Abstract
Background
Cognitive impairment (CI) increases cardiac mortality among very elderly patients. Percutaneous coronary intervention (PCI) for ischemic heart disease (IHD) patients is considered a favorable strategy for decreasing cardiac mortality. Here, we investigated the influence of CI on cardiac mortality after PCI in very elderly patients.
Methods
We performed a retrospective observational analysis of patients who received PCI between 2012 and 2014 at the South Miyagi Medical Center, Japan. IHD patients over 80 years old who underwent the Mini-Mental State Examination for CI screening during hospitalization and/or who had been diagnosed with CI were included. Participants were divided into CI and non-CI groups, and cardiac mortality and incidence of adverse cardiac events in a 3-year follow-up period were compared between groups. Statistical analyses were performed using the t-test, χ2 test, and multivariable Cox regression analysis, with major comorbid illness and conventional cardiac risk factors as confounders.
Results
Of 565 patients, 95 were included (41 CI, 54 non-CI). Cardiac mortality during the follow-up period was significantly higher in the CI group (36%) compared with the non-CI group (13%) (OR = 4.3, 95% CI: 1.56–11.82, P < 0.05). CI was an independent cardiac prognostic factor after PCI and, for CI patients, living only with a CI partner was an independent predictor of cardiac death within three years.
Conclusions
CI significantly affected cardiac prognosis after PCI in very elderly patients, particularly those living with a CI partner. To improve patients' prognoses, social background should be considered alongside conventional medical measures.
Keywords: Cognitive impairments, Family background, Mortality, Octogenarians, Percutaneous coronary intervention
1. Introduction
Percutaneous coronary intervention (PCI) for very elderly patients with ischemic heart disease (IHD) is considered a favorable therapeutic strategy compared with optimal pharmacological therapy.[1]–[5] However, the development of IHD increases with age, and the prognosis of these patients is poor.[4],[6],[7] One possible reason for this declining prognosis with age is the prevalence of cognitive impairment (CI), which is reported to be present in approximately 20% of patients aged > 80 years, while reported all-cause mortality due to cardiovascular disease, respiratory disease and external causes is higher in CI patients compared with that in non-CI patients.[8]–[10]
For patients over 80 years old, PCI is considered to be favorable for decreasing mortality.[1] However, few studies have examined the association between CI and prognosis in very elderly patients undergoing PCI. Because patients with CI tend to exhibit a range of pathophysiological problems, the evaluation of factors influencing prognosis is challenging.[11],[12] However, it is important to elucidate the factors influencing prognosis after PCI in very elderly patients based on conventional medical and social factors. Clarification of the effects of medical and social background factors on prognosis after PCI, including the presence or absence of CI, presence or absence of comorbid illness, and presence or absence of a domestic partner, as well as conventional cardiac prognostic factors, may contribute to decision-making regarding the most appropriate therapeutic strategy.
In the current study, we sought to identify the effects of prognostic factors in very old CI patients to inform proactive intervention.
2. Methods
This single-center case control study retrospectively analyzed consecutive elderly patients at South-Miyagi Medical Center, Miyagi, Japan, for whom PCI was indicated. This study was approved by the institutional review board of South Miyagi Medical Center and was conducted in accordance with the tenets of the Declaration of Helsinki. All patients provided written informed consent prior to enrollment.
2.1. Study population
We retrospectively analyzed an electronic database of consecutive patients with IHD who underwent PCI at the South-Miyagi Medical Center, Miyagi, Japan from 2012 to 2014. Among these patients, very elderly patients (over 80 years old) were included. We obtained patients' information from the hospital, the outpatient department, and/or the patients' home doctor within the past three years. Patients who underwent both emergent and elective PCI were included in this study. To handle missing data and the loss of follow-up cases, we excluded patients who could not be diagnosed as either CI or non-CI throughout hospitalization, and patients for whom it was not possible to obtain follow-up information at the end of the 3-year period after discharge. Because this was a single center study in a rural area, the social background of the patients may not be representative of the general population.
2.2. Definition of adverse events
We compared all-cause mortality, cardiac mortality, and development of congestive heart failure (CHF) between groups within 1-3 years after PCI (Table 1). Cardiac death was defined as death due to CHF, involving cardiac shock and sudden death. CHF was defined based on the Framingham criteria.[13] According to these criteria, patients who exhibited two major findings or one major and two minor findings were diagnosed with CHF. Cases of probable and questionable CHF were not included. We referred to findings from X-ray examination, echocardiography and laboratory data when we obtained information on adverse cardiac events such as cardiac death or the development of CHF. Furthermore, we obtained information from clinical records, discharge records, death certificate, the home doctor, and telephone calls to the patient or patient's family.
Table 1. Comparison of clinical outcomes between the CI group and non-CI group for three years after PCI with univariate analyses.
| Variables | CI (n = 41) | Non-CI (n = 54) | OR (95% CI) | P-value |
| All-cause mortality | 17 (18%) | 13 (14%) | NS | NS |
| Cardiac mortality | ||||
| Within the 1st year | 12 (29%) | 5 (9%) | 4.1 (1.29–12.67) | < 0.05 |
| Within the 2nd year | 12 (29%) | 6 (11%) | 3.7 (1.27–10.87) | < 0.05 |
| Within the 3rd year | 15 (36%) | 7 (13%) | 4.3 (1.56–11.82) | < 0.05 |
| Adverse cardiac events | 16 (39%) | 9 (17%) | 3.2 (1.23–8.29) | < 0.05 |
CI: cognitive impairment; NS: no significance; OR: odds ratio; PCI: percutaneous coronary intervention.
2.3. Definition of CI
Cognitive impairment was diagnosed at initial admission using the Mini-Mental State Examination (MMSE) during hospitalization for IHD.[14] In the current study, patients with MMSE scores < 20 points were diagnosed with CI. Differential diagnosis regarding the cause of CI, such as Alzheimer's disease or cerebrovascular disease, was not considered in this study. These patients and/or those receiving pharmacological treatment for CI were also enrolled in the CI group without undergoing the MMSE. Note that the MMSE was not administered for all elderly patients, but was performed depending on the physician's decision. Patients who exhibited delirium during hospitalization before and after PCI were excluded because the MMSE was not performed.
2.4. Definition of IHD
Patients who reported severe chest pain and/or showed ST-segment elevation on electrocardiography, and those with significant stenosis or occlusion of a coronary artery, were diagnosed with acute coronary syndrome. Patients who had a history of myocardial infarction (MI) with electrocardiographic evidence of MI and abnormal wall motion on ultrasound echocardiography (UCG) were diagnosed with old MI. Patients who exhibited symptoms (i.e., chest oppression) and/or obvious ischemic findings on electrocardiography, UCG, stress test, and cardiac catheterization were diagnosed with angina pectoris. Asymptomatic patients with positive findings in the aforementioned examinations were diagnosed with silent myocardial ischemia.
2.5. Co-morbidity
Data regarding co-morbidity at the moment of admission were mainly obtained from clinical records. In this study, diseases that were still under medical treatment were considered co-morbidities. Cardiac disease, cerebrovascular disease, respiratory disease, malignant disease, orthopedic disease, physical disability with any cause, and prevalence of conventional cardiac risk factors such as diabetes mellitus, hypertension, dyslipidemia, chronic kidney disease, were considered co-morbidities, as shown in Table 2. This information was obtained from clinical records and/or interviews with patients or their families. Note that recovered illness, such as conditions requiring minor surgical treatment, was found to have little effect in the present study, so is not included in the table.
Table 2. Clinical characteristics of all patients with or without CI.
| Variables | CI (n = 41) | Non-CI (n = 54) | P-value |
| Mean follow-up period, days | 775 ± 495 | 1493 ± 785 | < 0.05 |
| Age, yrs | 85.2 ± 3.4 | 84.3 ± 3.1 | NS |
| Male sex | 23 (52%) | 32 (57%) | NS |
| BMI, kg/m2 | 23.2 ± 4.0 | 22.4 ± 3.6 | NS |
| Emergent PCI | 20 (45%) | 21 (37%) | NS |
| Prevalence of CHF on admission | 20 (45%) | 16 (29%) | NS |
| Comorbidity | |||
| Cardiac disease | 11 (39%) | 7 (18%) | NS |
| AF | 6 (15%) | 4 (7%) | NS |
| Conduction disturbance | 0 (0%) | 3 (6%) | NS |
| IHD | 0 (0%) | 2 (4%) | NS |
| Chronic heart failure | 6 (15%) | 2 (4%) | NS |
| Cerebrovascular disease | 5 (11%) | 4 (4%) | NS |
| Respiratory disease | 2 (4%) | 5 (9%) | NS |
| Malignant disease | 1 (2%) | 1 (2%) | NS |
| Major bleeding | 2 (4%) | 4 (4%) | NS |
| Hypertension | 33 (75%) | 44 (78%) | NS |
| Diabetes | 14 (32%) | 19 (36%) | NS |
| Chronic kidney disease | 23 (57%) | 24 (44%) | |
| Prevalence of physical disability on admission | 20 (50%) | 13 (24%) | < 0.05 |
| Dyslipidemia | 22 (50%) | 42 (75%) | < 0.05 |
| Laboratory data | |||
| Hemoglobin, g/dL | 12.3 ± 1.82 | 12.5 ± 1.69 | NS |
| Lymphocyte subset, % | 21.1 ± 11.1 | 23.5 ± 8.0 | NS |
| Albumin, g/mL | 3.76 ± 0.44 | 3.84 ± 0.53 | NS |
| eGFR, mL/min·1.73 m2 | 56 ± 18.4 | 61.3 ± 21.6 | NS |
| BNP on admission, pg/mL | 402 ± 528 | 277 ± 544 | NS |
| BNP on chronic phase, pg/mL | 426 ± 870 | 105 ± 115 | < 0.05 |
| Serum Na, mEq/L | 140 ± 4.56 | 139 ± 4.23 | NS |
| Cardiac function on admission | |||
| LVEF, % | 55 ± 1.9 | 61 ± 10.7 | < 0.05 |
| LAD, mm | 43 ± 8.4 | 42 ± 6.4 | NS |
| E/A | 0.89 ± 0.52 | 0.71 ± 0.24 | NS |
| LVH (IVS + PW > 21 mm) | 8 (18%) | 8 (14%) | NS |
| Cardiac function at the chronic phase | |||
| LVEF, % | 56 ± 12.9 | 63 ± 9.7 | < 0.05 |
| LAD, mm | 44.3 ± 7.07 | 41 ± 6.8 | NS |
| E/A | 0.69 ± 0.24 | 0.72 ± 0.29 | NS |
| LVH (IVS + PW > 21 mm) | 8 (18%) | 8 (14%) | NS |
| Medication | |||
| Antiplatelet | 42 (95%) | 54 (98%) | NS |
| ACE/ARB | 29 (66%) | 40 (71%) | NS |
| Diuretics | 15 (34%) | 15 (26%) | NS |
| Beta blocker | 24 (54%) | 28 (50%) | NS |
| Anti-aldosterone agent | 10 (22%) | 8 (14%) | NS |
| Statin | 28 (52%) | 42 (75%) | NS |
Data are presented as means ± SD or n (%). ACE: angiotensin-converting enzyme; AF: atrial fibrillation; ARB: angiotensin II receptor blocker; BMI: body mass index; BNP: brain natriuretic peptide; CHF: congestive heart failure; CI: cognitive impairment; eGFR: estimated glomerular filtration rate; IHD: ischemic heart disease; IVS: interventricular septum; LAD: left atrial dimension; LVEF: left ventricular ejection fraction; LVH: left ventricular hypertrophy; NS: no significance; PCI: percutaneous coronary intervention; PW: posterior wall.
2.6. Data characteristics
The physiological and biochemical data were obtained within seven days prior to PCI, except in emergency cases, in which the data were obtained several days after PCI. Diabetes mellitus was defined as hemoglobin A1c > 6.3% according to the standards proposed in the National Glycohemoglobin Standardization Program.[15] Chronic kidney disease was defined according to an estimated glomerular filtration rate < 60 mL/min per 1.73 m2. Hypertension was defined as systolic blood pressure > 140 mmHg during the period of admission or being administered antihypertensive drugs. Dyslipidemia was defined as a low-density lipoprotein cholesterol level of 140 mg/dL or being administered with cholesterol-lowering drugs. The smoking status of patients could not be included in the analysis because of unclear description in the records. The data obtained from UCG findings after PCI, such as left ventricle ejection fraction (LVEF), left atrial dimension (LAD), wall thickness, and the ratio of the early (E) to late (A) ventricular filling velocities, were included in the analyses as indicators of cardiac function. UCG data during the acute and chronic phases were obtained on admission and approximately one year after PCI, respectively.
2.7. Social background
We obtained information from clinical records and/or interviews with patients or their families at the time of admission. The situation of certification of eligibility for nursing care, and the patient's family structure were examined in this study. Family structure was divided into five patterns: living alone, living with a non-CI partner, living with a CI partner, living with children, and living with children and their family.
2.8. Definition of frailty
We obtained information about the progression of frailty from clinical records and/or physical examination after approximately one year after PCI. We referred to clinical records for information regarding frailty.[16] We defined as progression of frailty when the frailty of the patient was changed from vulnerable to mild frailty or higher within one year.
2.9. Statistical analyses
All data were retrospectively analyzed. Continuous data were expressed as mean ± SD, while categorical variables were expressed as numbers and percentages. Comparisons between the two groups were performed using Student's t-tests for continuous variables, and the χ2 test for categorical variables. Following the univariate analyses, multivariable logistic regression analysis was performed by adjusting the factors potentially related to adverse cardiac events with P < 0.1 in the univariate analyses. Not all of the established prognostic factors affecting adverse cardiac events were included as confounding factors when the factor did not reach P < 0.1 in univariate analyses. A level of P < 0.05 in the multivariable logistic regression analysis was considered to indicate statistical significance in all comparisons. All statistical analyses were performed using JMP® 14 software (SAS Institute Inc., Cary, NC, USA).
3. Results
3.1. Patient characteristics
A total of 103 patients of 565 IHD patients who performed PCI from 2012 to 2014 were identified as being over 80 years old. Among the 103 patients, eight patients were excluded because it was not possible to obtain information about their CI or non-CI status throughout hospitalization for delirium (n = 2), or to acquire follow-up information during the 3-year period after PCI (n = 6). Thus, we retrospectively examined a final sample of 95 consecutive patients. The CI and non-CI groups included 41 patients (43%) and 54 patients (57%), respectively. The mean follow-up period was 1134 ± 300 days. The baseline patient characteristics are shown in Table 2. There were no significant differences between groups in terms of co-morbidity, such as cardiac disease, cerebrovascular disease, and conventional cardiac risk factors including hypertension and diabetes, which are possible risk factors for cardiac events. The presence of physical disability due to any cause was higher in the CI group than in the non-CI group. Regarding laboratory data, brain natriuretic peptide (BNP) in the chronic phase was significantly higher in the CI group (BNP: 426 ± 870 pg/mL in the CI group vs. 105 ± 115 pg/mL in the non-CI group, P < 0.05) although the other data showed no significant differences between the groups. Importantly, BNP on admission was no different between groups. According to UCG data on admission, indices of cardiac function were no different, except LVEF (LVEF: 55% vs. 61%, P < 0.05). In the chronic phase (i.e., approximately one year after PCI), LVEF was significantly lower in the CI group versus non-CI group (LVEF: 57% vs. 62%, P < 0.05), and LAD also tended to be larger in the CI group than the non-CI group, although this difference was not significant. In terms of medication, there were no significant differences between the groups.
3.2. Clinical outcomes
Table 1 shows the clinical outcomes. All-cause mortality was not significantly different in the non-CI group versus the CI group. The main causes of death in the non-CI group were fracture, cerebral hemorrhage and pneumonia during 1-3 year follow-up period. In contrast, the main cause of death in the CI group was cardiac death resulting from development of CHF and/or cardiac shock during the 1-3 year follow-up period. The odds ratios (ORs) for one, two, and three years were 4.1 (95% CI: 1.29–12.67, P < 0.05), 3.7 (95% CI: 1.26–10.87, P < 0.05), and 4.3 (95% CI: 1.56–11.83, P < 0.05), respectively (Table 1). Adverse cardiac events (i.e., development of CHF) were also more common in the CI group versus the non-CI group (OR = 3.2, 95% CI: 1.23–8.29). Importantly, no patients expired or developed CHF due to major complications of PCI itself. Figure 1 shows a Kaplan–Meier curve, showing that the difference in cardiac mortality between groups steadily increased throughout the whole follow-up period (log-rank test P < 0.05).
Figure 1. Cardiac mortality after PCI in patients with and without CI.
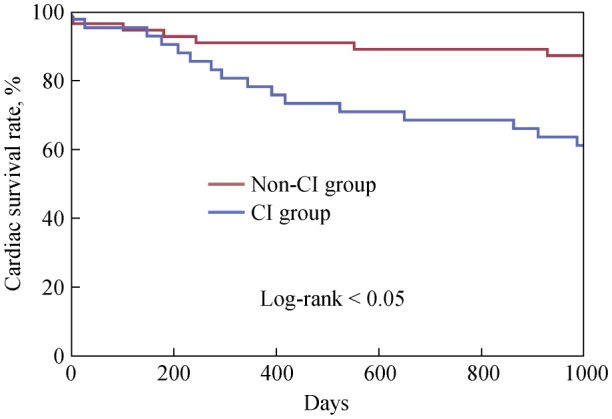
Kaplan-Meier curve comparing cardiac mortality after PCI between CI and non-CI patients in the 3-year follow-up period. CI: cognitive impairment; PCI: percutaneous coronary intervention.
3.3. Progression of frailty between CI and non-CI patients after PCI
We estimated the difference in the progression of frailty within one year after PCI between CI and non-CI patients. We found a higher prevalence of progressed frailty in the CI group compared with the non-CI group: 59% (n = 20 of 34) vs. 36% (n = 18 of 50), respectively, with an OR of 2.5 (95% CI: 1.03–6.21, P < 0.05). Note that, data regarding frailty were obtained from only 83% of CI patients and 92% of non-CI patients.
3.4. Independent predictors of cardiac mortality throughout the whole follow-up period
Subsequently, we evaluated whether CI was an independent predictor of cardiac prognosis using multivariate regression analysis. We selected confounding factors that could potentially influence cardiac prognosis (P < 0.1 in the univariate analyses). Age, comorbidity with cardiac disease, presence of physical disability on admission, presence of CHF on admission, and estimated glomerular filtration rate were selected as confounding factors, as well as the presence of CI. In all patients, CI was an independent prognostic factor of cardiac mortality within three years after PCI (Table 3).
Table 3. Factors influencing cardiac death in all following periods after PCI with multivariate logistic analyses.
| Variables | OR (95% CI) | P-value |
| Prevalence of CI | 3.8 (1.19–12.32) | 0.02 |
| Age | 1.2 (1.19–12.32) | 0.04 |
| eGFR on admission | 1.0 (0.97–1.03) | 0.86 |
| Comorbid with cardiac disease | 2.3 (0.55–9.48) | 0.24 |
| Physical disability on admission | 1.1 (0.30–3.97) | 0.88 |
| Prevalence of CHF on admission | 2.9 (0.92–9.35) | 0.06 |
CHF: congestive heart failure; CI: cognitive impairment; eGFR: estimated glomerular filtration rate; OR: odds ratio; PCI: percutaneous coronary intervention.
3.5. Involvement of social circumstances in cardiac mortality among patients with CI
To explore the factors responsible for the poor cardiac prognosis of CI patients versus non-CI patients, we examined patients' social background, particularly their family structure. Family structures of CI patients were divided into five patterns: living alone, 2% (n = 1); living with a non-CI partner, 24% (n = 10); living with a CI partner, 17% (n = 7); living with children, 15% (n = 6); and living with children and their family or living at a care facility, 41% (n = 17). We found that CI patients living only with a CI partner exhibited higher cardiac mortality rates throughout the whole follow-up period after PCI compared with those living with a non-CI partner, living with children and their family, or living at a care facility [86% in CI patients living with CI partner (n = 6) vs. 29% in patients living with a person who could support the CI patient (n = 10), OR = 14.4 (95% CI: 1.5–135.5, P < 0.05)]. Subsequently, we performed multivariate regression analysis with confounding factors, possibly influencing cardiac death. Age, comorbidity with cardiac disease, physical disability on admission, and BNP on admission were selected as confounding factors through univariate analyses, revealing that living only with a CI partner was an independent prognostic factor for cardiac death in CI patients within a 3-year follow-up period after PCI (Table 4).
Table 4. Factors influencing cardiac death after PCI in all following periods in the CI group with multivariate logistic analyses.
| Variables | OR (95% CI) | P-value |
| Living with CI partner | 30.7 (1.24–763) | 0.03 |
| Age | 1.4 (0.98–2.22) | 0.06 |
| BNP on admission | 1.0 (0.99–1.00) | 0.73 |
| Comorbid with cardiac disease | 9.9 (1.27–77.35) | 0.02 |
| Physical disability on admission | 2.0 (0.32–12.81) | 0.45 |
BNP: brain natriuretic peptide; CI: cognitive impairment; OR: odds ratio; PCI: percutaneous coronary intervention.
4. Discussion
PCI for very elderly patients with IHD has recently been proposed as a favorable therapeutic strategy versus optimal medical therapy. In this retrospective study, we explored the influence of CI on cardiac prognosis after PCI in very elderly patients.
The results revealed five major findings. Firstly, very elderly status among patients with CI was associated with poor cardiac prognosis compared with non-CI patients within three years after PCI. Moreover, CI was an independent cardiac prognostic factor throughout the whole 3-year follow-up period. Secondly, the difference in cardiac mortality between CI and non-CI patients increased over time throughout the whole follow-up period. Thirdly, the incidence of adverse cardiac events after PCI (i.e., development of CHF) was higher in the CI group versus the non-CI group. Fourthly, the difference in cardiac function between groups, which was indicated by UCG findings and BNP values, became larger within one year after PCI. Last but not least, CI patients living with a CI partner after PCI were exposed to a higher risk of cardiac death. In contrast, the prognosis of CI patients living with a large family (i.e., children and grandchildren) was equivalent to that in non-CI patients.
Numerous studies have reported that very elderly patients undergoing PCI are at risk of developing complications, such as major bleeding and cerebral thrombosis.[17],[18] Moreover, very elderly patients often have comorbidities (i.e., chronic illnesses), and the functionality of their cardiovascular and other systems may be compromised.[19],[20] Furthermore, among very elderly patients with IHD, coronary artery lesions are often complicated by severe calcification and/or multi-vessel disease. Therefore, treating physicians are reported to be hesitant regarding performing PCI in very elderly patients.[17]–[20] However, recent improvements in medical devices (i.e., drug-eluting stents) and sophistication of techniques has facilitated the use of PCI. Importantly, it was recently reported that patients with coronary vascular disease treated with PCI were less likely to die because of heart disease.[21]
However, in the current study, cardiac mortality after PCI among CI patients was found to be higher compared with non-CI patients, as shown in Table 1. Regardless of the improvements in the therapeutic system, the present results indicated that cardiac death among very elderly CI patients treated with PCI increased over time. Furthermore, CI was an obvious independent cardiac prognostic factor within a 3-year follow-up period.
Based on these results, we speculate that poor cardiac prognosis after PCI among CI patients may not only be attributed to lesion complexity or the PCI procedure. In the past decade, several studies have emphasized the importance of the relationship between cardiac mortality and frailty. In the current study, the results revealed that the progression of frailty within one year after PCI was substantially more severe among CI patients compared with non-CI patients with an OR of 2.5 (95% CI: 1.03–6.21). In general, very elderly patients with CI have a fragile physical condition, often due to physical disability, insufficient control of co-morbid chronic illness or other illness, and social isolation.[22] These factors can result in insufficient management of nutrition, and consequent proneness to frailty.[23] The causes of frailty are heterogeneous, however, and frailty could be an indicator of multisystem disorders such as chronic inflammation and immune activation, musculoskeletal system disorders, or endocrine system disorders.[24]–[29] In addition, frailty can be suggestive of features of patient's living circumstances. These multisystem disorders may also cause the development of cardiovascular disease. Both frailty and CI have been reported to correlate strongly with higher prevalence of coronary artery disease and/or valvular heart disease, respectively,[19],[21] as found in the current study. This is consistent with the current finding that CI patients with a higher risk of developing frailty, were exposed to a significantly higher risk of cardiac mortality after PCI compared with non-CI patients, and it is conceivable that CI is an independent prognostic factor for cardiac mortality. These results suggest that comprehensive care for preventing frailty as well as the treatment of conventional cardiac risk factors are necessary to reduce cardiac mortality among CI patients after PCI.
In the current study, we focused on patients' social environments, including family structure. An analysis of family structure revealed that very elderly patients with CI after PCI living only with a CI partner were at a higher risk of adverse cardiac events. Importantly, patients with CI living with a large family showed lower mortality compared with those living with a small family. Given that a CI partner may not be able to provide sufficient support for CI patients, these findings suggest the importance of taking into account the comprehensive daily support provided by family members or other individuals as a strong factor influencing the cardiac prognosis of CI patients after PCI. Thus, if a CI patient is thought to have insufficient daily support (i.e., living only with a CI partner, restriction due to financial limitations, or lack of knowledge about the disease causing poor drug adherence) attention must be paid to providing sufficient social support after PCI. As PCI was found to be a favorable strategy for very elderly IHD patients, determining the appropriateness of PCI for very elderly CI patients suspected of having insufficient social support requires further investigation.
For further clarification of influential cardiac prognostic factors after PCI for CI patients, a prospective cohort study involving larger populations of very elderly patients is warranted. Additionally, future studies should examine the influence of social background factors other than family structure on cardiac prognosis in CI patients after PCI.
4.1. Limitations
The current study involved several limitations that should be considered. Firstly, this was a retrospective, observational, single-center study including a small patient population. Secondly, regarding the diagnosis of CI, we obtained information from the clinical records of MMSE, which is a widely used tool for screening global cognition. However, the sensitivity and specificity of the MMSE are not sufficient for determining CI. In addition, we did not independently consider the phenotypes of CI (i.e., Alzheimer's disease, dementia with Lewy bodies, and vascular dementia). Therefore, it is necessary for future studies to evaluate CI using diversified methods. Thirdly, we did not determine the prevalence of frailty one year after PCI for all patients, because in 2012–2014, frailty was not regarded as an important factor for cardiac mortality among cardiologists. Thus, we did not directly examine the prevalence of frailty for very elderly patients on admission and/or in the chronic phase. To address this issue, prospective studies are required to evaluate the phenotype (i.e., cognitive or physical) and severity of frailty. Fourthly, we were unable to acquire data regarding the nutritional status of patients using physical findings or laboratory data. Patients with CI may exhibit insufficient nutrition, resulting in frailty that could lead to death.[30],[31] Therefore, it will be important for future studies to examine the correlation between nutrition and cardiac prognosis after PCI. Last but not least, social background, including family structure, was evaluated on admission, but these factors may change during the follow-up period. Further studies will be needed to elucidate the effect of changes in social background, including family structure.
4.2. Conclusions
The current results suggest that CI is an important cardiac prognostic factor after PCI among very elderly patients and family support is necessary to improve cardiac prognosis. Prior to performing PCI, cardiologists should consider the social background of very elderly patients with CI, as well as conventional measures of the indication of PCI, maximizing the likelihood of improving cardiac prognosis in very elderly patients with CI.
Acknowledgments
The authors thank the medical staff at the South-Miyagi Medical Center, Miyagi, Japan for their cooperation in the present study. All authors had no conflicts of interest to disclose.
References
- 1.Tegn N, Abdeinoor M, Aaberge L, et al. Invasive versus conservative strategy in patients aged 80 years or older with non-ST-elevation myocardial infarction or unstable angina pectoris (After Eighty study): an open-label randomized controlled trial. Lancet. 2016;387:1057–1065. doi: 10.1016/S0140-6736(15)01166-6. [DOI] [PubMed] [Google Scholar]
- 2.Shanmugam VB, Harper R, Meredith I, et al. An overview of PCI in the very elderly. J Geriatr Cardiol. 2015;12:174–184. doi: 10.11909/j.issn.1671-5411.2015.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arisha MJ, Ibrahim DA, Abouarab AA, et al. Percutaneous coronary intervention in the elderly: current updates and trends. Vessel Plus. 2018;2:14. [Google Scholar]
- 4.Johnman C, Oldroyd KG, Mackay DF, et al. Percutaneous coronary intervention in the elderly: changes in case-mix and periprocedural outcomes in 31758 patients treated between 2000 and 2007. Circ Cardiovasc Interv. 2010;3:341–345. doi: 10.1161/CIRCINTERVENTIONS.109.928705. [DOI] [PubMed] [Google Scholar]
- 5.Schwarz AK, Zahn R, Hochadel M, et al. Age-related differences in antithrombotic therapy, success rate and in-hospital mortality in patients undergoing percutaneous coronary intervention: results of the quality control registry of the Arbeitsgemeinschaft Leitende Kardiologische Krankenhausärzte (ALKK) Clin Res Cardiol. 2011;100:773–780. doi: 10.1007/s00392-011-0311-6. [DOI] [PubMed] [Google Scholar]
- 6.An J, Li H, Tang Z, et al. Cognitive impairment and risk of all-cause and cardiovascular disease mortality over 20-year follow-up: results from the BLSA. J Am Heart Assoc. 2018;7:e008252. doi: 10.1161/JAHA.117.008252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O'Donnell M, Teo K, Gao P, et al. Cognitive impairment and risk of cardiovascular events and mortality. Eur Heart. 2012;33:1777–1786. doi: 10.1093/eurheartj/ehs053. [DOI] [PubMed] [Google Scholar]
- 8.Bae JB, Han JW, Kwak KP, et al. Impact of mild cognitive impairment on mortality and cause of death in the elderly. J Alzheimers Dis. 2018;64:607–616. doi: 10.3233/JAD-171182. [DOI] [PubMed] [Google Scholar]
- 9.Dardiotis E, Giamouzis G, Mastrogiannis D, et al. Cognitive impairment in heart failure. Cardiol Res Pract. 2012;2012:595821. doi: 10.1155/2012/595821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cacciatore F, Abete P, Ferrara N, et al. Congestive heart failure and cognitive impairment in an older population. J Am Geriatr Soc. 1998;46:1343–1348. doi: 10.1111/j.1532-5415.1998.tb05999.x. [DOI] [PubMed] [Google Scholar]
- 11.Leto L, Feola M. Cognitive impairment in heart failure patients. J Geriatr Cardiol. 2014;11:316–328. doi: 10.11909/j.issn.1671-5411.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rise IV, Haro JM, Gjervan B, et al. Clinical features, comorbidity, and cognitive impairment in elderly bipolar patients. Neuropsychiatr Dis Treat. 2016;12:1203–1213. doi: 10.2147/NDT.S100843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McKee PA, Castelli WP, McNamara PM, et al. The natural history of congestive heart failure: The Framingham study. N Engl J Med. 1971;285:1441–1446. doi: 10.1056/NEJM197112232852601. [DOI] [PubMed] [Google Scholar]
- 14.Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 15.Little RR, Rohlfing CL, Wiedmeyer HM, et al. The national glycohemoglobin standardization program: a five-year progress report. Clin Chem. 2001;47:1985–1992. [PubMed] [Google Scholar]
- 16.Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173:489–495. doi: 10.1503/cmaj.050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnman C, Oldroyd KG, Mackay DF, et al. Percutaneous coronary intervention in the elderly. Circ Cardiovasc Interv. 2010;3:341–345. doi: 10.1161/CIRCINTERVENTIONS.109.928705. [DOI] [PubMed] [Google Scholar]
- 18.Shanmugasundaram M. Percutaneous coronary intervention in elderly patients: is it beneficial? Tex Heart Inst J. 2011;38:398–403. [PMC free article] [PubMed] [Google Scholar]
- 19.Veronese N, Sigeirsdottir K, Eiriksdottir G, et al. Frailty and risk of cardiovascular diseases in older persons: the age, gene/environment susceptibility-reykjavik study. Rejuvenation Res. 2017;20:517–524. doi: 10.1089/rej.2016.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wennberg DE, Malenka DJ, Sengupta A, et al. Percutaneous transluminal coronary angioplasty in the elderly: epidemiology, clinical risk factors, and in-hospital outcomes. Am Heart J. 1999;137:639–645. doi: 10.1016/s0002-8703(99)70216-4. [DOI] [PubMed] [Google Scholar]
- 21.Gerber RT, Arri SS, Mohamed MO, et al. Age is not a bar to PCI: insights from the long-term outcomes from off-site PCI in a real-world setting. J Interv Cardiol. 2017;30:347–355. doi: 10.1111/joic.12400. [DOI] [PubMed] [Google Scholar]
- 22.Fried LP, Ferrucci L, Darer J, et al. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol A Biol Sci Med Sci. 2004;59:255–263. doi: 10.1093/gerona/59.3.m255. [DOI] [PubMed] [Google Scholar]
- 23.Steptoe A, Shankar A, Demakakos P. Social isolation, loneliness, and all-cause mortality in older men and women. Proc Natl Acad Sci USA. 2013;110:5797–5801. doi: 10.1073/pnas.1219686110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vellas B, Sourdet S. Prevention of frailty in aging. J Frailty Aging. 2017;6:174–177. doi: 10.14283/jfa.2017.42. [DOI] [PubMed] [Google Scholar]
- 25.Robertson DA, Savva GM, Kenny RA. Frailty and cognitive impairment: a review of the evidence and causal mechanisms. Aging Res Rev. 2013;12:840–851. doi: 10.1016/j.arr.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 26.Chen X, Mao G, Leng SX. Frailty syndrome: an overview. Clin Inter Aging. 2014;9:433–441. doi: 10.2147/CIA.S45300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murali-Krishnan R, Iqbel J, Rowe R, et al. Impact of frailty on outcomes after percutaneous coronary intervention: a prospective cohort study. Open Heart. 2015;2:e000294. doi: 10.1136/openhrt-2015-000294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kleipol EE, Hoogendijk EO, Trappemburg MC, et al. Frailty in older adults with cardiovascular disease: cause, effect or both? Aging Dis. 2018;9:489–497. doi: 10.14336/AD.2017.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hao Q, Dong B, Yang M, et al. Frailty and cognitive impairment in predicting mortality among oldest-old people. Front Aging Neurosci. 2018;10:295. doi: 10.3389/fnagi.2018.00295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cruz-Jentoft AJ, Banhat G, Bauer J, et al. Sarcopenia: revised European consensus definition and diagnosis. Age Ageing. 2019;48:16–31. doi: 10.1093/ageing/afy169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vellas B, Fielding R, Bhasin S, et al. Sarcopenia trials in specific diseases: report by the International Conference on Frailty and Sarcopenia Research Task Force. J Frailty Aging. 2016;5:194–200. doi: 10.14283/jfa.2016.110. [DOI] [PubMed] [Google Scholar]


