Regulation of cells with cytotoxic functionality is crucial to provide an effective and proportional response to immune challenges. CD8 T cells and NK cells are responsible for differentiating infected or tumorigenic host cells from healthy self-cells and removing them from the host. IL-15 signaling promotes the survival, maturation, and proliferation of CD8 T cells and NK cells.1 In a recent work, Zhou et al. identified a novel regulatory checkpoint that inhibits activation through the IL-15 signaling pathway.2 This study dissects the mechanism through which the deubiquitinase Otub1 negatively regulates CD8 T cell and NK cell activation in response to IL-15. These findings identify an intriguing and previously undescribed checkpoint that can potentially be manipulated to modulate the immune response. Here, the authors demonstrate that by removing Otub1 inhibition, immune tolerance to self-cells and tumor antigens can be broken.
IL-15 is trans-presented in complex with IL-15Rα to nearby cells and recognized through the heterodimeric IL15Rβ/common γ chain (γC) receptor complex (IL15R) to initiate signaling (Fig. 1). Both CD8 T cells and NK cells exhibit high-level surface expression of IL15R and rely upon IL-15 signaling for homeostatic maintenance.1 In CD8 T cells, IL-15 plays a critical role in the survival of effector T cells as they transition into memory cells. At homeostasis, T cells undergo homeostatic proliferation in response to IL-15, and in the absence of this cytokine, maintenance of the memory population is impaired. NK cells also require IL-15 for their development, homeostatic survival, proliferation, and maturation. Downstream of the IL-15 receptor complex, ubiquitinated AKT is activated through phosphorylation by PI3K. AKT activation regulates the overall activation state of the cell and promotes metabolic reprogramming to meet energy demands. Zhou et al. showed that in response to IL-15 signaling, Otub1 complexes with AKT at the cell membrane and inactivates AKT.2 AKT activation is mediated through localization to the cell membrane after ubiquitination. As a deubiquitinase, Otub1 acts as an inhibitor of AKT by removing ubiquitin and inhibiting its localization at the cell membrane. Interestingly, although Otub1 is highly expressed in CD4 T cells, it complexes with AKT at the membrane of CD8 T cells and NK cells only following IL-15 stimulation, providing a regulatory mechanism restricted to specific immune cell types.
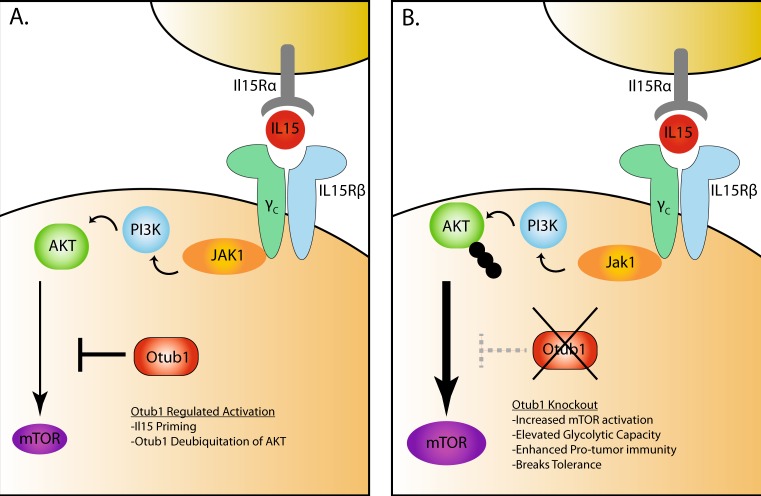
Fig. 1.
Otub1-mediated regulation of the IL-15 signaling pathway. a The heterodimeric IL-15Rβ /common γ chain (γc) receptor complex on CD8 T cells and NK cells recognizes IL-15 in complex with IL-15Rα from IL-15-producing cells. Engagement of the IL-15 receptor complex initiates a number of signaling cascades, including activation of PI3K. Ubiquitinated AKT is recruited to the membrane surface, where it activates mTOR, leading to downstream changes in cellular activation. Zhou et al. showed that Otub1 negatively regulates the IL-15 signaling pathway by deubiquitinating AKT, limiting mTOR activation. b When Otub1 is removed from CD8 T cells and NK cells, this pathway is left unchecked and causes increased signaling through mTOR. Otub1-deficient cells have enhanced effector function and clear pathogens and tumors more effectively. Otub1 KO also causes a break in self-tolerance and increases susceptibility to autoimmunity. Changes in Otub1-deficient cells are dependent upon intact IL-15 signaling
The authors go on to show that Otub1 specifically regulates the AKT axis downstream of IL-15 stimulation, leaving STAT5 phosphorylation intact, thus providing a highly specific regulatory checkpoint. Deubquitination of AKT via Otub1 reduces PIP3/AKT binding and inhibits mTOR activation. By deleting Otub1, Zhou and colleagues were able to enhance cellular proliferation, effector function, and metabolic reprogramming in CD8 T cells. These changes were completely abrogated in IL-15ra−/− recipient mice. The absence of Otub1 enhanced glycolytic metabolism in activated cells but had no effect on oxidative phosphorylation. Given that Otub1 deletion promoted a more activated immune phenotype, the authors asked whether this can alter pathogen clearance. Indeed, Otub1-deficient mice had a significantly enhanced capacity to clear Listeria monocytogenes infection.
IL-15 signaling has been demonstrated to sensitize T cells to increase responsiveness to self-antigen. Given that Otub1 negatively regulates IL-15 signaling in CD8 T cells, the authors hypothesized that deletion of Otub1 can break tolerance to self-antigens. To test this hypothesis, Otub1-deficient Pmel1 mice that contain T cells specific for gp100, a self-antigen expressed by melanocytes, were generated. In this system, Otub1 -deficient mice developed more severe autoimmune vitiligo with increased CD8 T cell proliferation and activation. Breaking tolerance to tumor antigens is a critical goal in developing effective cancer immunotherapy techniques. Thus, this study identifies Otub1 as a novel regulatory target to enhance the anticancer immune response. Mice in which Otub1 was inducibly deleted displayed slower B16 tumor growth and increased survival that was dependent on increases in tumor-infiltrating CD8 T cells and NK cells. Furthermore, the authors injected Otub1-deficient CD8 T cells into wild-type animals to demonstrate antitumor efficacy in the context of immunotherapy. Mice produced using this adoptive transfer strategy also showed increased antitumor immunity, CD8 T cell activation, and tumor infiltration.
The work by Zhou expands our current knowledge of how IL-15 signaling is regulated and provides a potentially novel therapeutic target, Otub1, to regulate the immune response. Importantly, the presented experiments eloquently identify Otub1 as a potential immunomodulatory target that specifically regulates CD8 T cells and NK cells. This inherent specificity could be useful in fine-tuning the immune response. The current study demonstrated that Otub1 depletion can stimulate a robust immune response and may be clinically useful as a target during adoptive T cell therapies. However, it is also possible that overexpression of Otub1 can be exploited to dampen an overactive immune response, thereby reducing immunopathology or autoimmunity. Future studies are required to understand the intricacies of this newly described regulatory pathway.
Several IL-15 therapies have been proposed as a way to stimulate the immune response and enhance tumor immunotherapy.3 Stimulating the IL-15 pathway has been shown to increase cellular proliferation and sustain the antitumor response; in contrast, blocking the IL-15/IL-15 receptor can dampen the immune response to treat autoimmunity.1,4 The high level of interest in therapeutic interventions targeting the IL-15 signaling pathway significantly increases the translational appeal of the current work. Importantly, this work by Zhou et al. identifies regulatory mechanisms downstream of the IL-15 receptor complex that can differentially modulate the arms of the IL-15 signaling pathway. Deletion of Otub1 was shown to inhibit AKT activation while leaving IL-15 activation of the STAT5 signaling pathway unperturbed.2 This finding provides the potential for a more precise point of intervention in the IL-15 pathway that could yield a more targeted outcome. Currently, much regarding IL-15 signaling remains unknown. For example, while IL-15 signaling is commonly described as proinflammatory, this is not always the case. In certain contexts, IL-15 stimulation can also promote a regulatory phenotype in NK cells by inducing IL-10 production that can protect the host from immunopathology.1 Future work investigating Otub1 and other regulators of IL-15 will help to better understand which combination of signals determines the outcome of cytokine stimulation. Furthermore, there is clearly still more to learn regarding regulation of the IL-15 signaling pathway.
References
- 1.Leonard, W. J., Lin, J. X. & O’Shea, J. J. Immunity. 10.1016/j.immuni.2019.03.028 (2009).
- 2.Zhou, X. et al. Nat. Immunol.20, 879–889 (2019). [DOI] [PMC free article] [PubMed]
- 3.Rautela, J. & Huntington, N. D. Curr. Opin. Immunol.44, 1–6 (2017). [DOI] [PubMed]
- 4.Waldmann, T. A. & Tagaya, Y. Annu. Rev. Immunol.17, 19–49 (1999). [DOI] [PubMed]