Abstract
Introduction
Several therapeutic options are available to manage anogenital warts (AGWs). However, no hierarchy of treatments is provided in the latest European and American recommendations. This study aimed to determine the efficacy and safety of local treatments for the management of AGWs.
Methods
A search was conducted through 12 databases from inception to August 2018. All randomized controlled trials (RCTs) in which at least one parallel treatment group composed of immunocompetent adults with AGWs received at least one provider-administered or patient-administered treatment were included. Risk of bias assessment and meta-analyses of aggregated study data were performed on the basis of the Cochrane Handbook, and quality of evidence evaluation followed the Grading of Recommendation Assessment, Development and Evaluation (GRADE) approach. Primary endpoints were complete clearance and recurrence at 3 months.
Results
Seventy RCTs (9931 patients) were included. All but four RCTs had a high risk of bias. CO2 laser was slightly more efficacious than cryotherapy [risk ratio (RR) 2.05; 95% confidence interval (CI) 1.61–2.62], with fewer recurrences at 3 months (RR 0.28; 95% CI 0.09–0.89). Electrosurgery was slightly more efficacious than cryotherapy. No differences in efficacy or side effects were found between cryotherapy and imiquimod or trichloroacetic acid. Podophyllotoxin gel was slightly more efficacious than podophyllotoxin cream. 5-Fluorouracil (5-FU) was slightly more efficacious and caused less erosion than CO2 laser (RR 1.37; 95% CI 1.11–1.70).
Conclusion
The vast majority of included RCTs had a low level of evidence, thereby preventing the establishment of a hierarchy of treatments. Nevertheless, our results provide an overview of the main AGW treatments available for general practitioners and specialists. While provider-administered treatments are superior, patient-administered treatments (e.g., imiquimod, podophyllotoxin) are useful solutions for compliant patients.
Protocol registration
PROSPERO-CRD42015025827.
Electronic supplementary material
The online version of this article (10.1007/s13555-019-00328-z) contains supplementary material, which is available to authorized users.
Keywords: Anogenital warts, Condyloma, Cryotherapy, Genital, Meta-analysis, Systematic review
Introduction
Anogenital warts (AGWs) are benign epithelial skin lesions that typically occur on the external genitalia. They are one of the most common sexually transmitted diseases [1], with an overall prevalence rate of around 1–5% depending on world region [1]. AGWs are usually painless, but are often physically uncomfortable. Their burden is relatively high, as they affect quality of life (QOL) and incur significant healthcare costs [2, 3]. Different options are available for first-line management of AGWs, including (1) provider-administered treatments [trichloroacetic acid (TCA), electrosurgery, CO2 laser, surgical excision, podophyllin, bleomycin, intracondyloma injection] and (2) patient-administered treatments [imiquimod, potassium hydroxide (KOH), 5-fluorouracil (5-FU), sinecatechins, podophyllotoxin], which can be prescribed by general practitioners.
The efficacy of these various therapies is variable, and in the case of patient-administered treatments, notable side effects may impact compliance. Vaccination campaigns, which focus mainly on oncogenic anti-human papillomavirus, have been too narrow in scope to control these infections [4]. In addition, evidence-based indications of AGW treatment efficacy are limited. The latest European and American guidelines do not provide a hierarchy of first-line treatments for AGWs in immunocompetent adults [5, 6]. According to these guidelines, therapeutic decisions should take into account patient preference, physician experience, treatment costs, anatomic site, and the size and number of AGWs. The latest systematic review, which includes randomized controlled trials (RCTs) up to September 2014, concludes that ablative techniques are clinically more efficacious at completely clearing AGWs, but that they cannot prevent recurrence. This review also found podophyllotoxin 0.50% solution to be the most cost-effective treatment from the perspective of the UK National Health Service [7]. It should be noted, however, that several RCTs of AGW treatments have since been published.
Our recent pooled analysis provided an overview of available treatments, but did not include any comparative statistical analysis; consequently, our results were less robust than they would have been using direct comparisons [8].
The aim of the present meta-analyses was to assess the efficacy and safety of local treatments and ablative procedures for the management of AGWs.
Methods
This systematic review was registered with Prospero (No. CRD42015025827). Recommendations of the PRISMA statement for systematic review and meta-analyses were followed [9].
Search Strategy, Study Selection, Risk of Bias Assessment, and Data Synthesis
The methodology of this systematic review, including registration, databases, search strategy (reference lists of review articles [5–7, 10–12] were searched to identify additional studies), study selection, outcomes of interest, bias assessment [13], data extraction, and data synthesis, was described in a previous article [14]. Inclusion criteria were then extended to include studies that compared provider-administered treatments, patient-administered treatments, or both, and in which at least one treatment arm received the treatment of interest.
Statistical Analyses
Dichotomous outcomes were analyzed according to a fixed effects or random effects model with the Mantel–Haenszel method using Review Manager v5.3 (http://ims.cochrane.org/revman). Estimates of the effects of interventions were given as risk ratios (RR) (95% CI). A random effects model was used when heterogeneity was detected. Heterogeneity was estimated clinically (e.g., age, sex, location and number of AGWs, etc.), methodologically (blinding, randomization, etc.), and statistically when Higgins’ I2 > 50% [15]. Subgroup analyses were performed to explore the potential sources of heterogeneity. Cochrane’s test for heterogeneity and the I2 statistic were used to evaluate the significance of estimated discrepancies in treatment efficacy between the various trials. The Grading of Recommendation Assessment, Development and Evaluation (GRADE) [16] approach was applied.
Ethics Guidelines
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Results
Study Screening
After duplicates were removed, queries of our 12 computerized databases retrieved 4768 references (Fig. 1). A total of 169 full-text articles remained after screening of titles and abstracts. Finally, after the full papers were read, 70 unique RCTs involving 9931 individual patients with a mean of 142 participants per study fulfilled the inclusion criteria of our systematic review [17–86] (Appendices S2–S3 in the supplementary material).
Fig. 1.
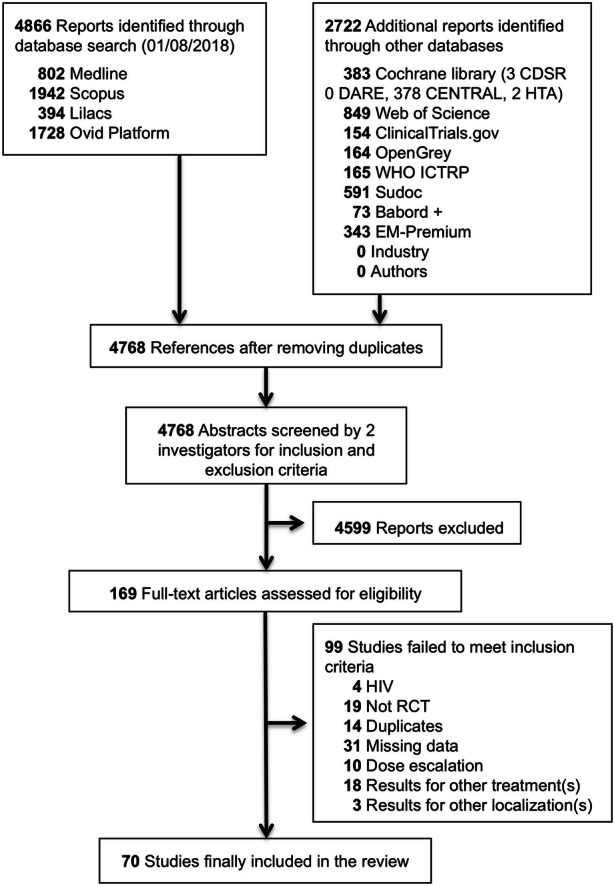
Flow diagram of randomized controlled trials (RCTs) selected for treatment of anogenital warts. CDSR Cochrane Database of Systematic Reviews, DARE Database of Abstract of Reviews of Effects, HTA Health Technology Assessment, WHO ICTRP World Health Organization International Clinical Trials Registry Platform, EM Elsevier Masson, HIV human immunodeficiency virus
Description of Included Studies
The 70 included RCTs assessed 46 provider-administered or patient-administered treatments. Parallel design was used in 45 studies with 2 arms [17–61], 21 studies with 3 arms [62–82], 3 studies with 4 arms [65, 83, 84], and 1 study with 5 arms [85]. The risk of bias assessment of included studies is described in a previous study [87]. The 66 RCTs that presented more than one criterion for uncertain or high risk of bias (based on the Cochrane Risk of Bias Tool) were described as having a high risk of bias. The remaining four studies [44, 63, 68, 86] were classified as having a low risk of bias. The 25 trials that received drugs from pharmaceutical laboratories were classified as having a high risk of bias [21, 22, 28–33, 38, 39, 46, 56, 57, 63–69, 72, 76, 80, 82, 83]. The 35 trials that provided no information on financial support were classified as having an unclear risk of bias [18, 20, 23–26, 35–37, 40–42, 45, 47, 48, 50–54, 58–62, 70, 71, 73–75, 77–79, 81, 85, 87]. However, four studies [17, 19, 43, 49] that provided no information on financial support were considered to have a low risk of bias because they compared two provider-administered treatments.
Results of Meta-analyses
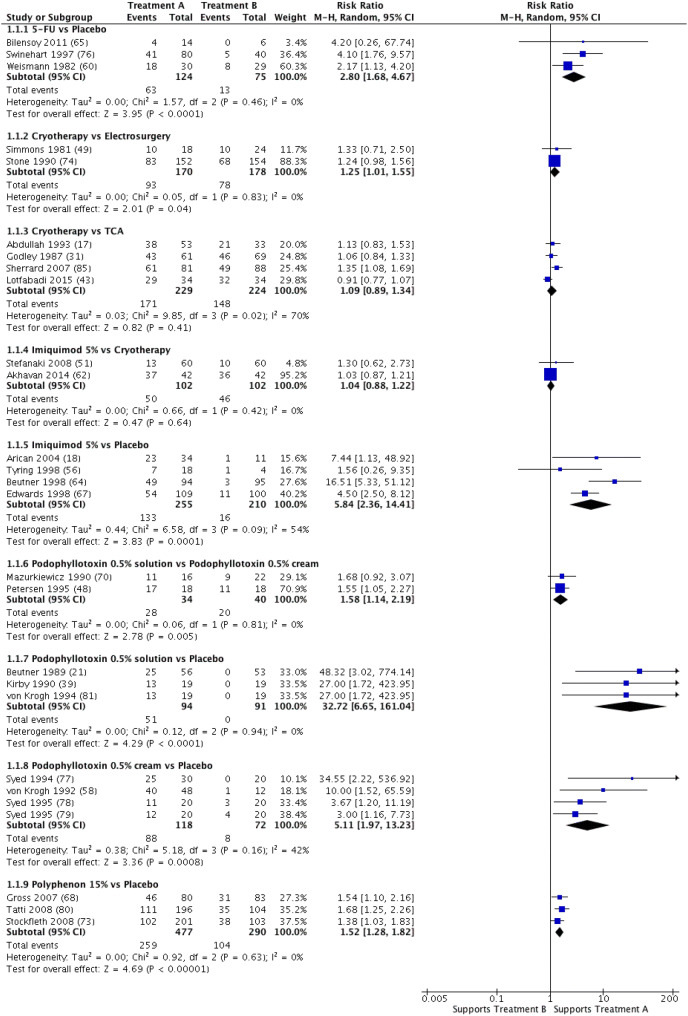
Our main results on AGW clearance are reported in Fig. 2, with the numerator representing the effect of treatment A. Placebo was systematically found to have fewer side effects than comparators. Patient satisfaction, QOL during treatment, and cost/efficacy ratio were not examined in the included RCTs. Detailed results on podophyllin, which is no longer in use, are not reported.
Fig. 2.
Forest plot of anogenital wart clearance after two first-line treatments. Random effects analysis. CI confidence interval, TCA trichloroacetic acid, M–H Mantel–Haenszel method. Reference numbers are given in parentheses
5-FU Cream
A meta-analysis of data from three RCTs (n = 299) [60, 65, 76] comparing 5-FU to a placebo estimated the pooled RR at 2.80 (95% CI 1.68–4.67; χ2 = 1.57; df = 2; P = 0.46; I2 = 0%) in favor of 5-FU. Nevertheless, recurrence at 3 months could not be estimated for two of these three studies [65, 76]. In one RCT (n = 289) [71], a statistically significant difference in clearance slightly favored 5-FU over CO2 laser (RR 1.37; 95% CI 1.11–1.70), with 5-FU causing less erosion. However, no differences in recurrence were found between the two treatments. Lastly, no differences were found between 5-FU and KOH (one RCT; n = 60) [34] or between 5-FU and podophyllin 20–25% (one RCT; n = 42) [59], except for the fact that podophyllin 20–25% caused less erosion than 5-FU.
CO2 Laser
One RCT (n = 50) found no differences in clearance between CO2 laser and ablative procedures [26]. In another RCT (n = 289) [71], 5-FU was associated with higher clearance and with less erosion than CO2 laser, but no differences in recurrence were found. In a third RCT (n = 160) [19], CO2 laser was associated with higher clearance (RR 2.05; 95% CI 1.61–2.62) and lower recurrence at 3 months (RR 0.28; 95% CI 0.09–0.89) than cryotherapy, but was found to cause more erosion. Studies comparing CO2 laser to CO2 laser + 5-FU (two RCTs; n = 186; RR 0.82; 95% CI 0.34–1.98) [24, 71], photodynamic therapy (PDT) (one RCT; n = 86) [25], or CO2 laser + PDT (one RCT; n = 211) [54] found no differences in clearance.
Electrosurgery
In one RCT (n = 99) [20], electrosurgery was associated with significantly higher clearance than placebo (RR 59.37; 95% CI 3.73–943.92), but recurrence at 6 months was not estimated. Another RCT (n = 296) [74] compared electrosurgery to podophyllin 20–25%. A meta-analysis of data from two RCTs (n = 348) [49, 74] comparing electrosurgery to cryotherapy estimated the pooled RR at 1.25 (95% CI 1.01–1.55) in favor of electrosurgery with no heterogeneity (χ2 = 0.05; df = 1, P = 0.83, I2 = 0%). This same meta-analysis found no differences in side effects or recurrence at 3 months.
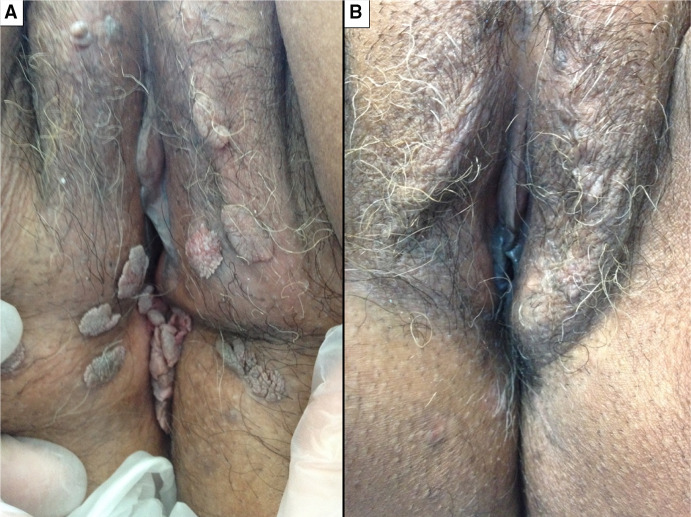
Cryotherapy (Fig. 3)
Fig. 3.
Efficacy of cryotherapy in one patient before (a) and after (b) four sessions at 1-month intervals
No differences were found between cryotherapy and imiquimod (two RCTs; n = 204) [51, 62], TCA (four RCTs; n = 453) [17, 31, 43, 85], KOH (one RCT; n = 48) [23], or podophyllin (three RCTs; n = 542) [62, 74, 85]; however, KOH was associated with less erythema and pain. CO2 laser (one RCT; n = 160) [19] and electrosurgery (two RCTs; n = 348) [49, 74] were associated with higher clearance and lower recurrence at 3 months than cryotherapy, but CO2 laser was shown to cause more erosion. No clinical improvement was obtained by combining cryotherapy with polyphenon (one RCT; n = 42) [46] (RR 1.50; 95% CI 0.49–4.56) or with podophyllotoxin 0.15% (one RCT; n = 140) [30] (RR 1.31; 95% CI 0.95–1.80).
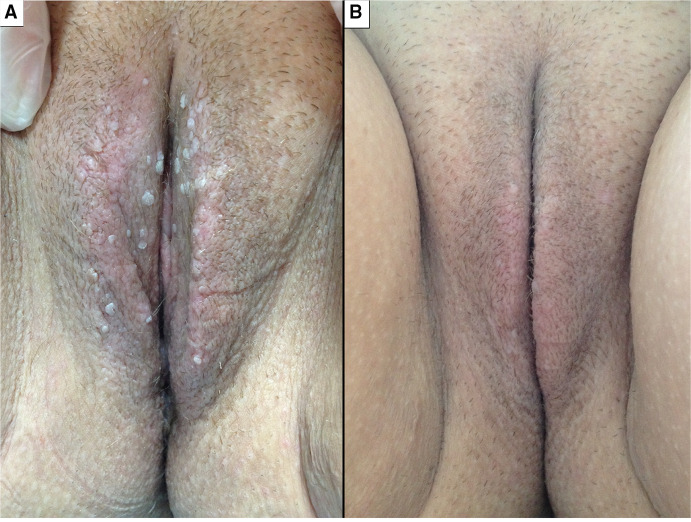
Imiquimod 5% (Fig. 4)
Fig. 4.
Efficacy of imiquimod in one patient before (a) and after (b) 6 weeks of use three times a week
One RCT (n = 255) [72] comparing imiquimod 5% to ablative procedures favored the latter, with significant differences in clearance (RR 1.43; 95% CI 1.25–1.62) and recurrence at 3 months (RR 0.39; 95% CI 0.16–0.98). A meta-analysis of data from four RCTs (n = 465) [18, 56, 64, 67] comparing imiquimod 5% to a placebo estimated the pooled RR at 5.84 (95% CI 2.36–14.41; χ2 = 6.58; df = 3; P = 0.09; I2 = 54%) in favor of imiquimod 5%. A subgroup analysis (without the study by Beutner et al. [64] on daily imiquimod application) estimated the pooled RR at 4.48 (95% CI 2.61–7.68; χ2 = 1.61; df = 2; P = 0.45; I2 = 0%). A meta-analysis of data from three RCTs (n = 130) [18, 64, 67] comparing recurrence at 3–6 months between imiquimod 5% and a placebo estimated the pooled RR at 1.15 (95% CI 0.41–3.27; χ2 = 3.11; df = 2; P = 0.21; I2 = 36%) in favor of imiquimod 5%. No differences in clearance, recurrence, and side effects were found between imiquimod 5% and cryotherapy (two RCTs; n = 204) [51, 62] or between imiquimod 5% and podophyllotoxin 0.50% solution (one RCT; n = 51) [40]. No differences in clearance were found between imiquimod and intralesional Bacillus Calmette–Guerin (one RCT; n = 90) [41] or between imiquimod and podophyllin 20–25% gel (two RCTs; n = 144) [47, 62]; however, imiquimod 5% was found to cause less erosion and ulceration than both treatments.
Podophyllotoxin 0.50% Solution
A meta-analysis of data from three RCTs (n = 185) [21, 39, 81] comparing podophyllotoxin 0.50% solution to a placebo favored podophyllotoxin 0.50% solution, with an estimated pooled RR at 32.72 (95% CI 6.65–161.04; χ2 = 0.63; df = 3; P = 0.089; I2=0%). A meta-analysis of data from two RCTs (n = 74) [48, 70] comparing podophyllotoxin 0.50% solution to podophyllotoxin 0.50% cream estimated the pooled RR at 1.58 in favor of the solution (95% CI 1.14–2.19; χ2 = 0.06; df = 1; P = 0.81; I2 = 0%); this same meta-analysis found similar side effects for both treatments. One RCT (n = 28) [48] found no differences in recurrence at 1.5 month between podophyllotoxin 0.50% solution and podophyllotoxin 0.50% cream. A meta-analysis of data from three RCTs (n = 417) [66, 69, 75] comparing podophyllotoxin 0.50% solution to podophyllotoxin 0.15% cream favored the solution, with an estimated pooled RR at 1.14 (95% CI 1.02–1.29; χ2 = 1.03; df = 2; P = 0.60; I2 = 0%). A significant difference in clearance favored podophyllotoxin 0.50% solution over podophyllin 20–25% (five RCTs; n = 671) [28, 38, 42, 69, 70]. However, no significant differences in clearance were found between podophyllotoxin 0.50% solution and podophyllotoxin 0.30% cream (two RCTs; n = 180) [66, 75] or between podophyllotoxin 0.50% solution and imiquimod 5% (one RCT; n = 51) [40].
Podophyllotoxin 0.50% Cream
A meta-analysis of data from four RCTs (n = 190) [58, 77–79] comparing podophyllotoxin 0.50% cream to a placebo estimated the pooled RR at 5.11 (95% CI 1.97–13.23; χ2 = 5.18; df = 3; P = 0.16; I2 = 42%) in favor of podophyllotoxin 0.50% cream; it also found recurrence to be similar between the two treatments (two RCTs; n = 80) [58, 78]. In two RCTs (n = 74) [48, 70], podophyllotoxin 0.50% solution was associated with higher clearance than podophyllotoxin 0.50% cream, but no differences in recurrence or side effects were found. Two RCTs (n = 98) [33, 70] found no differences in clearance between podophyllotoxin 0.50% cream and podophyllin 20–25%.
Polyphenon 15%
A meta-analysis of data from three RCTs (n = 767) [68, 73, 80] comparing polyphenon 15% to a placebo estimated the pooled RR at 1.52 (95% CI 1.28–1.82; χ2 = 0.92; df = 2; P = 0.63; I2 = 0%) in favor of polyphenon 15%. However, no differences in recurrence at 3 months were found, and polyphenon 15% was shown to cause more ulcerations.
Surgery
No differences in clearance or side effects were found between surgery and podophyllin 20–25% (two RCTs; n = 82) [35, 37], but surgery was associated with lower recurrence.
TCA
Four RCTs (n = 453) [17, 31, 43, 85] comparing TCA to cryotherapy and three RCTs (n = 387) [45, 55, 85] comparing TCA to podophyllin 20–25% found no differences in clearance, recurrence, or side effects.
KOH
While no differences in clearance were found between KOH and cryotherapy (one RCT; n = 27) [24] or between KOH and 5-FU (one RCT; n = 44) [34], KOH was shown to cause less erythema and pain than cryotherapy.
Grade
The level of evidence was found to be very low for all outcome measures and treatments studied. The only exception was the study comparing high-grade local side effects between polyphenon 15% and a placebo, which was classified as having a low level of evidence. No high level of evidence was reported (Appendices S4–S9 in the supplementary material).
Discussion
Despite a low level of evidence, our systematic review with meta-analyses found that electrosurgery and CO2 laser are slightly more efficacious than cryotherapy, but that CO2 laser causes more erosion. No differences in efficacy and side effects were found between cryotherapy and imiquimod or between cryotherapy and TCA. Podophyllotoxin gel was shown to be slightly more efficacious than podophyllotoxin cream. Imiquimod 5% was found to be more efficacious than a placebo, which is in line with a Cochrane review from 2014 [10]. The slight quantitative differences with our results may be explained by the fact that the Cochrane review examined all imiquimod concentrations (1%, 5%) and that it likely considered overlapping studies as different studies [63, 64]. In addition, imiquimod was associated with higher recurrence in our review, likely because the Cochrane review included an intention-to-treat (ITT) analysis that identified patients lost to follow-up as presenting no recurrence. Our findings for patient-administered treatments were similar to those of a recent systematic review by Werner et al. [88]. However, that review included only 18 RCTs (from Europe and North America) and was restricted to patient-administered treatments. Moreover, it did not evaluate 5-FU and, most importantly, podophyllin, despite the fact that the latter remains the older standard to which most other therapeutic strategies are compared. Lastly, our results are globally consistent with those of Thurgar et al. [7]; in our review, however, 5-FU was associated with higher clearance and lower recurrence than CO2 laser, and the two treatments induced the same local mild-grade side effects. Note, however, that we considered only immunocompetent adults, whereas Thurgar et al. indiscriminately included immunocompetent and human immunodeficiency virus-positive patients. Moreover, unlike these authors, we examined polyphenon and KOH, even though the paucity of RCTs and the high risk of bias prevented us from determining their respective efficacies.
Given the low-level evidence of the RCTs examined in our review, we wish to make the following recommendations for future studies of AGW treatments. First, recurrence at 3, 6, and 12 months, patient satisfaction, and QOL should be properly addressed in future RCTs, as they constitute important clinical outcomes. Second, side effects that induce treatment interruption should be better characterized, with data on post-intervention intensity and duration, impact on QOL, and impact on compliance (in the case of patient-administered treatments). Third, efficacy analyses should be conducted not on AGWs but on patients themselves [89–93] for two reasons: on the one hand, the primary goal of therapy is complete healing of the patient; on the other, the observed heterogeneity of outcome measures statistically impedes direct and indirect comparisons and, therefore, the development of general recommendations based on available RCTs. Fourth, split studies should not be used to design new AGW treatments, both for statistical reasons and because of the biases induced by the lack of participant blinding [94]. Indeed, given the prevalence of performance biases identified in our review, future RCTs should ensure that outcome evaluation is systematically blinded via different approaches [95, 96] and that outcomes are assessed by an independent committee unaware of treatment group assignment. Fifth, treatments with clearly demonstrated lower efficacy (e.g., podophyllin 20–25%) should be definitively excluded from future RCTs. Lastly, medical-economic evaluation of AGW treatments should be systematically performed.
Limitations
The main limitation of this systematic review is the high risk of bias of the overwhelming majority (66/70, 94%) of included RCTs [14], which prevented us from developing a clinically meaningful hierarchy of first-line treatments. Note, however, that ITT analysis was performed whenever possible as it comes closest to real-life practices. The lack of information on older therapies or AGW location and characteristics (flat, keratinized, etc.) made it impossible to analyze efficacy based on these criteria. Similarly, sensitivity analyses and assessments of publication bias [97] were not attempted because of the paucity of RCTs. As was the case in other systematic reviews, authors and pharmaceutical companies could not be contacted to obtain unpublished information [98, 99]. Another important limitation was restricted access to Chinese databases. While direct comparisons are statistically more robust than pooled analyses, the paucity of RCTs comparing several therapies also prevented the establishment of a hierarchy of treatments. In spite of these limitations, our pooled study found lower recurrence at 12 months for patient-administered treatments, suggesting that these are more relevant than provider-administered treatments as a global therapeutic response [8].
Conclusion
The vast majority of included RCTs had a low level of evidence, preventing the establishment of a clinically meaningful hierarchy of treatments. Nevertheless, our systematic review provides an overview of the main AGW treatments available to general practitioners and specialists. While provider-administered treatments (e.g., surgery, CO2 laser) are superior, patient-administered treatments (e.g., imiquimod, podophyllotoxin) are useful solutions for compliant patients.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank Prof. Rodolphe Thiebaut for his continuous support; the research librarian Evelyne Mouillet; dermatologists from the “French Group of Dermato-Infectiology and Sexually Transmitted Diseases of the Société Française de Dermatologie”; “l’Association des Dermatologues des Alpes du Sud”; and our copy editor Arianne Dorval.
Funding
This study was supported by grants from the “Allocation jeunes chercheurs hospitaliers” 2015 and the “Programme Hospitalier de Recherche Clinique Interrégional” (no. 13 069). The Rapid Service Fee was funded by the Department of Research and Innovation of the Centre Hospitalier Universitaire de La Réunion.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authors’ Contributions
Christian Derancourt, Brigitte Milpied, and Nicolas Dupin conceptualized and designed the study. Antoine Bertolotti, Christian Derancourt, and Brigitte Milpied participated in the acquisition, analysis, and interpretation of data. Antoine Bertolotti and Christian Derancourt drafted the initial manuscript. André Cabié, Sébastien Fouéré, Nicolas Dupin, and Brigitte Milpied critically reviewed the manuscript. All authors read and approved the final manuscript.
Disclosures
Antoine Bertolotti, André Cabié, Sébastien Fouéré, Nicolas Dupin, Brigitte Milpied, and Christian Derancourt have nothing to disclose.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Data Availability
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Footnotes
Enhanced Digital Features
To view enhanced digital features for this article go to 10.6084/m9.figshare.9785996.
References
- 1.Patel H, Wagner M, Singhal P, et al. Systematic review of the incidence and prevalence of genital warts. BMC Infect Dis. 2013;13:39. doi: 10.1186/1471-2334-13-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Qi SZ, Wang SM, Shi JF, et al. Human papillomavirus-related psychosocial impact of patients with genital warts in China: a hospital-based cross-sectional study. BMC Public Health. 2014;14:739. doi: 10.1186/1471-2458-14-739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Woodhall SC, Jit M, Soldan K, et al. The impact of genital warts: loss of quality of life and cost of treatment in eight sexual health clinics in the UK. Sex Transm Infect. 2011;87(6):458–463. doi: 10.1136/sextrans-2011-050073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bruni L, Diaz M, Barrionuevo-Rosas L, et al. Global estimates of human papillomavirus vaccination coverage by region and income level: a pooled analysis. Lancet Glob Health. 2016;4(7):e453–e463. doi: 10.1016/S2214-109X(16)30099-7. [DOI] [PubMed] [Google Scholar]
- 5.Gilson R, Nugent D, Werner RN, Ballesteros J. 2018 European guideline for the management of anogenital warts. https://www.iusti.org/regions/Europe/pdf/2019/IUSTIguidelinesHPV2019.pdf. Accessed 16 May 2019. [DOI] [PubMed]
- 6.Centers for Disease Control and Prevention. Anogenital warts—2015 STD treatment guidelines. https://www.cdc.gov/std/tg2015/warts.htm. Accessed 17 July 2017.
- 7.Thurgar E, Barton S, Karner C, et al. Clinical effectiveness and cost-effectiveness of interventions for the treatment of anogenital warts: systematic review and economic evaluation. Health Technol Assess. 2016;20(24):1–486. doi: 10.3310/hta20240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bertolotti A, Milpied B, Fouéré S, Cabié A, Dupin N, Derancourt C. Local management of anogenital warts in immunocompetent adults: systematic review and pooled analysis of randomized-controlled trial data. J Am Acad Dermatol. 2019. 10.1016/j.jaad.2019.04.008. [DOI] [PubMed]
- 9.Shamseer L, Moher D, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;349:g7647. doi: 10.1136/bmj.g7647. [DOI] [PubMed] [Google Scholar]
- 10.Batista CS, Atallah AN, Saconato H, et al. 5-FU for genital warts in non-immunocompromised individuals. Cochrane Database Syst Rev. 2010;4:CD006562. doi: 10.1002/14651858.CD006562.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grillo-Ardila CF, Angel-Müller E, Salazar-Díaz LC, et al. Imiquimod for anogenital warts in non-immunocompromised adults. Cochrane Database Syst Rev. 2014;11:CD010389. doi: 10.1002/14651858.CD010389.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bouscarat F, Pelletier F, Fouéré S, et al. External genital warts (condylomata) Ann Dermatol Venereol. 2016;143(11):741–745. doi: 10.1016/j.annder.2016.09.013. [DOI] [PubMed] [Google Scholar]
- 13.Higgins JPT, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bertolotti A, Dupin N, Bouscarat F, et al. Cryotherapy to treat anogenital warts in nonimmunocompromised adults: systematic review and meta-analysis. J Am Acad Dermatol. 2017;77(3):518–526. doi: 10.1016/j.jaad.2017.04.012. [DOI] [PubMed] [Google Scholar]
- 15.Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 16.Guyatt GH, Oxman AD, Schünemann HJ, et al. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol. 2011;64(4):380–382. doi: 10.1016/j.jclinepi.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 17.Abdullah AN, Walzman M, Wade A. Treatment of external genital warts comparing cryotherapy (liquid nitrogen) and trichloroacetic acid. Sex Transm Dis. 1993;20(6):344–345. doi: 10.1097/00007435-199320060-00008. [DOI] [PubMed] [Google Scholar]
- 18.Arican O, Guneri F, Bilgic K, et al. Topical imiquimod 5% cream in external anogenital warts: a randomized, double-blind, placebo-controlled study. J Dermatol. 2004;31(8):627–631. doi: 10.1111/j.1346-8138.2004.tb00568.x. [DOI] [PubMed] [Google Scholar]
- 19.Azizjalali M, Ghaffarpour G, Mousavifard B. CO2 laser therapy versus cryotherapy in treatment of genital warts; a randomized controlled trial (RCT) Iran J Microbiol. 2012;4(4):187–190. [PMC free article] [PubMed] [Google Scholar]
- 20.Benedetti Panici P, Scambia G, Baiocchi G, et al. Randomized clinical trial comparing systemic interferon with diathermocoagulation in primary multiple and widespread anogenital condyloma. Obstet Gynecol. 1989;74(3 Pt 1):393–397. [PubMed] [Google Scholar]
- 21.Beutner KR, Conant MA, Friedman-Kien AE, et al. Patient-applied podofilox for treatment of genital warts. Lancet. 1989;1(8642):831–834. doi: 10.1016/S0140-6736(89)92282-4. [DOI] [PubMed] [Google Scholar]
- 22.Bornstein J, Pascal B, Zarfati D, et al. Recombinant human interferon-beta for condylomata acuminata: a randomized, double-blind, placebo-controlled study of intralesional therapy. Int J STD AIDS. 1997;8(10):614–621. doi: 10.1258/0956462971918878. [DOI] [PubMed] [Google Scholar]
- 23.Camargo CLDA, Belda W, Jr, Fagundes LJ, et al. A prospective, open, comparative study of 5% potassium hydroxide solution versus cryotherapy in the treatment of genital warts in men. An Bras Dermatol. 2014;89(2):236–241. doi: 10.1590/abd1806-4841.20141702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carpiniello VL, Malloy TR, Sedlacek TV, et al. Results of carbon dioxide laser therapy and topical 5-fluorouracil treatment for subclinical condyloma found by magnified penile surface scanning. J Urol. 1988;140(1):53–54. doi: 10.1016/S0022-5347(17)41484-4. [DOI] [PubMed] [Google Scholar]
- 25.Chen K, Chang BZ, Ju M, et al. Comparative study of photodynamic therapy vs CO2 laser vaporization in treatment of condylomata acuminata: a randomized clinical trial. Br J Dermatol. 2007;156(3):516–520. doi: 10.1111/j.1365-2133.2006.07648.x. [DOI] [PubMed] [Google Scholar]
- 26.Duus BR, Philipsen T, Christensen JD, et al. Refractory condylomata acuminata: a controlled clinical trial of carbon dioxide laser versus conventional surgical treatment. Genitourin Med. 1985;61(1):59–61. doi: 10.1136/sti.61.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Edwards A, Atma-Ram A, Thin RN. Podophyllotoxin 0.50% v podophyllin 20% to treat penile warts. Genitourin Med. 1988;64(4):263–265. doi: 10.1136/sti.64.4.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eron LJ, Alder MB, O’Rourke JM, et al. Recurrence of condylomata acuminata following cryotherapy is not prevented by systemically administered interferon. Genitourin Med. 1993;69(2):91–93. doi: 10.1136/sti.69.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gabriel G, Thin RN. Treatment of anogenital warts. Comparison of trichloracetic acid and podophyllin versus podophyllin alone. Br J Vener Dis. 1983;59(2):124-126. [DOI] [PMC free article] [PubMed]
- 30.Gilson RJC, Ross J, Maw R, et al. A multicentre, randomised, double-blind, placebo controlled study of cryotherapy versus cryotherapy and podophyllotoxin cream as treatment for external anogenital warts. Sex Transm Infect. 2009;85(7):514–519. doi: 10.1136/sti.2009.038075. [DOI] [PubMed] [Google Scholar]
- 31.Godley MJ, Bradbeer CS, Gellan M, et al. Cryotherapy compared with trichloroacetic acid in treating genital warts. Genitourin Med. 1987;63(6):390–392. doi: 10.1136/sti.63.6.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Greenberg MD, Rutledge LH, Reid R, et al. A double-blind, randomized trial of 0.50% podofilox and placebo for the treatment of genital warts in women. Obstet Gynecol. 1991;77(5):735–739. [PubMed] [Google Scholar]
- 33.Hellberg D, Svarrer T, Nilsson S, et al. Self-treatment of female external genital warts with 0.50% podophyllotoxin cream (Condyline) vs weekly applications of 20% podophyllin solution. Int J STD AIDS. 1995;6(4):257–261. doi: 10.1177/095646249500600407. [DOI] [PubMed] [Google Scholar]
- 34.Işik S, Koca R, Sarici G, et al. A comparison of a 5% potassium hydroxide solution with a 5-fluorouracil and salicylic acid combination in the treatment of patients with anogenital warts: a randomized, open-label clinical trial. Int J Dermatol. 2014;53(9):1145–1150. doi: 10.1111/ijd.12505. [DOI] [PubMed] [Google Scholar]
- 35.Jensen SL. Comparison of podophyllin application with simple surgical excision in clearance and recurrence of perianal condylomata acuminata. Lancet. 1985;2(8465):1146–1148. doi: 10.1016/S0140-6736(85)92677-7. [DOI] [PubMed] [Google Scholar]
- 36.Keay S, Teng N, Eisenberg M, et al. Topical interferon for treating condyloma acuminata in women. J Infect Dis. 1988;158(5):934–939. doi: 10.1093/infdis/158.5.934. [DOI] [PubMed] [Google Scholar]
- 37.Khawaja HT. Podophyllin versus scissor excision in the treatment of perianal condylomata acuminata: a prospective study. Br J Surg. 1989;76(10):1067–1068. doi: 10.1002/bjs.1800761027. [DOI] [PubMed] [Google Scholar]
- 38.Kinghorn GR, McMillan A, Mulcahy F, et al. An open, comparative, study of the efficacy of 0.50% podophyllotoxin lotion and 25% podophyllotoxin solution in the treatment of condylomata acuminata in males and females. Int J STD AIDS. 1993;4(4):194–199. doi: 10.1177/095646249300400403. [DOI] [PubMed] [Google Scholar]
- 39.Kirby P, Dunne A, King DH, et al. Double-blind randomized clinical trial of self-administered podofilox solution versus vehicle in the treatment of genital warts. Am J Med. 1990;88(5):465–469. doi: 10.1016/0002-9343(90)90424-C. [DOI] [PubMed] [Google Scholar]
- 40.Komericki P, Akkilic-Materna M, Strimitzer T, et al. Efficacy and safety of imiquimod versus podophyllotoxin in the treatment of anogenital warts. Sex Transm Dis. 2011;38(3):216–218. doi: 10.1097/OLQ.0b013e3181f68ebb. [DOI] [PubMed] [Google Scholar]
- 41.Kumar P, Dar L, Saldiwal S, et al. Intralesional injection of Mycobacterium w vaccine vs imiquimod, 5%, cream in patients with anogenital warts: a randomized clinical trial. JAMA Dermatol. 2014;150(10):1072–1078. doi: 10.1001/jamadermatol.2014.794. [DOI] [PubMed] [Google Scholar]
- 42.Lassus A. Comparison of podophyllotoxin and podophyllin in treatment of genital warts. Lancet. 1987;2(8557):512–513. doi: 10.1016/S0140-6736(87)91823-X. [DOI] [PubMed] [Google Scholar]
- 43.Lotfabadi P, Maleki F, Gholami A, et al. Liquid nitrogen cryotherapy versus 70% trichloroacetic acid in the treatment of anogenital warts: a randomized controlled trial. Iran J Dermatol. 2015;18:151–155. [Google Scholar]
- 44.Mahajan BBA, Tilak Raj RB, Kumar R. A comparative evaluation of therapeutic efficacy and safety of the cryotherapy (liquid nitrogen) with topical 20% podophyllin v/s intralesional bleomycin with topical 5% Placentrex gel in the treatment of condyloma acuminata. Asian J Pharm Clin Res. 2014;7(1):36–42. [Google Scholar]
- 45.Nath D, Kumar B, Sharma VK, et al. Comparison of podophyllin and trichloroacetic acid for the treatment of genital warts. Indian J Dermatol Venereol Leprol. 1990;56(1):22–24. [Google Scholar]
- 46.On SC, Linkner RV, Haddican M, et al. A single-blinded randomized controlled study to assess the efficacy of twice daily application of sinecatechins 15% ointment when used sequentially with cryotherapy in the treatment of external genital warts. J Drugs Dermatol. 2014;13(11):1400–1405. [PubMed] [Google Scholar]
- 47.Padhiar BB, Karia UK, Aggarwal R, et al. A comparative study of efficacy of imiquimod 5% versus podophyllin 20% in treatment of external and genital warts (60 patients) Indian J Sex Transm Dis. 2006;27(2):671–679. [Google Scholar]
- 48.Petersen CS, Agner T, Ottevanger V, et al. A single-blind study of podophyllotoxin cream 0.50% and podophyllotoxin solution 0.50% in male patients with genital warts. Genitourin Med. 1995;71(6):391–392. doi: 10.1136/sti.71.6.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Simmons PD, Langlet F, Thin RN. Cryotherapy versus electrocautery in the treatment of genital warts. Br J Vener Dis. 1981;57(4):273–274. doi: 10.1136/sti.57.4.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Snoeck R, Bossens M, Parent D, et al. Phase II double-blind, placebo-controlled study of the safety and efficacy of cidofovir topical gel for the treatment of patients with human papillomavirus infection. Clin Infect Dis. 2001;33(5):597–602. doi: 10.1086/322593. [DOI] [PubMed] [Google Scholar]
- 51.Stefanaki C, Katzouranis I, Lagogianni E, et al. Comparison of cryotherapy to imiquimod 5% in the treatment of anogenital warts. Int J STD AIDS. 2008;19(7):441–444. doi: 10.1258/ijsa.2007.007196. [DOI] [PubMed] [Google Scholar]
- 52.Syed TA, Ahmadpour OA, Ahmad SA, et al. Management of female genital warts with an analog of imiquimod 2% in cream: a randomized, double-blind, placebo-controlled study. J Dermatol. 1998;25(7):429–433. doi: 10.1111/j.1346-8138.1998.tb02429.x. [DOI] [PubMed] [Google Scholar]
- 53.Syed TA, Hadi SM, Qureshi ZA, et al. Treatment of external genital warts in men with imiquimod 2% in cream. A placebo-controlled, double-blind study. J Infect. 2000;41(2):148–151. doi: 10.1053/jinf.2000.0709. [DOI] [PubMed] [Google Scholar]
- 54.Szeimies RM, Schleyer V, Moll I, et al. Adjuvant photodynamic therapy does not prevent recurrence of condylomata acuminata after carbon dioxide laser ablation—a phase III, prospective, randomized, bicentric, double-blind study. Dermatol Surg. 2009;35(5):757–764. doi: 10.1111/j.1524-4725.2009.01125.x. [DOI] [PubMed] [Google Scholar]
- 55.Tabari S, Javadian M, Barat S. The efficacy of podophylin 20% and thricholoroacetic acid %30 [sic] in the treatment of genital wart. Casp J Intern Med. 2010;1(1):16–19. [Google Scholar]
- 56.Tyring SK, Arany I, Stanley MA, et al. A randomized, controlled, molecular study of condylomata acuminata clearance during treatment with imiquimod. J Infect Dis. 1998;178(2):551–555. doi: 10.1086/517472. [DOI] [PubMed] [Google Scholar]
- 57.Tyring S, Edwards L, Cherry LK, et al. Safety and efficacy of 0.50% podofilox gel in the treatment of anogenital warts. Arch Dermatol. 1998;134(1):33–38. doi: 10.1001/archderm.134.1.33. [DOI] [PubMed] [Google Scholar]
- 58.von Krogh G, Hellberg D. Self-treatment using a 0.50% podophyllotoxin cream of external genital condylomata acuminata in women. A placebo-controlled, double-blind study. Sex Transm Dis. 1992;19(3):170–174. doi: 10.1097/00007435-199205000-00012. [DOI] [PubMed] [Google Scholar]
- 59.Wallin J. 5-Fluorouracil in the treatment of penile and urethral condylomata acuminata. Br J Vener Dis. 1977;53(4):240–243. doi: 10.1136/sti.53.4.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Weismann K, Kassis V. Treatment of condyloma acuminatum with 0.50% 5-fluorouracil-solution, a double-blind clinical trial. Z Für Hautkrankh. 1982;57(11):810–816. [PubMed] [Google Scholar]
- 61.Welander CE, Homesley HD, Smiles KA, et al. Intralesional interferon alfa-2b for the treatment of genital warts. Am J Obstet Gynecol. 1990;162(2):348–354. doi: 10.1016/0002-9378(90)90383-I. [DOI] [PubMed] [Google Scholar]
- 62.Akhavan S, Mohammadi SR, Modarres Gillani M, et al. Efficacy of combination therapy of oral zinc sulfate with imiquimod, podophyllin or cryotherapy in the treatment of vulvar warts. J Obstet Gynaecol Res. 2014;40(10):2110–2113. doi: 10.1111/jog.12457. [DOI] [PubMed] [Google Scholar]
- 63.Baker DA, Ferris DG, Martens MG, et al. Imiquimod 3.75% cream applied daily to treat anogenital warts: combined results from women in two randomized, placebo-controlled studies. Infect Dis Obstet Gynecol. 2011;2011:806105. [DOI] [PMC free article] [PubMed]
- 64.Beutner KR, Tyring SK, Trofatter KF, et al. Imiquimod, a patient-applied immune-response modifier for treatment of external genital warts. Antimicrob Agents Chemother. 1998;42(4):789–794. doi: 10.1128/AAC.42.4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bilensoy EA, Moroy PB, Çirpanli YA, et al. A double-blind placebo-controlled study of 5-fluorouracil: cyclodextrin complex loaded thermosensitive gel for the treatment of HPV induced condyloma. J Incl Phenom Macrocycl Chem. 2011;69:309–313. doi: 10.1007/s10847-010-9734-3. [DOI] [Google Scholar]
- 66.Claesson U, Lassus A, Happonen H, et al. Topical treatment of venereal warts: a comparative open study of podophyllotoxin cream versus solution. Int J STD AIDS. 1996;7(6):429–434. doi: 10.1258/0956462961918400. [DOI] [PubMed] [Google Scholar]
- 67.Edwards L, Ferenczy A, Eron L, et al. Self-administered topical 5% imiquimod cream for external anogenital warts. HPV Study Group. Human papillomavirus. Arch Dermatol. 1998;134(1):25–30. doi: 10.1001/archderm.134.1.25. [DOI] [PubMed] [Google Scholar]
- 68.Gross G, Meyer KG, Pres H, et al. A randomized, double-blind, four-arm parallel-group, placebo-controlled phase II/III study to investigate the clinical efficacy of two galenic formulations of polyphenon E in the treatment of external genital warts. J Eur Acad Dermatol Venereol. 2007;21(10):1404–1412. doi: 10.1111/j.1468-3083.2007.02441.x. [DOI] [PubMed] [Google Scholar]
- 69.Lacey CJN, Goodall RL, Tennvall GR, et al. Randomised controlled trial and economic evaluation of podophyllotoxin solution, podophyllotoxin cream, and podophyllin in the treatment of genital warts. Sex Transm Infect. 2003;79(4):270–275. doi: 10.1136/sti.79.4.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mazurkiewicz W, Jablońska S. Clinical efficacy of condyline (0.50% podophyllotoxin) solution and cream versus podophyllin in the treatment of external condylomata acuminata. J Dermatol Treat. 1990;1(3):123–125. doi: 10.3109/09546639009086712. [DOI] [Google Scholar]
- 71.Relakis K, Cardamakis E, Korantzis A, et al. Treatment of men with flat (FC) or acuminata (CA) condylomata with interferon alpha-2a. Eur J Gynaecol Oncol. 1996;17(6):529–533. [PubMed] [Google Scholar]
- 72.Schöfer HA, van Ophoven AB, Henke UA, et al. Randomized, comparative trial on the sustained efficacy of topical imiquimod 5% cream versus conventional ablative methods in external anogenital warts. Eur J Dermatol. 2006;16(6):642–648. [PubMed] [Google Scholar]
- 73.Stockfleth E, Beti H, Orasan R, et al. Topical Polyphenon® E in the treatment of external genital and perianal warts: a randomized controlled trial. Br J Dermatol. 2008;158(6):1329–1338. doi: 10.1111/j.1365-2133.2008.08520.x. [DOI] [PubMed] [Google Scholar]
- 74.Stone KM, Becker TM, Hadgu A, et al. Treatment of external genital warts: a randomised clinical trial comparing podophyllin, cryotherapy, and electrodesiccation. Genitourin Med. 1990;66(1):16–19. doi: 10.1136/sti.66.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Strand A, Brinkeborn RM, Siboulet A. Topical treatment of genital warts in men, an open study of podophyllotoxin cream compared with solution. Genitourin Med. 1995;71(6):387–390. doi: 10.1136/sti.71.6.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Swinehart JM, Skinner RB, McCarty JM, et al. Development of intralesional therapy with fluorouracil/adrenaline injectable gel for management of condylomata acuminata: two phase II clinical studies. Genitourin Med. 1997;73(6):481–487. doi: 10.1136/sti.73.6.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Syed TA, Lundin S, Ahmad SA. Topical 0.30% and 0.50% podophyllotoxin cream for self-treatment of condylomata acuminata in women. A placebo-controlled, double-blind study. Dermatology. 1994;189(2):142–145. doi: 10.1159/000246818. [DOI] [PubMed] [Google Scholar]
- 78.Syed TA, Cheema KM, Khayyami M, et al. Human leukocyte interferon-alpha versus podophyllotoxin in cream for the treatment of genital warts in males. A placebo-controlled, double-blind, comparative study. Dermatology. 1995;191(2):129–132. doi: 10.1159/000246530. [DOI] [PubMed] [Google Scholar]
- 79.Syed TA, Khayyami M, Kriz D, et al. Management of genital warts in women with human leukocyte interferon-alpha vs. podophyllotoxin in cream: a placebo-controlled, double-blind, comparative study. J Mol Med (Berl) 1995;73(5):255–258. doi: 10.1007/BF00189926. [DOI] [PubMed] [Google Scholar]
- 80.Tatti S, Swinehart JM, Thielert C, et al. Sinecatechins, a defined green tea extract, in the treatment of external anogenital warts: a randomized controlled trial. Obstet Gynecol. 2008;111(6):1371–1379. doi: 10.1097/AOG.0b013e3181719b60. [DOI] [PubMed] [Google Scholar]
- 81.von Krogh G, Szpak E, Andersson M, et al. Self-treatment using 0.25%–0.50% podophyllotoxin-ethanol solutions against penile condylomata acuminata: a placebo-controlled comparative study. Genitourin Med. 1994;70(2):105–109. doi: 10.1136/sti.70.2.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.White DJ, Billingham C, Chapman S, et al. Podophyllin 0.50% or 2.0% v podophyllotoxin 0.50% for the self treatment of penile warts: a double blind randomised study. Genitourin Med. 1997;73(3):184–187. doi: 10.1136/sti.73.3.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ormerod AD, van Voorst Vader PC, Majewski S, et al. Evaluation of the efficacy, safety, and tolerability of 3 dose regimens of topical sodium nitrite with citric acid in patients with anogenital warts: a randomized clinical trial. JAMA Dermatol. 2015;151(8):854–861. doi: 10.1001/jamadermatol.2015.0381. [DOI] [PubMed] [Google Scholar]
- 84.Reichman RC, Oakes D, Bonnez W, et al. Treatment of condyloma acuminatum with three different interferons administered intralesionally. A double-blind, placebo-controlled trial. Ann Intern Med. 1988;108(5):675–679. doi: 10.7326/0003-4819-108-5-675. [DOI] [PubMed] [Google Scholar]
- 85.Sherrard J, Riddell L. Comparison of the effectiveness of commonly used clinic-based treatments for external genital warts. Int J STD AIDS. 2007;18(6):365–368. doi: 10.1258/095646207781024711. [DOI] [PubMed] [Google Scholar]
- 86.Vance JC, Bart BJ, Hansen RC, et al. Intralesional recombinant alpha-2 interferon for the treatment of patients with condyloma acuminatum or verruca plantaris. Arch Dermatol. 1986;122(3):272–277. doi: 10.1001/archderm.1986.01660150050014. [DOI] [PubMed] [Google Scholar]
- 87.Bertolotti A, Milpied B, Fouéré S, Cabié A, Dupin N, Derancourt C. Methodological gaps and risk of bias in randomized controlled trials of local anogenital warts treatments. J Am Acad Dermatol. 2019. 10.1016/j.jaad.2019.03.080. [DOI] [PubMed]
- 88.Werner RN, Westfechtel L, Dressler C, et al. Self-administered interventions for anogenital warts in immunocompetent patients: a systematic review and meta-analysis. Sex Transm Infect. 2017;93(3):155–161. doi: 10.1136/sextrans-2016-052768. [DOI] [PubMed] [Google Scholar]
- 89.Liang J, Lu XN, Tang H, et al. Evaluation of photodynamic therapy using topical aminolevulinic acid hydrochloride in the treatment of condylomata acuminata: a comparative, randomized clinical trial. Photodermatol Photoimmunol Photomed. 2009;25:293–297. doi: 10.1111/j.1600-0781.2009.00467.x. [DOI] [PubMed] [Google Scholar]
- 90.Mi X, Chai W, Zheng H, et al. A randomized clinical comparative study of cryotherapy plus photodynamic therapy vs. cryotherapy in the treatment of multiple condylomata acuminata. Photodermatol Photoimmunol Photomed. 2011;27(4):176–180. doi: 10.1111/j.1600-0781.2011.00592.x. [DOI] [PubMed] [Google Scholar]
- 91.Monsonego J, Cessot G, Ince SE, et al. Randomised double-blind trial of recombinant interferon-beta for condyloma acuminatum. Genitourin Med. 1996;72(2):111–114. doi: 10.1136/sti.72.2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sharma N, Sharma S, Singhal C. A comparative study of liquid nitrogen cryotherapy as monotherapy versus in combination with podophyllin in the treatment of condyloma acuminata. J Clin Diagn Res. 2017;11(3):WC01–WC05. doi: 10.7860/JCDR/2017/23797.9339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liu HA, Zhang PA, An XA, et al. CO2 laser plus photodynamic therapy versus CO2 laser in the treatment of condyloma acuminatum: a randomized comparative study. J Innov Opt Health Sci. 2012;5(1):1–6. doi: 10.1142/S1793545811500088. [DOI] [Google Scholar]
- 94.Lesaffre E, Philstrom B, Needleman I, et al. The design and analysis of split-mouth studies: what statisticians and clinicians should know. Stat Med. 2009;28(28):3470–3482. doi: 10.1002/sim.3634. [DOI] [PubMed] [Google Scholar]
- 95.Spigt MG, Knipschild PG, van Schayck CP, et al. The validity and ethics of giving placebo in a randomized nonpharmacologic trial was evaluated. J Clin Epidemiol. 2005;58(4):350–356. doi: 10.1016/j.jclinepi.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 96.Boter H, van Delden JJM, de Haan RJ, Rinkel GJ, Home Evaluation of Stroke Induced Aid Study Group Patients’ evaluation of informed consent to postponed information: cohort study. BMJ. 2004;329(7457):86. doi: 10.1136/bmj.38041.636250.EE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Choi SW, Lam DMH. Funnels for publication bias—have we lost the plot? Anaesthesia. 2016;71:338–341. doi: 10.1111/anae.13355. [DOI] [PubMed] [Google Scholar]
- 98.Gilbody S, House A. Publication bias and meta-analysis. Br J Psychiatry. 1995;167(2):266. doi: 10.1192/bjp.167.2.266a. [DOI] [PubMed] [Google Scholar]
- 99.Nassir Ghaemi S, Shirzadi AA, Filkowski M. Publication bias and the pharmaceutical industry: the case of lamotrigine in bipolar disorder. Medscape J Med. 2008;10(9):211. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets analyzed during the current study are available from the corresponding author on reasonable request.