Introduction
Allergen-specific immunotherapy (AIT) is a therapeutic strategy to restore the normal immune response by suppressing inflammatory effector cells and inducing regulatory cells specific to the culprit allergen. Conventionally, AIT is achieved by administering escalating doses of allergen over a long treatment course. However, the use of unmodified allergens for AIT often results in severe anaphylactic side effects, and alternative approaches such as hypoallergens and T-cell epitopes with enhanced safety have been considered. The use of mimotopes in AIT is a relatively new concept, but earlier proof-of-concept studies showed promising results for mimotopes, with decent safety profiles and immunomodulatory capacity1. While the treatment of cancer with mimotope-based immunotherapy has already been tested in clinical trials2, mimotope-based AIT remains largely in the preclinical stage. Here, we revisit the pros and cons of mimotope-based AIT and describe recent findings concerning the use of mimotopes in AIT.
Benefits and pitfalls of mimotope-based AIT
The term “mimotope” was first coined by Mario Geysen in 1986 to describe peptides that mimic epitopes3. By mimicking the structural or physiochemical characteristics of epitopes, mimotopes are able to inhibit the binding of antibodies to the native antigen or to induce an epitope-specific antibody response when coupled to an immunogenic carrier. Such properties are of special interest in AIT, where mimotopes could be used to induce blocking antibodies that competitively inhibit the binding of specific IgE to allergens. The major advantage of mimotope-based AIT is that mimotopes lack allergen-specific T-cell epitopes; thus, late-phase allergic side effects caused by repeated stimulation of allergen-specific T cells are less likely to occur. Moreover, since mimotopes are monovalent peptides with low crosslinking capacity, mimotope-based AIT is considerably safer than AIT based on unmodified allergens (Fig. 1). Compared with native allergens or hypoallergens, mimotopes are also able to induce epitope-specific humoral immune responses that result in antibodies with increased blocking capacity. In addition, mimotopes can be conjugated to different immunogenic carriers that confer different beneficial effects. Some peptide carriers, such as coat proteins from bacteriophages or virus-like particles, are known to mediate a Th1-biased immune response, which would be beneficial in AIT4. In contrast, the major concern regarding mimotope-based AIT is the weak immunogenicity of mimotopes. Mimotopes must be conjugated to a carrier to be immunogenic, and the safety and efficacy of mimotope-carrier constructs must be evaluated case by case.
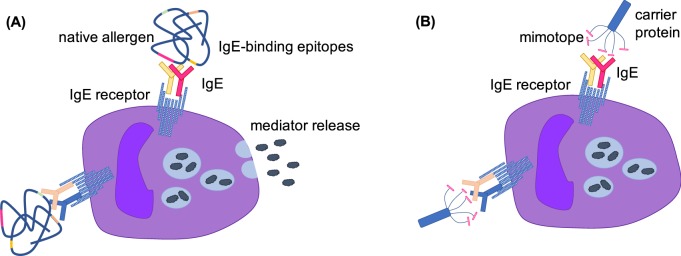
Fig. 1.
Illustration of the increased safety of mimotopes compared with native allergens. a Crosslinking of IgE receptors on mast cells requires polyclonal IgE binding to two different epitopes on the same allergen. b A mimotope displayed on a carrier protein (e.g., a filamentous phage coat protein) would only bind to IgE from a single colony and would thus fail to crosslink the IgE receptors on mast cells
Recent advances in mimotope-based AIT
Biopanning phage displayed5 or screening of one-bead-one-compound combinatorial peptide libraries6 are high-throughput approaches to identify mimotopes. Via screening of these peptide libraries with allergen-specific IgE, the mimotopes obtained can be subsequently mapped to the three-dimensional structure of the allergen using bioinformatic algorithms to identify the particular epitopes that they mimic. These methods are technically undemanding and cost effective. Many IgE epitopes of foods and inhalant allergens have been identified using these approaches over the last decade. However, few of these mimotopes were tested for use in AIT, except for a few inhalant allergens. Two studies investigated the allergenicity and safety of mimotopes fused with coat proteins of filamentous phages and reported that these mimotope constructs failed to induce CD63 expression in a basophil activation assay7,8. In addition, these studies demonstrated that the phage coat proteins carrying the mimotopes stimulated a Th1 response when cocultured with PBMCs from the patients. The therapeutic potential of mimotopes was also evaluated in mouse models of allergic asthma9,10, and these studies demonstrated a significant decrease in inflammatory cell infiltration and Th2 cytokine production in lung tissues and bronchoalveolar lavage fluid upon mimotope treatment. Collectively, the results from these studies tend to support the use of mimotope-based AIT. However, three of the four abovementioned studies used phage coat proteins as the carrier, the clinical use of which is limited due to their fast clearance in the human body and strict regulatory control8. The other study utilized keyhole limpet hemocyanin (KLH) as the mimotope carrier, but the allergenicity and safety of the construct was not addressed. Although KLH has frequently been used as a peptide carrier in clinical trials, there are still safety concerns, as the mimotopes could crosslink IgE and activate mast cells when displayed at a very high density on the KLH carrier. A safe but immunogenic peptide carrier, possibly with beneficial T-cell-modulating properties, is therefore desirable. In addition, inconsistent results were observed in the above animal studies. One study reported a decrease in specific IgE and an increase in IgG110, but the specific antibody levels were not altered in another study9. More importantly, changes in biomarkers commonly used to assess the efficacy of AIT, such as regulatory cells (Treg and Breg) and cytokines (IL-10 and TGF-β), have yet to be examined in mimotope-based AIT studies. Clearly, further advancements are necessary to understand the underlying mechanisms of mimotope-based AIT for potential clinical applications.
Competing interests
The authors declare no competing interests.
References
- 1.Knittelfelder R, Riemer AB, Jensen-Jarolim E. Mimotope vaccination – from allergy to cancer. Expert Opin. Biol. Ther. 2009;9:493–506. doi: 10.1517/14712590902870386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Makhoul I, et al. Moving a carbohydrate mimetic peptide into the clinic. Hum. Vaccines Immunother. 2015;11:37–44. doi: 10.4161/hv.34300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Geysen HM, Rodda SJ, Mason TJ. A priori delineation of a peptide which mimics a discontinuous antigenic determinant. Mol. Immunol. 1986;23:709–715. doi: 10.1016/0161-5890(86)90081-7. [DOI] [PubMed] [Google Scholar]
- 4.Zahirović A, Lunder M. Microbial delivery vehicles for allergens and allergen-derived peptides in immunotherapy of allergic diseases. Front. Microbiol. 2018;9:1449. doi: 10.3389/fmicb.2018.01449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luzar J, Štrukelj B, Lunder M. Phage display peptide libraries in molecular allergology: from epitope mapping to mimotope-based immunotherapy. Allergy. 2016;71:1526–1532. doi: 10.1111/all.12965. [DOI] [PubMed] [Google Scholar]
- 6.Leung NYH, et al. Screening and identification of mimotopes of the major shrimp allergen tropomyosin using one-bead-one-compound peptide libraries. Cell. Mol. Immunol. 2017;14:308–318. doi: 10.1038/cmi.2015.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luzar J, et al. Identification and characterization of major cat allergen Fel d 1 mimotopes on filamentous phage carriers. Mol. Immunol. 2016;71:176–183. doi: 10.1016/j.molimm.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 8.Zahirović A, et al. Identification of bee venom Api m 1 IgE epitopes and characterization of corresponding mimotopes. J. Allergy Clin. Immunol. 2019;143:791–794. doi: 10.1016/j.jaci.2018.10.003. [DOI] [PubMed] [Google Scholar]
- 9.Wallmann J, et al. Mimotope vaccination for therapy of allergic asthma: anti-inflammatory effects in a mouse model. Clin. Exp. Allergy. 2009;40:650–658. doi: 10.1111/j.1365-2222.2009.03392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li J, et al. Alternaria B cell mimotope immunotherapy alleviates allergic responses in a mouse model. J. Immunol. 2019;203:31–38. doi: 10.4049/jimmunol.1801182. [DOI] [PubMed] [Google Scholar]