Abstract
Background
Sarcopenia is a progressive and generalized skeletal muscle disorder that is associated with an increased likelihood of adverse outcomes, including falls, fractures, physical disability, and mortality. However, there have been few systematic studies of the prevalence and prognostic values of sarcopenia in older patients with coronary heart disease (CHD). This study aimed to investigate the prevalence of sarcopenia in hospitalized older patients with CHD, and to prospectively evaluate the effect of sarcopenia on the short-term prognosis of these patients.
Methods
Patients aged ≥ 65 years, with the diagnosis of CHD from Peking Union Medical College Hospital between December 2017 and November 2018, were included. Sarcopenia was diagnosed according to consensus of the Asian Working Group for Sarcopenia in 2014. Follow-up items included unscheduled return visits, occurrence of major adverse cardiac and cerebral events (MACCE), and all-cause mortality. The MACCE-free survival curve of sarcopenic and non-sarcopenic older patients with CHD was estimated by the Kaplan-Meier method. Cox regression analysis was used to analyze the association between sarcopenia and an unscheduled return visits, MACCE, and all-cause mortality.
Results
A total of 345 older patients with CHD were enrolled in the study, with a median age of 74 years. Among the patients, 78 (22.6%) were diagnosed with sarcopenia. During the follow-up time, there were significantly more unscheduled return visits in sarcopenic patients than in non-sarcopenic patients (34.2% vs. 21.8%, χ2 = 4.418, P = 0.036), while there was no significant difference in the occurrence of MACCE (χ2 = 2.869, P = 0.09) or all-cause mortality (χ2 = 1.673, P = 0.196) between these patient groups. The Kaplan-Meier curve showed that the MACCE-free survival time of sarcopenic patients was significantly shorter than that in non-sarcopenic patients (χ2 = 4.102, P = 0.043). After adjusting for sex, age, and the Charlson comorbidity index, sarcopenia was not an independent risk factor of unscheduled return visits (HR = 1.002, 95% CI: 0.556–1.807). However, the complication of anxiety and depression was an independent risk factor (HR = 1.876, 95% CI: 1.012–3.477, P = 0.046) for unscheduled return visits in older patients with CHD.
Conclusions
There is a high prevalence of sarcopenia among hospitalized older adults with CHD. A shorter MACCE-free survival time and more unscheduled return visits are found in sarcopenic older patients with CHD. Clinicians should pay more attention to the functional status of older patients with CHD, as well as identification and management of geriatric syndromes.
Keywords: Coronary heart disease, Older adult, Prognosis, Sarcopenia
1. Introduction
China has entered an aging society. By the end of 2016, the number of older adults aged 60 and older reached 230 million. This number is projected to reach 418 million by 2035 and peak at 487 million by 2053.[1] Coronary heart disease (CHD) is one of the main reasons affecting the health of the older population (especially older people aged ≥ 80 years old) and its prevalence increases with age. In China, the mortality of acute myocardial infarction increases almost exponentially since 40 years old, and it is more prominent in people aged ≥ 80 years.[2]
Sarcopenia, which is defined as a low muscle mass and low muscle function and/or reduced physical performance,[3] is a major public health consideration. Sarcopenia leads to serious adverse health consequences, such as functional disabilities, frailty, fatigue, falls, fractures, hospitalizations, multiple comorbidities (osteoporosis, diabetes mellitus),[4] mortality, and compromised quality of life.[5]
The effect of low muscle mass and reduced muscle strength on quality of life and prognosis of older patients with cardiovascular disease is not well understood, and there are few related clinical studies. A propensity score-matched analysis of a single center all-comer cohort study included 475 patients with coronary artery disease who underwent successful percutaneous coronary intervention.[6] Muscle mass was measured using the cross-sectional areas of skeletal muscle at the first lumbar (L1) vertebral level and was presented as the L1 skeletal muscle index (L1 SMI, normalized for height). During the three-year follow-up, the incidence of major adverse cardiovascular events (MACEs) (39.6% vs. 11.8%, P < 0.001) and all-cause mortality (23.7% vs. 5.9%, P < 0.001) were significantly higher in patients with a low L1 SMI than in those with a high L1 SMI. Moreover, a low L1 SMI was an independent predictor of a higher risk of all-cause mortality [hazard ratio (HR) = 4.07, P < 0.001] and MACEs (HR = 3.76, P < 0.001). However, this study did not assess muscle strength and physical performance of the patients, and analyzed the prognostic implications of sarcopenia as a whole in patients with coronary artery disease.
There is no published clinical study on the prevalence of sarcopenia in older patients with CHD (including stable coronary heart disease and acute coronary syndrome), and the effect of sarcopenia on the prognosis of older patients with CHD. To bridge this important knowledge gap, this single-center study aimed to examine the prevalence and prognostic value of sarcopenia for older patients with CHD. We also aimed to accumulate clinical data for following intervention studies for older patients with CHD and sarcopenia in China.
2. Methods
This was a prospective, observational study of hospitalized older patients with CHD. The study protocol was approved by the institutional review board of Peking Union Medical College Hospital (S–589). Study procedures were performed in accordance with the Declaration of Helsinki ethical principles for medical research involving human subjects.
2.1. Study population
Patients with CHD who were aged ≥ 65 years and admitted to the Cardiology and Geriatrics Departments, Peking Union Medical College Hospital from December 2017 to November 2018 were enrolled in this study. Patients with the following conditions were considered for inclusion: (1) selective coronary angiography identified one or more major coronary artery stenosis ≥ 50%; (2) clinical symptoms, an electrocardiogram, and myocardial markers were in accordance with the 2014 American College of Cardiology/American Heart Association (ACC/AHA) guideline for diagnosis and treatment of acute coronary syndrome (ACS); (3) stable coronary heart disease; (4) New York Heart Association classes I-II; and (5) patients willing to participate in this study and sign informed consent. ACS included ST segment elevation myocardial infarction, non-ST segment elevation myocardial infarction, and unstable angina pectoris. Patients with the following conditions were exclusion: (1) patients with aphasia, delirium, and inability to communicate because of severe cognitive impairment; (2) patients complicated by Parkinson's disease, severe knee or hip osteoarthritis, lumbar spinal canal stenosis, or tumors; (3) New York Heart Association classes III-IV; and (4) patients with an unstable condition, not willing to participate in this study, and incomplete information and assessment data.
2.2. Data collection
A clinical database was established to record age, sex, height, and weight of the patients in detail, and the body mass index (BMI) was calculated. Underlying diseases, such as diabetes mellitus, hypertension, anxiety, and depression, of the patients were also recorded. Fasting blood samples were taken the next morning after admission. White blood cell count, neutrophil count, and hemoglobin and platelet count were determined, as well as other laboratory parameters, such as serum creatinine, total cholesterol, triglyceride, low-density lipoprotein cholesterol, serum albumin, prealbumin, and high-sensitivity C-reactive protein levels. Evaluation of patients with stable CHD was conducted on the second day after hospitalization. Evaluation of patients with ACS was conducted after complete remission of myocardial ischemia symptoms.
2.3. Assessment of sarcopenia
A Jamar handgrip dynamometer was used to measure grip strength. Walking speed was measured by the 6-m walking speed test. Sarcopenia was diagnosed according to consensus of the Asian Working Group for Sarcopenia in 2014 as follows: (1) the walking speed cut point was a 6-m walking speed ≤ 0.8 m/s; and (2) the grip strength cut point was < 26 kg for men and < 18 kg for women.[3] When (1) and/or (2) was found, muscle mass, including skeletal muscle mass of the limbs and the whole body, of the patient was further measured by bioelectrical impedance analysis, and the appendicular skeletal muscle mass index (ASMI) was calculated. Patients with an ASMI < 7.0 kg/m2 (men) or < 5.7 kg/m2 (women) were diagnosed with sarcopenia. Measurements were conducted by the InBody720 Body Composition Analyzer (Biospace Co., Ltd., Korea).
2.4. Assessment of physical function and other assessments
Activities of daily living (ADL) of the patients were assessed by the Katz ADL Scale.[7] Instrumental activity of daily living (IADL) of the patients was assessed by the Lawton IADL scale.[8] Comorbidity of the patients was evaluated by the Charlson comorbidity index.[9] We recorded long-term medication use and assessed whether there was polypharmacy. We examined the nutritional status of the patients by the Mini Nutritional Assessment-Short Form (MNA-SF). An MNA-SF score ≤ 7 indicates malnutrition and MNA-SF scores 8-11 indicate a risk of malnutrition.[10] In a balance test, we evaluated the full tandem stance time of the patients, and < 10 s indicated that the patient failed the test.[11] The history of falls over the previous year, urinary incontinence, and living conditions of the patients were recorded.
2.5. Follow-up
Through a clinical visit or telephone consultation, the time and specific reasons for occurrence of the following were recorded in detail: (1) unscheduled return visits; (2) major adverse cardiac and cerebral events (MACCE), including myocardial infarction, stroke, new-onset or worsened heart failure, and death from cardiac and cerebrovascular disease, and (3) all-cause mortality during the follow-up period.
2.6. Statistical analyses
SPSS software version 24.0 was used for statistical analysis. Normally distributed data are expressed as mean ± SD and the t-test was used for comparison between two groups. Non-normally distributed data are expressed as median (interquartile range) and the Wilcoxon rank sum test was used for comparison between two groups. Count data are expressed by frequency (percentage), and comparison between groups was performed by the chi-square test or Fisher's exact test. Cox regression analysis was used to analyze the association between sarcopenia and unscheduled return visits, MACCE, and all-cause mortality. Survival rate was estimated by the Kaplan-Meier method and comparison between the two survival curves was performed by the log-rank test. All of the tests were two-sided and the difference was statistically significant when P < 0.05.
3. Results
There were 414 older patients with CHD who were admitted to the Cardiology and Geriatrics Departments, Peking Union Medical College Hospital from December 2017 to November 2018. Of them, 69 patients were excluded according to exclusion criteria. A total of 345 patients with CHD with complete data aged ≥ 65 years were included in this study. The patients were aged between 65 and 96 years old, with a median age of 74 years. There were 250 cases of stable CHD and 95 cases of ACS. Among these patients, 78 (22.6%) were diagnosed with sarcopenia according to the consensus issued by the Asian Working Group for Sarcopenia. Patients with sarcopenia were significantly older in age and had a lower BMI compared with non-sarcopenic patients (both P < 0.001). The Charlson comorbidity index and long-term medication use were significantly greater in sarcopenic patients compared with non-sarcopenic patients (both P < 0.05). The proportion of ACS was significantly lower and that of anxiety and depression was higher in sarcopenic patients than in non-sarcopenic patients (both P < 0.05). MNA-SF scores in sarcopenic patients were significantly lower than those in non-sarcopenic patients (P < 0.001). The proportions of urinary incontinence, history of falls in the previous year, and living alone in sarcopenic patients were significantly higher than those in non-sarcopenic patients (P < 0.05 for all). In patients with sarcopenia, the ADL score and IADL score were significantly lower than those in non-sarcopenic patients (both P < 0.001). The percentage of those who failed to complete the full tandem stance test was significantly higher in sarcopenic patients than in non-sarcopenic patients (P < 0.001). High-sensitivity C-reactive protein levels were higher, and albumin and prealbumin levels were lower in sarcopenic patients than in non-sarcopenic patients (all P < 0.05) (Table 1).
Table 1. Comparison of clinical data of sarcopenic and non-sarcopenic older patients with CHD.
| Variable | All cases (n = 345) | Sarcopenia group (n = 78) | Non-sarcopenia group (n = 267) | χ2 or t-value | P-value |
| Median age, yrs | 74 (69, 79) | 79.5 (75, 84) | 72 (68, 77) | −6.875 | < 0.001** |
| Male | 208 (60.3%) | 32 (41%) | 176 (65.9%) | 15.623 | < 0.001** |
| Body mass index, kg/m2 | 24.93 ± 4.42 | 23.52 ± 4.26 | 25.42 ± 3.04 | −4.401 | < 0.001** |
| History of falls | 92 (26.7%) | 28 (35.9%) | 64 (24%) | 4.391 | 0.036* |
| Urinary incontinence | 76 (22%) | 33 (42.3%) | 43 (16.1%) | 24.13 | < 0.001** |
| Living alone | 33 (9.6%) | 12 (15.4%) | 21 (7.9%) | 3.925 | 0.048* |
| Median MNA-SF score | 12 (11, 13) | 11.5 (9, 12.25) | 12 (11, 14) | −3.977 | < 0.001** |
| Acute coronary syndrome | 95 (27.5%) | 14 (17.9%) | 81 (30.3%) | 4.643 | 0.031* |
| Complicated with type 2 diabetes mellitus | 144 (41.7%) | 38 (48.7%) | 106 (39.7%) | 2.019 | 0.155 |
| Complicated with anxiety and depression | 35 (10.1%) | 13 (16.7%) | 22 (8.2%) | 4.703 | 0.030* |
| Median Charlson comorbidity index | 1 (0, 2) | 1.5 (1, 3) | 1 (0, 2) | −2.708 | 0.007* |
| Median long-term medication use | 7 (5, 9) | 7 (6, 10) | 7 (5, 9) | −2.228 | 0.026* |
| Median ADL score | 6 (5, 6) | 5 (4, 6) | 6 (6, 6) | −6.99 | < 0.001** |
| Median IADL score | 8 (7, 8) | 6 (4, 8) | 8 (8, 8) | −8.637 | < 0.001** |
| Median grip strength, kg | 26.9 (20.1, 33.4) | 17.6 (14.88, 23.7) | 29.7 (23.3, 35.1) | −10.27 | < 0.001** |
| Median walking speed, m/s | 0.86 (0.67, 1.01) | 0.6 (0.44, 0.71) | 0.92 (0.81, 1.05) | −10.211 | < 0.001** |
| Failure to complete full tandem stance | 85 (24.6%) | 45 (57.7%) | 40 (15%) | 59.308 | < 0.001** |
| Median albumin, g/L | 40 (38, 43) | 40 (37, 42) | 41 (38, 43) | −2.164 | 0.030* |
| Prealbumin, mg/L | 225.86 ± 49.01 | 207.64 ± 52.07 | 231.18 ± 46.86 | 3.803 | < 0.001** |
| Median glycosylated hemoglobin, % | 6.1 (5.7, 7.1) | 6.2 (5.7, 7.2) | 6.1 (5.7, 7) | −0.697 | 0.486 |
| Median uric acid, mmol/L | 340 (283.5, 405) | 348 (298.5, 439.75) | 337 (281, 395) | −1.589 | 0.112 |
| Median high-sensitivity C-reactive protein, mg/L | 1.2 (0.51, 2.75) | 1.85 (0.75, 4.92) | 1.09 (0.49, 2.35) | −2.915 | 0.004* |
| Median triglycerides, mmol/L | 1.16 (0.87, 1.59) | 1.15 (0.87, 1.61) | 1.17 (0.86, 1.57) | −0.383 | 0.702 |
| Median low-density lipoprotein cholesterol, mmol/L | 1.95 (1.59, 2.4) | 2.01 (1.64, 2.43) | 1.95 (1.57, 2.4) | −0.825 | 0.409 |
Data are presented as means ± SD or n (%) or median (interquartile range). *P < 0.05, **P < 0.001. ADL: activities of daily living; CHD: coronary heart disease; IALD: instrumental activity of daily living; MNA-SF: Mini Nutritional Assessment-Short Form.
The follow-up time of older patients with CHD ranged from 90 days to 446 days and the median follow-up time was 351 days (300, 394). There were three cases of loss of follow-up (two cases in the sarcopenia group and one case in the non-sarcopenia group). During the follow-up period, 84 (24.6%) patients had unscheduled visits. There were significantly more unscheduled visits in sarcopenic patients than in non-sarcopenic patients (χ2 = 4.418, P = 0.036) (Table 2).
Table 2. Comparison of unscheduled return visits for sarcopenic and non-sarcopenic patients with CHD during follow-up.
| Variable | All patients (n = 342) | Sarcopenia group (n = 76) | Non-sarcopenia group (n = 266) | P-value |
| Unscheduled visit | 84 (24.6%) | 26 (34.2%) | 58 (21.8%) | 0.027* |
| Infectious disease | 17 (20.2%) | 9 (34.6%) | 8 (13.8%) | 0.005* |
| Angina/acute myocardial infarction | 19 (22.6%) | 6 (23.1%) | 13 (22.4%) | 0.497 |
| Arrhythmia | 5 (5.9%) | 3 (11.5%) | 2 (3.4%) | 0.140 |
| Congestive heart failure | 2 (2.4%) | 1 (3.8%) | 1 (1.7%) | 0.935 |
| Stroke | 4 (4.8%) | 1 (3.8%) | 3 (5.2%) | 1.000 |
| Acute intestinal obstruction | 3 (3.6%) | 2 (7.7%) | 1 (1.7%) | 0.255 |
| Fracture after falling | 6 (7.1%) | 1 (3.8%) | 5 (8.6%) | 1.000 |
| Other causes | 28 (33.3%) | 3 (11.5%) | 25 (43.1%) | 0.116 |
*P < 0.05. CHD: coronary heart disease.
During the follow-up period, there was no significant difference in the rate of MACCE between the two groups (χ2 = 2.869, P = 0.09) (Table 3). There was also no significant difference in all-cause mortality between the two groups (χ2 = 1.673, P = 0.196) (Table 4).
Table 3. Comparison of MACCE in sarcopenic and non-sarcopenic patients during follow-up.
| Variable | All patients (n = 342) | Sarcopenia group (n = 76) | Non-sarcopenia group (n = 266) | P-value |
| Major adverse cardiac and cerebral events | 20 (5.8%) | 8 (10.5%) | 12 (4.5%) | 0.050 |
| Death from cardiac and cerebrovascular diseases | 11 (55%) | 4 (50%) | 7 (58.3%) | 0.251 |
| Stroke | 4 (20%) | 1 (12.5%) | 3 (25%) | 0.856 |
| Acute myocardial infarction | 3 (15%) | 2 (25%) | 1 (8.3%) | 0.063 |
| New-onset or worsen heart failure | 2 (10%) | 1 (12.5%) | 1 (8.3%) | 0.320 |
MACCE: major adverse cardiac and cerebral event.
Table 4. Comparison of all-cause mortality in sarcopenic and non-sarcopenic patients with CHD during follow-up.
| Variable | All patients (n = 342) | Sarcopenia group (n = 76) | Non-sarcopenia group (n = 266) | P-value |
| All-cause mortality | 19 (5.56%) | 7 (9.21%) | 12 (4.51%) | 0.101 |
| Cardiac and cerebrovascular diseases | 11 (57.9%) | 4 (57.1%) | 7 (58.3%) | 0.251 |
| Multiple organ failure due to severe pneumonia | 4 (21.1%) | 2 (28.6%) | 2 (16.7%) | 0.179 |
| Tumor | 1 (5.3%) | 1 (14.3%) | 0 | 0.061 |
| Diabetic ketoacidosis | 1 (5.3%) | 0 | 1 (8.3%) | 0.592 |
| Unknown causes | 2 (10.5%) | 0 | 2 (16.7%) | 0.448 |
CHD: coronary heart disease.
The median MACCE-free survival time in patients with sarcopenia was 337 days (282, 389) and that in non-sarcopenic patients was 361 days (304, 398). The Kaplan-Meier curve showed that the MACCE-free survival time of sarcopenic patients was significantly shorter than that in non-sarcopenic patients (χ2 = 4.102, P = 0.043) (Figure 1).
Figure 1. Comparison of MACCE-free survival curves between sarcopenic patients and non-sarcopenic patients.
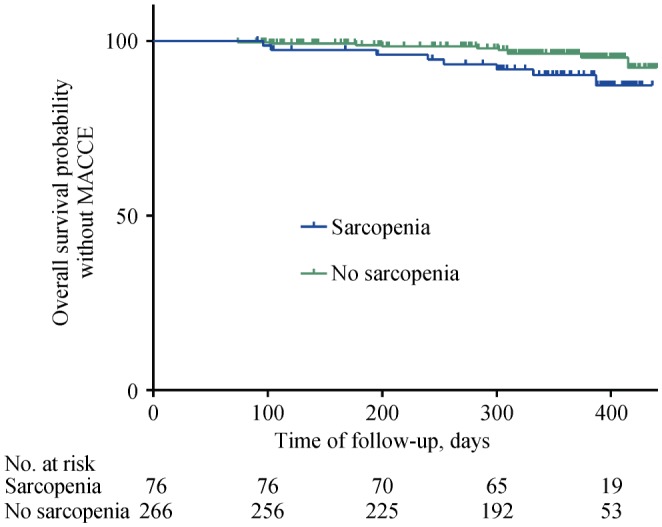
MACCE: major adverse cardiac and cerebral event.
After adjusting for sex, age, and the Charlson comorbidity index, we found that sarcopenia was not an independent risk factor of unscheduled return visits (HR = 1.002, 95% CI: 0.556–1.807), MACCE (HR = 0.875, 95% CI: 0.469–1.633), and all-cause mortality (HR = 0.409, 95% CI: 0.126–1.33). However, the complication of anxiety and depression was an independent risk factor (HR = 1.876, 95% CI: 1.012–3.477, P = 0.046) for unscheduled return visits in older patients with CHD.
4. Discussion
In recent years, the effect of sarcopenia as assessed with various methods on the prognosis of patients with diabetes mellitus, liver cirrhosis, and tumors has been previously studied.[12]–[18] These studies have also reported that sarcopenia assessed with various methods was closely associated with subclinical features of CHD, such as coronary artery calcification and subclinical coronary artery stenosis.[19]–[21] However, the effect of CHD on sarcopenia remains unclear. This study showed that there was a high prevalence of sarcopenia in hospitalized older adults with CHD. The incidence of unscheduled return visits in sarcopenic older patients with CHD was higher than that in those without sarcopenia. Moreover, the MACCE-free survival time in patients with sarcopenia was significantly shorter than that in patients without sarcopenia.
The current study showed that during follow-up, 24.6% of older patients with CHD had unscheduled return visits. Among them, angina pectoris/myocardial infarction and infectious disease were the most common reasons for those visits. Furthermore, these reasons were also the most common reasons for unscheduled return visits in sarcopenic older patients with CHD. The incidence of unscheduled return visits in patients with sarcopenia was significantly higher than that in patients without sarcopenia. These results suggest that the rate of unscheduled return visits of older patients with CHD, especially those complicated by sarcopenia, is high after discharge. Therefore, clinicians should provide detailed health instructions to patients and their families when they are discharged from hospital.
Our study showed that there were no significant differences in the rates of MACCE and all-cause mortality between sarcopenic and non-sarcopenic patients with CHD. After adjusting for age, sex, and comorbid diseases, sarcopenia was not an independent risk factor of unscheduled return visits. Because of the small sample size, particularly the short follow-up time, and the fact that this was a single-center study, the association between sarcopenia and prognosis of older patients with CHD cannot be fully determined. To understand the effect of sarcopenia on the long-term prognosis of CHD, the sample size should be further expanded. Additionally, further follow-up observations and multicenter clinical studies should be conducted.
In our study, 10.1% of older patients with CHD were complicated by anxiety and depression. Cox regression analysis showed that the complication of anxiety and depression was an independent risk factor for unscheduled visits in older patients with CHD. Mental illness itself is an independent risk factor for the onset and exacerbation of CHD. Watkins, et al.[22] showed that anxiety increased all-cause mortality by 1.6 times in patients with CHD. For older patients with CHD complicated by anxiety and depression, a multidisciplinary team of geriatrics should be included in ward rounds. Furthermore, whether patients should be prescribed anti-anxiety and depression drugs should be determined under joint discussion of geriatricians, psychiatrists, and clinical pharmacists. Drug interactions between prescribed anti-anxiety and depression drugs and the drugs currently being taken by patients should also be evaluated.
The current study showed that the MACCE-free survival time in sarcopenic patients was significantly shorter than that in non-sarcopenic patients. Cox regression analysis showed that sarcopenia was not an independent risk factor for MACCE. This difference in findings might have been caused by the degree of coronary artery lesions, age, sex, comorbid diseases or geriatric syndromes of the patients. Further studies are required to determine this issue.
In treatment of sarcopenia, progressive exercise schemes of at least three times per week (including resistance exercise) can improve physical function of older patients with sarcopenia and frailty, and reduce the occurrence of adverse events, such as falls.[23]–[25] For older patients with CHD and sarcopenia or frailty, a corresponding rehabilitation exercise scheme should be developed according to the cardio-pulmonary function of patients with the assistance of therapists. The therapeutic effects of nutritional interventions, such as supplementation of protein, vitamin D, and antioxidants, on sarcopenia remain controversial. The PROVIDE study was a randomized, double-blind, placebo-controlled study, which enrolled 380 older patients with reduced muscle mass, but self-care in daily life.[26] The active group (n = 184) received a vitamin D and leucine-enriched whey protein nutritional supplement for consumption twice daily for 13 weeks. The control group (n = 196) received an iso-caloric control product for consumption twice daily for 13 weeks. Primary outcomes of handgrip strength and the Short Physical Performance Battery score, and secondary outcomes of the chair-stand test, gait speed, balance score, and appendicular muscle mass were measured at baseline, week 7, and week 13 of the intervention. This previous study showed that handgrip strength and the Short Physical Performance Battery score were improved in both groups without significant between-group differences. The active group improved more in the chair-stand test compared with the control group [between-group effect, 95% CI: −1.01 (−1.77–−0.19), P = 0.018]. The active group gained more appendicular muscle mass than did the control group [between-group effect, 95% CI: 0.17 (0.004–0.338), P = 0.045]. In our study, nutritional-related indices (MNA-SF score, albumin levels, and prealbumin levels) in patients with sarcopenia were significantly lower than those in patients without sarcopenia. Therefore, attention should be paid to the nutritional status of older patients with CHD complicated by decreased physical function. For older patients with CHD complicated by malnutrition and nutritional risk, a nutritional prescription should be provided, and body fitness training should be carried out simultaneously to improve their physical function.
This study was an observational cohort study and proposed no intervention measures for sarcopenia. In future studies, we plan to design a prospective cohort study based on comprehensive geriatric assessment, and focus on intervention of a geriatric interdisciplinary team, including geriatricians, cardiologists, dieticians, therapists, clinical pharmacists, psychologists, and social workers. We aim to investigate the effects of comprehensive geriatric assessment and use of a geriatric interdisciplinary team on prognosis and quality of life of older patients with cardiovascular disease complicated by sarcopenia.
4.1. Limitations
There were several limitations in this study. Firstly, the sample size of this study was small and the follow-up time was relatively short. In the future, the sample size should be further expanded, and the effect of sarcopenia on long-term prognosis of older patients with CHD should be studied. Secondly, the degree of underlying coronary lesions was not included, which may have affected the outcome. Thirdly, because of the small sample size, different types of CHD were not analyzed separately to investigate the effects of sarcopenia on the prognosis of older patients with ACS and stable CHD. Last but not least, secondary prophylactic therapy in CHD and percutaneous intervention/coronary artery bypass grafting treatment between the sarcopenia and non-sarcopenia groups were not analyzed in this study, which should be investigated in the future.
4.2. Conclusions
Our study shows that there is a high prevalence of sarcopenia in hospitalized older adults with CHD. The incidence of unscheduled return visits in sarcopenic older patients with CHD is higher than that in patients without sarcopenia. There is no difference in the incidence of MACCE and all-cause mortality between older patients with CHD and sarcopenia and those without sarcopenia. Sarcopenia is not an independent risk factor of short-term prognosis in older patients with CHD. However, the complication of anxiety and depression is an independent risk factor of unscheduled return visits in older patients with CHD. The MACCE-free survival time in patients with sarcopenia is significantly shorter than that in patients without sarcopenia.
Acknowledgments
This study was supported by the CAMS Innovation Fund for Medical Sciences (CIFMS #2018-I2M-1-002). All authors had no conflicts of interest to disclose.
References
- 1.Li X, Li F, Leng SX. The aging tsunami and senior healthcare development in China. J Am Geriatr Soc. 2018;66:1462–1468. doi: 10.1111/jgs.15424. [DOI] [PubMed] [Google Scholar]
- 2.Shang P, Liu GG, Zheng X, et al. Association between medication adherence and 1-year major cardiovascular adverse events after acute myocardial infarction in China. J Am Heart Assoc. 2019;8:e011793. doi: 10.1161/JAHA.118.011793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen LK, Liu LK, Woo J, et al. Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc. 2014;15:95–101. doi: 10.1016/j.jamda.2013.11.025. [DOI] [PubMed] [Google Scholar]
- 4.Beaudart C, Rizzoli R, Bruyère O, et al. Sarcopenia: burden and challenges for public health. Arch Public Health. 2014;72:45. doi: 10.1186/2049-3258-72-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsekoura M, Kastrinis A, Katsoulaki M, et al. Sarcopenia and its impact on quality of life. Adv Exp Med Biol. 2017;987:213–218. doi: 10.1007/978-3-319-57379-3_19. [DOI] [PubMed] [Google Scholar]
- 6.Kang DO, Park SY, Choi BG, et al. Prognostic impact of low skeletal muscle mass on major adverse cardiovascular events in coronary artery disease: a propensity score-matched analysis of a single center all-comer cohort. J Clin Med. 2019;8:712. doi: 10.3390/jcm8050712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arik G, Varan HD, Yavuz BB, et al. Validation of Katz index of independence in activities of daily living in Turkish older adults. Arch Gerontol Geriatr. 2015;61:344–350. doi: 10.1016/j.archger.2015.08.019. [DOI] [PubMed] [Google Scholar]
- 8.Bier N, Belchior Pda C, Paquette G, et al. The instrumental activity of daily living profile in aging: a feasibility study. J Alzheimers Dis. 2016;52:1361–1371. doi: 10.3233/JAD-150957. [DOI] [PubMed] [Google Scholar]
- 9.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 10.Koren-Hakim T, Weiss A, Hershkovitz A, et al. Comparing the adequacy of the MNA-SF, NRS-2002 and MUST nutritional tools in assessing malnutrition in hip fracture operated elderly patients. Clin Nutr. 2016;35:1053–1058. doi: 10.1016/j.clnu.2015.07.014. [DOI] [PubMed] [Google Scholar]
- 11.Chen TY, Janke MC. Predictors of falls among community-dwelling older adults with cancer: results from the health and retirement study. Support Care Cancer. 2014;22:479–485. doi: 10.1007/s00520-013-2000-7. [DOI] [PubMed] [Google Scholar]
- 12.Fukuda T, Bouchi R, Takeuchi T, et al. Sarcopenic obesity assessed using dual energy X-ray absorptiometry (DXA) can predict cardiovascular disease in patients with type 2 diabetes: a retrospective observational study. Cardiovasc Diabetol. 2018;17:55. doi: 10.1186/s12933-018-0700-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kang SH, Jeong WK, Baik SK, et al. Impact of sarcopenia on prognostic value of cirrhosis: going beyond the hepatic venous pressure gradient and MELD score. J Cachexia Sarcopenia Muscle. 2018;9:860–870. doi: 10.1002/jcsm.12333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakashima Y, Saeki H, Nakanishi R, et al. Assessment of sarcopenia as a predictor of poor outcomes after esophagectomy in elderly patients with esophageal cancer. Ann Surg. 2018;267:1100–1104. doi: 10.1097/SLA.0000000000002252. [DOI] [PubMed] [Google Scholar]
- 15.Caan BJ, Cespedes Feliciano EM, Prado CM, et al. Association of muscle and adiposity measured by computed tomography with survival in patients with nonmetastatic breast cancer. JAMA Oncol. 2018;4:798–804. doi: 10.1001/jamaoncol.2018.0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shachar SS, Williams GR, Muss HB, et al. Prognostic value of sarcopenia in adults with solid tumours: a meta-analysis and systematic review. Eur J Cancer. 2016;57:58–67. doi: 10.1016/j.ejca.2015.12.030. [DOI] [PubMed] [Google Scholar]
- 17.Hu X, Dou WC, Shao YX, et al. The prognostic value of sarcopenia in patients with surgically treated urothelial carcinoma: a systematic review and meta-analysis. Eur J Surg Oncol. 2019;45:747–754. doi: 10.1016/j.ejso.2019.03.003. [DOI] [PubMed] [Google Scholar]
- 18.Feliciano EMC, Kroenke CH, Meyerhardt JA, et al. Association of systemic inflammation and sarcopenia with survival in nonmetastatic colorectal cancer: results from the CSCANS study. JAMA Oncol. 2017;3:e172319. doi: 10.1001/jamaoncol.2017.2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ko BJ, Chang Y, Jung HS, et al. Relationship between low relative muscle mass and coronary artery calcification in healthy adults. Arterioscler Thromb Vasc Biol. 2016;36:1016–1021. doi: 10.1161/ATVBAHA.116.307156. [DOI] [PubMed] [Google Scholar]
- 20.Bahrami H, Budoff M, Haberlen SA, et al. Inflammatory markers associated with subclinical coronary artery disease: the multicenter AIDS cohort study. J Am Heart Assoc. 2016;5:e003371. doi: 10.1161/JAHA.116.003371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tibuakuu M, Zhao D, Saxena A, et al. Low thigh muscle mass is associated with coronary artery stenosis among HIV-infected and HIV-uninfected men: the multicenter AIDS cohort study (MACS) J Cardiovasc Comput Tomogr. 2018;12:131–138. doi: 10.1016/j.jcct.2018.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Watkins LL, Koch GG, Sherwood A, et al. Association of anxiety and depression with all-cause mortality in individuals with coronary heart disease. J Am Heart Assoc. 2013;2:e000068. doi: 10.1161/JAHA.112.000068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mangione KK, Miller AH, Naughton IV. Cochrane review: improving physical function and performance with progressive resistance strength training in older adults. Phys Ther. 2010;90:1711–1715. doi: 10.2522/ptj.20100270. [DOI] [PubMed] [Google Scholar]
- 24.Peterson MD, Rhea MR, Sen A, et al. Resistance exercise for muscular strength in older adults: a meta-analysis. Ageing Res Rev. 2010;9:226–237. doi: 10.1016/j.arr.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Finnegan S, Seers K, Bruce J. Long-term follow-up of exercise interventions aimed at preventing falls in older people living in the community: a systematic review and meta-analysis. Physiotherapy. 2019;105:187–199. doi: 10.1016/j.physio.2018.09.002. [DOI] [PubMed] [Google Scholar]
- 26.Bauer JM, Verlaan S, Bautmans I, et al. Effects of a vitamin D and leucine-enriched whey protein nutritional supplement on measures of sarcopenia in older adults, the PROVIDE study: a randomized, double-blind, placebo-controlled trial. J Am Med Dir Assoc. 2015;16:740–747. doi: 10.1016/j.jamda.2015.05.021. [DOI] [PubMed] [Google Scholar]


