Abstract
Although the recent advances in stem cell engineering have gained a great deal of attention due to their high potential in clinical research, the applicability of stem cells for preclinical screening in the drug discovery process is still challenging due to difficulties in controlling the stem cell microenvironment and the limited availability of high-throughput systems. Recently, researchers have been actively developing and evaluating three-dimensional (3D) cell culture-based platforms using microfluidic technologies, such as organ-on-a-chip and organoid-on-a-chip platforms, and they have achieved promising breakthroughs in stem cell engineering. In this review, we start with a comprehensive discussion on the importance of microfluidic 3D cell culture techniques in stem cell research and their technical strategies in the field of drug discovery. In a subsequent section, we discuss microfluidic 3D cell culture techniques for high-throughput analysis for use in stem cell research. In addition, some potential and practical applications of organ-on-a-chip or organoid-on-a-chip platforms using stem cells as drug screening and disease models are highlighted.
Keywords: Stem cell, Microfluidic technology, Three-dimensional cell culture, High-throughput screening
Core tip: A recent advance of microfluidic techniques using stem cells for high-throughput assay is described. Induced pluripotent stem cells and the innovative organ-on-a-chip or organoid-on-a-chip have led to progress in in vitro drug screening platforms. We summarized the various examples of microfluidic techniques, including organ-on-a-chip or organoid-on-a-chip using stem cells for high-throughput screening, and discussed the current challenges and future perspectives of microfluidic technologies in stem cell research.
INTRODUCTION
Stem cell engineering, the interface of engineering with the world of stem cells, has emerged over the last decade and covers fields from the basic science to engineered approaches[1]. With the significant advances in the development of stem cells technologies, many approaches have been introduced for modeling genetic diseases, and these models have been made available for applications, such as in vitro drug tests[2-5]. Usually, immortalized cell lines lack the differentiated functions of specific organs, and they may not display the disease-specific or patient-specific phenotypes. Also, these cell lines may include oncogenic factors, such as SV40, during the transformation[6]. Stem cells self-renew extensively and have pluripotency in that they can differentiate into all types of cells in an organism. Thus, stem cells have gained significant attention in providing a variety of specialized cells that are relevant for modeling human development and disease as well as applications in regenerative medicine[7-9]. However, stem cells tend to be very sensitive to various biochemical and physiological cures, and their fate is altered easily by their microenvironment. Also, stem cells themselves cannot recapitulate the microenvironment that is physiologically relevant to the complex structure of human organs.
Recently, emphasis has been placed on the roles of the three-dimensional (3D) cell culture techniques that can precisely control multiple cues in the biological microenvironment of stem cells. The 3D cell culture systems are comprised of organ-specific cells and their microenvironments, so they were able to mimic human physiology more accurately. Indeed, organ-on-a-chip platforms consist of tissue-specific cells and their extracellular matrixes (ECMs) that can remodel 3D tissue architectures and also mimic the physiological conditions, such as shear stress and fluidic flow[10,11]. In this regard, microfluidic devices are ideally suited for stem cell cultures and their maintenance by providing a way to recreate a microenvironment in vivo. Also, this system has flexibility and feasibility that can be coupled to robust hardware systems that are capable of high-throughput analysis, rapid sampling, and liquid handling, allowing them to process hundreds of samples[12,13]. Such advantages have led to the innovative development of organ-on-a-chip or organoid-on-a chip systems based on stem cells and their applications in high-throughput drug screening[9,14-16].
In this review, we discuss the most recent advances in 3D microfluidic technology in the field of stem cell research and their applications for high-throughput screening (HTS). Also, we review the progress that has been made to generate organ-on-a-chip platforms and, more recently, organoid-on-a-chip, particularly with an emphasis on important innovations of different microfluidic aspects to improve stem cell research for high-throughput analysis. Then, we discuss how these technologies combined with high-throughput analysis might be enhanced in the future.
3D MICROFLUIDIC CELL CULTURE
It is difficult to maintain the cellular functions in conventional two-dimensional (2D) cell cultures for prolonged periods of time because these cultures lack the physiological microenvironment of in vivo tissue. Such cell systems may not be able to prove the real cellular response to drugs due to their inability to control and mimic the microenvironment of complicated organs. Also, drug diffusion kinetics is not modeled accurately in a 2D cell culture. Therefore, 2D cell cultures increase the chances of providing misleading and non-predictive preclinical results for in vivo test[17,18]. On the other hand, in vivo animal tests have traditionally been the gold standard models for preclinical efficacy tests in the drug discovery process, but various issues still exist, such as ethical issues and genetic differences between species. In addition, animal models have many drawbacks, such as high cost and uncertainties in the interpretation of the results in many pathological studies. Due to these weaknesses of the traditional models, an alternative cell culture model that corresponds to an in vivo system is required in order to obtain better predictions of the preclinical response to drugs.
In recent years, advances in microfluidic technology in 3D cell cultures have resulted in promising alternative methods to the conventional in vivo and in vitro models in the field of drug development[4,15,19-23]. In nature, the fate of in vivo cells is affected largely by external physical and chemical factors, and cell-cell and cell-ECM interact actively with each other. The 3D microfluidic cell culture platform is considered to precisely control these external cues in vitro, thereby producing more reliable and predictive preclinical data than either animal models or conventional 2D cell-based models[5,20]. This is consistent with the trend toward more physiologically-relevant models, such as 3D organs or organoid-on-a-chips, for use in the early phase of drug discovery and development.
Over the past few decades, advances in microfluidic technologies have accelerated the development rate of the 3D cell culture or tissue model by virtue of the following significant features[19,24,25]. First, the microscale dimensions of microfluidic platforms are suitable for creating the biological microenvironment of in vivo tissues that have high complexity and spatial heterogeneity. Also, the physical structure of microfluidic channels can provide a well-controlled hydrodynamic environment, such as a chemical gradient or fluidic flow[26,27]. Second, the small scale of the systems requires only a small amount of cells and reagents in the experiments, which lowers the cost as the research progress from bio-analysis to drug development. Third, microfluidic technology can integrate the multiple and subsequent steps of bioanalysis, from culture and liquid handling to detection and analysis[28,29]. In addition, this technology is amenable to high-resolution, real-time monitoring, as well as the analysis of biochemical, genetic, and metabolic processes under conditions that closely resemble in vivo conditions. With these advantages, various approaches using microfluidic technology have been suggested in association with the study of stem cells, such as the cell culture, identification, and screening of cells as well as modeling diseases. We discuss the 3D microfluidic technologies in more detail because they provide potential solutions for problems in stem cell engineering.
3D MICROFLUIDICS IN STEM CELL ENGINEERING
Microfluidic chips provide a new platform with unique advantages to mimic complex physiological microenvironments in vivo. Since some groups started to use microfluidic technology for patterning or capturing stem cells in the early 2000s[30-32], the use of this technology in stem cell research has increased significantly. The emerging and rapid development of microfluidic technologies has presented an ideal solution in stem cell engineering, as summarized in Table 1. Many studies have been reported that focused on the application of microfluidic devices for stem cell research, such as culture, differentiation, patterning, tissue engineering, recreating organs, drug discovery, and therapeutics. In stem cell culture, it is important to control the biochemical microenvironment of cells to regulate the basic cell functions and biological processes, such as differentiation, development, and immune response. The temporal and spatial control over defined gradients of soluble factors or immobilized factors[33] provided by microchannel-based microfluidic devices can be an important advantage in stem cell research. For patterning cells or ECMs in desired locations, the patterned channel or the polydimethylsiloxane (PDMS) microwell generated using the soft lithography technique are simple and traditional method, while stably interfacing with other supporting cells[34,35]. The benefits of combining biomaterial engineering and microfluidics for stem cell applications are clear. Microfluidic technology could be used to mimic the spatial heterogeneity of stem cell microenvironment[36]. In particular, the chemical gradient in a microfluidic channel is one of the unique features that allows for this heterogeneous microenvironment. Some groups have used microfluidic approaches in which cells within hydrogels were exposed to desirable soluble gradients in 3D microenvironments[26,37]. Also, chemical gradient generators that use multiple microfluidic channels with flow control have been suggested to investigate the neural stem cell differentiation by the chemokine (CXCL12) gradient generated within a single device[38]. To study cell-cell or cell-ECM interactions, the spatially-isolated compartments in a microfluidic device are also useful in investigating the differentiation or migration behavior of stem cells[39], and they help visualize their biological processes within a microscale device.
Table 1.
Features of microfluidic techniques for stem cell engineering
| Features of microfluidic technology | Solutions | Stem cell applications | Ref. |
| Cell or ECM patterning | Microchannel | Co-culture of hMSCs and hNSCs | Yang et al[34], 2015 |
| Microwell-patterned substrate | Co-culture of hESCs and fibroblasts | Khademhosseini et al[35], 2006 | |
| Chemical gradient | Multichannel array | Regulation of hematopoietic stem cell fates | Mahadik et al[26], 2014 |
| Overlapping gradients | Neuronal commitment of mouse ESCs | Cosson et al[37], 2013 | |
| Sink and source channel with continuous flow | Chemotaxis of NSCs | Xu et al[38], 2013 | |
| Cell-cell or cell-ECM interaction | Microchannel-groove | Monitoring of differentiation and migration of NSCs derived from hESCs | Lee et al[39], 2013 |
| Shear stress | Flow | Behavior observation of MSCs | Zheng et al[40], 2012 |
| Droplet | Encapsulation and emulsion | Construction of ADSC microenvironment | Sakai et al[41], 2011 |
| Construction of pre-hatching embryo | Agarwal et al[42], 2013 | ||
| Sorting and separation | Optical tweezer | Sorting of hESCs | Wang et al[43], 2013 |
| Electrical impedance flow | Identifying the differentiation of state of single cell | Song et al[44], 2013 | |
| Multiple dielectrophoresis | Sorting of hMSCs | Song et al[45], 2015 | |
| Hydrodynamic trapping | Chamber array | EB-trap array | Suri, et al[46], 2013 |
| Integration and automation | Multi-arrayed chips and integrated systems (e.g., liquid handler, cell chamber, imaging system, software) | Automatic culture of stem cell-derived dopaminergic neurons | Kane et al[47], 2019 |
| Investigation of dynamic changes of hematopoietic stem cell condition | Dettinger et al[48], 2018 | ||
| Clonal analysis of hESCs differentiation pattern | Sikorski et al[49], 2015 | ||
| Mechanical and electrical actuators | Mechanical stretch | Stretch-activated stem cell differentiation | He et al[51], 2018 |
| Magnetic resonance | Quantification of metabolic flux in leukemia stem cells | Jeong et al[52], 2017 |
ECMs: Extracellular matrixes; hMSC: Human mesenchymal stem cell; hESC: Human embryonic stem cell; NSC: Neural stem cell; ADSC: Adipose-derived stem cell; EB: Embryoid body.
Despite the high potential impact of stem cell technologies, there are some technical challenges associated with culturing and differentiating stem cells for use in drug discovery and development. With a conventional well plate or dish, it is difficult to mimic the physiological complexity of the stem cell niche because it is a microenvironment that provides a variety of stimuli. Flow is one of the most important stimuli since some organs are affected by the shear flow induced by the blood stream. Microfluidic devices are the only platform capable of supplying flow, thereby inducing the important flow shear stress. This provides a way of observing stem cells by the effect of physical stimuli[40].
A 3D co-culture for niche construction can be achieved with droplet-based technology. By varying the ratio of the flow rates of the two cell streams, the ratio of the concentrations of the two types of cells can be altered within the microgel[41,42]. This technique enables the cells to be compartmentalized into a mono-dispersed and physicochemically-defined 3D matrix. Another advantage of this technique is its generation of high-throughput and microscale cell-matrix environments. For example, Sakai et al[41] reported the enclosed rat-adipose-derived stem cell aggregates in gelatin microbeads using a microfluidic droplet technique in which the stem cells were re-coated with additional supporting cells to construct a heterogeneous tissue structure.
Microfluidic devices combined with electrics and physics have been used to separate single cells[43-45]. Optical tweezers, electrical impedance, and dielectrophoresis techniques combined with microfluidic technologies can be used to sort or separate cells. For instance, Song et al[45] have developed a method to identify the differentiated state of human embryonic stem cells (ESCs) using electrical impedance in a microfluidic channel. Numerous other approaches have been tried by combining microfluidic technology with different analysis methods and by integrating various structures and functions. Recent advances in microfluidic technology using hydrodynamic trapping have resulted in an array culture method that enables precise and standardized tools that are controllable, constituent and high-throughput. Throughout the drug discovery and development process, a human stem cell-based cell culture system can be important in screening, validating candidate compounds and preclinical studies, such as the toxicity test, efficacy test, and the mechanism studies (e.g., integration and automation[46-50], mechanical and electrical actuator[51,52]). Next, we discuss the microfluidic technologies in more detail for the high-throughput analysis of stem cells.
HIGH-THROUGHPUT ANALYSIS TECHNIQUES FOR STEM CELL ENGINEERING
The development of drugs requires a series of complex procedures that involves preclinical and clinical studies with well-established regulatory compliance. Developing a new drug, i.e., from the discovery stage to approval by the United States Food and Drug Administration, generally takes more than 10 years and costs more than two billion dollars, and only about 10% of the compounds progress successfully through clinical development[6,20]. Current standard drug discovery traditionally starts with the 2D cell culture-based screening of compounds, followed by animal model testing and clinical trials. While 2D cell-based assays are used extensively because they have certain advantages, such as lower cost and higher throughput than animal tests, they also have limitations. These limitations include the lack of a cell-cell or a cell- ECM, which results in failure to reconstitute the in vivo cellular microenvironments, which means they cannot maintain the differentiated functions of the cells. Animal tests also cause errant pharmacokinetic predictions due to the differences between animal and human species that make it impossible to directly translate the findings in animal models to human biology. Therefore, there is a considerable need for new approach with a more accurate and cost-effective system that is representative of humans to efficiently screen and validate the potential drug candidates in the early stages of drug development[53].
Miniaturized, high-throughput techniques using microfluidics are required to identify efficient and cost-effective compounds using stem cell-based models and to gain insight into the possible underlying mechanism[23,54,55]. Microfluidic devices with micro-sized scale, automatic operation, and large-scale integration possibly can offer many unique benefits, including high-throughputs, low cost, and high efficiency in drug development. Also, due to the nature of microfluidic devices, quantitative analysis can be a useful tool in combinatorial mixing and processing samples[28,56,57] In drug discovery, HTS is a major instrumental technique. HTS commonly uses well plates ranging from 96 to 1536 plates, and these plates enable parallel and simultaneous testing of multiple factors. This allows rapid analysis of thousands of chemicals and biochemical using genetic or pharmacological tests in parallel, and this allows us to identify specific compounds for specific biological processes. Among these systems, the development of fast and automated microscopes, such as the high-content screening (HCS) microscope, has been accelerated by hardware advances and innovations in the software for analyzing images[13]. This system uses an automated liquid handler to simultaneously process hundreds of biological samples, and it provides the unbiased, multiple-parametric data with the high-spatiotemporal resolution from the acquired images, and it does so at the levels of individual proteins, organelles, whole cells, or even entire organisms. Therefore, this approach has been used to understand the complexity and dynamics of the cell biological processes that occur in cells and to identify a plethora of quantitative phenotypes of varying complexity in numerous different models.
With such advances in the scientific equipment, different approaches have been suggested for stem cell-based screening platforms using microfluidic devices. Table 2 provides a summary of some examples of microfluidic systems that have high-throughput capability for stem cell research. Miniaturization of the microfluidic platform increases the throughput of assays used to analyze stem cells because the small scale of the samples reduces both the consumption of reagents and the number of cells required[58-60]. Lee et al[61] and Du et al[57] suggested the microarray technique (1080 chips) and the microfluidic droplet array technique (342 droplets), respectively, for generating miniaturized cell array systems using cancer cell lines for the high-throughput testing of drugs. These techniques also can be applied to stem cell research since they provide rapid and cost-effective testing for a wide range of applications that involve in high-throughput toxicity tests[60,62]. With the advent of robotic spotting technology and microfabrication, it is possible to generate the pattern of cells that are encapsulated in a 3D ECM matrix and that support cell growth at the microscale[59,62]. One of the powerful techniques of microfluidics for high-throughput 3D cell generation is the flow focusing technique, which is used for the encapsulation of cells in the ECM or hydrogel beads[42,63,64]. To understand the fate of stem cells, it is important to regulate the stem cell niche. Gobaa et al[65] reported microengineered niche spotting that was comprised of a hydrogel array for controlling the stiffness of the gel. As a similar example, Beachley et al[66] reported a 3D microtissue array when they used the spotting technique to investigate the tissue-specific response based on the composition of the ECM.
Table 2.
High-throughput screening analysis for stem cell engineering
| Techniques for high-throughput screening | Advantages | Applications | Cell types | Ref. |
| Microarray technique | Cell encapsulation in hydrogel-matrix spots; minimal consumption of cells and reagents | Toxicity and phenotypic screening of NPCs | NPCs | Nierode et al[60], 2016 |
| Studying of the expansion of mouse ESC | ESCs | Fernandes et al[62], 2009 | ||
| Microencapsulation using microfluidic flow focusing | Multiple generation of 3D cells | Study of embryogenesis | iPSCs | Agarwal et al[42], 2013 |
| Encapsulation of cells and ECMs; controlled physicochemical properties of gel beads | Study of ESC expansion | mESCs | Allazetta et al[63], 2013 | |
| Co-culture of pancreatic islets and hMSCs | hMSCs | Headen et al[64], 2014 | ||
| Silicon stamp for spotting protein | Control of gel stiffness for stem cell fates | Study of stem cell niche | hMSCs | Gobaa et al[65], 2011 |
| ECM array | Mimicking of microenvironments | Study of stem cell niche | hASCs | Beachley et al[66], 2015 |
| Microwell array using thermoformed cyclic olefin polymer | Round-bottom array, uniform size of well array | EB generation | mESCs | Vrij et al[67], 2016 |
| Micro droplet array; hydrophobic-hydrophilic surface | Robotics-free sample handling; high throughput; low reagent consumption; high-content readouts | Screening of iPSC pluripotency and proliferation | iPSCs | Zhang et al[68], 2016 |
| Serial dilution generator | Generation of different concentration, combination and temporal sequence of drugs | Effect of cytokine (Tgfβ3) on hBM-MSC | hBM-MSC | Occhetta et al[69], 2015 |
| Microraft array | Mimicking of microenvironments and enhancement of contact | Study of stem cell fate by mimicking niche | Intestinal stem cells | Gracz et al[70], 2015 |
| Micropattern-well hybrid | Compatibility of HCS | Screening of stem cell differentiation and drug screening | NPCs | Yu et al[71], 2018 |
ECMs: Extracellular matrixes; iPSC: Induced pluripotent stem cell; NPC: Neural progenitor cell; mESC: Mouse embryonic stem cell; hASC: Human adipose-derived stem cell; HCS: High-content screening.
Also, soft lithography can be used to fabricate an array of wells with physically-defined dimensions, allowing for the cellular aggregates in the wells. The defined the sizes of wells can control the size of the cell aggregates and offer an attractive solution for controlling the fate of stem cells. Vrij et al[67] used optically-clear, cyclic olefin polymer (COP) films based on a thermoforming technique to develop a round-bottom, 96 microwell array for the generation of uniform-sized embryoid bodies (EBs). As a combined technique, arrayed microwell fabrication using PDMS soft lithography technique and droplet generation of cell suspension using surface tension due to hydrophobic and hydrophilic difference enable the formation of induced pluripotent stem cell (iPSC) arrays in a 512 well[68]. As another example, Occhetta et al[69] suggested that a high-throughput serial dilution generator could be used for making different concentrations and combinations of cytokine to investigate the effect of cytokines on the expansion and differentiation of embryonic stem cells (ESCs).
A major focus of the miniaturized HTS of stem cells is on screening for drugs, compounds and small molecules that could affect the properties of stem cells, such as differentiation, self-renewal, and expansion. 3D HTS platform also can be used to recreate the stem cell niche for mimicking the in vivo environment. Co-culturing different, interdependent types of cells is an important part of stem cell niche[70]. In general, HTS requires the use of robotics due to the multiple pipetting steps, and it consumes large quantities of reagents and valuable cells, resulting in the experiments having high costs. Despite the disadvantages, including the labor and time required, HTS technology using well plates is used extensively for developing various protocols for cell cultures and 2D and 3D screening of cells because the microplate is still a well-established platform for HTS applications so many research groups view it as a user-friendly approach. For this reason, Yu et al[71] developed the well plate-based gel unit array for HTS analysis. This platform has a unique feature in that it has hydrogel-incorporating compartments integrated in a well to culture 3D tissue with uniform thickness while co-culturing with other neighboring cells in a single well. This can be used as HCS integrated with a co-culture model.
3D TISSUE MODEL FOR STEM CELL ENGINEERING
Stem cells have their unique ability of self-renewal and the potential to differentiate into many specific types of cells. Immortalized cell lines are capable of extended proliferation but exhibit fewer organ-specific activities than primary cells or stem cells. Moreover, primary cells are functional, but have limited cell number and a finite lifespan. Therefore, stem cells that was able to differentiate into specific organs are considered to be more functional, and an ideal source to mimic the architecture and specific activity of human organs, and are more likely to be accurate with respect to human bodies. As a more reliable and sustainable human source that represents phenotypical characteristics of the inherited disease or genetic disorders, patient-specific cells are needed. Recently, iPSCs and their organoid techniques have undergone a rapid increase in popularity. These techniques allow reprogramming of fibroblasts into stem cells that can be differentiated into various tissues, such as neurons, cardiomyocytes (CM), and several types of blood cells. The iPSC technology provides a new and powerful tool for drug-screening for personalized medicines, and it allows the use of cells with the same genetic background as the patients. Furthermore, these sources of cells allow the recapitulation of various inherited diseases in vitro, and allow researchers to study the genotypic differences. For these reasons, iPSCs were have been used extensively in recent 3D in vitro organ models[22].
There are the two distinct strategies in generating in vitro 3D tissue and organ models, i.e., the bottom-up and the top-town approaches[2]. A key example of the use of the top-down approaches is an organ-on-a-chip model, the aim of which is to engineer individual components of tissue environments, such as cells and ECMs in a microfluidic device, and this work is conducted mostly by bioengineers. Bottom-up approaches rely on biological self-organization, which refers to intrinsic abilities of biological systems, and they are led largely by stem cell biologists. These two approaches both have the same goal, i.e., achieving the generation of high-fidelity 3D tissue. However, both approaches have their own limitations. For instance, organoid systems have low controllability for recreating the biochemical and biophysical microenvironment of 3D organoids, while organ-on-a-chip systems have limitations when reconstituting the biological complexity of tissue development. Thus, by combining the strengths of both two approaches, the organoid-on-a-chip platform has emerged as a synergistic approach to recapitulate both the physiological and biochemical features of in vivo tissue[10,14]. In this section, we introduce examples of stem cell-based organ-on-a-chip and organoid-on-a-chip system using microfluidic technologies for high-throughput analysis.
ORGAN-ON-A-CHIP AND ORGANOID-ON-A-CHIP FOR HIGH-THROUGHPUT ANALYSIS
With the development of the generation of iPSCs, tissue models and disease models based on organ-on-a-chip technology have been proposed, and they are expected to serve as a platform for cell-based, high-throughput assays during the drug discovery and development. The organ-on-a-chip, which utilizes the microfluidic approach to mimic the architecture and function of 3D tissue, consists of microengineered biomimetic systems that represent key functional units of living human organs. Also, recent advances in microfabrication, cell engineering, and imaging technologies have led organ-on-a-chip to become an innovative technology that is capable of reproducing physiological cell behaviors in vitro. These systems include important design considerations for developing systems, i.e., (1) Organizing the spatial distribution of multiple types of tissues; (2) functional tissue-tissue interfaces; and (3) organ-specific mechanical and biochemical microenvironments.
Stem cell-derived organoid systems that are 3D self-organized tissue models provide new biological models for the development of new drugs. Organoids have been generated from both pluripotent stem cells and tissue-resident adult stem cells by mimicking the biochemical and physical cues of tissue development and homeostasis[72,73]. Because of these unique features, conventional 3D organoid systems may be more advantageous in some aspects than organ-on-a-chip systems in drug discovery. One of the important applications of organoid cultures is to model pathologies of diseases. Organoid-on-a-chip engineering has been emerged recently based on the integration of the two distinct approaches of organoid and chip technology.
Stem cells, including iPSCs, have the potential to serve as a source of cells that can be engineered to suit specific needs in the development of organ-on-chips[5,6]. In recent years, the organ- and organoid-on-a-chip approaches using stem cells have been used extensively to establish the new microengineered models that recapitulate the structure and functional complexity of human organs, such as the liver[74-77], heart[78-86], brain[71,87-94], intestine[95-97], kidney[98-100], and bone[101-103]. Recently, organ-on-a chip technology has been able to integrate multiple organ or tissue models to simulate the human body, and multi-organ systems generated using stem cells have been developed for a human body-on-a chip system[16,75,104,105] It is possible for such a system to provide a predictive model for pharmacokinetics of drugs by mimicking the activities of the human body such as absorbing, distributing, metabolizing, and eliminating drugs.
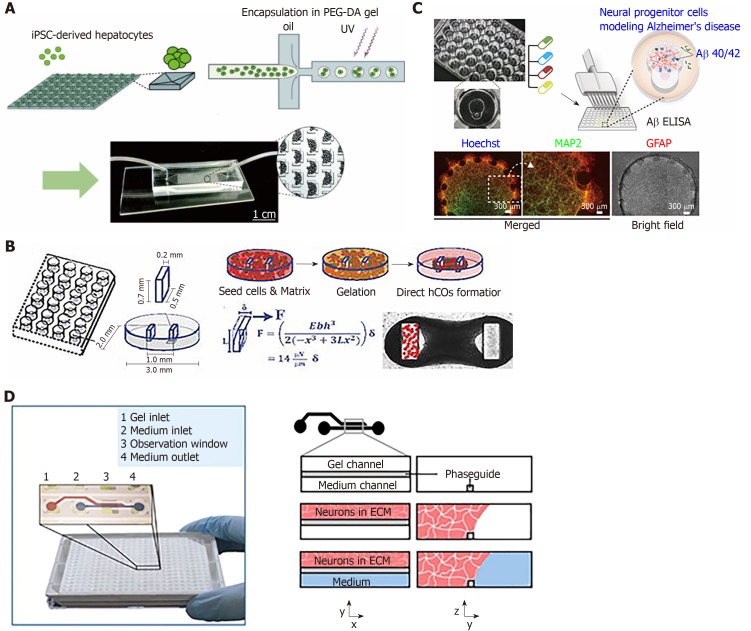
We introduce examples of organ- and organoid-on-a-chip platform using stem cells for high-throughput assay, as summarized in Table 3. In general, the primary focus of organ-on-a-chip has been on the microengineered liver due to the importance of its central role relative to hepatic drug toxicity and metabolism[74-76]. Ware et al[74] demonstrated the possibility of a high-throughput hepatotoxicity test on iPSC-derived hepatocytes co-cultured with fibroblasts, which were micropatternd islands using the soft-lithography technique. In another approach, Schepers et al[76] developed a liver-on-a-chip using human iPSCs from a patient. This cell was cultured as 3D organoids using a perfusable system, and the organoids that were constructed were integrated in a chip with multiple patterned C-traps, as shown in Figure 1A. The liver organoids were long-term cultured for 28 d during perfusion. Recently, researchers have begun to explore the potential of heart-on-a-chip as a HTS tool for the monitoring of contractile functions and cardiomyopathy using iPSC-derived CMs[83,84,86]. For instance, Mills et al[86] developed a 96 well-type screening platform for screening functions in hiPSC-derived cardiac organoids to reveal the cardiac metabolic mechanism, as shown in Figure 1B.
Table 3.
High-throughput screening-based three-dimensional organ- or organoid-on-a-chip
| Organ type | Platform type | Cell type | Applications | Ref. |
| Liver | Organ-on-a-chip | Human iPSC -hepatocytes | Screening of hepatotoxic drugs | Ware et al[74], 2015 |
| Organ-on-a-chip | Hepatocyte-ESC line | Co-culture of multi-organ | Materne et al[75], 2015 | |
| Organoid-on-a-chip | iPSCs | Tests of liver function | Schepers et al[76], 2016 | |
| Heart | Organ-on-a-chip | Human iPSC -cardiomyocytes | Screening of molecular inducer related to cardiac myocyte proliferation | Titmarsh et al[83], 2016 |
| Human iPSC - cardiomyocytes | Modeling of mitochondrial cardiomyopathy of Barth syndrome | Wang et al[84], 2014 | ||
| Organoid-on-a-chip | hPSCs | Study of cardiac maturation | Mills et al[86], 2017 | |
| Brain | Organ-on-a-hip | NPCs | Toxicity test with in vitro brain model of Alzheimer’s disease | Park et al[91], 2015 |
| NPCs | Calcium assay | Lai et al[92], 2012 | ||
| NPCs | In vitro test for Alzheimer’s disease | Yu et al[71], 2018 | ||
| Organoid-on-a-chip | Human iPSC | Model of neurodevelopment disorder by prenatal nicotine exposure | Wang et al[93], 2018 | |
| Organ-on-a-chip | Human iPSC | High-throughput compound evaluation on three-dimensional networks of neurons and glia | Wevers et al[94], 2016 | |
| Intestine | Organoid-on-a-chip | Human iPSC | Study of response to exogenous stimuli | Workman et al[97], 2018 |
| Kidney | Organoid-on-a-chip | Human iPSC | High-throughput screening format organoids for multidimensional phenotypic screening | Czerniecki et al[100], 2018 |
iPSC: Induced pluripotent stem cell; NPC: Neural progenitor cell; ESC: Embryonic stem cell.
Figure 1.
Representative examples of high-throughput screening microfluidic systems using stem cells based on organ-on-a-chip or organoid-on-a-chip. A: An engineered perfusable liver platform using induced pluripotent stem cell (iPSC). The iPSC-derived hepatocytes were aggregated as three-dimensional (3D) organoids and encapsulated in Poly (ethylene glycol) diacrylate hydrogel. These cells were loaded in a C-trap chip subjected to perfusion for a long-term culture (Reproduced from Ref[76] with permission from the Royal Society of Chemistry); B: A miniaturized 96 well-type human iPSC-derived cardiac organoid (hCOs) screening platform, which is called heart dynamometer (Heart-Dyno), facilitates the automated formation of hCOs (Reproduced from Ref[86] with permission from the National Academy of Science); C: Well plate-micropattern hybrid platform for NPC differentiation for modeling Alzheimer’s disease (Reproduced from Ref[71] with permission form the Royal Society of Chemistry). This hybrid-platform is compatible with high-throughput screeninganalysis; D: Organo-plate® comprising 96 microfluidic tissue chips and experimental outline for culturing 3D neuronal-glial networks (reproduced from Ref[94] with permission from the Nature Research).
Similarly, there have been recent developments in brain-on-a-chip for HTS. The attention for brain models fits in a wider trend towards attention for neural progenitor-cell-derived brain models for diseases, such as Alzheimer’s disease[71,91]. This follows the more generic increase in the popularity of iPSC techniques and progress in controlling the stem cell niche of differentiated tissues. Wang et al[93], developed brain-organoids using iPSCs to model neurodevelopment disorders under prenatal nicotine exposure and showed the potential of drug testing. In addition, the 96-well plate-based, HTS-compatible 3D cell culture platform for the brain model was developed for preclinical drug screening applications[71,92]. Especially, Yu et al[71] developed a micropattern array platform combined with conventional well-plate for HTS drug screening to show the proof-of-concept for the Alzheimer’s disease model using neural progenitor cells, as shown in Figure 1C. Wevers et al[94] showed 3D ECM-embedded neuronal-glial networks in a microfluidic platform using iPSC-derived neural stem cells. The iPSC-derived mature neurons and astrocytes were cultured in the microfluidic channel-based OrganoPlate, which is the integrated microtiter plate that is comprised of 96 tissue chips developed by MIMETAS, lnc., as shown in Figure 1D. An HTS-compatible platform also was developed in kidney-on-a-chip by Czerniecki et al[100]. The iPSCs were cultured and differentiated on this platform with fully-automated and HTS-compatible formats for multi-dimensional phenotypic screening.
CHALLENGES AND FUTURE PROSPECTS
In the field of drug screening, the need for a 3D stem cell platform will become more pressing because it provides a more efficient approach in the early, preclinical stage of drug development. Although 3D microfluidic technology provides significant potential for creating a highly complex, well-controlled 3D dynamic environment as an in vivo system, there are certain to be technical challenges in both the engineering and biological technologies of this platform. In general, microfabricated devices contain various complex designs within small areas, and this limits biochemical experiments and requires advanced skill and optimization. Under such physical conditions, certain types of stem cells can be very sensitive to the excessively high shear stress induced by flow, which might cause phenotypic changes or adversely affect cell viability in microfluidic devices during long-term cultures[106]. In addition, high adsorption of proteins on the PDMS or the plastic walls of microfluidic devices also can hinder the accurate evaluation of the effects of drugs. Currently, several of these problems are being addressed by simpler designs, and stem-cell-specific changes in the design of the devices. Recent approaches that have relatively simpler hybrid systems that combine traditional cell culture plates with microfluidic compartments by decoupling the handling of cells from handling of microfluidic liquids could be alternative approaches[37]. Also, the various microfluidic designs, such as low perfusion, deeper chambers, and large input/output reservoirs to avoid handling the tubes, could be solutions.
In the aspects of high-throughput analysis, the nature of microfluidic systems, which require complicated handling and multiple processes for a series of biological processes, present barriers to high-throughput analysis[19,28,50,53]. This is especially important in the case of primary patient-derived stem cells with time constraints that could be cultured outside of the organism due to rapid changes in their microenvironments during in vitro culture. For this reason, miniaturized screening compartments, systemized cell manipulation, and robotic liquid handling must be developed.
In addition, many proposed systems, as with many other HTS platforms, are focusing largely relying on biomolecular engineering techniques coupled with microscopy-based imaging. However, practical in vitro systems require a system that both observe and analyze a variety of biochemical and physiological responses[54].
Despite of these challenging issues, the high demand for microfluidic devices for HTS of stem cells is uncontroversial. Microfluidic technology is still evolving to overcome these current issues, and the techniques are becoming more sophisticated and acceptable for miniaturization, automation, and versatile testing of all critical parameters for stem cell research. The combination of microfluidic technologies with stem cell analysis may fill the gaps between the present knowledge about stem cells and an in-depth understanding of the underlying mechanisms for their broad applications. By using these techniques in the future, in-vivo-like culture of stem cells and their drug discovery applications can be improved, and the prediction of drug responses will be more reliable.
ACKNOWLEDGEMENTS
This work was supported by the National Research Foundation of Korea (NRF) (NRF-2017R1C1B2002377, NRF-2016R1A5A1010148, and NRF2019R1A2C1003111) funded by the Ministry of Science and ICT (MSIT) and partly supported by the Technology Innovation Program (No.10067787) funded by the Ministry of Trade, Industry & Energy (MOTE, Korea).
Footnotes
Conflict-of-interest statement: The authors have no conflicts of interest to report.
Manuscript source: Invited Manuscript
Peer-review started: March 19, 2019
First decision: June 27, 2019
Article in press: July 29, 2019
Specialty type: Cell and tissue engineering
Country of origin: South Korea
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Labusca L, Miyagoe-Suzuki Y S-Editor: Cui LJ L-Editor: A E-Editor: Wu YXJ
Contributor Information
Jeong Ah Kim, Research Center for Bioconvergence Analysis, Korea Basic Science Institute, Cheongju 28119, South Korea; Department of Bio-Analytical Science, University of Science and Technology, Daejeon 34113, South Korea.
Soohyun Hong, Research Center for Bioconvergence Analysis, Korea Basic Science Institute, Cheongju 28119, South Korea; Program in Biomicro System Technology, Korea University, Seoul 02841, South Korea.
Won Jong Rhee, Division of Bioengineering, Incheon National University, Incheon 22012, South Korea; Department of Bioengineering and Nano-Bioengineering, Incheon National University, Incheon 22012, South Korea. wjrhee@inu.ac.kr.
References
- 1.Nerem RM. Stem cell engineering. Tissue Eng Part A. 2014;20:893–894. doi: 10.1089/ten.tea.2013.0764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hutmacher DW, Holzapfel BM, De-Juan-Pardo EM, Pereira BA, Ellem SJ, Loessner D, Risbridger GP. Convergence of regenerative medicine and synthetic biology to develop standardized and validated models of human diseases with clinical relevance. Curr Opin Biotechnol. 2015;35:127–132. doi: 10.1016/j.copbio.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 3.Jackson EL, Lu H. Three-dimensional models for studying development and disease: moving on from organisms to organs-on-a-chip and organoids. Integr Biol (Camb) 2016;8:672–683. doi: 10.1039/c6ib00039h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huh D, Hamilton GA, Ingber DE. From 3D cell culture to organs-on-chips. Trends Cell Biol. 2011;21:745–754. doi: 10.1016/j.tcb.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu C, Oikonomopoulos A, Sayed N, Wu JC. Modeling human diseases with induced pluripotent stem cells: from 2D to 3D and beyond. Development. 2018:145. doi: 10.1242/dev.156166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hung SSC, Khan S, Lo CY, Hewitt AW, Wong RCB. Drug discovery using induced pluripotent stem cell models of neurodegenerative and ocular diseases. Pharmacol Ther. 2017;177:32–43. doi: 10.1016/j.pharmthera.2017.02.026. [DOI] [PubMed] [Google Scholar]
- 7.Rhim JH, Luo X, Xu X, Gao D, Zhou T, Li F, Qin L, Wang P, Xia X, Wong ST. A High-content screen identifies compounds promoting the neuronal differentiation and the midbrain dopamine neuron specification of human neural progenitor cells. Sci Rep. 2015;5:16237. doi: 10.1038/srep16237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kshitiz, Kim DH, Beebe DJ, Levchenko A. Micro- and nanoengineering for stem cell biology: the promise with a caution. Trends Biotechnol. 2011;29:399–408. doi: 10.1016/j.tibtech.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yin X, Mead BE, Safaee H, Langer R, Karp JM, Levy O. Engineering Stem Cell Organoids. Cell Stem Cell. 2016;18:25–38. doi: 10.1016/j.stem.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Skardal A, Shupe T, Atala A. Organoid-on-a-chip and body-on-a-chip systems for drug screening and disease modeling. Drug Discov Today. 2016;21:1399–1411. doi: 10.1016/j.drudis.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim C, Lee KS, Bang JH, Kim YE, Kim MC, Oh KW, Lee SH, Kang JY. 3-Dimensional cell culture for on-chip differentiation of stem cells in embryoid body. Lab Chip. 2011;11:874–882. doi: 10.1039/c0lc00516a. [DOI] [PubMed] [Google Scholar]
- 12.Tronser T, Popova AA, Levkin PA. Miniaturized platform for high-throughput screening of stem cells. Curr Opin Biotechnol. 2017;46:141–149. doi: 10.1016/j.copbio.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 13.Mattiazzi Usaj M, Styles EB, Verster AJ, Friesen H, Boone C, Andrews BJ. High-Content Screening for Quantitative Cell Biology. Trends Cell Biol. 2016;26:598–611. doi: 10.1016/j.tcb.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 14.Takebe T, Zhang B, Radisic M. Synergistic Engineering: Organoids Meet Organs-on-a-Chip. Cell Stem Cell. 2017;21:297–300. doi: 10.1016/j.stem.2017.08.016. [DOI] [PubMed] [Google Scholar]
- 15.Ronaldson-Bouchard K, Vunjak-Novakovic G. Organs-on-a-Chip: A Fast Track for Engineered Human Tissues in Drug Development. Cell Stem Cell. 2018;22:310–324. doi: 10.1016/j.stem.2018.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Skardal A, Murphy SV, Devarasetty M, Mead I, Kang HW, Seol YJ, Shrike Zhang Y, Shin SR, Zhao L, Aleman J, Hall AR, Shupe TD, Kleensang A, Dokmeci MR, Jin Lee S, Jackson JD, Yoo JJ, Hartung T, Khademhosseini A, Soker S, Bishop CE, Atala A. Multi-tissue interactions in an integrated three-tissue organ-on-a-chip platform. Sci Rep. 2017;7:8837. doi: 10.1038/s41598-017-08879-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Edmondson R, Broglie JJ, Adcock AF, Yang L. Three-dimensional cell culture systems and their applications in drug discovery and cell-based biosensors. Assay Drug Dev Technol. 2014;12:207–218. doi: 10.1089/adt.2014.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baharvand H, Hashemi SM, Kazemi Ashtiani S, Farrokhi A. Differentiation of human embryonic stem cells into hepatocytes in 2D and 3D culture systems in vitro. Int J Dev Biol. 2006;50:645–652. doi: 10.1387/ijdb.052072hb. [DOI] [PubMed] [Google Scholar]
- 19.Li XJ, Valadez AV, Zuo P, Nie Z. Microfluidic 3D cell culture: potential application for tissue-based bioassays. Bioanalysis. 2012;4:1509–1525. doi: 10.4155/bio.12.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Breslin S, O'Driscoll L. Three-dimensional cell culture: the missing link in drug discovery. Drug Discov Today. 2013;18:240–249. doi: 10.1016/j.drudis.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 21.Esch EW, Bahinski A, Huh D. Organs-on-chips at the frontiers of drug discovery. Nat Rev Drug Discov. 2015;14:248–260. doi: 10.1038/nrd4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Duinen V, Trietsch SJ, Joore J, Vulto P, Hankemeier T. Microfluidic 3D cell culture: from tools to tissue models. Curr Opin Biotechnol. 2015;35:118–126. doi: 10.1016/j.copbio.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 23.Wu MH, Huang SB, Lee GB. Microfluidic cell culture systems for drug research. Lab Chip. 2010;10:939–956. doi: 10.1039/b921695b. [DOI] [PubMed] [Google Scholar]
- 24.Ren K, Zhou J, Wu H. Materials for microfluidic chip fabrication. Acc Chem Res. 2013;46:2396–2406. doi: 10.1021/ar300314s. [DOI] [PubMed] [Google Scholar]
- 25.Ertl P, Sticker D, Charwat V, Kasper C, Lepperdinger G. Lab-on-a-chip technologies for stem cell analysis. Trends Biotechnol. 2014;32:245–253. doi: 10.1016/j.tibtech.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 26.Mahadik BP, Wheeler TD, Skertich LJ, Kenis PJ, Harley BA. Microfluidic generation of gradient hydrogels to modulate hematopoietic stem cell culture environment. Adv Healthc Mater. 2014;3:449–458. doi: 10.1002/adhm.201300263. [DOI] [PubMed] [Google Scholar]
- 27.Choi NW, Cabodi M, Held B, Gleghorn JP, Bonassar LJ, Stroock AD. Microfluidic scaffolds for tissue engineering. Nat Mater. 2007;6:908–915. doi: 10.1038/nmat2022. [DOI] [PubMed] [Google Scholar]
- 28.Jensen EC, Stockton AM, Chiesl TN, Kim J, Bera A, Mathies RA. Digitally programmable microfluidic automaton for multiscale combinatorial mixing and sample processing. Lab Chip. 2013;13:288–296. doi: 10.1039/c2lc40861a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chabert M, Viovy JL. Microfluidic high-throughput encapsulation and hydrodynamic self-sorting of single cells. Proc Natl Acad Sci USA. 2008;105:3191–3196. doi: 10.1073/pnas.0708321105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chung BG, Flanagan LA, Rhee SW, Schwartz PH, Lee AP, Monuki ES, Jeon NL. Human neural stem cell growth and differentiation in a gradient-generating microfluidic device. Lab Chip. 2005;5:401–406. doi: 10.1039/b417651k. [DOI] [PubMed] [Google Scholar]
- 31.Khademhosseini A, Yeh J, Jon S, Eng G, Suh KY, Burdick JA, Langer R. Molded polyethylene glycol microstructures for capturing cells within microfluidic channels. Lab Chip. 2004;4:425–430. doi: 10.1039/b404842c. [DOI] [PubMed] [Google Scholar]
- 32.Tan W, Desai TA. Microfluidic patterning of cells in extracellular matrix biopolymers: effects of channel size, cell type, and matrix composition on pattern integrity. Tissue Eng. 2003;9:255–267. doi: 10.1089/107632703764664729. [DOI] [PubMed] [Google Scholar]
- 33.Liu Z, Xiao L, Xu B, Zhang Y, Mak AF, Li Y, Man WY, Yang M. Covalently immobilized biomolecule gradient on hydrogel surface using a gradient generating microfluidic device for a quantitative mesenchymal stem cell study. Biomicrofluidics. 2012;6:24111–2411112. doi: 10.1063/1.4704522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang K, Park HJ, Han S, Lee J, Ko E, Kim J, Lee JS, Yu JH, Song KY, Cheong E, Cho SR, Chung S, Cho SW. Recapitulation of in vivo-like paracrine signals of human mesenchymal stem cells for functional neuronal differentiation of human neural stem cells in a 3D microfluidic system. Biomaterials. 2015;63:177–188. doi: 10.1016/j.biomaterials.2015.06.011. [DOI] [PubMed] [Google Scholar]
- 35.Khademhosseini A, Ferreira L, Blumling J, 3rd, Yeh J, Karp JM, Fukuda J, Langer R. Co-culture of human embryonic stem cells with murine embryonic fibroblasts on microwell-patterned substrates. Biomaterials. 2006;27:5968–5977. doi: 10.1016/j.biomaterials.2006.06.035. [DOI] [PubMed] [Google Scholar]
- 36.Lutolf MP, Gilbert PM, Blau HM. Designing materials to direct stem-cell fate. Nature. 2009;462:433–441. doi: 10.1038/nature08602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cosson S, Lutolf MP. Hydrogel microfluidics for the patterning of pluripotent stem cells. Sci Rep. 2014;4:4462. doi: 10.1038/srep04462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu H, Heilshorn SC. Microfluidic investigation of BDNF-enhanced neural stem cell chemotaxis in CXCL12 gradients. Small. 2013;9:585–595. doi: 10.1002/smll.201202208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee N, Park JW, Kim HJ, Yeon JH, Kwon J, Ko JJ, Oh SH, Kim HS, Kim A, Han BS, Lee SC, Jeon NL, Song J. Monitoring the differentiation and migration patterns of neural cells derived from human embryonic stem cells using a microfluidic culture system. Mol Cells. 2014;37:497–502. doi: 10.14348/molcells.2014.0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zheng W, Xie Y, Zhang W, Wang D, Ma W, Wang Z, Jiang X. Fluid flow stress induced contraction and re-spread of mesenchymal stem cells: a microfluidic study. Integr Biol (Camb) 2012;4:1102–1111. doi: 10.1039/c2ib20094e. [DOI] [PubMed] [Google Scholar]
- 41.Sakai S, Ito S, Inagaki H, Hirose K, Matsuyama T, Taya M, Kawakami K. Cell-enclosing gelatin-based microcapsule production for tissue engineering using a microfluidic flow-focusing system. Biomicrofluidics. 2011;5:13402. doi: 10.1063/1.3516657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Agarwal P, Zhao S, Bielecki P, Rao W, Choi JK, Zhao Y, Yu J, Zhang W, He X. One-step microfluidic generation of pre-hatching embryo-like core-shell microcapsules for miniaturized 3D culture of pluripotent stem cells. Lab Chip. 2013;13:4525–4533. doi: 10.1039/c3lc50678a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang X, Chen S, Kong M, Wang Z, Costa KD, Li RA, Sun D. Enhanced cell sorting and manipulation with combined optical tweezer and microfluidic chip technologies. Lab Chip. 2011;11:3656–3662. doi: 10.1039/c1lc20653b. [DOI] [PubMed] [Google Scholar]
- 44.Song H, Wang Y, Rosano JM, Prabhakarpandian B, Garson C, Pant K, Lai E. A microfluidic impedance flow cytometer for identification of differentiation state of stem cells. Lab Chip. 2013;13:2300–2310. doi: 10.1039/c3lc41321g. [DOI] [PubMed] [Google Scholar]
- 45.Song H, Rosano JM, Wang Y, Garson CJ, Prabhakarpandian B, Pant K, Klarmann GJ, Perantoni A, Alvarez LM, Lai E. Continuous-flow sorting of stem cells and differentiation products based on dielectrophoresis. Lab Chip. 2015;15:1320–1328. doi: 10.1039/c4lc01253d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Suri S, Singh A, Nguyen AH, Bratt-Leal AM, McDevitt TC, Lu H. Microfluidic-based patterning of embryonic stem cells for in vitro development studies. Lab Chip. 2013;13:4617–4624. doi: 10.1039/c3lc50663k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kane KIW, Moreno EL, Hachi S, Walter M, Jarazo J, Oliveira MAP, Hankemeier T, Vulto P, Schwamborn JC, Thoma M, Fleming RMT. Automated microfluidic cell culture of stem cell derived dopaminergic neurons. Sci Rep. 2019;9:1796. doi: 10.1038/s41598-018-34828-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dettinger P, Frank T, Etzrodt M, Ahmed N, Reimann A, Trenzinger C, Loeffler D, Kokkaliaris KD, Schroeder T, Tay S. Automated Microfluidic System for Dynamic Stimulation and Tracking of Single Cells. Anal Chem. 2018;90:10695–10700. doi: 10.1021/acs.analchem.8b00312. [DOI] [PubMed] [Google Scholar]
- 49.Sikorski DJ, Caron NJ, VanInsberghe M, Zahn H, Eaves CJ, Piret JM, Hansen CL. Clonal analysis of individual human embryonic stem cell differentiation patterns in microfluidic cultures. Biotechnol J. 2015;10:1546–1554. doi: 10.1002/biot.201500035. [DOI] [PubMed] [Google Scholar]
- 50.Gómez-Sjöberg R, Leyrat AA, Pirone DM, Chen CS, Quake SR. Versatile, fully automated, microfluidic cell culture system. Anal Chem. 2007;79:8557–8563. doi: 10.1021/ac071311w. [DOI] [PubMed] [Google Scholar]
- 51.He L, Si G, Huang J, Samuel ADT, Perrimon N. Mechanical regulation of stem-cell differentiation by the stretch-activated Piezo channel. Nature. 2018;555:103–106. doi: 10.1038/nature25744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jeong S, Eskandari R, Park SM, Alvarez J, Tee SS, Weissleder R, Kharas MG, Lee H, Keshari KR. Real-time quantitative analysis of metabolic flux in live cells using a hyperpolarized micromagnetic resonance spectrometer. Sci Adv. 2017;3:e1700341. doi: 10.1126/sciadv.1700341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kimura H, Sakai Y, Fujii T. Organ/body-on-a-chip based on microfluidic technology for drug discovery. Drug Metab Pharmacokinet. 2018;33:43–48. doi: 10.1016/j.dmpk.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 54.Barata D, van Blitterswijk C, Habibovic P. High-throughput screening approaches and combinatorial development of biomaterials using microfluidics. Acta Biomater. 2016;34:1–20. doi: 10.1016/j.actbio.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 55.Gupta N, Liu JR, Patel B, Solomon DE, Vaidya B, Gupta V. Microfluidics-based 3D cell culture models: Utility in novel drug discovery and delivery research. Bioeng Transl Med. 2016;1:63–81. doi: 10.1002/btm2.10013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Junkin M, Tay S. Microfluidic single-cell analysis for systems immunology. Lab Chip. 2014;14:1246–1260. doi: 10.1039/c3lc51182k. [DOI] [PubMed] [Google Scholar]
- 57.Du GS, Pan JZ, Zhao SP, Zhu Y, den Toonder JM, Fang Q. Cell-based drug combination screening with a microfluidic droplet array system. Anal Chem. 2013;85:6740–6747. doi: 10.1021/ac400688f. [DOI] [PubMed] [Google Scholar]
- 58.Du G, Fang Q, den Toonder JM. Microfluidics for cell-based high throughput screening platforms - A review. Anal Chim Acta. 2016;903:36–50. doi: 10.1016/j.aca.2015.11.023. [DOI] [PubMed] [Google Scholar]
- 59.Fernandes TG, Diogo MM, Clark DS, Dordick JS, Cabral JM. High-throughput cellular microarray platforms: applications in drug discovery, toxicology and stem cell research. Trends Biotechnol. 2009;27:342–349. doi: 10.1016/j.tibtech.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nierode GJ, Perea BC, McFarland SK, Pascoal JF, Clark DS, Schaffer DV, Dordick JS. High-Throughput Toxicity and Phenotypic Screening of 3D Human Neural Progenitor Cell Cultures on a Microarray Chip Platform. Stem Cell Reports. 2016;7:970–982. doi: 10.1016/j.stemcr.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee MY, Kumar RA, Sukumaran SM, Hogg MG, Clark DS, Dordick JS. Three-dimensional cellular microarray for high-throughput toxicology assays. Proc Natl Acad Sci USA. 2008;105:59–63. doi: 10.1073/pnas.0708756105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fernandes TG, Kwon SJ, Bale SS, Lee MY, Diogo MM, Clark DS, Cabral JM, Dordick JS. Three-dimensional cell culture microarray for high-throughput studies of stem cell fate. Biotechnol Bioeng. 2010;106:106–118. doi: 10.1002/bit.22661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Allazetta S, Hausherr TC, Lutolf MP. Microfluidic synthesis of cell-type-specific artificial extracellular matrix hydrogels. Biomacromolecules. 2013;14:1122–1131. doi: 10.1021/bm4000162. [DOI] [PubMed] [Google Scholar]
- 64.Headen DM, Aubry G, Lu H, García AJ. Microfluidic-based generation of size-controlled, biofunctionalized synthetic polymer microgels for cell encapsulation. Adv Mater. 2014;26:3003–3008. doi: 10.1002/adma.201304880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gobaa S, Hoehnel S, Roccio M, Negro A, Kobel S, Lutolf MP. Artificial niche microarrays for probing single stem cell fate in high throughput. Nat Methods. 2011;8:949–955. doi: 10.1038/nmeth.1732. [DOI] [PubMed] [Google Scholar]
- 66.Beachley VZ, Wolf MT, Sadtler K, Manda SS, Jacobs H, Blatchley MR, Bader JS, Pandey A, Pardoll D, Elisseeff JH. Tissue matrix arrays for high-throughput screening and systems analysis of cell function. Nat Methods. 2015;12:1197–1204. doi: 10.1038/nmeth.3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vrij EJ, Espinoza S, Heilig M, Kolew A, Schneider M, van Blitterswijk CA, Truckenmüller RK, Rivron NC. 3D high throughput screening and profiling of embryoid bodies in thermoformed microwell plates. Lab Chip. 2016;16:734–742. doi: 10.1039/c5lc01499a. [DOI] [PubMed] [Google Scholar]
- 68.Zhang P, Zhang J, Bian S, Chen Z, Hu Y, Hu R, Li J, Cheng Y, Zhang X, Zhou Y, Chen X, Liu P. High-throughput superhydrophobic microwell arrays for investigating multifactorial stem cell niches. Lab Chip. 2016;16:2996–3006. doi: 10.1039/c6lc00331a. [DOI] [PubMed] [Google Scholar]
- 69.Occhetta P, Centola M, Tonnarelli B, Redaelli A, Martin I, Rasponi M. High-Throughput Microfluidic Platform for 3D Cultures of Mesenchymal Stem Cells, Towards Engineering Developmental Processes. Sci Rep. 2015;5:10288. doi: 10.1038/srep10288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gracz AD, Williamson IA, Roche KC, Johnston MJ, Wang F, Wang Y, Attayek PJ, Balowski J, Liu XF, Laurenza RJ, Gaynor LT, Sims CE, Galanko JA, Li L, Allbritton NL, Magness ST. A high-throughput platform for stem cell niche co-cultures and downstream gene expression analysis. Nat Cell Biol. 2015;17:340–349. doi: 10.1038/ncb3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yu YJ, Kim YH, Na K, Min SY, Hwang OK, Park DK, Kim DY, Choi SH, Kamm RD, Chung S, Kim JA. Hydrogel-incorporating unit in a well: 3D cell culture for high-throughput analysis. Lab Chip. 2018;18:2604–2613. doi: 10.1039/c8lc00525g. [DOI] [PubMed] [Google Scholar]
- 72.Ho BX, Pek NMQ, Soh BS. Disease Modeling Using 3D Organoids Derived from Human Induced Pluripotent Stem Cells. Int J Mol Sci. 2018:19. doi: 10.3390/ijms19040936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ranga A, Gjorevski N, Lutolf MP. Drug discovery through stem cell-based organoid models. Adv Drug Deliv Rev. 2014;69-70:19–28. doi: 10.1016/j.addr.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 74.Ware BR, Berger DR, Khetani SR. Prediction of Drug-Induced Liver Injury in Micropatterned Co-cultures Containing iPSC-Derived Human Hepatocytes. Toxicol Sci. 2015;145:252–262. doi: 10.1093/toxsci/kfv048. [DOI] [PubMed] [Google Scholar]
- 75.Materne EM, Ramme AP, Terrasso AP, Serra M, Alves PM, Brito C, Sakharov DA, Tonevitsky AG, Lauster R, Marx U. A multi-organ chip co-culture of neurospheres and liver equivalents for long-term substance testing. J Biotechnol. 2015;205:36–46. doi: 10.1016/j.jbiotec.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 76.Schepers A, Li C, Chhabra A, Seney BT, Bhatia S. Engineering a perfusable 3D human liver platform from iPS cells. Lab Chip. 2016;16:2644–2653. doi: 10.1039/c6lc00598e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ware BR, Khetani SR. Engineered Liver Platforms for Different Phases of Drug Development. Trends Biotechnol. 2017;35:172–183. doi: 10.1016/j.tibtech.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Conant G, Lai BFL, Lu RXZ, Korolj A, Wang EY, Radisic M. High-Content Assessment of Cardiac Function Using Heart-on-a-Chip Devices as Drug Screening Model. Stem Cell Rev. 2017;13:335–346. doi: 10.1007/s12015-017-9736-2. [DOI] [PubMed] [Google Scholar]
- 79.Ghafar-Zadeh E, Waldeisen JR, Lee LP. Engineered approaches to the stem cell microenvironment for cardiac tissue regeneration. Lab Chip. 2011;11:3031–3048. doi: 10.1039/c1lc20284g. [DOI] [PubMed] [Google Scholar]
- 80.Mannhardt I, Breckwoldt K, Letuffe-Brenière D, Schaaf S, Schulz H, Neuber C, Benzin A, Werner T, Eder A, Schulze T, Klampe B, Christ T, Hirt MN, Huebner N, Moretti A, Eschenhagen T, Hansen A. Human Engineered Heart Tissue: Analysis of Contractile Force. Stem Cell Reports. 2016;7:29–42. doi: 10.1016/j.stemcr.2016.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Marsano A, Conficconi C, Lemme M, Occhetta P, Gaudiello E, Votta E, Cerino G, Redaelli A, Rasponi M. Beating heart on a chip: a novel microfluidic platform to generate functional 3D cardiac microtissues. Lab Chip. 2016;16:599–610. doi: 10.1039/c5lc01356a. [DOI] [PubMed] [Google Scholar]
- 82.Mathur A, Loskill P, Shao K, Huebsch N, Hong S, Marcus SG, Marks N, Mandegar M, Conklin BR, Lee LP, Healy KE. Human iPSC-based cardiac microphysiological system for drug screening applications. Sci Rep. 2015;5:8883. doi: 10.1038/srep08883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Titmarsh DM, Glass NR, Mills RJ, Hidalgo A, Wolvetang EJ, Porrello ER, Hudson JE, Cooper-White JJ. Induction of Human iPSC-Derived Cardiomyocyte Proliferation Revealed by Combinatorial Screening in High Density Microbioreactor Arrays. Sci Rep. 2016;6:24637. doi: 10.1038/srep24637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang G, McCain ML, Yang L, He A, Pasqualini FS, Agarwal A, Yuan H, Jiang D, Zhang D, Zangi L, Geva J, Roberts AE, Ma Q, Ding J, Chen J, Wang DZ, Li K, Wang J, Wanders RJ, Kulik W, Vaz FM, Laflamme MA, Murry CE, Chien KR, Kelley RI, Church GM, Parker KK, Pu WT. Modeling the mitochondrial cardiomyopathy of Barth syndrome with induced pluripotent stem cell and heart-on-chip technologies. Nat Med. 2014;20:616–623. doi: 10.1038/nm.3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ribas J, Sadeghi H, Manbachi A, Leijten J, Brinegar K, Zhang YS, Ferreira L, Khademhosseini A. Cardiovascular Organ-on-a-Chip Platforms for Drug Discovery and Development. Appl In Vitro Toxicol. 2016;2:82–96. doi: 10.1089/aivt.2016.0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mills RJ, Titmarsh DM, Koenig X, Parker BL, Ryall JG, Quaife-Ryan GA, Voges HK, Hodson MP, Ferguson C, Drowley L, Plowright AT, Needham EJ, Wang QD, Gregorevic P, Xin M, Thomas WG, Parton RG, Nielsen LK, Launikonis BS, James DE, Elliott DA, Porrello ER, Hudson JE. Functional screening in human cardiac organoids reveals a metabolic mechanism for cardiomyocyte cell cycle arrest. Proc Natl Acad Sci USA. 2017;114:E8372–E8381. doi: 10.1073/pnas.1707316114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Choi J, Kim S, Jung J, Lim Y, Kang K, Park S, Kang S. Wnt5a-mediating neurogenesis of human adipose tissue-derived stem cells in a 3D microfluidic cell culture system. Biomaterials. 2011;32:7013–7022. doi: 10.1016/j.biomaterials.2011.05.090. [DOI] [PubMed] [Google Scholar]
- 88.Korhonen P, Malm T, White AR. 3D human brain cell models: New frontiers in disease understanding and drug discovery for neurodegenerative diseases. Neurochem Int. 2018;120:191–199. doi: 10.1016/j.neuint.2018.08.012. [DOI] [PubMed] [Google Scholar]
- 89.Rocha DN, Carvalho ED, Pêgo AP. High-throughput platforms for the screening of new therapeutic targets for neurodegenerative diseases. Drug Discov Today. 2016;21:1355–1366. doi: 10.1016/j.drudis.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 90.Wang YI, Abaci HE, Shuler ML. Microfluidic blood-brain barrier model provides in vivo-like barrier properties for drug permeability screening. Biotechnol Bioeng. 2017;114:184–194. doi: 10.1002/bit.26045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Park J, Lee BK, Jeong GS, Hyun JK, Lee CJ, Lee SH. Three-dimensional brain-on-a-chip with an interstitial level of flow and its application as an in vitro model of Alzheimer's disease. Lab Chip. 2015;15:141–150. doi: 10.1039/c4lc00962b. [DOI] [PubMed] [Google Scholar]
- 92.Lai Y, Kisaalita WS. Performance evaluation of 3D polystyrene 96-well plates with human neural stem cells in a calcium assay. J Lab Autom. 2012;17:284–292. doi: 10.1177/2211068212442503. [DOI] [PubMed] [Google Scholar]
- 93.Wang Y, Wang L, Zhu Y, Qin J. Human brain organoid-on-a-chip to model prenatal nicotine exposure. Lab Chip. 2018;18:851–860. doi: 10.1039/c7lc01084b. [DOI] [PubMed] [Google Scholar]
- 94.Wevers NR, van Vught R, Wilschut KJ, Nicolas A, Chiang C, Lanz HL, Trietsch SJ, Joore J, Vulto P. High-throughput compound evaluation on 3D networks of neurons and glia in a microfluidic platform. Sci Rep. 2016;6:38856. doi: 10.1038/srep38856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bein A, Shin W, Jalili-Firoozinezhad S, Park MH, Sontheimer-Phelps A, Tovaglieri A, Chalkiadaki A, Kim HJ, Ingber DE. Microfluidic Organ-on-a-Chip Models of Human Intestine. Cell Mol Gastroenterol Hepatol. 2018;5:659–668. doi: 10.1016/j.jcmgh.2017.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kim HJ, Li H, Collins JJ, Ingber DE. Contributions of microbiome and mechanical deformation to intestinal bacterial overgrowth and inflammation in a human gut-on-a-chip. Proc Natl Acad Sci USA. 2016;113:E7–15. doi: 10.1073/pnas.1522193112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Workman MJ, Gleeson JP, Troisi EJ, Estrada HQ, Kerns SJ, Hinojosa CD, Hamilton GA, Targan SR, Svendsen CN, Barrett RJ. Enhanced Utilization of Induced Pluripotent Stem Cell-Derived Human Intestinal Organoids Using Microengineered Chips. Cell Mol Gastroenterol Hepatol. 2017;5:669–677.e2. doi: 10.1016/j.jcmgh.2017.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Musah S, Mammoto A, Ferrante TC, Jeanty SSF, Hirano-Kobayashi M, Mammoto T, Roberts K, Chung S, Novak R, Ingram M, Fatanat-Didar T, Koshy S, Weaver JC, Church GM, Ingber DE. Mature induced-pluripotent-stem-cell-derived human podocytes reconstitute kidney glomerular-capillary-wall function on a chip. Nat Biomed Eng. 2017:1. doi: 10.1038/s41551-017-0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wilmer MJ, Ng CP, Lanz HL, Vulto P, Suter-Dick L, Masereeuw R. Kidney-on-a-Chip Technology for Drug-Induced Nephrotoxicity Screening. Trends Biotechnol. 2016;34:156–170. doi: 10.1016/j.tibtech.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 100.Czerniecki SM, Cruz NM, Harder JL, Menon R, Annis J, Otto EA, Gulieva RE, Islas LV, Kim YK, Tran LM, Martins TJ, Pippin JW, Fu H, Kretzler M, Shankland SJ, Himmelfarb J, Moon RT, Paragas N, Freedman BS. High-Throughput Screening Enhances Kidney Organoid Differentiation from Human Pluripotent Stem Cells and Enables Automated Multidimensional Phenotyping. Cell Stem Cell. 2018;22:929–940.e4. doi: 10.1016/j.stem.2018.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Torisawa YS, Mammoto T, Jiang E, Jiang A, Mammoto A, Watters AL, Bahinski A, Ingber DE. Modeling Hematopoiesis and Responses to Radiation Countermeasures in a Bone Marrow-on-a-Chip. Tissue Eng Part C Methods. 2016;22:509–515. doi: 10.1089/ten.TEC.2015.0507. [DOI] [PubMed] [Google Scholar]
- 102.Marturano-Kruik A, Nava MM, Yeager K, Chramiec A, Hao L, Robinson S, Guo E, Raimondi MT, Vunjak-Novakovic G. Human bone perivascular niche-on-a-chip for studying metastatic colonization. Proc Natl Acad Sci USA. 2018;115:1256–1261. doi: 10.1073/pnas.1714282115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sieber S, Wirth L, Cavak N, Koenigsmark M, Marx U, Lauster R, Rosowski M. Bone marrow-on-a-chip: Long-term culture of human haematopoietic stem cells in a three-dimensional microfluidic environment. J Tissue Eng Regen Med. 2018;12:479–489. doi: 10.1002/term.2507. [DOI] [PubMed] [Google Scholar]
- 104.Oleaga C, Bernabini C, Smith AS, Srinivasan B, Jackson M, McLamb W, Platt V, Bridges R, Cai Y, Santhanam N, Berry B, Najjar S, Akanda N, Guo X, Martin C, Ekman G, Esch MB, Langer J, Ouedraogo G, Cotovio J, Breton L, Shuler ML, Hickman JJ. Multi-Organ toxicity demonstration in a functional human in vitro system composed of four organs. Sci Rep. 2016;6:20030. doi: 10.1038/srep20030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhang YS, Aleman J, Shin SR, Kilic T, Kim D, Mousavi Shaegh SA, Massa S, Riahi R, Chae S, Hu N, Avci H, Zhang W, Silvestri A, Sanati Nezhad A, Manbohi A, De Ferrari F, Polini A, Calzone G, Shaikh N, Alerasool P, Budina E, Kang J, Bhise N, Ribas J, Pourmand A, Skardal A, Shupe T, Bishop CE, Dokmeci MR, Atala A, Khademhosseini A. Multisensor-integrated organs-on-chips platform for automated and continual in situ monitoring of organoid behaviors. Proc Natl Acad Sci USA. 2017;114:E2293–E2302. doi: 10.1073/pnas.1612906114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Toh YC, Voldman J. Fluid shear stress primes mouse embryonic stem cells for differentiation in a self-renewing environment via heparan sulfate proteoglycans transduction. FASEB J. 2011;25:1208–1217. doi: 10.1096/fj.10-168971. [DOI] [PMC free article] [PubMed] [Google Scholar]