Abstract
The objective of the study was to develop optimum Headspace-Solid Phase Microextraction (HS-SPME) conditions for determining volatile compounds of margarine. The effects of fiber type, extraction temperature, extraction time on extraction rates of 2,3 butandione, dimethyl disulfide, nonanal, butanoic acid, hexanoic acid, octanoic acid, γ-nonalactone, δ-decalactone, total area and number of volatile compounds were investigated. A response surface methodology was applied using three independent variables such as fiber type, extraction temperature and extraction time. The fiber type, extraction temperature and extraction time were arranged with Box–Behnken design. The results shows that extraction temperature and extraction time affect both total area and number of volatile compounds but fiber type effect total area of volatile compounds. The optimum HS-SPME conditions were determined as 47.54 °C extraction temperature and 33.63 min extraction time using DVB/CAR/PDMS fiber type.
Keywords: SPME, Optimization, Response surface methodology, Margarine, Volatile compounds
Introduction
Margarine was invented by Me`ge Mourie`s in 1896 as an alternative to butter, also to fullfill the need for butter which has limited supply (Laia et al. 2000; Liu et al. 2010). In early years margarines were formulated from animal fats but today margarines are produced from vegetable oils. Table margarines, bakery margarines, specialized puff pastry margarines and low calorie spreads take place in the margarine product range (Laia et al. 2000). Margarine has an increasing production and consumption around the world so margarine has become a significant food item. Nowadays, margarines are produced by adding butter aroma to be a better substitute of butter, since aroma is one of the important criterions of consumer acceptability of margarine. To determine volatile compounds of foods various sample preparation methods have been used such as high vacuum steam distillation, simultaneous distillation–extraction, steam distillation under reduced pressure, purge and trap (Mondello et al. 2005). These methods are time consuming and laborious. Solid phase microextraction (SPME) was developed in 1989 by Pawliszyn as a rapid sample preparation technique (Pawliszyn 1997; Peña et al. 2008). SPME has been the most popular approach for volatile compounds analysis and it has a lot of advantages compared to traditional techniques. SPME has high sensitivity, low cost, solvent-free extraction, provide quantification of a large number of volatiles with low detection limits, extraction and concentration take place in one step and it needs small sample volume (Arthur et al. 1992; Lanças 2003; Zhu and Chai 2005; Pinho et al. 2006; Rodrigues et al. 2008; Charry-Parra et al. 2011). In SPME, volatile compounds are adsorbed from sample matrix onto a fused-silica fiber coated with polymeric phase by headspace (HS) extraction (Pellati et al. 2005). Type of fiber, extraction temperature, pH, stirring speed, sample volume, extraction time and ionic strength are the factors related to SPME. Among these factors type of fiber, extraction temperature and extraction time are important for volatile compound analysis. These parameters are dependent on compound properties and matrix characteristics (Roberts et al. 2000; Rega et al. 2003; Pawliszyn 2009). In most of the studies some of these parameters are evaluated separately. Evaluation of these parameters separately is time consuming and interactions between factors are not investigated. Response surface methodology (RSM) explores many explanatory variables and their interactions on the response variables and reduces number of experiments. RSM has been widely used in optimization of conditions and processes (Rodriguez-Bencomo et al. 2012). Moreover, RSM has been successfully applied for the optimization of SPME conditions. Well-balanced extraction rate and sensitivity can be obtained by optimized extraction conditions (Balasubramanian and Panigrahi 2011; Ma et al. 2013). In previous studies, the volatile compounds of margarine, butter and cream were investigated using SPME. On the other hand SPME conditions were not optimized in these studies and fiber type, extraction temperature, extraction time were varied (Adahchour et al. 1999; Povolo and Contarini 2003; Adahchour et al. 2005; Lozano et al. 2007; Chen et al. 2010; Kurtovic et al. 2011; Shiota et al. 2011). Therefore, the optimization of SPME conditions for volatile compounds of margarin would be useful.
The aim of this study was to optimize HS-SPME method for analysis of volatile compounds of margarine with butter aroma. The effect of HS-SPME conditions namely fiber type, extraction temperature and extraction time were evaluated by using Box–Behnken design.
Materials and methods
Samples
Margarine samples obtained from Sana, Unilever were used for optimization of the SPME method. Margarine with butter aroma was prepared by adding butter aroma mixture (Aromsa, Inc.) at 0.82% (w/w) level. Margarine samples were stored at 4 °C up to 1 week prior to analyses.
Solid phase microextraction
Extraction of volatile compounds was carried out using SPME fibers with manual SPME holder. The SPME fibers were purchased from Supelco (Bellefonte, PA, USA), a stableflex fiber core coated with 50/30 µm divinylbenzene/carboxen/polydimethylsiloxane (DVB/CAR/PDMS), 65 μm polydimethylsiloxane (PDMS/DVB) and 85 µm carboxene/polydimethylsiloxane (CAR/PDMS). All fibers were conditioned prior to analysis according to the instructions of the manufacturer by insertion into GC injector port.
Samples were prepared by weighing 10 g of margarine into 40 ml flat bottom vials and 1 μl 2,3-pentandione was added as internal standard. The vial was sealed with a Teflon coated silicone septum. Vials were placed on a block heater and fiber was exposed to the headspace of the margarine sample at a specified temperature (30–50 °C) for a specified time (20–40 min).
Gas chromatography–mass spectrometry (GC–MS) analysis
GC–MS analysis was performed using a Hewlett-Packard (HP) 6890 GC/HP 5973 MS (Agilent Technologies). The volatiles were separated on a fused silica capillary column DB-WAX (60 m × 0.25 mm, 0.50 μm film thickness, Agilent Technologies). Thermal desorption of the volatiles from the fiber was carried out in the GC injector at 250 °C. The inlet was operated in the splitless mode. The flow of the carrier gas Helium was 1.0 mL/min. The oven temperature program began with an initial temperature of 50 °C for 5 min, followed by an increase of 8 °C/min to 240 °C and held at 240 °C for 10 min. The electron impact mass spectra were recorded at 70 eV ionization voltage. The mass range acquisition was m/z 50–550. The volatile compounds were identified by comparing their mass spectra with the WILEY and NIST mass spectral libraries. The Kovats indexes were calculated for each volatile compound by using the retention times of a homologous series of C7–C30. To confirm the identity of volatile compounds Kovats indexes were compared with compounds analysed in similar conditions in the literature.
Experimental design for Headspace Solid Phase Microextraction
The independent variables such as fiber type (f), extraction temperature (T), extraction time (t), selected for the HS-SPME process were evaluated using response surface methodology. The optimization of the HS-SPME conditions for volatiles of margarine with butter aroma was implemented by using Box–Behnken design. A total of 17 treatments were performed for optimization based on 3 level design and 5 centre points to estimate the repeatability of the method. Also the experiments were done in randomized order. By using this design three factors were assessed at 3 different experimental levels: fiber type as DVB/CAR/PDMS, PDMS/DVB and CAR/PDMS; extraction temperature at 30, 40 and 50 °C; extraction time at 20, 30 and 40 min. The factor levels and experimental domains are given in Table 1.
Table 1.
Factors and their levels for Box–Behnken design
| Factor | Coded levels | ||
|---|---|---|---|
| − 1 | 0 | + 1 | |
| Fiber type, f | PDMS/DVB | DVB/CAR/PDMS | CAR/PDMS |
| Extraction temperature, T (°C) | 30 | 40 | 50 |
| Extraction time, t (min) | 20 | 30 | 40 |
The optimization of HS-SPME conditions was implemented using two responses namely: R1, total area of volatile compounds and R2, number of volatile compounds. The optimum HS-SPME conditions providing the maximum volatile compound area and maximum number of volatile compounds were estimated using the desirability function method, used for simultaneous optimization of the multiple responses. The experimental design and data were analyzed using the statistical software (Design Expert-version 10.0 Stat-Ease Inc., Minneapolis, MN, USA). Models for total volatile compound area and number of volatile compounds created for regression analysis were given below (Eq. 1).
| 1 |
In this model R is response value, β0 is a constant, βi linear, βii quadratic and βij interaction coefficients, x values are the independent variables.
Results and discussion
Experimental design for optimization of SPME parameters
Fiber type, extraction time and extraction temperature are the main factors which influenced the HS-SPME efficiency for volatile compound analysis. The effect of these independent variables and their interactions were evaluated. The volatile compounds of butter aroma mixture used in margarine and their sensory terms are listed in Table 2. A total of 8 volatile compounds were determined in margarine namely; 2,3 butandione, dimethyl disulfide, nonanal, butanoic acid, hexanoic acid, octanoic acid, γ-nonalactone, δ-decalactone.
Table 2.
Volatile compounds of margarine determined by HS-SPME-GS-MS
| Volatile compound | Kovats indexa | Sensory descriptionb | Library score (%) |
|---|---|---|---|
| 2,3 butandione | 989 | Buttery | 85 |
| Dimethyl disulfide | 1086 | Cauliflower, garlic | 87 |
| Nonanal | 1412 | Mushroom-like, floral, green, waxy, fatty | 91 |
| Butanoic acid | 1643 | Rancid cheesy, putrid, sweaty, fecal | 96 |
| Hexanoic acid | 1865 | Doughy, pungent, blue cheese, sour, sweaty | 88 |
| Octanoic acid | 2084 | Goaty, waxy, soapy, musty, rancid, fruity, sweaty, cheesy | 89 |
| γ-Nonalactone | 2114 | Peach, coconut-like | 87 |
| δ-Decalactone | 2201 | Waxy, sweet | 87 |
R1 (Total area of volatile compounds) and R2 (number of volatile compounds) response values obtained from Box–Behnken experimental design conditions of HS-SPME are shown in Table 3. Each volatile compound area divided by the area of the internal standard was summed up to calculate the total area.
Table 3.
Experimental conditions and response values of Box–Behnken design for butter volatile compounds of margarine extraction by HS-SPME
| Exp. no | Fiber type | Extraction temperature, T (°C) | Extraction time, t (min) | R1 (Total area of volatile compoundsa) | R2 (Number of volatile compounds) |
|---|---|---|---|---|---|
| 1 | CAR/PDMS | 30 | 30 | 1.06655 ± 0.2097 | 6 |
| 2 | DVB/CAR/PDMS | 50 | 20 | 2.94353 ± 0.4163 | 7 |
| 3 | PDMS/DVB | 50 | 30 | 3.54877 ± 0.1159 | 7 |
| 4 | PDMS/DVB | 30 | 30 | 2.33744 ± 0.0908 | 5 |
| 5 | CAR/PDMS | 50 | 30 | 2.88108 ± 0.4386 | 7 |
| 6 | PDMS/DVB | 40 | 40 | 3.02459 ± 0.1981 | 7 |
| 7 | DVB/CAR/PDMS | 40 | 30 | 2.80404 ± 0.3222 | 7 |
| 8 | DVB/CAR/PDMS | 40 | 30 | 3.26585 ± 0.2949 | 8 |
| 9 | DVB/CAR/PDMS | 50 | 40 | 3.65450 ± 0.2989 | 8 |
| 10 | PDMS/DVB | 40 | 20 | 2.24293 ± 0.2863 | 7 |
| 11 | CAR/PDMS | 40 | 20 | 1.19712 ± 0.1467 | 7 |
| 12 | DVB/CAR/PDMS | 40 | 30 | 3.28538 ± 0.2266 | 8 |
| 13 | DVB/CAR/PDMS | 30 | 40 | 1.87774 ± 0.0688 | 6 |
| 14 | DVB/CAR/PDMS | 40 | 30 | 3.07491 ± 0.1098 | 8 |
| 15 | CAR/PDMS | 40 | 40 | 1.79668 ± 0.2342 | 7 |
| 16 | DVB/CAR/PDMS | 40 | 30 | 3.17000 ± 0.1905 | 8 |
| 17 | DVB/CAR/PDMS | 30 | 20 | 1.32387 ± 0.4358 | 3 |
aSum of the ratio of peak areas to internal standard
The effects of fiber type, extraction temperature and extraction time on optimization responses were determined by ANOVA (Analysis of Variance). The ANOVA results and regression coefficients of the models were determined by using the Design Expert software (Table 4). The statistical significances of all factors, their interaction, and square effect are shown in Table 4. The calculated models of each response were statistically significant (p < 0.05) while the lack of fit of models were insignificant (p > 0.05). Quadratic models with R2 > 0.90 were obtained for volatile compounds, total area and number of volatile compounds. On the other hand 2,3 butandione and dimethyl disulfide were better explained with linear model.
Table 4.
ANOVA results for each response variables of optimization process (x1:f; x2:T; x3:t)
| 2,3 Butandione | Dimethyl disulfide | Nonanal | Butanoic acid | Hexanoic acid | Octanoic acid | γ-Nonalactone | δ-Decalactone | R1 (Total area of volatile compounds) | R2 (Number of volatile compounds) | |
|---|---|---|---|---|---|---|---|---|---|---|
| p value | p value | p value | p value | p value | p value | p value | p value | p value | p value | |
| Model | < 0.0001 | 0.0057 | 0.0004 | 0.0037 | 0.0011 | 0.0067 | 0.0266 | 0.0119 | < 0.0001 | 0.0059 |
| x1 | < 0.0001 | 0.0039 | 0.0002 | 0.0005 | 0.0011 | 0.7171 | 0.2795 | 0.3862 | < 0.0001 | 0.3757 |
| x2 | 0.0092 | 0.0229 | 0.0021 | 0.1501 | 0.0001 | 0.0001 | 0.0008 | 0.0004 | < 0.0001 | 0.0007 |
| x3 | 0.7009 | 0.6876 | 0.0003 | 0.1691 | 0.0781 | 0.0161 | 0.0257 | 0.0156 | 0.0005 | 0.0218 |
| x1x2 | 0.0034 | 0.9920 | 0.8123 | 0.6760 | 0.5729 | 0.6396 | 0.0982 | 0.1026 | ||
| x1x3 | 0.0008 | 0.9337 | 0.4780 | 0.7409 | 0.4069 | 0.2044 | 0.7186 | 0.0562 | ||
| x2 x3 | 0.5625 | 0.1010 | 0.1089 | 0.1974 | 0.3463 | 0.0444 | 0.5380 | 0.0676 | ||
| x22 | 0.0018 | 0.0140 | 0.0059 | 0.7032 | 0.5668 | 0.0989 | 0.0405 | 0.0017 | ||
| x23 | 0.023 | 0.0015 | 0.0008 | 0.1454 | 0.3210 | 0.9787 | 0.0002 | 0.0542 | ||
| Lack of fit | 0.0833 | 0.5884 | 0.7049 | 0.0982 | 0.1189 | 0.0537 | 0.0615 | 0.0518 | 0.3757 | 0.4734 |
| R2 | 0.8531 | 0.8776 | 0.9882 | 0.9712 | 0.9824 | 0.9632 | 0.9335 | 0.9531 | 0.9938 | 0.9651 |
Effect of fiber type
Three different fibers were used, namely; DVB/CAR/PDMS, CAR/PDMS, PDMS/DVB. The fiber type significantly affects the total volatile compound area while it does not impinge on the number of volatile compounds. Also fiber type had a significant effect on each volatile compound except octanoic acid, γ-nonalactone and δ-decalactone. DVB/CAR/PDMS, CAR/PDMS, PDMS/DVB fibers were suitable for volatile compounds in the molecular range of 40–275, 3–225 and 50–300, respectively (Anonymus 2017). So, CAR/PDMS was used for low molecular weight volatile compounds like 2,3 butandione. Moreover, polarity of the fiber was an important criterion for extraction. The compounds in the non-polar structure were better extracted with the non-polar type fiber and the polar compounds with polar fiber coating. The fibers DVB/CAR/PDMS, CAR/PDMS, PDMS/DVB are bipolar (Kataoka et al. 2000; Pawliszyn 2009). The low molecular weight compounds were better extracted with CAR/PDMS fiber compared to DVB/CAR/PDMS fiber. Similarly Salum et al. (2017) used CAR/PDMS fiber efficiently to extract low molecular weight volatile compounds compared to DVB/CAR/PDMS. On the other hand DVB/CAR/PDMS fiber is bipolar and extracts both polar and non-polar analytes, hence highest area and number of volatile compounds was obtained with DVB/CAR/PDMS in the experimental design (Exp. no 9).
Effect of extraction temperature and time
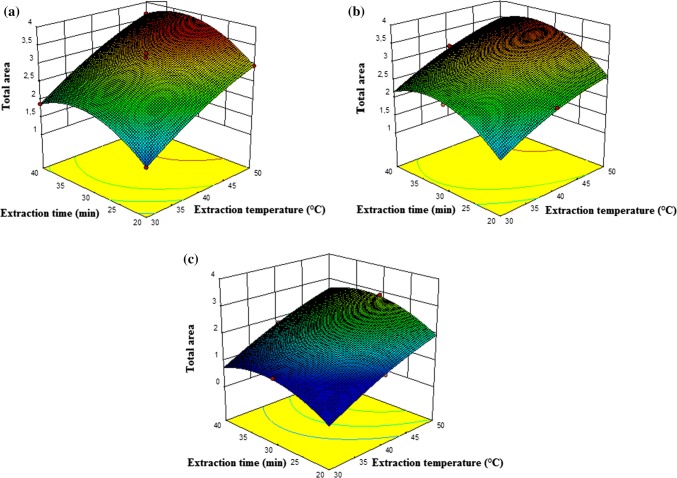
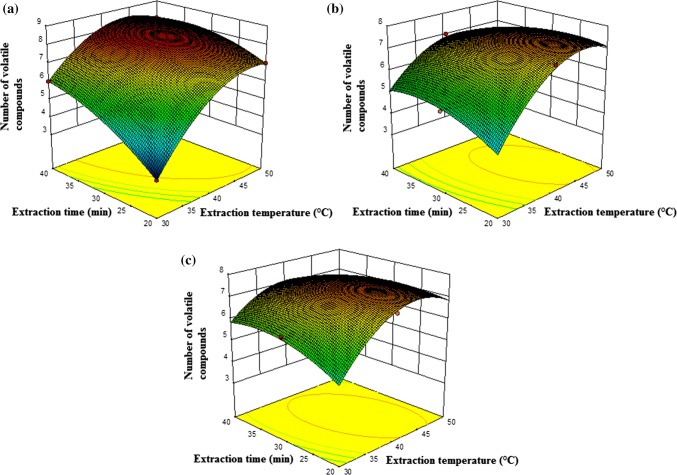
In SPME technique the extraction temperature and extraction time are two important factors effecting the vapour pressure and equilibrium of the volatile compounds in the headspace of the vial (Ho et al. 2006). Optimum extraction temperature highly depends on sample properties. Extraction temperature determined to have a statistically important positive effect on total area and number of volatile compounds. The counter plots of the predicted model for total area of volatile compounds are illustrated in Fig. 1 and the number of volatile compounds is illustrated in Fig. 2.
Fig. 1.
Calculated effect of extraction time (min) and extraction temperature (°C) on total area of volatile compounds using DVB/CAR/PDMS (a), PDMS/DVB (b), CAR/PDMS (c)
Fig. 2.
Calculated effect of extraction time (min) and extraction temperature (°C) on number of volatile compounds using DVB/CAR/PDMS (a), PDMS/DVB (b), CAR/PDMS (c)
Extraction temperature affects all volatile compounds except butanoic acid (p < 0.05). Food matrices with a high level of fat like margarine can trap volatile compounds, so using high temperature provides a better distribution in the headspace. Conversely, the use of high temperatures can cause formation of thermal degradation products and artefacts in the volatile profile (Ruiz et al. 1998; Wagner and Franco 2012).
The interaction effects of variables were not significantly important for all responses except for nonanal and δ-decalactone (p > 0.05). The interaction between fiber type and extraction temperature, fiber type and extraction time affected nonanal. Moreover interaction between extraction time and extraction temperature influenced δ-decalactone (p < 0.05).
The extraction time was found to have a statistically important positive effect on total area and number of volatile compounds. The increase in extraction time improves the efficiency of extraction of compounds having high boiling point and increases the detected peak area. However, the increase in extraction time does not show the same effect on compounds with low boiling point (Ho et al. 2006). Similarly, in this study the increase in extraction time increased the extraction of high boiling point compounds such as nonanal, octanoic acid, δ-decalactone, γ-nonalactone.
Optimization
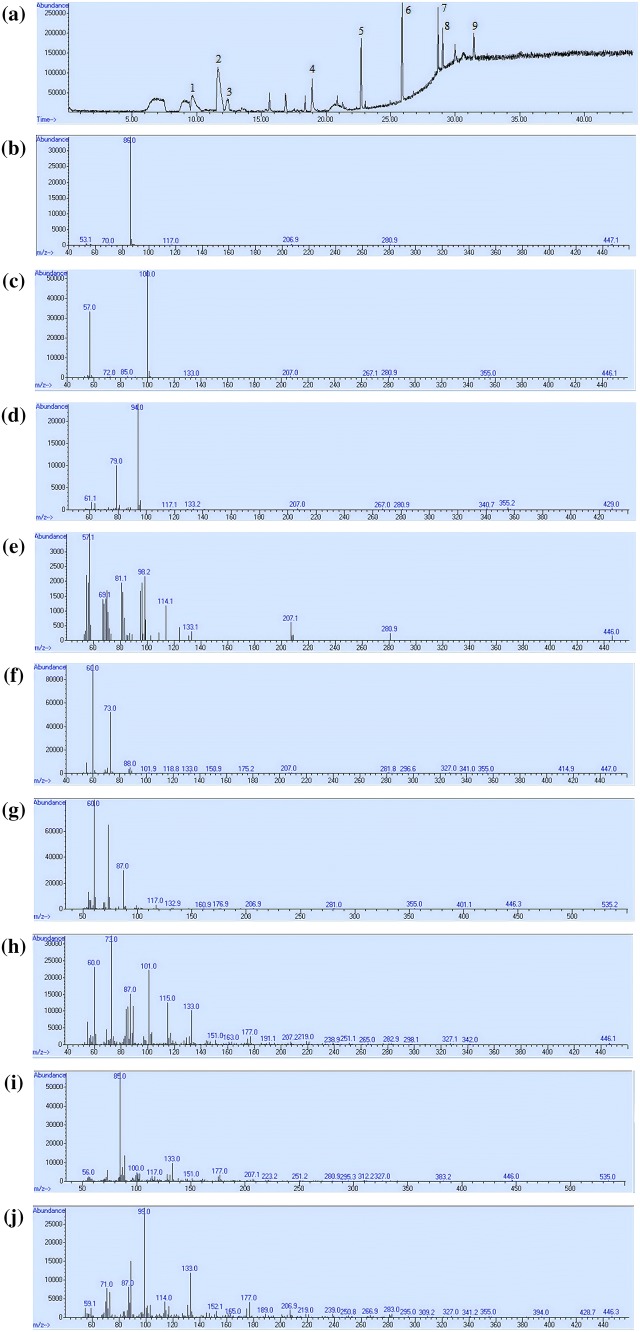
The optimum HS-SPME conditions namely fiber type, extraction temperature and extraction time were determined to attain maximum total area and number of volatile compounds. Optimization was implemented using DVB/CAR/PDMS, PDMS/DVB, CAR/PDMS fibers at ranges of 30–50 °C extraction temperature and 20–40 min extraction time. Numerical optimization was carried out for these independent variables to obtain the optimum HS-SPME conditions for margarine with butter aroma. Design Expert—version 10.0 software (Stat-Ease Inc., MN, USA) was used to perform simultaneous optimization of the multiple responses. The desirability function approach was applied to obtain highest area and number of volatile compounds. The optimum SPME conditions were determined as DVB/CAR/PDMS fiber type, 47.54 °C extraction temperature and 33.63 min extraction time. Five verification experiments were performed at optimum extraction conditions. The verification experiment results are listed in Table 5 and experimental total area of volatile compounds, number of volatile compounds were not significantly different from the predicted values (p > 0.05). Total ion chromatogram and mass spectra of volatiles which were extracted using optimum SPME conditions are shown in Fig. 3.
Table 5.
Results of statistical analysis for verification of optimization
| Reponses | Predicted value | Experimental valuea | SEb | Difference | % errorc | p value |
|---|---|---|---|---|---|---|
| Total area of volatile compounds | 3.764 | 3.6310 ± 0.3550 | 0.1587 | 0.1333 | − 3.66 | 0.4480 |
| Number of volatile compounds | 8.363 | 7.800 ± 0.4472 | 0.200 | − 0.563 | − 7.21 | 0.1100 |
aExperimental values were expressed as mean ± standard deviation
bMean standard error
cThe % error = (|yexp − ypre|/yexp) × 100
Fig. 3.
a Total ion chromatogram of margarine volatiles (1: 2,3 butandione, 2: 2,3 pentandione (internal standard), 3: dimethyl disulfide, 4: nonanal, 5: butanoic acid, 6: hexanoic acid, 7: octanoic acid, 8: γ-nonalactone, 9: δ-decalactone). Mass spectra of 2,3 butandione (b), 2,3 pentandione (c), dimethyl disulfide (d), nonanal (e), butanoic acid (f), hexanoic acid (g), octanoic acid (h), γ-nonalactone (i), δ-decalactone (j)
DVB/CAR/PDMS fiber was yielding optimum extraction as a result of optimization conditions. Similarly, Povolo and Contarini (2003) as well as Shiota et al. (2011) used the same fiber type for volatile compound analysis in butter so as in the analysis of the aroma mix in margarine respectively.
In the literature, volatile compound analysis of butter and milk cream using HS-SPME has been investigated but the optimization study was not applied. In this study, the optimum extraction temperature was determined as 47.54 °C, very close to 45 °C which was the extraction temperature used for volatile compound extraction of butter and milk cream by SPME technique (Povolo and Contarini 2003; Kurtovic et al. 2011). On the other hand this extraction temperature was just a little higher than the 40 °C extraction temperature used by Adahchour et al. (1999, 2005) and Lozano et al. (2007) for volatile compounds of butter. In SPME technique, the extraction temperature and duration are closely related to each other. In volatile compounds analyses performed with SPME using butter, cream and margarine samples, the duration of the extraction varies between 20 and 60 min depending on other extraction conditions (Povolo and Contarini 2003; Adahchour et al. 2005; Chen et al. 2010; Shiota et al. 2011). Similarly, the optimum extraction time was determined to be 33.63 min in our study.
Conclusion
The effect of SPME parameters in terms of fiber type, extraction temperature and extraction time were optimized to obtain the maximum total area and number of volatile compounds of margarine. Also the effects on of fiber type, extraction temperature and extraction time on each volatile compound of margarine was investigated. Extraction of volatile compounds of margarine by SPME showed that both total area and number of volatile compounds were influenced by extraction temperature and extraction time. Interestingly, fiber type only affects the total area of volatile compounds in margarine. As a result, it was found that the optimum extraction condition of margarine was maintained at 47.54 °C for 33.63 min using DVB/CAR/PDMS fiber. These results can be used as a reference for forthcoming studies to investigate butter or margarine volatile compounds by HS-SPME. This study will also be useful in the selection of HS-SPME parameters including fiber type, extraction temperature and extraction time for volatile compounds of margarine.
Acknowledgements
This study was supported by Ege University Scientific Research Projects Coordination Unit (Project No: 16/MUH/028). The authors would also like to thank Aromsa, Inc. for supplying the aroma used in this research.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Acree T, Arn H (2016) Flavornet and human odor space. http://www.flavornet.org/flavornet.html. Accessed 15 June 2017
- Adahchour M, Vreuls RJJ, van der Heijden A, Brinkman UAT. Trace-level determination of polar flavour compounds in butter by solid-phase extraction and gas chromatography–mass spectrometry. J Chromatogr A. 1999;844:295–305. doi: 10.1016/S0021-9673(99)00351-9. [DOI] [PubMed] [Google Scholar]
- Adahchour M, Wiewel J, Verdel R, Vreuls RJJ, Brinkman UAT. Improved determination of flavour compounds in butter by solid-phase (micro) extraction and comprehensive two-dimensional gas chromatography. J Chromatogr A. 2005;1086:99–106. doi: 10.1016/j.chroma.2005.05.094. [DOI] [PubMed] [Google Scholar]
- Anonymus (2017) Selection Guide for Supelco SPME Fibers. http://www.sigmaaldrich.com/technical-documents/articles/analytical/selecting-spme-fibers.html. Accessed 19 June 2017
- Arthur L, Killam L, Buchholz K, Pawliszyn J. Automation and optimization of solid-phase microextraction. Anal Chem. 1992;64:1960–1966. doi: 10.1021/ac00041a034. [DOI] [Google Scholar]
- Balasubramanian S, Panigrahi S. Solid-phase microextraction (SPME) techniques for quality characterization of food products: a review. Food Bioprocess Technol. 2011;4:1–26. doi: 10.1007/s11947-009-0299-3. [DOI] [Google Scholar]
- Charry-Parra G, DeJesus-Echevarria M, Perez FJ. Beer volatile analysis: optimization of HS/SPME coupled to GC/MS/FID. J Food Sci. 2011;76:C205–C211. doi: 10.1111/j.1750-3841.2010.01979.x. [DOI] [PubMed] [Google Scholar]
- Chen Y, Shirey RE, Sidisky LM. Determination of diacetyl in butter and air samples by SPME coupled with GC–MS. Chromatographia. 2010;72:999–1004. doi: 10.1365/s10337-010-1739-y0009-5893/10/11. [DOI] [Google Scholar]
- Ho CW, Wan Aida WM, Maskat MY, Osman H. Optimization of headspace solid phase microextraction (HS-SPME) for gas chromatography mass spectrometry (GC–MS) analysis of aroma compound in palm sugar (Arenga pinnata) J Food Compost Anal. 2006;19:822–830. doi: 10.1016/j.jfca.2006.05.003. [DOI] [Google Scholar]
- Kataoka H, Lord HL, Pawliszyn J. Applications of solid-phase microextraction in food analysis. J Chromatogr A. 2000;880:35–62. doi: 10.1016/S0021-9673(00)00309-5. [DOI] [PubMed] [Google Scholar]
- Kurtovic I, Marshall SN, Miller MR, Zhao X. Flavour development in dairy cream using fish digestive lipases from Chinook salmon (Oncorhynchus tshawytscha) and New Zealand hoki (Macruronus novaezealandiae) Food Chem. 2011;127:1562–1568. doi: 10.1016/j.foodchem.2011.02.018. [DOI] [Google Scholar]
- Laia OM, Ghazalia HM, Cho F, Chong CL. Physical and textural properties of an experimental table margarine prepared from lipase-catalysed transesterified palm stearin: palmkernel olein mixture during storage. Food Chem. 2000;71:173–179. doi: 10.1016/S0308-8146(00)00084-4. [DOI] [Google Scholar]
- Lanças F. The role of the separation sciences in the 21th century. J Braz Chem Soc. 2003;14:183–197. doi: 10.1590/S0103. [DOI] [Google Scholar]
- Lecanu L, Ducruet V, Jouquand C, Gratadoux JJ, Feigenbaum A. Optimization of headspace solid-phase microextraction (spme) for the odor analysis of surface-ripened cheese. J Agric Food Chem. 2002;50:3810–3817. doi: 10.1021/jf0117107. [DOI] [PubMed] [Google Scholar]
- Liu Y, Meng Z, Zhang F, Shan L, Wang X. Influence of lipid composition, crystallization behavior and microstructure on hardness of palm oil-based margarines. Eur Food Res Technol. 2010;230:759–767. doi: 10.1007/s00217-010-1217-7. [DOI] [Google Scholar]
- Lozano PR, Miracle ER, Krause AJ, Drake M, Cadwallader KR. Effect of cold storage and packaging material on the major aroma components of sweet cream butter. J Agric Food Chem. 2007;55:7840–7846. doi: 10.1021/jf071075q. [DOI] [PubMed] [Google Scholar]
- Ma QL, Hamid N, Bekhit AED, Robertson J, Law TF. Optimization of headspace solid phase microextraction (HS-SPME) for gas chromatography mass spectrometry (GC–MS) analysis of aroma compounds in cooked beef using response surface methodology. Microchem J. 2013;111:16–24. doi: 10.1016/j.microc.2012.10.007. [DOI] [Google Scholar]
- Mallia S, Escher F, Schlichtherle-Cerny H. Aroma-active compounds of butter: a review. Eur Food Res Technol. 2008;2263:315–325. doi: 10.1007/s00217-006-0555-y. [DOI] [Google Scholar]
- Mondello L, Costa R, Tranchida PQ, Chiofalo B, Zumbo A, Dugo P, Dugo G. Determination of flavor components in Sicilian goat cheese by automated HS-SPME-GC. Flavour Fragr J. 2005;20:659–665. doi: 10.1002/ffj.1529. [DOI] [Google Scholar]
- Pawliszyn JB. Solid-phase microextraction. Theory and practice. New York: Wiley-VCH; 1997. [Google Scholar]
- Pawliszyn J. Handbook of solid phase microextraction. Ontario: Chemical Industry Press of China; 2009. [Google Scholar]
- Pellati F, Benvenuti S, Yoshizaki F, Bertelli D, Rossi MC. Headspace solid-phase microextraction-gas chromatography–mass spectrometry analysis of the volatile compounds of Evodia species fruits. J Chromatogr A. 2005;1087:265–273. doi: 10.1016/j.chroma.2005.01.060. [DOI] [PubMed] [Google Scholar]
- Peña RM, Barciela J, Herrero C, García-Martín S. Headspace Solid-Phase microextraction gas chromatography–mass spectrometry analysis of volatiles in orujo spirits from a defined geographical origin. J Agric Food Chem. 2008;56:2788–2794. doi: 10.1021/jf073481f. [DOI] [PubMed] [Google Scholar]
- Peterson DG, Reineccius GA. Determination of the aroma impact compounds in heated sweet cream butter. Flavour Fragr J. 2003;18:320–324. doi: 10.1002/ffj.1228. [DOI] [Google Scholar]
- Peterson DG, Reineccius GA. Characterization of the volatile compounds that constitute fresh sweet cream butter aroma. Flavour Fragr J. 2003;18:215–220. doi: 10.1002/ffj.1192. [DOI] [Google Scholar]
- Pinho O, Ferreira I, Santos L. Method optimization by solid-phase micro-extraction in combination with gas chromatography with mass spectrometry for analysis of beer volatile fraction. J Chromatogr A. 2006;1121:145–153. doi: 10.1016/j.chroma.2006.04.013. [DOI] [PubMed] [Google Scholar]
- Povolo M, Contarini G. Comparison of solid-phase microextraction and purge-and trap methods for the analysis of the volatile fraction of butter. J Chromatogr A. 2003;985:117–125. doi: 10.1016/S0021-9673(02)01395-X. [DOI] [PubMed] [Google Scholar]
- Rega B, Fournier N, Guichard E. Solid Phase Microextraction (SPME) of orange juice flavor: odor representativeness by direct gas chromatography olfactometry (D-GC-O) J Agric Food Chem. 2003;51:7092–7099. doi: 10.1021/jf034384z. [DOI] [PubMed] [Google Scholar]
- Roberts DD, Pollien P, Milo C. Solid-phase microextraction method development for headspace analysis of volatile flavor compounds. J Agric Food Chem. 2000;48:2430–2437. doi: 10.1021/jf991116l. [DOI] [PubMed] [Google Scholar]
- Rodrigues F, Caldeira M, Camara JS. Development of a dynamic headspace solid-phase microextraction procedure coupled to GC–qMSD for evaluation the chemical profile in alcoholic beverages. Anal Chim Acta. 2008;609:82–104. doi: 10.1016/j.aca.2007.12.041. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Bencomo JJ, Muñoz-González C, Martín-Álvarez PJ, Lázaro E, Mancebo R, Castañé X, Pozo-Bayón MA. Optimization of a HS-SPME-GC-MS procedure for beer volatile profiling using response surface methodology: application to follow aroma stability of beers under different storage conditions. Food Anal Methods. 2012;5:1386–1397. doi: 10.1007/s12161-012-9390-x. [DOI] [Google Scholar]
- Ruiz J, Cava R, Ventanas J, Jensen MT. Headspace solid phase microextraction for the analysis of volatiles in a meat Product: dry-cured Iberian ham. J Agric Food Chem. 1998;46:4688–4694. doi: 10.1021/jf980139h. [DOI] [Google Scholar]
- Salum P, Erbay Z, Kelebek H, Selli S. Optimization of Headspace Solid-Phase Microextraction with different fibers for the analysis of volatile compounds of white-brined cheese by using Response Surface Methodology. Food Anal Methods. 2017;10:1956–1964. doi: 10.1007/s12161-016-0774-1. [DOI] [Google Scholar]
- Shiota M, Isogai T, Iwasawa A, Kotera M. Model studies on volatile release from different semisolid fat blends correlated with changes in sensory perception. J Agric Food Chem. 2011;59:4904–4912. doi: 10.1021/jf104649y. [DOI] [PubMed] [Google Scholar]
- Wagner R, Franco MRB. Effect of the variables time and temperature on volatile compounds extraction of salami by solid phase microextraction. Food Anal Methods. 2012;5:1186–1195. doi: 10.1007/s12161-012-9362-1. [DOI] [Google Scholar]
- Zellner BD, Dugo P, Dugo G, Mondello L. Gas chromatography–olfactometry in food flavour analysis. J Chromatogr A. 2008;1186:123–143. doi: 10.1016/j.chroma.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Zhu JY, Chai XS. Some recent developments in headspace gas chromatography. Curr Anal Chem. 2005;1:79–83. doi: 10.2174/1573411052948488. [DOI] [Google Scholar]