Abstract
The study investigated the use of batch and continuous thermosonication for pasteurization of pumpkin (Cucurbita moschata) juice emphasizing on its microbial, physicochemical and sensorial quality parameters. Batch thermosonication (40, 50, 60 °C, 37 kHz, 150 W) of pumpkin juice was compared with the ultrasonication (23 °C) and conventional heat treatments (40, 50, 60 °C). For batch thermosonication, maximum inactivation of Escherichia coli K-12 was 6.62 ± 0.00 log cfu/mL, meanwhile, it was 3.64 ± 0.19 log cfu/mL for heat treatment. In addition, only 0.37 ± 0.21 log cfu/mL inactivation in E. coli K-12 was obtained by ultrasonication. The designed continuous thermosonication system (0.029 L/min, 60 °C) reduced E. coli K-12 by 6.23 ± 0.34 cfu/mL log after cycle 3 (34.15 min of processing). Color properties (L*, a*, b*, ∆E), pH, total titratable acidity, total soluble solids content, turbidity and non-enzymatic browning index were determined for batch and continuously thermosonicated, ultrasonicated and heat-treated pumpkin juices. Total color change of continuously thermosonicated samples were higher than the batch thermosonicated (60 °C) ones but, lower than the conventional heat treated (60 °C) samples. Sensory panel showed general acceptance scores of fresh, batch (60 °C) and continuously thermosonicated pumpkin juice samples have no significant (P < 0.05) difference. Continuous treatment results supported by the batch ones revealed that thermosonication could be effectively used for pasteurization of pumpkin juice producing a safe product with minimum changes in physicochemical and sensorial properties.
Keywords: Pumpkin juice, Thermosonication, Ultrasound, Heat treatment
Introduction
Pumpkin is a popular crop with an approximate world production rate of 26.5 million tons (including squash and gourds) in 2016 (FAOSTAT 2018). Cucurbita moschata used in this study belongs to the genus Cucurbita and Cucurbitaceae family which are usually used in the production of jams, jellies, purees, alcoholic beverages and local desserts (Kim et al. 2012). Pumpkin was reported to be a good source of carotenoids and β-carotene (vitamin A precursor) and phenolics, flavonoids, vitamins (A and C), amino acids as well which were mostly responsible for the antioxidant activity of pumpkins (Nakhon et al. 2017). Consumption of carotenoids were reported to prevent degenerative and cardiovascular diseases, cancer and other chronic diseases that makes pumpkin juice a suitable beverage for the people interested in consuming healthy food in their daily diet (Rao and Rao 2007).
As is well known, due to their sugar content and initial microbial load, fruit and vegetable juices are prone to rapid deterioration with the contribution of intensive enzymatic activities of endogenous enzymes released during the processing of these juices. Conventional heat treatment (CH) is the most common microbial inactivation method that is used to ensure extended shelf-life and stability of fruit and vegetable juices up to date. Nevertheless, undesirable effects of thermal treatment on the sensorial, physicochemical and nutritional properties of food and increasing demand of consumers to the fresh-like food with high sensorial and nutritional quality have led the scientists to search for alternative food preservation techniques (Anaya-Esparza et al. 2017). Ultrasonication (US) of food is a promising technology. The changes in pressure due to ultrasonic waves caused cavitation. Cavitation has bactericidal effect through the thinning of peptidoglycan layer of cell wall membranes, generation of local hotspots and production of free radicals (Paniwnyk 2017). On the other hand, many researchers revealed that ultrasound treatment combined with the moderate heat, which is called thermosonication (TS), is more effective than solely using US (Herceg et al. 2013). With the combination of US and heat, a synergistic effect on the microbial inactivation is possible due to the stress factors generated for the target microorganism within the frame of the hurdle concept (Chemat et al. 2011). However, it was also reported that increasing the temperature of medium may reduce the effect of cavitation by hampering the formation of large bubbles (Guerrero et al. 2001). In case of long time and temperature exposure of fruit and vegetable juices during processing, emergence of Maillard reactions are also possible that will primarily affect the color and flavor attributes of juice in an adverse way (Jaeger et al. 2010). Food and Drug Administration (FDA) has reported that the technologies developed to have a stable product should ensure the reduction of the target bacteria (such as Escherichia coli and Listeria monocytogenes) by at least five log (FDA 2004). Therefore, in the light of these listed information, it is necessary to optimize the thermosonication conditions, to produce fruit or vegetable juices having acceptable microbial, physicochemical and nutritional quality. Acidification (pH < 4.6) of low-acid (pH > 4.6) vegetable juices such as pumpkin juice (pH: 5.65 to 5.82) enables the use of pasteurization (below 100 °C) process that is more sensitive to sensorial and nutritional quality of vegetable juices (Wu and Chen 2011). On the other hand, reduced pH of pumpkin juice is advantageous for microbial inactivation that low pH value of the medium was reported to reduce the resistance of cell to TS by altering the cell membrane permeability and structure (Marx et al. 2011).
After the successes obtained on the use of TS for microbial inactivation of pathogenic or spoilage bacteria in liquid food by batch treatments, laboratory scale studies evolved to the continuous flow ultrasonic systems that will enable scale-up of TS to industrial scale. Several previous studies used continuous flow-through ultrasonic systems to inactivate microorganisms in various fruit and vegetable juices (Zenker et al. 2003; Valero et al. 2007; Mohideen et al. 2015). However, to the best of the Authors’ knowledge, there is no reports on the thermosonication of pumpkin juice for microbial inactivation using a batch or continuous flow system. Studies on the preservation of pumpkin juice are limited.
The objective of this study is to evaluate the effects of batch ultrasonication, thermosonication and conventional thermal treatment on the microbial and physicochemical properties of pumpkin (Cucurbita moschata) juice. In addition, a newly designed continuous-flow thermosonication system was tested for the inactivation of inoculated E. coli K-12 and physicochemical quality attributes of the pumpkin juice.
Materials and methods
Pumpkin juice extraction
Pumpkins (Cucurbita moschata) were purchased from a local greengrocer at Osmaniye, Turkey. Pumpkins were selected according to their pale orange, undamaged skin and characteristic elongated pear shape. Pumpkins with decayed flesh were not used in this study. Washed pumpkins were peeled by a hand knife, their seeds and fibers in the center were discarded. Pumpkin juice was extracted from the fleshes of the pumpkin by a home-type juice extractor (J700, Braun, Germany). Produced pumpkin juice was processed or analyzed in the same day immediately after acidification by lemon juice (manually squeezed) until pH around 4.3.
Microbial analyses
E. coli K-12 (ATCC 25253), a non-pathogenic surrogate of E. coli O157:H7 was selected as the target microorganism to determine the microbial inactivation and gradually adapted to pH 4.3 using citric acid. Nutrient broth (peptone from meat 5.0 g/L; meat extract 3.0 g/L) (Merck, Germany) was used as the enrichment medium. To inoculate the pumpkin juice samples, cells propagated in tryptic soy broth with 0.75% glucose were harvested. Before E. coli K-12 inoculation, pumpkin juice was pasteurized at 90 °C (internal temperature) in 100 mL Pyrex bottle for 10 min using a water bath (Precisdig, JP Selecta S.A., Spain) to eliminate background microflora. Adequate tubes of suspended E. coli K-12 cells were inoculated into the pasteurized and cooled pumpkin juice to reach inoculum level of 106−7 cfu/mL. Enumeration was done by surface plating on tryptic soy agar in duplicate as described in Unluturk et al. (2008).
Physicochemical analyses
CIE L* (lightness), a* (redness) and b* (yellowness) color parameters were measured using a Konica Minolta CR 400 Chromometer (Konica Inc., Japan) in triplicate. Total color change (ΔE) was calculated by Eq. 1 with untreated pumpkin juice accepted as the reference.
| 1 |
pH of the juice samples was determined by a pH meter (HI 2211, Hanna Instruments, USA) at 25 °C. Total titratable acidity was determined and calculated according to Tomadoni et al. (2017) and expressed in % anhydrous citric acid (ACA). The non-enzymatic browning index (NEBI) was quantified by adding 5 ml of ethyl alcohol to 5 ml of sample, centrifuging for 20 min at 3000 rpm and the absorbance of the supernatant was read at 420 nm in a spectrophotometer (SP 3000 nano, Optima, Japan). Total soluble solids content (°Bx) and turbidity of the juice samples was measured at room temperature by a digital refractometer (Krüss DR6000, Krüss Optronic GmbH, Germany) and turbidimeter (HACH 2100 N, HACH Company, USA), respectively. Turbidity results were given as nephelometric turbidity unit (NTU). Density and viscosity of pumpkin juice were measured by a digital density meter (Kyoto DA650, Kyoto electronics manufacturing Co., Ltd, Japan) at 20 °C and by Brookfield Viscometer LVDV-II + PRo Extra (Brookfield Engineering Laboratories, USA) with SC4-18 spindle and 13RP sample chamber at 22 °C, respectively.
Ultrasonication, thermosonication and conventional heat treatment
The pumpkin juice samples were treated in 10 mL plastic capped sterile glass test tubes by immersing in the geometric center of the ultrasonic bath (Elmasonic ultrasonic E-100H, Germany) with 37 kHz frequency and 150 W effective ultrasonic power or static water bath (Precisdig, JP Selecta S.A., Spain). The duration of treatments (30 min for TS and US, 15 min for CH) were selected because inacceptable color observed in the pumpkin juice (preliminary studies) after 30 min of TS and 15 min of CH at 60 °C. The temperatures of pumpkin juice samples were kept constant at 23 ± 1 for US and 40 ± 3, 50 ± 3, 60 ± 3 °C for TH and CH treatments. Temperature monitoring was done by a K-type thermocouple that was replaced in an extra test tube of pumpkin juice. After US, TS or CH treatments test tubes were immediately immersed into ice bath to avoid excess heat. All treatments were performed in dark to avoid any possible interference with light. The following codes were used to describe the different treatments in this study: control (freshly prepared or no treatment); US (ultrasonicated); TS40, TS50 or TS60 (thermosonicated at 40, 50 or 60 °C respectively); CH40, CH50 or CH60 (conventionally heat-treated at 40, 50 or 60 °C respectively).
Continuous thermosonication system and flow dynamics
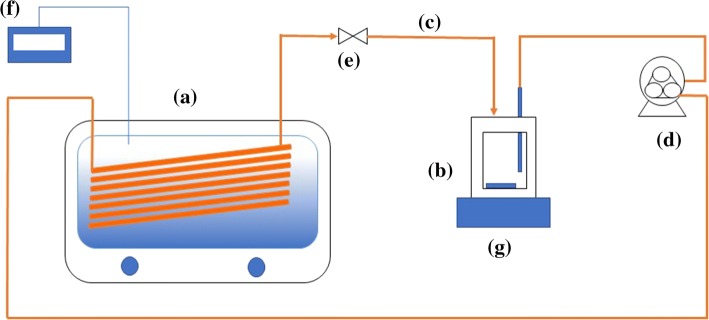
Pumpkin juice was circulated in the designed continuous system (Fig. 1). Inoculation of E. coli K-12 (106−7 cfu/mL) was done after the temperature of pumpkin juice balanced to 60 °C in the reservoir flask (250 mL). The inoculum distributed homogenously by a Teflon® magnetic stir bar and a magnetic stirrer placed under the reservoir. The flow rate was adjusted to 0.029 L/min via a peristaltic pump (Percom N-M, Selecta, Spain). Pumpkin juice was exposed to thermosonication (Fig. 1A) as it flows through the silicone pipe (244 cm) that was completely immersed in the ultrasonic bath (at 60 ± 3 °C). K-type thermocouple and infrared thermometer (Fluke IR Thermometer 568) were used to monitor temperatures of water in the ultrasonic bath and pumpkin juice in the reservoir flask and silicone pipes (Ø 5 mm). Samples were aseptically taken using Bunsen burner flame. Sampling times were 0, 3.65 (cycle 1), 18.9 (cycle 2) and 34.15 (cycle 3) minutes. For each cycle, juice residence time was 2.87 min in the ultrasonic bath. Reservoir and piping were autoclaved (121 °C, 15 min) before and after each treatment.
Fig. 1.
Continuous thermosonication system (a: ultrasonic bath, b: pumpkin juice reservoir flask, c: insulated silicone pipes, d: peristaltic pump, e: sampling valve, f: thermocouple on digital thermometer, g: magnetic stirrer)
The viscosity (µ), density (ρ), average rate of flow (ν) of pumpkin juice were; 1.84 × 10−2 Pa s, 1041.84 kg/m3, 0.0246 m/s, respectively. Average rate of flow was calculated by the volumetric flow rate (m3/s) divided by cross-sectional area of tube (m2). The diameter of tube (D) was 5 × 10−3 m. Using Eq. 2, Reynolds number was calculated as 7.0. Then a laminar type of flow can be referred, as the Reynolds number less than 2100 indicates laminar flow for Newtonian liquids.
| 2 |
Sensory evaluation
A panel consisting of 20 untrained panelists was established. 30 mL of fresh, batch thermosonicated and continuously thermosonicated pumpkin juice samples were served to the panelists under room temperature. Batch thermosonication was conducted at 60 °C as mentioned in Thermosonication section. Continuously thermosonicated (0.029 L/min, 60 °C) pumpkin juice samples were produced using the designed continuous thermosonication system (Fig. 1) by collecting the juice from the third cycle. Sensory panel was carried out immediately after the production of fresh, batch and continuously thermosonicated samples. Samples were coded by 3-digit random numbers. Panelists were asked to rate odor, aroma, color, flavor, appearance, sweetness, sourness parameters and general acceptance of samples using a 5-point hedonic scale, where 1 indicated “very much disliked”, 3 indicated “neither like nor dislike” and 5 indicated “liked very much”. For eliminating the residual taste between samples, water and unsalted cracker was provided to panelists.
Statistical analysis
All treatments (batch or continuous) and analyses were carried out in triplicate. The results of microbial, physicochemical and sensorial analyses were evaluated by one-way analysis of variance (ANOVA) test using Minitab software (Minitab Inc., State College, PA, USA). The differences among means were compared by Tukey test. Significance level was 0.05 for the p value.
Results and discussion
Microbial inactivation of E. coli K-12 in batch treatments
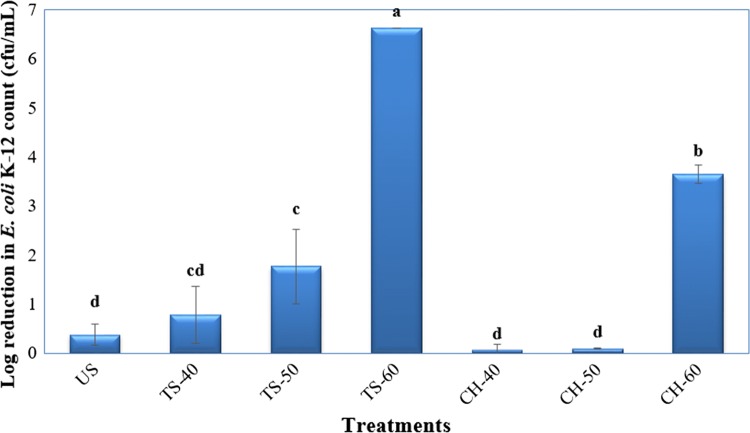
FDA’s 5 log reduction in the target microorganism criterion was well accepted by many researchers and emerging food stabilization techniques were designed and tested to approach this goal. Within this perspective, microbial inactivation performances of ultrasonication, thermosonication and conventional heat treatment methods were evaluated via log reduction in the number of target microorganism E. coli K-12 for the pasteurization of pumpkin juice and illustrated in Fig. 2. The results in Fig. 2 revealed that ultrasonication was able to inactivate E. coli K-12 only by less than 1 log, however, thermosonication at 60 °C complied with the FDA’a 5 log criterion by 6.62 ± 0.00 log cfu/mL reduction in the target microorganism. Thermal treatment at 60 °C could only inactivate E. coli K-12 by 3.64 ± 0.19 log cfu/mL that was not appropriate to reach FDA’s 5 log criterion. During thermosonication, acoustic cavitation may enhance heat transfer throughout the ultrasound bath and inside the sample due to improved critical heat flux and increased heat transfer coefficient (Zhang et al. 2015).
Fig. 2.
Effects of ultrasonication, thermosonication and conventional heat treatments on log reduction in E. coli K-12 count in pumpkin juice. US: 23 °C, 30 min; TS-40, 50, 60: thermosonication at 40, 50 and 60 °C, 30 min; CH-40, 50, 60: conventional heat treatment at 40, 50 and 60 °C, 15 min. Different letters above the bars represent significant differences (P < 0.05) among treatments according to Tukey test
The microbial inactivation performance of thermosonication increased as the treatment temperature increased from 40 °C to 60 °C (Fig. 2). It is possible that the cell walls of E. coli K-12 were weakened by ultrasound and became more sensitive to heat treatment (Anaya-Esparza et al. 2017). Another reason will be the low pH of studied pumpkin juice (pH > 4.3) that may reduce the resistance of E. coli K-12 cells to thermosonication via affecting membrane permeability (Marx et al. 2011). Abid et al. (2014a) similarly observed an insignificant reduction in natural microflora of apple juice by ultrasonication, however, whole inactivation could be achieved by thermosonication at 60 °C.
Physicochemical changes in batch treatments
Major goal of preservation methods for fruit or vegetable juices is to eliminate microbial risks for the consumer health. However, physicochemical, organoleptic and nutritional properties of juices should also meet consumers’ acceptance criteria. Therefore, effect of ultrasonication, thermosonication and conventional heat treatment methods on the physicochemical properties of pumpkin juice was determined. Color is the key criterion for the consumers for the decision of accepting or rejecting the fruit or vegetable juices (Abid et al. 2014a). Total color change (∆E) of conventional heat treatment (CH-40, 50, 60) samples were significantly (P < 0.05) higher compared to the ultrasonicated (US) and thermosonicated (TS-40, 50, 60) samples (Table 1). The differences in total color change were observed as a result of the significantly (P < 0.05) higher a* and b* values of CH-40, 50 and 60 samples compared to US, TS-40, 50 and 60 samples (Table 1). Any change in a* value of fruit or vegetable juices will be associated to the browning fact as well as the degradation of carotenoids responsible for the specific color of that juice (Pokhrel et al. 2017). Then, insignificant changes in a* and b* values of TS pumpkin juice samples indicated insignificant degradation of carotenoids. Treatment type has no significant effect (P < 0.05) on the L* value of pumpkin juice (Table 1). According to Cserhalmi et al. (2006) color change in US and TS (TS-40, 50, 60) samples were slightly noticeable, whereas it was well visible for the CHT samples (CH-40, 50, 60).
Table 1.
Color parameters and non-enzymatic browning index of pumpkin juice after batch treatments
| Treatment | Color parameters | NEBI | |||
|---|---|---|---|---|---|
| L* value | a* value | b* value | ∆E | ||
| Fresh | 40.88 ± 0.18ab | 1.96 ± 0.06f | 22.16 ± 0.49ef | 0.20 ± 0.00c | |
| US | 40.73 ± 0.13ab | 2.06 ± 0.14ef | 23.15 ± 0.65cdef | 1.04 ± 0.61b | 0.18 ± 0.01c |
| TS-40 | 41.24 ± 0.26a | 2.28 ± 0.08e | 22.82 ± 0.44def | 0.89 ± 0.38b | 0.20 ± 0.01c |
| TS-50 | 40.44 ± 0.47ab | 2.02 ± 0.01ef | 22.03 ± 0.84ef | 1.02 ± 0.31b | 0.24 ± 0.02b |
| TS-60 | 40.32 ± 0.59ab | 2.21 ± 0.12ef | 21.60 ± 0.76f | 1.24 ± 0.29b | 0.33 ± 0.02a |
| CH-40 | 41.20 ± 0.19a | 3.66 ± 0.06d | 26.07 ± 0.57a | 4.29 ± 0.52a | 0.34 ± 0.02a |
| CH-50 | 41.13 ± 0.08a | 4.02 ± 0.03d | 25.44 ± 0.09ab | 4.20 ± 0.07a | 0.28 ± 0.00b |
| CH-60 | 40.07 ± 0.29ab | 4.33 ± 0.06c | 24.81 ± 0.33abc | 3.66 ± 0.28a | 0.27 ± 0.00b |
Values are means ± standard deviations of triplicate determinations
Fresh: no treatment; US: ultrasonicated at 23 °C for 30 min; TS-40, 50, 60: thermosonicated at 40, 50, 60 °C for 30 min; CH-40, 50, 60: conventional thermal treatment at 40, 50, 60 °C for 15 min, NEBI: non-enzymatic browning index
Values with different superscript letters in a column are significantly different (P < 0.05)
Non-enzymatic browning was defined as the browning of fruit or vegetable juices as a result of reactions that may cause undesired effects on the color and nutritional properties of the food (Caminiti et al. 2011). Non-enzymatic browning index values of heat treated samples were relatively higher than that of fresh, ultrasonicated and thermosonicated samples (Table 1), in compatible with the effect of treatments on the a* values of pumpkin juices (Table 1). Non-enzymatic browning index of fresh, ultrasonicated and 40 °C-thermosonicated samples have no significant (P < 0.05) difference showing the cavitation during the sonication did not induced the Maillard reactions (Caminiti et al. 2011). However, as the thermosonication temperature raised from 40 to 60 °C (Table 1), NEBI of pumpkin juice samples increased significantly (P < 0.05) possibly due to the formation or release of brown pigments in the pumpkin juice. Maran et al. (2015) stated extraction yield of natural pigments (betacyanin and betaxanthin) from a plant source (Bougainvillea glabra flower) increased with the increasing temperature due to accelerated solubility and diffusivity of solid from the plant material using ultrasound-assisted extraction techniques.
On the other hand, NEBI of heat-treated pumpkin juice samples reduced with the increasing temperature (Table 1), indicating the degradation of pigment compounds as similarly observed by Cerón-García et al. (2018) during the heat-assisted extraction of carotenoids from microalgae Tetraselmis suecica and Protoceratium reticulatum.
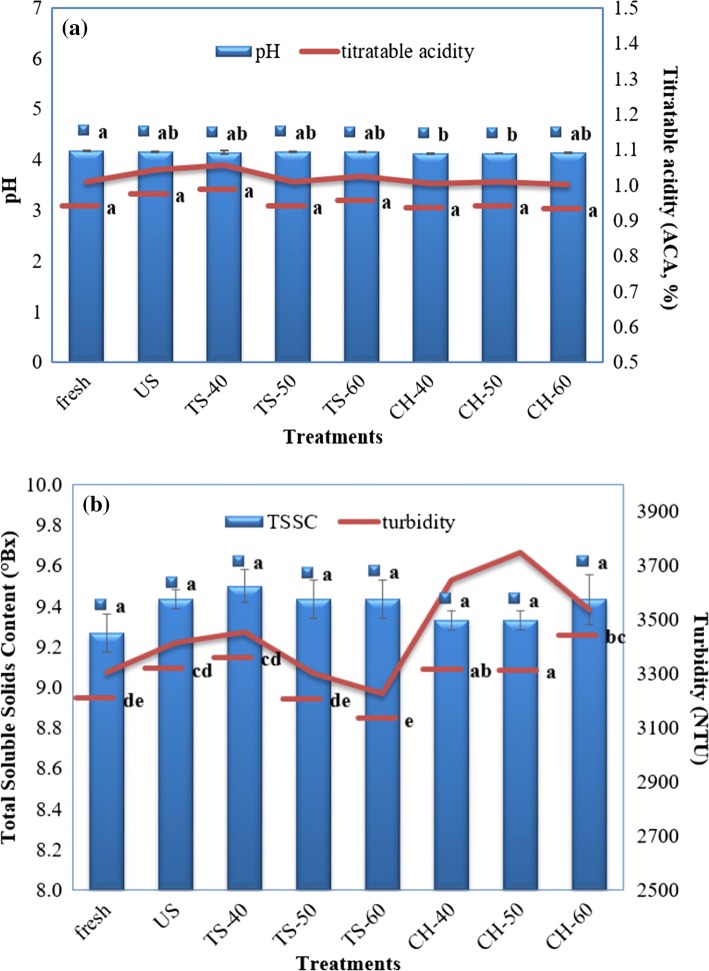
Figure 3a, b illustrated the effect of ultrasonication, thermosonication and conventional heat treatment on the pH and titratable acidity of pumpkin juice. The treatments did not induce any significant change (P < 0.05) in the pH of pumpkin juice. Cruz-Cansino et al. (2015) reported no significant difference (P < 0.05) between the pH values of thermosonicated (at 47.5 and 50.0 °C) and fresh purple cactus pear juice samples. According to Fig. 3a, pH values of all treated samples were in the range of 4.12–4.17. pH change is directly related to the sensorial quality of a fruit or vegetable juice. Then, the non-significant change (P < 0.05) in the pH of TS-60 (the most successful E. coli K-12 inactivation treatment) with respect to the fresh pumpkin juice was a positive result for the manufacturers’ choice of thermosonication. Additionally, there was no significant difference (P < 0.05) between the ultrasonicated, thermosonicated or heat-treated samples and the fresh pumpkin juice (Fig. 3a) with respect to titratable acidity (% ACA). This result was in accordance with the findings of Tomadoni et al. (2017) who have observed non-significant effects of heat treatment (90 °C, 60 s) and ultrasonication (10 min) with respect to control on the total acidity of strawberry juice.
Fig. 3.
Effects of US, TS and CH treatments on the a pH and titratable acidity and b total soluble solids content and turbidity of pumpkin juice. Fresh: no treatment; US: ultrasonicated at 23 °C for 30 min; TS-40, 50, 60: thermosonicated at 40, 50,60 °C for 30 min. Different letters above the bars represent significant differences (P < 0.05) among treatments according to Tukey test
Pumpkin juice samples having 9.2–9.6°Bx (Fig. 3b) is compatible with the ones reporting 12°Bx for apple juice (Abid et al. 2014a), 7.8°Bx for pulpy carrot juice (Ordóñez-Santos et al. 2017) and 10.3°Bx for strawberry juice (Tomadoni et al. 2017). The applied treatments had no significant effect (P < 0.05) on the total soluble solids content of pumpkin juice (Fig. 3b). Similarly, non-significant effect of ultrasonication, thermosonication and conventional heat treatment on the total soluble solids content of fruit or vegetable juices was observed in various studies (Abid et al. 2014a; Adiamo et al. 2017). Santhirasegaram et al. (2013) stated that low power ultrasound applications (used in the present study) may enhance clarity due to reduction in viscosity and pectin content. However, in the present study, the turbidity of thermosonicated or ultrasonicated samples did not differ significantly (P < 0.05) from the fresh pumpkin juice (Fig. 3b). Turbidity levels of conventional heat treated pumpkin juice samples were significantly higher (P < 0.05) than the fresh, ultrasonicated and thermosonicated ones (Fig. 3b). Increased turbidity in orange-carrot juice blend after heat treatment (98 °C, 21 s) with respect to untreated juice was also observed by Rivas et al. (2006). Ugarte-Romero et al. (2006) reported higher turbidity for heat treated apple cider samples compared to the thermosonicated (40 and 60 °C) ones due to probable effect of relatively long (> 17.7 min) thermosonication time causing turbidity loss.
Microbial inactivation of E. coli K-12 in continuous thermosonication system
As the microbial and physicochemical results in batch treatment revealed the potential use of thermosonication for the pasteurization of pumpkin juice, a bench-top continuous thermosonication system was designed, set up and used for the same purpose to create an information infrastructure for the industrial production process of pumpkin juice.
Maximum log reduction in E. coli K-12 number among the batch treatments was obtained at 60 °C thermosonication (Fig. 2), therefore the continuous thermosonication system was experimented at 60 °C for the pasteurization of pumpkin juice (Fig. 1). Inactivation of E. coli K-12 inoculated to the 250 mL pumpkin juice was monitored (Table 2) by sampling at cycle (pass) 1, cycle 2 and cycle 3 during the flow at a rate of 0.029 L/min through the designed continuous thermosonication system at 60 °C. Table 2 indicates after cycle 1, log reduction in E. coli K-12 number was 2.49 log ± 0.22 cfu/mL. The log reduction in E. coli K-12 increased as the cycle number increased and reached to 6.23 ± 0.34 cfu/mL inactivation after cycle 3 (Table 2). The circulation of pumpkin juice was terminated after cycle 3 as the microbial inactivation of the target microorganism complied with the FDA’s 5 log reduction criteria. For each cycle, pumpkin juice was subjected to thermosonication for 172 s (mean residence time within the ultrasonic bath at 60 °C), which is equal to 8.6 min of total thermosonication at the end of cycle 3. The difference between the batch thermosonication (Fig. 2) at 60 °C (TS-60) and continuous thermosonication at 60 °C (cycle 3) was insignificant (P < 0.05) with respect to log reduction in E. coli K-12 number, however, thermosonication time was reduced by 71% that makes continuous system more economic allowing the user to pasteurize relatively higher volume of pumpkin juice in a shorter time.
Table 2.
Microbial and physicochemical changes in continuous thermosonication of pumpkin juice
| Microbial or physicochemical property | Fresh | 1. Cycle | 2. Cycle | 3. Cycle |
|---|---|---|---|---|
| Log reduction in E. coli K-12 count (cfu/mL) | – | 2.49 ± 0.22c | 4.00 ± 0.24b | 6.23 ± 0.34a |
| L* | 42.12 ± 0.16b | 43.58 ± 0.37a | 43.70 ± 0.45a | 43.86 ± 0.68a |
| a* | 3.54 ± 0.04b | 4.01 ± 0.23ab | 4.56 ± 0.25a | 4.73 ± 0.46a |
| b* | 31.41 ± 0.34a | 32.07 ± 0.52a | 32.37 ± 0.10a | 32.41 ± 0.65a |
| Total color change (∆E) | – | 1.77 ± 0.35a | 2.17 ± 0.19a | 2.36 ± 0.99a |
| pH | 4.41 ± 0.00a | 4.42 ± 0.03a | 4.43 ± 0.01a | 4.46 ± 0.01a |
| Titratable acidity (%, ACA) | 0.99 ± 0.01a | 0.90 ± 0.00c | 0.99 ± 0.01a | 0.97 ± 0.00b |
| Total soluble solids content (°Bx) | 9.13 ± 0.05a | 9.00 ± 0.16a | 9.20 ± 0.16a | 9.37 ± 0.05a |
| Turbidity (NTU) | 3606.00 ± 61.19b | 4295.00 ± 188.23a | 4464.00 ± 199.06a | 4501.00 ± 53.24a |
| Non-enzymatic browning index (A420 nm) | 0.31 ± 0.02a | 0.26 ± 0.02a | 0.28 ± 0.03a | 0.24 ± 0.01a |
Values are means ± standard deviations of triplicate determinations
Values with different superscript letters in a row are significantly different (P < 0.05)
There are limited reports on the use of continuous systems for pasteurization of juices by ultrasonication or thermosonication. Among these Mohideen et al. (2015) reported to obtain 1.36 log reduction in aerobic plate count in the blueberry juice flowing in the continuous ultrasonication system with 93.5 mL/min (100% amplitude). Similarly Valero et al. (2007) could achieve negligible reductions of microbial count after continuous ultrasonic treatment of orange juice with a flow rate of 3000 L/h. In a pilot scale ultrasound-assisted thermal treatment system designed by Zenker et al. (2003), target microorganism E. coli K12 DH 5 α inoculated in phosphate buffer (pH 7.0) was inactivated by 3.8 log flowing with 26 L/h at 60 °C.
Physicochemical changes in continuous thermosonication system
The designed continuous thermosonication system was able to meet FDA’s 5 log inactivation criterion in the inactivation of E. coli K-12, however, besides the microbial safety, manufacturers should also guarantee physicochemical and nutritional properties of the processed fruit or vegetable juices. Therefore, some important physicochemical properties of pumpkin juice during its flow in the designed continuous thermosonication system was determined (Table 2).
In the present study, lightness (L* value) of the pumpkin juice thermosonicated in the continuous system was significantly higher than the control (Table 2). Contrary to this result, Mohideen et al. (2015) found that ultrasonication slightly reduced the lightness of blueberry juice in a continuous system. According to Tiwari et al. (2009), the reduction of lightness in juices indicates browning and a darker color. It was also observed that no significant difference (P < 0.05) in the lightness of pumpkin juice occurred during its circulation (between cycle 1, 2 or 3) in the system. In accordance with the results of batch experiments (Table 2), redness (a*) values of the continuously thermosonicated pumpkin juice samples were relatively higher than the control. However, contrary to the results of batch experiments, continuous thermosonication achieved to fix the non-enzymatic browning index values of the pumpkin juice samples compared to the control. This result is also in accordance with the slightly improved L* values of the continuously thermosonicated samples compared to the control (Table 2). Continuous thermosonication (cycle 1, 2 or 3) did not cause a significant difference (P < 0.05) in the yellowness (b*) of the pumpkin juice samples with respect to the control.
A non-significant difference (P < 0.05) was obtained between the total color change (∆E) values of the pumpkin juice samples taken from the cycles 1, 2 and 3. The ∆E value at the end of cycle 3 was observed as 2.36 ± 0.99, that is classified as noticeable (1.5 < ∆E < 3.0) according to Cserhalmi et al. (2006). Batch thermosonicated (60 °C) samples were in the slightly noticeable (0.5 < ∆E < 1.5) group (Table 2), indicating continuous thermosonication at 60 °C resulted in relatively higher change in total color. Orange juice treated by a continuous ultrasound-assisted thermal system for 30 s was found to have 2.56 of ∆E (Zenker et al. 2003), that is in accordance with the ∆E value of pumpkin juice obtained after cycle 3 of the present study (Table 2).
Continuous thermosonication (60 °C) (for cycles 1, 2 and 3) did not significantly (P < 0.05) change the pH and total soluble solids content of pumpkin juice samples compared to control (Table 2) compatible with the findings of Mohideen et al. (2015). A fluctuation was observed between the total titratable acidity values of the thermosonicated (for cycles 1, 2 and 3) and control samples (Table 2). Circulation of the pumpkin juice in the continuous thermosonication system did not significantly (P < 0.05) change the turbidity of the samples, on the other hand, the mentioned values were significantly (P < 0.05) higher than the control (Table 2). No measured turbidity value could be found in the literature for any continuously thermosonicated fruit or vegetable juice. However, the increase in turbidity of the continuously thermosonicated pumpkin juice samples with respect to control may be caused by the slight increase in the total color change, likewise Donahue et al. (2004) have reported of a correlation between turbidity and color of juices. Continuous thermosonication (for cycles 1, 2 and 3) did not significantly (P < 0.05) changed non-enzymatic browning index of pumpkin juice samples compared to control, however, Valero et al. (2007) reported significantly (P < 0.05) increased NEBI values after continuous ultrasonication of orange juice with respect to the fresh samples.
Effect of treatments on sensory properties of pumpkin juice
Consumers’ choice of acceptance or rejection of juices mainly depend on the satisfaction of sensory expectations. Therefore, effect of batch (60 °C 30 min) and continuous (0.029 L/min, 60 °C) thermosonication on the organoleptic properties of pumpkin juice was evaluated by a sensory panel. The sensory scores of batch and continuously thermosonicated samples were compared to fresh ones. Sensory analysis results were given in Table 3. The batch and continuous thermosonication significantly (P < 0.05) affected aroma, color, flavor and sweetness of pumpkin juice samples. Abid et al. (2014b) reported ultrasonication treatment enhances release of sugar from ultrasound-disrupted cells. However, panelist scores for sweetness were lower for batch and continuously thermosonicated samples compared to fresh one. The panelists’ liking for the sourness of both thermosonicated samples was lower than the fresh ones (Table 3). Tomadoni et al. (2017) also reported of ultrasonicated strawberry juice having higher acidity scores than the untreated samples. On the other hand, color of batch thermosonicated samples were superior to the continuously thermosonicated samples, which is also compatible with the total color change (∆E) results where, continuously thermosonicated samples (Table 2) had higher ∆E values than the batch ones (Table 1). No significant (P < 0.05) difference can be found between the three types of samples with respect to odor, appearance, sourness and general acceptance. However, batch or continuously thermosonicated samples had lower scores compared to the fresh pumpkin juice in general. These results showed that thermosonication treatment had a slight effect on the sensory properties of pumpkin juice.
Table 3.
Sensory evaluation of fresh, batch and continuously thermosonicated pumpkin juice
| Sensorial parameter | Fresh | Batch thermosonicated (TS-60) | Continuously thermosonicated |
|---|---|---|---|
| Odor | 3.0 ± 1.3a | 2.9 ± 1.0a | 2.3 ± 1.1a |
| Aroma | 3.7 ± 1.2a | 2.8 ± 1.0ab | 2.5 ± 1.2b |
| Color | 4.1 ± 1.3ab | 4.4 ± 1.1a | 3.2 ± 0.9b |
| Flavor | 3.7 ± 1.7a | 2.5 ± 1.2b | 2.3 ± 1.1b |
| Appearance | 4.0 ± 1.1a | 4.2 ± 1.3a | 3.7 ± 0.8a |
| Sweetness | 3.6 ± 1.4a | 2.8 ± 1.1ab | 2.1 ± 1.2b |
| Sourness | 3.4 ± 1.5a | 2.7 ± 1.3a | 3.1 ± 1.4a |
| General acceptance | 3.4 ± 1.5a | 3.3 ± 1.2a | 2.7 ± 1.2a |
Values are means ± standard deviations of 20 determinations
Fresh: no treatment; Batch thermosonicated: 60 °C, 30 min, Continuously thermosonicated: at 60 °C, from cycle 3
Values with different superscript letters in a row are significantly different (P < 0.05)
Conclusion
This study demonstrated the potential use of batch thermosonication for the pasteurization of pumpkin juice compared with the ultrasonication and conventional heat treatment. In addition, a novel bench-top continuous thermosonication system (60 °C of process temperature, 0.029 L/min flow rate, 34.15 min of total process time) was tested for the treatment of pumpkin juice. Both batch (60 °C) and continuous thermosonication by the designed bench-top system have complied with the FDA’s criterion of 5 log reduction in the target microorganism. In general, the physicochemical properties such as color of batch thermosonicated pumpkin juices were superior to ultrasound and heat-treated ones. Sensorial color properties of batch thermosonicated pumpkin juices had higher scores compared to the fresh and continuously thermosonicated ones, whereas the difference between general acceptance of all three tested samples were statistically (P < 0.05) insignificant. Promising microbial, physicochemical and sensory evaluation results showed that continuous thermosonication system is a useful technology to obtain a safe and minimally processed pumpkin juice. Additionally, further research would be done on the optimization of the process conditions to validate and construct an informational base for the design of a pilot scale thermosonication process.
Acknowledgement
This work was financially supported by OKÜBAP (Scientific Research Projects Unit of Osmaniye Korkut Ata University) with the project number OKÜBAP-2017-PT3-031. Prof. Dr. T. Koray Palazoğlu (Mersin University, Turkey) is kindly acknowledged for the viscosity measurement.
Compliance with ethical standards
Conflict of interest
The Authors declare that there are no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abid M, Jabbar S, Hu B, et al. Thermosonication as a potential quality enhancement technique of apple juice. Ultrason Sonochem. 2014;21:984–990. doi: 10.1016/j.ultsonch.2013.12.003. [DOI] [PubMed] [Google Scholar]
- Abid M, Jabbar S, Wu T, Hashim MM, et al. Sonication enhances polyphenolic compounds, sugars, carotenoids and mineral elements of apple juice. Ultrason Sonochem. 2014;21(1):93–97. doi: 10.1016/j.ultsonch.2013.06.002. [DOI] [PubMed] [Google Scholar]
- Adiamo OQ, Ghafoor K, Al-Juhaimi F, et al. Effects of thermosonication and orange by-products extracts on quality attributes of carrot (Daucus carota) juice during storage. Int J Food Sci Technol. 2017;52:2115–2125. doi: 10.1111/ijfs.13490. [DOI] [Google Scholar]
- Anaya-Esparza LM, Velázquez-Estrada RM, Roig AX, et al. Thermosonication: an alternative processing for fruit and vegetable juices. Trends Food Sci Technol. 2017;61:26–37. doi: 10.1016/j.tifs.2016.11.020. [DOI] [Google Scholar]
- Caminiti IM, Noci F, Muñoz A, et al. Impact of selected combinations of non-thermal processing technologies on the quality of an apple and cranberry juice blend. Food Chem. 2011;124:1387–1392. doi: 10.1016/j.foodchem.2010.07.096. [DOI] [Google Scholar]
- Cerón-García MC, González-López CV, Camacho-Rodríguez J, et al. Maximizing carotenoid extraction from microalgae used as food additives and determined by liquid chromatography (HPLC) Food Chem. 2018;257:316–324. doi: 10.1016/j.foodchem.2018.02.154. [DOI] [PubMed] [Google Scholar]
- Chemat F, Zill-E-Huma M, Khan MK. Applications of ultrasound in food technology: processing, preservation and extraction. Ultrason Sonochem. 2011;18:813–835. doi: 10.1016/j.ultsonch.2010.11.023. [DOI] [PubMed] [Google Scholar]
- Cruz-Cansino NDS, Ramírez-Moreno E, León-Rivera JE, et al. Shelf life, physicochemical, microbiological and antioxidant properties of purple cactus pear (Opuntia ficus indica) juice after thermoultrasound treatment. Ultrason Sonochem. 2015;27:277–286. doi: 10.1016/j.ultsonch.2015.05.040. [DOI] [PubMed] [Google Scholar]
- Cserhalmi Z, Sass-Kiss Á, Tóth-Markus M, Lechner N. Study of pulsed electric field treated citrus juices. Innov Food Sci Emerg Technol. 2006;7:49–54. doi: 10.1016/j.ifset.2005.07.001. [DOI] [Google Scholar]
- Donahue D, Canitez N, Bushway A. UV inactivation of E. coli O157:H7 in apple cider: quality, sensory and shelf-life analysis. J Food Process Preserv. 2004;28:268–287. doi: 10.1111/j.1745-4549.2004.23062.x. [DOI] [Google Scholar]
- FAOSTAT (2018) Production share of pumpkin (incl. squash and gourds) in 2016. http://www.fao.org/faostat/en/#data/QC/visualize
- FDA (2004) Guidance for industry: juice HACCP hazards and controls guidance, 1st ed. Food and Drug Administration of United States of America. Final Guidance
- Guerrero S, López-Malo A, Alzamora S. Effect of ultrasound on the survival of Saccharomyces cerevisiae: influence of temperature, pH and amplitude. Innov Food Sci Emerg Technol. 2001;2:31–39. doi: 10.1016/S1466-8564(01)00020-0. [DOI] [Google Scholar]
- Herceg Z, Markov K, Šalamon BS, et al. Effect of high intensity ultrasound treatment on the growth of food spoilage bacteria. Food Technol Biotechnol. 2013;51:352–359. [Google Scholar]
- Jaeger H, Janositz A, Knorr D. The Maillard reaction and its control during food processing. The potential of emerging technologies. Pathol Biol. 2010;58:207–213. doi: 10.1016/j.patbio.2009.09.016. [DOI] [PubMed] [Google Scholar]
- Kim MY, Kim EJ, Kim YN, et al. Comparison of the chemical compositions and nutritive values of various pumpkin (Cucurbitaceae) species and parts. Nutr Res Pract. 2012;6:21–27. doi: 10.4162/nrp.2012.6.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maran JP, Priya B, Nivetha CV. Optimization of ultrasound-assisted extraction of natural pigments from Bougainvillea glabra flowers. Ind Crops Prod. 2015;63:182–189. doi: 10.1016/j.indcrop.2014.09.059. [DOI] [Google Scholar]
- Marx G, Moody A, Bermúdez-Aguirre D. A comparative study on the structure of Saccharomyces cerevisiae under nonthermal technologies: high hydrostatic pressure, pulsed electric fields and thermo-sonication. Int J Food Microbiol. 2011;151:327–337. doi: 10.1016/j.ijfoodmicro.2011.09.027. [DOI] [PubMed] [Google Scholar]
- Mohideen FW, Solval KM, Li J, et al. Effect of continuous ultra-sonication on microbial counts and physico-chemical properties of blueberry (Vaccinium corymbosum) juice. LWT Food Sci Technol. 2015;60:563–570. doi: 10.1016/j.lwt.2014.07.047. [DOI] [Google Scholar]
- Nakhon PP na S, Jangchud K, Jangchud A, Prinyawiwatkul W. Comparisons of physicochemical properties and antioxidant activities among pumpkin (Cucurbita moschata L.) flour and isolated starches from fresh pumpkin or flour. Int J Food Sci Technol. 2017;52:2436–2444. doi: 10.1111/ijfs.13528. [DOI] [Google Scholar]
- Ordóñez-Santos LE, Martínez-Girón J, Arias-Jaramillo ME. Effect of ultrasound treatment on visual color, vitamin C, total phenols, and carotenoids content in Cape gooseberry juice. Food Chem. 2017;233:96–100. doi: 10.1016/j.foodchem.2017.04.114. [DOI] [PubMed] [Google Scholar]
- Paniwnyk L. Applications of ultrasound in processing of liquid foods: a review. Ultrason Sonochem. 2017;38:794–806. doi: 10.1016/j.ultsonch.2016.12.025. [DOI] [PubMed] [Google Scholar]
- Pokhrel PR, Bermúdez-Aguirre D, Martínez-Flores HE, et al. Combined effect of ultrasound and mild temperatures on the inactivation of E. coli in fresh carrot juice and changes on its physicochemical characteristics. J Food Sci. 2017;82:2343–2350. doi: 10.1111/1750-3841.13787. [DOI] [PubMed] [Google Scholar]
- Rao AV, Rao LG. Carotenoids and human health. Carotenoids Hum Heal. 2007;55:207–2016. doi: 10.1016/j.phrs.2007.01.012. [DOI] [PubMed] [Google Scholar]
- Rivas A, Rodrigo D, Martínez A, et al. Effect of PEF and heat pasteurization on the physical-chemical characteristics of blended orange and carrot juice. LWT Food Sci Technol. 2006;39:1163–1170. doi: 10.1016/j.lwt.2005.07.002. [DOI] [Google Scholar]
- Santhirasegaram V, Razali Z, Somasundram C. Effects of thermal treatment and sonication on quality attributes of Chokanan mango (Mangifera indica L.) juice. Ultrason Sonochem. 2013;20:1276–1282. doi: 10.1016/j.ultsonch.2013.02.005. [DOI] [PubMed] [Google Scholar]
- Tiwari BK, O’Donnell CP, Cullen PJ. Effect of non thermal processing technologies on the anthocyanin content of fruit juices. Trends Food Sci Technol. 2009;20:137–145. doi: 10.1016/j.tifs.2009.01.058. [DOI] [Google Scholar]
- Tomadoni B, Cassani L, Viacava G, et al. Effect of ultrasound and storage time on quality attributes of strawberry juice. J Food Process Eng. 2017;40:1–8. doi: 10.1111/jfpe.12533. [DOI] [Google Scholar]
- Ugarte-Romero E, Feng H, Martin SE, et al. Inactivation of Escherichia coli with power ultrasound in apple cider. J Food Sci. 2006;71:E102–E108. doi: 10.1111/j.1365-2621.2006.tb08890.x. [DOI] [Google Scholar]
- Unluturk S, Atilgan MR, Handan Baysal A, Tari C. Use of UV-C radiation as a non-thermal process for liquid egg products (LEP) J Food Eng. 2008;85:561–568. doi: 10.1016/j.jfoodeng.2007.08.017. [DOI] [Google Scholar]
- Valero M, Recrosio N, Saura D, et al. Effects of ultrasonic treatments in orange juice processing. J Food Eng. 2007;80:509–516. doi: 10.1016/j.jfoodeng.2006.06.009. [DOI] [Google Scholar]
- Wu JSB and Chen S-C . In: Handbook of vegetables and vegetable processing. Sinha NK, editor. Hoboken: Wiley-Blackwell; 2011. p. 772. [Google Scholar]
- Zenker M, Heinz V, Knorr D. Application of ultrasound-assisted thermal processing for preservation and quality retention of liquid foods. J Food Prot. 2003;66:1642–1649. doi: 10.4315/0362-028X-66.9.1642. [DOI] [PubMed] [Google Scholar]
- Zhang YN, Zhang YN, Du XZ, Xian HZ. Enhancement of heat and mass transfer by cavitation. IOP Conf Ser Mater Sci Eng. 2015;72:12002. doi: 10.1088/1757-899X/72/1/012002. [DOI] [Google Scholar]