Abstract
Objectives
The software “SyMRI” generates different MR contrasts and characterizes tissue properties based on a single acquisition of a multi-dynamic multi-echo (MDME)-FLAIR sequence. The aim of this study was to assess the applicability of “SyMRI” in the assessment of myelination in preterm and term-born neonates. Furthermore, “SyMRI” was compared with conventional MRI.
Methods
A total of 30 preterm and term-born neonates were examined at term-equivalent age using a standardized MRI protocol. MDME sequence (acquisition time, 5 min, 24 s)–based post-processing was performed using “SyMRI”. Myelination was assessed by scoring seven brain regions on quantitative T1-/T2-maps, generated by “SyMRI” and on standard T1-/T2-weighted images, acquired separately. Analysis of covariance (ANCOVA) (covariate, gestational age (GA) at MRI (GAMRI)) was used for group comparison.
Results
In 25/30 patients (83.3%) (18 preterm and seven term-born neonates), “SyMRI” acquisitions were successfully performed. “SyMRI”-based myelination scores were significantly lower in preterm compared with term-born neonates (ANCOVA: T1: F(1, 22) = 7.420, p = 0.012; T2: F(1, 22) = 5.658, p = 0.026). “SyMRI”-based myelination scores positively correlated with GAMRI (T1: r = 0.662, n = 25, p ≤ 0.001; T2: r = 0.676, n = 25, p ≤ 0.001). The myelination scores based on standard MRI did not correlate with the GAMRI. No significant differences between preterm and term-born neonates were detectable.
Conclusions
“SyMRI” is a highly promising MR technique for neonatal brain imaging. “SyMRI” is superior to conventional MR sequences in the visual detection of delayed myelination in preterm neonates.
Key Points
• By providing multiple MR contrasts, “SyMRI” is a time-saving method in neonatal brain imaging.
• Differences concerning the myelination in term-born and preterm infants are visually detectable on T1-/T2-weighted maps generated by “SyMRI”.
• “SyMRI” allows a faster and more sensitive assessment of myelination compared with standard MR sequences.
Electronic supplementary material
The online version of this article (10.1007/s00330-019-06325-2) contains supplementary material, which is available to authorized users.
Keywords: Magnetic resonance imaging, Brain, Newborn, Gestational age, Software
Introduction
Myelin forms a spiral layer around the nerve fibers in the central nervous system [1]. Myelination is the structural basis for fast transmission of information, and is therefore, integral in developmental and neurobiological processes [1, 2]. The myelin sheath is composed of several layers that encompass different biochemical substances, such as lipids and proteins. Above all, the cholesterol fraction allows the detection of myelin in magnetic resonance imaging (MRI) sequences [3–5]. Brain myelination proceeds step-by-step and essential developmental stages can be recorded by MRI [6–8]. Thus, myelin can be visualized on conventional T1- and T2-contrasts and serves as a non-invasive imaging biomarker for brain maturation. Preterm birth interferes with the process of white matter maturation [9, 10], resulting in a delay of myelination [11]. Neonatal brain MRI is sensitive for the prognostic assessment of subtle cerebral pathologies of preterm neonates [12–14]. However, conventional MRI is a highly time-consuming procedure and currently lacks diagnostic sensitivity for the assessment of myelination.
Quantitative T1- and T2-mapping approaches allow the direct estimation of relaxation parameters of specific tissue types [15–18]. In these tissue-specific maps, disturbing influences of T2-weighted signals on T1-weighted images are eliminated and vice versa, which facilitates neuroradiological assessment [19–23]. As it has been demonstrated in children aged 3 months and older, quantitative maps may allow a highly accurate assessment of brain myelination compared with conventional MR images [24]. However, multi-echo mapping sequences have required acquisition times beyond 10 min, thus limiting their application in a clinical neonatal imaging setting [19, 24, 25].
The MR data post-processing software “SyMRI”, combined with a multi-dynamic multi-echo (MDME)-FLAIR sequence, enables the acquisition of different MR contrasts within a vastly shortened examination time compared with standard MRI sequences [26–28]. Moreover, this technique offers the possibility to exhibit and further define myelination through the automated calculation of myelin fractions [26, 29]. Using the quantitative MDME sequence, specific parameters, such as the T1-relaxation constants, the T2-relaxation constants, as well as the proton density (PD) of the examined tissue, can be quantified [16, 28, 30–35]. The MDME sequence acquires all the required parameters for image post-processing in less than 6 min [29]. Extrinsic scan parameters, such as the repetition time (TR), echo time (TE), and inversion time (TI), are not predefined in this procedure, as these factors can be defined and modulated retrospectively [27, 31]. Once intrinsic tissue parameters are acquired and extrinsic scan parameters are defined, “SyMRI” provides T1-weighted, T2-weighted, PD-weighted, as well as inversion recovery contrasts within less than 1 min [29]. Hence, “SyMRI” is advantageous when multiple MR contrasts are needed, which is common in clinical routine. It has been shown recently that image data generated by “SyMRI” are comparable with conventional T1- and T2-images [27, 30]. Apart from conventional MR contrasts, “SyMRI” allows to generate quantitative MR maps within a few seconds [29]. The aim of this study was to evaluate the feasibility of “SyMRI” in the assessment of myelination. For this purpose, we compared brain myelination in preterm and term-born neonates based on visual assessment on conventional MR images and quantitative maps, generated by “SyMRI”. In addition, visual neuroradiological assessment of myelination based on “SyMRI” and conventional T1- and T2-weighted images was compared in a cohort of preterm and term-born neonates.
Materials and methods
Ethical approval
The local Ethics Commission approved the protocol of this study, which was performed in accordance with the Declaration of Helsinki.
Study cohort
A total of 30 preterm and term-born neonates were examined at the Neuroradiology Department of a tertiary care hospital between June 2017 and June 2018. All newborns imaged in this study were referred for MRI examination by the Neonatology Department, Intensive Care Unit. General indications for neonatal MRI included extreme premature delivery (prior to 28 weeks (w) of gestation), intraventricular hemorrhage, hypoxic-ischemic encephalopathy, and epileptic seizures. Table 1 gives a detailed overview of the clinical data of the subjects included in this study. In the majority of cases, premature infants were examined at approximately term-equivalent age. In contrast, term-born neonates were studied post-natally between 2 days and 48 days post-partum. The gestational age (GA) at MRI (GAMRI) indicates the total of GA and post-partum period up to the date of MRI. In terms of preterm neonates, GAMRI is used synonymously with term-equivalent age in this study. The clinical data of the subjects were retrospectively obtained using the electronic patient management system of the hospital.
Table 1.
Participant demographics
| Neonates | |
|---|---|
| n = 25 | |
| Term-born | |
| n = 7 | |
| Clinical characteristics | |
| Male/female | 1/6 |
| GA (w)a | 39 + 6, SD = 1 + 2 |
| Post-natal period to MRI (d)b | 17.3, SD = 18.3 |
| GAMRI (w)a | 42 + 2, SD = 2 + 2 |
| Clinical diagnosis | |
| Inconspicuousc | n = 4 |
| Expired infarctionc | n = 1 |
| Subarachnoid hemorrhage/hypoxiac | n = 1 |
| Subdural/subarachnoid hemorrhagec | n = 1 |
| Preterm | |
| n = 18 | |
| Clinical characteristics | |
| Male/female | 9/9 |
| GA (w)a | 25 + 4, SD = 1 + 6 |
| Post-natal period to MRI (d)b | 94, SD = 27.3 |
| GAMRI (w)a | 38 + 1, SD = 2 + 6 |
| Clinical diagnosis | |
| Inconspicuousc | n = 10 |
| Microbleeding (cerebellar)c | n = 4 |
| Intraventricular hemorrhage (grade I)c,d | n = 1 |
| Intraventricular hemorrhage (grade II)c,d | n = 1 |
| Intraventricular hemorrhage (grade III/IV)c,d | n = 2 |
aData represented as mean (weeks (w)) and standard deviation (SD)
bData represented as mean (days (d)) and standard deviation (SD)
cData represented as total number
dAccording to Deutsche Gesellschaft fuer Ultraschall in der Medizin (DEGUM) criteria
GA gestational age, GAMRI gestational age at MRI, MRI magnetic resonance imaging
Image acquisition, MDME sequence, and post-processing
Infants were fed or slightly sedated (chloral hydrate, 30 mg/kg to 60 mg/kg or chloral hydrate, 30 mg/kg combined with midazolam, 0.1 mg/kg) prior to the MRI examination and bedded in a vacuum mattress to prevent movement artifacts. All neonates were examined in the same Philips Ingenia 1.5-T MR system using a standardized neonatal MRI protocol, which consisted of an axial T1 spin echo (SE) sequence, a T2 turbo spin echo (TSE) sequence (in three orthogonal planes), a diffusion-weighted imaging (DWI) sequence, a susceptibility-weighted imaging (SWI) sequence, and a T1 3D sequence. An MDME sequence (one plane) was also acquired, using two repeated acquisition phases [29, 36]. First phase: one slice was saturated by a slice-selective saturation pulse (flip angle, 120°). Second phase: a train of spin echoes was generated for another slice by a series of slice-selective refocusing pulses (flip angle, 180°) and a slice-selective excitation pulse (flip angle, 90°) [28, 29, 36]. The mismatch between the image slice and the saturated slice allowed to acquire a matrix with variable effects of both relaxation rates [29, 36]. T1- and T2-relaxation parameters were estimated by echo trains characterized by different saturation delays [28, 29, 36]. Based on the estimated T1-relaxation constants, the local B1 field was calculated, which was used to correct effects of flip angle deviations [29]. Longitudinal and transverse relaxation parameters as well as B1 also allow to retrieve the unsaturated magnetization, which is needed to calculate the PD [28]. Based on the acquired parameters, “SyMRI” (Synthetic MR AB, Version 8.0.4) was applied to generate quantitative T1- and T2-maps. The generation of quantitative maps was based on the assignment of voxels characterized by ascertained relaxation parameters to a tissue that showed corresponding relaxation parameters [29]. Hence, voxels were assigned according to their relaxation constants to unmyelinated gray and white matter, myelinated structures, as well as cerebrospinal fluid (CSF). A color-coding according to the T1- and T2-relaxation constants allowed an identification of the different tissues, primarily myelin (Supplementary Figure 1). Technical information about the individual sequences are shown in Table 2.
Table 2.
Neonatal MRI protocol and technical data
| Sequence | Plane | FOV (mm) | Voxel size (mm) | Matrix (slices) | TE (ms) | TR (ms) | AT |
|---|---|---|---|---|---|---|---|
| 2D T1 SE | Transversal | 120 × 120 × 90 | 0.83 × 1.05 × 3 | 144 × 115 × 30 | 15 | 400 | 3:07 |
| 2D T2 TSE | Transversal | 120 × 120 × 102 | 0.94 × 1.06 × 3 | 128 × 113 × 34 | 140 | 3000 | 1:48 |
| 2D T2 TSE | Coronal | 110 × 110 × 108 | 0.94 × 1.06 × 3 | 116 × 103 × 36 | 140 | 3000 | 1:48 |
| 2D T2 TSE | Sagittal | 120 × 120 × 108 | 0.94 × 1.06 × 3 | 128 × 113 × 36 | 140 | 3000 | 1:48 |
| 2D DWI | Transversal | 200 × 200 × 92 | 1.14 × 1.15 × 3 | 176 × 170 × 28 | 90 | 4066 | 1:34 |
| 2D SWI | Transversal | 170 × 139 × 90 | 0.85 × 1 × 2 | 200 × 138 × 90 | 12 | 51 | 3:35 |
| 3D T1 | Sagittal | 120 × 120 × 99 | 0.75 × 0.75 × 2 | 160 × 160 × 99 | 7.6 | 25 | 3:46 |
| 2D MDME | Transversal | 200 × 165 × 109 | 0.9 × 1 × 4 | 224 × 159 × 22 | 13 | 3309 | 5:24 |
AT acquisition time, DWI diffusion-weighted imaging, FOV field of view, MDME multi-dynamic multi-echo-FLAIR sequence, SE spin echo, SWI susceptibility-weighted imaging, TE echo time, TR repetition time, TSE turbo spin echo
Myelin score and myelin assessment
To determine myelination, we developed a myelin total score (MTS), based on existing MR myelination scores [6, 8, 37]. A total of seven brain regions were evaluated for myelination on axial T1- and T2-imaging data: medulla oblongata, mesencephalon, thalamus, internal capsule, optic tract, frontal lobe (cortical and subcortical white matter), and central region (cortical and subcortical white matter). Based on existing myelination scores, a minimum of zero and a maximum of four points were allocated per region [6, 8, 37, 38]. Criteria about how the points for myelin assessment were allocated are shown in Table 3 and supplementary Figure 1. Regions of interest placement are shown in supplementary Figure 2, based on conventional T1-weighted images. Myelin assessment was performed in the right hemisphere by default. In case of a right-sided pathology, myelin evaluation was performed in the left hemisphere. Subsequently, the values of the individual brain areas were totaled, resulting in MTS values for T1- and T2-imaging data. The myelin assessment was performed by two experienced and independent neuroradiologists (rater 1, 15 years of experience with neonatal MRI and rater 2, 6 years of experience with neonatal MRI), who were blinded to the GAMRI and GA of the neonates. Myelination was evaluated on both conventional T1-weighted images (T1 SE/T1 3D sequence)/T2-weighted images, as well as on quantitative T1-/T2-maps, generated by “SyMRI”. During myelin assessment, rater 1 performed a critical visual review of the image data. Neonates were excluded from the study if myelin assessment was not possible, for instance due to motion artifacts.
Table 3.
Criteria for the assessment of myelination
| Myelination score per region | T1-imagea | T2-imageb | T1-mapc | T2-mapd |
|---|---|---|---|---|
| 0 | Slightly hypointense/isointense to surrounding tissue | Isointense to surrounding tissue | Color-coding corresponding to a T1-relaxation constante of 1750 to 2000 ms | Color-coding corresponding to a T2-relaxation constantf of 175 to 200 ms |
| 1 | Slightly hyperintense to surrounding tissue/hyperintense to CSF | Slightly hypointense to surrounding tissue/hypointense to CSF | Color-coding corresponding to a T1-relaxation constante of 1500 to 1750 ms | Color-coding corresponding to a T2-relaxation constantf of 150 to 175 ms |
| 2 | Hyperintense to surrounding tissue/clearly hyperintense to CSF | Hypointense to surrounding tissue/clearly hypointense to CSF | Color-coding corresponding to a T1-relaxation constante of 1250 to 1500 ms | Color-coding corresponding to a T2-relaxation constantf of 125 to 150 ms |
| 3 | Clearly hyperintense to surrounding tissue/considerably hyperintense to CSF | Clearly hypointense to surrounding tissue/considerably hypointense to CSF | Color-coding corresponding to a T1-relaxation constante of 1000 to 1250 ms | Color-coding corresponding to a T2-relaxation constantf of 100 to 125 ms |
| 4 | Considerably hyperintense to surrounding tissue | Considerably hypointense to surrounding tissue | Color-coding corresponding to a T1-relaxation constante of < 1000 ms | Color-coding corresponding to a T2-relaxation constantf of < 100 ms |
Statistical analyses
Preterm and term-born neonates were divided into two groups for comparison. Based on the generally accepted division in preterm and term-born newborns, infants included in this study were allocated according to the GA at the time of birth. Thus, all neonates born < 37 w of gestation were allocated to the preterm neonate group, and subjects born ≥ 37 w were allocated to the term-born neonate group [39]. Statistical analyses were performed using SPSS Statistics for Macintosh, Version 25.0 (IBM Corp, 2017) and XLSTAT 2017, Version 20.5 at a significance level of alpha (α) = 5% (p < 0.05). Graphs were created using XLSTAT 2017, Version 20.5. In order to detect concordances of the myelin measurements and the calculated MTS values of both raters, an intra-class correlation (ICC) analysis was performed. ICC values of 0.75 or above were considered a strong correlation [40]. In case of high concordance, the results of rater 1 are shown. To assess correlations between the MTS and the GAMRI, a Pearson’s correlation analysis was performed. In order to detect statistical differences between the groups, an analysis of covariance (ANCOVA) (covariate, GAMRI) was performed. Preterm and term-born neonate groups were compared using the corresponding MTS, which was assessed by both raters based on conventional T1-/T2-weighted images, as well as on quantitative T1-/T2-maps, generated by “SyMRI” by both raters. Thus, preterm and term-born neonates were compared eight times in total; rater 1: preterm versus (vs.) term-born neonates based on conventional T1- and T2-images, preterm vs. term-born neonates based on quantitative T1- and T2-maps; rater 2: preterm vs. term-born neonates based on conventional T1- and T2-images, preterm vs. term-born neonates based on quantitative T1- and T2-maps.
Results
Feasibility of the application of the MR post-processing software “SyMRI”
After careful visual review, non-motion-degraded image data were suitable for “SyMRI”-based post-processing in 25/30 subjects (83.3%), 18 preterm (mean GA, 25 + 4 w, SD = 1 + 6 w; mean GAMRI, 38 + 1 w, SD = 2 + 6 w) and seven term-born neonates (mean GA, 39 + 6 w, SD = 1 + 2 w; mean GAMRI, 42 + 2 w, SD = 2 + 2 w). In 5/30 subjects (one preterm and four term-born neonates), myelin assessment using “SyMRI” was not possible; in 1/5 subjects, image quality was too poor for inclusion due to movement artifacts, 3/5 subjects were excluded due to bilateral pathological tissue devastation and 1/5 was excluded due to both poor image quality caused by movement artifacts and a highly devastated brain anatomy. Quantitative T1- and T2-maps of preterm and term-born neonates are shown in Figs. 1 and 2.
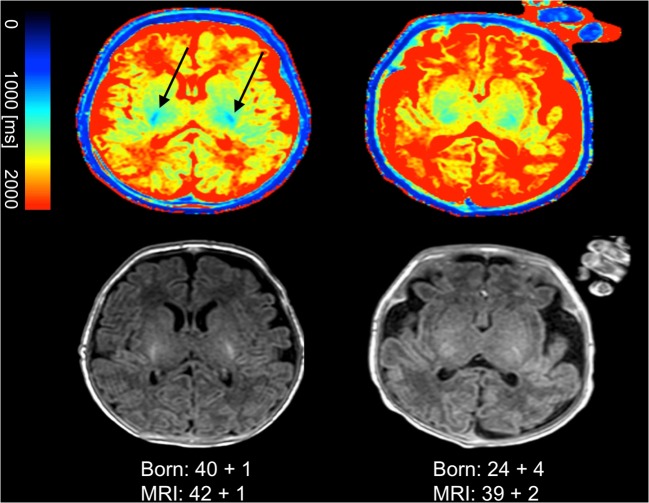
Fig. 1.
T1-maps, generated by “SyMRI”, are shown in the upper row. Conventional T1-images are shown in the bottom row. The left column shows the quantitative T1-map and the conventional image of a term-born neonate. The right column shows the quantitative T1-map and the conventional T1-image of a former premature infant. T1-relaxation constants are represented by the colored bar. The bluish signal in the posterior limb of the internal capsule in the term-born infant indicates myelin (arrows). The corresponding signal is absent in the premature infant. The color-coding of the tissue in the quantitative maps enables an easier distinction between preterm and term-born neonates compared with conventional images
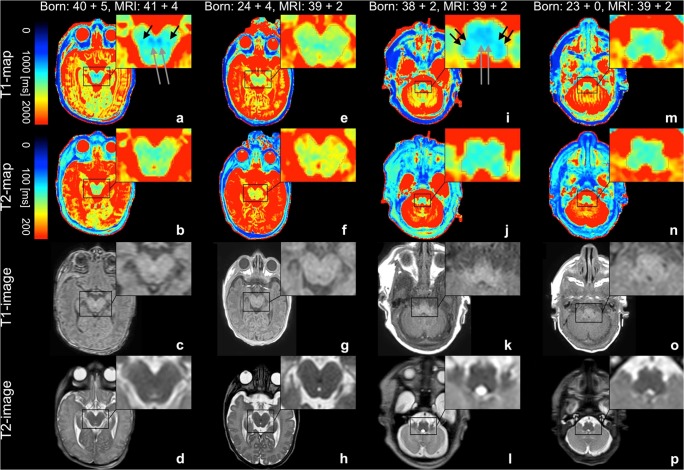
Fig. 2.
Quantitative T1-/T2-maps are shown in the upper row. T1- and T2-relaxation constants are represented by the colored bars. Conventional T1-/T2-weighted images are shown in the bottom row. a–d and i–l Data from a term-born infant. e–h and m–p Data from a former premature infant. The quantitative T1-map of the midbrain of the term-born neonate (a) shows a distinct myelination of the brachium conjunctivum (long arrows) and medial lemniscus (short arrows). A corresponding signal is far less detectable on the T2-map (b) and completely absent in the preterm neonate (e, f). Conventional MR images of the midbrain are shown for comparison (c, d, g, h). The quantitative T1-map of the medulla oblongata of the term-born neonate (i) shows a distinct myelination of the inferior cerebellar peduncle (short double arrows) and medial lemniscus (long double arrow). A corresponding signal is far less detectable on the T2-map (j) and completely absent in the preterm neonate (m, n). Conventional MR images of the medulla oblongata are shown for comparison (k, l, o, p)
Interrater statistics
There was a high degree of concordance between the T1 and the T2 MTS values assessed by both raters on quantitative MR maps, generated by the MR data post-processing software “SyMRI”: the average measured ICC for the T1 MTS was 0.866 with a 95% confidence interval from 0.696 to 0.941 (F(24, 24) = 7.463, p ≤ 0.001); the average measured ICC for the T2 MTS was 0.810 with a 95% confidence interval from 0.568 to 0.916 (F(24, 24) = 5.251, p ≤ 0.001). There was no concordance between the T1 and the T2 MTS values assessed by both raters on conventional MR images: the average measured ICC for the T1 MTS was 0.353 with a 95% confidence interval from − 0.468 to 0.715 (F(24, 24) = 1.546, p = 0.146); the average measured ICC for the T2 MTS was 0.386 with a 95% confidence interval from − 0.393 to 0.729 (F(24, 24) = 1.629, p = 0.120).
Pearson’s correlation analysis
The MTS based on quantitative maps showed a positive correlation (Fig. 3) with the GAMRI (T1: r = 0.662, n = 25, p ≤ 0.001; T2: r = 0.676, n = 25, p ≤ 0.001). The MTS based on standard T1-/T2-weighted images did not correlate (Fig. 3) with the GAMRI (rater 1: T1: r = 0.181, n = 25, p = 0.386; T2: r = 0.259, n = 25, p = 0.211/rater 2: T1: r = 0.337, n = 25, p = 0.100; T2: r = 0.199, n = 25, p = 0.341).
Fig. 3.
Pearson’s correlation between GAMRI and the T1 (left)/T2 (right) MTS calculated by rater 1. T1-map: r = 0.662, n = 25, p ≤ 0.001; T2-map: r = 0.676, n = 25, p ≤ 0.001. T1-image: r = 0.181, n = 25, p = 0.386; T2-image: r = 0.259, n = 25, p = 0.211
Preterm vs. term-born neonates
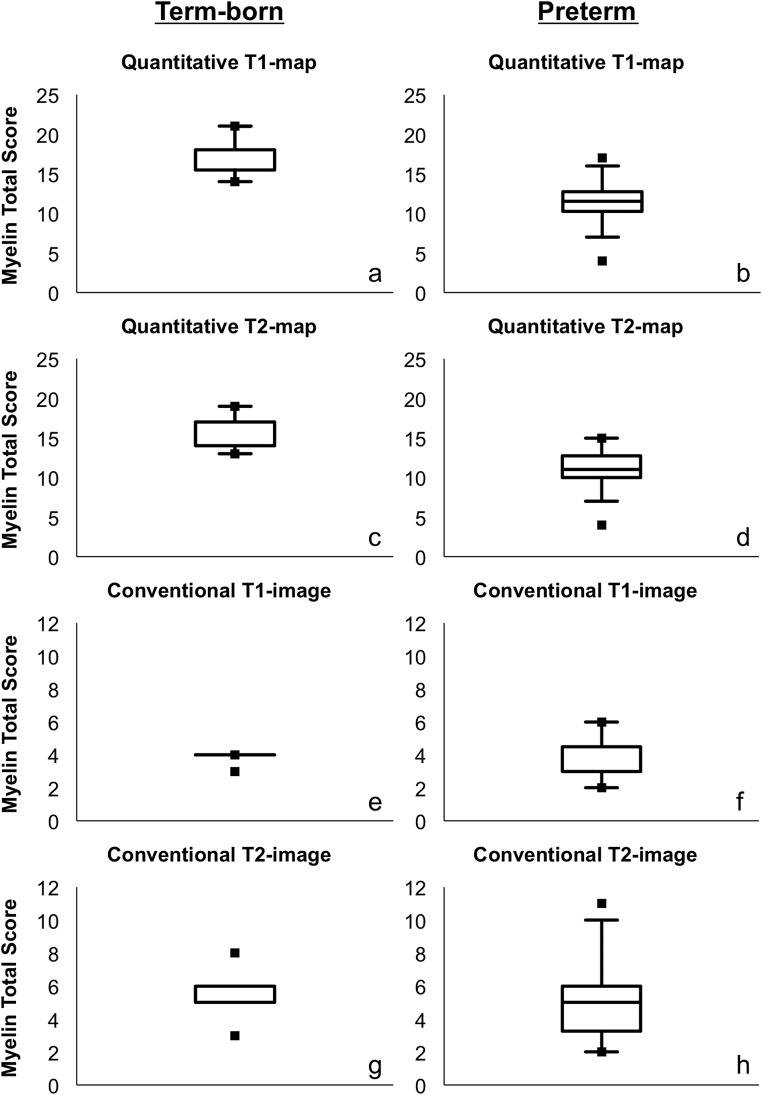
MTS values based on quantitative maps were significantly lower in preterm compared with term-born neonates (T1: F(1, 22) = 7.420, p = 0.012; T2: F(1, 22) = 5.658, p = 0.026). No significant differences between preterm and term-born neonates were detectable based on conventional T1-/T2-images (rater 1: T1: F(1, 22) = 0.116, p = 0.736; T2: F(1, 22) = 0.066, p = 0.799/rater 2: T1: F(1, 22) = 1.141, p = 0.297; T2: F(1, 22) = 0.045, p = 0.834). Descriptive data for the MTS values are shown in Fig. 4 and supplementary Table 1.
Fig. 4.
Data from term-born infants are shown in the left column (a, c, e, g). The right column shows data from preterm infants (b, d, f, h). The boxplots show descriptive data for the MTS values of quantitative T1-/T2-maps (a–d) and conventional T1-/T2-weighted images (e–h) assessed by rater 1. Detailed information on the descriptive data is shown in supplementary Table 1
Discussion
Prematurity may be associated with diminished cognitive abilities in up to 35% [41–43] of patients. Myelination positively correlates with higher neurocognitive functions, whereas reduced myelination has been associated with cognitive deficits during later life [44]. In pediatric neuroradiology, the determination of the state of myelination in neonates is currently derived from scores based on the assessment of T1-weighted and T2-weighted MRI sequences [6, 8, 38]. Any tool that ultimately allows for a better characterization of the early stages of myelination may help clinicians to understand and predict cognitive deficits in later life, and thus, identify patients in need of intensified therapeutic support and closer follow-up. In this study, a novel MRI technique—“SyMRI”—was successfully applied in preterm and term-born neonates. The “SyMRI” software creates maps based on T1- and T2-relaxation constants and the PD, which differ depending on the measured tissue [16, 19, 28, 33–35, 45–47]. This facilitates the visualization of different tissue types and enables the quantification of myelin, resulting in a better assessment of brain development and demyelinating diseases [29, 48]. In the present study, “SyMRI” was more efficient and superior to conventional MRI in the visualization of myelin and in the detection of delayed myelination in preterm neonates. Two radiologists independently rated images to consistently detect subtle differences in myelin content in preterm compared with term-born neonates, which were not detectable on conventional T1- and T2-weighted sequences, using “SyMRI”-generated quantitative maps. Myelin could be better distinguished from non-myelinated gray and white matter using quantitative maps than by conventional images (Figs. 1 and 2). The advantages of “SyMRI”-based maps became particularly evident when assessing myelination of brain stem structures, such as the superior and inferior cerebellar peduncles and the medial lemniscus (Fig. 2), since these structures are already myelinated at the normal, expected due date [6, 38]. Numerous studies have examined brain maturity in preterm neonates based on conventional MR images [49–51]. The results of these studies often varied, indicating a lack of reliability in the assessment of neonatal brain maturity and, in a broader sense, unreliable information regarding neurodevelopmental outcome based on standard T1- and T2-weighted images [43, 49]. Various promising imaging techniques have been studied in recent years to assess brain maturity and myelin content, especially diffusion tensor imaging (DTI), DWI, computational morphometry, and resting-state functional MRI [43, 49]. Based on fractional anisotropy values, a significantly reduced myelination of the brain of premature infants was demonstrated on the basis of DTI data [52]. Several studies detected alterations of functional connectivity in preterm compared with term-born neonates, which might be caused by decelerated brain maturation [53–55]. However, the above-mentioned imaging methods are highly time-consuming and have not been studied on an individual or routine patient-level basis [26–28]. Especially in clinical practice, time is an important parameter. Conventional methods for MR mapping would take between 15 and 30 min, even by means of modern techniques [19, 29, 56]. “SyMRI” is a quantitative imaging method that detects myelination-related differences on an individual level, beyond the neonatal brain imaging protocol. Moreover, it generally allows a reduction of examination time while providing multiple MR contrasts, including T1, T2, PD, and inversion recovery. Thus, “SyMRI” offers the possibility to provide quantitative maps as well as various MR contrasts in one-third of the time needed for conventional methods for quantitative mapping. This opens further possibilities in diagnostic neonatal brain imaging, which were not the subject of the present study. Our study has several limitations. “SyMRI”-generated maps and conventional MRI differed in resolution and slice thickness, which limits a direct comparison with a certain extent. The investigated cohort, consisting of 18 preterm and seven term-born neonates, is small and heterogeneous, with different referral reasons. Not all examined infants showed unremarkable brain findings. It must, therefore, be assumed that changes in myelin content were partly related to brain pathologies in both included groups of preterm and term-born neonates. However, this limitation did not influence the clinical radiological assessment or the primary outcome of the study. Although the assessment of myelination using quantitative maps and conventional imaging data is a matter of subjective judgment by the evaluating radiologist, this study was conducted as a qualitative assessment alone, as this most closely resembles clinical practice. This study mainly addressed the added potential of “SyMRI” in the assessment of myelin-associated signal changes. The accuracy and reliability of “SyMRI”-generated T1-weighted and T2-weighted images in the detection and analysis of neonatal brain injuries (hypoxic-ischemic injury, infection, trauma, etc.) was outside the scope of this study, but should be the topic of future investigations.
In summary, our results indicate that assessing the maturity and myelination of the neonatal brain using quantitative MR maps is a highly viable, reliable, and easy-to-apply method. Compared with conventional T1- and T2-weighted images, the myelin content can be assessed more accurately, within a highly reduced examination time. We conclude that quantitative T1- and T2-maps allow a rapid and valid assessment of brain myelination, and therefore provide a highly reliable imaging biomarker.
Electronic supplementary material
(DOCX 796 kb)
Abbreviations
- CSF
Cerebrospinal fluid
- DTI
Diffusion tensor imaging
- DWI
Diffusion-weighted imaging
- GA
Gestational age
- GAMRI
Gestational age at MRI
- ICC
Intra-class correlation
- MDME
Multi-dynamic multi-echo-FLAIR sequence
- MRI
Magnetic resonance imaging
- MTS
Myelin total score
- PD
Proton density
- SE
Spin echo
- SWI
Susceptibility-weighted imaging
- TE
Echo time
- TI
Inversion time
- TR
Repetition time
- TSE
Turbo spin echo
Funding
Open access funding provided by Medical University of Vienna. The authors state that this work has not received any funding.
Compliance with ethical standards
Guarantor
The scientific guarantor of this publication is Gregor Kasprian, MD.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was not required for this study because data were retrospectively reviewed.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• Retrospective
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Barkovich AJ. Concepts of myelin and myelination in neuroradiology. AJNR Am J Neuroradiol. 2000;21:1099–1109. [PMC free article] [PubMed] [Google Scholar]
- 2.Baumann N, Pham-Dinh D. Biology of oligodendrocyte and myelin in the mammalian central nervous system. Physiol Rev. 2001;81:871–927. doi: 10.1152/physrev.2001.81.2.871. [DOI] [PubMed] [Google Scholar]
- 3.Dobbing J, Sands J. Quantitative growth and development of human brain. Arch Dis Child. 1973;48:757–767. doi: 10.1136/adc.48.10.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koenig SH. Cholesterol of myelin is the determinant of gray-white contrast in MRI of brain. Magn Reson Med. 1991;20:285–291. doi: 10.1002/mrm.1910200210. [DOI] [PubMed] [Google Scholar]
- 5.Miot-Noirault E, Barantin L, Akoka S, Le Pape A. T2 relaxation time as a marker of brain myelination: experimental MR study in two neonatal animal models. J Neurosci Methods. 1997;72:5–14. doi: 10.1016/S0165-0270(96)00148-3. [DOI] [PubMed] [Google Scholar]
- 6.Barkovich A J, Kjos B O, Jackson D E, Norman D. Normal maturation of the neonatal and infant brain: MR imaging at 1.5 T. Radiology. 1988;166(1):173–180. doi: 10.1148/radiology.166.1.3336675. [DOI] [PubMed] [Google Scholar]
- 7.Flechsig P. Developmental (myelogenetic) localisation of the cerebral cortex in the human subject. Lancet. 1901;158:1027–1030. doi: 10.1016/S0140-6736(01)01429-5. [DOI] [Google Scholar]
- 8.van der Knaap MS, Valk J. MR imaging of the various stages of normal myelination during the first year of life. Neuroradiology. 1990;31:459–470. doi: 10.1007/BF00340123. [DOI] [PubMed] [Google Scholar]
- 9.Childs AM, Ramenghi LA, Cornette L, et al. Cerebral maturation in premature infants: quantitative assessment using MR imaging. AJNR Am J Neuroradiol. 2001;22:1577–1582. [PMC free article] [PubMed] [Google Scholar]
- 10.Felderhoff-Mueser U, Rutherford MA, Squier WV, et al. Relationship between MR imaging and histopathologic findings of the brain in extremely sick preterm infants. AJNR Am J Neuroradiol. 1999;20:1349–1357. [PMC free article] [PubMed] [Google Scholar]
- 11.Schiffmann R, van der Knaap MS. Invited article: an MRI-based approach to the diagnosis of white matter disorders. Neurology. 2009;72:750–759. doi: 10.1212/01.wnl.0000343049.00540.c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rutherford M, Pennock J, Schwieso J, Cowan F, Dubowitz L. Hypoxic-ischaemic encephalopathy: early and late magnetic resonance imaging findings in relation to outcome. Arch Dis Child Fetal Neonatal Ed. 1996;75:145–151. doi: 10.1136/fn.75.3.F145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benders MJ, Kersbergen KJ, de Vries LS. Neuroimaging of white matter injury, intraventricular and cerebellar hemorrhage. Clin Perinatol. 2014;41:69–82. doi: 10.1016/j.clp.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 14.de Vries LS, Benders MJ, Groenendaal F. Progress in neonatal neurology with a focus on neuroimaging in the preterm infant. Neuropediatrics. 2015;46:234–241. doi: 10.1055/s-0035-1554102. [DOI] [PubMed] [Google Scholar]
- 15.Brix G, Schad LR, Deimling M, Lorenz WJ. Fast and precise T1 imaging using a TOMROP sequence. Magn Reson Imaging. 1990;8:351–356. doi: 10.1016/0730-725X(90)90041-Y. [DOI] [PubMed] [Google Scholar]
- 16.Whittall KP, MacKay AL, Graeb DA, Nugent RA, Li DK, Paty DW. In vivo measurement of T2 distributions and water contents in normal human brain. Magn Reson Med. 1997;37:34–43. doi: 10.1002/mrm.1910370107. [DOI] [PubMed] [Google Scholar]
- 17.Menon RS, Rusinko MS, Allen PS. Multiexponential proton relaxation in model cellular systems. Magn Reson Med. 1991;20:196–213. doi: 10.1002/mrm.1910200204. [DOI] [PubMed] [Google Scholar]
- 18.Beaulieu C, Fenrich FR, Allen PS. Multicomponent water proton transverse relaxation and T2-discriminated water diffusion in myelinated and nonmyelinated nerve. Magn Reson Imaging. 1998;16:1201–1210. doi: 10.1016/S0730-725X(98)00151-9. [DOI] [PubMed] [Google Scholar]
- 19.Deoni SC, Peters TM, Rutt BK. High-resolution T1 and T2 mapping of the brain in a clinically acceptable time with DESPOT1 and DESPOT2. Magn Reson Med. 2005;53:237–241. doi: 10.1002/mrm.20314. [DOI] [PubMed] [Google Scholar]
- 20.Williamson P, Pelz D, Merskey H, et al. Frontal, temporal, and striatal proton relaxation times in schizophrenic patients and normal comparison subjects. Am J Psychiatry. 1992;149:549–551. doi: 10.1176/ajp.149.4.549. [DOI] [PubMed] [Google Scholar]
- 21.Pitkänen A, Laakso M, Kälviäinen R, et al. Severity of hippocampal atrophy correlates with the prolongation of MRI T2 relaxation time in temporal lobe epilepsy but not in Alzheimer’s disease. Neurology. 1996;46:1724–1730. doi: 10.1212/WNL.46.6.1724. [DOI] [PubMed] [Google Scholar]
- 22.Bartzokis G, Sultzer D, Cummings J, et al. In vivo evaluation of brain iron in Alzheimer disease using magnetic resonance imaging. Arch Gen Psychiatry. 2000;57:47–53. doi: 10.1001/archpsyc.57.1.47. [DOI] [PubMed] [Google Scholar]
- 23.Larsson HB, Frederiksen J, Petersen J, et al. Assessment of demyelination, edema, and gliosis by in vivo determination of T1 and T2 in the brain of patients with acute attack of multiple sclerosis. Magn Reson Med. 1989;11:337–348. doi: 10.1002/mrm.1910110308. [DOI] [PubMed] [Google Scholar]
- 24.Deoni S. C. L., Mercure E., Blasi A., Gasston D., Thomson A., Johnson M., Williams S. C. R., Murphy D. G. M. Mapping Infant Brain Myelination with Magnetic Resonance Imaging. Journal of Neuroscience. 2011;31(2):784–791. doi: 10.1523/JNEUROSCI.2106-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McKenzie CA, Chen Z, Drost DJ, Prato FS. Fast acquisition of quantitative T2 maps. Magn Reson Med. 1999;41:208–212. doi: 10.1002/(SICI)1522-2594(199901)41:1<208::AID-MRM30>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 26.McAllister A, Leach J, West H, Jones B, Zhang B, Serai S. Quantitative synthetic MRI in children: normative intracranial tissue segmentation values during development. AJNR Am J Neuroradiol. 2017;38:2364–2372. doi: 10.3174/ajnr.A5398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tanenbaum LN, Tsiouris AJ, Johnson AN, et al. Synthetic MRI for clinical neuroimaging: results of the magnetic resonance image compilation (MAGiC) prospective, multicenter, multireader trial. AJNR Am J Neuroradiol. 2017;38:1103–1110. doi: 10.3174/ajnr.A5227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Warntjes JB, Leinhard OD, West J, Lundberg P. Rapid magnetic resonance quantification on the brain: optimization for clinical usage. Magn Reson Med. 2008;60:320–329. doi: 10.1002/mrm.21635. [DOI] [PubMed] [Google Scholar]
- 29.Hagiwara A, Warntjes M, Hori M, et al. SyMRI of the brain: rapid quantification of relaxation rates and proton density, with synthetic MRI, automatic brain segmentation, and myelin measurement. Invest Radiol. 2017;52:647–657. doi: 10.1097/RLI.0000000000000365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bobman S A, Riederer S J, Lee J N, Suddarth S A, Wang H Z, MacFall J R. Synthesized MR images: comparison with acquired images. Radiology. 1985;155(3):731–738. doi: 10.1148/radiology.155.3.4001377. [DOI] [PubMed] [Google Scholar]
- 31.Bobman SA, Riederer SJ, Lee JN, Suddarth SA, Wang HZ, MacFall JR (1985) Cerebral magnetic resonance image synthesis. AJNR Am J Neuroradiol 6:265–269 [PMC free article] [PubMed]
- 32.Riederer SJ, Suddarth SA, Bobman SA, Lee JN, Wang HZ, MacFall JR. Automated MR image synthesis: feasibility studies. Radiology. 1984;153:203–206. doi: 10.1148/radiology.153.1.6089265. [DOI] [PubMed] [Google Scholar]
- 33.Deichmann R. Fast high-resolution T1 mapping of the human brain. Magn Reson Med. 2005;54:20–27. doi: 10.1002/mrm.20552. [DOI] [PubMed] [Google Scholar]
- 34.Henderson E, McKinnon G, Lee TY, Rutt BK. A fast 3D look-locker method for volumetric T1 mapping. Magn Reson Imaging. 1999;17:1163–1171. doi: 10.1016/S0730-725X(99)00025-9. [DOI] [PubMed] [Google Scholar]
- 35.Neeb H, Zilles K, Shah NJ. A new method for fast quantitative mapping of absolute water content in vivo. Neuroimage. 2006;31:1156–1168. doi: 10.1016/j.neuroimage.2005.12.063. [DOI] [PubMed] [Google Scholar]
- 36.Kang KM, Choi SH, Kim H et al (2018) The effect of varying slice thickness and interslice gap on T1 and T2 measured with the multidynamic multiecho sequence. Magn Reson Med Sci. 10.2463/mrms.mp.2018-0010 [DOI] [PMC free article] [PubMed]
- 37.Vossough A, Limperopoulos C, Putt ME, et al. Development and validation of a semiquantitative brain maturation score on fetal MR images: initial results. Radiology. 2013;268:200–207. doi: 10.1148/radiol.13111715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yakovlev P, Lecours A. The myelogenetic cycles of regional maturation of the brain. In: Minkowski A, editor. Regional development of the brain in early life. Oxford: Blackwell; 1967. pp. 3–70. [Google Scholar]
- 39.Quinn JA, Munoz FM, Gonik B, et al. Preterm birth: case definition & guidelines for data collection, analysis, and presentation of immunisation safety data. Vaccine. 2016;34:6047–6056. doi: 10.1016/j.vaccine.2016.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cicchetti D. Guidelines, criteria, and rules of thumb for evaluating normed and standardized assessment instruments in psychology. Psychol Assess. 1994;6:284–290. doi: 10.1037/1040-3590.6.4.284. [DOI] [Google Scholar]
- 41.Ibrahim John, Mir Imran, Chalak Lina. Brain imaging in preterm infants <32 weeks gestation: a clinical review and algorithm for the use of cranial ultrasound and qualitative brain MRI. Pediatric Research. 2018;84(6):799–806. doi: 10.1038/s41390-018-0194-6. [DOI] [PubMed] [Google Scholar]
- 42.Mathur A, Inder T. Magnetic resonance imaging--insights into brain injury and outcomes in premature infants. J Commun Disord. 2009;42:248–255. doi: 10.1016/j.jcomdis.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Parikh NA. Advanced neuroimaging and its role in predicting neurodevelopmental outcomes in very preterm infants. Semin Perinatol. 2016;40:530–541. doi: 10.1053/j.semperi.2016.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saab Aiman S, Nave Klaus-Armin. Myelin dynamics: protecting and shaping neuronal functions. Current Opinion in Neurobiology. 2017;47:104–112. doi: 10.1016/j.conb.2017.09.013. [DOI] [PubMed] [Google Scholar]
- 45.Clare S, Jezzard P. Rapid T(1) mapping using multislice echo planar imaging. Magn Reson Med. 2001;45:630–634. doi: 10.1002/mrm.1085. [DOI] [PubMed] [Google Scholar]
- 46.Ordidge RJ, Gibbs P, Chapman B, Stehling MK, Mansfield P. High-speed multislice T1 mapping using inversion-recovery echo-planar imaging. Magn Reson Med. 1990;16:238–245. doi: 10.1002/mrm.1910160205. [DOI] [PubMed] [Google Scholar]
- 47.Zhu DC, Penn RD. Full-brain T1 mapping through inversion recovery fast spin echo imaging with time-efficient slice ordering. Magn Reson Med. 2005;54:725–731. doi: 10.1002/mrm.20602. [DOI] [PubMed] [Google Scholar]
- 48.West H, Leach JL, Jones BV, et al. Clinical validation of synthetic brain MRI in children: initial experience. Neuroradiology. 2017;59:43–50. doi: 10.1007/s00234-016-1765-z. [DOI] [PubMed] [Google Scholar]
- 49.Doria V, Arichi T, Edwards DA. Magnetic resonance imaging of the preterm infant brain. Curr Pediatr Rev. 2014;10:48–55. doi: 10.2174/157339631001140408120821. [DOI] [PubMed] [Google Scholar]
- 50.Dyet LE, Kennea N, Counsell SJ, et al. Natural history of brain lesions in extremely preterm infants studied with serial magnetic resonance imaging from birth and neurodevelopmental assessment. Pediatrics. 2006;118:536–548. doi: 10.1542/peds.2005-1866. [DOI] [PubMed] [Google Scholar]
- 51.Woodward LJ, Anderson PJ, Austin NC, Howard K, Inder TE. Neonatal MRI to predict neurodevelopmental outcomes in preterm infants. N Engl J Med. 2006;355:685–694. doi: 10.1056/NEJMoa053792. [DOI] [PubMed] [Google Scholar]
- 52.Li BX, Liu GS, Ling XY, Chen HF, Luo XQ. Evaluation of white matter myelination in preterm infants using DTI and MRI. Zhongguo Dang Dai Er Ke Za Zhi. 2016;18:476–481. doi: 10.7499/j.issn.1008-8830.2016.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Doria V, Beckmann CF, Arichi T, et al. Emergence of resting state networks in the preterm human brain. Proc Natl Acad Sci U S A. 2010;107:20015–20020. doi: 10.1073/pnas.1007921107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Smyser CD, Inder TE, Shimony JS, et al. Longitudinal analysis of neural network development in preterm infants. Cereb Cortex. 2010;20:2852–2862. doi: 10.1093/cercor/bhq035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.He Lili, Parikh Nehal A. Brain functional network connectivity development in very preterm infants: The first six months. Early Human Development. 2016;98:29–35. doi: 10.1016/j.earlhumdev.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 56.Bouhrara Mustapha, Spencer Richard G. Rapid simultaneous high-resolution mapping of myelin water fraction and relaxation times in human brain using BMC-mcDESPOT. NeuroImage. 2017;147:800–811. doi: 10.1016/j.neuroimage.2016.09.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 796 kb)