conduct of the study; in collection, analysis, and interpretation of data; or in preparation, review, or approval of the manuscript | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "6 unions (clusters) in each district (stratum) were randomly allocated to either intervention or control by blindly pulling pieces of paper, each representing 1 union, from a bottle. The allocation sequence had been decided on before drawing the papers" Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote: "the nature of the intervention means that allocation was not masked" Comment: since this is a cluster‐randomised trial, allocation concealment should not be an issue, as in this design, all clusters are randomised at once |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: blinding of participants not possible due to nature of study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "the board, as well as the implementation and in country monitoring and evaluation teams, were blind to the allocation arm on both occasions" Comment: probably yes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition was 1% and reasons were reported |
| Selective reporting (reporting bias) | Low risk | Comment: this is a registered trial, and the study has reported all outcomes mentioned in the protocol |
| Other bias | Low risk | Comment: study seems to be free from other biases |
Ijumba 2015.
| Methods | Community randomised trial in an urban informal settlement, KwaZulu‐Natal, South Africa | |
| Participants | Participants included all consenting pregnant women < 17 years of age and their newborns residing in clusters. In the intervention (n = 15) and control (n = 15) clusters (total = 30), community health workers (CHWs) identified all pregnant women living in their cluster. CHWs were women who resided in the neighbourhoods and were literate. Data were available for 1659 intervention and 1902 mother‐infant pairs at 12 weeks postpartum | |
| Interventions | Intervention: CHWs were trained and implemented the Goodstart intervention package through a structured home visiting programme consistent with existing programmes to "prevent mother‐to‐child transmission" of HIV, Integated Management of Childhood Illness, lactation counselling, and newborn care guidelines. Motivational interviewing techniques were used for breastfeeding counselling ‐ to assist women to engage in social supports (e.g. family members, community resources). Women in the intervention arm were scheduled to receive 7 home‐based visits (1 within 48 hours of delivery, during days 3 to 4 and 10 to 14, and during weeks 3 to 4 and 7 to 8) (n = 1659) Control: women were provided with information and support by CHWs for accessing social welfare grants and also conducted 3 home‐based visits (1 during pregnancy, 2 during weeks 4‐6 and 10‐12 post delivery). CHWs provided home‐based education and support for families to understand how to obtain the grant, the necessary papers and procedures. (n = 1,902) |
|
| Outcomes | Primary outcomes: exclusive and appropriate breastfeeding at 12 weeks postnatally, HIV‐free infant survival Secondary outcomes: uptake of postnatal clinic visit within 7 days of life, coverage of care and behavioural indicators, post‐intervention levels of maternal depressed mood at 12 weeks postpartum |
|
| Notes | Funding: funded through a grant to Save the Children’s Saving Newborn Lives programme, from the Bill & Melinda Gates Foundation. No funding bodies had any role in study design, data collection and analysis, or decision to publish, nor in preparation of the manuscript | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...neither stratification nor matching was performed. We used simple computer‐generated randomisation, with clusters assigned in a 1:1 allocation ratio" Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Comment: this study was a cluster‐randomised effectiveness trial; allocation concealment should not have been an issue due to the randomisation process |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: could not blind participants or personnel to the intervention due to training and receipt of treatment |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there was loss to follow‐up in 2 periods of the study: first in pregnant women (attrition rate 2.1%) and then following birth (attrition rate 9.5%) |
| Selective reporting (reporting bias) | Low risk | Comment: this is not a registered trial, but outcomes mentioned in the methods are reported in the results |
| Other bias | Low risk | Study seems to be free from other biases |
Jokhio 2005.
| Methods | Cluster‐randomised controlled trial in Pakistan | |
| Participants | All pregnant women were eligible for inclusion. Seven subdistricts (talukas) were randomly assigned to intervention (n = 3) or control (n = 4). Total numbers of participants were 10,114 and 9443 in intervention and control arms, respectively (total = 19,557). Completed follow‐up data of 42 days resulted in 10,093 women in intervention and 9432 in control (total = 19,525) | |
| Interventions | Intervention: traditional birth attendants (TBAs) were trained by obstetricians and female paramedics and issued disposable delivery kits. Lady Health Workers linked TBAs to established services and documented processes and outcomes. Obstetrical teams provided outreach clinics for antenatal care. TBAs were asked to visit each woman at least 3 times during pregnancy for danger signs Control: women received standard care by Lady Health Workers; TBAs did not receive additional training nor disposable delivery kits |
|
| Outcomes | Primary outcomes: perinatal and maternal mortality Secondary outcomes: major complications of pregnancy (haemorrhage, obstructed labor, puerperal sepsis, eclampsia, abortion), referral by TBA for emergency obstetrical care, types and places of delivery, delivery attendant |
|
| Notes | Funding: supported by a grant from Family Health Project of the Sindh government’s health department (for capital costs) and by the University of Birmingham (for data entry). There is no statement relating to involvement of these funding parties in the design and conduct of the study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "with a simple cluster‐randomisation sampling scheme, and with a computer‐generated procedure, Larkana’s seven talukas were allocated to intervention or control groups" Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Comment: since this is a cluster‐randomised trial, allocation concealment should not be an issue, as in this design, all clusters are randomised at once |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "although the traditional birth attendants and Lady Health Workers could not be blinded to the intervention, observer bias is unlikely to have affected the reporting of the primary outcomes of perinatal and maternal mortality" Comment: probably not done |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Lady Health Workers who recorded outcomes could not be blinded to the intervention status of the women but were not made aware of the main study objective or the outcome measures for the planned comparison" Comment: probably not done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: acknowledged loss to follow‐up but no reasons were given; attrition rate was 2% in the intervention arm and 1.2% in the control arm, and reasons were not provided |
| Selective reporting (reporting bias) | Low risk | Comment: not a registered trial; however, outcomes reported in the methods section were reported in the results section |
| Other bias | Low risk | Study seems to be free from other biases |
Kirkwood 2013.
| Methods | Cluster‐randomised trial (NEWHINTs) undertaken in Ghana | |
| Participants | 49 zones were randomly assigned to intervention and 49 to control. From the control arm, n = 10,096 pregnant women were recruited, and n = 9885 pregnant women were recruited from the NEWHINTs zones (intervention). Data analysis included all pregnancies that result in live birth or stillbirth (total of 16,329 deliveries during trial period) | |
| Interventions | Community‐based surveillance volunteers (CBSVs) in intervention zones were trained to identify pregnant women in their community and to counsel and solve problems related to key essential newborn care behaviours. CBSVs were trained to make 2 home visits during pregnancy, and 3 in the first week of life, to promote newborn care practices, to weigh and assess babies for danger signs, and to refer if necessary (n = 8035 eligible deliveries) Control: routine maternal and child health care, including antenatal clinics, access to free fertility delivery, postpartum checkups, infant welfare clinics, and routine CBSV activities.(n = 8294 eligible deliveries) |
|
| Outcomes | Primary outcome: all‐cause neonatal mortality rate (all deaths that happen in the first 28 days of life), % of mothers practising the Newhints recommended behaviours Secondary outcomes: age‐specific and cause‐specific neonatal mortality rates |
|
| Notes | Funding: WHO; Save the Children’s Saving Newborn Lives Programme from the Bill & Melinda Gates Foundation; and UK Department for International Development provided funding. Funders had no role in data gathering, data analysis, or writing of the report | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated restricted randomisation using stratified sampling (1‐to‐1 ratio) Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Comment: since this is a cluster‐randomised trial, allocation concealment should not be an issue, as in this design, all clusters are randomised at once |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: could not blind community‐based surveillance volunteers as they were trained |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: attrition (4.9%) and reasons were reported |
| Selective reporting (reporting bias) | Low risk | Comment: this is a registered trial, and this study has reported all outcomes mentioned in the protocol |
| Other bias | High risk | Comment: some pregnant women swapped groups (intervention or control) during the trial |
Kumar 2008.
| Methods | Three‐arm cluster‐randomised trial done in Shivgarh, a rural area in Uttar Pradesh, India | |
| Participants | Pregnant women, mothers‐in‐law, other female members who played supportive role, male members including fathers‐in‐law and husbands, family's immediate support group including neighbours and relatives who influenced family behaviours and helped with delivery (n = 3762 births) | |
| Interventions | Intervention 1 (n = 1537): essential newborn care only Control (n = 1115): usual services of governmental and non‐governmental organisations in the area Essential preventive newborn care includes home visits and group meetings of stakeholders about birth preparedness, hygienic delivery, and immediate newborn care, including clean umbilical cord, and skin care and thermal care including skin‐to‐skin care, breastfeeding, and care‐seeking from trained providers. The second intervention group received essential newborn care plus use of a liquid crystal sticker that indicates hypothermia by changing colour. All messages were designed to promote newborn care practices to align with existing cultural values and traditions of the local area. Saksham Sahayak (community health worker) were recruited and received classroom‐based and apprenticeship‐based field training on knowledge, attitudes, and practices about essential newborn care, behaviour change management, and trust building. Saksham Sahayaks first engaged with community stakeholders in community meetings to seek their approval, sensitise them toward the importance of their role in newborn survival, encourage shared learning, and create a supportive environment in community. Pregnant women were identified by Saksham Sahayak, self‐reporting of pregnant women, and other community health workers. Intervention was delivered by 2 antenatal visits (60 days and 30 days before expected delivery) and 2 postnatal visits (first within 24 hours of birth and second on day 3). No treatment was offered to sick neonates; however, they were advised to seek care at nearest health facility |
|
| Outcomes | Primary outcomes: miscarriages, stillbirths, live births, neonatal deaths Secondary outcomes: other pregnancy outcomes, neonatal care outcomes |
|
| Notes | We included in this analysis data for Intervention 1 (i.e. essential newborn care) vs control Funding: study was funded by the United States Agency for International Development, Delhi Mission, and the Saving Newborn Lives program of Save the Children US through a grant from the Bill and Melinda Gates Foundation. There is no statement about the influence these funding sources had on design and cohort of the study |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "stratified cluster randomisation was done at Johns Hopkins University using Stata 7.0 (StataCorp, College Station, TX, USA) to allocate the 39 cluster units randomly to the three study groups, yielding three allocation sequences of 13 clusters each" Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Comment: since this is a cluster‐randomised trial, allocation concealment should not be an issue, as in this design, all clusters are randomised at once |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: because of the nature of the intervention, blinding did not occur |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition (3.9%) was given along with reasons |
| Selective reporting (reporting bias) | Low risk | Comment: this is a registered trial, and this study has reported all outcomes mentioned in the protocol |
| Other bias | Low risk | Study seems to be free from other biases |
Lewycka 2013.
| Methods | Factorial, cluster‐randomised controlled trial in rural Mawali (Mai Mwana trial) | |
| Participants | A cohort of women that of child‐bearing age (10 to 49 years) was defined at a baseline survey (n = 43,719), and those who consented were enrolled to participate. 48 clusters were defined, with populations of approximately 3000 people, and these clusters were randomly allocated to 1 of the 4 study groups. Monitored outcomes of 26,262 births between 2005 and 2009 | |
| Interventions | Four study groups were included: (1) women's groups and volunteer peer counselling, (2) only women's groups, (3) only volunteer peer counselling, (4) no intervention Facilatator guided women's groups through a community action cycle, to tackle maternal and child health problems (n = 207 groups) Trained volunteer peer counsellors made home visits at 5 time points during pregnancy and after birth to support breastfeeding and infant care. (n = 75 female volunteer peer counsellors) |
|
| Outcomes | Primary outcomes: women's groups: maternal, perinatal, neonatal, and infant mortality rates; peer counsellors: infant mortality rates, exclusive breastfeeding rates Secondary outcomes: maternal and infant morbidity; skilled antenatal, delivery, and postnatal care; tetanus toxoid immunisation; use of malaria prophylaxis; insecticide‐treated bed nets during pregnancy; PMTCT services; infant immunisations; early BF; reduced use of prelacteal feeds; neonatal mortality and infant morbidity rates; caretaker practices; timing of initiation of breastfeeding; family planning (condom use) |
|
| Notes | Funding: Saving Newborn Lives, UK Department for International Development, and Wellcome Trust. There is no statement related to involvement of these funding parties in design and conduct of the study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "clusters of villages, rather than individual villages, were used as the unit of randomisation to further reduce rates of travel across cluster boundaries and the possibility of contamination"; "allocated clusters with a random number sequence generated in Stata" Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote: "the interventions made masking of allocation impossible at the participant level, allocation was masked for data analysis" Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: the women knew their intervention allocation due to activities that they undertook |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: attrition (14.5%) was given with reasons |
| Selective reporting (reporting bias) | Low risk | Comment: this is a registered trial, and this study has reported all outcomes mentioned in the protocol |
| Other bias | Low risk | Study seems to be free from other biases |
Magoma 2013.
| Methods | Cluster‐randomised trial in Ngorongoro district, Arusha region in Tanzania, involving 16 health units to determine the effectiveness of birth plans | |
| Participants | 16 health units were randomly assigned to control or intervention (8 health units per arm). 905 pregnant women at > 24 weeks' gestation (intervention arm, n = 404) were enrolled in the study | |
| Interventions | Intervention arm: introduction and promotion of birth plans by care providers during antenatal care to prepare women and their families for birth and complication readiness. This involved discussions on topics of delivery (planned place, importance of skilled delivery, transport arrangements to delivery site or during an emergency, funding arrangements/emergency care services, identification of possible blood donors, identification of birth companion, support for looking after the household) and strategies for overcoming barriers and recognising danger signs. Providers in the intervention arm were given a birth plan intervention guide with instructions on how to assist women to develop and achieve their birth plans. These providers also received practical and didactic training for 2.5 days Control arm: providers in the control units were trained for a half‐day on how to collect required information (n = 501 women) |
|
| Outcomes | Primary outcome: delivery by individual woman in a health unit Secondary outcomes: postnatal care attendance, satisfaction of women and providers with care received and provided, respectively |
|
| Notes | Funding: World Health Organization/HRP (UNDP/UNFPA/WHO/World Bank Special Programme of Research, Development and Research Training in Human Reproduction). There is no statement related to involvement of these funding parties in design and conduct of the study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "the facilities were randomly assigned to the intervention or control arms in a 1:1 ratio using computer‐generated random numbers" Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Comment: since this is a cluster‐randomised trial, allocation concealment should not be an issue, as in this design, all clusters are randomised at once |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "the study did not allow blinding of either providers or women who participated in the study to the treatment allocation" Comment: probably not done |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "the data collectors were people from the same villages as the study participants, which facilitated the identification of women’s location for the follow‐up interview" Comment: probably not done |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: 0% attrition rate reported, perhaps due to use of health units as clusters |
| Selective reporting (reporting bias) | Low risk | Comment: not a registered trial, but outcomes mentioned in the methods were reported in the results |
| Other bias | Low risk | Study seems to be free from other biases |
Manandhar 2004.
| Methods | Cluster‐randomised controlled trial conducted in Makwanpur district of Nepal. Between 1998 and 2000, local community leaders and interested parties were taken into confidence | |
| Participants | Inclusion criteria included 15 to 49 years of age, married, and potential to conceive within the study period Exclusion criteria were age under 15 or over 49 years, unmarried, permanently separated or widowed, and no potential for conception within study period. A village development committee (VDC) was taken as a unit of randomisation. 42 rural VDC were matched to 21 pairs on the basis of geography, ethnicity, and population. Total number of participants was 28,931 women |
|
| Interventions | Intervention (n = 2972): monthly meetings of mothers in groups to identify maternal and neonatal problems; prioritisation of problems; possible solutions, planning, implementation, and monitoring of those solutions; and sharing information with others. Primary cycle consisted of series of 10 meetings Control (n = 3303): there was no active intervention in the control area. However, benefits to control clusters were improvements in equipment and training provided at all levels of already existing government healthcare system Married women of reproductive age were identified through a door‐to‐door baseline survey. A community surveillance system was put in place. This system was responsible for monthly visits by local women for enumerations and to monitor pregnancy status of women in the cohort. After identification of pregnancy, interviews were carried out by VDC interviewer at 7 months of gestation and 1 month postpartum. All pregnancies occurring within the cohort were followed at least 6 weeks after delivery. In the first year, facilitation team's skills were developed and groundwork was laid by exploring ideas about child birth |
|
| Outcomes | Primary outcomes: neonatal mortality rate, perinatal mortality rate Secondary outcomes: antenatal care services usage, perinatal illness, birth practices, health care‐seeking behaviour, newborn care practices, breastfeeding practices, infant mortality |
|
| Notes | Perinatal birth attendants were available in all localities Funding: representatives of the UK Department for International Development (DFID) suggested that no healthcare activities should be carried out in parallel with existing government services and that—for sustainability reasons—no funding should be available for women’s group activities. Apart from these issues, sponsors of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "pairing was based on a process of topographic stratification"; "we used a list of random numbers to select 12 pairs"; "we randomly allocated one cluster in each pair to either intervention or control on the basis of a coin toss" Comment: probably done |
| Allocation concealment (selection bias) | Low risk | This is a cluster‐randomised controlled trail so allocation concealment is not an issue |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "because of the nature of the intervention the trial allocation was not masked" Comment: probably not done |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Exclusion and attrition (9.5%) were described with reasons |
| Selective reporting (reporting bias) | Low risk | Comment: this is a registered trial, and this study has reported all outcomes mentioned in the protocol |
| Other bias | Low risk | Study seems to be free from other biases |
McConnell 2016.
| Methods | Randomised trial of community health workers using a postnatal checklist to increase health‐seeking behaviours and knowledge in new mothers in Kiambu County, Kenya | |
| Participants | Women were recruited after a normal delivery just before discharge home. A total of 104 women were enrolled in the study; 41 were assigned to the community health worker (CHW) phone call group, 32 to the CHW home visit, and 31 to standard care | |
| Interventions | Randomised into 1 of 3 groups: 1. Early postnatal care 3 days after delivery provided in‐person with a CHW using a simple checklist 2. Care provided by phone with a CHW using the same checklist 3. Standard care (control) CHWs in the intervention groups were trained to screen for maternal and neonatal danger signs, to deliver targeted postnatal health education, and to refer mothers and their newborns to facility‐based care guided by a checklist. For all referrals, a nurse conducted a follow‐up by phone call to ensure that mother and newborn received appropriate care |
|
| Outcomes | Primary outcomes: 3 days postpartum (maternal or newborn complications detected or referred), day 10 and 9‐week survey outcomes (care‐seeking behaviours for mothers and newborns, knowledge and practice of infant care, nutrition, feeding, recognition of danger signs) | |
| Notes | Funding: this research was supported by Grand Challenges Canada, funding award number 0166–03. There is no statement related to involvement of these funding parties in the design and conduct of the study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were individually randomised prior to enrolment using numeric patient identifiers assigned by Jacaranda Health"; "Randomization was conducted by assignment of each of these unique identifiers to one of the three central treatment groups with equal probability"; "Random assignment of patient identifiers was done using a randomisation sequence generated by the principal investigators with STATA 11" Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Comment: since this is a cluster‐randomised trial, allocation concealment should not be an issue, as in this design, all clusters are randomised at once |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "our study also had significant attrition, both in the implementation of the interventions and tracking of respondents at the ten‐day follow‐up survey and nine week follow‐up survey" |
| Selective reporting (reporting bias) | Low risk | Comment: this is a registered trial, and this study has reported all outcomes mentioned in the protocol |
| Other bias | High risk | Comment: mention of reporting bias due to self‐reporting method related to cord care and exclusive breastfeeding |
Memon 2015.
| Methods | Study of exploratory quasi‐experimental design to determine the impact of community‐based perinatal and newborn preventive care package on perinatal and neonatal mortality in Northern Parkistan | |
| Participants | Overall population for the study district was 283,324, comprising 35,641 households; the intervention area comprised 16,802 households and a population of 137,781; the control area covered 18,659 households with a population of 145,543. A total 165 LHWs and CHWs were trained with additional curriculum on essential newborn care to deliver the intervention package | |
| Interventions | Intervention package: Lady Health Workers and community health workers were trained on causes of perinatal and newborn mortality and risky maternal and newborn care practices, and they were trained to educate families on these topics. They were trained to deliver the intervention package that involves developing awareness related to positive maternal and newborn healthcare practices at a household level, involving the importance of seeking antenatal care, adequate nutrition during pregnancy and lactation, and skilled birth attendance. The intervention package was implemented through monthly household visits, one‐to‐one counselling sessions with pregnant women, and video sessions in communities (group education) (n = 849 total births in intervention areas post intervention) Control: these areas received the routine services of government and non‐government‐provided services (n = 863 total births in control areas post intervention) | |
| Outcomes | Outcome measures: change in maternal and newborn practices, perinatal and neonatal mortality rates | |
| Notes | Funding: jointly funded by University Research Council, Aga Khan University, Pakistan, and Saving Newborn Lives Initiative, Save the Children, US. There is no statement related to involvement of these funding parties in design and conduct of the study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "the overall population of 283,324 comprising 35,641 households located in the study district was allocated to intervention and control areas based on geographical proximity to avoid contamination and manage logistics and undertake the study with limited resources available"; "forty‐eight villages were randomly selected for this phase" Comment: probably not done |
| Allocation concealment (selection bias) | High risk | Comment: this is a quasi‐experimental design |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Attrition of pre‐intervention and post‐intervention populations 4.4%; no explanation for losses to follow‐up Comment: insufficient information to permit judgement |
| Selective reporting (reporting bias) | Low risk | Comment: this is a registered trial, and this study has reported all outcomes mentioned in the protocol |
| Other bias | Low risk | Study seems to be free from other biases |
Mersal 2013.
| Methods | Experimental randomised controlled study evaluating effects of prenatal counselling on compliance with health promotion and teenage pregnancy outcomes in Egypt | |
| Participants | A sample of 86 teenage pregnant women attending the maternal and child health centre, with 43 mothers and their newborns in the control group and the other 43 mothers and newborns in the educational intervention group | |
| Interventions | Educational intervention: a prenatal counselling programme was designed by researchers and 3 to 4 sessions were conducted per pregnant woman. A pre‐test on knowledge about health promotion was conducted before counselling and a post‐test was conducted on all participants 3 months after the pre‐test. Pregnany outcome was assessed in 3 to 6 days at home post delivery (n = 47 pregnant teenagers) Control: participants were recruited from the same health centre; further details of their care were not provided (n = 46) |
|
| Outcomes | Outcomes: knowledge regarding health promotion pre‐counselling and post‐counselling, pre‐counselling and post‐counselling compliance regarding aspects of health promotion (e.g. antenatal follow‐up, hygiene), pregnancy outcomes (gestational age at labour, type of delivery, full‐term/pre‐term baby, general condition of baby, birth weight, breastfeeding, umbilicus) | |
| Notes | Funding: no mention of funding | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Comment: this is a randomised controlled study; however the randomisation process is not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "4 were excluded from the study group and 3 from the controls owing to incomplete data" Comment: explanation was included; attrition 7.5% |
| Selective reporting (reporting bias) | Unclear risk | Comment: this is not a registered trial; outcomes mentioned in the methods section are reported in the results section |
| Other bias | Low risk | Study seems to be free from other biases |
Midhet 2010.
| Methods | Cluster‐randomised controlled trial implemented during 1998 to 2002 in 32 village clusters in Khuzdar, a rural district of Balochistan province in Pakistan | |
| Participants | Women of reproductive age and their husbands (n = 2561) | |
| Interventions | Information and Education for Empowerment and Change (IEEC) (n = 836) for women was designed to increase awareness of safe motherhood and neonatal health. Each facilitator initially invited 10 to 12 women from close villages to participate in a support group. Local traditional birth attendants (TBAs) ‐ who deliver over 90% of all births in the project area ‐ were trained in clean home delivery and in recognising common obstetrical and newborn emergencies. The project also facilitated timely referral and transportation of obstetrical and newborn emergencies to the district hospital. A typical support group started with a discussion of the problems faced by women during pregnancy and childbirth. Participants were then asked to look at their booklets while listening to a cassette tape that guided them through the pictures in the booklet. The pictures formed part of the dramatised stories recorded on the tape, thus creating an audiovisual effect. The booklet covered the following topics: family planning; nutrition; preparation for pregnancy and delivery; and danger signs during pregnancy, at delivery, and postpartum. Typically, the booklet was finished in 6 sessions of 1 to 2 hours each, after which participants were entitled to have their personal copy of the booklet and audiocassette. The husbands' IEEC (n = 703)was implemented in 8 village clusters randomly selected from the 16 intervention clusters. Husbands' booklets and audiocassettes were designed after formative research with married men. Then in each village cluster, 20 to 30 male community volunteers were identified who distributed the materials among husbands of the women who had participated in the support groups The control group received standard care (n = 1022) |
|
| Outcomes | Primary outcomes: neonatal mortality, perinatal mortality Secondary outcomes: iron supplementation, tetanus immunisation |
|
| Notes | Funding: funded by NICHD, USAID, UNICEF, World Health Organization, British Council, Government of Japan, and The Asia Foundation, and implemented by The Asia Foundation’s Islamabad office. There is no statement related to the involvement of these funding parties in design and conduct of the study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomisation took place separately within each of the three zones; equal numbers of village clusters were randomly allocated to the intervention or control sites (by blindly drawing village cluster names written on folded chits)" Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote: "randomisation took place separately within each of the three zones; equal numbers of village clusters were randomly allocated to the intervention or control sites (by blindly drawing village cluster names written on folded chits)" Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: insufficient information to permit any judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: insufficient information to permit any judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: insufficient information to permit any judgement |
| Selective reporting (reporting bias) | Unclear risk | Comment: this is not a registered trial, but outcomes mentioned in the methods section are reported in the results section |
| Other bias | Low risk | Study seems to be free from other biases |
More 2012.
| Methods | Cluster‐randomised controlled trial in 24 intervention and 24 control settlements covering a population of 283,000 in India | |
| Participants | Key participants were women who joined groups in 24 intervention clusters to be compared with 24 control clusters (n = 15,192 births) | |
| Interventions | Intervention (n = 7656 births): study recruited 1 full‐time facilitator in each intervention cluster of about 1000 households. This sakhi (friend) was a local woman with secondary education and leadership skills, preferably married with children. Her role was to conduct meetings with women, attend planning and supervision of meetings, and support group action. After training, she began by profiling her settlement and building rapport with local stakeholders. She also attended regular training on a range of healthcare topics. Over about 6 months, she set up 10 women's groups, formative work having shown that women's mobility tended to be confined to their own alley. The groups met fortnightly, and she met weekly with other sakhis and her supervisor. The intervention followed a 36‐meeting cycle that was pre‐determined in general but developed iteratively in detail. There was no set point at which women had to join a group, and women of all ages, pregnant and non‐pregnant, were welcome to participate. Study took a participatory approach with emphasis on sharing and peer learning, rather than on the sakhi as an expert resource, and used the change method of appreciative inquiry to focus on the positive and to build energy for action through identification of the strengths of participants and their families and neighbourhoods Control (n = 7536): standard care |
|
| Outcomes | Perinatal care, maternal morbidity, extended perinatal mortality | |
| Notes | Funding: interventions involved in the City Initiative for Newborn Health were funded by the ICICI Foundation for Inclusive Growth – Centre for Child Health and Nutrition. Evaluative aspects of the trial were funded from 2007 by The Wellcome Trust. DO was funded by a Wellcome Trust Fellowship (081052/Z/06/Z). The funding agency had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "in a transparent process, social workers external to the trial drew lots to select 48 in blocks of eight per ward, and then to allocate four clusters per block to the intervention" Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote: "the nature of the intervention precluded allocation concealment" Comment: since it was a cluster randomised trial, allocation concealment should not be an issue, as in this design, all clusters are randomised at once |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "emphasis on participation and demystification of research" Comment: probably not done |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up was around 18% in both intervention and control arms, and reasons for loss to follow‐up were similar across both arms |
| Selective reporting (reporting bias) | Low risk | Comment: this is a registered trial, and this study has reported all outcomes mentioned in the protocol |
| Other bias | Low risk | Comment: study seems to be free from other biases |
Pasha 2013.
| Methods | Community‐based, 2‐arm, cluster‐randomised trial that tested whether a community‐ and facility‐based approach can improve pregnancy outcomes in low‐resource settings where there is limited access to quality obstetrical and neonatal care | |
| Participants | This trial included all pregnancies of residents in 106 clusters, ranging from 6 clusters in Argentina to 24 in Pakistan, which were randomised with 53 in each treatment group. Outcomes were recorded for all women with newborns at ≥ 1000 grams and/or > 28 weeks who consented to be included in the study. A total of 70,351 pregnant women were screened in the intervention clusters and 66,830 in the control clusters (59,189 and 57,929 women in the intervention and control clusters, respectively, were eligible and consented) | |
| Interventions | Intervention: each cluster included a cluster team that comprised healthcare providers, local residents, and study personnel to develop and implement comprehensive interventions to improve the quality of obstetrical and neonatal care. These teams worked within their community and local healthcare system to facilitate a multi‐faceted intervention that involved 2 components. The first component involved community mobilisation to establish village core groups and strengthen community capacity to identify needs and barriers related to obstetrical and neonatal care. Each village core group was trained to move through a cycle to organise, plan, explore, act on, and evaluate perinatal and maternal outcomes in their community. The second component consisted of Home‐based Life Saving Skills (HBLSS) training for birth attendants and families to recognise danger signs (e.g. prolonged labor, infection) and appropriate stabilisation methods that can be employed in homes and in first‐level care facilities. This component also involved improvement of quality care through a combination of facility staff Emergency Obstetric and Newborn Care (EmONC) training for clinical care for the major causes of perinatal and maternal mortality. This EmONC trial utilised a train‐the‐trainer model, the aforementioned 2 components of intervention training were combined, and this trial emphasised the Community Action Cycle and relevant HBLSS modules Control: some clusters were assigned to control with no intervention, and data were collected for comparison (no extensive detail for methods) |
|
| Outcomes | Primary outcome: perinatal mortality (defined: composite of stillbirth and 7‐day neonatal mortality per 1000 births among births occurring at > 28 weeks' gestation or > 1000 g) Secondary outcomes: rate of stillbirth (fresh and macerated), 7‐day neonatal mortality, 28‐day neonatal mortality, maternal death |
|
| Notes | Funding: this trial was funded by grants from the US National Institutes of Health (NIH). The NIH programme officers participated in protocol development and study monitoring and reviewed the manuscript | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomization was performed at the cluster level, stratifying by rates of the primary outcome (stillbirth and early neonatal death) and number of deliveries. The data coordinating centre produced a computer‐generated randomisation algorithm which assigned clusters at a 1:1 ratio within each stratum" Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Comment: since this was a cluster‐randomised trial, allocation concealment should not be an issue |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "because of the nature of the intervention, there was no masking" Comment: probably not done |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "an additional 3,139 (4.5%) and 2,862 (4.3%) deliveries in intervention and control clusters were excluded at delivery due to missing data or other ineligibility criteria" |
| Selective reporting (reporting bias) | Low risk | Comment: this is a registered trial, and this study has reported all outcomes mentioned in the protocol |
| Other bias | Low risk | Comment: study seems to be free from other biases |
Penfold 2014.
| Methods | Cluster‐randomised controlled trial investigating the effect of home‐based counselling on newborn care practices based in southern Tanzania | |
| Participants | 6 districts that consisted of 132 wards; 65 of these wards were allocated to the home‐based counselling intervention with routine care, and 67 received routine care only. After randomisation, there were no exclusion criteria for clusters, households, or women. All villages in the intervention wards recruited volunteers to implement the counselling intervention. Over 800 women who volunteered were recruited from and by their communities and were trained to make contact with every pregnant woman in their village A household survey sample size was based on the number of women age 13 to 49 who had given birth in the year before the survey and had received counselling or routine care. It was assumed that 1 woman of reproductive age resided in each household. 131 of the 132 wards were visited, and a total of 5217 households were visited. Of these households 4989 (96%) household heads agreed to participate ‐ 2491 in intervention areas and 2498 in comparison areas. For the final analysis, there were 512 women (n = 257 in intervention, n = 255 in comparison) from 128 subvillages who had delivered 521 live births during the study period and who answered the detailed survey questions |
|
| Interventions | Intervention: for every pregnant woman identified, a village volunteer was expected to make 3 visits to her home during pregnancy and 2 in the early neonatal period, with additional visits for small babies born. Counselling involved one‐on‐one interaction between volunteer and mother; there were also discussions with other family members (fathers and mother‐in‐laws) involving decision‐making around childbirth and newborn care. Counselling included behaviour change messages, specifically, hygiene during delivery (e.g. birth assistants using gloves), initiation of and exclusive breastfeeding, and identification of and care for small babies. Additional behaviour messages included birth preparedness (importance of health facility delivery, cleanliness of materials and money), delayed bathing of baby, and putting nothing on the cord. Postnatal counselling visits focused on reinforcing and supporting mothers to implement appropriate neonatal care. Volunteers also utilised Mtunzi counselling tools during their visits, including picture‐based cards that illustrated the counselling messages for each visit; these were left with the family to spread the message to other family members and to assist with retention. Volunteers also used a locally made doll to demonstrate the breastfeeding position and skin‐to‐skin care Control: routine care | |
| Outcomes | Primary outcomes: breastfeeding within an hour of delivery, birth attendants for home deliveries washing hands before childbirth or wearing gloves, babies fed only breast milk for the first 3 days Secondary outcomes: other behaviours promoted during counselling visits to maximise newborn health (e.g. birth preparedness, skilled attendance for childbirth, immediate drying and wrapping of baby, clean cord care, delayed bathing of the baby) |
|
| Notes | Funding: funded by the Bill & Melinda Gates Foundation through the Saving Newborn Lives programme of Save the Children, Unicef, the Laerdal Foundation, and the Batchworth Trust. Funders had no role in design and conduct of the study; in collection, analysis, and interpretation of data; nor in preparation, review, or approval of the manuscript | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "114 wards in 5 districts with baseline data...were randomised using implicit stratification to maximise balance in intervention and comparison groups... allocated the wards in each 'pair' to intervention of control using random numbers generated by Microsoft Excel"; "We used multi‐stage sampling to select households" Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Comment: since this is a cluster‐randomised trial, allocation concealment should not be an issue, as in this design, all clusters are randomised at once |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "the nature of the intervention prevented blinding researchers, community members or health staff to the allocation" Comment: probably not done |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: insufficient information to permit judgement |
| Selective reporting (reporting bias) | Low risk | Comment: this is a registered trial, and this study has reported all outcomes mentioned in the protocol |
| Other bias | Low risk | Comment: study seems to be free from other biases |
Persson 2013.
| Methods | Cluster‐randomised controlled trial conducted in Quang Ninh province, Vietnam | |
| Participants | A cluster constituted the geopolitical unit known as a commune (which includes a Commune Health Centre (CHC) in each commune); 99 communes were included in the trial and 44 were allocated to intervention and 46 to control. All mother‐newborn pairs within the study area with births from July 2008 to June 2012 were eligible to be included in the trial. 22,561 births were registered in the study area during the trial period | |
| Interventions | Intervention: consisted of facilitated work of maternal and child stakeholder groups on the commune level that included identification of local perinatal health problems followed by a problem‐solving cycle. Lay women were recruited from Women's Unions in the province to act as facilitators in supporting CHC staff and key commune stakeholders in improving perinatal healthcare practices. Training of facilitators involved theoretical sessions, group discussions, role‐plays, and field practices, which covered topics of group dynamics, quality improvement methods, and basic evidence‐based perinatal care. Each intervention commune included a Maternal and Newborn Health Group, which consisted of 8 members (including 3 CHC staff, 1 village health worker of the commune, 1 population collaborator, the chairperson or vice chairperson of the commune, and 2 WU representatives). Facilitators mainly mobilised these groups by using the plan‐do‐study‐act cycle to identify and prioritise local perinatal health problems and to accomplish improvement cycles Control: no details were provided (n = 10,655 births in control communes) |
|
| Outcomes | Primary outcome: neonatal mortality Secondary outcomes: care‐seeking behaviour (attendance to antenatal care, tetanus immunisation during antenatal care, reported material and financial preparedness for birth delivery, institutional delivery), exclusive breastfeeding (initiation of breastfeeding within 1 hour), temperature control at delivery, home visit by midwife first week after delivery, perinatal health knowledge of primary health staff |
|
| Notes | Funding: funded by the Swedish International Development Cooperation Agency (Sida), Swedish Research Council, and Uppsala University. The funding agency had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "an individual randomisation was not possible, due to the intervention on the commune level"; "A random number list was used to subsequently allocate "intervention" or "control" to the list of communes" Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote: "the sequence was concealed until the intervention was assigned; otherwise the allocation was not masked" Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "the training program of the facilitators included theoretical sessions, group discussions, role‐plays, and field practice" Comment: blinding of personnel was not possible due to the training element of the trial; there was no mention of blinding of participants; this was probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: all communes were accounted for; due to the nature of the trial, the attrition rate cannot be calculated (i.e. recorded only birth outcomes with no mention of loss to follow‐up for mothers). Not enough information |
| Selective reporting (reporting bias) | Low risk | Comment: this is a registered trial, and this study has reported all outcomes mentioned in the protocol |
| Other bias | Low risk | Comment: study seems to be free from other biases |
Soofi 2017.
| Methods | Community‐based cluster‐randomised trial in rural Pakistan | |
| Participants | All households in the selected districts were included in this study. There were 27 randomised clusters, including 35 healthcare facilities and their affiliated Lady Health Workers (LHWs), with the entire population of the clusters included in the trial. 13 clusters were allocated to the intervention group (n = 242,740) and 14 to the control group (n = 256,985). During the trial intervention period, 51,436 pregnancies were identified | |
| Interventions | Intervention: package focused on immediate household management of intrapartum events, recognition of low birth weight and suspected serious neonatal infection, and prompt referral to public sector hospitals LHWs from the intervention clusters were trained at their affiliated health facility and were provided with a bag and mask for neonatal resuscitation and oral amoxicillin. LHWs were also given pictorial guides depicting the management of asphyxia, thermal care, co‐bedding, breastfeeding of low birth weight babies, and recognition of suspected pneumonia and administration of oral amoxicillin before referral. LHWs were also encouraged to maintain links with traditional birth attendants, to keep records of expected births, and to attend home deliveries. Pregnant women in the intervention clusters were provided with clean delivery kits during health education sessions delivered by LHWs and the importance of provision of urgent neonatal care at birth. LHWs were also trained as per national and project guidelines to do additional postnatal visits (days 3, 5, 14, 28 after birth). With regard to traditional birth attendants in the intervention arm, a programme in basic immediate maternal and newborn care was organised, and attendants were trained in the use of clean delivery kits. Separate training sessions on health education and community mobilisation were held for male community members, during which meetings aimed to promote antenatal and postnatal care and facility births (n = 19,984 deliveries) Control: received routine care through the existing national programme, with the LHW programme functioning as usual (n = 18,325 deliveries) |
|
| Outcomes | Primary outcome: all‐cause neonatal mortality Secondary outcomes: cause‐specific neonatal mortality due to intrapartum events, prematurity, and sepsis; stillbirth rate |
|
| Notes | Funding: Saving Newborn Lives, Save the Children USA. There is no statement related to involvement of these funding parties in design and conduct of the study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "to ensure reasonable balance between the two arms, we used stratified, restricted randomisation to allocate clusters"; "1 million random allocation schemes were generated by the study statistician (SC), who used a computer algorithm" Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Comment: as this is a cluster‐randomised trial, allocation concealment should not be an issue because the clusters are randomised at once |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "delivery of the intervention was not blinded for practical reasons" Comment: probably not done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "verbal autopsies of all stillbirths and neonatal deaths were done by a separate team of trained anthropologists within 2–16 weeks of the event with standard WHO recommended instruments" Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reasons given (migrated or lost to follow‐up). Attrition (%) was 9.5% in each of intervention and control |
| Selective reporting (reporting bias) | Low risk | Comment: this is a registered trial, and this study has reported all outcomes mentioned in the protocol |
| Other bias | Low risk | Comment: study seems to be free from other biases |
Srinivasan 1995.
| Methods | Randomised controlled field trial in rural south India | |
| Participants | 4 primary health centres were selected from each of 3 subcentres: therefore 12 subcentres in Karur health unit district, Tamil Nadu. 3 packages (2 intervention and 1 control) were implemented and included 294 pregnant women in the high‐risk package, 242 in the uniform package recommended by the Tamil Nadu Government, and 335 in the control package | |
| Interventions | Intervention: all packages were implemented by trained female auxiliary nurse midwives (ANMs). (1) The high‐risk package involved ANMs detecting pregnancies between the 12th and 18th weeks of gestation, registering them, and collecting baseline data. Screenings in the high‐risk package included at registration and at subsequent visits (20, 28, 34, 38 weeks), where the ANM undertook a clinical examination and distributed folic acid (dose dependent on haemoglobin). All high‐risk women were advised to give birth in a hospital. Postnatally, ANMs were expected to visit each new mother 3 times (3, 10, 40 postnatal days), when they were expected to detect and treat infections in mother and neonate, and to refer to the hospital those she could not diagnose/treat. (2) The Tamil Nadu Government (TNG) uniform package involved a set of routine antenatal care services recommended by the local government. These services were similar to the high‐risk package with regard to registration and visits by the ANM; however there was no high‐risk approach. Therefore all women were treated uniformly. Five visits postnatally (1, 3, 7, 15, 30) were to be made by the ANMs to all women who gave birth Control: implementation of the TNG package was the responsibility of general health services; therefore no special input was provided by project staff (e.g. ANMs). Data from pregnant women in this group represent outcomes under routine programme conditions |
|
| Outcomes | Preventable maternal morbidity, preventable perinatal morbidity, preventable neonatal morbidity | |
| Notes | Funding: no funding was acknowledged | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "from each Primary Health Centre, three sub centres were selected at random from among those beyond a radius of 10 km from the PHC: one each was randomly allocated to the high‐risk package and the Tamil Nadu Government package and the third served as the control" Comment: this is a randomised controlled trial, the the unit of random allocation was the subcentre; however the method of randomisation has not been disclosed |
| Allocation concealment (selection bias) | Unclear risk | Comment: there is no mention of allocation concealment, although this is a randomised controlled field trial; information is insufficient to support a judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "...69% in the HR series, 72% in the TNG series, and 77% in the control series could be considered for analysis..." Comment: reasons were given (migrated, abortion, and refusal), resulting in an attrition rate of 21% |
| Selective reporting (reporting bias) | Unclear risk | Comment: this is not a registered trial, but outcomes mentioned in the methods are reported in the results |
| Other bias | Low risk | Study seems to be free from other biases |
Tripathy 2010.
| Methods | Cluster‐randomised controlled trial conducted in Jharkhand and Orissa in India | |
| Participants | Pregnant women aged 15 to 49 years, residing in the project area, who had given birth during the study. From 36 clusters in Jharkhand and Orissa (mean cluster population 6338), 18 clusters were randomly assigned to intervention or control via stratified allocation. Total number of participants was 228,186 (n = 4672 births) | |
| Interventions | In intervention clusters (n = 2457 births), a facilitator convened 13 groups every month to support participatory action and learning for women, and facilitated the development and implementation of strategies to address maternal and newborn health problems Groups took part in a participatory learning and action cycle Control clusters (n = 2235) given standard care |
|
| Outcomes | Neonatal mortality rate, maternal depression, stillbirth, maternal and perinatal deaths, uptake of antenatal and delivery services, home care practices during and after delivery, health care‐seeking behaviour | |
| Notes | Analysis was by intention‐to‐treat Funding: Health Foundation, UK Department for International Development, Wellcome Trust, and the Big Lottery Fund (UK). Funders had no role in design of the study, data collection, data analysis or interpretation, or writing up of study findings, although they made a site visit early in the study implementation |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "we assigned 18 clusters to intervention or control using stratified randomizations" Comment: insufficient information to permit judgement |
| Allocation concealment (selection bias) | Low risk | Comment: since this was a cluster‐randomised trial, allocation concealment should not be an issue in this design, as all clusters are randomised at once |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "neither the intervention team nor the participants were masked to group assignment during the trial" Comment: no blinding occurred due to the nature of the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "the intervention and surveillance teams were not unaware of allocation" Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition (19%) was reported along with reasons |
| Selective reporting (reporting bias) | Low risk | Comment: this is not a registered trial, but outcomes mentioned in the methods are reported in the results |
| Other bias | Low risk | Study seems to be free from other biases |
Tripathy 2016.
| Methods | Cluster‐randomised controlled trial conducted in rural eastern India (Jharkhand and Odisha) | |
| Participants | Study participants were women of reproductive age (15 to 49 years) who gave birth during the study period. From 30 clusters in Jharkhan and Odisha (estimated population 156,519), 15 clusters were randomly assigned to intervention or control. In the intervention group, 3700 births were identified compared to 3519 births in the control group. A total of 152 Accredited Social Health Activists (ASHA) helped facilitate the women's groups | |
| Interventions | Intervention: a cycle of women's groups led by ASHA, which involved participatory learning and action with a 4‐phase structure. The first phase involved identifying and prioritising maternal and newborn health problems (picture cards and participatory voting utilised). The second phase involved listening to stories of the identified areas to consider causes and possible solutions. The third phase involved implementation of the chosen strategy and learning about other practical actions that could be taken. The final phase involved evaluation of the meeting cycle and progress against strategies (n = 1635 mothers at baseline) Control: this group did not have participatory women's groups facilitated by ASHAs (n = 1609 mothers at baseline) Village health sanitation and nutrition committees were another aspect of this study in both intervention and control areas, attempting to carrying out at least 1 village health sanitation and nutrition committee meeting about rights and entitlements per village |
|
| Outcomes | Primary outcome: neonatal mortality Secondary outcomes: stillbirths, perinatal mortality, maternal mortality, home care, care‐seeking practices |
|
| Notes | Intention‐to‐treat Funding: Big Lottery Fund (UK). The funding agency had no role in designing the study, data collection and analysis, the decision to publish, or preparation of this manuscript |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomisation was stratified by district, with six clusters in each district allocated to either control or intervention in a public randomisation meeting ....public randomisation technique used with small plastic balls (e.g. "...allocated a number to each cluster, wrote these numbers on small plastic balls, and placed balls in a dark bag")" Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Comment: since this is a cluster‐randomised trial, allocation concealment should not be an issue, as in this design, all clusters are randomised at once |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "because of the nature of the intervention being tested, the intervention team could not be masked to allocation" Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "the data collection team was masked to allocation, both at the cluster and at the individual level" Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "one cluster in the intervention group was lost to follow‐up due to law and order problems"; "Loss to follow‐up as a result of migration or refusal to be interviewed was 35 (< 1%) of 3735 births in the intervention clusters and 28 (< 1%) of 3547 births in the control clusters" Comment: reasons were given, and attrition rate was 0.9% |
| Selective reporting (reporting bias) | Low risk | Comment: this is not a registered trial, but outcomes mentioned in the methods are reported in the results |
| Other bias | Low risk | Study seems to be free from other biases |
Waiswa 2015.
| Methods | Cluster‐randomised controlled trial in Iganga and Mayuge districts, eastern Uganda (The Ugandan Newborn Study) | |
| Participants | This study was implemented within the Health Demographic Surveillance Site (HDSS) in eastern Uganda, which comprises 65 villages. The cluster unit for the study was the village; 63 of these villages were randomly allocated to intervention (n = 31) and control (n = 32). The trial included all consenting pregnant women and their newborns residing in the HDSS. A baseline survey was conducted involving women with a live birth within 4 months of the survey (intervention = 194, control = 201), and an end line survey was given amongst women who had had a live birth within 12 months of the survey (intervention = 894, control = 893) | |
| Interventions | Intervention: the UNEST package involved training community health workers for 5 days on the package, which involved identifying pregnant women in their community and undertaking 2 home visits during pregnancy and 3 visits after birth, at or close to 1, 3, and 7 days. These visits by CHWs offered women preventive and promotive care as well as counselling. Health system strengthening was also undertaken for all public and private health facilities in and around the study area. Training modules included goal‐oriented antenatal care, management of maternal complications, infection prevention, management of normal labour and partograph use, neonatal resuscitation, care of the sick newborn, and extra care for small babies via kangaroo mother care (n = 894 women gave birth at end line) Control: women and their newborns in comparison villages had access to standard health services, overseen by the district health team, in addition to improved health facilities (n = 893 women gave birth at end line) |
|
| Outcomes | Primary outcomes: improved coverage of services for antenatal care, birth preparedness, skilled attendance at delivery, postnatal care, increase in healthy practices (breastfeeding, thermal care, hygiene) | |
| Notes | Funding: funds provided by Save the Children through a grant from the Bill & Melinda Gates Foundation. This supplement was funded by Save the Children’s Saving Newborn Lives programme through a grant from the Bill & Melinda Gates Foundation. There is no statement related to involvement of these funding parties in design and conduct of the study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "each of the 63 villages in the HDSS was randomly allocated to the intervention or control arm, without any stratification or matching due to the relatively large number of study units. Computer‐generated restricted randomisation was done in a one‐to‐one ratio..." Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Comment: there is no mention of concealing or masking; however this is a cluster‐randomised controlled trial, and randomisation of clusters and therefore allocation concealment should not be an issue |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: due to the nature of the intervention package, it does not appear realistic to employ blinding; however there is insufficient information |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: loss to follow‐up and attrition rate were not reported and cannot be calculated due to the method of baseline and end line comparisons |
| Selective reporting (reporting bias) | Low risk | Comment: this is not a registered trial, but outcomes mentioned in the methods are reported in the results |
| Other bias | Low risk | Study seems to be free from other biases |
Wu 2011.
| Methods | Community‐based randomised controlled trial in rural China | |
| Participants | This study was based in a rural county with a total of 55 townships; of these, 10 townships were assigned to the intervention group and each was paired with a control, resulting in a total of 20 study townships. Pairing was based on the township's socioeconomic development, perinatal health, and maternal care utilisation and provision. Pregnant women were enrolled in this study ‐ 673 in the intervention townships and 591 in the control | |
| Interventions | The intervention had 3 components: 1. Training township hospital midwives and instructing them on how to provide systematic maternal care. 2. Informing women in the community of the importance of prenatal care. 3. Providing basic medical instruments to hospitals, if needed 1. The material that midwives were trained on was based on “Maternal Care Management Approaches for Rural Women”. Topics related to prenatal care included health education, routine checkups, lab tests, measurements, referral for high‐risk pregnancy, and instructions for safe delivery. The township midwives were asked to give each pregnant woman a prenatal care card at their first meeting ‐ in order to record their pregnancy progress and use of maternal care services. 2. A leaflet on maternal care was provided to pregnant women; it advised them to seek prenatal care and to have their delivery in the township hospital. A set of educational posters was also distributed around the townships; these provided information on daily life during pregnancy (food, nutrition, sleep, work, activities, etc.) and when and where to have prenatal care and delivery (hospital delivery) (n = 673 intervention, n = 591 control; total women for both years of the study) |
|
| Outcomes | Maternal care utilisation, content of prenatal care, impact of trial on pregnancy and perinatal outcomes | |
| Notes | Both qualitative (researchers’ observations, interviews, group discussions, field visits) and quantitative data (community‐based survey of mothers, survey conducted by midwives at township hospitals of women giving birth, and routine pregnancy and birth records) were used in this study Funding: financially supported by a grant from the Academy of Finland and a doctoral scholarship from the Finnish Ministry of Education (DPPH Programme). Analysis and reporting stage was partially funded by the European Commission INCO Programme, “Structural hinders to and promoters of good maternal care in rural China" ‐ C HIMACA (015396) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "one township was assigned to the intervention and one to control group in each matched pair by the toss of a coin. The randomisation was done by the investigators" |
| Allocation concealment (selection bias) | Low risk | Comment: randomisation was by community (cluster); therefore allocation concealment should not have been an issue |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: insufficient information to permit judgement |
| Selective reporting (reporting bias) | Low risk | Comment: this is not a registered trial, but outcomes mentioned in the methods are reported in the results |
| Other bias | High risk | Comment: there was acknowledgement of possible contamination between intervention and control townships, perhaps related to midwives communicating with each other and pregnant women visiting relatives in different townships |
ANM: axillary nurse midwife.
ASHA: accredited social health activist.
AWW: Anganwadi worker.
BF: breastfeeding.
CBSV: community‐based surveillance volunteer.
CBW: community‐based worker.
CC: community care.
CHW: community health worker.
HC: home care.
HEW: health extension worker
iCCM: Integrated community case management
IEEC: Information and Education for Empowerment and Change.
IMNCI: Integrated management of neonatal and childhood illness.
LHW: Lady Health Worker; local health worker.
PMTCT: Prevention of mother‐to‐child transmission
PSBI: Possible serious bacterial infections
RCT: randomised controlled trial.
TBA: traditional birth attendant.
TMP‐SMX: trimethoprim‐sulfamethoxazole.
VDC: village development committee.
VHT: Village Health Team.
VLC: village‐level committee.
WHO: World Health Organization.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Agboatwalla 1997 | This was a non‐randomised controlled trial and therefore was excluded |
| Ahluwalia 2000 | This was a non‐randomised cohort study conducted in Georgia State (USA) and therefore was excluded |
| Alexandre 2007 | This was a non‐randomised cohort study conducted in France and therefore was excluded |
| Ayiasi 2015b | This trial reported only on use of contraceptive during the trial period; no other relevant outcomes on neonatal survival, therefore this trial was excluded |
| Bang 1990 | This was a non‐randomised controlled trial and therefore was excluded |
| Bang 1999 | This was a non‐randomised controlled trial and therefore was excluded |
| Baqui 2015 | This trial was mainly on antibiotic provision for young infants and had no educational component. Also this study did not report on neonatal outcomes only and therefore was excluded |
| Bartington 2006 | This cohort study was conducted in hospitals of the UK and therefore was excluded |
| Bhandari 2003 | This was a cluster‐randomised controlled trial in which infants were enrolled within 72 hours (70%) and 1 week (90%) of life. However, outcomes were assessed at 6 months of age, and the trial was therefore excluded |
| Bhandari 2004 | This was a cluster‐randomised controlled trial in which neonates were enrolled. Main intervention was to promote appropriate complementary feeding practices during infancy. However, outcomes were assessed at 3, 6, 9, and 18 months of age. Because outcomes were not assessed in neonates, this study was excluded |
| Bhattacharya 1988 | Target population was mothers of children younger than 6 years of age, and outcomes were assessed for children younger than 6 years of age. Main intervention was education of mothers and family members about identification of diarrhoea, signs of dehydration, and rehydration with home‐made salt‐sugar solution. This study was excluded because it did not mention any disaggregated data for neonates |
| Bhopal 2017 | This is an abstract that provides insufficient information on outcomes for neonates only; therefore it was excluded |
| Bhutta 2009 | The primary objective of this study was to study the impact of multiple micronutrient supplementation interventions among women of reproductive age; it was therefore excluded |
| Bland 2008 | This was a non‐randomised cohort study in which exclusive breastfeeding was promoted. Outcomes were assessed at 6 months of age; therefore this study was excluded |
| Bolam 1998 | This study was conducted in a 250‐bed hospital and was therefore excluded |
| Boulvain 2004 | This randomised controlled trial was conducted in Switzerland and was therefore excluded |
| Carlo 2010 | This was a before‐and‐after study and was therefore excluded |
| Castrucci 2007 | This cross‐sectional study was conducted in a hospital in Philadelphia and was therefore excluded |
| Chapman 2004 | This study was conducted in a hospital setting of a developed country (USA) and was therefore excluded |
| Coombs 1998 | This study was conducted in a developed country (USA) and was therefore excluded |
| Coskun 2009 | This study was conducted in a hospital setting and was therefore excluded |
| Davies‐Abetugbo 1996 | Education provided involved promotion of breastfeeding alone; therefore this study was excluded |
| Dearden 2002 | Education provided involved promotion of breastfeeding alone; therefore this study was excluded |
| Dennis 2001 | This was a literature review and was therefore excluded |
| Di Napoli 2004 | This study was conducted in a developed country (Italy) and was therefore excluded |
| Escobar 2001 | This randomised controlled trial was conducted in a developed country (USA) and was therefore excluded |
| Flax 2009 | This study focused only on breastfeeding and was therefore excluded |
| Foreit 1993 | This randomised controlled trial was conducted in a hospital setting and was therefore excluded |
| Forster 2004 | This randomised controlled trial was conducted in a hospital setting in a developed country (Australia) and was therefore excluded |
| Froozani 1999 | This was a quasi‐experimental hospital‐based study and was therefore excluded |
| Gill 2007 | This quasi‐experimental study was conducted in the maternity clinics of a developed country (USA) and was therefore excluded |
| Gill 2011 | This study had no component on educational intervention and therefore was excluded |
| Gross 2009 | This cross‐sectional study was conducted in a developed country; it was therefore excluded |
| Grossman 2009 | This quasi‐randomised study was conducted in a hospital setting in a developed country (USA) and was therefore excluded |
| Guise 2003 | This systematic review examined whether primary care‐based interventions improve initiation and duration of breastfeeding. The review included studies from developed countries only and was therefore excluded |
| Haider 1996 | This was a hospital‐based study and was therefore excluded |
| Haider 2000 | This randomised controlled study was conducted to promote breastfeeding alone and was therefore excluded |
| Hannula 2008 | This systematic review was conducted in a developed country to examine professional support interventions for breastfeeding in a hospital setting and was therefore excluded |
| Hoare 1999 | In an educational session, mothers of children 3 years of age were included to participate; this study was therefore excluded |
| Hoddinott 2006 | This study was conducted in a developed country (Scotland) and was therefore excluded |
| Hotz 2005 | All mothers with children 4 to 23 months of age in these communities were invited to participate in nutrition education; therefore this study was excluded |
| Ickovics 2007 | This study was conducted in a hospital‐based setting in a developed country (USA) and was therefore excluded |
| Ingram 2004 | This study was conducted in a developed country (UK) and was therefore excluded |
| Ingram 2009 | This study was conducted in a developed country (UK) and was therefore excluded |
| Jang 2008 | This quasi‐randomised study was conducted in a hospital setting and was therefore excluded |
| Johnson 2017 | The intervention was based on contraceptive use only and the study included no outcomes on neonatal survival; therefore it was excluded |
| Kadam 2005 | This randomised controlled trial was conducted in a hospital setting and was therefore excluded |
| Kafatos 1989 | This randomised controlled trial was conducted to study the effect of a nutritional intervention programme among pregnant women and was conducted in a developed country (Greece); therefore it was excluded |
| Kafatos 1991 | This study was from a developed country (Greece) and was therefore excluded |
| Kimani‐Murage 2015 | This study did not report on primary or secondary outcomes and therefore was excluded |
| Kools 2005 | This cluster‐randomised trial was conducted to study promotion and support of breastfeeding in a developed country (Netherlands) and was therefore excluded |
| Lin 2008 | This quasi‐experimental study on the effectiveness of an antenatal education programme for breastfeeding outcomes was conducted in a developed country (Taiwan) and was therefore excluded |
| MacArthur 2009 | This study was conducted in a developed country (UK) and was therefore excluded |
| Martens 2002 | This study was conducted in a developed country (USA) and was therefore excluded |
| Merewood 2003 | This study was conducted in a hospital setting of a developed country (USA) and was therefore excluded |
| Moran 2006 | This was a cross‐sectional study and was therefore excluded |
| Mottl‐Santiago 2008 | This retrospective cohort was conducted at a tertiary care centre in a developed country (USA) and was therefore excluded |
| Murihead 2006 | This study was conducted in a developed country (Scotland) and was therefore excluded |
| Nabulsi 2014 | This is a study protocol with an outcome of knowledge of breastfeeding at 6 months; it was therefore excluded |
| Nair 2017 | This is a randomised controlled trial focused on infant and child outcomes and was therefore excluded |
| Nichols 2009 | This study was conducted in a hospital setting of a developed country (Australia) and was therefore excluded |
| Petrova 2009 | This study was conducted in a developed country (USA) and was therefore excluded |
| Philipp 2001 | This quasi‐randomised study was conducted in a developed country (USA) and was therefore excluded |
| Pobocik 2000 | This study was from a developed country (Guam) and was therefore excluded |
| Quinn 2005 | This was not an experimental study; therefore it was excluded |
| Raghupathy 1996 | In this study, national data were compared and analysed; the study was therefore excluded |
| Rahman 2008 | The objective of this study was not to improve neonatal survival; therefore it was excluded |
| Raj 2016 | The objective of this study was to improve gender equity and family planning; therefore it was excluded |
| Rawat 2017 | This study explored complementary feeding and was therefore excluded |
| Rishel 2005 | This retrospective study was conducted in a developed country (USA) and was therefore excluded |
| Rosato 2006 | This was a qualitative review and was therefore excluded |
| Rosen 2008 | This retrospective cohort study was conducted in a hospital setting of a developed country (USA) and was therefore excluded |
| Rossiter 1994 | This study was conducted in a hospital setting of a developed country (Australia) and was therefore excluded |
| Roy 2007 | This was a randomised controlled trial in which children aged 6 to 9 months were recruited; therefore it was excluded |
| Russell 1999 | This retrospective study was conducted in a hospital setting of a developed country (USA) and was therefore excluded |
| Ryser 1999 | This non‐randomised controlled trial was conducted in a developed country (USA) and was therefore excluded |
| Ryser 2004 | This trial was from a developed country (USA) and was therefore excluded |
| Sachdeva 1994 | This study focused on nutrition in infants and therefore was excluded |
| Sandy 2009 | This study was conducted in a developed country (USA) and was therefore excluded |
| Schneider 2001 | This study was conducted in a developed country (Australia) and was therefore excluded |
| Seema 1997 | This prospective study was conducted in hospitalised infants and was therefore excluded |
| Serwint 1996 | This randomised controlled trial was conducted in a hospital setting and was therefore excluded |
| Shaw 1999 | This study was conducted in a developed country (USA) and was therefore excluded |
| Shinwell 2006 | This prospective cohort study was conducted in a hospital setting and was therefore excluded |
| Shrestha 2016 | Counselling was provided to women in a hospital; therefore this study was excluded |
| Sloan 2008 | Both groups received intervention on essential newborn care, with additional intervention on kangaroo mother care in the intervention arm; therefore this study was excluded |
| Stille 2001 | This study was from a developed country (USA) and was therefore excluded |
| Susin 2008 | This controlled clinical trial was conducted in a hospital setting and was therefore excluded |
| Svenson 2009 | This randomised controlled trial was conducted in a hospital setting of a developed country (Australia) and was therefore excluded |
| Syed 2006 | This was not a randomised controlled trial; therefore it was excluded |
| Syed 2008 | This was a review and was therefore excluded |
| Tshefu 2015 | This study had no educational component and was therefore excluded |
| Tylleskär 2011 | This randomised controlled study was conducted to promote breastfeeding alone and was therefore excluded |
| Volpe 2000 | This study was conducted in a developed country (USA) and was therefore excluded |
| Warren 2010 | This was a before‐and‐after study; therefore it was excluded |
| Whitelaw 1988 | This study was from a developed country (UK) and was therefore excluded |
| Wong 2007 | This study was conducted in a hospital setting of a developed country (Hong Kong) and was therefore excluded |
| Yun 2010 | This study was conducted in a developed country (USA) and was therefore excluded |
| Zaidi 2012 | This study had no educational component and was therefore excluded |
| Zaidi 2013 | This study had no educational component and was therefore excluded |
Characteristics of studies awaiting assessment [ordered by study ID]
Mazumder 2017.
| Methods | Impact of community‐initiated kangaroo mother care (KMC) on survival of low birth weight infants: study protocol for a randomised controlled trial |
| Participants | Randomised controlled trial in Palwal and Faridabad districts in the State of Haryana, India. Surveillance workers conduct door‐to‐door surveys in study areas. All identified pregnancies are allocated to the pregnancy follow‐up and screening and enrolment (PSE) team. All infants are visited by ASHAs at days 1, 3, 7, 14, 21, 28, and 42 for counselling on newborn care, identification of illnesses, and referral of ill infants |
| Interventions | Participants include babies between 1500 and 2250 grams, with the enrolment window within 3 days of birth. All newborns and mothers are screened within 3 days of delivery; babies born at home and babies born in health facilities are included if KMC was not initiated in the facility. Exclusion criteria: inability to feed, breathing problems, gross congenital malformations, less than normally active (these babies are referred to the hospital) Once an infant is allocated to the intervention group, a pair of workers (ANMs and ASHAs) make the first visit to explain KMC and to support the mother in doing it. The team provides visual aids (photographs), helps the mother with placing the newborn in the KMC position, and observes breastfeeding. Family members (husbands and mothers‐in‐law) are also taught the procedure and are encouraged to perform skin‐to‐skin contact during the periods the mother is not doing so. If the mother is struggling with performing KMC or needs counselling for breastfeeding, the ANM will visit her house and assist. Visits end at 28 days or earlier if the baby no longer wants to be kept in KMC |
| Outcomes | Primary outcomes:
Secondary outcomes:
|
| Notes | Currently, only the protocol is available for this study; therefore no results have been published |
Morrison 2011.
| Methods | Single‐centre unmasked cluster‐randomised controlled trial from Nepal |
| Participants | Participants will be HMC members but may also be community members whom HMCs invite to participate |
| Interventions | The intervention has 2 components: community mobilisation through women's groups, and HMC strengthening. Both interventions will be implemented for 2 years in 21 intervention clusters Community mobilisation through women's groups: A government health cadre, the female community health volunteer (FCHV), runs 1 women's group per month. At least 9 FCHVs are running 9 women's groups per cluster (N = 189). FCHVs are supported by 7 supervisors, who provide general field support and conduct monthly training and feedback meetings with FCHVs to discuss the upcoming women's group agenda and to monitor their progress. Women's groups work through a participatory action cycle to identify local problems preventing women from delivering in an institution or at home with a trained health worker, then implement and evaluate strategies to address these problems Health management committee strengthening: One health institution is included per cluster, and each institution has a health management committee. 4 appreciative inquiry facilitators and trained representatives from the District Public Health Office conduct 3‐day workshops with HMCs from each health facility (N = 21). Other participants such as community representatives and health workers may also join workshops. The workshops take an appreciative planning and action approach, whereby participants are encouraged to build on their strengths to take action to improve health facilities. Facilitators will follow up on progress at HMC meetings approximately 2 months after they have completed their planning workshop and at regular intervals thereafter |
| Outcomes | Primary outcomes:
Secondary outcomes:
|
| Notes | Currently, only the protocol is available for this study; therefore no results have been published |
Pell 2016.
| Methods | Cluster‐randomised trial in Kwale County, Kenya |
| Participants | Participants are recruited by CHWs during a home visit during their third trimester |
| Interventions | This trial consists of 4 arms: (1) control, (2) neonatal kit, (3) stimulation, (4) neonatal kit and stimulation The intervention is delivered by CHWs after consent is received at the routine third trimester visit; appropriate intervention(s) and associated education are delivered Neonatal kit: consists of a clean birth kit, 4% chlorhexidine solution, sunflower oil emollient, ThermoSpot™, Mylar infant sleeve, and a reusable instant heat pack Stimulation programme: teaches 3 key messages to enhance caregivers' current practices (e.g. making eye contact and talking to their children, engaging in responsive feeding). These messages are taught at three 15‐minute sessions that take place in participants' homes. They are taught verbally by CHWs, and participants follow the messages via pictorial illustrations of behaviours associated with these messages Control: the current standard of community‐based prenatal care is delivered by CHWs |
| Outcomes | Primary outcome:
Secondary outcomes:
|
| Notes | Currently, only the protocol is available for this study; therefore no results have been published |
Shrestha 2011 .
| Methods | Study is a cluster‐randomised controlled trial involving 60 village development committee clusters, allocated 1:1 to 2 interventions in a factorial design conducted in Nepal |
| Participants | Women and newborns |
| Interventions | Mother and infant research activities (MIRA): Dhanusha community groups: female community health volunteers (FCHVs) are supported in convening monthly women's groups. 9 groups per cluster (270 in total) work through 2 action research cycles in which they (1) identify local issues around maternity, newborn health, and nutrition, (2) prioritise key problems, (3) develop strategies to address them, (4) implement these strategies, and (5) evaluate their success. Cycle 1 focuses on maternal and newborn health, and cycle 2 on nutrition in pregnancy and infancy and associated postpartum care practices. MIRA Dhanusha sepsis management: FCHVs are trained to care for vulnerable newborn infants. They (1) identify local births, (2) identify low birth weight infants, (3) identify possible newborn infection, (4) manage the process of treatment with oral antibiotics and referral to a health facility to receive parenteral gentamicin, and (5) follow up on infants and support families |
| Outcomes | Neonatal mortality rates MIRA Dhanusha community group: stillbirth, infant and under‐2 mortality rates, care practices and health care‐seeking behaviour, maternal diet, breastfeeding and complementary feeding practices, maternal and under‐2 anthropometric status MIRA Dhanusha sepsis management: identification and treatment of neonatal sepsis by community health volunteers, infection‐specific neonatal mortality |
| Notes | Currently, only the protocol is available for this study; therefore no results have been published |
Su 2016.
| Methods | Quasi‐randomised controlled trial in rural China |
| Participants | Participants were local pregnant women at any gestational age; to be included, had to own a cell phone in the household and visit a local maternal and child health centre for antenatal care |
| Interventions | Four study arms include (1) good household prenatal practice messages (advice on nutrition, exercise, self‐awareness of depression, breastfeeding, etc.), (2) care‐seeking messages (information about government‐subsidised programmes, warning signs of potential problems, importance of care‐seeking during illness), (3) both types of messaging, and (4) a limited set of ‘status‐quo’ messages about pregnancy (control) At baseline, a survey was conducted. Text messages are sent from time of enrolment until delivery, with information related to their week of gestation. A final survey is conducted a month after delivery to assess postpartum depression. Data from medical records are collected at baseline, during pregnancy, at birth, and 1 month after birth for all participants. The group assigned to intervention 1 (good household) and the control group receive 6 reminders with brief information (prenatal visits and hospital delivery). The intervention 2 (care‐seeking) and combination group (3) receives 6 similar reminders with greater detail and 2 additional messages. Text message topics include faetal development, reminders for prenatal visits and hospital delivery, recognition of danger signs, reminders for government‐subsidised projects, healthy lifestyle, mental health during pregnancy, pain management, labour, and breastfeeding; topics and numbers were dependent on the group assignment |
| Outcomes | Primary outcome:
Secondary outcomes:
|
| Notes | Currently, only the protocol is available for this study; therefore no results have been published |
Turab 2013.
| Methods | Community‐based cluster‐randomised controlled trial from Pakistan |
| Participants | Target population includes all pregnant women (15 to 49 years) or women who will conceive during the course of the study and their newborns in the 20 Union Councils |
| Interventions | This study has 2 arms: the intervention arm, which includes the "emergency obstetric and newborn care" (EmONC) package; and the control arm, which consists of standard care All participants will receive a household cross‐sectional survey to collect information on socio‐demographic characteristics, maternal and neonatal morbidity, and mortality. Another survey to assess knowledge, attitudes, and practices of communities regarding maternal and newborn health problems and care‐seeking patterns will be nested within the baseline survey The EmONC package comprises a maternal and neonatal health pack, enhanced training of health workers, and community mobilisation. The health pack includes 4% CHX solution, emollient (sunflower seed oil), and a clean delivery kit (CDK) and a health messages brochure, which will be delivered to intervention mothers in their third trimester. Lady Health Workers will be employed to deliver the package and to explain health education messages, focusing on antenatal, natal, and postnatal care, along with instructions on how to use the pack. LHWs, community midwives, and traditional birth attendants in the intervention arm will receive training on basic obstetrics and newborn care, recognition of danger signs, and early referrals. The community mobilisation component will consist of health sessions facilitated by LHWs for women and their husbands. Specifically, the sessions aim to deliver awareness on antenatal care, birth preparedness, essential and immediate newborn care, recognition of danger signs, management of low birth weight, and sepsis, with early and appropriate referral. Health SMSs related to maternal and neonatal and child health will be delivered to the woman's cell phone or to family members throughout pregnancy, childhood, and the postpartum period Control: women allocated to the control group will receive standard care through the existing health system |
| Outcomes | Primary outcome:
|
| Notes | Currently, only the protocol is available for this study; therefore no results have been published |
Var 2015.
| Methods | Randomised controlled trial in the province Takeo, Cambodia |
| Participants | All health centres in the trial will be included in the intervention but will be randomised to 1 of 16 start dates. All live births occurring in the study area will be eligible, and pregnant women in the last trimester will be recruited from health centre catchment areas using the stepped wedge design |
| Interventions | The intervention is a community and health facility linked to improve health outcomes for newborns, specifically, the Newborn Infection Control and Care Initiative (NICCI) package. The package is implemented at health centre, community, and household levels and is designed to improve care practices and care‐seeking for newborns. The intervention will address infection control in the perinatal period in health facilities, promote infection prevention and control practices in health centres and homes, and improve the timeliness of referrals for newborns with suspected infection. This will be done by linking families to medical systems through a network of community‐based volunteers (from Village Health Support Groups ‐ VHSG), who will make home visits to families in the first week of life |
| Outcomes | Primary outcomes:
Secondary outcomes:
|
| Notes | There are also primary outcomes from the evaluation process. Currently, only the protocol is available for this study; therefore no results have been published |
ANM: axillary nurse midwife.
ASHA: accredited social health activist.
CDK: clean delivery kit.
CHW: community health worker.
FCHV: female community health volunteer.
HMC: health management committee.
KMC: kangaroo mother care.
LBW: low birth weight.
LHW: Lady Health Worker; local health worker.
MIRA: mother and infant research activities.
NICCI: Newborn Infection Control and Care Initiative.
PCM‐IT: Protocol for Child Monitoring – Infant/Toddler version
PSE: pregnancy follow‐up and screening and enrolment.
VHSG: Village Health Support Group.
Differences between protocol and review
The current review has a few differences from the original protocol.
Title changed from "Community health educational interventions on maternal and newborn care for improving neonatal health and survival in low‐ and middle‐income countries" to "Community‐based maternal and newborn educational care packages for improving neonatal health and survival in low‐ and middle‐income countries".
Replaced the word "developing countries" from the title with "low‐ and middle‐income countries".
Objective: change the terms "..on neonatal mortality, neonatal morbidity, access to health care, and cost" to "...on neonatal health and survival", as those were the primary outcomes. Morbidity, access to health care, and cost are still reported in the review as secondary outcomes.
Outcomes: perinatal mortality was moved from secondary outcomes to primary outcomes, as perinatal mortality also encompasses early neonatal mortality.
GRADE assessment has been added.
Subgroup analysis was moved from under the objective to separate the heading in the methods section.
Subgroup analysis comparing one‐to‐one and group counselling was not possible, as none of the included studies compared these approaches.
Removed "duration and frequency of the intervention" from the subgroup analysis, as this was not reported at all in the included studies and was not making any sense.
Due to the heterogeneity of studies, we used the random‐effects method instead of the anticipated fixed‐effect approach.
Contributions of authors
The review was conducted by Zohra Lassi (ZSL) and Sophie Kedzior (SK) under the guidance of Dr Zulfiqar A Bhutta (ZAB).
Sources of support
Internal sources
Aga Khan University, Pakistan.
External sources
-
Vermont Oxford Network, USA.
Cochrane Neonatal Reviews are produced with support from Vermont Oxford Nework, a worldwide collaboration of health professionals dedicated to providing evidence‐based care of the highest quality for newborn infants and their families.
Declarations of interest
ZL has no interest to declare.
SK has no interest to declare.
ZB has no interest to declare.
New
References
References to studies included in this review
Ayiasi 2016 {published data only}
- Ayiasi RM, Atuyambe LM, Kiguli J, Orach CG, Kolsteren P, Criel B. Use of mobile phone consultations during home visits by community health workers for maternal and newborn care: community experiences from Masindi and Kiryandongodistricts, Uganda. BMC Public Health 2015;15:560. [DOI: 10.1186/s12889-015-1939-3; PUBMED: 26084369] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayiasi RM, Kolsteren P, Batwala V, Criel B, Orach CG. Effect of village health team home visits and mobile phone consultations on maternal and newborn care practices in Masindi and Kiryandongo, Uganda: a community‐intervention trial. PLoS ONE 2016;11(4):e0153051. [DOI: 10.1371/journal.pone.0153051; PUBMED: 27101379] [DOI] [PMC free article] [PubMed] [Google Scholar]
Azad 2010 {published data only}
- Azad K, Barnett S, Banerjee B, Shaha S, Khan K, Rego AR, et al. Effect of scaling up women's groups on birth outcomes in three rural districts in Bangladesh: a cluster‐randomised controlled trial. Lancet 2010;375(9721):1193‐202. [DOI: 10.1016/S0140-6736(10)60142-0; PUBMED: 20207412] [DOI] [PubMed] [Google Scholar]
Baqui 2008 (a) {published data only}
- Baqui AH, El‐Arifeen S, Darmstadt GL, Ahmed S, Williams EK, Seraji HR, et al. Effect of community‐based newborn‐care intervention package implemented through two service‐delivery strategies in Sylhet district, Bangladesh: a cluster‐randomised controlled trial. Lancet 2008;371(9628):1936‐44. [DOI: 10.1016/S0140-6736(08)60835-1; PUBMED: 18539225] [DOI] [PubMed] [Google Scholar]
- LeFevre AE, Shillcutt SD, Waters HR, Haider S, Arifeen S, Mannan I, et al. Economic evaluation of neonatal care packages in a cluster‐randomized controlled trial in Sylhet, Bangladesh. Bulletin World Health Organization 2013;91(10):736–45. [DOI: 10.2471/BLT.12.117127; PUBMED: 24115797] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannan I, Rahman SM, Sania A, Seraji HR, Arifeen SE, Winch PJ, et al. Can early postpartum home visits by trained community health workers improve breastfeeding of newborns?. Journal of Perinatology 2008;28(9):632–40. [DOI: 10.1038/jp.2008.64; PUBMED: 18596714] [DOI] [PMC free article] [PubMed] [Google Scholar]
Baqui 2008 (b) {published data only}
- Baqui AH, Rosecrans AM, Williams EK, Agrawal PK, Ahmed S, Darmstadt GL, et al. NGO facilitation of a government community‐based maternal and neonatal health programme in rural India: improvements in equity. Health Policy and Planning 2008;23(4):234–43. [DOI: 10.1093/heapol/czn012; PUBMED: 18562458] [DOI] [PubMed] [Google Scholar]
- Baqui AH, Williams EK, Rosecrans AM, Agrawal PK, Ahmed S, Darmstadt GL, et al. Impact of an integrated nutrition and health programme on neonatal mortality in rural northern India. Bulletin of the World Health Organization 2008;86(10):796‐804. [PUBMED: 18949217] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bashour 2008 {published data only}
- Bashour HN, Kharouf MH, Abdulsalam AA, Asmar K, Tabbaa MA, Cheikha SA. Effect of postnatal home visits on maternal/infant outcomes in Syria: a randomized controlled trial. Public Health Nursing 2008;25(2):115‐25. [DOI: 10.1111/j.1525-1446.2008.00688.x; PUBMED: 18294180] [DOI] [PubMed] [Google Scholar]
Bhandari 2012 {published data only}
- Bhandari N, Mazumder S, Taneja S, Sommerfelt H, Strand TA, IMNCI Evaluation Study Group. Effect of implementation of Integrated Management of Neonatal and Childhood Illness (IMNCI) programme on neonatal and infant mortality: cluster randomised controlled trial. BMJ 2012;344:e1634. [DOI: 10.1136/bmj.e1634; PUBMED: 22438367] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bhutta 2008 {published data only}
- Bhutta ZA, Memon ZA, Soofi S, Salat MS, Cousens S, Martines J. Implementing community‐based perinatal care: results from a pilot study in rural Pakistan. Bulletin of the World Health Organization 2008;86(6):452‐9. [PUBMED: 18568274] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bhutta 2011 {published data only}
- Bhutta ZA, Soofi S, Cousens S, Mohammad S, Memon ZA, Ali I, et al. Improvement of perinatal and newborn care in rural Pakistan through community‐based strategies: a cluster‐randomised effectiveness trial. Lancet 2011;377(9763):403‐12. [DOI: 10.1016/S0140-6736(10)62274-X; PUBMED: 21239052] [DOI] [PubMed] [Google Scholar]
Colbourn 2013 {published data only}
- Colbourn R, Nambiar B, Bondo A, Makwenda C, Tsetekanib E, Makonda‐Ridley A, et al. Effects of quality improvement in health facilities and community mobilization through women’s groups on maternal, neonatal and perinatal mortality in three districts of Malawi: MaiKhanda, a cluster randomized controlled effectiveness trial. International Health 2013;5(3):180‐95. [DOI: 10.1093/inthealth/iht011; PUBMED: 24030269] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colbourn T, Pulkki‐Brännström A, Nambiar B, Kim S, Bondo A, BandaL, Makwenda C, et al. Cost‐effectiveness and affordability of community mobilisation through women’s groups and quality improvement in health facilities (MaiKhanda trial) in Malawi. Cost Effectiveness and Resource Allocation 2015;13(1):1. [DOI: 10.1186/s12962-014-0028-2; PUBMED: 25649323] [DOI] [PMC free article] [PubMed] [Google Scholar]
Darmstadt 2010 {published data only}
- Bari S, Mannan I, Rahman MA, Darmstadt GL, Seraji MHR, Baqui AH, et al. Trends in use of referral hospital services for care of sick newborns in a community‐based intervention in Tangail District, Bangladesh. Journal of Health, Population, and Nutrition 2006;24(4):519‐29. [PUBMED: 17591349 ] [PMC free article] [PubMed] [Google Scholar]
- Darmstadt GL, Choi Y, Arifeen SE, Bari S, Rahman SM, Mannan I, et al. Evaluation of a cluster‐randomized controlled trial of a package of community‐based maternal and newborn interventions in Mirzapur, Bangladesh. PLoS ONE 2010;5(3):e9696. [DOI: 10.1371/journal.pone.0009696; PUBMED: 20352087] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darmstadt GL, Arifeen S, Choi Y, Bari S, Rahman SM, Mannan I, et al. Household surveillance of severe neonatal illness by community health workers in Mirzapur, Bangladesh: coverage and compliance with referral. Health Policy and Planning 2010;25(2):112‐24. [DOI: 10.1093/heapol/czp048; PUBMED: 19917652] [DOI] [PMC free article] [PubMed] [Google Scholar]
Degefie 2017 {published data only}
- Degefie Hailegebriel T, Mulligan B, Cousens S, Mathewos B, Wall S, Bekele A, et al. Effect on neonatal mortality of Newborn Infection Management at Health Posts when referral is not possible: a cluster‐randomized trial in Rural Ethiopia. Global Health: Science and Practice 2017;5(2):202‐16. [DOI: 10.9745/GHSP-D-16-00312; PUBMED: 28611102] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fottrell 2013 {published data only}
- Fottrell E, Azad K, Kuddus A, Younes L, Shaha S, Nahar T, et al. The effect of increased coverage of participatory women’s groups on neonatal mortality in Bangladesh. JAMA Pediatrics 2013;167(9):816‐25. [DOI: 10.1001/jamapediatrics.2013.2534; PUBMED: 23689475] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houweling TAJ, Azad K, Younes L, Kuddus A, Shaha S, Haq B, et al. The effect of participatory women’s groups on birth outcomes in Bangladesh: does coverage matter? Study protocol for a randomised controlled trial. Trials 2011;12:208. [DOI: 10.1186/1745-6215-12-208; PUBMED: 21943044] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ijumba 2015 {published data only}
- Ijumba P, Doherty T, Jackson D, Tomlinson M, Sanders D, Swanevelder S, et al. Effect of an integrated community‐based package for maternal and newborn care on feeding patterns during the first 12 weeks of life: a cluster‐randomized trial in a South African township. Public Health Nutrition 2015;18(14):2660–8. [DOI: 10.1017/S1368980015000099; PUBMED: 25660465] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nsibande S, Doherty T, Ijumba P, Tomlinson M, Jackson D, Sanders D, et al. Assessment of the uptake of neonatal and young infant referrals by community health workers to public health facilities in an urban informal settlement, KwaZulu‐Natal, South Africa. BMC Health Services Research 2013;13:47. [DOI: 10.1186/1472-6963-13-47; PUBMED: 23388385] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson M, Doherty T, Ijumba P, Jackson D, Lawn J, Persson L, et al. Goodstart: a cluster randomised effectiveness trial of an integrated, community‐based package for maternal and newborn care, with prevention of mother‐to‐child transmission of HIV in a South African township. Tropical Medicine and International Health 2014;19(3):256‐66. [DOI: 10.1111/tmi.12257; PUBMED: 24433230] [DOI] [PubMed] [Google Scholar]
- Tomlinson M, Doherty T, Jackson D, Lawn JE, Ijumba P, Colvin T, et al. An effectiveness study of an integrated, community‐based package for maternal, newborn, child and HIV care in South Africa: study protocol for a randomized controlled trial. Trials 2011;12:236. [DOI: 10.1186/1745-6215-12-236; PUBMED: 22044553] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jokhio 2005 {published data only}
- Jokhio AH, Winter HR, Cheng KK. An intervention involving traditional birth attendants and perinatal and maternal mortality in Pakistan. New England Journal of Medicine 2005;352(20):2091‐9. [DOI: 10.1056/NEJMsa042830; PUBMED: 15901862] [DOI] [PubMed] [Google Scholar]
Kirkwood 2013 {published data only}
- Kirkwood BR, Manu A, Asbroek AH, Soremekun S, Weobong B, Gyan T, et al. Effect of the NEWHINTS home‐visits intervention on neonatal mortality rate and care practices in Ghana: a cluster randomised controlled trial. Lancet 2013;381(9884):2184–9. [DOI: 10.1016/S0140-6736(13)60095-1; PUBMED: 23578528] [DOI] [PubMed] [Google Scholar]
- Kirkwood BR, Manu A, Tawiah‐Agyemang C, Asbroek G, Gyan T, Weobong B, et al. NEWHINTS cluster randomised trial to evaluate the impact on neonatal mortality in rural Ghana of routine home visits to provide a package of essential newborn care interventions in the third trimester of pregnancy and the first week of life: trial protocol. Trials 2010;11:58. [DOI: 10.1186/1745-6215-11-58; PUBMED: 20478070] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manu A, Hill Z, Asbroek AHA, Soremekun S, Weobong B, Gyan T, et al. Increasing access to care for sick newborns: evidence from the Ghana NEWHINTS cluster‐randomised controlled trial. BMJ Open 2016;6(6):e008107. [DOI: 10.1136/bmjopen-2015-008107; PUBMED: 27297006] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kumar 2008 {published data only}
- Darmstadt GL, Kumar V, Yadav R, Singh V, Singh P, Mohanty S, et al. Introduction of community based skin‐to‐skin care in rural Uttar Pradesh, India. Journal of Perinatology 2006;26(10):597‐604. [DOI: 10.1136/bmjopen-2015-008107; PUBMED: 27297006] [DOI] [PubMed] [Google Scholar]
- Kumar V, Kumar A, Das V, Srivastava NM, Baqui AH, Santosham M, et al. Community‐driven impact of a newborn‐focused behavioral intervention on maternal health in Shivgarh, India. International Journal of Gynecology and Obstetrics 2012;117(1):48‐55. [DOI: 10.1016/j.ijgo.2011.10.031; PUBMED: 22281244] [DOI] [PubMed] [Google Scholar]
- Kumar V, Mohanty S, Kumar A, Misra RP, Santosham M, Awasthi S, et al. Effect of community based behaviour change management on neonatal mortality in Shivgarh, Uttar Pradesh, India: a cluster‐randomised controlled trial. Lancet 2008;372(9644):1151‐62. [DOI: 10.1016/S0140-6736(08)61483-X; PUBMED: 18926277] [DOI] [PubMed] [Google Scholar]
- Willis JR, Kumar V, Mohanty S, Singh V, Kumar A, Singh JV, et al. Impact of community‐based behaviour‐change management on perceived neonatal morbidity: a cluster‐randomized controlled trial in Shivgarh, Uttar Pradesh, India. Journal of Tropical Pediatrics 2012;58(4):286‐91. [DOI: 10.1093/tropej/fmr097; PUBMED: 22147281] [DOI] [PubMed] [Google Scholar]
Lewycka 2013 {published data only}
- Lewycka S, Mwansambo C, Kazembe P, Phiri T, Mganga A, Rosato M, et al. A cluster randomised controlled trial of the community effectiveness of two interventions in rural Malawi to improve health care and to reduce maternal, newborn and infant mortality. Trials 2010;11:88. [DOI: 10.1186/1745-6215-11-88; PUBMED: 20849613] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewycka S, Mwansambo C, Rosato M, Kazembe P, Phiri T, Mganga A, et al. Effect of women’s groups and volunteer peer counselling on rates of mortality, morbidity, and health behaviours in mothers and children in rural Malawi (MaiMwana): a factorial, cluster randomised controlled trial. Lancet 2013;381(9879):1721–35. [DOI: 10.1016/S0140-6736(12)61959-X; PUBMED: 23683639] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosato M, Lewycka A, Mwansambo C, Kazembe P, Phiri T, Chapota H, et al. Research report‐volunteer infant feeding and care counselors: a health education intervention to improve mother and child health and reduce mortality in rural Malawi. Malawi Medical Journal 2012;24(2):39‐42. [PUBMED: 23638270] [PMC free article] [PubMed] [Google Scholar]
Magoma 2013 {published data only}
- Magoma M, Requejo J, Campbell O, Cousens S, Merialdi M, Filippi V. The effectiveness of birth plans in increasing use of skilled care at delivery and postnatal care in rural Tanzania: a cluster randomised trial. Tropical Medicine and International Health 2013;18(4):435–43. [DOI: 10.1111/tmi.12069; PUBMED: 23383733] [DOI] [PubMed] [Google Scholar]
Manandhar 2004 {published data only}
- Manandhar DS, Osrin D, Shrestha BP, Mesko N, Morrison J, Tumbahangphe KM, et al. Effect of participatory intervention with women’s groups on birth outcomes in Nepal: cluster randomised controlled trial. Lancet 2004;364(9438):970‐9. [DOI: 10.1016/S0140-6736(04)17021-9; PUBMED: 15364188] [DOI] [PubMed] [Google Scholar]
- Morrison J, Tamang S, Mesko N, Osrin D, Shrestha B, Manandhar M, et al. Women's health groups to improve perinatal care in rural Nepal. BMC Pregnancy and Childbirth 2005;5(1):6. [DOI: 10.1186/1471-2393-5-6; PUBMED: 15771772] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osrin D, Mesko N, Shrestha BP, Shrestha D, Tamang S, Thapa S, et al. Implementing a community‐based participatory intervention to improve essential newborn care in rural Nepal. Transaction of the Royal Society of Tropical Medicine and Hygiene 2003;97(1):18‐21. [PUBMED: 12886798] [DOI] [PubMed] [Google Scholar]
- Wade A, Osrin D, Shrestha BP, Sen A, Morrison J, Tumbahangphe KM, et al. Behaviour change in perinatal care practices among rural women exposed to a women's group intervention in Nepal. BMC Pregnancy and Childbirth 2006;6:20. [DOI: 10.1186/1471-2393-6-20; PUBMED: 16776818] [DOI] [PMC free article] [PubMed] [Google Scholar]
McConnell 2016 {published data only}
- McConnell M, Ettenger A, Watt Rothschild C, Muigai F, Cohen J. Can a community health worker administered postnatal checklist increase health‐seeking behaviors and knowledge? evidence from a randomized trial with a private maternity facility in Kiambu County, Kenya. BMC Pregnancy and Childbirth 2016;16(1):136. [DOI: 10.1186/s12884-016-0914-z; PUBMED: 27260500] [DOI] [PMC free article] [PubMed] [Google Scholar]
Memon 2015 {published data only}
- Memon ZA, Khan GN, Soofi SB, Baig IY, Bhutta ZA. Impact of a community‐based perinatal and newborn preventive care package on perinatal and neonatal mortality in a remote mountainous district in Northern Pakistan. BMC Pregnancy and Childbirth 2015;15:106. [DOI: 10.1186/s12884-015-0538-8; PUBMED: 25925407] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mersal 2013 {published data only}
- Mersal FA, Esmat OM, Khalil GM. Effect of prenatal counselling on compliance and outcomes of teenage pregnancy. Eastern Mediterranean Health Journal 2013;19(1):10‐7. [PUBMED: 23520900] [PubMed] [Google Scholar]
Midhet 2010 {published data only}
- Midhet F, Becker S. Impact of community‐based interventions on maternal and neonatal health indicators: results from a community randomized trial in rural Balochistan, Pakistan. Reproductive Health 2010;7:30. [DOI: 10.1186/1742-4755-7-30; PUBMED: 21054870] [DOI] [PMC free article] [PubMed] [Google Scholar]
More 2012 {published data only}
- More NS, Bapat U, Das S, Alcock G, Patil S, Porel M, et al. Community mobilization in Mumbai slums to improve perinatal care and outcomes: a cluster randomized controlled trial. PLoS Medicine 2008;9(7):e1001257. [DOI: 10.1371/journal.pmed.1001257; PUBMED: 22802737] [DOI] [PMC free article] [PubMed] [Google Scholar]
- More NS, Bapat U, Das S, Alcock G, Patil S, Porel M, et al. Community mobilization in Mumbai slums to improve perinatal care and outcomes: a cluster randomized controlled trial. PLoS Medicine 2012;9(7):e1001257. [DOI: 10.1371/journal.pmed.1001257; PUBMED: 22802737] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pasha 2013 {published data only}
- Pasha O, Goldenberg RL, McClure EM, Saleem S, Goudar SS, Althabe F, et al. Communities, birth attendants and health facilities: a continuum of emergency maternal and newborn care (the global network’s EmONC trial). BMC Pregnancy and Birth 2010;10:82. [DOI: 10.1186/1471-2393-10-82; PUBMED: 21156060] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasha O, McClure EM, Wright LL, Saleem S, Goudar SS, Chomba E, Patel A, et al. A combined community‐ and facility‐based approach to improve pregnancy outcomes in low‐resource settings: a Global Network cluster randomized trial. BMC Medicine 2013;11:215. [DOI: 10.1186/1741-7015-11-215; PUBMED: 24090370] [DOI] [PMC free article] [PubMed] [Google Scholar]
Penfold 2014 {published data only}
- Hanson C, Manzi F, Mkumbo E, Shirima K, Penfold S, Hill Z, et al. Effectiveness of a home‐based counselling strategy on neonatal care and survival: a cluster‐randomised trial in six districts of Rural Southern Tanzania. PLoS Medicine 2015;12(9):e1001881. [DOI: 10.1371/journal.pmed.1001881; PUBMED: 26418813] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mkumboa E, Hansona C, Penfold S, Manzia F, Schellenbergc J. Innovation in supervision and support of community health workers for better newborn survival in southern Tanzania. International Health 2014;6(4):339‐41. [DOI: 10.1093/inthealth/ihu016; PUBMED: 24711599] [DOI] [PubMed] [Google Scholar]
- Penfold S, Manzi F, Mkumbo E, Temu S, Jaribu J, Shamba DD, et al. Effect of home‐based counselling on newborn care practices in southern Tanzania one year after implementation: a cluster‐randomised controlled trial. BMC Pediatrics 2014;14:187. [DOI: 10.1186/1471-2431-14-187; PUBMED: 25052850] [DOI] [PMC free article] [PubMed] [Google Scholar]
Persson 2013 {published data only}
- Persson LA, Nga NT, Malqvist M, Thi Phuong Hoa D, Eriksson L, et al. Effect of facilitation of local maternal‐and‐newborn stakeholder groups on neonatal mortality: cluster‐randomized controlled trial. PLoS Medicine 2013;10(5):e1001445. [DOI: 10.1371/journal.pmed.1001445; PUBMED: 23690755] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallin L, Målqvist M, Nga NT, Eriksson L, Persson LA, Hoa DP, et al. Implementing knowledge into practice for improved neonatal survival; a cluster‐randomised, community‐based trial in Quang Ninh province, Vietnam. BMC Health Services Research 2011;11:239. [DOI: 10.1186/1472-6963-11-239; PUBMED: 21951770] [DOI] [PMC free article] [PubMed] [Google Scholar]
Soofi 2017 {published data only}
- Soofi S, Cousens S, Turab A, Wasan Y, Mohammed S, Ariff S, et al. Effect of provision of home‐based curative health services by public sector health‐care providers on neonatal survival: a community‐based cluster‐randomised trial in rural Pakistan. Lancet Global Health 2017;5(8):796–806. [DOI: 10.1016/S2214-109X(17)30248-6; PUBMED: 28716351] [DOI] [PMC free article] [PubMed] [Google Scholar]
Srinivasan 1995 {published data only}
- Srinivasan V, Radhakrishna S, Sudha R, Malathi MV, Jabbar S, Ramakrishnan R, et al. Randomised controlled field trial of two antenatal care packages in rural South India. Indian Journal of Medical Research 1995;102:86‐94. [PUBMED: 8834820] [PubMed] [Google Scholar]
Tripathy 2010 {published data only}
- Houweling TAJ, Tripathy P, Nair N, Rath S, Rath S, Gope R, et al. The equity impact of participatory women’s groups to reduce neonatal mortality in India: secondary analysis of a cluster‐randomised trial. International Journal of Epidemiology 2013;42(2):520‐32. [DOI: 10.1093/ije/dyt012; PUBMED: 23509239] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathy P, Nair N, Barnett S, Mahapatra R, Borghi J, Rath S, et al. Effect of a participatory intervention with women's groups on birth outcomes in Jharkhand and Orissa, India: a cluster‐randomised controlled trial. Lancet 2010;375(9721):1182‐92. [DOI: 10.1016/S0140-6736(09)62042-0; PUBMED: 20207411] [DOI] [PubMed] [Google Scholar]
Tripathy 2016 {published data only}
- Sinha RK, Haghparast‑Bidgoli H, Tripathy P, Nair N, Gope R, Rath S, et al. Economic evaluation of participatory learning and action with women’s groups facilitated by Accredited Social Health Activists to improve birth outcomes in rural eastern India. Cost Effectiveness and Resource Allocation 2017;15:2. [DOI: 10.1186/s12962-017-0064-9; PUBMED: 28344517] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathy P, Nair N, Sinha R, Rath S, Gope RK, Rath S, et al. Effect of participatory women’s groups facilitated by Accredited Social Health Activists on birth outcomes in rural eastern India: a cluster‐randomised controlled trial. Lancet Global Health 2016;4(2):119‐28. [DOI: 10.1016/S2214-109X(15)00287-9; PUBMED: 26823213] [DOI] [PubMed] [Google Scholar]
Waiswa 2015 {published data only}
- Waiswa P, Pariyo G, Kallander K, Akuze J, Namazzi G, Ekirapa‐Kiracho E, et al. Effect of the Uganda Newborn Study on care‐seeking and care practices: a cluster‐randomised controlled trial. Global Health Action 2015;8:24584. [DOI: 10.3402/gha.v8.24584; PUBMED: 25843498] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waiswa P, Peterson SS, Namazzi G, Ekirapa EK, Naikoba S, Byaruhanga R, et al. The Uganda Newborn Study (UNEST): an effectiveness study on improving newborn health and survival in rural Uganda through a community‐based intervention linked to health facilities ‐ study protocol for a cluster randomized controlled trial. Trials 2013;13:213. [DOI: 10.1186/1745-6215-13-213; PUBMED: 23153395] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wu 2011 {published data only}
- Wu Z, Viisainen K, Wang Y, Hemminki E. Evaluation of a community‐based randomized controlled prenatal care trial in rural China. BMC Health Services Research 2011;11(1):92. [DOI: 10.1186/1472-6963-11-92; PUBMED: 21542939] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Agboatwalla 1997 {published data only}
- Agboatwalla M, Akram DS. Impact of health education on mothers' knowledge of preventive health practices. Tropical Doctor 1997;27(4):199‐202. [DOI: 10.1177/004947559702700405; PUBMED: 9316359] [DOI] [PubMed] [Google Scholar]
Ahluwalia 2000 {published data only}
- Ahluwalia IB, Tessaro I, Grummer‐Strawn LM, MacGowan C, Benton‐Davis S. Georgia's breastfeeding promotion program for low‐income women. Pediatrics 2000;105(6):e86. [PUBMED: 10835098] [DOI] [PubMed] [Google Scholar]
Alexandre 2007 {published data only}
- Alexandre C, Bomy H, Bourdon E, Truffert P, Pierrat V. Lactation counselling support provided to mothers of preterm babies who intend to breastfeed. Evaluation of an educational intervention in a level III perinatal unit [Accompagnement des mères de nouveau‐nés prématurés dans leur projet d’allaitement maternel. Évaluation d'un programme de formation dans une unité périnatale de niveau III]. Archives de Pédiatrie 2007;14(12):1413–9. [DOI: 10.1016/j.arcped.2007.08.017; PUBMED: 17997289] [DOI] [PubMed] [Google Scholar]
Ayiasi 2015b {published data only}
- Ayiasi RM, Muhumuza C, Bukenya J, Orach CG. The effect of prenatal counselling on postpartum family planning use among early postpartum women in Masindi and Kiryandongo districts, Uganda. Pan African Medical Journal. 2015;21:1388. [DOI: 10.11604/pamj.2015.21.138.7026; PUBMED: 26327975] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bang 1990 {published data only}
- Bang AT, Bang RA, Morankar VP, Sontakke PG, Solanki JM. Pneumonia in neonates: can it be managed in the community?. Archives of Disease in Childhood 1993;68(5 Spec No):550‐6. [PUBMED: 8323354] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang AT, Bang RA, Sontakke PG. Management of childhood pneumonia by traditional birth attendants. The SEARCH Team. Bulletin of the World Health Organization 1994;72(6):897‐905. [PUBMED: 7867135] [PMC free article] [PubMed] [Google Scholar]
- Bang AT, Bang RA, Tale O, Sontakke P, Solanki J, Wargantiwar R, et al. Reduction in pneumonia mortality and total childhood mortality by means of community‐based intervention trial in Gadchiroli, India. Lancet 1990;336(8709):201‐6. [PUBMED: 1973770] [DOI] [PubMed] [Google Scholar]
Bang 1999 {published data only}
- Bang AT, Bang RA, Baitule SB, Reddy HM, Deshmukh MD. Management of birth asphyxia in home deliveries in rural Gadchiroli: the effect of two types of birth attendants and of resuscitating with mouth‐to‐mouth, tube‐mask or bag–mask. Journal of Perinatology 2005;25 Suppl 1:S82‐91. [DOI: 10.1038/sj.jp.7211275; PUBMED: 15791282] [DOI] [PubMed] [Google Scholar]
- Bang AT, Bang RA, Baitule SB, Reddy MH, Deshmukh MD. Effect of home‐based neonatal care and management of sepsis on neonatal mortality: field trial in rural India. Lancet 1999;354(9194):1955‐61. [DOI: 10.1016/S0140-6736(99)03046-9; PUBMED: 10622298] [DOI] [PubMed] [Google Scholar]
- Bang AT, Bang RA, Reddy HM, Deshmukh MD, Baitule SB. Reduced Incidence of neonatal morbidities: effect of home‐based neonatal care in rural Gadchiroli, India. Journal of Perinatology 2005;25 Suppl 1:S51‐61. [DOI: 10.1038/sj.jp.7211274; PUBMED: 15791279] [DOI] [PubMed] [Google Scholar]
- Bang AT, Reddy HM, Deshmukh MD, Baitule SB, Bang RA. Neonatal and infant mortality in the ten years (1993 to 2003) of the Gadchiroli Field Trial: effect of home‐based neonatal care. Journal of Perinatology 2005;25 Suppl 1:S92–107. [DOI: 10.1038/sj.jp.7211277; PUBMED: 15791283] [DOI] [PubMed] [Google Scholar]
Baqui 2015 {published data only}
- Baqui AH, Saha SK, Ahmed NU, Shahidullah M, Quasem I, Roth DE, et al. Safety and efficacy of alternative antibiotic regimens compared with 7 day injectable procaine benzylpenicillin and gentamicin for outpatient treatment of neonates and young infants with clinical signs of severe infection when referral is not possible: a randomised, open‐label, equivalence trial. Lancet Global Health 2015;3(5):279–87. [DOI: 10.1016/S2214-109X(14)70347-X; PUBMED: 25841891] [DOI] [PubMed] [Google Scholar]
- Baqui AH, Saha SK, Ahmed NU, Shahidullah M, Quasem I, Roth DE, et al. Safety and efficacy of simplified antibiotic regimens for outpatient treatment of serious infection in neonates and young infants 0–59 days of age in Bangladesh. Pediatric Infectious Disease Journal 2013;32(9):S12–8. [DOI: 10.1097/INF.0b013e31829ff790; PUBMED: 23945570] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bartington 2006 {published data only}
- Bartington S, Griffiths LJ, Tate AR, Dezateux C, Millennium Cohort Study Child Health Group. Are breastfeeding rates higher among mothers delivering in baby friendly accredited maternity units in the UK?. International Journal of Epidemiology 2006;35(5):1178–86. [DOI: 10.1093/ije/dyl155; PUBMED: 16926214] [DOI] [PubMed] [Google Scholar]
Bhandari 2003 {published data only}
- Bhandari N, Bahl R, Mazumdar S, Martines J, Black ER, Bhan KM, Infant Feeding Study Group. Effect of community‐based promotion of exclusive breastfeeding on diarrhoeal illness and growth: a cluster randomised controlled trial. Lancet 2003;361(9367):1418‐23. [DOI: 10.1016/S0140-6736(03)13134-0; PUBMED: 12727395] [DOI] [PubMed] [Google Scholar]
Bhandari 2004 {published data only}
- Bhandari N, Mazumder S, Bahl B, Martines J, Robert EB, Bhan MK, Infant Feeding Study Group. An educational intervention to promote appropriate complementary feeding practices and physical growth in infants and young children in rural Haryana, India. Journal of Nutrition 2004;134(9):2342‐8. [DOI: 10.1093/jn/134.9.2342; PUBMED: 15333726] [DOI] [PubMed] [Google Scholar]
Bhattacharya 1988 {published data only}
- Bhattacharya R, Kaur P, Reedy DC. Impact of education in the knowledge and practices of rural mothers and key family members on diarrhoea and its treatment at home. Journal of Diarrheal Diseases Research 1988;6(1):15‐20. [PUBMED: 3251936] [PubMed] [Google Scholar]
Bhopal 2017 {published data only}
- Bhopal S, Verma D, Divan G, Hill Z, Barlow J, Roy R, et al. Early life stress of infants in the SPRING home visits intervention promoting child growth and development in rural India (SPRING‐ELS): development of stress measures for administration at scale. Lancet Poster 2017;389 Suppl 1:S26. [DOI: ] [Google Scholar]
Bhutta 2009 {published data only}
- Bhutta ZA, Rizvi A, Raza F, Hotwani S, Zaidi S, Hossain SM, et al. A comparative evaluation of multiple micronutrient and iron–folic acid supplementation during pregnancy in Pakistan: impact on pregnancy outcomes. Food and Nutrition Bulletin 2009;30(4):S496‐505. [DOI: 10.1177/15648265090304S404; PUBMED: 20120791] [DOI] [PubMed] [Google Scholar]
Bland 2008 {published data only}
- Bland RM, Little KE, Coovadiad HM, Coutsoudis A, Rollinse NC, Newella ML. Intervention to promote exclusive breast‐feeding for the first 6 months of life in a high HIV prevalence area. AIDS 2008;22(7):883‐91. [DOI: 10.1097/QAD.0b013e3282f768de; PUBMED: 18427207] [DOI] [PubMed] [Google Scholar]
Bolam 1998 {published data only}
- Bolam A, Manandhar DS, Shrestha P, Ellis M, Costello AM. The effects of postnatal health education for mothers on infant care and family planning practices in Nepal: a randomised controlled trial. BMJ 1998;316(7134):805–11. [PUBMED: 9549449] [DOI] [PMC free article] [PubMed] [Google Scholar]
Boulvain 2004 {published data only}
- Boulvain M, Perneger TV, Othenin‐Girard V, Petrou S, Berner M, Iriona O. Home‐based versus hospital‐based postnatal care: a randomised trial. BJOG 2004;3(8):807–13. [DOI: 10.1111/j.1471-0528.2004.00227.x; PUBMED: 15270928] [DOI] [PubMed] [Google Scholar]
Carlo 2010 {published data only}
- Carlo WA, McClure EM, Chomba E, Chakraborty H, Hartwell T, Harris H, et al. Newborn care training of midwives and neonatal and perinatal mortality rates in a developing country. Pediatrics 2010;126(5):e1064. [DOI: 10.1542/peds.2009-3464; PUBMED: 20937659] [DOI] [PubMed] [Google Scholar]
Castrucci 2007 {published data only}
- Castrucci BC, Hoover KL, Lim S, Maus KC. Availability of lactation counselling services influences breastfeeding among infants admitted to neonatal intensive care units. American Journal of Health Promotion 2007;21(5):410‐5. [DOI: 10.4278/0890-1171-21.5.410; PUBMED: 17515004] [DOI] [PubMed] [Google Scholar]
Chapman 2004 {published data only}
- Chapman DJ, Damio G, Young S, Pe´rez‐Escamilla R. Effectiveness of breastfeeding peer counseling in a low‐income, predominantly Latina population. Archives of Pediatrics and Adolescent Medicine 2004;158(9):897‐902. [DOI: 10.4278/0890-1171-21.5.410; PUBMED: 17515004] [DOI] [PubMed] [Google Scholar]
Coombs 1998 {published data only}
- Coombs DW, Reynolds K, Joyner G, Son MB. A self‐help program to increase breastfeeding among low‐income women. Journal of Nutrition Education 1998;30(4):203‐9. [DOI: ] [Google Scholar]
Coskun 2009 {published data only}
- Coskun AM, Karakaya E, Yaser Y. A safe motherhood education and counselling programme in Istanbul. European Journal of Contraception and Reproductive Health Care 2009;14(6):424‐36. [DOI: 10.3109/13625180903274460; PUBMED: 19929646] [DOI] [PubMed] [Google Scholar]
Davies‐Abetugbo 1996 {published data only}
- Davies‐Adetugbo AA. Promotion of breastfeeding in the community: impact of health education program in rural communities in Nigeria. Journal of Diarrhoeal Diseases 1996;14(1):5‐11. [PUBMED: 8708336] [PubMed] [Google Scholar]
Dearden 2002 {published data only}
- Dearden K, Altaye M, Maza I, Oliva M, Stone‐Jimenez M, Burkhalter BR, et al. The impact of mother‐to‐mother support on optimal breast‐feeding: a controlled community intervention trial in peri‐urban Guatemala City, Guatemala. Revista Panamericana de Salud Publica 2002;12(3):193‐201. [PUBMED: 12396638] [DOI] [PubMed] [Google Scholar]
Dennis 2001 {published data only}
- Dennis CL. Breastfeeding initiation and duration: a 1990‐2000 literature review. Journal of Obstetric, Gynecologic and Neonatal Nursing 2002;31(1):12–32. [PUBMED: 11843016] [DOI] [PubMed] [Google Scholar]
Di Napoli 2004 {published data only}
- Napoli A, Lallo D, Fortes C, Franceschelli C, Armeni E, Guasticchi G. Home breastfeeding support by health professionals: findings of a randomised controlled trial in a population of Italian women. Acta Paediatrica 2004;93(8):1108‐14. [PUBMED: 15456204] [PubMed] [Google Scholar]
Escobar 2001 {published data only}
- Escobar GJ, Braveman PA, Ackerson L, Odouli R, Coleman‐Phox K, Capra AM, et al. A randomized comparison of home visits and hospital‐based group follow‐up visits after early postpartum discharge. Pediatrics 2001;108(3):719–27. [PUBMED: 11533342] [DOI] [PubMed] [Google Scholar]
Flax 2009 {published data only}
- Flax VL, Negerie M, Ibrahim AU, Leatherman S, Daza EJ, Bentley ME. Integrating group counseling, cell phone messaging, and participant‐generated songs and dramas into a microcredit program increases Nigerian women's adherence to international breastfeeding recommendations. Journal of Nutrition 2009;144(7):1120–4. [DOI: 10.3945/jn.113.190124; PUBMED: 24812071] [DOI] [PMC free article] [PubMed] [Google Scholar]
Foreit 1993 {published data only}
- Foreit KG, Foreit JR, Lagos G, Guzman A. Effectiveness and cost‐effectiveness of postpartum IUD insertion in Lima, Peru. International Family Planning Perspectives 1993;19(1):19‐33. [DOI: 10.2307/2133378] [DOI] [Google Scholar]
Forster 2004 {published data only}
- Forster D, McLachlan H, Lumley J, Beanland C, Waldenstrom U, Amir L. Two mid‐pregnancy interventions to increase the initiation and duration of breastfeeding: a randomized controlled trial. Birth 2004;31(3):176‐82. [DOI: 10.1111/j.0730-7659.2004.00302.x; PUBMED: 15330879] [DOI] [PubMed] [Google Scholar]
Froozani 1999 {published data only}
- Froozani MD, Permehzadeh K, Dorosty AR, Golestan B. Effect of breastfeeding education on the feeding pattern and health of infants in their first 4 months in the Islamic Republic of Iran. Bulletin of the World Health Organization 1999;77(5):381‐5. [PUBMED: 10361754] [PMC free article] [PubMed] [Google Scholar]
Gill 2007 {published data only}
- Gill SL, Reifsnider E, Lucke JF. Effects of support on the initiation and duration of breastfeeding. Western Journal of Nursing Research 2007;29(6):708‐23. [DOI: 10.1177/0193945906297376; PUBMED: 17557933] [DOI] [PubMed] [Google Scholar]
Gill 2011 {published data only}
- Gill CJ, Phiri‐Mazala G, Guerina NG, Kasimba J, Mulenga C, MacLeod WB, et al. Effect of training traditional birth attendants on neonatal mortality (Lufwanyama Neonatal Survival Project): randomised controlled study. BMJ 2011;342:346. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gross 2009 {published data only}
- Gross SM, Resnik AK, Cross‐Barnet C, Nanda JP, Augustyn M, Paige DM. The differential impact of WIC peer counseling programs on breastfeeding initiation across the state of Maryland. Journal of Human Lactation 2009;25(4):435‐43. [DOI: 10.1177/0890334409342070; PUBMED: 19652195] [DOI] [PubMed] [Google Scholar]
Grossman 2009 {published data only}
- Grossman X, Chaudhuri J, Feldman‐Winter L, Abrams J, Newton KN, Philipp BL, et al. Hospital Education in Lactation Practices (Project HELP): does clinician education affect breastfeeding initiation and exclusivity in the hospital?. Birth 2009;36(1):54‐9. [DOI: 10.1111/j.1523-536X.2008.00295.x; PUBMED: 19278384] [DOI] [PubMed] [Google Scholar]
Guise 2003 {published data only}
- Guise JM, Palda V, Westhoff C, Chan BK, Helfand M, Lieu TA. The effectiveness of primary care‐based interventions to promote breastfeeding: systematic evidence review and meta‐analysis for the US Preventive Services Task Force. Annals of Family Medicine 2003;1(2):70‐8. [PUBMED: 15040435] [DOI] [PMC free article] [PubMed] [Google Scholar]
Haider 1996 {published data only}
- Haider R, Islam A, Hamadani J, Amin NJ, Kabir, Malek MA, et al. Breast‐feeding counselling in a diarrhoeal disease hospital. Bulletin of the World Health Organization 1996;74(2):173‐9. [PUBMED: 8706233] [PMC free article] [PubMed] [Google Scholar]
Haider 2000 {published data only}
- Haider R, Ashworth A, Kabir I, Huttly SR. Effect of community‐based peer counsellors on exclusive breastfeeding practices in Dhaka, Bangladesh: a randomised controlled trial. Lancet 2000;356(9242):1643–7. [PUBMED: 11089824] [DOI] [PubMed] [Google Scholar]
Hannula 2008 {published data only}
- Hannula L, Kaunonen M, Tarkka MT. A systematic review of professional support interventions for breastfeeding. Journal of Clinical Nursing 2008;17(9):1132–43. [DOI: 10.1111/j.1365-2702.2007.02239.x; PUBMED: 18416790] [DOI] [PubMed] [Google Scholar]
Hoare 1999 {published data only}
- Hoare K, Hoare S, Rhodes D, Erinosos OH, Weaver LT. Effective health education in rural Gambia. Journal of Tropical Pediatrics 1999;45(4):208‐14. [DOI: 10.1093/tropej/45.4.208; PUBMED: 10467831] [DOI] [PubMed] [Google Scholar]
Hoddinott 2006 {published data only}
- Hoddinott P, Lee AJ, Pill R. Effectiveness of a breastfeeding peer coaching intervention in rural Scotland. Birth 2006;33(1):27‐36. [DOI: 10.1111/j.0730-7659.2006.00071.x; PUBMED: 16499529] [DOI] [PubMed] [Google Scholar]
Hotz 2005 {published data only}
- Hotz C, Gibson RS. Participatory nutrition education and adoption of new feeding practices are associated with improved adequacy of complementary diets among rural Malawian children: a pilot study. European Journal of Clinical Nutrition 2005;59(2):226‐37. [DOI: 10.1038/sj.ejcn.1602063; PUBMED: 15483634] [DOI] [PubMed] [Google Scholar]
Ickovics 2007 {published data only}
- Ickovics JR, Kershaw TS, Westdahl C, Magriples U, Massey Z, Reynolds H, et al. Group prenatal care and perinatal outcomes: a randomized controlled trial. Obstetrics and Gynecology 2007;110(2 Pt 1):330–9. [DOI: 10.1097/01.AOG.0000275284.24298.23; PUBMED: 17666608] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ingram 2004 {published data only}
- Ingram J, Johnson D. A feasibility study of an intervention to enhance family support for breast feeding in a deprived area in Bristol, UK. Midwifery 2004;20(4):367‐79. [DOI: 10.1016/j.midw.2004.04.003; PUBMED: 15571885] [DOI] [PubMed] [Google Scholar]
Ingram 2009 {published data only}
- Ingram J, Johnson D. Using community maternity care assistants to facilitate family‐focused breastfeeding support. Maternal and Child Nutrition 2009;5(3):276‐81. [DOI: 10.1111/j.1740-8709.2008.00175.x; PUBMED: 19531048] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jang 2008 {published data only}
- Jang GJ, Kim SH, Jeong KS. Effect of postpartum breast‐feeding support by nurse on the breast‐feeding prevalence. Taehan Kanho Hakhoe Chi 2008;38(1):172‐9. [PUBMED: 18323730] [DOI] [PubMed] [Google Scholar]
Johnson 2017 {published data only}
- Johnson D, Jurasb R, Rileyc P, Chatterji M, Sloanec P, Choid SK, et al. A randomized controlled trial of the impact of a family planning mHealth service on knowledge and use of contraception. Contraception 2017;95(1):90‐7. [DOI: 10.1016/j.contraception.2016.07.009; PUBMED: 27421767] [DOI] [PubMed] [Google Scholar]
Kadam 2005 {published data only}
- Kadam S, Binoy S, Kanbur W, Mondkar JA, Fernandez A. Feasibility of kangaroo mother care in Mumbai. Indian Journal of Pediatrics 2005;72(1):35‐8. [PUBMED: 15684446] [DOI] [PubMed] [Google Scholar]
Kafatos 1989 {published data only}
- Kafatos AG, Vlachonikolis IG, Codrington CA. Nutrition during pregnancy: the effects of an educational intervention program in Greece. American Journal of Clinical Nutrition 1989;50(5):979‐9. [DOI: 10.1093/ajcn/50.5.970; PUBMED: 2816804] [DOI] [PubMed] [Google Scholar]
Kafatos 1991 {published data only}
- Kafatos AG, Tsitoura S, Pantelakis SN, Doxiadis SA. Maternal and infant health education in a rural Greek community. Hygie 1991;10(1):32‐7. [PubMed] [Google Scholar]
Kimani‐Murage 2015 {published data only}
- Kimani‐Murage EW, Kimiywe J, Kabue M, Wekesah F, Matiri E, Muhia N, et al. Feasibility and effectiveness of the baby friendly community initiative in rural Kenya: study protocol for a randomized controlled trial. Trials 2015;16:431. [DOI: 10.1186/s13063-015-0935-3] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maingi M, Kimiywe J, Iron‐Segev S. Effectiveness of Baby Friendly Community Initiative (BFCI) on complementary feeding in Koibatek, Kenya: a randomized control study. BMC Public Health 2018;18(1):600. [10.1186/s12889‐018‐5519‐1] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kools 2005 {published data only}
- Kools EJ, Thijs C, Kester ADM, Brandt PA, Vries H. A breast‐feeding promotion and support program: a randomised trial in the Netherlands. Preventive Medicine 2005;40(1):60‐70. [DOI: 10.1016/j.ypmed.2004.05.013; PUBMED: 15530582] [DOI] [PubMed] [Google Scholar]
Lin 2008 {published data only}
- Lin SS, Chien LY, Tai CJ, Lee CF. Effectiveness of a prenatal education programme on breastfeeding outcomes in Taiwan. Journal of Clinical Nursing 2008;17(3):296–303. [DOI: 10.1111/j.1365-2702.2006.01927.x; PUBMED: 17931376] [DOI] [PubMed] [Google Scholar]
MacArthur 2009 {published data only}
- MacArthur C, Jolly K, Ingram L, Freemantle N, Dennis CL, Hamburger R, et al. Antenatal peer support workers and initiation of breastfeeding: cluster randomised controlled trial. BMJ 2009;338:b131. [DOI: 10.1136/bmj.b131; PUBMED: 19181730] [DOI] [PMC free article] [PubMed] [Google Scholar]
Martens 2002 {published data only}
- Martens PJ. Increasing breastfeeding initiation and duration at a community level: an evaluation of Sagkeeng First Nation's community health nurse and peer counselor programs. Journal of Human Lactation 2002;18(3):236‐46. [DOI: 10.1177/089033440201800305; PUBMED: 12192958] [DOI] [PubMed] [Google Scholar]
Merewood 2003 {published data only}
- Merewood A, Philipp BL, Chawla N, Cimo S. The baby‐friendly hospital initiative increases breastfeeding rates in a US neonatal intensive care unit. Journal of Human Lactation 2003;19(2):166‐71. [DOI: 10.1177/0890334403252475; PUBMED: 12744533] [DOI] [PubMed] [Google Scholar]
Moran 2006 {published data only}
- Moran AC, Sangli G, Dineen R, Rawlins B, Yaméogo M, Baya B. Birth‐preparedness for maternal health: findings from Koupéla District, Burkina Faso. Journal of Health, Population and Nutrition 2006;24(4):489‐97. [PUBMED: 17591346] [PMC free article] [PubMed] [Google Scholar]
Mottl‐Santiago 2008 {published data only}
- Mottl‐Santiago J, Walker C, Ewan J, Vragovic O, Winder S, Stubblefield P. A hospital‐based doula program and childbirth outcomes in an urban, multicultural setting. Maternal and Child Health Journal 2008;12(2):372‐7. [DOI: 10.1007/s10995-007-0245-9; PUBMED: 17610053] [DOI] [PubMed] [Google Scholar]
Murihead 2006 {published data only}
- Muirhead PE, Butcher G, Rankin J, Munley A. The effect of a programme of organised and supervised peer support on the initiation and duration of breastfeeding: a randomised trial. British Journal of General Practice 2006;56(524):191‐7. [PUBMED: 16536959] [PMC free article] [PubMed] [Google Scholar]
Nabulsi 2014 {published data only}
- Nabulsi M, Hamadeh H, Tamim H, Kabakian T, Charafeddine L, Yehya N, et al. A complex breastfeeding promotion and support intervention in a developing country: study protocol for a randomized clinical trial. BMC Public Health 2014;14:36. [DOI: 10.1186/1471-2458-14-36; PUBMED: 24428951] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nair 2017 {published data only}
- Nair N, Tripathy P, Sachdev HS, Pradhan H, Bhattacharyya S, Gope R, et al. Effect of participatory women’s groups and counselling through home visits on children’s linear growth in rural eastern India (CARING trial): a cluster‐randomised controlled trial. Lancet Global Health 2017;5(10):1004–16. [DOI: 10.1016/S2214-109X(17)30339-X; PUBMED: 28911749] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nichols 2009 {published data only}
- Nichols J, Schutte NS, Brown RF, Dennis CL, Price I. The impact of a self‐efficacy intervention on short‐term breast‐feeding outcomes. Health Education & Behavior 2009;36(2):250‐8. [DOI: 10.1177/1090198107303362; PUBMED: 17893124] [DOI] [PubMed] [Google Scholar]
Petrova 2009 {published data only}
- Petrova A, Ayers C, Stechna S, Gerling JA, Mehta R. Effectiveness of exclusive breastfeeding promotion in low‐income mothers: a randomized controlled study. Breastfeeding Medicine 2009;4(2):63‐9. [DOI: 10.1089/bfm.2008.0126; PUBMED: 19239405] [DOI] [PubMed] [Google Scholar]
Philipp 2001 {published data only}
- Philipp BL, Merewood A, Miller LW, Chawla N, Murphy‐Smith MM, Gomes JS, et al. Baby‐friendly hospital initiative improves breastfeeding initiation rates in a US hospital setting. Pediatrics 2001;108(3):677‐83. [PUBMED: 11533335] [DOI] [PubMed] [Google Scholar]
Pobocik 2000 {published data only}
- Pobocik SR, Benaventea JC, Schwab AC, Boudreau N, Morris C, Houston MS. Effect of a breastfeeding education and support program on breastfeeding initiation and duration in a culturally diverse group of adolescents. Journal of Nutrition Education 2000;32(3):139‐45. [DOI: ] [Google Scholar]
Quinn 2005 {published data only}
- Quinn VJ, Guyon AB, Schubert JW, Stone‐Jiménez M, Hainsworth MD, Martin LH. Improving breastfeeding practices on a broad scale at the community level: success stories from Africa and Latin America. Journal of Human Lactation 2005;21(3):345‐54. [DOI: 10.1177/0890334405278383; PUBMED: 16113023] [DOI] [PubMed] [Google Scholar]
Raghupathy 1996 {published data only}
- Raghupathy S. Education and the use of maternal health in Thailand. Social Science and Medicine 1996;43(4):459‐71. [PUBMED: 8844947] [DOI] [PubMed] [Google Scholar]
Rahman 2008 {published data only}
- Rahman A, Malik A, Sikander S, Roberts C, Creed F. Cognitive behaviour therapy‐based intervention by community health workers for mothers with depression and their infants in rural Pakistan: a cluster‐randomised controlled trial. Lancet 2008;372(9642):902‐9. [DOI: 10.1016/S0140-6736(08)61400-2; PUBMED: 18790313] [DOI] [PMC free article] [PubMed] [Google Scholar]
Raj 2016 {published data only}
- Raj A, Ghule M, Ritter J, Battala M, Gajanan V, Nair S, et al. Cluster randomized controlled trial evaluation of a gender equity and family planning intervention for married men and couples in Rural India. PLoS ONE 2016;11(5):e0153190. [DOI: 10.1371/journal.pone.0153190; PUBMED: 27167981] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yore J, Dasgupta A, Ghule M, Battala M, Nair S, Silverman J, et al. CHARM, a gender equity and family planning intervention for men and couples in rural India: protocol for the cluster randomized controlled trial evaluation. Reproductive Health 2016;13:14. [DOI: 10.1186/s12978-016-0122-3; PUBMED: 26897656] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rawat 2017 {published data only}
- Rawat R, Nguyen PH, Tran LM, Hajeebhoy N, Nguyen HV, Baker J, et al. Social franchising and a nationwide mass media campaign increased the prevalence of adequate complementary feeding in Vietnam: a cluster‐randomized program evaluation. Journal of Nutrition 2017;147(4):670–9. [DOI: 10.3945/jn.116.243907; PUBMED: 28179488] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rishel 2005 {published data only}
- Rishel PEN, Sweeney P. Comparison of breastfeeding rates among women delivering infants in military treatment facilities with and without lactation consultants. Military Medicine 2005;170(5):435‐8. [PUBMED: 15974214] [DOI] [PubMed] [Google Scholar]
Rosato 2006 {published data only}
- Rosato M, Mwansambo CW, Kazembe PN, Phiri T, Soko QS, Lewycka S, et al. Women’s groups' perceptions of maternal health issues in rural Malawi. Lancet 2006;368(9542):1180‐8. [DOI: 10.1016/S0140-6736(06)69475-0; PUBMED: 17011945] [DOI] [PubMed] [Google Scholar]
Rosen 2008 {published data only}
- Rosen IM, Krueger MV, Carney LM, Graham JA. Prenatal breastfeeding education and breastfeeding outcomes. MCN. American Journal of Maternal Child Nursing 2008;33(5):315‐9. [DOI: 10.1097/01.NMC.0000334900.22215.ec; PUBMED: 18758336] [DOI] [PubMed] [Google Scholar]
Rossiter 1994 {published data only}
- Rossiter JC. The effect of a culture‐specific education program to promote breastfeeding among Vietnamese women in Sydney. International Journal of Nursing Studies 1994;31(4):369‐79. [PUBMED: 7928125] [DOI] [PubMed] [Google Scholar]
Roy 2007 {published data only}
- Roy KS, Jolly SP, Shafique S, Fuchs GJ, Mahmud Z, Chakraborty B, et al. Prevention of malnutrition among young children in rural Bangladesh by a food‐health‐care educational intervention: a randomised, controlled trial. Food and Nutrition Bulletin 2007;28(4):375‐83. [DOI: 10.1177/156482650702800401d; PUBMED: 18274163] [DOI] [PubMed] [Google Scholar]
Russell 1999 {published data only}
- Russell BK, Aviles M, Brion LP. Relationship between perinatal counseling and incidence of breastfeeding in an inner‐city population. Journal of Perinatology 1999;19(7):501‐4. [PUBMED: 10685299] [DOI] [PubMed] [Google Scholar]
Ryser 1999 {published data only}
- Ryser FG. Breastfeeding Attitudes, Intention and Initiation in Low‐income Women: The Effect of the Best Start Program. Denton, TX: Texas Woman's University, College of Nursing, 1999. [DOI] [PubMed] [Google Scholar]
Ryser 2004 {published data only}
- Ryser FG. Breastfeeding attitudes, intention, and initiation in low‐income women: the effect of the best start program. Journal of Human Lactation 2004;20(3):300. [DOI: 10.1177/0890334404266985; PUBMED: 15296584] [DOI] [PubMed] [Google Scholar]
Sachdeva 1994 {published data only}
- Sachdeva R, Mann SK. Impact of nutrition counselling and supplements on the mineral nutriture of rural pregnant women and their neonates. Indian Pediatrics 1994;31(6):643‐9. [PUBMED: 7896386] [PubMed] [Google Scholar]
Sandy 2009 {published data only}
- Sandy JM, Anisfeld E, Ramirez E. Effects of a prenatal intervention on breastfeeding initiation rates in a Latina immigrant sample. Journal of Human Lactation 2009;25(4):404‐11. [DOI: 10.1177/0890334409337308; PUBMED: 19487705] [DOI] [PubMed] [Google Scholar]
Schneider 2001 {published data only}
- Schneider Z. Antenatal education classes in Victoria: what the women said. Australian Journal of Midwifery 2001;14(3):14‐20. [PUBMED: 12760007] [DOI] [PubMed] [Google Scholar]
Seema 1997 {published data only}
- Seema, Patwari AK, Satyanarayana L. Relactation: an effective intervention to promote exclusive breastfeeding. Journal of Tropical Pediatrics 1997;43(4):213‐6. [DOI: 10.1093/tropej/43.4.213; PUBMED: 9283123] [DOI] [PubMed] [Google Scholar]
Serwint 1996 {published data only}
- Serwint JR, Wilson ME, Vogelhut JW, Repke JT, Seidel HM. A randomized control trial of prenatal pediatric visits for urban low‐income families. Pediatrics 1996;98(6):1069‐75. [PUBMED: 8951255] [PubMed] [Google Scholar]
Shaw 1999 {published data only}
- Shaw E, Kaczorowski J. The effect of a peer counseling program on breastfeeding initiation and longevity in a low‐income rural population. Journal of Human Lactation 1999;15(1):19‐25. [DOI: 10.1177/089033449901500108; PUBMED: 10578771] [DOI] [PubMed] [Google Scholar]
Shinwell 2006 {published data only}
- Shinwell ES, Churgin Y, Shlomo M, Shani M, Flidel‐Rimon O. The effect of training nursery staff in breastfeeding guidance on the duration of breastfeeding in healthy term infants. Breastfeeding Medicine 2006;1(4):247‐52. [DOI: 10.1089/bfm.2006.1.247; PUBMED: 17661605] [DOI] [PubMed] [Google Scholar]
Shrestha 2016 {published data only}
- Shrestha S, Adachi K, Petrini MA, Shrestha S, Khagi BR. Development and evaluation of a newborn care education programme in primiparous mothers in Nepal. Midwifery 2016;42:21–8. [DOI: 10.1016/j.midw.2016.09.006; PUBMED: 27710817] [DOI] [PubMed] [Google Scholar]
Sloan 2008 {published data only}
- Sloan NL, Ahmed S, Mitra SN, Choudhury N, Chowdhury M, Rob U, et al. Community‐based kangaroo mother care to prevent neonatal and infant mortality: a randomized, controlled cluster trial. Pediatrics 2008;121(5):e1047‐59. [DOI: 10.1542/peds.2007-0076; PUBMED: 18450847] [DOI] [PubMed] [Google Scholar]
Stille 2001 {published data only}
- Stille CJ, Christison‐Lagay J, Bernstein BA, Dworkin PH. A simple provider‐based educational intervention to boost infant immunization rates: a controlled trial. Clinical Pediatrics 2001;40(7):365‐73. [DOI: 10.1177/000992280104000701; PUBMED: 11491130] [DOI] [PubMed] [Google Scholar]
Susin 2008 {published data only}
- Susin LR, Giugliani ERJ. Inclusion of fathers in an intervention to promote breastfeeding: impact on breastfeeding rates. Journal of Human Lactation 2008;24(4):386‐92. [DOI: 10.1177/0890334408323545; PUBMED: 18784322] [DOI] [PubMed] [Google Scholar]
Svenson 2009 {published data only}
- Svensson J, Barclay L, Cooke M. Randomised‐controlled trial of two antenatal education programmes. Midwifery 2009;25(2):114–25. [DOI: 10.1016/j.midw.2006.12.012; PUBMED: 17459542] [DOI] [PubMed] [Google Scholar]
Syed 2006 {published data only}
- Syed U, Asiruddin, Helal SI, Mannan II, Murray J. Immediate and early postnatal care for mothers and newborns in Rural Bangladesh. Journal of Health, Population and Nutrition 2006;24(4):508‐18. [PUBMED: 17591348] [PMC free article] [PubMed] [Google Scholar]
Syed 2008 {published data only}
- Syed U, Khadka N, Khan A, Wall S. Care‐seeking practices in South Asia: using formative research to design program interventions to save newborn lives. Journal of Perinatology 2008;28 Suppl 2:S9‐13. [DOI: 10.1038/jp.2008.165; PUBMED: 19057572] [DOI] [PubMed] [Google Scholar]
Tshefu 2015 {published data only}
- Tshefu A, Lokangaka A, Ngaima S, Engmann C, Esamai F, Gisore P, et al. Oral amoxicillin compared with injectable procaine benzylpenicillin plus gentamicin for treatment of neonates and young infants with fast breathing when referral is not possible: a randomised, open‐label, equivalence trial. Lancet 2015;385(9979):1758–66. [DOI: 10.1016/S0140-6736(14)62285-6; PUBMED: 25842223] [DOI] [PubMed] [Google Scholar]
- Tshefu A, Lokangaka A, Ngaima S, Engmann C, Esamai F, Gisore P, et al. Simplified antibiotic regimens compared with injectable procaine benzylpenicillin plus gentamicin for treatment of neonates and young infants with clinical signs of possible serious bacterial infection when referral is not possible: a randomised, open‐label, equivalence trial. Lancet 2015;385(14):1767–76. [DOI: 10.1016/S0140-6736(14)62284-4; PUBMED: 25842221] [DOI] [PubMed] [Google Scholar]
Tylleskär 2011 {published data only}
- Tylleskär T, Jackson D, Meda N, Engebretsen IM, Chopra M, Diallo AH, et al. Exclusive breastfeeding promotion by peer counsellors in sub‐Saharan Africa (PROMISE‐EBF): a cluster‐randomised trial. Lancet 2011;378(9789):420‐7. [DOI] [PubMed] [Google Scholar]
Volpe 2000 {published data only}
- Volpe EM, Bear M. Enhancing breastfeeding initiation in adolescent mothers through the Breastfeeding Educated and Supported Teen (BEST) Club. Journal of Human Lactation 2000;16(3):196‐200. [DOI: 10.1177/089033440001600303; PUBMED: 11153152] [DOI] [PubMed] [Google Scholar]
Warren 2010 {published data only}
- Warren C, Mwangi A, Oweya E, Kamunya R, Koskei N. Safeguarding maternal and newborn health: improving the quality of postnatal care in Kenya. International Journal for Quality in Health Care 2010;22(1):24‐30. [DOI: 10.1093/intqhc/mzp050; PUBMED: 19946120] [DOI] [PubMed] [Google Scholar]
Whitelaw 1988 {published data only}
- Whitelaw A, Heisterkamp G, Sleath K, Acolet D, Richards M. Skin to skin contact for very low birthweight infants and their mothers. Archives of Disease in Childhood 1988;63(11):1377‐81. [PUBMED: 3060024] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wong 2007 {published data only}
- Wong EH, Nelson E, Choi KC, Wong KP, Ip C, Ho LC. Evaluation of a peer counselling programme to sustain breastfeeding practice in Hong Kong. International Breastfeeding Journal 2007;2:12. [DOI: 10.1186/1746-4358-2-12; PUBMED: 17883851] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yun 2010 {published data only}
- Yun S, Liu Q, Mertzlufft K, Kruse C, White M, Fullerand P, et al. Evaluation of the Missouri WIC (Special Supplemental Nutrition Program for Women, Infants, and Children) breast‐feeding peer counselling programme. Public Health Nutrition 2010;13(2):229‐37. [DOI: 10.1017/S1368980009990668; PUBMED: 19607746] [DOI] [PubMed] [Google Scholar]
Zaidi 2012 {published data only}
- Zaidi AM, Tikmani SS, Warraich HJ, Darmstadt GL, Bhutta ZA, Sultana S, et al. Community‐based treatment of serious bacterial infections in newborns and young infants: a randomized controlled trial assessing three antibiotic regimens. Pediatric Infectious Disease Journal 2012;31(7):667‐72. [DOI: 10.1097/INF.0b013e318256f86c; PUBMED: 22481421] [DOI] [PubMed] [Google Scholar]
Zaidi 2013 {published data only}
- Zaidi AK, Tikmani SS, Sultana S, Baloch B, Kazi M, Rehman H, et al. Simplified antibiotic regimens for the management of clinically diagnosed severe infections in newborns and young infants in first‐level facilities in Karachi, Pakistan. Pediatric Infectious Disease Journal 2013;32(9):S19‐25. [DOI: 10.1097/INF.0b013e31829ff7aa; PUBMED: 23945571] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
Mazumder 2017 {published data only}
- Mazumder S, Taneja S, Dalpath SK, Gupta R, Dube B, Sinha B, et al. Impact of community‐initiated Kangaroo Mother Care on survival of low birth weight infants: study protocol for a randomized controlled trial. Trials 2017;18(1):262. [NCT02653534] [DOI] [PMC free article] [PubMed] [Google Scholar]
Morrison 2011 {published data only}
- Morrison J, Tumbahangphe KM, Budhathoki B, Neupane R, Sen A, Dahal K, et al. Community mobilisation and health management committee strengthening to increase birth attendance by trained health workers in rural Makwanpur, Nepal: study protocol for a cluster randomised controlled trial. Trials 2011;12:128. [DOI: 10.1186/1745-6215-12-128; PUBMED: 21595902] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pell 2016 {published data only}
- Pell LG, Bassani DG, Nyaga L, Njagi I, Wanjiku C, Thiruchselvam T, et al. Effect of provision of an integrated neonatal survival kit and early cognitive stimulation package by community healthworkers on developmental outcomes of infants in Kwale County, Kenya: study protocol for a cluster randomized trial. BMC Pregnancy and Childbirth 2016;16:265. [DOI: 10.1186/s12884-016-1042-5; PUBMED: 27608978] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shrestha 2011 {published data only}
- Clarke K, Saville N, Bhandari B, Giri K, Ghising M, Jha M, et al. Understanding psychological distress among mothers in rural Nepal: a qualitative grounded theory exploration. BMC Psychiatry 2014;14:60. [DOI: 10.1186/1471-244X-14-60; PUBMED: 24581309] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrestha BP, Bhandari B, Manandhar DS, Osrin D, Costello A, Saville N. Community interventions to reduce child mortality in Dhanusha, Nepal: study protocol for a cluster randomized controlled trial. Trials 2011;12:136. [DOI: 10.1186/1745-6215-12-136; PUBMED: 21635791] [DOI] [PMC free article] [PubMed] [Google Scholar]
Su 2016 {published data only}
- Su Y, Yuan C, Zhou Z, Heitner J, Campbell B. Impact of an SMS advice programme on maternal and newborn health in rural China: study protocol for a quasi‐randomised controlled trial. BMJ Open 2016;6(8):e011016. [DOI: 10.1136/bmjopen-2015-011016; PUBMED: 27515750] [DOI] [PMC free article] [PubMed] [Google Scholar]
Turab 2013 {published data only}
- Turab A, Ariff S, Habib MA, Ahmed I, Hussain M, Rashid A, et al. Improved accessibility of emergency obstetrics and newborn care (EmONC) services for maternal and newborn health: a community based project. BMC Pregnancy and Childbirth 2013;13:136. [DOI: 10.1186/1471-2393-13-136; PUBMED: 23800194] [DOI] [PMC free article] [PubMed] [Google Scholar]
Var 2015 {published data only}
- Var C, Bazzano AN, Srivastav SK, Welty JC, Ek NI, Oberhelman RA. Newborn Infection Control and Care Initiative for health facilities to accelerate reduction of newborn mortality (NICCI): study protocol for a randomized controlled trial. Trials 2015;16:257. [DOI: 10.1186/s13063-015-0771-5; PUBMED: 26044715] [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Ahmed 2001
- Ahmed S, Sobhan F, Islam A, Barkat‐e‐Khuda. Neonatal morbidity and care seeking behaviour in rural Bangladesh. Journal of Tropical Pediatrics 2001;47(2):98‐105. [DOI: 10.1093/tropej/47.2.98; PUBMED: 11336143] [DOI] [PubMed] [Google Scholar]
Atuoye 2015
- Atuoye KN, Dixon J, Rishworth A, Galaa SZ, Boamah SA, Luginaah I. Can she make it? Transportation barriers to accessing maternal and child health care services in rural Ghana. BMC Health Services Research 2015;15(1):333. [DOI: 10.1186/s12913-015-1005-y; PUBMED: 26290436] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bang 2001
- Bang AT, Bang RA, Baitule S, Deshmukh M, Reddy MH. Burden of morbidities and the unmet need for health care in rural neonates ‐ a prospective observational study in Gadchiroli, India. Indian Pediatrics 2001;38(9):952‐65. [PUBMED: 11568371] [PubMed] [Google Scholar]
Barzgar 1997
- Barzgar MA, Sheikh MR, Bile MK. Female health workers boost primary care. World Health Forum 1997;18(2):202‐10. [PUBMED: 9393010] [PubMed] [Google Scholar]
Benova 2018
- Benova L, Tunçalp Ö, Moran AC, Campbell OM. Not just a number: examining coverage and content of antenatal care in low‐income and middle‐income countries. BMJ Global Health 2018;3(2):e000779. [DOI: 10.1136/bmjgh-2018-000779; PUBMED: 29662698] [DOI] [PMC free article] [PubMed] [Google Scholar]
Beun 2003
- Beun MH, Wood SK. Acceptability and use of clean home delivery kits in Nepal: a qualitative study. Journal of Health, Population and Nutrition 2003;21(4):367‐73. [PUBMED: 15038592] [PubMed] [Google Scholar]
Bhardwaj 1995
- Bhardwaj N, Hasan SB, Zaheer M. Maternal care receptivity and its relation to perinatal and neonatal mortality. A rural study. Indian Pediatrics 1995;32(4):416‐23. [PUBMED: 8635804] [PubMed] [Google Scholar]
Black 2010
- Black RE, Cousens S, Johnson HL, Lawn JE, Rudan I, Bassani DG, et al. Global, regional, and national causes of child mortality in 2008: a systematic analysis. Lancet 2010;375(9730):1969–87. [DOI: 10.1016/S0140-6736(10)60549-1; PUBMED: 20466419] [DOI] [PubMed] [Google Scholar]
Bohren 2014
- Bohren MA, Hunter EC, Munthe‐Kaas HM, Souza JP, Vogel JP, Gülmezoglu AM. Facilitators and barriers to facility‐based delivery in low‐ and middle‐income countries: a qualitative evidence synthesis. Reproductive Health 2014;11(1):71. [DOI: 10.1186/1742-4755-11-71; PUBMED: 25238684] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bryanton 2010
- Bryanton J, Beck CT. Postnatal parental education for optimising infant general health and parent‐infant relationships. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD004068.pub3] [DOI] [PubMed] [Google Scholar]
Chukwuma 2017
- Chukwuma A, Wosu AC, Mbachu C, Weze K. Quality of antenatal care predicts retention in skilled birth attendance: a multilevel analysis of 28 African countries. BMC Pregnancy and Childbirth 2017;17(1):152. [DOI: 10.1186/s12884-017-1337-1; PUBMED: 28545422] [DOI] [PMC free article] [PubMed] [Google Scholar]
de Zoysa 1998
- Zoysa I, Bhandari N, Akhtar N, Bhan MK. Care seeking for illness in young infants in an urban slum in India. Social Science and Medicine 1998;47(12):2101‐11. [PUBMED: 10075250] [DOI] [PubMed] [Google Scholar]
Emond 2002
- Emond, A, Pollock J, Costa N, Maranhao T, Macedo A. The effectiveness of community‐based interventions to improve maternal and infant health in the Northeast of Brazil. Revista Panamericana de Salud Publica 2002;12(2):101‐10. [PUBMED: 12243695] [DOI] [PubMed] [Google Scholar]
Ensor 2004
- Ensor T, Cooper S. Overcoming barriers to health service access: influencing the demand side. Health Policy and Planning 2004;19(2):69‐79. [PUBMED: 14982885] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Lassi 2015
- Lassi ZS, Bhutta ZA. Community‐based intervention packages for reducing maternal and neonatal morbidity and mortality and improving neonatal outcomes. Cochrane Database of Systematic Reviews 2015, Issue 3. [DOI: 10.1002/14651858.CD007754.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lassi 2016
- Lassi ZS, Kumar R, Bhutta ZA. Community‐based care to improve maternal, newborn, and child Health. Disease Control Priorities. Vol. 2nd, Washington, DC: The International Bank for Reconstruction and Development/The World Bank, 2016:263‐84. [PubMed] [Google Scholar]
Lawn 2004
- Lawn JE, Cousens S, Darmstadt GL, Paul V, Martines J. Why are 4 million newborn babies dying every year?. Lancet 2004;364(9450):2020. [DOI] [PubMed] [Google Scholar]
Lawn 2005
- Lawn JE, Cousens S, Zupan J, Lancet Neonatal Survival Steering Team. 4 million neonatal deaths: when? Where? Why?. Lancet 2005;365(9462):891‐900. [DOI: 10.1016/S0140-6736(05)71048-5; PUBMED: 15752534] [DOI] [PubMed] [Google Scholar]
Mangala 2001
- Mangala S, Gopinath D, Narasimhamurthy NS, Shivaram C. Impact of educational intervention on knowledge of mothers regarding home management of diarrhoea. Indian Journal of Pediatrics 2001;68(5):393‐7. [PUBMED: 11407151] [DOI] [PubMed] [Google Scholar]
Nair 2010
- Nair N, Tripathy P, Prost A, Costello A, Osrin D. Improving newborn survival in low‐income countries: community‐based approaches and lessons from South Asia. PLoS Medicine 2010;7(4):e1000246. [DOI: 10.1371/journal.pmed.1000246; PUBMED: 20386728] [DOI] [PMC free article] [PubMed] [Google Scholar]
O'Rourke 1998
- O'Rourke K, Howard‐Grabman L, Seoane G. Impact of community organization of women on perinatal outcomes in rural Bolivia. Revista Panamericana de Salud Publica 1998;3(1):9‐14. [PUBMED: 9503957] [DOI] [PubMed] [Google Scholar]
Osrin 2003
- Osrin D, Mesko N, Shrestha BP, Shrestha D, Tamang S, Thapa S, et al. Implementing a community‐based participatory intervention to improve essential newborn care in rural Nepal. Transactions of the Royal Society of Tropical Medicine and Hygiene 2003;97(1):18‐21. [PUBMED: 12886798] [DOI] [PubMed] [Google Scholar]
PATH 2005
- PATH. Clean delivery kits: simple kits save lives, 2005. www.path.org/projects/clean‐delivery_kit.php (accessed November 2012).
RevMan 2011 [Computer program]
- Nordic Cochrane Centre: The Cochrane Collaboration. Review Manager (RevMan). Copenhagen: Nordic Cochrane Centre: The Cochrane Collaboration, 2011; Vol. 5.1.
Riaz 2015
- Riaz A, Zaidi S, Khowaja AR. Perceived barriers to utilizing maternal and neonatal health services in contracted‐out versus government‐managed health facilities in the rural districts of Pakistan. International Journal of Health Policy and Management 2015;4(5):279‐84. [DOI: 10.15171/ijhpm.2015.50; PUBMED: 25905478] [DOI] [PMC free article] [PubMed] [Google Scholar]
UN 2015
- United Nations, Department of Economic and Social Affairs, Population Division. World Fertility Patterns 2015 – Data Booklet. www.un.org/en/development/desa/population/publications/pdf/fertility/world‐fertility‐patterns‐2015.pdf (accessed 9 April 2018).
UN 2015a
- United Nations, Department of Economic and Social Affairs, Population Division. Trends in Contraceptive Use Worldwide 2015. www.un.org/en/development/desa/population/publications/pdf/family/trendsContraceptiveUse2015Report.pdf (accessed July 2018).
UNICEF 2010
- UNICEF. Progress for children: achieving the MDGs with equity, 2010. www.childinfo.org/files/PFC_No.9_EN_090710.pdf. (accessed 26 November 2012).
UNICEF 2017
- UNICEF. Levels & trends in child mortality: report 2007. www.unicef.org/publications/files/Child_Mortality_Report_2017.pdf. (accessed 9 April 2018). [Google Scholar]
WHO 2006
- WHO. Reproductive health indicators: reproductive health and research guidelines for their generation, interpretation and analysis for global monitoring. whqlibdoc.who.int/publications/2006/924156315X_eng.pdf. 2006 (accessed January 2013).
WHO 2006a
- WHO. Reproductive health: the mother baby package, 2004. www.who.int/entity/reproductivehealth/publications/maternal_perinatal_health/WHO_FHE_MSM_94_11/en/index.html (accessed November 2012).
WHO 2018
- WHO. Newborns: reducing mortality, 2018. www.who.int/news‐room/fact‐sheets/detail/newborns‐reducing‐mortality (accessed July 2018).
World Bank 2018
- World Bank. World Bank List of Country Economies. The World Bank Group 2018; Vol. http://data.worldbank.org/about/country‐classifications/countryand‐lending‐groups, issue accessed 2018.
References to other published versions of this review
Thaver 2009
- Thaver D, Zaidi AKM, Owais A, Haider BA, Bhutta ZA. The effect of community health educational interventions on newborn survival in developing countries. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD007647] [DOI] [Google Scholar]


